Abstract
Enzymes are the dominating class of biocatalysts, which are extensively employed in food industries. Immobilization of enzymes on inactive and not soluble supports practically increases their efficiency owning to their high stability and multiple reuses, while it can negatively impact enzyme activity. The characteristics of immobilized enzymes are based on the procedure of immobilization and achieved beneficial properties, such as biocompatibility, chemical and thermal stability, the impossibility to dissolve (leak) in the reaction liquids, reconstitution, recyclability, and cost efficiency. Various immobilized enzymatic systems, like proteases, amino acylase, glucose isomerase, β-galactosidase, aspartase, lipases, or glucosidase have been shown to be techno-economically used in food industries on a multi-ton’s scale per year. This chapter provides a general survey of the benefits and applications of the immobilization enzymes with a major focus on the food industries.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Immobilization is a process used for transformation of biocatalysts (cells and enzymes) or bioactive components from a soluble phase to an insoluble state by using an insoluble carrier or by encapsulating them within a matrix material (Van de Velde et al., 2002; Chen et al., 2016). As a consequence, the properties of the native catalysts may change significantly, and in order to be able to explain the observed reaction behavior the laws of heterogeneous kinetics have to be applied. The immobilization of enzymes has become a major process in manufacture, medication, and biotechnology over the past decades. Scientists have developed many processes based on methods from physical adsorption and covalent binding to encapsulation or entrapment into polymers (Ispas et al., 2009; Alkan et al., 2009; Koszelewski et al., 2010; Klein et al., 2011; Defaei et al., 2018). Already in 2010, the market volume of immobilized enzymes amounted to almost 6 × 109 US-$ with a currently clear upwards trend, with food/beverage production and pharmaceutical applications constituting the major fields of application, each of both occupying about 21% of the entire market for immobilized enzymes (DiCosimo et al., 2013).
In contrast to free, dissolved enzymes, immobilized enzymes are of higher stability and more resilient against inhibition by heavy metals and other chemicals, non-optimal conditions of pH-value, temperature, or salinity in the environment, and turbulent flow regimes. Importantly, the high variety of reported immobilized enzyme systems provides a range of beneficial aspects, such as convenient recovery of enzymes and products, which reduces the number of process steps, recyclability of enzymes, continuous operation mode of enzymatic reactions, high overall productivity, possibility of sudden termination of reaction on demand, contribution to sophisticated development of enhanced bioreactor designs (“immobilized enzyme reactors”), and decreased environmental pollution and economic costs (Sheldon, 2007a; Defaei et al., 2018). Drawbacks of using immobilized enzymes encompass lower activity and reaction rates compared to free enzymes, susceptibility to putrefaction (“fouling”), additional cost for supports (carriers), or the need for disposing spent (exhausted) immobilized enzymes (Basso & Serban, 2019).
To date, various types of matrices and supports have been utilized to immobilize enzymes. Polysaccharides, glass beads, nano-metals, petro-plastics, and biopolymers are the most frequently used matrices. To immobilize enzymes, the binding strength between support and enzyme depends on the type of chemical or physical reaction, which heavily depends on the nature of the support surface, and the interaction between individual enzyme molecules (Kahraman et al., 2007; Asgher et al., 2014; Rana et al., 2014; Reshmi et al., 2006; Dey et al., 2002; Defaei et al., 2018). Especially the immobilization of enzymes on nanosized supports, such as nanofibers, nanobeads, carbon nanotubes (CNTs), and other nanoparticles for different reactions driven by biocatalysts, is nowadays emerging as an innovative research area (Sheldon, 2007a; Klein et al., 2011; Reshmi et al., 2006; Defaei et al., 2018).
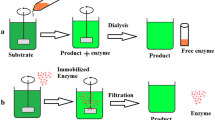
Also enzymes of importance for food technology have been immobilized on various matrices. Some examples are given in Fig. 15.1. The immobilized food enzyme that was the first to be industrially utilized already in the 1970s was amino acylase (EC 3.5.1.14), a hydrolase which was implemented for the production of racemic mixtures of d- and l-amino acids from N-acyl-amino acids as substrates. Industrially, this reaction took and still takes place in columns carrying the immobilized enzyme, while the substrate solution is washed thoroughly. Two other effectually immobilized enzymes were the glycosidase invertase (EC 3.2.1.26), an approved food additive (E 1103) which is used in fructose-rich corn syrup production, and lipases (EC 3.1.1.x), a versatile group of amphiphilic that are applied in the hydrolysis and transesterification of oily products, which, beyond food industry, is also a novel route toward biodiesel production from waste lipids. The advantages of immobilized enzymes in food industries are increased productivity, reduction of product recovery cost, and ultimately increased yields for diverse food products in the future (DiCosimo et al., 2013; Homaei et al., 2013).
15.2 Methods of Enzyme Immobilization
A number of methods can be used to immobilize a given enzyme; these methods encompass simple reversible physical adsorption, formation of ionic bonds between enzymes and supports, and generation of firm covalent linkages (Fig. 15.2). Such immobilization methods are categorized into reversible (adsorption, ionic binding, disulfide formation, affinity binding) and irreversible (covalent binding, cross-linking, physical entrapment [matrix entrapment and encapsulation]) methods. Apart from this classification, immobilization methods can also be classified according to the type of chemical reaction involved in binding as support binding and entrapment methods (Homaei et al., 2013).
15.2.1 Irreversible Methods
In irreversible enzyme immobilization, the biocatalyst that binds to the matrix cannot be detached without causing an effect on the biological enzyme activity or the structural properties of the support. The most usual methods of irreversible enzyme immobilization encompass covalent binding, cross-linking, and entrapment or encapsulation.
15.2.1.1 Immobilization via Formation of Covalent Binding
This method is widely utilized for enzyme immobilization. Based on this procedure, covalent bonds are created between the chemical groups of the enzyme molecule and the chemical groups present on the support. This method leads to formation of a stable bond between enzyme and support, resulting in the prevention of detachment of enzyme from the support when in use. This covalent bond is typically formed between the side-chain amino acids of biocatalysts, such as aspartic acid, arginine, histidine, and the functional groups, like imidazole, indolyl, phenolic, and hydroxyl present on the support. This method is used when the complete absence of enzyme in the product is desirable. However, it leads to chemical modification of enzymes and, as a result, the activity of the enzyme can get reduced (Homaei et al., 2013). As an option to increase activity of enzymes immobilized via covalent linking, linear spacers are often used to combine enzyme and support, giving the enzyme enough space for mobility and reaction (De Maio et al., 2003). An important example in this context is the carboxyl- and amine-reactive linker 1-ethyl-3-3- (3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), which can link hydrophobic polymeric supports with enzymes (Mohan et al., 2015).
15.2.1.2 Cross-Linking
Crosslinking resorts to covalent linking between the enzyme and active molecules, and is used to generate biocatalytically active polymeric particles, which can conveniently be used for given reactions. This mechanism is also known as “copolymerization”. Enzymes bind to each other with the aid of bi-functional reagents, such as glutaraldehyde, glutardialdehyde, glyoxal, diisocyanates, hexamethylene diisocyanate, and toluene diisocyanate, which build bridges between individual enzyme molecules. The major drawback of this procedure is that the multifunctional reagents used for cross-linking the enzyme may reconstruct or change the structure of the enzyme, which again causes the loss of catalytic activity (Albayrak & Yang, 2002).
15.2.1.3 Physical Entrapment
This method involves the physical trapping of biocatalysts into a film, gel, fiber, coating, or microencapsulation (Costa et al., 2005). In this procedure, the enzyme or reactive molecule can be mixed with a polymer to attach to it, resulting in creation of a lattice structure that encapsulates/entraps the enzyme. The advantages of this immobilization method include generation of a vast biocatalytically active surface area generated by the substrate and the enzyme, in only a low volume. The most important disadvantages of this method are the probable inactivation of the enzyme during microencapsulation, enzyme leakage into the environment, and the need for typically high concentrations of the enzyme. The most frequently used supports that are used for encapsulation of enzymes are polymers, such as cellulose, collagen, polyacrylamide gel, gelatin, alginate, starch, silicone, and rubber (Nisha et al., 2012).
15.2.2 Reversible Methods
15.2.2.1 Adsorption
The adsorption of enzymes on supports, such as activated charcoal, alumina, and ion exchange resins is among the simplest techniques used to limit enzyme mobility (Brady & Jordaan, 2009). Depending on the nature of amino acids present on the surface of enzymes and the chemical nature of the support, the enzyme is fixed by non-covalent binding trough ionic and hydrophobic interactions, or by formation of hydrogen bonds. This method can be achieved by mixing an aqueous enzyme solution with a matrix for a defined time, followed by a washing step to remove the remaining free enzyme from the immobilization matrix. This method of immobilization is simple and has little effect on enzyme activity and can be repeatedly applied by adding fresh enzyme solution (“recharging” of the support with biocatalyst). However, in this method, shortcomings, like fast enzyme desorption from the support, or loss of activity by changing pH-value, temperature, solvent, and ionic strength of the surrounding environment, can hardly be avoided (Costa et al., 2005).
15.2.2.2 Ionic Bond Formation
Immobilization by generation of ionic bonds is primarily based on the formation of ionic bonds between the enzyme molecules or reactive molecules and a solid matrix that has a charged ionic surface. The main advantage of ionic binding in comparison to physical binding is the strength of generated bonds that is stronger in case of ionic bonds than the power responsible for formation of covalent bonds (Torres et al., 2002). Such non-covalent immobilization assays can be reversed/deactivated by alterations in the temperature, solvent polarity, and ionic strength conditions (Nisha et al., 2012).
15.2.2.3 Immobilization via Disulfide Bridges
This method involves the configuration of disulfide (-S-S-) bridges between the enzyme and the support. The major benefit of this method is the ability to recover the bonds created between the activated solid surface and the thiol groups on the enzyme, because the leakage of bounded protein leads to the release of high amounts of low-molecular-weight thiol from disulfide bridges. The feasibility of reusing the polymeric matrix after enzyme deactivation may facilitate the actual large-scale immobilization of enzymes in industrial procedures, where their use is currently not economical because of the high cost of support materials (Ovsejevi et al., 2013).
15.2.2.4 Affinity Binding
Affinity immobilization combines the properties of the enzyme to maintain under varied physiological conditions. This can be done via two routes of support pre-junction by an affinity ligand for desired enzyme, or enzyme attachment to the organism that has an affinity toward the support. The benefits of this procedures are that the enzyme is not exposed to any unusual chemical conditions, minimal conformational changes occur during immobilization, and the high activity of immobilized enzyme is maintained (Sardar et al., 2000).
15.3 Classification of Different Support Materials for Immobilization of Food Enzymes
The reaction occurring between the enzyme and a carrier generates an immobilized enzyme with particular structural, biochemical, physical, and kinetic characteristics. Carriers can be divided into different groups based on their appearance or their chemical components. The support can be a synthetic or biological organic polymer, or an inorganic solid. The support must display certain features, like extended surface-to-volume ratio, high permeability (mass transference), acceptable functional groups for enzyme binding under non-denaturing conditions, hydrophilic moieties, insolubility in water, chemical and thermal stability, mechanical strength, high recalcitrance, applicable particle shape, resistance to microbial attack, regenerability, biological safety, and low or acceptable price (Sheldon, 2007b; Garcia-Galan et al., 2011). Furthermore, multi-enzyme biocatalysis on only one support, especially processes resorting to multi-enzyme cascades, is an emerging approach to generate high-value chemicals on an industrial scale (Xu et al., 2020).
15.3.1 Classification of Different Support Materials Based on the Chemical Structure
15.3.1.1 Inorganic Solid Supports
Different inorganic solids, such as zeolites, alumina, silica, and mesoporous silicas, are possible to use for enzyme immobilization. Silica-based supports are the best applicable matrices for immobilization of enzymes in the industrial manufacturing of enzymatically produced products, in addition to research purposes (Vianello et al., 2006; Pierre, 2004; Hudson et al., 2008). In general, the high surface area provided via silica gel matrixes is nonpareil. Moreover, silica gel can be facilely treated to obtain the desired shape, pore size, and microchannels to allow reaction between substrates and ligands. Furthermore, silica gel is mechanically stable and chemically inactive; it is therefore environmentally benign for manufacturing and industrial procedures (Blanco et al., 2004). The simplest and cheapest methods for enzyme immobilizing, namely, attachment on silica, are realized by simple adsorption. For instance, this method is utilized for generation of enzyme formulations in detergent powders that release the enzyme into the washing liquid during the washing process (Deere et al., 2002).
15.3.1.1.1 Polymers
A recent method of enzyme immobilization is based on covalent linkage of such enzymes to polymers that undergo significant structural conformation changes in response to even minor environmental changes in terms of pH-value, temperature, and ionic strength (Klouda & Mikos, 2008). A studied sample is poly (N-isopropyl acrylamide) (poly NIPAM), a thermo- and bio-compatible polymer (Klis et al., 2009). Aqueous poly NIPAM has its critical solution temperature (CST) at around 32 °C. Above the CST, it becomes dissolvable because of release of water molecules from the polymer fibers. Thus, the bio-conversion can take place under states that maintain the enzyme solubility, thereby minimizing diffusional restriction. Subsequently, an increase in temperature above the CST leads to detachment of the immobilized enzyme, thus the enzyme is recovered and can be reused (Virtanen & Tenhu, 2000; Virtanen et al., 2000; Lozinsky et al., 2003). Polyurethane has recently been proposed as an entrapping polymer that retains the bioactivity of biocatalysts for long times. The usage of this polymer resulted in a remaining crude oil degradation capacity of 44.31% by a microbial consortium after more than 6 months (Kazemzadeh et al., 2020).
Polymers that possess electrical conductivity have already been successfully synthesized and utilized in different areas, including biotechnology. Recently, a new class of polymers has been proposed as novel electro-active conjugated polymers. This kind of supports exhibit interesting electrical and optical characteristics previously reported only for inorganic systems. Electronically directing polymers are different from all the familiar inorganic crystalline semiconductors, such as silicon. They are molecular in nature and long chains are absent in them. Immobilization of enzymes and biosensor construction are two applications of these polymers (Cirpan et al., 2003). Lots of theoretical models have also been associated with the electrochemical entrapment of enzymes to evaluate the polymer thickness, enzyme configuration, and the level of enzyme loading in the biosensor design (Gerard et al., 2002).
15.3.1.1.2 Nanomaterials
The headway of nanotechnology in the 1990s was preceded by the quick evolvement of nanobiotechnology including the construction of nanobiocatalysts. In the early methods applied in nanobiocatalysis, enzymes were immobilized on various nanostructured materials utilizing conventional procedures, like simple adsorption and covalent linkage. This method attracted attention for immobilizing enzymes on a wide range of nanostructured matrices, like porous nanomaterials, electroconductive nanofibers, and magnetic nanoparticles (MNPs). This large area provides better enzyme loading, which in turn improves the enzyme mobility in comparison to immobilized enzyme systems on conventional matrices. One of the special advantages of nanostructured materials is that the pore size in nanopores, nanofibers, or nanotubes can be controlled at nanometer scale (Homaei et al., 2013).
Recently, nanobiocatalytic methods have evolved from simple strategies for immobilization of enzymes (Homaei et al., 2013). By rapid advancement in nanotechnology, MNPs are presently extremely interesting. The physico-chemical characteristics of MNPs can widely differ from the properties of the bulk material from which the nanoparticle is made; this has attracted attention in these materials also for enzyme immobilization. For instance, magnetizing a particle in a particular direction by magnetic anisotropy is usually done on the surface of a particle (Schellenberger et al., 2002). Nanoparticles constructed by ultra-small superparamagnetic iron oxide (Kooi et al., 2003; Keller et al., 2004), cross-linked iron oxide (CLIO), and mono-crystalline iron oxide (Krause et al., 2004) all were fabricated as imaging elements in magnetic resonance imaging (MRI). Magnetic particles are used for enzyme immobilization for the purpose of increasing the stability of the biocatalyst, to maintain the stability of the catalyst, and, importantly, they can conveniently be separated from the interaction environment and recovered by applying an external magnetic field. (Bilal & Iqbal, 2019). MNPs perform best at typical sizes ranging between 10 and 20 nm, where superparamagnetism emerges (Netto et al., 2013). Such magnetic particles have been suggested for biotechnological applications (Kluchova et al., 2009; Defaei et al., 2018) or for developing analytical systems, like biosensors (Bilal & Iqbal, 2019; Kouassi et al., 2005).
Nanostructured metal oxides (NMOs) recently became of interest in the area of enzyme immobilization because these materials have the best structural revising and high bioactivity, which leads to elevated sensing properties (Antony et al., 2016). As a part of NMOs, MNPs have been widely utilized in enzyme immobilization because of their advantageous properties, such as their size, magnitude, higher safety levels, better reusability, wide surface, and large capacity of enzyme loading. Because they have an inactive surface that limits direct binding to enzymes, protective molecules must be coated on MNPs to supply dynamic functional groups for immobilization of enzymes (Amirbandeh & Taheri-Kafrani, 2016; Mehnati-Najafabadi et al., 2018). With all these benefits in mind, MNPs are highly inhibited in acidic and oxidative conditions. Therefore, coating of the outer protective surface is so vital to sustain the consistency of MNPs (Landarani-Isfahani et al., 2015). In a recent study, Defaei et al. (2018) immobilized the hydrolase α-amylase (EC 3.2.1.1) onto naringin-functionalized MNPs by ionic reactions. The MNPs were covered with naringin, which is a biocompatible flavonoid. The appearance, structure, and features of functionalized MNPs and the immobilization status of the nanocomposite were determined by analytical instruments, like thermogravimetry (TGA), vibrating sample magnetometer (VSM), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy with energy dispersive X-ray (SEM-EDX), and transmission electron microscopy (TEM). In addition, the optimum conditions of temperature, pH-value, interaction time, and enzyme tendency for better immobilization were evaluated. The results evaluated the optimum conditions for α-amylase immobilization on the synthesized nanocarrier at pH 6.5 and a temperature of 55 °C. Reuse experiments showed high maintenance of immobilized α-amylase activity even after 10 reaction repeats. Furthermore, the storage consistency of enzyme immobilization was repaired by immobilization compared to that of the free enzyme and it retained 60% of its original activity even after 6 weeks of storage at 4 °C. Improving the catalytic properties of enzymes through immobilization has made this nanobiocatalyst a feasible tool in bio-industrial systems.
Another field of application of MNPs as supports for immobilized enzymes is the pharma sector; here, it was shown that the enzyme penicillin G acylase (PGA; EC 3.5.1.11) can be immobilized to functionalized MNPs (epoxy-activated magnetic cellulose beads) due to the cavity and affinity forces in the matrix of activated cellulose, and applied for hydrolytic removal of the side chain of penicillin G molecules, generating 6-aminopenicillanic acid as product. Improved biocatalytic activity and stability of the enzyme were reported for this process in comparison to the use of free enzyme (Luo & Zhang, 2010). In recent years, this process was improved by various research groups globally, such as by Liu and colleagues, who covalently immobilized PGA on hydroxy- and aldehyde-functionalized magnetic Fe2O3/Fe3O4 nanoparticles (Liu et al., 2020), or Zhaoyu et al., who used novel di-functional magnetic “nanoflowers,” equipped with epoxy groups and hydrophilic catechol as well as with phthaloquinone groups enabling the covalent coupling of penicillin G acylase (Zhaoyu et al., 2020).
15.3.1.2 Organic Solid Supports
Different biological polymers, especially hardly water-soluble or de facto water-insoluble polysaccharides, like agarose, cellulose, starch, and carrageenans, have been heavily utilized as matrices for enzyme immobilization. These polymers create highly inactive aqueous gels, with the property of high gel formation even at low concentrations (Van de Velde et al., 2002).
The best described and most widely applied synthetic polymers used to carry the immobilized enzymes are acrylic resins, like sepabeads or eupergit. These macroporous polymers are copolymerized by using N,N′-methylene-bi-(methacrylamide), glycidyl ether, methacrylamide, allyl methacrylate, and glycidyl methacrylate. They are very hydrophilic and highly chemically and mechanically stable in the entire pH-range of 0 to 14 and are not inactivated even at a sudden and tremendous pH-change. A basic disadvantage of hydrophilic resins are diffusion limitations that have been shown in kinetically monitored procedures. Immobilization by covalent binding to acrylic resins has been successfully utilized for a variety of enzymes in industrial operations (Katchalski-Katzir & Kraemer, 2000; Boller et al., 2002).
Enzymes may also be immobilized in biological or artificial hydro- or cryo-gels in an insoluble environment. For example, poly (vinyl alcohol) (PVA) crucibles made by melting ice are frequently used for whole-cell immobilization (Tripathi et al., 2010). Also, because of their minor size, free enzymes can spread from the gel matrix and get dissolved in an aqueous medium. To trap free enzymes, the enzyme size should be enlarged by mechanisms, such as cross-linking. Another option to increase the biocatalytic enzyme size is to design a composite material with a polyelectrolyte. Because of their ampholytic nature, proteins are released either as polycations or polyanions, based on the environmental pH-value. Therefore, the typically can form complexes with polyelectrolytes of opposite charges (Homaei et al., 2013).
15.4 Multi-Enzyme Immobilization
Multi-enzyme immobilization is a technique that co-establishes more than one enzyme on appropriate supports/carriers, or joins enzymes using a linking agent (cross-linker) with no carriers (Ren et al., 2019). Enzymes will be adjacent to each other, and the total material-transport restriction will be decreased during co-immobilization, which has been shown to boost the activity of enzymes by matrix canaling and raising the consistency and ability to reuse them. As support materials can mainly influence the characteristics of enzymes, selection of support has been mentioned as a hot subject in the majority of reports on enzyme immobilization techniques. To date, different matrices, like CNTs, graphene, metal-organic frameworks (MOFs), DNA nanocomposites, silica, and polymers, have been used for immobilization of multi-enzyme, which can efficiently shelter enzyme activity from biological challenges, such as heavy metals or high temperature (Sheldon & Woodley, 2018; Ren et al., 2019). There are three major types for co-immobilization of enzymes, such as random and positional co-immobilization, and compartmentalization (Hwang & Lee, 2019).
Biocatalytic transformation by a cascade of enzymes (“multi-enzyme catalysis”) is an emerging technology to manufacture various industrially valuable compounds; briefly, it mimics the cascadic catalytic steps of pathways in living cells. In this context, positional multi-enzyme immobilization has been demonstrated as a viable tool for effective immobilization of co-enzymes, which can trigger the rates of coenzyme cascade reactions by fine-tuning the sequence of immobilized enzymes, and which, first of all, improves channeling of the substrate flow, and avoids the formation of unwanted side products, thus leading to higher overall yields. Moreover, such positional multi-enzyme immobilization approaches result in high stability and reusability of involved enzymes. Polymers, DNA nanostructures, graphene, or CNTs are the most frequently used supports for positional multi-enzyme immobilization grace to their expedient capability to manage the relative positions of enzymes by usual reactions (Xu et al., 2020).
As an imitation of the organization of natural enzymes in cellular environments, compartmentalization can divide enzymes by varied features and ratios spatially, which can inhibit enzymes proteolysis, biological decomposition, or exposure to toxic agents (Marguet et al., 2013). As an example, a compartmentalized co-enzyme operation was created on the base of inorganic nanocrystal–protein complexes via a simple precipitation technique that showed increased overall catalytic performance in comparison to the use of free enzymes. For this purpose, the versatile enzyme horseradish peroxidase (HRP; EC 1.11.1.7), a well-known oxidoreductase, was combined with CuSO4 in aqueous environment in order to form HRP-incorporated complexes; subsequently, glucose oxidase (GOx; EC 1.1.3.4) was attached on the surface of the complexes via the cooperative reaction between Cu2+ and protein amino acids (Li et al., 2014). Different organic and inorganic bases can be applied for immobilization of multi-enzymes as described in Fig. 15.3.
DNA nanotechnology has turned out to be a feasible method to construct complex bio-molecular nanocomposites because of the ability of programmed DNA hybridization. For immobilization of multi-enzymes, it is important to decrease the mass transfer consistency by managing the relative location and directions of a variety of enzymes in a limited space. Therefore, DNA nanotechnology has been used as an effective instrument for the multi-enzyme immobilization in which notably addressable DNA nanocomposites can ease the suitable self-aggregation of varied enzymes and optimize the substrate penetration (Xu et al., 2020).
15.5 Kinetics of Enzyme Immobilization
Compared to measurements of the kinetics of enzymes in absolute solution, the interactions of immobilized enzymes have only scarcely been shown under spatially sole substrate and uniform conditions, such as pH-value. In addition, immobilization can change the innate kinetics of the enzyme by altering the structure of the enzyme and surrounding microenvironment. To diminish the effect of immobilization on the enzyme catalytic activity, the molecular configuration of the enzyme should be considered (Gonzalez-Saiz & Pizarro, 2001).
Immobilization usually enhances enzyme maintenance at the expense of lower catalytic function (Sheldon, 2007a; Garcia-Galan et al., 2011). Nevertheless, the appropriate choice of enzyme immobilization techniques may reduce or increase the enzyme activity (Rodrigues et al., 2013, 2011; Mateo et al., 2007). For example, enzyme immobilization on highly active supports can improve the multipoint covalent linkage and prevent the enzyme from being inactive after immobilization (Pedroche et al., 2007; Mohan et al., 2015). Similarly, the selection of enzyme loading and immobilization matrix may prefer the partition of H+ or OH− ions, and change the local pH-value in the support. The changed pH-value may cause the immobilized enzyme to work under conditions close to the pH optimum (Pedroche et al., 2007). The conformation of the enzyme molecule across the immobilization process may also affect its function in the presence of detergents. MCA immobilization of lipases can enhance their activity because the enzyme is immobilized in an active combination (Fernández-Lorente et al., 2006).
Omitting standard metrics for enzymatic activity assessment, such as Km, Vmax, and kcat, or extracting separated function metrics from data sets other than interaction progress curves can be prevented using the available literature to lead to rational choose of immobilization schemes (Herzog et al., 2005; Koh & Pishko, 2005). To enhance an acceptable comparison between the studies about kinetics, minimal reporting standards (STRENDA) have been reported that contain features of model election and error diagnosis (Tipton et al., 2014; Gardossi et al., 2010).
15.6 The Usage of Enzyme Immobilization for Production of Different Food Products
Unlike pharmaceutical industries and some chemical industries, the food industry requires the production of vast quantities of commercial products. For this purpose, the cost of the biocatalyst should be lowered, thus, using immobilized enzymes show acceptable operational consistency that allows lots of repetitive production cycles to be carried out. In the food sector, continuous fermentation processes are preferred to batch processes, especially when large quantities of material are manufactured. Examples of broad-scale usages of immobilized enzymes for production of food products are explained in the subsequent paragraphs of the chapter at hand.
15.6.1 d-Glucose/Xylose Isomerase for Production of High Fructose Corn Syrup
The use of immobilized d-glucose/xylose isomerase (EC 5.3.1.5) in the preparation of highly fructose-rich corn syrup (HFCS) shows a largely applied commercial procedure encompassing an enzyme immobilization step, with a high quantity of enzyme that is used, and expedient product yield (Crabb & Shetty, 1999). Generally, the enzyme, which belongs to the top three industrially used enzymes (others being proteases and amylases), catalyzes the interconversion of d-glucose to d-fructose and d-xylose to d-xylulose, respectively (Gaikwad et al., 1992). HFCS is predominantly used in fructose production that is applied as a sweetener for beverages and foods, or utilized directly as a food and beverage ingredient. While d-xylose is the native substrate for d-glucose/xylose isomerase function, the enzyme has a wide substrate spectrum, and in its industrial application it produces d-fructose from d-glucose in an efficient manner (Bhosale et al., 1996).
Today, HFCS production processes are performed in fixed bed reactors that are ordered in parallel and operated continuously (Nedwin et al., 2014). d-glucose syrup that is stemming from corn is converted into a mixture that is generally named as HFCS-42. This syrup contains approximately 42%, 50%, 6%, and 2% d-fructose, d-glucose, maltose, and maltotriose, respectively, as well as low quantities of other sugars. Higher concentrations of fructose that are obtained by chromatographic enrichment of the 42% syrup to a final 90% d-fructose (HFCS-90) content is used in soft drinks. The two commercially available immobilized d-glucose/xylose isomerase preparations, most commonly used for these processes, are on the basis of inexpensive inorganic supports, like bentonite clay and diatomaceous earth with cross-linking the enzyme by glutaraldehyde. The resulting composite is dewatered and then dried in a fluidized bed dryer. The obtained immobilized d-glucose/xylose isomerase preparations are very consistent, with a half-life of more than 1 year and are used in a packed bed reactor at 60 °C (Basso & Serban, 2019).
15.6.2 Epimerase for Production of Allulose
d-allulose (d-psicose or “pseudofructose”) is a low-calorie monosaccharide sweetener recommended due to its sweeting similarity to dextrose, and about 70% of the sweetness of sucrose. d-allulose is the C3-epimer of d-fructose. The difference between allulose and fructose is that allulose is hardly metabolized in the human body and has almost zero calories (only 0.007 kcal/g)). Globally leading food and beverage manufacturers are the main target markets for allulose that replace dextrose, fructose or HFCS in their products, which is important to decrease calories in parallel with maintenance of the properties obtained by the sugar ingredient, such as browning, bulking, texture, and sweetness. Allulose is also supposed to display potential antihyperglycemic effects, and was shown to prevent postprandial hyperglycemia in humans. More than 0.2 g per day human allulose intake is estimated when consuming naturally found materials, such as processed cane and beet molasses, coffee treated via steam, wheat products, and HFCS. In 2012, allulose was labeled as generally recognized as safe (GRAS) by the FDA and approved as sweetener in food products (not yet approved in, e.g., the European Union!), so it is commercially utilized as food additive in some parts of the world. Moreover, ketose-3-epimerase (EC 5.1.3.31), an isomerase which is found in various microorganisms, can interconvert fructose to allulose and vice versa (Basso & Serban, 2019).
15.6.3 β-Galactosidase for Production of Tagatose
Another emerging sweetener is the ketohexose tagatose, which can be isolated from animal origins. Tagatose is a kind of sweetener with 92% fructose sweetness, but contains only 38% of fructose calories. Its catabolic route is different from sucrose; therefore, it has insignificant effect on insulin and blood glucose levels. Moreover, tagatose is considered a “tooth-friendly” compound for dental care products. Tagatose can be gained from lactose with only 16% of the sweetening of the later. The disaccharide lactose (β-d-galactopyranosyl-(1 → 4)-d-glucose) is a natural sugar found in milk and normally makes up 2–8% of its total mass. The process of tagatose production utilizing the immobilized glucosidase β-galactosidase (3.2.1.23) has been reported in 2014. This process suggests preparation and valorization of lactose present in whey at a concentration of 18 wt.-%. It is possible to obtain tagatose by lactose hydrolysis using immobilized β-galactosidase to produce the monomers glucose and galactose. Then glucose is removed from the mixture by deglycosylation using baker’s yeast; now, tagatose is obtained by epimerization of galactose with aerated Ca(OH)2 (Basso & Serban, 2019). There have also been reports on the utilization of immobilized l-arabinose isomerase (EC 5.3.1.4) to obtain tagatose in stirred tank reactors or continuous flow systems (Lim et al., 2008; Oh, 2007).
15.6.4 Other Sweeteners Produced by Immobilized Enzymes
Another sweetener for food and beverage industry, also accessible by means of biocatalysis, is the canonic amino acid l-aspartate (2-aminobutanedioic acid). Aspartate production is performed by amination of fumaric acid; this reaction is catalyzed by the lyase enzyme aspartase (aspartate ammonia-lyase, EC 4.3.1.1). Already in 1973, Tosa et al. reported the application of aspartase from Escherichia coli for aspartate production from ammonium fumarate; these authors immobilized the enzyme by different methods: ionic binding on cellulose derivatives or sephadex, physical adsorption to silica gel or calcium phosphate gel, covalent binding to cellulose azide or diazonium derivatives of cellulose, or entrapping in polyacrylamide gel lattice. The authors obtained the most active immobilized aspartase by the entrapping process; regarding optimum pH-value, temperature, and ion concentration, no differences were observed between the immobilized and free enzyme in terms of kinetic constants and heat stability. Excellent conversion yields were reported for this process when operated in continuous mode using columns packed with the immobilized aspartase (Tosa et al., 1973). Later, the same group of authors immobilized E. coli entrapped in a polyacrylamide gel lattice as whole-cell biocatalyst for continuous aspartate production in a packed column reactor (Tosa et al., 1974). Currently, aspartate is produced on an annual scale of 104 tons by cross-linked whole cells or by the isolated enzyme immobilized by different methods (reviewed by DiCosimo et al., 2013).
Moreover, aspartame is another sweetener produced as a dipeptide of l-aspartate and l-phenylalanine. For this purpose, in 1981, Oyama et al. immobilized the hydrolase enzyme thermolysin (EC 3.4.24.4) by different methods: physical adsorption to Amberlite XAD-7 and XAD-8, ionic binding to the ionic ion exchange resin Amberlite IRA-94, adsorption on glass beads, and covalent linking to a hydrophilic ethylenediamine-derivatized polymer gel by glutaraldehyde. The immobilized enzyme assays prepared via these different ways were tested for formation of aspartame by reaction of the precursors N-(benzyloxycarbonyl)-l-aspartic acid with l-phenylalanine methyl ester by incubating the mixture of the substrates in the solvent ethyl acetate, which normally would denature the enzyme. Substrates moved from the organic phase to the aqueous phase in the support, where the reaction took place, and the product (aspartame) diffused back to the organic phase, from which it could be recovered. Among the different tested immobilization strategies, physical adsorption to Amberlite XAD-7 and XAD-8 resulted in the best yields (Oyama et al., 1981). Later, this immobilization technique for thermolysin was successfully used by Miyanaga et al. for continuous production of the aspartame precursor N-(benzyloxycarbony1)-l-aspartyl-l-phenylalanine methyl ester from N-(benzyloxycarbony1)-l-aspartic acid and l-phenylalanine methyl ester in a mixed organic solvent system consisting of tert-amyl alcohol and ethyl acetate in a column reactor. Here, excellent conversion yields of 99% were achieved under optimized conditions (Miyanaga et al., 1995).
15.6.5 Enzyme Immobilization for Debittering of Citrus Fruit Juices
The polyphenol naringin, a flavonoid, is responsible for the bitter taste of citrus fruits. There is increased interest in fruit juice industry by using highly efficient immobilized enzymes for debittering of citrus fruit juices (Puri et al., 2008). Already in 1979, Olson and co-workers reported the immobilization of commercially available naringinase (mixture of the hydrolases α-rhamnosidase, EC 3.2.1.40, and β-glucosidase, EC 3.2.1.21; hydrolyzes naringin to naringenin, glucose, and rhamnose) in a reactor system consisting of polysulfone hollow fibers; immobilization took place by ultrafiltration of the enzymes into the sponge region of the hollow fibers. After 210 min of continuous operation, 50% of naringin contained in grapefruit juice was hydrolyzed at 25 °C and a flow rate of 300 mL/min (Olson et al., 1979). Other approaches to immobilize naringinase encompass entrapping in alginate, which resulted in 60% debittering of kinnow juice after 3 h when using a total enzyme activity of 30 U (Puri et al., 1996), immobilization on electrospun cellulose acetate nanofibers (Huang et al., 2017), on chitin (Tsen, 1984) or chitosan microspheres (Bodakowska-Boczniewicz & Garncarek, 2019) by linking with glutaraldehyde, or adsorption of the enzyme on mesoporous molecular sieves via glutaraldehyde for naringin hydrolysis in white grapefruit juice (Lei et al., 2011). A rather bizarre protocol for naringinase immobilization was developed by Puri and colleagues, who attached the enzyme on chicken egg white beads obtained by cross-linking the protein with glutaraldehyde; debittering of Kinnow juice achieved an efficiency of 68% (Puri et al., 2001). In addition, Busto et al. immobilized thermophilic Aspergillus niger naringinase by entrapping it into a PVA hydrogel matrix, which was cryostructured in liquid nitrogen, to generate beads biocatalytically active for naringin hydrolysis. Authors reported high stability of the beads; after storage at 4 °C for 2 months, they retained 75% of initial activity (Busto et al., 2007).
15.6.6 Lipases for Production of Vitamin C Esters and Cocoa Butter Analogs
One of the main water-soluble natural antioxidants is l-ascorbic acid (vitamin C). l-ascorbic acid and its derivatives act as free radical scavengers, reacting with oxygen, and destroying it. Moreover, hydrophobic long-chain fatty acid ester derivatives of l-ascorbic acid are used as antioxidants in fat-rich food because of their higher ability to dissolve in fats in comparison to the typical hydrophilic compound vitamin C, which is insoluble in oils (Burham et al., 2009). In this context, ascorbic palmitate and stearate are currently prepared by reaction between ascorbic acid with sulfuric acid, followed by re-esterification with the corresponding fatty acid; finally, a purification step by re-crystallization is carried out (Ferreira-Dias et al., 2013). In a biocatalytic approach, immobilization of Candida antarctica B lipase (CalB) was utilized for generation of ascorbyl esters. The biocatalytic conversion can reach a yield of approximately 95%, depending on process temperature, the level of removal of the side product water, and fatty acid chain length. In spite of the fact that enzymatic synthesis suggests some benefits to the current chemical procedures, such as interaction in the lower temperatures than chemical reactions temperatures, higher material purity, and decreased downstream processing expenditure, many of the manufactures of ascorbyl esters still carry out this synthesis by chemical processes, because of the long interaction time needed by the enzymatic procedure and the high price of the immobilized enzymes in contrast to the chemical catalysts (Villeneuve, 2007).
The source of vegetable oils, such as palm, rapeseed, canola, and sunflower, specifies the physical characteristics of fats and oils present in food products, because each oil has a different arrangement and type of saturated mono- and polyunsaturated fatty acids in the 1, 2, and 3 locations of triacylglycerides. To obtain the suitable melting properties of fats and oils, especially in the generation of margarine and baking fat, chemical hydrogenation, fractioning, and esterification have been applied. The enzymatic transesterification of food oils and fats is one of the benefits because of the option to better monitor the product composition compared to chemically transesterified products due to the removal of the hydrogenated trans fats that have important health challenges (Marangoni & Rousseau, 1998; Asif, 2011).
Enzymatic transesterification was first investigated to produce an equivalent of cocoa that used the sn-1,3 specificity of different fungal lipases. Cocoa butter homologs are semisolid oils that commonly have a melting temperature of 37 °C. They are obtained from more cost-effective origins than cocoa, like palm, sunflower, or rapeseed oil. A variety of commercial processes have been developed to produce the equivalent of cocoa butter with elevated amount of the demanded triglycerides, 1(3)-palmitoyl-3(1)-stearoyl-2-monooleine, and 1,3-distearoyl-2-monooleine, required for chocolate production. Most systems are made by using fungal lipases immobilized by surface adsorption or encapsulation in liposomes (Basso & Serban, 2019).
15.6.7 β-Galactosidase for Lactose Hydrolysis
Bovine milk contains 4.3–4.5 wt.-% lactose that exposes 38–40% of the whole milk solids. Lactose in milk and milk products is not hydrolyzed in the stomach or in the initial part of the small intestine; it enters to other parts of the intestine and gets hydrolyzed into the monosaccharides d-galactose and d-glucose by the glycosidase β-galactosidase (lactase, EC 3.2.1.23) excreted by the intestinal microflora. About 65% of the entire human population (up to 90% in some Asian countries) are unable to secrete sufficient quantities of β-galactosidase, causing many health disorders. Elimination of lactose from milk and milk products makes them suitable for consumption by people with lactose intolerance (hypolactasia), so the dairy industry has demonstrated great interest to develop advanced lactose hydrolysis processes based on β-galactosidase. Because the sweetening potential of lactose, glucose, and galactose is 20, 70, and 58%, respectively, of sucrose, lactose-hydrolyzed milk is sweeter than pristine milk (Panesar et al., 2010).
The simplest but most expensive solution for this problem is to add free β-galactosidase to whole milk. Enzyme activity is stopped after complete substrate hydrolysis, typically combined with pasteurization. Another procedure is the usage of immobilized β-galactosidase for processing of skimmed milk; after completion of hydrolysis, the fat fraction is added again to the hydrolyzed milk to reassemble its nutritious components. This technique, of course, displays the benefits of recycling and reusing the immobilized enzyme in contrast to adding free enzyme, and the final product is free from additional ingredients, like enzymes or components or the enzyme formulation that can constitute putative allergens.
The techno-economic evaluation of lactose removal by immobilized β-galactosidase from the fungus Aspergillus niger dates back as far as to 1990; that time, Axelsson and Zacchi calculated the cost for a tank reactor operated in batch mode, free and immobilized β-galactosidase, a continuously operated stirred tank reactor (CSTR), and for a plug-flow tubular reactor (PFTR). For all cases, the mass transfer behavior and enzyme deactivation were considered. As outcome, the authors concluded that enzyme immobilization indeed is economically more feasible when compared with application of free enzymes, although the high cost for enzyme immobilization itself still constitute an obstacle. The lowest cost of 0.48 Swedish crowns (SEK) per kg lactose to be hydrolyzed, calculated for a half-life time of 80 days, were calculated for immobilized enzyme in the PFTR; however, calculated costs for using immobilized β-galactosidase in a batch reactor were only insignificantly higher with 0.66 SEK/kg lactose. In any case, these two modes of using immobilized enzyme were considerably lower in costs than for the case of free enzyme in a batch reactor (2.10 SEK/kg lactose) (Axelsson & Zacchi, 1990).
In 2003, Roy and Gupta used the commercially available β-galactosidase preparation Lactozym™ (Novozymes, Denmark) from the yeast Kluyveromyces fragilis. This preparation is GRAS for hydrolysis of whey to produce lactose-poor milk. The authors immobilized Lactozym™ on cellulose beads via covalent epichlorohydrin coupling. In a column serving as fluidized bed reactor, whey lactose was hydrolyzed by >90% within 5 h, while the same enzyme, when applied in continuous batch mode, took 48 h for the same hydrolysis outcome. It was possible to reuse the immobilized enzyme three times without decrease in the biocatalytic performance of the fluidized bed reactor column. In the same fluidized bed reactor, also lactose in whole milk was converted to glucose and galactose up to 60% within 5 h (Roy & Gupta, 2003).
A more recent example for the use of an immobilized β-galactosidase is the enzyme isolated from the yeast Saccharomyces lactis. This enzyme was immobilized by entrapment in cellulose triacetate fibers. The entrapped enzyme was reused for 50 times with the reduction of enzyme activity less than 9% in a rotary horizontal column reactor; 10 tons milk were processed per day via this process on industrial scale (reviewed by Basso & Serban, 2019). Moreover, Aspergillus oryzae β-galactosidase was immobilized by covalently binding the enzyme to an ion exchange resin based on polyphenolic formaldehyde (Hirohara et al., 1981; reviewed by Basso & Serban, 2019).
15.7 The Usage of Immobilized Enzymes in Transforming Food Waste
A notable amount (approximately 40%) of all types of food are disposed as waste (Godfray et al., 2010), and these losses not only lead to environmental pollution but also affect the entire food chain. This amount varies between different geographic regions, and one should differentiate between food waste sensu stricto and agricultural waste. These waste streams are responsible for a major global challenge both in causing environmental pollution and ethical concern considering the huge number of people starving worldwide. With the global population expected to increase to 9.8 billion until 2050, suitable technological solutions should be developed to solve this problem. Some technical proposals are represented at the food processing level. Liquid food processing waste contains numerous organic carbonaceous compounds; therefore, it has high biological oxygen demand (BOD) that causes problems for direct disposal of them to wastewater removal plants. Here, the disposal of about one million liters of lactose-rich whey per day only on the Northern Italian region constitutes a prime example (Koller et al., 2016). Hence, the lipid, carbohydrate, and protein contents of food and agricultural waste liquids are leading to high BOD; however, at the same time, they have the potential to be converted to valuable products, thus upgrading waste liquids into potential recoverable sources. Examples of such conversions include oxidation, hydrolysis, acylation, and phosphorylation of carbohydrates as well as glycosylation and deamination of amino acids, and esterification and hydrogenation of lipids. In particular, esterification processes are widely used for production of different value-added food and agricultural products. Waste oils and animal waste lipids from the slaughtering and rendering industry can be transesterified with alcohol to generate biofuels (Koller et al., 2018). Esterified sugars can be applied as surfactants, and esterified starch may be used as biodegradable plastics and adhesives. Esterification of flavonoids was reported to increase their life time, health, and acceptance characteristics (Walle, 2009). Traditional processes to these transformations require significant amounts of chemical catalysts and energy resources that have limited reactivity, and lead to formation of by-products, especially when done in complex matrices, like food waste liquids (Alissandratos & Halling, 2012; Fang et al., 2002).
15.7.1 Carbohydrate Wastes
Food processing waste streams which are carbohydrate-rich can easily be converted by the enzymatic valorization catalyzed by hydrolases and isomerases into more valuable products, like sweeteners and prebiotics. In fact, some of the best accepted, well-known procedures in food and agricultural systems begin with the use of carbohydrate substrates. In this context, immobilized thermophilic enzymes (“thermozymes”), which have been studied for the production of high fructose corn syrup, could be progressed and used for valorization of food waste liquids that are carbohydrate rich (Andler & Goddard, 2018). Emtiazi et al. (2001) used immobilized cellulase enzymes from Aspergillus terreus to decrease chemical oxygen demand (COD) by cellulose removal (40–80%) from pulp manufacturing waste.
15.7.2 Lipid Wastes
According to the significant impact of environmental and economic waste stream valorization, waste oil can be converted to value-added products, such as biodiesel, surfactants, and lubricants, by the use of enzymes. Lipases, like most other enzymes, can be mined from different microbial sources with different performance properties. For instance, lipases produced by Thermomyces lanuginosus and Candida antarctica were used for lipid hydrolysis and esterification, respectively, yielding valuable products, such as biofuel, from waste cooking oil. More than 90% conversion was obtained after 10 h of hydrolysis and 10 h of esterification reactions. Noteworthy, after 5 catalysis repeats, the lipase from C. antarctica retained its activity, while the lipase from T. lanuginosus lost some of its activity after each use (Vescovi et al., 2016). In another study, Rhizomucor miehei lipase and C. antarctica lipases were immobilized on silica particles that were epoxy-functionalized and used to enhance the performance of biofuel generation from waste cooking oil. A 91.5% conversion rate was achieved during 10 h (Babaki et al., 2017).
Other examples for immobilization of C. antarctica lipase for conversion of lipid substrates to value-added products encompass the use of support materials as diverse as core–shell MNPs for conversion of waste cooking oil to biodiesel (Mehrasbi et al., 2017), immobilization by adsorption on poly (styrene) nanoparticles (Miletić et al., 2010), covalent attachment on chitosan-based hydrogels (Silva et al., 2012), or adsorption to green coconut fibers (Brígida et al., 2007).
15.7.3 Proteinaceous Wastes
Proteinaceous food waste may stem from different origins like dairy products (whey retentate), grains (Zhi et al., 2017), oilseeds (Doshi et al., 2014), soybeans (Laskar et al., 2018), eggs (Hong et al., 2019), or even poultry feathers (Pernicova et al., 2019). Proteases are used for hydrolyzing proteins from waste streams and converting them to biological peptides or useful chemicals, such as the monomers that build up polymers. Here, enzymatic pathways are more favorable than chemical reactions, which are not easily controllable, as observed for the degradation of tryptophan via acid hydrolysis (Kumar et al., 2015). Immobilized trypsin has been used for hydrolysis of dairy waste, such as whey protein (retentate fraction remaining after ultrafiltration of full whey), as an alternative to well-established acidic hydrolysis (Koller et al., 2019). Immobilization of bovine pancreas trypsin on porous polymethacrylate with a pore size of 2.1 μm has led to 9.68% hydrolysis. The hydrolysis degree had reached ~6% under the same conditions when using free trypsin, which indicates the need for optimization of the immobilization process. Most significantly, the peptide analysis differed between the immobilized and free trypsin that showed the effect of immobilization methods on enzyme selectivity for hydrolysis of amino acid sequences (Mao et al., 2017). Trypsin has also been immobilized on reusable matrices containing spent grain and lignocellulose for hydrolysis of whey protein (Tavano, 2013; Bassan et al., 2016).
15.8 Commercialization of Immobilized Enzymes for Usage in Food Industries
For successful commercialization of procedures based on immobilized enzymes, the overall process cost is the decisive factor. Some of the major factors determining the cost of immobilization, such as the used matrix (support), the enzyme itself, the chemicals used in the immobilization procedure, and the special equipment that may be needed should be considered. Current requests for immobilized enzymes make up only a small portion of the whole enzyme market (DiCosimo et al., 2013). Different uses of immobilized enzymes may be developed in food manufacturing, medical applications, and food research studies (Homaei et al., 2013). Other examples for commercial usages of immobilized enzymes are synthesis of organic materials on laboratory scale as well as analytical and pharmaceutical usage. Moreover, apart from the food sector, the application of immobilized enzymes for removal of eco-pollutants from aqueous environment might be an important future field of research and development, as it was suggested for the removal of endocrine-disrupting compounds from wastewater by covalently immobilizing HRP on filtration membranes (Mohan et al., 2015; Rathner et al., 2017). For each commercial application, the appropriate selection of a particular immobilized enzyme or strategy of immobilization should be based on the advantages and disadvantages of the performance of free and immobilized enzymes, respectively. This shows that the establishment and use of immobilized enzymes needs profound understanding of affecting functional and economic factors, in-depth kinetic studies, plus the knowledge about the current market requirements and trends (DiCosimo et al., 2013).
15.9 Conclusions
Enzymes usage in soluble form for food processing is well established. Although a high variety of enzymes have already been immobilized and used in different food manufacturing industries, only few procedures have become practical and economical, and succeeded in getting established on the long term. Numerous recent concepts have been attempted or are being used in this field. The future of such applications and operations will depend mainly on their cost and also political decisions. Despite the fact that not all established transformations involved in food processing and food production can to date be replaced by biocatalytic techniques resorting to immobilized enzymes, and although many immobilization processes holding promise in lab-scale experiments are not yet scalable to industrial dimensions, the outlook for immobilized enzymes in food industry is indeed promising considering current food industry trends to become more efficient and sustainable, combined with the rapid progression of immobilized enzyme techniques. In any case, due to the growing human population on Earth and a remarkable decrease in limited natural food resources, the future use of immobilized enzymes may elevate significantly in order to produce higher amounts of food products, and even to unlock alternative food sources for enhanced global food security.
References
Albayrak, N., & Yang, S. T. (2002). Immobilization of β-galactosidase on fibrous matrix by polyethyleneimine for production of galacto-oligosaccharides from lactose. Biotechnology Progress, 18(2), 240–251.
Alissandratos, A., & Halling, P. J. (2012). Enzymatic acylation of starch. Bioresource Technology, 115, 41–47.
Alkan, S., Gür, A. Y. C. A. N., Ertan, M., Savran, A., Gür, T., & Genel, Y. (2009). Immobilization of catalase via adsorption into natural and modified active carbon obtained from walnut in various methods. African Journal of Biotechnology, 8(11).
Amirbandeh, M., & Taheri-Kafrani, A. (2016). Immobilization of glucoamylase on triazine-functionalized Fe3O4/graphene oxide nanocomposite: Improved stability and reusability. International Journal of Biological Macromolecules, 93, 1183–1191.
Andler, S. M., & Goddard, J. M. (2018). Transforming food waste: How immobilized enzymes can valorize waste streams into revenue streams. NPJ Science of Food, 2(1), 1–11.
Antony, N., Balachandran, S., & Mohanan, P. V. (2016). Immobilization of diastase α-amylase on nano zinc oxide. Food Chemistry, 211, 624–630.
Asgher, M., Shahid, M., Kamal, S., & Iqbal, H. M. N. (2014). Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. Journal of Molecular Catalysis B: Enzymatic, 101, 56–66.
Asif, M. (2011). Process advantages and product benefits of interesterification in oils and fats. International Journal of Nutrition, Pharmacology, Neurological Diseases, 1(2), 134.
Axelsson, A., & Zacchi, G. (1990). Economic evaluation of the hydrolysis of lactose using immobilized β-galactosidase. Applied Biochemistry and Biotechnology, 24(1), 679.
Babaki, M., Yousefi, M., Habibi, Z., & Mohammadi, M. (2017). Process optimization for biodiesel production from waste cooking oil using multi-enzyme systems through response surface methodology. Renewable Energy, 105, 465–472.
Bassan, J. C., de Souza Bezerra, T. M., Peixoto, G., Da Cruz, C. Z. P., Galán, J. P. M., Vaz, A. B. D. S., et al. (2016). Immobilization of trypsin in lignocellulosic waste material to produce peptides with bioactive potential from whey protein. Materials, 9(5), 357.
Basso, A., & Serban, S. (2019). Industrial applications of immobilized enzymes—A review. Molecular Catalysis, 479, 110607.
Bhosale, S. H., Rao, M. B., & Deshpande, V. V. (1996). Molecular and industrial aspects of glucose isomerase. Microbiological Reviews, 60(2), 280–300.
Bilal, M., & Iqbal, H. M. (2019). Sustainable bioconversion of food waste into high-value products by immobilized enzymes to meet bio-economy challenges and opportunities–A review. Food Research International, 123, 226–240.
Blanco, R. M., Terreros, P., Fernández-Pérez, M., Otero, C., & Díaz-González, G. (2004). Functionalization of mesoporous silica for lipase immobilization: Characterization of the support and the catalysts. Journal of Molecular Catalysis B: Enzymatic, 30(2), 83–93.
Bodakowska-Boczniewicz, J., & Garncarek, Z. (2019). Immobilization of naringinase from Penicillium decumbens on chitosan microspheres for debittering grapefruit juice. Molecules, 24(23), 4234.
Boller, T., Meier, C., & Menzler, S. (2002). Eupergit oxirane acrylic beads: How to make enzymes fit for biocatalysis. Organic Process Research & Development, 6(4), 509–519.
Brady, D., & Jordaan, J. (2009). Advances in enzyme immobilization. Biotechnology Letters, 31(11), 1639.
Brígida, A. I., Pinheiro, Á. D., Ferreira, A. L., & Gonçalves, L. R. (2007). Immobilization of Candida antarctica lipase B by adsorption to green coconut fiber. In Biotechnology for fuels and chemicals (pp. 293–307). Humana Press.
Burham, H., Rasheed, R. A. G. A., Noor, N. M., Badruddin, S., & Sidek, H. (2009). Enzymatic synthesis of palm-based ascorbyl esters. Journal of Molecular Catalysis B: Enzymatic, 58(1–4), 153–157.
Busto, M. D., Meza, V., Ortega, N., & Perez-Mateos, M. (2007). Immobilization of naringinase from Aspergillus niger CECT 2088 in poly (vinyl alcohol) cryogels for the debittering of juices. Food Chemistry, 104(3), 1177–1182.
Chen, Y., Yu, B., Lin, J., Naidu, R., & Chen, Z. (2016). Simultaneous adsorption and biodegradation (SAB) of diesel oil using immobilized Acinetobacter venetianus on porous material. Chemical Engineering Journal, 289, 463–470.
Cirpan, A., Alkan, S., Toppare, L. E. V. E. N. T., Hepuzer, Y., & Yagci, Y. (2003). Immobilization of invertase in conducting copolymers of 3-methylthienyl methacrylate. Bioelectrochemistry, 59(1–2), 29–33.
Costa, S. A., Azevedo, H. S., & Reis, R. L. (2005). Enzyme immobilization in biodegradable polymers for biomedical applications. In R. L. Reis & J. S. Roma (Eds.), Biodegradable systems in tissue engineering and regenerative medicine (pp. 301–323). CRC Press, LLC.
Crabb, W. D., & Shetty, J. K. (1999). Commodity scale production of sugars from starches. Current Opinion in Microbiology, 2(3), 252–256.
Deere, J., Magner, E., Wall, J. G., & Hodnett, B. K. (2002). Mechanistic and structural features of protein adsorption onto mesoporous silicates. The Journal of Physical Chemistry B, 106(29), 7340–7347.
Defaei, M., Taheri-Kafrani, A., Miroliaei, M., & Yaghmaei, P. (2018). Improvement of stability and reusability of α-amylase immobilized on naringin functionalized magnetic nanoparticles: A robust nanobiocatalyst. International Journal of Biological Macromolecules, 113, 354–360.
De Maio, A., El-Masry, M. M., Portaccio, M., Diano, N., Di Martino, S., Mattei, A., et al. (2003). Influence of the spacer length on the activity of enzymes immobilised on nylon/polyGMA membranes: Part 1. Isothermal conditions. Journal of Molecular Catalysis B: Enzymatic, 21(4–6), 239–252.
Dey, G., Nagpal, V., & Banerjee, R. (2002). Immobilization of α-amylase from Bacillus circulans GRS 313 on coconut fiber. Applied Biochemistry and Biotechnology, 102(1–6), 303–313.
DiCosimo, R., McAuliffe, J., Poulose, A. J., & Bohlmann, G. (2013). Industrial use of immobilized enzymes. Chemical Society Reviews, 42(15), 6437–6474.
Doshi, P., Srivastava, G., Pathak, G., & Dikshit, M. (2014). Physicochemical and thermal characterization of nonedible oilseed residual waste as sustainable solid biofuel. Waste Management, 34(10), 1836–1846.
Emtiazi, G., Naghavi, N., & Bordbar, A. (2001). Biodegradation of lignocellulosic waste by Aspergillus terreus. Biodegradation, 12(4), 257–261.
Fang, J. M., Fowler, P. A., Tomkinson, J., & Hill, C. A. S. (2002). The preparation and characterisation of a series of chemically modified potato starches. Carbohydrate Polymers, 47(3), 245–252.
Fernández-Lorente, G., Palomo, J. M., Mateo, C., Munilla, R., Ortiz, C., Cabrera, Z., et al. (2006). Glutaraldehyde cross-linking of lipases adsorbed on aminated supports in the presence of detergents leads to improved performance. Biomacromolecules, 7(9), 2610–2615.
Ferreira-Dias, S., Sandoval, G., Plou, F., & Valero, F. (2013). The potential use of lipases in the production of fatty acid derivatives for the food and nutraceutical industries. Electronic Journal of Biotechnology, 16(3), 12–12.
Gaikwad, S. M., Rao, M. B., & Deshpande, V. V. (1992). d-Glucose/xylose isomerase from Streptomyces: Differential roles of magnesium and cobalt ions. Enzyme and Microbial Technology, 14(4), 317–320.
Gardossi, L., Poulsen, P. B., Ballesteros, A., Hult, K., Švedas, V. K., Vasić-Rački, Đ., et al. (2010). Guidelines for reporting of biocatalytic reactions. Trends in Biotechnology, 28(4), 171–180.
Garcia-Galan, C., Berenguer-Murcia, Á., Fernandez-Lafuente, R., & Rodrigues, R. C. (2011). Potential of different enzyme immobilization strategies to improve enzyme performance. Advanced Synthesis & Catalysis, 353(16), 2885–2904.
Gerard, M., Chaubey, A., & Malhotra, B. D. (2002). Application of conducting polymers to biosensors. Biosensors and Bioelectronics, 17(5), 345–359.
Godfray, H. C. J., Beddington, J. R., Crute, I. R., Haddad, L., Lawrence, D., Muir, J. F., et al. (2010). Food security: The challenge of feeding 9 billion people. Science, 327(5967), 812–818.
Gonzalez-Saiz, J. M., & Pizarro, C. (2001). Polyacrylamide gels as support for enzyme immobilization by entrapment. Effect of polyelectrolyte carrier, pH and temperature on enzyme action and kinetics parameters. European Polymer Journal, 37(3), 435–444.
Herzog, G., Gorgy, K., Gulon, T., & Cosnier, S. (2005). Electrogeneration and characterization of photoactivable films and their application for enzyme grafting. Electrochemistry Communications, 7(8), 808–814.
Hirohara, H., Yamamoto, H., Kawano, E., & Nabeshima, S. (1981) Immobilized lactase, its preparation and use. EP 0037667B1.
Homaei, A. A., Sariri, R., Vianello, F., & Stevanato, R. (2013). Enzyme immobilization: An update. Journal of Chemical Biology, 6(4), 185–205.
Hong, Y. G., Moon, Y. M., Hong, J. W., Choi, T. R., Jung, H. R., Yang, S. Y., Jang, D.-W., Park, Y.-R., Brigham, C. J., Kim, J.-S., Lee, Y.-K., & Lee, Y. K. (2019). Discarded egg yolk as an alternate source of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). Journal of Microbiology and Biotechnology, 29(3), 382–391.
Huang, W., Zhan, Y., Shi, X., Chen, J., Deng, H., & Du, Y. (2017). Controllable immobilization of naringinase on electrospun cellulose acetate nanofibers and their application to juice debittering. International Journal of Biological Macromolecules, 98, 630–636.
Hudson, S., Cooney, J., & Magner, E. (2008). Proteins in mesoporous silicates. Angewandte Chemie International Edition, 47(45), 8582–8594.
Hwang, E. T., & Lee, S. (2019). Multienzymatic cascade reactions via enzyme complex by immobilization. ACS Catalysis, 9(5), 4402–4425.
Ispas, C., Sokolov, I., & Andreescu, S. (2009). Enzyme-functionalized mesoporous silica for bioanalytical applications. Analytical and Bioanalytical Chemistry, 393(2), 543–554.
Kahraman, M. V., Bayramoğlu, G., Kayaman-Apohan, N., & Güngör, A. (2007). α-Amylase immobilization on functionalized glass beads by covalent attachment. Food Chemistry, 104(4), 1385–1392.
Katchalski-Katzir, E., & Kraemer, D. M. (2000). Eupergit® C, a carrier for immobilization of enzymes of industrial potential. Journal of Molecular Catalysis B: Enzymatic, 10(1–3), 157–176.
Kazemzadeh, S., Naghavi, N. S., Emami-Karvani, Z., Fouladgar, M., & Emtiazi, G. (2020). Gas chromatography-mass spectrometry analyses of crude oil bioremediation by the novel Klebsiella variicola SKV2 immobilized in polyurethane polymer scaffold and two-layer microcapsulation. Bioremediation Journal, 24(2–3), 129–149.
Keller, T. M., Michel, S. C., Fröhlich, J., Fink, D., Caduff, R., Marincek, B., & Kubik-Huch, R. A. (2004). USPIO-enhanced MRI for preoperative staging of gynecological pelvic tumors: Preliminary results. European Radiology, 14(6), 937–944.
Klein, M. P., Scheeren, C. W., Lorenzoni, A. S. G., Dupont, J., Frazzon, J., & Hertz, P. F. (2011). Ionic liquid-cellulose film for enzyme immobilization. Process Biochemistry, 46(6), 1375–1379.
Klis, M., Karbarz, M., Stojek, Z., Rogalski, J., & Bilewicz, R. (2009). Thermoresponsive poly (N-isopropylacrylamide) gel for immobilization of laccase on indium tin oxide electrodes. The Journal of Physical Chemistry B, 113(17), 6062–6067.
Klouda, L., & Mikos, A. G. (2008). Thermoresponsive hydrogels in biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics, 68(1), 34–45.
Kluchova, K., Zboril, R., Tucek, J., Pecova, M., Zajoncova, L., Safarik, I., et al. (2009). Superparamagnetic maghemite nanoparticles from solid-state synthesis–Their functionalization towards peroral MRI contrast agent and magnetic carrier for trypsin immobilization. Biomaterials, 30(15), 2855–2863.
Koh, W. G., & Pishko, M. (2005). Immobilization of multi-enzyme microreactors inside microfluidic devices. Sensors and Actuators B: Chemical, 106(1), 335–342.
Koller, M., Hesse, P., & Braunegg, G. (2019). Application of whey retentate as complex nitrogen source for growth of the polyhydroxyalkanoate producer Hydrogenophaga pseudoflava strain DSM1023. The EuroBiotech Journal, 3(2), 78–89.
Koller, M., Marsalek, L., & Braunegg, G. (2016). PHA Biopolyester production from surplus whey: Microbiological and engineering aspects. Recent Advances in Biotechnology, 1, 100–172.
Koller, M., Shahzad, K., & Braunegg, G. (2018). Waste streams of the animal-processing industry as feedstocks to produce polyhydroxyalkanoate biopolyesters. Applied Food Biotechnology, 5(4), 193–203.
Kooi, M. E., Cappendijk, V. C., Cleutjens, K. B. J. M., Kessels, A. G. H., Kitslaar, P. J. E. H. M., Borgers, M., et al. (2003). Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation, 107(19), 2453–2458.
Koszelewski, D., Müller, N., Schrittwieser, J. H., Faber, K., & Kroutil, W. (2010). Immobilization of ω-transaminases by encapsulation in a sol–gel/celite matrix. Journal of Molecular Catalysis B: Enzymatic, 63(1–2), 39–44.
Kouassi, G. K., Irudayaraj, J., & McCarty, G. (2005). Activity of glucose oxidase functionalized onto magnetic nanoparticles. BioMagnetic Research and Technology, 3(1), 1.
Krause, M. H., Kwong, K. K., Gragoudas, E. S., & Young, L. H. (2004). MRI of blood volume with superparamagnetic iron in choroidal melanoma treated with thermotherapy. Magnetic Resonance Imaging, 22(6), 779–787.
Kumar, M. B., Gao, Y., Shen, W., & He, L. (2015). Valorisation of protein waste: An enzymatic approach to make commodity chemicals. Frontiers of Chemical Science and Engineering, 9(3), 295–307.
Landarani-Isfahani, A., Taheri-Kafrani, A., Amini, M., Mirkhani, V., Moghadam, M., Soozanipour, A., & Razmjou, A. (2015). Xylanase immobilized on novel multifunctional hyperbranched polyglycerol-grafted magnetic nanoparticles: An efficient and robust biocatalyst. Langmuir, 31(33), 9219–9227.
Laskar, I. B., Rajkumari, K., Gupta, R., Chatterjee, S., Paul, B., & Rokhum, L. (2018). Waste snail shell derived heterogeneous catalyst for biodiesel production by the transesterification of soybean oil. RSC Advances, 8(36), 20131–20142.
Lei, S., Xu, Y., Fan, G., Xiao, M., & Pan, S. (2011). Immobilization of naringinase on mesoporous molecular sieve MCM-41 and its application to debittering of white grapefruit. Applied Surface Science, 257(9), 4096–4099.
Li, Z., Zhang, Y., Su, Y., Ouyang, P., Ge, J., & Liu, Z. (2014). Spatial co-localization of multi-enzymes by inorganic nanocrystal–protein complexes. Chemical Communications, 50(83), 12465–12468.
Lim, B. C., Kim, H. J., & Oh, D. K. (2008). Tagatose production with pH control in a stirred tank reactor containing immobilized L-arabinose isomerase from Thermotoga neapolitana. Applied Biochemistry and Biotechnology, 149(3), 245–253.
Liu, R., Huang, W., Pan, S., Li, Y., Yu, L., & He, D. (2020). Covalent immobilization and characterization of penicillin G acylase on magnetic Fe2O3/Fe3O4 heterostructure nanoparticles prepared via a novel solution combustion and gel calcination process. International Journal of Biological Macromolecules, 162, 1587–1596.
Lozinsky, V. I., Simenel, I. A., Kulakova, V. K., Kurskaya, E. A., Babushkina, T. A., Klimova, T. P., et al. (2003). Synthesis and studies of N-vinylcaprolactam/N-vinylimidazole copolymers that exhibit the “proteinlike” behavior in aqueous media. Macromolecules, 36(19), 7308–7323.
Luo, X., & Zhang, L. (2010). Immobilization of penicillin G acylase in epoxy-activated magnetic cellulose microspheres for improvement of biocatalytic stability and activities. Biomacromolecules, 11(11), 2896–2903.
Marangoni, A. G., & Rousseau, D. (1998). Chemical and enzymatic modification of butterfat and butterfat-canola oil blends. Food Research International, 31(8), 595–599.
Marguet, M., Bonduelle, C., & Lecommandoux, S. (2013). Multicompartmentalized polymeric systems: Towards biomimetic cellular structure and function. Chemical Society Reviews, 42(2), 512–529.
Mehnati-Najafabadi, V., Taheri-Kafrani, A., & Bordbar, A. K. (2018). Xylanase immobilization on modified superparamagnetic graphene oxide nanocomposite: Effect of PEGylation on activity and stability. International Journal of Biological Macromolecules, 107, 418–425.
Mao, Y., Černigoj, U., Zalokar, V., Štrancar, A., & Kulozik, U. (2017). Production of β-lactoglobulin hydrolysates by monolith based immobilized trypsin reactors. Electrophoresis, 38(22–23), 2947–2956.
Mateo, C., Palomo, J. M., Fernandez-Lorente, G., Guisan, J. M., & Fernandez-Lafuente, R. (2007). Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme and Microbial Technology, 40(6), 1451–1463.
Mehrasbi, M. R., Mohammadi, J., Peyda, M., & Mohammadi, M. (2017). Covalent immobilization of Candida antarctica lipase on core-shell magnetic nanoparticles for production of biodiesel from waste cooking oil. Renewable Energy, 101, 593–602.
Miletić, N., Abetz, V., Ebert, K., & Loos, K. (2010). Immobilization of Candida antarctica lipase B on polystyrene nanoparticles. Macromolecular Rapid Communications, 31(1), 71–74.
Miyanaga, M., Tanaka, T., Sakiyama, T., & Nakanishi, K. (1995). Synthesis of aspartame precursor with an immobilized thermolysin in mixed organic solvents. Biotechnology and Bioengineering, 46(6), 631–635.
Mohan, T., Rathner, R., Reishofer, D., Koller, M., Elschner, T., Spirk, S., et al. (2015). Designing hydrophobically modified polysaccharide derivatives for highly efficient enzyme immobilization. Biomacromolecules, 16(8), 2403–2411.
Nedwin, G. E., Sharma, V., & Shetty, J. K. (2014). Alpha-amylase blend for starch processing and method of use thereof. US 2014/0087429 A1, 2014.
Netto, C. G., Toma, H. E., & Andrade, L. H. (2013). Superparamagnetic nanoparticles as versatile carriers and supporting materials for enzymes. Journal of Molecular Catalysis B: Enzymatic, 85, 71–92.
Nisha, S., Karthick, S. A., & Gobi, N. (2012). A review on methods, application and properties of immobilized enzyme. Chemical Science Review and Letters, 1(3), 148–155.
Oh, D. K. (2007). Tagatose: Properties, applications, and biotechnological processes. Applied Microbiology and Biotechnology, 76(1), 1.
Olson, A. C., Gray, G. M., & Guadagni, D. G. (1979). Naringin bitterness of grapefruit juice debittered with naringinase immobilized in a hollow fiber. Journal of Food Science, 44(5), 1358–1361.
Ovsejevi, K., Manta, C., & Batista-Viera, F. (2013). Reversible covalent immobilization of enzymes via disulfide bonds. In Immobilization of enzymes and cells (pp. 89–116). Humana Press.
Oyama, K., Nishimura, S., Nonaka, Y., Kihara, K., & Hashimoto, T. (1981). Synthesis of an aspartame precursor by immobilized thermolysin in an organic solvent. The Journal of Organic Chemistry, 46(25), 5241–5242.
Panesar, P. S., Kumari, S., & Panesar, R. (2010). Potential applications of immobilized β-galactosidase in food processing industries. Enzyme Research, 2010, 473137.
Pedroche, J., del Mar Yust, M., Mateo, C., Fernández-Lafuente, R., Girón-Calle, J., Alaiz, M., et al. (2007). Effect of the support and experimental conditions in the intensity of the multipoint covalent attachment of proteins on glyoxyl-agarose supports: Correlation between enzyme–support linkages and thermal stability. Enzyme and Microbial Technology, 40(5), 1160–1166.
Pernicova, I., Enev, V., Marova, I., & Obruca, S. (2019). Interconnection of waste chicken feather biodegradation and keratinase and mcl-PHA production employing Pseudomonas putida KT2440. Applied Food Biotechnology, 6(1), 83–90.
Pierre, A. C. (2004). The sol-gel encapsulation of enzymes. Biocatalysis and Biotransformation, 22(3), 145–170.
Puri, M., Kaur, A., Singh, R., & Kanwar, J. (2008). Immobilized enzyme technology for debittering citrus fruit juices (pp. 91–103). Application of new technologies.
Puri, M., Marwaha, S. S., & Kothari, R. M. (1996). Studies on the applicability of alginate-entrapped naringiase for the debittering of kinnow juice. Enzyme and Microbial Technology, 18(4), 281–285.
Puri, M., Seth, M., Marwaha, S. S., & Kothari, R. M. (2001). Debittering of kinnow mandarin juice by covalently bound naringinase on hen egg white. Food Biotechnology, 15(1), 13–23.
Rana, M., Kumari, A., Chauhan, G. S., & Chauhan, K. (2014). Modified chitosan microspheres in non-aggregated amylase immobilization. International Journal of Biological Macromolecules, 66, 46–51.
Rathner, R., Petz, S., Tasnádi, G., Koller, M., & Ribitsch, V. (2017). Monitoring the kinetics of biocatalytic removal of the endocrine disrupting compound 17α-ethinylestradiol from differently polluted wastewater bodies. Journal of Environmental Chemical Engineering, 5(2), 1920–1926.
Ren, S., Li, C., Jiao, X., Jia, S., Jiang, Y., Bilal, M., & Cui, J. (2019). Recent progress in multienzymes co-immobilization and multienzyme system applications. Chemical Engineering Journal, 373, 1254–1278.
Reshmi, R., Sanjay, G., & Sugunan, S. (2006). Enhanced activity and stability of α-amylase immobilized on alumina. Catalysis Communications, 7(7), 460–465.
Roy, I., & Gupta, M. N. (2003). Lactose hydrolysis by Lactozym™ immobilized on cellulose beads in batch and fluidized bed modes. Process Biochemistry, 39(3), 325–332.
Sardar, M., Roy, I., & Gupta, M. N. (2000). Simultaneous purification and immobilization of Aspergillus niger xylanase on the reversibly soluble polymer EudragitTM L-100. Enzyme and Microbial Technology, 27(9), 672–679.
Schellenberger, E. A., Bogdanov Jr, A., Högemann, D., Tait, J., Weissleder, R., & Josephson, L. (2002). Annexin V–CLIO: A nanoparticle for detecting apoptosis by MRI. Molecular Imaging, 1(2), 15353500200202103.
Sheldon, R. A. (2007a). Cross-linked enzyme aggregates (CLEA® s): Stable and recyclable biocatalysts. Biochemical Society Transactions, 35(6), 1583–1587.
Sheldon, R. A. (2007b). Enzyme immobilization: The quest for optimum performance. Advanced Synthesis & Catalysis, 349(8–9), 1289–1307.
Sheldon, R. A., & Woodley, J. M. (2018). Role of biocatalysis in sustainable chemistry. Chemical Reviews, 118(2), 801–838.
Silva, J. A., Macedo, G. P., Rodrigues, D. S., Giordano, R. L. C., & Gonçalves, L. R. B. (2012). Immobilization of Candida antarctica lipase B by covalent attachment on chitosan-based hydrogels using different support activation strategies. Biochemical Engineering Journal, 60, 16–24.
Rodrigues, R. C., Berenguer-Murcia, Á., & Fernandez-Lafuente, R. (2011). Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Advanced Synthesis & Catalysis, 353(13), 2216–2238.
Rodrigues, R. C., Ortiz, C., Berenguer-Murcia, Á., Torres, R., & Fernández-Lafuente, R. (2013). Modifying enzyme activity and selectivity by immobilization. Chemical Society Reviews, 42(15), 6290–6307.
Tavano, O. L. (2013). Protein hydrolysis using proteases: An important tool for food biotechnology. Journal of Molecular Catalysis B: Enzymatic, 90, 1–11.
Tipton, K. F., Armstrong, R. N., Bakker, B. M., Bairoch, A., Cornish-Bowden, A., Halling, P. J., et al. (2014). Standards for reporting enzyme data: The STRENDA consortium: What it aims to do and why it should be helpful. Perspectives in Science, 1(1–6), 131–137.
Torres, R., Mateo, C., Fuentes, M., Palomo, J. M., Ortiz, C., Fernández-Lafuente, R., & Guisan, J. M. (2002). Reversible immobilization of invertase on Sepabeads coated with polyethyleneimine: Optimization of the biocatalyst’s stability. Biotechnology Progress, 18(6), 1221–1226.
Tosa, T., Sato, T., Mori, T., & Chibata, I. (1974). Basic studies for continuous production of L-aspartic acid by immobilized Escherichia coli cells. Applied Microbiology, 27(5), 886–889.
Tosa, T., Sato, T., Mori, T., Matuo, Y., & Chibata, I. (1973). Continuous production of L-aspartic acid by immobilized aspartase. Biotechnology and Bioengineering, 15(1), 69–84.
Tripathi, A., Sami, H., Jain, S. R., Viloria-Cols, M., Zhuravleva, N., Nilsson, G., Jungvid, H., & Kumar, A. (2010). Improved bio-catalytic conversion by novel immobilization process using cryogel beads to increase solvent production. Enzyme and Microbial Technology, 47(1–2), 44–51.
Tsen, H. Y. (1984). Factors affecting the inactivation of naringinase immobilized on chitin during debittering of fruit juice. Journal of Fermentation Technology, 62(3), 263–267.
Van de Velde, F., Lourenço, N. D., Pinheiro, H. M., & Bakker, M. (2002). Carrageenan: A food-grade and biocompatible support for immobilisation techniques. Advanced Synthesis & Catalysis, 344(8), 815–835.
Vescovi, V., Rojas, M. J., Baraldo, A., Botta, D. C., Santana, F. A. M., Costa, J. P., et al. (2016). Lipase-catalyzed production of biodiesel by hydrolysis of waste cooking oil followed by esterification of free fatty acids. Journal of the American Oil Chemists’ Society, 93(12), 1615–1624.
Vianello, F., Ragusa, S., Cambria, M. T., & Rigo, A. (2006). A high sensitivity amperometric biosensor using laccase as biorecognition element. Biosensors and Bioelectronics, 21(11), 2155–2160.
Villeneuve, P. (2007). Lipases in lipophilization reactions. Biotechnology Advances, 25(6), 515–536.
Virtanen, J., Baron, C., & Tenhu, H. (2000). Grafting of poly (N-isopropylacrylamide) with poly (ethylene oxide) under various reaction conditions. Macromolecules, 33(2), 336–341.
Virtanen, J., & Tenhu, H. (2000). Thermal properties of poly (N-isopropylacrylamide)-g-poly (ethylene oxide) in aqueous solutions: Influence of the number and distribution of the grafts. Macromolecules, 33(16), 5970–5975.
Walle, T. (2009). Methylation of dietary flavones increases their metabolic stability and chemopreventive effects. International Journal of Molecular Sciences, 10(11), 5002–5019.
Xu, K., Chen, X., Zheng, R., & Zheng, Y. (2020). Immobilization of multi-enzymes on support materials for efficient biocatalysis. Frontiers in Bioengineering and Biotechnology, 8.
Zhaoyu, Z., Ping, X., Keren, S., Weiwei, Z., Chunmiao, H., & Peng, L. (2020). Di-functional magnetic nanoflowers: A highly efficient support for immobilizing penicillin G acylase. Journal of the Chinese Chemical Society, 67, 1591–1601.
Zhi, Y., Wu, Q., & Xu, Y. (2017). Production of surfactin from waste distillers’ grains by co-culture fermentation of two Bacillus amyloliquefaciens strains. Bioresource Technology, 235, 96–103.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Naghavi, N.S., Sanei, N., Koller, M. (2022). Enzyme Immobilization and Its Application Strategies in Food Products. In: Dutt Tripathi, A., Darani, K.K., Srivastava, S.K. (eds) Novel Food Grade Enzymes . Springer, Singapore. https://doi.org/10.1007/978-981-19-1288-7_15
Download citation
DOI: https://doi.org/10.1007/978-981-19-1288-7_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1287-0
Online ISBN: 978-981-19-1288-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)