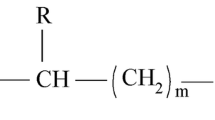Abstract
In the present time, polyhydroxyalkanoates have established itself as the alternatives of petroleum-based synthetic polymers due to their biodegradability and eco-friendly nature. Several efforts have been done toward this direction by using microorganisms. Since the last two decades, several scientists have engaged in search of cost-effective alternatives of producing polyhydroxyalkanoates at larger scales. Therefore, many plant species have been genetically engineered for this purpose. The major obstacles in producing PHA polymers in transgenic plants are the regulation of the appropriate monomer’s composition and ratio synthesized in their cells. Efforts are on the way to encounter these difficulties as soon as possible. Among the targeted cell organelles, plastids have been considered as the best sites for higher production of polyhydroxyalkanoates because of its maternal inheritance and it is unaffected by gene silencing. The research is also going on for enhancing the production and accumulation of these biopolymers in transgenic plants. Polyhydroxyalkanoate production technologies are still costly, but these could be cost-effective in the near future. The present chapter describes about the current status of transgenic plants developed for the production of polyhydroxyalkanoates at cheaper costs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Over the past 60 years, the development of synthetic polymers has reduced the men’s dependence upon the utilization of plant product-based polymers such as rubber, cotton, wood, etc. But the excessive and ever-growing demands of utilizing synthetic polymers raise the global environmental concerns. Therefore, the scientific community has engaged to discover alternatives of synthetic polymers. As an alternative of synthetic polymers, polyhydroxyalkanoates (PHAs) came into existence. These are the natural products of certain bacterial cells. Therefore, scientists decided to produce biological polymers by using bacterial cells as bioreactors. This was first time exploited in 1980 by Imperial Chemical Industries (Anderson and Dawes 1990). This industry generated a large setup for the bulk production of polyhydroxyalkanoates using polymer-accumulating bacterial strains such as Ralstonia eutropha. But the cost of producing PHAs through this way was very high. So again there were also the requirements of cost-effective alternatives for producing biopolymers. The development of advanced biotechnological tools attracted the concern of global scientists toward the utilization of plant bioreactors for producing renewable biological polymers. Therefore, the scientific community moved toward the utilization of plant bioreactors for generating polyhydroxyalkanoates (PHAs) in a cost-effective and eco-friendly manner. For this purpose, a variety of transgenic plant species including Arabidopsis, tobacco, rapeseed, cotton, alfalfa, flax, sugarcane, Camelina, and oil palm were tested at larger scales and generated new dimensions of producing biopolymers.
Polyhydroxyalkanoates (PHAs) are biopolyesters that are synthesized naturally in a broad range of bacterial cells such as Alcaligenes eutrophus and many other species as an inert carbon and energy reserve accumulated in the cytoplasm up to about 80% of the total dry weight in the form of round-shaped granules with a diameter of 0.2–1.0 μm (Sabbagh and Muhamad 2017). These polymers are made up of about 600–35,000 identical monomer units. Polyhydroxyalkanoates act as water-insoluble storage compounds which are synthesized under environmental stress conditions in the excess of carbon and the limiting quantities of important growth nutrients such as nitrogen, phosphorus, iron, magnesium, potassium, sulfur, zinc, or oxygen (Masood et al. 2014). These biopolymers are depolymerized during the exhausted carbon source conditions. Thus, the degraded products could be used by microbes as an energy and carbon source (Anderson and Dawes 1990).
Polyhydroxyalkanoates (PHAs) are considered similar to the conventional plastics in reference of its properties such as thermoplastic and polypropylene nature (Anjum et al. 2016). Instead of petrochemical plastics, PHAs are natural, nontoxic, biodegradable, and renewable (Sharma et al. 2016). These properties make PHA an attractive alternative of petrochemical plastic. In the near future, it is hopefully projected that the production of synthetic plastic polymers could possibly reached up to about eight hundred ten million tons (810 million tons) by the end of 2050 (Gumel et al. 2013). As it is well known that plastic pollution has been an unbeatable burning issue across the globe, it has been an urgent necessity to find out the eco-friendly alternatives of synthetic plastics. That’s why, the scientific community and industries are engaging to produce synthetic polymers through natural means. Generally microbial bioreactors are utilized in producing PHAs at larger scales, but the whole process of bioplastic polymer production is still highly expensive than the process of producing petrochemical-based synthetic polymers due to the cost of the nutrition for microbial cultures (Baikar et al. 2017). So the scientific community has engaged in optimizing transgenic plants as novel bioreactors for production of PHAs at cheaper costs. The present chapter summarizes the information about PHA, its structure, biosynthesis, and the current status of transgenic plants which were engineered for producing PHAs at cheaper costs.
2 PHA Structure and Biosynthesis
Polyhydroxyalkanoates are generally linear polyesters consisting of several 3-(R)-hydroxy fatty acid monomers (HA) linked together by ester bonds. These ester bonds are produced by the linkage of carboxylic group of one monomer unit to the hydroxyl group of another monomer unit (Sudesh et al. 2000; Lenz and Marchessault 2005). On the basis of the presence of carbon atoms in the monomers, polyhydroxyalkanoates are generally categorized into two major groups. The first group is called short chain length polyhydroxyalkanoates (scl-PHAs), and the second group is medium chain length polyhydroxyalkanoates (mcl-PHAs). Short chain length polyhydroxyalkanoates (scl-PHAs) generally consist of 3–5 carbon atoms, whereas the medium chain length polyhydroxyalkanoates (scl-PHAs) have 6–14 carbons. Under natural conditions, the short chain length polyhydroxyalkanoates (scl-PHAs) are synthesized in Cupriavidus necator, while the medium chain length polyhydroxyalkanoates (scl-PHAs) are accumulated in Pseudomonas species. The examples of short chain length PHAs are P(3HB) [poly-3-hydroxybutyrate], P(4HB) [poly-4-hydroxybutyrate], and P(3HV) [Poly(3-hydroxyvalerate)] or the copolymer P(3HB-co-3HV), whereas the P(3HHx) [poly-3-hydroxyhexanoate], P(3HO) [poly-3-hydroxyoctanoate], and copolymer P(3HHx-co-3HO) are considered as medium chain length PHAs (Kim and Lenz 2001). Each polyhydroxyalkanoate (PHA) polymer generally consists of about 1000–10,000 monomers, but most of them are synthesized by short chain length (SCL) monomer units (Van der walle et al. 2001). The chemical structure of polyhydroxyalkanoates (PHAs) is depicted in Fig. 15.1.
The chemical structure of polyhydroxyalkanoates (PHAs). The pendant R groups (shaded boxes) vary in chain length from 1 carbon (C1) to over 14 carbons (C14). Structures shown here are poly-3-hydroxybutyrate (PHB) [R = methyl], poly-3-hydroxyvalerate (PHV) [R = ethyl], and poly-3-hydroxyhexanoate (PHH) or poly-4-hydroxybutyrate (P4HB) [R = propyl]. (Adapted from Suriyamongkol et al. 2007)
Most of our knowledge about biosynthesis of polyhydroxyalkanoate is mainly based upon the studies on the production of polyhydroxybutyrate (PHB) in cytoplasm of a gram-negative soil bacterium Ralstonia eutrophus or Alcaligenes eutrophus bacteria. This bacterium has the capability of producing polyhydroxyalkanoate in a natural way and could accumulate the polymer up to 85% of its total dry body weight when grown on culture media with excess of glucose. In this way, it acts as an energy source, but the production of this polymer is limited when there is growth-limiting conditions such as lack of macroelements such as nitrogen, phosphorus, and trace elements or the lack of oxygen in culture media (De Koning 1995). Previous studies reported that the polyhydroxybutyrate (PHB) could be depolymerized into acetoacetate and further into acetyl coenzyme A (CoA) by applying growth-limiting conditions (Steinbuchel and Valentin 1995). Therefore, it is clearly demonstrated that the acetyl coenzyme A acts as a precursor of polyhydroxybutyrate biosynthesis in bacterial cell. Polyhydroxybutyrate (PHB) decomposes into 3-hydrobutyrate (3-HB) monomers that can be used by fungi and bacteria as carbon sources. The biosynthesis of PHB was first time described in 1973 in a bacterium Ralstonia eutrophus by Gottingen and Hull (Senior and Dawes 1973). There are three key enzymes, namely, acetoacetyl-CoA reductase, 3-ketothiolase, and PHA synthase which leads to the production of polyhydroxybutyrate by using acetyl-CoA. PHA synthase uses CoA thioester of (R)-hydroxy fatty acids as substrate. The enzyme popularly known as 3-ketothiolase encoded by gene phaA or phbA is mainly responsible for catalyzing the reversible condensation of two molecules of acetyl-CoA into acetoacetyl-CoA molecule. The acetoacetyl-CoA reductase encoded by phaB or phbB gene reduces acetoacetyl-CoA into R-(−)-3-hydroxybutyryl-CoA. After that the R-(−)-3-hydroxybutyryl-CoA finally polymerizes into polyhydroxybutyrate (PHB) by the action of PHA synthase enzyme encoded by a gene called phaC or phbC (Yunus et al. 2008; Kosseva and Rusbandi 2018). The polyhydroxyalkanoates (PHAs) biosynthesis pathway is schematically depicted in Fig. 15.2. Polyhydroxyalkanoates are generally biosynthesized through two possible routes. The first route is based upon β-oxidation pathway intermediates and also on alkanoic acids. In this process, the levorotatory S-3-hydroxyacyl-CoA is converted into R-3-hydroxyacyl-CoA, a dextrorotatory enantiomer by the action of an enzyme epimerase. In the second route, the fatty acid biosynthesis intermediates such as R-3-hydroxyacyl-ACP are used. In this process, the acyl carrier protein (ACP) is replaced by coenzyme A using an important enzyme 3-hydroxyacyl-CoA-ACP transacylase. Both these processes are completed by a gene called phaC [Kosseva and Rusbandi 2018].
The medium chain length polyhydroxyalkanoates (mcl-PHA) biosynthesis pathways occur in the peroxisomes of transgenic plants. Here, enzyme ACD = acyl-CoA dehydrogenase; ECH = enoyl-CoA hydratase; HCD = l-3-hydroxyacyl-CoA dehydrogenase; and KT = β-ketothiolase. (Reproduced from Dobrogojski et al. 2018)
3 PHA Production in Transgenic Plants
The production of PHAs by using microorganisms is costly because of various factors such as variety and amount of nutrition supplied for microbes, optimized growth environment, and sterilized conditions (Din et al. 2012; Mozejko-Ciesielska and Kiewisz 2016). Therefore in comparison to microbes, transgenic plants are considered as cheaper eco-friendly alternatives. The biosynthesis of PHAs in transgenic plants mainly depends upon mineral salts, water, light, and carbon dioxide (CO2). The PHA production in transgenic plants is generally based upon the availability of acetyl-CoA, a primary substrate for PHA biosynthesis, because the plant cells do not have the abilities to degrade PHA as the microbes do. Acetyl-CoA is the main metabolite of plant’s catabolic and anabolic processes. The plant cellular compartments such as cytoplasm, mitochondria, peroxisomes, and plastids are rich in acetyl-CoA. Therefore, the scientists targeted these compartments as the major sites for producing and accumulating various PHAs in transgenic plants. The literature showed that the first experimental research attempt for producing PHA was successfully achieved in the cytoplasm of transgenic Arabidopsis thaliana. After this work several another research experiments were also conducted using various plant species. But the deficiency of acetyl-CoA and acetoacetyl-CoA because of their utilization in plant hormone and steroid biosynthesis pathways limited the production of PHAs inside plant cell cytoplasm. Like cytoplasm, mitochondria also have the limitations of the deficiency of acetyl-CoA because of its utilization during cellular respiration. Plastids appear to the best site for PHA biosynthesis in plants because there acetyl-CoA is present in higher concentrations and mainly utilized for the biosynthesis of fatty acids. The plastids are the organelles which work properly despite the structural changes and have the ability to store larger starch granules. But the plastids do not have the stocks of beta ketothiolases. The beta ketothiolases are located in the cell’s cytoplasm. This problem could be overcome by applying specific DNA-encoding plastid-targeted sequences inserting in the vectors. The peroxisomes are also considered as high potential sites for the production of PHAs in transgenic plant cells because of having high reductive strength of NADH and their beta oxidation of fatty acids. Peroxisomes are important cell organelles because of synthesizing medium chain length polyhydroxyalkanoates (mcl-PHAs). Since the last two decades, several scientists are doing research on producing PHAs in transgenic plants. The detailed information regarding the current status of transgenic plants developed for producing polyhydroxyalkanoates are given in Table 15.1.
4 Conclusion and Future Prospects
The environmental pollution generated through petroleum-based synthetic polymers has become a very big global challenge. The production of synthetic polymers is increasing day by day, and now it has appeared in an unbeatable form of pollutants. The management of the plastic and its products is not an easy task; it takes several hundreds of years to be decomposed. Therefore, it is a need of present time to find out eco-friendly biodegradable alternatives. Polyhydroxyalkanoates (PHAs) have appeared as a smart choice of scientific community as well as industry in the form of plastic alternatives. PHAs are the major class of biodegradable biopolymers which are biosynthesized by microorganisms in a natural way. The production of PHAs by using microorganisms is costly because of various factors such as variety and amount of nutrition supplied for microbes, optimized growth environment, and sterilized conditions. Therefore in comparison to microbes, transgenic plants are considered as cheaper eco-friendly alternatives. The biosynthesis of PHAs in transgenic plants mainly depends upon mineral salts, water, light, and carbon dioxide (CO2). Some cellular compartments such as cytoplasm, mitochondria, peroxisomes, and plastids have been targeted as important sites for producing and accumulating PHAs in transgenic plants. Since the last two decades, several scientists have engaged in research for optimizing transgenic plants as bioreactors for producing PHAs. Several plant species including Arabidopsis thaliana, Camelina, tobacco, sugarcane, maize, rapeseed, flax, cotton, and oil palm have been genetically engineered for producing PHAs. But till date, no one plant species is released for commercial production of biopolymers. The research is on the way, things are optimizing, and we hopefully expect that the transgenic plants would be available in the near future for producing PHAs at commercial scales.
References
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Anderson D, Gnanasambandam A, Mills E, O’Shea M, Nielsen L, Brumbley S (2011) Synthesis of short-chain length/medium chain length polyhydroxyalkanoate (PHA) copolymers in peroxisomes of transgenic sugarcane plants. Trop Plant Biol 4(3):170–184
Anjum A, Zuber M, Zia KM, Noreen A, Anjum MN, Tabasum S (2016) Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: a review of recent advancements. Int J Biol Macromol 89:161–174
Arai Y, Nakashita H, Doi Y, Yamaguchi I (2001) Plastid targeting of polyhydroxybutyrate biosynthetic pathway in tobacco. Plant Biotechnol 18:289–293
Arai Y, Nakashita H, Suzuki Y, Kobayashi Y, Shimizu T, Yasuda M, Doi Y, Yamaguchi I (2002) Synthesis of a novel class of polyhydroxyalkanoates in Arabidopsis peroxisomes and their use in monitoring short-chain-length intermediates of β-oxidation. Plant Cell Physiol 43(5):555–562
Arai Y, Shikanai T, Doi Y, Yoshida S, Yamaguchi I, Nakashita H (2004) Production of polyhydroxybutyrate by polycistronic expression of bacterial genes in tobacco plastid. Plant Cell Physiol 45(9):1176–1184
Ariffin N, Abdullah R, Muad MR, Lourdes J, Emran NA, Ismail MR, Ismail I, Fadzil MFM, Ling KL, Siddiqui Y, Amir AA, Berahim Z, Omar MH (2011) Constructions of expression vectors of polyhydroxybutyrate-co-hydroxyvalerate (PHBV) and transient expression of transgenes in immature oil palm embryos. Plasmid 66:136–143
Baikar V, Rane A, Deopurkar R (2017) Characterization of polyhydroxyalkanoate produced by Bacillus megaterium VB89 isolated from Nisargruna biogas plant. Appl Biochem Biotechnol 183:241–253
Bohmert K, Balbo I, Kopka J, Mittendorf V, Nawrath C, Poirier Y, Tischendorf G, Trethewey RN, Willmitzer L (2000) Transgenic Arabidopsis plants can accumulate polyhydroxybutyrate to up to 4% of their fresh weight. Planta 211:841–845
Bohmert K, Balbo I, Steinbuchel A, Tischendorf G, Willmitzer L (2002) Constitutive expression of the beta-ketothiolase gene in transgenic plants: a major obstacle for obtaining polyhydroxybutyrate-producing plants. Plant Physiol 128:1282–1290
Bohmert-Tatarev K, McAvoy S, Daughtry S, Peoples OP, Snell KD (2011) High levels of bioplastic are produced in fertile transplastomic tobacco plants engineered with a synthetic operon for the production of polyhydroxybutyrate. Plant Physiol 155(4):1690–1708
Brumbley SM, Petrasovits LA, Bonaventura PA, Oshea MJ, Purnell MP, Nielsen LK (2003) Production of polyhydroxyalkanoates in sugarcane. In: Proc. of Int. Soc. of Sugarcane Tech., Molecular Biology Workshop, Montpellier, France, vol 4, p 31
Dalton DA, Ma C, Shrestha S, Kitin P, Strauss SH (2011) Trade-offs between biomass growth and inducible biosynthesis of polyhydroxybutyrate in transgenic poplar. Plant Biotechnol J 9(7):759–767
De Koning G (1995) Physical properties of bacterial poly((R)-3-hydroxyalkanoates). Can J Microbiol 41:303–309
Din MF, Mohanadoss P, Ujang Z, Van Loosdrecht M, Yunus SM, Chelliapan S, Zambare V, Olsson G (2012) Development of Bio-PORec® system for polyhydroxyalkanoates (PHA) production and its storage in mixed cultures of palm oil mill effluent (POME). Bioresour Technol 124:208–216
Dobrogojski J, Spychalski M, Lucinski R, Borek S (2018) Transgenic plants as a source of polyhydroxyalkanoates. Acta Physiol Plant 40:162–178
Endo N, Yoshida K, Akiyoshi M, Manji S (2006) Hybrid fiber production: a wood and plastic combination in transgenic rice and Tamarix made by accumulating poly-3-hydroxybutyrate. Plant Biotechnol 23:99–109
Fuad FAA, Ismail I, Sidik N, Zain CRCM, Abdullah R (2008) Super binary vector system enhanced transformation frequency and expression level of polyhydroxyvalerate gene in oil palm immature embryo. Asian J Plant Sci 7(6):526–535
Gumel AM, Annuar MSM, Chisti Y (2013) Recent advances in the production, recovery and applications of polyhydroxyalkanoates. J Polym Environ 21:580–605
Hahn JJ, Eschenlauer AC, Sleytr UB, Somers DA, Srienc F (1999) Peroxisomes as sites for synthesis of polyhydroxyalkanoates in transgenic plants. Biotechnol Prog 15:1053–1057
Houmiel KL, Slater S, Broyles D (1999) Poly (β-hydroxybutyrate) production in oilseed leukoplasts of Brassica napus. Planta 209:547–550
Ismail I, Iskandar NF, Chee GM, Abdullah R (2010) Genetic transformation and molecular analysis of polyhydroxybutyrate biosynthetic gene expression in oil palm (Elaeis guineensis Jacq. var Tenera) tissues. Plant Omics 3:18–27
John ME, Keller G (1996) Metabolic pathway engineering in cotton: biosynthesis of polyhydroxybutyrate in fiber cells. Proc Natl Acad Sci U S A 93:12768–12773
Kim YB, Lenz RW (2001) Polysters from microorganisms. Adv Biochem Eng Biotechnol 71:51–79
Kosseva MR, Rusbandi R (2018) Trends in the biomanufacture of polyhydroxyalkanoates with focus on downstream processing. Int J Biol Macromol 107:762–778
Kourtz L, Dillon K, Daughtry S, Madison LL, Peoples O, Snell KD (2005) A novel thiolase-reductase gene fusion promotes the production of polyhydroxybutyrate in Arabidopsis. Plant Biotechnol J 3:435–447
Kourtz L, Dillon K, Daughtry S, Madison LL, Peoples O, Snell KD (2007) Chemically inducible expression of the PHB biosynthetic pathway in Arabidopsis. Transgenic Res 16:759–769
Kulma A, Skorkowska-Telichowska K, Kostyna K, Szatkowskia M, Skała J, Drulis-Kawa Z, Preisnera M, Zuk M, Szperlika J, Wang YF, Szopaa J (2015) New flax producing bioplastic fibers for medical purposes. Ind Crop Prod 68:80–90
Lenz RW, Marchessault RH (2005) Bacterial polyesters: biosynthesis, biodegradable plastics and biotechnology. Biomacromolecules 6:1–8
Lossl A, Eibl C, Harloff HJ, Jung C, Koop HU (2003) Polyester synthesis in transplastomic tobacco (Nicotiana tabacum L.): significant contents of polyhydroxybutyrate are associated with growth reduction. Plant Cell Rep 21:891–899
Lossl A, Bohmert K, Harloff H, Eibl C, Muhlbauer S, Koop H (2005) Inducible trans-activation of plastid transgenes: expression of the R. eutropha phb operon in transplastomic tobacco. Plant Cell Physiol 46(9):1462–1471
Malik MR, Yang W, Patterson N, Tang J, Wellinghoff RL, Preuss ML, Burkitt C, Sharma N, Ji Y, Jez JM, Peoples OP, Jaworski JG, Cahoon EB, Snell KD (2015) Production of high levels of poly-3-hydroxybutyrate in plastids of Camelina sativa seeds. Plant Biotechnol J 13(5):675–688
Masood F, Yasin T, Hameed A (2014) Polyhydroxyalkanoates—what are the uses? Current challenges and perspectives. Crit Rev Biotechnol 35:514–521
Matsumoto K, Nagao R, Murata T, Arai Y, Kichise T, Nakashita H, Taguchi S, Shimada H, Doi Y (2005) Enhancement of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production in the transgenic Arabidopsis thaliana by the in vitro evolved highly active mutants of polyhydroxyalkanoate (PHA) synthase from Aeromonas caviae. Biomacromolecules 69(4):2126–2130
Matsumoto K, Arai Y, Nagao R, Murata T, Takase K, Nakashita H, Taguchi S, Shimada H, Doi Y (2006) Synthesis of short-chain-length/medium-chain-length polyhydroxyalkanoate (PHA) copolymers in peroxisome of the transgenic Arabidopsis thaliana harboring the PHA synthase gene from Pseudomonas sp. 61-3. J Polym Environ 14:369–374
Matsumoto K, Murata T, Nagao R, Nomura CT, Arai S, Arai Y, Takase K, Nakashita H, Taguchi S, Shimada H (2009) Production of short-chain-length/medium-chain-length polyhydroxyalkanoate (PHA) copolymer in the plastid of Arabidopsis thaliana using an engineered 3-ketoacyl-acyl carrier protein synthase III. Biomacromolecules 10(4):686–690
Matsumoto K, Morimoto K, Gohda A, Shimada H, Taguchi S (2011) Improved polyhydroxybutyrate (PHB) production in transgenic tobacco by enhancing translation efficiency of bacterial PHB biosynthetic genes. J Biosci Bioeng 111(4):485–488
McQualter RB, Fong Chong B, O’Shea M, Meyer K, van Dyk DE, Viitanen PV, Brumbley SM (2005) Initial evaluation of sugarcane as a production platform for a p-hydroxybenzoic acid. Plant Biotechnol J 3(1):29–41
McQualter RB, Petrasovits LA, Gebbie LK, Schweitzer D, Blackman DM, Chrysanthopoulos P, Hodson MP, Plan MR, Riches JD, Snell KD, Brumbley SM, Nielsen LK (2015) The use of an acetoacetyl-CoA synthase in place of a β-ketothiolase enhances poly-3-hydroxybutyrate production in sugarcane mesophyll cells. Plant Biotechnol J 13(5):700–707
Menzel G, Harloff HJ, Jung C (2003) Expression of bacterial poly (3-hydroxybutyrate) synthesis genes in hairy roots of sugar beet (Beta vulgaris L.). Appl Microbiol Biotechnol 60:571–576
Mitsky TA, Slater S, Reiser SE, Hao M, Houmiel KL (2003) Multigene expression vectors for the biosynthesis of products via multienzyme biological pathways. US Patent Application, 2003/0182678
Mittendorf V, Robertson EJ, Leech RM, Kruger N, Steinbuchel A, Poirier Y (1998) Synthesis of medium-chain-length polyhydroxyalkanoates in Arabidopsis thaliana using intermediates of peroxisomal fatty acid β-oxidation. Proc Natl Acad Sci U S A 95:13397–13402
Mozejko-Ciesielska J, Kiewisz R (2016) Bacterial polyhydroxyalkanoates: still fabulous? Microbiol Res 192:271–282
Mozes-Koch R, Tanne E, Brodezki A, Yehuda R, Gover O, Rabinowitch HD, Sela I (2017) Expression of the entire polyhydroxybutyrate operon of Ralstonia eutropha in plants. J Biol Eng 11(1):44
Nakashita H, Arai Y, Yoshioka K, Fukui T, Doi Y, Usami R, Horikoshi K, Yamaguchi I (1999) Production of biodegradable polyester by a transgenic tobacco. Biosci Biotechnol Biochem 63:870–874
Nakashita H, Arai Y, Shikanai T, Doi Y, Yamaguchi I (2001a) Introduction of bacterial metabolism into higher plants by polycistronic transgene expression. Biosci Biotechnol Biochem 65:1688–1691
Nakashita H, Arai Y, Suzuki Y, Yamaguchi I (2001b) Molecular breeding of transgenic tobacco plants which accumulate polyhydroxyalkanoates. RIKEN Rev 42:67–70
Nawrath C, Poirier Y, Somerville C (1994) Targeting of the polyhydroxybutyrate biosynthetic-pathway to the plastids of Arabidopsis thaliana results in high-levels of polymer accumulation. Proc Natl Acad Sci U S A 91:12760–12764
Omar WSW, Willis LB, Rha C, Sinskey AJ, Ramli US, Matyunus AM, Parveez GKA, Sambanthamurthi RD (2008) Isolation and utilization of acetyl-coa carboxylase from oil palm (Elaeis guineensis) mesocarp. J Oil Palm Res 2:97–107
Omidvar V, Akmar ASN, Marziah M, Maheran AA (2008) A transient assay to evaluate the expression of polyhydroxybutyrate genes regulated by oil palm mesocarp-specific promoter. Plant Cell Rep 27(9):1451–1459
Parveez GKA, Bahariah B, Nur Hanin A, Masani AMY, Tarmizi AH, Zamzuri I (2008) Transformation of PHB and PHBV genes driven by maize ubiquitin promoter into oil palm for the production of biodegradable plastics. J Oil Palm Res 2:76–86
Parveez GKA, Bahariah B, Ayub NH, Masani MYA, Rasid OA, Tarmizi AH, Ishak Z (2015) Production of polyhydroxybutyrate in oil palm (Elaeis guineensis Jacq.) mediated by microprojectile bombardment of PHB biosynthesis genes into embryogenic calli. Front Plant Sci 6:598
Patterson N, Tang J, Cahoon EB, Jaworski JG, Wang W, Peoples OP, Snell KD (2011) Generation of high polyhydroxybutyrate producing oilseeds. Int Patent Appl, WO/2011/034945
Petrasovits LA, Purnell MP, Nielsen LK, Brumbley SM (2007) Production of polyhydroxybutyrate in sugarcane. Plant Biotechnol J 5(1):162–172
Petrasovits LA, Zhao L, McQualter RB, Snell KD, Somleva MN, Patterson NA, Nielsen LK, Brumbley SM (2012) Enhanced polyhydroxybutyrate production in transgenic sugarcane. Plant Biotechnol J 10(5):569–578
Petrasovits LA, McQualter RB, Gebbie LK, Blackman DM, Nielsen LK, Brumbley SM (2013) Chemical inhibition of acetyl coenzyme a carboxylase as a strategy to increase polyhydroxybutyrate yields in transgenic sugarcane. Plant Biotechnol J 11(9):1146–1151
Poirier Y, Gruys KJ (2001) Production of PHAs in transgenic plants. In: Babel W, Steinbuchel A (eds) Biopolyesters. Springer, New York, NY, pp 209–240
Poirier Y, Dennis DE, Klomparens K, Somerville C (1992) Polyhydroxybutyrate a biodegradable thermoplastic, produced in transgenic plants. Science 256:520–523
Poirier Y, Somerville C, Schechtman LA, Satkowski MM, Noda I (1995) Synthesis of high-molecular-weight poly ([R]-(-)-3-hydroxybutyrate) in transgenic Arabidopsis thaliana plant cells. Int J Biol Macromol 17:7–12
Purnell MP, Petrasovits LA, Nielsen LK, Brumbley DW (2007) Spatiotemporal characterisation of polyhydroxybutyrate accumulation in sugarcane. Plant Biotechnol J 5:173–184
Romano A, Vreugdenhil D, Jamar D, Vander Plas LHW, De Roo G, Witholt B, Eggink G, Mooibroek H (2003) Evidence of medium-chain-length polyhydroxyoctanoate accumulation in transgenic potato lines expressing the Pseudomonas oleovorans Pha-C1 polymerase in the cytoplasm. Biochem Eng J 16(2):135–143
Romano A, Van der Plas LHW, Witholt B, Eggink G, Mooibroek H (2005) Expression of poly-3-(R)-hydroxyalkanoate (PHA) polymerase and acyl-CoA-transacylase in plastids of transgenic potato leads to the synthesis of a hydrophobic polymer, presumably medium-chain-length PHAs. Planta 3(220):455–464
Sabbagh F, Muhamad I (2017) Production of polyhydroxyalkanoate as secondary metabolite with main focus on sustainable energy. Renew Sust Energ Rev 72:95–104
Saruul P, Srienc F, Somers DA, Samac DA (2002) Production of a biodegradable plastic polymer, poly-beta-hydroxybutyrate in transgenic alfalfa. Crop Sci 42:919–927
Schnell JA, Treyvaud-Amiguet V, Arnason JT, Johnson DA (2012) Expression of polyhydroxybutyric acid as a model for metabolic engineering of soybean seed coats. Transgenic Res 4(21):895–899
Senior PJ, Dawes EA (1973) The regulation of poly ß-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem J 134:225–238
Sharma L, Srivastava JK, Singh AK (2016) Biodegradable polyhydroxyalkanoate thermoplastics substituting xenobiotic plastics: a way forward for sustainable environment. In: Singh A, Prasad S, Singh R (eds) Plant responses to xenobiotics. Springer, Singapore, pp 317–346
Slater S, Mitsky TA, Houmiel KL (1999) Metabolic engineering of Arabidopsis and Brassica for poly (3-hydroxybutyrateco-3-hydroxyvalerate) copolymer production. Nat Biotechnol 17:1011–1016
SomlevaM, Ali A (2010) Propagation of transgenic plants. Int Patent Appl WO/2010/102220
Somleva MN, Snell KD, Beaulieu JJ, Peoples OP, Garrison BR, Patterson NA (2008) Production of polyhydroxybutyrate in switchgrass, a value-added co-product in an important lignocellulosic biomass crop. Plant Biotechnol J 6(7):663–678
Somleva M, Chinnapen H, Ali A, Snell KD, Peoples OP, Patterson N, Tang J, Bohmert-Tatarev K (2012) Increasing carbon flow for polyhydroxybutyrate production in biomass crops. US Patent Appl 2012/0060413
Steinbuchel A, Valentin HE (1995) Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett 128:219–228
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25:1503–1555
Suriyamongkol P, Weselake R, Narine S, Moloney M, Shah S (2007) Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants—a review. Biotechnol Adv 25:148–175
Suzuki Y, Kurano M, Arai Y, Nakashita H, Doi Y, Usami R, Horikoshi K, Yamaguchi I (2002) Enzyme inhibitors to increase poly-3-hydroxybutyrate production by transgenic tobacco. Biosci Biotechnol Biochem 66:2537–2542
Szopa J, Wrobel-Kwiatkowska M, Kulma A, Zuk M, Skorkowska-Telichowska K, Dyminska L, Maczka M, Hanuza J, Zebrowski J, Preisner M (2009) Chemical composition and molecular structure of fibers from transgenic flax producing polyhydroxybutyrate, and mechanical properties and platelet aggregation of composite materials containing these fibers. Compos Sci Technol 69(14):2438–2446
Tilbrook K, Gebbie L, Schenk PM, Poirier Y, Brumbley SM (2011) Peroxisomal polyhydroxyalkanoate biosynthesis is a promising strategy for bioplastic production in high biomass crops. Plant Biotechnol J 9(9):958–969
Tilbrook K, Poirier Y, Gebbie L, Schenk PM, McQualter RB, Brumbley SM (2014) Reduced peroxisomal citrate synthase activity increases substrate availability for polyhydroxyalkanoates biosynthesis in plant peroxisomes. Plant Biotechnol J 12(8):1044–1052
Valentin HE, Broyles DL, Casagrande LA (1999) PHA production, from bacteria to plants. Int J Biol Macromol 25:303–306
Van der Walle GAM, De Koning GJM, Weusthuis RA, Eggink G (2001) Properties, modifications and applications of biopolyesters. Adv Bioch Eng/Biotechnol 71:263–291
Wang YH, Wu ZY, Zhang XH, Chen GQ, Wu Q, Huang CL, Yang Q (2005) Synthesis of medium-chain-length-polyhydroxyalkanoates in tobacco via chloroplast genetic engineering. Chin Sci Bull 50(11):1113–1120
Wrobel-Kwiatkowska M, Zebrowski J, Szopa J (2004) Polyhydroxybutyrate synthesis in transgenic flax. J Biotechnol 107(1):41–54
Wrobel-Kwiatkowska M, Zebrowski J, Starzycki M, Oszmianski J, Szopa J (2007) Engineering of PHB synthesis causes improved elastic properties of flax fibers. Biotechnol Prog 23:269–277
Wrobel-Kwiatkowska M, Skorkowska-Telichowska K, Dyminska L, Mączka M, Hanuza J, Szopa J (2009) Biochemical, mechanical, and spectroscopic analyses of genetically engineered flax fibers producing bioplastic (poly-β-hydroxybutyrate). Biotechnol Prog 25:1489–1498
Wrobel-Kwiatkowska M, Kropiwnicki M, Zebrowski J, Beopoulos A, Dyminska L, Hanuza J, Rymowicz W (2019) Effect of mcl-PHA synthesis in flax on plant mechanical properties and cell wall composition. Transgenic Res 28(1):77–90
Yokoo T, Matsumoto K, Ooba T, Morimoto K, Taguchi S (2015) Enhanced poly (3-hydroxybutyrate) production in transgenic tobacco BY-2 cells using engineered acetoacetyl-CoA reductase. Biosci Biotechnol Biochem 79(6):986–988
Yunus AMM, Parveez GKA, Ho CL (2008) Transgenic plants producing polyhydroxyalkanoates. Asia Pac J Mol Biol Biotechnol 16(1):1–10
Zhang J, Su N, Zhang Z, Zhao H, Zhu S, Song Y (2002) Expressing PHB synthetic genes through chloroplast genetic engineering. Chin Sci Bull 47(16):1373–1376
Zhong H, Teymouri F, Chapman B, Maqbool SB, Sabzikar R, El-Maghraby Y, Dale B, Sticklen MB (2003) The pea (Pisum sativum L.) rbcS transit peptide directs the Alcaligenes eutrophus polyhydroxybutyrate enzymes into the maize (Zea mays L.) chloroplasts. Plant Sci 165(3):455–462
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sharma, M.K., Singh, S., Kapoor, N., Tomar, R.S. (2022). Polyhydroxyalkanoate Production in Transgenic Plants: Green Plastics for Better Future and Environmental Sustainability. In: Kumar, P., Tomar, R.S., Bhat, J.A., Dobriyal, M., Rani, M. (eds) Agro-biodiversity and Agri-ecosystem Management. Springer, Singapore. https://doi.org/10.1007/978-981-19-0928-3_15
Download citation
DOI: https://doi.org/10.1007/978-981-19-0928-3_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0927-6
Online ISBN: 978-981-19-0928-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)