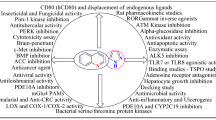
Abstract
Over the past two centuries, fused N-heterocyclic compounds are extensively utilized as valuable entities for the expansion of pharmacological significant agents and deliberated as one of the advantaged scaffolds. Among the numerous fused N-heterocyclic compounds, cinnoline, quinoxalines and quinazolines are significant pharmacological agents, and a noteworthy amount of study has been conducted regarding this type of compounds. In medicinal chemistry, these N-heterocyclic compounds have broad range of biological properties and used as synthetic intermediates, potential drug candidates and chemical probes. Since they are indispensable moieties to treat infectious diseases, in the past years, there is a surge in the significance of designing innovative cinnoline, quinoxalines and quinazolines derivatives, sightseeing auspicious methods to access these moieties, examining their different properties and potential applications. The aim of this chapter is to highpoint the topical studies made by chemist on numerous biological activities and synthetic methods of cinnoline, quinoxalines and quinazolines via sustainable synthetic approaches described in the literature. It would buoyantly be useful for the researchers who have fascinates in evolving innovative therapeutic agents and associated valuable chemical probes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
9.1 Introduction
Heterocycles engage a middle arrangement in synthetic organic chemistry. Over the past two centuries, heterocyclic chemistry has been soundly established due to the agricultural, pharmaceutical and industrial importance of the majority of organic-based value-added chemicals. Heterocyclic entities play an essential position in Natural systems and show a noteworthy involvement in maintaining livelihood (Brahmachari 2015). They are extensively scattered in “Universe” which is crucial for existence such as oxygen transporting pigment “hemoglobin,” photosynthetic pigment “chlorophyll,” plant alkaloids (e.g., Strychnine, Flavones), vitamins (e.g., vitamin B6, vitamin E), enzymes, polysaccharides, anthocyanins, energy carrier (e.g., ATP, ADP), neurotransmitter (e.g., Serotonin, Histamine) and nucleic acid (DNA and RNA) (Shalini et al. 2010; Keri et al. 2014; Afzal et al. 2015; Shinde and Haghi 2020; Hussaini et al. 2019). Furthermore, numerous amino acids, alkaloids (e.g., nicotine and caffeine), carbohydrates, hormones, pheromones, antibiotics, antioxidants, flavorings and perfumes, etc. are also value-added heterocyclic compounds that are important for our life (Berger 2007; Ramsewak et al. 1999; Padwa et al. 1992; Festa et al. 2019; Ameta et al 2014; Lambat et al. (2020); Liu and Fu (2012); Dandia et al. 2011, 2012, 2013a, 2014, 2020a; b) (Fig. 9.1).
9.1.1 N-Heterocyclic Compounds
As life has developed in universe over last centuries, nature has expanded an assortment of valuable heterocyclic compounds that provide as chemical couriers that activate biological feedbacks and regulate biological course of actions, more commonly. N-Heterocyclic compounds have become an imperative contributor of everyday life during last the century and it plays a major role in our environment (Verma 2020). The chemistry of “N-Heterocycles” attracts particular concentration in science basically due to its enormous significance to our everyday life. N-Heterocyclic compounds have diverse uses such as in life saving drugs, optoelectronics, flavoring agents, polymers, herbicides, preservatives, anticorrosive agent, light-emitting diode, fragrances, fabric whiteners, fertilizers, conductivity-based sensors, pesticides, agrochemicals and modifier for rockets propellant fuels (Dandia et al. 2012, 2013c, 2015, 2016, 2017a, b, 2018a, 2019a, 2020a, b, c, 2021a, b, c).
Owing to their marvelous impact of N-heterocyclic compounds in our humanity, there are constant demands to decrease unpreserved resources and expenses to have less disadvantageous impact to the atmosphere. The fused N-heterocyclic compounds have an immense significance in therapeutic chemistry (Aher et al. 2014; Mermer et al. 2021; Bhardwaj et al. 2021). One of the most imperative fused scaffolds in medicinal chemistry is cinnolines, quinoxalines and quinazolines (Scheme 9.1).
They are well-known heterocycles for their extensive pharmacological properties counting anticancer, anticonvulsant, anti-allergic, anti-inflammatory, antidiabetic, antimalarial, antibacterial, antitumor, antitubercular, antihypertensive, as anti-hypertensive, antihistamine and antihypertensive (Taek et al. 2017).
The N-heterocyclic fused ring has drained an enormous deliberation because of their prolonged applications in field of therapeutic chemistry. This chapter assembles the modern work on fused 6-membered N-heterocyclic scaffolds with two N-atoms like quinoxalines, cinnoline and quinazolines reported in literature by researchers (Keneford et al. 1950; Mathew et al. 2017; Mamedov 2016; Chandra et al. 2014). These N-heterocyclic compounds are manufactured from combination of pyrimidine and benzene ring. One benzene ring contains two N-atoms. On the basis of position of the two nitrogen atoms these fused N-heterocyclic compounds are named as cinnoline, quinoxalines and quinazolines (Fig. 9.2).
9.2 Cinnoline
9.2.1 Introduction
Cinnoline is an organic N-heterocyclic compound having molecular formula C8H6N2. Cinnoline (1,2-benzodiazine) has fascinated a great covenant of attention owing to their friendship with a variety of pharmaceutical and biological properties (Lewgowd and Stanczak 2007; Abdelrazek et al. 2006; Somei and Ura 1978). It is a fused N-heterocyclic compound in which two N-atoms are present at 1,2 position (benzene and pyrimidine ring). It is an isomeric form of quinoline or isoquinoline and also with phthalazine.
As a outcome of widespread range of biological significance’s for example anti-inflammatory, analgesic, anxiolytic, antitumor, antimalarial, antifungal and antibacterial activities of cinnoline (1,2-benzodiazine) derivatives, enormous endeavor has been made to construct these bioactive molecules (Fig. 9.3). For that reason, construction of these biologically active N-heterocyclic moieties has increased enormous significance in organic synthesis. The plenty implication of Cinnoline (1,2-benzodiazine) derivatives have influence them to meet via the development of various organic transformations (Alvarado et al. 2006; Tian et al. 2021; Rinderspacher et al. 2021; Kandeel et al. 2018; Bommagani et al. 2020). To date many procedures have been described to access these scaffolds.
9.2.2 Various Approaches for the Preparation of Cinnolines
An exceedingly proficient microwave-assisted method to access bioactive molecules of cinnoline derivatives by the reaction of 4-Alkylpyridazine and nitrostyrene in dioxane/piperidine at 100 °C was presented by Hameed et al. (2017) (Scheme 9.2).
Feng et al. (Li et al. 2021a) designed a straightforward and palladium-catalyzed sustainable pathway to afford biologically important cinnoline derivatives via one-pot dual C–H activation strategy in AcOH at 80 °C (Scheme 9.3).
Mekheimer et al. (Nazmy et al. 2020) described a microwave-induced reaction in dioxane/piperidine to generate densely functionalized cinnolines derivatives at 100 °C. The synthesized cinnolines derivatives show in vitro anticancer biological activity through apoptosis generation (Scheme 9.4).
An competent and green Neber Bossel preparation of several cinnolines from diazotization of (2-aminophenyl)-hydroxyacetate under acidic conditions at 0 °C was introduced by Henry et al. (1960) The reaction was followed by intramolecular condensation and aromatization (Scheme 9.5).
Barber et al. (1961) have been demonstrated catalytic behavior of TiCl4 to afford a library of biologically relevant compounds cinnolines by intramolecular cyclization of phenylhydrazone-linked acid chloride under Friedel–Crafts condition (Scheme 9.6).
A proficient pathway for the production biologically important cinnolines utilizing PPA catalyst at 100–120 °C was explained by Mubarak and co-workers (Awad et al. 2012). The cinnoline is formed through hydrazone intermediate. The author observed that the formed cinnoline derivatives show anticancer and antibacterial activity (Scheme 9.7).
Cu(I) catalyzed production of various functionalized cinnolines derivatives through tandem C–N bond forming reaction using K2CO3 and DMEDA in dioxane at 90 °C was described by Willis and co-workers (Ball et al. 2012). The reported method is based on diazotization chemistry (Scheme 9.8).
Zhang et al. (2012) used Cu(II) as green catalyst for the aerobic dehydrogenative cyclization of hydrazine to afford pharmacologically important cinnoline derivatives followed by the Csp3-H oxidation, cyclization and aromatization sequence in DMF under air atmosphere at 110 °C (Scheme 9.9).
A proficient formal [2 + 2 + 2] cycloaddition of arynes, tosylhydrazine and α-bromoketones to access cinnoline derivatives catalyzed by CsF in CH3CN at 90 °C was developed by Shu co-workers (Shu et al. 2016). In this transition-metal-free reaction, two C-N bond and one C–C bond are formed via one-pot succession (Scheme 9.10).
Au(I)-induced hydroarylation of N-propargyl-N’-arylhydrazines has been successfully applied to generate 4-exo-methylene-1,2-dihydrocinnoline derivatives via hydroarylation process in refluxing nitromethane under catalytic acidic conditions were reported by Gagosz et al. (2011) In this reaction [XPhosAu(NCCH3)SbF6] is used as Au complex (Scheme 9.11).
9.3 Quinazoline
9.3.1 Introduction
Quinazoline is well-known N-containing heterocyclic compounds for their extensive pharmacological properties including anticancer, antifungal, antibacterial, antiulcer, anticonvulsant, antiinflammatory, antidiabetic, antimalarial, antitumor, antitubercular, antihypertensive, antihistamine and antihypertensive (Wang et al. 2013; Kuneš et al. 2000; Ravez et al. 2015; Alafeefy et al. 2010; Marzaro et al. 2012). They are also found in numerous natural products like L-vasicinone, ispinesib, luotonin E, circumdatin F and sclerotingenin and pharmaceutical drugs. Numerous quinazolines are presently in utilized for therapeutic motive owing to their superior pharmaceutical proficiency (Abuelizz et al. 2017; El-Azab et al. 2010).
Quinazolinone alkaloids (derivative of quinazoline) are widely present in nature. Quinazolinones have also been provided as constructive synthetic intermediates. Furthermore, these quinazolinone motifs are of attention as COX-2 inhibitors, herbicidal agents, anti-allergic, antipsychotic, melatonin receptor MT1 and agonists (Ahmad et al. 2017; Rehuman et al. 2021; Li et al. 2021b; Zheng et al. 2020; Selvam et al. 2011) (Fig. 9.4).
Owing to their remarkable properties, traditionally various synthetic approaches have been the subject of several publications for the production of derivatives of quinazoline.
9.3.2 Various Approaches for the Preparation of quinazolines
To date many procedures have been developed to prepare these compounds.
t-butylhydroperoxide is used as a competent and efficient catalyst by Asif et al. (2014) to generate quinazoline derivatives from aminobenzophenones and benzylamines in acetonitrile (Scheme 9.12).
Pd is used as a competent catalyst by Wang and co-workers (Wang et al. 2011) to synthesize 4-aminoquinazoline derivatives from isonitriles and N-aryl amidines in toluene. This reaction takes place via intramolecular C(sp2)-H amidination (Scheme 9.13).
An exceedingly proficient microwave-promoted synthesis of bioactive molecules of quinazoline derivatives from o-phenyl oximes and aldehydes was presented by Portela-Cubillo and co-workers (Portela-Cubillo et al. 2009). For this microwave-promoted reactions, ZnCl2 is used as efficient catalyst with PF4 (Scheme 9.14).
Ferrini et al. (2007) illustrated new microwave-induced cyclization reactions to afford quinazolines through Fries rearrangement reactions of anilides catalyzed by ammonium formate. For this Fries rearrangement reaction, salicylamides are formed by the acylation of o-aminoacyl benzene derivatives (Scheme 9.15).
Ligand-free copper has been successfully used as cheap and willingly accessible catalyst to generate 2-phenylquinazolines (quinazoline derivatives) from 2-bromophenyl methyl amines and amindes was described by Zhang et al. (2010). In this method, K2CO3 is used as a mild base (Scheme 9.16).
Dandia and co-workers (2019b) envisaged a commercially available Cu(I)-catalyzed novel flexible strategy for selective production of quinazolinones utilizing smoothly accessible anthranilonitrile and benzyl bromides. This reaction is complete through N-benzylation/CSp3-H oxidation/CN hydrolysis/cyclization sequence (Scheme 9.17).
Dandia et al. (2018b) designed a new regioselective water-assisted strategy to access substituted quinazolinones from o-aminobenzamides with benzyl alcohols catalyzed by NaCl under microwave irradiation. In this approach, NaCl plays a deceive role for the C–N bond formation through “kosmotropes perturbation” (Scheme 9.18).
A novel and environmental friendly visible-light photoredox procedure to access quinazolinones in the presence of Cu decked ZnS nano-photocatalyst in CH3CN was reported by Dandia et al. (2020d). This reaction completed through amide intermediate (Scheme 9.19).
Peng et al. (2018) reported a convenient Pd-catalyzed carbonylative cyclization reaction to give 2,3-disubstituted quinazolinones derivatives utilizing Mo metal containing carbonyl complex as a reductant and a CO supplier (Scheme 9.20).
Ding et al. (Ren et al. 2018) demonstrated one-pot Pd(PPh3)4-initiated cross-coupling of 2-azidobenzamides and isocyanides to prepare biological active quinazolinones derivatives in DMF at room temperature (Scheme 9.21).
Trifluoroacetic acid (TFA) has been successfully used as cheap and willingly accessible CF3 source to generate trifluoromethyl substituted quinazolinones by narrative and sensible chronological cascade pathway was introduced by Almeida and co-workers (2018) (Scheme 9.22).
Huang et al. (Lin et al. 2019) developed an environmentally benign electrochemical process to afford substituted 2-aryl-quinazolinones derivatives through cascade cyclization of o-aminobenzamides with alcohols utilizing manganese(II) sulfate as a catalyst in CH3CN/H2O medium (Scheme 9.23).
Salehi et al. (2005) accomplished a versatile way for the production of disubstituted quinazolinones by the reaction of commercially available reactants utilizing silica sulfuric acid at 80 °C temperature (Scheme 9.24).
Alper et al. (Zheng et al. 2008) demonstrated Pd(OAc)2/PPh3/CO-catalyzed cyclo-carbonylation of iodoanilines and acid chlorides to generate a series of quinazolinones proceed by in situ construction of an amidine (Scheme 9.25).
Lanthanide triflate [Yb(OTf)3] catalyzed production of pharmaceutical vital quinazolinones from 2-aminobenzoic acid, ortho-esters and amine was developed by Wang and co-workers (Wang et al. 2003) (Scheme 9.26).
A broad and proficient process to synthesized a variety of quinazolinones from the condensation of aldehydes and anthranilamide catalyzed by the complex of Ir metal in aqueous medium was developed by Feng co-workers (Li et al. 2015) (Scheme 9.27).
[Cu(Py)4(OTf)2] is found as an competent catalyst by Kapdi et al. (Gholap et al. 2017) to afford substituted quinazolinones derivatives through one-pot sequential way (Scheme 9.28).
[Cp*RhCl2] is found as competent catalyst by Xiong et al. (2018) to afford substituted quinazolinones derivatives through one-pot successive regioselective ortho-C–H amidation and cyclization of N-methoxybenzamide and dioxazolones under nitrogen atmosphere. In this reaction AgSbF6 is used as acid catalyst (Scheme 9.29).
Bi-SO3H-functionalized ionic liquids (ILs) provoked aerobic oxidation approach to afford quinazolinones via solvation-induced proton transfer under air atmosphere described by Yu and co-workers (Yu et al. 2017) (Scheme 9.30).
9.4 Quinoxaline
9.4.1 Introduction
Quinoxaline is isomeric form of quinoline or isoquinoline, phthalazine, cinnoline and also with phthalazine. It is also called a benzopyrazine. Quinoxalines are well-known fused N-containing heterocyclic compounds for their extensive pharmacological properties including anticancer, anticonvulsant, antineoplastic, antitubercular, antiamoebic, anti-HIV agent, antidepressant, antibacterial, antifungal, antimalarial, anti-inflammatory, antileishmanial, herbicidal, antiprotozoal, fungicidal, insecticidal, antioxidant and anti-ebola activities (Ahmed et al. 2018; Loughran et al. 2016; Ibrahim et al. 2017; Achutha et al. 2013; Carta et al. 2001; Shekhar et al. 2014; Ali et al. 2017; Corona et al. 2009).
Quinoxaline derivatives also exist in many natural compounds, for example vitamin B2, izumiphenazines A-C, cyclic peptide triostin A, hunanamycin A (Zhang et al. 2014; Abdelfattah et al. 2010; Henriques et al. 2010; Shingare et al. 2013; Hu et al. 2013; Refat et al. 2011) and DNA cleavage agents and functional materials (Dandia et al. 2012, 2013b, 2015, 2016, 2017a, 2020b, 2021a; b) (Aher et al. 2014; Mermer et al. 2021; Bhardwaj et al. 2021; Taek et al. 2017; Keneford et al. 1950; Mathew et al. 2017; Mamedov 2016). They are also important in the fields of technology for example chemical switches, cavitands, fluorescent dying agents, semiconductors and electroluminescent materials (Jaung 2006; Zhang et al. 2008; Thomas et al. 2005; Crossley and Johnston 2002; Dailey et al. 2001; Katoh et al. 2000; Sessler et al. 2002) (Fig. 9.5).
9.4.2 Various Approaches for the Preparation of Quinoxalines
To date many procedures have been developed to prepare these compounds.
Kundu and co-workers (Shee et al. 2020) described a straightforward and proficient NiBr2/1,10-phenanthroline system-promoted approach to generate quinoxalines using cesium carbonate at 150 °C. The used catalytic system is reusable for the next seventh cycle (dehydrogenative coupling reaction) (Scheme 9.31).
Alumina-supported heteropolyoxometalates (AlMoVP) has been successfully used as cheap and reusable catalyst to generate a series of quinoxalines from 1,2-dicarbonyls and 1,2-diamines at 25 °C was introduced by Romanelli and co-workers (Ruiz et al. 2012) (Scheme 9.32).
An earth-abundant manganese(I) complex (Mn(CO)5Br) is demonstrated to be a competent catalyst for the preparation of functionalized quinoxalines and quinazolines by the dehydrogenative annulation reaction described by Balaraman et al. (Mondal et al. 2020). In this dehydrogenative annulation reaction, only H2O and H2 is formed as side product (Scheme 9.33).
Co-phen/C-800 have been successfully used as an innovative, selective, reusable and efficient catalyst to generate a series of quinoxaline derivatives via coupling reaction between diamines and diols at 150 °C was described by Kundu et al. (Panja et al. 2020) (Scheme 9.34).
In situ formed alcohols and nitroarenes using tricarbonyl (η4-cyclopentadienone) iron complex have been successfully used as simple and efficient reactant for the Pictet-Spengler-type annulation/oxidation reaction to access the quinoxaline derivatives at 160 °C was developed by Hong et al. (Chun et al. 2020) (Scheme 9.35).
Zahouily et al. (Dânoun et al. 2020) depicted eco-friendly synthesis of functionalized quinoxalines via nanostructured Na2PdP2O7 catalyzed condensation reaction of aryl 1,2-dicarbonyl and diamines in EtOH at room temperature. The used bifunctional heterogeneous catalyst is reusable for the next five consecutive cycles (Scheme 9.36).
Chen et al. (Xie et al. 2016) developed an environmentally benign process to afford quinoxaline derivatives through one-pot domino reaction of 2-pyrrol-1-ylaniline[2-(1H-pyrrol-1-yl)phenyl]amine and a variety of β-diketones catalyzed by Brønsted acid (TsOH·H2O) in DMSO at 110 °C (Scheme 9.37).
Gi et al. (Cho and Oh 2006) demonstrated a ruthenium RuCl2(PPh3)3-catalyzed reaction of o-phenylene diamines and vicinal diols to generate a series of quinoxalines in the presence of KOH and diglyme at reflux (Scheme 9.38).
Lindsley et al. (Zhao et al. 2004) described a microwave-induced protocol to generate functionalized quinoxaline in 9:1 MeOH-HOAc at160 ℃ (Scheme 9.39).
Magnetically separable Fe3O4 nanoparticles were found a proficient catalyst to access substituted 2 quinoxalines in water was introduced by Zhang and co-workers (Lü et al., 2010). The used nano-catalyst is reusable (Scheme 9.40).
9.5 Conclusion
This chapter emphasizes on effective and miscellaneous biological activities and synthetic methods of the fused N-heterocyclic compounds for example cinnoline, quinoxalines and quinazolines derivatives described in literature. It offers an viewpoint on modern advances of cinnoline, quinoxalines and quinazolines consuming numerous biological activities such as anticonvulsant, antifungal, antibacterial, antiulcer, antiinflammatory, anticancer, antimalarial, antitumor, antitubercular, antihypertensive, antihistamine, antidiabetic and antihypertensive. This chapter could be useful for other scientist to advanced important drugs having these moieties for the treatment of numerous deadly syndromes in future. Cinnoline, quinoxalines and quinazolines derivatives are considered as significant precursor to synthesize numerous biologically important scaffolds. In this chapter, we hope to deliver an overview of the significant common approaches for manufacturing these moieties and current progresses toward their biological activity and exposed the entrance for upcoming research in this field.
References
Abdelfattah MS, Kazufumi T, Ishibashi M (2010) Izumiphenazines A-C: isolation and structure elucidation of phenazine derivatives from streptomyces sp. IFM 11204. J Nat Prod 73:1999–2002
Abdelrazek FM, Metz P, Metwally NH et al (2006) Synthesis and molluscicidal activity of new cinnoline and pyrano [2,3-c] pyrazole derivatives. Archiv Der Pharmazie Int J Pharm Med Chem 339:456–460
Abuelizz HA, Marzouk M, Ghabbour H et al (2017) Synthesis and anticancer activity of new quinazoline derivatives. Saudi Pharm J 25:1047–1054
Achutha L, Parameshwar R, Reddy BM et al (2013) Microwave-assisted synthesis of some quinoxaline-incorporated Schiff bases and their biological evaluation. J Chem 578438
Afzal O, Kumar S, Haider MR et al (2015) A review on anticancer potential of bioactive heterocycle quinoline. Eur J Med Chem 97:871–910
Aher SB, Muskawar PN, Thenmozhi K et al (2014) Recent developments of metal N-heterocyclic carbenes as anticancer agents. Eur J Med Chem 81:408–419
Ahmad I (2017) An insight into the therapeutic potential of quinazoline derivatives as anticancer agents. Med Chem Comm 8:871–885
Ahmed HEA, Ihmaid SK, Omar AM et al (2018) Design, synthesis, molecular docking of new lipophilic acetamide derivatives affording potential anticancer and antimicrobial agents. Bioorg Chem 76:332–342
Alafeefy AM, Kadi AA, Al-Deeb OA et al (2010) Synthesis, analgesic and anti-inflammatory evaluation of some novel quinazoline derivatives. Eur J Med Chem 45:4947–4952
Ali I, Lee J, Go A et al (2017) Discovery of novel [1,2,4]triazolo[4,3-a]quinoxaline aminophenyl derivatives as BET inhibitors for cancer treatment. Bioorg Med Chem Lett 27:4606–4613
Almeida S, Marti R, Vanoli E et al (2018) One-pot synthesis of trifluoromethylated quinazolin-4(3 H)-ones with trifluoroacetic acid as CF3 source. J Org Chem 83:5104–5113
Alvarado M, Barceló M, Carro L et al (2006) Synthesis and biological evaluation of new quinazoline and cinnoline derivatives as potential atypical antipsychotics. Chem Biodivers 3:106–117
Ameta KL, Kumar S, Rathi P, Kishore D (2014) Green chemistry approach using heterogeneous catalysts in the heterocyclic synthesis. In: Dandia A, Ameta KL (eds) In green chemistry: synthesis of bioactive heterocycles, 1st edn. Springer, New Delhi, pp 367–392
Asif M (2014) Chemical characteristics, synthetic methods, and biological potential of quinazoline and quinazolinone derivatives. Int J Med Chem:1–27
Awad ED, El-Abadelah MM, Matar S (2012) Synthesis and biological activity of some 3-(4-(substituted)- piperazin-1-yl)cinnolines. Molecules 17:227–239
Ball CJ, Gilmore J, Willis MC (2012) Copper-catalyzed tandem C–N bond formation: an efficient annulative synthesis of functionalized cinnolines. Angew Chem Int Ed 51:5718–5722
Barber HJ, Washbourn K, Wragg WR et al (1961) A new cinnolinesynthesis. Part I. cyclisation of mesoxalyl chloride phenylhydrazones to give substituted 4-hydroxycinnoline-3-carboxylic acids. J Chem Soc 552:2828–2843
Berger RG (ed) (2007) Flavours and fragrances: chemistry, bioprocessing and sustainability. Springer Science & Business Media, Germany
Bhardwaj N, Pathania A, Kumar P (2021) Naturally available nitrogen-containing fused heterocyclics as prospective lead molecules in medicinal chemistry. Curr Tradit Med 7:5–27
Bommagani MB, Mokenapelli S, Yerrabelli JR et al (2020) Novel 4-(1H–1, 2, 3-triazol-4-yl) methoxy) cinnolines as potent antibacterial agents: Synthesis and molecular docking study. Synth Commun 50:1016–1025
Brahmachari G (2015) Green synthetic approaches for biologically relevant heterocycles: an overview, 1st edn. In: Brahmachari G (ed) Green synthetic approaches for biologically relevant heterocycles. Elsevier, Netherlands, p 1–6
Carta A, Sanna P, Gherardini L (2001) Novel functionalized pyrido[2,3-g]quinoxalinones as antibacterial, antifungal and anticancer agents. Il Farmaco 56:933–938
Chandra Shekhar A, Ravi Kumar A, Sathaiah G (2014) Aqueous hydrofluoric acid catalyzed facile synthesis of 2, 3, 6-substituted quinoxalines. J Heterocycl Chem 51:1504–1508
Cho CS, Oh SG (2006) A new ruthenium-catalyzed approach for quinoxalines from o-phenylenediamines and vicinal-diols. Tetrahedron Lett 47:5633–5636
Chun S, Ahn J, Putta RR et al (2020) Direct synthesis of pyrrolo [1, 2-α] quinoxalines via iron-catalyzed transfer hydrogenation between 1-(2-nitrophenyl) pyrroles and alcohols. J Org Chem 85:15314–15324
Corona P, Carta A, Loriga M et al (2009) Synthesis and in vitro antitumor activity of new quinoxaline derivatives. Eur J Med Chem 44:1579–1591
Crossley MJ, Johnston LA (2002) Laterally-extended porphyrin systems incorporating a switchable unit. Chem Commun 10:1122–1123
Dailey S, Feast WJ, Peace RJ et al (2001) Synthesis and device characterisation of side-chain polymer electron transport materials for organic semiconductor applications. J Mater Chem 11:2238–2243
Dandia A, Parewa V, Jain AK et al (2011) Step-economic, efficient, ZnS nanoparticle-catalyzed synthesis of spirooxindole derivatives in aqueous medium via Knoevenagel condensation followed by Michael addition. Green Chem 13:2135–2145
Dandia A, Parewa V, Rathore KS (2012) Synthesis and characterization of CdS and Mn doped CdS nanoparticles and their catalytic application for chemoselective synthesis of benzimidazoles and benzothiazoles in aqueous medium. Catal Commun 28:90–94
Dandia A, Gupta SL, Parewa V et al (2013) “On-water” synthesis of 3-substituted indoles via Knoevenagel/Michael addition sequence catalyzed by Cu doped ZnS NPs. Tetrahedron Lett 54:5711–5717
Dandia A, Parewa V, Sharma A et al (2013) Co-doped ZnS nanoparticles as a recyclable catalyst for aqueous mediated synthesis of 2,4,5-triaryl-1H-imidazoles under ultrasonic irradiation. Eur Chem Bull 2:971–974
Dandia A, Parewa V, Gupta SL et al (2013) Cobalt doped ZnS nanoparticles as a recyclable catalyst for solvent-free synthesis of heterocyclic privileged medicinal scaffolds under infrared irradiation. J Mol Catal A Chem 373:61–71
Dandia A, Parewa V, Sharma A (2014) An approach towards green switch through nanocatalysis for the synthesis of biodynamic heterocycles. In: Dandia A, Ameta KL (eds) In green chemistry: synthesis of bioactive heterocycles. Springer, New Delhi, pp 129–161
Dandia A, Parewa V, Gupta SL et al (2015) Microwave-assisted Fe3O4 nanoparticles catalyzed synthesis of chromeno [1, 6] naphthyridines in aqueous media. Catal Commun 61:88–91
Dandia A, Parewa V, Kumari S et al (2016) Imposed hydrophobic interactions by NaCl: accountable attribute for the synthesis of spiro [acenaphthylene-1,5′-pyrrolo [1,2-c] thiazole] derivatives via 1,3-dipolar cycloaddition reaction in aqueous medium. Green Chem 18:2488–2499
Dandia A, Khan S, Parewa V et al (2017) In multicomponent reactions. In: Dandia A, Ameta KL (eds) Modern synthesis of bioactive heterocycles via IMCR modification, 1st edn. CRC Press Taylor & Francis, United States, pp 229–280
Dandia A, Gupta SL, Indora A et al (2017) Ag NPs decked GO composite as a competent and reusable catalyst for ‘ON WATER’ chemoselective synthesis of pyrano [2,3-c:6,5-c′] dipyrazol]-2-ones. Tetrahedron Lett 58:1170–1175
Dandia A, Bansal S, Sharma R et al (2018) Water-triggered metal-free synthesis of pyranopyrazoles via one-pot oxidation/Michael addition/cyclization/dehydration sequence. Chem Sel 3:9785–9789
Dandia A, Sharma R, Indora A et al (2018) Kosmotropes perturbation and ambiphilic dual activation: responsible features for the construction of C–N bond towards the synthesis of quinazolin-4(3H)-ones in water. ChemistrySelect 3:8285–8290
Dandia A, Mahawar DK, Sharma R et al (2019a) Graphene oxide-catalyzed CSp3–H activation of methylarenes in aqueous medium: a unified metal-free access to amides and benzimidazoles. Appl Organomet Chem 33:e5232
Dandia A, Saini P, Bansal S et al (2019b) One-pot copper (I)-catalyzed synthesis of 2-aryl-quinazolin-4(3H)-ones via N-benzylation/Csp3–H oxidation/CN hydrolysis/cyclization. ChemistrySelect 4:9871–9877
Dandia A, Saini P, Sharma R et al (2020a) Green organic synthesis by photochemical protocol, 1st edn. In: Inamuddin, Boddula R, Asiri AM (ed) In green sustainable process for chemical and environmental engineering and science. Elsevier, Netherlands, pp 155–198
Dandia A, Saini P, Sharma R et al (2020b) Visible light driven perovskite-based photocatalysts: a new candidate for green organic synthesis by photochemical protocol. Curr Res Green Sustain Chem:100031
Dandia A, Gupta SL, Saini P et al (2020c) Structure couture and appraisal of catalytic activity of carbon nitride (g-C3N4) based materials towards sustainability. Curr Res Green Sustain Chem 3:100039
Dandia A, Bansal S, Sharma R et al (2020d) Nanoporous Cu doped ZnS nanoparticles an efficient photo catalyst for the chemoselective synthesis of 2-substituted azoles via CN arylation/CSp3-H oxidation/cyclization/dehydration sequence in visible light. J Photochem Photobiol A 389:112242
Dandia A, Saini P, Kumar K et al (2021a) Synergetic effect of functionalized graphitic carbon nitride catalyst and ultrasound in aqueous medium: an efficient and sustainable synthesis of 1,3,5-trisubstituted hexahydro-1,3,5-triazines. Curr Res Green Sustain Chem 4:100170
Dandia A, Saini P, Sethi M et al (2021b) Nanocarbons in quantum regime: an emerging sustainable catalytic platform for organic synthesis. Catal Rev:1–55
Dandia A, Mahawar DK, Saini P et al (2021) Site-specific role of bifunctional graphitic carbon nitride catalyst for the sustainable synthesis of 3,3-spirocyclic oxindoles in aqueous media. RSC Adv 11:28452–28465
Dânoun K, Essamlali Y, Amadine O et al (2020) Eco-friendly approach to access of quinoxaline derivatives using nanostructured pyrophosphate Na2 PdP2O7 as a new, efficient and reusable heterogeneous catalyst. BMC Chem 14:1–13
El-Azab AS, Al-Omar MA, Alaa AM et al (2010) Design, synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: molecular docking study. Eur J Med Chem 45:4188–4198
Ferrini S, Ponticelli F, Taddei M (2007) Convenient synthetic approach to 2,4-disubstituted quinazolines. Org Lett 9:69–72
Festa AA, Voskressensky LG, Van der Eycken EV (2019) Visible light-mediated chemistry of indoles and related heterocycles. Chem Soc Rev 48:4401–4423
Gholap AV, Maity S, Schulzke C et al (2017) Synthesis of Cu-catalysed quinazolinones using a C sp3–H functionalisation/cyclisation strategy. Org Biomol Chem 15:7140–7146
Hameed AA, Ahmed EK, Fattah AAA et al (2017) Green and efficient synthesis of polyfunctionally substituted cinnolines under controlled microwave irradiation. Res Chem Intermed 43:5523–5533
Henriques BJ, Olsen RK, Bross P et al (2010) Emerging roles for riboflavin in functional rescue of mitrochondrial betaoxidation flavoenzymes. Curr Med Chem 17:3842–3854
Henry E, Baumgarten PLC (1960) Cinnolines VII. The Neber-Bosselsynthesis 1,2. J Am Chem Soc 82:4634–4638
Hussaini SY, Haque RA, Razali MR (2019) Recent progress in silver (I)-, gold (I)/(III)-and palladium (II)-N-heterocyclic carbene complexes: a review towards biological perspectives. J Org Chem 882:96–111
Hu Y, Wang K, MacMillan JB et al (2013) an antibiotic from amarine-derived Bacillus hunanensis. Org Lett 15:390–393
Ibrahim MK, Eissa IH, Abdallah AE et al (2017) Design, synthesis, molecular modeling and anti-hyperglycemic evaluation of novel quinoxaline derivatives as potential PPARγ and SUR agonists. Bioorg Med Chem 25:1496–1513
Jaung JY (2006) Synthesis and halochromism of new quinoxaline fluorescent dyes. Dyes Pigm 71:245–250
Jurberg ID, Gagosz F (2011) Formation of cinnoline derivatives by a gold (I)-catalyzed hydroarylation of N-propargyl-N′-arylhydrazines. J Organomet Chem 696:37–41
Kandeel MM, Kamal AM, Naguib BH et al (2018) Design, synthesis, cytotoxic activity and apoptosis-inducing action of novel cinnoline derivatives as anticancer agents. Anticancer Agents Med Chem ANTI-Cancer Agent Me 18:1208–1217
Katoh A, Yoshida T, Ohkanda J (2000) Synthesis of quinoxaline derivatives bearing the styryl and phenylethynyl groups and application to a fluorescence derivatization reagent. Heterocycles 52:911–920
Keneford JR, Morley JS, Simpson JCE et al (1950) The chemistry of simple heterocyclic systems. Part V. A comparative study of some 4-substituted cinnolines, quinazolines, and quinolines. J Chem Soc (resumed) 227:1104–1111
Keri RS, Budagumpi S, Pai RK et al (2014) Chromones as a privileged scaffold in drug discovery: a review. Eur J Med Chem 78:340–374
Kuneš J, Bažant J, Pour M et al (2000) Quinazoline derivatives with antitubercular activity. Il Farmaco 55:725–729
Lambat TL, Chopra PKP, Mahmood SH (2020) Microwave: a green contrivance for the synthesis of N-heterocyclic compounds. Curr Org Chem 24:2527–2554
Lewgowd W, Stanczak A (2007) Cinnoline derivatives with biological activity. Archiv Der Pharmazie Int J Pharm Chem 340:65–80
Li F, Lu L, Ma J (2015) Acceptorless dehydrogenative condensation of o-aminobenzamides with aldehydes to quinazolinones in water catalyzed by a water-soluble iridium complex [Cp* Ir (H2O)3][OTf]2. Org Chem Front 2:1589–1597
Li H, Zhao J, Yi S et al (2021a) Consequent construction of C–C and C–N bonds via palladium-catalyzed dual c–h activation: synthesis of benzo [c] cinnoline Derivatives. Organometallics 40:880–889
Li Z, Qin T, Li Z et al (2021b) Discovery of quinazoline derivatives as a novel class of potent and in vivo efficacious LSD1 inhibitors by drug repurposing. Eur J Med Chem 225:113778
Lin DZ, Lai YL, Huang JM (2019) Mn-catalyzed electrochemical synthesis of quinazolinones from primary alcohols/benzyl ethers and o-aminobenzamides. ChemElectroChem 6:4188–4193
Liu T, Fu H (2012) Copper-catalyzed synthesis of N-heterocyclic compounds. Synthesis 44:2805–2824
Loughran HM, Han Z, Wrobel JE et al (2016) Quinoxaline-based inhibitors of ebola and Marburg VP40 egress. Bioorg Med Chem Lett 26:3429–3435
Lü HY, Yang SH, Deng J et al (2010) Magnetic Fe3O4 nanoparticles as new, efficient, and reusable catalysts for the synthesis of quinoxalines in water. Aust J Chem 63:1290–1296
Mamedov VA (2016) Quinoxalines synthesis, reactions, mechanisms and structure. Springer, Switzerland
Marzaro G, Guiotto A, Chilin A (2012) Quinazoline derivatives as potential anticancer agents: a patent review (2007–2010). Expert Opin Ther Pat 22:223–252
Mathew T, Papp AÁ, Paknia F et al (2017) recent synthetic advances. Chem Soc Rev 46:3060–3094
Mermer A, Keles T, Sirin Y (2021) Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: a review. Bioorg Chem:105076
Mondal A, Sahoo MK, Subaramanian M, Balaraman E et al (2020) Manganese (I)-catalyzed sustainable synthesis of quinoxaline and quinazoline derivatives with the liberation of dihydrogen. J Org Chem 85:7181–7191
Nazmy MH, Mekheimer RA, Shoman ME et al (2020) Densely functionalized cinnolines: controlled microwave-assisted facile one-pot multi-component synthesis and in vitro anticancer activity via apoptosis induction. Bioorg Chem 101:103932
Padwa A, Ishida M, Muller CL et al (1992) 2, 3-Dihalo-1-(phenylsulfonyl)-1-propenes as versatile reagents for the synthesis of annulated furans and cyclopentenones. J Org Chem 57:1170–1178
Panja D, Paul B, Balasubramaniam B et al (2020) Application of a reusable Co-based nanocatalyst in alcohol dehydrogenative coupling strategy: synthesis of quinoxaline and imine scaffolds. Catal Commun 137:105927
Peng JB, Geng HQ, Wang W et al (2018) Palladium-catalyzed four-component carbonylative synthesis of 2,3-disubstituted quinazolin-4(3H)-ones: convenient methaqualone preparation. J Catal 365:10–13
Portela-Cubillo F, Scott JS, Walton JC (2009) Microwave-promoted syntheses of quinazolines and dihydroquinazolines from 2-aminoarylalkanone O-phenyl oximes. J Org Chem 74:4934–4942
Ramsewak RS, Nair MG, Strasburg GM et al (1999) Biologically active carbazole alkaloids from Murraya k oenigii. J Agric Food Chem 47:444–447
Ravez S, Castillo-Aguilera O, Depreux P et al (2015) Quinazoline derivatives as anticancer drugs: a patent review (2011–present). Expert Opin Ther Pat 25:789–804
Refat MS, Moussa MAA, Mohamed SF (2011) Synthesis, spectroscopic characterization, thermal analysis and electrical conductivity studies of Mg(II), Ca(II), Sr(II) and Ba(II) vitamin B2 complexes. J Mol Struct 994:194–201
Rehuman NA, Al-Sehemi AG, Parambi DGT et al (2021) Current progress in quinazoline derivatives as acetylcholinesterase and monoamine oxidase inhibitors. ChemistrySelect 6:7162–7182
Ren ZL, Kong HH, Lu WT et al (2018) One-pot synthesis of quinazolin-4(3H)-ones and fused quinazolinones by a palladium-catalyzed domino process. Tetrahedron 74:184–193
Rinderspacher KA (2021) Six-membered ring systems: diazines and benzo derivatives. In: Gribble GW, Joule JA (eds) In progress in heterocyclic chemistry. Elsevier, Netherlands, vol 32, pp 431–466
Ruiz DM, Autino JC, Quaranta N et al (2012) An efficient protocol for the synthesis of quinoxaline derivatives at room temperature using recyclable alumina-supported heteropolyoxometalates. Sci World J 2012:1–8
Salehi P, Dabiri M, Zolfigol MA et al (2005) A new approach to the facile synthesis of mono-and disubstituted quinazolin-4(3H)-ones under solvent-free conditions. Tetrahedron Lett 46:7051–7053
Selvam TP, Kumar PV (2011) Quinazoline marketed drugs—a review research in pharmacy. Res Pharm 1:1–21
Sessler JL, Maeda H, Mizuno TV et al (2002) Quinoxaline-bridged porphyrinoids. J Am Chem Soc 124:13474–13479
Shalini K, Sharma PK, Kumar N (2010) Imidazole and its biological activities: a review. Der Chemica Sinica 1:36–47
Shee S, Panja D, Kundu S (2020) Nickel-catalyzed direct synthesis of quinoxalines from 2-nitroanilines and vicinal diols: identifying nature of the active catalyst. J Org Chem 85:2775–2784
Shekhar AC, Rao PS, Narsaiah B et al (2014) Emergence of pyrido quinoaxalines as a new family of antimalarial agents. Eur J Med Chem 77:280–287
Shinde RS, Haghi AK (2020) Modern green chemistry and heterocyclic compounds: molecular design, synthesis, and biological evaluation. CRC Press, United States
Shingare RD, Velayudham R, Gawade JR (2013) First total synthesis of hunanamycin A. Org Lett 15:4556–4559
Shu WM, Ma JR, Zheng KL et al (2016) Multicomponent coupling cyclization access to cinnolines via in situ generated diazene with arynes, and bromoketones. Org Lett 18:196–199
Somei M, Ura K (1978) Ring enlargement reaction of 1-aminoindoles to cinnoline derivatives. Chem Lett 7:707–708
Taek Han Y, Jung JW, Kim NJ (2017) Recent advances in the synthesis of biologically active cinnoline, phthalazine and quinoxaline derivatives. Curr Org Chem 21:1265–1291
Thomas KRJ, Velusamy M, Lin Jiann T et al (2005) Chromophore-labeled quinoxaline derivatives as efficient electroluminescent materials. Chem Mater 17:1860–1866
Tian C, Yang C, Wu T et al (2021) Discovery of cinnoline derivatives as potent PI3K inhibitors with antiproliferative activity. Bioorg Med Chem Lett 48:128271
Verma C, Rhee KY, Quraishi MA et al (2020) Pyridine based N-heterocyclic compounds as aqueous phase corrosion inhibitors: a review. J Taiwan Inst Chem Eng 117:265–277
Wang D, Gao F (2013) Quinazoline derivatives: synthesis and bioactivities. Chem Cent J 7:1–15
Wang L, Xia J, Qin F et al (2003) Yb (OTf) 3-catalyzed one-pot synthesis of quinazolin-4(3H)-ones from anthranilic acid, amines and ortho esters (or formic acid) in solvent-free conditions. Synthesis 8:1241–1247
Wang Y, Wang H, Peng J et al (2011) Palladium-catalyzed intramolecular C(sp2)-H amidination by isonitrile insertion provides direct access to 4-aminoquinazolines from N-arylamidines. Org Lett 13:4596–4599
Xie C, Feng L, Li W et al (2016) Efficient synthesis of pyrrolo [1,2-a] quinoxalines catalyzed by a Brønsted acid through cleavage of C–C bonds. Org Biomol Chem 14:8529–8535
Xiong H, Xu S, Sun S et al (2018) Cp*Rh (iii)-catalyzed annulation of N-methoxybenzamide with 1,4,2-bisoxazol-5-one toward 2-aryl quinazolin-4(3H)-one derivatives. J Org Chem Front 5:2880–2884
Yu ZY, Chen MY, He JX et al (2017) Controllable Brønsted acid-promoted aerobic oxidation via solvation-induced proton transfer: metal-free construction of quinazolinones and dihydroquinazolinones. Mol Catal 434:134–139
Zhang C, Kong L, Lei X et al (2014) Advance in the research on quinomycins biosynthesis. Chin J Org Chem 34:1240–1252
Zhang G, Miao J, Zhao Y et al (2012) Copper-catalyzed aerobic dehydrogenativecyclization of N-methyl-N-phenylhydrazones: synthesis of cinnolines. Angew Chem Int Ed 51:8318–8321
Zhang J, Zhu D, Yu C et al (2010) A simple and efficient approach to the synthesis of 2-phenylquinazolines via sp3 C−H functionalization. Org Lett 12:2841–2843
Zhang QY, Liu BK, Chen WQ et al (2008) A green protocol for synthesis of benzo-fused N, S-, N, O- and N, N-heterocycles in water. Green Chem 10:72–977
Zhao Z, Wisnoski DD, Wolkenberg SE et al (2004) General microwave-assisted protocols for the expedient synthesis of quinoxalines and heterocyclic pyrazines. Tetrahedron Lett 45:4873–4876
Zheng YG, Zhang WQ, Meng L et al (2020) Design, synthesis and biological evaluation of 4-aniline quinazoline derivatives conjugated with hydrogen sulfide (H2S) donors as potent EGFR inhibitors against L858R resistance mutation. Eur J Med Chem 202:112522
Zheng Z, Alper H (2008) Palladium-catalyzed cyclocarbonylation of o-iodoanilines with imidoyl chlorides to produce quinazolin-4(3H)-ones. Organic Lett 10:829–832
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Saini, P. et al. (2022). An Overview of Cinnolines, Quinazolines and Quinoxalines: Synthesis and Pharmacological Significance. In: Ameta, K.L., Kant, R., Penoni, A., Maspero, A., Scapinello, L. (eds) N-Heterocycles. Springer, Singapore. https://doi.org/10.1007/978-981-19-0832-3_9
Download citation
DOI: https://doi.org/10.1007/978-981-19-0832-3_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0831-6
Online ISBN: 978-981-19-0832-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)