Abstract
In many power plants, several types of fuel (coal with fuel oil, coal with gas, gas with fuel oil) are burned simultaneously. This practice is sometimes justified by the presence of a fuel imbalance at the stations and an attempt to solve the problem of stabilizing the torch of the burner. At the same time, the most important task is to determine the dew point of the exhaust gases, because this temperature has a decisive effect on the efficiency of the steam boiler. Under normal operating conditions, losses of heat with exhaust gases are large even in comparison with the total losses. In this article, a methodology is proposed which allows a more detailed study of the effect of co-combustion of gaseous and liquid fuels on the intensity of nitrogen and sulfur oxides formation. This technique makes it possible to develop recommendations for optimizing the co-combustion process when burning gas and fuel oil. A comparative analysis of the results obtained from the numerical studies and the data obtained from the readings of the devices suggests the suitability of the proposed methodology for wide use.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
1 Introduction
The issue of ensuring reliable and economical operation of thermal power plants is one of the issues of relevance for Russian energy. This issue is of particular importance in the modern state of Russian thermal power engineering due to the exhaustion of the design resource of the main standard equipment, the expansion of the use of non-design or non-ignited fuels, the need for the development of energy, and environmental saving technologies for fuel use.
In many power plants, especially during peak loads, contrary to technological regulations, it is necessary to simultaneously burn several types of fuels (coal with fuel oil, coal with gas, gas with fuel oil). This practice is justified not only by the presence of a fuel imbalance at the enterprise but also by an attempt to solve the problem of stabilizing the burner flare. Nowadays, there are no methods for a comprehensive assessment of the impact of such parameters on the efficiency of boilers: dew-point temperature of exhaust gases, a specific concentration of NOx and SOx when organizing the combined combustion of gas and fuel oil in steam boiler furnaces.
2 Literature Review
Unfortunately, the question of finding the dew-point temperature of the exhaust gases when burning together various types of fuels is not sufficiently covered in the literature. Although this temperature has a decisive effect on the efficiency of the steam boiler, a decrease in the temperature of the exhaust gases by 12–16 °C leads to an increase in the efficiency of the boiler by about 1%.
Sulfuric acid generated by the combustion of high sulfur fuels (fuel oil, and some coals) is one of the significant reasons for the decrease in the reliability and economy of boilers. Therefore, it is important to accurately determine the dew-point temperature of the exhaust gases. Numerous theoretical studies carried out by various scientific groups [1,2,3,4,5,6,7] cover in sufficient detail the issue of the production of sulfurous anhydride when burning separate fuels, and empirical dependencies are given for them. Also, for particular cases, graphical dependencies of SO3 generation were obtained when burning various types of fuels, depending on excess air, load, flare temperature, boiler grade, etc. In addition, work was carried out to determine the temperature of the sulfate dew point of the exhaust gases, but when burning only certain fuels, such as coal, gas, and fuel oil.
The practical implementation of mixed combustion of gas and fuel oil has set another important task for researchers to estimate the value of specific emissions (or concentrations) of NOx in the joint combustion of fuels (gas and fuel oil). The started developments in this area in the 70s–80s of the last century led to the creation of methodological guidelines [8], however, by the time they were approved, it was not possible to collect sufficient information on NOx emissions for the joint combustion of fuels. It was decided to use the principle of additivity but, with respect to the task of mixed combustion, it showed large discrepancies with experimental data up to 25–30%. Subsequently developed by the group of authors method of calculation of emissions of nitrogen oxides [9], taking into account the duration of combustion products stay in the zone of active combustion, made it possible to correlate experimental and calculated data.
Work [10] shows a reduction of NOx concentration level by 30–50% during gas combustion together with fuel oil (relative to the level of the highest concentration of nitrogen oxides realized during combustion of fuel oil alone), which is confirmed in practice.
3 Materials and Methods
With mixed combustion of gas and fuel oil, an additional effect is achieved by a more complete filling of the volume of the combustion chamber with a luminous flare, which causes a noticeable change in the flare temperature in the active combustion zone. The gas burns at the start of the flare at a higher rate than fuel oil. This causes the active combustion zone to “stretch” and further reduces the maximum temperature in the flare core. The reduction of NOx in the combined combustion of gas and fuel oil requires additional analysis. It is possible to study in-depth the theory of the processes of power units passing in combustion chambers when organizing joint combustion of gas and fuel oil, possibly during numerical experiments using modern computational complexes and applied programs that allow modeling various conditions, while creating a complete and objective picture of the process.
The complex solution of problems: reducing the concentration of NOx and finding the dew-point temperature of the stack gases, and as a consequence of this, the optimal alignment of the combustion mode and finding the corridor of the optimal permissible temperatures at which the stack gases will have the lowest possible temperature (at which the formation of sulfuric acid can’t yet begin and the associated low-temperature corrosion of the tail heating surfaces), and a minimum concentration of nitrogen oxide, will increase both the efficiency and improve the environmental picture of the process as a whole.
To solve this problem, the work proposes a method for calculating the dew-point temperature of exhaust gases during mixed combustion in various proportions of gas and fuel oil in boiler furnaces. Calculations were carried out on the example of the Ulyanovsk CHP-2. Tests were carried out on boilers of type TPE-429; during the tests, gas analyzers of type TESTO-33 were used.
Below is a summary table of the measurements (Fig. 1).
The developed technique is as follows:
The true steam consumption of the steam generator is determined by the formula [1]:
Dn—steam consumption as indicated by the steam meter, t/h; Va—actual specific volume of superheated steam; Vc—specific volume of the steam parameters at calibration (calibration temperature \(t_{\text{n}}^{{\text{calib}}}\) and calibration pressure \(P_{\text{n}}^{{\text{calib}}}\) according to the instrument passport).
Next, the heat load of the boiler unit is determined:
heat content of superheated steam, kJ/kg (determined from the table depending on the parameters of superheated steam, i.e. superheated steam pressure Pn and superheated steam temperature ts); ifw—heat content of feedwater, kJ/kg, (we find from tables for parameters of feedwater, Pfwtfw—pressure and temperature of feedwater obtained as a result of measurements); ibw, kJ/kg—the heat content of the blowdown water, is also determined depending on the parameters of the blowdown water: pressure and temperature determined, respectively, as a result of measurements by instruments; Gbw—blowdown water consumption, t/h (determined empirically), \(D_{\text{t}}^{\text{b}}\)—true steam consumption.
The adjusted fuel consumption is calculated [2] as
where \(\eta_{\text{b}}\)—boiler efficiency determined by mode cards; \(Q_{{\text{ng}}}^{\text{c}}\)—specific heat of combustion of the natural gas, kJ/m3, according to the Ulyanovsk TPP-2 laboratory; \(Q_{{\text{ng}}}^{\text{c}}\)—specific heat of combustion of the fuel oil, kJ/kg, laboratory analysis data.
The proportion of the gas in the mixture with the fuel oil in terms of heat release is determined according to the ratio:
where dg—the proportion of the gas by the heat release; Bg—the natural gas consumption.
The specific volumes of air, the flue gases, and water vapor in the flue gases are found from
where \(V_{\text{gg }}^{\text{g}}\)—the specific volume of the flue gases during combustion of 1 m3 of the natural gas; \(V_{{\text{gf}}}^0\)—the specific volume of the flue gases during combustion of 1 kg of the fuel oil; \(V_{{\text{wg}}}^0\)—the specific volume of air required for combustion of 1 m3 of the natural gas m3/m3; \(V_{\text{wf }}^0\)—the specific volume of air required for combustion of 1 kg of the fuel oil, m3/kg.
Excess air α in the operating section behind the regenerative air heater (RAH) is determined [3, 4] as
where αp—leaked-in air along the exhaust duct; \({\Delta }\alpha \sqrt {\frac{{D_{{\text{sn}}} }}{{D_{{\text{st}}} }}} \)—leaked-in air in RAH.
According to operational regulation (OR) and normative calculation for RAH Δα = 0.25, and for tubular-air heater (TAH) value Δα = 0.1; in turn, the leaked-in air along the exhaust duct can be calculated from the ratio:
where Kg, Kf—coefficients, respectively, for gas Kg = 0.05, for the fuel oil Kf = 0.1; O2—excess oxygen concentration in the operating section.
The weighted average volume of the water vapor by fuel mass during combustion of mixed fuel is determined from the ratio:
where \(V_{{\text{H}}_2 {\text{O}}_{\text{g}} }^0\)—the specific volume of the water vapor in the flue gases, m3/m3; \( V_{{\text{H}}_2 {\text{O}}_{\text{f}} }^0\)—the specific volume of the water vapor in the flue gases when burning the fuel oil, m3/kg.
The determination of the condensation temperature of the water vapor of the flue gases is carried out according to the formula obtained from an approximate dependence of the form:
\(\ln P = \frac{A + B_t }{{C + t}}\), where the value of the coefficients A, B, C was obtained as a result of approximation in the temperature range which determines the smallest error for our case (0 < t < 100 °C)
Ps = Pg \(r_{{\text{H}}_2 {\text{O}}_{\text{g}} }\) = \(\frac{{V_{{\text{H}}_2 {\text{O}}} }}{{V_{\text{g}} }}\) Pg—the saturation pressure of water vapor of flue gases (for boilers with balanced draft); Pg—absolute flue gas pressure taking into account the flue gas rarefaction behind the air heater.
The formula for determining the temperature of sulfuric acid dew point in the flue gases has the form [3, 4]:
where \(S^{\text{r}} = \frac{{\overline{S}^{\text{c}} }}{Q_{\text{n}}^{\text{c}} }\)—reduced sulfur content of fuel, \(\overline{Q}_{\text{n}}^{\text{c}}\)—weighted average by the calorific value of the fuel calorific value, which is directly proportional to the coefficient \(K_{\text{g}}^Q\), \(\overline{s}^{\text{c}}\) = Sc \(\frac{{B_{\text{f}} }}{{B_{\text{g}} + B_{\text{f}} }}\)—weighted average content of sulfur by fuel mass, and \(S^{\text{c}}\)—sulfur content in the fuel oil.
where
4 Results
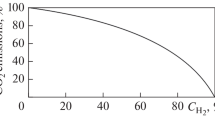
Comparative analysis of the results obtained when carrying out numerical studies of the dew-point temperature (Fig. 2) and the data obtained from the readings of the devices (Fig. 1) gives the right to speak about the suitability of the proposed method for calculation. The discrepancy between the calculated values and the experimental data is insignificant, despite the fact that the calculations were carried out at different loads and with different quantitative ratios of fuels in the total mixture.
The results of calculations using this technique are listed in Fig. 2.
For the first time, a general view of the curve was obtained that characterizes the change in the dew-point temperature, from the gas fraction by heat release (Fig. 3).
5 Conclusions
Analysis of the proposed method will help to study in more detail the effect of co-combustion of gaseous and liquid fuels on harmful emissions, and develop recommendations to ensure the combustion process with co-combustion.
References
Instructions and guidelines for carrying out express operational tests of boiler units. CCNIT (1974)
Lipov, Y., Samoilov, O.: Layout and thermal calculation of a steam boiler. Energoatomizdat (1988)
Yureniev, V., Lebedev, P.: Thermal engineering reference. Energy M 2(2) (1976)
Khzmalen, D.: Theory of furnace processes. Energoatomizdat (1990)
Methodological guidelines for drawing up a report for electric power plants RD 34.08.5552-95. Service of best practices OGRES, Moscow (1995)
Ashurov, S.: Determination of the excess air ratio when burning natural gas together with other types of fuel. Ind. Heat Power Eng. 5 (1987)
Reznikov, N., Lipov, Y.: Boiler Plants of Power Plants 3 (1990)
Guidelines for calculating emissions of nitrogen oxides with flue gases from boilers, RD 34.02.304-88. ATEI (1989)
Kotler, V.R.: Emissions of nitrogen oxides during co-combustion of coal with gas or fuel oil. Heat Power Eng. 5 (1996)
Fatkullin, R., Pakhomov, A., Arslanov, R., Valiev, A.: Reducing nitrogen oxide emissions at PK-41 boilers by rational organization of co-firing of gas and fuel oil. Heat Power Eng. 12 (1998)
Yörük, C.R., Meriste, T., Sener, S., Kuusik, R., Trikkel, A.: Thermogravimetric analysis and process simulation of oxy-fuel combustion of blended fuels including oil shale, semicoke, and biomass. Int. J. Energy Res. 42 (2018)
Sun, B., Guo, P., Zhang, W.: Research on prediction of dew point pressure of condensate gas reservoir based on gwo-lssvm and ace models. Fresenius Environ. Bull. 29(12) (2021)
Xu, M.-X., Wu, H.-B., Wu, Y.-C., Ouyang, H.-D., Lu, Q.: Design and evaluation of a novel system for the flue gas compression and purification from the oxy-fuel combustion process. Appl. Energy 285 (2021)
Burgass, R., Chapoy, A., Filho, V.D.O.C.: Development of a new method for measurement of the water dew/frost point of gas. Fluid Phase Equilib. 530 (2021)
Seebold, J.G., Chevron: Quantifying the products of incomplete combustion from petroleum, petrochemical & chemical sector gas-fired process heaters & industrial boilers. Ind. Combust. 15 (2015)
Novikova, O.V., Erastov, A.E., Livshits, S.A.: Features of evaluating the efficiency indicators of the electric power enterprise. E3S Web Conf. 124 (2019)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Livshits, S., Yudina, N., Lebedev, R., Mantserova, T., Galiakhmetova, A. (2022). The Method of Calculating the Dew-Point Temperature of Stack Gases in the Compound Firing of Gases and Fuel Oil in the Boiler Furnace. In: Irina, A., Zunino, P. (eds) Proceedings of the International Symposium on Sustainable Energy and Power Engineering 2021. SUSE 2021. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-16-9376-2_11
Download citation
DOI: https://doi.org/10.1007/978-981-16-9376-2_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-9375-5
Online ISBN: 978-981-16-9376-2
eBook Packages: EngineeringEngineering (R0)