Abstract
Physical activity (PA) boosts mental health and well-being in both healthy and diseased populations. As regards to the latter, its therapeutic effects have been noted in patients diagnosed with various neurodegenerative disorders, and in this chapter we summarize these effects. The neuroprotective effects of PA are conferred via improved neuronal hormones, neurotransmitters, and neurotrophic factor production. These changes are effected through several cellular and molecular mechanisms. PA also leads to enhanced neuroplasticity and neuronal survival, as well as the optimization of physiological and neuroendocrine responses to physical and psychosocial stressors. PA also contributes to the sensitization of the autonomic nervous system, central nervous system, and parasympathetic nervous system. This is done via the promotion of angiogenesis, autophagy, neurogenesis, and synaptic plasticity, amongst other neurological processes. Altogether, PA confers neuroprotective and neuropreventive effects, including improved cognition, memory, sleep, and angiogenesis in the nervous system, and reduced anxiety, insulin resistance, neuro-inflammation, and stress.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Physical activity (PA)
- Neurodegenerative diseases
- Neuroprotective and neuropreventive effects
- Neuronal hormones
- Neurotransmitters
- Neurotrophic factors
8.1 Introduction
Neuroprotection is the process of interfering with the processes responsible for cellular dysfunction and death. This is done to avert neuronal cell death. The notion of neuroprotection has piqued the scientific community’s interest in the search for new medicines that can assist maintenance of brain tissue while also improving overall outcomes [1]. In the elderly, the most prominent risk factor for neurological illnesses is aging [2]. Physical activity reduces the risk of Alzheimer’s disease (AD) and dementia by 45% and 28%, respectively, according to epidemiological research [3, 4].
Physical activity (PA) has garnered much attention as a potential neurological disease-modifying therapeutic method, based on prior studies [5,6,7]. PA has appropriately been described as a non-drug therapy for a variety of disorders. These include cardiovascular, metabolic, neurological and psychiatric diseases [8]. For example, a study by Lu et al. investigated the effect of treadmill-mediated physical activity on cognitive function in a rat model of AD caused by streptozotocin. They found a significant inhibition of neuronal apoptosis in the rat hippocampal Cornu ammonis (CA1) [9]. Furthermore, Lu et al. showed that the induction of angiogenesis probably occurred due to the upregulation of MT1-MMP expression caused by the treadmill exercise. This, in turn, conferred neuroprotection to the rat models of AD against cerebral ischemia [10]. Also, there are considerable data from various in vivo studies on neurological disorders and physical activity that indicate the therapeutic potential of exercise for improving cognition [11, 12].
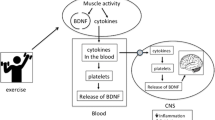
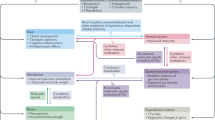
Various PA-induced molecules involved in neurological processes have been discovered, due to considerable breakthroughs in molecular methods [13]. The identified neurological molecules include brain-derived neurotrophic factor (BDNF), endothelial nitric oxide synthase), insulin-like growth factor (IGF), nerve growth factor, superoxide dismutase (SOD), and vascular endothelial growth factor (VEGF), whose levels are increased in the brain hippocampus. In contrast, there occurs a decline in the production of free radicals that are detrimental to neurological functions. Together, these are involved in memory [14]. Figure 8.1 illustrates the neuroprotective and neuropreventive effects of PA for various neurodegenerative disorders.
PA has been shown to slow the progression of neurodegeneration and is known to help reduce the risk of dementia and other neurodegenerative disorders such as Parkinson’s disease (PD), AD, and others [15]. In a meta-analysis, PA was found to be a safe and efficient additional therapy for improving attention, cognition, and memory, impairment of which is associated with various neurological disorders such as AD, PD, Huntington’s disease, multiple sclerosis, schizophrenia, and unipolar depression. PA also improves psychomotor speed, and quality of life, with no complications [16]. Authors of another study reported that PA in midlife maintains functions associated with cognition and minimizes or postpones the risk of dementia in later life [17]. Furthermore, PA and diet modulate the substrates involved in brain neuroplasticity, including antioxidant defense, inflammation, neurogenesis, neurotropic signaling, and stress response. As a result, these are regarded as crucial therapeutic alternatives for age-related disorders, including dementia [18]. Furthermore, Bass et al. found that PA was positively correlated to the academic performance of schoolchildren [19]. In addition, individuals who exercise aerobically improve their attention, executive function, memory, and processing speed, according to a meta-analysis of randomized controlled studies [20]. Exercise also causes an increased blood flow to the hippocampus and reduced neuro-inflammation [21, 22]. Moreover, numerous biological pathways are affected by PA. In particular, it optimizes the physiological and neuroendocrine responses to physical and psychosocial stressors, acts as an armor against stress in general or stress associated with chronic diseases, promotes a state of anti-inflammation, and enhances the expression of growth factor and neuroplasticity [23]. PA affects brain functioning and causes structural alteration as reported in a neuroanatomical study. Here, there was a significant improvement in the cortical tissue density of the frontal, temporal, and parietal cortices, which are otherwise known to be reduced with age (55 to 79 years). This could be attributed to the cardiovascular fitness levels associated with the PA in the study group [24]. Similar findings were reported in an in vivo study, wherein arborization, spine density, and spine morphology were altered among rats that performed voluntary long-term running on the wheel [25]. Interestingly, neuropathology related to AD was attenuated and cognitive functions (hippocampus-mediated) were improved with PA, particularly in the early stages of disease progression. However, specific PA guidelines are yet to be reported [26].
PA, when performed regularly, alleviates the symptoms of AD, as evidenced from animal studies and human clinical trials [23]. PA is also advantageous to PD patients, leading to improved balance, gait, physical functioning, strength, and quality of life, and reduction in the occurrence of PD [27, 28]. In this chapter, we have summarized the neuroprotective and neuropreventive effects of PA for neurodegenerative diseases to aid researchers and medical professionals interested in this area (Table 8.1).
8.2 Role of PA in Neurodegenerative Disease
A sedentary lifestyle with insufficient exercise may increase the risk of AD, PD, and stroke [133]. Aerobic exercise improves cognitive function in elderly people [134]. This could be attributed to decreased chronic oxidative stress while increasing mitochondrial biogenesis and autophagy upregulation, and the neurotransmitters and trophic factors that are stimulated by PA. These include BDNF, fibroblast growth factor 2, glial-derived neurotrophic factor (GDNF), and IGF-1 [28].
Autophagy, anti-oxidant defense mechanisms, neurogenesis, neural plasticity, and other neurophysiological features and pathways are all affected by PA, along with a reduction in neurodegeneration and neural apoptosis. Neuro-plastic changes in the brain are induced by PA, although there is a lot of variation across people [15]. Regular PA enhances neurological function and promotes autophagy [10, 135]. Also, it stimulates mitochondrial biogenesis and lowers chronic oxidative stress. In the hippocampus, there occurs an enhanced expression of neurotrophic factors (BDNF and GDNF) and neurotransmitters (irisin and dopamine [DA]), while BAX and neuro-inflammatory cytokines are suppressed [136]. PA regulates BDNF, which performs crucial functions that include neuronal stress resistance, synaptic transmission and plasticity, neuronal plasticity, activation of DA and NFκB in the neurons, and neuronal differentiation and maturation [13, 137].
AD is perhaps the most common form of dementia and a major healthcare concern [138]. AD patients are often treated with a combination of pharmacological drugs and counselling to retard disease progression [7, 139]. PA prevents cognitive decline and lowers AD risk [140]. It aids in the stabilization and improvement of cognitive functions as well as the prevention and delay of severe neuropsychological symptoms such as apathy, disorientation, and depression in AD patients [141]. Anti-inflammatory and neurotrophic factors have also been found to be induced by PA [142, 143]. In vivo studies have shown that PA can avert damage to white matter (induced by obesity) via suppression of vascular dysfunction and neuro-inflammation. These effects were evident even when there was weight gain in the study animals [144]. Aerobic exercise, in particular, enhances ABCA1 mRNA expression, which in turn may cause improved cognition via alleviating and avoiding symptoms of AD [145]. The above reports provide strong evidence for the therapeutic utility of PA for age-related neurodegenerative disorders such as AD.
PD is the second most common age-related neurodegenerative disease [146]. PD is characterized by α-synuclein accumulation (cytosolic protein) and dopaminergic degeneration at the cellular level [27]. Many efforts have been undertaken to utilize various ways to address its therapeutic element. However, despite numerous advancements in treatment that have slowed the disease’s development and reduced locomotor impairment, clinical management remains a problem [147]. Only high-intensity PA has been shown to be beneficial in alleviating the motor symptoms in PD patients [148]. Furthermore, mood, fatigue, aerobic fitness, motor function, and quality of life have been improved in PD patients [149]. In PD patients, 8 weeks of multi-component PA have improved functional status and gait speed [150]. Another study showed an increase in the concentrations of BDNF, DJ-1, and Hsp70, while aggregation of α-synuclein decreased, in the brains of mice who performed voluntary activity on a running wheel, in contrast to a control group. This provides compelling evidence that the PA can reduce the progression of PD by preventing aberrant protein aggregation in the brain [151]. According to a recent simulation study, PA such as horseback riding improves balance and cognitive impairment in PD-affected elderly [152]. Numerous studies have shown that PA can improve brain function while also reducing the risk of neurodegeneration [153]. PA is also known to improve neuroplasticity through synaptic structural alterations and functional changes in different brain regions. Multiple systems concerning the regulation of neuroinflammation and glial activation are also modulated [153]. Furthermore, using food additives (for example, carvacrol) in combination with PA has led to a reduction in both rotational behavior and aversive memory deficit when observed in rat models of PD. This study also demonstrated a decline in the levels of lipid peroxidation together with an increase in the hippocampus concentration of total thiol in rat models of PD [154]. These observations strengthen the notion that a combined PA-carvacrol therapy may be a promising therapeutic approach for PA patients suffering from impaired neurobehavioral characteristics [154]. PA is also known to benefit benefits PD patients’ health by improving the patient’s ability to adjust to impediments encountered during gait [155].
In a pilot study, coordination and manipulation therapy led to improved cardiac function and balance, and reduced mobility disorder, in PD patients over the control group [156]. In another study, the changes in lifestyle concerning PA and including natural anti-oxidants in the diet alleviates dopaminergic neuronal deterioration. However, this requires strategizing PA and dietary incorporation of oxygen radical scavengers as well as iron-binding agents [157]. PAs such as running on a treadmill improve stability in posture and gait activity, and promote α-synuclein and dopaminergic homeostasis in vivo However, in the same study PA did not significantly induce cerebral alkaline phosphatase [158].
8.3 Neurological Diseases and the Underlying Mechanisms of PA Intervention
8.3.1 PA-Mediated Regulation of the Neuroendocrine System
If the activity is of sufficient intensity and/or duration, PA serves as a stressor for the human body and acts as a neuroendocrine system activator. Chronic exercise training causes neuroendocrine system modifications, such as a reduction in the hormone stress response to submaximal activity [159]. Many substantial alterations in hormone concentrations (β-endorphin, cortisol, vasopressin, adreno-corticotropic hormone) are induced by PA as compared to resting levels. The higher the PA duration and intensity, the larger is the neuroendocrine response [160]. PA triggers various physiological responses, including stimulation of the sympathetic nervous system and hypothalamic-pituitary-adrenal axis, which causes optimal metabolic substrate selection and use. The stimulation of the hypothalamus-pituitary-adrenal axis by PA relies upon myriad attributes, including activity type, when it is performed, dietary intake, and characteristics of the individual [161].
8.3.2 PA and Regulation of Neurotransmitters
The central serotonergic, dopaminergic, and noradrenergic systems are all affected by PA [162]. PA gives rise to peripheral physiological adaptations to compensate for the activity-stimulated disruption in homeostasis in the resting state. Alterations in neurotransmitters and monoamine synthesis and metabolism take place during PA, as documented in various studies that used homogenized tissues to evaluate the levels of the neurotransmitters [162]. The use of voltammetry and microdialysis has revealed that PA influences the release of most of the neurotransmitters in vivo [162]. DA, noradrenaline, and serotonin or hydroxytryptamine (5-HT) are altered by PA, causing an increase in their release, and also affecting their extracellular levels along with γ-aminobutyric acid (GABA), and glutamate (GLU) [163]. Brain DA upregulation has been reported to be associated with PA-induced elevated serum calcium levels. Consequently, calcium/calmodulin-dependent DA production is influenced via tyrosine hydroxylase enzyme activation [164]. Furthermore, PA improves DA-receptor binding affinity [165, 166]. Also, in response to unpredictable stress, PA causes neural adaptation [167]. The galanin expression in the locus coeruleus is responsible for the PA-mediated anti-stress protective mechanism [168]. The expressed galanin, in turn, causes hyperpolarization of noradrenergic neurons, leading to neuronal firing inhibition by the locus coeruleus. This ultimately suppresses norepinephrine (NE) release [169]. It is well-documented that memory consolidation and retrieval are also aided by NE [170]. In comparison to sedentary controls, elevated levels of NE in the pons and medulla of the spinal cord were observed in chronic treadmill running and wheel running-based activities [171]. PA also elevates the endogenous NE activity levels, indicating an association between PA-mediated improved cognition and NE [163]. PA affects the HT system, however it depends on the region of the brain and is influenced by the intensity and duration of the activity. For example, moderate treadmill activity (4 weeks) caused a decline in the hippocampus levels of 5-HT while its metabolism remained unaffected [172]. In contrast, a high-intensity treadmill activity (1 week) led to a significant elevation of hippocampus levels of 5-HT [173].
8.3.3 PA and Neural Insulin Signaling
Insulin signaling in the brain is necessary for the survival of neurons and restoration of critical brain functions. Also, it causes aversion and reversal of BDNF transport abnormalities [174]. Abnormalities in the pathways associated with neural insulin signaling are associated with learning, memory impairment and neurodegenerative disorders, while its deregulation is related to cardiovascular diseases, diabetes, hypertension, and obesity [175]. The pyramidal cell axons of the hippocampal-CAl and other brain regions associated with cognition, memory, and learning have overexpressed insulin receptors [176].
The concentration of IRs is comparatively higher in the cerebral cortex, hippocampus, and hypothalamus regions of the brain [177, 178]. BDNF, insulin, IGF-1, IGF-2, and VEGF are actively involved in intracellular hippocampal neuronal signal transmission under normal physiological conditions. This maintains hippocampal neuronal integrity and functionality [179]. The risk of AD development becomes higher when these are suppressed [179]. A decline in aversive memory, elevation in inflammatory markers (interleukin-1(IL1-β), tissue necrosis factor-alpha (TNFα), and NF-kβ), and decline in anti-inflammatory markers (IL-4) have been observed in the rat models with aging. In the same study, histone H4 acetylation levels were found to have decreased. However, PA caused a reversal in the observed levels [180]. Improved hippocampal neuronal insulin signaling and anti-inflammatory effects have been shown to be exerted by PA, along with the elicitation of insulin-sensitizing effects in the peripheral nervous system (PNS) [179, 181]. Researchers have therefore speculated that PA confers neuroprotection and induces similar effects in the central nervous system (CNS) [182]. Many more investigational pieces of evidence suggest that PA assists in neuroprotection by acting on both CNS and PNS. Insulin-independent glucose uptake in the peripheral tissues is promoted by PA through activation of protein kinase. This is achieved by mammalian targets of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) - mediated activation. By contrast, in the CNS, cognition, synaptic plasticity, angiogenesis, and neurogenesis are improved by PA [183,184,185,186]. Furthermore, neurotransmitter synthesis and degradation are also regulated by PA [187, 188].
8.3.4 BDNF-Signaling and PA
BDNF is a hippocampal neurotrophin and critical regulator of neuronal and synaptic plasticity, and neuronal stress resistance. It is involved in learning and memory-related processes, and may be a key player in depression [189, 190]. It is well known for stimulating the differentiation and maturation of developing neurons [191]. However, positive regulation of the synaptic transmission and plasticity is undertaken in the mature neurons [192]. As a result, BDNF helps with memory and learning [193]. Brain size in humans and PA endurance are positively correlated, which is suggestive of cognition and locomotion co-evolution [194]. Furthermore, brain BDNF expression is elevated by endurance-based PA, and brain growth (of the hippocampus, in particular) is enhanced by improved PA capacity [195]. PA such as running on the treadmill has been found to ameliorate peri-neuronal net disorganization (specifically on the axotomized motoneurons) and synaptic stripping in peripheral nerve injury. Although this is credited to PA-mediated BDNF increases, the underlying molecular mechanisms remain unclear [196]. The hippocampal- and amygdala-associated neuronal functions are enhanced with PA. AD onset could also be delayed with PA as studied in double transgenic mouse models of (aged 1.5–4 months) AD. In this study, 10 weeks of treadmill training elevated the memory associated with the hippocampus while the amygdala-associated memory was restored. Also, the dendritic arbor of amygdala basolateral neurons was restored while those of CA1 and CA3 neurons increased in vivo. The amygdala and hippocampal phosphorylated- protein kinase B, phosphorylated-protein kinase C, and p-TrkB (phosphorylated-tropomyosin receptor kinase B) levels (all signaling molecules of BDNF/TrkB) increased due to PA while the soluble amyloid-β levels declined in vivo [197]. Treadmill and running wheel exercises in vivo (in mouse models aged approximately 4 weeks) significantly elevated the mRNA and protein levels of BDNF and synaptic load in the dentate gyrus. Also, the exercises caused alterations in astrocyte morphology and the orientation of their projections. These could be due to astrocytic TrkB receptor level elevation [198]. The DA content in the neurons and their release are pivotal for neuronal survival as well as learning and memory. All these were modulated by BDNF [199].
8.3.5 Production and Secretion of Irisin and PA
PA induces the muscle protein FNDC5 (fibronectin type III domain containing 5), which in turn is cleaved and secreted as a myokine called irisin [200, 201]. Irisin is known to promote thermogenesis while improving glucose homeostasis and related obesity. There occurred an enhanced BDNF expression due to a forced neuronal FNDC5 expression [200]. Additionally, elevated blood irisin-induced BDNF and hippocampal neuroprotective gene expression were observed upon adenovirus-mediated peripheral FNDC5 delivery to the liver. It has been suggested that the brain’s BDNF expression, endurance-based PA, and metabolic mediators are all linked [200]. It has been further suggested that irisin may serve as a link between motivation and reward mechanisms, and PA. These are, in turn, associated with DA that is activated via BDNF [199]. The neuronal injury induced by ischemia has also been ameliorated by irisin. This was achieved via Akt and ERK1/2 signaling pathway activation. Therefore, it appears that irisin aids the PA-induced neuroprotection against cerebral ischemia. There could be a possible irisin-mediated association between cardio-cerebrovascular disorders and metabolism [202]. Further, irisin has been shown to ameliorate neuronal injury induced by deprivation of oxygen and glucose. This is achieved via inhibition of the ROS-NLRP-3 (reactive oxygen species-Nod-like receptor pyrin-3) signaling pathway (involved in inflammation), which indicates therapeutic effects of irisin in the case of ischemic stroke [203]. Other therapeutic PA effects include neuropathic pain reduction as observed in rat models (male) of chronic constriction injury. In this study, it was observed that the pain threshold increased upon acute administration of irisin while the neuronal number was still reduced [204]. In vitro studies reported that a 12-hour irisin pretreatment conferred neuroprotection against amyloid-β toxicity. Here, IL-6 and IL-1β release was also attenuated along with the reduction in COX-2 expression, and AKT phosphorylation in cultured astrocytes. There occurred a reduction in the activation of NFκB in amyloid-β exposed astrocytes due to abrogation of IκBα phosphorylation and loss. These convincing findings suggest irisin as a potential therapeutic candidate for AD and memory dysfunction associated with diabetes mellitus [205].
8.3.6 PA-Mediated Neuronal Responses: Anti-Inflammatory and Oxidative Responses
To maintain homeostasis, the hypothalamic-pituitary-adrenal axis and the autonomic nervous system are activated in response to PA. Consequently, the plasma levels of catecholamine and cortisol increase. There occurs a stimulation of prolactin and growth hormone secretion. This, in turn, stimulates the TH2 (T-helper cells) response profile and might impact the immune response generated [206]. Attempts have been made to discover novel biomarkers for characterizing PA-induced responses and unraveling the molecular mechanisms underlying neurodegenerative disorders. This would also be beneficial in assessing the effects of PA in these conditions. Kurgan et al. performed proteomic analysis (liquid chromatography-tandem mass spectroscopy) post-2D-gel electrophoresis on the samples obtained from six patients. A significant alteration was observed in the serum levels of 20 proteoforms post high-intensity PA at durations of 5 and 60 min, respectively. These proteoforms included apolipoproteins, protease inhibitors (serpins), and immune system proteins with known anti-inflammatory and antioxidant effects. These are also documented to have important roles in neuro- and cardio-protection, and lipid clearance [207].
Numerous studies have been performed to determine the synergistic and neuroprotective effects of anti-oxidants and PA on neurons in neurological disorders, such as PD. A combination neuroprotection strategy that involved NAC (N-Acetyl-L-cysteine, an anti-oxidant) and PA revealed its neuroprotective effects on mouse models of PD. Later, it was found that only NAC was responsible for conferring this neuroprotection in vivo [75]. PA is also known to induce the production of heat shock proteins (iHSP70, intracellular and eHSP70, extracellular). The iHSP70 activation is essential for anti-inflammatory mechanisms, cellular protection, and promotion of tissue repair while eHSP70 participates in immune system activation. In general, the internalization of eHSP70 (chaperones) by the motor neurons occurs as a stress response to attain cellular protection against oxidative damage and protein denaturation. Furthermore, neurodegenerative disorders (Amyotrophic lateral sclerosis, AD, PD, and Huntington’s disease) are often characterized by lower expression levels of iHSP70. Therefore, it is important to elucidate their functional attributes and the neuroprotective effects of PA [208]. In response to PA, the anti-oxidant enzyme SOD is also released [28]. Together, these delay the onset of neurodegenerative disorders such as PD by retarding neural apoptosis, promoting neuroplasticity, and delaying neurodegenerative processes [133].
8.3.7 Effects of PA on Survival and Apoptosis of Neurons
PA is known to effectuate brain cell activity, survival, and apoptosis. PA, when performed voluntarily under favorable conditions, has caused cognition improvement, brain microvasculature, and neurogenesis promotion in hypobaric hypoxia exposed rat models. These effects were observed to be mediated via VEGF signaling [209]. PA, in the early stages of life, has been observed to induce prolonged neuronal (cortical) and hippocampal morphological changes in rat models. These in vivo effects were noticeable in a subsequent sedentary period. This study’s authors speculated that neuronal growth promotion and neurotrophic factor expression are enhanced by PA, which replenishes the neuronal reservoir for later use in life. Also, there occurred PA-induced elevation in the neuronal (cortical) and hippocampal cellular population along with dendritic arborization [210]. Additionally, survival protein expression increased. These included hippocampal BDNF and cortical mTOR [210]. Reportedly, BDNF promotes PA-induced neuroprotective effects against type-II diabetes and dementia [211]. PA training type determined alterations in brain cell survival and inflammatory protein levels and their expression in rat models (aged rats). In particular, PA such as aerobics enhanced brain (cortex) Akt, p38, p70S6k, and ERK protein expression levels [212]. PA such as running improved spatial learning and memory in APP/PS1 transgenic mouse models (middle-aged) of AD. This was attributed to the neurogenesis and neuroprotection conferred by the PA in the dentate gyrus of these mouse models [213].
Further, PA such as treadmill exercise retarded Aβ-42 deposition via β-secretase (BACE-1) and C-99 inhibition and checked memory impairment (PS2 mutation-induced) in the cortex and the hippocampal region of PS2 mutant mouse models (aged). Also, there occurred a downregulation of protein disulfide (PDI) and glucose-related protein/binding immunoglobulin protein (GRP78/Bip) expression and abrogation of activating transcription factor-alpha (ATF6α), eukaryotic initiation factor-2α (eIF2α), Jun N-terminal kinases-p38- mitogen-activated protein kinases (JNK-p38 MAPK), protein kinase R-like endoplasmic reticulum kinase (PERK), and spliced X-box binding protein 1 (sXBP1). PA also led to Bcl-2 upregulation, CHOP, caspase-3, and caspase-12 activation, and BAX downregulation in PS2 mutant mouse models (aged) [214]. PA with varied intensities was observed to produce distinct effects on the nervous system. For instance, moderate intensity PA (treadmill) conferred neuroprotection in rat models of ischemia over a high-intensity workout, which causes downregulation of the neurotrophic factors influencing cell cycle-related protein expression levels [215]. PA that involved voluntary running stimulated progenitor cell proliferation in the dentate gyrus, and neurogenesis [216]. Reportedly, PA confers protection to the injury-susceptible retinal ganglion cells. This could be due to neuronal functional restoration and survival via thwarting of synaptic elimination (complement-mediated), and abrogation of retinal BDNF loss by the PA [217]. Finally, PA was observed to positively affect BDNF resting serum levels and cognition in adolescent mouse models (male) that were exposed to aerobics-based PA (moderate to high intensity) [218].
8.3.8 PA and Its Effects on Neural Autophagy
Under conditions of stress, such as restricted food supplies, evolution favored species with greater cognitive and physical abilities. This is suggestive of the fact that brain function can be improved by PA and dietary restrictions. Autophagy, DNA-repair proteins, mitochondrial biogenesis, neurotrophic factors, and protein chaperones are all involved in the neuronal signaling pathways for stress-response under energy limitations. The risk of neurodegenerative disorders, such as AD, PD, depression, and stroke might increase due to dietary malpractices, suppressed cellular adaptive stress responses, and lack of PA [133]. Furthermore, brain functions have been shown to improve in vivo with PA and dietary regulations, which checked the neurodegenerative processes. PA along with dietary regulations stimulate the signaling pathways for cellular adaptive stress responses, which, in turn, promote proteostasis, DNA repair, mitochondrial biogenesis, and neurotrophic signaling [219]. Aerobics-based PA and food deprivation have been observed to activate the neuronal signaling pathways involving PGC-1α, NFκB, CREB, and Ca2+. These, in turn, induce mitochondrial biogenesis and cellular stress responses [220].
Autophagy is the cell’s natural, conserved breakdown process, which removes unwanted or malfunctioning components via a lysosome-dependent, controlled mechanism. It enables the breakdown and recycling of cellular components in a controlled manner. Autophagic dysfunction leads to an increased sensitivity to stress conditions such as oxidative damage or starvation, loss of stem cells, neurodegeneration, and a rapid deterioration in neuromuscular function in vivo [221]. Autophagy plays a pivotal role in the production of β-amyloid and therefore its dysfunction can cause AD progression. Under genetically hyper-activated autophagic conditions, there occurs a significant decline in the accumulation of β-amyloid in knock-in mouse models of AD (Becn1F121A). A restoration in the cognitive decline and survival was also observed in this study. This could be due to the mutated Becn 1 (Becn1F121A), which led to a significant decline in the BECN 1 and BCL2 (inhibitor) interaction. Consequently, there occurred a constitutive autophagy activation. The amyloid-β-oligomers were found to be segregated inside the brain autophagosomes in vivo. Finally, PA was observed to be a physiological inducer of autophagy, which confers neuroprotective effects similar to those of Becn1. These included the removal of amyloid-β and improved memory in vivo [222].
8.4 Conclusion
Physical activities have been shown to improve people’s overall health and well-being when they participate in them regularly. Regular exercisers reap the benefits in every part of the body in some way. When it comes to the effects on neuronal cells and brain function, numerous studies show that the PA has neuroprotective effects. The neuroprotective effects of physical activity are elicited by signaling processes that have yet to be fully understood. However, hormones such as irisin, neurotransmitters such as DA, and neurotrophins such as BDNF are known to directly participate in these signaling mechanisms. Furthermore, PA improves balance, cognition, and gait in PD patients, and retards disease progression by preventing brain aggregation of the protein. Furthermore, disease progression is retarded and the onset of neuropsychological symptoms is delayed in AD patients, along with improved cognition and memory. PA affects different neurophysiological aspects in afflicted patients. These include anti-inflammatory and anti-oxidant responses, autophagy, cell survival, apoptotic pathways, and hippocampal insulin signaling. PA is also known to upregulate BDNF expression that contributes to its neuroprotective effects. These neuroprotective mechanisms also involve Akt are DA, GABA, and irisin. In conclusion, PA is an excellent therapy for patients diagnosed with various neurological disorders when used in combination with other well-established treatment regimens.
References
Majid A. Neuroprotection in stroke: past, present, and future. ISRN Neurol. 2014;2014:515716.
Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–81.
Rovio S, Kareholt I, Viitanen M, Winblad B, Tuomilehto J, Soininen H, et al. Work-related physical activity and the risk of dementia and Alzheimer's disease. Int J Geriatr Psychiatry. 2007;22(9):874–82.
Tan ZS, Spartano NL, Beiser AS, DeCarli C, Auerbach SH, Vasan RS, et al. Physical activity, brain volume, and dementia risk: the Framingham study. J Gerontol A Biol Sci Med Sci. 2017;72(6):789–95.
Hoffmann K, Sobol NA, Frederiksen KS, Beyer N, Vogel A, Vestergaard K, et al. Moderate-to-high intensity physical exercise in patients with Alzheimer's disease: a randomized controlled trial. J Alzheimers Dis. 2016;50(2):443–53.
Groot C, Hooghiemstra AM, Raijmakers PG, van Berckel BN, Scheltens P, Scherder EJ, et al. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res Rev. 2016;25:13–23.
Frederiksen KS, Gjerum L, Waldemar G, Hasselbalch SG. Effects of physical exercise on Alzheimer’s disease biomarkers: a systematic review of intervention studies. J Alzheimers Dis. 2018;61(1):359–72.
Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl 3):1–72.
Lu Y, Dong Y, Tucker D, Wang R, Ahmed ME, Brann D, et al. Treadmill exercise exerts neuroprotection and regulates microglial polarization and oxidative stress in a Streptozotocin-induced rat model of sporadic Alzheimer's disease. J Alzheimers Dis. 2017;56(4):1469–84.
Tang Y, Zhang Y, Zheng M, Chen J, Chen H, Liu N. Effects of treadmill exercise on cerebral angiogenesis and MT1-MMP expression after cerebral ischemia in rats. Brain Behav. 2018;8(8):e01079.
Allard JS, Ntekim O, Johnson SP, Ngwa JS, Bond V, Pinder D, et al. APOEepsilon4 impacts up-regulation of brain-derived neurotrophic factor after a six-month stretch and aerobic exercise intervention in mild cognitively impaired elderly African Americans: a pilot study. Exp Gerontol. 2017;87(Pt A):129–36.
Stranahan AM, Martin B, Maudsley S. Anti-inflammatory effects of physical activity in relationship to improved cognitive status in humans and mouse models of Alzheimer's disease. Curr Alzheimer Res. 2012;9(1):86–92.
Liu Y, Yan T, Chu JM, Chen Y, Dunnett S, Ho YS, et al. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab Invest. 2019;99(7):943–57.
Paillard T, Rolland Y, de Souto BP. Protective effects of physical exercise in Alzheimer's disease and Parkinson's disease: a narrative review. J Clin Neurol. 2015;11(3):212–9.
Mullers P, Taubert M, Muller NG. Physical exercise as personalized medicine for dementia prevention? Front Physiol. 2019;10:672.
Dauwan M, Begemann MJH, Slot MIE, Lee EHM, Scheltens P, Sommer IEC. Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: a transdiagnostic systematic review and meta-analysis of randomized controlled trials. J Neurol. 2021;268(4):1222–46.
Chang M, Jonsson PV, Snaedal J, Bjornsson S, Saczynski JS, Aspelund T, et al. The effect of midlife physical activity on cognitive function among older adults: AGES—Reykjavik study. J Gerontol A Biol Sci Med Sci. 2010;65(12):1369–74.
Phillips C. Lifestyle modulators of neuroplasticity: how physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plast. 2017;2017:3589271.
Bass RW, Brown DD, Laurson KR, Coleman MM. Physical fitness and academic performance in middle school students. Acta Paediatr. 2013;102(8):832–7.
Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–52.
Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, et al. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31(32):11578–86.
Young MF, Valaris S, Wrann CD. A role for FNDC5/Irisin in the beneficial effects of exercise on the brain and in neurodegenerative diseases. Prog Cardiovasc Dis. 2019;62(2):172–8.
Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. 2014;4(5):20140040.
Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58(2):176–80.
Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1017–22.
Phillips C, Baktir MA, Das D, Lin B, Salehi A. The link between physical activity and cognitive dysfunction in Alzheimer disease. Phys Ther. 2015;95(7):1046–60.
Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008;23(5):631–40.
Monteiro-Junior RS, Cevada T, Oliveira BR, Lattari E, Portugal EM, Carvalho A, et al. We need to move more: neurobiological hypotheses of physical exercise as a treatment for Parkinson's disease. Med Hypotheses. 2015;85(5):537–41.
Wu PL, Lee M, Huang TT. Effectiveness of physical activity on patients with depression and Parkinson's disease: a systematic review. PLoS One. 2017;12(7):e0181515.
Bhalsing KS, Abbas MM, Tan LCS. Role of physical activity in Parkinson's disease. Ann Indian Acad Neurol. 2018;21(4):242–9.
Lauze M, Daneault JF, Duval C. The effects of physical activity in Parkinson's disease: a review. J Parkinsons Dis. 2016;6(4):685–98.
Fan B, Jabeen R, Bo B, Guo C, Han M, Zhang H, et al. What and how can physical activity prevention function on Parkinson's disease? Oxid Med Cell Longev. 2020;2020:4293071.
Bouca-Machado R, Rosario A, Caldeira D, Castro Caldas A, Guerreiro D, Venturelli M, et al. Physical activity, exercise, and physiotherapy in Parkinson's disease: defining the concepts. Mov Disord Clin Pract. 2020;7(1):7–15.
Mantri S, Fullard ME, Duda JE, Morley JF. Physical activity in early Parkinson disease. J Parkinsons Dis. 2018;8(1):107–11.
Cusso ME, Donald KJ, Khoo TK. The impact of physical activity on non-motor symptoms in Parkinson's disease: a systematic review. Front Med. 2016;3:35.
Hughes KC, Gao X, Molsberry S, Valeri L, Schwarzschild MA, Ascherio A. Physical activity and prodromal features of Parkinson disease. Neurology. 2019;93(23):e2157–e69.
Fang X, Han D, Cheng Q, Zhang P, Zhao C, Min J, et al. Association of levels of physical activity with risk of Parkinson disease: a systematic review and meta-analysis. JAMA Netw Open. 2018;1(5):e182421.
Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39(1):3–11.
Malczynska P, Piotrowicz Z, Drabarek D, Langfort J, Chalimoniuk M. The role of the brain-derived neurotrophic factor (BDNF) in neurodegenerative processes and in the neuroregeneration mechanisms induced by increased physical activity. Postepy Biochem. 2019;65(1):2–8.
Biddiscombe KJ, Ong B, Kalinowski P, Pike KE. Physical activity and cognition in young-onset Parkinson's disease. Acta Neurol Scand. 2020;142(2):151–60.
Quinn L, Macpherson C, Long K, Shah H. Promoting physical activity via telehealth in people with Parkinson disease: the path forward after the COVID-19 pandemic? Phys Ther. 2020;100(10):1730–6.
van der Heide A, Meinders MJ, Bloem BR, Helmich RC. The impact of the COVID-19 pandemic on psychological distress, physical activity, and symptom severity in Parkinson's disease. J Parkinsons Dis. 2020;10(4):1355–64.
LaHue SC, Comella CL, Tanner CM. The best medicine? The influence of physical activity and inactivity on Parkinson's disease. Mov Disord. 2016;31(10):1444–54.
Vizzi L, Padua E, D'Amico AG, Tancredi V, D'Arcangelo G, Cariati I, et al. Beneficial effects of physical activity on subjects with neurodegenerative disease. J Funct Morphol Kinesiol. 2020;5:4.
Schirinzi T, Di Lazzaro G, Salimei C, Cerroni R, Liguori C, Scalise S, et al. Physical activity changes and correlate effects in patients with Parkinson's disease during COVID-19 lockdown. Mov Disord Clin Pract. 2020;
Fayyaz M, Jaffery SS, Anwer F, Zil EAA, Anjum I. The effect of physical activity in Parkinson's disease: a mini-review. Cureus. 2018;10(7):e2995.
Pradhan S, Kelly VE. Quantifying physical activity in early Parkinson disease using a commercial activity monitor. Parkinsonism Relat Disord. 2019;66:171–5.
Shih CH, Moore K, Browner N, Sklerov M, Dayan E. Physical activity mediates the association between striatal dopamine transporter availability and cognition in Parkinson's disease. Parkinsonism Relat Disord. 2019;62:68–72.
Amara AW, Chahine L, Seedorff N, Caspell-Garcia CJ, Coffey C, Simuni T, et al. Self-reported physical activity levels and clinical progression in early Parkinson's disease. Parkinsonism Relat Disord. 2019;61:118–25.
Aktar B, Donmez Colakoglu B, Balci B. Does the postural stability of patients with Parkinson's disease affect the physical activity? Int J Rehabil Res. 2020;43(1):41–7.
Hermanns M, Haas BK, Lisk J. Engaging older adults with Parkinson's disease in physical activity using technology: a feasibility study. Gerontol Geriatr Med. 2019;5:2333721419842671.
Urell C, Zetterberg L, Hellstrom K, Anens E. Factors explaining physical activity level in Parkinson s disease: a gender focus. Physiother Theory Pract. 2021;37(4):507–16.
Feliciano JS, Rodrigues SMA, de Carvalho LR, Polese JC. Predictors of physical activity levels in individuals with Parkinson's disease: a cross-sectional study. Neurol Sci. 2021;42(4):1499–505.
Hunter H, Lovegrove C, Haas B, Freeman J, Gunn H. Experiences of people with Parkinson's disease and their views of physical activity interventions: a qualitative systematic review protocol. JBI Database System Rev Implement Rep. 2017;15(11):2619–23.
Gorzkowska A, Cholewa J, Malecki A, Klimkowicz-Mrowiec A, Cholewa J. What determines spontaneous physical activity in patients with Parkinson's disease? J Clin Med. 2020;9:5.
Mantri S, Wood S, Duda JE, Morley JF. Comparing self-reported and objective monitoring of physical activity in Parkinson disease. Parkinsonism Relat Disord. 2019;67:56–9.
Krishnamurthi N, Fleury J, Belyea M, Shill HA, Abbas JJ. ReadySteady intervention to promote physical activity in older adults with Parkinson's disease: study design and methods. Contemp Clin Trials Commun. 2020;17:100513.
Oveisgharan S, Dawe RJ, Leurgans SE, Yu L, Schneider JA, Bennett DA, et al. Total daily physical activity, brain pathologies, and parkinsonism in older adults. PLoS One. 2020;15(4):e0232404.
Swank C, Shearin S, Cleveland S, Driver S. Auditing the physical activity and Parkinson disease literature using the behavioral epidemiologic framework. PM R. 2017;9(6):612–21.
Mantri S, Wood S, Duda JE, Morley JF. Understanding physical activity in veterans with Parkinson disease: a mixed-methods approach. Parkinsonism Relat Disord. 2019;61:156–60.
Terashi H, Taguchi T, Ueta Y, Mitoma H, Aizawa H. Association of daily physical activity with cognition and mood disorders in treatment-naive patients with early-stage Parkinson's disease. J Neural Transm (Vienna). 2019;126(12):1617–24.
Penko AL, Barkley JE, Rosenfeldt AB, Alberts JL. Multimodal training reduces fall frequency as physical activity increases in individuals with Parkinson's disease. J Phys Act Health. 2019;16(12):1085–91.
Nero H, Franzen E, Stahle A, Benka Wallen M, Hagstromer M. Long-term effects of balance training on habitual physical activity in older adults with Parkinson's disease. Parkinsons Dis. 2019;2019:8769141.
Alwardat M, Schirinzi T, Di Lazzaro G, Sancesario GM, Franco D, Imbriani P, et al. Association between physical activity and dementia's risk factors in patients with Parkinson's disease. J Neural Transm (Vienna). 2019;126(3):319–25.
Yang F, Trolle Lagerros Y, Bellocco R, Adami HO, Fang F, Pedersen NL, et al. Physical activity and risk of Parkinson's disease in the Swedish National March Cohort. Brain. 2015;138(Pt 2):269–75.
Alausa A, Ogundepo S, Olaleke B, Adeyemi R, Olatinwo M, Ismail A. Chinese nutraceuticals and physical activity; their role in neurodegenerative tauopathies. Chinas Med. 2021;16(1):1.
Nguy V, Barry BK, Moloney N, Hassett LM, Canning CG, Lewis SJG, et al. The associations between physical activity, sleep, and mood with pain in people with Parkinson's disease: an observational cross-sectional study. J Parkinsons Dis. 2020;10(3):1161–70.
Baumeister S, Meisinger C, Leitzmann M, Teumer A, Bahls M, Karch A, et al. Physical activity and Parkinson's disease: a two-sample Mendelian randomisation study. J Neurol Neurosurg Psychiatry. 2021;92(3):334–5.
Galperin I, Herman T, Assad M, Ganz N, Mirelman A, Giladi N, et al. Sensor-based and patient-based assessment of daily-living physical activity in people with Parkinson's disease: do Motor subtypes play a role? Sensors (Basel). 2020;20:24.
Hiorth YH, Larsen JP, Lode K, Tysnes OB, Godfrey A, Lord S, et al. Impact of falls on physical activity in people with Parkinson's disease. J Parkinsons Dis. 2016;6(1):175–82.
Aktar B, Balci B, Donmez CB. Physical activity in patients with Parkinson's disease: a holistic approach based on the ICF model. Clin Neurol Neurosurg. 2020;198:106132.
Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64(4):664–9.
Dontje ML, de Greef MH, Speelman AD, van Nimwegen M, Krijnen WP, Stolk RP, et al. Quantifying daily physical activity and determinants in sedentary patients with Parkinson's disease. Parkinsonism Relat Disord. 2013;19(10):878–82.
Speelman AD, van de Warrenburg BP, van Nimwegen M, Petzinger GM, Munneke M, Bloem BR. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol. 2011;7(9):528–34.
Gil-Martinez AL, Cuenca L, Sanchez C, Estrada C, Fernandez-Villalba E, Herrero MT. Effect of NAC treatment and physical activity on neuroinflammation in subchronic parkinsonism; is physical activity essential? J Neuroinflammation. 2018;15(1):328.
Jeng B, Cederberg KL, Lai B, Sasaki JE, Bamman MM, Motl RW. Step-rate threshold for physical activity intensity in Parkinson's disease. Acta Neurol Scand. 2020;142(2):145–50.
Daneault JF, Sadikot AF, Barbat-Artigas S, Aubertin-Leheudre M, Jodoin N, Panisset M, et al. Physical activity in advanced Parkinson's disease: impact of subthalamic deep brain stimulation. J Parkinsons Dis. 2015;5(1):85–93.
Oveisgharan S, Yu L, Dawe RJ, Bennett DA, Buchman AS. Total daily physical activity and the risk of parkinsonism in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2020;75(4):702–11.
Duncan RP, Van Dillen LR, Garbutt JM, Earhart GM, Perlmutter JS. Low back pain–related disability in parkinson disease: impact on functional mobility, physical activity, and quality of life. Phys Ther. 2019;99(10):1346–53.
Roland KP, Theou O, Jakobi JM, Jones GR. Physical activity across frailty phenotypes in females with Parkinson's disease. J Aging Res. 2012;2012:468156.
Coe S, Franssen M, Collett J, Boyle D, Meaney A, Chantry R, et al. Physical activity, fatigue, and sleep in people with Parkinson's disease: a secondary per protocol analysis from an intervention trial. Parkinsons Dis. 2018;2018:1517807.
Nero H, Benka Wallen M, Franzen E, Conradsson D, Stahle A, Hagstromer M. Objectively assessed physical activity and its association with balance, physical function and dyskinesia in Parkinson's disease. J Parkinsons Dis. 2016;6(4):833–40.
Logroscino G, Sesso HD, Paffenbarger RS Jr, Lee IM. Physical activity and risk of Parkinson's disease: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2006;77(12):1318–22.
Santos D, Mahoney JR, Allali G, Verghese J. Physical activity in older adults with mild parkinsonian signs: a cohort study. J Gerontol A Biol Sci Med Sci. 2018;73(12):1682–7.
Annesi JJ. Effects of a group protocol on physical activity and associated changes in mood and health locus of control in adults with Parkinson disease and reduced mobility. Perm J. 2019;23:18–128.
Bryant MS, Kang GE, Protas EJ. Relation of chair rising ability to activities of daily living and physical activity in Parkinson's disease. Arch Phys Ther. 2020;10(1):22.
Shih IF, Liew Z, Krause N, Ritz B. Lifetime occupational and leisure time physical activity and risk of Parkinson's disease. Parkinsonism Relat Disord. 2016;28:112–7.
Galperin I, Hillel I, Del Din S, Bekkers EMJ, Nieuwboer A, Abbruzzese G, et al. Associations between daily-living physical activity and laboratory-based assessments of motor severity in patients with falls and Parkinson's disease. Parkinsonism Relat Disord. 2019;62:85–90.
Colon-Semenza C, Latham NK, Quintiliani LM, Ellis TD. Peer coaching through mHealth targeting physical activity in people with Parkinson disease: feasibility study. JMIR Mhealth Uhealth. 2018;6(2):e42.
Lamont RM, Daniel HL, Payne CL, Brauer SG. Accuracy of wearable physical activity trackers in people with Parkinson's disease. Gait Posture. 2018;63:104–8.
Thacker EL, Chen H, Patel AV, McCullough ML, Calle EE, Thun MJ, et al. Recreational physical activity and risk of Parkinson's disease. Mov Disord. 2008;23(1):69–74.
Snider J, Muller ML, Kotagal V, Koeppe RA, Scott PJ, Frey KA, et al. Non-exercise physical activity attenuates motor symptoms in Parkinson disease independent from nigrostriatal degeneration. Parkinsonism Relat Disord. 2015;21(10):1227–31.
Ito H, Yokoi D, Kobayashi R, Okada H, Kajita Y, Okuda S. The relationships between three-axis accelerometer measures of physical activity and motor symptoms in patients with Parkinson's disease: a single-center pilot study. BMC Neurol. 2020;20(1):340.
Shih IF, Starhof C, Lassen CF, Hansen J, Liew Z, Ritz B. Occupational and recreational physical activity and Parkinson's disease in Denmark. Scand J Work Environ Health. 2017;43(3):210–6.
Ellis T, Motl RW. Physical activity behavior change in persons with neurologic disorders: overview and examples from Parkinson disease and multiple sclerosis. J Neurol Phys Ther. 2013;37(2):85–90.
Palasz E, Bak A, Gasiorowska A, Niewiadomska G. The role of trophic factors and inflammatory processes in physical activity-induced neuroprotection in Parkinson's disease. Postepy Hig Med Dosw (Online). 2017;71(1):713–26.
Abrantes AM, Friedman JH, Brown RA, Strong DR, Desaulniers J, Ing E, et al. Physical activity and neuropsychiatric symptoms of Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):138–45.
van Nimwegen M, Speelman AD, Overeem S, van de Warrenburg BP, Smulders K, Dontje ML, et al. Promotion of physical activity and fitness in sedentary patients with Parkinson's disease: randomised controlled trial. BMJ. 2013;346:f576.
Srulijes K, Klenk J, Schwenk M, Schatton C, Schwickert L, Teubner-Liepert K, et al. Fall risk in relation to individual physical activity exposure in patients with different neurodegenerative diseases: a pilot study. Cerebellum. 2019;18(3):340–8.
Gronek P, Haas AN, Czarny W, Podstawski R, Delabary MDS, Clark CC, et al. The mechanism of physical activity-induced amelioration of Parkinson's disease: a narrative review. Aging Dis. 2021;12(1):192–202.
van Uem JMT, Cerff B, Kampmeyer M, Prinzen J, Zuidema M, Hobert MA, et al. The association between objectively measured physical activity, depression, cognition, and health-related quality of life in Parkinson's disease. Parkinsonism Relat Disord. 2018;48:74–81.
Bril A, Perez-Lloret S, Rossi M, Farina S, Morisset P, Sorrentino L, et al. A multifactorial study on nutritional status, binge eating and physical activity as main factors directly influencing body weight in Parkinson's disease. NPJ Parkinsons Dis. 2017;3:17.
Paul SS, Ellis TD, Dibble LE, Earhart GM, Ford MP, Foreman KB, et al. Obtaining reliable estimates of ambulatory physical activity in people with Parkinson's disease. J Parkinsons Dis. 2016;6(2):301–5.
Garber CE, Friedman JH. Effects of fatigue on physical activity and function in patients with Parkinson's disease. Neurology. 2003;60(7):1119–24.
Jimenez-Pardo J, Holmes JD, Jenkins ME, Johnson AM. An examination of the reliability and factor structure of the physical activity scale for individuals with physical disabilities (PASIPD) among individuals living with Parkinson's disease. J Aging Phys Act. 2015;23(3):391–4.
Lana Rde C, de Araujo LN, Cardoso F, Rodrigues-de-Paula F. Main determinants of physical activity levels in individuals with Parkinson's disease. Arq Neuropsiquiatr. 2016;74(2):112–6.
Wu PF, Lu H, Zhou X, Liang X, Li R, Zhang W, et al. Assessment of causal effects of physical activity on neurodegenerative diseases: a Mendelian randomization study. J Sport Health Sci. 2021;10(4):454–61.
Cai G, Huang Y, Luo S, Lin Z, Dai H, Ye Q. Continuous quantitative monitoring of physical activity in Parkinson's disease patients by using wearable devices: a case-control study. Neurol Sci. 2017;38(9):1657–63.
Borrione P, Tranchita E, Sansone P, Parisi A. Effects of physical activity in Parkinson's disease: a new tool for rehabilitation. World J Methodol. 2014;4(3):133–43.
Loprinzi PD, Danzl MM, Ulanowski E, Paydo C. A pilot study evaluating the association between physical activity and cognition among individuals with Parkinson's disease. Disabil Health J. 2018;11(1):165–8.
Kim R, Park S, Yoo D, Jun JS, Jeon B. Association of Physical Activity and APOE genotype with longitudinal cognitive change in early Parkinson disease. Neurology. 2021;96(19):e2429–e37.
Khalil H, Alomari MA, Khabour O, Al-Hieshan A, Bajwa JA. The association between physical activity with cognitive function and brain-derived neurotrophic factor in people with Parkinson's disease: a pilot study. J Aging Phys Act. 2017;25(4):646–52.
Elbers R, van Wegen EE, Rochester L, Hetherington V, Nieuwboer A, Willems AM, et al. Is impact of fatigue an independent factor associated with physical activity in patients with idiopathic Parkinson's disease? Mov Disord. 2009;24(10):1512–8.
Santos PC, Gobbi LT, Orcioli-Silva D, Simieli L, van Dieen JH, Barbieri FA. Effects of leg muscle fatigue on gait in patients with Parkinson's disease and controls with high and low levels of daily physical activity. Gait Posture. 2016;47:86–91.
von Rosen P, Hagstromer M, Franzen E, Leavy B. Physical activity profiles in Parkinson's disease. BMC Neurol. 2021;21(1):71.
Ravenek MJ, Schneider MA. Social support for physical activity and perceptions of control in early Parkinson's disease. Disabil Rehabil. 2009;31(23):1925–36.
Lim I, van Wegen E, Jones D, Rochester L, Nieuwboer A, Willems AM, et al. Does cueing training improve physical activity in patients with Parkinson's disease? Neurorehabil Neural Repair. 2010;24(5):469–77.
Nero H, Benka Wallen M, Franzen E, Stahle A, Hagstromer M. Accelerometer cut points for physical activity assessment of older adults with Parkinson's disease. PLoS One. 2015;10(9):e0135899.
Stanojlovic M, Pallais JP, Kotz CM. Inhibition of orexin/Hypocretin neurons ameliorates elevated physical activity and energy expenditure in the A53T mouse model of Parkinson's disease. Int J Mol Sci. 2021;22:2.
van Nimwegen M, Speelman AD, Smulders K, Overeem S, Borm GF, Backx FJ, et al. Design and baseline characteristics of the ParkFit study, a randomized controlled trial evaluating the effectiveness of a multifaceted behavioral program to increase physical activity in Parkinson patients. BMC Neurol. 2010;10:70.
Porta M, Pilloni G, Pili R, Casula C, Murgia M, Cossu G, et al. Association between objectively measured physical activity and gait patterns in people with Parkinson's disease: results from a 3-month monitoring. Parkinsons Dis. 2018;2018:7806574.
Ng SY, Chia NS, Abbas MM, Saffari ES, Choi X, Heng DL, et al. Physical activity improves anxiety and apathy in early Parkinson's disease: a longitudinal follow-up study. Front Neurol. 2020;11:625897.
Delikanaki-Skaribas E, Trail M, Wong WW, Lai EC. Daily energy expenditure, physical activity, and weight loss in Parkinson's disease patients. Mov Disord. 2009;24(5):667–71.
Saaksjarvi K, Knekt P, Mannisto S, Lyytinen J, Jaaskelainen T, Kanerva N, et al. Reduced risk of Parkinson's disease associated with lower body mass index and heavy leisure-time physical activity. Eur J Epidemiol. 2014;29(4):285–92.
Speelman AD, van Nimwegen M, Bloem BR, Munneke M. Evaluation of implementation of the ParkFit program: a multifaceted intervention aimed to promote physical activity in patients with Parkinson's disease. Physiotherapy. 2014;100(2):134–41.
Sutcu G, Ayvat E, Kilinc M. Effects of fatigue and kinesiophobia on functional capacity, physical activity and quality of life in Parkinson's disease. Int J Rehabil Res. 2021;44(1):65–8.
Fleming A, Cook KF, Nelson ND, Lai EC. Proxy reports in Parkinson's disease: caregiver and patient self-reports of quality of life and physical activity. Mov Disord. 2005;20(11):1462–8.
Benka Wallen M, Franzen E, Nero H, Hagstromer M. Levels and patterns of physical activity and sedentary behavior in elderly people with mild to moderate Parkinson disease. Phys Ther. 2015;95(8):1135–41.
Hoff JI, Van Hilten JJ, Middelkoop HA, Roos RA. Fatigue in Parkinson's disease is not associated with reduced physical activity. Parkinsonism Relat Disord. 1997;3(1):51–4.
Cugusi L, Solla P, Zedda F, Loi M, Serpe R, Cannas A, et al. Effects of an adapted physical activity program on motor and non-motor functions and quality of life in patients with Parkinson's disease. NeuroRehabilitation. 2014;35(4):789–94.
Martignon C, Ruzzante F, Giuriato G, Laginestra FG, Pedrinolla A, Di Vico IA, et al. The key role of physical activity against the neuromuscular deterioration in patients with Parkinson's disease. Acta Physiol (Oxf). 2021;231(4):e13630.
Balci B, Aktar B, Buran S, Tas M, Donmez CB. Impact of the COVID-19 pandemic on physical activity, anxiety, and depression in patients with Parkinson's disease. Int J Rehabil Res. 2021;44(2):173–6.
Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16(6):706–22.
Erickson KI, Gildengers AG, Butters MA. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. 2013;15(1):99–108.
Moreira OC, Estebanez B, Martinez-Florez S, de Paz JA, Cuevas MJ, Gonzalez-Gallego J. Mitochondrial function and Mitophagy in the elderly: effects of exercise. Oxid Med Cell Longev. 2017;2017:2012798.
Di Liegro CM, Schiera G, Proia P, Di Liegro I. Physical activity and brain health. Genes (Basel). 2019;10:9.
Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 2015;11(6):1164–78.
Jo T, Nho K, Saykin AJ. Deep learning in Alzheimer's disease: diagnostic classification and prognostic prediction using neuroimaging data. Front Aging Neurosci. 2019;11:220.
Taylor MK, Swerdlow RH, Sullivan DK. Dietary Neuroketotherapeutics for Alzheimer's disease: an evidence update and the potential role for diet quality. Nutrients. 2019;11:8.
Li B, Liang F, Ding X, Yan Q, Zhao Y, Zhang X, et al. Interval and continuous exercise overcome memory deficits related to beta-amyloid accumulation through modulating mitochondrial dynamics. Behav Brain Res. 2019;376:112171.
Kouloutbani K, Karteroliotis K, Politis A. The effect of physical activity on dementia. Psychiatriki. 2019;30(2):142–55.
Lavie CJ, Church TS, Milani RV, Earnest CP. Impact of physical activity, cardiorespiratory fitness, and exercise training on markers of inflammation. J Cardiopulm Rehabil Prev. 2011;31(3):137–45.
Alomari MA, Khabour OF, Alzoubi KH, Alzubi MA. Forced and voluntary exercises equally improve spatial learning and memory and hippocampal BDNF levels. Behav Brain Res. 2013;247:34–9.
Graham LC, Grabowska WA, Chun Y, Risacher SL, Philip VM, Saykin AJ, et al. Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiol Aging. 2019;80:154–72.
McGurran H, Glenn JM, Madero EN, Bott NT. Prevention and treatment of Alzheimer's disease: biological mechanisms of exercise. J Alzheimers Dis. 2019;69(2):311–38.
Mhyre TR, Boyd JT, Hamill RW, Maguire-Zeiss KA. Parkinson's disease. Subcell Biochem. 2012;65:389–455.
Cucarian JD, Berrio JP, Rodrigues C, Zancan M, Wink MR, de Oliveira A. Physical exercise and human adipose-derived mesenchymal stem cells ameliorate motor disturbances in a male rat model of Parkinson's disease. J Neurosci Res. 2019;97(9):1095–109.
Schenkman M, Moore CG, Kohrt WM, Hall DA, Delitto A, Comella CL, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with De novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. 2018;75(2):219–26.
Uc EY, Doerschug KC, Magnotta V, Dawson JD, Thomsen TR, Kline JN, et al. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology. 2014;83(5):413–25.
Gazmuri-Cancino M, Regalado-Vasquez E, Pavez-Adasme G, Hernandez-Mosqueira C. Multicomponent physical training in patients with Parkinson disease. Rev Med Chil. 2019;147(4):465–9.
Zhou W, Barkow JC, Freed CR. Running wheel exercise reduces alpha-synuclein aggregation and improves motor and cognitive function in a transgenic mouse model of Parkinson's disease. PLoS One. 2017;12(12):e0190160.
Goudy LS, Rigby BR, Silliman-French L, Becker KA. Effects of simulated horseback riding on balance, postural sway, and quality of life in older adults with Parkinson's disease. Adapt Phys Activ Q. 2019;36(4):413–30.
Mee-Inta O, Zhao ZW, Kuo YM. Physical exercise inhibits inflammation and microglial activation. Cell. 2019;8:7.
Hamzehloei L, Rezvani ME, Rajaei Z. Effects of carvacrol and physical exercise on motor and memory impairments associated with Parkinson's disease. Arq Neuropsiquiatr. 2019;77(7):493–500.
Combs-Miller SA, Dugan EL, Beachy A, Derby BB, Hosinski AL, Robbins K. Physiological complexity of gait between regular and non-exercisers with Parkinson's disease. Clin Biomech (Bristol, Avon). 2019;68:23–8.
Zhao M, Hu C, Wu Z, Chen Y, Li Z, Zhang M. Effects of coordination and manipulation therapy for patients with Parkinson disease. Int J Neurosci. 2017;127(9):762–9.
Aaseth J, Dusek P, Roos PM. Prevention of progression in Parkinson's disease. Biometals. 2018;31(5):737–47.
Minakaki G, Canneva F, Chevessier F, Bode F, Menges S, Timotius IK, et al. Treadmill exercise intervention improves gait and postural control in alpha-synuclein mouse models without inducing cerebral autophagy. Behav Brain Res. 2019;363:199–215.
Hackney AC. Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expert Rev Endocrinol Metab. 2006;1(6):783–92.
Viru A. Plasma hormones and physical exercise. Int J Sports Med. 1992;13(3):201–9.
Leal-Cerro A, Gippini A, Amaya MJ, Lage M, Mato JA, Dieguez C, et al. Mechanisms underlying the neuroendocrine response to physical exercise. J Endocrinol Invest. 2003;26(9):879–85.
Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Med. 1995;20(3):160–88.
Lin TW, Kuo YM. Exercise benefits brain function: the monoamine connection. Brain Sci. 2013;3(1):39–53.
Sutoo D, Akiyama K. Regulation of brain function by exercise. Neurobiol Dis. 2003;13(1):1–14.
MacRae PG, Spirduso WW, Cartee GD, Farrar RP, Wilcox RE. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolite levels. Neurosci Lett. 1987;79(1-2):138–44.
MacRae PG, Spirduso WW, Walters TJ, Farrar RP, Wilcox RE. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolites in presenescent older rats. Psychopharmacology (Berl). 1987;92(2):236–40.
Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HE, Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience. 2003;120(1):269–81.
Sciolino NR, Holmes PV. Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci Biobehav Rev. 2012;36(9):1965–84.
Pieribone VA, Xu ZQ, Zhang X, Grillner S, Bartfai T, Hokfelt T. Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience. 1995;64(4):861–74.
Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117(1):131–43.
Dunn AL, Reigle TG, Youngstedt SD, Armstrong RB, Dishman RK. Brain norepinephrine and metabolites after treadmill training and wheel running in rats. Med Sci Sports Exerc. 1996;28(2):204–9.
Chen HI, Lin LC, Yu L, Liu YF, Kuo YM, Huang AM, et al. Treadmill exercise enhances passive avoidance learning in rats: the role of down-regulated serotonin system in the limbic system. Neurobiol Learn Mem. 2008;89(4):489–96.
Chennaoui M, Grimaldi B, Fillion MP, Bonnin A, Drogou C, Fillion G, et al. Effects of physical training on functional activity of 5-HT1B receptors in rat central nervous system: role of 5-HT-moduline. Naunyn Schmiedebergs Arch Pharmacol. 2000;361(6):600–4.
Park HS, Park SS, Kim CJ, Shin MS, Kim TW. Exercise alleviates cognitive functions by enhancing hippocampal insulin signaling and neuroplasticity in high-fat diet-induced obesity. Nutrients. 2019;11:7.
Dodd GT, Tiganis T. Insulin action in the brain: roles in energy and glucose homeostasis. J Neuroendocrinol. 2017;29:10.
Freychet P. Insulin receptors and insulin actions in the nervous system. Diabetes Metab Res Rev. 2000;16(6):390–2.
Ketterer C, Tschritter O, Preissl H, Heni M, Haring HU, Fritsche A. Insulin sensitivity of the human brain. Diabetes Res Clin Pract. 2011;93(Suppl 1):S47–51.
McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93(4):546–53.
Kuga GK, Botezelli JD, Gaspar RC, Gomes RJ, Pauli JR, Leme JACA. Hippocampal insulin signaling and neuroprotection mediated by physical exercise in Alzheimer’s disease. Motriz: Revista de Educação Física. 2017;23:5.
Lovatel GA, Elsner VR, Bertoldi K, Vanzella C, Moyses Fdos S, Vizuete A, et al. Treadmill exercise induces age-related changes in aversive memory, neuroinflammatory and epigenetic processes in the rat hippocampus. Neurobiol Learn Mem. 2013;101:94–102.
Pauli JR, Cintra DE, Souza CT, Ropelle ER. New mechanisms by which physical exercise improves insulin resistance in the skeletal muscle. Arq Bras Endocrinol Metabol. 2009;53(4):399–408.
Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol Dis. 2013;57:47–55.
Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–90.
Diegues JC, Pauli JR, Luciano E, de Almeida Leme JA, de Moura LP, Dalia RA, et al. Spatial memory in sedentary and trained diabetic rats: molecular mechanisms. Hippocampus. 2014;24(6):703–11.
Nokia MS, Lensu S, Ahtiainen JP, Johansson PP, Koch LG, Britton SL, et al. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol. 2016;594(7):1855–73.
Van der Borght K, Kobor-Nyakas DE, Klauke K, Eggen BJ, Nyakas C, Van der Zee EA, et al. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19(10):928–36.
Paillard T. Preventive effects of regular physical exercise against cognitive decline and the risk of dementia with age advancement. Sports Med Open. 2015;1(1):20.
Gligoroska JP, Manchevska S. The effect of physical activity on cognition - physiological mechanisms. Mater Sociomed. 2012;24(3):198–202.
von Bohlen Und Halbach O, von Bohlen Und Halbach V. BDNF effects on dendritic spine morphology and hippocampal function. Cell Tissue Res. 2018;373(3):729–41.
Marosi K, Kim SW, Moehl K, Scheibye-Knudsen M, Cheng A, Cutler R, et al. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem. 2016;139(5):769–81.
Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22(3):123–31.
Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76(2):99–125.
Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neuroscientist. 2008;14(2):147–56.
Raichlen DA, Gordon AD. Relationship between exercise capacity and brain size in mammals. PLoS One. 2011;6(6):e20601.
Mattson MP. Evolutionary aspects of human exercise--born to run purposefully. Ageing Res Rev. 2012;11(3):347–52.
Arbat-Plana A, Cobianchi S, Herrando-Grabulosa M, Navarro X, Udina E. Endogenous modulation of TrkB signaling by treadmill exercise after peripheral nerve injury. Neuroscience. 2017;340:188–200.
Lin TW, Shih YH, Chen SJ, Lien CH, Chang CY, Huang TY, et al. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer's disease (APP/PS1) transgenic mice. Neurobiol Learn Mem. 2015;118:189–97.
Fahimi A, Baktir MA, Moghadam S, Mojabi FS, Sumanth K, McNerney MW, et al. Physical exercise induces structural alterations in the hippocampal astrocytes: exploring the role of BDNF-TrkB signaling. Brain Struct Funct. 2017;222(4):1797–808.
Zsuga J, Tajti G, Papp C, Juhasz B, Gesztelyi R. FNDC5/irisin, a molecular target for boosting reward-related learning and motivation. Med Hypotheses. 2016;90:23–8.
Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, et al. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013;18(5):649–59.
Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience. 2013;240:155–62.
Li DJ, Li YH, Yuan HB, Qu LF, Wang P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism. 2017;68:31–42.
Peng J, Deng X, Huang W, Yu JH, Wang JX, Wang JP, et al. Irisin protects against neuronal injury induced by oxygen-glucose deprivation in part depends on the inhibition of ROS-NLRP3 inflammatory signaling pathway. Mol Immunol. 2017;91:185–94.
Dameni S, Janzadeh A, Yousefifard M, Nasirinezhad F. The effect of intrathecal injection of irisin on pain threshold and expression rate of GABAB receptors in peripheral neuropathic pain model. J Chem Neuroanat. 2018;91:17–26.
Wang K, Li H, Wang H, Wang JH, Song F, Sun Y. Irisin exerts neuroprotective effects on cultured neurons by regulating astrocytes. Mediators Inflamm. 2018;2018:9070341.
Mastorakos G, Pavlatou M, Diamanti-Kandarakis E, Chrousos GP. Exercise and the stress system. Hormones (Athens). 2005;4(2):73–89.
Kurgan N, Noaman N, Pergande MR, Cologna SM, Coorssen JR, Klentrou P. Changes to the human serum proteome in response to high intensity interval exercise: a sequential top-down proteomic analysis. Front Physiol. 2019;10:362.
Krause M, Rodrigues-Krause JC. Extracellular heat shock proteins (eHSP70) in exercise: possible targets outside the immune system and their role for neurodegenerative disorders treatment. Med Hypotheses. 2011;76(2):286–90.
Koester-Hegmann C, Bengoetxea H, Kosenkov D, Thiersch M, Haider T, Gassmann M, et al. High-altitude cognitive impairment is prevented by enriched environment including exercise via VEGF signaling. Front Cell Neurosci. 2018;12:532.
Serra FT, Carvalho AD, Araujo BHS, Torres LB, Cardoso FDS, Henrique JS, et al. Early exercise induces long-lasting morphological changes in cortical and hippocampal neurons throughout of a sedentary period of rats. Sci Rep. 2019;9(1):13684.
Pedersen BK, Pedersen M, Krabbe KS, Bruunsgaard H, Matthews VB, Febbraio MA. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol. 2009;94(12):1153–60.
Henrique JS, Franca EF, Cardoso FDS, Serra FT, de Almeida AA, Fernandes J, et al. Cortical and hippocampal expression of inflammatory and intracellular signaling proteins in aged rats submitted to aerobic and resistance physical training. Exp Gerontol. 2018;110:284–90.
Chao F, Jiang L, Zhang Y, Zhou C, Zhang L, Tang J, et al. Stereological investigation of the effects of treadmill running exercise on the hippocampal neurons in middle-aged APP/PS1 transgenic mice. J Alzheimers Dis. 2018;63(2):689–703.
Kang EB, Kwon IS, Koo JH, Kim EJ, Kim CH, Lee J, et al. Treadmill exercise represses neuronal cell death and inflammation during Abeta-induced ER stress by regulating unfolded protein response in aged presenilin 2 mutant mice. Apoptosis. 2013;18(11):1332–47.
Zhao YN, Li JM, Chen CX, Li SX, Xue CJ. Effect on intensity of treadmill running on learning, memory and expressions of cell cycle-related proteins in rats with cerebral ischemia. Oncotarget. 2017;8(25):40633–42.
Mastrorilli V, Scopa C, Saraulli D, Costanzi M, Scardigli R, Rouault JP, et al. Physical exercise rescues defective neural stem cells and neurogenesis in the adult subventricular zone of Btg1 knockout mice. Brain Struct Funct. 2017;222(6):2855–76.
Chrysostomou V, Galic S, van Wijngaarden P, Trounce IA, Steinberg GR, Crowston JG. Exercise reverses age-related vulnerability of the retina to injury by preventing complement-mediated synapse elimination via a BDNF-dependent pathway. Aging Cell. 2016;15(6):1082–91.
Jeon YK, Ha CH. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ Health Prev Med. 2017;22(1):27.
Mattson MP. Interventions that improve body and brain bioenergetics for Parkinson's disease risk reduction and therapy. J Parkinsons Dis. 2014;4(1):1–13.
Raefsky SM, Mattson MP. Adaptive responses of neuronal mitochondria to bioenergetic challenges: roles in neuroplasticity and disease resistance. Free Radic Biol Med. 2017;102:203–16.
Maruzs T, Simon-Vecsei Z, Kiss V, Csizmadia T, Juhasz G. On the fly: recent Progress on autophagy and aging in drosophila. Front Cell Dev Biol. 2019;7:140.
Rocchi A, Yamamoto S, Ting T, Fan Y, Sadleir K, Wang Y, et al. A Becn1 mutation mediates hyperactive autophagic sequestration of amyloid oligomers and improved cognition in Alzheimer's disease. PLoS Genet. 2017;13(8):e1006962.
Acknowledgments
This work was supported by the Regional Innovation Cooperation between Sichuan and Guangxi Provinces (2020YFQ0019), the COVID-19 Research Projects of West China Hospital Sichuan University (Grant no. HX-2019-nCoV-057) and the National Natural Science Foundation of China (32070671).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Joon, S., Singla, R.K., Shen, B. (2022). Physical Activities and Prevention of Neurodegenerative Diseases. In: Shen, B. (eds) Translational Informatics . Springer, Singapore. https://doi.org/10.1007/978-981-16-9162-1_8
Download citation
DOI: https://doi.org/10.1007/978-981-16-9162-1_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-9161-4
Online ISBN: 978-981-16-9162-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)