Abstract
Secondary metabolites of plants are rich source of highly diverse chemical compounds with biological characteristics for food and pharmaceutical use. Among polyphenolic compounds, flavonoids are potential naturally occurring group present in fruit, vegetables, and beverages which are derived from plant sources. Structurally, flavonoids’ skeleton consists of C-15 carbon with framework of C6-C3-C6. Favones, favonols, favanones, favanonols, favan-3-ols, chalcones, anthocyanidins, and isofavones are important subgroups of flavonoids. The linkage position between the B ring and the C ring delineates the basic flavonoids. The biosynthesis of such bioactive compounds to meet the increasing market demands is difficult due to the high chemical structural complexity, lack of direct extraction techniques from plants, use of harsh chemicals, and slow plants’ growth. Therefore, microbial biosynthesis is superior alternative to sustainable and economic production of flavonoids due to rapid and easy growth of microbes, genetically tradable, friendly approach to the environment, and less use of harsh chemical. Escherichia coli and Saccharomyces cerevisiae are well-known and studied organisms have been used in food, beverages, and pharmaceutical industries. This chapter explains the importance of flavonoids compounds and their contribution to health, the advantages of utilizing microbes in biosynthesizing flavonoids over plants, and explaining the microbial biosynthesis pathways of several abundant flavonoids’ compounds. Finally, the important advances of microbial biosynthesis of flavonoids in food and beverages industries are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nutraceuticals are the significant class of molecules due to their potential effects in health-promoting, treating, and preventing human’s diseases. Nutraceuticals involve bioactive compounds that have distinct functional and structural features and can trigger health benefits and physiological effect in long term (Wang et al. 2016). They can be produced from diverse sources including plants (phytochemicals and vitamins), animals (polysaccharides), and microorganisms (poly amino acids), and they are superior antioxidants, anti-inflammatory, and prevent gastrointestinal, arthritis, osteoporosis, cancer, cardiovascular, and chronic diseases. Nutraceuticals market has been greatly increased due to the growing demand on promoting human health through diet rather than drugs. Global Information Inc. estimated that nutraceuticals market in 2019 will reach $241.1 billion, and by 2022, $1121 (Jain and Ramawat 2013; Yuan and Alper 2019). Flavonoids are the secondary metabolic phenolic substances, which are widely produced in all vascular and medicinal plants (Karak 2019; Jucá et al. 2020). Flavonoids are the major diverse class of phenolic compounds in plants kingdom (Nabavi et al. 2020). They contribute several health benefits to human such as, anti-inflammatory, antioxidant, anticancer, antimicrobial functions, and add color and taste to food and beverages (Karak 2019; Jucá et al. 2020). However, various issues need to be addressed in order to fulfill the expanding demand of flavonoid nutraceuticals market. For example, limited direct extraction approaches, high raw materials cost, short-term sustainability and stability (Wang et al. 2016; Yuan and Alper 2019), and low amount of produced and purified (flavonoids) nutraceuticals. Therefore, microbial production of nutraceuticals (flavonoids) can be promising strategy as an attractive alternative approach of chemical synthesis and extractions (Yuan and Alper 2019). E-coli strains and food grade S. cerevisiae are great examples of factorial cells that have been widely utilized in developing nutraceuticals (Wang et al. 2016). Therefore, biosynthetic pathways of microbes to produce nutraceuticals (flavonoids) can provide nonfood lignocellulosic feedstocks, low cost of raw materials, and safe use in pharmaceutical and food industries (Yuan and Alper 2019). Taditional host of microorganisms such as Escherichia coli, some strains of Saccharomyces cerevisiae, and Corynebacterium glutamicum have been investigated. Furthermore, they are generally recognized as safe microbes other than traditional microbes such as, the oleaginous organisms Yarrowia lipolytica, which are commercially available for their ability to produce high quantities of omega-3 polyunsaturated fatty acid. Microbial cocultures or synthetic consortia were recognized as the best way to produce a highly complex molecule of nutraceuticals (flavonoids) such as, glycosides (Yuan and Alper 2019). Plants, fungi, and microorganisms are living organisms that can produce crucial compounds and are called primary metabolites. Primary metabolites are important for various vital processes involving photosynthesis, production/expenditure of energy, metabolism process of carbohydrate, fat, and protein. Beside fundamental primary metabolites, there are broad secondary metabolites such as, polyketides, terpenoids, phenylpropanoids, and alkaloids.
Administered enzymatic reactions facilitate secondary metabolites’ biosynthesis in cellular systems. It is crucial to understand the metabolic process to control the potential of desired plant phytochemicals’ biosynthesis in food and pharmaceuticals industries. Secondary metabolites production can be regulated by investigating the compromised genes in the metabolic pathways and environmental and physiological processes (Nabavi et al. 2020). Thus, this chapter will discuss the importance of flavonoids, the general biosynthetic pathway of flavonoids in plants, the microbial biosynthesis of several flavonoid substances including naringenin, apigenin and genkwanin, flavone, isoflavones, and anthocyanin. The importance of flavonoids microbial biosynthesis in food, beverages, and pharmaceutical industries is discussed, and the recent modifications in the field of flavonoids microbial biosynthesis are highlighted.
2 Overview of Flavonoids
Phenolic compounds are the highly diverse phytochemicals existed in plant kingdom. Benzoic and cinnamic acid, coumarins, tannins, lignins, lignans, and flavonoids are simple phenols found in plant food. They offered significant health benefits because they prevent oxidative stress, which eventually reduces the risk of inflammation, cancer, diabetics, heart diseases, and cells mutagenesis. The global market of flavonoids is estimated by 2022 to reach $1121 due to their physiological effects and diverse structures (Yuan and Alper 2019). Ecological and physiological pressures can partly synthesize phenolic compounds in plants (Khoddami et al. 2013). Flavonoids are the secondary metabolic phenolic substances, which are widely produced in all vascular plants such as, fruit and vegetables, and medicinal plants (glycoside and methylated derivatives) (Karak 2019; Jucá et al. 2020). Flavonoids are the major diverse class of phenolic compounds in plants kingdom (Marranzano et al. 2018; Nabavi et al. 2020). They possess several biological activities due to the fact that they have diverse complex chemical structures. It has been reported that flavonoids offer strong antioxidant activities and protect plants and human cells against free radical activities (ROS). The functional group arrangement of flavan nucleus is responsible for strong antioxidant properties (Heim et al. 2002; Kukić et al. 2006; Zhang et al. 2014; Marranzano et al. 2018; Jucá et al. 2020).
Flavonoids protect cells and tissues from injury, pathogen infections, damage, chemical irradiation, and overall immune system, and they potentially prevent several diseases including leukemia, sepsis, asthma, sclerosis, atherosclerosis, psoriasis, allergic rhinitis, ileitis/colitis, and rheumatoid arthritis (Marranzano et al. 2018). In addition, flavonoids and anthocyanidins have demonstrated anticancer properties, which reduce or prevent the chance of cancer’s initiation, promotion, and progression due to their pharmaceutical characteristics (Prasad et al. 2010; Marranzano et al. 2018; Jucá et al. 2020). Several studies have demonstrated antimicrobial effects of several flavonoids including Apigenin, galangin, flavone and flavonol glycosides, isoflavones, flavanones, and chalcones due to the fact they are formed in plants as a result of microbial infections. Flavonoids can inactivate adhesion, protein transportation, enzymes of microorganisms, and interrupt microbial membranes (Mishra et al. 2009; Pandey et al. 2012; Mishra et al. 2013; Marranzano et al. 2018; Jucá et al. 2020). Moreover, they significantly contribute color and taste characteristics to the food and beverages are derived from plants industries, and they have potential effect in improving the nutritional properties of food. Flavonoids are the hydroxylated substances from a large group of phenolic compounds. In response to microbial infections in plants, flavonoids are synthesized Aglycones, glycosides, and methylated derivatives that are the three forms of flavonoids in plans (Karak 2019; Jucá et al. 2020).
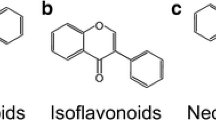
From chemical standpoint, flavonoids are structurally built from 15-carbon skeleton and A and B benzenic ring linked by 3-carbon. Favonoids subdivision into diverse groups relies on the carbon that attach (C ring to B ring), degree of C ring’ unsaturation, and oxidation. For example, the three-carbon linking chain characterized as C6-C3-C6 compounds and called chalcones (Fig. 17.1). Chalcones are precursors for most classes of flavonoids. Isoflavone can be formed by linking the carbon at position three of (C ring) to (B ring) (Fig. 17.2). However, a heterocyclic pyran or pyrone ring (C ring) can be further formed from the linking chain in many flavonoids (Fig. 17.2) (Nabavi et al. 2020). Flavones are found in steam, leaves, and roots, and they are converted by favone synthase (FNS) from favanones via the present of single double bound between the carbon atoms at second and third position. Anthocyanins are another important class of natural colorant and preservatives flavonoids, which are derived from favonols and possess the favylium ion basic structure at the lack of a ketone oxygen fourth position (Shah et al. 2019).
3 Biosynthetic Pathways of Flavonoids in Plants
The acetate (A ring) and the shikimate pathways (B ring) with the linking chain (C ring) forming the C6-C3 components are the two biosynthesis pathways of flavonoids (Nabavi et al. 2020). Figure 17.3 shows the full biosynthetic pathway of flavonoids in plants. The flavylium ion, which is the core of all flavonoids that exist in the upstream pathway transformations of glucose, generates three malonyl-CoA molecules that form (A ring) and one of 4-coumaroyl-CoA (Petrussa et al. 2013). Phenylalanine produces 4-coumaroyl-CoA through shikimate pathways to form (B ring) (Nabavi et al. 2020). Two enzymes are involved in the condensation of two steps to produce naringenin chalcone synthase (CHS) and chalcone isomerase (CHI). The dihydrokaempferol (colorless dihydroflavonol) is produced via the oxidation of naringenin, which is a colorless flavanone via flavanone 3-hydroxylase (F3H). Subsequently, flavonoid 3′-hydroxylase (F3′H) or flavonoid 3′,5′-hydroxylase (F3′5′H) produces dihydroquercetin or dihydromyricetin, respectively, by hydroxylation of dihydrokaempferol on the 3′ or 5′ position of (B ring). F3′H or F3′5′H can directly hydroxylated naringenin to deliver eriodictyol and pentahydroxy-flavanone, respectively.
Eriodictyol and pentahydroxy-flavanone are further hydroxylated to produce dihydroquercetin and dihydromyricetin. Unsaturation and oxidation of (C ring) forms the further divers’ structures of flavonoids such as, (2-phenylchromenyliums(anthocyanidins/anthocyanins); 2-phenylochromones (flavones, flavonols, flavanones, di-OH-flavonols); 2-phenylchromanes (flavans, flavan-3-ols, and flavan-3,4-diols (proanthocyanidins)); chalcones/dihydrochalcones; 2-benzylidene coumaranones (Fig. 17.3) (Nabavi et al. 2020). Anthocyanidins are formed via dihydroflavonol reductase (DFR) and leucoanthocyanidin oxidase (LDOX), which catalyze reactions, “The (DFR) converts dihydroquercetin, dihydrokaempferol, and dihydromyricetin to leucocyanidin, leucopelargonidin, and leucodelphinidin (colorless flavan-3,4-cis-diols), respectively. Subsequently, (LDOX) catalyzes the oxidation of leucocyanidin, leucopelargonidin, and leucodelphinidin to cyanidin (red-magenta anthocyanidin), pelargonidin (orange anthocyanidin), and delphinidin (purple-mauve anthocyanidin), respectively”(Petrussa et al. 2013).
Chalcone is produced by condensation of (A ring) and (B ring). The later compound goes through isomerase-catalyzed cyclization to form flavanone, which is the budding blocs for other flavonoids. Approximately 7000 of flavonoids have the same basic structure since most of them are biosynthesized in this pathway. However, the simple structure of flavonoids that includes (C ring) is flavan. Therefore, flavonoids are characterized into two categories due to their structural diversity including flavanones, flavanols, flavones, and flavonol; on the other hand, isoflavones, biflavonoids, flavonolignans, prenylflavonoids, flavonoid glycosidoesters, aurones, and chalcones. Two different forms of flavonoids are found in plants’ free aglycones form and glycoside-bound form. The later form is the major ingested form of flavonols and flavones by human (Nabavi et al. 2020). However, the production of flavonoids from plant sources is very difficult to be accomplished for large-scale production due to the long extraction process, expensive extraction approaches, and harsh chemical and environmental conditions. Thus, microbial biosynthesis of flavonoids can be a superior and sustainable alternative.
4 Importance of Microbial Biosynthetic Pathways of Flavonoids
The flavonoids as secondary metabolites are having several distinguishable characteristics among substances of primary metabolites. First, they have an ability to accumulate on organs or tissues such as, the accumulation of flavanols on skin of grabs (Mu et al. 2014). Second, they have limited distribution of specific taxonomic entities. As an example, the biosynthesis of isoflavones in Fabaceae plant species (Reynaud et al. 2005). Third, flavonoids production can be regulated when the tissue cultures are unable to form the secondary metabolites since the important genetic information possess in plant cells. Fourth, they demonstrated several biological activities either in the interaction between organism-organism or in diverse organisms (Trantas et al. 2015). Fifth, the culture suspensions have lower complexity than solid matrices of plants as well as low separation cost and carbon footprint. Finally, levels of flavonoids production from plants relay on crop’s season. On the other hand, microbial productions have very short life cycles and do not rely on season. Microbial production requires simple feedstock such as, carbon source (glucose and oxygen and/or carbon dioxide of initial input and nutrients and will be able to meet the demand of production) (Ng et al. 2019).
The metabolomics improvements can offer a great chance to discover new chemicals formed through organisms. Moreover, the biosynthetic of flavonoids has been improved. Due to the fact that the advancement of structural and biology including deputation of gene and mutation, which are the main responsible process for the enzymatic modification in microbial flavonoid’s biosynthesis. Diverse array of compounds could be produced through this enzymatic action and complex of enzymes on the metabolic pathway basic structure. As it is obvious, the enzymes involving in the flavonoid’s biosynthesis pathway are progressing in a way to result in catalysis to glycosylation, acylation, prenylation, sulfation, methylation, isomerization, or condensation to specific region (Trantas et al. 2015).
A core molecule can be synthesized by the flavonoids, which are produced by organisms such as, flavanone naringenin. Several enzymes (such as, hydroxylases, isomerases) further proceeded the core molecule downstream to achieve the end product. Utilizing enzymes that can act on different substrates is a common feature of different organisms. For instance, the oxidation of flavanonol to flavono is catalyzed via flavonol synthase utilizing substrate dihydrokaempferol or dihydroquercetin to produce kaempferol or quercetin, respectively (Trantas et al. 2015). Bacterial, yeast, or cell biotransformation of plants is an innovative emerging strategy for production of rare, natural, inexpensive, and low environmental pollution products in in vivo biosynthesis under fully controlled conditions; moreover, it helps in easier recovery of the formed products than the extractions of natural products or chemically synthesized products due to generation of less waste and side products. A lot of efforts have been made to produce array of diverse natural compounds, analogs, and useful intermediates class including isoprenoids, flavonoids, stilbenes, polysaccharides and glycoproteins, and alcohols, and these compounds possess potential pharmaceutical applications (Abdullah et al. 2008;) (Otero and Nielsen 2010; Van Summeren-Wesenhagen and Marienhagen 2013; Trantas et al. 2015) such as, antimicrobial, anticancer, cardioprotective, antioxidant, anti-inflammatory, and immune system-promoting effects (Cazarolli et al. 2008; Tungmunnithum et al. 2018).
For instance, the number of hydroxyl substituents that constituted the flavonoids structure is highly associated with the increased antioxidants’ capacity and scavenging of free radical caused by reactive oxygen species in human body (Jucá et al. 2020). For example, (Ocimum basilicum L.) and oregano (Origanum vulgare L.) are natural antioxidant and preservatives extracted from basil (Pitaro et al. 2012; Jucá et al. 2020). In addition, cardiovascular disease caused by atherosclerosis’ clinical events led to increased morbidity and mortality rate, such as myocardial infarction, embolisms, and cerebrovascular accidents which can be prevented via antioxidant and free radical scavenging properties of flavonoids. The chronic or acute inflammatory process could be prevented by the presence of substances such as, the active compound campferol3-O-methyl of ginger extract (Zingiber zerumbet), which decrease itric oxide and prostaglandin E2 production (Soares et al. 2015; Jucá et al. 2020). Flavonoids are natural chemical factors that can promote the immune system and body defense against diseases. It has been scientifically proven that propolis possesses antimicrobial properties against microorganisms due to the presence of flavonoids galangina and pinocembrin. Obviously, the oxidative stress and free radical activities are the major causes of most disease’ initiation and progression. Therefore, high antioxidant capacity of flavonoids can promote human health and prevent or treat several diseases such as, aging, cancer, cardiovascular diseases, arteriosclerosis, and neurodegenerative diseases (Tapas et al. 2008; Sandhar et al. 2011; Lima et al. 2015; Jucá et al. 2020).
5 Microbial Biosynthetic Pathways of Flavonoids
Traditional laboratories, expensive extraction methods, environmental and seasonal conditions of plants, toxic catalysts utilization, harsh chemical conditions, and presence of many compounds, such as chiral centers and labile connectivities, cannot meet the growing demand on flavonoids production from plants source in large scale (Chouhan et al. 2017; Nabavi et al. 2020). Also, individual compound of polyphenols cannot be achieved in large quantities due to the complex mixture of plants’ polyphenols (Milke et al. 2018). Therefore, utilizing microorganisms in biotechnological production of plant secondary metabolites could be an ideal solution (Nabavi et al. 2020), and they can be grown with high rate very easily (Chouhan et al. 2017). High quality, economical, ecofriendly, short time, less harsh chemical conditions, less of intermediate pathways loss, glycerol, and cellulose use as cheap carbon source are converted to high chemicals, and a diverse generation of synthesized natural products has been produced by microorganisms using biology tools and novel enzymes (Chouhan et al. 2017).
Biotechnological production of plants secondary metabolites requires several techniques involving, choosing the most effective host strains, gene manipulation objects determination, and clear understanding of biosynthesis enzymes. The primary metabolism of plants and microorganism is very similar, which means polyphenol precursor molecules including malonylCoA and aromatic amino acids can be produced by microbial metabolism (Milke et al. 2018). Saccharomyces cerevisiae and Escherichia coli are the major considered microorganisms as cellular factories of flavonoids such as, flavones, flavonols, flavanones, and isoflavones, and other species such as Streptomyces venezuelae could be used in some cases (Trantas et al. 2015; Nabavi et al. 2020).
The platform of flavonoids production mainly requires manipulation and controlling the pathways of two rings:(A) malonyl-CoA pathway and (B) 4-coumaroyl-CoA pathway (Pandey et al. 2016; Trantas et al. 2015; Nabavi et al. 2020). Flavonoids can be originated using microorganism by the universal phenylpropanoid route leading to form (B ring) starting from aromatic amino acid phenylalanine or tyrosine (Yuan and Alper 2019; Nabavi et al. 2020). The phenylalanine ammonia-lyase (PAL), Cinnamic acid 4-hydroxylase (C4H), coumarate CoA ligase (4CL), chalcone synthase (CHS), and chalcone isomerase (CHI) genes are required in the case of using Saccharomyces cerevisiae organism to complete the pathway and form naringenin.
6 Naringenin
Naringenin, a (2S)-flavanone, is the major citrus bioactive compound found in grabs and oranges and is demonstrated to have several health benefits such as, antioxidant and anti-inflammatory effects. In addition, it can act as a modulator of immune system and promoter of carbohydrates metabolisms (Yıldız et al. 2009; Leonardi et al. 2010; Qin et al. 2011; Thapa et al. 2019). In order to achieve hydroxylation step from cinnamate to an electron-donor (p-coumarate), a cytochrome P450 reductase (CPR) requires the presence of C4H as coexpression. Naringenin can be also biosynthesized in Escherichia coli via the aromatic amino acids (a precursor tyrosine) with the presence of the coumarate CoA ligase (4CL), chalcone synthase (CHS), and chalcone isomerase (CHI) genes (Nabavi et al. 2020). The formation of several flavonoid compounds (naringenin, apigenin, kaempferol, and quercetin) utilizing p-coumarate as a precursor has been extensively reported (Leonard et al. 2007, 2008; Nabavi et al. 2020). Coumarate CoA ligase (4CL), chalcone synthase (CHS), and chalcone isomerase (CHI) genes are used to achieve kaempferol and quercetin. Flavanone 3β-hydroxylases (FLS, F3H) are the further genes used in the pathway for kaempferol, while quercetin can further use (FLS, F3H and F3′,5′H lavonoid-3′,5′- hydroxylase. F3′,5′H) bonded to a cytochrome P450 reductase (CPR), which requires to obtain hydroxylation steps on (B ring) (Nabavi et al. 2020). Because of the enzymes and enzyme complex involving in flavonoids production, specific region catalyst condensation, glycosylation, acylation, prenylation, sulfation, methylation, or isomerization can be achieved, and these enzymes enable to form flavanone naringenin. In order to produce the flavonoids’ end products, the core molecules flavanone naringenin via multiple enzymes such as, hydroxylases and isomerases further regulated downstream (Trantas et al. 2015).
Another approach of flavonoids (naringenin and pinocembrin) production via yeast or bacteria is D-glucose as a simple source of carbon (Nabavi et al. 2020) as it is shown in (Fig. 17.4) since it decreases the cost of production in large scale (Jones et al. 2016). In the case of using the glucose as a supplementation for the microbial cells, shikimate pathway originates phenylalanine and tyrosine (aromatic amino acid precursors). The shikimate route in microorganisms could be imposed accordingly, and the enzyme versions’ resistant will be introduced to feedback inhibition evacuated via phenylalanine or tyrosine when the 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP) synthase level is reached. For phenylalanine, the chorismate is mutase/prephenate dehydratase (CM/PDT), while tyrosine is the chorismate mutase/prephenate dehydrogenase (CM/PDH). Modular metabolic strategy could optimize the production of (2S)-pinocembrin via D-glucose in E. coli through four molecules comprising 1. First, “the aroF gene encoding a 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP) synthase and the pheA gene encoding a resistant version of chorismate mutase/prephenate dehydratase (CM/PDT). Second, phenylalanine ammonia-lyase (PAL) and 4-coumarate CoA ligase (4CL). Third, chalcone synthase (CHS) and chalcone isomerase (CHI). Forth, matC encoding a malonate carrier protein and matB encoding a malonyl-CoA synthase to increase the intracellular pool of malonyl-CoA” (Nabavi et al. 2020).
In another study, a combination of three molecules, which are module 1: tyrosine ammonia-lyase (TAL) and 4-coumarate CoA ligase (4CL), module 2: chalcone synthase (CHS) and chalcone isomerase (CHI), module 3: matC and matB, and Two genes, aroG encoding (DAHP) synthase and tyrA encoding a resistant version of chorismate mutase/prephenate dehydratase (CM/PDH), were used to optimize E. coli de novo synthesis of (2S)-naringenin from D-glucose (Wu et al. 2014). Controlling the malonylCoA intracellular pool is a very important feature in the formation of (A ring) in flavonoids biosynthesis because the malonylCoA is the intersection compound that links pathway of flavonoids and biosynthesis of fatty acids. Upstream and downstream pathways could be controlled via the intracellular pool of malonyl-CoA regulation (Nabavi et al. 2020). Overexpression of the acetate assimilation pathway’ key enzyme, which is the acetyl-CoA carboxylase complex (ACC), can increase malonyl-CoA concentration. In flavonoids’ production from E. coli, the ACC gene and the biotin ligase (BirA) gene were used (Leonard et al. 2007; Fowler and Koffas 2009; Nabavi et al. 2020).
7 Apigenin and Genkwanin
Apigenin is a typical flavone that is biosynthesized from naringenin using flavone synthase (FNS), which acts as a substrate for (S)-naringenin. O-methylation is the derivative of Apigenin, from which genkwanin can be generated. Apigenin and genkwanin have several biological activities such as anti-inflammatory, antioxidant, anticancer etc. (Lee et al. 2015). The biosynthesis of genkwanin from apigenin was accomplished by glucose source using E. coli with presence of four genes (Os4CL, PeCHS, MtCHI, and FNS) as it is shown. Four enzymes, 4-coumarate CoA ligase. (4CL), chalcone synthase (CHS), chalcone isomerase (CHI), and flavone synthase (FNS), and four genes (Os4CL, PeCHS, MtCHI, and FNS) are introduced to synthesize apigenin via p-coumaric acid pathway. Subsequently, naringenin chalcone converts to naringenin. Three genes (Os4CL, PeCHS, and FNS) are introduced to E. coli as well as the CHI. The naringenin chalcone transformed to (S)-naringenin, and the CHI could be utilized as FNS’ substrate. 4CL and CHS coordinated expression is an important step for apigenin end product. For genkwain’s biosynthesis from E. coli, two additional genes tyrosine ammonium lyase.
(TAL) and apigenin 7-Omethyltransferase (POMT7)) are required. Tyrosine ammonia-lyase (TAL) is critical to produce p-coumaric acid from tyrosine, while (POMT7) is required to catalyze the reaction of apigenin conversion to genkwanin (Lee et al. 2015).
8 Flavones
Flavones are the first generated compounds of all other flavonoids, which represent pharmaceutical characteristics. Also, it is demonstrated as the largest group of flavonoids compounds and provides several biological, physiological, and nutritional functions (Jiang et al. 2016). The structure of flavones is shown in (Fig. 17.2). The flavone backbone biosynthesis in most species generates from phenylpropanoid pathway followed by the biosynthetic branch of flavonoid (Fig. 17.4) The phenylalanine ammonia-lyase (PAL) deaminates the phenylalanine to cinnamic acid. Cinnamic acid 4-hydroxylase (C4H) can catalyze hydroxyl group introduction to the phenyl ring, which forms p-coumaric acid from cinnamic acid. P-coumaroyl-coenzyme A (CoA) forms through activation of the carboxyl group of p-coumaric acid. A thioester bond with (CoA) to form p-coumaroyl-CoA ligase and (4CL) catalyzes the process (Jiang et al. 2016). The chalcone synthase (CHS) enzyme generates chalcone through condensation reaction of three molecules of Malonyl-CoA, which is the first step for core flavonoids molecule production (Fig. 17.4). The (CHS) is acting as a starter molecule. Acysteine residue attaches to p-coumaroyl-CoA at chalcone synthase (CHS) active site. Subsequently, a series of decarboxylative condensation reactions produces tetraketide intermediate. The intramolecular cyclization of the tetraketide intermediate forms the chalcone (4,2′,4′,6′-tetrahydroxychalcone). (Ververidis et al. 2007; Jiang et al. 2016; Trantas et al. 2015). Then, chalcone isomerase (CHI) is applied for consequent isomeration of chalcone into flavones.
Flavones group is produced via flavone synthase I (FNS I) action. In further steps of flavonoids biosynthetic metabolism of flavanones downstream, a flavanone 3β–hydroxylase (F3H) enzyme creates Dihydroflavonols. This enzyme (F3H) assists in transformation of flavanones to flavonols via a flavonol synthase (FLS) enzymatic action (Ververidis et al. 2007; Jiang et al. 2016; Trantas et al. 2015).
9 Isoflavones
Isoflavones are known for their ability to attach oestrogen receptors (ER) based on their structures. They share common structural features with the mammalian oestrogen oestradiol-17β such as, similar distance separation of hydroxyl groups and they exhibit oestrogenicity (Cassidy et al. 2000). The structure of isoflavones is shown in (Fig. 17.2). To date, more than 1600 derivatives of isoflavones have been investigated. For heterologous isoflavonoids production, the most common hosts are E. coli and S. cerevisiae. The main biosynthetic region of isoflavones in the flavonoids is (B ring). After the formation of chalcone as it is shown in (Fig. 17.4), a combination of an isoflavone synthase (IFS)-encoded gene expressed in soybean with cytochrome P450 oxidoreductase (CPR) enzymatic action isoflavones group is generated (Ververidis et al. 2007; Trantas et al. 2015) via introduction to yest S. cerevisiae. Then, 7,40 - dihydroxyflavone (liquiritigenin) and naringenin are converted through a probable 2-hydroxyiso-favanone intermediate to daidzein and genistein, respectively. In S. cerevisiae, Glycine max co-expressed isoflavone synthase (IFS), cytochrome P450 oxidoreductase (CPR), chalcone isomerase, and (CHI), which led to transfer chalcone substrates to 2-hydroxyiso-favanones and isoflavones group, respectively, due to CHI and IFS functions. (Song et al. 2014). Also, isoflavones could be biosynthesized in E. coli with improved turnover rate via the fusion of isoflavone synthase (IFS) and cytochrome P450 oxidoreductase (CPR) enzymes and E. coli (P450) and (IFS) in bacterial P450BM-3 in order to mimic its intrinsic architecture, and daidzein and genistein were produced with high yields (Leonard and Koffas 2007; Song et al. 2014). Cocultures can significantly improve the yield of daidzein and genistein in either yeast or microorganisms. For example, E. coli and S. cerevisiae co-incubation is a great strategy since S. cerevisiae possessed (IFS), while E. coli possessed phenylalanine ammonia lyase (PAL), ScCCL, chalcone synthase (CHS), chalcone isomerase (CHI), and acetyl-CoA carboxylase (ACC) (Song et al. 2014; Teplova et al. 2018).
10 Anthocyanins
It is one of useful and desirable flavonoids compounds groups in food industry since it provides color pigments of red, orange, purple, and blue, and it is a common natural colorant in food and beverages industries. Moreover, it could contribute several health benefits to human such as, prevention and protective effect against cardiovascular diseases, improvement of vision, scavenging of free radical activities, and protection from cancer, diabetics, and inflammation (Pojer et al. 2013; Cortez et al. 2017; Eichenberger et al. 2018; Nabavi et al. 2020). The structure of anthocyanin is shown in (Fig. 17.2). Depending on the initial precursor, dihydroflavonol 4-reductase (DFR) and anthocyanin synthase (ANS) combined action or ANS sole of action require to build flavylium cation (Fig. 17.4). Leucoanthocyanidin reductase (LAR) and Flavanone 3β-hydroxylase (FHT) are catalyzing enzymes that involve the biosynthesis to produce anthocyanin. Decorating enzymes are important to control the (B ring) of anthocyanin such as, anthocyanin-O-glucosyltransferases (GT) and anthocyanin-O-methyltransferases (AOMT). Respectively, uridine diphosphate.
(UDP)-glucose (UDPG) and S-adenosyl-L-methionine (SAM) are used as donors of glucose or methyl (Nabavi et al. 2020). For instance, in E. coli strain, Cn 3-G were produced from naringenin by the action of three genes, which are ANS, DFR, and 3-GT (Yan et al. 2005; Eichenberger et al. 2018; Nabavi et al. 2020); further modifications of anthocyanidins to form anthocyanin through glycosylation, acylation, and methylation. Then, the anthocyanin is transported to vacuoles. The color of anthocyanin is highly dependent on the flavonoid’s structures and the flavones and flavanols present. For anthocyanin heterologous production, it is difficult to express the required multiple plant CYP enzymes for full biosynthesis pathway in bacterial hosts such as, E. coli; therefore, yeast such as, Saccharomyces cerevisiae is considered the best (Eichenberger et al. 2018).
11 Microbial Biosynthesis in Food and Beverages Industries
There are growing demand of protective nutraceuticals such as flavonoids in a form of food or drugs due to the fact that flavonoids potentially can reduce the risks of several chronic and aging diseases such as, Alzheimer, cancer, and inflammation. Flavonoids’ global market has been estimated to reach US $200 yearly by 2022 (Xu et al. 2020), due to the fact that they are considered potential natural colorant, preservatives, and antimicrobial candidates in food, beverages, and pharmaceutical industries (Marranzano et al. 2018).
Rei Ng et al. (2019) analyzed the effect of secreted phenolic metabolites from a naringenin, which resulted from Saccharomyces cerevisiae strain (a GRAS organism), and their potentials as preservatives with high antimicrobial and antioxidant characteristics. Phenolic metabolites biosynthesized using Saccharomyces cerevisiae strain were compared to naturally produced flavonoid naringenin and its prenylated derivatives. The results showed that the biosynthesized flavonoid naringenin from yeast exhibited superior antimicrobial against major pathogens, which cause food borne illness such as, (Campylobacter sp., Salmonella sp., S. aureus, E. coli, Listeria monocytogenes, C. botulinum). Phenylacetaldehyde, homogentisic acid, and phloretic acid are the key identified metabolites that increase the bioactivities. In addition, it showed superior antioxidant effect via DMPD. + scavenger the free radical against the naturally extracted flavonoid naringenin. Therefore, the biosynthesized phenolic metabolites naringenin from yeast could be innovative natural preservative since it offers economic, maintainable, and safe to environment over large-scale extraction process from plants.
Also, complex of flavonoids, which are produced by complex cluster of genes and precursors, can be biosynthesized via microbial cocultures (Ganesan et al. 2017; Akdemir et al. 2019). Naringenin has been rapidly produced through the engineered coculture of two strains of Escherichia coli. Coculture biosynthetic pathway results in decreasing the metabolic stress and improved catalytic reaction, and associated coculture strains biosynthetic steps were simply regulated (Ganesan et al. 2017). Escherichia coli cocultures were able to increase flavan-3-ol titer up to 970-fold over monoculture strain when the carbon source, temperature, point of indication, compatibility of strains, and ratio of inculcation were optimized (Jones et al. 2016).
For example, hydroxyphenyl-pyranoanthocyanins are important natural colorants that have been used in low-to-medium acidic beverages and food industries, and they extensively present in fruit and red wine. They are more stable under variation of pH than due to the fact that they consist of (D ring) or pyran their anthocyanin precursors. Extraction of pyranoanthocyanins is a very difficult task because they present in very low concentrations. Cocultures of two strains of Escherichia coli were accomplished, and 4-vinylphenol and 4-vinylcatechol producer modules were engineered.
Then, each strain was cocultured with yanidin-3-O-glucoside producer recombinant cells to achieve pyranocyanidin-3-O-glucoside-phenol (cyanidin-3-O-glucoside with vinylphenol adduct) and pyranocyanidin-3-O-glucoside-catechol (cyanidin-3-O-glucoside with vinylcatechol adduct). The results show that pyranoanthocyanins was produced with higher yield, titer, and stability in compression to the one produced from plants (Akdemir et al. 2019).
3-Deoxyanthocyanidins is another rare type of natural colorant derived from anthocyanin with interesting chemical and biochemical characteristics, including antioxidant, anticancer, and antimicrobial, and stable under environmental stress and conditions. 3-Deoxyanthocyanidins pigmies have potential applications in food and beverages industries due to the fact that C-3 position lacks hydroxyl group (Xiong et al. 2019). Coculture is a potential strategy for increasing yields of complex chemical compounds such as, flavonoids allowing division and optimization to achieve the complete pathways, controlling, and balancing the metabolic efflux ratio between the strains. The functional overexpression of genes can be optimized in all pathways and enhanced the utilization of substrates to achieve maximum yields of flavonoids. Therefore, simple mixing and matching of different microbial strains facilitate various chemical compounds production (Wang et al. 2020).
In Jones et al. (2016) study, phenylpropanoic acid precursors were used in the production of flavan-3-ols via six enzymatic phases in E-Coli, which were 4-coumaroylCoA ligase (4CL), chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3β-hydroxylase (F3H), dihydroflavonol 4-reductase (DFR), and leucoanthocyanidin reductase (LAR) as shown in (Fig. 17.5). The upstream and downstream modules were partitioned to complete the pathway with three genes. The separation of the pathways was according to malonylCoA (upstream) and NADPH (downstream) because they are important cofactors in the pathway. Coculture genetic optimization of each strain enhances the important cofactor efflux, substrates, and reduce the efflux of unwanted side of interest. 4CL, CHS, and CHI were used in upstream strain, and F3H, DFR, and LAR were used in downstream strain genetic optimization. Source of carbon, temperature, compatibility of strain, and point of indication are important factors in genetic optimization for efficient flavonoids production in vivo (Jones et al. 2016).
Lv et al. (2019)’ study utilized the chassis (Y. lipolytica) to biosynthesized flavonoids by constructing two models: synthesis and hydroxylation modules. Synthesis module consists of malonyl-CoA and chalcone precursors to produce the shikimic acid. The hydroacylation module consists of cytochrome c P450 (CYP) flavonoid 3′-hydroxylase 129 (F3’H) and cytochrome c P450 reductase (CPR) since they are important in phenyl ring oxidation and producing hydroxylated flavonoids. P-coumaric acid-CoA ligase (4CL), chalcone synthase (CHS), and chalcone isomerase (CHI) were used in synthesis pathway. The results showed that for heterologous Y. lipolytica, it is an ideal platform to produce flavonoids with high value.
Thus, microbial biosynthesis is a great alternative for producing sustainable, cost-effective, enhanced functionality, and nutritional values of bioactive compounds (flavonoids).
12 Conclusion and Recommendation
Plants’ Secondary metabolites are rich source of highly diverse chemical compounds with biological characteristics for food, beverages, and pharmaceutical use (Song et al. 2014). Among polyphenolic compounds, flavonoids are potential naturally occurring group present in fruit, vegetables, and beverages that are derived from plant sources. Due to the fact that flavonoids have essential role in heath promoting, treating, and preventing several diseases, they are involved in diverse food, beverages, and pharmaceutical applications in industries as nutraceuticals. In addition, they produce series of substances including flavones, flavonols, flavanones, isoflavones, flavanols, and anthocyanins. These compounds exhibit great biological activities such as, antioxidants and free radical scavenging, anticancer, anti-inflammatory, antidiabetic, antiviral, antimicrobial, and antiallergic activities (Karak 2019). Due to high antioxidant capacity of flavonoids, they have received great attention as promising pharmaceutical compounds. However, the extraction of these chemically complex compounds is difficult for large-scale production and economically impractical. Therefore, microbial biosynthesis of flavonoids by using microbial hosts such as, Escherichia coli, Saccharomyces cerevisiae, and Streptomyces species is a great alternative and offers various advantages over plants production. The microbes are rapidly grown in friendly way to the environment. Also, the genetic manipulation of microbes is easy and cost effective, and the microbes’ developed metabolic engineering tools are well-established (Song et al. 2014). Recent advance of microbial biosynthesis of flavonoids reported that several phenylpropanoid enzymes such as, PAL, C4H, 4CL, CHS, and CHI heterologous expression are required for microbial biosynthesis of flavonoids in E. Coli. However, PAL and C4H (the cytochrome P450 enzyme), along with a cytochrome P450 reductase (CPR), were reported to be sufficient in feeding carbon flux into the phenylpropanoid pathway in the case of Saccharomyces cerevisiae (Shah et al. 2019). Producing flavonoids via microbes is a great strategy in meeting the growing market demands of such bioactive compounds for food, beverages, and pharmaceutical industries, and this will offer sustainability and cost-efficiency for global scale production. Cocultures such as, E. coli-E. coli and E. coli-other species can be also utilized to face the challenges and improve the microbial biosynthesis of flavonoids.
References
Abdullah MA, Rahmah A, Sinskey AJ, Rha CK (2008) Cell engineering and molecular pharming for biopharmaceuticals. Open Med Chem J 2:49–61. https://doi.org/10.2174/1874104500802010049
Akdemir H, Silva A, Zha J, Zagorevski DV, Koffas MA (2019) Production of pyranoanthocyanins using Escherichia coli co-cultures. Metab Eng Commun 55:290–298. https://doi.org/10.1016/j.ymben.2019.05.008
Cassidy A, Hanley B, Lamuela-Raventos RM (2000) Isoflavones, lignans and stilbenes–origins, metabolism and potential importance to human health. J Sci Food Agric 80(7):1044–1062. https://doi.org/10.1002/(SICI)1097-0010(20000515)80:7<1044::AID-JSFA586>3.0.CO;2-N
Cazarolli LH, Zanatta L, Alberton EH, Bonorino Figueiredo MSR, Folador P, Damazio RG, Barreto Silva FRM (2008) Flavonoids: prospective drug candidates. Mini Rev Med Chem 8(13):1429–1440. https://doi.org/10.2174/138955708786369564
Chouhan S, Sharma K, Zha J, Guleria S, Koffas MA (2017) Recent advances in the recombinant biosynthesis of polyphenols. Front Microbiol 8:2259. https://doi.org/10.3389/fmicb.2017.02259
Cortez R, Luna-Vital DA, Margulis D, Gonzalez de Mejia E (2017) Natural pigments: stabilization methods of anthocyanins for food applications. Compr Rev Food Sci Food Saf 16(1):180–198. https://doi.org/10.1111/1541-4337.12244
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. Nutr Biochem 13(10):572–584. https://doi.org/10.1016/S0955-2863(02)00208-5
Eichenberger M, Hansson A, Fischer D, Dürr L, Naesby M (2018) De novo biosynthesis of anthocyanins in Saccharomyces cerevisiae. FEMS Yeast Res 18(4):foy046. https://doi.org/10.1093/femsyr/foy046
Fowler ZL, Koffas MA (2009) Biosynthesis and biotechnological production of flavanones: current state and perspectives. Appl Microbiol Biotechnol 83(5):799–808
Ganesan V, Li Z, Wang X, Zhang H (2017) Heterologous biosynthesis of natural product naringenin by co-culture engineering. Synth Syst Biol 2(3):236–242. https://doi.org/10.1016/j.synbio.2017.08.003
Jain N, Ramawat KG (2013) Nutraceuticals and antioxidants in prevention of diseases. In: Natural products. Springer, Berlin, pp 2559–2580. https://doi.org/10.1007/978-3-642-22144-6_70
Jiang N, Doseff AI, Grotewold E (2016) Flavones: from biosynthesis to health benefits. Plan Theory 5(2):27. https://doi.org/10.3390/plants5020027
Jones JA, Vernacchio VR, Sinkoe AL, Collins SM, Ibrahim MH, Lachance DM et al (2016) Experimental and computational optimization of an Escherichia coli co-culture for the efficient production of flavonoids. Metab Eng 35:55–63. https://doi.org/10.1016/j.ymben.2016.01.006
Jucá MM, Cysne Filho FMS, de Almeida JC, Mesquita DDS, Barriga JRDM, Dias KCF et al (2020) Flavonoids: biological activities and therapeutic potential. Nat Prod Res 34(5):692–705. https://doi.org/10.1080/14786419.2018.1493588
Karak P (2019) Biological activities of flavonoids: an overview. Int J Pharm Sci 10(4):1567–1574. https://doi.org/10.13040/IJPSR.0975-8232.10(4).1567-74
Khoddami A, Wilkes MA, Roberts TH (2013) Techniques for analysis of plant phenolic compounds. Molecules 18(2):2328–2375. https://doi.org/10.3390/molecules18022328
Kukić J, Petrović S, Niketić M (2006) Antioxidant activity of four endemic Stachys taxa. Biol Pharm Bull 29(4):725–729. https://doi.org/10.1248/bpb.29.725
Lee H, Kim BG, Kim M, Ahn JH (2015) Biosynthesis of two flavones, apigenin and genkwanin in Escherichia coli. J Microbiol Biotechnol 25(9):1442–1448. https://doi.org/10.4014/jmb.1503.03011
Leonard E, Lim KH, Saw PN, Koffas MA (2007) Engineering central metabolic pathways for high-level flavonoid production in Escherichia coli. Appl Environ Microbiol 73(12):3877–3886. https://doi.org/10.1128/AEM.00200-07
Leonard E, Koffas MA (2007) Engineering of artificial plant cytochrome P450 enzymes for synthesis of isoflavones by Escherichia coli. Appl Environ Microbiol 73(22):7246–7251. https://doi.org/10.1128/AEM.01411-07
Leonardi T, Vanamala J, Taddeo SS, Davidson LA, Murphy ME, Patil BS, Turner ND (2010) Apigenin and naringenin suppress colon carcinogenesis through the aberrant crypt stage in azoxymethane treated rats. Exp Biol Med 235:710–717. https://doi.org/10.1258/ebm.2010.009359
Leonard E, Yan Y, Fowler ZL, Li Z, Lim CG, Lim KH, Koffas MA (2008) Strain improvement of recombinant Escherichia coli for efficient production of plant flavonoids. Mol Pharm 5(2):257–265. https://doi.org/10.1021/mp7001472
Lima EBC, Sousa CNS, Meneses LN, Ximenes NC, Júnior S, Vasconcelos GS et al (2015) Cocos nucifera (L.)(Arecaceae): a phytochemical and pharmacological review. Braz J Med Biol Res 48(11):953–964. https://doi.org/10.1590/1414-431x20154773
Lv Y, Marsafari M, Koffas M, Zhou J, Xu P (2019) Optimizing oleaginous yeast cell factories for flavonoids and hydroxylated flavonoids biosynthesis. ACS Synth Biol 8(11):2514–2523. https://doi.org/10.1021/acssynbio.9b00193
Marranzano M, Rosa RL, Malaguarnera M, Palmeri R, Tessitori M, Barbera AC (2018) Polyphenols: plant sources and food industry applications. Curr Pharm Des 24(35):4125–4130. https://doi.org/10.2174/1381612824666181106091303
Milke L, Aschenbrenner J, Marienhagen J, Kallscheuer N (2018) Production of plant-derived polyphenols in microorganisms: current state and perspectives. Appl Microbiol Biotechnol 102(4):1575–1585. https://doi.org/10.1007/s00253-018-8747-5
Mishra AK, Mishra A, Kehri HK, Sharma B, Pandey AK (2009) Inhibitory activity of Indian spice plant Cinnamomum zeylanicum extracts against Alternaria solani and Curvularia lunata, the pathogenic dematiaceous moulds. Ann Clin Microbiol Antimicrob 8(1):1–7. https://doi.org/10.1186/1476-0711-8-9
Mishra A, Kumar S, Pandey AK (2013) Scientific validation of the medicinal efficacy of Tinospora cordifolia. Sci World J. https://doi.org/10.1155/2013/292934
Mu L, He J-J, Pan Q-H, He F, Duan C-Q (2014) Tissue-specific accumulation of flavonoids in grape berries is related to transcriptional expression of VvF3 H and VvF3 5 H. S Afr J Enol Vitic 35(1):68–81
Nabavi SM, Šamec D, Tomczyk M, Milella L, Russo D, Habtemariam S et al (2020) Flavonoid biosynthetic pathways in plants: versatile targets for metabolic engineering. Biotechnol Adv 38:107316. https://doi.org/10.1016/j.biotechadv.2018.11.005
Ng KR, Lyu X, Mark R, Chen WN (2019) Antimicrobial and antioxidant activities of phenolic metabolites from flavonoid-producing yeast: potential as natural food preservatives. Food Chem 270:123–129. https://doi.org/10.1016/j.foodchem.2018.07.077
Otero JM, Nielsen J (2010) Industrial systems biology. Biotechnol Bioeng 105:439–460. https://doi.org/10.1002/bit.22592
Pandey AK, Mishra AK, Mishra A (2012) Antifungal and antioxidative potential of oil and extracts derived from leaves of Indian spice plant Cinnamomum tamala. Cell Mol Biol 58(1):142–147. https://doi.org/10.1170/T933
Pandey RP, Parajuli P, Koffas MA, Sohng JK (2016) Microbial production of natural and non-natural flavonoids: pathway engineering, directed evolution and systems/synthetic biology. Biotechnol Adv 34(5):634–662. https://doi.org/10.1016/j.biotechadv.2016.02.012
Petrussa E, Braidot E, Zancani M, Peresson C, Bertolini A, Patui S, Vianello A (2013) Plant flavonoids—biosynthesis, transport and involvement in stress responses. Int J Mol Sci 14(7):14950–14973. https://doi.org/10.3390/ijms140714950
Pitaro SP, Fiorani LV, Jorge N (2012) Potencial antioxidante dos extratos de manjericão (Ocimum basilicum Lamiaceae) e orégano (Origanum vulgare Lamiaceae) em óleo de soja. Rev Bras Plantas Med 14(4):686–691. https://doi.org/10.1590/S1516-05722012000400017
Pojer E, Mattivi F, Johnson D, Stockley CS (2013) The case for anthocyanin consumption to promote human health: a review. Compr Rev Food Sci Food Saf 12(5):483–508. https://doi.org/10.1111/1541-4337.12024
Prasad KN, Xie H, Hao J, Yang B, Qiu S, Wei X et al (2010) Antioxidant and anticancer activities of 8-hydroxypsoralen isolated from wampee [Clausena lansium (Lour.) Skeels] peel. Food Chem 118(1):62–66. https://doi.org/10.1016/j.foodchem.2009.04.073
Qin L, Jin L, Lu L, Lu X, Zhang C, Zhang F, Liang W (2011) Naringenin reduces lung metastasis in a breast cancer resection model. Protein Cell 2:507–516. https://doi.org/10.1007/s13238-011-1056-8
Reynaud J, Guilet D, Terreux R, Lussignol M, Walchshofer N (2005) Isoflavonoids in non-leguminous families: an update. Nat Prod Rep 22(4):504–515. https://doi.org/10.1039/B416248J
Sandhar HK, Kumar B, Prasher S, Tiwari P, Salhan M, Sharma P (2011) A review of phytochemistry and pharmacology of flavonoids. Int Pharm Sci 1(1):25–41
Shah FLA, Ramzi AB, Baharum SN, Noor NM, Goh HH, Leow TC et al (2019) Recent advancement of engineering microbial hosts for the biotechnological production of flavonoids. Mol Biol Rep 46(6):6647–6659. https://doi.org/10.1007/s11033-019-05066-1
Soares DJ, de Moura Neto LG, Damaceno MN, de Souza PA, Braga RC, Brasil IM (2015) Atividade antiinflamatória de frutas. Rev Saude Publica 15(39):33–45. https://doi.org/10.15600/2238-1244/sr.v15n39p33-45
Song MC, Kim EJ, Kim E, Rathwell K, Nam SJ, Yoon YJ (2014) Microbial biosynthesis of medicinally important plant secondary metabolites. Nat Prod Rep 31(11):1497–1509. https://doi.org/10.1039/c4np00057a
Tapas AR, Sakarkar DM, Kakde RB (2008) Flavonoids as nutraceuticals: a review. Trop J Pharm Res 7(3):1089–1099. https://doi.org/10.4314/tjpr.v7i3.14693
Teplova VV, Isakova EP, Klein OI, Dergachova DI, Gessler NN, Deryabina YI (2018) Natural polyphenols: biological activity, pharmacological potential, means of metabolic engineering. Appl Biochem Microbiol 54(3):221–237. https://doi.org/10.1134/S0003683818030146
Thapa SB, Pandey RP, Bashyal P, Yamaguchi T, Sohng JK (2019) Cascade biocatalysis systems for bioactive naringenin glucosides and quercetin rhamnoside production from sucrose. Appl Microbiol Biotechnol 103(19):7953–7969. https://doi.org/10.1007/s00253-019-10060-5
Trantas EA, Koffas MA, Xu P, Ververidis F (2015) When plants produce not enough or at all: metabolic engineering of flavonoids in microbial hosts. Front Plant Sci 6:7. https://doi.org/10.3389/fpls.2015.00007
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines 5(3):93. https://doi.org/10.3390/medicines5030093
Van Summeren-Wesenhagen PV, Marienhagen J (2013) Putting bugs to the blush: metabolic engineering for phenylpropanoid-derived products in microorganisms. Bioengineered 4:355–362. https://doi.org/10.4161/bioe.23885
Ververidis F, Trantas E, Douglas C, Vollmer G, Kretzschmar G, Panopoulos N (2007) Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: chemical diversity, impacts on plant biology and human health. Biotechnol J 2(10):1214–1234. https://doi.org/10.1002/biot.200700084
Wang J, Guleria S, Koffas MA, Yan Y (2016) Microbial production of value-added nutraceuticals. Curr Opin Biotechnol 37:97–104. https://doi.org/10.1016/j.copbio.2015.11.003
Wang R, Zhao S, Wang Z, Koffas MA (2020) Recent advances in modular co-culture engineering for synthesis of natural products. Curr Opin Biotechnol 62:65–71. https://doi.org/10.1016/j.copbio.2019.09.004
Wu J, Du G, Zhou J, Chen J (2014) Systems metabolic engineering of microorganisms to achieve large-scale production of flavonoid scaffolds. J Biotechnol 188:72–80. https://doi.org/10.1016/j.jbiotec.2014.08.016
Xiong Y, Zhang P, Warner RD, Fang Z (2019) 3-Deoxyanthocyanidin colorant: nature, health, synthesis, and food applications. Compr Rev Food Sci Food Saf 18(5):1533–1549. https://doi.org/10.1111/1541-4337.12476
Xu P, Marsafari M, Zha J, Koffas M (2020) Microbial coculture for flavonoid synthesis. Trends Biotechnol 38(7):686–688. https://doi.org/10.1016/j.tibtech.2020.01.008
Yan Y, Kohli A, Koffas MA (2005) Biosynthesis of natural flavanones in Saccharomyces cerevisiae. Appl Environ Microbiol 71(9):5610–5613
Yıldız SZ, Küçükislamoğlu M, Tuna M (2009) Synthesis and characterization of novel flavonoid-substituted phthalocyanines using (±) naringenin. J Org Chem 694:4152–4161. https://doi.org/10.1016/j.jorganchem.2009.09.012
Yuan SF, Alper HS (2019) Metabolic engineering of microbial cell factories for production of nutraceuticals. Microb Cell Fact 18(1):46. https://doi.org/10.1186/s12934-019-1096-y
Zhang J, Wu Y, Zhao X, Luo F, Li X, Zhu H et al (2014) Chemopreventive effect of flavonoids from Ougan (Citrus reticulata cv. Suavissima) fruit against cancer cell proliferation and migration. J Funct Foods 10:511–519. https://doi.org/10.1016/j.jff.2014.08.006
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Alessa, F.M. (2022). Transfer of Plant Biosynthetic Pathways to Microbes for the Production of Nutraceuticals. In: Belwal, T., Georgiev, M.I., Al-Khayri, J.M. (eds) Nutraceuticals Production from Plant Cell Factory. Springer, Singapore. https://doi.org/10.1007/978-981-16-8858-4_17
Download citation
DOI: https://doi.org/10.1007/978-981-16-8858-4_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8857-7
Online ISBN: 978-981-16-8858-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)