Abstract
The increasing occurrence of contaminants such as pharmaceutically active compounds, endocrine-disrupting compounds, nanomaterials, surfactants, personal care products is an emerging concern in the water sector. Some of these emerging contaminants are toxic to all life forms, are bio-resistant, and can sustain after primary and secondary wastewater treatment. Conventional water treatment processes are also ineffective in removing these compounds. Advanced oxidation is a potential technique and can degrade and mineralize complex organic molecules. Advanced oxidation processes (AOPs) rely on the in-situ generation of reactive chemical species (RCS) such as hydroxyl radicals for degradation. Most of the RCS with higher oxidizing potential is short-lived, and hence the effective production of these compounds is crucial for this technology’s success. The plasma-mediated AOP is an emerging technology superior to other conventional AOPs due to its ability to generate RCS at a controlled rate without using chemical agents. Moreover, the nonthermal plasma can also produce the RCS at controlled temperature and ambient pressure and, therefore, very suitable for commercial-scale processing. The plasma produces a cocktail of reactive species whose collective effect enhances the efficacy of the process. However, before translating this technology to the commercial scale, it is essential to make it affordable and energy-efficient. In this regard, significant studies are being carried focusing on reactor design and its optimization. The chapter reviews the recent developments in plasma-based reactors and their application in the degradation of emerging contaminants. The chapter also highlights the current challenges and prospects of plasma-based technology in treating emerging contaminants and various operating parameters influencing the process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The decline in quality and quantity of fresh water is one of the most challenging problems faced by humanity in the twenty-first century. If adequate measures are not taken, these problems would aggravate in the coming decades and threaten the sustenance of human life and the ecosystem. According to a recent WHO report, at least 2 billion people lack access to clean water globally (WHO 2019). The United Nations World Water Development Report (UN WWDR) projected that by 2050, 6 billion people would face freshwater scarcity globally (Boretti and Rosa 2019). Further, the increasing use of chemicals in industrial processing, agriculture, and other daily needs has polluted many freshwater resources and are unavailable for direct use. The growing occurrence of new contaminants in the water is an emerging concern in the water sector. Some of these contaminants of emerging concern (ECs) are toxic to all life forms, are bio-resistant, flame retardants, and can sustain after primary and secondary wastewater treatment. In many cases, traces of these pollutants can pose serious health concerns (Rout et al. 2021). The widespread occurrence of ECs in water and their chemical toxicity demands the development of effective, efficient, and eco-friendly management and treatment strategies.
The NORMAN project, which enables the exchange of information and validation of ECs under the European Commission, has listed 1036 ECs by 2016 and is further labelled into 30 classes based on their origin and type (Dey et al. 2019). The ECs include pesticides, pharmaceutical active compounds (PhACs), disinfection by products, personal care products (PCPs), food additives, artificial sweeteners, flame retardants, endocrine-disrupting compounds (EDCs), nanomaterials, and surfactants (Lin et al. 2020). These contaminants exhibit very different physical and chemical properties, thus challenging to detect and remove from water. Many conventional treatment methods such as coagulation and flocculation, filtration, membrane treatment, and chlorination are ineffective in addressing ECs in water (Mondal et al. 2018). The high chemical usage and sludge production, biofouling, high energy requirements, and/or difficulty in operation and maintenance further limit their use. The frequent reports on the presence of ECs in treated water also support that the conventional treatment techniques are ineffective and cannot guarantee complete removal of ECs in water (Khetan and Collins 2007; Magureanu et al. 2015).
Advanced oxidation process (AOP) is a promising technique and can provide a potential route to degrade and mineralize complex organic molecules. The process relies on the in-situ generation of reactive chemical species (RCS) such as hydroxyl radicals, atomic oxygen, ozone, perhydroxyl radical, and hydrogen peroxide. These RCS can degrade or mineralize the ECs into less toxic, simple, and treatable compounds. Some existing AOPs include the Fenton and photo-Fenton processes, photocatalysis, sonolysis, catalytic wet air oxidation processes, and combinations thereof (Ribeiro et al. 2015). However, limitations like the addition of excess chemicals, catalyst regeneration, sludge generation, and low penetration efficiency of UV photons, formation of toxic byproducts, the negative influence of water quality parameters persist (Klamerth et al. 2010). Figure 15.1 summarizes the studies from 2000 to 2021 utilizing various AOPs to treat ECs in water.
Plasma-assisted AOP is an emerging technology that can simultaneously generate a wide variety of short and long-lived RCS at a controlled rate in an ambient environment. The technology also demonstrates good potential for commercial-scale processing (Pankaj et al. 2018). The significant attraction lies in the ability of plasma to produce a cocktail of reactive species without any additional chemical/catalyst (Gorbanev et al. 2018). The collective effect of plasma-generated RCS enhances the efficacy of the process. However, before translating this technology to the commercial scale, it is essential to make it affordable and energy-efficient. Significant efforts are being paid in this direction by developing novel reactors and hybridizing plasma technology with other approaches (Snoeckx and Bogaerts 2017). The following sections will outline the recent development in plasma-based reactors and their application in the degradation of ECs in water. The chapter will conclude by providing the current challenges and prospects of plasma-based technology in treating emerging contaminants.
2 Emerging Contaminants in the Aquatic Environment: Origin, Health Effects, Occurrence, Fate, and Transport
The significant levels of ECs in the aquatic system have become a serious global concern. They can enter the aquatic environment from the point or non-point sources, including wastewater treatment plants (WWTP), agricultural activities, surface runoff, air transportation and precipitation, adsorption onto soil, and sewage biosolids. Inadequate information on interaction mechanisms and toxic effects of ECs at sub-lethal concentrations pose a severe threat to environmental and public health (Brausch and Rand 2011). A few examples of ECs detected in surface and groundwater of different countries during 2010–2021 are shown in Table 15.1.
ECs like propranolol, ketoprofen, naproxen, E2, gemfibrozil, EE2, and ibuprofen are susceptible to photolysis (Khetan and Collins 2007). Recent studies report the occurrence of metabolites such as 3-hydroxycarbofuran, acephate, clethodim, acetochlor ethane sulfonic acid, and acetochlor oxanilic acid in water bodies. These metabolites are listed in contaminant candidate list 3 (Epa 2009). The human body cannot absorb drugs completely, and a good portion of the drug is often excreted with its metabolites. For instance, ibuprofen was found to be present as a parent chemical (1%) in surface water along with some of its metabolites like (+)-2–40-(2-carboxypropyl)-phenyl propionic acid (37%), (+)-2–4′-(2-hydroxy-2-methylpropyl)-phenyl propionic acid (25%) and conjugated ibuprofen (14%) (Kasprzyk-Hordern et al. 2008). Numerous metabolites are generated through various biodegradation pathways, and their toxicity is not often studied. Sorption of ECs onto dissolved organic matter is also responsible for the presence of ECs in an aqueous medium (Petrie et al. 2015).
Globally, only limited studies are available on the toxicity of ECs on humans and the environment. ECs like bisphenol A, organophosphate and brominated flame retardants, perchlorate, phthalate, polycyclic siloxanes, and triclosan can adversely affect human health (Covaci et al. 2012). Bisphenol A, a universal plasticizer, is responsible for severe endocrine disorders in salivary glands, thyroid, and men's genetic systems. Hormonal imbalance due to bisphenol A can lead to cancer (Bolong et al. 2009). Musk compound like xylol can indirectly affect the human tissues and leads to brain damage (Bolong et al. 2009; Lei et al. 2015). Prolonged exposure to herbicides like atrazine destroys adrenal glands and impairs steroid hormone metabolism (Debnath et al. 2019). About 70% increase in streptomycin, penicillin and tetracycline-resistant bacteria was identified in a dairy farm's soil manure (Qiao et al. 2011). Intake of phthalates leads to increased insulin resistance, abdominal obesity, and neurobehavioral disorder (Dewalque et al. 2014). EDCs like nonylphenol can cause feminization of aquatic organisms and hinder the hormonal system (Saidulu et al. 2021). Analysis of metabolites, in-depth survey of ECs existence, toxicology studies, risk assessment, mitigation measures, and awareness in public is required to plan appropriate mitigative measures and reduce the health impacts from ECs (Fig. 15.2).
Summary of effects of emerging contaminants on human health. The image is adapted with modifications from Rathi et al. (2021) with the permission from Elsevier
3 Advanced Oxidation Process for Removal of ECs in Water
Owing to the widespread occurrence and possible toxic effects on humans and other living organisms, removing ECs from water is imperative. However, most conventional treatment technologies are ineffective in removing ECs due to their complex nature and various physicochemical properties. Even popular technologies such as adsorption and membrane filtration are not attractive due to ECs complex nature and generation of contaminated sludge. In this context, developing more reliable and effective technologies is the need of the hour. The recent advancement in AOP promises a potential treatment option for removing ECs in water. The AOP can degrade and mineralize the ECs to simple carbon dioxide, water, and inorganic acids or ions. The technique utilizes ROS like hydroxyl radicals (•OH), hydrogen peroxide (H2O2), ozone (O3), nascent oxygen (•O), and hydroperoxyl (•HO2) for degradation. Among these, the •OH is the most reactive radical with an oxidation potential of 2.8 V. The highlights of its properties are shown in Fig. 15.3 (Brillas et al. 2009).
Most of the higher oxidizing potential radicals are short-lived; therefore, in-situ production of RCS is a prime requirement for efficient processing. Many AOPs such as the Fenton process, heterogeneous photocatalysis, sonolysis, and ozonation along with hybridized techniques such as O3/photo-Fenton process, O3/UV, O3/H2O2, UV/H2O2, catalytic ozonation, plasma treatment, and O3/UV/H2O2 are developed. A description of pathways of radical production in these processes is shown in Table 15.2.
Though AOPs are promising, the radical generation pathway, water matrices, and experimental conditions significantly influence the degradation/mineralization of ECs. Despite higher oxidation potential, low mineralization values are reported depending on the persistence of the pollutant. For example, Zhao et al. achieved complete degradation of 2.5 μM indomethacin with ozone dosage of 2–35 mg/L in 7 min (Zhao et al. 2017). However, only 50% mineralization was achieved even after extending the treatment to 30 min. Similarly, 0.38 mM of propranolol was degraded entirely in 8 min using the ozone dosage of 0.47 mM. Still, the total organic carbon (TOC) value remained close to 5% even after 60 min exposure with ozone dosages of 3.54 mM (Dantas et al. 2011). Even though ozonation is a proven technique, the limitations like inefficient mass transfer and the formation of secondary intermediates restrict operational capability (Shin et al. 1999). The presence of organic matter in WWTP effluent or surface waters can significantly demand higher ozone dosage and reduce the process efficacy (Almomani et al. 2016).
Fenton and photo-Fenton are extensively studied AOPs. Though the process is promising, large chemical requirements, the generation of chemical sludge, and the high sensitivity of the process are major concerns (Diya’uddeen and Daud 2012). Photocatalysis is also a promising AOPs suitable for removing complex organic molecules in water. However, catalyst fouling, slow rate of degradation, and low penetration of UV photons are some of the disadvantages of photocatalysis despite the less operational cost. The plasma-assisted AOP is an emerging approach that can offer an efficient and eco-friendly way to degrade and mineralize ECs in a relatively lower treatment time. The plasma-assisted AOP can produce short and long-lived RCS simultaneously, and its combined effect can significantly enhance the efficacy of the process (Nippatla and Philip 2019). The plasma can also be nonthermal and can produce RCS at ambient pressure, which is very suitable for commercial-scale processing. It can also eliminate the use of any additional precursor for the generation of RCS. The following section discusses the plasma-assisted AOP technique in detail and the recent studies on the degradation of emerging contaminants. The current challenges and prospects of plasma-based technology in treating emerging contaminants and various operating parameters influencing the process are also discussed.
4 Introduction to Plasma
As shown in Fig. 15.4, the phase transition from the solid-state to the gaseous state of matter can occur by increasing the system's internal energy. The addition of energy in the gaseous system enhances inelastic collisions that can prompt ionization and dissociation processes. Under appropriate conditions, this shall lead to the transition from the gaseous phase to the plasma, often referred to as the fourth state of matter. For the first time (1928), the “Plasma” (“mouldable substance” or “jelly” in Greek) (Fridman 2008) term was coined by well-known chemist Irving Langmuir to describe the ionized gas system, which is macroscopically neutral and exhibits collective behaviour. All ionized systems cannot be classified as plasma, and the plasma state must fulfil a set of criteria to ensure bulk quasi properties. One of the most exciting properties of plasma is bulk quasi neutrality. In simple terms, quasi neutrality means that the density of negatively charged species is equal to the density of positively charged species. To achieve quasi neutrality, the smallest dimension (L) defining the volume occupied by the plasma should be much larger than the Debye length (λd), i.e. (L ≫ λd). The plasma is a collection of charged particles. Therefore, the effective potential due to charge inside plasma at a point is screened Coulomb potential. This screening can be parameterized through the Debye length. The self-screening effect of plasma limits the influence of any given charge particle within the Debye sphere (a sphere with a radius λd) and allows quasi neutrality in bulk plasma. The λd depends on species temperature and density and can be expressed in Eq. 15.1 (Mozetiˇc et al. 2019).
For further details of basic properties, the readers are requested to refer the standard textbooks on introduction to plasma (Fridman 2008; Chen 2003). The plasma state can be characterized through some basic plasma parameters associated with the temperature and density of plasma constituents. Since the electron-induced kinetics is dominant in low-temperature plasma (LTP), the electron temperature (Te) and electron density (ne) are crucial plasma parameters. Another key parameter is the gas temperature to ensure the non-thermal nature of the plasma during processing. However, the most exciting feature of the plasma state is that it provides an environment where electrons, positive ions, photons, reactive species (excited molecules and atoms), and radicals can coexist simultaneously. This makes plasma a very suitable candidate for a wide range of applications, including material deposition, etching, surface modification, waste management, material processing, plasma cutting, plasma spraying, biomedical, energy, etc. (Samukawa et al. 2012). A more detailed discussion on various plasma applications is available elsewhere, and this chapter provides the application of plasma for the degradation of emerging contaminants in water.
4.1 Classification and Definition of Plasma
Plasma can be classified based on the fundamental plasma parameter related to the temperature (or average energy) of species such as ions, neutrals, and electrons. If the average thermal energy of ions (Ti), neutrals (Tg), and electrons (Te) are of a similar order, then they can be classified as hot plasmas (HP). The stars and plasma generated in fusion reactors come under HP. Most laboratory plasmas fall in the LTP category, where electron temperature is higher than other heavy mass species’ temperatures. The LTPs significantly deviate from equilibrium nature and can also be sustained at ambient pressure and temperature. In few cases where the electron density is very high such as welding or laser-induced plasmas, Local Thermal Equilibrium (LTE) may exist (Mal 2019; Rezaei et al. 2020). Further, in some plasmas, such as surface wave discharges or rotating gliding arcs, the partial local thermal equilibrium may also exist for higher-lying levels due to lower energy spacing (Ananthanarasimhan et al. 2021). Table 15.3 presents the classification of the plasmas commonly described in the literature (Fridman 2008; Yuri et al. 1997).
In recent years, the application domain of non-thermal plasma (NTP) assisted processing is significantly increasing. The major attraction lies in its ability to generate reactive species (ions, metastable, radicals, neutrals) at a relatively lower temperature and often close to room temperature (Shashurin et al. 2008). The generation of such plasmas is challenging as the operating condition are relatively very limited. However, over the years, various schemes to sustain the NTP are proposed. The following section will overview this NTP generation scheme for water treatment application.
4.2 Brief Note of Nonthermal Plasma/Discharges
The critical factor in sustaining the NTP is to limit the collisions between low mass (electron) and heavy mass (ion, neutral) species (Foster 2017). The mass difference leads to different mobilities, and plasma can simultaneously attain different equilibrium temperatures for other mass species. It is worth mentioning that this non-equilibrium regime is susceptible to plasma environment and operating conditions. Therefore, for water treatment application one needs to design the plasma generation scheme carefully so that plasma water interaction (direct or indirect) does not disturb the nonthermal characteristics of the plasma. Typically, a high voltage signal is applied between the two electrodes. When the voltage reaches above the inter-medium breakdown potential, an avalanche of electrons is being produced in the form of streamers. Once the streamers are generated, there are high chances of the thermal runaway due to the electric field application for long durations; this leads to the transition of non-thermal to the thermal regime. Here, the collision frequency of electron-neutral/ion increases and causes gas heating phenomena (Foster 2017; Wang et al. 2013). Thus, through such collisions, a gradual merge can be observed in the electron and gas/ion temperatures. To avoid the transition from non-thermal to thermal plasma or from streamer to arc discharge transition, one can employ a variety of techniques: (1) use of dielectric barriers, (2) shot pulse voltage signal, (3) gas composition, (4) flow rate, (5) variation in frequency, etc. (Bruggeman et al. 2016; Vanraes and Bogaerts 2018).
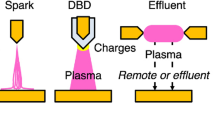
Over the years, numerous plasma reactors for water treatment applications were reported. Therefore, the classification of these reactors is challenging (Vanraes and Bogaerts 2018). Various criteria can be used to classify these plasmas. For example, based on typical current–voltage characteristics, these plasmas can be classified into three discharge regimes viz. Townsend, glow, and arc discharges (Yuri et al. 1997). Similarly, one can also classify these plasmas based on the applied signal such as DC, low frequency (kHz range), radiofrequency (MHz range), microwave (GHz range), or pulsed wave discharges. In context to plasma water treatment application, the most popular way is to classify the plasma based on the plasma interaction with the water surfaces. In this approach, the plasma can be divide into three categories: (1) Direct (discharge inside the water), (2) Indirect (discharge at the surface of the water without contact), and (3) Hybrid (multi-phase) discharges (Bruggeman et al. 2016). Most of the plasmas employed for the AOP process in water treatment are non-equilibrium discharges that are sustained at high-pressure conditions. Therefore, the present discussion will be limited to such discharge.
In the direct discharge or liquid-phase discharge, the discharge is sustained inside the water. To maintain such discharge, a very high electric field of the order of ~1000 kV/cm is applied between the electrodes. The liquid-phase discharges are energy-intensive, and to limit the heating and electrode erosion, the short pulses of micro-second order are applied. As shown schematically through Fig. 15.5 (a), (b), the pin-to-pin and pin-to-plate are the most common schemes to generate liquid phase discharges. The discharge is highly transient and often comes under the steamer or corona discharge category. Compare to other classes, the electron density in liquid-phase discharge is at least 5 to 6 orders (1024–1026/m3) higher and also produces 3 to 4 times higher radical density (~1024 m−3 s for OH radical) (Bruggeman et al. 2016; Locke and Thagard 2012).
Illustration of different reactors based on plasma water interactions: a, b direct discharge (liquid phase discharge); c, d indirect discharge (gas phase discharge); e, f hybrid discharge (multi-phase discharge). The image is adapted with modifications from Vanraes et al. (2016) (open
In the indirect or gas-phase discharge, the discharge is sustained in contact with water. As shown schematically in Fig. 15.5 (c), (d), the discharge is often created between the electrode plate and water surface or remotely generated above the water surface without direct contact. Normally for water treatment applications, the employed gas-phase discharges are based on dielectric barrier discharge concepts. However, spark or corona discharges can also be operated using pin-water electrode configuration. In recent years, remote discharges such as the atmospheric pressure plasma jets (APPJs) and surface dielectric barrier discharge are becoming popular choices because the discharge characteristics are independent of the properties of the treated water sample. The gas-phase discharge can be sustained by a wide range of high voltage signals ranging from DC, kHz, MHz to GHz frequencies (Foest et al. 2005). Compare to liquid-phase discharges, the gas phase discharges consume low power. Consequently, the electron density is of the order of 1018–1021 m−3 and the radical density of the order of 1019–1023 m−3 s (Bobkova et al. 2014; Wang et al. 2021a).
In the hybrid or multi-phase discharge category, the widely used approach is to sustain the discharge in the dispersed gas (Bubbles) inside the liquid. The water treatment can also be achieved by dispersing the water droplets (aerosols) in the gas phase plasmas. The schematic representation of both the approaches is shown in Fig. 15.5 (e), (f). The discharge is formed inside the bubbles by creating a sufficiently high electric field gradient around the bubbles. The studies reported that the discharge could sustain for ~100 ps before the bubble breaks off. The discharge is of steamer type and initiated at the bubble water interface. The electron density in hybrid discharges is reported as high as 1022 cm−3 (Bruggeman et al. 2010). The reactive species are mainly being produced in gas-phase in both hybrid as well as indirect discharges. One of the major advantages of the hybrid discharge approach is that reactive species are immediately dispersed in the water. The highest oxidizing potential species such as OH radicals are short-lived, and therefore the immediate dispersion can significantly enhance the efficacy of water treatment process.
It is worth mentioning that only brief details of the reactors and underlying discharge mechanism are given here and for further information, the readers are direct to published articles (Bruggeman et al. 2016; Vanraes and Bogaerts 2018). In fact, over the years, hundreds of geometries under the categories mentioned above are reported to address the engineering aspect as well as to improve performance (Samukawa et al. 2012; Vanraes et al. 2016).
4.3 Plasma Induced Reactive Species
The advanced oxidation process is achieved by the plasma water interaction, which is the source of the generation of various reactive chemical species (RCS). One of the significant advantages is that the RCS generation does not require any additional consumables. Figure 15.6 schematically shows the generation of RCS through plasma-water interactions (Samukawa et al. 2012). The RCS generated immediately after collision with electrons from the plasma is called primary species. Electrons (e−), excited neutrals (M*), ion (M+), neutrals (M), radicals (•OH, N, O), UV photon, etc. are primary species produced in plasma-gas phase interaction. These species usually have less lifetime. For example, the lifetimes of OH radicals, NO, and O2* are 2.7 µs ~1.2 µs, 1.4 µs, and ~1.3 µs respectively (Attri et al. 2015). However, due to higher reactivity rates, these species get quickly consumed, and secondary species are formed such as H2O2, NO2, NO3, and O3. The secondary species have relatively long lifetimes and are dissolved in water media. The secondary species radicals further oxides to form tertiary species such as O3, H2O2, NO3 that can last up to several days (Kondeti et al. 2018). The RCS generated through plasma water interaction either in gas-phase or inside liquid can broadly be classified into reactive oxygen species (ROS) and reactive nitrogen species (RNS). Sometimes both classes can simultaneously be referred to as reactive nitrogen and oxygen species (RONS).
Schematic representation of the formation of reactive chemical species through plasma-water interactions. The image is adapted with modifications from Barjasteh et al. (2021)
4.3.1 Reactive Oxygen Species
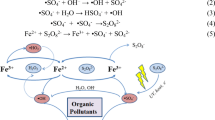
Diatomic oxygen (O2) contains two unpaired electrons in the valance shell, limiting its scope to react and degrade ECs in water. An electron or energy transfer process can convert O2 into ROS, atoms or molecules with at least one unpaired electron. These include both radicals and non-radical such as hydroxyl radical (•OH), hydrogen peroxide (H2O2), singlet oxygen (1O2), superoxide anion (•O2−) and ozone (O3). (Nosaka and Nosaka 2017). Figure 15.7 represents an overview of various possible reaction routes for ROS production during the plasma-assisted advanced oxidation process. A few of the significant ROS species are discussed below.
Overview of the mechanisms of RCS production during plasma assisted advanced oxidation process. Reprinted from Mitra et al. (2019)
Hydroxyl radical (•OH): Hydroxyl radicals are the most potent ROS with the oxidation potential value of 2.8 V, which is only next to the oxidation potential of fluorine (3.03 V). The high oxidising potential and short lifetime (~1 ns) allow OH radicals to react with most organic molecules, including ECs in water and degrade them. In the plasma-mediated treatment of water, the OH radicals are produced by the interaction of high energetic plasma electrons with water molecules by dissociating them into •OH and H• and relatively less energetic secondary electrons. Ozone generated during the plasma process can react with water molecules and produces diatomic oxygen and OH radicals. Plasma also produces UV light along with a variety of RCS. This UV light lies in VUV to UV region and break H2O2 into two OH radicals (Mitra et al. 2019).
Superoxide anion (•O2−): The monovalent reduction of diatomic oxygen (O2) by electron impact ionisation produces a superoxide anion (•O2−). It is one of the few molecules among the ROS that are considered radical and anion with low reactivity. Due to its unstable nature and low reactivity, it quickly disintegrates into two weak potential reactive species, namely oxygen and hydroperoxide anion (HO2−), as shown below (Hayyan et al. 2016).
Singlet oxygen (1O2): Singlet oxygen is not radical but highly reactive. It is usually formed when the two unpaired valance electrons attain anti-parallel spin and occupy the same orbital. It is the first excited state of diatomic oxygen (O2), and the removal of spin restriction makes it a powerful enough oxidant. Singlet oxygen is thus capable of reacting with electrons of all spin orientations, unlike molecular oxygen. The primary source of 1O2 is the photoexcitation process (Wu et al. 2011).
Hydrogen peroxide (H2O2): Hydrogen peroxide (1.75 V) is a potent oxidizing agent in removing ECs in water. The relatively high solubility and stability help it participate in reactions with molecules distant from the point of their origin. Its ability to reproduce OH radicals upon reacting with photons make it suitable to degrade a wide range of contaminants (Kehrer et al. 2010). The hydrogen peroxide is typically formed in aqueous media subjected to plasma treatment is by the recombination of OH radicals as per the reaction. They can also form when plasma is produced from gases containing O and H2, but the route is perilous as both the reactants are highly combustible. When plasma is in direct contact with water, it can entail hydrogen peroxide by the relatively less probable reaction mechanism, as shown below (Nosaka and Nosaka 2017).
Ozone (O3): Ozone (2.07 V) is a powerful oxidising agent extensively employed in water and wastewater treatment. Ozone is produced through a three-body recombination mechanism where the atomic oxygen is reacted with the diatomic oxygen in the presence of a third colliding body produces ozone, where the electron-O2 collisions produce the atomic oxygen (Zangouei and Haynes 2019).
4.3.2 Types of Reactive Nitrogen Species
Nitrogen molecules have numerous excited states and metastable states, which often leads to many interactions with the liquid media. As a result, it is not easy to understand the mechanism of nitrogen plasmas or plasmas produced with ambient air. Nitrogen subjected to excitation has high vibrational energies ranging from a few 100–1000 K and less rotational energies lying around room temperature. This distinctive characteristic results from the low probability for collisions between neutral-vibrationally excited N2 molecules. As the dissociation energies lie well below the excitation energy of N2, the collision between these exited atoms leads to ionization, resulting in the formation of positive ions and electrons inside the plasma. Due to the richness in exited states and metastable stable states, nitrogen species are formed with oxygen available in ambient air or liquid media. One explanation for this phenomenon is that the dissociation energy of water molecules and oxygen lies par below some nitrogen species’ excitation energy (Bradu et al. 2020).
Nitric oxide (•NO): During the gas phase discharge of N2 in the presence of O2 or ambient air, the RNS reacts with ROS and leads to the formation of nitric oxide, which is one of the prominent secondary species of nitrogen (Tian and Kushner 2014).The key mechanisms through which •NO produced are given in Eqs. (15.12)–(15.16).
Nitrogen dioxide (•NO2): In oxygen-rich plasmas, nitric oxide produced can interact with species that can donate oxygen or O-donors such as •OH, •O and O3 to produce nitrogen radicals. In dry gases, the ozone plays a significant role in nitrogen dioxide formation, whereas in humid gases, it is substantially less (Chen and Davidson 2002; Chen and Wang 2005). The possible chemical pathways of •NO2 formation are:
Nitrous and nitric acids (HNO2, HNO3): Discharges happening during the humid air or N2/O2 mixtures form nitric and nitrous acids through the following reactions (Tas et al. 1997; Chase and Hunt 2002)
Nitrogen species in aqueous media: Alongside the humid air, the interaction of plasmas formed in nitrogen-rich environments can also produce RNS in the liquid–gas phase and inside the liquid phase. The most dominating ones are the nitrogen oxy, and per-oxy molecules, nitric acids, nitrites and nitrates are formed from the interaction of water molecules and oxygen radicals such as •OH, •O with the dissolved nitrogen oxides produced in gas phase interactions (Bruggeman et al. 2008; Parvulescu et al. 2012).The interaction of nitrogen dioxide (•NO2) species with oxygen radicals such as •OH forms the nitric acid in aqueous media similar to the reaction in the gas phase. Peroxy-nitrous acid can be formed by the interaction of •NO2 with H2O. Hydrogen peroxide and several other ROS can also produce peroxy-nitrous acid by reacting with nitrites, nitrogen dioxide and nitric oxide, which are listed below (Tian and Kushner 2014).
4.4 Quantification of Reactive Chemical Species
The quantification of whole range of RCS is a challenging task due to uncertainty in formation pathways of these chemical species. So, the quantification of the radicals like •OH, 1O2, H2O2, and O3 through well-known characterisation methods is discussed briefly in the following section.
4.4.1 Quantification of OH Radicals
Direct measurement of OH radicals in an aqueous solution is difficult due to its low concentrations and short lifetime. One must rely on relatively more straightforward methods, like the chemical probe method, to quantify the OH radicals. Among all the available techniques, sodium terephthalate as OH radical probe has many advantages. Its symmetric structure forms only a single ring compound, 2- hydroxy terephthalic acid, upon interaction with OH radical, as given in Fig. 15.8. In addition to this, it has low limits of detection, typically 2 nM and strongly fluorescent (Gonzalez et al. 2018; Tampieri et al. 2021).
Another popular technique used to detect the presence of OH radicals is Electron Spin Resonance (ESR). It is a conventional spin trapping method for which DMPO (5,5-dimethyl-1-pyrroline N-oxide) is used as a spin trapping compound. During the plasma treatment of water, the OH radicals react with DMPO to form stable DMPO-OH radicals and can be detected by ESR spectroscopy (Dvoranová et al. 2014).
4.4.2 Quantification of H2O2
Hydrogen peroxide is typically quantified by a colorimetric method using an aqueous titanium reagent solution as an indicator. Metal complexes are formed in acidic solution, resulting in the formation of pale-yellow solution whose absorbance can be measured using a UV–Visible spectrophotometer at 410 nm (Eisenberg 1943).
With titanium-oxy-sulphate solution as a colorimetric probe, other probes such as N, N-diethyl-p-phenylenediamine (DPD) method are based on the oxidation of DPD on reacting with H2O2 catalysed using horseradish peroxidase. The reactant DPD generated during the reaction can form long-lasting colour and exhibit an absorption peak at 551 nm (Wu et al. 1999).
4.4.3 Quantification of (1O2)
Sterically hindered cyclic amines such as 4-hydroxy-2,2,6,6- tetramethylpiperidine (HTMP) generate 1-oxyl radical by reacting with singlet oxygen. The reaction produces stable compounds such as 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl, also called nitroxide radical. The chemical reaction is shown below. This can be detected using ESR spectroscopy (Dimitrijevic et al. 2009; Brezová et al. 2005) (Fig. 15.9).
4.4.4 Quantification of O3
In recent years a rapid detection method for the ozone based on N-diethy1-1,4-phenylen-ediammonium sulphate coupled with spectrophotometry is proposed. When ozone reacts with potassium iodide in a buffered solution (pH 3.35) iodine is formed. This iodine would undergo a reaction with DPD reagent and produces a characteristic pink colour which gives an absorption peak at 510 nm (Song et al. 2000).
5 Plasma Assisted Degradation Process for ECs
The past decade has witnessed several works from the scientific community to enhance plasmas efficiency, especially NTP, for removing ECs in water. Phenols, azo dyes, pharmaceutical compounds, and pesticides have dominated this spectrum primarily due to their extensive usage in household and commercial purposes. Knowledge of plasma operating parameters like input voltage, electrode distance, flow rate, gas composition, reactor geometry, and type of discharge is essential. The water quality parameters such as pH, conductivity, concentration of organic contaminants, and background ions can also affect the process. The effect of these parameters on the plasma process is discussed in the following subsections.
5.1 Effect of Initial Pollutant Concentration
The influence of initial pollutant concentration on degradation kinetics is systematically studied. It is found that the rate of reaction increases with an increase in initial pollutant concentration but fails to keep up when the concentration further increased. The observed decrease in the rate constant at higher pollutant concentrations is attributed to the limited generation of RCS. Wang et al. studied the effect of triclocarban (TCC) concentration on the degradation efficiency in a DBD reactor. They observed a significant improvement in reaction rate when the pollutant concentration is varied from 2 to 7 mg/L. However, the increase in TCC above 7 mg/L did not keep up the trend (Wang et al. 2016). A similar study was performed on Rhodamine B (RhB) solution by Panomsuwan et al. by changing the initial concentration from 1 to 15 mg/L. The first-order kinetic model fits well for the lower concentrations than the higher concentrations (Panomsuwan et al. 2016). The data reveals that there is much competition between dye molecules and their byproducts at higher pollutant concentrations to react with available RCS, which leads to the deviation of the first-order kinetics model.
5.2 Effect of pH
pH is one of the critical factors that decide the efficiency of the plasma degradation process. Previous studies report a proportional relationship between the pH of the sample and the decomposition rate (Reddy et al. 2013). We can explain this phenomenon based on the change in OH radicals’ production when medium pH changes from alkaline to acidic. In an alkaline medium, ozone decomposes to form more powerful OH radicals because of the favorable presence of OH− ions, as shown in the equations below (Peleg 1976).
In alkaline conditions, the ozone can be further reacted with water to form a superoxide ion and the recombination of OH radicals to form hydrogen peroxide. In acidic media ozone participates in degradation process more actively compared to basic medium because of relatively higher oxidation potential ranging from 1.4 to 2.8 V. Chen et al. have studied the influence of pH on methylene blue (MB) degradation (Chen et al. 2019). They reported that the shorted lived species have limited penetration inside the water; the long-lived species performed better under acidic conditions. OH radicals dominate the higher pH, but the degradation efficiency is suffered from the recombination of OH radicals to form peroxides. Although the initial pH is high while the experiment is initiated, due to the presence of N2, O2 in feeder gas produces secondary and tertiary species like HNO2, HNO3 along with peroxy-nitrous acid, which results in the reduction of pH values as low as 2. Peroxy-nitrous acid is a powerful oxidant and very unstable in acidic conditions, thus enhances the degradation of MB.
Ghodbane et al. reported that in acidic conditions, increased efficiency of AB25 dye is due to the protonation of SO3− results in increased hydrophobic character of the dye molecules, which leads to the greater reactivity with reactive species generating higher degradation rates (Ghodbane et al. 2015; Ghodbane and Hamdaoui 2009). Zhao et al. performed the degradation of p-nitrophenol (PNP) using the microwave ambient air discharge observed that in the first minute of treatment the pH of the solution dropped significantly from 5.5 to 4 (Zhao et al. 2021). It slowed down during the next 11 min of treatment time. Further analysis with IC confirmed that the drop in the pH is due to the formation of tertiary compounds like acetic acid and formic acid when PNP is reacted with secondary species formed from the N2 discharge interacting with the liquid phase.
Wu et al. have also studied the effect of pH on the degradation of MB using a dielectric barrier discharge and observed that pH of all the samples reached a specific value, which is in the range of pH 2–4 (Wu et al. 2019). It is inferred that the fall in pH is due to the solubility of nitrogen species in water and forming nitrous and nitric acid, as shown in Fig. 15.6.
5.3 Effect of Water Conductivity
The effect of the initial conductivity of the water sample significantly affects the discharge propagation, initiation in underwater discharges, and the number density of species. Hamdan et al. studied the effect of water conductivity using microwave underwater bubble discharge using argon as a trace gas (Hamdan et al. 2020). They have reported that the plasma electron density has increased with an increase in the conductivity of water from 10 to 100 µS/cm. No significant change in degradation was observed with a further increase in conductivity of sample from 100 to 1000 µS/cm. However, the further increase in the conductivity did not show any significant impact on the degradation due to the interaction of electrons with ions available in liquid media, leading to the formation of lower oxidative species.
The conductivity of the solution can also be increased by plasma exposure. Rancev et al. investigated the degradation of reactive orange 16 in a nonthermal multipin electrode atmospheric plasma reactor as a function of plasma exposure time and initial dye concentrations (Rancev et al. 2018). The conductivity of the sample was increased from 11.1 to 77.4 µS/cm within a minute of plasma exposure time. In some cases, the sample having high initial concentrations of the pollutant upon subjected to plasma treatment accumulates reactive nitrogen species like nitrite, nitrate ions and peroxy-nitric acid as discussed in the above Sect. 4.3 will increases the system conductivity and shifts the pH towards acidic.
5.4 Effect of Feed Gas Composition and Flow Rate
The choice of the feed gas is crucial in the plasma process as it influences the choice of input voltage, reactor configuration, electrode gap, input waveform etc. It can also affect the density of RCS formed during the plasma operation. It is observed that the use of oxygen as feeder gas results better degradation efficiencies followed by noble gas mixtures and ambient air. The flow rate of the feed gas is another important parameter that decides the total number of reactive chemical species being generated at a particular instant of time. The higher the flow rate, the more species will be generated as long as the applied electric field has enough strength to ionize more atoms. This increased species density results in a higher removal rate. Yamatake et al. studied the influence of gas flow at different velocities with inlet diameter changing from 200 to 300 µm (Yamatake et al. 2006). The degradation rate increases with an increase in a gas velocity proportional to the flow rate. The system's efficiency is higher at inlet diameters 200 µm compared to 300 µm because of a substantial increase in gas velocity due to smaller diameters. A possible conclusion from this study is that the generation and lifetime of ROS like •OH and •O are in the range of milliseconds, so they need to be transported fast to achieve faster degradation rates.
5.5 Effect of Reactor Configuration and Plasma Discharge
The last decade has witnessed several reactor configurations deployed to study degradation of ECs in terms of power consumption, formation of byproducts, toxicity of plasma-treated water and complete mineralization. It can be observed that DBD reactors have been most successful and leading the battle against the removal of ECs from the water with low power consumption and breaking of ECs into less nontoxic byproducts and time required to mineralize the target contaminant completely.
In summary, numerous reactors under various plasma conditions were employed for water treatment; commonly used configurations are summarized in Fig. 15.5. Further, the reactors mentioned in Table 15.4 are being often utilized for the removal of ECs. The DBD discharges, also called silent discharges, are quite energy-efficient. DBDs can be produced by covering either or both the electrodes using a dielectric material. The role of dielectric is to prevent the transition from glow/Townsend mode to arc mode. A schematic configuration is illustrated in Fig. 15.5 (c) (Magureanu et al. 2011). In DBDs the electron density is of the order of 1018–1021 m−3 (Bruggeman et al. 2016). Micro-arc discharges both in-liquid and above the liquid surface are energy-intensive. However, they produce a high amount of RCS of the order of ~1024 m−3 s (Chen et al. 2019). Nevertheless, electrode erosion during this type of direct plasma treatment is a significant concern. A schematic discharge of this category can be seen in Fig. 15.5 (a). Bubble discharges are hybrid discharges and similar to those mentioned in Fig. 15.5 (e). The hybrid discharges are not pure in-liquid or in-gas phase discharges but discharges induced in liquid media inside the gas bubble (Shimizu et al. 2010). In hybrid discharge, the energy consumption is relatively lower than the direct discharges inside the liquid. The electron density in hybrid discharge is in the range of 1021–1023 m−3 (Bruggeman et al. 2010). Gliding arc discharges are formed between two electrodes exposed to open air, one of which is ground and another connected to a high voltage source. At atmospheric pressure, this discharge can produce high electron densities (1019–1021 m−3) (Bruggeman et al. 2016; Du et al. 2007).
Further, some innovative reactor designs were also reported to enhance the degradation efficiency, including plasma-catalyst hybrid processes. Shang et al. has studied the DBD reactor with the addition of persulfate (PS) (Shang et al. 2017). The PS greatly enhanced the degradation efficiency of acid orange 7 (AO7) by almost 60%. The interaction of plasma with PS enhanced the production of •OH and •SO4− radicals. It also improved the TOC removal efficiency. Ajo et al. (2017) have proposed a pulsed corona discharge (PCD) reactor consisting of a vertical wire plate configuration where the water is sprayed using five atomizers from the top and plasma directly contact the water. It is reported that the gas–liquid interface is a barrier for the effective decomposition of ECs in aqueous media by OH radical. The generation of OH radicals is possible through the enhanced interaction of plasma-liquid interface through which high energetic plasma electrons directly collide with water molecules to generate •OH. Raji et al. added ZnO nanoparticles in a non-thermal atmospheric pressure plasma jet and compared the efficiency under various feed gases like oxygen, argon, and ambient air. The degradation of their pharmaceutical compound valsartan has decomposed more effectively by the addition of ZnO nanoparticles (Raji et al. 2020).
5.6 Effect of the Electrode Gap
An optimal electrode gap is crucial to achieving the maximum degradation capacity of the given configuration. The higher or lower gap can have adverse effects on degradation efficiency. Sugiarto and Sato have investigated the impact of electrode gap in removing phenols from water in the needle to plate configuration (Sugiarto and Sato 2001). Three configurations with 45, 15 and, 6 mm electrode gaps were investigated, and the 6 mm electrode gap yielded the highest removal rate. The formation of multiple small steamers to single spark transition creates shock waves, intense UV radiation, and high-energy electrons produce copious amounts of radicals. At 45 mm electrode gap, it is observed that there are multiple streamers, yielding less amount of RCS and lower degradation efficiency. Shang et al. studied the effect of different air discharge gaps in the degradation of acid orange 7 (Shang et al. 2017). The study reported that the degradation efficiency increases as the electrode gap increases from 2 to 4 mm. The further increase in the gap from 4 to 6 mm showed no significant improvement in the degradation efficiency. It is worth mentioning that at a low electrode gap, the discharge can significantly disturb by the water surface turbulence. Chen et al. has pointed out cathode fall as an important factor in producing reactive species (Chen et al. 2019).
5.7 Effect of Input Power
Several studies have been conducted to explore the input power or voltage influence on the degradation efficiency of ECs in water. As the input voltage increases, it is reported that the overall degradation efficiency increases. Energy yield increases proportion to the applied voltage up to a certain point and decreases, because production of reactive species reaches to saturation stage which cannot be further enhanced unless external parameters like feeder gas composition and flow rate are varied. The increase in removal rate can be attributed to high electric field strength and associated increase in electron temperature. High collision rates with the gas molecules lead to the rise in the concentration of reactive species, high-intensity UV-light and induced ionization of oxygen and water molecules.
Wu et al. studied the removal of tetracycline antibiotics (TCs) from water using a DBD reactor (Wu et al. 2021). The reactor was operated at different voltages (12, 15 and 18 kV) to investigate the influence of voltage on degradation kinetics of TC. The degradation efficiency increases proportionally to the rise in voltage. Reddy et al. also studied methylene blue (MB) removal using a non-thermal DBD discharge at different operating voltages (14, 16 and 18 kV). It is observed that 90% decolorization is achieved with an initial concentration of MB 100 mg/L in the first 25 min of plasma exposure at an applied voltage of 14 kV. The efficiency slightly increased to 94% as the voltage increased to 18 kV. The TOC removal % also slightly improved from 18 to 20% (Reddy et al. 2013). Pankaj et al. also reported the degradation kinetics of various dyes like methyl red, crystal violet and fast green FCF by deploying a high voltage atmospheric air and modified air cold plasma (Pankaj et al. 2017). It was reported that all the dyes showed higher removal rates at a maximum applied voltage of 80 kV.
6 Conclusion and Future Prospects
The widespread occurrence of emerging contaminants in water bodies is a serious concern in the water sector worldwide. Unlike conventional water pollutants, these contaminants are complex, diverse in their physicochemical properties. As a result, traditional water and wastewater treatment processes are ineffective in removing emerging contaminants from water. However, many of these compounds are potentially toxic to all life forms, and their removal from water is imperative to protect public health and the ecosystem. Considering the various physicochemical properties and complex fate and transport, a thorough understanding of various aspects of emerging contaminants, including their source, occurrence, classification, fate and transport, detection method, and treatment techniques, are essential in developing an effective management strategy.
The advanced oxidation process is a promising treatment technology for the removal of ECs in water. Among advanced oxidation processes, NTP looks attractive due to its ability to generate RCS at a controlled rate and ambient conditions. The process is also attractive due to its ability to produce reactive species like ROS and RONS, non-selective behaviour towards target pollutants, and sludge-free operation. However, the application of NTP in removing organic contaminants is mainly limited to small-scale laboratory experiments. The biggest challenge in scale-up is high energy consumption and lack of information on the process that dictates liquid plasma interaction. To overcome these issues, new models that include all the effects ranging from transport phenomenon, fluid dynamics effects, complex plasma chemistry, and fundamental collision process need to be set up, explaining the plasma liquid interaction phenomenon alongside the experimental observations. However, the plasma modelling studies with experimental validation are limited. The plasma diagnostics in the liquid phase are also challenging due to the interference of many species; most of the available methods are not selective. Moreover, physical techniques like optical emission spectroscopy (OES) and optical absorption spectroscopy (OAS) are limited to estimating the species densities in the gas phase. The liquid surrounding the plasma or species poses a severe challenge in quantification using spectroscopic techniques. Besides, radical transport in liquid and liquid surfaces is still unknown. The effect of UV radiation and shockwaves produced by plasma on intermediate compounds and the plasma decomposition of water is clueless. For plasma technology to become dominant, these complex problems have to be addressed.
References
Abdelmalek F, Ghezzar MR, Belhadj M, Addou A, Brisset JL (2006) Bleaching and degradation of textile dyes by nonthermal plasma process at atmospheric pressure. Ind Eng Chem Res 45:23–29. https://doi.org/10.1021/ie050058s
Ajo P, Kornev I, Preis S (2017) Pulsed corona discharge induced hydroxyl radical transfer through the gas-liquid interface. Sci Rep 7:1–6. https://doi.org/10.1038/s41598-017-16333-1
Almomani FA, Shawaqfah M, Bhosale RR, Kumar A (2016) Removal of emerging pharmaceuticals from wastewater by ozone-based advanced oxidation processes. Environ Prog Sustain Energy 35:982–995. https://doi.org/10.1002/EP.12306
Ananthanarasimhan J, Gangwar RK, Leelesh P, Srikar PSNSR, Shivapuji AM, Rao L (2021) Estimation of electron density and temperature in an argon rotating gliding arc using optical and electrical measurements. J Appl Phys 129:223301. https://doi.org/10.1063/5.0044014
Asghar A, Raman AAA, Daud WMAW (2015) Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: a review. J Clean Prod 87:826–838. https://doi.org/10.1016/J.JCLEPRO.2014.09.010
Attri P, Kim YH, Park DH, Park JH, Hong YJ, Uhm HS, Kim KN, Fridman A, Choi EH (2015) Generation mechanism of hydroxyl radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. Sci Rep 5:1–8. https://doi.org/10.1038/srep09332
Bagal MV, Gogate PR (2014) Wastewater treatment using hybrid treatment schemes based on cavitation and fenton chemistry: a review. Ultrason Sonochem 21:1–14. https://doi.org/10.1016/J.Ultsonch.2013.07.009
Barjasteh A, Dehghani Z, Lamichhane P, Kaushik N, Choi EH, Kaushik NK (2021) Recent progress in applications of non-thermal plasma for water purification, bio-sterilization, and decontamination. Appl Sci 11:3372 11:3372 . https://doi.org/10.3390/APP11083372
Bobkova ES, Smirnov SA, Zalipaeva Y V., Rybkin V V. (2014) Modeling chemical composition for an atmospheric pressure dc discharge in air with water cathode by 0-D model. Plasma Chem Plasma Process 34:721–743. https://doi.org/10.1007/s11090-014-9539-z
Bolong N, Ismail AF, Salim MR, Matsuura T (2009) A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 239:229–246. https://doi.org/10.1016/j.desal.2008.03.020
Boretti A, Rosa L (2019) Reassessing the projections of the world water development report. npj Clean Water 21(2):1–6 . https://doi.org/10.1038/s41545-019-0039-9
Bradu C, Kutasi K, Magureanu M, Puač N, Živković S (2020) Reactive nitrogen species in plasma-activated water: generation, chemistry and application in agriculture. J Phys D Appl Phys 53. https://doi.org/10.1088/1361-6463/ab795a
Brausch JM, Rand GM (2011) A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere 82:1518–1532. https://doi.org/10.1016/j.chemosphere.2010.11.018
Brezová V, Gabčová S, Dvoranová D, Staško A (2005) Reactive oxygen species produced upon photoexcitation of sunscreens containing titanium dioxide (an EPR study). J Photochem Photobiol B Biol 79:121–134. https://doi.org/10.1016/j.jphotobiol.2004.12.006
Brillas E, Sirés I, Oturan MA (2009) Electro-fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev 109:6570–6631. https://doi.org/10.1021/cr900136g
Bruggeman P, Degroote J, Vierendeels J, Leys C (2008) Dc electrical breakdown between a metal electrode and a water surface. In: GD 2008—17th Int Conf Gas Discharges Their Appl 36:321–324
Bruggeman P, Verreycken T, González MÁ, Walsh JL, Kong MG, Leys C, Schram DC (2010) Optical emission spectroscopy as a diagnostic for plasmas in liquids: opportunities and pitfalls. J Phys D Appl Phys 43:124005. https://doi.org/10.1088/0022-3727/43/12/124005
Bruggeman PJ, Kushner MJ, Locke BR, Gardeniers JGE, Graham WG, Graves DB, Hofman-Caris RCHM, Maric D, Reid JP, Ceriani E, Fernandez Rivas D, Foster JE, Garrick SC, Gorbanev Y, Hamaguchi S, Iza F, Jablonowski H, Klimova E, Kolb J, Krcma F, Lukes P, MacHala Z, Marinov I, Mariotti D, Mededovic Thagard S, Minakata D, Neyts EC, Pawlat J, Petrovic ZL, Pflieger R, Reuter S, Schram DC, Schröter S, Shiraiwa M, Tarabová B, Tsai PA, Verlet JRR, Von Woedtke T, Wilson KR, Yasui K, Zvereva G (2016) Plasma-liquid interactions: a review and roadmap. Plasma Sourc Sci Technol 25:053002. https://doi.org/10.1088/0963-0252/25/5/053002
Carmona E, Andreu V, Picó Y (2014) Occurrence of acidic pharmaceuticals and personal care products in Turia river Asin: from waste to drinking water. Sci Total Environ 484:53–63. https://doi.org/10.1016/J.SCITOTENV.2014.02.085
Chase WJ, Hunt JW (2002) Solvation time of the electron in polar liquids. Water and alcohols. J Phys Chem 79:2835–2845 . https://doi.org/10.1021/J100593A007
Chen, Francis F. JPC (2003) Principles of plasma processing
Chen J, Davidson JH (2002) Ozone production in the positive DC corona discharge: model and comparison to experiments. Plasma Chem Plasma Process 22:495–522. https://doi.org/10.1023/A:1021315412208
Chen J, Wang P (2005) Effect of relative humidity on electron distribution and ozone production by DC coronas in air. IEEE Trans Plasma Sci 33:808–812. https://doi.org/10.1109/TPS.2005.844530
Chen Q, He B, Ma Y, Wang X, Xiong Q, Li J, Liu QH (2019) Influence of the pH value on the degradation of an azo dye of methyl orange by air discharge plasma. Plasma Process Polym 16:1800152. https://doi.org/10.1002/ppap.201800152
Covaci A, Geens T, Roosens L, Ali N, Eede Van den N, Ionas AC, Malarvannan G, Dirtu AC (2012) Human exposure and health risks to emerging organic contaminants, 20:243–305. https://doi.org/10.1007/978-3-642-28132-7
Cristale J, García Vázquez A, Barata C, Lacorte S (2013) Priority and emerging flame retardants in rivers: Occurrence in water and sediment, daphnia magna toxicity and risk assessment. Environ Int 59:232–243. https://doi.org/10.1016/J.ENVINT.2013.06.011
da Silva CP, Emídio ES (2015) Marchi MRR de (2015) The occurrence of UV filters in natural and drinking water in São Paulo State (Brazil). Environ Sci Pollut Res 2224(22):19706–19715. https://doi.org/10.1007/S11356-015-5174-3
Dantas RF, Sans C, Esplugas S (2011) Ozonation of propranolol: transformation, biodegradability, and toxicity assessment. J Environ Eng 137:754–759. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000377
De Gerónimo E, Aparicio VC, Bárbaro S, Portocarrero R, Jaime S, Costa JL (2014) Presence of pesticides in surface water from four sub-basins in Argentina. Chemosphere 107:423–431. https://doi.org/10.1016/J.CHEMOSPHERE.2014.01.039
De la Cruz N, Esquius L, Grandjean D, Magnet A, Tungler A, de Alencastro LF, Pulgarín C (2013) Degradation of emergent contaminants by UV, UV/H2O2 and neutral photo-Fenton at pilot scale in a domestic wastewater treatment plant. Water Res 47:5836–5845. https://doi.org/10.1016/J.WATRES.2013.07.005
Debnath D, Gupta AK, Ghosal PS (2019) Recent advances in the development of tailored functional materials for the treatment of pesticides in aqueous media: a review. J Ind Eng Chem 70:51–69. https://doi.org/10.1016/j.jiec.2018.10.014
Dewalque L, Charlier C, Pirard C (2014) Estimated daily intake and cumulative risk assessment of phthalate diesters in a Belgian general population. Toxicol Lett 231:161–168. https://doi.org/10.1016/j.toxlet.2014.06.028
Dey S, Bano F, Malik A (2019) Pharmaceuticals and personal care product (PPCP) contamination-a global discharge inventory. Elsevier Inc.
Dimitrijevic NM, Rozhkova E, Rajh T (2009) Dynamics of localized charges in dopamine-modified TiO and their effect on the formation of reactive oxygen species. J Am Chem Soc 131:2893–2899. https://doi.org/10.1021/ja807654k
Dindarsafa M, Khataee A, Kaymak B, Vahid B, Karimi A, Rahmani A (2017) Heterogeneous sono-Fenton-like process using martite nanocatalyst prepared by high energy planetary ball milling for treatment of a textile dye. Ultrason Sonochem 34:389–399. https://doi.org/10.1016/J.ULTSONCH.2016.06.016
Diya’uddeen BH, A.R. AA, Daud WMAW (2012) On the limitation of Fenton oxidation operational parameters: a review. Int J Chem React Eng 10. https://doi.org/10.1515/1542-6580.2913
Du CM, Yan JH, Cheron BG (2007) Degradation of 4-chlorophenol using a gas-liquid gliding arc discharge plasma reactor. Plasma Chem Plasma Process 27:635–646. https://doi.org/10.1007/s11090-007-9092-0
Dvoranová D, Barbieriková Z, Brezová V (2014) Radical intermediates in photoinduced reactions on TiO2 (An EPR spin trapping study). Molecules 19:17279–17304. https://doi.org/10.3390/molecules191117279
Eisenberg GM (1943) Colorimetric determination of hydrogen peroxide. Ind Eng Chem Anal Ed 15:327–328. https://doi.org/10.1021/i560117a011
Epa U, of Water O, of Groundwater O, Water D (2009) Office of water (4607M) EPA EPA 815F09001 fact sheet: final third drinking water contaminant candidate list (CCL 3)
Foest R, Kindel E, Ohl A, Stieber M, Weltmann KD (2005) Non-thermal atmospheric pressure discharges for surface modification. Plasma Phys Control Fusion 47. https://doi.org/10.1088/0741-3335/47/12B/S38
Foster JE (2017) Plasma-based water purification: Challenges and prospects for the future. Phys Plasmas 24:055501. https://doi.org/10.1063/1.4977921
Fridman A (2008) Plasma Chemistry (Cambridge 2008)-Cambridge University Press (2008), 1st ed. Cambridge University Press
Gani KM, Kazmi AA (2016) Contamination of emerging contaminants in Indian aquatic sources: first overview of the situation. J Hazard Toxic Radioact Waste 21:04016026. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000348
Ghodbane H, Hamdaoui O (2009) Degradation of acid blue 25 in aqueous media using 1700 kHz ultrasonic irradiation: ultrasound/Fe(II) and ultrasound/H2O2 combinations. Ultrason Sonochem 16:593–598. https://doi.org/10.1016/j.ultsonch.2008.11.006
Ghodbane H, Hamdaoui O, Vandamme J, Van Durme J, Vanraes P, Leys C, Nikiforov AY (2015) Degradation of AB25 dye in liquid medium by atmospheric pressure non-thermal plasma and plasma combination with photocatalyst TiO2. Open Chem 13:325–331. https://doi.org/10.1515/chem-2015-0040
Gligorovski S, Strekowski R, Barbati S, Vione D (2018) Addition and correction to environmental implications of hydroxyl radicals (•OH). Chem Rev 118:2296. https://doi.org/10.1021/ACS.CHEMREV.7B00742
Gong J, Duan D, Yang Y, Ran Y, Chen D (2016) Seasonal variation and partitioning of endocrine disrupting chemicals in waters and sediments of the pearl river system, south china. Environ Pollut 219:735–741. https://doi.org/10.1016/J.ENVPOL.2016.07.015
Gonzalez DH, Kuang XM, Scott JA, Rocha GO, Paulson SE (2018) Terephthalate probe for hydroxyl radicals: Yield of 2-hydroxyterephthalic acid and transition metal interference. Anal Lett 51:2488–2497. https://doi.org/10.1080/00032719.2018.1431246
Gonzalez-Rey M, Tapie N, Le Menach K, Dévier MH, Budzinski H, Bebianno MJ (2015) Occurrence of pharmaceutical compounds and pesticides in aquatic systems. Mar Pollut Bull 96:384–400. https://doi.org/10.1016/J.MARPOLBUL.2015.04.029
Gorbanev Y, Privat-Maldonado A, Bogaerts A (2018) Analysis of short-lived reactive species in plasma-air-water systems: the dos and the do nots. Anal Chem 90:13151–13158. https://doi.org/10.1021/acs.analchem.8b03336
Hamdan A, Profili J, Cha MS (2020) Microwave plasma jet in water: effect of water electrical conductivity on plasma characteristics. Plasma Chem Plasma Process 40:169–185. https://doi.org/10.1007/s11090-019-10034-5
Hayyan M, Hashim MA, Alnashef IM (2016) Superoxide ion: generation and chemical implications. Chem Rev 116:3029–3085. https://doi.org/10.1021/acs.chemrev.5b00407
Huerta-Fontela M, Galceran MT, Ventura F (2011) Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res 45:1432–1442. https://doi.org/10.1016/J.WATRES.2010.10.036
Itikawa Y, Mason N (2005) Cross sections for electron collisions with water molecules. J Phys Chem Ref Data 34:1–22. https://doi.org/10.1063/1.1799251
Lewis J, Thomas Joyce A, Hadaya M, Ebrahimi F, Dragiev I, Giardetti N, Yang J, Fridman G, Rabinovich A, Fridman A, McKenzie R, EM Sales C (2020) Rapid degradation of PFAS in aqueous solutions by reverse vortex flow gliding arc plasma. Environ Sci Water Res Technol 6:1044–1057. https://doi.org/10.1039/C9EW01050E
Jose J, Philip L (2019) Degradation of chlorobenzene in aqueous solution by pulsed power plasma: mechanism and effect of operational parameters. J Environ Chem Eng 7:103476. https://doi.org/10.1016/j.jece.2019.103476
Joseph CG, Li Puma G, Bono A, Krishnaiah D (2009) Sonophotocatalysis in advanced oxidation process: a short review. Ultrason Sonochem 16:583–589. https://doi.org/10.1016/J.ULTSONCH.2009.02.002
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2008) The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res 42:3498–3518. https://doi.org/10.1016/j.watres.2008.04.026
Kehrer JP, Robertson JD, Smith CV (2010) Free radicals and reactive oxygen species. Compr Toxicol Second Ed 1–14:277–307. https://doi.org/10.1016/B978-0-08-046884-6.00114-7
Khetan SK, Collins TJ (2007) Human pharmaceuticals in the aquatic environment: a challenge to green chemisty. Chem Rev 107:2319–2364. https://doi.org/10.1021/cr020441w
Kim U-J, Kannan K (2018) Occurrence and distribution of organophosphate flame retardants/plasticizers in surface waters, tap water, and rainwater: Implications for human exposure. Environ Sci Technol 52:5625–5633. https://doi.org/10.1021/ACS.EST.8B00727
Klamerth N, Malato S, Maldonado MI, Agüera A, Fernández-Alba AR (2010) Application of photo-fenton as a tertiary treatment of emerging contaminants in municipal wastewater. Environ Sci Technol 44:1792–1798. https://doi.org/10.1021/es903455p
Kleywegt S, Pileggi V, Yang P, Hao C, Zhao X, Rocks C, Thach S, Cheung P, Whitehead B (2011) Pharmaceuticals, hormones and bisphenol A in untreated source and finished drinking water in Ontario, Canada—occurrence and treatment efficiency. Sci Total Environ 409:1481–1488. https://doi.org/10.1016/j.scitotenv.2011.01.010
Kondeti VSSK, Phan CQ, Wende K, Jablonowski H, Gangal U, Granick JL, Hunter RC, Bruggeman PJ (2018) Long-lived and short-lived reactive species produced by a cold atmospheric pressure plasma jet for the inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Free Radic Biol Med 124:275–287. https://doi.org/10.1016/j.freeradbiomed.2018.05.083
Krause H, Schweiger B, Schuhmacher J, Scholl S, Steinfeld U (2009) Degradation of the endocrine disrupting chemicals (EDCs) carbamazepine, clofibric acid, and iopromide by corona discharge over water. Chemosphere 75:163–168. https://doi.org/10.1016/J.CHEMOSPHERE.2008.12.020
Le Thi MT, Nguyen Phuoc D, Dinh Quoc T, Ngo HH, Do Hong Lan C (2016) Presence of e-EDCs in surface water and effluents of pollution sources in Sai Gon and Dong Nai river basin. Sustain Environ Res 26:20–27. https://doi.org/10.1016/J.SERJ.2015.09.001
Lee S, Jeong W, Kannan K, Moon HB (2016) Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res 103:182–188. https://doi.org/10.1016/J.WATRES.2016.07.034
Lee J, Lee CW, Yong HI, Lee HJ, Jo C, Jung S (2017) Use of atmospheric pressure cold plasma for meat industry. Korean J Food Sci Anim Resour 37:477–485. https://doi.org/10.5851/kosfa.2017.37.4.477
Lei M, Zhang L, Lei J, Zong L, Li J, Wu Z, Wang Z (2015) Overview of emerging contaminants and associated human health effects. Biomed Res Int 2015:1–12. https://doi.org/10.1155/2015/404796
Lin X, Xu J, Keller AA, He L, Gu Y, Zheng W, Sun D, Lu Z, Huang J, Huang X, Li G (2020) Occurrence and risk assessment of emerging contaminants in a water reclamation and ecological reuse project. Sci Total Environ 744:140977. https://doi.org/10.1016/j.scitotenv.2020.140977
Liu Y, Zhang H, Sun J, Liu J, Shen X, Zhan J, Zhang A, Ognier S, Cavadias S, Li P (2018) Degradation of aniline in aqueous solution using non-thermal plasma generated in microbubbles. Chem Eng J 345:679–687. https://doi.org/10.1016/J.CEJ.2018.01.057
Locke BR, Thagard SM (2012) Analysis and review of chemical reactions and transport processes in pulsed electrical discharge plasma formed directly in liquid water. Plasma Chem Plasma Process 32:875–917. https://doi.org/10.1007/s11090-012-9403-y
Magureanu M, Mandache NB, Parvulescu VI (2015) Degradation of pharmaceutical compounds in water by non-thermal plasma treatment. Water Res 81:124–136. https://doi.org/10.1016/j.watres.2015.05.037
Magureanu M, Piroi D, Mandache NB, David V, Medvedovici A, Bradu C, Parvulescu VI (2011) Degradation of antibiotics in water by non-thermal plasma treatment. Water Res 45:3407–3416. https://doi.org/10.1016/j.watres.2011.03.057
Mahamuni NN, Adewuyi YG (2010) Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: a review with emphasis on cost estimation. Ultrason Sonochem 17:990–1003. https://doi.org/10.1016/J.ULTSONCH.2009.09.005
Mal E (2019) Laser induced plasma studies on W, Mo and alloys of Cu in air via time and space resolved LIBS and application in single line calibration free -LIBS. Doctoral dissertation, Indian Institute of Technology Guwahati
Mededovic S, Locke BR (2007) Side-chain degradation of atrazine by pulsed electrical discharge in water. Ind Eng Chem Res 46:2702–2709. https://doi.org/10.1021/IE070020A
Mitra S, Nguyen LN, Akter M, Park G, Choi EH, Kaushik NK (2019) Impact of ROS generated by chemical, physical, and plasma techniques on cancer attenuation. Cancers 11: 1030 11:1030 . https://doi.org/10.3390/CANCERS11071030
Mondal S, Purkait MK, De S (2018) Advances in dye removal technologies. Springer Singapore, Singapore
Montes-Grajales D, Fennix-Agudelo M, Miranda-Castro W (2017) Occurrence of personal care products as emerging chemicals of concern in water resources: a review. Sci Total Environ 595:601–614. https://doi.org/10.1016/J.SCITOTENV.2017.03.286
Mozetiˇc M, Vesel A, Gregor P, Zaplotnik R (2019) Introduction to plasma and plasma diagnostics. In: Non-thermal plasma technology for polymeric materials, 1st ed. Elsevier, p 459
Nath A, Vendan SE, Priyanka JKS, Singh CK, Kumar S (2014) Carcinogenic pesticides residue detection in cow milk and water samples from Patna, India. Curr Trends Biotechnol Chem Res 3:1–7
Nippatla N, Philip L (2019) Electrocoagulation-floatation assisted pulsed power plasma technology for the complete mineralization of potentially toxic dyes and real textile wastewater. Process Saf Environ Prot 125:143–156. https://doi.org/10.1016/J.PSEP.2019.03.012
Nosaka Y, Nosaka AY (2017) Generation and detection of reactive oxygen species in photocatalysis. Chem Rev 117:11302–11336. https://doi.org/10.1021/acs.chemrev.7b00161
Pankaj SK, Wan Z, Keener KM (2018) Effects of cold plasma on food quality: a review. Foods 7:1–21. https://doi.org/10.3390/foods7010004
Pankaj SK, Wan Z, Colonna W, Keener KM (2017) Degradation kinetics of organic dyes in water by high voltage atmospheric air and modified air cold plasma. Water Sci Technol 76:567–574. https://doi.org/10.2166/wst.2017.169
Panomsuwan G, Morishita T, Kang J, Rujiravanit R, Ueno T, Saito N (2016) Degradation of synthetic dye in water by solution plasma process. J Korean Soc Mar Eng 40:888–893. https://doi.org/10.5916/jkosme.2016.40.10.888
Parvulescu VI, Magureanu M, Lukes P (2012) Plasma chemistry and catalysis in gases and liquids. Plasma Chem Catal Gases Liq. https://doi.org/10.1002/9783527649525
Peleg M (1976) The chemistry of ozone in the treatment of water. Water Res 10:361–365. https://doi.org/10.1016/0043-1354(76)90052-X
Petrie B, Barden R, Kasprzyk-Hordern B (2015) A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res 72:3–27. https://doi.org/10.1016/j.watres.2014.08.053
Picó Y, Campo J, Alfarhan AH, El-Sheikh MA, Barceló D (2021) A reconnaissance study of pharmaceuticals, pesticides, perfluoroalkyl substances and organophosphorus flame retardants in the aquatic environment, wild plants and vegetables of two Saudi Arabia urban areas: environmental and human health risk assessment. Sci Total Environ 776:145843. https://doi.org/10.1016/J.SCITOTENV.2021.145843
Qiao T, Zhengrong Yu, Xihui Zhang TAuDW (2011) Occurrence and fate of pharmaceuticals and personal care products in drinking water in southern China. J Environ Monit 13:3097–3103. https://doi.org/10.1039/C1EM10318K
Radwan EK, Ibrahim MBM, Adel A, Farouk M (2019) The occurrence and risk assessment of phenolic endocrine-disrupting chemicals in Egypt’s drinking and source water. Environ Sci Pollut Res 272(27):1776–1788. https://doi.org/10.1007/S11356-019-06887-0
Raji A, Pandiyaraj KN, Vasu D, Ramkumar MC, Deshmukh RR, Kandavelu V (2020) Non-equilibrium atmospheric pressure plasma assisted degradation of the pharmaceutical drug valsartan: Influence of catalyst and degradation environment. RSC Adv 10:35709–35717. https://doi.org/10.1039/d0ra05608a
Rancev S, Petrovic M, Bojic A, Radivojevic D, Maluckov C, Radovic M (2018) Degradation of reactive orange 16 using a prototype atmospheric-pressure non-thermal plasma reactor. Facta Univ Ser Phys Chem Technol 16:285–295. https://doi.org/10.2298/fupct1803285r
Rathi BS, Kumar PS, Show PL (2021) A review on effective removal of emerging contaminants from aquatic systems: current trends and scope for further research. J Hazard Mater 409:124413. https://doi.org/10.1016/j.jhazmat.2020.124413
Reddy PMK, Subrahmanyam C (2012) Green approach for wastewater treatment—degradation and mineralization of aqueous organic pollutants by discharge plasma. Ind Eng Chem Res 51:11097–11103. https://doi.org/10.1021/IE301122P
Reddy P, Rama Raju B, Karuppiah J, Linga Reddy E, Subrahmanyam C (2013) Degradation and mineralization of methylene blue by dielectric barrier discharge non-thermal plasma reactor. Chem Eng J 217:41–47. https://doi.org/10.1016/j.cej.2012.11.116
Rezaei F, Cristoforetti G, Tognoni E, Legnaioli S, Palleschi V, Safi A (2020) A review of the current analytical approaches for evaluating, compensating and exploiting self-absorption in laser induced breakdown spectroscopy. Spectrochim Acta Part B At Spectrosc 169:105878. https://doi.org/10.1016/j.sab.2020.105878
Ribeiro AR, Nunes OC, Pereira MFR, Silva AMT (2015) An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched directive 2013/39/EU. Environ Int 75:33–51. https://doi.org/10.1016/j.envint.2014.10.027
Rout PR, Zhang TC, Bhunia P, Surampalli RY (2021) Treatment technologies for emerging contaminants in wastewater treatment plants: a review. Sci Total Environ 753:141990. https://doi.org/10.1016/j.scitotenv.2020.141990
Saidulu D, Gupta B, Gupta AK, Ghosal PS (2021) A review on occurrences, eco-toxic effects, and remediation of emerging contaminants from wastewater: special emphasis on biological treatment based hybrid systems. J Environ Chem Eng 9:105282. https://doi.org/10.1016/j.jece.2021.105282
Salimi M, Esrafili A, Gholami M, Jafari AJ, Kalantary RR, Farzadkia M, Kermani M, Sobhi HR (2017) Contaminants of emerging concern: a review of new approach in AOP technologies. Environ Monit Assess 1898(189):1–22 . https://doi.org/10.1007/S10661-017-6097-X
Samukawa S, Hori M, Rauf S, Tachibana K, Bruggeman P, Kroesen G, Whitehead JC, Murphy AB, Gutsol AF, Starikovskaia S, Kortshagen U, Boeuf JP, Sommerer TJ, Kushner MJ, Czarnetzki U, Mason N (2012) The 2012 plasma roadmap. J Phys D Appl Phys 45. https://doi.org/10.1088/0022-3727/45/25/253001
Sarangapani C, Misra NN, Milosavljevic V, Bourke P, O’Regan F, Cullen PJ (2016) Pesticide degradation in water using atmospheric air cold plasma. J Water Process Eng 9:225–232. https://doi.org/10.1016/J.JWPE.2016.01.003
Sathishkumar P, Mangalaraja RV, Anandan S (2016) Review on the recent improvements in sonochemical and combined sonochemical oxidation processes—a powerful tool for destruction of environmental contaminants. Renew Sustain Energy Rev 55:426–454. https://doi.org/10.1016/j.rser.2015.10.139
Shang K, Wang X, Li J, Wang H, Lu N, Jiang N, Wu Y (2017) Synergetic degradation of acid orange 7 (AO7) dye by DBD plasma and persulfate. Chem Eng J 311:378–384. https://doi.org/10.1016/j.cej.2016.11.103
Shashurin A, Keidar M, Bronnikov S, Jurjus RA, Stepp MA (2008) Living tissue under treatment of cold plasma atmospheric jet. Appl Phys Lett 93:181501. https://doi.org/10.1063/1.3020223
Shimizu K, Muramatsu S, Sonoda T, Blajan M, Supply APP (2010) Water treatment by low voltage discharge in water. Int J Plasma Environ Sci Technol 4:58–64
Shin WT, Mirmiran A, Yiacoumi S, Tsouris C (1999) Ozonation using microbubbles formed by electric fields. Sep Purif Technol 15:271–282. https://doi.org/10.1016/S1383-5866(98)00107-5
Singh KP, Rai P, Singh AK, Verma P (2014) Gupta S (2014) Occurrence of pharmaceuticals in urban wastewater of north Indian cities and risk assessment. Environ Monit Assess 18610(186):6663–6682. https://doi.org/10.1007/S10661-014-3881-8
Singh RK, Philip L, Ramanujam S (2016) Rapid removal of arbofuran from aqueous solution by pulsed corona discharge treatment: kinetic study, oxidative, reductive degradation pathway, and toxicity assay. Ind Eng Chem Res 55:7201–7209. https://doi.org/10.1021/ACS.IECR.6B01191
Slamani S, Abdelmalek F, Ghezzar MR, Addou A (2018) Initiation of Fenton process by plasma gliding arc discharge for the degradation of paracetamol in water. J Photochem Photobiol A Chem 359:1–10. https://doi.org/10.1016/J.JPHOTOCHEM.2018.03.032
Snoeckx R, Bogaerts A (2017) Plasma technology-a novel solution for CO2 conversion? Chem Soc Rev 46:5805–5863. https://doi.org/10.1039/c6cs00066e
Song Y, Cai S, Zhang W (2000) Rapid determination of the ozone in water. J Hyg Res 29:151–153
Sorensen JPR, Lapworth DJ, Nkhuwa DCW, Stuart ME, Gooddy DC, Bell RA, Chirwa M, Kabika J, Liemisa M, Chibesa M, Pedley S (2015) Emerging contaminants in urban groundwater sources in Africa. Water Res 72:51–63. https://doi.org/10.1016/J.WATRES.2014.08.002
Sugiarto AT, Sato M (2001) Pulsed plasma processing of organic compounds in aqueous solution. Thin Solid Films 386:295–299. https://doi.org/10.1016/S0040-6090(00)01669-2
Sun B, Aye NN, Gao Z, Lv D, Zhu X, Sato M (2012) Characteristics of gas-liquid pulsed discharge plasma reactor and dye decoloration efficiency. J Environ Sci 24:840–845. https://doi.org/10.1016/S1001-0742(11)60837-1
Tampieri F, Ginebra MP, Canal C (2021) Quantification of plasma-produced hydroxyl radicals in solution and their dependence on the pH. Anal Chem 93:3666-3670. https://doi.org/10.1021/acs.analchem.0c04906
Tas MA, Van Hardeveld R, Van Veldhuizen EM (1997) Reactions of NO in a positive streamer corona plasma. Plasma Chem Plasma Process 17:371–391. https://doi.org/10.1023/A:1021818313047
Tian W, Kushner MJ (2014) Atmospheric pressure dielectric barrier discharges interacting with liquid covered tissue. J Phys D Appl Phys 47. https://doi.org/10.1088/0022-3727/47/16/165201
Vanraes P, Bogaerts A (2018) Plasma physics of liquids—a focused review. Appl Phys Rev 5:031103. https://doi.org/10.1063/1.5020511
Vanraes P, Nikiforov, Anton Y, Christophe L (2016) Electrical discharge in water treatment technology for micropollutant decomposition. In: Mieno T (ed) Plasma Science and Technology: Progress in Physical States and Chemical Reactions. InTech, pp 457–506
Vulliet E, Cren-Olivé C, Grenier-Loustalot M-F (2009) Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters. Environ Chem Lett 91(9):103–114 .https://doi.org/10.1007/S10311-009-0253-7
Wang H, Li J, Quan X, Wu Y (2008) Enhanced generation of oxidative species and phenol degradation in a discharge plasma system coupled with TiO2 photocatalysis. Appl Catal B Environ 83:72–77. https://doi.org/10.1016/J.APCATB.2008.02.004
Wang Y, Sun J, Wang D (2013) Modelling of atmospheric pressure plasmas. Low Temp Plasma Technol 61–96. https://doi.org/10.1201/b15153
Wang J, Sun Y, Feng J, Xin L, Ma J (2016) Degradation of triclocarban in water by dielectric barrier discharge plasma combined with TiO2/activated carbon fibers: effect of operating parameters and byproducts identification. Chem Eng J 300:36–46. https://doi.org/10.1016/J.CEJ.2016.04.041
Wang J, Simeni Simeni M, Rong M, Bruggeman P (2021) Absolute OH density and gas temperature measurements by laser induced fluorescence in a microsecond pulsed discharge generated in a conductive NaCl solution. Plasma Sourc Sci Technol 30:075016. https://doi.org/10.1088/1361-6595/abf71c
Wang X, Luo J, Huang Y, Mei J, Chen Y (2021) Degradation of pharmaceutical contaminants by bubbling gas phase surface discharge plasma combined with g-C 3 N 4 photocatalysis. Environ Sci Water Res Technol 7:610–621. https://doi.org/10.1039/D0EW00985G
Wee SY, Aris AZ, Yusoff FM, Praveena SM (2020) Occurrence of multiclass endocrine disrupting compounds in a drinking water supply system and associated risks. Sci Rep 101 10:1–12. https://doi.org/10.1038/s41598-020-74061-5
WHO (2019) Drinking-water. https://www.who.int/en/news-room/fact-sheets/detail/drinking-water. Accessed 1 Aug 2021
Williams M, Kookana RS, Mehta A, Yadav SK, Tailor BL, Maheshwari B (2019) Emerging contaminants in a river receiving untreated wastewater from an Indian urban centre. Sci Total Environ 647:1256–1265. https://doi.org/10.1016/J.SCITOTENV.2018.08.084
Wu T, Liu G, Zhao J, Hidaka H, Serpone N (1999) Evidence for H2O2 generation during the TiO2-assisted photodegradation of dyes in aqueous dispersions under visible light illumination. J Phys Chem B 103:4862–4867. https://doi.org/10.1021/jp9846678
Wu H, Song Q, Ran G, Lu X, Xu B (2011) Recent developments in the detection of singlet oxygen with molecular spectroscopic methods. TrAC Trends Anal Chem 30:133–141. https://doi.org/10.1016/j.trac.2010.08.009
Wu L, Xie Q, Lv Y, Wu Z, Liang X, Lu M, Nie Y (2019) Degradation of methylene blue via dielectric barrier discharge plasma treatment. Water (Switzerland) 11. https://doi.org/10.3390/w11091818
Wu H, Fan J, Liu F, Shu L, Yin B (2021) Degradation of tetracycline in aqueous solution by persulphate assisted gas-liquid dielectric barrier discharge. Water Environ J 35:902–912. https://doi.org/10.1111/wej.12678
Yamatake A, Fletcher J, Yasuoka K, Ishii S (2006) Water treatment by fast oxygen radical flow with dc-driven microhollow cathode discharge. IEEE Trans Plasma Sci 34:1375–1381. https://doi.org/10.1109/TPS.2006.877249
Yamazaki E, Yamashita N, Taniyasu S, Lam J, Lam PKS, Moon HB, Jeong Y, Kannan P, Achyuthan H, Munuswamy N, Kannan K (2015) Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol Environ Saf 122:565–572. https://doi.org/10.1016/J.ECOENV.2015.09.029
Yan JH, Liu YN, Bo Z, Li XD, Cen KF (2008) Degradation of gas–liquid gliding arc discharge on Acid Orange II. J Hazard Mater 157:441–447. https://doi.org/10.1016/J.JHAZMAT.2008.01.007
York N (2014) Water quality monitoring data for pesticides on long island, NY, 1–172
Yuri, P R, John, E A, V, I K (1997) Gas discharge Physics, 1st ed. Springer-verlag
Zangouei M, Haynes BS (2019) The role of atomic oxygen and ozone in the plasma and post-plasma catalytic removal of N2O. Plasma Chem Plasma Process 39:89–108. https://doi.org/10.1007/s11090-018-9926-y
Zhang Y, Qin P, Lu S, Liu X, Zhai J, Xu J, Wang Y, Zhang G, Liu X (2020) Wan Z (2020) Occurrence and risk evaluation of organophosphorus pesticides in typical water bodies of Beijing. China. Environ Sci Pollut Res 282(28):1454–1463. https://doi.org/10.1007/S11356-020-10288-Z
Zhao Y, Kuang J, Zhang S, Li X, Wang B, Huang J, Deng S, Wang Y, Yu G (2017) Ozonation of indomethacin: Kinetics, mechanisms and toxicity. J Hazard Mater 323:460–470. https://doi.org/10.1016/J.JHAZMAT.2016.05.023
Zhao C, Xue L, Zhou Y, Zhang Y, Huang K (2021) A microwave atmospheric plasma strategy for fast and efficient degradation of aqueous p-nitrophenol. J Hazard Mater 409:124473 . https://doi.org/10.1016/j.jhazmat.2020.124473
Zhou R, Zhang T, Zhou R, Mai-Prochnow A, Ponraj SB, Fang Z, Masood H, Kananagh J, McClure D, Alam D, Ostrikov K (Ken), Cullen PJ (2021) Underwater microplasma bubbles for efficient and simultaneous degradation of mixed dye pollutants. Sci Total Environ 750:142295. https://doi.org/10.1016/J.SCITOTENV.2020.142295
Acknowledgements
Authors gratefully acknowledge the Government of India, Ministry of Science and Technology, Department of Science and Technology (DST), Technology Mission Division [Grant no: DST/TM/WTI/WIC/2K17/82(C)] and SERB-DST New Delhi, Government of India for financial support through Grant Nos. CRG/2018/000419 and CVD/2020/000458 for supporting the work. All the authors also thank IIT Tirupati for the support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Allabakshi, S.M., Srikar, P.S.N.S.R., Gangwar, R.K., Maliyekkal, S.M. (2022). Application of Plasma-Assisted Advanced Oxidation Processes for Removal of Emerging Contaminants in Water. In: P. Singh, S., Agarwal, A.K., Gupta, T., Maliyekkal, S.M. (eds) New Trends in Emerging Environmental Contaminants. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-16-8367-1_15
Download citation
DOI: https://doi.org/10.1007/978-981-16-8367-1_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8366-4
Online ISBN: 978-981-16-8367-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)