Abstract
Food safety and quality is one of the scientific disciplines that have gained the concern and attention of people. For the most part, food safety tends to be considered by end consumers first before the nutrients provided by the food, as food contaminants can have a significant, direct and often irreversible health effect. There is, therefore, a need for rapid and on-site sensing technologies or devices to be established to rapidly identify and sort contaminated foods. The inability to rapidly identify and isolate infected food from the food supply chain will lead to foodborne disease outbreaks. After consuming these contaminated foods, about 10% of the world’s population gets sick, according to the WHO. Consequently, this issue promotes the design and development of miniaturized (portable), cost-effective and, at the same time, sensitive, precise, and reliable technologies and methods for the identification of food contaminants such as acrylamides, microbes, heavy metals, food preservatives, toxins, pesticides, and antibiotics. These food contaminants are foreign substances commonly contained in consumable food products that, after their absorption into the human body, could potentially cause short- and long-term health effects. Different detection technologies and instruments for food quality assessment, such as chromatography, spectroscopy, magnetic, electrochemical, and optical methods, are available to date. Optical methods of detection that have been developed in recent years to identify targeted food contaminants have been studied in this chapter. Analytical performance such as LOD, LOQ, linear range and time of analysis, detection cost, advantages, disadvantages, and limitations of different approaches to optical sensing are presented. The comparison between different types of optical sensing approaches are outlined as well.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
The rise in living standards has led to the need to develop rapid and on-site advanced sensing technologies to ensure food safety and quality, beginning from the production line, packaging and distribution to the end consumer. Food safety and quality have become the criteria for consumers to think about before making any purchases in the last few decades. It has also been one of the variables to be checked before making decisions by the food manufacturers and policymakers. To assess our level of health, the amount and types of food we eat are essential factors. It impacts the development and function of our body’s hormones, enzymes, and other metabolic process regulators. Food safety and quality has since been a concern of scientists and the public until today, as food quality is closely related to health quality and unsafe food poses global threats to health.
Carcinogens, dangerous pathogens (bacteria, viruses, parasites), heavy metals, chemical compounds, toxins, pesticides, and other substances that could cause various diseases ranging from diarrhea to cancer are found in unsafe or contaminated foods. Nearly 1 in 10 individuals in the world get sick after eating contaminated food, according to the World Health Organization (WHO), and about 42,000 die every year (WHO 2020). The most common foodborne disease is diarrheal disease, which causes 550 million people to fall sick and about 230,000 die each year (WHO 2020). Foodborne disease has created a problem for health care and creates an economic burden worldwide. As the world’s population is growing exponentially, the growing demand for food could pose a challenge to food safety due to the escalation of industrialization in agriculture and meat production. Food can become contaminated at all of these points in the food supply chain, beginning from the food production line, the transport and distribution of food, the storage of food up to the handling of food in the kitchen, if no precautionary measures have been taken.
People have been grappling with food safety problems dating back three decades, including the appearance of novel foodborne illnesses, uncertain long-term effects of genetically modified foods (GMOs), adulterated foods, the presence of food contaminants, and food spoilage. A consistent and efficient quality management approach, or regular screening, should be developed and enforced to ensure food safety and food quality for customers. There are several up-to-date methods for detecting target contaminants in foods, ranging from laborious and time-consuming traditional bacterial detection methods based on culture, colony counting, chromatography and immunoassay, amplification based on nuclear acid sequence, polymerase chain reaction (PCR) to non-destructive instrumental imaging techniques such as optical biosensors, electrochemical biosensors, and spectroscopy (Bhardwaj et al. 2017; Hameed et al. 2018).
The emphasis of this chapter will be on the occurrence of these different target contaminants in food and an overview of available optical detection methods, including attractive optical biosensors, certain optical detection methods based on spectroscopy, and other advanced techniques used in optical detection to trace the existence of target contaminants in food and reduce the incidence of foodborne diseases. Optical detection can be defined as a non-destructive, automated technique focused on the interaction of light and matter for food quality evaluation, resulting in the conversion of incoming light rays (optical energy) into electronic signals. Eventually, the presence of contaminants in food can be determined and quantified through examination, analysis, and interpretation of the visual images and spectral characteristics provided by an integrated measuring system. The operating theory of an optical detector is that the targets from an emitting diode will reflect or disrupt the coming light beam. A measuring system responds to the changes to the light beam and interprets them for information. For food safety and quality assurance, because of its sensitivity, precision, and performance, optical detection is often a better option than sensory evaluation or traditional bacterial detection.
The contaminated food issue, following the alarming statistics announced by the WHO, prompts the production and design of compact, cost-effective, responsive, quick and shortest-time-as-possible technologies and methods for detecting these food contaminants as the rise in population year by year will exacerbate the condition if the speed of contaminant detection and separation could not be preserved. There are many up-to-date techniques available for detecting food contaminants, such as chromatography, spectroscopy, magnetic, and electrochemical methods. This chapter studies optical detection methods for food contaminants (carcinogens, microbes, heavy metals, food preservatives, toxins, pesticides, and antibiotics) that have been established in recent years. Carefully discussed are the analytical performance (LOD, LOQ, linear range, analysis time), cost, advantages, disadvantages, and limitations of different optical detection methods for the food contaminants mentioned above. The comparison between different types of methods of optical detection as a whole is also outlined.
6.2 Food Contaminants: Sources, Removal Problems, and Effective Corrective Approaches
Carcinogenic substances in food can be classified into two classes, genotoxic and non-genotoxic. Genotoxic carcinogens are chemicals that can cause DNA damage, lead to cell mutation, and thus increase the risk of tumors. It is dangerous and presents a risk of cancer even when exposed to a very low dose, so there is no threshold value or dose deemed safe for exposure to genotoxic carcinogens. Natural food constituents such as ethyl carbamate, phytotoxins or contaminants such as acrylamide (AA), heterocyclic amines (HCAs), polycyclic aromatic hydrocarbons (PAHs), and products of cholesterol oxidation (COPs) may be genotoxic carcinogens found in the diet (Cuevas-González et al. 2020). On the contrary, non-genotoxic carcinogens is assumed to have a threshold dose at which concentration surpass the threshold value would induce cancers through diverse underlying mechanisms including cell proliferation, tumor promoters, cytotoxicity, endocrine modifiers, receptor mediators, or immunosuppressants other than mutation in the previous case (Hartwig et al. 2020; Hernández et al. 2009; Nohmi 2018). Phenobarbital, carbon tetrachloride, diethylstilbestrol, and others are the examples of non-genotoxic carcinogens (Nohmi 2018).
Most of the dietary carcinogens evolved during the process of food storage, preparation or processing, while some of the dietary carcinogens may be the natural constituents of the food (Cuevas-González et al. 2020). Food thermal treatment is known to produce carcinogens by accelerating lipid and protein oxidative reactions due to increased production of free radicals that react rapidly with atmospheric oxygen and decreased protection of food antioxidants. It is important to note that in addition to giving the desirable aromas, colors, and flavors to the food, different carcinogenic compounds could be produced in the single thermal treatment of food. Therefore, decreasing dietary carcinogens while preserving the organoleptic properties and palatability of food is a significant challenge for the food industry (Koszucka and Nowak 2018).
Food serves as a medium for microbes’ growth, but not all of it is harmful. For fermentation, some microbes such as fungi and probiotic bacteria are beneficial, giving the food stronger organoleptic properties. Yet certain bacteria, such as Escherichia coli (E. coli) O157:H7, Staphylococcus aureus (S. aureus), Salmonella sp., Listeria monocytogenes, Clostridium perfringens, Campylobacter jejuni, Vibrio sp., Clostridium botulinum, Bacillus sp., Shigella sp. and Streptococcus pyogenes are responsible for food poisoning (Bhardwaj et al. 2017; Mukama et al. 2017). Various food bacteria, including both Gram-positive and Gram-negative, can secrete toxins that cause foodborne infection, from less severe gastrointestinal disorder to more severe paralysis and even death (Abebe et al. 2020).
The high survivability and adaptability characteristics of microbes allow them to grow and reproduce in different environments, so the eradication of microbes is a challenge to the food industry. Since at different stages and phases the microbes can reach the food chain and food distribution line, it poses a new food safety problem. Some foodborne microbial pathogens manifest diseases within a few days, while others, in some cases, remain latent in the body after consumption of microbial-contaminated foods prior to disease manifestation. Although rigorously heat-sterilizing the food could kill the microbial environment, the taste and texture of the food could likely alternate at the same time. Nevertheless, some pathogens, especially in microbial-contaminated pork or bacon, are not killed even after being subjected to high-temperature cooking. Those three S. typhimurium (ATCC 14028, I33, and I116 strain), S. derby B4373, S. Potsdam I133, S. Menston I79, S. eppendorf I66, and S. kingston I124 are examples of these high heat resistant pathogen strains that could be contained in pork meat (Quintavalla et al. 2001). That is why Muslims refuse to consider non-halal pork. In addition, the emergence of antibiotic resistance bacteria is also one of the global threats that contributes to prolonged hospitalization and increased mortality that should not be undermined. Therefore, the growth of multi-drug resistant bacteria has also contributed to the development of rapid and efficient bacteria detection techniques (Locke et al. 2020).
One of the commonly debated concerns is also the inclusion of heavy metals in food because heavy metals are non-biodegradable, toxic even at small concentrations and do not fully excrete from the human body (Sharma et al. 2018). The source of heavy metals in food may be heavy metal accumulation in the soil where crops are grown (Sharma et al. 2018), or contamination of the abiotic ecosystem in which the animal origins are produced, causing bioaccumulation across various tropical levels along the food chain (Ali and Khan 2018), or the leaching of heavy metals from cookware and foodstuffs during food preparation and storage (Aderemi et al. 2017). Long-term health problems such as improper function of endocrine glands, hypertension, neurological disorders, respiratory diseases, immunological disorders, and others could be caused by the ingestion of food containing heavy metals (Sharma et al. 2018).
Heavy metal transmission from soil-food crops is the contributing factor for the presence of heavy metals in food. The presence of heavy metals in the soil matrix has a few sources; it may be due to atmospheric deposition, animal manure, irrigation with wastewater or polluted sewage, and pesticides and herbicides containing heavy metals. In addition, heavy metals from point sources such as thermal power plants, coal or gold mining, textile industries, and others may also contaminate the soil. Improper discharge of waste material (sludge) or wastewater containing heavy metals may disrupt the soil environment and the food safety problem with respect to heavy metals is therefore one of the challenges to address. Reliable and effective sewage water treatment is critical for reducing heavy metal leaching to food crops from wastewater discharged from factories. Remote real-time sensing is required in order to track agricultural activities from any heavy metal contaminated soils and water irrigation system. In addition, stringent regulatory implementation should be enforced to prevent any irresponsible or illegal disposal of industrial discharges.
Food preservatives are substances used to protect food by the prevention or suppression or delay of changes induced by microorganisms and oxidation reactions, resulting in spoilage. Tert-butylhydroquinone (TBHQ) is a strong phenolic antioxidant added to prevent oxidative deterioration. The addition of this compound to the food does not cause the color, taste, or odor to change. The optimal daily intake of TBHQ is 0–0.2 mg/kg of body weight, according to the Joint FAO/WHO (Balram et al. 2021). TBHQ is used in numerous food products such as unsaturated vegetable oils, animal fats, and meat products as well as cosmetic products. Overdose intake of TBHQ can result in vision disturbances, contact dermatitis, medullary paralysis, seizures, and potential immune system damage (Balram et al. 2021). Other common preservatives used in food packaging, cosmetics, biodiesel, and pharmaceutical preparation to prevent the oxidation process are butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT). The reasonable daily intake of BHA via food is 0–0.5 mg/kg/kg, according to the Joint FAO/WHO (Manoranjitham and Narayanan 2020). While for BHT, the acceptable daily intake is 0.25 mg/kg according to the European Food Safety and Authority (EFSA) (Wang and Kannan 2019). Excessive intake of BHA may cause cellular damage and proliferation in the fore stomach and promote cancer formation (Williams et al. 1999). While for BHT, it might exert adverse effect on the lungs, kidneys, myocardial cells, lipid metabolism in the liver and others (Babich 1982). Other common preservatives used in drinks, foods, cosmetics, personal hygiene products, and pharmaceuticals are benzoic acid and parabens (4-hydroxybenzoic acid esters). Due to their carcinogenic nature, benzoic acids have attracted public attention (Balram et al. 2021).
The Staphylococcus aureus bacterium is commonly found in foods that have been improperly handled and subsequently stored at a high temperature, such as ham, meats, and dairy products, as S. aureus has high salt tolerance, high heat resistance and is not easily destroyed through cooking. Staphylococcal entertotoxins (SEs) secreted by S. aureus are the major cause of staphylococcal food poisoning (SFP) (Argudín et al. 2010; Denayer et al. 2017; Permyakov et al. 2017) (Locke et al. 2020). An effective and rapid detection technique should therefore be established in order to detect the presence of toxins in food before it reaches the end consumers.
The presence of pesticide residue in the food could be highly toxic to human health. Pesticides are widely used in agriculture to destroy or control pests. It has been estimated that less than 0.01% of pesticides reaches its purposes of pest control, the remainder would enter the environment and pollute it, and eventually enter the food chain and end up in the food we consume. Consequently, the pesticide residue may be absorbed through the human digestive system. Examples of pesticides are organophosphate pesticides, acetamiprid, carbamate pesticides, and others (Nagabooshanam et al. 2020; Xu et al. 2017).
Antibiotic contamination has become a worldwide epidemic as it is persistent and exists in many environmental samples, foods, dairy products, and beverages that require more attention to prevent any dire consequences. Antibiotics are drugs used to treat infections by inhibiting the growth of germs and bacteria. But over time, when germs or bacteria have established resistance to them, it may be less efficient. If they enter the food chain, it could cause adverse health effect by decreasing blood cells (red and white) causing weakness, headache, diarrhea, muscle pain, blurred vision, temporary vision loss, and hypertension. Tetracycline, penicillin, fluroquinolone, and sulfonamide are examples of common antibiotics. Manufacturing industries, hospitals, and veterinary facilities discharge these antibiotics into groundwater and river water, resulting in the spread of antibiotic resistance (Tarannum et al. 2020).
6.3 Optical Detection Toward Accurate Food Quality Assessment
6.3.1 Optical Detection of Food Carcinogens
Carcinogens are substances that may cause the development of cancers upon exposure. During the food heating and processing process, it will induce the formation of carcinogens such as heterocyclic aromatic amines (HAA), polycyclic aromatic hydrocarbons (PAH), and acrylamide. It is difficult to avoid carcinogens, especially those that are produced during food processing operations. In this section, only acrylamide will be addressed since this compound has been extensively reported. Other food carcinogens such as 3-chloropropane-1,2-diol (3-MCPD) may also be detected by biosensing approaches, but not many similar studies have been reported (Wong et al. 2020).
Acrylamide (2-propanamide), an unsaturated amide formed when reducing sugars and protein containing asparagine are heated (such as frying and baking) at elevated temperatures (Pundir et al. 2019; Sani et al. 2018), to give food color and flavor through the Maillard reaction (Pan et al. 2020). The thermal processing methods, the temperature range at which food is heated will affect the acrylamide content in carbohydrate-rich food. The acrylamide content is directly proportional to the processing time and temperature (Pan et al. 2020). In addition, there is also a direct correlation between the production of acrylamide and food color development (Wang et al. 2013). Since the tolerable daily intake (TDI) reported for acrylamide is 40 and 2.6 μg/kg day, respectively, in terms of neurotoxicity and carcinogenicity (Tardiff et al. 2010), therefore, for food quality assessment, accurate, sensitive, and robust methods and techniques for the detection of acrylamide in food should be developed and adopted.
Optical instrumental analysis techniques such as high-performance ultraviolet liquid chromatography (HPLC-UV) are still the common and widely used methods of detecting the presence of acrylamide in foodstuffs to date (Norouzi et al. 2018; Saraji and Javadian 2019; Wang et al. 2013). Many studies have been conducted using these methods to detect the acrylamide content in food. High accuracy, sensitivity, good reproducibility, and stability are shown by these analytical methods. The drawbacks are expensive instrumentation and high costs of detection (Wei et al. 2020). Other than that, a meticulous and tedious process for sample preparation and purification (clean-up process) is required to increase the sensitivity and accuracy of acrylamide detection as the food matrix is quite complex (Pan et al. 2020; Rashidi Nodeh et al. 2018).
One research was conducted to detect acrylamide in food using dummy molecularly imprinted silica nanoparticles (DMISNPs) as a dispersant to extract acrylamide in food samples by matrix solid phase dispersion (MSPD). Molecularly imprinted polymers (MIPs) are porous materials that are man-made and have unique binding sites for certain molecules. DMISNPs have high acrylamide affinity, mechanical and chemical robustness, simplicity in synthesis procedure, low preparation costs, and low consumption of toxic organic solvent during the synthesis process. Acrylamide can easily recognize DMISNPs and enter the cavities and form hydrogen bonds with their functional groups. The content of acrylamide can be quantified by integrating high-performance liquid chromatography with UV detection (HPLC-UV). For this method, the detection limit (LOD) and quantification limit (LOQ) are 15.3 ng/g and 40.3 ng/g, respectively, with a linear range between 0.05 and 5.0 μg/g (Arabi et al. 2016; Pan et al. 2020).
In another paper, the presence of acrylamide extracted from snack, seasoning, and refreshment food samples was detected using an iOS gadget-based digital imaging calorimeter (iOS gadgets-based DIC). The acrylamide is converted to vinyl amine through Hofmann reaction prior analysis. The vinyl amine reacts with fluorescein to give fluorescence emission at 590 nm with the excited wavelength at 470 nm. It enables the acrylamide content in the food sample to be determined simultaneously and synchronously by simply exposing the sample that has been undergone Hofmann reaction along with the fluorescein to emit fluorescence light. Using an iOS gadget, the emitted fluorescence light is detected and captured. The captured digital images can be used for estimation of the acrylamide concentration of the sample solutions by processing the color values of the digital images. Besides, the color value of the standard acrylamide solutions can be plotted as the calibration graph and thus the unknown concentration of the acrylamide can be derived from the calibration plot. The method has 0.53 and 1.78 mg/L LOD and LOQ, respectively, with a linearity range between 1.00 and 10.0 mg/L (Wongthanyakram et al. 2020).
An instrument-free approach to determine the concentration of acrylamide in food samples is through a filtration-assisted approach. Acrylamide extract was mixed and interacted with the silver nanoparticles (AgNPs)-EDT system. EDT will react with AgNPs before the addition of acrylamide, resulting in the formation of the aggregate of AgNPs due to reduced electrostatic repulsion and cross-linkage. Owing to the presence of an aggregate of AgNPs, the color of the aggregate changes from yellow to gray. Using ImageJ software, the gray color intensity can be quantified and analyzed. It is possible to use the filter membrane to isolate aggregate AgNPs from unaggregated AgNPs. The aggregates are vacuum-filtered and retained on the filter membrane. Acrylamide will form complex with EDT upon introduction of acrylamide into the AgNPs-EDT framework and thus reduce the content of EDT. Consequently, there are less aggregate AgNPs. With greater acrylamide concentration, the color of the aggregate is more yellowish. Or in other words, the acrylamide-containing sample appears to have a lower gray intensity. This technique enables optical detection by naked eyes through the intensity of the gray color or can be subjected to ImageJ software analysis. It is possible to use UV-Vis spectrophotometers to validate the results obtained. Using this filtration-assisted method, the LOD and LOQ are 3.7 ng/mL and 11.1 ng/mL, respectively (Lin et al. 2021).
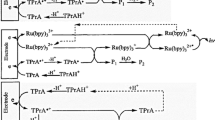
Other than that, electrochemiluminescence (ECL) can be used to detect acrylamide in food and this method is believed to be simple, convenient and has high stability and reproducibility. For this detection technique, Ru(bpy)32+ was used as luminophore. Oxidized Ru(bpy)32+ and oxidized acrylamide (at scanning potential close to 1.1 V) react with each other to obtain an excited state of Ru(bpy)32+*. This excited state of Ru(bpy)32+* is unstable and tends to return to the ground state with release of energy and give the ECL signal. The ECL signals can be monitored and captured by cyclic voltammetry (CV). An increasing in ECL intensity is accompanied by an increased acrylamide concentration. The digital images of ECL emission are collected using a remote wireless camera at which Red, Green, Blue (RGB) model is converted to Hue, Saturation, Value (HSV) using MATLAB software. The V shows a linear relationship with the acrylamide concentration. This method offers a LOD of 1.2 μM with a linear range of 5–10 μM (Yang 2019).
Following the digital era, the future trend for the identification of carcinogens will rely more on digital optical images, where the concentration of targeted analytes is directly or even inversely proportional to parameters such as the intensity of color derived from digital images. The concentration of the targeted carcinogenic molecules can be measured using tools such as ImageJ or the RGB (Red, Green, Blue) of digital images can be transformed to HSV (Hue, Saturation and Value) using MATLAB and others for quantification by simply taking a real-time and on-site digital image of the optical emission from the contaminated food sample solution using a smartphone camera. The above optical sensing approaches of the food carcinogen acrylamide are summarized in Table 6.1.
6.3.2 Optical Detection Methods of Microbes
Bacteria are one of the causes of cases of food poisoning that have haunted us for decades, typically due to inappropriate techniques of food handling. E. coli, S. aureus, and Salmonella sp. are examples of common bacteria commonly found in food. There might be no distinction in terms of appearance, taste or smell of the microbe-contaminated food from the non-microbe-contaminated-food. Therefore, in order to detect and distinguish microbe-contaminated foods from non-microbe-contaminated foods, cost-effective methods that could be carried out in the shortest time possible should be developed.
For the detection of E. coli bacteria, mesoporous silicon (immunosensor) biosensors can be used. Nanostructured porous silicon (Psi) as an optical transducer of the biosensor functionalized with specific antibodies against E. coli has been used to capture and real-time detection of E. coli in food samples. E. coli bacteria detection is based on a shift in optical data when target analytes are captured or bound to the surface of the biosensor based on an antibody-antigen interaction. In other words, the white light is directed to the biosensor, and the film’s average refractive index is recorded throughout the experiment and the wavelength change in the reflectivity spectrum is recorded. The reflectivity spectrum is then transformed to a single peak (Intensity versus Effective Optical Thickness (EOT)) by applying a fast Fourier transform (FFT). Biosensors capture of bacteria will reduce the intensity of the single peak. In this case, pre-treatment of the sample leading to prior lysis of the bacteria cell to extract the targeted protein and DNA fragments is not required for the direct capture of the target bacteria to the biosensor. This method of optical detection enables E. coli to be detected and quantified rapidly. However, because antibodies are used as a capture element, the sensor is confined to targeted analytes containing antibodies. In addition, the chemical stability of this sensor in corrosive environments should also be considered, as it is possible to observe a baseline drift in non-neutral environments. The LOD is 103 cells/mL for this method, with a linear range of 103–105 cells/mL (Massad-Ivanir et al. 2016).
A modified lectin-coupled porous-silicon-based biosensor was used in other studies to detect two types of commonly found food bacteria, E. coli and S. aureus, by reflectometric Fourier transform spectroscopy interference (RIFTS). Owing to its unique carbohydrate-binding (glycan moieties of glycoproteins or glycolipids) properties and relatively inexpensive compared to other antibodies, lectin is selected rather than an antibody. First, the fresh PSi is oxidized to form oxidized PSi, followed by dipping to form APTES modified PSi in ethanolic (3-aminopropyl)triethoxysilane solution (APTES). APTES modified PSi is converted to glutaraldehyde modified PSi, which is then ready for lectin immobilization to form lectin modified PSi. A reflection spectrum is obtained for penetration of analytes into the pores of PSi. Reflection spectrum data is transformed to the Fourier transform (FFT) intensity. After the addition of target analytes, the amplitude of the peak decreases. The result showed that ConA (Concanavalin A) and WGA (Wheat Germ Agglutinin) lectins have greater binding affinity to E. coli and S. aureus. Lectin, as compared to antibodies, is inexpensive and more stable, but less specific than antibodies. But still, because lectin has more than one (polyvalent) binding sites, less specificity can be improved and compensated (Yaghoubi et al. 2020).
In addition, a fiber optic surface plasmon resonance (FOSPR) sensor based on antimicrobial peptides (AMP), Magainin I, and silver nanoparticles-reduced graphene oxide (AgNPs-rGO) has been developed. AgNPs-rGO is secured to the optic fiber surface followed by surface covering with AuNPs. Next, the Magainin I is immobilized onto the fiber optic probe. The advantages of such sensor are improved performance of SPR response and protection of AgNPs from oxidation. Magainin I acts as the recognition element that could specifically capture E. coli and results in the change of the refractive on the optical fiber surface and wavelength shift of the SPR absorption peak. While AgNPs-rGO acts as signal amplification and allows for low limits of detections of 5.0 × 102 cfu/mL (Zhou et al. 2018a, b).
Based on poly(carboxybetaine acrylamide) (pCBAA) brushes, biotinylated secondary antibodies and streptavidin-coated gold nanoparticles, another SPR biosensor was developed to specifically detect low levels of E. coli and Salmonella sp. in complex food samples such as cucumbers and hamburgers. The biotinylated secondary antibodies are immobilized on the pCBAA coating mounted on the SPR chip. The antibodies capture and bind to the bacteria (target analyte). The captured bacteria are then bound by secondary (2°) biotinylated antibodies. The binding of streptavidin-coated gold nanoparticles to the 2° antibodies is then followed to enhance the detection/sensor response signals (Vaisocherová-Lísalová et al. 2016). Table 6.2 summarizes the optical sensing approaches described above for potential microbes present in food.
6.3.3 Optical Detection Methods for Heavy Metals
Heavy metals, due to irrigation with contaminated water, mining or industrial emissions, are commonly found in agricultural food crops such as fruits and vegetables, as a result of the introduction of heavy metals to the soil matrix. Heavy metals are also found in the food during cooking as a result of leaching from the cookware.
Atomic Absorption Spectroscopy (AAS) has been widely used to detect heavy metals in food samples. Co, Ni, Cu, Zn, Cd, Mn, Mg, Fe, and Ca in Sage Tea have been determined by Flame Atomic Absorption Spectroscopy (FAAS). The sample is subjected to acid dissolution prior to analysis by FAAS.
By using FAAS coupled with MnO2/3MgO nanocomposite, a sorbent for solid phase extraction for preconcentration, trace copper and lead in food can be detected. The analytes were desorbed from the nanocomposites after extraction using ethylenediaminetetraacetic acid. The extract is then analyzed using FAAS for copper and lead concentrations (Khayatian et al. 2018).
A novel method of in-syringe solvent dispersive solid phase extraction (ISSADSPE) method followed by FAAS to detect the nickel in the water and food samples has been developed. The sample solution was placed in the syringe barrel, followed by the addition of sorbent material to the sample solution. The analytes are deposited on the sorbent and, through the syringe membrane, the sorbent is separated from the sample solution. Next, ethanol was then used to extract the deposited targeted analytes on the sorbent prior to analysis using FAAS (Nakhaei et al. 2019).
Heavy metals can be detected using laser-induced breakdown spectroscopy (LIBS). LIBS is an in situ detector that enables the detection of solid, liquid, or gas samples with minimal sample preparation on different food matrices. In addition, it allows rapid and multiple elements detection. The high-power pulsed laser source is focused on the sample, which subsequently causes the sample molecules to break down into atoms. The laser energy allows a small number of samples to turn into a vapor and form a plasma at high temperatures. The atoms are excited by this high-temperature settings, and the unstable atoms relax to the ground state by emitting light radiation, which is in turn detected by the spectrometer. However, further studies need to be performed on its interaction with the food matrices, plasma formation, radiation emission, and signal analysis (Sezer et al. 2017). Studies on fresh vegetables contaminated with heavy metals by LIBS have been reported (Yao et al. 2017). Another LIBS study was carried out on a pork sample to detect the heavy metal chromium (Huang et al. 2016). In order to improve stability and sensitivity, it is necessary to eliminate the water content and some organics while using these techniques, as these compounds would affect the LIBS spectrum, such as higher noise and background signals (Huang et al. 2016; Yao et al. 2017). Since this approach is very new, further studies are needed to understand its interaction with different food matrices, plasma formation, emission of radiation and signal analysis. Table 6.3 provides a summary of the discussed optical detection methods for heavy metals present in food.
6.3.4 Optical Detection Methods for Food Preservatives
Food preservatives have been added to food for decades to prevent deterioration and spoilage and to lengthen the shelf life of the food. Excessive use has, however, been documented to promote cancer and affect metabolism (Prapainop et al. 2019). Therefore, quality control should also be in place to ensure food safety and quality.
Benzoic acid and paraben derivatives are common food-added preservatives. They can be detected using mercaptosuccinic acid capped cadmium telluride quantum dots (MSA capped CdTe). For the parabens, hydrolysis is performed to transform them to p-hydroxybenzoic acid (PHBA) as parabens are unable to interact directly with the quantum dots. Addition of MSA capped CdTe QDs to the target analytes solution in 1:1 ratio, the mixture is subject to fluorescent analysis. Benzoic acid and PHBA will result in fluorescent quenching of the MSA-CdTe QDs. In addition, upon addition of benzoic acid, there is a color change of MSA-CdTe QDs, therefore UV-Vis spectrophotometer can also be used for benzoic acid and parabens determination, where the increasing trend of the absorbance peak at approximately 456.50 nm has been perfectly shown by increasing from 0 mg/L to 100 mg/L benzoic acid. This approach gives a detection limit for benzoic acid and PHBA of 0.3 mg/L and 0.1 mg/L, respectively, with a linearity range from 1.0 mg/L to 500.0 mg/L (Prapainop et al. 2019).
In recent years, another tert-butylhydroquinone (TBHQ) food preservative has gained a lot of attention because it causes potential liver damage. In food manufacturing, TBHQ is popular as it can prevent putrefaction and deterioration of edible oils and lipids. Based on the competitive reaction between the photo-induced electron transfer (PET) effect and the complexation reaction between phenolic hydroxyl groups with the Fe(III) ions in TBHQ, a ‘on-off-on’ fluorescent sensor has been developed. For the fluorescence intensity measurement of the sample solutions, an excited wavelength at 360 nm was set. The “on-off-on” mechanism process is as follows (Yue et al. 2016):
-
1.
On: The synthesized quantum dots (CDs) act as fluorophores, giving a certain fluorescence intensity at 360 nm when the excited electrons return to the ground state due to instability.
-
2.
Off: In the presence of Fe(III) ions, the fluorescence intensity of CDs is quenched due to the photo-induced electron transfer effect between Fe(III) and CDs. The introduction of Fe(III) ions would lead to the entry of excited CD electrons into the unfilled Fe(III) ion orbital. This process deactivates the excited electrons and subsequently decrease fluorescence intensity and is referred to as fluorescence “quenching”.
-
3.
On: Next, with the introduction of TBHQ, fluorescence intensity gradually increases due to the strong complexation of TBHQ with Fe(III) ions. TBHQ will compete Fe(III) with CDs.
This “on-off-on” fluorescence sensor gives a linearity range from 0.5 to 80 μg/mL with a low detection limit of 0.01 μg/mL (Yue et al. 2016).
Other commonly used preservatives in food are formaldehyde, hydrogen peroxide, and sodium carbonate. For the detection of the above-mentioned preservatives, a fiber optic sensor is used based on the change in the refractive index of the outer region of the fiber cladding or on the concentration of the medium surrounding the sensing area. This technique can be used for liquid foods such as milk, juice, etc. and can detect changes in the refractive index of less than 0.4% (Saracoglu and Hayber 2016).
A compact, selective, and on-site detection method should be developed for food preservatives in the future. A bent fiber optic sensor may be an option because it is compact, but not all target analytes are subject to change in the refractive index and one targeted analyte is not clearly distinguished from another by this method. Due to its simple instrumental setup in which an iOS gadget such as a smartphone equipped with a camera can be used to capture digital images of the sample optical emission, a digital image based on the parameters of the images captured to determine the preservative concentration can also be developed in the near future. In addition, it is also a rapid detection in which the digital image can be acquired in 1 s and then analyzed using software or applications such as MATLAB. Table 6.4 summarizes the above optical sensing methods for food preservatives.
6.3.5 Optical Detection Methods for Toxins
Some bacteria and fungi in food could secrete substances that are toxic to humans and these toxin-containing foods should be immediately detected and removed instantly from the consumer food supply chain to ensure food safety and quality.
Two types of toxins are available: bacteriotoxin and mycotoxin (secreted by fungi). One of the mycotoxins commonly found in maize, nuts, and spices is aflatoxin. Aflatoxin can be found in many forms, but aflatoxin B1 is the most toxic. In one study, almonds were used as almond matrices, showing a significant intrinsic fluorescence emission in the observed spectral range. High-performance fluorescence detection liquid chromatography (HPLC/FLD) coupled with machine learning algorithms—a binary classification model based on Support Vector Machine (SVM) was used for the total aflatoxin (B1 and B2) detection produced by the Aspergillus flavus bacteria in the slurry almond. Prior to HPLC-FLD analysis, the slurry almond was then extracted and filtered. The excitation wavelength used for the detection of fluorescence is 365 nm, while 435 nm is for emission. This technique’s LOD is 0.2 ng/g. The accuracy value (ACC) and False Negative Rate (FNR) were calculated for the binary classification using SVM. In contaminant analysis, FNR is an important parameter which provides an indication of the misclassification of the contaminated food sample as a safe food sample. In developing countries, this technique is suitable because it is a simple setup with minimal sample preparation (Bertani et al. 2020).
Shiga toxin (Stx) is one of the potential E. coli toxin-producing bacteriotoxins produced by Shiga (STEC). The toxin affects the intestinal tract, causing diseases that range from hemorrhagic colitis to hemolytic-uremic syndrome (HUS). For the detection of this toxin, a portable and real-time optical sensing system has been developed. The sensing device consists of optical components: light sources, a Stx sample loading platform and a highly sensitive light detector, a photomultiplier tube (PMT) that detects and measures fluorescence from fluorescently tagged Stx, a control and data acquisition platform consisting of an amplifier-equipped microcontroller unit (MCU) and a display component board. The advantage of this system is that it is battery-powered and thus compact, weighing around 770 g excluding battery, with a total size of 17 × 13 × 9 cm3. Moreover, it does not require any peripheral computer or external display equipment. The light source is directed to the samples and the light emitted from the samples is directed to the PMT, where the light for the LCD display is converted to current and voltage. This sensing device provides a low limit of detection of 110 pM (D’Auria et al. 2020).
Another label-free detection technique for mycotoxins is using absolute internal reflection ellipsometry (TIRE), which is a combination of spectroscopic ellipsometry (SE) and surface plasmon resonance (SPR). Apparently, this aptamer assay is more sensitive than SPR alone and can classify certain analytes of low molecular weight such as ochratoxin A (OTA), aflatoxin B1, alkyl-phenols, and microcystin. In this study, OTA with a low molecular weight of 403.8 Da was detected. For the preparation of aptamers, gold and chromium layers have been evaporated and immobilized into the standard glass slide followed by binding to the DNA-based aptamers specific to OTA on the gold surface. Then, the TIRE method was used for the detection and measurement. The increasing introduction of OTA will trigger a progressive blue shift (shift to shorter wavelength of the TIRE spectra). This is explained by the decrease in the molecular layer thickness or refractive index upon OTA binding. This label-free model is capable of detecting OTA concentrations down to 0.01 ppb (Al Rubaye et al. 2018).
Among these optical methods, portable fluorescence device with PMT and LCD is worth further investigating for other toxins found in food. The portability of this device could provide on-site detection and could instantly separate those toxin-contaminated from others. Table 6.5 summarizes the optical sensing methods discussed in this section.
6.3.6 Optical Detection Methods for Pesticides
Pesticide residues in agricultural products are detrimental to human health. In order to ensure food safety and quality, detecting the level of pesticides in food is critical and enables regulation and monitoring of the use of pesticides in agriculture at the same time.
One of the useful direct pesticide detection techniques that can be used to ensure food safety and quality is surface-enhanced Raman spectroscopy (SERS). The unique and characteristic vibrational fingerprints provided by the molecules are the basis of this non-destructive detection. Prior to SERS analysis, extraction and clean-up of the sample is required. Density Functional Theory (DFT) is used to predict molecular conformation and spectral data (Xu et al. 2017). An in situ SERS detection for pesticides (thiram and thiabendazole) in fruits and vegetables has been developed in one study. For detection, jelly-like and slightly sticky Ag nanoparticles deposited on nanocellulose (Ag/NC substrate) were used. As it is abundant, environmentally friendly and has a nano-scale radius, nanocellulose (made from woods) is chosen. Stirring of AgNPs and NC produces the jelly-like appearance of the Ag/NC substrate. By only smearing the jelly-like Ag/NC substrate on the pesticide-containing fruit peels or vegetable surface, SERS measurement can achieve in situ and non-invasive detection. Besides, by observing the clear, sharp, and specific “fingerprint-like” Raman peaks, SERS allows multiple detection of different pesticides in the sample. The lowest reported detection limit for thiram is 0.5 ng/cm2 and for thiabendazole is 5 ng/cm2 (Chen et al. 2019).
One of the organophosphorus (OP) pesticides extensively used in agriculture to protect crops against pests and insects is O,O-Diethyl O-[4-methyl-6-(propan-2-yl)pyrimidin-2-yl] phosphorothioate or also known as diazinon. In order to detect trace concentrations of OPs in the food sample based on the fluorescence quenching effect of copper ions (Cu2+) on lanthanide-doped conversion nanoparticles (UCNPs), a sensitive fluorescence biosensor was developed and reported. UCNPs own some distinctive properties and advantages such as having large anti-Stokes shifts, high quantum yields, long lifetimes, no photobleaching and non-blinking emissions, high chemical stability, and low cytotoxicity that enable them to be selected as the fluorophores over those conventional down-conversion organic luminescence materials. A fluorescence of high intensity would be given by UCNPs capped with branched polyethylenimine (PEI) itself. Upon addition of Cu2+ ions, the fluorescence is quenched due to the coordination of Cu2+ ions with the amino group of PEI of UCNPs, which is termed as “off” or known as fluorescence quenching. It is followed by the addition of acetylcholinesterase (AChE) and acetylthiocholine (ATCh) into the UCNPs-Cu2+ system. AChE is catalytically hydrolyzed by ATCh to form thiocholine (TCh). This TCh has a high affinity to bind with Cu2+ ions from the UCNPs-Cu2+ system, resulting in fluorescence intensity restoration and this phenomenon is referred to as “on.” It was then followed by the addition of OPs pesticides that serve as an enzyme inhibitor and thus suppress the formation of TCh, resulting in fluorescence quenching again, which is “off.” This “off-on-off” method gives a linear detection range from 0.1 to 50 ng/mL with LOD of 0.05 ng/mL (Wang et al. 2019).
A rapid detection of pesticides residues has also been developed by using flexible, transparent, and adhesive commercial tapes with SERS. The procedure is relatively simple, following the “paste, peel off, and paste again” steps. The pesticides analytes can be easily extracted from surfaces such as fruits and vegetables by simply pasting the adhesive tape onto the sample. After peeling off, the adhesive tape loaded with analytes was then pasted onto the Al2O3-coated silver nanorod (AgNR@Al2O3) which serves as SERS substrate where SERS signal could be read and obtained from there. In this study, tetramethylthiuram disulfide (TMTD) was identified as analytes. The LOD of this method is 28.8 ng/cm3 or ~0.1 μg/g with a linear range from 10−5 to 10−6 M (Jiang et al. 2018).
SERS is a promising optical method among these, as it could provide rapid on-site and multiplexed detection of analytes. Other extraction methods for other pesticides or herbicides present on the surface of fruits and vegetables, such as acetamiprid, may be developed for future research. Table 6.6 summarizes optical detection methods for pesticides in foodstuffs.
6.3.7 Optical Detection Methods for Antibiotics
Antibiotics such as penicillin, streptomycin, and kanamycin (KANA) have been commonly used in livestock breeding. Excessive use could establish antibiotic resistance, changing the response of bacteria to these antibiotics.
A SERS-based aptasensor for KANA has been proposed and developed. It is based on the principle of embedded and attached DNAs to form Au NPs-ssDNA on the surface of Au nanoparticles. Thiol groups form covalent bonds between these two compounds. The encapsulation of Au NPs-ssDNA with Ag shells was followed to form Au@Ag NPs-ssDNA. DNA aptamer was then added to form Au@Ag NPs-dsDNA. Au@Ag NPs-dsDNA is ready to be mixed with milk samples containing different concentrations of KANA and scanned for Raman spectra. The intensity of the Raman spectrum decreases with increasing KANA concentration. The lowest detection limit for this method is 0.90 pg/mL (Jiang et al. 2019).
Tetracycline (TC) is commonly used in food-producing animal as a growth promoter. It can be found in foods such as milk, eggs, and poultry. A fluorescent nanosensor has been developed for detection of TC antibiotics based on molecularly imprinted polymers (MIP) coated on graphene quantum dots (GQD). The GQD-MIPs solution is prepared using the sol-gel method. The addition of GQD-MIPs with carbonyl groups to different concentrations of TC was then followed, and subsequently summited for fluorescence measurement. Quenching of fluorescence intensity is observed upon the addition of TC. The authors have also demonstrated the effect of the GQDs-MIPs compared to the non-imprinted particles (GQD-NIPs) acting as reference. Both GQD-MIPs and NIPs have been reported to show fluorescence quenching, with the impact of GQD-MIPs on fluorescence response reduction being more significant than GQD-NIPs. Moreover, since the molecular imprinting technique was used to create the binding sites consistent with the targeted analytes in terms of shape, size, and functional groups, the selective recognition of the GQD-MIPs against TC is very satisfactory and effective. The LOD of this detection method is 1 μg/L with a linear range of 1–104 μg/L (Zhou et al. 2018a).
In another study, kanamycin (KANA) and neomycin (NEO) (classified as aminoglycoside group antibiotics) were detected using an ellipsometric aptamer-based sensor using spectroscopic ellipsometry (SE) and SPR-enhanced (SPRe) total internal reflection ellipsometry (TIRE) techniques. Thio-functionalized anti-kanamycin/ anti-neomycin aptamers were immobilized on cleaned sensor chip surfaces made of 50 nm Au film-coated glass slides, while amine-functionalized anti-kanamycin/ anti-neomycin aptamers were immobilized on a surface-modified silicon (Si) wafer. For those aptamers immobilized on Si surfaces, the detection of antibiotics KANA and NEO is through immersion of the aptamers in a solution containing specific antibiotics, followed by spectroscopic ellipsometer analysis (SE). The presence of antibiotics causes surface thickness to build up and the refractive index to change. While for those aptamers immobilized onto Au surfaces, KANA and NEO solutions were brought into contact with those aptamers, a total internal reflection device (SPRe-TIRE) and a flow system were required. A flow cell (to which the target analyte solutions are injected to) consists of a glass prism coupled with a refractive-index-matching-oil. The antibiotics amount captured on the aptasensors is determined by the change in the angle of light reflected through the prism. The LOD of these two sensor platforms is between 0.1 and 1 nmol/L with a linear range between 0.1 and 1000 nmol/L (Caglayan 2020).
Aptamer is one of the advanced technologies that could specifically bind to the targeted molecules. Most of the aptamer is DNA or RNA based, which is relatively expensive. In the case of rapid and on-site detection, SERS is often a better option, as it is portable. Future research on the advancement of cost-effective extraction of target analytes for detection, such as using adhesive tape or filter syringe, could be developed. However, since they are not automated, these two extraction methods are inappropriate for large-scale detection. The aforementioned optical sensing techniques for antibiotics that are likely to be present in food are summarized in Table 6.7.
6.4 Comparison Between Different Optical Detection Techniques for Food Safety and Quality Assessment
Various optical detection techniques are available for food safety and quality assessment. The selection of one optical approach over the other mainly depends on the properties and characteristics of the target analytes. For example, if the target analyte has a high fluorescence activity or a high binding ability with the readily available fluorescein, the fluorescence sensing method may be appropriate for this analyte. While for other target analytes containing certain bio-recognizing elements, SPR, biosensor, and aptamer may be a considerable option for detection.
In terms of analysis time or detection time, excluding the sample preparation, the fluorometer can provide output data for each sample in less than 5 min by scanning through a certain wavelength range. Therefore, it offers a relatively rapid analysis. The capital cost of the fluorometer, HPLC-UV instrument and FAAS instrument may be a challenge for scientists, but the sample can be collected and sent to the testing laboratory where a sample which cost only around USD 10–50. It costs around USD 2000–3000 for the portable SERS detector, which is rather cost-effective as the detector can be used repeatedly to detect multiple samples and is ideal for routine screening of contaminated food samples. Biosensors, on the other hand, are a promising tool for real-time and on-site analysis, since biosensors can be made portable for field sensing. Relatively high accuracy, sensitivity, and selectivity could be given by biosensors. The novel LIBS technique has the ability for multiplexed target analytes (element) analysis in which the target analytes are break downed into atoms by high powered lasers and the analytes are determined and quantified from the LIBS spectra. This technique has recently been miniaturized into a portable LIBS for on-site and real-time analysis. It is worth to mention that SERS also allows multiplexed analytes detection based on the unique and characteristic vibrational fingerprints of the target analytes.
Few commercially portable devices, such as handheld fluorometers or portable biosensors, are available on the market. Keeping pace with the digitalized era, our smartphone, the iOS gadget, can also be used as an optical detection device in which the applications or apps such as Color Analysis or Colorimeter for color measurement that can be installed and utilized for detection and measurement. The accuracy of analysis using these portable devices is considered to be high, but nevertheless it is still unacceptable for multiplexed target analysis. Comparisons are summarized and outlined in Table 6.8 between the various optical detection methods for food quality assessment.
6.5 Conclusion and Future Perspective
The presence of food contaminants is associated to food safety and quality, while food safety and quality are correlated to our health in turn. The issue of food contaminants should, therefore, not be treated indifferently. There is a need to develop portable devices that rapidly detect targeted contaminants in food and are cost-effective at the same time. Fluorescence, ECL sensor, SPR, SERS, biosensor, and aptamers, among the various optical methods discussed, have the potential to be miniaturized and become portable devices or systems that could provide on-site monitoring and detection convenience. In food quality assessment, on-site sensing is crucial as it allows the contaminated foodstuffs to be instantly detected and separated. While optical detection, due to its non-destructive and non-cell damaging properties, will be a promising option over other techniques, it allows rapid and sensitive detection at the same time. Future research could be conducted to integrate artificial intelligence into optical sensors, such as machine learning algorithms and deep learning or chemometrics. The Internet of Things (IoT) makes it possible to capture real-time sensor data and has the ability to store historical data massively in a database. Therefore, it is an encouraging alternative to quick, on-site and real-time routine screening of foodstuffs for food safety assessment.
References
Abebe E, Gugsa G, Ahmed M (2020) Review on major food-borne zoonotic bacterial pathogens. J Trop Med 2020:1–19. https://doi.org/10.1155/2020/4674235
Aderemi TA, Adenuga AA, Oyekunle JAO, Ogunfowokan AO (2017) High level leaching of heavy metals from colorful ceramic foodwares: a potential risk to human. Environ Sci Pollut Res 24(20):17116–17126. https://doi.org/10.1007/s11356-017-9385-7
Al Rubaye A, Nabok A, Catanante G, Marty J-L, Takacs E, Szekacs A (2018) Detection of ochratoxin A in aptamer assay using total internal reflection ellipsometry. Sensors Actuators B Chem 263:248–251. https://doi.org/10.1016/j.snb.2018.01.220
Ali H, Khan E (2018) Trophic transfer, bioaccumulation, and biomagnification of non-essential hazardous heavy metals and metalloids in food chains/webs—concepts and implications for wildlife and human health. Hum Ecol Risk Assess Int J 25(6):1353–1376. https://doi.org/10.1080/10807039.2018.1469398
Ali MH, Elsherbiny ME, Emara M (2019) Updates on aptamer research. Int J Mol Sci 20(10):2511. https://doi.org/10.3390/ijms20102511
Amin NU, Siddiqi HM, Kun Lin Y, Hussain Z, Majeed N (2020) Bovine serum albumin protein-based liquid crystal biosensors for optical detection of toxic heavy metals in water. Sensors 20(1):298. https://doi.org/10.3390/s20010298
Arabi M, Ghaedi M, Ostovan A (2016) Development of dummy molecularly imprinted based on functionalized silica nanoparticles for determination of acrylamide in processed food by matrix solid phase dispersion. Food Chem 210:78–84. https://doi.org/10.1016/j.foodchem.2016.04.080
Argudín MÁ, Mendoza MC, Rodicio MR (2010) Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2(7):1751–1773. https://doi.org/10.3390/toxins2071751
Babich H (1982) Butylated hydroxytoluene (BHT): a review. Environ Res 29(1):1–29. https://doi.org/10.1016/0013-9351(82)90002-0
Balram D, Lian K-Y, Sebastian N, Rasana N (2021) Ultrasensitive detection of cytotoxic food preservative tert-butylhydroquinone using 3D cupric oxide nanoflowers embedded functionalized carbon nanotubes. J Hazard Mater 406:124792. https://doi.org/10.1016/j.jhazmat.2020.124792
Bertani FR, Businaro L, Gambacorta L, Mencattini A, Brenda D, Di Giuseppe D et al (2020) Optical detection of aflatoxins B in grained almonds using fluorescence spectroscopy and machine learning algorithms. Food Control 112:107073. https://doi.org/10.1016/j.foodcont.2019.107073
Bhardwaj N, Bhardwaj SK, Nayak MK, Mehta J, Kim K-H, Deep A (2017) Fluorescent nanobiosensors for the targeted detection of foodborne bacteria. TrAC Trends Anal Chem 97:120–135. https://doi.org/10.1016/j.trac.2017.09.010
Bruzas I, Lum W, Gorunmez Z, Sagle L (2018) Advances in surface-enhanced Raman spectroscopy (SERS) substrates for lipid and protein characterization: sensing and beyond. Analyst 143(17):3990–4008. https://doi.org/10.1039/c8an00606g
Caglayan MO (2020) Aptamer-based ellipsometric sensor for ultrasensitive determination of aminoglycoside group antibiotics from dairy products. J Sci Food Agric 100(8):3386–3393. https://doi.org/10.1002/jsfa.10372
Chen J, Huang M, Kong L, Lin M (2019) Jellylike flexible nanocellulose SERS substrate for rapid in-situ non-invasive pesticide detection in fruits/vegetables. Carbohydr Polym 205:596–600. https://doi.org/10.1016/j.carbpol.2018.10.059
Cuevas-González PF, González-Córdova AF, Vallejo-Cordoba B, Aguilar-Toalá JE, Hall FG, Urbizo-Reyes UC et al (2020) Protective role of lactic acid bacteria and yeasts as dietary carcinogen-binding agents – a review. Crit Rev Food Sci Nutr 62:160–180. https://doi.org/10.1080/10408398.2020.1813685
D’Auria S, Kim J, Park J-Y, Park Y-J, Park S-Y, Lee M-S, Koo C (2020) A portable and high-sensitivity optical sensing system for detecting fluorescently labeled enterohaemorrhagic Escherichia coli Shiga toxin 2B-subunit. PLoS One 15(7):e0236043. https://doi.org/10.1371/journal.pone.0236043
Denayer S, Delbrassinne L, Nia Y, Botteldoorn N (2017) Food-borne outbreak investigation and molecular typing: high diversity of Staphylococcus aureus strains and importance of toxin detection. Toxins 9(12):407. https://doi.org/10.3390/toxins9120407
Hameed S, Xie L, Ying Y (2018) Conventional and emerging detection techniques for pathogenic bacteria in food science: a review. Trends Food Sci Technol 81:61–73. https://doi.org/10.1016/j.tifs.2018.05.020
Hartwig A, Arand M, Epe B, Guth S, Jahnke G, Lampen A et al (2020) Mode of action-based risk assessment of genotoxic carcinogens. Arch Toxicol 94(6):1787–1877. https://doi.org/10.1007/s00204-020-02733-2
Hernández LG, van Steeg H, Luijten M, van Benthem J (2009) Mechanisms of non-genotoxic carcinogens and importance of a weight of evidence approach. Mutat Res 682(2–3):94–109. https://doi.org/10.1016/j.mrrev.2009.07.002
Huang L, Chen T, He X, Yang H, Wang C, Liu M, Yao M (2016) Determination of heavy metal chromium in pork by laser-induced breakdown spectroscopy. Appl Opt 56(1):8148. https://doi.org/10.1364/ao.56.000024
Jiang J, Zou S, Ma L, Wang S, Liao J, Zhang Z (2018) Surface-enhanced Raman scattering detection of pesticide residues using transparent adhesive tapes and coated silver Nanorods. ACS Appl Mater Interfaces 10(10):9129–9135. https://doi.org/10.1021/acsami.7b18039
Jiang Y, Sun D-W, Pu H, Wei Q (2019) Ultrasensitive analysis of kanamycin residue in milk by SERS-based aptasensor. Talanta 197:151–158. https://doi.org/10.1016/j.talanta.2019.01.015
Khayatian G, Moradi M, Hassanpoor S (2018) MnO2/3MgO nanocomposite for preconcentration and determination of trace copper and Lead in food and water by flame atomic absorption spectrometry. J Anal Chem 73(5):470–478. https://doi.org/10.1134/s1061934818050088
Koszucka A, Nowak A (2018) Thermal processing food-related toxicants: a review. Crit Rev Food Sci Nutr 59(22):3579–3596. https://doi.org/10.1080/10408398.2018.1500440
Lin Z, Gao S, Yang JS, Qu Y, Zhang Z, He L (2021) A filtration-assisted approach to enhance optical detection of analytes and its application in food matrices. Food Chem 338:127814. https://doi.org/10.1016/j.foodchem.2020.127814
Locke A, Fitzgerald S, Mahadevan-Jansen A (2020) Advances in optical detection of human-associated pathogenic bacteria. Molecules 25(22):5256. https://doi.org/10.3390/molecules25225256
Manoranjitham JJ, Narayanan SS (2020) Electrochemical sensor for determination of butylated hydroxyanisole (BHA) in food products using poly O-cresolphthalein complexone coated multiwalled carbon nanotubes electrode. Food Chem 342:128246. https://doi.org/10.1016/j.foodchem.2020.128246
Massad-Ivanir N, Shtenberg G, Raz N, Gazenbeek C, Budding D, Bos MP, Segal E (2016) Porous silicon-based biosensors: towards real-time optical detection of target bacteria in the food industry. Sci Rep 6(1):38099. https://doi.org/10.1038/srep38099
Mukama O, Sinumvayo JP, Shamoon M, Shoaib M, Mushimiyimana H, Safdar W et al (2017) An update on aptamer-based multiplex system approaches for the detection of common foodborne pathogens. Food Anal Methods 10(7):2549–2565. https://doi.org/10.1007/s12161-017-0814-5
Nagabooshanam S, Sharma S, Roy S, Mathur A, Krishnamurthy S, Bharadwaj LM (2020) Development of field deployable sensor for detection of pesticide from food chain. IEEE Sensors J 21(4):4129–4134. https://doi.org/10.1109/jsen.2020.3030034
Nakhaei JM, Jamali MR, Sohrabnezhad S, Rahnama R (2019) In-syringe solvent-assisted dispersive solid phase extraction followed by flame atomic absorption spectrometry for determination of nickel in water and food samples. Microchem J 144:88–92. https://doi.org/10.1016/j.microc.2018.08.063
Nguyen H, Park J, Kang S, Kim M (2015) Surface plasmon resonance: a versatile technique for biosensor applications. Sensors 15(5):10481–10510. https://doi.org/10.3390/s150510481
Nohmi T (2018) Thresholds of genotoxic and non-genotoxic carcinogens. Toxicol Res 34(4):281–290. https://doi.org/10.5487/TR.2018.34.4.281
Norouzi E, Kamankesh M, Mohammadi A, Attaran A (2018) Acrylamide in bread samples: determining using ultrasonic-assisted extraction and microextraction method followed by gas chromatography-mass spectrometry. J Cereal Sci 79:1–5. https://doi.org/10.1016/j.jcs.2017.09.011
Pan M, Liu K, Yang J, Hong L, Xie X, Wang S (2020) Review of research into the determination of acrylamide in foods. Foods 9(4):524. https://doi.org/10.3390/foods9040524
Permyakov EA, Regenthal P, Hansen JS, André I, Lindkvist-Petersson K (2017) Thermal stability and structural changes in bacterial toxins responsible for food poisoning. PLoS One 12(2):e0172445. https://doi.org/10.1371/journal.pone.0172445
Prapainop K, Mekseriwattana W, Siangproh W, Chailapakul O, Songsrirote K (2019) Successive detection of benzoic acid and total parabens in foodstuffs using mercaptosuccinic acid capped cadmium telluride quantum dots. Food Control 96:508–516. https://doi.org/10.1016/j.foodcont.2018.10.009
Pundir CS, Yadav N, Chhillar AK (2019) Occurrence, synthesis, toxicity and detection methods for acrylamide determination in processed foods with special reference to biosensors: a review. Trends Food Sci Technol 85:211–225. https://doi.org/10.1016/j.tifs.2019.01.003
Quintavalla S, Larini S, Mutti P, Barbuti S (2001) Evaluation of the thermal resistance of different Salmonella serotypes in pork meat containing curing additives. Int J Food Microbiol 67(1–2):107–114. https://doi.org/10.1016/s0168-1605(01)00430-5
Rashidi Nodeh H, Wan Ibrahim WA, Kamboh MA, Sanagi MM (2018) Magnetic graphene sol–gel hybrid as clean-up adsorbent for acrylamide analysis in food samples prior to GC–MS. Food Chem 239:208–216. https://doi.org/10.1016/j.foodchem.2017.06.094
Sani NDM, Heng LY, Marugan RSPM, Rajab NF (2018) Electrochemical DNA biosensor for potential carcinogen detection in food sample. Food Chem 269:503–510. https://doi.org/10.1016/j.foodchem.2018.07.035
Saracoglu O, Hayber S (2016) Bent fiber sensor for preservative detection in milk. Sensors 16(12):2094. https://doi.org/10.3390/s16122094
Saraji M, Javadian S (2019) Single-drop microextraction combined with gas chromatography-electron capture detection for the determination of acrylamide in food samples. Food Chem 274:55–60. https://doi.org/10.1016/j.foodchem.2018.08.108
Sezer B, Bilge G, Boyaci IH (2017) Capabilities and limitations of LIBS in food analysis. TrAC Trends Anal Chem 97:345–353. https://doi.org/10.1016/j.trac.2017.10.003
Sharma S, Nagpal AK, Kaur I (2018) Heavy metal contamination in soil, food crops and associated health risks for residents of Ropar wetland, Punjab, India and its environs. Food Chem 255:15–22. https://doi.org/10.1016/j.foodchem.2018.02.037
Tarannum N, Khatoon S, Dzantiev BB (2020) Perspective and application of molecular imprinting approach for antibiotic detection in food and environmental samples: a critical review. Food Control 118. https://doi.org/10.1016/j.foodcont.2020.107381
Tardiff RG, Gargas ML, Kirman CR, Leigh Carson M, Sweeney LM (2010) Estimation of safe dietary intake levels of acrylamide for humans. Food Chem Toxicol 48(2):658–667. https://doi.org/10.1016/j.fct.2009.11.048
Vaisocherová-Lísalová H, Víšová I, Ermini ML, Špringer T, Song XC, Mrázek J et al (2016) Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens Bioelectron 80:84–90. https://doi.org/10.1016/j.bios.2016.01.040
Villena Gonzales W, Mobashsher A, Abbosh A (2019) The progress of glucose monitoring—a review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors 19(4):800. https://doi.org/10.3390/s19040800
Wang W, Kannan K (2019) Quantitative identification of and exposure to synthetic phenolic antioxidants, including butylated hydroxytoluene, in urine. Environ Int 128:24–29. https://doi.org/10.1016/j.envint.2019.04.028
Wang H, Feng F, Guo Y, Shuang S, Choi MMF (2013) HPLC-UV quantitative analysis of acrylamide in baked and deep-fried Chinese foods. J Food Compos Anal 31(1):7–11. https://doi.org/10.1016/j.jfca.2013.02.006
Wang P, Li H, Hassan MM, Guo Z, Zhang Z-Z, Chen Q (2019) Fabricating an acetylcholinesterase modulated UCNPs-Cu2+ fluorescence biosensor for ultrasensitive detection of organophosphorus pesticides-Diazinon in food. J Agric Food Chem 67(14):4071–4079. https://doi.org/10.1021/acs.jafc.8b07201
Wei Q, Liu T, Pu H, Sun D-W (2020) Determination of acrylamide in food products based on the fluorescence enhancement induced by distance increase between functionalized carbon quantum dots. Talanta 218:121152. https://doi.org/10.1016/j.talanta.2020.121152
WHO (2020) Food safety. https://www.who.int/news-room/fact-sheets/detail/food-safety
Williams GM, Iatropoulos MJ, Whysner J (1999) Safety assessment of butylated hydroxyanisole and butylated Hydroxytoluene as antioxidant food additives. Food Chem Toxicol 37(9–10):1027–1038. https://doi.org/10.1016/s0278-6915(99)00085-x
Wong SF, Low KH, Khor SM (2020) Differential-based biosensor array for fluorescence-chemometric discrimination and the quantification of subtle chloropropanols by cross-reactive serum albumin scaffolding. Talanta 218:121169. https://doi.org/10.1016/j.talanta.2020.121169
Wongthanyakram J, Kheamphet P, Masawat P (2020) Fluorescence determination of acrylamide in snack, seasoning, and refreshment food samples with an iOS gadget–based digital imaging colorimeter. Food Anal Methods 13(12):2290–2300. https://doi.org/10.1007/s12161-020-01835-y
Xu M-L, Gao Y, Han XX, Zhao B (2017) Detection of pesticide residues in food using surface-enhanced Raman spectroscopy: a review. J Agric Food Chem 65(32):6719–6726. https://doi.org/10.1021/acs.jafc.7b02504
Yaghoubi M, Rahimi F, Negahdari B, Rezayan AH, Shafiekhani A (2020) A lectin-coupled porous silicon-based biosensor: label-free optical detection of bacteria in a real-time mode. Sci Rep 10(1):16017. https://doi.org/10.1038/s41598-020-72457-x
Yang X (2019) Novel detection of acrylamide by electrochemiluminescence sensor and optical imaging analysis. Int J Electrochem Sci 14:7380–7388. https://doi.org/10.20964/2019.08.31
Yao M, Yang H, Huang L, Chen T, Rao G, Liu M (2017) Detection of heavy metal Cd in polluted fresh leafy vegetables by laser-induced breakdown spectroscopy. Appl Opt 56(14):4070. https://doi.org/10.1364/ao.56.004070
Yue X, Zhu W, Ma S, Yu S, Zhang Y, Wang J et al (2016) Highly sensitive and selective determination of tertiary butylhydroquinone in edible oils by competitive reaction induced “on–off–on” fluorescent switch. J Agric Food Chem 64(3):706–713. https://doi.org/10.1021/acs.jafc.5b05340
Zhou T, Halder A, Sun Y (2018a) Fluorescent nanosensor based on molecularly imprinted polymers coated on graphene quantum dots for fast detection of antibiotics. Biosensors 8(3):82. https://doi.org/10.3390/bios8030082
Zhou C, Zou H, Li M, Sun C, Ren D, Li Y (2018b) Fiber optic surface plasmon resonance sensor for detection of E. coli O157:H7 based on antimicrobial peptides and AgNPs-rGO. Biosens Bioelectron 117:347–353. https://doi.org/10.1016/j.bios.2018.06.005
Acknowledgement
This work was financially supported by the Fundamental Research Grant Scheme (FRGS) from the Ministry of Higher Education of Malaysia (MOHE) (FRGS/1/2019/STG01/UM/02/6).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ng, P.C., Khor, S.M. (2022). Optical Detection of Targets for Food Quality Assessment. In: Chandra, P. (eds) Biosensing and Micro-Nano Devices. Springer, Singapore. https://doi.org/10.1007/978-981-16-8333-6_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-8333-6_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8332-9
Online ISBN: 978-981-16-8333-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)