Abstract
Oligodendrocytes (OLs) are the myelinating cells of the central nervous system (CNS) and myelinate the axons facilitating and boosting the propagation and speed of nerve conduction. In addition, the OLs also provide metabolic support to neurons and enhance their viability, regulate ion and water homeostasis and play a crucial role in learning and memory via white matter (WM) plasticity. Loss of myelin/demyelination is common in many demyelinating and neurodegenerative disorders. The myelin loss may occur either due to direct damage to myelin sheath or indirectly by disruption or death of OLs due to autoimmune attack, injury, stroke and toxic insult or genetic defects involving intrinsic abnormalities in the production and maintenance of myelin. Loss of OLs usually triggers a regenerative remyelination causing the differentiation of OPCs into myelinating OLs and restoration of the myelin sheath. However, the regeneration is poor in many demyelinating disorders. Thus, enhancing remyelination strategies is of human health importance and is under active research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Oligodendrocytes
Oligodendrocytes (OLs), the myelinating cells of the CNS , are found in all vertebrates and are fundamental to myelin formation during development and critical for myelin regeneration following injury and demyelinating diseases. Myelin provides an evolutionary advantage in vertebrates by increasing axial resistance of the axonal surface in addition to reducing its capacitance (Castelfranco and Hartline 2015) and contributes to the emergence of complex and plastic behaviours (Tomassy et al. 2016). Oligodendrocytes are generated from neuroepithelial cells (NEPs ) of the neural tube through a well-concerted process of migration, proliferation and differentiation (Davis and Temple 1994; Rogister et al. 1999; Bradl and Lassmann 2010). The fate specification of the NEPs to oligodendrocyte precursor cells (OPCs) is specifically regulated by the gradients of sonic hedgehog protein during early embryogenesis (Orentas et al. 1999; Cai et al. 2005). A number of transcription factors and epigenetic regulators, microRNAs and intracellular signalling pathways are known to drive the lineage progression of OLs (Emery and Lu 2015; Galloway and Moore 2016; Gaesser and Fyffe-Maricich 2016). Recent advances suggest the role of neuronal activity in the origin, proliferation and differentiation of OPCs and myelin remodelling (Barres and Raff 1993; Hughes et al. 2018). In the developing CNS , not all the OPCs that are generated get differentiated into mature myelinating glia; some undergo apoptosis as they fail to contact an appropriate axon , while others form a significant pool of adult OPCs (Tongatta and Miller 2016). OPCs represent as highly proliferative and migratory bipolar cell population, evenly distributed in WM and grey matter, although less abundant in grey matter (Dawson et al. 2003). In the developing CNS , an appreciable number of WM OPCs differentiate into myelinating OLs as compared to OPCs in the grey matter which persist as NG2+ progenitors (Dimou et al. 2008; Kang et al. 2010). The NG2+ progenitors express chondroitin sulphate proteoglycans, are highly dynamic and proliferative in adult CNS and maintain their population by self-renewal, differentiation and self-repulsion (Dimou et al. 2008; Kang et al. 2010; Hughes et al. 2013). The OPCs , which remain undifferentiated, are stored as potential backup progenitor pool and comprise majority of the proliferating cells in the adult CNS (Dawson et al. 2003). The proliferation and survival of OPCs are mediated by platelet-derived growth factor-α (PDGF-α) produced by astrocytes and neurons, via receptor for PDGF-α (PDGFR-α) expressed by OPCs ; thus, PDGFR-α act as the best characterized marker for them (Noble et al. 1988; Calver et al. 1998). OPCs lose their bipolarity, differentiate into pre-OLs by expressing myelin-specific 2′-3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase ) and the cell surface (O4 and O1) markers (Sommer and Schachner 1981; Braun et al. 1988) and start contacting the target axon to myelinate. Further differentiation into the mature oligodendrocytes is associated with the production of myelin and expression of myelin proteins, myelin basic protein (MBP), proteolipid protein (PLP ), myelin-associated glycoprotein (MAG ), galactocerebroside (GalC) and myelin-oligodendrocyte glycoprotein (MOG ) (Kuhn et al. 2019). A number of intracellular and extracellular signalling molecules are involved in maintaining the balance between OPC proliferation and differentiation (Hughes et al. 2013).
1.1 Myelination
Myelin is a multilamellar lipid structure that wraps around the axons, enables efficient saltatory conduction of nerve impulses and reduces axonal energy consumption (Huxley and Stampfli 1949). In addition to myelination and facilitating propagation and speed of nerve conduction, OLs also provide metabolic support to neurons to enhance their viability, regulate ion and water homeostasis and thus play a crucial role in learning and memory via WM plasticity and activity-dependent adaptive responses in myelin-forming cells that affect the formation of neural circuitry (Funfschilling et al. 2012; Lee et al. 2012; Philips and Rothstein 2017; Monje 2018; Stadelmann et al. 2019).
Pre-oligodendrocytes, the mitotically active OPCs , are the most prominent cell population during 18–28 weeks of human gestation. These cells subsequently become post-mitotic and switch to immature oligodendrocytes, start contacting axons and initiate myelination during 28–40 weeks of gestation (Craig et al. 2003; Dean et al. 2011). Thus, the gestation period between 23 and 32 weeks is considered to be the critical period of oligodendroglial maturation because it is when the OLs are highly vulnerable to infections, hypoxic-ischaemic injury and other insults (Hagberg et al. 2002; Semple et al. 2013). WM is especially more susceptible than grey matter in the pre-term and term infants and also in children, with infections and hypoxic-ischaemic injury or both in conjunction as the most common cause of WM lesions (Hagberg et al. 2002). The myelination in rodents occurs between postnatal days (PND) 10 and 14 and peaks around PND20, when most of the mature OLs expressing specific markers required for myelination and paranodal loop formation are frequently seen (Wiggins et al. 1986; Cahoy et al. 2008). The myelination in mice is completed in first two postnatal months, and the myelination of specific brain areas well correlates with the development of the cognitive functions and clearly depends on the brain area, time course of life, type of neurons, axonal diameter and environmental milieu (Tomassy et al. 2016). Moreover, the myelinated axons also differ in the number, distribution, internodal length and thickness of myelin sheath that defines the precise conduction times and nervous system plasticity (Fields 2015; Klingseisen and Lyons 2018). Development and differentiation of OPCs into myelinating oligodendrocytes can occur independent of axons (Almeida and Lyons 2016) as reported by in vitro studies where OLs can differentiate, mature and efficiently extend processes independent of neurons, suggesting their default potential for differentiation, and launch a programme of myelin gene expression (Simons et al. 2000; Klingseisen and Lyons 2018).
Myelination during development occurs in a conserved and region-specific pattern and follows a complex spatiotemporal sequence depending on the position of the system in the functional hierarchy, initiated in areas dedicated to basic homeostasis, progressing to areas involved in more complex tasks and finally in the areas required for higher-order functions (Yakovlev and Lecours 1967; Brody et al. 1987; Kinney et al. 1988). Moreover, the peripheral nervous system, brainstem and spinal cord are myelinated earlier than the brain, generally advancing from inferior to superior and caudal to rostral (Inder and Huppi 2000). Regions of the occipital lobe are myelinated first and then the temporal and followed by the frontal lobe (Brody et al. 1987; Tasker 2006; Volpe 2000). More so, myelination is faster and finished early in regions that are myelinated first. Early researchers believed that myelination is complete by 3–5 years in humans along with many of the major tracts well myelinated by early childhood (Dietrich et al. 1988; Nakagawa et al. 1998). But more recently it has been reported that axons continue to be myelinated by the second to third decades of the human life, contributing to a net linear increase in the total WM volume by 12% between the ages of 4 and 22 years (Giedd et al. 1999). Thus, the pattern of myelination in the nervous system is a complex process and requires a continuous signalling between the axons and the OLs to select the axons and part of axons that are to be myelinated. The factors mediating such signalling are not well elucidated. In fact, the OLs in culture can myelinate paraformaldehyde-fixed axons (Rosenberg et al. 2008) and even inert axon-shaped fibres (Mei et al. 2016). Moreover, the sheath-like structure covering the inert axon-shaped fibres resembled that of compact myelin, but the inert fibres only with a diameter of 0.4 μm and more were myelinated in vitro, similar to an in vitro observation (Remahl and Hideberg 1982; Lee et al. 2012; Bechler et al. 2015). Using the inert axon-like fibre model, Bechler and group (2015) further reported that when oligodendrocytes were cultured on inert fibres of mixed diameter from 0.4 to 4.0 μm, the myelin sheath was more and even longer around larger diameter fibres, again similar to an in vivo observation made more than a century back by Donaldson and Hoke in 1905. These studies suggested that the length of the myelin sheath may be regulated by the axon and its diameter (Donaldson and Hoke 1905). However, the mechanisms by which the axons differentiate into the fibres of varying diameters and regulate the myelin length and thickness just by contact remain a matter of speculation. Moreover, the dendrites are never myelinated, indicating that diameter alone may not be responsible for the selection of axons by oligodendrocytes for myelination. The complete myelination requires electrically active neurons and healthy axons. It is now clear that extrinsic signals are required for the precise selection of axons for myelination as the oligodendrocytes show the bias only for axons, avoid inappropriate targets and show preference for more active axons (Wake et al. 2015; Koudelka et al. 2016). Once the internodal myelin sheath is formed, it grows both radially and longitudinally to a specific thickness and length that provide specific conduction property to the axon (Ford et al. 2015). The role of neuregulin-ErbB signalling is primarily important for the myelination and identification of axon calibre in the peripheral nervous system by Schwann cells (Birchmeier and Nave 2008), while its role is insignificant in the CNS myelination (Brinkmann et al. 2008). Moreover, Schwann cells are not able to myelinate the inert fibres (Bechler et al. 2015). Although the role of electrical signalling, glutamate release and neuregulin signalling or interactions with extracellular matrix molecules have been reported to modulate myelination in a limited way (reviewed by Stadelmann et al. 2019), further research is required to explore in more detail the factors that would correlate the signals with the axon calibre to determine myelination.
Myelination is developmentally prolonged in humans than in non-human primates. In chimpanzees, density of myelinated axons reaches to the maximum level by adolescence in most cortical areas. However, in the human cerebral cortex, only a few numbers of axons are myelinated by birth; the myelination is slower during childhood and extended beyond adolescence to early adulthood. Moreover, the primary cortical areas are myelinated earlier than the association cortical areas followed by the late maturing brain areas such as pre-frontal cortex in the last (Yakovlev and Lecours 1967; Knickmeyer et al. 2010; Shaw et al. 2008; Miller et al. 2012). Most of the association areas continue to be myelinated in the third decade of life (Lebel et al. 2012; Williamson and Lyons 2018). The dynamic maturation of association and projection pathways involved in maintaining cortical and brainstem integration occurs during adolescence and is accompanied by the maturation of critical cognitive functions (Asato et al. 2010; Kumar et al. 2013). Thus, the gradual maturation of WM from childhood to early adulthood is fast and dynamic with remarkable increase in fibre density and the myelination (Lebel et al. 2019). WM structure becomes more or less static by mid-adulthood and subsequently shows degenerative changes during the latter part of life (Lebel et al. 2012).
The development of an individual is associated with the maturation of WM pathways that connect distant and proximal brain regions and are essential elements of higher-order cognitive processing (Fields 2010; Buyanova and Arsalidau 2021). About more than 50% of the adult brain volume represents WM and is crucial for sensory (Chang et al. 2016), motor (Hollund et al. 2017) and higher-order executive functions (Ohlhauser et al. 2018). Communication of neural signals is essentially required for humans to move, think, feel and respond. WM consists of neuronal fibres with varying degrees of myelination that allow the transfer of signals across different brain regions at different rates. Moreover, most glia are generated during either late embryogenesis or postnatal life, suggesting that development continues beyond birth and even adolescence. With glia being the dynamic cells, the continued active neuron-glia interactions actively chisel and remodel the nervous system throughout life.
1.2 Structure and Composition of Myelin
Myelin is a large spade-like extension of the plasma membrane of the myelinating glia that wraps around the axons to form a multi-layered stack visualized as a periodic structure of alternating major dense and intraperiod lines at the tightly apposed and compacted cytoplasmic and outer membrane surfaces, respectively, resulting in the periodicity of ~12 nm (Aggarwal et al. 2011; Nave and Werner 2014). The compacted myelin is devoid of cytoplasmic components except at the edges, where it forms a continuous network of clustered cytoplasmic channels and forms complex axo-glial junctions between the terminal ends of the myelin sheath, ~4 μm long, called as paranodal loops and 10–15-μm-long inner tongue that runs along the axon under the myelin sheath called as juxtaparanode (Hildebrand et al. 1993; Nave 2010; Stadelmann et al. 2019). In between the paranodes of the two adjacent myelin sheaths is the nodal region which is covered by paranodal astrocytic processes. The axon is usually constricted in the nodal region and is strikingly visible in larger fibres (Hildebrand 1971). The paranodal axon-glial junctions provide electrical insulation by restricting current flow beneath the myelin sheath and help to segregate the voltage-gated sodium channels at the nodes from the potassium channels at the juxtaparanodes (Rasband et al. 2001).
Three integral myelin proteins, myelin basic protein (MBP), 2′-3′cyclic nucleotide-3 phosphodiesterase (CNP ) and proteolipid protein (PLP ), play significant roles in the myelin architecture. MBP is essential for the compaction of two cytoplasmic interfaces of myelin lamellae and formation of major dense line (Wolf et al. 2021). PLP , a transmembrane protein in CNS myelin, contributes to the tight apposition of the extracellular surfaces to each other forming a double intraperiod line and also mediates the closure of cytoplasmic channels (Snaidero and Simons 2014). CNP helps in maintaining the functional cytoplasmic-rich compartment in myelin and mediates the interactions with the actin cytoskeleton to keep the channels open (Snaidero et al. 2017). Other important CNS myelin proteins include myelin-associated glycoprotein (MAG ) involved in axo-myelin interactions; myelin oligodendrocyte glycoprotein (MOG ) located on the surface of the compacted myelin, involved in adhesion and interactions between adjacent sheaths within axon fascicles to provide structural integrity to myelin sheath; and myelin oligodendrocyte basic protein (MOBP) located in the major dense lines, which play a role in the compaction and stabilization of myelin (Montague et al. 2006; Pronker et al. 2016). Claudin-11 is essential for the formation of radial component of CNS myelin, which is a network of interlamellar tight junctions involved in mediating adhesion between myelin membranes and potentiates the insulative properties of myelin (Devaux and Gow 2008; Denninger et al. 2015).
About 70–75% of the dry weight of myelin is lipid, rich in saturated long-chain fatty acids that affect membrane thickness and the packing density of lipids in the myelin. Major myelin lipids are cholesterol, phospholipids, galactolipids and plasmalogens in the ratio of 2:2:1:1, respectively (Norton and Poduslo 1973; Schmitt et al. 2015). Cholesterol is an essential component of CNS myelin membranes and accounts for about 80% of the total brain cholesterol (Dietschy 2009). In the brain, cholesterol is not imported from the blood circulation; rather, it is synthesized by the cells of the brain and switches from neurons during embryogenesis to oligodendrocytes during postnatal life to astrocytes during adulthood (Morell and Jurevics 1996; Saher et al. 2015). The bulk of the cholesterol incorporated into the myelin is synthesized by oligodendrocytes. Thus, cholesterol is the only integral myelin component, and its availability in oligodendrocytes is a rate-limiting factor for brain maturation as shown in mutant mouse or zebrafish lacking enzymes essential for cholesterol synthesis (Saher 2005, 2015). Saher and associates (2015) also emphasized that cholesterol is involved in many aspects of myelin biogenesis and an interference with cholesterol homeostasis in the brain would affect the synthesis and maintenance of myelin. Myelination is also affected either directly or indirectly in disorders that interfere with synthesis or intracellular trafficking of cholesterol.
By using live in vivo imaging in zebrafish using electron microscopy, Snaidero and his associates, in 2014, explained the mechanism of myelin layer wrap around the axons. They showed that the newly generated myelin layers settle by constant coiling of the innermost tongue of the myelin sheath over the axon along with the lateral spread of myelin along the axon . The dynamic changes in actin assembly facilitate the development of myelinic channels in uncompacted myelin that help in the transportation of metabolites from oligodendrocyte cytoplasm to the myelin and also to the neurons (Zuchero et al. 2015; Snaidero et al. 2017). Compromised metabolic support from oligodendrocytes to neurons causes the degeneration of certain neuronal subpopulations and neurodegenerative disorders. This suggests the importance of oligodendrocytes as metabolic supporters of neurons and neuronal homeostasis (Philips and Rothstein 2017).
Recent research suggests that myelination occurs in two phases: (a) genetically pre-defined intrinsic phase, occurring around birth to early childhood that proceeds in a precise spatiotemporal order, and (b) adaptive myelination driven as per the need of the neural network, which can be modified by experience leading to myelination variability in different individuals (Fields 2008; Chang et al. 2016; Mount and Monje 2017; Bechler et al. 2018). Adaptive myelination occurs more prominently in brain areas involved in complex behaviour, where the continuous myelination helps to fine-tune neuronal network function by synchronizing the firing pattern (De Hoz and Simons 2015; Filley and Fields 2016) and shape the myelination as per the requirement of the neuron and its network. Human MRI studies pointing to experience-based changes in WM and ultra-microscopic analysis of changes in myelin structure upon learning new tasks in animal models clearly indicate that myelination is influenced by experience (Oztürk et al. 2002; Bengtsson et al. 2005; Steele et al. 2013; McKenzie et al. 2014; Xiao et al. 2016). Thus, myelin deposition is a dynamic and plastic process showing adaptive changes in response to neuronal activity (Chang et al. 2016; Xiao et al. 2016; Gibson et al. 2014; Fields 2015). Neuregulin-ErbB signalling regulates the switch between intrinsic and adaptive myelination (Lundgaard et al. 2013). The oligodendrocytes are intrinsically capable of generating myelin, but the neuronal activity finally helps in adjusting the myelin thickness, suggesting that the role of electrical activity helps in myelin remodelling (Gibson et al. 2014). In addition, oligodendrocytes in different CNS regions have intrinsic differences (Crawford et al. 2016; Dimou and Simons 2017) with spinal cord oligodendrocytes forming longer sheath than cortical oligodendrocytes (Bechler et al. 2015).
Astrocytes also participate in the process of myelination by secreting soluble growth factors. Astrocyte-derived PDGF-α and leukaemia inhibitory factor (LIF )-like proteins are essential for the survival and differentiation of OPCs (Noble et al. 1988; McKinnon et al. 2005; Gard et al. 1995). In addition, astrocytes also transport lipids to oligodendrocytes for the generation of large myelin sheaths (Abrams 2017), and failure to this leads to persistent myelin deficits (Camargo et al. 2017). Astrocytes couple with oligodendrocytes through gap junctions formed by connexins and also with nodes of Ranvier and thus provide metabolic support to axons (Abrams 2017). Neonatal microglia promote myelination via insulin-like growth factor (Wlodarczyk et al. 2017).
Research information gathered during the last two decades suggests that in both humans and mice, the myelination is an ongoing process and continues throughout adult life either by generating new myelinating oligodendrocytes from their precursors (OPCs ) or by remodelling of prevailing myelin to renew growth long after its initial formation for enhanced cell function (Young et al. 2013, 2014; Snaidero et al. 2014; Jeffries et al. 2016). Substantial changes in WM have been considered as a key regulatory factor for higher-order brain functions. This was well demonstrated in a mice model of social isolation stress imposed during critical period of postnatal development and myelination or beyond adulthood, which resulted in defects in myelination and associated behaviour (Makinoden et al. 2012; Liu et al. 2012), while motor learning in animal models was seen to promote the differentiation of adult OPCs and myelination (McKenzie et al. 2014). The proliferation of OPCs /NG2+ glia is also intensified in conditions of demyelination, traumatic injury to CNS and chronic neurodegenerative diseases (Magnus et al. 2008; Kang et al. 2010). The WM volume also decreases gradually with normal ageing (Sowell et al. 2003), and the frequent observations are changes in myelinated nerve fibre morphology and degenerative changes in myelin sheaths (Peters 2002). The ageing WM is extremely vulnerable to degeneration and loss of myelin. In ageing humans, the loss of myelin integrity correlates well with the decline of cognitive functions (Bastin et al. 2010). Although the WM degeneration is not uniform throughout the CNS with the association tracts more susceptible to myelin loss than the projection tracts, the myelin deficits in the corpus callosum are commonly seen in all men and women at old age and might be a crucial factor for age-associated impairment in cognition and working memory (Sullivan et al. 2001; Kohama et al. 2012; Hedden et al. 2016). Other factors like nutritional deficiencies, early life infections and stress have also been found to cause myelin deficits. In an intra-generational protein deprivation rat model, we have also reported drastic changes in oligodendrogenesis in terms of reduction in oligodendrocyte progenitor pool, reduced expression of myelin genes leading to hypo-myelination, disorganized myelin fibre alignments, reduced corpus callosum calibre and associated behavioural deficits persisting through pre-adolescence to late adulthood (Patro et al. 2019). Early life exposure to LPS-induced bacterial infection also results in demyelinating changes during adulthood and senility by altering the expression of myelin proteins resulting in motor coordination deficits (Singh et al. 2017).
1.2.1 Defects in Myelination and Neuropathologies
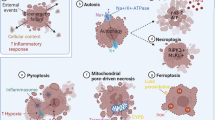
Developmental myelination and remyelination in adult CNS are high energy-demanding tasks as the synthesis of myelin sheath requires vast quantities of lipids and proteins (Baron and Hoekstra 2010). To meet this demand, oligodendrocytes consume a large amount of metabolites, like glucose and lactate, which are supplied from the diet or from the stored protein, fat and glycogen source, which needs to be continuously refuelled. This high energy demand imposed on oligodendrocytes makes them susceptible to oxidative stress, cytotoxic by-products and excitotoxic factors leading to their altered functions (Juurlink et al. 1998; Matute et al. 1997, 2007). Oligodendrocyte pathology is clearly seen in many disorders like Alzheimer’s disease, multiple sclerosis (MS ), schizophrenia, traumatic injuries and ischaemia, some leukodystrophies or autoimmune attacks and demyelinating disorders (Fancy et al. 2011; Traka et al. 2016; Torii et al. 2014; Duncan and Radcliff 2016; McAleese et al. 2017). In addition, the ageing is also associated with WM atrophy, gradual impairment in motor learning and reduced remyelination ability (Sim et al. 2002; Ruckh et al. 2012).
Myelin has recently been reported to provide metabolic support to neurons crucial for axonal integrity and neuronal activity, indicating its persistent role in neural circuit formation and functions throughout life (Saab et al. 2016). This emphasizes that disruption of myelin and myelin-forming oligodendrocytes in CNS may have significant neurological manifestations and may lead to neurodevelopmental, neurodegenerative and neuropsychiatric pathologies, viz. leukodystrophies, schizophrenia, multiple sclerosis, amyotrophic lateral sclerosis and others (Compston and Coles 2008; Franklin et al. 2012; Pouwels et al. 2014; Jin et al. 2015; Miyata et al. 2015). Multiple factors have been reported to variously affect the process of myelination, notably its proportion and timing. Intra-generational protein malnutrition negatively affects overall development of the brain, oligodendrocyte development and maturation, impaired myelination and motor deficits at adolescence and later (Patro et al. 2019). Other nutritional deficiencies, viz. acquired or genetic B12 or folate deficiencies, also lead to delayed myelination or even white matter disturbances, cortical insults and peripheral neuropathy (Prado and Dewey 2014; Kobayashi et al. 2016). Thyroid hormone is required for the terminal differentiation of OPCs into myelinating cells, and thus hypothyroidism also leads to delayed myelination (Barres et al. 1994; Lee and Petratos 2016). In addition, early life exposure to viral (our data under publication) and bacterial infections also leads to demyelinating lesions in the adult brain leading to poor motor coordination (Singh et al. 2017).
Myelin diseases are generally grouped into three types, demyelinating, dysmyelinating and hypomyelinating disorders, caused by loss of myelin, abnormal myelin production or compromised myelin production, respectively. In demyelinating disorders, the myelin loss may occur either due to direct damage to myelin sheath or indirectly by disruption or death of OLs consequent upon inflammation or toxic insults and also by axonal injury through Wallerian degeneration. The dysmyelinated diseases primarily occur due to genetic defects involving intrinsic abnormalities in the production and maintenance of myelin and appear early in life during childhood or adolescence. In the hypomyelinating conditions, the axons are either unmyelinated or myelinated with thin myelin sheaths, mainly due to the genetic defects or epigenetic adversities. The myelin pathologies have also been categorized into inherited and acquired based on their origin whether genetic, inflammatory or toxic (Duncan and Radcliff 2016). Antibody-mediated diseases, primary axonal pathologies and structural protein defects are preferentially associated with myelin damage, while viral infection and genetic and metabolic deficiencies negatively influence OL survival.
1.2.2 Primary Demyelinating Diseases
1.2.2.1 Multiple Sclerosis
Multiple sclerosis (MS ) is the most common and well-researched acquired demyelinating human disease affecting CNS myelin. It affects individuals of all age groups but most prevalent in young adults (Reich et al. 2018) and forms the foremost cause of nontraumatic neurological disability in the young and middle-aged population. The occurrence is 2.3 times more prevalent in females, and the susceptibility is strongly related to the human leukocyte antigen (HLA) locus, and homozygosity at the HLA-DRB1*15 gene locus increases the incidence to develop the disease (Stadelmann et al. 2019). Moreover, the polymorphism in genes regulating both the innate and adaptive immunity, regulatory and cytotoxic T-cell and microglia functions present higher risk of disease (Sawcer et al. 2011).
MS is mediated by autoreactive immune cells, myelin-specific CD8+ T cells, targeting the myelin and the OLs, initiating myelin damage and axonal injury and leading to sensory-motor and/or visual impairments (Noseworthy et al. 2000; Trapp and Nave 2008; Filippi et al. 2018). Clinically, the disease may be classified into relapsing-remitting MS , primary progressive MS and secondary progressive MS . The hallmarks of MS are demyelinating lesions in the CNS , characterized by immune cell infiltration across the blood-brain barrier (BBB ), triggering an inflammatory response, myelin damage, activation of astrocytes and microglia and axonal injury (Matveeva et al. 2018). With disease progression, more striking grey and white matter lesions are seen with the persistent gliosis throughout the course of the disease (Dendrou et al. 2015). Moreover, in the MS patients, the components of the myelin sheath, like MBP , MOG and PLP , have been identified as autoantigens mainly by the CD4+ T cells (Androutsou et al. 2018).
Following focal demyelination, the consequences of the autoimmune attack are progressive, leaving the axons exposed to toxic environment, inefficient and vulnerable to degeneration, ultimately leading to axonal loss and neuronal death, a pathological hallmark of MS (De Stefano et al. 1998; Bradl and Lassmann 2010; Duncan and Radcliff 2016). Metabolic dysfunction is commonly associated with the pathogenesis of MS along with impaired mitochondrial functioning, oxidative stress resulting in axonal energy failure and subsequent neurodegeneration (Adiele and Adiele 2017). Impaired energy metabolism also leads to oligodendrogliopathy by either lack of blood supply to lesions or the production of toxic metabolites (Lassmann 2003).
Remyelination is triggered during the early phase of the disease by the generation of new mature OLs, which help in restoring the myelin to sheath the denervated axons and recover the saltatory conduction, axonal integrity and functional deficits (Kierstead and Blakemore 1999; Franklin and French-Constant 2008). Recently, Duncan and associates (2018) have indicated that mature OLs can also help in remyelination. However, in later progressive phase of MS , remyelination is not sufficient to recondition the severe demyelination, leading to remyelination failure and exacerbating to chronic relapsing progressive MS (Franklin 2002; Goldenberg 2012; Gruchot 2019). There are two potential sources of myelinating OPCs that participate in the remyelination of MS axons: the parenchymal OPCs and the endogenous NSCs . Parenchymal OPCs are more sensitive to endogenous growth factors produced by resident CNS cells than the mature OLs. The proliferation, differentiation and maturation of the OPCs to mature OLs are strongly influenced by the extracellular milieu and crucial to regain homeostasis and replace the lost OLs (Duncan et al. 2021). But the absence of the appropriate signalling factors in the MS environment inhibits the differentiation of OPCs to mature OLs and prevents successful remyelination (Franklin 2002; Gruchot 2019). Moreover, poor clearance and accumulation of the myelin debris at the MS lesion sites may also impair the differentiation of oligodendroglial lineage cells (Kotter et al. 2006; Baer et al. 2009).
Multiple molecular pathways have been identified that block the differentiation of OPCs into mature OLs. These pathways are potentially targeted to explore therapeutic interventions to promote repair. Some of these pathways include the dysregulation of Wnt pathway (Fancy et al. 2009), signalling through glycosaminoglycan hyaluronan that accumulates at the site of MS lesions (Back et al. 2005; Sloane et al. 2010) and axonally derived neuregulin (Vartanian et al. 1999) that prevents the differentiation of OL precursors and contributes to the remyelination failure. Chronic metabolic stress in MS lesions also contributes to remyelination failure because of OPC dysfunction and dying back of the OL terminal processes leading to the destabilization of myelin/axon interactions (Rone et al. 2016). Together, these findings suggest that the impaired differentiation of OPCs is the major cause of remyelination failure in MS , not the deficiency of OPCs (Kuhlmann et al. 2008).
There are many diseases that are associated with MS and are listed under demyelinating disorders: (a) neuromyelitis optica (Devic’s disease), a variant of MS or a separate demyelinating disease which primarily attacks the optic nerve and spinal cord; (b) Balo’s disease (concentric sclerosis), a rare demyelinating disorder in which the CNS myelin is damaged; (c) Schilder’s disease (diffuse sclerosis), an acute rapidly progressive, degenerative, demyelinating disease of the CNS , seen in childhood; and (d) Marburg’s disease, a variant of MS , characterized as acute, fatal, fulminant or malignant MS showing acute demyelination.
Several murine models mimicking MS have been developed, viz. (1) inflammation-dependent, experimental autoimmune encephalitis (EAE) and viral encephalomyelitis and (2) inflammation-independent, chemical and toxin-induced demyelination using EtBr, lysolecithin, cuprizone, etc. These models have been useful in replicating various clinical, immunological and microscopic aspects of the human MS and helped to understand the role of immune components in CNS myelination and repair as well as the complex nature of the CNS environment to enable to develop novel therapeutic modalities (Miller et al. 2001; Bergmann et al. 2006; Mecha et al. 2013; Borjini et al. 2016).
1.2.3 Secondary Demyelinating Diseases
1.2.3.1 Acute Disseminated Encephalomyelitis (ADEM)
ADEM is an acute inflammatory demyelinating disease of the CNS , caused mainly due to the exposure of antigens through upper respiratory and gastrointestinal infections or after immunizations (Scolding 2014). Clinicopathological features of ADEM show multiple demyelinating lesions confined to the perivenular tissue in specific brain regions, with minimal signs of progressive neurodegeneration that are typical of MS , suggesting different pathogenic mechanisms of the two diseases. The perivascular inflammatory infiltrate consists of T cells, foamy macrophages, granulocytes and eosinophils (Hart and Earle 1975; Young et al. 2010).
Although the mechanism of ADEM pathogenesis is not clear, antibody reactivity against myelin proteins has been found to be critical. Antigens such as MBP , PLP and MOG are the targets of the reactive T cells, as was revealed from a study reporting the presence of IgG antibodies reacting to various myelin proteins in the CSF of patients (Cole et al. 2019). IgG antibodies against MOG are frequently found in the serum of patients with inflammatory demyelination and paediatric ADEM (Mader et al. 2011; Hoftberger and Lassmann 2017), and thus MOG detection serves as a reliable diagnostic marker of these diseases. MOG antibodies initiate demyelination and cell death by activating the complement cascade and contribute to the disease pathogenesis. Perivascular demyelinating lesions are usually surrounded by macrophages containing residual myelin proteins and infiltrates of T and B cells, granulocytes, plasma cells and activated astrocytes and microglia (Scolding 2014; Popescu and Lucchinetti 2011). Axons are mainly spared, but finally show features of injury during acute demyelination in ADEM , and the exudate from the wall of the blood vessels leads to necrosis of the neighbouring tissue showing the conjoining features of ADEM and acute haemorrhagic leukoencephalopathies (Hoftberger and Lassmann 2017). Most patients respond to treatment and recover, but others survive with mild to moderate neurological deficits (Cole et al. 2019).
1.2.3.2 Neuromyelitis Optica (NMO)/Neuromyelitis Optica Spectrum Disorder (NMOSD)
NMO also known NMOSD or Devic’s syndrome is a severe inflammatory demyelinating disease of unknown aetiology that predominantly affects the optic nerves and spinal cord, causing blindness and motor paralysis (Lennon et al. 2004; Weinshenker and Wingerchuk 2017). The disease is basically allocated to AQP4 serum antibodies (NMO-IgG) that target primarily the astrocytes, progressing to oligodendrocyte and myelin damage as well as substantial axonal loss (Misu et al. 2007; Parratt and Prineas 2010; Wingerchuk et al. 2015). The disease pathogenesis involves the potential of anti-AQP4 antibodies causing the local activation of the complement system leading to astrocyte death and early loss of oligodendrocytes and OPCs , subsequently leading to loss of myelin and formation of NMO typical lesions, distinct from MS (Parratt and Prineas 2010; Wrzos et al. 2014; Tradtrantip et al. 2017). The near-complete absence of astrocytes and oligodendrocytes in the NMO lesions fails to support the oligodendrocyte regeneration and myelin repair (Parratt and Prineas 2010). Axons are preserved in early NMO lesions, but in the chronic disease stage, axon loss and spinal cord atrophy are usually intense (Wrzos et al. 2014; Herwerth et al. 2016).
1.2.4 Leukodystrophies
1.2.4.1 Demyelination Due to Mutations and Defects in Oligodendrocyte- and Myelin-Related Genes
Genetic mutations in OL-specific genes, viz. the genes encoding structural myelin proteins, enzymes involved in various metabolic pathways and proteins involved in myelin development and maintenance, are associated with hypomyelination, dys−/demyelination or myelin swelling (van der Knapp and Bugiani 2017).
The Pelizaeus-Merzbacher disease (PMD) and X-linked severe spastic paraplegia (SPG2) are inherited myelin disorders caused by mutation in PLP1 gene that encodes for the structural myelin membrane protein, PLP , and its alternatively spliced form DM20. About 60–70% of the PMD patients show duplication of PLP1 gene, while the rest of the PMD cases reveal missense or point mutations, insertions and deletions (Torii et al. 2014). As PLP is essentially required for the apposition of the myelin sheaths, any defects in its expression may be deleterious for the CNS functioning. PLP duplication generally causes classical PMD with patients showing hypomyelination in the cerebral and cerebellar WM , while the grey matter is comparatively preserved (Harding et al. 1995). Mature OLs are completely absent, because of massive apoptosis due to the altered myelin membrane composition, abnormal level and accumulation of PLP and associated lipids and toxic gain in function in OLs (Simons et al. 2002; Karim et al. 2007; Sima et al. 2009; Torii et al. 2014). PLP null mutations also result in hypomyelination, but the myelin loss is much less than the PLP duplication in PMD patients. Although the WM appears to be well myelinated, axonal swelling and Wallerian degeneration are frequently present (Garbern et al. 2002; Sima et al. 2009). This suggests that axonal survival is crucially dependent on proper myelin function (Nave and Werner 2014). Moreover, several point mutations in PLP1 gene have also been reported to cause demyelination and oligodendrocyte loss ranging from mild to severe connatal forms (Calloux et al. 2000; Hübner et al. 2005).
The Pelizaeus-Merzbacher-like disease (PMLD) is a recessive inherited demyelinating disease caused by mutations in GJC2 gene encoding the OL-specific connexin , Cx47, involved in gap junctional communication for myelin development and maintenance (Menichella et al. 2003; Abrams and Orthmann-Murphy 2013). Demyelination in PMLD patients occurs due to the loss of function of gap junctional communication in mature oligodendrocytes, oligodendrocyte apoptosis and defective myelin development and maintenance.
Mutations in genes related to lipid metabolism are also associated with many leukodystrophies. Metachromatic leukodystrophy (MLD) is an autosomal recessive leukodystrophy caused by mutation in ARSA gene encoding for the lysosomal protein arylsulphatase A (ASA) or mutation in PSAP gene encoding prosaporin, an activator of ASA (Cesani et al. 2016). ASA plays a crucial role in the metabolism of sulphatides, a major myelin lipid, and the defects in its metabolism lead to its impaired degradation and intra-lysosomal accumulation in the nervous system and other visceral organs (Eckhardt 2008). Lipid accumulation directly causes the demyelination leading to the death of OLs accompanied by myelin destruction and severe axonal damage (Kohlschütter 2013). However, the pathophysiological mechanisms linking the metabolic alterations and myelin abnormalities with axonal dysfunction are still ambiguous.
1.2.4.2 Mutations in Microglial and Astrocytic Genes and Leukodystrophies
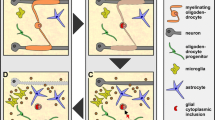
Microglia , the resident immune cells of the CNS , are now well documented to play a role in myelin homeostasis and development as is clear from two hereditary diseases, diffuse leukoencephalopathy with spheroids (HDLS ) and the Nasu-Hakola disease (NHD ), caused by mutations in microglial genes. The affected genes [colony-stimulating factor 1 receptor (CSF1R) in HDLS and TREM2 and DAP12 in NHD ] involved in both the diseases are key regulators of microglial activation and neuroinflammatory pathways (Rademakers et al. 2012; Paloneva et al. 2000, 2001). Clinically both the diseases present similar features with demyelination and axonal loss in defined brain areas, axonal swellings and spheroids, myelin vacuolization in demyelinating areas, widespread astrogliosis and tau-positive neurites in the cortex (Baba et al. 2006; Paloneva et al. 2001; Robinson et al. 2015). The mechanism linking the microglial dysfunction and demyelination is not clearly resolved. However, there are reports that suggest that demyelination might arise as a consequence of primary neuroaxonal damage and microglial dysfunction, impaired OPC homeostasis and reduced microglial number affecting myelin clearance and persistent demyelination (Stadelamnn et al. 2019).
Research in the last decade has revealed a highly specialized role of astrocytes in the physiology and pathology of the nervous system ranging from protective to destructive role (Pekny et al. 2016). Although there is only scanty information about the role of astrocytes in OL health and myelin development, maintenance and pathology, the demyelinating disorders caused by the astrocyte-specific genes clearly indicate the intercellular interactions between these two cell types. Mutation in GFAP gene, encoding an intermediate filament protein expressed in astrocytes, causes an autosomal dominant disorder, Alexander disease. GFAP overexpression might be involved in the pathogenesis of Alexander disease through gain-of-toxic function of GFAP and astrocyte dysfunction (Messing et al. 1998). The mechanism linking the astrocyte dysfunction with demyelination might include (a) the increased expression of CXCL10 in astrocytes that may directly affect OLs or exert immune response triggering demyelination (Olabarria and Goldman 2017), (b) loss of gap junctional communication and altered buffering capacity of astrocytes that may lead to intramyelinic oedema (Sosunov et al. 2013) and (c) extracellular deposition of hyaluronan, an astrocytic protein that deposits in extracellular space and might inhibit OPC differentiation and maturation (Bugiani et al. 2013). More recent research proposed that GFAP mutation defects may directly interfere in the cellular development and OPC differentiation from neural progenitor cells expressing GFAP (Gomez-Pinedo et al. 2017).
Vanishing white matter disease (VWM), which is one of the most prevalent leukodystrophies with a central role of astrocytes in its pathogenesis, mainly affects the CNS . The disease is inherited in an autosomal recessive manner and is characterized by childhood onset chronic neurological deterioration, signified by cerebellar ataxia. It is caused by mutation in any of the genes encoding the five subunits of the eukaryotic translation initiation factor 2B (eIF2B; Bugiani et al. 2011, 2018). Defective maturation and dysfunction of astrocytes due to abnormal composition of its cytoskeletal protein GFAP and an upregulation in the heat shock protein αB-crystallin along with the co-existence of increased density of pre-myelinating OL progenitors might be involved in the loss of WM in VWM (Bugiani et al. 2011). In their later publication, Bugiani and his group (2018) stressed that the astrocytes play a central role in the pathogenesis of VWM with the secondary effects on oligodendrocytes and axons; thus, VWM may be grouped with astrocytopathies in which the loss of essential function and gain of detrimental function by astrocytes could be driving the WM degeneration and VWM pathogenesis (van der Knapp and Bugiani 2017).
1.2.4.3 Viral Encephalopathies
The most common human demyelinating diseases with viral aetiology include progressive multifocal leukoencephalopathy (PML), acquired immunodeficiency syndrome (AIDS) caused by human immunodeficiency virus (HIV) and subacute sclerosing panencephalitis (SSPE). The common mechanism of demyelination in most viral CNS infections involves OL damage/loss and the breakdown of the cellular machinery required for myelin synthesis.
PML is the most studied fatal demyelinating disease caused by JC virus (JCV) infection, a double-stranded DNA polyomavirus, commonly seen to affect the CNS of individuals on immune-modulatory therapies (Major 2010). JCV induces demyelination by infection, followed by lysis of the OLs, which subsequently infects the surrounding OLs and results in focal demyelination. The infected OLs contain inclusion bodies with viral particles in the nuclei and show nuclear swelling and loss of chromatin leading to their demise (Richardson 1961). The neurons and astrocytes are also infected to some extent (Wollebo et al. 2015). The infected astrocytes appear hypertrophied with irregular and lobulated nuclei and give bizarre appearance, and their presence in areas without apparent demyelinating lesions might be involved in viral propagation (Seth et al. 2004). Demyelination finally results in axonal dysfunction leaving the axons susceptible to toxic products released by the surrounding glial cells causing the retrograde loss of neuronal cell body. Microglia and macrophages are not infected by JCV but are seen in the centre of the demyelinating lesions (Ferenczy et al. 2012).
The patients with AIDS show tendency to develop multiple CNS infections with high incidence to develop PML . The pathogenesis of HIV-associated encephalomyelitis may involve an immune-mediated “bystander effect” with myelin destruction via cytokines released by activated monocytes and lymphocytes (Corral et al. 2004). In HIV-PML patients, massive necrotic demyelinating lesions are seen with the infiltration of HIV-infected macrophages and microglia (Wiley et al. 1988). Persistent viral infection caused by defective measles virus causes another progressive neurological disorder known as SSPE, a progressive fatal demyelinating disease. The virus destroys the host cells, including oligodendrocytes, and initiate inflammatory responses resulting in demyelination.
1.2.4.4 Vascular (Hypoxia/Ischaemia)
WM abnormalities in the elderly individuals usually result from ischaemia, secondary to the damage of the cerebral arteries. The degree of WM abnormality directly correlates with the impaired motor and cognitive abilities. Other vascular WM abnormalities include postanoxic encephalopathy developed after a severe anoxic episode, reversible posterior leukoencephalopathy and Binswanger’s disease.
1.2.4.5 Metabolic/Nutritional
Alcoholism, malnutrition, disability and other debilitating conditions also lead to the demyelination, commonly called as central pontine myelinolysis involving severe damage to the myelin sheath of nerve cells in the pons. This condition is also known to be associated with liver or kidney failure, diabetes mellitus, immunosuppressive therapy and long-term usage of some other drugs. Demyelination is usually seen without inflammatory response sparing the blood vessels, most neurons and axons. Alcoholism and nutritional deficiencies are also known to cause sustained demyelination of the corpus callosum. Thiamine deficiency leads to severe memory impairment and anterograde amnesia, a hallmark of the Wernicke encephalopathy .
1.2.4.6 Other Concerns for Demyelination
A variety of chemical substances are also known to damage myelin sheath or OLs or both. Most of them are known as myelinotoxic or glia toxic chemicals and include hexachlorophene (HCP), triethyltin (TET), lysolecithin, ethidium bromide, zymosan and cuprizone. Most of them are used experimentally to create animal models, but some of them are known to affect human beings through exposure (Duncan and Radcliff 2016). There are other toxins and nutritional deficiencies that cause myelin vacuolation disorders. Most of the toxin are used experimentally and include cycloleucine, sodium cyanate, actinomycin D, isoniazid, 6-amino-recotinamide, etc. In addition, myelin vacuolation is also noted in cases of vitamin B12 deficiency, genetic deletion of Cx30 or Cx40, knockout of the enzyme UDP-galactose:ceramide galactosyltransferase and duplication of laminin 1 gene in mouse.
Traumatic injury of the brain (TBI ) or spinal cord can also lead to death of oligodendrocytes and demyelination (Plemel et al. 2014; Mierzwa et al. 2015). In both the cases, damage to the myelin may be caused by ischaemic effect on OLs primarily due to excitotoxicity and glutamate elevation (Tsutsui and Stys 2013). Radiation therapy commonly used to treat brain tumours can also cause demyelination by killing the OPCs (Panagiotakos et al. 2007). In addition, there are a variety of other demyelinating diseases and WM disorders in which myelin is damaged and are being increasingly identified because of the modern imaging techniques and the next-generation sequencing to identify the mutant gene; these are beyond the scope of this chapter.
1.2.4.7 Remyelination Strategies
As detailed vide supra, the disruption of OLs or the myelin sheath following injury and disease bears direct consequences on the function of neurons. In addition to demyelination, long-term effects of OL death include axonal atrophy and neuronal loss and form the major cause of many neurological disorders, including MS , inherited leukodystrophies of the CNS and the neuropathies of peripheral nervous system (PNS) (Nave 2010). Demyelination is a common factor in most of these diseases, thus becoming a therapeutic target with enormous potential. However, the endogenous myelin repair/remyelination will occur in a long time (Duncan et al. 2020). Moreover, in the remyelinated tissue, the myelin sheaths are shorter and thinner with lower conduction amplitude than the developmental myelin sheath (Gallo and Deneen 2014; Almeida 2018). Although the endogenous remyelination does occur in both CNS and PNS , the process is much less impressive in CNS (Franklin and French-Constant 2008). Moreover, remyelination being a time-consuming process, acute demyelination can finally lead to cell death before the remyelination happens. OPCs are the largest source of endogenous progenitors in CNS . During remyelination, the OPCs become multipotent and proliferative, migrate to the site of lesion, exit the cell cycle and differentiate into myelin producing OLs (Silveira et al. 2021). Although the mature OLs in remyelination have also been reported (Duncan et al. 2018; Macchi et al. 2020), their contribution is still a matter of speculation and needs further clarification. Thus, OPCs provide the majority of remyelination. Moreover, there are many barriers of endogenous remyelination that limit the regeneration in the CNS . Thus, treatments and therapies targeting OLs and remyelination may have widespread potential for application. Most treatment strategies used are targeted to prevent progressive demyelination that contributes to chronic disability, but a few can promote remyelination. Various translational approaches have been devised using several animal species and models for TBI /SCI and MS . These include (a) extrinsic and intrinsic factors that act as either the inhibitors or stimulators of OPC differentiation (Gruchot et al. 2019); (b) use of monoclonal antibodies targeted to enhance axonal regeneration and suppress neuroinflammation and against the endogenous CNS myelin inhibitory molecules; (c) a gene therapy RNA interference approach; and (d) cell replacement strategies. However, the human trials are very limited with poor success rate so far.
References
Abrams CK (2017) Diseases of connexins expressed in myelinating glia. Neurosci Lett 695:91–99
Abrams CK, Orthmann-Murphy J (2013) Connexin mutations in Pelizaeus-Merzbacher-like disease, oculodentodigital dysplasia and related diseases. In: Dere E (ed) Gap junctions in the brain. Academic, San Diego, pp 165–187
Adiele RC, Adiele CA (2017) Metabolic defects in multiple sclerosis. Mitochondrion 44:7–14. https://doi.org/10.1016/j.mito.2017.12.005
Aggarwal S, Yurlova L, Snaidero N, Reetz C, Frey S, Zimmermann J, Pähler G, Janshoff A, Friedrichs J, Müller DJ, Goebel C (2011) A size barrier limits protein diffusion at the cell surface to generate lipid-rich myelin-membrane sheets. Dev Cell 21:445–456
Almeida R, Lyons D (2016) Oligodendrocyte development in the absence of their target axons in vivo. PLoS One 11:e0164432
Almeida RG (2018) The rules of attraction in central nervous system myelination. Front Cell Neurosci 12:367. https://doi.org/10.3389/fncel.2018.00367
Androutsou ME, Tapeinou A, Vlamis-Gardikas A, Tselios T (2018) Myelin oligodendrocyte glycoprotein and multiple sclerosis. Med Chem 14:120–128. https://doi.org/10.2174/1573406413666170906123204
Asato MR, Terwilliger R, Woo J, Luna B (2010) WM development in adolescence: a DTI study. Cereb Cortex 20:2122–2131. https://doi.org/10.1093/cercor/bhp282
Baba Y, Ghetti B, Baker MC, Uitti RJ, Hutton ML, Yamaguchi K, Bird T, Lin W, De Lucia MW, Dickson DW, Wszolek ZK (2006) Hereditary diffuse leukoencephalopathy with spheroids: clinical, pathologic and genetic studies of a new kindred. Acta Neuropathol 111:300–311. https://doi.org/10.1007/s00401-006-046-z
Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF Jr, Rao MS, Sherman LS (2005) Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med 11:966–972
Baer AS, Syed YA, Kang SU, Mitteregger D, Vig R, Ffrench-Constant C, Franklin RJ, Altmann F, Lubec G, Kotter MR (2009) Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain 132:465–481
Baron W, Hoekstra D (2010) On the biogenesis of myelin membranes: sorting, trafficking and cell polarity. FEBS Lett 584:1760–1770
Barres BA, Lazar MA, Raff MC (1994) A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120:1097–1108
Barres BA, Raff MC (1993) Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361:258–260
Bastin ME, Munoz Maniega S, Ferguson KJ, Brown LJ, Wardlaw JM, MacLullich AM, Clayden JD (2010) Quantifying the effects of normal ageing on white matter structure using unsupervised tract shape modelling. Neuroimage 51:1–10
Bechler ME, Byrne L (2015) CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr Biol 25:2411–2416
Bechler ME, Swire M, Ffrench-Constant C (2018) Intrinsic and adaptive myelination-a sequential mechanism for smart wiring in the brain. Dev Neurobiol 78:68–79. https://doi.org/10.1002/dneu.22518
Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F (2005) Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8:1148–1150. https://doi.org/10.1038/nn1516
Bergmann CC, Lane TE, Stohlman SA (2006) Coronavirus infection of the central nervous system: host–virus stand-off. Nat Rev Microbiol 4:121–132. https://doi.org/10.1038/nrmicro1343
Birchmeier C, Nave KA (2008) Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 56:1491–1497
Borjini N, Fernández M, Giardino L, Calzà L (2016) Cytokine and chemokine alterations in tissue, CSF, and plasma in early presymptomatic phase of experimental allergic encephalomyelitis (EAE), in a rat model of multiple sclerosis. J Neuroinflammation 13:291. https://doi.org/10.1186/s12974-016-0757-6
Bradl M, Lassmann H (2010) Oligodendrocytes: biology and pathology. Acta Neuropathol 119:37–53
Braun PE, Sandillon F, Edwards A, Matthieu JM, Privat A (1988) Immunocytochemical localization by electron microscopy of 2′,3’-cyclic nucleotide 3’-phosphodiesterase in developing oligodendrocytes of Normal and mutant brain. J Neurosci 8:3057–3066
Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Müller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, Radyushkin K (2008) Neuregulin-1/ErbB signalling serves distinct functions in myelination of the peripheral and central nervous system. Neuron 59:581–595
Brody BA, Kinney HC, Kloman AS, Gilles FH (1987) Sequence of central nervous system myelination in human infancy- an autopsy study of myelination. J Neuropathol Exp Neurol 46:283–301
Bugiani M, Boor I, van Kollenburg B, Postma N, Polder E, van Berkel C, van Kesteren RE, Windrem MS, Hol EM, Scheper GC, Goldman SA (2011) Defective glial maturation in vanishing white matter disease. J Neuropathol Exp Neurol 70:69–82
Bugiani M, Postma N, Polder E, Dieleman N, Scheffer PG, Sim FJ, van der Knaap MS, Boor I (2013) Hyaluronan accumulation and arrested oligodendrocyte progenitor maturation in vanishing white matter disease. Brain 136:209–222
Bugiani M, Vuong C, Breur M, van der Knaap Marjo S (2018) Vanishing white matter: a leukodystrophy due to astrocytic dysfunction. Brain Pathol 2018:408–421
Buyanova IS, Arsalidou M (2021) Cerebral White matter myelination and relations to age, gender, and cognition: a selective review. Front Hum Neurosci 15:662031. https://doi.org/10.3389/fnhum.2021.662031
Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278
Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M (2005) Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron 45:41–53
Cailloux F, Gauthier-Barichard F, Mimault C, Isabelle V, Courtois V, Giraud G, Dastugue B, Boespflug-Tanguy O (2000) Genotype-phenotype correlation in inherited brain myelination defects due to proteolipid protein gene mutations. Clinical European network on brain Dysmyelinating disease. Eur J Hum Genet 8:837–845
Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD (1998) Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron 20:869–882
Camargo N, Goudriaan A, van Deijk AL, Otte WM, Brouwers JF, Lodder H, Gutmann DH, Nave KA, Dijkhuizen RM, Mansvelder HD, Chrast R (2017) Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol 15:e1002605
Castelfranco AM, Hartline DK (2015) The evolution of vertebrate and invertebrate myelin: a theoretical computational study. J Comput Neurosci 38:521–538
Cesani M, Lorioli L, Grossi S, Amico G, Fumagalli F, Spiga I, Filocamo M, Biffi A (2016) Mutation update of ARSA and PSAP genes causing metachromatic Leukodystrophy. Hum Mutat 37:16–27. https://doi.org/10.1002/humu.22919
Chang KJ, Redmond SA, Chan JR (2016) Remodeling myelination: implications for mechanisms of neural plasticity. Nat Neurosci 19:190–197. https://doi.org/10.1038/nn.4200
Cole J, Evans E, Mwangi M, Mar S (2019) Acute disseminated encephalomyelitis in children: an updated review based on current diagnostic criteria. Pediatr Neurol 100:26–34. https://doi.org/10.1016/j.pediatrneurol.2019.06.017
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372:1502–1517
Corral I, Quereda C, García-Villanueva M, Casado JL, Pérez-Elías MJ, Navas E, Ariza A, Moreno S (2004) Focal monophasic demyelinating leukoencephalopathy in advanced HIV infection. Eur Neurol 52:36–41
Craig A, Luo NL, Beardsley DJ, Wingate-Pearse N, Walker DW, Hohimer AR, Back SA (2003) Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp Neurol 181:231–240
Crawford AH, Tripathi RB, Richardson WD, Franklin RJ (2016) Developmental origin of oligodendrocyte lineage cells determines response to demyelination and susceptibility to age-associated functional decline. Cell Rep 15:761–773
Davis AA, Temple S (1994) A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature 372:263–266
Dawson MR, Polito A, Levine JM, Reynolds R (2003) NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24:476–488
De Hoz L, Simons M (2015) The emerging functions of oligodendrocytes in regulating neuronal network behaviour. Bioessays 37:60–69. https://doi.org/10.1002/bies.201400127
De Stefano N, Matthews PM, Fu L, Narayanan S, Stanley J, Francis GS, Antel JP, Arnold DL (1998) Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain 121(8):1469–1477
Dean JM, Moravec MD, Grafe M, Abend N, Ren J, Gong X, Volpe JJ, Jensen FE, Hohimer AR, Back SA (2011) Strain-specific differences in perinatal rodent oligodendrocyte lineage progression and its correlation with human. Dev Neurosci 33:251. https://doi.org/10.1159/000327242
Dendrou CA, Fugger L, Friese MA (2015) Immunopathology of multiple sclerosis. Nat Rev Immunol 15:545–558. https://doi.org/10.1038/nri3871
Denninger AR, Breglio A, Maheras KJ, LeDuc G, Cristiglio V, Deme B, Gow A, Krischner DA (2015) Claudin-11 tight junctions in myelin are a barrier to diffusion and lack strong adhesive properties. Biophys J 109(7):1387–1397. https://doi.org/10.1016/j.bpj.2015.08.012
Devaux J, Gow A (2008) Tight junctions potentiate the insulative properties of small CNS myelinated axons. J Cell Biol 183:909–921. https://doi.org/10.1083/jcb.200808034
Dietrich RB, Bradley WG, Zaragoza EJ 4th, Otto RJ, Taira RK, Wilson GH, Kangarloo H (1988) MR evaluation of early myelination patterns in normal and developmentally delayed infants. Am J Roentgenol 150:889–896
Dietschy JM (2009) Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol Chem 390:287–293
Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M (2008) Progeny of Olig2-expressing progenitors in the grey and white matter of the adult mouse cerebral cortex. J Neurosci 28:10434–10442
Dimou L, Simons M (2017) Diversity of oligodendrocytes and their progenitors. Curr Opin Neurobiol 47:73–79. https://doi.org/10.1038/nature11314
Donaldson HH, Hoke GW (1905) On the areas of the axis cylinder and medullary sheath as seen in cross sections of the spinal nerves of vertebrates. J Comp Neurol 15:1–16
Duncan GJ, Manesh SB, Hilton BJ, Assinck P, Liu J, Moulson A, Plemel JR, Tetzlaff W (2018) Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nat Commun 9:3066. https://doi.org/10.1038/s41467-018-05473-1
Duncan GJ, Manesh SB, Hilton BJ, Assinck P, Plemel JR, Tetzlaff W (2020) The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. Glia 68:227–245
Duncan GJ, Simkins TJ, Emery B (2021) Neuron-oligodendrocyte interactions in the structure and integrity of axons. Front Cell Dev Biol 9:653101. https://doi.org/10.3389/fcell.2021.653101
Duncan ID, Radcliff AB (2016) Inherited and acquired disorders of myelin: the underlying myelin pathology. Exp Neurol 283:452–475
Eckhardt M (2008) The role and metabolism of sulfatide in the nervous system. Mol Neurobiol 37:93–103. https://doi.org/10.1007/s12035-008-8022-3
Emery B, Lu QR (2015) Transcriptional and epigenetic regulation of oligodendrocyte development and myelination in the central nervous system. Cold Spring Harb Perspect Biol 7(9):a020461
Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH (2009) Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev 23:1571–1585
Fancy SPJ, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, Franklin RJ (2011) Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci 14:1009–1016
Ferenczy MW, Marshall LJ, Nelson CDS, Atwood WJ, Nath A, Khalili K, Major EO (2012) Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev 2012:471–506
Fields RD (2008) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31:361–370. https://doi.org/10.1016/j.tins.2008.04.001
Fields RD (2010) Change in the brain’s WM. Science 330:768–769. https://doi.org/10.1126/science.1199139
Fields RD (2015) A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci 16(12):756–767
Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, Rocca AM (2018) Multiple sclerosis. Nat Rev Dis Primers 4:1–27. https://doi.org/10.1038/s41572-018-0041-4
Filley CM, Fields RD (2016) White matter and cognition: making the connection. J Neurophysiol 116:2093–2104. https://doi.org/10.1152/jn.00221.2016
Ford MC, Alexandrova O, Cossell L, Stange-Marten A, Sinclair J, Kopp-Scheinpflug C, Pecka M, Attwell D, Grothe B (2015) Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat Commun 6:1–14
Franklin RJ (2002) Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci 3:705–714. https://doi.org/10.1038/nrn917
Franklin RJ (2008) Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci 9:839–855. https://doi.org/10.1038/nrn2480
Franklin RJ, Edgar JM, Smith KJ (2012) Neuroprotection and repair in multiple sclerosis. Nat Rev Neurol 8:624–634
Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517–521
Gaesser JM, Fyffe-Maricich SL (2016) Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp Neurol 283:501–511
Gallo V, Deneen B (2014) Glial development: the crossroads of regeneration and repair in the CNS. Neuron 83:283–308. https://doi.org/10.1016/j.neuron.2014.06.010
Galloway DA, Moore CS (2016) miRNAs as emerging regulators of oligodendrocyte development and differentiation. Front Cell Dev Biol 4:59
Garbern JY, Yool DA, Moore GJ, Wilds IB, Faulk MW, Klugmann M, Nave KA, Sistermans EA, van der Knaap MS, Bird TD, Shy ME, Kamholz JA, Griffiths IR (2002) Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain 125:551–561. https://doi.org/10.1093/brain/awf043
Gard AL, Burrell MR, Pfeiffer SE, Rudge JS, Williams WC (1995) Astroglial control of oligodendrocyte survival mediated by PDGF and leukemia inhibitory factor-like protein. Development 121:2187–2197
Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M (2014) Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344:1252304. https://doi.org/10.1126/science.1252304
Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL (1999) Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2:861–863
Goldenberg MM (2012) Multiple sclerosis review. PT 37(3):175–184
Gómez-Pinedo U, Sirerol-Piquer MS, Durán-Moreno M, García-Verdugo JM, Matias-Guiu J (2017) Alexander disease mutations produce cells with coexpression of glial fibrillary acidic protein and NG2 in neurosphere cultures and inhibit differentiation into mature oligodendrocytes. Front Neurol 8:255. https://doi.org/10.3389/fneur.2017.00255
Gruchot J, Weyers V, Göttle P, Förster M, Hartung HP, Küry P, Kremer D (2019) The molecular basis for remyelination failure in multiple sclerosis. Cell 8:825. https://doi.org/10.3390/cells8080825
Hagberg H, Peebles D, Mallard C (2002) Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev 8:30–38
Harding B, Ellis D, Malcolm S (1995) A case of Pelizaeus-Merzbacher disease showing increased dosage of the proteolipid protein gene. Neuropathol Appl Neurobiol 21:111–115. https://doi.org/10.1111/j.1365-2990.1995.tb01036.x
Hart MN, Earle KM (1975) Haemorrhagic and perivenous encephalitis: a clinical-pathological review of 38 cases. J Neurol Neurosurg Psychiatry 38:585–591. https://doi.org/10.1136/jnnp.38.6.585
Hedden T, Schultz AP, Rieckmann A, Mormino EC, Johnson KA, Sperling RA, Buckner RL (2016) Multiple brain markers are linked to age-related variation in cognition. Cereb Cortex 26:1388–1400
Herwerth M, Kalluri SR, Srivastava R, Kleele T, Kenet S, Illes Z, Merkler D, Bennett JL, Misgeld T, Hemmer B (2016) In vivo imaging reveals rapid astrocyte depletion and axon damage in a model of neuromyelitis optica related pathology. Ann Neurol 79:794–805. https://doi.org/10.1002/ana.24630
Hildebrand C (1971) Ultrastructural and light-microscopic studies of the developing feline spinal cord white matter. I. the nodes of Ranvier. Acta Physiol Scand Suppl S364:81–107. https://doi.org/10.1111/j.1365-201X.1971.tb10979.x
Hildebrand C, Remahl S, Persson H, Bjartmar C (1993) Myelinated nerve fibres in the CNS. Prog Neurobiol 40:319–384
Höftberger R, Lassmann H (2017) Inflammatory demyelinating diseases of the central nervous system. Handb Clin Neurol 145:263–283. https://doi.org/10.1016/B978-0-12-802395-2.00019-5
Hollund IM, Olsen A, Skranes J, Brubakk AM, Håberg AK, Eikenes L, Evensen KA (2017) WM alterations and their associations with motor function in young adults born preterm with very low birth weight. Neuroimage Clin 17:241–250. https://doi.org/10.1016/j.nicl.2017.10.006
Hübner CA, Orth U, Senning A, Steglich C, Kohlschütter A, Korinthenberg R, Gal A (2005) Seventeen novel PLP1 mutations in patients with Pelizaeus-Merzbacher disease. Hum Mutat 25:321–322. https://doi.org/10.1002/humu.9314
Hughes EG, Kang SH, Fukaya M, Bergles DE (2013) Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci 16:668–676
Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE (2018) Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat Neurosci 21:696–706
Huxley AF, Stämpfli R (1949) Evidence for saltatory conduction in peripheral myelinated nerve fibres. J Physiol 108:315–339. https://doi.org/10.1113/jphysiol.1949.sp004335
Inder TE, Huppi PS (2000) In vivo studies of brain development by magnetic resonance techniques. Ment Retard Dev Disabil Res Rev 6:59–67
Jeffries MA, Urbanek K, Torres L, Wendell SG, Rubio ME, Fyffe-Maricich SL (2016) ERK1/2 activation in pre-existing oligodendrocytes of adult mice drives new myelin synthesis and enhanced CNS function. J Neurosci 36:9186–9200
Jin J, Peng Q, Hou Z, Jiang M, Wang X, Langseth AJ, Tao M, Barker PB, Mori S, Bergles DE, Ross CA (2015) Early white matter abnormalities, progressive brain pathology and motor deficits in a novel knock-in mouse model of Huntington’s disease. Hum Mol Genet 24:2508–2527
Juurlink BHJ, Thorburne SK, Hertz L (1998) Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia 22:371–378
Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE (2010) NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68:668–681
Karim SA, Barrie JA, McCulloch MC, Montague P, Edgar JM, Kirkham D, Anderson TJ, Nave KA, Griffiths IR, McLaughlin M (2007) PLP overexpression perturbs myelin protein composition and myelination in a mouse model of Pelizaeus-Merzbacher disease. Glia 55:341–351. https://doi.org/10.1002/glia.20465
Keirstead HS, Blakemore WF (1999) The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination. Adv Exp Med Biol 468:183–197
Kinney HC, Brody BA, Kloman AS, Gilles FH (1988) Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol 47:217–234. https://doi.org/10.1097/00005072-198805000-00003
Klingseisen A, Lyons DA (2018) Axonal regulation of central nervous system myelination: structure and function. Neuroscientist 24(1):7–21
Knickmeyer RC, Styner M, Short SJ, Lubach GR, Kang C, Hamer R, Coe CL, Gilmore JH (2010) Maturational trajectories of cortical brain development through the pubertal transition: unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cereb Cortex 20(5):1053–1063
Kobayashi Y, Tohyama J, Akiyama T, Magara S, Kawashima H, Akasaka N, Nakashima M, Saitsu H, Matsumoto N (2016) Severe leukoencephalopathy with cortical involvement and peripheral neuropathy due to FOLR1 deficiency. Brain Dev 39:266–270
Kohama SG, Rosene DL, Sherman LS (2012) Age-related changes in human and non-human primate white matter: from myelination disturbances to cognitive decline. Age 34:1093–1110
Kohlschütter A (2013) Lysosomal leukodystrophies: Krabbe disease and metachromatic leukodystrophy. Handb Clin Neurol 113:1611–1618. https://doi.org/10.1016/B978-0-444-59565-2.00029-0
Kotter MR, Li WW, Zhao C, Franklin RJ (2006) Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci 26:328–332
Koudelka S, Voas MG, Almeida RG, Baraban M, Soetaert J, Meyer MP, Talbot WS, Lyons DA (2016) Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr Biol 26:1447–1455
Kuhlmann T, Miron V, Cuo Q, Wegner C, Antel J, Brück W, Kuhlmann T (2008) Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131(7):1749–1758
Kuhn S, Gritti L, Crooks D, Dombrowski Y (2019) Oligodendrocyte in development, myelin generation and beyond. Cell 8:1424
Kumar R, Chavez AS, Macey PM, Woo MA, Harper RM (2013) Brain axial and radial diffusivity changes with age and gender in healthy adults. Brain Res 1512:22–36. https://doi.org/10.1016/j.brainres.2013.03.028
Lassmann H (2003) Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J Neurol Sci 206:187–191
Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C (2012) Diffusion tensor imaging of WM tract evolution over the lifespan. Neuroimage 60:340–352. https://doi.org/10.1016/j.neuroimage.2011.11.094
Lebel C, Treit S, Beaulieu C (2019) A review of diffusion MRI of typical WM development from early childhood to young adulthood. NMR Biomed 32(4):e3778. https://doi.org/10.1002/nbm.3778
Lee JY, Petratos S (2016) Thyroid hormone signaling in oligodendrocytes: from extracellular transport to intracellular signal. Mol Neurobiol 53:6568–6583
Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487:443–448. https://doi.org/10.1038/nature11314
Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364:2106–2112. https://doi.org/10.1016/S0140-6736(04)17551-X
Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J (2012) Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci 15:1621–1623
Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HO, Franklin RJ, Attwell D, Káradóttir RT (2013) Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol 11:e1001743. https://doi.org/10.1371/journal.pbio.1001743
Macchi M, Magalon K, Zimmer C, Peeva E, El Waly B, Brousse B, Jaekel S, Grobe K, Kiefer F, Williams A, Cayre M (2020) Mature oligodendrocytes bordering lesions limit demyelination and favor myelin repair via heparan sulfate production. eLife 9:e51735. https://doi.org/10.7554/eLife.51735
Mader S, Gredler V, Schanda K, Rostasy K, Dujmovic I, Pfaller K, Lutterotti A, Jarius S, Di Pauli F, Kuenz B, Ehling R (2011) Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation 8:184
Magnus T, Carmen J, Deleon J, Xue H, Pardo AC, Lepore AC, Mattson MP, Rao MS, Maragakis NJ (2008) Adult glial precursor proliferation in mutant SOD1G93A mice. Glia 56(2):200–208
Major EO (2010) Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med 61:35–47. https://doi.org/10.1146/annurev.med.080708.082655
Makinodan M, Rosen KM, Ito S, Corfas G (2012) A critical period for social experience–dependent oligodendrocyte maturation and myelination. Science 337:1357–1360
Matute C, Sánchez-Gómez MV, Martínez-Millán L, Miledi R (1997) Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proc Natl Acad Sci U S A 94:8830–8835
Matute C, Torre I, Pérez-Cerdá F, Pérez-Samartín A, Alberdi E, Etxebarria E, Arranz AM, Ravid R, Rodríguez-Antigüedad A, Sánchez-Gómez M, Domercq M (2007) P2X 7 receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci 27:9525–9533
Matveeva O, Bogie JFJ, Hendriks JJA, Linker RA, Haghikia A, Kleinewietfeld M (2018) Western lifestyle and immunopathology of multiple sclerosis. Ann N Y Acad Sci 1417:71–86. https://doi.org/10.1111/nyas.13583
McAleese KE, Walker L, Graham S, Moya EL, Johnson M, Erskine D, Colloby SJ, Dey M, Martin-Ruiz C, Taylor JP, Thomas AJ (2017) Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol 134:459–473
McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD (2014) Motor skill learning requires active central myelination. Science 346:318–322. https://doi.org/10.1126/science.1254960
McKinnon RD, Waldron S, Kiel ME (2005) PDGFα-receptor signal strength controls an RTK rheostat that integrates phosphoinositol 3-kinase and phospholipase C pathways during oligodendrocyte maturation. J Neurosci 25:3499–3508
Mecha M, Carrillo-Salinas FJ, Mestre L, Feliú A, Guaza C (2013) Viral models of multiple sclerosis: neurodegeneration and demyelination in mice infected with Theiler’s virus. Prog Neurobiol 101-102:46–64. https://doi.org/10.1016/j.pneurobio.2012.11.003
Mei F, Lehmann-Horn K, Shen YA, Rankin KA, Stebbins KJ, Lorrain DS, Pekarek K, Sagan SA, Xiao L, Teuscher C, von Büdingen HC (2016) Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. Elife 5:e18246
Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL (2003) Connexins are critical for normal myelination in the CNS. J Neurosci 23:5963–5973
Messing A, Head MW, Galles K, Galbreath E, Goldman JE, Brenner M (1998) Fatal encephalopathy with astrocyte inclusions in GFAP transgenic mice. Am J Pathol 152:391–398
Mierzwa AJ, Marion CM, Sullivan GM, McDaniel DP, Armstrong RC (2015) Components of myelin damage and repair in the progression of white matter pathology after mild traumatic brain injury. J Neuropathol Exp Neurol 74:218–232
Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AM, Sestan N, Wildman DE, Lipovich L, Kuzawa CW, Hof PR, Sherwood CC (2012) Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci U S A 109:16480–16485
Miller SD, Olson JK, Croxford JL (2001) Multiple pathways to induction of virus-induced autoimmune demyelination: lessons from Theiler’s virus infection. J Autoimmun 16:219–227. https://doi.org/10.1006/jaut.2000.0489
Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, Watanabe S, Takahashi T, Nakashima I, Takahashi H, Itoyama Y (2007) Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 130(Pt 5):1224–1234
Miyata S, Hattori T, Shimizu S, Ito A, Tohyama M (2015) Disturbance of oligodendrocyte function plays a key role in the pathogenesis of schizophrenia and major depressive disorder. Biomed Res Int 2015:492367
Monje M (2018) Myelin plasticity and nervous system function. Annu Rev Neurosci 41:61–76. https://doi.org/10.1146/annurev-neuro-080317-061853
Montague P, McCallion AS, Davies RW, Griffiths IR (2006) Myelin-associated oligodendrocytic basic protein: a family of abundant CNS myelin protein in search of a function. Dev Neurosci 28:479–487. https://doi.org/10.1159/000095110
Morell P, Jurevics H (1996) Origin of cholesterol in myelin. Neurochem Res 21:463–470
Mount CW, Monje M (2017) Wrapped to adapt: experience-dependent myelination. Neuron 95:743–756. https://doi.org/10.1016/j.neuron.2017.07.009
Nakagawa H, Iwasaki S, Kichikawa K, Fukusumi A, Taoka T, Ohishi H, Uchida H (1998) Normal myelination of anatomic nerve fiber bundles: MR analysis. Am J Neuroradiol 19:1129–1136
Nave KA (2010) Myelination and the trophic support of long axons. Nat Rev Neurosci 11:275–283. https://doi.org/10.1038/nrn2797
Nave KA, Werner HB (2014) Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol 30:503–533. https://doi.org/10.1146/annurev-cellbio-100913-013101
Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P (1988) Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature 333:560–562. https://doi.org/10.1038/333560a0
Norton WT, Poduslo SE (1973) Myelination in rat brain: changes in myelin composition during brain maturation. J Neurochem 21:759–773
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG (2000) Multiple sclerosis. N Engl J Med 343:938–952. https://doi.org/10.1056/NEJM200009283431307
Ohlhauser L, Parker AF, Smart CM, Gawryluk JR (2018) WM and its relationship with cognition in subjective cognitive decline. Alzheimers Dement (Amst) 11:28–35
Olabarria M, Goldman JE (2017) Disorders of astrocytes: Alexander disease as a model. Annu Rev Pathol 12:131–152. https://doi.org/10.1146/annurev-pathol-052016-100218
Orentas DM, Hayes JE, Dyer KL, Miller RH (1999) Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development 126:2419–2429
Oztürk AH, Tasçioglu B, Aktekin M, Kurtoglu Z, Erden I (2002) Morphometric comparison of the human corpus callosum in professional musicians and non-musicians by using in vivo magnetic resonance imaging. J Neuroradiol 29:29–34
Paloneva J, Autti T, Raininko R, Partanen J, Salonen O, Puranen M, Hakola P, Haltia M (2001) CNS manifestations of Nasu-Hakola disease: a frontal dementia with bone cysts. Neurology 56:1552–1558. https://doi.org/10.1212/WNL.56.11.1552
Paloneva J, Kestilä M, Wu J, Salminen A, Böhling T, Ruotsalainen V, Hakola P, Bakker ABH, Phillips JH, Pekkarinen P, Lanier LL, Timonen T, Peltonen L (2000) Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet 25:357–361. https://doi.org/10.1038/77153
Panagiotakos G, Alshamy G, Chan B, Abrams R, Greenberg E, Saxena A, Bradbury M, Edgar M, Gutin P, Tabar V (2007) Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS One 2:e588
Parratt JD, Prineas JW (2010) Neuromyelitis optica: a demyelinating disease characterized by acute destruction and regeneration of perivascular astrocytes. Mult Scler 16:1156–1172. https://doi.org/10.1177/1352458510382324
Patro N, Naik AA, Patro IK (2019) Developmental changes in oligodendrocyte genesis, myelination, and associated behavioral dysfunction in a rat model of intra-generational protein malnutrition. Mol Neurobiol 56:595–610
Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol 131:323–345. https://doi.org/10.1007/s00401-015-1513-1
Peters A (2002) The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol 31:581–593. https://doi.org/10.1023/A:1025731309829
Philips T, Rothstein JD (2017) Oligodendroglia: metabolic supporters of neurons. Clin Investig 127(9):3271–3280. https://doi.org/10.1172/JCI90610
Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, Tetzlaff W (2014) Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol 117:54–72
Popescu BFG, Lucchinetti CF (2011) Pathology of demyelinating diseases. Annu Rev Pathol Mech Dis 7:185–217. https://doi.org/10.1146/annurev-pathol-011811-132443
Pouwels PJ, Vanderver A, Bernard G, Wolf NI, Dreha-Kulczewksi SF, Deoni SC, Bertini E, Kohlschutter A, Richardson W, French-Constant C, Kohler W, Rowitch D, Barkovich AJ (2014) Hypomyelinating leukodystrophies: translational research progress and prospects. Ann Neurol 76:5–19
Prado EL, Dewey KG (2014) Nutrition and brain development in early life. Nutr Rev 72:267–284
Pronker MF, Lemstra S, Snijder J, Heck AJR, Thies-Weesie DME, Pasterkamp RJ, Janssen BJC (2016) Structural basis of myelin-associated glycoprotein adhesion and signalling. Nat Commun 7:13584
Rademakers R, Baker M, Nicholson AM, Rutherford NJ, Finch N, Soto-Ortolaza A, Lash J, Wider C, Wojtas A, DeJesus-Hernandez M, Adamson J, Kouri N, Sundal C, Shuster EA, Aasly J, MacKenzie J, Roeber S, Kretzschmar HA, Boeve BF, Knopman DS, Petersen RC, Cairns NJ, Ghetti B, Spina S, Garbern J, Tselis AC, Uitti R, Das P, Van Gerpen JA, Meschia JF, Levy S, Broderick DF, Graff-Radford N, Ross OA, Miller BB, Swerdlow RH, Dickson DW, Wszolek ZK (2012) Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet 44:200–205. https://doi.org/10.1038/ng.1027
Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS (2001) Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A 98:13373–13378. https://doi.org/10.1073/pnas.231376298
Reich DS, Lucchinetti CF, Calabresi PA (2018) Multiple sclerosis. N Engl J Med 378:169–180. https://doi.org/10.1056/NEJMra1401483
Remahl S, Hildebrand C (1982) Changing relation between onset of myelination and axon diameter range in developing feline white matter. J Neurol Sci 54:33–45
Richardson EPJ (1961) Progressive multifocal leukoencephalopathy. N Engl J Med 265:815–823
Robinson JL, Suh E, Wood EM, Lee EB, Coslett HB, Raible K, Lee VMY, Trojanowski JQ, Van Deerlin VM (2015) Common neuropathological features underlie distinct clinical presentations in three siblings with hereditary diffuse leukoencephalopathy with spheroids caused by CSF1R p.Arg782His. Acta Neuropathol Commun 3:42. https://doi.org/10.1186/s40478-015-0219-x
Rogister B, Ben-Hur T, Dubois-Dalcq M (1999) From neural stem cells to myelinating oligodendrocytes. Mol Cell Neurosci 14:287–300
Rone MB, Cui QL, Fang J, Wang LC, Zhang J, Khan D, Bedard M, Almazan G, Ludwin SK, Jones R, Kennedy TE (2016) Oligodendrogliopathy in multiple sclerosis: low glycolytic metabolic rate promotes oligodendrocyte survival. J Neurosci 36:4698–4707
Rosenberg SS, Kelland EE, Tokar E, La Torre De AR, Chan JR (2008) The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci U S A 105:14662–14667
Ruckh JM, Zhao JW, Shadrach JL, Van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJM (2012) Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10:96–103
Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Möbius W, Goetze B, Jahn HM, Huang W, Steffens H (2016) Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91:119–132
Saher G, Brugger B, Lappe-Siefke C, Mobius W, Ryu-ichi T, Wehr MC, Wieland F, Shun Ishibashi S, Klaus-Armin Nave KA (2005) High cholesterol level is essential for myelin membrane growth. Nat Neurosci 8(4):468–475
Saher G, Stumpf SK (2015) Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim Biophys Acta 1851(8):1083–1094
Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, Edkins S (2011) International multiple sclerosis genetics consortium; Wellcome Trust case control consortium 2 genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476:214–219. https://doi.org/10.1038/nature10251
Schmitt S, Castelvetri LC, Simons M (2015) Metabolism and functions of lipids in myelin. BiochimBiophys Acta 1851:999–1005. https://doi.org/10.1016/j.bbalip.2014.12.016
Scolding N (2014) Acute disseminated encephalomyelitis and other inflammatory demyelinating variants, 1st edn. Elsevier, Amsterdam
Semple BD, Blomgren K, Gimlin K, Ferriero M, Noble-Haeusslein LJ (2013) Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 2013:1–16
Seth P, Diaz F, Tao-Cheng JH, Major EO (2004) JC virus induces non apoptotic cell death of human central nervous system progenitor cell-derived astrocytes. J Virol 78:4884–4891. https://doi.org/10.1128/JVI.78.9.4884-4891.2004
Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN (2008) Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28:3586–3594
Silveira AH, Patil N, Brickner MA, Parr AM (2021) Strategies for oligodendrocyte and myelin repair in traumatic CNS injury. Front Cell Neurosci 14:619707. https://doi.org/10.3389/fncel.2020.619707
Sim FJ, Zhao C, Penderis J, Franklin RJM (2002) The age related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci 22:2451–2459
Sima AA, Pierson CR, Woltjer RL, Hobson GM, Golden JA, Kupsky WJ, Schauer GM, Bird TD, Skoff RP, Garbern JY (2009) Neuronal loss in Pelizaeus-Merzbacher disease differs in various mutations of the proteolipid protein 1. Acta Neuropathol 118:531–539. https://doi.org/10.1007/s00401-009-0562-8
Simons M, Kramer EM, Macchi P, Rathke-Hartlieb S, Trotter J, Nave KA, Schulz JB (2002) Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: implications for Pelizaeus-Merzbacher disease. J Cell Biol 157:327–336. https://doi.org/10.1083/jcb.200110138
Simons M, Krämer EM, Thiele C, Stoffel W, Trotter J (2000) Assembly of myelin by association of proteolipid protein with cholesterol- and galactosylceramide-rich membrane domains. J Cell Biol 151:143–154
Singh K, Patro N, Pradeepa M, Patro I (2017) Neonatal lipopolysaccharide infection causes demyelination and behavioral deficits in adult and senile rat brain. Ann Neurosci 24:146–154
Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T (2010) Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A 107:11555–11560
Snaidero N, Möbius W, Czopka T, Hekking LH, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, Nave KA (2014) Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156:277–290
Snaidero N, Simons M (2014) Myelination at a glance. J Cell Sci 127:2999–3004
Snaidero N, Velte C, Myllykoski M, Raasakka A, Ignatev A, Werner HB, Erwig MS, Möbius W, Kursula P, Nave KA, Simons M (2017) Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Rep 18(2):314–323
Sommer I, Schachner M (1981) Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol 83:311–327
Sosunov AA, Guilfoyle E, Wu X, McKhann GM 2nd, Goldman JE (2013) Phenotypic conversions of “protoplasmic” to “reactive” astrocytes in Alexander disease. J Neurosci 33:7439–7450
Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003) Mapping cortical change across the human life span. Nat Neurosci 6:309–315. https://doi.org/10.1038/nn1008
Stadelmann C, Timmler S, Barrantes-Freer A, Simons M (2019) Myelin in the central nervous system: structure, function and pathology. Physiol Rev 99:1381–1431
Steele CJ, Bailey JA, Zatorre RJ, Penhune VB (2013) Early musical training and white matter plasticity in the corpus callosum: evidence for a sensitive period. J Neurosci 33:1282–1290. https://doi.org/10.1523/JNEUROSCI.3578-12.2013
Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A (2001) Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport 12:99–104
Tasker RC (2006) Changes in white matter late after severe traumatic brain injury in childhood. Dev Neurosci 28:302–308
Tomassy GS, Lori Bowe Dershowitz LB, Arlotta P (2016) Diversity matters: a revised guide to myelination. Trends Cell Biol 26(2):135–147. https://doi.org/10.1016/j.tcb.2015.09.002
Tongatta R, Miller RH (2016) Contribution of the oligodendrocyte lineage to CNS repair and neurodegenerative pathology. Neuropharmacology 110(B):539–547
Torii T, Miyamoto Y, Yamauchi J, Tanoue A (2014) Pelizaeus-Merzbacher disease: cellular pathogenesis and pharmacologic therapy. Pediatr Int 56:659–666. https://doi.org/10.1111/ped.12450
Tradtrantip L, Yao X, Su T, Smith AJ, Verkman AS (2017) Bystander mechanism for complement initiated early oligodendrocyte injury in neuromyelitis optica. Acta Neuropathol 134:35–44. https://doi.org/10.1007/s00401-017-1734-6
Traka M, Podojil JR, McCarthy DP, Miller SD, Popko B (2016) Oligodendrocyte death results in immune-mediated CNS demyelination. Nat Neurosci 19:65–74
Trapp BD, Nave KA (2008) Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci 31:247–269. https://doi.org/10.1146/annurev.neuro.30.051606.094313
Tsutsui S, Stys PK (2013) Metabolic injury to axons and myelin. Exp Neurol 246:26–34
van der Knaap MS, Bugiani M (2017) Leukodystrophies: a proposed classification system based on pathological changes and pathogenetic mechanisms. Acta Neuropathol 134:351–382
Vartanian T, Fischbach G, Miller R (1999) Failure of spinal cord oligodendrocyte development in mice lacking neuregulin. Proc Natl Acad Sci U S A 96:731–735
Volpe JJ (2000) Overview: normal and abnormal human brain development. Ment Retard Dev Disabil Res Rev 6:1–5
Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MC, Fields RD (2015) Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat Commun 6:7844
Weinshenker BG, Wingerchuk DM (2017) Neuromyelitis spectrum disorders. Mayo Clin Proc 92:663–679. https://doi.org/10.1016/j.mayocp.2016.12.014
Wiggins RC (1986) Myelination: a critical stage of development. Neurotoxicology 7:103–120
Wiley CA, Grafe M, Kennedy C, Nelson JA (1988) Human immunodeficiency virus (HIV) and JC virus in acquired immune deficiency syndrome (AIDS) patients with progressive multifocal leukoencephalopathy. Acta Neuropathol 76:338–346
Williamson JM, Lyons DA (2018) Myelin dynamics throughout life: an ever-changing landscape? Front Cell Neurosci 12:424. https://doi.org/10.3389/fncel.2018.00424
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, Greenberg B, Jacob A, Jarius S, Lana-Peixoto M, Levy M, Simon JH, Tenembaum S, Traboulsee AL, Waters P, Wellik KE, Weinshenker BG (2015) International panel for NMO diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85:177–189. https://doi.org/10.1212/WNL.0000000000001729
Wlodarczyk A, Holtman IR, Krueger M, Yogev N, Bruttger J, Khorooshi R, Benmamar-Badel A, de Boer-Bergsma JJ, Martin NA, Karram K, Kramer I (2017) A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J 36:3292–3308
Wolf NI, Ffrench-Constant C, van der Knaap MS (2021) Hypomyelinating leukodystrophies- unravelling myelin biology. Nat Rev Neurol 17(2):88–103
Wollebo HS, White MK, Gordon J, Berger JR, Khalili K (2015) Persistence and pathogenesis of the neurotropic polyomavirus JC. Ann Neurol 77:560–570. https://doi.org/10.1002/ana.24371
Wrzos C, Winkler A, Metz I, Kayser DM, Thal DR, Wegner C, Brück W, Nessler S, Bennett JL, Stadelmann C (2014) Early loss of oligodendrocytes in human and experimental neuromyelitis optica lesions. Acta Neuropathol 127:523–538. https://doi.org/10.1007/s00401-013-1220-8
Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, Emery B, Li H, Richardson WD (2016) Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci 19:1210–1217. https://doi.org/10.1038/nn.4351
Yakovlev PI, Lecours A (1967) The myelogenetic cycles of regional maturation of the brain. In: Minkowski A (ed) Regional development of the brain in early life. Blackwell Science, Oxford, pp 3–70
Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, Druid H (2014) Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159:766–774
Young KM, Psachoulia K, Tripathi RB, Dunn S-J, Cossell L, Attwell D, Tohyama K, Richardson WD (2013) Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodelling. Neuron 77:873–885
Young NP, Weinshenker BG, Parisi JE, Scheithauer B, Giannini C, Roemer SF, Thomsen KM, Mandrekar JN, Erickson BJ, Lucchinetti CF (2010) Perivenous demyelination: association with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain 133:333–348. https://doi.org/10.1093/brain/awp321
Zuchero JB, Fu MM, Sloan SA, Ibrahim A, Olson A, Zaremba A, Dugas JC, Wienbar S, Caprariello AV, Kantor C, Leonoudakis D (2015) CNS myelin wrapping is driven by actin disassembly. Dev Cell 34(2):152–167
Acknowledgements
The Department of Biotechnology’s support through the DBT-National Initiative on Glial Cell Research in Health and Disease is gratefully acknowledged. We are thankful to the Department of Biotechnology, the Department of Science and Technology and the Indian Council of Medical Research, Govt. of India, for their financial support through various grants. We are thankful to Dr. Urmishree Bedamatta for critically evaluating the manuscript for its English.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Patro, N., Patro, I., Tandon, P.N. (2022). Oligodendrocyte: Structure, Function and Pathology. In: Patro, I., Seth, P., Patro, N., Tandon, P.N. (eds) The Biology of Glial Cells: Recent Advances. Springer, Singapore. https://doi.org/10.1007/978-981-16-8313-8_3
Download citation
DOI: https://doi.org/10.1007/978-981-16-8313-8_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8312-1
Online ISBN: 978-981-16-8313-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)