Abstract
Lectins are group of proteins which specifically binds to carbohydrate on the cell surface. Microbial lectins are the glycoproteins present in microorganisms which assists them to bind to host cell surfaces and the association among themselves. These properties render them to be an important tool in the diverse fields such as immunology, oncology, biotechnology, and microbiology. Lectins are extremely helpful to microbes since it assists their adherence to cell surface. When this function is prevented, it will lead to curtailing of several human microbial diseases. Adhesion property of lectins can be the basis of many upcoming applications and approaches in biomedical sciences. The interactions of lectins with carbohydrate moieties trigger the neutrophils to invade the infection site and it in turn initiates immune responses in humans. In contrast, the microbial lectins generally are incapable of eliciting immune response. There are several classes of microbial lectins based on their location, carbohydrate specificity, structure, and origin with diverse functions and applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Lectins are group of glycoproteins which are capable of interacting specifically with carbohydrate moeities on the cell surfaces. Lectins are ubiquitous in nature. Microbial lectins are the lectins present in or produced by microorganisms including bacteria, fungi, virus, and protozoans. These lectins are instrumental in binding of these microorganisms to host cell surfaces (infection) and the interaction among themselves, such as adhesion and inhibition of other microbes (Singh et al. 2011). These properties render them to be an important tool in the diverse fields such as immunology, oncology, biotechnology, and microbiology. Microbial lectins also have diverse functional applications in various bioprocesses such as bioremediation, bio flocculation, etc.
Till 1970s, only few lectins were isolated. Later, importance of lectins was realized and it led to extensive studies on lectins from microorganisms (Paiva et al. 2010). First identified microbial lectin was isolated from influenza virus by Alfred Gottschalk in 1950 and it was revealed that the primary function of this lectins were to mediate the interaction with host cells which is the initial step to cause infection (Shen et al. 2007). The adherence of the microbes to host cell surface is assisted by the lectins and hence they are important in initiating infections and cell–cell interactions. When this function is prevented, it will lead to curtailing of several human microbial diseases.
This specific binding property of the lectins is exploited in many applications and approaches in biomedical sciences such as drug delivery in cancer therapy, protein purification by affinity chromatography, bioflocculation, bioremediation of heavy metals, etc. (Singh et al. 2019). The interactions of lectins with carbohydrate moieties trigger the neutrophils to invade the infection site and it in turn initiates immune responses in humans.
Only a few among protozoal and fungal lectins are studied in detail. One of the most studied protozoal lectin is the galactose specific lectin in Entamoeba histolytica which mediates the adhesion of this parasite to human intestinal mucin glycoproteins and initiates contact dependent cytosis which eventually leads to infection (Abd Alla et al. 2012).
The microbial lectins are incapable of eliciting immune response and in turn they mediate the interaction of microbes with host cell surfaces and also the interaction among themselves to form biofilms. Microbial lectins are of different types, based on their carbohydrate specificity, amino acid sequence, three-dimensional structure, and molecular weight. There are only limited reports available regarding their classification, functional aspects, and structural peculiarities (Santos et al. 2014; Esko and Sharon 2009).
Lectins usually contain a carbohydrate recognition domain (CRD) which recognizes and binds to the carbohydrate moieties. In some lectins there are metal-binding sites to which the metal ions like Ca2+ ions binds which mediates the binding of lectins (Shen et al. 2007). Mostly lectins exhibit hemagglutination and antimicrobial activities (Singh et al. 2011). Thus, lectins have a promising role in the field of life sciences, and this has made the lectin research a hot cake among scientific fraternity.
7.2 Microbial Lectins
The first microbial lectin to be isolated was from the influenza virus in the early 1950s by Alfred Gottschalk (Nizet et al. 2017). The bacterial lectins were studied for the first time in 1970s (Esko and Sharon 2009). Later on, extensive research was carried out in this field which unraveled the potential applications of microbial lectins (Nizet et al. 2017; Esko and Sharon 2009; Slifkin and Doyle 1990). The first fungal lectin to be studied on the crystal structure was Aleuria aurantia (Wimmerova et al. 2003).
Microbes initiate their attachment to host cells through lectins by specific cell adhesion and hence microbial lectins are also known as adhesins. The adsorption and attachment of the microbes eventually leads to their colonization, pathogenesis, and infection to the host. Hence, disease resistance can be manipulated by modulating the activity of lectins (Ofek and Doyle 1994; Sharon and Ofek 2000). Microbial lectins are non-immunogenic in nature and a very little studies have been reported regarding their structure and classification (Santos et al. 2014). Broadly, the microbial lectins can be classified as lectins from bacteria, fungi, viruses, and protozoa.
7.2.1 Bacterial Lectins
The bacterial lectins present on the bacterial surfaces serve as adhesins to bind to the host cell receptors which in turn may initiate infection. They have the ability to specifically recognize complex carbohydrate moieties present on host cell surfaces and can also inhibit other microbes as well (Imberty and Varrot 2008; Sharon 1996; Springer and Gagneux 2013). Bacteria possess fimbriae and pili, the appendages that help them to attach to the host surfaces, serves as lectins or adhesins which binds to the glycoprotein receptors on the host cells. It was reported that Escherichia coli bearing type 1 fimbriae which is specific for mannose could agglutinate erythrocytes (Ofek and Doyle 1994). The other bacterial lectins reported from different strains of E. coli are P fimbriae and F-17 fimbriae which specifically binds to galabiose and N-acetyl glucosamine, respectively. Type 1 fimbriae can bind to the glycoprotein uroplakin Ia in epithelial cells of urinary bladder and hence it is important in urinary tract infections (Faris et al. 1980). Type P fimbriae of E. coli are specific for Galα4Gal and Type II fimbriae of oral actinomycetes are specific for βgalactosides (Goldstein and Hayes 1978).
A bacterial lectin, LecB was identified in Pseudomonas aeruginosa which can bind with l-Fucose in the presence of Ca2+ ions (Mitchell et al. 2002). A bacteriocin, L1pA, with a lectin-like property, i.e. with two β-domains, was produced by the gram negative Proteobacteria (Ghequire et al. 2018). In a study reported by Kehr et al. (2006), Microcystic aeruginosa, a cyanobacteria secreted microvirin, which is a mannan binding lectin that helped them in colonization by attachment (Kehr et al. 2006). The bioluminescent bacteria, Photorhabdus asymbiotica was reported to secrete a PHL, a novel fucose binding lectin, which had an antimicrobial activity and phenoloxidase activity (Jančaříková et al. 2017).
The carbohydrate specificity of the bacterial lectins depends on the interaction of lectins with other surface structures of bacteria as well as the primary structure of the lectins. The knowledge on the specificity of lectin binding can help in describing the range of susceptible tissues in the host. Multiple lectins were identified in a wide variety of different species of bacteria with different carbohydrate specificity (Table 7.1).
7.2.2 Fungal Lectins
Fungal lectins are produced by unicellular yeasts as well as multicellular molds and many of them play a pivotal role in human infection. The fungal lectins are isolated from fruiting bodies, spores, conidia, and mycelium. Both filamentous and non-filamentous fungi are reported to possess carbohydrate specific adhesins or lectins that interact with host cell surfaces such as buccal and vaginal epithelium and even in ocular cells as in case of ophthalmic mycosis and several other fungal infections (Ballal and Inamdar 2018).
Aspergillus and Candida are the major human fungal pathogens and they cause aspergillosis and candidiasis, respectively. Aspergillus is a filamental fungus while candida is a non-filamentous fungus but both are opportunistic pathogens. AFL, a conidial lectin expressed by Aspergillus fumigatus, is involved in the pathogenesis during early stage of infection (Houser et al. 2013). A mucin-binding fungal lectin was purified by Singh et al. (2011) from A. nidulans by two step purification process of ion exchange and gel filtration chromatography. Another mucin-binding lectin FleA was purified from the conidia of A. fumigatus which is responsible for lung infections. But when it binds to the respiratory epithelial cells, the FleA is recognized by the macrophages which in turn elicit immune response in host against the pathogen (Kerr et al. 2016). The fungal lectins secreted by Aspergillus nidulans, Cephalosporium, and R. bataticola were described to exhibit mitogenic potential (Pujari et al. 2010). A novel GlcNAc-binding lectin, Paracoccin, secreted by Paracoccidioides brasiliensis was described to elicit fungal pathogenesis in man (Coltri et al. 2006). Many lectins have been identified in different microfungi (Table 7.2).
7.2.3 Viral Lectins
Only a few viral lectins have been reported till date. The most studied viral lectins are hemagglutinins of influenza viruses and they binds specifically to sialic acid. This interaction of virus to their host cells initiates viropexis (internalization of the viral particles by endocytosis). Though the affinity of the interaction is low, the adsorption of viral particles is directly proportional to the abundance of the receptors human and avian influenza viruses binds to N-acetylneuraminic acid (Neu5Acα) 2–6Gal- and Neu5Acα2–3Gal- receptors of the host cell, respectively, whereas porcine influenza viruses bind to both type of receptors (Nizet et al. 2017). Lectins with anti-HIV activity were reported from a green algae and the mechanism of its antiviral activity is not yet elucidated. The viral C-type lectins, CpBV produced by a wasp, Cotesia plutellae could induce immune suppression in their hosts (Lee et al. 2008). Szymanski et al. (2017) reported that the glycoproteins in the Herpes simplex virus are specific to 3-O-sulfated heparan sulfate, while the capsid proteins of entero viruses, gp120 V3 loop of HIV and envelope protein of Dengue viruses are specific for heparan sulfate. Some corona viruses possess lectins with both the hemagglutinin and receptor destroying activity.
7.2.4 Protozoal Lectins
Numerous parasitic protozoa possess lectins which mediate the adhesion of parasites to host cells based on their carbohydrate specificities. This kind of interactions could be used for developing novel therapeutics targeting the adherence and thus it is helpful in preventing the wide spread of various protozoan diseases.
A 260 kDa heterodimeric lectin was isolated from Entamoeba histolytica, which could identify and bind to terminal Gal/Gal NAc residues present on the intestinal epithelium of host. The extent of this interaction determines the virulence of the parasite as it mediates the attachment and invasion to host cell surfaces which leads to the development of infection. Moreover it may function in binding of E. histolytica to bacteria as a source of food (Abd Alla et al. 2012). The adhesion may also bring out protective immunity and is a potential target to manage the infection caused by E. histolytica.
Malaria is developed as a result of interaction of an adhesion, erythrocyte-binding antigen-175 (EBA-175), in Plasmodium falciparum merozoites mainly with the Neu5Ac sialic acid residues on the red blood cells (erythrocytes) of the host (Nawrot et al. 2014; Persson et al. 2013). Adhesin-glycan binding triggers the invasion of the merozoites into red blood cells, where they mature into schizonts which then rupture and release newly formed merozoites into the bloodstream, thus facilitating the development of infection.
The C-type lectin receptors constitute a superfamily of more than thousand proteins and it is classified into 17 groups based on their domain organization and phylogeny. Most CLRs possess one or more C-type lectin domains.
Mannose receptor (MR) is a C-type lectin. It is a transmembrane glycoprotein with eight C-type lectin-like domain that is expressed on the surface of various cell types (Takebe et al. 2013). MR mediates the binding and internalization of mannosylated glycoproteins and participates in the endocytosis of different pathogens having mannose residues on their surface.
7.3 Roles of Microbial Lectins
7.3.1 Biofilm Formation
The first and foremost function of microbial lectins is to initiate adhesion which surges with time and leads to the formation of biofilms. The lectins interact with the carbohydrates-glycocalyx on the cell surface and help the bacteria to adhere to the host surface and initiates colonization which eventually leads to the biofilm formation. The biofilm formation is the first step leading to the development of infection.
Biofilms are the aggregation of bacteria at the host surfaces which is characterized by the production of carbohydrate mucous layer that further helps in the attachment of other bacteria and maintaining the coherence of the biofilm (Blaser 2005). The communication of bacteria through lectin-mediated biofilm adhesion is known as quorum sensing (Mack et al. 2008).
7.3.2 Antimicrobial Activity
The microbial lectins are reported to have antimicrobial activity which inhibits the colonization of other bacteria. A 30 kDa lectin isolated from Acinetobacter baumannii could inhibit both Gram-positive and Gram-negative bacteria. The antibacterial activity of the lectin was instrumental in inhibiting multidrug resistant pathogenic bacteria (Alyousef et al. 2018).
The Proteobacteria secreted a lectin, L1pA, could inhibit other bacteria from colonization in a competition for nutrients and space, by contact-dependent killing (Ghequire et al. 2018). The lectin-like bacteriocin microviridin produced by the cyanobacteria, Microcystic aeruginosa enhances the bloom formation by facilitating the colonization of Microcystic aeruginosa over other species of phytoplanktons (Kehr et al. 2006).
Lectins from various fungi were also found to possess potent antimicrobial and antifungal activities. Lectins from several Penicillium species and A. gorakhpurensis exhibited antibacterial activity against Bacillus cereus, Staphylococcus aureus, and E. coli. In addition, A. gorakhpurensis was reported to possess anti-fungal activity against Saccharomyces cerevisiae (Singh et al. 2014).
7.3.3 Antitumor Activity
Microbial lectins exhibit antiproliferative activity and this property can be exploited for the development of therapeutic agents to treat cancerous growths. The tumor cells express specific altered glycoconjugates at their surfaces which can be recognized by the lectins and bind to them. The binding of lectin with cancer cells triggers various signal transduction pathways which eventually arrests the cellular activities ultimately leading to cell death. The proliferation of HeLa cells was reported to be inhibited by a 30 kDa lectin isolated from Acinetobacter baumannii as revealed by the MTT assay (Alyousef et al. 2018).
7.3.4 Mitogenic Activity
Several microbial lectins are reported to exhibit mitogenic potential and trigger the binding of T cell receptor complex with the ligand which in turn initiates the mitosis of the cells by a signal transduction pathway (Kilpatrick 1999). Such mitogenic activity was reported in the lectin produced by the fungus, Rhizoctonia bataticola. The mitogenic potential of the fungal lectins secreted by Aspergillus nidulans, Cephalosporium, and R. bataticola were described to have applications in histochemistry, glycobiology, and oncology (Pujari et al. 2010). A novel mucin-binding microfungal lectin was purified by Singh et al. (2011) from A. nidulans was reported to exhibit mitogenic potential which helped in elucidating the biochemical changes of immune cells.
Lectins purified from the fungi Hericium erinaceus (Li et al. 2018), Trametes versicolor (Singh et al. 2019), and Hygrophorus russula (Suzuki et al. 2012) exhibited mitogenic activity in murine splenocytes. Mannose binding lectins produced by Pseudomonas spp. induced T cell proliferation and lectin-mediated phagocytosis (Abraham et al. 1988; Sharon 1984). Among bacterial lectins, Pseudomonas spp. exhibit lectins that are mostly mitogenic.
7.3.5 Bioflocculation
In microorganisms, role of lectin-mediated aggregation in bio flocculation of activated sludge was revealed by hemagglutination and inhibition assays on extracellular polymeric substances (EPS) derived from several activated sludges. It revealed strong hemagglutination with trypsin-treated human red blood cells and the agglutination was inhibited by several glycoproteins, indicating that glycoprotein specific lectins are present in activated sludge.
7.3.6 Bioremediation
Lectins bind the cell together and form cluster of cells. This is because microbial glycol conjugation reacts with their specific lectin. This property serves as an important tool in bioremediation process. There is a lot more to be studied and applied in this field.
7.4 Future Perspectives and Applications
Bacterial lectins are capable of binding specifically to different carbohydrates present on different cells of the human body and this specificity is a causative factor that leads to an infection. This specific adhesion property can be utilized in the synthesis of antiadhesive drugs. However, bacteria and viruses possess different types of lectins and they bind selectively to various carbohydrates for adherence. Therefore, researchers face highly challenging task for developing antiadhesive therapy.
The mitogenic potential of the fungal lectins secreted by Aspergillus nidulans, Cephalosporium, and R. bataticola were described to have applications in histochemistry, glycobiology, and oncology. Horizontal gene transfer occurs as a result of this amoeba-lectin-mediated internalization thus provides a useful microbiome homeostasis model. These lectins can also be used to identify the infectious organisms that cause tissue damage without using any specific diagnostic tool.
The specific and augmented adhesive property of microbial lectins can also be exploited in the large-scale application in bioremediation. Biosorption of cuB2 from contaminated areas was enhanced by the aggregates of yeast cells which was facilitated by the presence of lectins as the heavy melts could occupy the binding sites of lectin.
A laboratory scale system was set up consisting of an activated sludge. This set up, arranged by Park and Novak (2009) was effective in checking the efficiency and ease of process using lectins. This process revealed that lectins play an important role in bio flocculation and increase the rate of process. In 1998 Murthy observed the lectin-like proteins matrix which in turn results in bio flocculation.
The bactericidal and bacteriostatic effects of various lectins may open a new way for antimicrobial research. Currently, antibiotics are widely used for the treatment of various diseases which may induce allergic responses and nonspecific reactions. The unethical use of antibiotics may lead to several serious threats to the public health including development of new multidrug resistant strains and loss of beneficial intestinal flora. Recent studies of deadly multidrug resistant microbial pathogens reveal that lectin-based medicines could be an invaluable alternative to the antibiotics and may also be used for targeted drug delivery and its implication needs further studies.
Recently, fluorescent staining technique has gained more importance and popularity because it is easy to handle, and has high specificity. Also, this method avoids the long radioactive permission procedure. A particular species can be detected from a mixture of different microbes by using lectin-based fluorescence staining (Fife et al. 2000). A similar technique to this was used by Sizemore et al. (1990) to selectively differentiate moderately thermophilic and acidophilic mining bacteria in mixtures that contain Thiobacillus ferrooxidans. This was made possible by binding of wheat germ agglutinin to the n-acetyl glucosamine residue in the peptidoglycan layer of Gram-positive bacteria.
Probiotic Bacterial Lectins (PBL) are lectins produced by certain bacteria such as Lactobacilli which is instrumental in maintaining the relationship between the gut microbes and the host. When PBLs were tested against certain clinical pathogens such as Candida and Staphylococcus, the results showed that PBLs have inhibitory effect on the growth of the various strains along with its proteolysis (Lakhtin et al. 2012).
7.5 Conclusion
Lectins are prevalent and are produced by both prokaryotic and eukaryotic organisms. The tremendous properties of lectins attracted researchers to focus more on lectins and hence carried out enormous studies on lectins from bacteria, fungus, viruses, protozoan which revealed the importance of lectins. With the advancement of molecular biology techniques, more microbial lectins are expected to be studied and analyzed for its biomedical and industrial applications.
Microbial lectins are core factors which involved in the host–pathogen interaction. Lectins are hope of scientists for the discovery of new highly effective drugs which can prevent severe infections in humans. Lectins are proteins that recognize and bind to specific carbohydrate target on host cell therefore structure, specificity, and composition are attributes of lectin–cellular interaction. Studies on lectin–carbohydrate interaction indicate that lectins play a pivotal role and that are discussed in this chapter. Lectins have role in bacterial communication, antimicrobial activity which ensures competitive advantage for nutrition and space, i.e., growth and survival. Cellular interaction mostly ends up in infection of host.
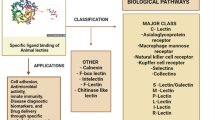
Lectins are specific carbohydrate binding protein, which makes special interest of glycobiologists to concentrate on this topic. The important applications of microbial lectins can be attributed to various fields such as bioremediation, bioflocculation, biomedical applications, fluorescent staining techniques, antiadhesive drug development, and targeted drug delivery (Fig. 7.1). However, further studies in this area will shed light to more potential advanced applications of the microbial lectins in diverse fields.
Abbreviations
- CLR:
-
C-type lectin receptor
- CRD:
-
Carbohydrate recognition domain
- EPS:
-
Extracellular polymeric substance
- HIV:
-
Human immunodeficiency virus
- MR:
-
Mannose receptor
- PBL:
-
Probiotic bacterial lectins
References
Abd Alla MD, Wolf R, White GL, Kosanke SD, Cary D, Verweij JJ, Zhang MJ, Ravdin JI (2012) Efficacy of a Gal-lectin subunit vaccine against experimental Entamoeba histolytica infection and colitis in baboons (Papio sp.). Vaccine 30(20):3068–3075. https://doi.org/10.1016/j.vaccine.2012.02.066
Abraham SN, Sun D, Dale JB, Beachey EH (1988) Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature 336(6200):682
Alyousef AA, Alqasim A, Aloahd MS (2018) Isolation and characterization of lectin with antibacterial, antibiofilm and antiproliferative activities from Acinetobacter baumannii of environmental origin. J Appl Microbiol 124(5):1139–1146
Andersson B, Dahmen J, Frejd T, Leffler H, Magnusson G, Noori G, Svanborg-Eden C (1983) Identification of an active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J Exp Med 158:559–570
Ballal S, Inamdar SR (2018) An overview of lectin–glycan interactions: a key event in initiating fungal infection and pathogenesis. Arch Microbiol 200:371–382
Blaser MJ (2005) An endangered species in the stomach. Sci Am 292(2):38–45
Coltri KC, Casabona-Fortunato AS, Gennari-Cardoso ML, Pinzan CF, Ruas LP, Mariano VS, Martinez R, Rosa JC, Panunto-Castelo A, Roque-Barreira MC (2006) Paracoccin, a GlcNAc-binding lectin from Paracoccidioides brasiliensis, binds to laminin and induces TNF-a production by macrophages. Microb Infect 8(3):704–713
Cormack BP, Ghori N, Falkow S (1999) Anadhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 285:578–582
Drake D, Taylor KG, Bleiweis AS, Doyle RJ (1988) Specificity of the glucanbinding lectin of Streptococcus cricetus. Infect Immun 56:1864–1872
Duguid JP, Old DC (1980) Adhesive properties of Enterobacteriaceae. In: Beachey EH (ed) Bacterial adherence (receptors and recognition), vol 6. Chapman and Hall, London, pp 185–217
Esko JD, Sharon N (2009) Microbial lectins: hemagglutinins, adhesins, and toxins. Cold Spring Harbor Laboratory Press
Esquenazi D, Alviano CS, de Souza W, Rozental S (2004) The influence of surface carbohydrates during in vitro infection of mammalian cells by the dermatophyte Trichophyton rubrum. Res Microbiol 155:144–153
Faris A, Lindahl M, Wadstrom T (1980) GMZ like glycoconjugate as possible receptor for the CFA/I and K99 hemagglutinins of entero-toxigenic Escherichia coli. FEMS Microbiol Lett 7:265–269
Fife DJ, Bruhn DF, Miller KS, Stoner DL (2000) Evaluation of a fluorescent lectin-based staining technique for some acidophilic mining bacteria. Appl Environ Microbiol 66(5):2208–2210
Ghequire MG, Swings T, Michiels J, Buchanan SK, De Mot R (2018) Hitting with a BAM: selective killing by lectin-like bacteriocins. MBio 9(2):e02138-17
Gilboa-Garber H (1986) Lectins of Pseudomonas aeruginosa. Properties, effects and applications. In: Mirelman D (ed) Microbial lectins and agglutinins. Properties and biological activity. Wiley, New York, pp 255–269
Goldstein IJ, Hayes CE (1978) The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem 35:127–340
Gunnarson A, Mardh PA, Lundblad A, Svensson S (1984) Oligosaccharide structures mediating agglutination of sheep erythrocytes by staphylococcus saprophyticus. Infect Immun 45:41–46
Hansson GC, Karlsson KA, Larson G, Lindberg A, Stromberg N, Thurin J (1983) Lactosylceramide is the probable adhesion site for major indigenous bacteria of the gastrointestinal tract. In: Chester MA, Heingegard D, Lundblad A, Svensson S (eds) Glycoconjugates. 7th international symposium on glycoconjugates. Lund, Sweden, July 17–23, p 631
Houser J, Komarek J, Kostlanova N, Cioci G, Varrot A, Kerr SC et al (2013) A soluble fucose-specific lectin from Aspergillus fumigatus conidia-structure, specificity and possible role in fungal pathogenicity. PLoS One 8:e83077
Imberty A, Varrot A (2008) Microbial recognition of human cell surface glycoconjugates. Curr Opin Struct Biol 18:567–576
Ishikawa H, Isayama Y (1987) Evidence for sialyl glycoconjugates as receptors for Bordetella bronchiseptica on swine nasal mucosa. Infect Immun 55:1607–1609
Jančaříková G, Houser J, Dobeš P, Demo G, Hyršl P, Wimmerová M (2017) Characterization of novel bangle lectin from Photorhabdus asymbiotica with dual sugar-binding specificity and its effect on host immunity. PLoS Pathog 13(8):e1006564
Jimenez-Lucho V, Ginsburg V, Krivan HC (1990) Cryptococcus neoformans, Candida albicans, and other fungi bind specifically to the Glycosphingolipid Lactosylceramide (GalIl-4GlcI3-lCer), a possible adhesion receptor for yeasts. Infection and Immunity, pp 2085–2090
Jones GW, Freter R (1976) Adhesive properties of vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun 14:240–245
Karlsson KA (1989) Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem 58:309–350
Kehr JC, Zilliges Y, Springer A, Disney MD, Ratner DD, Bouchier C, Seeberger PH, De Marsac NT, Dittmann E (2006) A mannan binding lectin is involved in cellecell attachment in a toxic strain of Microcystis aeruginosa. Mol Microbiol 59(3):893–906
Kerr SC, Fischer GJ, Sinha M, McCabe O, Palmer JM, Choera T et al (2016) FleA expression in Aspergillus fumigatus is recognized by Fucosylated structures on mucins and macrophages to prevent lung infection. PLoS Pathog 12(4):e1005555. https://doi.org/10.1371/journal.ppat.1005555
Kilpatrick DC (1999) Mechanisms and assessment of lectin-mediated mitogenesis. Mol Biotechnol 11(1):55
Krivan HC, Roberts DD, Ginsburg V (1988a) Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GA1NAcß1,4Gal found in some glycolipids. Proc Natl Acad Sci U S A 85:6157–6161
Krivan HC, Ginsburg V, Roberts DD (1988b) Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialoGM1) and gangliotetraosylceramide (asialo GM2). Arch Biochem Biophys 260:493–496
Lakhtin M, Lakhtin Bajrakova A, Aleshkin A, Afanasiev S, Aleshkin V (2012) Lectin systems imitating probiotics: potential and prospects for biotechnology and medical microbiology. PRO 32:417
Landale EC, McCabe MM (1987) Characterization by affinity electrophoresis of an a-1, 6-glucan-binding protein from Streptococcus sobrinus. Infect Immun 55:3011–3016
Lee S, Nalini M, Kim Y (2008) A viral lectin encoded in Cotesia plutellae bracovirus and its immunosuppressive effect on host hemocytes. Comp Biochem Physiol Mol Integr Physiol 149(4):351–361
Leffler H, Svanborg-Eden C (1986) Glycolipids as receptors for Escherichi coli lectins or adhesins. In: Mirelman D (ed) Microbial lectins and agglutinins: properties and biological activity. Wiley, New York, pp 83–111
Li IC, Lee LY, Tzeng TT et al (2018) Neurohealth properties of Hericium erinaceus mycelia enriched with erinacines. Behav Neurol 2018:5802634. https://doi.org/10.1155/2018/5802634
Lindstedt R, Baker N, Falk P, Hull R, Hull S, Karr J, Leffler K, SvanborgEden C, Larson G (1989) Binding specificities of wild-type and cloned Escherichia coli strains that recognize globo-A. Infect Immun 57:3389–3394
Lindstedt R, Larson G, Falk P, Jodal U, Leffler H, Svanborg C (1991) The receptor repertoire defines the host range for attaching Escherichia coli strains that recognize globo-a. Infect Immun 59:1086–1092
Mack D, Davies AP, Harris LG, Knobloch JK, Rohde H (2008) Staphylococcus epidermidis biofilms: functional molecules, relation to virulence, and vaccine potential. Glycosci Microbial Adhesion 288:157–182
Matsumura K, Higashida K, Hata Y, Kominami J, Nakamura-Tsuruta S, Hirabayashi J (2009) Comparative analysis of oligosaccharide specificities of fucose-specific lectins from Aspergillus oryzae and Aleuria aurantia using frontal affinity chromatography. Anal Biochem 386(2):217–221
Mitchell E, Houles C, Sudakevitz D, Wimmerova M, Gautier C et al (2002) Structural basis for oligosaccharide-mediated adhesion of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Nat Struct Biol 9:918–921
Murray PA, Levine MJ, Tobak LA, Reddy MS (1982) Specificity of salivary—bacterial interactions: II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAca2,3, Galf3l,3Ga1NAc sequence. Biochem Biophys Res Commun 106:390–396
Nawrot R, Barylski J, Nowicki G, Broniarczyk J, Buchwald W, Goździcka-Józefiak A (2014) Plant antimicrobial peptides. Folia Microbiol (Praha) 59(3):181–196. https://doi.org/10.1007/s12223-013-0280-4
Nilsson G, Svensson S, Lindberg AA (1983) The role of the carbohydrate portion of glycolipids for the adherence of Escherichia coli K88’ to pig intestine. In: Chester MA, Heinegard D, Lundbead A, Svensson S (eds), Glycoconjugates: 7th international symposium on glycoconjugates, Lund, pp 637–638
Nizet V, Varki A, Aebi M (2017) Microbial lectins: hemagglutinins, adhesins, and toxins. Essentials of glycobiology. 3rd edn, vol 37. Cold Spring Harbor Laboratory Press, New York, NY
Ofek I, Doyle RJ (1994) Principles of bacterial adhesion. Bacterial adhesion to cells and tissues. Springer, Boston, pp 1–15
Park C, Novak JT (2009) Characterization of lectins and bacterial adhesins in activated sludge flocs. Water Environ Res 81(8):755–764
Parkkinen J, Rogers GN, Korhonen T, Dahr W, Finne J (1986) Identification of 0-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for Sfimbriated Escherichia coli. Infect Immun 54:37–42
Paiva PMG, Gomes FS, Napoleão TH, Sá RA, Correia MTS, Coelho LCBB (2010) Antimicrobial activity of secondary metabolites and lectins from plants. In: Vilas AM (ed) Current research technology and education topics in applied microbiology and microbial biotechnology
Persson KE, Fowkes FJ, McCallum FJ, Gicheru N, Reiling L, Richards JS, Wilson DW, Lopaticki S, Cowman AF, Marsh K, Beeson JG (2013) Erythrocyte-binding antigens of Plasmodium falciparum are targets of human inhibitory antibodies and function to evade naturally acquired immunity. J Immunol 191(2):785–794. https://doi.org/10.4049/jimmunol.1300444
Pujari R, Nagre NN, Chachadi VB, Inamdar SR, Swamy BM, Shastry P (2010) Rhizoctonia bataticola lectin (RBL) induces mitogenesis and cytokine production in human PBMC via p38 MAPK and STAT-5 signaling pathways. Biochim Biophys Acta 1800(12):1268–1275
Ramphal R, Carnoy C, Fievre S, Michalski J-C, Houdret N, Lamblin G, Strecker G, Roussel P (1991) Pseudomonas aeruginosa recognizes carbohydrate chains containing type 1 (Galß1–3G1cNAc) or type 2 (Galß1–4G1cNAc) disaccharide units. Infect Immun 59:700–704
Saitoh T, Natomi H, Zhao W, Okuzumi K, Sugano K, Iwamori M, Nagai Y (1991) Identification of glycolipid receptors for Helicobacter pylori by TLC-immunostaining. FEBS Lett 282:385–387
Santos AF, da Silva MD, Napoleão TH, Paiva PM, Correia MT, Coelho LC (2014) Lectins: function, structure, biological properties and potential applications. Curr Top Pept Protein Res 15:41–62
Sharon N (1984) Carbohydrates as recognition determinants in phagocytosis and in lectin mediated killing of target cells. Biol Cell 51(2):239–245
Sharon N (1996) Carbohydrate-lectin interactions in infectious disease. Adv Exp Med Biol 408:1–8
Sharon N, Ofek I (2000) Safe as mother’s milk: carbohydrates as future anti-adhesion drugs for bacterial diseases. Glycoconj J 17(7–9):659–664
Shen Z, Huang M, Xiao C, Zhang Y, Zeng X, Wang PG (2007) Nonlabeled quartz crystal microbalance biosensor for bacterial detection using carbohydrate and lectin recognitions. Anal Chem 79(6):2312–2319. https://doi.org/10.1021/ac061986j
Singh RS, Bhari R, Singh J, Tiwary AK (2011) Purification and characterization of a mucinbinding mycelial lectin from Aspergillus nidulans with potent mitogenic activity. World J Microbiol Biotechnol 27(3):547–554
Singh RS, Kaur HP, Singh J (2014) Purification and characterization of a mucin specific mycelial lectin from Aspergillus gorakhpurensis: application for mitogenic and antimicrobial activity. PLoS One 9:e109265
Singh SS, Wong JH, Ng TB, Singh WS, Thangjam R (2019) Biomedical applications of lectins from traditional Chinese medicine. Curr Protein Pept Sci 20(3):220–230. https://doi.org/10.2174/1389203719666180612081709
Sizemore RK, Caldwell JJ, Kendrick AS (1990) Alternate gram staining technique using a fluorescent lectin. Appl Environ Microbiol 56(7):2245–2257
Slifkin M, Doyle RJ (1990) Lectins and their application to clinical microbiology. Clin Microbiol Rev 3(3):197–218
Smit H, Gaastra W, Kamerling JP, Vliegenthart JFG, DeGraaf FK (1984) Isolation and structural characterization of the equine erythrocyte receptor for enterotoxigenic Escherichia coli K99 fimbrial adhesin. Infect Immun 46:578–584
Springer SA, Gagneux P (2013) Glycan evolution in response to collaboration, conflict, and constraint. J Biol Chem 288:6904–6911
Stromberg N, Deal C, Nyberg G, Normark S, So M, Karlsson K-A (1988) Identification of carbohydrate structures that are possible receptors for Neisseria gonorrhoeae. Proc Natl Acad Sci U S A 85:4902–4906
Stromberg N, Marklund B-I, Lund B, Ilver D, Hamers A, Gaastra W, Karlsson K-A, Normark S (1990) Host-specificity of uropathogenic Escherichia coli depends on differences in binding specificity to Gala1–4Gal-containing isoreceptors. EMBO J 9:2001–2010
Suzuki T, Sugiyama K, Hirai H, Ito H, Morita T, Dohra H, Murata T, Usui T, Tateno H, Hirabayashi J, Kobayashi Y, Kawagishi H (2012) Mannose-specific lectin from the mushroom Hygrophorus russula. Glycobiology 22(5):616–629. https://doi.org/10.1093/glycob/cwr187
Szymanski CM, Schnaar RL, Aebi M (2017) Bacterial and viral infections. In: Essentials of glycobiology, vol 37, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Takebe Y, Saucedo CJ, Lund G, Uenishi R, Hase S, Tsuchiura T et al (2013) Antiviral lectins from red and blue-green algae show potent In Vitro and In Vivo activity against hepatitis C virus. PLoS ONE 8(5):e64449. https://doi.org/10.1371/journal.pone.0064449
Tuomanen E, Towbin H, Rosenfelder G, Braun D, Larson G, Hansson GC, Hill R (1988) Receptor analogues and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med 168:267–277
Wimmerova M, Mitchell E, Sanchez JF, Gautier C, Imberty A (2003) Crystal structure of fungal lectin: six-bladed beta-propeller fold and novel fucose recognition mode for Aleuria aurantia lectin. J Biol Chem 278:27059–27067
Yu L, Lee KK, Ens K, Doig PC, Carpenter MR, Staddon W, Hodges RS, Paranchych W, Irvin RT (1994) Partial characterization of a Candida albicans fimbrial adhesin. Infect Immun 62:2834–2842
Zupancic ML, Frieman M, Smith D, Alvarez RA, Cummings RD (2008) Cormack BP Glycan microarray analysis of Candida glabrata adhesin ligand specificity. Mol Microbiol 68(3):547–559
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rubeena, A.S., Abraham, A., Aarif, K.M. (2021). Microbial Lectins. In: Elumalai, P., Lakshmi, S. (eds) Lectins. Springer, Singapore. https://doi.org/10.1007/978-981-16-7462-4_7
Download citation
DOI: https://doi.org/10.1007/978-981-16-7462-4_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7461-7
Online ISBN: 978-981-16-7462-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)