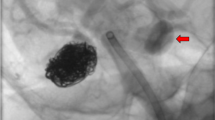
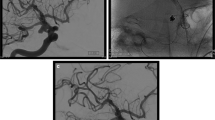
Abstract
Cerebral aneurysms are relatively common, and if found incidentally (unruptured aneurysm), have a relatively benign clinical course with a low annual risk of rupture. Subarachnoid hemorrhage following aneurysmal bleed lead to significant morbidity and mortality, even with the best possible care. Our understanding of the pathogenesis, natural history, diagnostic imaging, treatment modalities and outcomes of cerebral aneurysms has significantly increased in recent years. Despite these advances, providing optimal management requires consideration of several factors and has to be tailored for each patient. This chapter will provide the caretakers involved in the management of cerebral aneurysms with an insight into the recent advances in cerebral aneurysms and review the recent advances made in various aspects of cerebral aneurysms from pathogenesis to management. The different functional pathways and their histological/molecular markers contributing to the development of cerebral aneurysms are reviewed. The advances made in imaging modalities like vessel wall imaging and computational flow dynamics are elaborated. This chapter provides an update on the debate between the two primary modalities of treatment, clipping, and coiling. The recent advances made in microneurosurgery for the cerebral aneurysm to make it more safe and acceptable are described. Endovascular interventions continue to evolve, and this chapter throws some light on the latest advances in next-generation endovascular techniques for treating cerebral aneurysms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cerebral aneurysm
- Subarachnoid haemorrhage
- Pathogenesis
- Natural history
- Diagnosis
- Imaging
- Treatment
- Development
14.1 Introduction
Several advances were made in all the aspects of cerebral aneurysms in the recent past. The pathophysiology and natural history of cerebral aneurysms were extensively studied with particular emphasis on identifying risk factors for rupture, enabling personalized aneurysm care to the patient. Advances in imaging have led to the development of promising diagnostic and prognostic tools. Advances in Microneurosurgery and Endovascular techniques have made the treatment of complex aneurysms more safe and effective. This chapter throws light on these recent advances made in the management of cerebral aneurysms.
14.2 Pathophysiology of Aneurysms-Advanced Concepts
A cerebral aneurysm is an outpouching of an arterial wall due to focal disruption of the internal elastic lamina with inflammation [1]. There are many factors (luminal and extraluminal) involved in the process of aneurysm formation, growth, and rupture (Fig. 14.1). These factors contribute to the three main precursors of aneurysm formation: Focal hemodynamic stress, weakened vessel wall (congenital/environmental), and inflammation [2]. Extensive research on the etiopathogenesis of cerebral aneurysms in recent times has elucidated the mechanism of formation, growth, and rupture of cerebral aneurysms [3]. The hemodynamic stress due to abnormal flow patterns of blood induces several changes in the vessel wall. The vascular endothelial cells transform into pro-inflammatory cells secreting a cocktail of inflammation mediators, which further recruit leukocytes (Macrophages, Lymphocytes) and cause phenotypic modification of the medial cells from a contractile phenotype to a pro-inflammatory phenotype. These modified pro-inflammatory cells along with the recruited leukocytes release a plethora of inflammatory mediators, which bring about vascular remodeling (breakage of the internal elastic lamina, thinning of media, degradation of extracellular matrix), and aneurysm formation [4].
Pathophysiology of intracranial aneurysms formation. ADPKD autosomal dominant polycystic kidney disease; FMD fibromuscular dysplasia; ADAMTS2 A-disintegrin and metalloproteinase with thrombospondin motifs 2 genes; PDGFRB platelet-derived growth factor receptor β gene; THSD1 thrombospondin type 1 domain-containing protein 1; MMP2 matrix metalloproteinase 2 gene; SNP’s single nucleotide polymorphisms; VCAM vascular cell adhesion molecule; ICAM intercellular adhesion molecule; MCP-1 monocyte chemoattractant protein 1; TNF α tumor necrosis factor α; IL-1β Interleukin 1β; IL-6 Interleukin 6; EC’s endothelial cells; VSMC’s vascular smooth muscle cells; MMP matrix metallo proteinase; ROS reactive oxygen species; TGF β transforming growth factor β; VEGF vascular endothelial growth factor; PGE2 Prostaglandin E2; NO nitric oxide; PDE-4 phosphodiesterase 4; TLR4 Toll-like receptor 4; IEL Internal elastic lamina; ECM extracellular matrix
The most prominent hemodynamic factors responsible for aneurysm formation are Wall shear stress (WSS), Wall shear stress gradient (WSSG) and Oscillatory shear index (OSI) [5]. Although aneurysm formation has been linked to regions of high WSS, the hemodynamics causing the growth and rupture of aneurysms is more complex and controversial. Based on several Computational fluid dynamics (CFD) studies and animal experiments, two phenotypes of cerebral aneurysms are recognized, the thin, weak walled phenotype and the hyperplastic atherosclerotic phenotype [6]. Thin, weak walled aneurysms arise parallel to the flow of the artery, experience high WSS, high WSSG, low OSI, have faster-impinging flow and are caused due to endothelial injury and vessel wall degeneration. The thicker walled hyperplastic aneurysms arise perpendicular to the flow stream, experience low WSS, high OSI, have stagnant circulatory flow and are caused due to atherosclerosis, thrombosis, and inflammation [7]. With the advancements in imaging, small incidental unruptured aneurysms are being reported with increasing frequency. Still, the five-year risk of rupture (3%) is lower than the risk associated with prophylactic treatment [8]. Researchers have tried various parameters to stratify the risk of rupture and identify this small subset of unruptured aneurysms prone to rupture without convincing results [9]. The risk factors for rupture of an aneurysm can be classified into clinical, morphological, radiological, and hemodynamic aspects (Fig. 14.2). All these factors need to be considered before treating the aneurysm, and the treatment has to be individualized to give a personalized aneurysm treatment [10].
Pathophysiology of intracranial aneurysm enlargement and rupture. CFD computational fluid dynamics; WSS wall shear stress; OSI oscillatory shear index; LSAR low wall shear stress area ratio; AR aspect ratio; SR size ratio; H/W ratio height-width ratio; AVM arteriovenous malformation; PHASES population, hypertension, age, size, earlier subarachnoid hemorrhage, site; UIATS unruptured intracranial aneurysm treatment score; ELAPSS earlier subarachnoid hemorrhage, location, age, population, size, shape. *basilar bifurcation, internal carotid-posterior communicating artery, anterior communicating artery †Finnish and Japanese
14.3 Advances in Imaging
Imaging of intracranial aneurysms has advanced substantially and plays a central role in the screening, diagnosis, management, and post-treatment surveillance of intracranial aneurysms. 2D-Digital subtraction angiography (DSA) with 3D rotational angiography is the investigation of choice in the imaging of intracranial aneurysms [11] gives the highest spatial and temporal resolution. Computed tomography angiography (CTA) is the investigation of choice for aneurysm detection in acute SAH [12]. The Magnetic Resonance Angiography (MRA) is the investigation of choice for non-emergent detection for screening intracranial aneurysms in high-risk populations and patients where contrast, ionizing radiation is contraindicated [13]. Technological advances like dual-energy CTA and three-dimensional time-of-flight (3D-TOF) MRA have significantly improved the spatial and temporal resolution of these modalities.
MR-vessel wall imaging (VWI) is a novel technique that suppresses the signals from the vessel lumen and CSF and highlights the structure of the vessel wall [14]. Wall enhancement in VWI helps in identifying aneurysms that are prone to rupture, helps in identifying the culprit aneurysm in a patient with multiple aneurysms and also helps in identifying the point of rupture in a multilobulated aneurysm [15].
Macrophage imaging is a diagnostic tool utilizing the phagocytic activity of macrophages. Macrophages play a pivotal role in the pathogenesis of aneurysms, and Ferumoxytol, a contrast agent used in MRI, is engulfed by the macrophages in the aneurysm wall. Enhancement of aneurysms with Ferumoxytol after 24 h of administration is associated with rupture of an aneurysm within 6 months of imaging [16].
Computational fluid dynamics (CFD) is a post-processing technique that utilizes images from CTA, MRA, and 3D Rotational angiography and replicates the hemodynamic conditions inside the aneurysm [17]. CFD analyses help in assessing the risk of rupture in the cerebral aneurysm and also predict the characteristics of the aneurysm wall. The hemodynamic parameters associated with increased risk of aneurysm rupture are elevated maximum WSS, low WSS with high OSI, high Pmax or maximum pressure, high OSI with high PD (pressure difference) [18].
14.4 Medical Management of Cerebral Aneurysms
A subset of patients with unruptured aneurysms who are categorized as aneurysms with a low risk of rupture by various criteria (PHASES, UIATS, ELAPSS) are managed conservatively and followed up with serial MRA imaging. The two most essential components of conservative management of unruptured aneurysms are blood-pressure reduction and Acetylsalicylic acid (ASA), which are being studied in a prospective, randomized, phase III trial titled PROTECT-U [19]. The incidence of Cerebral vasospasm (CVS) following aneurysmal SAH is high and has been associated with delayed cerebral ischemia leading to increased morbidity and mortality. Nimodipine is the only conventional drug to improve outcomes and decrease mortality prophylactically. Recent animal studies and clinical trials involving various emerging medical therapies (Cilostazol, Fasudil, Clazosentan, Rosiglitazone, Tenascin-C knockout, Sildenafil, Erythropoietin) have given contrasting results, and the search for an ideal drug to prevent/treat vasospasm is far from over [20].
14.5 Clipping Versus Coiling
The quest to choose the optimal treatment for aneurysmal SAH between Endovascular technique and microsurgical clipping is never ending. Ten years follow-up of International Subarachnoid Aneurysm Trial (ISAT) and Barrow Ruptured Aneurysm Trial (BRAT) gave contrasting results suggesting the need for a new perspective intent-to-treat trial to reach a conclusion [21, 22]. Recent metanalysis concluded that clipping is appropriate for ruptured aneurysms and coiling is superior for unruptured aneurysms [23]. Among the endovascular options available for unruptured aneurysms patients treated with flow diverters fared better than those treated with coiling [24].
14.6 Advances in Microneurosurgery
The introduction of advanced microneurosurgery hardware and techniques has revolutionized the treatment of aneurysms. These advances in neurosurgical techniques have prompted neurosurgeons to innovate surgical tools and methods (Fig. 14.3) to make neurosurgery safer, cosmetically appealing, and less invasive [25].
Pterional craniotomy has been the main workhorse for clipping of anterior circulation aneurysms. The choice of craniotomies for these aneurysms has expanded with the addition of the minipterional craniotomy, lateral supraorbital craniotomy (LSO), Supraorbital keyhole approach (SOKHA) [26], f-SOKHA [27] and extradural minipterional approach [28]. These approaches are equally safe and effective as pterional craniotomy with shorter operative time and good cosmetic results.
Endoscopic-assisted microsurgery allows visualization of the blind spots to the microscope. Endoscopic ICG-VA combines the advantages of both ICG-VA and endoscope and enables the visualization of perforating arteries hidden in blind spots [29]. Purely Endoscopic approaches for clipping aneurysms are reported as small case series and need further studies to confirm the safety and efficacy before recommending broad application of these approaches [30].
Intraoperative ICG-VA is a complementary tool that increases the aneurysm occlusion rate. The use of intraoperative ICG-VA revealed unexpected residual aneurysms in 9% and an intraoperative clip modification rate of 15% after an apparent complete occlusion under microscopic visualization [31]. Flow 800 is a microscope-integrated visualization tool that gives an objective analysis of ICG-VA rather than subjective assessment and gives a better idea of the vasculature, especially where ICG is ambiguous [32].
Ultrasonic transit-time flowmetry provides quantitative intraoperative measurements of arterial blood flow using a microflow probe. It is a valuable tool for clipping complex aneurysms and maintaining adequate flow (>50% of baseline), reducing the risk of postoperative ischemic events [33].
Intraoperative neurophysiological monitoring (IOM) (somatosensory-evoked potentials, motor-evoked potentials) reduces the incidence of ischemic complications and development of new motor deficit in monitored patients, more so in patients with the middle cerebral artery (MCA) aneurysms [34]. A small case series of 30 patients underwent clipping of aneurysm in awake condition and found three patients who developed neurological deficits without associated changes in neuromonitoring. This study revealed a potential advantage of awake aneurysm surgery, but additional studies are needed to establish the safety of this approach [35].
Transient cardiac standstill (Adenosine, Rapid ventricular pacing) softens the aneurysm sac, avoids intraoperative rupture, bleeding and facilitates permanent clip placement without the need for temporary clipping [36].
Cerebral revascularization is a crucial tool in the armamentarium of the cerebrovascular surgeon used to treat complex intracranial aneurysms that are difficult to manage with traditional surgical or endovascular methods. Apart from the primary EC-IC bypass, several creative and innovative bypasses (“the third generation” bypasses/in situ intracranial-intracranial bypasses, Reimplantation/Reanastomoses, and “the fourth generation” bypasses/double reimplantation using three end-to-side anastomoses) have been invented and used with good outcomes in patients with complex VA, PICA, MCA, and DACA aneurysms [37].
14.7 Advances in Endovascular Management of Aneurysms
Guglielmi introduced the detachable coil system in the 1990s, and this marked the development of a new field of Endovascular Neurosurgery [38]. The early results of coiling were not convincing. Still, significant technological advances were made to alter the coil properties and various devices were introduced to assist coil embolization (Fig. 14.4) and improve occlusion rates [39].
14.7.1 Advances in Coils
Many advances have been made in the design and deployment technique of coils to improve the outcomes of aneurysm coiling (Fig. 14.5). Soft Nano-type coils with increased conformability are used to fill out residual spaces post coiling and to treat small aneurysms. Longer coils with larger coil diameters are available to address larger aneurysms. Coils are coated with materials like polyglycolic/polylactic acid (PGLA) microfilament and hydrophilic acrylic copolymer to increase the thrombogenic effect (Bioactive coils) [40]. Another advancement in coils includes coils containing a hydrogel polymer (HES coils) that expands to fill the coil lumen once it makes contact with blood. Several trials (HELPS, PRET, GREAT, and HEAT) have demonstrated promising outcomes and lesser recurrences in patients managed with HES coils [41].
A 42-year-old woman with a large aneurysm of the supraclinoid segment of the left internal carotid artery was coiled with Microplex coils (Microvention, USA). (a) oblique view of the left internal carotid artery injection showing the large aneurysm of the supraclinoid segment of the left internal carotid artery (arrows). (b) frontal view of the left internal carotid artery injection after aneurysm coil embolization. (c) lateral view of the left internal carotid artery injection after aneurysm coil embolization. Showing the aneurysm was completely occluded (arrows)
14.7.2 Balloon-Assisted Coiling
Balloon-assisted coiling involves the temporary inflation of a balloon catheter across the aneurysm neck to prevent herniation of coils back into the parent artery and acts as a rescue in case of aneurysm rupture. Advanced super compliant balloons (HyperForm, HyperGlide, TransForm, Scepter), double-lumen balloons (ECLIPSE 2L) have replaced low-compliance balloons. Balloon-assisted coiling simplified and made coiling safe by reducing the procedural time [42] (Fig. 14.6).
A 41-year-old man presented with an unruptured ophthalmic aneurysm of the internal carotid artery. (a) 3-D reconstruction of the right internal carotid artery injection showing an aneurysm of the ophthalmic artery segment (arrow). (b) Unsubtracted image showing the aneurysm was coiled (black arrow) with the assistance of a 4 mm × 20 mm Hyperglide balloon catheter (Medtronic ev3, USA) (white arrow). (c) Lateral view of the right internal carotid artery injection showing the aneurysm was completely occluded (arrow)
14.7.3 Stent-Assisted Coiling
Stent-assisted coiling (SAC) uses stents to stabilize coils inside the aneurysmal sac and prevent herniation back into the parent artery, maintaining the patency of the parent vessel. Unlike in balloon-assisted coiling, stents are left inside the vessels, require chronic antiplatelet therapy and carry the risk of delayed stenosis/occlusion. The stents used to depend on the type of SAC technique employed. In the Jailed coiling technique, a microcatheter is first inserted into the aneurysm sac, and then the stent is deployed to jail the microcatheter. Usually, resheathable closed-cell stents are used like LEO (Balt Extrusion, Montmorency, France), Enterprise stent (Codman Neurovascular, Raynham, MA, USA) and LVIS (MicroVention Inc., Aliso Viejo, CA, USA) (Figs. 14.7 and 14.8). Trans-cell coiling involves the stent deployment and advancement of the microcatheter into the aneurysm through the open cells. The trans-cell coiling technique is done with open-cell stents like Neuroform Atlas (Stryker Neurovascular, Fremont, CA, USA) [43].
A 62-year-old woman presented with a recurrent aneurysm after 2 years of coil embolization. (a) Lateral view of the left carotid artery injection showing recanalization of a supraclinoid aneurysm of the internal carotid artery (arrow). (b) Roadmap image of the left carotid artery injection showing coils was introduced (arrow) after the 4.5 mm × 25 mm Leo stent (Balt, France) placement. (c) Lateral view of the unsubtracted image showing the Leo stent (arrow) and coil mass. (d) Oblique view of the left carotid artery injection showing the aneurysm was completely occluded after treatment
A 54-year-old man presented with Hunt-Hess grade 1 subarachnoid hemorrhage. (a) Frontal view of the left vertebral artery injection showing a basilar artery aneurysm at the origin of the left superior cerebellar artery (arrow). (b) Frontal view of the right vertebral artery injection showing the aneurysm was treated with 2.5 mm × 23 mm LVIS-junior stent (Microvention, USA) and coils (black arrow). (c) Frontal view of the right vertebral artery injection showing the aneurysm was occluded completely (arrow)
Advances in stent-assisted coiling include Temporary Bridging Devices and Bifurcation support devices. Temporary bridging devices support coil packing without compromising blood flow aided by their compliant mesh design. They are retrieved once the coils are deployed and obviate the need for chronic antiplatelet therapy. The Comaneci device (Rapid Medical, Israel) and Cascade (Perflow Medical Ltd., Netanya, Israel) are examples of temporary bridging devices [44]. Bifurcation support devices offer support for coil mass as well as neck reconstruction in bifurcation aneurysms and have flow diversion properties. These are novel stent-like, self-expanding, nitinol devices with two components, a component each for the parent vessel and aneurysm sac. They need chronic antiplatelet therapy, and examples include pCANVAS (Phenox, Bochum, Germany), The PulseRider Device (Pulsar Vascular, Inc., Los Gatos, CA, USA), and eCLIPs (Evasc Medical Systems Corp, Vancouver, Canada) [45].
14.7.4 Flow Diverter Devices
Flow Diverter Devices (FDDs) are a novel breakthrough in the endovascular management of cerebral aneurysms and are rapidly evolving as the first-line of treatment modality for various complex aneurysms. The mesh of FDD creates an impedance that disrupts the blood flow into and out of the aneurysm. The substantial reduction in velocity of blood flow inside the aneurysm activates platelets to form a stable thrombus which over a few months to years transform into collagen, leading to complete occlusion of the aneurysm [46]. There are two types of flow diverters Intraluminal FDD and Intrasaccular FDD.
Intraluminal FDD involves the placement of a semipermeable stent in the parent artery, which redirects blood away from the aneurysm, causing flow stasis and thrombosis. The pipeline embolization device (PED; ev3/Covidien, Irvine, CA, USA) (Fig. 14.9), The Silk flow diverter device (Silk, Balt Extrusion, Montmorency, France) (Fig. 14.10), The Surpass flow diverter device (Stryker Neurovascular, Fremont, CA, USA), The flow redirection endoluminal device system (FRED; MicroVention, Tustin, CA, USA) and The Tubridge flow diverter (MicroPort Medical Company Shanghai, China) are examples of intraluminal FDD [47] (Fig. 14.11). The role of flow diversion for aneurysm treatment has expanded, and various recent trials (PREMIER, SAFE, SCENT, PARAT) have proved their favorably low complication and high cure rates compared with alternative treatments [47].
A 53-year-old woman presented with a blurred vision of her left eye. (a) Axial MR imaging showing a round flow void signal near the left optic chiasm (arrow). (b) Frontal view of the left internal carotid artery injection showing a large aneurysm of the supraclinoid internal carotid artery (arrow). (c) 3-D reconstruction of the left internal carotid artery injection showing a 16 mm aneurysm of the supraclinoid internal carotid artery (arrow). (d) Frontal view of the unsubtracted image showing the aneurysm was treated with 4.0 mm × 20 mm Pipeline flow diversion and coils (Axium, Medtronic-ev3, USA) (arrow). (e) Lateral view of the left internal carotid artery injection after treatment. (f) frontal view of the left internal carotid artery injection after treatment
A 37-year-old man suffered from swallowing difficulty and numbness of his left limbs. (a) Magnetic resonance imaging, sagittal view, T2-weighted, showing a giant saccular vascular lesion compressing the brain stem (arrow). (b) Frontal view of the right vertebral artery injection showing a giant aneurysm (arrow). (c) Lateral view of the left vertebral artery injection, unsubtraction image, showing a giant saccular aneurysm of the vertebra-basilar junction (arrow). (d) Unsubtraction image showing a 3.0 mm × 25 mm Silk flow diversion (Balt, France) was placed in the left vertebral artery (arrow), and the right vertebral artery was occluded using coils. (e) Frontal view of the right vertebral artery showing the right vertebral artery was occluded. (f) Lateral view of the left vertebral artery injection showing the aneurysm was completely thrombosed after flow diversion and additional coils treatment
A 72-year-old man presented with an incidental aneurysm of the supraclinoid internal carotid artery. (a) Lateral of the right internal carotid artery injection showing an aneurysm arising from the supraclinoid internal carotid artery (arrow), which was treated with Tubridge flow diversion (MicroPort Medical Company, Shanghai, China). (b) Lateral view of the unsubtracted image showing the 3.5 mm × 25 mm Tubridge flow diversion. (c) Lateral view of the right internal carotid artery injection after flow diversion treatment showing the intra-aneurysm contrast stagnation (arrow). (d) Picture showing the Tubridge flow diversion system
Intrasaccular flow diverter/flow disrupters are deployed within the aneurysm and do not require the problematic catheterization of bifurcation branches nor the use of chronic antiplatelet therapy. Woven EndoBridge device (WEB, Sequent Medical, Aliso Viejo, California, USA), Luna aneurysm embolization device (AES; NFocus Neuromedical, Palo Alto, California), and Medina embolization device (MED, Medtronic, Irvine, California, USA) are examples of intrasaccular flow diverters. Several trials (WEBCAST 2, WEB-IT) have proven the safety and have shown adequate occlusion rates of the aneurysm [48].
14.8 Conclusions
Significant advances were made in the last decade in various aspects of cerebral aneurysms. Future research should convincingly identify aneurysms at risk of rupture by using serum/genetic/imaging markers to give personalized aneurysm care. New Endovascular innovations to tackle complex aneurysms should be developed and extensively studied to confirm efficacy and safety. New training modules should be invented to give haptic feedback to beginners doing endovascular work to better the outcomes. The role of microneurosurgery in the management of cerebral aneurysms cannot be ignored. Microneurosurgery with innovative revascularisation techniques will continue to be a significant treatment modality for complex aneurysms till time tested endovascular alternatives emerge.
References
Stehbens WE. Etiology of intracranial berry aneurysms. J Neurosurg. 1989 Jun;70(6):823–31.
Krings T, Mandell DM, Kiehl TR, Geibprasert S, Tymianski M, Alvarez H, et al. Intracranial aneurysms: from vessel wall pathology to therapeutic approach. Nat Rev Neurol. 2011 Sep 20;7(10):547–59.
Cebral J, Ollikainen E, Chung BJ, Mut F, Sippola V, Jahromi BR, et al. Flow conditions in the intracranial aneurysm lumen are associated with inflammation and degenerative changes of the aneurysm wall. AJNR Am J Neuroradiol. 2017 Jan;38(1):119–26.
Kim DH, Santiago-Sim T. Pathobiology of intracranial aneurysms. In: Richard Winn H, editor. Youmans and Winn neurological surgery. 7th ed. Philadelphia, PA: Elsevier; 2017. p. 3221–31.
Byrne G, Mut F, Cebral J. Quantifying the large-scale hemodynamics of intracranial aneurysms. AJNR Am J Neuroradiol. 2014 Feb;35(2):333–8.
Soldozy S, Norat P, Elsarrag M, Chatrath A, Costello JS, Sokolowski JD, et al. The biophysical role of hemodynamics in the pathogenesis of cerebral aneurysm formation and rupture. Neurosurg Focus. 2019 Jul 1;47(1):E11. https://doi.org/10.3171/2019.4.FOCUS19232.
Cebral JR, Detmer F, Chung BJ, Choque-Velasquez J, Rezai B, Lehto H, et al. Local hemodynamic conditions associated with focal changes in the intracranial aneurysm wall. AJNR Am J Neuroradiol. 2019 Mar;40(3):510–6.
Chen J, Liu J, Zhang Y, Tian Z, Wang K, Zhang Y, et al. China intracranial aneurysm project (CIAP): protocol for a registry study on a multidimensional prediction model for rupture risk of unruptured intracranial aneurysms. J Transl Med. 2018 Sep 26;16(1):263.
Feghali J, Gami A, Caplan JM, Tamargo RJ, McDougall CG, Huang J. Management of unruptured intracranial aneurysms: correlation of UIATS, ELAPSS, and PHASES with referral center practice. Neurosurg Rev. 2020. https://doi.org/10.1007/s10143-020-01356-6.
Darsaut TE, Desal H, Cognard C, Januel AC, Bourcier R, Boulouis G, et al. Comprehensive aneurysm management (CAM): an all-inclusive care trial for unruptured intracranial aneurysms. World Neurosurg. 2020 Sep;141:e770–7.
Brinjikji W, Gupta V, Vibhute P. Imaging of intracranial aneurysms. In: Ringer AJ, editor. Intracranial aneurysms. London: Academic Press An imprint of Elsevier; 2018. p. 59–83.
Pradilla G, Wicks RT, Hadelsberg U, Gailloud P, Coon AL, Huang J, et al. Accuracy of computed tomography angiography in the diagnosis of intracranial aneurysms. World Neurosurg. 2013 Dec;80(6):845–52.
Sailer AM, Wagemans BA, Nelemans PJ, de Graaf R, van Zwam WH. Diagnosing intracranial aneurysms with MR angiography: systematic review and meta-analysis. Stroke. 2014 Jan;45(1):119–26.
Mandell DM, Mossa-Basha M, Qiao Y, Hess CP, Hui F, Matouk C, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American society of neuroradiology. AJNR Am J Neuroradiol. 2017 Feb;38(2):218–29.
Texakalidis P, Hilditch CA, Lehman V, Lanzino G, Pereira VM, Brinjikji W. Vessel wall imaging of intracranial aneurysms: systematic review and meta-analysis. World Neurosurg. 2018;117:453–58.e1.
Shimizu K, Kushamae M, Aoki T. Macrophage imaging of intracranial aneurysms. Neurol Med Chir (Tokyo). 2019;59(7):257–63.
Can A, Du R. Association of hemodynamic factors with intracranial aneurysm formation and rupture: systematic review and meta-analysis. Neurosurgery. 2016 Apr;78(4):510–20.
Jirjees S, Htun ZM, Aldawudi I, Katwal PC, Khan S. Role of morphological and hemodynamic factors in predicting intracranial aneurysm rupture: a review. Cureus. 2020;12(7):e9178. https://doi.org/10.7759/cureus.9178.
Etminan N, Dörfler A, Steinmetz H. Unruptured intracranial aneurysms—pathogenesis and individualized management. Dtsch Arztebl Int. 2020 Apr 3;117(14):235–42.
Li K, Barras CD, Chandra RV, Kok HK, Maingard JT, Carter NS, et al. A review of the management of cerebral vasospasm after aneurysmal subarachnoid haemorrhage. World Neurosurg. 2019 Jun;126:513–27.
Hua X, Gray A, Wolstenholme J, Clarke P, Molyneux AJ, Kerr RSC, Clarke A, Sneade M, Rivero-Arias O. Survival, dependency, and health-related quality of life in patients with ruptured intracranial aneurysm: 10-year follow-up of the United Kingdom Cohort of the International Subarachnoid Aneurysm Trial. Neurosurgery. 2020 Oct 19. https://doi.org/10.1093/neuros/nyaa454
Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Nakaji P, Karis JP, Wallace RC. Ten-year analysis of saccular aneurysms in the Barrow ruptured aneurysm trial. J Neurosurg. 2019 Mar;8:1–6.
Jiang Z, Chen Y, Zeng C, Feng J, Wan Y, Zhang X. Neurosurgical clipping versus endovascular coiling for patients with intracranial aneurysms: a systematic review and meta-analysis. World Neurosurg. 2020 Jun;138:e191–222.
Xin WQ, Xin QQ, Yuan Y, Chen S, Gao XL, Zhao Y, Zhang H, Li WK, Yang XY. Comparison of flow diversion and coiling for the treatment of unruptured intracranial aneurysms. World Neurosurg. 2019 Aug;128:464–72.
Kalani MYS, Wanebo JE, Martirosyan NL, Nakaji P, Zabramski JM, Spetzler RF. A raised bar for aneurysm surgery in the endovascular era. J Neurosurg. 2017 May;126(5):1731–9.
Park J. Supraorbital keyhole approach for intracranial aneurysms: transitioning from concerns to confidence. J Korean Neurosurg Soc. 2020 Jan;63(1):4–13.
Chandra PS, Tej M, Sawarkar D, Agarwal M, Doddamani RS. Fronto-orbital variant of supraorbital keyhole approach for clipping ruptured anterior circulation aneurysms (f-Sokha). Neurol India. 2020 Sep-Oct;68(5):1019–27.
Martinez-Perez R, Joswig H, Tsimpas A, Poblete T, Albiña P, Perales I, et al. The extradural minipterional approach for the treatment of paraclinoid aneurysms: a stepwise cadaver dissection and clinical case series. Neurosurg Rev. 2020 Feb;43(1):361–70.
Catapano G, Sgulò F, Laleva L, Columbano L, Dallan I, de Notaris M. Multimodal use of indocyanine green endoscopy in neurosurgery: a single-centre experience and review of the literature. Neurosurg Rev. 2018 Oct;41(4):985–98.
Martinez-Perez R, Hardesty DA, Silveira-Bertazzo G, Albonette-Felicio T, Carrau RL, Prevedello DM. Safety and effectiveness of endoscopic endonasal intracranial aneurysm clipping: a systematic review. Neurosurg Rev. 2020. https://doi.org/10.1007/s10143-020-01316-0.
Riva M, Amin-Hanjani S, Giussani C, De Witte O, Bruneau M. Indocyanine green video angiography in aneurysm surgery: systematic review and meta-analysis. Neurosurgery. 2018 Aug 1;83(2):166–80.
Chavan VS, Yamada Y, Chandratej K, Gowtham D, Riccardo S, Firuz S, et al. Intraoperative use of microscope-integrated flow 800 - a valuable tool in the surgical management of anterior communicating artery aneurysm: our institutional experience. Asian J Neurosurg. 2020 Feb 25;15(1):26–30.
Van Lanen RHGJ, Jacobi-Postma LAA, Veersema TJ, Teernstra OPM, Dings JTA. Clinical and radiological outcomes of intracranial aneurysm clipping aided by transit-time flowmetry. World Neurosurg. 2020 Apr;136:e660–70.
Nasi D, Meletti S, Tramontano V, Pavesi G. Intraoperative neurophysiological monitoring in aneurysm clipping: does it make a difference? A systematic review and meta-analysis. Clin Neurol Neurosurg. 2020 Sep;196:105954. https://doi.org/10.1016/j.clineuro.2020.105954.
Abdulrauf SI, Vuong P, Patel R, Sampath R, Ashour AM, Germany LM, et al. “Awake” clipping of cerebral aneurysms: report of initial series. J Neurosurg. 2017 Aug;127(2):311–8.
Meling TR, Lavé A. What are the options for cardiac standstill during aneurysm surgery? A systematic review. Neurosurg Rev. 2019 Dec;42(4):843–52.
Raper DMS, Rutledge WC, Winkler EA, Meisel K, Callen AL, Cooke DL, et al. Controversies and advances in adult intracranial bypass surgery in 2020. Oper Neurosurg (Hagerstown). 2020. https://doi.org/10.1093/ons/opaa276.
Guglielmi G, Viñuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: preliminary clinical experience. J Neurosurg. 1991 Jul;75(1):8–14.
Jiang B, Paff M, Colby GP, Coon AL, Lin LM. Cerebral aneurysm treatment: modern neurovascular techniques. Stroke Vasc Neurol. 2016 Oct 25;1(3):93–100.
Van Rooij WJ, de Gast AN, Sluzewski M. Results of 101 aneurysms treated with polyglycolic/polylactic acid microfilament nexus coils compared with historical controls treated with standard coils. AJNR Am J Neuroradiol. 2008 May;29(5):991–6.
Bendok BR, Abi-Aad KR, Ward JD, Kniss JF, Kwasny MJ, Rahme RJ, et al. The hydrogel endovascular aneurysm treatment trial (HEAT): a randomized controlled trial of the second-generation hydrogel coil. Neurosurgery. 2020 May 1;86(5):615–24.
Pop R, Harsan O, Martin I, Mihoc D, Richter JS, Manisor M, et al. Balloon-assisted coiling of intracranial aneurysms using the Eclipse 2L double-lumen balloon. Interv Neuroradiol. 2020 Jun;26(3):291–9.
Zhu Y, Zhang H, Zhang Y, Wu H, Wei L, Zhou G, et al. Endovascular metal devices for the treatment of cerebrovascular diseases. Adv Mater. 2019 Feb;31(8):e1805452. https://doi.org/10.1002/adma.201805452.
Sirakov S, Sirakov A, Minkin K, Karakostov V, Raychev R. Early clinical experience with Cascade: a novel temporary neck bridging device for embolization of intracranial aneurysms. J Neurointerv Surg. 2020 Mar;12(3):303–7.
Peach TW, Ricci D, Ventikos Y. A virtual comparison of the eCLIPs device and conventional flow-diverters as a treatment for cerebral bifurcation aneurysms. Cardiovasc Eng Technol. 2019 Sep;10(3):508–19.
Dandapat S, Mendez-Ruiz A, Martínez-Galdámez M, Macho J, Derakhshani S, Foa Torres G, et al. Review of current intracranial aneurysm flow diversion technology and clinical use. J Neurointerv Surg. 2020. https://doi.org/10.1136/neurintsurg-2020-015877.
Chancellor B, Raz E, Shapiro M, Tanweer O, Nossek E, Riina HA, et al. Flow diversion for intracranial aneurysm treatment: trials involving flow diverters and long-term outcomes. Neurosurgery. 2020 Jan 1;86(Suppl 1):36–45.
Kaya HE, Bakdık S, Keskin F, Erdi MF, Koç O. Endovascular treatment of intracranial aneurysms using the Woven EndoBridge (WEB) device: a retrospective analysis of a single-centre experience. Clin Imaging. 2020 Jan;59(1):25–9.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ramesh Chandra, V.V., Prasad, B.C.M., Goutham, T., Venkat, K., Sasank, D., Lv, X. (2022). Recent Advances in Cerebral Aneurysms. In: Lv, X. (eds) Endovascular Surgery of Cerebral Aneurysms. Springer, Singapore. https://doi.org/10.1007/978-981-16-7102-9_14
Download citation
DOI: https://doi.org/10.1007/978-981-16-7102-9_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7101-2
Online ISBN: 978-981-16-7102-9
eBook Packages: MedicineMedicine (R0)