Abstract
Nodal metastasis plays a crucial role in determining prognosis, management, and survival of colorectal cancer patients. Thus, adequate lymphadenectomy remains the corner stone for improving survival in colorectal cancers. This chapter expounds the following current concepts related to lymphadenectomy for colorectal cancers in the following arenas: (a) CME with CVL (complete mesocolic excision with central vascular ligation) (b) D2 versus D3 lymphadenectomy for colonic cancers (c) TME total mesorectal excision for rectal malignancies (d) Lateral lymph node dissection in colorectal cancers (e) Sentinel node biopsy in colonic cancers. The technique of laparoscopic resection for right sided and left sided colonic cancers is also elucidated.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Lymphadenectomy
- Colorectal cancers
- Complete mesocolic excision
- Central vascular ligation
- Total mesocolic excision
- Total mesorectal excision
- Sentinel node biopsy
- CME
- CVL
- TME
- Laparoscopic colectomy
Introduction
The mainstay management for colon cancer remains surgery. The pathological findings in the specimen are the most important predictor of further treatment and survival. Cancer staging depends upon the assessment of primary tumor [T], lymph node metastasis [N], and distant metastasis [M] and these variables are most important for pathologists and treating clinicians.
Nodal metastasis plays a crucial role in determining prognosis, management, and survival of colorectal cancer patients and consists of an important parameter in contemporary prognostic staging systems particularly the widely used tumor node metastasis [TNM] system proposed by the UICC/AJCC. The 5-year survival rates range between 70% and 80% in node negative disease, with the corresponding values in node positive disease being 30%–60%. Adjuvant chemotherapy improves the survival in node positive disease. Occult lymph node disease is present in 20%–30% cases which is apparently present in completely excised disease [1, 2]. Adjuvant chemotherapy is beneficial in such a subset of cases when identified. Some of the other prognostic variables over and above TNM which might affect disease spread, recurrence and eventually benefit from adjuvant chemotherapy in colorectal cancers are: (a) venous invasion, (b) perineural invasion, (c) tumour perforation, (d) serosal involvement and (e) incomplete resection [1, 2]. Incomplete resection particularly refers to both primary tumor and nodal resection. Therefore, to obtain an accurate staging a conscientiousness effort is required both by surgeons and pathologists alike.
This chapter further discusses the nodal staging and the concept of adequate lymphadenectomy in right and left sided colonic tumours and rectum with an emphasis on the techniques of adequate lymphadenectomy.
Nodal Staging
American Joint Committee on Cancer [AJCC] 6th edition suggested a range of 7–14 LNs that should be obtained. The corresponding 7th and 8th editions in their respective sections stated that it is important to obtain and examine at least 12 LNs [3,4,5,6,7]. Even if less than the suggested number of LNs is identified, actual N stage rather than Nx should be provided. The factors which highly impact LN recovery include
-
I.
Patient age
-
II.
Gender and body habitus
-
III.
Immune response to neo adjuvant treatment
-
IV.
Tumor site and size
-
V.
Length of colon resected
-
VI.
Experience of surgeon
-
VII.
Diligence and experience of a pathologist.
CAP [College of American pathologists] malignancy convention proposes that if less than 12 LNs are found, the specimen should be reconsidered for examination methods using lymph node enhancement techniques. In contrast to the sixth version, the seventh release further partitions N1 into N1a, N1b, and N1c; and N2 into N2a and N2b. N1c is a recently presented class in the seventh release, which is characterized by Tumour deposits (TD’s) in subserosa, mesentery, or nonperito-nealized pericolic or perirectal/mesorectal tissue with no local nodal metastasis. The eighth version does not have critical changes in N staging definitions in contrast to the seventh version. The master board endeavored to explain a few issues that have stayed testing in past versions, like TDs and micrometastasis.
Techniques of Colorectal Lymphadenectomy
Current Concepts
As mentioned, adequate lymphadenectomy remains the most important prognostic determinant for overall and disease-free survival. In the last three decades, the concept of lymphadenectomy in colorectal cancers has been revolutionized. The concept came more into practice with evolving minimal access surgery and centres across the world performing laparoscopic and robotic surgery. After two decades of the utilization of laparoscopic approach for colorectal surgeries, many randomized trials and systemic reviews have shown that the laparoscopic approach for colon malignancy is related with quicker recovery and less morbidity in contrast with the standard open methodology without influencing oncologic results [8,9,10,11,12,13,14,15].
Complete Mesocolic Excision [CME] and Central Vascular Ligation [CVL] with D3 Lymphadenectomy for Right Sided Colonic Cancers
Western Concept of Right Sided Colonic Cancers Lymphadenectomy
Hohenberger et al. promulgated the idea of complete mesocolic excision as the standard operative procedure for colonic malignancy with an emphasis that CME with CVL decreases local recurrence and improves survival rates particularly in stage III cancers [16]. Subsequent literature from different parts of the world likewise showed comparable critical decrease in local recurrences and improvement of oncological radicality [17,18,19].
The concept of complete mesocolic excision (CME) with central vascular ligation (CVL), paralleling the concept of total mesorectal excision (TME) described by Professor RJ Heald [20], entails dissection of entire mesocolon in the embryonic planes of fusion. In the intaruterine period, the colon along with its vascular supply and lymphatics is suspended in a dorsal mesentery, which subsequently fuses with the retroperitoneum in the region of caecum, ascending and descending colon. Thus, an avascular plane exists between the mesocolon and the retroperitoneum (Fig. 1). It is important to note that the peritoneal bilayer covering the mesocolon envelopes the entire colon and is not merely limited to the pelvis. The aim of CME with CVL technique is to separate these two planes and excise the tumour along with the colon, the mesocolon with its accompanying lymphatic and vascular supply in totality, ensuring an intact visceral fascial layer is maintained. It can be achieved by sharp dissection between the visceral and the parietal peritoneal layers. Appropriate knowledge of anatomy of the mesocolon as well as adequate surgical expertise is desirable for the purpose.
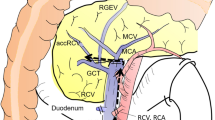
The essence of CME-CVL or D3 lymphadenectomy is the ligation of the ileocolic vein, right colic vein, Henle trunk, and middle colic vein at their point of drainage into the superior mesenteric vein (SMV). Corresponding with venous ligation, the ileocolic artery, right colic artery, and middle colic artery are divided from their origin on the superior mesenteric artery (SMA) (Fig. 2). CME-CVL is a technically demanding procedure due to the complex anatomy of the region and explicit knowledge of vascular anatomy (SMV and SMA) is vital to avoid iatrogenic complications. As regards oncological adequacy, this procedure is almost equivalent to eastern concept of Japanese D3 lymphadenectomy.
Eastern Concept of Right Sided Colonic Cancers Lymphadenectomy
The Japanese Society for Cancer of the Colon and Rectum (JSCCR) classification groups the nodes associated with lymphatic drainage of colon into three groups.
-
The main lymph nodes are situated at the source of the main feeding artery.
-
Intermediate lymph nodes lie between the initial and terminal branch of the main artery
-
Pericolic lymph nodes are stationed between the terminal branch of the main feeding artery and the colonic wall [21, 22].
In D2 lymphadenectomy the pericolic and intermediate groups lymph nodes are removed. D3 lymphadenectomy entails dissection of the main lymph nodes in addition to D2 lymphadenectomy. Thus, the western concept of CME-CVL is essentially comparable to definition of D3 lymphadenectomy by the JSCCR. However, in D3 dissection duodenal kocherization, and removal of the gastroepiploic and infrapyloric lymph nodes is not mentioned which has been described as a component of CME with CVL [21, 22].
The JSCCR guidelines recommend D3 lymphadenectomy for advanced T categories (T3/4) or node positive (N+) disease and D2 lymphadenectomy for early node negative cancers (T1N0). Whereas performance of either a D3 or D2 lymphadenectomy is suggested for T2N0 disease. Therefore, D3 lymphadenectomy is essentially recommended for stage II or III colon cancer in tertiary care centers.
Total Mesorectal Excision [TME]
The notion of total mesorectal excision [TME] for rectal cancer has been the most revolutionary concept that has evolved during the last three decades. Multiple studies noted a decrease in local recurrence to the tune of 6%–12%, and a 53%–87% improvement in 5-year survival after incorporation of TME [23,24,25].
In TME the rectum, along with its surrounding mesorectum comprising of lymphovascular fatty tissue (the first area of drainage of tumour cells), is excised using precise, sharp dissection in an avascular potential space between the visceral mesorectal fascia and parietal endopelvic fascia the so called “Holy plane” a term introduced by Heald [26]. TME minimizes the chances of leaving behind residual tumor and preserves nerve fibres which supply the urinary bladder, prostate, and vagina (Fig. 3).
The essence of the TME hypothesis is that lymph nodes randomly distributed within the mesorectum, which are not visible or palpable, are completely removed. The size of the normal lymph nodes in mesorectum in about 80% of cases is <3 mm. Most lymph nodes in the mesorectum are located posteriorly, and 90% of the posterior lymph nodes lie within the upper half of the upper 2/3 of the rectum [27].
Rectal cancers very rarely spread in a downward direction intramurally, but the lymphatic spread in the mesorectum i.e. extramural spread, appears to be bidirectional (both in distal and proximal directions), within the limits of fascia of mesorectum, emphasizing the need for a complete mesorectal excision. Whereas TME is a beneficial procedure to extirpate lymphatic spread in high rectal carcinomas located >5 cm above the dentate line, the same is not noted in lower rectal neoplasms [less than 5 cm from the dentate line] wherein around 15–20% cases there is lateral nodal involvement which lies outside the confines of TME. A lateral node dissection as described below may prove beneficial in such patients.
Lateral Lymph Node Dissection [LLND]
The lymphatic drainage from the rectum below the peritoneal reflection follows two major pathways [Fig. 4]:
-
1.
The superior rectal artery, inferior mesenteric artery, para-aortic corridor
-
2.
Middle and inferior rectal artery, obturator, internal iliac and external iliac corridor (the lateral nodal group).
Schematic diagram showing the Lateral pelvic lymph nodes. In the diagram marked vessels are 1. Inferior mesenteric artery, 2. Superior rectal artery, 3. Common Iliac artery, 4. External Iliac artery, 5. Internal Iliac artery, 6. Obturator artery, 7. Middle rectal artery, 8. Internal pudendal artery, 9. Inferior rectal artery. (Radjindrin A (2018) Does Lateral Pelvic Lymph node matters in rectal cancer Glob Surg, 2018 doi: https://doi.org/10.15761/GOS.1000196 )
Total Mesorectal Excision [TME] involves removal of the first pathway of lymph nodes [28]. Management of the lymph node stations in the second route of drainage (the lateral nodes) has been a topic of interest lately [29]. It needs to be emphasized that the internal iliac group of nodes is classified as regional disease (Stage III) whereas the external and common iliac nodes are grouped as metastatic disease (Stage IV) in the TNM classification. Despite the classification radiation oncologists often treat external and common iliac nodes in rectal cancer with curative intent in concordance with treatment of regional nodes [Table 1] [30]. The lateral lymph nodes can be treated with either a radiotherapy boost or surgically by lateral lymph node dissection [30]. The Japanese guidelines for colorectal cancer [2016] recommends LLND for all rectal tumours situated caudal to the peritoneal reflection [31]. According to the JSCCR, LLND decreases intrapelvic recurrence by 50% and offers a survival advantage of 8–9% [31]. A randomized controlled trial noted no increase in urinary dysfunction consequent to LLND though a tumor location below peritoneal reflection was proposed as a risk factor for the complication [32]. A multicentre non inferiority trial from Japan, JCOG2012, could not conclude a non-inferiority of TME alone over TME + LLND, however observed that the incidence of urinary and male sexual dysfunction was not significantly higher in the LLND group.[33] Nevertheless an increased morbidity has been observed following the procedure [32,33,34]. Mesorectal nodal metastasis has been proposed to be another important determinant of lateral lymph node metastasis [35].
Minimally Invasive LLND
In a study assessing feasibility of lateral pelvic lymph node dissection, when compared with the open approach the laparoscopic approach was considered safe, incurred a less blood loss, had lower hospital stay and had higher mean number of harvested nodes [35]. An autonomic nerve preserving approach for laparoscopic LLND based on vesical-hypogastric fascia and uretero-hypogastric nerve fascia has been proposed [36]. Robotic LLND has similar short-term outcomes and lymph node harvest, offering advantages in male narrow pelvis where manipulation of instruments becomes difficult in laparoscopic approach [37, 38].
Sentinel Lymph Node [SLN] Resection
The concept of sentinel lymph node biopsy [SLNB], which has significantly impacted the treatment of melanoma and breast cancer, is being investigated in colorectal cancers to enhance nodal staging accuracy especially in T1 disease. The sentinel lymph node is considered the lymph node[s] located the closest in the lymphatic mapping area. Despite a potential curative resection, 20–30% of node negative colorectal cancers develop distant metastasis presumably due to occult undetected nodal disease [39]. It has been noted that small <5 mm nodal deposits carry similar survival prognostication as >5 mm deposits emphasizing the importance of thorough examination of nodes [40]. Though yet controversial, micrometastatic deposits <2 mm may also benefit from postoperative adjuvant therapy.
Identifying patients who have tumor-negative nodes but are at high risk of regional or distant node metastasis who might benefit from adjuvant chemotherapy is challenging. The current histopathological evaluation of lymph nodes with standard Hematoxylin–Eosin [HE] pathological techniques is inadequate as large regions of the lymph nodes remains unexamined, with the subsequent risk of undetected residual micrometastases. Therefore, SLN mapping in colon cancer can help identify nodes that carry the higher risk of metastasis and such nodes can be subjected to detailed pathologic scrutiny, including more sections, immunohistochemistry and molecular diagnostic techniques thereby enhancing the staging accuracy.
Modification in Techniques of Sentinel Lymph Node Mapping
In Vivo Versus Ex Vivo Technique
The mapping can be performed in vivo or ex vivo using various substances: blue dyes, fluorescent dyes or radioactive tracers. Blue dye is the most commonly used dye both for in vivo and ex vivo techniques. The ex vivo technique can be also used in case the in vivo technique fails and has been noted to upstage the tumor in upto 12%. The results of the two techniques of mapping is reported to be similar. One advantage of the ex vivo technique is that the patient is spared from adverse reactions related to the dye but carries limitations due to the surgical disruption of the native lymphatic channels [41]. In vivo analysis involves injecting 1–2 mL of blue dye into the subserosa, around the tumor. The first blue-colored lymph node is removed after 5–10 min and sent separately to the pathologist. In ex vivo mapping, about 30 min after resection and before formalin fixation, 0.5–2 mL of blue dye is injected subserosally or circumferentially around the tumor (depends on the location of tumor i.e. above or below peritoneal reflection) and sites are massaged for five minutes to push the tracer into the lymphatic vessels. The first blue stained lymph node[s] is defined as the SLN [41]. Factors which influence the In vivo technique are: gender, age, BMI, tumor size, tumor location, previous abdominal surgery, nodal status, grade of tumour, tracer used, technique and preoperative chemoradiation [42].
Fluorescent Dye Technique
Recently fluorescence navigation with Indocyanine Green [ICG] has gained popularity for in vivo visualization of SLN. A near infra-red imaging camera system is used in laparoscopic surgeries. The tracer can be injected subserosally or submucosally around the tumor. Advantages of the technique is that it offers real time visualization of lymph nodal compartments and aids detection of aberrant lymphatic drainage. In a study evaluating this method, 96% identification rate was noted. The main deterrent of this procedure is the high cost [43].
Immunohistochemistry and Molecular Methods for Detection of Metastasis in Lymph Nodes
Use of immunohistochemistry and molecular diagnostic methods has been proposed for more accurate detection of micro metastasis in sentinel nodes. Immunohistochemical examination is more sensitive than Hematoxylin–Eosin [HE] whereas and molecular diagnostics (RTPCR/ one step nucleic acid amplification test) is more specific, and more accurate than immunohistochemistry [IHC] in detecting micrometastasis and isolated tumor cells [ITC]. The one step nucleic acid amplification test also decreases time to adjuvant chemotherapy. Ultra-staging with RT PCR demonstrated that node negative colon cancer patients who had recurrence had positive SLN [42]. Focused examination of sentinel node using CK-IHC and RT-PCR can identify micrometastases in 53% of patients whose SNs were labelled as negative by conventional histopathological techniques [44]. Among all the techniques used for the identification of the lymph nodes, the molecular one is the most expensive, but appears to provide the most accurate up staging [44].
Laparoscopic Right Colonic Resections with CVL
Though there are numerous laparoscopic techniques described in literature. The common approaches described are:
-
Medial to lateral approach,
-
Lateral to medial approach
-
Caudo-cranial approach
We prefer the caudo-cranial approach [also called the initial retrocolic endoscopic approach IRETA APPROACH]. All procedures are done in the modified lithotomy position under general anaesthesia, and table position modified in accordance with the area to be mobilized.
Placement of Trocars
Pneumoperitoneum is established with open or closed technique. A diagnostic laparoscopy is initially performed through a 10 mm/12 mm umbilical port. Subsequently the camera port is shifted to suprapubic region to facilitate viewing of the retroperitoneal tunnel that will be created. Two other ports, a 5 mm working port is placed in the region of right iliac fossa and another 5 mm port placed in the left subcostal region to retract small bowel and colon (Fig. 5). Later, the camera port can be transferred to the umbilicus for enabling better visualization during superior dissection along hepatic flexure and transverse colon. The procedure is performed in head down, right up position.
Mobilization of the Retro Colic Colon and Complete Mesocolic Excision [CME]
In the initial retrocolic tunnel approach (IRETA), dissection begins with incision on the inferior border of terminal ileal mesentery (ileocolic fold) and is continued upwards laterally to behind the caecum and cranially and anteriorly in the avascular plane which separates the right ureter, right gonadal vessels and IVC posteriorly from the small bowel mesentery and retroperitonealized right mesocolon anteriorly (Fig. 1). It is important not to dissect free the lateral attachments of the colon at this stage to maintain retraction and keep open the retroperitoneal tunnel by preventing the colon from falling into the operative field. A retroperitoneal tunnel is thus created between two layers of embryologic fusion. Superiorly the dissection continues anterior to Gerota’s fascia laterally, and the duodenum and pancreas cranially (Fig. 6).
Central Vascular Ligation (Figs. 7 and 8)
Tenting the ileocolic mesentery by lifting it up is a useful technique that helps in identifying the ileocolic vessels which are dissected and traced to their origin from the superior mesenteric vessels and clipped. The right colic artery is thereafter addressed. It needs to be noted that it is inconsistently present. Further attention is directed to the middle colic vessels that can be identified traversing the transverse mesocolon vertically up when the transverse colon is lifted towards the abdominal wall. There are variations in drainage of right colic and middle colic veins which may be encountered in the process. In conventional right hemicolectomy only the right branch of middle colic artery is ligated at its origin.
Mobilization of Transverse Colon and Hepatic Flexure
After completion of CVL the following sequence of steps is adopted (1) lesser sac entry by dividing gastrocolic ligament along with omentectomy (2) dissection of hepatocolic ligament and mobilization of hepatic flexure of colon (3) The attachments of the mesoclon dissected from anterior surface of duodenum and pancreas (4) The ascending colon dissected from its lateral attachments to abdominal wall and retroperitoneum.
Resection of Specimen and Anastomosis
If an extracorporeal anastomosis is planned the bowel is delivered through a plastic sheath, by extending the umbilical port and resection as well as anastomosis (handsewn/stapled) is performed outside (Fig. 9). In a totally laparoscopic approach, the transection of the colon and anastomosis is performed intracorporeally using Endo GIA stapler, conversion of the 10 mm port to 12 mm is needed for the purpose or initially a 12 mm umbilical port may be inserted (Fig. 10). Side to side ileo- transverse anastomosis is preferred anastomosis.
Advantages of Initial Retro Colic Approach
-
There is minimal initial handling of colon thereby decreasing chances of tumor dissemination and bowel injury
-
Easy creation of retroperitoneal tunnel and excellent retroperitoneal view
-
The lateral attachments of the colon are dissected last thereby eliminating need for retraction of colon and preventing colon from falling into the operative field, particularly useful for bulky disease
-
Easy early access to vascular pedicles near the origin
Laparoscopic Left Sided Colonic Resections with Total Mesorectal Excision [TME]
The approaches frequently described in literature for the left colon are:
-
Medial to lateral approach
-
Lateral to medial approach
All procedures are done in the modified lithotomy position under general anaesthesia.
Placement of Trocars
The surgeon and the camera assistant are stationed to the right of the patient. A 10mm camera port is inserted at the umbilicus. The procedure is performed with 4 or 5 ports: two 5-mm ports are introduced on either side and another 12-mm port (for stapler) is placed at 2 cm above and medial to the right anterior superior iliac spine, additional 5-mm port can be inserted for bowel retraction (Fig. 11). At the commencement of operation, a diagnostic laparoscopy is performed to assess for metastatic disease. The procedure is performed with patient placed in Trendelenburg position and the table tilted to left up.
Pedicle Ligation: (Fig. 12)
The omentum is displaced superiorly over the liver. A useful manoevure commonly practised for retraction of the uterus anteriorly is slinging the uterus using a percutaneous suture loop passed directly and tied above the skin over a piece of gauze. Retraction of the sigmoid colon to the left and anteriorly helps in identification of the sigmoid vessels and inferior mesenteric artery.
The peritoneum is incised caudal and to the right of the vascular trunk, at the level of sacral promontory and further dissection proceeds cranially to the origin of the vascular trunk (Fig. 13). Care should be taken to make the tunnel anterior to the ureter and hypogastric nerve plexuses which lie in close proximity. The superior rectal artery is lifted cranially and all vessels are skeletonized, and ligated separately using endoscopic clips (Fig. 14). In high ligation the inferior mesenteric artery is ligated at its origin whereas in low ligation, inferior mesenteric artery is ligated distal to its left colic branch (Fig. 12).
Total Mesorectal Excision and Rectal Mobilization
The dissection for TME is initially done posteriorly and laterally then subsequently anteriorly. The lateral peritoneal attachments of the rectum are incised down to the level of peritoneal reflection. The sigmoid colon is retracted ventrally to open the retrorectal space and dissection is carried out in the avascular presacral plane between the parietal and visceral pelvic fascia. The hypogastric autonomic nerves which lie posteriorly, comes close to the mesorectum inferiorly and supply branches to the rectum where they should be carefully dissected by sparing the pelvic branches. Vessels entering the rectum can be addressed with harmonic or vessel sealing devices. Caudally the dissection is continued to the rectosacral fascia following which the rectum curves anteriorly to the pelvic floor (Fig. 15)
Anteriorly the peritoneum is incised to the level of rectovesical or rectovaginal pouch. Traction counter traction remains an integral part of TME. Usually, a gauze piece can be tied around rectum to pull the rectum out of the pelvis and provide traction and counter traction. Dissection proceeds anterior to the Denonvillier’s fascia, posterior to the seminal vesicles in male patients and in the rectovaginal septum in females.
Division of the Rectum
After ensuring complete circumferential mobilization to the pelvic floor, the mesorectum is dissected to the rectal wall and the rectum is divided at least 2 cm below the lesion using endostaplers.
Mobilisation of the Left Colon, Splenic Flexure and Anastomosis
Proceeding in medio-lateral fashion the left and sigmoid mesocolon is dissected of the retroperitoneum and then the lateral peritoneal attachments of the colon along the white line of Toldt is released. Mobilization upto splenic flexure may be done if necessary to obtain an adequate length for anastomosing the proximal sigmoid to the distal rectum. Specimens are usually extracted through suprapubic incision and end to end colorectal anastomosis is performed using circular staplers (Fig. 16).
Advantages of Laparoscopic TME/CME
Laparoscopic resections for colorectal cancer offers the advantage of the improved visibility due to magnification and angled optics as also good illumination of the operation field and can aid in better delineation and preservation of the pelvic autonomic nerves.
Lateral Lymph Node Dissection
After completion of TME and rectal transection, the lateral pelvic nodes are addressed. They are grouped into three regions:
-
common iliac region: comprising of the common iliac & external iliac nodes,
-
hypogastric region: internal iliac nodes
-
obturator region: obturator nodes (Fig. 4).
The procedure begins by dissecting fibrofatty tissue around the aortic bifurcation at the origin of the common iliac vessels. The common iliac and external iliac nodes are dissected, thereafter, the hypogastric group is addressed by exposing the hypogastric nerve, external and internal iliac vessels, and ureter which are laid bare on the lateral pelvic wall up to the iliac bifurcation. The dissection proceeds to address the lymphatic tissues between the urinary bladder and the pelvic wall which are cleared. The lymphatic tissue along internal iliac vessels cleared upto the middle hemorrhoidal vessels. The obturator fossa is cleared of lymphoareolar tissue to lay bare the obturator nerve and vessels (Fig. 17).
Complications
Common concerns following complete mesocolic excision have been rates of
-
Bleeding or vascular injury,
-
Chyle leak,
-
Anastomotic leakage,
-
Duodenal or gastric perforations and
-
Clavien Dindo grade 3 & 4 postoperative complications.
Bleeding/Vascular Injury
A recent metanalysis reported an increased risk of vascular injury with CME as compared to conventional colonic resection [45]. A higher intraoperative blood loss has also been noted in CME group as compared to non-CME [46]. Other metanalysis did not observe a higher blood loss or vascular injury with CME [47, 48]. Contrarily laparoscopic CME has been attributed to have less blood loss than open CME [19, 49].
Anastomotic Leak
Anastomotic leak rates are not found to be different following CME CVL as compared to conventional hemicolectomy, though delayed gastric emptying has been noted [50, 51, 52].
Chyle Leak
One of the chief concerns in extended lymphadenectomy is the possibility of chyle leak. Chyle leak can lead to malnutrition, electrolyte imbalance and a theoretical risk of malignant recurrences. A recent systematic review on chyle leak/chylous ascites following colonic surgery for malignancies found it to be a rare complication (5.5%). Most chyle leaks are discovered during the index admission and can be managed conservatively (diet change, total parental nutrition, drainage, somatostatin analogues) and reoperation is rarely needed [53]. Tumour location in right colon, extended lymphadenectomy and number of lymph nodes retrieved are proposed as independent associates for chyle leak after colonic resections [53, 54].
Severe Complications and Risk Factors for Complications
Some studies have reported a higher postoperative complication rate following complete mesocolic excision [52]. The rates of Clavien-Dindo Grade 1 complications is reported to be to the tune of 40% whereas severe grade 4 complications reported is 2.7% after laparoscopic right CME. The cited independent risk factors in multivariate analysis being: age ≥ 65 years, body mass index (BMI) ≥ 28 kg/m(2) [55]. In another study on risk factors for severe complications after radical colonic surgery it was observed that anemia, elevated body mass index, and open surgery were important predictors in multinomial logistic regression [56].
Conclusion
Lymph node metastasis is an important prognostic factor in colorectal malignancies. The western concept of complete mesocolic excision with central vasculature ligation is similar to D3 lymphadenectomy practised in the east for colonic cancers. Total mesorectal excision is an established standard of care for operable rectal cancers. Laparoscopic mesocolic excision for colonic cancer and laparoscopic total mesorectal excision for rectal cancers can be performed safely with few postoperative complications and good oncological outcome. Lateral lymph node dissection is an important addition to TME for rectal cancers and has been shown to influence survival. Sentinel node biopsy with fluorescent imaging appears to be promising in early node negative colonic malignancies.
Key Clinical Points
-
1.
In colorectal cancer, lymph node metastasis is a key factor for deciding prognosis, management, and survival of the patients. Lymphadnectomy remains the mainstay of surgical management for colorectal cancers to improve the prognosis and outcomes.
-
2.
Laparoscopic CME with CVL is established western practice in management of colonic cancers.
-
3.
The aim of CME with CVL technique is to dissect the embryonic fusion planes and excise in totality the tumour along with its lymphovascular contents enclosed in the mesocolon as a single entity.
-
4.
D2 lymphadenectomy entails removal of the pericolic lymph nodes and intermediate lymph group of nodes, whereas D3 lymphadenectomy involve dissection of the main lymph nodes in addition to D2
-
5.
Western CME-CVL is comparable to Eastern D3 lymphadenectomy.
-
6.
Laparoscopic total mesorectal excision remains the standard of care for rectal cancers and allows better preservation of nerves and vessels ensuring complete removal of lymph nodes.
-
7.
The size of the normal mesorectum lymph nodes in about 80% of cases is <3 mm. Most mesorectum lymph nodes are located posteriorly, and 90% of the posterior lymph nodes lie within the upper half of the upper 2/3 of the rectum. Metastasis in mesorectal node is bidirectional i.e. both superiorly and inferiorly therefore necessitating complete mesorectal excision.
-
8.
Lateral lymph node resection is advised in mid and lower rectal cancer to improve the prognosis by reducing local recurrence but is still not the standard of care across all centres.
-
9.
Laparoscopic sentinel lymph node biopsy can be used to detect micrometastasis and improve the staging in T1/T2 disease of colon cancer. Standardized use of sentinel lymph node removal still remains controversial as expensive instrumentation is required.
-
10.
Flourescence imaging and molecular staging are the two new methods to enhance detection of tumor deposits in sentinel lymph nodes.
References
Fang SH, Efron JE, Berho ME, Wexner SD. Dilemma of stage II colon cancer and decision making for adjuvant chemotherapy. J Am Coll Surg. 2014;219:1056–69 [PMID: 25440029. https://doi.org/10.1016/j.Jamcollsurg.2014.09.010]
Dienstmann R, Salazar R, Tabernero J. Personalizing colon cancer adjuvant therapy: selecting optimal treatments for individual patients. J Clin Oncol. 2015;33:1787–96 [PMID: 25918287: https://doi.org/10.1200/JCO.2014.60.0213]
Nagtegaal ID, Quirke P. Colorectal tumour deposits in the mesorectum and pericolon; a critical review. Histopathology. 2007;51:141–9 [PMID: 17532768: https://doi.org/10.1111/j.1365-2559.2007.02720.x]
Quirke P, Williams GT, Ectors N, Ensari A, Piard F, Nagtegaal I. The future of the TNM staging system in colorectal cancer: time for a debate? Lancet Oncol. 2007;8:651–7 [PMID: 17613427: https://doi.org/10.1016/S1470-2045[07]70205-X]
Nagtegaal ID, Quirke P, Schmoll HJ. Has the new TNM classification for colorectal cancer improved care? Nat Rev Clin Oncol. 2012;9:119–23 [PMID: 22009076: https://doi.org/10.1038/nrclinonc.2011.157]
Esser S, Reilly WT, Riley LB, Eyvazzadeh C, Arcona S. The role of sentinel lymph node mapping in staging of colon and rectal cancer. Dis Colon Rectum. 2001;44(6):850–4.; discussion 854–856. https://doi.org/10.1007/BF02234707.
Smith J, Hwang H, Wiseman KW, Filipenko D, Phang PT. Ex vivo sentinel lymph node mapping in colon cancer: improving the accuracy of pathologic staging? Am J Surg. 2006;191(5):665–8. https://doi.org/10.1016/j.amjsurg.2006.01.045.
Bonjer HJ, Hop WC, Nelson H, Sargent DJ, Lacy AM, Castells A, Guillou PJ, Thorpe H, Brown J, Delgado S, Kuhrij E, Haglind E, Påhlman L; Transatlantic Laparoscopically Assisted vs Open Colectomy Trials Study Group. Laparoscopically assisted vs open colectomy for colon cancer: a meta-analysis. Arch Surg. 2007 Mar;142(3):298–303. https://doi.org/10.1001/archsurg.142.3.298. PMID: 17372057.
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM; MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005 May 14–20;365(9472):1718–26. https://doi.org/10.1016/S0140-6736(05)66545-2. PMID: 15894098.
Clinical Outcomes of Surgical Therapy Study Group, Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Ota D. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004 May 13;350(20):2050–59. https://doi.org/10.1056/NEJMoa032651. PMID: 15141043.
Bertelsen CA, Bols B, Ingeholm P, Jansen JE, Neuenschwander AU, Vilandt J. Can the quality of colonic surgery be improved by standardization of surgical technique with complete mesocolic excision? Colorectal Dis. 2011 Oct;13(10):1123–29. https://doi.org/10.1111/j.1463-1318.2010.02474.x. PMID: 20969719.
Ma Y, Yang Z, Qin H, Wang Y. A meta-analysis of laparoscopy compared with open colorectal resection for colorectal cancer. Med Oncol. 2011 Dec;28(4):925–33. https://doi.org/10.1007/s12032-010-9549-5. Epub 2010 May 11. PMID: 20458560.
Colon Cancer Laparoscopic or Open Resection Study Group, Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009 Jan;10(1):44–52. https://doi.org/10.1016/S1470-2045(08)70310-3. Epub 2008 Dec 13. PMID: 19071061.
Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Nelson H; Clinical Outcomes of Surgical Therapy Study Group. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007 Oct;246(4):655–662.; discussion 662–4. https://doi.org/10.1097/SLA.0b013e318155a762. PMID: 17893502.
Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010 Nov;97(11):1638–45. https://doi.org/10.1002/bjs.7160. PMID: 20629110.
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009 May;11(4):354–64; discussion 364–5. https://doi.org/10.1111/j.1463-1318.2008.01735.x. Epub 2009 Nov 5. PMID: 19016817.
Bae SU, Saklani AP, Lim DR, Kim DW, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol. 2014 Jul;21(7):2288–94. https://doi.org/10.1245/s10434-014-3614-9. Epub 2014 Mar 7. PMID: 24604585.
Gouvas N, Pechlivanides G, Zervakis N, Kafousi M, Xynos E. Complete mesocolic excision in colon cancer surgery: a comparison between open and laparoscopic approach. Colorectal Dis. 2012 Nov;14(11):1357–64. https://doi.org/10.1111/j.1463-1318.2012.03019.x. PMID: 22390358.
Negoi I, Hostiuc S, Negoi RI, Beuran M. Laparoscopic vs open complete mesocolic excision with central vascular ligation for colon cancer: A systematic review and meta-analysis. World J Gastrointest Oncol. 2017 Dec 15;9(12):475–91. https://doi.org/10.4251/wjgo.v9.i12.475. PMID: 29290918; PMCID: PMC5740088.
Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986 Jun 28;1(8496):1479–82. https://doi.org/10.1016/s0140-6736(86)91510-2. PMID: 2425199.
Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018 Feb;23(1):1–34. https://doi.org/10.1007/s10147-017-1101-6. Epub 2017 Mar 27. PMID: 28349281; PMCID: PMC5809573.
Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Takiuchi H, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012 Feb;17(1):1–29. https://doi.org/10.1007/s10147-011-0315-2. Epub 2011 Oct 15. PMID: 22002491.
Hill GL, Rafique M. Extrafascial excision of the rectum for rectal cancer. Br J Surg. 1998 Jun;85(6):809–12. https://doi.org/10.1046/j.1365-2168.1998.00735.x. PMID: 9667714.
Ross A, Rusnak C, Weinerman B, Kuechler P, Hayashi A, MacLachlan G, Frew E, Dunlop W. Recurrence and survival after surgical management of rectal cancer. Am J Surg. 1999 May;177(5):392–5. https://doi.org/10.1016/s0002-9610(99)00080-x. PMID: 10365877.
Bjerkeset T, Edna TH. Rectal cancer: the influence of type of operation on local recurrence and survival. Eur J Surg. 1996 Aug;162(8):643–8. PMID: 8891623.
Heald RJ. The ‘Holy Plane’ of rectal surgery. J R Soc Med. 1988 Sept;81(9):503–8. PMID: 3184105; PMCID: PMC1291757.
Topor B, Acland R, Kolodko V, Galandiuk S. Mesorectal lymph nodes: their location and distribution within the mesorectum. Dis Colon Rectum. 2003 Jun;46(6):779–85. https://doi.org/10.1007/s10350-004-6656-4. PMID: 12794580.
Bell S, Sasaki J, Sinclair G, Chapuis PH, Bokey EL. Understanding the anatomy of lymphatic drainage and the use of blue-dye mapping to determine the extent of lymphadenectomy in rectal cancer surgery: unresolved issues. Colorectal Dis. 2009 Jun;11(5):443–9. https://doi.org/10.1111/j.1463-1318.2009.01769.x. Epub 2009 Jan 17. PMID: 19207711.
Kundagulwar GK, Pai VD, Saklani AP. Is there a role of lateral pelvic lymph node dissection in the current era of neoadjuvant chemoradiotherapy for rectal cancer? J Gastrointest Dig Syst. 2016;6:473. https://doi.org/10.4172/2161-069X.1000473.
Yahya JB, Herzig DO, Farrell MJ, Degnin CR, Chen Y, Holland J, Brown S, Jaboin J, Tsikitis VL, Lu K, Thomas CR Jr, Mitin T. Does a fine line exist between regional and metastatic pelvic lymph nodes in rectal cancer-striking discordance between national guidelines and treatment recommendations by US radiation oncologists. J Gastrointest Oncol. 2018 June;9(3):441–7. https://doi.org/10.21037/jgo.2018.02.05. PMID: 29998009; PMCID: PMC6006036.
Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, et al. Japanese Society for Cancer of the Colon and Rectum [JSCCR] guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1–34.
Ito M, Kobayashi A, Fujita S, Mizusawa J, Kanemitsu Y, Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, Yamaguchi T, Akasu T, Moriya Y; Colorectal Cancer Study Group of Japan Clinical Oncology Group. Urinary dysfunction after rectal cancer surgery: Results from a randomized trial comparing mesorectal excision with and without lateral lymph node dissection for clinical stage II or III lower rectal cancer (Japan Clinical Oncology Group Study, JCOG0212). Eur J Surg Oncol. 2018 Apr;44(4):463–8. https://doi.org/10.1016/j.ejso.2018.01.015. Epub 2018 Jan 17. PMID: 29428473.
Fujita S, Akasu T, Mizusawa J, Saito N, Kinugasa Y, Kanemitsu Y, Ohue M, Fujii S, Shiozawa M, Yamaguchi T, Moriya Y; Colorectal Cancer Study Group of Japan Clinical Oncology Group. Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol. 2012 Jun;13(6):616–21. https://doi.org/10.1016/S1470-2045(12)70158-4. Epub 2012 May 15. PMID: 22591948.
Saito S, Fujita S, Mizusawa J, Kanemitsu Y, Saito N, Kinugasa Y, Akazai Y, Ota M, Ohue M, Komori K, Shiozawa M, Yamaguchi T, Akasu T, Moriya Y; Colorectal Cancer Study Group of Japan Clinical Oncology Group. Male sexual dysfunction after rectal cancer surgery: Results of a randomized trial comparing mesorectal excision with and without lateral lymph node dissection for patients with lower rectal cancer: Japan Clinical Oncology Group Study JCOG0212. Eur J Surg Oncol. 2016 Dec;42(12):1851–58. https://doi.org/10.1016/j.ejso.2016.07.010. Epub 2016 Jul 30. PMID: 27519616.
Nagayoshi K, Ueki T, Manabe T, Moriyama T, Yanai K, Oda Y, Tanaka M. Laparoscopic lateral pelvic lymph node dissection is achievable and offers advantages as a minimally invasive surgery over the open approach. Surg Endosc. 2016 May;30(5):1938–47. https://doi.org/10.1007/s00464-015-4418-0. Epub 2015 Aug 15. PMID: 26275538.
Matsumoto A, Arita K. A technique of laparoscopic lateral pelvic lymph node dissection based on vesicohypogastric fascia and ureterohypogastric nerve fascia for advanced low rectal cancer. Surg Endosc. 2017 Feb;31(2):945–8. https://doi.org/10.1007/s00464-016-5014-7. Epub 2016 Jun 20. PMID: 27324330.
Kagawa H, Kinugasa Y, Shiomi A, Yamaguchi T, Tsukamoto S, Tomioka H, Yamakawa Y, Sato S. Robotic-assisted lateral lymph node dissection for lower rectal cancer: short-term outcomes in 50 consecutive patients. Surg Endosc. 2015 Apr;29(4):995–1000. https://doi.org/10.1007/s00464-014-3760-y. Epub 2014 Aug 19. PMID: 25135444.
Kim HJ, Choi GS, Park JS, Park SY, Lee HJ, Woo IT, Park IK. Selective lateral pelvic lymph node dissection: a comparative study of the robotic versus laparoscopic approach. Surg Endosc. 2018 May;32(5):2466–73. https://doi.org/10.1007/s00464-017-5948-4. Epub 2017 Nov 9. PMID: 29124406.
Lips DJ, Koebrugge B, Liefers GJ, van de Linden JC, Smit VT, Pruijt HF, Putter H, van de Velde CJ, Bosscha K. The influence of micrometastases on prognosis and survival in stage I-II colon cancer patients: the Enroute⊕ Study. BMC Surg. 2011 May 11;11:11. https://doi.org/10.1186/1471-2482-11-11. PMID: 21569373; PMCID: PMC3123166
Rodriguez-Bigas MA, Maamoun S, Weber TK, Penetrante RB, Blumenson LE, Petrelli NJ. Clinical significance of colorectal cancer: metastases in lymph nodes < 5 mm in size. Ann Surg Oncol. 1996 Mar;3(2):124–30. https://doi.org/10.1007/BF02305790. PMID: 8646511.
Tuech JJ, Pessaux P, Di Fiore F, Nitu V, Lefebure B, Colson A, Michot F. Sentinel node mapping in colon carcinoma: in-vivo versus ex-vivo approach. Eur J Surg Oncol. 2006 Mar;32(2):158–61. https://doi.org/10.1016/j.ejso.2005.11.004. Epub 2006 Jan 11. PMID: 16376515.
Funahashi K. Current status of sentinel lymph node-based nodal ultrastaging in colorectal cancer. Nihon Geka Gakkai Zasshi. 2009 Mar;110(2):73–7. Japanese. PMID: 19348197.
Ankersmit M, Bonjer HJ, Hannink G, Schoonmade LJ, van der Pas MHGM, Meijerink WJHJ. Near-infrared fluorescence imaging for sentinel lymph node identification in colon cancer: a prospective single-center study and systematic review with meta-analysis. Tech Coloproctol. 2019 Dec;23(12):1113–26. https://doi.org/10.1007/s10151-019-02107-6. Epub 2019 Nov 18. PMID: 31741099; PMCID: PMC6890578.
Bilchik AJ, Saha S, Wiese D, Stonecypher JA, Wood TF, Sostrin S, Turner RR, Wang HJ, Morton DL, Hoon DS. Molecular staging of early colon cancer on the basis of sentinel node analysis: a multicenter phase II trial. J Clin Oncol. 2001 Feb 15;19(4):1128–36. https://doi.org/10.1200/JCO.2001.19.4.1128. Erratum in: J Clin Oncol 2001 May 1;19(9):2583. PMID: 11181678
Kong JC, Prabhakaran S, Choy KT, Larach JT, Heriot A, Warrier SK. Oncological reasons for performing a complete mesocolic excision: a systematic review and meta-analysis. ANZ J Surg. 2021 Jan;91(1–2):124–31. https://doi.org/10.1111/ans.16518. Epub 2021 Jan 5. PMID: 33400369.
Wang C, Gao Z, Shen K, Shen Z, Jiang K, Liang B, Yin M, Yang X, Wang S, Ye Y. Safety, quality and effect of complete mesocolic excision vs non-complete mesocolic excision in patients with colon cancer: a systemic review and meta-analysis. Colorectal Dis. 2017 Nov;19(11):962–72. https://doi.org/10.1111/codi.13900. PMID: 28949060.
Anania G, Davies RJ, Bagolini F, Vettoretto N, Randolph J, Cirocchi R, Donini A. Right hemicolectomy with complete mesocolic excision is safe, leads to an increased lymph node yield and to increased survival: results of a systematic review and meta-analysis. Tech Coloproctol. 2021 Jun 12. https://doi.org/10.1007/s10151-021-02471-2. Epub ahead of print. PMID: 34120270.
Ferri V, Vicente E, Quijano Y, Duran H, Diaz E, Fabra I, Malave L, Agresott R, Isernia R, Cardinal-Fernandez P, Ruiz P, Nola V, de Nobili G, Ielpo B, Caruso R. Right-side colectomy with complete mesocolic excision vs conventional right-side colectomy in the treatment of colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021 May 13. https://doi.org/10.1007/s00384-021-03951-5. Epub ahead of print. PMID: 33983451.
Gavriilidis P, Davies RJ, Biondi A, Wheeler J, Testini M, Carcano G, Di Saverio S. Laparoscopic versus open complete mesocolic excision: a systematic review by updated meta-analysis. Updates Surg. 2020 Sep;72(3):639–48. https://doi.org/10.1007/s13304-020-00819-1. Epub 2020 May 29. PMID: 32472404.
Prevost GA, Odermatt M, Furrer M, Villiger P. Postoperative morbidity of complete mesocolic excision and central vascular ligation in right colectomy: a retrospective comparative cohort study. World J Surg Oncol. 2018 Oct 30;16(1):214. https://doi.org/10.1186/s12957-018-1514-3. PMID: 30376849; PMCID: PMC6208021.
Crane J, Hamed M, Borucki JP, El-Hadi A, Shaikh I, Stearns AT. Complete mesocolic excision versus conventional surgery for colon cancer: A systematic review and meta-analysis. Colorectal Dis. 2021 May 2. https://doi.org/10.1111/codi.15644. Epub ahead of print. PMID: 33934455.
Díaz-Vico T, Fernández-Hevia M, Suárez-Sánchez A, García-Gutiérrez C, Mihic-Góngora L, Fernández-Martínez D, Álvarez-Pérez JA, Otero-Díez JL, Granero-Trancón JE, García-Flórez LJ. Complete mesocolic excision and D3 lymphadenectomy versus conventional colectomy for colon cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2021 Jun 4. https://doi.org/10.1245/s10434-021-10186-9. Epub ahead of print. PMID: 34089109.
Ng ZQ, Han M, Beh HN, Keelan S. Chylous ascites in colorectal surgery: A systematic review. World J Gastrointest Surg. 2021 June 27;13(6):585–96. https://doi.org/10.4240/wjgs.v13.i6.585.
Sun YW, Chi P, Lin HM, Lu XR, Huang Y, Xu ZB, Huang SH. [Risk factors of postoperative chyle leak following complete mesocolic excision for colon cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2012 Apr;15(4):328–31. Chinese. PMID: 22539374.
Li MZ, Li KY, Shen J, Xie DH. Clavien-Dindo classification of complications after complete mesocolic excision in laparoscopic radical resection of right hemicolon cancer and analysis on its influencing factors. Zhonghua Wei Chang Wai Ke Za Zhi. 2020 Jan 25;23(1):51–5. Chinese. https://doi.org/10.3760/cma.j.issn.1671-0274.2020.01.009. PMID: 31958931.
Furnes B, Storli KE, Forsmo HM, Karliczek A, Eide GE, Pfeffer F. Risk factors for complications following introduction of radical surgery for colon cancer: a consecutive patient series. Scand J Surg. 2019 June;108(2):144–151. https://doi.org/10.1177/1457496918798208. Epub 2018 Sep 6. PMID: 30187819
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Editor’s Note
References: Main chapter references are included after the “References Editor’s Note” section.
Editor’s Note
Anatomy
One of the crucial steps in laparoscopic total mesocolic excision is an understanding of the embryological fusion planes and vascular anatomy of the mesocolon. The vascular anatomy is particularly pertinent for right colectomy as several variations exist.
Variations in Blood Supply and Venous Drainage of Right Colon as Pertinent to Laparoscopic Right Hemicolectomy
In a study evaluating variations in colonic blood supply from superior mesenteric artery it was noted that the middle colic artery and ileocolic artery were consistently present in most patients. Whereas the right colic artery was present in 12.2% to 55.0% cases only. The right colic artery has been noted to variably originate from superior mesenteric, ileocolic, middle colic and right branch of middle colic in various studies. It is important to note that the ileocolic artery can cross the superior mesenteric vein anteriorly or posteriorly. On the other hand, the right colic artery usually crosses the superior mesenteric vein anteriorly. Similarly, variations have been noted in the venous system. The ileocolic vein consistently drains into the superior mesenteric vein and is thus considered an important anatomical marker in laparoscopic right hemicolectomy. Of particular note is the “Trunk of Henle” which can present as a GTH (gastrocolic Trunk of Henle). GPCT (Gastropancreaticocolic trunk) or GPT (Gastro pancreatic Trunk), the latter being rare. Right colic veins rarely drain into superior mesenteric vein in only 19% whereas the middle colic veins drain into the superior mesenteric vein in 84% cases in the rest of the cases these veins drain into the trunk of Henle. The superior right colic vein is an inconsistent vein formed from tributaries of hepatic flexure and is also known as accessory right colic vein considered to be an important source of bleeding due to avulsion [1].
Emryological Fusion Planes Encountered in Laparoscopic Right Hemicolectomy
Four critical view planes have been proposed in the open book model for standardization of CME in right hemicolectomy. They are essentially derived from the embryological fusion planes of colon and mesocolon and are: (a) retroperitoneal plane, (b) ileocolic plane (c) transverse mesocolic plane and (d) mesogastric plane [2].
Metaanalyses on Mesocolic Excision Versus Non Mesocolic Excision
Table EN1 tabulates the crux of the results of various meta-analysis comparing mesocolic excision versus non mesocolic excision. An advantage regarding oncological outcome parameters viz: recurrences, diseases free and overall survival has been consistently reported in latest studies. Surrogate pathological parameters of a better oncological resection such as number of lymphnodes retrieved, length of bowel excised, area of the mesocolon in specimen, distance to high tie have all been reported to be higher in the CME group [3–12].
Meta Analyses on Laparoscopic and Open Mesocolic Excision
Table EN2 enlists the results of metanalysis comparing laparoscopic and open mesocolic excision. A better postoperative recovery, lower blood loss, less requirement for blood transfusion, lower overall postoperative complications, less wound infections, early recovery of gastrointestinal function and shorter hospital stay are some of the reported benefits of laparoscopic over open CME for colonic cancers [13–16].
Meta Analyses on Lateral Lymph Node Dissection in Rectal Cancers
The results of metanalyses pertaining to lateral lymph node dissection is shown in Table EN3. Most metaanalyses project a higher incidence of urinary dysfunction and male sexual dysfunction associated with lateral lymphnode dissection. Though there is no major survival benefit overall it may be helpful in patients with clinically positive lateral lymph node that persist after preoperative chemoradiotherapy or those who do not receive neoadjuvant chemoradiotherapy [17–21].
Metaanalyses on Sentinel Lymph Node Biopsy in Colorectal Cancers
Table EN4 depicts the results of recent metaanalyses on sentinel node biopsy in colorectal cancers. A high identification rate sensitivity and diagnostic accuracy has been observed especially for early stage lesions. Colonic cancers, use of laparoscopic procedures and indocyanine green for performance of sentinel node biopsy has been noted to have a better yield [22–24].
References for Editor’s notes
-
1.
Sun KK, Zhao H. Vascular anatomical variation in laparoscopic right hemicolectomy. Asian J Surg. 2020 Jan;43(1):9–12. https://doi.org/10.1016/j.asjsur.2019.03.013. Epub 2019 Apr 10. PMID: 30979567.
-
2.
Strey CW, Wullstein C, Adamina M, Agha A, Aselmann H, Becker T, Grützmann R, Kneist W, Maak M, Mann B, Moesta KT, Runkel N, Schafmayer C, Türler A, Wedel T, Benz S. Laparoscopic right hemicolectomy with CME: standardization using the “critical view” concept. Surg Endosc. 2018 Dec;32(12):5021–30. https://doi.org/10.1007/s00464-018-6267-0. Epub 2018 Oct 15. PMID: 30324463; PMCID: PMC6208708.
-
3.
Ow ZGW, Sim W, Nistala KRY, Ng CH, Koh FH, Wong NW, Foo FJ, Tan KK, Chong CS. Comparing complete mesocolic excision versus conventional colectomy for colon cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2021 Apr;47(4):732–7. https://doi.org/10.1016/j.ejso.2020.09.007. Epub 2020 Sep 12. PMID: 32951936.
-
4.
De Simoni O, Barina A, Sommariva A, Tonello M, Gruppo M, Mattara G, Toniato A, Pilati P, Franzato B. Complete mesocolic excision versus conventional hemicolectomy in patients with right colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021 May;36(5):881–92. https://doi.org/10.1007/s00384-020-03797-3. Epub 2020 Nov 10. PMID: 33170319.
-
5.
Kong JC, Prabhakaran S, Choy KT, Larach JT, Heriot A, Warrier SK. Oncological reasons for performing a complete mesocolic excision: a systematic review and meta-analysis. ANZ J Surg. 2021 Jan;91(1–2):124–31. https://doi.org/10.1111/ans.16518. Epub 2021 Jan 5. PMID: 33400369.
-
6.
Crane J, Hamed M, Borucki JP, El-Hadi A, Shaikh I, Stearns AT. Complete mesocolic excision versus conventional surgery for colon cancer: A systematic review and meta-analysis. Colorectal Dis. 2021 May 2. https://doi.org/10.1111/codi.15644. Epub ahead of print. PMID: 33934455.
-
7.
Díaz-Vico T, Fernández-Hevia M, Suárez-Sánchez A, García-Gutiérrez C, Mihic-Góngora L, Fernández-Martínez D, Álvarez-Pérez JA, Otero-Díez JL, Granero-Trancón JE, García-Flórez LJ. Complete Mesocolic Excision and D3 Lymphadenectomy versus Conventional Colectomy for Colon Cancer: A Systematic Review and Meta-Analysis. Ann Surg Oncol. 2021 Jun 4. https://doi.org/10.1245/s10434-021-10186-9. Epub ahead of print. PMID: 34089109.
-
8.
Anania G, Davies RJ, Bagolini F, Vettoretto N, Randolph J, Cirocchi R, Donini A. Right hemicolectomy with complete mesocolic excision is safe, leads to an increased lymph node yield and to increased survival: results of a systematic review and meta-analysis. Tech Coloproctol. 2021 Jun 12. https://doi.org/10.1007/s10151-021-02471-2. Epub ahead of print. PMID: 34120270.
-
9.
Ferri V, Vicente E, Quijano Y, Duran H, Diaz E, Fabra I, Malave L, Agresott R, Isernia R, Cardinal-Fernandez P, Ruiz P, Nola V, de Nobili G, Ielpo B, Caruso R. Right-side colectomy with complete mesocolic excision vs conventional right-side colectomy in the treatment of colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021 May 13. https://doi.org/10.1007/s00384-021-03951-5. Epub ahead of print. PMID: 33983451.
-
10.
Balciscueta Z, Balciscueta I, Uribe N, Pellino G, Frasson M, García-Granero E, García-Granero Á. D3-lymphadenectomy enhances oncological clearance in patients with right colon cancer. Results of a meta-analysis. Eur J Surg Oncol. 2021 Jul;47(7):1541–51. https://doi.org/10.1016/j.ejso.2021.02.020. Epub 2021 Feb 26. PMID: 33676793.
-
11.
Dai Q, Tu S, Dong Q, Chen B. Laparoscopic Complete Mesocolic Excision Versus Noncomplete Mesocolic Excision: A Systematic Review and Meta-analysis. Surg Laparosc Endosc Percutan Tech. 2020 Aug 5;31(1):96–103. https://doi.org/10.1097/SLE.0000000000000845. PMID: 32769740.
-
12.
Wang C, Gao Z, Shen K, Shen Z, Jiang K, Liang B, Yin M, Yang X, Wang S, Ye Y. Safety, quality and effect of complete mesocolic excision vs non-complete mesocolic excision in patients with colon cancer: a systemic review and meta-analysis. Colorectal Dis. 2017 Nov;19(11):962–72. https://doi.org/10.1111/codi.13900. PMID: 28949060.
-
13.
Gavriilidis P, Davies RJ, Biondi A, Wheeler J, Testini M, Carcano G, Di Saverio S. Laparoscopic versus open complete mesocolic excision: a systematic review by updated meta-analysis. Updates Surg. 2020 Sep;72(3):639–48. https://doi.org/10.1007/s13304-020-00819-1. Epub 2020 May 29. PMID: 32472404.
-
14.
Negoi I, Hostiuc S, Negoi RI, Beuran M. Laparoscopic vs open complete mesocolic excision with central vascular ligation for colon cancer: A systematic review and meta-analysis. World J Gastrointest Oncol. 2017 Dec 15;9(12):475–91. https://doi.org/10.4251/wjgo.v9.i12.475. PMID: 29290918; PMCID: PMC5740088.
-
15.
Athanasiou CD, Markides GA, Kotb A, Jia X, Gonsalves S, Miskovic D. Open compared with laparoscopic complete mesocolic excision with central lymphadenectomy for colon cancer: a systematic review and meta-analysis. Colorectal Dis. 2016 Jul;18(7):O224–35. https://doi.org/10.1111/codi.13385. PMID: 27187520.
-
16.
Baloyiannis I, Perivoliotis K, Ntellas P, Dadouli K, Tzovaras G. Comparing the safety, efficacy, and oncological outcomes of laparoscopic and open colectomy in transverse colon cancer: a meta-analysis. Int J Colorectal Dis. 2020 Mar;35(3):373–86. https://doi.org/10.1007/s00384-020-03516-y. Epub 2020 Jan 24. PMID: 31980872.
-
17.
Gao X, Wang C, Yu Y, Singh D, Yang L, Zhou Z. Lateral lymph node dissection reduces local recurrence of locally advanced lower rectal cancer in the absence of preoperative neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. World J Surg Oncol. 2020 Nov 23;18(1):304. https://doi.org/10.1186/s12957-020-02078-1. PMID: 33228677; PMCID: PMC7685653.
-
18.
Hajibandeh S, Hajibandeh S, Matthews J, Palmer L, Maw A. Meta-analysis of survival and functional outcomes after total mesorectal excision with or without lateral pelvic lymph node dissection in rectal cancer surgery. Surgery. 2020 Sep;168(3):486–96. https://doi.org/10.1016/j.surg.2020.04.063. Epub 2020 Jun 30. PMID: 32620303.
-
19.
Wang X, Qiu A, Liu X, Shi Y. Total mesorectal excision plus lateral lymph node dissection vs TME on rectal cancer patients: a meta-analysis. Int J Colorectal Dis. 2020 Jun;35(6):997–1006. https://doi.org/10.1007/s00384-020-03610-1. Epub 2020 May 1. PMID: 32356120.
-
20.
Yang X, Yang S, Hu T, Gu C, Wei M, Deng X, Wang Z, Zhou Z. What is the role of lateral lymph node dissection in rectal cancer patients with clinically suspected lateral lymph node metastasis after preoperative chemoradiotherapy? A meta-analysis and systematic review. Cancer Med. 2020 Jul;9(13):4477–89. https://doi.org/10.1002/cam4.2643. Epub 2020 Apr 30. PMID: 32352659; PMCID: PMC7333827.
-
21.
Ma P, Yuan Y, Yan P, Chen G, Ma S, Niu X, Xu M, Yang K, Cai H. The efficacy and safety of lateral lymph node dissection for patients with rectal cancer: A systematic review and meta-analysis. Asian J Surg. 2020 Sep;43(9):891–901. https://doi.org/10.1016/j.asjsur.2019.11.006. Epub 2020 Jan 9. PMID: 31926817.
-
22.
Burghgraef TA, Zweep AL, Sikkenk DJ, van der Pas MHGM, Verheijen PM, Consten ECJ. In vivo sentinel lymph node identification using fluorescent tracer imaging in colon cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021 Feb;158:103149. https://doi.org/10.1016/j.critrevonc.2020.103149. Epub 2020 Nov 11. PMID: 33450679.
-
23.
Qiao L. Sentinel lymph node mapping for metastasis detection in colorectal cancer: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2020 Sep;112(9):722–30. https://doi.org/10.17235/reed.2020.6767/2019. PMID: 32894022.
-
24.
Villegas-Tovar E, Jimenez-Lillo J, Jimenez-Valerio V, Diaz-Giron-Gidi A, Faes-Petersen R, Otero-Piñeiro A, De Lacy FB, Martinez-Portilla RJ, Lacy AM. Performance of Indocyanine green for sentinel lymph node mapping and lymph node metastasis in colorectal cancer: a diagnostic test accuracy meta-analysis. Surg Endosc. 2020 Mar;34(3):1035–47. https://doi.org/10.1007/s00464-019-07274-z. Epub 2019 Nov 21. PMID: 31754853.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rawat, S., Selvasekar, C., Bansal, S. (2022). Laparoscopic Lymphadenectomy for Colorectal Cancers: Concepts and Current Results. In: Sharma, D., Hazrah, P. (eds) Recent Concepts in Minimal Access Surgery. Springer, Singapore. https://doi.org/10.1007/978-981-16-5473-2_7
Download citation
DOI: https://doi.org/10.1007/978-981-16-5473-2_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5472-5
Online ISBN: 978-981-16-5473-2
eBook Packages: MedicineMedicine (R0)