Abstract
Purpose
The objective of the current study was to assess the therapeutic benefit of lymphadenectomy according to the extent of lymphadenectomy.
Methods
Patients undergoing colectomy for right‐sided colon cancer were identified. Distribution of lymph node metastases (DLNM) of 1, 2 and 3 were defined as lymph node metastasis (LNM) in the pericolic nodes, the intermediate nodes and the front of the SMV near the origin of the major artery, respectively. The therapeutic index (TI) was calculated based on the frequency of LNM and the 5 year overall survival (OS) rate of patients with LNM.
Results
Among 344 patients who met the inclusion criteria, roughly half had LNM (n = 150, 43.7%). While 107 (31.1%) and 30 (8.7%) patients had DLNM1 and DLNM2, respectively, only 13 patients (3.8%) were defined as DLNM3. However, there was no significant difference in 5 year OS by DLNM (DLNM1 71.1%, DLNM2 78.7%, DLNM3 50.4%, p = 0.61). Overall, the TI of lymphadenectomy for D3 area was approximately 1/10 of the TI for D1 (1.9 vs.22.1), given the low frequency of LNM (3.8%) and poor 5 year OS of patients with LNM (50.4%). This trend was consistent irrespective of primary tumor locations.
Conclusion
The survival benefit from central lymphadenectomy namely D3 was low among patients with right‐sided colon cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since total mesorectal excision (TME) in the surgical treatment of rectal cancer has improved oncological outcomes [1,2,3], several studies have reported complete mesocolic excision (CME) with central vascular ligation (CVL) following the same principles of TME surgery [4,5]. The principles of CME are the removal of all lymphatic, vascular and neural tissue in the drainage area of the tumor in a complete mesocolic envelope with intact mesentery, peritoneum and encasing fascia [6]. As with TME, the successful outcomes have been reported after CME with CVL for right‐sided colon cancers [7]. In particular, West et al. reported that CME was associated with more mesocolic plane resection and higher number of lymph nodes (LNs) examined, which might have contributed to a better 5 year survival than with standard excisions [4].
However, the optimal extent of CVL still remains controversial. Specifically, Kanemitsu et al. suggested D3 lymphadenectomy extended to the left edge of the superior mesenteric artery (SMA) for right‐sided colon cancers using the no‐touch isolation technique [8]. A separate study using the largest Japanese cohort noted that D3 lymphadenectomy was associated with a better overall survival (OS) than D2 lymphadenectomy, even after adjusting for confounders by the propensity score matching method [9]. Bertelsen et al., conversely, concluded that there was no theoretical explanation supporting a better oncological outcome after extended lymph node dissection for colon cancers in a systematic review [10]. To this end, there has been increasing interest in the optimal extent of central lymphadenectomy and its therapeutic value for right‐sided colon cancers.
The therapeutic index is a metric for evaluating the survival benefit of lymphadenectomy for patients undergoing surgery for gastroenterological cancers. This value is based on the rationale that lymphadenectomy may be most effective among patients with a high estimated frequency of LNM, as well as individuals who are most likely to gain a survival benefit from the ascertainment of nodal status information. This concept has recently been applied to patients with gastric [11,12] and colorectal cancers [13] as well as hepatopancreatobiliary malignancies [14,15,16]. Tokunaga et al. evaluated the therapeutic index for each LN station to determine whether or not to retrieve posterior pancreatic head LNs for advanced gastric cancer, with the results incorporated into the Japanese Classification of Gastric Carcinoma guidelines [17,18].
Given the above, the present study assessed the therapeutic benefit of lymphadenectomy by LN area (i.e. D1, D2 and D3) among patients with right‐sided colon cancers.
Methods
Study population
Patients undergoing colectomy and extended (D3) lymphadenectomy for Stage 1–3 right‐sided colon cancer from 1992 to 2012 were identified at two tertiary institutions in Japan: Yokohama City University Graduate School of Medicine and Yokohama City University Medical Center. All patients included in the study were histologically diagnosed with colon cancer and underwent ileocecal resection (ICR) or right hemi‐colectomy (RHC). Patients with multiple primary tumors, as well as individuals with T1 tumor, distant metastatic disease and microscopically positive surgical margins (i.e. R1 resection), were excluded from the analysis. Transvers colon cancers were only included those at the hepatic flexure. The study was approved by the Institutional Review Board of both participating institutions before the study was initiated. Due to the retrospective nature of the study, written informed consent was not obtained. We used an opt‐out approach to disclose the study information.
Patient demographic and clinicopathological data included the age, sex, body mass index (BMI), tumor location and size, surgical procedure and approach, T/N Stage, number of LN evaluated and metastasis, distribution of LN metastasis (LNM), tumor grade, lymph‐vascular and perineural invasion as well as adjuvant chemotherapy.
Surgical procedure for right‐sided colon cancers
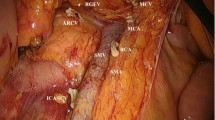
Laparoscopic colectomy was introduced in September 2000 at our institutions. Although laparoscopic right‐sided colectomy differs from the open approach in terms of mobilization (e.g. lateral or medial approach), the concepts are the same in the performance of dissection between the mesenteric plane and parietal fascia and the removal of the mesentery within a complete envelope of mesenteric fascia and visceral peritoneum that contains all LNs draining the tumor. As Fig. 1 shows, our central lymphadenectomy extends to the left edge of the superior mesenteric vein (SMV) and not to the SMA. The major veins, including the ileocolic, right colic and middle colic, are ligated and cut at the level of their root, only if those exist in the mesentery within 10 cm from the tumor. In the same manner, major arteries, such as the ileocolic, right colic and middle colic arteries, are ligated and cut at the level of the left edge of the SMV. Lymph nodes at the front of SMV were recognized by a titanium clip that ligated the roots of major veins (i.e. ICV, RCV), when lymph nodes were dissected individually from the adipose connective tissue of the specimen after resection.
A surgical schematic drawing displaying the extent of central lymphadenectomy for right-sided colon cancers in this study. RGEV: right gastricoepiploic vein; MCA, MCV: middle colic artery, vein; accRCV: accessary right colic vein; GCT: gastrocolic trunk; RCA, RCV: right colic artery, vein; SMA, SMV: superior mesenteric artery, vein; ICA, ICV: ileocolic artery, vein; ICV: ileocolic vein
Distribution of Lymph node metastases
Distribution of LNM 1, 2 and 3 were defined as LNM in the pericolic nodes, the intermediate nodes and the main nodes, respectively [19]. DLNM3 includes metastatic lymph nodes at the root of the ICV or RCV near the ligation clip.
Therapeutic index
The therapeutic index was first proposed by Sasako et al. to assess the survival benefit of lymphadenectomy for each lymph nodal basin and factors [20]. The therapeutic index of lymphadenectomy was calculated by multiplying the incidence of LNM by the 5 year OS rate of individuals with LNM among different patient cohorts, as previously reported. For example, if the frequency of LNM was 30.0% and the 5 year OS rate of patients with LNM was 80.0% in a particular patient group, the therapeutic index was 24.0 (0.300 × 80.0). In addition, the therapeutic index was calculated among different patient groups relative to the DLNM, as well as other clinicopathological factors. According to previous studies, lymphadenectomy was considered to be meaningful when the difference in the therapeutic index was ≥ 10 between groups [11,21].
Statistical analyses
Descriptive statistics were presented as the median [interquartile range (IQR)] and frequency (%) for continuous and categorical variables, respectively. Continuous variables were compared with the Mann–Whitney U or Kruskal–Wallis tests, as appropriate. Categorical variables were compared with the χ2 test or Fisher’s exact test, as appropriate. Statistical significance was assessed at α = 0.05 (two‐tailed). The OS calculated by creating Kaplan–Meier curves and differences was evaluated using the log‐rank test. The Cox regression analysis was performed to determine if DLNM3 is an independent prognostic factor of decreased OS adjusting covariates such as age, sex, and T stage. All statistical analyses were performed using the SPSS software program, version 25 (IBM Corp. Armonk, NY, USA) along with the JMP statistical software package, version 15 (SAS Institute Inc., Cary, NC, USA).
Results
Patient’s characteristics
A total of 344 patients met the inclusion criteria and were included in the final analytic cohort. The median age was 71 (IQR 62–78) years old, and most patients were female (n = 176, 51.1%). The median BMI and CEA were 21.7 (IQR 19.4–24.0) and 3.1 (IQR 1.9–7.6), respectively; the most common primary site was the ascending colon (n = 221, 64.2%). A majority of patients underwent RHC (n = 292, 84.9%), open colectomy (n = 222, 64.5%), had T3 tumor (n = 230, 66.9%) and had no LNM (n = 194, 56.4%). Of note, while 107 (31.1%) and 30 (8.7%) patients had DLNM1 and DLNM2, respective, only 13 patients (3.8%) had DLNM3. The median number of LNs evaluated and metastases were 29 (IQR 22–40) and 0 (0–2), respectively. Roughly half of all patients had lymphatic invasion (n = 167, 48.8%) and vascular invasion (n = 186, 54.4%). One‐third of the entire cohort received adjuvant chemotherapy (n = 71, 34.6%) (Table 1).
Impact of the DLNM on the survival
In a subgroup of patients with LNM (n = 150), patients with DLNM2 and 3 had higher proportions of T4 tumor (DLNM1 21.5% vs. DLNM2 46.7% vs. DLNM3 46.2%, p = 0.031) and N2 tumors (DLNM1 13.1% vs. DLNM2 70.0% vs. DLNM3 69.2%, p < 0.001) than those with DLNM1. Similarly, a higher proportion of patients with vascular invasion was noted in the DLNM2 and 3 groups than in the DLNM1 group (DLNM1 58.9% vs. DLNM2 73.3% vs. DLNM3 100.0%, p = 0.008) (Table 2). During the median follow‐up period of 67.5 months (IQR 54.8–93.9), while there was no significant difference in the 5 year OS by DLNM1‐3 (DLNM1 71.1%, DLNM2 78.7%, DLNM3 50.4%, p = 0.61) (Fig. 2a), N Stage was able to discriminate the 5 year OS well (N0 86.6%, N1 76.2%, N2 55.2%, p < 0.001) (Fig. 2b). According to a multivariable analysis after adjusting covariates including age, sex, and T stage, LND3 was not associated with a decreased OS (HR 1.21; 95% CI 0.51–2.88, p = 0.66).
As for adjuvant chemotherapy, among 84 patients with LNM and data on adjuvant chemotherapy, 64.4% of patients in DLNM1 (n = 38) received adjuvant chemotherapy, while 76.0% in DLNM2 or 3 (n = 19) (p = 0.44). According to a bivariate analysis, Adjuvant chemotherapy was not associated with prolonged OS in DLNM1 (HR 0.43; 95% CI 0.15–1.22, p = 0.11) or DLNM2/3 (HR 0.80; 95% CI 0.16–3.93, p = 0.79).
Therapeutic index by the DLNM and perioperative factors
Among all primary locations, the therapeutic index value of D1 lymphadenectomy was 22.1 based on a 33.1% LNM incidence and a 5 year OS of 71.1% among patients with LNM (0.331 multiplied by 71.1), whereas the therapeutic index of D2 lymphadenectomy was at least 10 points lower than that of D1 (D1 22.1 vs. D2 6.8) (Table 3) (Fig. 3). Of note, the therapeutic index was the lowest in D3 lymphadenectomy (1.9), due to the low frequency of LNM (3.8%) and poor 5 year OS of patients with LNM (50.4%). In addition, this trend was consistent among patients with primary sites at the cecum (D1 23.0 vs. D2 8.6 vs. D3 3.2) and ascending colon (D1 22.3 vs. D2 4.8 vs. D3 1.6). Among patients with transverse colon cancer, a therapeutic index difference of more than 10 was noted between D2 and D3 lymphadenectomy (D2 16.1 vs. D3 0) (Fig. 3).
Sites of recurrence after R0 resection and central lymphadenectomy
Of the 344 patients who met the inclusion criteria, after a median follow‐up of 67.5 months, 81 (23.8%) developed postoperative recurrence (Table 4). Including the duplicated counts when a patient had ≥ 2 recurrence sites, the most common recurrence site was the liver (n = 36, 10.5%), followed by the lung (n = 25, 7.3%) and peritoneum (n = 18, 5.2%). Of note, among patients with recurrence at LNs, only two patients had para‐SMA recurrence, and both LNs were located beyond the SMA (i.e. left side of SMA), indicating that the para‐SMA recurrence was not derived from insufficient central lymphadenectomy (Supplemental Fig. 1). Other detailed information on patients with para‐SMA recurrence are summarized in Supplemental Table 1.
Discussion
Surgery is the mainstay in the treatment of colon cancer, and the number of LNs examined is one of the most important prognostic factors. However, the optimal extent of central lymphadenectomy for right‐sided colon cancer remains unclear. In fact, whether the landmark of CVL should be SMV or SMA has not yet been well standardized. To this end, the current study was important because the survival benefit of lymphadenectomy was identified for each LN area (i.e. D1. D2, and D3) based on the therapeutic index reflecting the frequency of LNM and the overall survival among subgroups who underwent lymphadenectomy. Regarding the DLNM, approximately 1 in 30 patients (3.8%) had LNM in front of the SMV. According to the calculated therapeutic index, of interest, the survival benefit of central lymphadenectomy was almost 1/10 of that with pericolic lymphadenectomy (D3 1.9 vs. D1 22.1). Although two patients experienced local LN recurrence beyond the SMA after curative‐intent colectomy, no patients developed local recurrence at the right side or in front of the SMA.
The therapeutic index was first proposed by Sasako et al. in 1995 and used to assess the survival benefit of LN dissection in the second‐tier stations for advanced gastric cancers by multiplying the incidence of LNM and the 5 year OS among patients who had LNM in a particular LN station [20]. In the same manner, Ueno et al. first adapted this metric to colorectal cancer to identify subgroups likely to receive prognostic benefit from lateral pelvic node dissection for advanced lower rectal cancer [13]. Specifically, the therapeutic index for lateral dissection was calculated as 7.0, which was higher than that of lymphadenectomy in the superior rectal artery (SRA) area (1.6) and the inferior mesenteric artery area (IMA) (0.4) [13]. This is in line with the findings of the present study showing a low therapeutic index for the D3 area with a range of 0 to 3.2 (Fig. 3). In this context, the therapeutic index is a useful metric that can inform surgeons of the survival benefit of lymphadenectomy, which is intuitively challenging to recognize.
In addition, the conclusion that central lymphadenectomy to the left edge of the SMV resulted in low survival benefit can also be used to determine whether the landmark of CVL should be the SMV or SMA. Given that the farther the LN area is from the primary tumor, the lower the LNM rate is, the therapeutic benefit of removing LNs in front of the SMA should also be poor. According to the Japanese Classification of Colorectal Carcinoma guidelines [22], although radical surgery of right colon cancer requires D3 cleaning of the LN at the root of the colonic vessels, thus involving exposure of the SMA, the existing literature regarding the outcomes after lymphadenectomy beyond the SMV remains scarce [23]. Future studies are needed to clarify the impact of central lymphadenectomy extending to the SMA on the short‐ and long‐term outcomes in the treatment of right‐sided colon cancers.
Extended lymphadenectomy may play a major role in staging patients adequately as well as in removing potentially metastatic LN from an oncological perspective. Previous studies reported that the DLNM classified by the area of LNM has an important role in predicting the patient survival. Specifically, Huh et al. showed that the DLNM was an independent predictor of the survival of patients with sigmoid colon and rectal cancer and discriminated the 5 year OS rates well [24]. However, Kim et al. from the same group compared the prognostic significance of the number of LNMs versus the DLNM and concluded that the staging system incorporating the number of LNMs predicted the prognosis better than the DLNM [25]. Consistent with the study by Kim et al., the current study found the superiority of N staging (i.e. the number of LNMs) against the DLNM for predicting the 5 year OS of patients undergoing colectomy for right‐sided colon cancers. Regarding the number of LNMs, however, a randomized clinical trial revealed that an N‐positive status was more common in the D3 group than in the D2 group (46% vs. 26%), indicating the potential benefit of D3 lymphadenectomy on accurate staging [26]. Taken together, these findings suggest that central lymphadenectomy for right‐sided may be more beneficial for adequately predicting the prognosis than the therapeutic value. However, the median number of LNs evaluated was 29 in the current study, which was more than double the number (at least 10–14 LNs) recommended by the American Joint Committee of Cancer [27]. Therefore, the extent to which D3 lymphadenectomy contributes to adequate staging still remains to be fully elucidated.
The long‐term oncological effects of central lymphadenectomy should be clarified. Storli et al. noted that D3 mesenteric excision for T1/2 colon cancers was associated with a better OS and disease‐free survival after adjusting for confounders, including the age and T stage [28]. Similarly, a retrospective study using a Japanese cohort of 3425 patients compared the OS of patients with T3/4 colon cancers undergoing D2 lymphadenectomy versus D3. The propensity score‐matched analysis revealed that D3 lymphadenectomy was associated with a better OS as well as a higher number of LNs examined [9]. Nevertheless, the conclusions from these studies have been limited due to their retrospective nature and considerable number of confounders that were not adjusted for at the time of the study. For instance, in the study by Kotake et al., the D2 lymphadenectomy group had a higher proportion of patients with LNM even after PSM (n = 1,389, 40.6% vs. n = 1,290, 37.7%, p = 0.014), which presumably affected the difference in the OS. Furthermore, neither study accounted for advances in surgical techniques and multidisciplinary treatments over time, such as minimally invasive surgery and chemotherapy regimens.
Several limitations associated with the present study warrant mention. Due to the retrospective design, the current study was subject to information and selection biases. Furthermore, the utilized data were derived from two contributing hospitals, and the conclusions may not be generalizable to patients treated at other institutions. In addition, due to the nature of the metric, it was not feasible to determine the cut‐off value of the therapeutic index associated with a significant benefit of DLNM for a certain patient group. Finally, given that the therapeutic index was based on the frequency of LNM and the 5 year OS of a certain patient group, the therapeutic value may not be robust among small subgroups, such as patients with transverse colon cancer. In addition, despite the failure to demonstrate the impact of DLNM on OS in the current study, it might be due to a small number of the cohorts including DLNM2 or 3. Future studies are called for to validate the results on the therapeutic index using a larger number of patients.
In conclusion, based on the metric of the therapeutic index, the survival benefit from central lymphadenectomy namely D3 was low compared with D1 and D2 lymphadenectomy among patients with right‐sided colon cancers. Although several studies have proposed central lymphadenectomy beyond the SMV, it may nevertheless not be beneficial for the survival.
References
Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995;181(4):335–46.
Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch surg. 1998;133(8):894–9.
Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1(8496):1479–82.
West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J clin oncol. 2010;28(2):272–8.
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation–technical notes and outcome. Colorectal dis. 2009;11(4):354–64.
Emmanuel A, Haji A. Complete mesocolic excision and extended (D3) lymphadenectomy for colonic cancer: is it worth that extra effort? A review of the literature. Int J Colorectal Dis. 2016;31(4):797–804.
Tagliacozzo S, Tocchi A. Extended mesenteric excision in right hemicolectomy for carcinoma of the colon. Int J Colorectal Dis. 1997;12(5):272–5.
Kanemitsu Y, Komori K, Kimura K, Kato T. D3 Lymph Node Dissection in Right Hemicolectomy with a No-touch Isolation Technique in Patients With Colon Cancer. Dis Colon Rectum. 2013;56(7):815–24.
Kotake K, Mizuguchi T, Moritani K, Wada O, Ozawa H, Oki I, et al. Impact of D3 lymph node dissection on survival for patients with T3 and T4 colon cancer. Int J colorectal Dis. 2014;29(7):847–52.
Bertelsen CA, Kirkegaard-Klitbo A, Nielsen M, Leotta SM, Daisuke F, Gogenur I. Pattern of colon cancer Lymph node metastases in patients undergoing central mesocolic Lymph node excision: a systematic review. Dis Colon Rectum. 2016;59(12):1209–21.
Lin JX, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, et al. Is all advanced gastric cancer suitable for laparoscopy-assisted gastrectomy with extended lymphadenectomy? A case–control study using a propensity score method. Ann Surg Oncol. 2016;23(4):1252–60. https://doi.org/10.1245/s10434-015-4994-1.
Kosuga T, Ichikawa D, Okamoto K, Komatsu S, Shiozaki A, Fujiwara H, et al. Survival benefits from splenic hilar lymph node dissection by splenectomy in gastric cancer patients: relative comparison of the benefits in subgroups of patients. Gastric Cancer. 2011;14(2):172–7.
Ueno H, Mochizuki H, Hashiguchi Y, Ishiguro M, Miyoshi M, Kajiwara Y, et al. Potential prognostic benefit of lateral pelvic node dissection for rectal cancer located below the peritoneal reflection. Ann Surg. 2007;245(1):80–7.
Wu L, Sahara K, Tsilimigras DI, Maithel SK, Poultsides GA, Rocha FG, et al. Therapeutic index of lymphadenectomy among patients with pancreatic neuroendocrine tumors: A multi-institutional analysis. Jour Surg Oncology. 2019;120(7):1080–6.
Sahara K, Tsilimigras DI, Maithel SK, Abbott DE, Poultsides GA, Hatzaras I, et al. Survival benefit of lymphadenectomy for gallbladder cancer based on the therapeutic index: An analysis of the US extrahepatic biliary malignancy consortium. J Surg Oncology. 2020;121(3):503–10.
Sahara K, Tsilimigras DI, Pawlik TM. ASO author reflections: which patients benefit the most from lymphadenectomy during resection for intrahepatic cholangiocarcinoma? Ann Surg Oncology. 2019;26(9):2969–70.
Tokunaga MM, Ohyama MS, Hiki MN, Fukunaga MT, et al. Therapeutic value of lymph node dissection in advanced gastric cancer with macroscopic duodenum invasion is the posterior pancreatic head lymph node dissection beneficial. Ann Surg Oncol. 2009;16(5):1241–6. https://doi.org/10.1245/s10434-009-0345-4.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14(2):113–23.
Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal appendiceal, and anal carcinoma the 3d English Edition Secondary Publication. J Anus Rectum Colon. 2019;3(4):175–95.
Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82(3):346–51.
Sahara K, Tsilimigras DI, Merath K, Bagante F, Guglielmi A, Aldrighetti L, et al. Therapeutic index associated with lymphadenectomy among patients with intrahepatic cholangiocarcinoma: which patients benefit the most from nodal evaluation? Ann Surg Oncology. 2019;26(9):2959–68.
Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese society for cancer of the colon and rectum (jsccr) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncology. 2012;17(1):1–29.
Yi X, Li H, Lu X, Wan J, Diao D. "Caudal-to-cranial" plus "artery first" technique with beyond D3 lymph node dissection on the right midline of the superior mesenteric artery for the treatment of right colon cancer: is it more in line with the principle of oncology? Surg endos. 2019. https://doi.org/10.1007/s00464-019-07171-5.
Huh JW, Kim YJ, Kim HR. Distribution of lymph node metastases is an independent predictor of survival for sigmoid colon and rectal cancer. Ann Surg. 2012;255(1):70–8.
Kim CH, Huh JW, Kim HR, Kim YJ. Prognostic comparison between number and distribution of lymph node metastases in patients with right-sided colon cancer. Ann Surg Oncology. 2014;21(4):1361–8.
Karachun A, Panaiotti L, Chernikovskiy I, Achkasov S, Gevorkyan Y, Savanovich N, et al. Short-term outcomes of a multicentre randomized clinical trial comparing D2 versus D3 lymph node dissection for colonic cancer (COLD trial). BJS. 2020;107(5):499–508.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncology. 2010;17(6):1471–4.
Storli KE, Sondenaa K, Furnes B, Nesvik I, Gudlaugsson E, Bukholm I, et al. Short term results of complete (D3) vs standard (D2) mesenteric excision in colon cancer shows improved outcome of complete mesenteric excision in patients with TNM stages I-II. Tech coloproctol. 2014;18(6):557–64.
Funding
none.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
none.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sahara, K., Watanabe, J., Ishibe, A. et al. Optimal extent of central lymphadenectomy for right-sided colon cancers: is lymphadenectomy beyond the superior mesenteric vein meaningful?. Surg Today 51, 268–275 (2021). https://doi.org/10.1007/s00595-020-02084-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-020-02084-6