Abstract
Radiations are of different types and find use in diverse arenas. Medicine is benefitted by the invention of radiations both in diagnostic and therapeutic fields. The use of X-ray has become the axis of radiological diagnostics, while treatment with help of radiations is one of the prominent modalities of treatment of cancer. There are different types of radiotherapy used for this purpose like external beam radiation therapy and internal radiation therapy. Besides the conventional external beam radiation therapy, advent of its many newer modifications as well as internal radiation therapy has transformed the utility of radiotherapy for cancer patients. Radiations can now be administered to a localized area in a customized manner. Besides increasing the efficacy, this has helped in reducing the side effects. Exposure of body to any kind of radiation is associated with many adverse reactions including generation of oxidative stress. Increased oxidative stress, by oxidizing different biomolecules, produces a variety of harmful effects in the body. These effects are implicated in the causation of a number of diseases including cancer. Though radiotherapy is used with the intent of controlling tumor cells by producing free radicals and reactive oxygen/nitrogen species, the associated oxidative stress is an area of concern. This chapter is an effort to throw some light on this interrelationship of radiotherapy and oxidative stress.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
Human body is constantly under exposure of radiations. Ultraviolet (UV) radiation, in the form of sunlight, is the most common source of radiation to which all the living forms of life are exposed. The usefulness of radiation as a treatment modality was first considered after the discovery of X-rays by Wilhelm Conrad Röntgen from Germany in 1895. This understanding got a boost with Marie Curie establishing her position as a pioneer in the field of radiation therapy after winning a second Nobel Prize for her research into radium (Baskar et al. 2012).
Radiotherapy or radiation therapy (RT) is nowadays one of the standard modalities of treatment for cancer, the other two being surgery and chemotherapy. For treatment of cancer by RT, high doses of radiation are used to kill cancer cells and shrink tumor size. Low-dose radiation is used for X-ray, one of the radiological investigations frequently used in clinical diagnostics. Radiation therapy brings its effect on cancer cells by damaging their deoxyribonucleic acid (DNA) by a variety of mechanisms. Cancer cells, whose DNA is damaged beyond repair, stop proliferating or die. Radiation therapy does not bring about this effect right away but takes days or weeks of treatment and the cancer cells keep dying for weeks or months after therapy is completed (Núñez et al. 1996). Radiation can be administered with the intent of cure or of palliation. Other indications of radiotherapy are in combination with other treatment modalities like surgery, chemotherapy, or immunotherapy. When it is used before surgery to reduce the tumor size, it is termed as neoadjuvant therapy, and when used after surgery to take care of any leftover microscopic tumor cells, it is termed as adjuvant therapy. Different cancers vary in their sensitivity to RT as skin, prostate, lung, cervix, etc. are among good responders (Bernier et al. 2004).
Radiotherapy
Radiotherapy is the use of radiations for therapeutic purposes, mainly for treatment of cancer.
Types of Radiotherapy
Two principal types of RT are used, external beam radiation and internal RT (Adams and Warrington 2008).
Radiation administered to the patient depends on many factors like:
-
Type of cancer
-
Size of the tumor
-
Location of tumor
-
Closeness of tumor to radiosensitive healthy tissues
-
General health and medical history of the patient
-
Adjuvant or concomitant therapies
External Beam Radiation Therapy (EBRT)
As the name suggests, EBRT is the delivery of highly targeted radiation beams from some external source. It is administered in the form of daily fractions distributed over a period of few days to weeks. The radiation beam is generated, directed, and controlled by a machine (telecobalt/cobalt-60 teletherapy or linear accelerator). Cobalt-60 instrument is simple and is based on older technology. It utilizes high-energy gamma beam and requires the source to be changed every 7 years or so. Linear accelerator is the result of rapid progress in technology, design, ancillaries, and utility of the instrument. It makes use of high-frequency electromagnetic waves for acceleration of charged particles such as electrons to high energies through a linear tube. The high-energy electron itself can be used for treating superficial tumors, or it can be made to hit a target to produce X-rays (also called photons) for treating deep-seated tumors. It is capable of providing different varieties of EBRT, while telecobalt is used for conventional type only (Bernier et al. 2004).
Conventional External Beam Radiation Therapy
It is a tried and tested method of administering radiation to a tumor noninvasively. The radiation beams are regulated as they come out of the machine to ensure that they are carefully directed at the cancer site (Baskar et al. 2012).
Intensity-Modulated Radiation Therapy (IMRT)
IMRT is a form of EBRT which allows entry of large number of small radiation beams of different intensities at multitude of angles. This technique is quite precise and is able to deliver high doses of radiation without compromising the safety of healthy tissues in the vicinity of the tumor (Feng et al. 2007).
Image-Guided Radiation Therapy (IGRT)
IGRT facilitates delivering of treatment with supervision by constant imaging of the tissue. It is a feature of almost all IMRT techniques to target the tumor area accurately to the scale of millimeters. IGRT allows minute differences in tumor shape, size, and position to be considered to make the appropriate adjustments in delivery of radiation in different sessions. This allows targeted radiation delivery to malignant cells without causing damage to the surrounding healthy ones (Duma et al. 2010).
Stereotactic Radiosurgery (SRS)
SRS is specifically beneficial for cancers of the central nervous system (CNS) as is preferred for small-sized tumors having well-defined edges. In this, the tumor tissue is administered a single high dose of radiation in a highly accurate manner using constant ongoing imaging and planning techniques. The required complete stillness of the patient during RT is achieved using a head frame (Bijlani et al. 2013).
Stereotactic Body Radiation Therapy (SBRT)
SBRT is used to deliver very high-dose radiation focally to small tumors outside of the CNS region. Immobilization of the body is mandatory, although essential involuntary movements like respiratory movements, etc. are taken into account during planning. Therefore, SBRT needs to be delivered in form of several divided low-dose fractions over multiple sessions. SBRT may also be used for tumors of the CNS region, like SRS, but only in multiple lower-dose regimes in place of a single large-dose fraction. This approach of EBRT is still in a research phase but bears a potential to become popular and preferred modality of in future (Bijlani et al. 2013; Tipton et al. 2011).
Proton Therapy
Particle therapy, an advanced form of RT, has shown promising results in specific malignancies. Proton therapy is the most common form of particle therapy and may be very useful in the treatment of individuals having tumors near vital organs like brainstem and optic nerve posing a threat to sense of coordination and vision. It may be particularly advisable for the treatment of young patients where it is desirable to minimize the long-term effects of treatment like hormonal imbalances, mental and physical growth impairment, appearance of secondary malignancies, etc. (Schulz-Ertner and Tsujii 2007).
Internal Radiation Therapy
In this form of radiation therapy, the source of radiation is placed internally, i.e., inside the body. This source may be in solid or liquid form. The term “brachytherapy” is used for the internal radiation therapy using a solid source (“brachy” is Greek for short distance). The radiation source in the form of capsules, seeds, or ribbons is implanted within or close to the tumor area. Like EBRT, brachytherapy also targets only a specific part of the body as localized therapy. The radiation emitted by the radiation source placed in the body helps to eliminate the cancerous cells in the vicinity (Sadeghi et al. 2010).
The form of internal radiation therapy using a liquid source is called systemic therapy, i.e., the source of radiation administered circulates in the blood, detecting and eliminating malignant cells. The systemic radiation therapy may be administered per oral or parenterally. With this therapy, different body fluids like saliva, urine, and sweat may emit radiation for some duration (Sadeghi et al. 2010).
How Radiation Therapy Works?
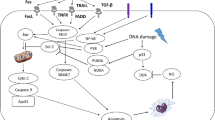
Radiation is one of the physical agents used to kill cancer cells. For this purpose, ionizing radiation is used which produces ions and transfers this energy to the cells which it traverses. This energy is able to destroy cancerous cells either directly or in an indirect manner by producing genetic changes. The ionizing radiation is more effective on actively dividing malignant cells as compared to the normal cells (Bernier et al. 2004). The ultimate biological target of this physical agent is DNA inside the nucleus of the cell (Fig. 1). The effects produced by radiation on DNA may be direct or indirect.
Direct Effects of Radiation
Radiation may affect the cellular DNA directly by producing a variety of damage in one or both of the DNA strands known as single strand breaks (SSBs) or double strand breaks (DSBs). Radiation-induced DSBs are considered the most dangerous type of DNA damage, which, if not get repaired, leads to cell death. Different pathways participate in the repair and maintenance of genome following exposure to ionizing radiation. Interestingly, a variety of proteins involved in cell death and DNA damage have been reported to lead to a decrease in the radioresistance of the rapidly multiplying cancer cells, while an increase in radioresistance of normal cells (Jorgensen 2009). Radioresistance is the term used for the process where tissues adapt to the RT-induced changes and turn resistant against it (Luo et al. 2017). Ionizing radiation used for treatment of cancer, therefore, produces diverse effects inside the cells. Some of these tend to revert the radiation-induced changes in the healthy cells, while others try to avoid the damage or if not able to do so induce cancer cell death (Jorgensen 2009).
The guardian of genome, p53, is a transcription factor which reacts by arresting the cell cycle, causing DNA repair and inducing apoptosis to protect the cells against ionizing radiation. Because of these actions, it is also termed as an “anti-oncogene” or tumor suppressor gene (Brosh and Rotter 2009). However, whether p53 will induce apoptosis or will arrest the cell cycle depends, in part, on the cellular concentration of the p53 protein (low levels, generally, cause cell cycle arrest, while its high levels may induce apoptosis) (Lai et al. 2007). Different DNA repair processes present in the tumor cells try to counter the radiation-induced effects and may boost the radioresistance of malignant cells (Adams and Warrington 2008). Also, inhibition of proteins involved in DNA repair like ataxia telangiectasia-mutated (ATM) or DNA dependent protein kinase (DNA-PK) has been observed to augment radiosensitization of the cancer cells (Rainey et al. 2008).
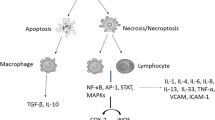
Types of Cell Death in Cancer
Radiotherapy, like most other anticancer treatment modalities, brings about its therapeutic effect by inducing cell death in the affected tissues. The cell death is achieved by a variety of mechanisms but predominantly by inducing apoptosis or mitotic catastrophe (Eriksson and Stigbrand 2010).
Apoptosis
Also known as programmed cell death, apoptosis is one of the major targeted processes in all types of cancer therapy, especially radiation therapy. Characteristic features of apoptotic process include shrinkage of cell volume and generation of apoptotic bodies. Common changes seen in the apoptotic cell include blebbing of cell membrane, condensed chromatin, nuclear margination and DNA fragmentation. Efficacy of radiation therapy against cancer is determined by its ability to induce apoptosis in malignant cells (Eriksson and Stigbrand 2010; Vakifahmetoglu et al. 2008).
Mitotic Catastrophe
This type of cell death is seen in response to aberrant mitosis and is brought about by mis-segregation of chromosomes forming giant cells with abnormal nuclear morphological features. These cells may show one or more micronuclei and overduplication of centrosome (Jonathan et al. 1999). Postirradiation, cell death observed in most solid tumors takes place principally due to abnormal mitotic processes in these cells (Hotchkiss et al. 2009).
Necrosis
In necrosis, cells swell visibly with breakdown of cell membrane unlike apoptosis. The changes observed in necrotic cells include atypical nuclei due to vacuolization, mitochondrial swelling, non-condensed chromatin, other damaged cellular organelles, and blebbing and rupture of plasma membrane resulting in release of intracellular contents. Necrosis is not a common type of cell death following radiation but is observed in some types of cancer cell lines or malignant tissues (Hotchkiss et al. 2009).
Senescence
Senescence in context of cell is defined as a permanent loss of its ability to proliferate. Thus, senescent cells are still viable but cannot divide, are larger in size, are flatter in shape, stop synthesizing DNA, and become more granular. Senescence in cancer cells may be a result of extensive cellular stress induced by radiation treatment which later may lead to apoptosis (Roninson 2003).
Autophagy
Autophagy is a phenomenon described more recently. It may be considered a genetically regulated form of apoptosis which is characterized by appearance of autophagic/lysosomal compartment in the cytoplasm leading to formation of double membrane vacuoles for sequestering cellular organelles. The cell appears to digest its own contents and is a typical form of radiation-induced cell death seen in cancer cells (Kuwahara et al. 2011).
Indirect Effects of Radiation
The other way to produce effect on DNA by radiation is in an indirect manner. Radiation-induced oxidative stress is responsible for this process. It is termed “indirect” as free radicals (reactive entities having free electron) are produced first which then react with the genetic material. The damage to genetic material by the free radicals is mediated by the process of ionization or excitation of the water present in the cells, known as radiolysis of water which may be explained in Fig. 2.
The process of oxidative damage is not restricted only to the irradiated cells but gets transmitted to their progeny also. These changes may continue to manifest for long periods stretching to days and months after the initial exposure, due to ongoing production of reactive oxygen (ROS) and nitrogen (RNS) species. In addition, nontargeted bystander cells in the vicinity of radiated ones may also show features of increased oxidative stress transmitted through different mechanisms of intercellular communication. The problem does not stop here; rather the progeny of these bystander cells also faces conditions of increased oxidative stress manifesting in a variety of consequences such as peroxidation of lipids and other macromolecules, protein carbonylation, and increased frequency of spontaneous gene mutations and subsequent malignant transformation of affected cells. These effects, if persist for long duration, may cause untoward effects in progeny cells having paramount long-term health implications and risks like appearance of a second malignancy or even recurrence subsequent to radiation therapy. The oxidative damage to genetic material manifesting as mutations in key tumor suppressor genes like P53 and Retinoblastoma (Rb) may be the underlying cause for appearance of these malignancies. This may twist the ultimate motive of administering the radiations at the first instance. Therefore, in-depth knowledge of the molecular and biochemical events responsible for producing a plethora of oxidative damage processes in irradiated cells/tissues will be crucial for anticipating and arresting health effect risks associated with exposure to ionizing radiation (Azzam et al. 2011).
Radiolysis of Water and Generation of ROS
Water is one of the predominant (constituting ∼80%) components of cells. It can undergo ionization or excitation on exposure to radiation known as water radiolysis. The process of water radiolysis needs to be understood in detail to be able to associate it with various radiobiological consequences. The free radicals produced as a result of water radiolysis can attack other molecules of critical significance inside the cell with diverse manifestations (indirect effect).
The reactive species produced by the radiolysis of pure and deaerated water, quantitatively, include e−, •OH, H•, H2, and H2O2. When exposed to oxygen, e− and H• atoms, rapidly, get converted to superoxide or perhydroxyl (O2•− or HO2•) radicals. Therefore, in an aerobic environment at physiological pH, the major reactive oxygen species include O2•−, •OH, and H2O2 inside the cell. H2 is observed not to play a significant role in the radiolysis of aqueous solutions as most of it evaporates out of the solution (Fig. 2).
Some organic radicals (R•) are also produced by the biologic systems, most commonly due to initiation by •OH radicals. These carbon-centered radicals usually react rapidly with O2 to produce peroxyl radicals (RO2•), which are more efficient oxidizing agents. The RO2• radicals may extract H• from other molecules to generate hydroperoxides (ROOH), believed to be the main constituent reaction responsible for lipid peroxidation. Thus, the radiation damage in cells gets augmented by constant lipid peroxidation reactions in association with protein inactivation (Azzam et al. 2011; Ferradini and Jay-Gerin 1999) (Fig. 2).
Radiation-Induced Production of Reactive Nitrogen Species (RNS)
The stimulation of inducible nitric oxide synthase (iNOS) activity by ionizing radiation produces large amounts of nitric oxide (NO•) in the target cells. Though NO• is considered to be chemically inert toward a number of molecules in the cell, it reacts with O2•− to produce highly reactive peroxynitrite anion (ONOO−) (Fig. 3). ONOO− is capable of targeting a wide range of cellular components like lipids, proteins, and nitrogen bases of nucleic acid, etc. (Azzam et al. 2011; Mikkelsen and Wardman 2003).
In contrast to peroxynitrite anion (ONOO−), the lesser reactivity of H2O2 and O2•− permits them to permeate farther from the originating site (the diffusion coefficients of H2O2 and O2•− being 2.3 × 10−9 and 1.75 × 10−9, respectively). When exposed to redox metal ions like iron and copper, these entities generate OH• radicals with help of Fenton and Haber-Weiss reactions (Fig. 4), which may induce extensive tissue damage (Halliwell and Gutteridge 1992).
Therefore, it is imperative to understand that the radiolysis of water and activation of nitric oxide synthase by ionizing radiation are the predominant sources of production of ROS/RNS in irradiated cells under aerobic conditions. The availability of these products and their concentrations have important bearing on the extent and nature of radiation-induced DNA damages. ROS/RNS may target cellular DNA causing alterations like DNA breaks, cross-links, nitrogen base damage, modification of sugars, and dysfunction of telomeres. These alterations, if left unrepaired or not repaired properly, may result in mutations with consequent malignant transformation or necrosis of the cells (Azzam et al. 2011; Halliwell and Gutteridge 1992).
Role of Radiation in Chronic Inflammation
The presence of constant oxidative perturbations inside the cell is found to be associated with the process of chronic inflammation. This holds great significance in terms of radiotherapy and radiation protection perspectives. This knowledge is reinforced by the fact that increased levels of a number of inflammatory markers like interleukin-6 (IL-6), C-reactive protein (CRP), sialic acid, and white blood cell count were observed in survivors of the atom bomb even after very long duration following the tragedy (Neriishi et al. 2001).
Chronic inflammation is a dynamic, complex, and progressive process. Cells involved in this process, especially macrophages and neutrophils, are known to generate ROS and RNS at the sites of inflammation. These species cause a variety of oxidative changes in the nucleic acids that may result in mutations as well as DNA cross-links in the nearby cells. It is interesting to notice that circulating inflammatory cells in patients that received partial body irradiation are able to produce DNA alteration at distant areas away from the target site. Therefore, the overall effects of targeted radiation administration in a cell remain not so much localized as anticipated. Out of the expected effects, some of the genetic as well as epigenetic changes may be observed shortly after exposure, while others require several generations to be expressed. These changes can produce genomic instability in targeted as well as nontargeted cells leading to serious health consequences including cancer (Azzam et al. 2011; Sedelnikova et al. 2010).
Cellular Defenses to Handle DNA Alterations
Even in the absence of exposure to ionizing radiation and other mutagens, the body is under constant oxidative stress. The reactive species generated as a result of normal cellular metabolic reactions (chiefly O2•− and H2O2) cause DNA alterations in the form of depurinations, depyrimidinations, and oxidation of bases. The extent of ROS generated postirradiation is quite akin to that encountered during metabolic processes except somewhat with dissimilarity. Principally radicals like O2•− and H2O2 are produced by endogenous processes, while •OH is generated by radiation. Also, release of endogenous ROS is a chronic process, while instantaneous production is seen during irradiation.
Several defense mechanisms are induced to restore DNA integrity. In this regard, cells first activate cell cycle checkpoints to give sufficient time to cellular repair machinery to rectify the DNA damage. Out of various DNA repair mechanisms, base excision repair (BER) is responsible for identifying and repairing spontaneous nitrogen base modifications, aprurinic/apyrimidinic sites, and SSBs. Others like nucleotide excision repair (NER) and homologous recombination repair, etc. take care of other types of DNA damage (Weinberg and Chandel 2009; Friedberg et al. 2004).
Radioprotection of Normal Cells
Protecting the normal tissue during treatment of cancerous cells by radiotherapy is a major concern. With recent techniques, precise and targeted radiation therapy to the tumor tissue can be achieved, but still it is not possible to abolish a tumor without harming the surrounding healthy tissue. In both normal and cancer cells, ionizing radiation can generate ROS, mitochondrial respiratory chain being the major source (Baskar et al. 2012). Mutation in mitochondrial DNA (mtDNA) has been found associated with increased metastatic potential of tumor cells. Therefore, mitochondrial targeting has become one of the essential steps to therapeutic approach for the cancer control (Fulda et al. 2010). The neoplastic cells, on virtue of their altered metabolism, generate higher levels of ROS as compared to normal cells. As ROS is the mediator of the cellular damage induced by radiation, compounds regulating ROS production might act as potential targets for protecting normal cells. Some such compounds suggested are dichloroacetate and inhibitors of glutamate-cysteine ligase. Dichloroacetate might induce mitochondrial oxidative phosphorylation and, hence, ROS production, selectively, in neoplastic cells, while the inhibitors of glutamate-cysteine ligase can markedly increase the radiosensitivity of cancer cells by inhibiting cellular glutathione synthesis (Diehn et al. 2009).
Mitochondria are very important in this regard. Mitochondrial damage in the form of altered permeability has been reported to change the morphology, increase production of ROS, and decrease ATP generation induced by radiation in cells. The enzyme manganese superoxide dismutase (MnSOD) is found exclusively in the mitochondria and scavenges harmful superoxide radicals produced by radiation and may play a role in the protection of normal cells and in promoting radiosensitization of the tumor cells (Borrelli et al. 2009). Administration of MnSOD plasmid liposomes (MnSOD-PL) carrying DNA damage control/repair genes has proved effective in local protection against radiation to a variety of cells. MnSOD has been proposed to be a potential game changer in this regard as its overexpression in cancer cells helps in increasing their radiosensitization, while in normal cells, its overexpression imparts protection against harmful effects of irradiation (Epperly et al. 2011).
Antioxidant Status and Radiotherapy
It has been reported in literature that the concurrent administration of antioxidant vitamins during treatment with radiotherapy might adversely affect its efficacy and enhance the risk of recurrence of cancer, as oxidative stress, in the form of production of ROS and RNS, is involved in bringing about the therapeutic effects of radiotherapy. This might lead to an attempt to induce generation of enzymatic antioxidants in the body, but not to such an extent as to counterbalance the increased oxidative stress observed in the patients receiving radiation therapy. During the ongoing treatment, the antioxidant capability might be expected to be restored with improvement in disease (Dahiya et al. 2012). As a general rule, antioxidant status is not rapidly replenished in a spontaneous manner; rather the occurrence is converse. As per a research carried out at Leiden University Medical Center, Netherlands, it was found that the levels of the endogenous antioxidant compounds remained subnormal for several months following irradiation. This was characterized as “a failure of the antioxidant defense mechanism against oxidative damage” in response to cytotoxic treatments and radiotherapy (Moss 2007).
Peptides glutathione (GSH) and thioredoxin (Trx) are the two important endogenously existing antioxidants. Their synthesis depends on the availability of nicotinamide adenine dinucleotide phosphate (NADPH) which keeps glutathione reductase (GR) and thioredoxin reductase (ThxR) in a reduced state. In cancer cells, major sources of NADPH are hexose monophosphate (HMP) shunt pathway and the malic enzyme. It has been demonstrated experimentally that Auranofin, a potent ThxR inhibitor and an antirheumatic agent, could be used as a radiosensitizer in cancer patients. Auranofin, in doses below the cytotoxic levels, may render cancer cells protected against oxidative injury caused by radiotherapy (Sonveaux 2017).
Advising antioxidant supplementation along with radiotherapy, always, is a cause of perplexity for radiation oncologists. It is so because antioxidants tend to protect normal cells against ROS, but the same effect in cancer cells may decrease the efficacy of radiotherapy. Antioxidants tend to prevent tissue damage and risk of malignant transformation in healthy cells. Newer studies claiming that antioxidants protect cancer patients against untoward side effects of radiation treatment may render these supplements beneficial in the future in the form of adjuvant treatment, but the current status is not clear yet (Borek 2004).
Conclusion
There is continual generation of ROS inside the body which not only are required to drive different regulatory pathways but are also involved in several pathological conditions including cancer. ROS may promote as well as suppress the survival of cancer cells. ROS regulate different processes like different steps of tumor development (transformation, survival, proliferation, invasion, metastasis, and angiogenesis), chronic inflammation as major mediator of cancer, various signaling molecules required for cell cycle progression, and the expression of various tumor suppressor genes and may mediate action of majority of chemotherapeutic and radiotherapeutic agents against cancer (Azzam et al. 2011). Ionizing radiation, delivered in the form of radiotherapy, controls or treats cancer by generating ROS and RNS. Though with the improved and advanced techniques, radiotherapy-related toxicities on normal cells have become minimal, still these pose a considerable challenge as effect of radiation on intercellular communications between healthy and malignant cells is not yet completely understood. Latest molecular biological approaches applied to radiobiological field are able to explain several mechanisms by which healthy and cancer cells respond differently to ionizing radiation. Antioxidant supplementation, though considered beneficial in patients of cancer, is not very advisable in patients being treated for cancer by radiotherapy. Antioxidants in low dose might help the patients without compromising the effect of radiotherapy. This claim is still controversial, and a difference of opinion is seen in practicing radiation oncologists (Singh et al. 2017).
References
Adams EJ, Warrington AP (2008)A Comparison between cobalt and linear accelerator-based treatment plans for conformal and intensity-modulated radiotherapy. Br J Radiol 81(964):304–310
Azzam EI, Jay-Gerin JP, Pain D (2011) Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 327(1–2):48–60
Baskar R, Yap SP, Chua KL, Itahana K (2012) The diverse and complex roles of radiation on cancer treatment: therapeutic target and genome maintenance. Am J Cancer Res 2(4):372–382
Bernier J, Hall EJ, Giaccia A (2004) Radiation oncology: a century of achievements. Nat Rev Cancer 4:737–747
Bijlani A, Aguzzi G, Schaal DW, Romanelli P (2013) Stereotactic radiosurgery and stereotactic body radiation therapy cost-effectiveness results. Front Oncol 3:77
Borek C (2004) Antioxidants and radiation therapy. J Nutr 134(11):3207S–3209S
Borrelli A, Schiattarella A, Mancini R, Morrica B, Cerciello V, Mormile M, d'Alesio V, Bottalico L, Morelli F, D'Armiento M, D'Armiento FP, Mancini A (2009) A recombinant MnSOD is radioprotective for normal cells and radiosensitizing for tumor cells. Free Radic Biol Med 46:110–116
Brosh R, Rotter V (2009) When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer 9:701–713
Dahiya K, Dhankhar R, Madaan H, Singh V, Arora K (2012) Nitric oxide and antioxidant status in head and neck carcinoma before and after radiotherapy. Ann Clin Lab Sci 42(1):94–97
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF (2009) Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458:780–783
Duma MN, Kampfer S, Wilkens JJ, Schuster T, Molls M, Geinitz H (2010) Comparative analysis of an image-guided versus a non-image-guided setup approach in terms of delivered dose to the parotid glands in head-and-neck cancer IMRT. Int J Radiat Oncol Biol Phys 77:1266–1273
Epperly MW, Wang H, Jones JA, Dixon T, Montesinos CA, Greenberger JS (2011) Antioxidant-chemoprevention diet ameliorates late effects of total-body irradiation and supplements radioprotection by MnSOD-plasmid liposome administration. Radiat Res 175:759–765
Eriksson D, Stigbrand T (2010) Radiation-induced cell death mechanisms. Tumour Biol 31:363–372
Feng FY, Kim HM, Lyden TH, Haxer MJ, Feng M, Worden FP, Chepeha DB, Eisbruch A (2007) Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Phys 68:1289–1298
Ferradini C, Jay-Gerin JP (1999) Radiolysis of water and aqueous solutions: history and present state of the science. Can J Chem 77:1542–1575
Friedberg EC, McDaniel LD, Schultz RA (2004) The role of endogenous and exogenous DNA damage and mutagenesis. Curr Opin Genet Dev 14:5–10
Fulda S, Galluzzi L, Kroemer G (2010) Targeting mitochondria for cancer therapy. Nat Rev Drug Discov 9:447–464
Halliwell B, Gutteridge JM (1992) Biologically relevant metal ion-dependent hydroxyl radical generation. An Update FEBS Lett 307:108–112
Hotchkiss RS, Strasser A, McDunn JE, Swanson PE (2009) Cell death. N Engl J Med 361:1570–1583
Jonathan EC, Bernhard EJ, McKenna WG (1999) How does radiation kill cells? Curr Opin Chem Biol 3:77–83
Jorgensen TJ (2009) Enhancing radiosensitivity: targeting the DNA repair pathways. Cancer BiolTher 8:665–670
Kuwahara Y, Oikawa T, Ochiai Y, Roudkenar MH, Fukumoto M, Shimura T, Ohtake Y, Ohkubo Y, Mori S, Uchiyama Y, Fukumoto M (2011) Enhancement of autophagy is a potential modality for tumors refractory to radiotherapy. Cell Death Dis 2:e177
Lai PBS, Chi TY, Chen GG (2007) Different levels of p53 induced either apoptosis or cell cycle arrest in a doxycycline-regulated hepatocellular carcinoma cell line in vitro. Apoptosis 12:387–393
Luo M, Ding L, Li Q, Yao H (2017) miR-668 enhances the radioresistance of human breast cancer cell by targeting I kappa B alpha. Breast Cancer 24:673–682
Mikkelsen RB, Wardman P (2003) Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene 22:5734–5754
Moss RW (2007) Do Antioxidants interfere with radiation therapy for cancer? Integr Cancer Ther 6(3):281–292
Neriishi K, Nakashima E, Delongchamp RR (2001) Persistent subclinical inflammation among A-bomb survivors. Int J Radiat Biol 77:475–482
Núñez MI, McMillan TJ, Valenzuela MT, Ruiz de Almodóvar JM, Pedraza V (1996) Relationship between DNA damage, rejoining and cell killing by radiation in mammalian cells. Radiother Oncol 39:155–165
Rainey MD, Charlton ME, Stanton RV, Kastan MB (2008) Transient inhibition of ATM kinase is sufficient to enhance cellular sensitivity to ionizing radiation. Cancer Res 68:7466–7474
Roninson I (2003) Tumor cell senescence in cancer treatment. Cancer Res 63:2705–2715
Sadeghi M, Enferadi M, Shirazi A (2010) External and internal radiation therapy: past and future directions. J Can Res Ther 6:239–248
Schulz-Ertner D, Tsujii H (2007) Particle radiation therapy using proton and heavier ion beams. J Clin Oncol 25:953–964
Sedelnikova OA, Redon CE, Dickey JS, Nakamura AJ, Georgakilas AG, Bonner WM (2010) Role of oxidatively induced DNA lesions in human pathogenesis. Mutat Res 704:152–159
Singh K, Bhori M, Kasu YA, Bhat G, Marar T (2017) Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity - exploring the armoury of obscurity. Saudi Pharm J 26(2):177–190
Sonveaux P (2017) ROS and radiotherapy: more we care. Oncotarget 8(22):35482–35483
Tipton K, Launders JH, Inamdar R, Miyamoto C, Schoelles K (2011) Stereotactic body radiation therapy: scope of the literature. Ann Intern Med 154:737–745
Vakifahmetoglu H, Olsson M, Zhivotovsky B (2008) Death through a tragedy: mitotic catastrophe. Cell Death Differ 15:1153–1162
Weinberg F, Chandel NS (2009) Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci 66:3663–3673
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
Dhankhar, R., Dahiya, K. (2022). Radiotherapy-Induced Augmentation of Cellular Oxidative Stress. In: Chakraborti, S. (eds) Handbook of Oxidative Stress in Cancer: Therapeutic Aspects. Springer, Singapore. https://doi.org/10.1007/978-981-16-5422-0_257
Download citation
DOI: https://doi.org/10.1007/978-981-16-5422-0_257
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5421-3
Online ISBN: 978-981-16-5422-0
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences