Abstract
Flavonoids are bioactives that are seen abundantly in various parts of plants, especially in fruits and vegetables. Flavonoids are classified into different subclasses, including anthocyanins, flavanols, chalcones, flavanones, flavones, and isoflavones. Many dietary flavonoids exhibit health-promoting effects, mainly disease preventive actions such as antioxidant, antimicrobial, cardioprotective, hepatoprotective, neuroprotective, antidiabetic, anti-inflammatory, antiproliferative, and anti-cancer. Dietary flavonoids typically help to reduce cellular oxidative stress and modulate dysregulated signaling pathways and thereby gene expression. Oxidative stress regulation is an essential factor in cancer treatment. Normal and cancerous cells mainly differ in their redox status. In cancer cells, there are increased levels of reactive oxygen species; hence, several anti-cancer therapies largely focus on modulating these reactive oxygen species levels. Recent studies suggest that small molecules with bioactive properties support to reduce the side effects of anti-cancer drugs. This chapter will briefly discuss the pro-apoptotic effects of some of the major dietary flavonoids such as quercetin, morin, EGCG, genistein, daidzein, apigenin, luteolin, and myricetin in oxidative stress-induced cancers.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Cancer
- Oxidative stress
- Reactive oxygen species
- Dietary flavonoids
- Phenolic compounds
- Antioxidant
- Apoptosis
Introduction
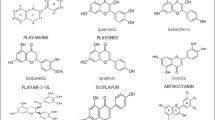
Flavonoids are mostly plant secondary metabolites which help in the defense system and to attract pollinating insects. Flavonoids are categorized under the class of polyphenols, which possess a C15 flavone skeleton. Phenolic compounds are known to be synthesized in plants as secondary metabolites and are categorized as phenolic acids, flavonoids, and proanthocyanidins. Flavonoids, which constitute a substantial portion, have a general structure with two aromatic rings with a minimum of one hydroxyl group connected to a six-membered heterocyclic pyran ring by a bridge formed by three carbon atoms. The major subclasses of flavonoids are anthocyanidins, flavan-3-ols, flavonols, flavanones, flavones, and isoflavones (Ibrahim et al. 2012). The different subclasses of flavonoids differ in the connections between the aromatic ring and the heterocyclic pyran rings. Within each flavonoid subclass, the individual compounds have characteristic hydroxylation and conjugation patterns. Flavonoids are synthesized through two biosynthetic pathways such as the shikimic acid pathway and the acetate pathway (Nabavi et al. 2020).
Flavonoids typically originate in plant-based food products such as wine, fruit juices, tea, coffee, chocolate, etc., which are generally termed dietary flavonoids. Berries and drupes are the richest sources of flavonoids among fruits, while in vegetables mainly red onion, shallots, broad bean pods, olives, spinach, etc. Seeds like beans, soy, and soy-derived food products are also rich sources of flavonoids. Beverages like green tea, black tea, and cocoa are reported to have high flavonoid content. Although the above dietary sources are rich in flavonoids, the type of flavonoid and its amount vary from one source to another. Therefore, a diet rich in fruits, vegetables, nuts, and cereals can ensure sufficient amounts of flavonoids required for the normal well-being of our body (Liu et al. 2018).
In plants, flavonoids are mostly found either in glycoside bound or as free aglycone form. The flavones and flavanols consumed in the diet are commonly in the glycoside bound form. The beneficial effect of flavonoids depends largely on their concentration and bioavailability. Once flavonoids enter the oral cavity, their metabolism and absorption begin in the digestive tract. Gut microbiota also play a vital role in the metabolism and absorption of flavonoids via the production of absorbable aglycone form in the large intestine. However, the absorption and bioavailability of flavonoids are increased or decreased by various factors. Typical high-fat diet increases the absorption of flavonoids due to increased secretion of bile acids, resulting in the formation of flavonoid-rich micelles. However, a protein-rich diet reduces the bioavailability of flavonoids, thereby decreasing its antioxidant activity and the digestion of proteins. Flavonoids are also shown to inhibit glycolytic enzymes and modulate glucose absorption when a diet rich in carbohydrates is consumed. The bioavailability of flavonoids also differs depending on their source and type. For example, quercetin glycoside is one of the most common flavonoid consumed through the diet, but its bioavailability varies from one source to another. Quercetin obtained from apple shows lower availability when compared to quercetin from onion (Rodriguez-Mateos et al. 2014).
Flavonoids possess a wide range of biological and pharmacological activities, thereby altering multiple mechanisms in the cell leading to their beneficial effects. Flavonoids were shown to modify enzymes and signaling pathways related to oxidative stress and inflammation. The antioxidant potential of flavonoids largely attributed to their structure due to the presence of hydroxyl groups. The detoxifying enzymes especially cytochrome p450 (CYC p450) are reported for activating chemical carcinogens, and flavonoids are shown to inhibit the CYC p450, thereby inhibiting carcinogenesis. Tea catechins are also identified to induce detoxifying and antioxidant enzymes present in the cells like superoxide dismutase (SOD), catalase, etc. The anti-inflammatory effects of flavonoids are due to their inhibitory effect on cyclooxygenase (COX) and activation of NADPH oxidase (NOX) enzymes.
Oxidative Stress and Reactive Oxygen Species
Maintaining a balance between generation and elimination of oxidants or reactive chemical species is termed a redox state. Oxidative stress is the destruction of redox equilibrium resulted due to increased levels of reactive oxygen species (ROS) further leading to damage to biomacromolecules (Pizzino et al. 2017). ROS are reactive molecules which contain oxygen either in radical form or non-radical derivatives. For a healthy environment, it is significant to retain the equilibrium of ROS in the cell between its production due to cellular metabolism and control by endogenous antioxidant enzymes. Oxidative stress leads to various clinical conditions such as inflammatory diseases, diabetes, cardiovascular disorders, cancer, etc.
Regulation of ROS at Enzymatic Level
ROS are unstable, partially reduced forms of specific metabolic products, which are repetitively produced and eliminated from cells. Some of the notable ROS in the biological system includes superoxide anion radical (O2•-), hydroxyl radical (•OH), singlet oxygen, hydrogen peroxide (H2O2), peroxynitrite, etc. ROS has an unpaired electron and shortened life span. Under stable physiological conditions, the stability among intracellular ROS and intercellular antioxidants is strictly delimited with a capable defense mechanism that wedges unwanted ROS production. In the presence of antioxidant defense mechanism, simple and grouped nucleic ROS levels allow sovereign regulation of mitochondrial, cytoplasmic ROS signals (Jones 2008).
SOD is an endogenous enzyme and is the central key for the defense system against oxidative stress. SOD are a group of metalloprotein enzymes found in all organisms, which can rapidly act as a free radical scavenger of oxygen. Joe McCord and Irwin Fridovich discovered the action of SOD in 1968, which catalyzes the partitioning or dispute the SOD resilient H2O2 by enzymatic Haber-Weiss reaction. In mammals, SODs are classified into three types, namely, copper/zinc SOD (SOD 1), manganese SOD (SOD 2), and extracellular SOD (Ec SOD 3). SOD 1 and SOD 2 are present in the cytosol, while SOD 3 is located in the extracellular matrix. In mitochondria, O2•- is the initiative molecule for the production of oxidant toxicity and ROS. Mitochondrial enzyme Mn-SOD provides essential protection against O2•-, SOD coupled with specific other proteins such as catalase, peroxidase, glutathione peroxidase, and glutathione S-transferase (GST), which finally convert H2O2 to water. All these antioxidant enzymes act on ROS to prevent the Fenton reaction and increasing H2O2 concentration, as well as •OH formation (Thomas et al. 2009).
Besides SOD, other enzymes also take part in the ROS detoxification. Glutathione (g-L-glutamyl-L-cysteinyl glycine), an intracellular thiol family of Phase II detoxification flavoprotein, is formed with a combination of glycine, cysteine, and glutamic acid. It is expressed more commonly by reduced form as glutathione (GSH) or oxidized form as glutathione disulfide (GSSG); the thiol mechanism gives its core biochemical properties. GSH can expel ROS by solid chemical reaction or by reducing peroxide, as a cofactor of GSH peroxidase, which induced a cycle among the reduced form and dimerized oxidized form of glutathione. GSH reductase, a homodimeric cellular antioxidant, mainly presents NADPH in the reduced form involved in many detoxifying types of ROS compounds. The thioredoxin reductase system has ubiquitous functions like SOD and glutathione. Thioredoxin is a protein dithiol/disulfide active site that turns to aid the reduction of additional proteins by establishing disulfide bridges between cysteine residues. Such interactive basic instincts enable cells to thrive in an antioxidant oxidizing environment, carry out the obligatory biochemical processes, and use ROS as signaling molecule (Arnér and Holmgren 2000).
Regulation of ROS at Molecular Levels
Antioxidant protein expression is regulated by NF-𝜅B, a transcription factor which involves multiple cellular process defense against ROS such as inflammatory response, apoptosis, and cell adhesion. NF-𝜅B associates with Rel (c-Rel), RelB, p50/p105 (NF-𝜅B1), RelA (p65), and p52/p100 (NF-𝜅B2). Also, NF-𝜅B disorders have been confirmed to be correlated with cancer. Mitogen-activated protein kinase (MAPK) plays a central role in complex cellular processes such as replication, cell cycle, development, and apoptosis. It consists of extracellular signal-related kinases (ERK1/2), c-Jun N-terminal kinases (JNK), p38 kinases (p38), and a large MAP kinase 1 (BMK1/ERK5) pathway. Recent studies show that ROS could activate ERK and JNK pathways, which ultimately induces oxidative stress-induced cancer (Wei and Liu 2002).
Keap1-Nrf2-ARE and phosphoinositide-3-kinase(PI3K-)/Akt signaling pathways also have some significant roles related to oxidative stress-induced cancers. Keap1-Nrf2-ARE is involved in redox balance and metabolism and adaptive immune response, and deregulation leads to many disorders like diabetes, inflammatory disease, Alzheimer’s disease, and cancer. PI3K/Akt pathway possesses multiple cellular responses, including cell cycle, protein synthesis, proliferation, and apoptosis. It also has a drug resistance response and is involved in stimulating hormones and cytokines (Cahill et al. 2012). Upregulation of PI3K/Akt signaling leads to oxidative stress-induced cancers including lung, liver, ovarian, prostate, and breast cancer.
Apoptosis: Pathways and Proteins Involved
Apoptosis is precisely programmed cell death, controlled by a sequence of cellular proteins and signal transduction cascades. When there is a fault in this orderly cell death, it will lead to uncontrolled growth of cells such as in many disease conditions such as Parkinson’s syndrome, cancer, and diabetes. The two major apoptotic pathways are the intrinsic or mitochondrial pathway and the extrinsic or cytoplasmic pathway. The liberation of cytochrome C originates the intrinsic pathway to the mitochondria, which attaches to Apaf1, dATP, and procaspase-9. Extrinsic pathway is initiated by inducing cell surface death receptors by binding death signals to trimeric death ligands Fas, TNF, etc. Proteins involved in apoptosis can be categorized into two classes, pro-apoptotic and anti-apoptotic proteins. Pro-apoptotic proteins are those proteins that induce apoptosis, while anti-apoptotic proteins will try to prevent apoptosis. Pro-apoptotic proteins are further classified into caspases and BCL2 proteins. Caspases are cysteine-dependent aspartate-directed proteases. Based on the function, caspases are of three types of inflammatory, initiator, and effector caspases. Caspases that serve in the extrinsic pathway are caspase-8, caspase-3, caspase-6, and caspase-7, while caspases that help in intrinsic pathways are caspase-2, caspase-3, caspase-7, and caspase-9. BCL2 proteins regulate apoptosis by controlling the permeability of the mitochondrial outer membrane and thereby releasing cytochrome C. Bcl2 proteins that induce apoptosis in various ways, such as by inhibiting apoptotic inhibitors, e.g., Noxa, Puma, Arts, SMAC, etc., belong to BH3 only class. Bid protein takes part in the intermediate pathway of apoptosis. Examples of Bcl2 multidomain proteins are Bax and Bak. Anti-apoptotic proteins mainly are of two types: Bcl2 multidomain proteins and BIR domain proteins. DIVA, Bcl-Xl, Bcl-b, Bcl-w, etc. are examples of Bcl2 multidomain proteins and survivin, livin, bruce, etc. are examples of BIR domain proteins (Abotaleb et al. 2019).
Intrinsic or Mitochondria-Dependent Pathway
The intrinsic pathway of apoptosis depends on the trophic factors, also known as survival factors. Trophic factors such as nerve growth factor (NGF) bind to the receptors present in the cell surface and activate the phosphatidylinositol 3-kinase (PI3K) signaling. PI3K activate protein kinase B, which further induce phosphorylation of the Bad (BCL-2-associated agonist of cell death), that are present in the outer membrane of mitochondria. When cells fail to receive the trophic factors, all this cascade of reactions will be inhibited and Bad proteins will be free, binding to Bcl-2 or Bcl-xL and releasing Bak or Bax, which stay attached to Bcl-2 or Bcl-xL. Anti-apoptotic proteins. Bak or Bax oligomerizes and forms pores in the surface of mitochondria and releases cytochrome C. Cytochrome C binds to Apaf-1 and activates caspase-9. These executioner caspases induce activation of effector caspases such as caspase-3, caspase-6, and caspase-7 resulting in the cleavage of substrates and finally cell death (Ozben 2007).
Extrinsic or Death Receptor-Induced Pathway
The extrinsic pathway is stimulated by favorably influencing death signals such as tumor necrosis factor-α (TNF-α) and Fas/CD95 ligand. Macrophages generate TNF-α, while Fas ligands were released by cytotoxic T lymphocytes and natural killer cells. Fas ligand and TNF-α are trimeric proteins present in the surface of a cell that attach to the death receptors of another cell surface. These ligands can bring three receptors together and then bind to cytosolic proteins Fas-associated death domain protein known as FADD and TNF receptor-associated death domain protein known as TRADD from the inner cell and form an oligomeric complex. FADD activate caspase-8 which further activate other effector caspases such as caspase-3, caspase-6, and caspase-7. Caspase-8 also connect to the intrinsic pathway of apoptosis by cleaving Bid, bh3 interacting domain death agonist into fragment, namely, tBiD, which bind to Bcl2/Bcl-xL proteins resulting in the release of Bad or Bax leads to the formation of pores on the mitochondrial membrane, which will release cytochrome C and continues to the intrinsic pathway (Matés et al. 2012).
A wide range of proteins participate during apoptosis with significant function. X-linked inhibitors of apoptotic proteins (XIAPs) typically inhibit apoptotic pathways by binding to caspase-3 and caspase-7 or caspase-9. SMAC/DIABLO are proteins released from mitochondria through the pores created by Bak or Bax and bind to XIAPs, preventing them from attaching to caspases and inhibiting apoptotic pathways (Lodish et al. 2004).
Dietary Flavonoids with Pro-apoptotic Effect Against Experimental Cancers
As a preventive approach against carcinogenesis, cell proliferation should be inhibited and apoptosis should be induced. Apoptosis mediates a physiological mechanism, thereby facilitating to eliminate cancerous cells. Dietary flavonoids are shown to exhibit many biological activities especially pro-apoptotic action which is employed in cancer treatments; however, detailed exploration of signaling pathways are yet to be discovered. The pro-apoptotic effects of major dietary flavonoids mediated by increased ROS production in experimental cancers are listed in Table 1.
Quercetin
Quercetin (3, 3′, 4′, 5, 7-pentahydroxyflavone) is a dietary flavonoid commonly found in apples, onions, grapes, berries, cherry, citrus fruits, etc. Quercetin is the most abundant flavonoid present in the human diet (Erlund 2004) and well reported for its antioxidant and pro-apoptotic activity (Lee et al. 2003). In a study conducted by Yi et al. (2014), quercetin induced apoptosis of ovarian cancer cells (SKOV-3, OVCAR-3, TOV-21G, and HOSE) via ROS-mediated ER stress (Yi et al. 2014). In HL-60 cells, quercetin treatment increased intracellular oxidative stress and potentiated apoptosis through activation of ERK-mediated pathway (Lee et al. 2015). In another study, quercetin glycoside (hyperoside) treatment to breast cancer cells (MCF-7) inhibited NF-κB signaling and exhibited ROS-related apoptosis (Qiu et al. 2019). Quercetin treatment in combination with paclitaxel to prostate cancer cells (PC-3) increased ROS generation and induced apoptosis and ER stress (Zhang et al. 2020).
Morin
Morin (2′, 3, 4′, 5, 7-pentahydroxyflavone) is a dietary flavonoid majorly present in guava leaves (Psidium guajava), old fustic (Maclura tinctoria), Osage orange (Maclura pomifera), etc. (MadanKumar et al. 2014). Morin possesses numerous pharmacological activities such as antioxidant, antimicrobial, antibacterial, antiviral, hepatoprotective, neuroprotective, cardioprotective, antifibrotic, anti-cancer, etc. (MadanKumar et al. 2015; Perumal et al. 2017a). The molecular mechanism behind the anti-cancer potential of morin was studied in several cancer cells such as liver (HepG2), cervical (HeLa), breast (MCF-7), colon (HCT-116), etc. In human leukemic cells (U937), morin-induced cytotoxicity and internucleosomal cleavage associated with apoptosis (Park et al. 2015). Morin treatment to HepG2 cells induced nucleosomal DNA fragmentation and promoted apoptosis via Mst1-JNK-caspase-mediated mechanism (Perumal et al. 2017b). In a study conducted by Zhang et al. (2018), morin treatment led to ROS generation, G2/M cell cycle arrest, and induced apoptosis to HeLa cells (Zhang et al. 2018). In another study, morin suppressed miR-188-5p and thereby induced apoptosis of chronic myeloid leukemia cells (Nie et al. 2019). In colorectal cancer cells (SW480), morin treatment-induced apoptosis was associated with ROS generation and loss of mitochondrial membrane potential (mmp) (Sithara et al. 2017).
Epigallocatechin-3-Gallate
Tea (Camellia sinensis) is the most widely consumed beverage in the world and composed of many bioactive components, such as polyphenols and alkaloids. Major polyphenols found in tea are epigallocatechin-3-gallate (EGCG), epigallocatechin, epicatechin, etc. Green tea polyphenols, including EGCG, are reported for anti-cancer potential mainly due to its antioxidant, antiproliferative, anti-angiogenic, anti-metastatic, and pro-apoptotic activities (Lambert and Elias 2010). A study by Manohar et al. (2013) demonstrated the anti-cancer effect of EGCG in human endometrial cancer cells, where EGCG treatment potentiated apoptosis via increased ROS and activation of p38 MAPK (Manohar et al. 2013). Combinational treatment of EGCG and metformin to non-small cell lung cancer cells (NSCLC) increased ROS production, suppressed Nrf2/HO-1 signaling, and induced apoptosis (Yu et al. 2017). EGCG treatment to HepG2 cells induced oxidative stress via increased ROS and mitochondrial-mediated apoptosis (Khiewkamrop et al. 2018). Velavan et al. (2018) compared the cellular and molecular effects of EGCG and nano-EGCG in lung cancer cells (A549). Both EGCG and nano-EGCG induced cytotoxicity of A549 cells and increased ROS levels leading to loss of mmp and apoptosis (Velavan et al. 2018). In NSCLC, EGCG treatment exerted anti-cancer effect mediated by increased ROS production and downregulation of ERK1/2 expression (Chen et al. 2020).
Genistein
Genistein (5, 7, 4-trihydroxyisoflavone) is an isoflavonoid commonly found in soybeans and legumes. In a study conducted by Salti et al. (2000), genistein treatment to colon cancer cells (HT-29, SW-620, and SW-116) induced G2/M phase arrest, apoptosis, and topoisomerase II-mediated DNA cleavage (Salti et al. 2000). Genistein (100μM) treatment reduced cell proliferation and induced apoptosis mediated by decreased survivin expression in MCF-7 cells (Prietsch et al. 2014). In pancreatic cells (Mia-PaCa2 and PANC-1), genistein treatment increased ROS production and reduced mmp, thereby inducing apoptosis (Bi et al. 2018). Genistein treatment suppressed phosphorylation of PI3K/Akt/NF-κB and improved the effect of centchroman in breast cancer cells by increasing ROS levels and potentiating apoptosis (Kaushik et al. 2019). Recently, Hsieh et al. (2020) studied the anti-cancer effect of genistein in head and neck cancer cells and found genistein treatment induced oxidative stress and apoptosis of tumor cells via the upregulation of miR-34a (Hsieh et al. 2020).
Daidzein
Daidzein (7, 4’-dihydroxyisoflavone) is an isoflavone commonly found in soybeans and legumes. Genistein and daidzein are structurally similar; the only difference is the hydroxyl group in the fifth position of genistein. Daidzein reported for various pharmacological activities such as antidiabetic, antifibrotic, antiproliferative, anti-cancer, etc. Daidzein treatment induced apoptosis in MCF-7 cells by the intrinsic mitochondrial pathway of cell death. Daidzein treatment caused ROS generation leading to the destruction of mmp, upregulation of Bax, and downregulation of Bcl-2 resulting in the release of cytochrome C, thereby activating caspases and finally apoptosis (Jin et al. 2010). A similar effect was also observed in hepatic cancer cells (SK-HEP-1) upon daidzein treatment, where daidzein induced apoptosis via the upregulation of Bak, downregulation of Bcl-2 and Bcl-xL proteins releasing cytochrome C, and activation of caspases (Park et al. 2013). Daidzein, along with genistein, induced apoptosis in HT-29 colon cancer cells by inhibiting the accumulation of lipid droplets. Daidzein decreased the expression of PI3K and increased the expression of FOXO3a and caspase-8 (Liang et al. 2018). Recently, Kumar and Chauhan (2021) showed daidzein (50μm and 100μm) treatment to MCF-7 cells resulted in ROS-mediated apoptosis and inhibition of cell growth (Kumar and Chauhan 2021).
Apigenin
Apigenin (5, 7, 4-trihydroxyflavone) is a dietary flavone present in spinach, garlic, celery, vine, carrot, etc. The cytotoxic effect of apigenin against hematological cancers was studied using primary effusion lymphoma (PEL) and found that apigenin activated p53, induced catalase, and inhibited STAT3 leading to apoptosis (Granato et al. 2017). Souza et al. (2017) demonstrated the anti-cancer effect of apigenin against human cervical cancer cells, where apigenin treatment affected the cell viability and induced mitochondrial redox impairment and apoptosis of cervical cancer cells (Souza et al. 2017). In another study, the anti-cancer effect of apigenin was studied using two human malignant mesothelioma cell lines (MSTO-211H and H-2452). Treatment of apigenin exerted a cytotoxicity in MSTO-211H and H-2452 cells mediated by ROS-dependent necroptotic cell death (Lee et al. 2020). The pro-apoptotic effect of apigenin was also studied using glioblastoma cells (U87) and found that apigenin treatment potentiated apoptosis via increased ROS generation (Shendge et al. 2021). Recently, Adham et al. (2021) studied the anti-cancer and pro-apoptotic potentials of apigenin against multiple myeloma cells. In this study, apigenin treatment inhibited cell growth and induced apoptosis and cell cycle arrest in NCI-H929 cells (Adham et al. 2021). A combinational treatment of metformin and apigenin to pancreatic cancer cells (AsPC-1) increased ROS levels and thereby decreased MP and potentiated apoptosis. Also, metformin and apigenin markedly reduced the tumor size in AsPC-1-xenografted nude mice when orally administered (Warkad et al. 2021).
Luteolin
Luteolin (3’, 4’, 5, 7-tetrahydroxyflavone) naturally occurs in various fruits and vegetables such as celery, carrot, cabbage, thyme, and green peppers with potent antioxidant, antimicrobial, anti-inflammatory, anti-cancer, and neuroprotective properties. In rat hepatoma cells (H4IIE), luteolin exhibited cytotoxicity by induction of apoptosis. Luteolin treatment caused nuclear fragmentation and activation of caspases, thereby triggering mitochondrial pathways of apoptosis (Michels et al. 2005). In another study, luteolin reduced the cell viability of oral squamous cancer cells and downregulated expression of anti-apoptotic proteins, promoted expression of caspases, and increased level of cleaved poly-ADP-ribose polymerase (Yang et al. 2008). Administration of luteolin (1.2 mg/kg body weight/day) to azoxymethane-induced colorectal cancer bearing rats exhibited anti-cancer effects by modulation of GST-α, μ, and Nrf2 expression (Pandurangan et al. 2014). In human glioblastoma cells (U251MG and U87MG), the underlying mechanism of pro-apoptotic effect of luteolin was investigated. Treatment of luteolin increased intracellular ROS and induced apoptosis mediated by activation of ER stress (Wang et al. 2017). Seydi et al. (2018) studied the anti-cancer property of luteolin in diethylnitrosamine-induced hepatocellular carcinoma (HCC) in a rat model. Luteolin treatment resulted in ROS production, thereby inducing mmp loss and apoptosis in the mitochondria from hepatocytes of HCC group (Seydi et al. 2018).
Myricetin
Myricetin (3, 5, 7-trihydroxy-2-(3, 4, 5-trihydroxyphenyl) chromen-4-one) is a dietary flavonoid found majorly in berries, fruits, nuts, vegetables, tea, and wine. Myricetin inhibited the Akt activity, downregulated phosphorylation of Bad protein, and induced loss in mmp and apoptosis in UV-B irradiated skin cancer cells (HaCaT) (Kim et al. 2010). In human colon cancer cells (HCT-15), myricetin caused cytotoxicity and DNA condensation in a dose-dependent manner and promoted apoptosis-inducing mitochondrial factors (Kim et al. 2014). Yang et al. (2017) tested the effect of myricetin in two choriocarcinoma cell lines (JAR and JEG-3) and found that myricetin treatment potentiated apoptosis with enhanced ROS production, glutathione depletion, and loss of mmp (Yang et al. 2017). Treatment of myricetin to triple-negative breast cancer cells exhibited intracellular ROS accumulation, double-strand DNA breaks, ERK1/2 and p38 MAPK activation, mitochondrial membrane destabilization, and apoptosis (Knickle et al. 2018). In another study, myricetin treatment to canine osteosarcoma cells (D-17 and DSN) induced ROS generation, lipid peroxidation, and depolarization of mmp (Park et al. 2018). Recently, Rajendran et al. (2021) demonstrated the anti-cancer effect of myricetin in A549 cells. Myricetin (73μg/ml) treatment prevented A549 cell growth by inducing ROS-mediated apoptosis (Rajendran et al. 2021).
Hesperidin
Hesperidin is a bioflavanone glycoside found majorly in citrus fruits. Hesperidin was shown to possess many pharmacological properties such as antioxidant, antiviral, anti-inflammatory, antiproliferative, anti-cancer, etc. Hesperidin treatment induced apoptosis in HepG2 cells through mitochondrial and death receptor pathways in a dose-dependent manner. Hesperidin-treated cells showed loss of mmp and increased activity of caspases and expression of pro-apoptotic proteins (Banjerdpongchai et al. 2016). A study by Pandey et al. (2019) described the anti-cancer effect of hesperidin using human gall bladder carcinoma cells. Hesperidin treatment increased ROS production and led to caspase-3 activation and G2/M phase cell cycle arrest in a dose-dependent manner (Pandey et al. 2019). In prostate cancer cells (DU145), hesperidin treatment inhibited the cell growth and triggered apoptosis via ROS pathway (Ning et al. 2020). In a recent study performed with HeLa cells, hesperidin downregulated Jab1 and upregulated p27, thereby potentiating apoptosis via increased ROS production (Pandey et al. 2021).
Conclusion
Dietary flavonoids are potent anticarcinogenic bioactive principles that inhibit cancer development by interfering with cell proliferation, apoptosis, and metastasis. Dietary flavonoids have come out as a safe and effective alternative for cancer treatment by inducing apoptosis. Flavonoids possess various characteristics contributing to their pro-apoptotic effect, such as upregulation of caspases, release of cytochrome C, induction of cell cycle arrest, regulation of Bcl-2 family proteins, suppression of survival signals, etc. To replace anti-cancer drugs with dietary flavonoids, we have to explore more about the mechanism of their anti-cancer activity. Combination therapy of flavonoids with present anti-cancer drugs could significantly reduce the concentration of drugs administered, improve bioavailability, extend resistance to medication, etc.

Scheme representation of the mechanism of dietary flavonoid intervention in extrinsic and intrinsic apoptosis pathways
References
Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, Liskova A, Büsselberg D (2019) Flavonoids in cancer and apoptosis. Cancers (Basel). https://doi.org/10.3390/cancers11010028
Adham AN, Abdelfatah S, Naqishbandi AM, Mahmoud N, Efferth T (2021) Cytotoxicity of apigenin toward multiple myeloma cell lines and suppression of iNOS and COX-2 expression in STAT1-transfected HEK293 cells. Phytomedicine 80:153371
Arnér ESJ, Holmgren A (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267:6102–6109
Banjerdpongchai R, Wudtiwai B, Khaw-on P, Rachakhom W, Duangnil N, Kongtawelert P (2016) Hesperidin from Citrus seed induces human hepatocellular carcinoma HepG2 cell apoptosis via both mitochondrial and death receptor pathways. Tumor Biol 37:227–237
Bi YL, Min M, Shen W, Liu Y (2018 Jan 15) Genistein induced anticancer effects on pancreatic cancer cell lines involves mitochondrial apoptosis, G0/G1cell cycle arrest and regulation of STAT3 signalling pathway. Phytomedicine 39:10–16
Cahill CM, Rogers JT, Walker WA (2012) The role of phosphoinositide 3-Kinase signaling in intestinal inflammation. J Signal Transduct 2012:1–13
Chen A, Jiang P, Zeb F, Wu X, Xu C, Chen L, Feng Q (2020) EGCG regulates CTR1 expression through its pro-oxidative property in non-small-cell lung cancer cells. J Cell Physiol 235(11):7970–7981
Erlund I (2004) Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr Res 24:851–874
Granato M, Gilardini Montani MS, Santarelli R, D’Orazi G, Faggioni A, Cirone M (2017) Apigenin, by activating p53 and inhibiting STAT3, modulates the balance between pro-apoptotic and pro-survival pathways to induce PEL cell death. J Exp Clin Cancer Res 36:1–9
Hsieh PL, Liao YW, Hsieh CW, Chen PN, Yu CC (2020) Soy Isoflavone Genistein impedes cancer Stemness and Mesenchymal transition in head and neck cancer through activating miR-34a/RTCB Axis. Nutrients 12(7):1924
Ibrahim MH, Jaafar HZE, Karimi E, Ghasemzadeh A (2012) Primary, secondary metabolites, photosynthetic capacity and anti-oxidant activity of the Malaysian Herb Kacip Fatimah (Labisia pumila Benth) exposed to potassium fertilization under greenhouse conditions. Int J Mol Sci 13:15321–15342
Jin S, Zhang QY, Kang XM, Wang JX, Zhao WH (2010) Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann Oncol 21:263–268
Jones DP (2008) Radical-free biology of oxidative stress. Am J Phys Cell Physiol 295:C849
Kaushik S, Shyam H, Agarwal S, Sharma R, Nag TC, Dwivedi AK, Balapure AK (2019) Genistein potentiates Centchroman induced antineoplasticity in breast cancer via PI3K/Akt deactivation and ROS dependent induction of apoptosis. Life Sci 239:117073
Khiewkamrop P, Phunsomboon P, Richert L, Pekthong D, Srisawang P (2018) Epistructured catechins, EGCG and EC facilitate apoptosis induction through targeting de novo lipogenesis pathway in HepG2 cells. Cancer Cell Int 18:46
Kim W, Yang JH, Youn HS, Yun YJ, Seong KM, Youn B (2010) Myricetin inhibits Akt survival signaling and induces bad-mediated apoptosis in a low dose ultraviolet (UV)-B-irradiated hacat human immortalized keratinocytes. J Radiat Res 51:285–296
Kim ME, Ha TK, Yoon JH, Lee JS (2014) Myricetin induces cell death of human colon cancer cells via BAX/BCL2-dependent pathway. Anticancer Res 34:701–706
Knickle A, Fernando W, Greenshields AL, Rupasinghe HPV, Hoskin DW (2018) Myricetin-induced apoptosis of triple-negative breast cancer cells is mediated by the iron-dependent generation of reactive oxygen species from hydrogen peroxide. Food Chem Toxicol 118:154–167
Kumar V, Chauhan SS (2021) Daidzein induces intrinsic pathway of apoptosis along with ER α/β ratio alteration and ROS production. Asian Pac J Cancer Prev 22(2):603–610
Lambert JD, Elias RJ (2010) The anti-oxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys 501:65–72
Lee JC, Kim J, Park JK, Chung GH, Jang YS (2003) The anti-oxidant, rather than pro-oxidant, activities of quercetin on normal cells: quercetin protects mouse thymocytes from glucose oxidase-mediated apoptosis. Exp Cell Res 291:386–397
Lee WJ, Hsiao M, Chang JL et al (2015) Quercetin induces mitochondrial-derived apoptosis via reactive oxygen species-mediated ERK activation in HL-60 leukemia cells and xenograft. Arch Toxicol 89:1103–1117
Lee YJ, Park KS, Nam HS, Cho MK, Lee SH (2020) Apigenin causes necroptosis by inducing ROS accumulation, mitochondrial dysfunction, and ATP depletion in malignant mesothelioma cells. Korean J Physiol Pharmacol 24(6):493–502
Liang YS, Qi WT, Guo W, Wang CL, Bin HZ, Li AK (2018) Genistein and daidzein induce apoptosis of colon cancer cells by inhibiting the accumulation of lipid droplets. Food Nutr Res 62:1–9
Liu J, Wang X, Yong H, Kan J, Jin C (2018) Recent advances in flavonoid-grafted polysaccharides: synthesis, structural characterization, bioactivities and potential applications. Int J Biol Macromol 116:1011–1025
Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipursky L, Darnell J (2004) Molecular cell biology, 5th edn. W.H. Freeman and company, New York
MadanKumar P, NaveenKumar P, Manikandan S, Devaraj H, NiranjaliDevaraj S (2014) Morin ameliorates chemically induced liver fibrosis in vivo and inhibits stellate cell proliferation in vitro by suppressing Wnt/β-catenin signaling. Toxicol Appl Pharmacol 277(2):210–220
MadanKumar P, NaveenKumar P, Devaraj H, NiranjaliDevaraj S (2015) Morin, a dietary flavonoid, exhibits anti-fibrotic effect and induces apoptosis of activated hepatic stellate cells by suppressing canonical NF-κB signaling. Biochimie 110:107–118
Manohar M, Fatima I, Saxena R, Chandra V, Sankhwar PL, Dwivedi A (2013) (-)-Epigallocatechin-3-gallate induces apoptosis in human endometrial adenocarcinoma cells via ROS generation and p38 MAP kinase activation. J Nutr Biochem 24(6):940–947
Matés JM, Segura JA, Alonso FJ, Márquez J (2012) Oxidative stress in apoptosis and cancer: an update. Arch Toxicol 86:1649–1665
Michels G, Wätjen W, Niering P, Steffan B, Thi QHT, Chovolou Y, Kampkötter A, Bast A, Proksch P, Kahl R (2005) Pro-apoptotic effects of the flavonoid luteolin in rat H4IIE cells. Toxicology 206:337–348
Nabavi SM, Šamec D, Tomczyk M et al (2020) Flavonoid biosynthetic pathways in plants: versatile targets for metabolic engineering. Biotechnol Adv 38:107316
Nie ZY, Yang L, Liu XJ, Yang Z, Yang GS, Zhou J, Qin Y, Yu J, Jiang LL, Wen JK, Luo JM (2019) Morin inhibits proliferation and induces apoptosis by modulating the miR-188-5p/PTEN/AKT regulatory pathway in CML cells. Mol Cancer Ther 18(12):2296–2307
Ning L, Zhao W, Gao H, Wu Y (2020) Hesperidin induces anticancer effects on human prostate cancer cells via ROS-mediated necrosis like cell death. J BUON 25(6):2629–2634
Ozben T (2007) Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci 96:2181–2196
Pandey P, Sayyed U, Tiwari RK, Siddiqui MH, Pathak N, Bajpai P (2019) Hesperidin induces ROS-mediated apoptosis along with cell cycle arrest at G2/M phase in human gall bladder carcinoma. Nutr Cancer 71(4):676–687
Pandey P, Khan F, Maurya P (2021) Targeting Jab1 using hesperidin (dietary phytocompound) for inducing apoptosis in HeLa cervical cancer cells. J Food Biochem 45(7):e13800
Pandurangan AK, Ananda Sadagopan SK, Dharmalingam P, Ganapasam S (2014) Luteolin, a bioflavonoid inhibits Azoxymethane-induced colorectal cancer through activation of Nrf2 signaling. Toxicol Mech Methods 24(1):13–20
Park HJ, Jeon YK, You DH, Nam MJ (2013) Daidzein causes cytochrome c-mediated apoptosis via the Bcl-2 family in human hepatic cancer cells. Food Chem Toxicol 60:542–549
Park C, Lee WS, Il GS et al (2015) Morin, a flavonoid from moraceae, induces apoptosis by induction of bad protein in human leukemic cells. Int J Mol Sci 16:645–659
Park H, Park S, Bazer FW, Lim W, Song G (2018) Myricetin treatment induces apoptosis in canine osteosarcoma cells by inducing DNA fragmentation, disrupting redox homeostasis, and mediating loss of mitochondrial membrane potential. J Cell Physiol 233(9):7457–7466
Perumal N, Perumal M, Halagowder D, Sivasithamparam N (2017a) Morin attenuates diethylnitrosamine-induced rat liver fibrosis and hepatic stellate cell activation by co-ordinated regulation of Hippo/Yap and TGF-β1/Smad signaling. Biochimie 140:10–19
Perumal N, Perumal M, Kannan A, Subramani K, Halagowder D, Sivasithamparam N (2017b) Morin impedes Yap nuclear translocation and fosters apoptosis through suppression of Wnt/β-catenin and NF-κB signaling in Mst1 overexpressed HepG2 cells. Exp Cell Res 355(2):124–141
Pizzino G, Irrera N, Cucinotta M et al (2017) Oxidative stress: harms and benefits for human health. Oxidative Med Cell Longev 2017:8416763
Prietsch RF, Monte LG, Da Silva FA et al (2014) Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of pro-apoptotic factors and oxidative stress enzymes. Mol Cell Biochem 390:235–242
Qiu J, Zhang T, Zhu X, Yang C, Wang Y, Zhou N, Ju B, Zhou T, Deng G, Qiu C (2019) Hyperoside induces breast cancer cells apoptosis via ROS-mediated NF-κB signaling pathway. Int J Mol Sci 21(1):131
Rajendran P, Maheshwari U, Muthukrishnan A, Muthuswamy R, Anand K, Ravindran B, Dhanaraj P, Balamuralikrishnan B, Chang SW, Chung WJ (2021) Myricetin: versatile plant based flavonoid for cancer treatment by inducing cell cycle arrest and ROS-reliant mitochondria-facilitated apoptosis in A549 lung cancer cells and in silico prediction. Mol Cell Biochem 476(1):57–68
Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, Mena P, Daniele, Rio D, Crozier A (2014) Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. https://doi.org/10.1007/s00204-014-1330-7
Salti GI, Grewal S, Mehta RR, Das Gupta TK, Boddie AW, Constantinou AI (2000) Genistein induces apoptosis and topoisomerase II-mediated DNA breakage in colon cancer cells. Eur J Cancer 36:796–802
Seydi E, Salimi A, Rasekh HR, Mohsenifar Z, Pourahmad J (2018) Selective cytotoxicity of Luteolin and Kaempferol on cancerous hepatocytes obtained from rat model of hepatocellular carcinoma: involvement of ROS-mediated mitochondrial targeting. Nutr Cancer 70(4):594–604
Shendge AK, Chaudhuri D, Mandal N (2021) The natural flavones, acacetin and apigenin, induce Cdk-Cyclin mediated G2/M phase arrest and trigger ROS-mediated apoptosis in glioblastoma cells. Mol Biol Rep 48(1):539–549
Sithara T, Arun KB, Syama HP, Reshmitha TR, Nisha P (2017) Morin inhibits proliferation of SW480 colorectal cancer cells by inducing apoptosis mediated by reactive oxygen species formation and uncoupling of warburg effect. Front Pharmacol 8:1–16
Souza RP, Bonfim-Mendonça PS, Gimenes F, Ratti BA, Kaplum V, Bruschi ML, Nakamura CV, Silva SO, Maria-Engler SS, Consolaro ME (2017) Oxidative stress triggered by Apigenin induces apoptosis in a comprehensive panel of human cervical cancer-derived cell lines. Oxid Med Cell Longev 2017:1512745
Thomas C, Mackey MM, Diaz AA, Cox DP (2009) Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: implications for diseases associated with iron accumulation. Redox Rep 14:102–108
Velavan B, Divya T, Sureshkumar A, Sudhandiran G (2018) Nano-chemotherapeutic efficacy of (-) -epigallocatechin 3-gallate mediating apoptosis in A549 cells: involvement of reactive oxygen species mediated Nrf2/Keap1signaling. Biochem Biophys Res Commun 503(3):1723–1731
Wang Q, Wang H, Jia Y, Pan H, Ding H (2017) Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemother Pharmacol 79(5):1031–1041
Warkad MS, Kim CH, Kang BG, Park SH, Jung JS, Feng JH, Inci G, Kim SC, Suh HW, Lim SS, Lee JY (2021) Metformin-induced ROS upregulation as amplified by apigenin causes profound anticancer activity while sparing normal cells. Sci Rep 11(1):14002
Wei Z, Liu HT (2002) MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 12:9–18
Yang SF, Yang WE, Chang HR, Chu SC, Hsieh YS (2008) Luteolin induces apoptosis in oral squamous cancer cells. J Dent Res 87:401–406
Yang C, Lim W, Bazer FW, Song G (2017) Myricetin suppresses invasion and promotes cell death in human placental choriocarcinoma cells through induction of oxidative stress. Cancer Lett 399:10–19
Yi L, Zongyuan Y, Cheng G, Lingyun Z, Guilian Y, Wei G (2014) Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Sci 105(5):520–527
Yu C, Jiao Y, Xue J, Zhang Q, Yang H, Xing L, Chen G, Wu J, Zhang S, Zhu W, Cao J (2017) Metformin sensitizes non-small cell lung cancer cells to an Epigallocatechin-3-Gallate (EGCG) treatment by suppressing the Nrf2/HO-1 signaling pathway. Int J Biol Sci 13(12):1560–1569
Zhang Q, Zhang F, Thakur K, Wang J, Wang H, Hu F, Zhang JG, Wei ZJ (2018) Molecular mechanism of anti-cancerous potential of Morin extracted from mulberry in Hela cells. Food Chem Toxicol 112:466–475
Zhang X, Huang J, Yu C et al (2020) Quercetin enhanced paclitaxel therapeutic effects towards PC-3 prostate cancer through ER stress induction and ROS production. Onco Targets Ther 13:513–523
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
Vijay, V., Vijayan, N., Venkatiesh, V.P., Vallikannan, B., Perumal, M.K. (2022). Pro-apoptotic Effects of Dietary Flavonoids in Oxidative Stress-Induced Cancer. In: Chakraborti, S. (eds) Handbook of Oxidative Stress in Cancer: Therapeutic Aspects. Springer, Singapore. https://doi.org/10.1007/978-981-16-5422-0_151
Download citation
DOI: https://doi.org/10.1007/978-981-16-5422-0_151
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5421-3
Online ISBN: 978-981-16-5422-0
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences