Abstract
Brain and CNS-related cancers are rare; however, 0.3 million incidences and 0.24 million deaths in 2018 demonstrates the unrelenting associated dangers. Glioblastoma is a brain cancer of star-shaped glial cells. It is almost universally fatal within 2 years of diagnosis despite maximal medical therapies. This study aims to evaluate the in-depth anticancer activity of acacetin and apigenin on glioblastoma cells (U87). In the present report, we have isolated two flavonoids, acacetin and apigenin; and studied the in-depth anticancer activity on U87 cells. Selective cytotoxicity of acacetin and apigenin was observed towards the U87 cells (IC50: 43.73 ± 1.19 and 48.18 ± 1.37 μM, respectively). The flow cytometer-based result revealed the induction of G2/M phase arrest along with the increase in sub G1 population upon compound treatment. Annexin-V-FLUOS and DAPI staining also confirmed the apoptosis-inducing effects of compounds. Flow cytometer and confocal microscopy-based DCFH-DA staining showed ROS-inducing effect of the compounds. The up-regulation of p21 and down-regulation of Cyclin-A1, Cyclin-B1, and Cdk-1 revealed the G2/M phase arrest mechanism of acacetin and apigenin. Furthermore, western blotting result confirmed the activation of intrinsic pathway of apoptosis upon acacetin treatment and activation of both extrinsic and intrinsic pathways of apoptosis upon apigenin treatment through the regulation of Bax, t-Bid, caspase 8, caspase 9, caspase 3, and PARP. The obtained result showed a significant effect (P < 0.05) of acacetin and apigenin on U87 cells. Acacetin and apigenin-induced ROS is responsible for the induction of cell cycle arrest and activation of caspase-cascade pathways in U87 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abnormal cellular behavior and uncontrolled proliferation of cells are the fundamental characteristics of cancer. Almost every part of the human body is vulnerable to cancer development, and cancer is the second leading cause of death globally. In 2018 alone, nearly 18.1 million cases were diagnosed, and 9.6 million deaths were estimated. The brain and CNS-related cancers are rare; nearly 0.3 million cases were reported worldwide only in 2018 [1]. While the incidences of brain and other nervous system cancers are relatively less compared to other cancers, the high mortality rate makes such cancers more dangerous. For example, breast cancer, the most common type of cancer, has nearly 55.2% of crude incidences rate and 16.6% of crude mortality rate. In contrast, brain and CNS-related cancers have a 3.5% of crude incidence rate and 2.8% of crude mortality rate. This translates to almost 0.24 million people losing their lives from the 0.3 million brain and CNS-related cancer cases reported in 2018. In the USA, almost 23-thousand new cases were found, with 18-thousand deaths only in the year 2020. Brain and CNS-related cancer represent only 1.3% of all new cancer cases in the USA; however, it represents 3.0% of all cancer deaths [2].
The most common histological type of primary CNS cancer is glioma, a group of malignant brain tumors. Glioblastoma, a brain cancer of glial origin, is almost universally fatal within 2 years of diagnosis despite maximal surgical and medical therapy [3]. The primary cause of most adult brain and CNS related cancer is not known. However, certain genetic syndromes may increase the risk of a central nervous system tumor [4]. Although many drugs have been approved to treat the brain and CNS-related cancers, the 5-year survival rate for people with a brain or CNS tumor is almost 36%. Natural resources have a long history as drugs to treat various detrimental diseases, and they continue to be an inspiration source for drug discovery.
Natural resources include a diverse group of substances from numerous plants, fungi, algae, bacteria, insects, and marine organisms. Among the substances, flavonoids are secondary metabolites that play a significant role in color and aroma formation in the flowers. Many flavonoids have been previously reported for their numerous biological activities [5, 6]. The genus Clerodendrum is widely distributed throughout the tropical and subtropical regions of the world [7]. Several species of Clerodendrum have been used in traditional medicine [8, 9]. Some of them have been studied for antioxidants, iron chelation, anticancer, and anti-inflammatory potentials [10,11,12]. Therefore, this study aims to evaluate in-depth anticancer activity of acacetin and apigenin on a glioblastoma cell line (U87).
Materials and methods
Chemicals
Dulbecco’s Modified Eagle’s Medium (DMEM), antibiotics, and sodium bicarbonate were provided by HiMedia Laboratories Pvt. Ltd., Mumbai, India. HyClone Laboratories, Inc., Utah, USA, provided Fetal bovine serum (FBS). Roche Diagnostics (Mannheim, Germany) provided cell proliferation reagent (WST-1), Annexin-V-FLUOS staining kit, and polyvinyl difluoride (PVDF) membrane. Triton X-100, RNAase A, and 4′,6-diamidino-2-phenylindole (DAPI) were provided by MP Biomedicals, France. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) was purchased from Alexis Biochemicals, San Diego, USA. Anti-Cdk-1, anti-Cyclin A1, and anti-Cyclin B1 antibodies were provided by Bioss Antibodies Inc., Massachusetts, USA. The anti-Bcl-2 antibody was purchased from AnaSpec, Inc., USA. Anti-p21 (WAF1, Cip1) antibody was purchased from eBioscience San Diego, USA. Anti-Bax, anti-caspase 3, anti-caspase 9, anti-PARP, and anti-β-actin antibodies were purchased from OriGene Technologies, Inc., Rockville, USA. The Cell Signalling Technology Inc. (Danvers, Massachusetts, USA) provided the alkaline phosphatase-conjugated anti-rabbit secondary antibody. All the other used reagents and chemicals were purchased from reputed companies.
Sample collection
Clerodendrum viscosum leaves were collected from the Darjeeling and Siliguri region of West Bengal, India. The taxonomist, Prof. A. P. Das authenticated the collected sample, and the same has been stored at the Herbarium of Botany Department, University of North Bengal, India, with accession number 9617.
Isolation and structure elucidation of compounds
The C. viscosum leaves were collected and prepared the 70% methanolic extract and named as CVLME (C. viscosum leaves methanol extract). CVLME was further fractionated with various solvents according to the previously reported protocol [13]. The 31 g of collected CVLE (ethyl acetate fraction of C. viscosum) was then subjected to silica gel column chromatography and then eluted with increasing hexane: ethyl acetate solvent using a step gradient of 10% (100:0–0:100) and obtained 11 sub-fractions (EAF1–11). The detailed experimental design is provided in Fig. S1.
Isolation and structure elucidation of CE1
The collection of ethyl acetate sub-fraction 5 (EAF5) was obtained after applying 10 l of 25% ethyl acetate in hexane. EAF5 was further concentrated using a rotary evaporator at 37 °C. The concentrated sample itself gets crystallized in a round bottom flask. The sample was dissolved in 5 ml of 20% methanol in DCM at 40 °C and recrystallized it. The isolated crystals were stored at 4 °C by naming CE1. The CE1 was used for structure elucidation by using LC-MS, IR spectra, and finally, NMR. LC-MS spectra indicated the parent mass (m/z)-283.1, Melting Point—260–265 °C. IR spectra showed the characteristic peaks of the broad hydroxyl group at 3429, 3021 (Strong), methoxy group at 2960, 2927, ester carbonyl group at 1723, aromatic unsaturation at 1645 cm-1 (Fig. S2). Finally, the NMR technique 1H 1D NMR, 13C 1D NMR, and DEPT-135 1D NMR were used to elucidate the compound’s structure (Fig. S3). The structure of CE1 was confirmed to be an acacetin. The structure of acacetin is shown in Fig. 1a.
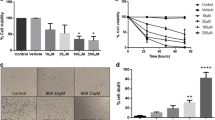
Structure and cytotoxic potential of isolated compounds from C. viscosum leaves. (a) Structure of acacetin, (b) structure of apigenin, (c) cytotoxic effect of acacetin on U87 and WI-38 cells, (d) cytotoxic effect of apigenin on U87 and WI-38 cells. The results were expressed as cell viability (% of control). All data were expressed as mean ± SD (n = 6). *p ˂ 0.05, **p ˂ 0.01 and ***p ˂ 0.001 vs. 0 μM
NMR data
1H NMR (500 MHz, DMSO d6)-δ: 3.85 (1H, s, C4′-OCH3), 6.205 (1H, d, j = 2.5, Ar-2′H), 6.506 (1H, d, j = 2, Ar-3′H, 5′H), 6.83 (1H, s, C3-H), 7.107 (1H, dd, J = 1.5, J = 1.5, C7-OH, C8), 8.016 (1H, dd, J = 1.5, J = 1.5, C5-OH, C6), 12.898 (1H, s, 6′H).
13C NMR (500 MHz, DMSO d6) δ: 55.61 (C-4′-OCH3), 94.14 (C-8), 98.99 (C-6), 103.54 (C-3), 103.79 (C-10), 114.697 (C-3′, C5′), 122.84 (C 1′), 128.39 (C-2′, C-6′), 157.42 (C-9), 161.48 (C-5), 162.39 (C-4′), 163.44 (C-2), 164.32 (C-7), 181.84 (C-4).
Isolation and structure elucidation of CE3
The collected EAF7 was further used for a re-column process followed by HPLC. We have collected one major peak using preparative HPLC at retention time of 32.8 min. The collected sample was further concentrated by using a rotary evaporator and stored at 4 °C by naming CE3. The detailed isolation and structure elucidation process of CE3 was described in a previously published report [14]. The structure of apigenin is showed in Fig. 1b.
In vitro anticancer study
Cell lines
The glioblastoma (U87) and normal lung fibroblast (WI-38) cell lines were purchased from the National Center for Cell Sciences (NCCS) Pune, India. Both the cell lines were supplied with DMEM containing 100 U/ml of penicillin G, 100 μg/ml of streptomycin, 50 μg/ml of gentamycin sulfate, 2.5 μg/ml of amphotericin-B, and 10% FBS. The cell lines were maintained in a 37 °C incubator with a constant supply of 5% CO2.
WST-1 cell viability assay
WST-1 cell proliferating reagent was used to evaluate the cytotoxic potential of acacetin and apigenin on U87 cells [15]. For the assay, 1 × 104 U87 cells were seeded in a 96-well plate and treated with different concentrations of acacetin and apigenin (0–50 μM). After the 24 h of treatment, the cells were washed with PBS (phosphate-buffered saline) and incubated with 10 μl of WST-1 containing media for 2–3 h. The absorbance was recorded at 460 nm on microplate ELISA reader MULTISKAN EX (Thermo Electron Corporation, USA).
Cell cycle phase distribution analysis
To study the cell cycle analysis, the U87 cells were seeded in a 12-well plate and treated with different concentrations of acacetin and apigenin (0–50 μM) for 24 h. After treatment, the cells were pelleted down and washed with PBS. The cells were further dislodged and incubated with 70% methanol for 3 min at −20 °C. After incubation, the cells were fully re-suspended in a 0.5 ml cold PBS solution containing RNase A and incubated at 37 °C for a minimum 1 h. The cells were further stained with propidium iodide (PI) dye, and cell cycle phase distribution was analyzed using a flow cytometer (FACS Verse, Becton Dickinson) [15].
Apoptosis study using Annexin-V-FLUOS staining kit
Apoptotic inducing effect of acacetin and apigenin (0–50 μM) on U87 cells was confirmed through Roche provided annexin-V-FLUOS staining kit following a standard protocol [15]. The acacetin and apigenin-treated U87 cells were analyzed using a flow cytometer (FACS Verse, Becton Dickinson), and the % of the apoptosis population was quantified.
Study the fragmentation of DNA using DAPI staining
The acacetin and apigenin-induced apoptosis were further confirmed through the confocal microscopy using a DAPI staining as described previously [16]. Briefly, the seeded cells were treated with acacetin and apigenin (0–50 μM) for 24 h. The cells were further fixed with 4% paraformaldehyde and incubated the plate in a CO2 incubator for 30 min. After gentle washing, the cells were permeabilized with 0.5% Triton X-100 for 10 min at room temperature. After PBS washing, the cells were stained with DAPI solution (10 μg/ml) and incubated for 30 min in the CO2 incubator. The morphological changes in the drug-treated and untreated nuclei were observed under a laser scanning confocal microscope (Leica TCS SP8, Illinois, United States).
ROS measurement using DCFH-DA staining
The effect of acacetin and apigenin treatment (0–50 μM) on ROS induction in U87 cells was evaluated through the DCFH-DA staining as described previously [17]. 1 × 105 cells were seeded in a 12-well culture plate and treated with acacetin and apigenin for 24 h. Upon incubation, the cells were washed with PBS and incubated with 20 μM DCFH-DA for 30 min. After PBS washes, the intensity of intracellular ROS was measured using a flow cytometer (FACS Verse, Becton Dickinson).
Western blotting analysis
Proteins of acacetin and apigenin-treated (50 μM) and untreated U87 cells were extracted using a cell lysis buffer, and total protein quantification was performed using a Folin Lowry method. Equal amounts of protein from each sample was used to resolve on 12% SDS-PAGE and transferred to PVDF membrane using a transfer buffer. Transferred blots were then blocked with a blocking buffer containing 5% skimmed milk and incubated at 4 °C overnight. After blocking, the blots were incubated with the following primary antibodies (Anti-Cyclin A1, anti-Cyclin B1, anti-Cdk-1, anti-p21, anti-Bax, anti-Bcl-2, anti-caspase 3, anti-caspase 9, anti-PARP, and anti-β-actin) at room temperature. Further, the blots were incubated with secondary antibody (alkaline phosphatase-conjugated anti-rabbit IgG) for 4 h at room temperature [15]. Finally, the blots were developed using the substrate (BCIP/NBT), and images were taken using the EC3 Chemi HR imaging system (UVP, USA). The β-actin was used as a loading control.
Statistical analysis
All the experimental data were reported as the mean ± SD of minimum three measurements. The statistical analysis was performed by using KyPlot (version 2.0 beta 15 (32 bit)). When A1 = IC50, Y = response (Y = 100% when X = 0), X = inhibitory concentration. Y = 100 × A1/(X + A1) formula was used to calculate the IC50 values. The IC50 values were compared by paired t-test and values with P < 0.05 were considered significant.
Results
In vitro anticancer study
Cell viability assay
Cell proliferation was quantified using the WST-1 cell proliferation reagent. It was found that acacetin and apigenin showed a potent cytotoxic effect towards the U87 cells with an IC50: 43.73 ± 1.19 and 48.18 ± 1.37 μM, respectively. Interestingly, none of the compounds showed cytotoxic effect on normal fibroblast cell line WI-38 (IC50: 964.27 ± 92.24 and 912.29 ± 36.40 μM, respectively) (Fig. 1c, d).
Analysis of cell cycle and apoptosis using flow cytometer
Cell cycle phase distribution of the acacetin and apigenin-treated U87 cells was analyzed through a flow cytometer. The results showed an increase in the G2/M phase population in U87 cells upon the treatment of both the compounds. The 18.94% G2/M population was observed in untreated U87 cells, while 30 μM concentration of acacetin-treatment increases the G2/M cell population to 27.18%. The 50 μM concentration of the apigenin showed a 25.31% G2/M cell population in U87 cells, which may be due to the excess death of the cells in high drug concentration. Moreover, acacetin increases the sub-G1 population in U87 cells from 0.54% to 28.32% (Fig. 2a). On the other hand, apigenin also induces G2/M phase cell cycle arrest in U87 cells. It increases G2/M phase population from 19.78% to 27.28% with increase of sub-G1 population from 0.74% to 22.29% (Fig. 3a). The annexin-V-FLUOS staining also confirmed the apoptotic effect of both the compounds on U87 cells. The annexin plot showed a negligible apoptotic population (0.04%) in untreated U87 cells, while the highest dose of acacetin and apigenin-treatment showed 33.18% and 28.00% apoptotic population in the U87 cells, respectively (Figs. 2b and 3b).
Cell cycle and apoptosis analysis of acacetin-treated U87 cells through a flow cytometer. (a) Sub G1, G1, S, and G2/M phases of U87 cells treated with the indicated doses of acacetin for 24 h. The graphical representation indicates the % cell population in different phases, (b) annexin-V-FLUOS and PI staining of acacetin treated U87 cells with increasing doses. Graph shows % of apoptotic population
Cell cycle and apoptosis analysis of apigenin-treated U87 cells through a flow cytometer. (a) Sub G1, G1, S, and G2/M phases of U87 cells treated with the indicated doses of apigenin for 24 h. The graphical representation indicates the % cell population in different phases, (b) annexin-V-FLUOS and PI staining of acacetin treated U87 cells with increasing doses. Graph shows % of apoptotic population
Morphological analysis using a confocal microscopy
Morphological analysis of the cell nuclei was done using a DAPI Staining and observed through the laser scanning confocal microscope. The Fig. 4a, b revealed that untreated U87 cells were found with normal morphology and intact nuclei. In contrast, the acacetin and apigenin-treated (50 μM) U87 cells showed condensed and fragmented nuclei, which are a characteristic of apoptosis.
Apoptosis and ROS inducing effect of acacetin and apigenin on U87 cells. (a) Nuclei of acacetin-treated/untreated U87 cells were stained with DAPI solution and observed under a confocal microscope (b) nuclei of apigenin-treated/untreated U87 cells were stained with DAPI solution and observed under a confocal microscope. The white arrows indicated in figure showed the fragmented and condensed nuclei of U87 cells upon acacetin and apigenin treatment, (c) ROS inducing ability of acacetin in U87 and WI-38 cells. The acacetin-treated/untreated cells were stained with DCFH-DA dye and observed through a flow cytometer, (d) acacetin-treated U87 cells were stained with DCFH-DA and observed under a confocal microscope, (e) ROS inducing ability of apigenin in U87 and WI-38 cells. The apigenin-treated/untreated cells were stained with DCFH-DA dye and observed through a flow cytometer, (f) apigenin-treated U87 cells were stained with DCFH-DA and observed under a confocal microscope
Selective induction of ROS in U87 cells
DCFH-DA is a fluorescent dye that is used to determine the intracellular ROS levels. The ROS-inducing effect of acacetin and apigenin was evaluated on the U87 cells. The flow cytometric observations showed the dose-dependent ROS induction effect of acacetin and apigenin in U87 cells. Nearly twofold increased ROS levels were observed in acacetin-treated U87 cells, while almost 2.5 fold increased were observed in apigenin-treated U87 cells. Interestingly, none of the compounds showed ROS induction in normal fibroblast cells (WI-38) (Fig. 4c, e). The results were further confirmed by visualizing U87 cells under a laser scanning confocal microscope by DCFH-DA staining, and visual results also support the data of a flow cytometer (Fig. 4d, f).
Western blotting analysis of cell cycle regulatory proteins
Western blotting was performed to analyze the expression of the cell cycle and apoptosis regulatory proteins. The western blotting results of cell cycle regulatory proteins also confirmed the previously reported G2/M phase arrest in U87 cells upon acacetin and apigenin-treatment. Acacetin and apigenin-treatment showed down-regulated expressions of Cyclin A1, Cyclin B1, and Cdk-1. Moreover, the activation of p21 was also observed upon the treatment with both the compounds. Along with the G2/M phase cell cycle arrest, the apoptosis regulatory proteins were also analyzed upon acacetin and apigenin-treatment on U87 cells. Results revealed that the up-regulation of Bax and down-regulation of Bcl-2 in a time-dependent manner led to the increase in the Bax/Bcl-2 ratio. Further activation of caspase 9 and subsequent activation of caspase 3 in acacetin-treated U87 cells showed the initiation of the intrinsic pathway of apoptosis (Fig. 5a). On the other hand, truncation of Bid, activation of caspase 8, caspase 9, and caspase 3 evidenced the initiation of both extrinsic and intrinsic apoptosis mechanisms in apigenin-treated U87 cells (Fig. 5b). Finally, the treatment of both compounds leads to the cleavage of PARP protein, which plays a vital role in the continuation of the apoptosis process.
The U87 cells were treated with or without acacetin/apigenin. The whole proteins were collected using a cell lysis buffer and analyzed the expression of cell cycle arrest and apoptosis-related proteins through a western blot technique. (a) The U87 cells were treated with 50 μM acacetin for different time intervals (0–24 h) and analyzed the expression of cell cycle arrest and apoptosis-related proteins, (b) The U87 cells were treated with 50 μM apigenin for different time intervals (0–24 h) and analyzed the cell cycle arrest and apoptosis-related proteins. The expression of β-actin was used as a protein loading control
Discussion
Cancer is an increasing burden on the scientific community due to late diagnoses, high metastasis, increased resistance, and the enormous side effects of available drugs. The Global Cancer Observatory (GCO) has projected annual diagnoses of nearly 24 million cases by 2030 and 29 million cases by 2040, corresponding to about 16 million deaths by cancer each year by 2040 [1]. Currently, numerous treatments are available for brain and other types of cancers, but their efficacies are limited due to their extensive side effects. As such, the scientific community is largely focused on the development of new drugs with mitigated side effects. More than 50% of cancer drugs have been developed from natural resources.
Since ages, many flavonoids have contributed to drug discoveries for many diseases, including cancers. Wang et al. recently reported the traditional uses and pharmacological properties of phytochemicals present in Clerodendrum. The review article has reported nearly 280 compounds from Clerodendrum genus. Among them, various phytochemicals have been reported for anti-inflammatory, antinociceptive, antioxidant, anti-hypertensive, anticancer, antimicrobial, anti-diarrheal, hepatoprotective, and neuroprotective properties. Clerodendrum trichotomum is widely studied for anticancer activities [18]. Uncinatone, Trichotomone, Mandarone E, Teuvincenone E, and (20R,22E,24R)-stigmasta-5,22,25-trien-3β,7β-diol have been isolated from C. trichotomum and studied for anticancer activities against various cancer cell lines [18,19,20,21,22,23]. In this report, we have isolated two natural flavonoids acacetin and apigenin from C. viscosum leaves and studied the in-depth anticancer mechanism on U87 glioblastoma cell line. The measurement of cell viability upon drug treatment is the primary mechanism for understanding the cytotoxic nature of drugs towards cancer and normal cells. Previously, other scientific groups have reported the cytotoxic effect of acacetin [24,25,26,27,28], and apigenin [14, 29,30,31] on various cancer cell lines. Some scientific groups also studied the effect of apigenin on glioblastoma cell lines GL-15, U251 [32], A172, and T98G [33]. Santos et al. reported the migration and invasion inhibitory activity as well as apoptosis death mechanism of apigenin on human glioblastoma cell lines [32]. However, very little is known about the effect of acacetin and apigenin on U87 cells. Chen et al. reported the reduction of viability and induction of apoptosis upon the apigenin-treatment. The report revealed the anticancer effect of apigenin through promoting miR-16 (microRNA-16) and suppression of BCL2 and NF-κB/MMP-9 [34]. Recently, Palma et al. also showed the effect of berberine on U87 cells, where berberine-treatment induces oxidative stress in U87 cells. The treatment also leads to the induction of G1 phase cell cycle arrest followed by apoptosis in U87 cells [35]. Correspondingly, our results also supported the cytotoxic nature of acacetin and apigenin on U87 cells (IC50 value: 43.73 ± 1.19 and 48.18 ± 1.37 μM respectively).
The cell cycle is the most crucial and controlled machinery for the growth of cellular organisms. The machinery is well-controlled by numerous factors, mainly cell cycle regulatory proteins [36]. However, dysregulation of cell cycle-related proteins can produce uncontrolled cell growth and proliferation. Hence, cell cycle regulatory factors are emerging as an attractive target for cancer therapies. Several reports are available in which a drug induces arrest in a specific cell cycle phase and hinders the further proliferation of the cells, ultimately leading to cell death [37, 38]. The previous report also showed the cell cycle arrest mechanism of acacetin in lung cancer (A549) [24], liver cancer (Hep G2) [27], and prostate cancer (DU145) [28]. Similarly, apigenin also showed a cell cycle arrest mechanism in cervical cancer (HeLa) [39], lung cancer (H460) [40], and liver cancer (Huh-7) [41]. We treated the U87 cells with acacetin and apigenin and found that both the compounds induce G2/M phase arrest in U87 cells.
Plasma membrane integrity is the major characteristics of healthy cells. The ruptured plasma membrane is the hallmark of necrosis, while the apoptosis mechanism leads to cell death without physical damage to the plasma membrane [42]. The plasma membrane is composed of lipids and proteins. Mainly, sphingomyelin and phosphatidylcholine present in the outer membrane, whereas the inner leaflet consists of phosphatidylserine and phosphatidylinositol. The symmetry of lipids is maintained by flippases and floppases enzymes involved in the translocation of phospholipids along the membrane [43]. During apoptosis, the membrane phospholipid, phosphatidylserine translocate to the outer leaflet of the plasma membrane from the inner leaflet. The translocation of phosphatidylserine is the major hallmark in the initiation of apoptosis. We have evaluated the acacetin and apigenin-induced apoptosis in U87 cells through the annexin-V-FLUOS staining kit. Figures 2b and 3b showed the dose-dependent increase of apoptosis population in U87 cells upon acacetin and apigenin-treatment. The release of phosphatidylserine is the initiation of the apoptosis process, which we have analyzed through a flow cytometer.
Moreover, cleavage and fragmentation of DNA are also characteristics of apoptosis. Mainly, endonucleases like caspase 3 activated DNase (CAD) carried out the process of cellular DNA cleavage in apoptotic cells [44]. To confirm the compound-mediated apoptosis, next, we have performed the DAPI staining. DAPI (4′,6-diamidino-2-phenylindole) is a fluorescent dye that binds to AT (adenine-thymine) rich region of the DNA. The stained U87 cells were observed under a confocal microscope, and results showed the fragmented DNA upon the treatment with acacetin and apigenin (Fig. 4a, b).
Furthermore, it has been well reported that balanced reactive oxygen species (ROS) are crucial for maintaining normal physiological functions [45]. However, excess ROS generation in the biological system is very detrimental for DNA and proteins, which may ultimately lead to cause cell death. It is very contradictory to discern whether ROS-elevating therapies are helpful in cancer treatment or not? However, many well-known anticancer drugs such as doxorubicin and cisplatin are responsible for the induction of oxidative stress and target the cancer cells [46,47,48]. Previous reports suggest that acacetin also induces ROS in gastric cancer cells (AGS) [49] and breast cancer (MCF-7) cells [26]. Moreover, apigenin also responsible for the induction of ROS in the cervical and lung cancer cells [31, 40]. Our data also supported the ROS inducing effect of acacetin and apigenin on U87 cells (Fig. 4c, e).
Targeting the cell cycle regulatory proteins is one of the most important steps towards cancer therapy. Several drugs cause cell cycle arrest, hinder the proliferation, and initiate the cancer cell death [37, 38]. CDKs and Cyclins are the fundamental molecules responsible for regulating cell cycle as well as other important functions. Most importantly, Cyclin A1/Cdk-1 and Cyclin B1/Cdk-1 complexes play a vital role in the transition of G2 phase to M phase of the cell cycle [50, 51]. As mentioned earlier, acacetin, and apigenin showed a G2/M phase cell cycle arrest in U87 cells. To confirm the compound-induced arrest mechanism, we evaluated the expression of cell cycle regulatory proteins through the western blot technique. The CDK inhibitor p21, a small 165 amino acid containing protein, is involved in diverse functions including, cell cycle regulation, and apoptosis. The overexpression of p21 inhibits the CDK1 and halts the cells in the G2/M phase [52]. Thus the protein expression of Cdk-1, Cyclin-A, Cyclin -B, and p21, clearly revealed the G2/M cell cycle phase arrest mechanism of acacetin and apigenin in U87 cells. Moreover, as described earlier, Annexin-V-FLUOS staining and DAPI staining also revealed the apoptosis mechanism of both the compounds in U87 cells.
To further clarify the results, we have evaluated the expression of apoptosis regulatory protein upon treatment of the compounds. The ratio of Bax (pro-apoptotic) and Bcl-2 (anti-apoptotic) proteins is essential during cell fate decisions. If the Bax/Bcl-2 ratio increases, the cell is supposed to undergo apoptosis, while if the ratio decreases, the cell is supposed to follow the survival mechanism [53, 54]. The results showed that the protein expression of Bax in compound-treated U87 cells was increased while Bcl-2 was decreased. Bax activates the intrinsic pathway of apoptosis through the interaction with the mitochondrial membrane. The interaction leads to the release of cytochrome c from mitochondria and activates the caspase 9 through the formation of the apoptosome complex. Activated caspase 9 (cleaved caspase 9) then activates executioner caspase, caspase 3. From Fig. 5, it was concluded that acacetin and apigenin-treatment in U87 cells was responsible for the activation of initiator caspase, caspase 9. Further activation of caspase 9 (cleaved caspase 9) activates the executioner caspase, caspase 3. Cleaved caspase 3 further carry out the cleavage of nuclear DNA, which was confirmed through the increased level of cleaved PARP. Relatedly, cleavage and fragmentation of nuclear DNA were previously confirmed through the DAPI staining.
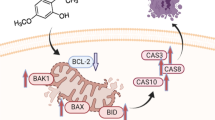
Bid (BH3 interacting-domain death agonist) is a pro-apoptotic protein and plays a vital role in the activation of the extrinsic pathway of apoptosis [55]. Truncated Bid (t-Bid) also connects the extrinsic pathway of apoptosis with the intrinsic. We have evaluated the effect of acacetin and apigenin on protein expression of Bid and t-Bid. Acacetin-treatment activates the intrinsic pathway of apoptosis in U87 cells. Interestingly, apigenin-treatment activates both the extrinsic and intrinsic pathways of apoptosis, which was confirmed through the activation of caspase 8 followed by t-Bid and activation of intrinsic pathway regulatory proteins, as mentioned above. The combined results reveal the potent anticancer effects of acacetin and apigenin towards the U87 glioblastoma cell line. We have also proposed the possible mechanism of action of acacetin and apigenin on U87 cell lines (Fig. 6). The report will also be helpful in the development of combination therapy or adjuvant therapy to treat the rare glioblastoma.
Conclusion
In conclusion, herein, we have reported the anticancer effect of acacetin and apigenin on the glioblastoma cell line, U87. Acacetin and apigenin-treatment induce ROS in U87 cells, which may increase oxidative stress. The oxidative stress may be responsible for the induction of G2/M phase cell cycle arrest. Acacetin-treatment further triggers the intrinsic pathway of apoptosis through activation of Bax, caspase 9, caspase 3, and PARP. Furthermore, apigenin-treatment triggers both extrinsic and intrinsic pathways of apoptosis through the activation of caspase 8, Bid, Bax, caspase 9, caspase 3, and PARP. The present report will help the scientific community to develop better and safer drugs against brain and CNS related cancers. Nevertheless, future investigation of synergistic effect of these two compounds will evince the diverse treatment option against rare glioblastoma.
References
World Health Organization. Global Cancer Observatory. International agency for research on cancer. http://gco.iarc.fr. Accessed 20 July 2020
National Institute of Health. The Surveillance, Epidemiology and End Results program. https://seer.cancer.gov/statfacts. Accessed 20 July 2020
Kazda T, Dziacky A, Burkon P et al (2018) Radiotherapy of glioblastoma 15 years after the landmark Stupp’s trial: more controversies than standards? Radiol Oncol 52:121–128
Vijapura C, Saad Aldin E, Capizzano AA et al (2017) Genetic syndromes associated with central nervous system tumors. Radiographics 37:258–280
Abotaleb M, Samuel SM, Varghese E et al (2019) Flavonoids in cancer and apoptosis. Cancers. https://doi.org/10.3390/cancers11010028
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci. https://doi.org/10.1017/jns.2016.41
Plants of the World Online: Clerodendrum L. Kew Science. http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30002447-2. Retrieved 18 Oct 2020
Nandi S, Mawkhlieng Lyndem L (2016) Clerodendrum viscosum: traditional uses, pharmacological activities and phytochemical constituents. Nat Prod Res 30(5):497–506
Wang JH, Luan F, He XD, Wang Y, Li MX (2018) Traditional uses and pharmacological properties of Clerodendrum phytochemicals. J Tradit Complement Med 8(1):24–38
Shendge AK, Basu T, Panja S et al (2018) An ellagic acid isolated from Clerodendrum viscosum leaves ameliorates iron-overload induced hepatotoxicity in Swiss albino mice through inhibition of oxidative stress and the apoptotic pathway. Biomed Pharmacother 106:454–465
Das A, Chaudhuri DI, Ghate NB et al (2013) Comparative assessment of phytochemicals and antioxidant potential of methanolic and aqueous extracts of Clerodendrum colebrookianum walp. leaf from North-East India. Int J Pharm Pharm Sci 5:420–427
Shrivastava N, Patel T (2007) Clerodendrum and healthcare: an overview. Medicinal Aromat Plant Sci Biotechnol 1:142–150
Shendge AK, Basu T, Chaudhuri D et al (2017) In vitro antioxidant and antiproliferative activities of various solvent fractions from Clerodendrum viscosum leaves. Pharmacogn Mag 13:344–353
Shendge AK, Chaudhuri D, Basu T et al (2020) A natural flavonoid, apigenin isolated from Clerodendrum viscosum leaves, induces G2/M phase cell cycle arrest and apoptosis in MCF-7 cells through the regulation of p53 and caspase-cascade pathway. Clin Transl Oncol. https://doi.org/10.1007/s12094-020-02461-0
Ghate NB, Chaudhuri D, Sarkar R et al (2013) An antioxidant extract of tropical lichen, Parmotrema reticulatum, induces cell cycle arrest and apoptosis in breast carcinoma cell line MCF-7. PLoS One. https://doi.org/10.1371/journal.pone.0082293
Ghate NB, Hazra B, Sarkar R, Mandal N (2014) In vitro anticancer activity of Spondias pinnata bark on human lung and breast carcinoma. Cytotechnology 66:209–218
Panja S, Ghate NB, Mandal N (2016) A microalga, Euglena tuba induces apoptosis and suppresses metastasis in human lung and breast carcinoma cells through ROS-mediated regulation of MAPKs. Cancer Cell Int. https://doi.org/10.1186/s12935-016-0330-5
Li LZ, Wang MH, Sun JB et al (2014) Abietane diterpenoids and other constituents from Clerodendrum trichotomum. Biochem Syst Ecol 56:218–220
Liu Q, Hu HJ, Li PF et al (2014) Diterpenoids and phenylethanoid glycosides from the roots of Clerodendrum bungei and their inhibitory effects against angiotensin converting enzyme and α-glucosidase. Phytochemistry 103:196–202
Wang WX, Xiong J, Tang Y (2013) Rearranged abietane diterpenoids from the roots of Clerodendrum trichotomum and their cytotoxicities against human tumor cells. Phytochemistry 89:89–95
Nan HH, Wu J, Yin H et al (2006) Terpenoid compounds from Clerodendrum inerme. Chin Tradit Herb Drug 37:508–509
Wang WX, Zhu JJ, Zou Y et al (2013) Trichotomone, a new cytotoxic dimeric abietane-derived diterpene from Clerodendrum trichotomum. Tetrahedron Lett 54:2549–2552
Xu RL, Wang R, Ding L et al (2013) New cytotoxic steroids from the leaves of Clerodendrum trichotomum. Steroids 78:711–716
Hsu YL, Kuo PL, Liu CF et al (2004) Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer Lett 212:53–60
Punia R, Raina K, Agarwal R et al (2017) Acacetin enhances the therapeutic efficacy of doxorubicin in non-small-cell lung carcinoma cells. PLoS One. https://doi.org/10.1371/journal.pone.0182870
Shim HY, Park JH, Paik HD et al (2007) Acacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria-mediated death signaling and SAPK/JNK1/2-c-Jun activation. Molecules Cells 24:95–104
Hsu YL, Kuo PL, Lin CC (2004) Acacetin inhibits the proliferation of Hep G2 by blocking cell cycle progression and inducing apoptosis. Biochem Pharmacol 67:823–829
Singh RP, Agrawal P, Yim D, Agarwal C et al (2005) Acacetin inhibits cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells: structure–activity relationship with linarin and linarin acetate. Carcinogenesis 26:845–854
Das S, Das J, Samadder A, Boujedaini N et al (2012) Apigenin-induced apoptosis in A375 and A549 cells through selective action and dysfunction of mitochondria. Exp Biol Med 7:1433–1448
Seo HS, Jo JK, Ku JM et al (2015) Induction of caspase-dependent extrinsic apoptosis by apigenin through inhibition of signal transducer and activator of transcription 3 (STAT3) signalling in HER2-overexpressing BT-474 breast cancer cells. Biosci Rep. https://doi.org/10.1042/BSR20150165
Xu Y, Xin Y, Diao Y et al (2011) Synergistic effects of apigenin and paclitaxel on apoptosis of cancer cells. PLoS One. https://doi.org/10.1371/journal.pone.0029169
Santos BL, Oliveira MN, Coelho PL et al (2015) Flavonoids suppress human glioblastoma cell growth by inhibiting cell metabolism, migration, and by regulating extracellular matrix proteins and metalloproteinases expression. Chem Biol Interact 242:123–138
Dixit D, Sharma V, Ghosh S et al (2012) Inhibition of Casein kinase-2 induces p53-dependent cell cycle arrest and sensitizes glioblastoma cells to tumor necrosis factor (TNF α)-induced apoptosis through SIRT1 inhibition. Cell Death Dis. https://doi.org/10.1038/cddis.2012.10
Chen XJ, Wu MY, Li DH et al (2016) Apigenin inhibits glioma cell growth through promoting microRNA-16 and suppression of BCL-2 and nuclear factor-κB/MMP-9. Mol Med Rep 14:2352–2358
Palma TV, Lenz LS, Bottari NB et al (2020) Berberine induces apoptosis in glioblastoma multiforme U87MG cells via oxidative stress and independent of AMPK activity. Mol Biol Rep
Murray AW (2004) Recycling the cell cycle: cyclins revisited. Cell 116:221–234
Otto T, Sicinski P (2017) Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. https://doi.org/10.1038/nrc.2016.138
Mills CC, Kolb EA, Sampson VB (2018) Development of chemotherapy with cell-cycle inhibitors for adult and pediatric cancer therapy. Cancer Res 78:320–325
Zheng PW, Chiang LC, Lin CC (2005) Apigenin induced apoptosis through p53-dependent pathway in human cervical carcinoma cells. Life Sci 76:1367–1379
Lu HF, Chie YJ, Yang MS et al (2011) Apigenin induces apoptosis in human lung cancer H460 cells through caspase-and mitochondria-dependent pathways. Hum Exp Toxicol 30:1053–1061
Kim EY, Kim AK (2012) Apigenin sensitizes Huh-7 human hepatocellular carcinoma cells to TRAIL-induced apoptosis. Biomol Ther. https://doi.org/10.4062/biomolther.2012.20.1.062
Zhang Y, Chen X, Gueydan C et al (2018) Plasma membrane changes during programmed cell deaths. Cell Res 28:9–21
Leventis PA, Grinstein S (2010) The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys 39:407–427
Larsen BD, Sorensen CS (2017) The caspase-activated DNase: apoptosis and beyond. FEBS J 284:1160–1170
Zhang J, Wang X, Vikash V et al (2016) ROS and ROS-mediated cellular signaling. Oxidative Med Cell Longev. https://doi.org/10.1155/2016/4350965
Kotamraju S, Chitambar CR, Kalivendi SV et al (2002) Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells role of oxidant-induced iron signaling in apoptosis. J Biol Chem 277:17179–17187
Marullo R, Werner E, Degtyareva N et al (2013) Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One. https://doi.org/10.1371/journal.pone.0081162
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378
Pan MH, Lai CS, Hsu PC et al (2005) Acacetin induces apoptosis in human gastric carcinoma cells accompanied by activation of caspase cascades and production of reactive oxygen species. J Agric Food Chem 53:620–630
Lim S, Kaldis P (2013) Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 140:3079–3093
Malumbres M (2014) Cyclin-dependent kinases. Genome Biol 15:1–10
Karimian A, Ahmadi Y, Yousefi B (2016) Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 42:63–71
Perlman H, Zhang X, Chen MW et al (1999) An elevated bax/bcl-2 ratio corresponds with the onset of prostate epithelial cell apoptosis. Cell Death Differ 6:48–54
Ghate NB, Das A, Chaudhuri D et al (2016) Sundew plant, a potential source of anti-inflammatory agents, selectively induces G2/M arrest and apoptosis in MCF-7 cells through upregulation of p53 and Bax/Bcl-2 ratio. Cell Death Discovery 2:1–10
Li H, Zhu H, Xu CJ et al (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 4:491–501
Acknowledgements
AKS and DC are grateful to the Council of Scientific and Industrial Research (CSIR) Govt. of India for providing the fellowships. The authors are also thankful to Mr. Ranjit Kumar Das for his technical assistance.
Funding
The present research did not receive any specific grant from the funding agencies.
Author information
Authors and Affiliations
Contributions
The AKS, DC, and NM designed the experiments. AKS performed the experiments. AKS, DC, and NM analyzed the data. AKS and NM wrote the paper. NM contributed to reagents/materials/analysis tools. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals.
Informed consent
The manuscript does not contain clinical studies or patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shendge, A.K., Chaudhuri, D. & Mandal, N. The natural flavones, acacetin and apigenin, induce Cdk-Cyclin mediated G2/M phase arrest and trigger ROS-mediated apoptosis in glioblastoma cells. Mol Biol Rep 48, 539–549 (2021). https://doi.org/10.1007/s11033-020-06087-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-06087-x