Abstract
The last discovered organ of the human body is microbiome which is present at different sites in it. Gut microbiome consists of about 1000–1500 bacterial species and as regulated by genetic makeup, lifestyle, and environmental conditions, the gut microbiota of a healthy individual can comprise approximately 160 species of bacteria. Majority of gut microbiome consists of Firmicutes, Actinobacteria, Bacteroidetes, and to a lesser extent Proteobacteria, Euryarchaeota, Fusobacteria, and Verrucomicrobia. The gut-lung axis is involved in the migration of immune cells from gut to respiratory tract through circulation and encourages the host’s ability to fight infections. The gut regulates the responses in lungs via host-acquired inflammatory mediators in the circulation. Dendritic cells located in the Peyer’s patches of the intestine, macrophages, and Langerhans cells are the major antigen-presenting cells that play a vital role in the modulation and development of innate immune response. Gut microbiota interacts via the regulation and development of adaptive immune response. B and T lymphocytes are the key players of adaptive immunity. CD4 + T cells after activation differentiate into four major kinds of cell classes: (1) regulatory T cells (Treg), (2) Th2, (3) Th1, and (4) Th17 cells. Gut microbial interactions can induce the production of various types of immune cells as demonstrated by various studies. For instance, Clostridia induces the formation of Treg cells. Likewise, Bacteroides fragilis inhabiting the gut can incite the production of Th1 cells and production of T17 cells is stimulated by segmental filamentous bacteria. Gut microbiota also plays a vital role in the physiology and metabolism leading to the synthesis of various immunoregulatory metabolites such as SCFAs, antimicrobial peptides (AMPs), amino acids, and polyamines. SARS-CoV-2 virus entry to the cell is via ACE2 receptor present in respiratory epithelium and gut epithelium. This receptor is highly expressed (100 times more than in the lung) in the epithelial cells of the stomach, duodenum, ileum, and rectum as well as cholangiocytes and hepatocytes. High level of ACE2 receptor expressing in the gastrointestinal epithelial cells along with high-level co-expression of TMPRSS2 (cellular serine peptidase) causes coronavirus to infect gastrointestinal tract along with lungs leading to altered intestinal permeability and enterocyte malabsorption with symptoms of diarrhea in patients of COVID-19. Hence, COVID-19 patients with gastrointestinal symptoms have significantly longer duration of illness and viral clearance time than patients without any gastrointestinal symptoms. Obese patients with gut dysbiosis have decreased population of Bacteroides species. COVID-19 patients with type 2 diabetics have increased population of Fusobacterium, Ruminococcus, and Blautia with decreased population of Bacteroides, Bifidobacterium, Faecalibacterium, Akkermansia, and Roseburia. Diet with low fiber, high fat, and high carbohydrate causes gut dysbiosis. Intake of high-fiber diet consisting of whole grains, vegetables, and fruits induces growth of Bifidobacterium, Bacteroides, and Lactobacilli. Probiotics are nonpathogenic live organisms which are safe to be taken as dietary supplements. The major genera of probiotics are Lactobacillus, Bifidobacterium, and Saccharomyces. These probiotics increase the activity of T cells, NK cell, and polymorphonuclear cells. Prebiotics in the form of maize fiber, inulin, and polydextrose improves digestion and immunity. Hence, healthy gut microbiome with its strong immune intervention may bring recovery in COVID-19 patients. However, so far no published studies have reported that probiotics can be used as an adjunctive therapy in our fight against the SARS-CoV-2 infection. A far-reaching approach should consist of randomized, multicenter, controlled trials to explore the potential benefits of gut microbiome and how changes in dietary habits can be used as an add-on strategy against the COVID-19 pandemic.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
19.1 Introduction
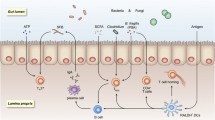
The last discovered organ of the human body is microbiome which is present at different sites in it. The microbiome of the human body is probably one of the significant factors playing a major role in the COVID-19 epidemic. The composition of microbiome in humans is trillions of microbes mostly consisting of tiny fauna, fungi, viruses, and other living entities which inhabit every part of the body. Gut microbiome consists of multispecies commensals having a strong impact on host immune homeostasis in the gut. The count of microbes residing in gastrointestinal tract (GIT) has been predicted to be more than 1014 and altogether their genomic content is reported to be 100 times the amount of total human genome (Bäckhed et al. 2005). Gut microbiome consists of about 1000–1500 bacterial species and as regulated by the genetic makeup, lifestyle, and environmental conditions, the gut microbiota of a healthy individual can comprise approximately 160 species of bacteria. Firmicutes and Bacteroidetes are among the most predominant genera found in the gut while lung microbiota is predominantly composed of Proteobacteria, Bacteroidetes, and Firmicutes (Zhang et al. 2020). Gut microbiota offers numerous benefits to its host which include direct inhibition of pathogens, maintaining gut integrity, metabolizing undigested compounds especially certain carbohydrates, as well as developing and strengthening the mucosal barrier along with intestinal epithelium (Natividad and Verdu 2013). Complex network of interactions exists between the gut microbiota and human immune system as approximately 70–80% of body’s total immunological components exist in the gut. Majority of gut microbiome consists of Firmicutes, Actinobacteria, Bacteroidetes, and to a lesser extent Proteobacteria, Euryarchaeota, Fusobacteria, and Verrucomicrobia. This community of microorganisms have evolved along with human species over millions of years. Due to this coevolution of prokaryotic bacteria and eukaryotic cells, the genomic functional complementarity with genetic reduction could occur among themselves. It is important to note that organs such as the lungs, stomach, esophagus, and intestine, also populated by the microbiota, are all embryologically derived from same germline endoderm. So, it is not surprising that these developmentally homologous organs are connected to each other in gut-lung axis. It is a bidirectional communication which means that microbial metabolites and endotoxins from gut can affect the lungs and vice versa (Dhar and Mohanty 2020). The gut-lung axis is involved in the migration of immune cells from gut to respiratory tract through circulation, where it encourages the host’s ability to fight infections. The gut regulates the responses in lungs via host-acquired inflammatory mediators in the circulation. Some studies have shown that changes do occur in the gut microbiota of mice during its infection of lung by respiratory syncytial virus infection (Groves et al. 2020). The interactions between gut and lung axis are caused either by the involvement of immune cells or via gut microbes or their products. However, microbes and their products which enter the intestinal mucosa are phagocytosed by the APCs and then moved to mesenteric lymph nodes which in turn might activate B and T cells. After activation, both B and T lymphocytes can either move to their original site, i.e., intestinal mucosa, or migrate to a different action site, e.g., lungs. The second proposed mechanism is that surviving bacteria or any bacterial products can enter the circulation and reach lungs either via blood or via lymphatic system generating a local or general immunological response that can further damage the lungs (Bingula et al. 2017). A study conducted by Fagundes et al. (2012) highlighted the impact of gut microbiota on lungs. In this study, mice lacking intestinal microbiota displayed much lower pathogenic clearance from lungs (Fagundes et al. 2012). Similarly, another study reported that intratracheal administration of lipopolysaccharide (LPS) might disturb the lung microbiota, which in turn can cause disruption of gut microbiota as well as increases the bacterial load (Sze et al. 2014). Various changes in the composition of microbiome in the gut and also of the lungs are connected to the immune response alterations and disease development in COVID-19 patients. For example, chronic obstructive pulmonary disease (COPD) typically occurs together with chronic diseases of the gastrointestinal tract. COVID-19 patients have symptoms of myalgia, fever, cough, fatigue, and pneumonia which may lead to acute respiratory distress syndrome with or without multiorgan dysfunction (Musa 2020). A lot of studies have reported the appearance of gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) as mild or severe during the course of COVID-19 infection. These symptoms can be caused either because of the ability of SARS-CoV-2 to directly bind, invade, and infect the enterocytes via gut-lung-microbiota axis or through immune-regulatory mechanisms (Dumas et al. 2018).
19.2 Gut-Lung Axis
Any change in gut microbiome is linked to a bidirectional deviation in the interaction between the gut and human organs which eventually may cause severe disease symptoms in COVID-19 patients. An increase in Clostridia species and reduction in Bifidobacteria in gut microbiome are linked to asthma in early life (Anand and Mande 2018). The gut-lung axis also causes the migration of immune cells from gut to respiratory tract through blood circulation, which helps the host’s ability to fight infections. Thus gut is able to regulate the immune responses in lungs via host-acquired inflammatory mediators (e.g., IL-6, TNFα) in the circulation. The elevated levels of these inflammatory mediators detected in the serum of patients with gut disorders influence immune responses in the lungs. The viral infections of respiratory tract can alter the intestinal microbiome in situations where the intestinal microbiome develops the adaptive immune responses against the respiratory pathogens which is necessary for priming the innate immune responses against those pulmonary infections. It is common during respiratory viral infections that the level of macrophage response to the respiratory viruses depends on the presence of healthy intestinal microbes (Hanada et al. 2018). This suggests that the lung and the gut are closely linked organs that affect each other’s homeostasis via an immunological coordination between them. Emerging data identifies the role of gut microbiota in improving the antiviral immunity (He et al. 2020). Certain reports also suggest the role of gut microbiota modulation in reducing enteritis and ventilator-associated pneumonia and reversing the side effects of antibiotics so as to reduce the replication of influenza virus in lung epithelium. However, at present no clinical evidence is available of gut microbiota modulation as a therapy for treating COVID-19. Few emerging reports have shown the role of targeting the gut microbiota as an adjuvant therapeutic option.
19.3 Gut Microbiome and Immunity
Gut microbiome has a role in the pathogenesis of various multifactorial diseases like inflammatory bowel disease (IBD), chronic heart diseases and kidney diseases, and type 2 diabetes mellitus (Wu and Wu 2012). The common microbial species found in gastrointestinal tract are Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria for providing nutrients and maintaining homeostasis in the host. Human intestine is exceptional in its own way as it possesses an outstanding mechanism for the development of host immune system. The immune system in gastrointestinal tract is an intestine mucosa immune system or gut-associated lymphoid tissue (GALT) with three different structures: epithelial and mucosal barrier, lamina propria, and Peyer’s patches (PPs). The epithelial and mucosal barriers are the small intestinal epithelium and Paneth cells which secrete antimicrobial peptides (AMPs). Immune cells of mucosal barrier contain innate lymphoid cells, natural killer or NK cells, intraepithelial lymphocytes (IEL), cytolytic and immunoregulatory αβ+ and γδ+ T cells, DCs, and Tregs (Johnson et al. 2019). The lamina propria and lower layer of intestinal epithelial cells carry a large number of innate lymphoid cells (ILCs) and B, NK, and T cells (γδ+ T, Th17). The key role of T cells seated in the lamina propria is to rapidly respond to the lumen signals and generate an appropriate anti-inflammatory response through secreting cytokines (IL17, IL22, IFN-γ, and IL26) and also inducing defensins and chemokine (Montalban-Arques et al. 2018; Bhagat et al. 2008). Intestinal homeostasis is achieved by the interaction and coordination of intestinal innate and adaptive immunity, both having mutually advantageous relationship among them. Gut microbiota exhibits their regulatory effect on innate immunity mainly via the antigen-presenting cells (APCs), for example, dendritic cells located in Peyer’s patches, Langerhans cells, and macrophages. These cells however have some immunogenic properties which enable them to be tolerant towards the gut microbiota. For instance, macrophages develop “inflammation anergy” (Smythies et al. 2005). Mast cells and NK cells are the major components of innate immune system that interacts with the gut microbiome cells. B and T lymphocytes are the major kinds of cells induced and involved whenever gut microbiome interacts via adaptive host immune response. B cells of gut are found mostly in the Payer’s patches. Gut microbiome may also have a role in the development of plasma cells since a study on germ-free mice revealed that they have lower levels of plasma (Ivanov et al. 2009). T cells constitute an essential component of adaptive immune response. CD4+ (cytotoxic) T cells after getting activated differentiate into four major types, namely Th1, Th2, Treg (regulatory t cells), and Th17 cells (Smythies et al. 2005). Gut microbial interactions can induce the production of various types of immune cells as demonstrated by various studies. For instance, Clostridia induces the formation of Treg cells. Likewise, Bacteroides fragilis inhabiting the gut can incite the production of Th1 cells and production of T17 cells is stimulated by segmental filamentous bacteria (Mazmanian et al. 2005; Nagano et al. 2012). Many studies have reported that synthesis and count of CD25+, CD4+, and NK cells and mononuclear leukocytes can be increased by Bifidobacterium lactis (Levy et al. 2017). In addition, gut microbiota plays a vital role in the physiology and metabolism leading to the synthesis of various immunoregulatory metabolites such as SCFAs, AMPs (antimicrobial peptides), amino acids, and polyamines (Gill et al. 2001). Therefore, gut microbiota plays a vital role in the development of adequate host immune responses. As mentioned before, many patients infected with SARS-CoV-2 display gastrointestinal symptoms due to the invasion of intestinal enterocytes by virus indicating that SARS-CoV-2 might be interacting and disturbing the balance of healthy gut microbiome.
19.4 Pathogenesis and COVID-19
COVID-19 is caused by SARS-CoV-2, a positive-sense, single-stranded RNA virus of family Coronaviridae and genus Betacoronavirus. The structure of SARS-CoV-2 virus consists of four structural proteins (membrane (M), envelope (E), nucleocapsid (N), spike (S)), 15 mature nonstructural proteins (nsp1−10 and nsp12−16), and 9 accessory proteins (Prates et al. 2020). The SARS-CoV-2 infection occurs by the entry of virus through ACE2 receptor present on the epithelial cell lining of lung, gut, and other organs. This receptor is highly expressed (100 times than that in the lung) in the epithelial cells of the stomach, duodenum, ileum, and rectum as well as in cholangiocytes and hepatocytes of the liver. However less expression of ACE2 receptor is found in esophageal mucosa. The high number expression of ACE2 receptor in the absorptive enterocytes of the ileum and colon suggests a reason for digestive symptoms such as diarrhea found in many COVID-19 patients. Butyric acid is a short-chain fatty acid (SCFA) produced by beneficial gut bacteria Clostridia species along with propionic and acetic acids (a fermentation product of dietary fiber) that plays a pivotal role in gut microbial metabolism. In elder persons altered gut microbiota predisposes COVID-19 patients to severe symptoms of diarrhea due to disrupted gut barrier (Kim 2021, Fig. 19.1). Next the new virions assembled in the enterocytes are released into the gastrointestinal tract (Dahiya et al. 2020; Vuille-Dit-Bille et al. 2020). These newly released virions SARS-CoV-2 destruct more epithelial cells, induce strong immune responses, and trigger cytokine storm in patients. The viral spike protein S1 mediates the attachment of virus to the host cell membrane and S2 spike protein favors the fusion of the cell membranes. This process also needs priming by host cellular enzyme serine proteases (TMPRSS2) which enables viral S-protein cleavage, thereby controlling the entire mechanism (D’Amico et al. 2020). Evidence also suggests that ACE2 receptor has protective anti-inflammatory effects and S-protein of the virus downregulates its expression via increased release of pro-inflammatory chemokines (MCP1, IP10, and MIP1α) and cytokines (TNF, IL-1β, IL-6, IL-8, G-CSF, and GM-CSF), thereby causing inflammation and increased vascular permeability in the lungs (Bonafè et al. 2020). By integrating the proteome structural analyses with the multi-omics data, a recent study suggests that COVID-19 not only involves a direct binding of SARS-CoV-2 to ACE2 for cell entry but also causes an imbalance of various other components of RAS. The expression profile of RAS genes from cells of bronchoalveolar lavage samples of COVID-19 patients suggested significantly upregulated angiotensin, renin, MAS, ACE, and ACE2. In addition, the expression data from several sources and tissues also suggest that the SARS-CoV-2 targets the host gastrointestinal system (Prates et al. 2020). Another study also showed presence of ACE2 receptor-rich lining in epithelial cells of the lower digestive tract (Wadman et al. 2020). Hence high level of ACE2 receptor expressing in the gastrointestinal epithelial cells along with high-level co-expression of TMPRSS2 (cellular serine peptidase) causes coronavirus to infect gastrointestinal tract along with lungs. This may lead to altered intestinal permeability and enterocyte malabsorption. Another role of intestinal ACE2 is involvement in dietary amino acid uptake and regulation of the expression of antimicrobial peptides (AMPs) to promote intestinal homeostasis (D’Amico et al. 2020). Evidences suggest that ACE2 plays a crucial non-catalytic role in the modulation of intestinal microbiota composition suggesting that the beneficial effects of ACE2 are partially mediated through the alteration in intestinal microbiome. For instance, ACE2-KO animals display altered gut microbial composition, decreased expression of AMPs, and declined levels of neutral amino acids in their serum with specific impairment of tryptophan (Trp) uptake, which can be restored by the tryptophan usage. To support this, the probiotics are shown to reduce the oxidative stress, positively alter the cholesterol levels, release vaso-deleterious ACE2-inhibiting peptides, and reduce stress-induced hyper-permeability (Cole-Jeffrey et al. 2015). ACE2 regulates innate immunity and also influences the composition of host intestinal microbiota (Perlot and Penninger 2013). Host tryptophan metabolites such as the melatonin, serotonin, and kynurenines and the bacterial tryptophan metabolites like indole, indolic acid, tryptamine, and skatole have effects on intestinal microbial composition, microbial metabolism, host immune system, host-microbiome interface, and host immune system-intestinal microbiota interactions. Thus, ACE2 has a role in regulating intestinal amino acid homeostasis, expression of AMPs, innate immunity, and gut microbial ecology. Another pathway for virus entry inside host cell is that the S-protein of SARS-CoV-2 can bind to another surface molecule, i.e., CD147 of the host cell. CD147 is mainly found on hematopoietic cells including red blood cells (RBCs) and neuronal and epithelial cells in humans. N-protein of SARS-CoV also binds to cyclophilin A in ACE2-expressing infected host cells. To validate it was found that in vitro inoculation of human lung epithelial cells with SARS-CoV-2 produces cytopathic effects of the lung epithelial cells (Gubernatorova et al. 2020). Hence, a strategy to develop therapeutics against the SARS-CoV-2 is by blocking the ACE2 or TMPRSS2 using compounds like baricitinib and ruxolitinib for ACE2 and camostat mesylate and nafamostat mesylate for TMPRSS2 or using monoclonal antibodies targeting the S-protein of SARS-CoV-2 that may inhibit the virus entry or membrane fusion into the host cell (Tay et al. 2020). The gene expression study via mRNA sequencing has found that the SARS-CoV-2 infection elicits specific cytokines and interferon-stimulated genes (ISGs) for type I and III interferon responses (Lamers et al. 2020).
19.5 Dysbiosis
Patients of COVID-19 with gastrointestinal symptoms have illness of longer duration with delayed viral clearance time than those patients without gastrointestinal involvement. Certain factors including poor diet, inadequate sanitation, superimposed infections, and antibiotic uptake may cause dysbiosis. Dysbiosis is also caused by host factors which are decreased immune function, impaired absorption, mucosal barrier failure, and pro-inflammatory response. This dysbiosis may cause over-reactive or under-reactive immune response leading to increase in severity of the infection. A shotgun sequencing and analysis of stool samples of 15 COVID-19 patients revealed that their fecal microbiome has undergone drastic changes as compared to controls and at least 23 bacterial taxa were associated with COVID-19 severity. The amount of beneficial microbes reduced and opportunistic pathogens have drastically increased in count. The gut dysbiosis in patients has not reversed even after the clearance of SARS-CoV-2 from throat swabs. It was found that at baseline, 23 taxa of bacteria were associated with COVID-19 severity and mainly belong to phylum Firmicutes (15 out of the 23 taxa). Among these, 8 taxa had displayed a positive correlation and 7 taxa had displayed a negative correlation with the severity of disease. Coprobacillus, Clostridium ramosum, and Clostridium hathewayi were the major bacterial genera which had a positive correlation with the severity of disease (Forrester and Spain 2014). Alistipes onderdonkii and Faecalibacterium prausnitzii were negatively correlated with COVID-19 severity. Faecalibacterium prausnitzii is considered to have anti-inflammatory properties and Alistipes plays a pivotal role in the maintenance of gut immune homeostasis. Also, Bacteroides dorei, Bacteroides ovatus, Bacteroides thetaiotaomicron, and Bacteroides massiliensis had a significant negative impact on fecal viral load in SARS-CoV-2 illness. All of these organisms are associated with the downregulation of ACE2 expression in the colon (Geva-Zatorsky et al. 2017). This study has demonstrated that when the count of certain species increases, expression of ACE2 receptors is upregulated while other species downregulate the ACE2 expression in COVID-19-infected individuals. There may also be a decrease in beneficial microbial species involved in gut-immune homeostasis and generation of anti-inflammatory responses required for the suppression of COVID-19 infection. Hence, in comparison to the healthy individuals, gut dysbiosis might increase the severity of illness in COVID-19 patients. Another study with analysis of 30 patients showed that the opportunistic pathogens such as Rothia, Streptococcus, Veillonella, and Actinomyces outnumbered the beneficial organisms during COVID-19 infection in patients (Yang et al. 2020; Gu et al. 2020). Obese persons of COVID-19 have gut dysbiosis with decreased population of Bacteroides species whereas type 2 diabetics have increased Ruminococcus, Fusobacterium, and Blautia. In them there is decreased population of Bacteroides, Bifidobacterium, Faecalibacterium, Akkermansia, and Roseburia. In elderly COVID-19 patients Bacteroidetes are increased and Firmicutes are decreased (Hu et al. 2021, Fig. 19.2). In the management of patients with preexisting digestive diseases such as IBD, COVID-19 infection may mimic IBD exacerbations. Hence, IBD patients should be tested for SARS-CoV-2 before assuming it a flare. It is also assumed that IBD patients are at higher risk of developing COVID-19 complications as the ACE2 expression and activity of host cell trypsin-like proteases are increased in inflamed gut of these patients (Queiroz et al. 2020).
19.6 Diet, Probiotics, and COVID-19
The content of low fiber, high fat, and abundant carbohydrate in daily diet leads to gut dysbiosis (Trompette et al. 2014). This may change the homeostasis with alteration in immune response of an individual. A study on mice showed that intake of high-fiber diet leads to higher levels of circulating short-chain fatty acids which protects against allergic inflammation in lungs. Hence a diet rich in fiber regulates gut microbiota as well as lung microbiota which shows an effect on lung immunity (Valdes et al. 2018). Intake of whole grains, vegetables, and fruits induces growth of Bacteroides, Bifidobacterium, and Lactobacilli in the gut. Gut microbiome is more stable and achieves microbial environment resembling the adults with greater resistance towards infections by 3 years of age (Heiman and Greenway 2016). It has been found that consumption of dietary fibers lowers down the serum levels of C-reactive protein (CRP), tumor necrosis factor-alpha (TNFα), interleukin IL-6, and IL-18, and thus has an inverse correlation with disease severity. High-fiber diet lowers blood glucose level and increases plasma concentrations of adiponectin (an insulin-sensitizing adipo-cytokine with anti-inflammatory properties) (Williams 2010). Hence, a balanced diet rich in cereals, legumes, fruits, whole grains, unsaturated fatty acids, and green vegetables is generally recommended to fight against SARS-CoV-2 infection and for those who are in quarantine as well as for patients who are asymptomatic or have mild symptoms to improve their chances of recovery. Thus, COVID-19 patients should adopt a diversified diet with high-fiber and plant-based foods (Trottein and Sokol 2020) to strengthen intestinal epithelial barrier, lower down the pro-inflammatory state, and increase the intestinal motility (Trottein and Sokol 2020).
Probiotics are the nonpathogenic live organism spores which are safe and can be supplemented with diet. The major classes of probiotics belong to the genera Bifidobacterium, Lactobacillus, and Saccharomyces. Probiotics increases the activity of T cells and NK cells and phagocytic activity of polymorphonuclear cells. Hence, probiotics have a significant role in maintaining the immunogenic homeostasis of the gut. Other functions of probiotics are to maintain the pH of the intestine and to lower the invasion or colonization of the pathogens in the gut. Probiotics have been found to be helpful in the recovery against various diseases, for instance ulcerative colitis, antibiotic-associated diarrhea (AADs), as well as infectious diarrhea and hepatic encephalopathy (Wilkins and Sequoia 2017). It has been found that probiotic species like Bifidobacterium breve and Lactobacillus rhamnosus are beneficial to mice in terms of modulating the innate immune response as well as maintaining the balance of inflammatory responses in mice. Probiotics intake influences the ACE2 receptors as their microbial fermentation produces ACE inhibitory peptides leading to decreased production of angiotensin-2 (Dave et al. 2016). Human cathelicidin, LL-37, is a human antimicrobial peptide with a broad range of activities against the bacterial and viral pathogens (Mookherjee et al. 2020). The immunomodulatory and protective effect of cathelicidin upregulates the count of Lactococcus lactis (Wong et al. 2012) which can be a step ahead in helping combat the COVID-19 infection by using antimicrobial peptide-expressing probiotics delivery system (Wong et al. 2012). Empirical use of antibiotics in the early phase of COVID-19 patients may lead to more unfavorable dysbiosis. Hence, in the early phase of COVID-19, patients can suffer from dysbiosis of gut microbiome. Therefore, intake of probiotics may restore the balance of colonic microbiota which may reduce the incidence of secondary coinfections. It was found that the dosage of probiotic species (Bacillus subtilis, Enterococcus faecalis, and Lactobacillus rhamnosus GG) to the ventilator-ridden patients with severe COVID-19 illness resulted in minimizing their ventilator requirements in comparison to placebo (Li et al. 2019). The increase in production of intestinal butyrate using probiotics is helpful to strengthen the gut epithelial cell health. The use of Bifidobacteria and Lactobacilli is considered beneficial for butyrate production. Similar role of F. prausnitzii in stimulating butyrate along with anti-inflammatory properties in the intestine has been found in facilitating the treatment for inflammatory bowel disease (Lopez-Siles et al. 2017).
Prebiotics like maize fiber and polydextrose are known to improve digestion and boost immunity via reconstructing the gut microbiome symbiosis especially among the elderly people. Prebiotics act closely in the growth and function of probiotics in the gut. Gut microbes, particularly belonging to probiotic genera, act upon prebiotics (fructan, glucan, arabinoxylan) and utilize them as their growth substrates to produce short-chain fatty acids (SCFAs), butyric acid, and propionic acid. These end products affect the differentiation or functions of T cells, macrophages, and dendritic cells. Prebiotics regulate various pro- and anti-inflammatory cytokines. They promote maturation, differentiation, and reproduction of macrophages and lymphocytes and activate reticuloendothelial cells. Complex carbohydrates found in whole grain are known to decrease the concentration of pro-inflammatory cytokines (IL-6). Likewise, butylated high-amylose maize starch has been shown to enhance the concentration of anti-inflammatory cytokines (IL-10) (Collado et al. 2018; Johnson et al. 2019). Gut microbes metabolize prebiotics and various dietary components and produce short-chain fatty acids (SCFAs), elevating their intestinal concentration, which in turn regulates the lymphoid (gastrointestinal as well as secondary) tissues (Agans et al. 2011). SARS COVID-19 virus after entering lungs activates lung immune system as well as through gut-lung axis does immune activation in gut microbiome. Healthy gut microbiome with its strong immune intervention may bring recovery in COVID-19 patients with correction of dysbiosis. Due to complex gut microbiota ecosystem, a single food item may not be helpful to dramatically shift its overall composition. The role of the Mediterranean diet has been found to boost immunity with a regular supply of dietary fibers in adequate quantity. Mediterranean diet is characterized by high consumption of whole grains, vegetables, legume, nuts, extra-virgin olive oil (rich in polyphenols), and fats mostly in the form of unsaturated fatty acids and low consumption of processed meats and refined sugars. This diet has favorable modulation of relative gut microbial abundance with diversity to maintain its homeostasis (De Filippis et al. 2016). On the other hand, paying attention to the effects of microbial-diet-host interactions, diet consisting of diversified food items such as fermented dairy products, vegetables, whole grains, and fermented soybeans naturally enriched with probiotics such as Lactobacillus bulgaricus and Streptococcus thermophilus is vital and beneficial in numerous ways as it creates a nurturing environment that helps maintain the host immunity (Ghosh et al. 2019). At this time when various drugs are being tested for the cure of COVID-19 disease with no clear success as yet the role of probiotics in various clinical trials is being analyzed to lessen the severity and incidence of COVID-19 infection. It may be that inter-individual variation in gut microbiome will have different efficacy on suggested various interventions for using gut microbiota in our fight against SARS-CoV-2 infection. So far, the International Scientific Association of Probiotics and Prebiotics (ISAPP) has mentioned that scientists and clinicians globally are studying to gain further insights of the relationship between the healthy gut microbiome and susceptibility to COVID-19 with assessment of the role of various probiotic strains to lower the viral load in patients. A multicenter randomized, double-blind, placebo-controlled phase 2 trial is being done to assess the role of probiotic Lactobacillus rhamnosus GG in the prevention and treatment of COVID-19 infection (Hu et al. 2021). More studies are being done to understand the host immunity to overcome and recover from COVID-19 illness either via manipulating the gut microbiota composition or via enhancing vaccine response. Diversified dietary strategies directed to restore beneficial microbiota may possibly suppress viral infection in the elderly and those with underlying health problems. A far-reaching approach should consist of randomized, multicenter, controlled trials to explore the potential benefits of gut microbiome and how changes in dietary habits can be used as an add-on strategy against the COVID-19 pandemic.
References
Agans R, Rigsbee L, Kenche H, Michail S, Khamis HJ, Paliy O (2011) Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol Ecol 77(2):404–412
Anand S, Mande SS (2018) Diet, microbiota and gut-lung connection. Front Microbiol 9:2147. https://doi.org/10.3389/fmicb.2018.02147
Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI et al (2005) Host-bacterial mutualism in the human intestine. Science 307(5717):1915–1920
Bhagat G, Naiyer AJ, Shah JG et al (2008) Small intestinal CD8+ TCRγδ+ NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J Clin Investig 118:281–293
Bingula R, Filaire M, Radosevic-Robin N, Bey M, Berthon JY, Bernalier-Donadille A et al (2017) Desired turbulence? Gut-Lung Axis, immunity, and lung cancer. J Oncol 15:5035371. https://doi.org/10.1155/2017/5035371
Bonafè M, Prattichizzo F, Giuliani A, Storci G, Sabbatinelli J, Olivieri F (2020) Inflamm-aging: why older men are the most susceptible to SARS-CoV-2 complicated outcomes. Cytokine Growth Factor Rev. https://doi.org/10.1016/j.cytogfr.2020.04.005
Cole-Jeffrey CT, Liu M, Katovich MJ, Raizada MK, Shenoy V (2015) ACE2 and microbiota: emerging targets for cardiopulmonary disease therapy. J Vardiovasc Pharmacol 66(6):540–550. https://doi.org/10.1097/FJC.0000000000000307
Collado MC, Engen PA, Bandín C, Cabrera-Rubio R, Voigt RM, Green SJ, Naqib A, Keshavarzian A, Scheer FA, Garaulet M (2018) Timing of food intake impacts daily rhythms of human salivary microbiota: a randomized, crossover study. FASEB J 32(4):2060–2072
D’Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L (2020) Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management. Clin Gastroenterol Hepatol. https://doi.org/10.1016/j.cgh.2020.04.001
Dahiya DS, Kichloo A, Albosta M, Pagad S, Wani F (2020) Gastrointestinal implications in COVID-19. J Invest Med. https://doi.org/10.1136/jim-2020-001559
Dave LA, Hayes M, Montoya CA, Rutherfurd SM, Moughan PJ (2016) Human gut endogenous proteins as a potential source of angiotensin-I-converting enzyme (ACE-I), renin inhibitory and antioxidant peptides. Peptides 76:30–44
De Filippis F, Pellegrini N, Vannini L et al (2016) High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65(11):1812–1821
Dhar D, Mohanty A (2020) Gut microbiota and Covid-19-possible link and implications. Virus Res 285:198018. https://doi.org/10.1016/j.virusres.2020.198018
Dumas A, Bernard L, Poquet Y, Villarino GL, Neyrolles O et al (2018) The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol 20(12):e12966. https://doi.org/10.1111/cmi.12966
Fagundes CT, Amaral FA, Vieira AT, Soares AC, Pinho V, Nicoli JR et al (2012) Transient TLR activation restores inflammatory response and ability to control pulmonary bacterial infection in germfree mice. J Immunol 188(3):1411–1420
Forrester JD, Spain DA (2014) Clostridium ramosum bacteremia: case report and literature review. Surg Infect (Larchmt) 15:343–346
Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, OrtizLopez A et al (2017) Mining the human gut microbiota for immunomodulatory organisms. Cell 168(5):928–943. https://doi.org/10.1016/j.cell.2017.01.022
Ghosh T, Beniwal A, Semwal A, Navani NK (2019) Mechanistic insights into probiotic properties of lactic acid bacteria associated with ethnic fermented dairy products. Front Microbiol 10:502
Gill HS, Rutherfurd KJ, Cross ML, Gopal PK (2001) Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr 74:833–839
Groves HT, Higham SL, Moffatt MF, Cox MJ, Tregoning JS (2020) Respiratory viral infection alters the gut microbiota by inducing inappetence. MBio. https://doi.org/10.1128/mBio.03236-19
Gu S, Chen Y, Wu Z, Chen Y, Gao H, Lv L, Guo F, Zhang X, Luo R, Huang C, Lu H (2020) Alterations of the gut microbiota in patients with COVID-19 or H1N1 influenza. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa709
Gubernatorova EO, Gorshkova EA, Polinova AI, Drutskaya MD (2020) IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Gowth Factor Rev. https://doi.org/10.1016/j.cytogfr.2020.05.009
Hanada S, Pirzadeh M, Carver KY, Deng JC (2018) Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol 9:2640. https://doi.org/10.3389/fimmu.2018.02640
He Y, Wang J, Li F, Shi Y (2020) Microbiota might play a role in SARS-CoV-2 infection. Front Microbiol 11:1302. https://doi.org/10.3389/fmicb.2020.01302
Heiman ML, Greenway FL (2016) A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol Metab 5(5):317–320
Hu J, Zhang L, Lin W, Tang W, FKL C, Ng SC (2021) Probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends Food Sci Technol 108(2021):187–196
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U et al (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139:485–498
Johnson AJ, Vangay P, Al-Ghalith GA, Hillmann BM, Ward TL, Shields-Cutler RR, Kim AD, Shmagel AK, Syed AN, Students PM, Walter J (2019) Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe 25(6):789–802
Kim HS (2021) Do an altered gut microbiota and an associated leaky gut affect COVID-19 severity? MBio 12:e03022-20. https://doi.org/10.1128/mBio.03022-20
Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Schayck JP, Mykytyn AZ, Duimel HQ et al (2020) SARS-CoV-2 productively infects human gut enterocytes. Science. https://doi.org/10.1126/science.abc1669
Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E (2017) Dysbiosis and the immune system. Nat Rev Immunol 17:219–232
Li N, Ma WT, Pang M, Fan QL, Hua JL (2019) The commensal microbiota and viral infection: a comprehensive review. Front Immunol 10:1551. https://doi.org/10.3389/fimmu.2019.01551
Lopez-Siles M, Duncan SH, Garcia-Gill LJ, Martinez-Madina M (2017) Faecalibacterium prausnitzii: from microbiology to diagnostics and prognostics. ISME J 11(4):841–852
Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL (2005) An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122:107–118
Montalban-Arques A, Chaparro M, Gisbert JP, Bernardo D (2018) The innate immune system in the gastrointestinal tract: role of intraepithelial lymphocytes and lamina propria innate lymphoid cells in intestinal inflammation. Inflamm Bowel Dis 24:1649–1659
Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ (2020) Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov 19(5):311–332
Musa S (2020) Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): what do we know till now? Arab J Gastroenterol 21(1):3–8
Nagano Y, Itoh K, Honda K (2012) The induction of Treg cells by gut indigenous clostridium. Curr Opin Immunol 24:392–397
Natividad JM, Verdu EF (2013) Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res 69(1):42–51
Perlot T, Penninger JM (2013) ACE2–from the renin–angiotensin system to gut microbiota and malnutrition. Microbes Infect 15(13):866–873. https://doi.org/10.1016/j.micinf.2013.08.003
Prates ET, Garvin MR, Pavicic M, Jones P, Shah M, Alvarez C, Kainer D, Demerdash O, Amos BK, Geiger A et al (2020) Functional immune deficiency syndrome via intestinal infection in COVID-19. bioRxiv. https://doi.org/10.1101/2020.04.06.028712
Queiroz NSF, Barros LL, Azevedo MFC, Oba J, Sobrado CW, Carlos AS, Milani LR, Sipahi AM, Damião AOMC (2020) Management of inflammatory bowel disease patients in the COVID-19 pandemic era: a Brazilian tertiary referral center guidance. Clinics 75. https://doi.org/10.6061/clinics/2020/e1909
Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH et al (2005) Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bactericidal activity. J Clin Invest 115:66–75
Sze MA, Tsuruta M, Yang SW, Oh Y, Man SF, Hogg JC et al (2014) Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS One 9(10):e111228. https://doi.org/10.1371/journal.pone.0111228
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF (2020) The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20:363–374. https://doi.org/10.1038/s41577-020-0311-8
Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C et al (2014) Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20:159–166
Trottein F, Sokol H (2020) Potential causes and consequences of gastrointestinal disorders during a SARS-CoV-2 infection. Cell Rep 32(3):107915
Valdes AM, Walter J, Segal E, Spector TD (2018) Role of the gut microbiota in nutrition and health. BMJ 13:–361. https://doi.org/10.1136/bmj.k2179
Vuille-Dit-Bille RN, Liechty KW, Verrey F, Guglielmetti LC (2020) SARS-CoV-2 receptor ACE2 gene expression in small intestine correlates with age. Amino Acids 52:1063–1065
Wadman M, Couzin-Frankel J, Kaiser J, Matacic C (2020) A rampage through the body. Science 368:356–360. https://doi.org/10.1126/science.368.6489.356
Wilkins T, Sequoia J (2017) Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician 96:170–178
Williams NT (2010) Probiotics. Am J Health Syst Pharm 67:449–458
Wong CCM, Zhang L, Li ZJ, Wu WKK et al (2012) Protective effects of cathelicidin-encoding Lactococcus lactis in murine ulcerative colitis. J Gastroenterol Hepatol 27(7):1205–1212
Wu HJ, Wu E (2012) The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3:4–14
Yang T, Chakraborty S, Saha P, Mell B, Cheng X, Yeo J-Y et al (2020) Gnotobiotic rats reveal that gut microbiota regulates colonic mRNA of ACE2, the receptor for SARS-CoV-2 infectivity. Hypertension 76(1):e1–e3. https://doi.org/10.1161/HYPERTENSIONAHA.120.15360
Zhang D, Li S, Wang N, Zhang Z, Feng Y et al (2020) The cross-talk between gut microbiota and lungs in common lung diseases. Front Microbiol. https://doi.org/10.3389/fmicb.2020.00301
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sharma, A.K. (2021). Gut Microbiome in COVID-19: New Insights. In: Sobti, R.C., Dhalla, N.S., Watanabe, M., Sobti, A. (eds) Delineating Health and Health System: Mechanistic Insights into Covid 19 Complications. Springer, Singapore. https://doi.org/10.1007/978-981-16-5105-2_19
Download citation
DOI: https://doi.org/10.1007/978-981-16-5105-2_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5104-5
Online ISBN: 978-981-16-5105-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)