Abstract
Three-dimensional (3D) bioprinting, consisting of computer-controlled deposition of scaffolds and cells into designed patterns, is an innovative and promising biofabrication strategy for creating artificial tissues and organs. Bioprinted skin has the potential for clinical transplantation, drug testing, cosmetic assaying as well as fundamental researches. Remarkable advancements have been done over the past decades in the improvement of engineered substitutes that mimic skin by applying the advances in polymer engineering, bioengineering, and nanomedicine. Prior to the printing stage, the pre-design of the process, selection of cell, and biofunctional inks are significant steps for the successful fabrication of 3D bioprinted skin constructs. It is crucial to seek and decide on an appropriate source of biofunctional ink capable of stimulating and supporting printed cells for tissue development. Based on this perspective, this chapter deals with the skin and wound structure, skin tissue engineering, the performance and properties of a broad range of biofunctional inks available for 3D bioprinting technologies to produce skin structures. Besides, the current challenges and advances in designing and developing biofunctional inks with desired properties are overviewed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Graphıcal Abstract

1 Introduction
Skin is one of the most significant part of the human body, acting as a barrier with protective, sensorial, and immunologic functions. Kind of external interventions on the skin tissue may result in various wounds [142]. As one of the most common injuries, skin wounds affect millions of people worldwide [10, 36, 72]. Due to the increment of diabetes and venous/arterial insufficiencies, the cases suffering from chronic wounds have been rising dramatically [101]. Indirect results of skin defects frequently cause bacterial infections, dehydration, and some serious complications which target the homeostasis and thereby the integrity of the skin [30].
To preserve the immediate reactions of the skin, wounds should be recovered or regenerated right after the injury as soon as possible [167]. Tissue engineering approaches suggest many promising treatments to overcome the devastating injuries via combining therapeutic agents, cells, and biomaterials in several methods [26, 40, 121]. As one of the eminent techniques, biofabrication, has emerged as a powerful and novel strategy to develop complex three-dimensional (3D) structures mimicking injured organs and tissues in the skin tissue engineering or outputs used as the basis for regenerative medicine [46, 77]. 3D bioprinting is an advanced biofabrication process that benefits from the computer-controlled deposition of biocompatible materials to produce living and functional 3D tissue constructs. To regenerate or substitute one of the body components, these constructs can be used in the body [23, 83]. 3D printing technology has paved the way for patient treatment plans that can best fit individual needs [191]. It has numerous advantages including the ability to design different kinds of porous natures [33], complex structures [195], combine growth factors [33], and multiple cells [99] mimicking native tissues. Bioink is the most critical parameter to reach the desired goals in mimicking the skin tissue. Depending on the technique used under biofabrication, bioinks can vary and deserves broad research. Applications of 3D printing are limited by the type of utilized bioinks. Numerous researchers have improved new bioinks to provide their utilization for characteristic 3D bioprinting [83].
Bioinks are the main building blocks for 3D bioprinted constructs [122, 197]. They are termed as cell-laden liquid materials that can include additional components like growth factors and/or signaling molecules in addition to the specific cells, to fabricate native-like structures [196]. To design and fabricate complex biofunctional inks, the bioinks should have critical features, like biodegradability, mechanical durability, biocompatibility, viscoelasticity, non-immunogenicity, and nontoxicity [20]. In addition, for maintaining cell viability, and induce/prevent cellular response, biofunctional ink materials have to be determined based on their mechanical, chemical, biological, and rheological features with best fitting one of the bioprinting techniques [136, 165].
In this chapter, a comprehensive overview of appropriate biofunctional inks utilized in 3D bioprinting for skin tissue engineering is provided. Finally, current challenges and advances in the development of biofunctional inks are also outlined.
2 The Structure and Function of Skin
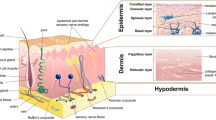
The skin tissue covers 15% of body weight and 1.8 m2 of the body area [3, 171]. It performs primarily as a barrier protecting the inner organs from the outer effects such as ultraviolet radiation, abrasion, and pathogens, also fulfills some functions like sensation, vitamin D synthesis, temperature/fluid homeostasis, immune protection, and self-healing [45, 98]. From outer to inner, the skin is composed of three main layers: epidermis, dermis, and hypodermis [6] (Fig. 1). The dermis is largely comprised of elastic and collagen fibrils found in a glycosaminoglycan structure, while the epidermis contains numerous cells [145]. The epidermis layer essentially consists of keratinocytes (95%) and also includes melanocytes, Merkel cells, and antigen-presenting dendritic Langerhans cells. The keratinocytes grow rapidly to transport urea and water throughout aquaporins, repair wounds, and play role in immunity via antimicrobial peptide secretion of the Langerhans cells. Also, they acquire skin color pigment, melanin, from melanocytes to filter out ultraviolet radiation from sunlight [21, 22, 175]. The epidermis has no intrinsic vascular network; therefore, it is nourished from microvascular networks of the dermal layer [6, 117]. The structures localized in the epidermis from the bottom to top are stratum basale (SB), stratum spinosum (SS), stratum granulosum (SG), stratum lucidum (SL), and the stratum corneum (SC) [145]. Generally, the differentiated cubic basal keratinocytes migrate from the bottom layer through the apical layers every 28 days [179]. Thus, they progressively get an ovoid shape, lose their nuclei, and ultimately detach from the epidermis [57]. During this process, lipids and keratin ensue which then undergoes terminal differentiation to constitute the upper layer (SC) [2]. This layer prevents the entry of foreign objects and microorganisms into the body [22]. This structure has two separate layers, consisting of the upper part, stratum disjunctum, and the lower part, stratum compactum [47, 51]. The latter layer is composed of 4–6 cell layers of the SC and includes solid components that provides a diffusion barrier. The more loosely structured upper part consists of ca. 8–15 cell layers with only lateral cell–cell junctions. Both of these sublayers maintain the cohesion of the stratum corneum [29, 53]. SB includes phospholipids while the upper epidermal layers are rich in lipid lamellae [55]. As a non-cellular layer, stratum basale includes fibrils, proteoglycans, and glycoproteins like laminin, collagen type IV, and VII. Under this structure, a reticular lamina including high concentrations of collagen type III is present [15]. Besides, the epidermis has various invaginations, including hair follicles connected to sweat glands and sebaceous glands (pilosebaceous units). The epithelial stem cells, changing into basal keratinocytes, are present in this pilosebaceous unit [21]. Then, as keratinocytes move towards the skin surface, they differentiate and mature. The keratinized cell layer on the skin contributes to the barrier function of the skin [22].
On the other hand, as the thickest layer, the dermis is located beneath the epidermis. It is a connective tissue including extracellular matrix (ECM), vascular endothelial cells, fibroblasts, hair follicles, sebaceous glands, sweat glands, nerve endings, and blood vessels [22]. The loose connective tissue layer is positioned under the epithelium. It harbors scarce cells with a high amount of the matrix material. Just beneath this layer, the dense connective layer is present. It includes collagen in high concentrations and sparse cells, typically single type-fibroblasts [145]. Fibroblasts are the most abundant cells in this layer and synthesize the ECM [6]. It is made of interconnected protein structures (elastin, fibronectin, collagen, and laminin), proteoglycans (chondroitin sulfate, heparan sulfate, and keratan sulfate), and glycosaminoglycans [128]. The ECM is like a scaffold providing flexibility and physical strength to the skin via extracellular structural proteins and glycosaminoglycans. In addition, it plays role in the hydration of tissue due to the high water-binding capacity of the hyaluronic acid component [3, 6, 166]. The lymphatic system in the dermal layer has various functions such as pressure maintenance in tissue by removal of interstitial fluid, waste byproducts, and also the regulation of immune responses [4, 56, 70, 80, 153]. On the other hand, the blood vessels are responsible for transferring oxygen and nutrients to the skin cells [8, 143]. The thin collagen fibers on the surface (superficial/papillary dermis) and much thicker collagen fibers in the deep (reticular dermis) provide mechanical strength to the skin [18]. Hair follicle stem cells are anticipated to be fundamental for the repairment of skin and regeneration of hair follicles, including specification into several types of hair follicle epithelial cells, epidermal cells, and sebaceous gland cells [109]. Moreover, sweat glands contribute to skin repair and are responsible for the thermo-regulation of the body [201].
The innermost layer of the skin is the hypodermis, underlying the reticular dermis [6]. The hypodermis consists of loose connective tissues and lipids, which provides insulation from cold and heat as a thermo-regulator. It also has several endocrine functions involved in inflammation, angiogenesis, food intake, glucose homeostasis, and lipid metabolism [45, 163].
3 Wound Types and Wound Healing Process
Damage or loss of integrity in skin layers can be caused by wounds. The wounds that resulted from blood circulation problems, burns, aging, surgical processes, or mechanical trauma, may disrupt the several functions of the skin [45]. They are grouped into chronic or acute wounds related to their underlying consequences and causes [92]. Skin damages often result in acute skin wounds. Acute wounds are healed through an organized repair process, and restoration of anatomical and functional integrity is provided to the tissue [50]. However, depending on the increment of the depth and size of a wound, the healing process may not complete properly, causing improper or delayed wound closure [157]. The wounds which do not progress through appropriate reparation of functional and structural integrity frequently result in chronic wounds [190]. Some factors like the presence of autoimmune/metabolic diseases, ongoing drug therapies, and patient age may influence the wound healing process and cause chronic wounds [106]. To restore the skin functions properly, rapid and exact wound healing are crucial.
The wound healing process includes closure of the wound to prevent infection, suppress pain, and recover the functionality of the skin. The epidermis has the capability for self-healing due to the presence of stem cells. However, in severe injuries, the healing mechanism is not adequate, thus resulting in a chronic wound [190]. Based on the injury’s depth, the wounds are categorized into four main groups consisting of epidermal, superficial partial-thickness (lose a part of the epidermis), deep partial-thickness (damage in deeper dermal layers and epidermis), and full-thickness skin wounds (damage in deeper tissue and subcutaneous fat) [135, 167]. The first two types are generally restored via self-healing processes. However, in the rest of the wound types, self-healing cannot be performed due to the destruction of the epithelial regenerative elements [152]. The healing process is a complex cascade system consisting of individual but overlapping stages. These are inflammation, hemostasis, proliferation, and remodeling (maturation) phases [25, 171] (Fig. 2). These dynamic and continuous processes are performed by various cellular components and molecular pathways and include the interactions of cells, ECM components, cytokines, and growth factors involved in repairing the wound [164]. The hemostasis phase reveals in a few seconds or minutes after the skin injury and is essentially mediated by platelets [145]. In this stage, the intravascular platelets are exposed to the subendothelial collagen by the skin injury, and thrombin is produced [177]. Then, in average patient profiles, fibrinogens are converted into fibrin fibers to prevent blood loss and form a fibrous scaffold for clotting blood. This blood plug temporarily prevents the loss of fluid and entry of pathogens [145, 190]. Besides, some cytokines and growth factors, such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), heparin-binding EGF-like growth factor, vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), transforming growth factor, alpha (TGF-α) and beta (TGF-β), are produced by activated platelets [16, 41, 105]. Then, the above-mentioned growth factors and cytokines diffuse into surrounding tissues and induce the migration of neutrophils and monocytes into the wound, within the first 24 h after the injury. Neutrophiles produce proteases and antimicrobial compounds [73, 145]. The monocytes differentiate into macrophages, and then the macrophages and also lymphocytes are attracted to the damaged layers to begin the inflammation phase [137, 145]. They digest the remaining matrix, cellular debris, and microorganisms to prevent infections (after about 48 h of injury) [21]. The blood circulation and expression of several pro-inflammatory factors, like colony-stimulating factor-1 (CSF-1) and tumor necrosis factor (TNF), are stimulated by these inflammatory cells. These molecules trigger the proliferation and migration of smooth muscle cells, keratinocytes, endothelial cells, and fibroblasts [146]. During this stage, collagen production is induced by the latter cells, and dead cells are surrounded by phagocytes [49, 104, 156]. The inflammatory phase prepares the damaged tissue for healing through restoring homeostasis and providing a barrier against pathogens [61, 114]. Production of novel tissue and angiogenesis (formation of blood vessels) begin with the third stage, namely, the proliferation phase. In this stage, due to the proliferation of the keratinocytes, re-epithelialization of the injured tissue occurs. Also, increased VEGF induces angiogenesis to generate the vascularized tissue [21]. The quantity of inflammatory cells is reduced during this stage, and PDGF and TGF-β chemotactically attract fibroblasts into the wound site [192]. Fibroblast growth factor (FGF) is continuously released by macrophages, and it induces the proliferation and activation of fibroblasts. ECM is produced by the latter cells [162]. Some proliferated fibroblasts produce type III collagen and glycosaminoglycans, like chondroitin-4-sulfate, heparin sulfate, dermatan sulfate, and hyaluronic acid [21, 168], which form a gel in which collagen fibers are deposited. The ECM is essential for the formation of novel tissue and formed by collagen and fibronectin [69]. Also, some fibroblasts change into myofibroblasts. The latter cells with their contractile function, reduce the wound size, and finally, contribute to the wound closure process [189]. In the final phase, the maturation (remodeling) stage, the ECM composition is changed by the reconstruction of collagen fibers type III to I. These changes and direction of fibers provide mechanical durability of the skin [67]. Besides, to reduce the blood vessel density and dermal cellularity, most of the cells that come from the previous stage encounter apoptosis. After all of these stages, by healing, the tissue gets its final appearance [21]. This phase is longer than the others, and generally begins 3 weeks post-injury and may continue for 2 years [145].
This native healing process gets severely destroyed in some pathophysiological cases. Because such critical cases result in loss of the skin tissue and this result in the interruption of wound healing [155]. Clinical treatments can be used in such situations to provide wound repair, and regeneration includes skin substitutes, allografts, autografts, cell therapy, and cytokine therapy [5, 17, 43, 133, 159]. Nevertheless, these conventional techniques are usually impeded by a small repair range, the availability of donor skin for grafting, high treatment cost, immune rejection, secondary injuries, and long healing time [59, 68]. Such situations can be overcome with current biofabrication techniques.
4 Skin Tissue Engineering
Damage to the function of skin integrity due to injuries and diseases represents a significant imbalance of physiological processes leading to disability. Common causes of skin damage include acute trauma, burns, chronic wounds, infections, genetic disorders, and surgical interventions [13]. Damages on the skin can be categorized according to their different thickness. These can be classified as epidermal, superficial partial-thickness, deep partial-thickness, and full-thickness skin wounds. The epidermis has an enormous wound healing capacity. However, there are situations where normal regeneration is insufficient and large areas of the epidermis need to be replaced. The regeneration capacity of the dermis is very low. The scar tissue formed in the absence of the dermis lacks the flexibility, elasticity, and strength of the natural dermis, also causes pain, limits movement, and is esthetically poor. There is a need for the development of engineering applications that mimic human skin for use as graft material after damage and wound, to restore skin function and facilitate wound healing [188]. Autologous grafts (autografts) taken from the patient’s own body are used to return the skin barrier to its normal function, facilitate wound healing after damage or injury, and prevent immune rejection. Unfortunately, autografts are insufficient for healing and wound closure of large and severe wounds or burns [111, 134, 158]. Skin tissue engineering develops applications to meet this need and to produce artificial skin using in vitro methods. The major important aim of skin tissue engineering is to regenerate the normal physiology and anatomy of natural skin [11]. Also, in skin tissue engineering, it is necessary to achieve effective recovery and full simulation of physiological skin, close to natural mechanical properties, without immune rejection or host toxicity. Artificial skin produced for skin regeneration should have a structural architecture to reinstitute the skin pigmentation, nerve, vascular plexus, and adnexa [89]. In recent years, researches have increased in skin tissue engineering applications, with the latest studies in materials science and the demand for artificial skin. The primary objective of skin tissue engineering is to produce a structure that provides skin regeneration and wound healing using various tissue engineering methods using suitable cells and biomaterials. Scaffolds that are produced for skin should have important properties such as biocompatibility, biodegradability, and non-toxic nature, and suitable mechanical properties [118]. Besides, skin tissue engineering scaffolds should be cost-effective and elicit minimal scarring and minimal inflammatory response [193]. Artificial skin, which is expected to replace and completely mimic the natural skin, is tried to be produced with many tissue engineering applications, including the three-dimensional bioprinting method.
5 Overview of 3D Bioprinting
The main aim of tissue engineering is to design cell-laden 3D structures that mimic natural tissue. So, designing and building biomaterial-based scaffolds is one of the most crucial parameters in tissue engineering [113]. Three-dimensional bioprinting (3D bioprinting), also known as additive manufacturing, steers novelties in many fields like engineering, manufacturing, medicine, art, and education. 3D bioprinting is an advanced manufacturing platform that enables the predefined deposition of living cells, biomaterials, and growth factors to manufacture customizable scaffolds via a layer-by-layer printing process using computer-aided design (CAD) [125]. The three-dimensional bioprinting technique has arisen as an alternative and easily applicable technique, especially in tissue engineering applications. With its broader definition, three-dimensional bioprinting can be defined as the computer-aided layer-by-layer modeling of bioinks, known as cells, DNA, drugs, growth factors, and biomaterials. This approach also includes toxicology studies and drug screening for the clinical use of artificial tissues created by the 3D printing method [119, 140].
Three-dimensional bioprinting can be described as a state-of-the-art product that aims to produce structures that are used for biological tissues with suitable and ideal hierarchical architecture. Living and functional tissues, which are much needed in tissue and organ transplantation, can be developed artificially. From this perspective, printing technologies are overwhelmingly accepted by researchers around the world as an alternative option to improve the lives of patients suffering from a disease [113]. Macro-scale architectures that can be achieved with bioprinting technology can perfectly mimic the natural extracellular matrix (ECM). Thus, it allows multiple cell types to bind and multiply at the same time [66]. Structures obtained by 3D printing method also have advantages such as adjustment of desired dimensional properties, mechanical strength, and simple drug loading [81]. The exact shapes and complex geometries of the desired tissues can be mimicked with the 3D bioprinting technology [178].
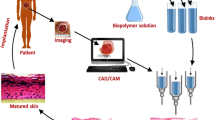
Generally, the 3D bioprinting process is separated into three main stages; pre-bioprinting (modeling), bioprinting, and post-bioprinting. Pre-bioprinting modeling is the process of designing the desired 3D model in a digital platform. It also includes the selection of bioink and biomaterials according to the desired tissue to be created and the type of 3D bioprinting model. Collecting complex tomography that will mimic natural tissue architecture is precisely composed using CAD software and stored as stereolithography (stl) files [147]. The designs of the printers are different from each other. In some printers, the 3D material file can be uploaded directly to the device, while in some printers, it is transferred to the printer after being converted into G-Code in the slicing program. The slicing program divides the shape into a stack of cross-sections and creates it by integrating it with the programmed fill pattern. After the printer reads the stl file, it deposits a layer of material to create the 3D model [34]. The bioprinting stage is the process of creating the desired structure with the desired features after making the necessary adjustments of many parameters. For tissue engineering applications, the printing process is divided according to two different procedures, with and without incorporated living cells. Printing with 3D printers is divided into three main categories according to their working strategies: extrusion-based, droplet-based, and laser-assisted bioprinting (mentioned in Sect. 5.2) [204]. Finally, post-printing is an important step in improving the mechanical support and biological functionality of generated structures [120]. Even more importantly, in vitro culture (inside in a bioreactor), in vivo implantation, and even in situ bioprinting can be used to improve the structure; hence, transform the formed structures into functional tissues [131].
5.1 3D Bioprinting Technologies
Scaffolds used in tissue engineering can be prepared according to two different approaches: bottom-up or top-down. Depending on the basic working principles for fabricating tissue structures, there are many different bioprinting strategies, such as inkjet bioprinting, laser-assisted bioprinting, pressure-supported (extrusion)-based bioprinting, acoustic bioprinting, stereolithography-based bioprinting, and magnetic bioprinting. These bioprinting methods can be applied with different combinations or alone, depending on the production goal [116]. Nowadays, in 3D bioprinters, three major methods are applied mostly: extrusion, droplet, and laser-based bioprinting (Fig. 3). The technique should be preferred depending on the characteristics of the cells and biomaterials to be used, the required sensitivity and speed, or the size and properties of the tissue to be printed.
5.1.1 Extrusion-Based 3D Bioprinting
The extrusion-based bioprinting technique is the most widespread application, especially due to its ability to produce greater extent 3D structures. This is the basis of all bioprinting techniques. The extrusion-based bioprinting technique is a combination of the fluid dispensing system and robotic system for bioprinting. Biological printing is distributed by the deposition system with the help of computer control. It results in precise deposition of encapsulated cells within cylindrical filaments of desired 3D-shaped structures. Thanks to this technique, the filaments are constantly deposited, which provides structural integrity [132]. During bioprinting, the dispensing head is moved along the X and Y axes to place the bioink onto a stage. As specified in CAD models, it is moved up and down along the Z-axis to create the scaffold by accumulating different layers [186]. Biological materials are extruded from the dispenser by pneumatic, mechanical (piston or screw assisted), or solenoid-based dispensing techniques [141]. Extrusion-based bioprinting is a suitable method for high viscosity materials that provide mechanical support, and low viscosity materials that support cellular bioactivity. This technique can print a very large class of biomaterials and cells, including both natural and synthetic hydrogel polymers, cell aggregates, and decellularized extracellular matrices. Compared to other bioprinting methods, it is more possible to deposit biomaterials with physiological cell density with the extrusion-based bioprinting technique. It can be preferred to produce large-scale scaffolds because it provides fast deposition speed [127]. Apart from the advantages provided by the extrusion-based bioprinting method, there are also some negative aspects. As higher pressures and smaller diameter nozzles are used in extrusion-based bioprinting, a decrease in cell viability has been observed due to process-induced stress [173]. In this technique, nozzle clogging caused by biomaterial solidification can completely interrupt the integrity of the created structure compared to nozzle-less techniques [127]. The other disadvantages are the lower resolution (≥100 μm) and less accuracy than other techniques [35].
5.1.2 Droplet-Based 3D Bioprinting
The droplet-based bioprinter was first presented in 1980s as the foundation of biological printing technologies [94]. The core of the droplet-based bioprinter is inkjet technology (also known as drop-on-demand printers). Inkjet printing is a technology derived from traditional 2D desktop inkjet printers. Inkjet printers that deliver controlled volumes of fluid to predefined locations are the widely preferred type of printer for non-biological and biological processes [182]. This non-contact bioprinting process relies on the formation and exact positioning of droplets on a computer-controlled substrate [151]. It has the same mechanism as the extrusion-based bioprinting technique [107]. The accumulated droplets create 3D scaffolds with the help of different chemical and physical cross-linking agents like pH, ultraviolet (UV) radiation, and cross-binding agents. With this method, cell viability is at least 70%, and this rate can be further increased by applying various procedures [64]. In inkjet bioprinters, drops created by heat and mechanical compression are often smaller than 30 µm, resulting in high resolution. Inkjet bioprinting sprays droplets onto the substrate using thermal, piezo, or acoustic forces.
A thermal inkjet bioprinter includes a heating element and an ink chamber with a nozzle. Thermal inkjet printers work by electrically heating the printhead to generate pressure pulses [24]. The thermal inkjet technique is more preferred because it is efficient, simple, and more economical. However, clogging of the nozzle due to bioink gelation disrupts the manufacturing of evenly sized drops and smooth printing operations [58]. Besides, the risk of exposing cells and materials to thermal and mechanical stress, low droplet orientation, non-uniform droplet size, unreliable cell encapsulation are major disadvantages [125]. In another technique, piezoelectric inkjet, the pressure pulse is produced by mechanically actuating piezoelectric crystals. Internal vibrations press the bioink droplet out of the nozzle. The pressure required to eject the droplets from the nozzle is achieved by using a voltage to the piezoelectric material, causing a quick change in structure [58]. Other inkjet printers can spray liquid droplets with the aid of the acoustic radiation force found in the ultrasound field. Acoustic inkjet printers have the advantages of generating and controlling a uniform droplet size and ejection directionality, as well as preventing cells from being exposed to heat and pressure stressors [150]. The main advantages of inkjet bioprinting are better resolution, affordable price, and the ability to easily model complex geometries. The main disadvantages are the lower viscosity range, longer printing time, which limits the bioink composition. Additionally, the technique used by the actuator to generate the drops can affect cell viability, especially the thermal mode [188].
5.1.3 Laser-Based 3D Bioprinting
Laser-based bioprinters are also called laser-assisted printers and laser direct printers. Although laser-based bioprinting systems are less popular compared to other printer techniques, over time they have been used more and more in tissue engineering applications. Laser-based printers have an ultraviolet (UV) laser, a hydrogel focusing principle that is made light-sensitive by the addition of a photoinitiator [95]. With the energy provided by the UV laser, covalent bonds are formed between neighboring polymer chains and allow the liquid to solidify [115]. This technology that is based on conventional laser-induced forward transfer is a nozzle-free technique. Laser-based printers include a pulsed laser beam, focusing system, donor slide, and collector substrate slide. The donor slide is covered with a laser-absorbing layer and bioink layer containing the biomaterials and cells to be transferred. The collector slide, which acts as the printing bed, is placed at distances ranging from millimeters to micrometer. Several lasers can be used in this technique to increase the printing speed. The main advantage of this method is that it has high sensitivity. The laser-based bioprinting technique allows more printing on small tissue surfaces and operates with lower viscosities (1–300 mega pascal-second [mPa · s]) than extrusion-based prints [65, 97, 154]. Because laser-assisted printers are a nozzle-free technique, it eliminates the risk of nozzle clogging and contamination, very widespread difficulties with nozzle-based bioprinting techniques [198]. Thanks to laser-based printers, high-resolution 2D and 3D patterns can be designed and different cell lines can be combined [163]. The most important advantage of laser-based printers is that cell encapsulated hydrogels accumulate high cell density (1 × 108 cells/ml) and high cell viability (>90%) at high print resolutions (80–140 mm) [96]. The desired mechanical performance in the produced structures is achieved by applying processes such as heating or photo-curing to fabricated parts. In laser-based printers, the thickness of each layer varies according to the exposure time to light energy. However, the laser-based printing technique is somewhat slow and costly compared to other techniques. The curing process and reaction kinetics are more complex. Besides, limited material types can be used in printing with this technique [12]. The use of metallic laser energy-absorbing layer in laser-based printers poses a high risk of metallic particle contamination. It is less efficient compared to other printing applications, and therefore the adaptation of this technique to the fabrication scale is limited [110].
6 3D Skin Bioprinting
It has now become possible to imitate and manufacture the skin, which is the body’s most complex, largest, and multi-layered organ, with 3D bioprinting applications. Tissue-engineered 3D skin structures have great potential as a graft for burnt skin replacement, in vitro human skin models, and wound healing for drug analysis [31]. Many researches so far have explored skin bioprinting methods to reconstruct functional skin tissue. The advantages of manufacturing skin structures with 3D bioprinting method are more than other traditional tissue engineering strategies. Especially with the skin bioprinting method, automation, standardization, and high sensitivity in the accumulation of cells can be achieved for clinical application. Although traditional tissue engineering applications, such as culturing cells in a scaffold and maturing in a bioreactor, achieve bioprinting-like results, there are many trends of the skin that require improvement in the manufacturing methods, including long production times to achieve larger areas. In situ bioprinting and in vitro bioprinting are two different strategies for how to apply print skin in wound treatments [187].
In situ bioprinting is the process of directly printing pre-cultured cells onto the wound site for wound healing. It is a mobile skin bioprinting system that includes a hand-held 3D scanner to determine the size and topography of the wound. The use of in situ bioprinting for wound regeneration allows for the precise accumulation of cells on the wound [131, 194]. In in vitro bioprinting, a skin structure (usually the dermis and epidermis) is made in vitro. The printed skin structure is then kept in a bioreactor for maturation. It is then transferred to the wound area of the patient or experimental animal [14]. The schematic illustration of 3D skin bioprinting is demonstrated in Fig. 4.
6.1 Design Considerations for Skin Bioprinting
6.1.1 Bio-Ink Development
The selection of bioink is an important parameter in skin bioprinting because it should provide the spectrum of biochemical (i.e., adhesion factors, growth factors, or signaling proteins, chemokines) and physical (i.e., interstitial flow, mechanical and structural properties of extracellular matrix) cues [63].
The bioink to be used must match the printability, biocompatibility, and mechanical strength properties of natural skin. The biomaterial to be used must have a fast gelation time for good quality bioprinting. The biomaterial to be used must have a fast gelation time for high throughput bioprinting. The complexity of natural ECM needs to be mimicked well enough to support cellular binding and proliferation. In addition, the deposition of thin bioink layers (1–2 mm) simplifies significant cell–cell interactions between adjacent printed cell layers. It is a considerable point in reducing cell sedimentation (Fung and Skalak 1982; [78]). There are different categories in the selection of bioink: cellular/non-cellular biomaterials as printing ink (functional scaffold bioprinting) and using only cells as printing ink (scaffold-free bioprinting) [125]. The produced biomaterial structures act as the extracellular matrix (ECM) that regulates tissue reconstruction. Other basic factors such as porosity, interconnection, pore sizes, and biological degradation kinetics are also taken into account in scaffolding [34].
6.1.2 Cell Sources and Selection
The proliferation of bioprinted cells is an important point when creating a 3D structure that will mimic the natural tissue most closely. There are two important parameters for cell selection; the first is how much the physiological state of bio-imprinted cells overlap with the cells of the natural tissue. The second is to what extent cells can maintain or improve their function in the optimized microenvironment [125]. Natural skin tissue contains multiple types of cells with specific and biological functions. Often choosing autologous cells minimizes the potential risk of rejection. Primary cells such as fibroblasts, melanocytes, and keratinocytes can be obtained from biopsies and can be used in bioprinting applications. The use of stem cells provides advantages in terms of differentiation and adaptation to natural tissue in skin bioprinting as in different areas [1, 102, 174].
7 Biofunctional Inks for Bioprinting in Skin Tissue Engineering
Three-dimension bioprinting is the important latest technology for the production of 3D structures for tissue engineering applications and regenerative medicine [62, 123]. Biofabrication is a powerful tool for developing biologically functional constructs [123]. The bioprinting consists of layer-by-layer deposition of living cells using computer-aided transfer processes to generate functional tissues and organs [62, 180].
The bioink properties are very important for choosing bioprinting techniques. Bioink formulations are the most important point in bioprinting process in aspect of rheological and biological properties to reach good printability [44]. Natural biomaterials are widely used in tissue engineering because of biodegradability, tunable properties, biocompatibility, abundantly available, and minimal inflammatory response in vivo [44, 87].
7.1 Natural Bioinks
The design and selection of ideal bioinks are important parameters for the bioprinting process. Bioink is a formulation of cells convenient for processing by an automated biofabrication technology. Biocompatibility, non-immunogenicity, nontoxicity, viscoelasticity, high mechanical integrity, convenient degradability are crucial parameters for providing ideal bioinks. Alginate, agarose, collagen, extracellular matrix derived from a special tissue, gelatin, or fibrin can be given as examples of natural bioinks [129].
7.1.1 Bioinks Based on Alginate
Alginate is an anionic linear copolymer polysaccharide consisting of (1,4)-linked α-l-guluronic acid (G) and β-d-mannuronic acid (M) residues harvested from Brown algae [20, 129]. The concentration of polymer, molecular weight, average chain subunit ratio, molecular weight distribution, pH of solution are critical parameters in the aspect of the viscosity of alginate [20, 27]. For cell bioprinting applications, the alginate is a remarkable material because of providing mechanical properties to the cell, biocompatibility, non-immunogenicity. Beside this, the gelation time is fast, and it is enable to fast encapsulation of cells and to hold water and other molecules, providing diffusion throughout the structure [20, 129]. Increasing the concentration of HMW alginates in bioink to increase structural stiffness is a common approach. If bioink material is modified with some parameters like viscosity, the concentration of alginate, and temperature, it will allow the user to optimize this material during several bioprinting techniques [20]. Despite its advantages, alginate has a relatively slow and unpredictable degradation rate [129]. To support cell adhesion and bioactivity, alginate is widely modified with functional groups, such as Arginylglycylaspartic acid peptides or blended with other polymers, such as collagen due to its bioinert nature [20]. Alginate was blended with different polymers such as hydroxyapatite [193], gelatin [193], polycaprolactone [37, 84, 160], and poloaxamer [7] to obtain different 3D printed structures for tissue engineering applications. For the first time, induced human pluripotent stem cells and human embryonic stem cells were bioprinted with alginate as bioink by Faulkner-Jones et al. [52]. In the other work, the effect of different combinations of HMW and LMW agaroses in creating 3D structures and supporting fibroblast cells was investigated by Park et al. The results showed that for bioprinting application area, combination of 2:1 ratio high molecular weight and LMW agarose polymer was good in aspect of its cell viability and process ability investigations for tissue engineering [138]. Nguyen et al. used hyaluronate-based nano fibrillated cellulose composite bioink and alginate-based nano fibrillated cellulose composite bioinks, it is also used to induce pluripotent stem cells for 3D bioprinting of cartilage tissue construct and compared with each other. The bioinks based on alginate with nanofibrillated cellulose composite presented higher cell proliferation [126].
7.1.2 Bioinks Based on Agarose
Agarose is a linear polysaccharide chain. It is obtained from seaweed. It is most commonly used in the bioprinting process among other natural polymers. Due to its advantageous gelation properties, it is used in a wide range of bioprinting applications. Agarose forms a hydrogel through hydrogen bonding, resulting in a gelled structure that will thermally degrade and cross-link naturally. Because of the gelation structure, the resulting bioink has high viscosity. This situation is a negative effect in the aspect of 3D printing. It can be used to lower viscosity bioinks, which are inherently less mechanically stable to overcome this problem. Agarose can be modified with, blended with different polymers, or bioactive groups to support biological activity and cell adhesion [20, 27]. Agarose-based bioink with collagen and agarose-based bioink with fibrinogen were used by Kreimendahl et al. It was reported that these agarose-based blend biomaterials were able to stable 3D structure and to support fibroblast and endothelial cell growth [100]. Another similar study was from Yang et al. for cartilage tissue engineering application. They used agarose collagen composition with sodium alginate as bioink. The results showed that the mechanical properties of biomaterial improved without affecting the gelling behavior [200]. Because of the advantageous properties of agarose, such as perfect gelation, biocompatibility, and rheological properties, it is a promising material for 3D bioprinting applications [82]. Daly et al. reported chemically modified agorose (carboxylated agarose) as a bioink for developing mechanically tunable 3D tissue structure. They used human mesenchymal stem cells for its evaluation. The results showed that the carboxylated agarose constructs yielded remarkable cell viability up to 95% according to the native agarose gel. The carboxylation degree may be changeable for obtainment of different gels with changing mechanical properties [37]. Daly et al. also reported Agorose was used to compare a range of commonly used hydrogel bioinks such as alginate, GelMA, and BioINK (loaded with mesenchymal stem cells) for their 3D printing biocompatibility properties for cartilage tissue engineering [38].
7.1.3 Bioinks Based on Collagen
Collagen is widely used for tissue engineering application area. Because its physiological properties of native skin and the main component of Extracellular Matrix are similar to each other [161, 185]. Besides this, because of its excellent biocompatible properties and low immunogenicity [112, 135], it is widely used in 3D bioprinting applications [62]. The basis of collagen bioink is a collagen hydrogel which is produced from the protein mass in the connective tissues of mammals [60, 130]. Collagen can be crosslinked with the change of temperature or changing of pH value or using vitamin Riboflavin [20, 62]. The mechanical properties of crosslinked collagen are better than the uncrosslinked one [54, 124]. Moreover, by adding various amounts of different polymers, for using it in 3D bioprinting, the mechanical properties of the collagen materials may be improved [62]. Bioink based on Collagen with sodium alginate was used to develop 3D structure by Yang et al. According to their report, the mechanical properties of the 3D structure suggest that alginate-collagen can be chosen for the field of cartilage tissue engineering. [199]. The other works about improving mechanical properties and biological activity were collagen-based, cell-loading bioink for 3D bioprinting application. The study showed increased biological activity and mechanical properties by adding of collagen [202]. Stratesteffen et al. used collagen with gelatin for producing 3D construct. It showed the ability of this collagen–gelatin combination to obtain stable 3D construct with good rheological properties and high biological activity [170].
7.1.4 Bioinks Based on Decellularized Extracellular Matrix
Extracellular Matrix (ECM) is a three-dimensional network of extracellular macromolecules such as collagen type II, glycosaminoglycans, chondroitin sulfate, elastin, fibronectin, laminin, tenascin, thrombospondin, vitronectin, fibrillin. Decellularized extracellular matrix (dECM) is obtained by removing the cells from desired organs or tissues by a sequential procedure leaving the ECM intact [62, 88]. The obtained components are pulverized, then dissolved in a suitable buffer solution. Moreover, several polymeric hydrogels can be added to this solution to improve the printability of the Decellularized Extracellular Matrix-based bioink [62]. However, there are some disadvantages about using of DECM for bioprinting applications. Because, the decellularization step is time-consuming and its use in organs or tissues is limited [20]. Variant polymeric hydrogels can be added to the solution for the increment of the printability of the Decellularized Extracellular Matrix-Based bioinks. For example, poly (caprolactone) was used to improve the printability of the Decellularized Extracellular Matrix-Based bioinks obtained from different type of tissues by Pati et al. The result of this work showed that good functionality of construct and high cell viability was obtained after the bioprinting [139]. For another work is related to 3D bioprinting for developing the cell-loading construct of tissue engineering applications. With controlling of heating and pH of the bioinks, they obtained 3D system which enable them to obtain the gelling form at 37 °C during 3D bioprinting [19].
7.1.5 Bioinks Based on Hyaluronic Acid
Hyaluronic acid (hyaluronan, HA) is a linear polysaccharide composed of d-glucuronic acid and N-acetyl-d-glucosamine. Hyaluronic acid is the basic component of Extracellular Matrix of cartilage and connective tissues [203]. Hyaluronic acid has remarkable properties for use in 3D bioprinting applications. These properties are non-immunogenic, biodegradable, and biocompatible [183, 185]. However, hyaluronic acid alone is not a viable polymer for 3D printing. Because Hyaluronic Acid has slow gelation time and its mechanical properties are not good. Because of this reason, there are a lot of studies in the literature about 3D printing application of HA blend-based bioinks. One of the works was to blend methacrylate with hyaluronic acid to form cross-linkable bioink by photo-crosslinking mechanism to increase mechanical properties and high stability after bioprinting [144]. The other study was declared by Stichler et al. They used hyaluronic acid with different synthetic polymers for producing a stable 3D construct with good cell viability. The ability of the hybrid 3D printed structures to improve chondrogenesis using a thiol-linked hyaluronic acid and polyglycidols gel with polycaprolactone [169]. There are a lot of works about the hyaluronic acid combination with synthetic polymers for 3D biorinting and various tissue engineering applications [74, 176].
7.1.6 Bioinks Based on Fibrin
Fibrin hydrogels are gelled by the enzymatic reaction of thrombin and fibrinogen [85, 185]. Thrombin and fibrinogen are the key proteins involved in blood clotting. It is an Extracellular Matrix part of the skin that promotes cell proliferation, vascularization, and differentiation [42]. Fibrin is highly biocompatible, biodegradable, and has capacity to bind cells, growth factors, and other Extracellular Matrix proteins [129]. Because of these promising properties, for tissue engineering applications, it is extensively used in 3D bioprinting. However, it has low viscosity and poor mechanical properties. Because of this reason, fibrin is widely blended with other polymers like HA, and during blending, the viscosity of bioink increases to provide the ability of 3D bioprinting [48]. The techniques have been based on the crosslinking of the other materials such as alginate, gelatin to generate 3D structures [108, 184]. For skin bioprinting applications, a bioink based on fibrin–gelatin blended hydrogel was declerated for using of a bio-paper. The results showed that fibrin–gelatin blended hydrogel ensures a natural scaffold for fibroblast embedding and culturing [71]. England et al. used fibrin combined with Hyaluronic acid to encapsulate Schwann cells for the aim of 3D printing. For supporting nerve generation, they researched both capabilities of bioink and in vitro characterizations [48].
7.1.7 Bioinks Based on Silk
Silk is a naturally derived hydrogel that includes ECM proteins, and it is used extensively to print different tissues. Silk fibroin is obtained from spiders or silkworm. Because of promising properties, such as tissue integration, biocompatibility, and cell permeability, it is widely used in tissue engineering applications area. Similar to the other polymer, fibrin can be often blended with other polymers. In the literature, there are various works about the blending of silk with other polymers. For example, Das et al. demonstrated a bioink based on silk–gelatin for investigation of 3D bioprinting application. Mesenchymal progenitor cells were used for the bioink formulation. The combination of silk and gelatin was crosslinked by sonication and enzymatic crosslinking methods [39]. Another similar study belongs to Rodriguez et al. They also used silk–gelatin blend for bioink application with the aim of increasing biocompatibility, tissue integration, and cell permeability in soft tissue integration, and in this work, glycerol was used for physical crosslinker [148]. The other blending method for silk with a polymer is silk and alginate combination. In this work, silk and alginate were crosslinked, thanks to calcium chloride [28]. Xiong et al. carried out the mechanism and efficiency of gelatin–silk-based ink to regenerate skin. In this study, they added fibroblast growth factor-2 in the ink before printing, and it is shown that there was an increment of the granulation and tissue regeneration in vitro and in vivo [181].
7.2 Bioinks Based on Synthetic Polymers
Natural polymer or hydrogels have many advantages such as the desired microenvironment mimicking the native Extracellular Matrix about proliferation and cell attachment for 3D bioprinting as bioinks. However, the tunable properties of the natural polymer are poor. Because of this reason, the natural polymer is used with synthetic or another natural polymer. Synthetic polymers are excellent candidates used in bioink formulations to improve printability, cross-linking, and mechanical properties [62].
7.2.1 Poly (Ethylene Glycol) (PEG)
PEG is a linear hydrophilic polyether compound and bioinert polymer. It is most widely used in 3D bioprinting. Polyethylene glycol may be functionalized with dimethacrylate and diacrylate to obtain the photo-cross-linkable Poly (ethylene glycol) diacrylate (PEGDA) and Poly (ethylene glycol) dimethacrylate (PEGDMA) [129]. Bioink-based poly(ethylene glycol) is used as polyethylene glycol-diacrylate and polyethylene glycol methacrylate in extrusion-based 3D bioprinting applications [32, 79]. Because poly (ethylene glycol) alone does not form a hydrogel [86]. It has a relatively low viscosity, and because of this reason, it is restricted to bioprint alone. As natural polymers-based bioinks, PEG can be used with different materials such as alginate, collagen, etc., for the aim of blending for 3D bioprinting applications to form different bioinks and to increase mechanical properties [75, 76, 86, 149]. Hockaday et al. used a Polyethlene-diacrylate with alginate blend as bioinks for printing aortic heart valve [75].
7.2.2 Pluronic®
Pluronic® is a block copolymer which consists of poly (ethylene oxide-b-propylene oxide-b-ethylene oxide). It is a hopeful bioink based on polymer type. Pluronic® is biocompatible with cells and tissues and is gel form at room temperature, and it is convenient for extrusion-based 3D bioprinting [86]. Pluronic® does not specifically support cell viability in long-term cell culture. However, there are some methods to improve the cell viability of Pluronic®-based bioinks. Khattak et al. reported that in the situation of Pluronic® with hydrocortisone, glucose, and glycerol as membrane-stabilizing agents, the viability of cells encapsulated in the matrix was increased [91]. Actually, Pluronic is not a bioink, technically due to their limited cell supporting capacity. However, it can be used as supporting ink [90].
7.2.3 Poly (N-isopropylacrylamide) (PNIPAAM)
Poly (N-isopropyl acrylamide) can be used in bioink area. PNIPAAM is a gel structure and thermoresponsive polymer. Poly (N-isopropyl acrylamide) has poor biodegradability and biocompatibility. In 3D bioprinting applications, PNIPAAM can be used in combination with different biopolymers and bioinks like HA and alginate [93, 172].
8 Current Challenges and Advances in Developing of Biofunctional Inks in Skin Tissue Engineering
There was a remarkable improvement in 3D bioprinting for the last few years to produce cellular constructs for skin tissue engineering. In the manufacture of artificial organs and tissues, the combination of bioinks based on natural or synthetic polymers and 3D bioprinting has significant potential for tissue engineering. For 3D bioprinting, the selection and design of bioinks are critical steps. Bioinks area consists of choosing of special cells and convenient materials designed for processing 3D structure. For producing tissue structure easily, 3D bioprintings have the remarkable properties. Nevertheless, bioinks need further improvement in the aspect of commercialization of the 3D printed products and developing more complex specific 3D constructs based on specific patients for an urgent medical situation.
In this chapter, different types of bioinks, the various selection parameters for bioinks, and properties of different bioinks types were discussed. The cell-loading hydrogels are widely preferred for the improvement of 3D structures during 3D bioprinting. 3D bioprinting methods have numerous advantages such as using special cell types, controlling biodegradation, adjusting mechanical properties, and design flexibility. For creating complex tissue structures, skin bioprinting has excellent advantages. It is a very promising alternative method, especially for correcting complex skin imperfections that are difficult to heal by normal clinical means.
The obtainment of a fully functional skin equivalent is the fundamental aim of 3D bioprinting, and it is transplanted and anastomosed with native blood circulation [9]. Another exciting development will be the 4D print [44]. Smart materials sensitive to stimuli can also provide special properties to bioprinted skin structures like triggered shape memory.
9 Conclusion
The skin, which is the body’s largest organ, has many functions such as protecting against toxins and microorganisms. There is no artificial skin model that mimics the natural skin and contains all its features. Therefore, one of the main goals of tissue engineering is to produce a universal skin substitute for skin damage. Three-dimension bioprinting is the important latest technology with excellent properties for the production of 3D structures among all tissue engineering techniques. Bioink formulations and properties are the most important points in the bioprinting process in aspects of rheological and biological properties to reach good printability. Bioink can be classified basically into two parts which are natural and synthetic. Natural bioinks have some good properties such as biocompatibility, viscoelasticity, high mechanical integrity, appropriate degradability, nontoxicity, and non-immunogenicity crucial for providing ideal bioinks. In this chapter, alginate, agarose, collagen, gelatin, decellularized matrix, fibrin-based bioink, hyaluronic acid-based bioink, silk-based bioink were given as natural types of bioink for skin bioprinting. The tunable properties of the natural polymer are poor. Because of this reason, the natural polymer is used with synthetic or another natural polymer. Synthetic polymers are excellent candidates used in bioink formulations to improve printability, cross-linking, and mechanical properties. The details of synthetic bioinks of Poly (Ethylene Glycol) (PEG), Pluronic®, and Poly (N-isopropyl acrylamide) (PNIPAAM) are given. This chapter deals with reviewing the utilization of bioinks for 3D bioprinting in skin tissue engineering, and experimental studies in the literature related to bioinks used for 3D bioprinting in tissue engineering were given.
References
Abbott RD, Kaplan DL (2015) Strategies for improving the physiological relevance of human engineered tissues. Trends Biotechnol 33(7):401–407
Abdo JM, Sopko NA, Milner SM (2020) The applied anatomy of human skin: a model for regeneration. Wound Med 28:100179
Albanna M, Holmes IV JH (2016) Skin tissue engineering and regenerative medicine. Academic Press
Alderfer L, Wei A, Hanjaya-Putra D (2018) Lymphatic tissue engineering and regeneration. J Biol Eng 12(1):32
Ali JM, Catarino P, Dunning J, Giele H, Vrakas G, Parmar J (2016, October) Could sentinel skin transplants have some utility in solid organ transplantation? In Transplantation proceedings, vol 48, no 8. Elsevier, pp 2565–2570
Amirsadeghi A, Jafari A, Eggermont L, Hashemi SS, Bencherif SA, Khorram M (2020) Vascularization strategies for skin tissue engineering. Biomater Sci 8(15):4073–4094
Armstrong JP, Burke M, Carter BM, Davis SA, Perriman AW (2016) 3D bioprinting using a templated porous bioink. Adv Healthcare Mater 5(14):1724–1730
Arroyo JA, Winn VD (2008, June) Vasculogenesis and angiogenesis in the IUGR placenta. In: Seminars in perinatology, vol 32, no 3. WB Saunders, pp 172–177
Augustine R (2018) Skin bioprinting: a novel approach for creating artificial skin from synthetic and natural building blocks. Prog Biomater 7(2):77–92
Balieva F, Kupfer J, Lien L, Gieler U, Finlay AY, Tomás‐Aragonés L, ... Halvorsen JA (2017) The burden of common skin diseases assessed with the EQ5D™: a European multicentre study in 13 countries. British J Dermatol 176(5):1170–1178
Bannasch H, Föhn M, Unterberg T, Bach AD, Weyand B, Stark GB (2003) Skin tissue engineering. Clin Plast Surg 30(4):573–579
Bedir T, Ulag S, Ustundag CB, Gunduz O (2020) 3D bioprinting applications in neural tissue engineering for spinal cord injury repair. Mater Sci Eng: C110:110741
Bhardwaj N, Chouhan D, Mandal BB (2018) 3D functional scaffolds for skin tissue engineering. In: Functional 3D tissue engineering scaffolds. Woodhead Publishing, pp 345–365
Binder KW, Zhao W, Aboushwareb T, Dice D, Atala A, Yoo JJ (2010) In situ bioprinting of the skin for burns. J Am Coll Surg 211(3):S76
Bouwstra JA, Pilgram GS, Ponec M (2006) Structure of the skin barrier. Skin barrier. Taylor & Francis, New York, pp 65–96
Braund R, Hook S, Medlicott NJ (2007) The role of topical growth factors in chronic wounds. Curr Drug Deliv 4(3):195–204
Brock WD, Bearden W, Tann T III, Long JA (2003) Autogenous dermis skin grafts in lower eyelid reconstruction. Ophthalmic Plast Reconstr Surg 19(5):394–397
Brown TM, Krishnamurthy K (2018) Histology, dermis. In: StatPearls [Internet]. StatPearls Publishing
Burdick JA, Prestwich GD (2011) Hyaluronic acid hydrogels for biomedical applications. Adv Mater 23(12):H41–H56
Camacho P, Busari H, Seims KB, Tolbert JW, Chow LW (2019) Materials as bioinks and bioink design. In: 3D bioprinting in medicine. Springer, Cham, pp 67–100
Casado-Díaz A, Quesada-Gómez JM, Dorado G (2020) Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: applications in skin wound healing.Front Bioeng Biotechnol 8
Chen D, Hou Q, Zhong L, Zhao Y, Li M, Fu X (2019) Bioactive molecules for skin repair and regeneration: progress and perspectives. Stem Cells Int 2019:6789823
Chimene D, Lennox KK, Kaunas RR, Gaharwar AK (2016) Advanced bioinks for 3D printing: a materials science perspective. Ann Biomed Eng 44(6):2090–2102
Choi CH, Lin LY, Cheng CC, Chang CH (2015) Printed oxide thin film transistors: a mini review. ECS J Solid State Sci Technol 4(4):P3044
Chouhan D, Dey N, Bhardwaj N, Mandal BB (2019) Emerging and innovative approaches for wound healing and skin regeneration: current status and advances. Biomaterials, 216:119267
Chua AWC, Khoo YC, Tan BK, Tan KC, Foo CL, Chong SJ (2016) Skin tissue engineering advances in severe burns: review and therapeutic applications. Burns Trauma 4:1–14
Chung JH, Naficy S, Yue Z, Kapsa R, Quigley A, Moulton SE, Wallace GG (2013) Bio-ink properties and printability for extrusion printing living cells. Biomater Sci 1(7):763–773
Compaan AM, Christensen K, Huang Y (2017) Inkjet bioprinting of 3D silk fibroin cellular constructs using sacrificial alginate. ACS Biomater Sci Eng 3(8):1519–1526
Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, ... Tazi-Ahnini R (2006). New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene–environment interactions. J Allergy Clin Immunol 118(1):3–21
Coyer F, Gardner A, Doubrovsky A, Cole R, Ryan FM, Allen C, McNamara G (2015) Reducing pressure injuries in critically ill patients by using a patient skin integrity care bundle (InSPiRE). Am J Crit Care 24(3):199–209
Cubo N, Garcia M, Del Cañizo JF, Velasco D, Jorcano JL (2016) 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication 9(1):015006
Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD (2012) Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A 18(11–12):1304–1312
Cui H, Zhu W, Nowicki M, Zhou X, Khademhosseini A, Zhang LG (2016a) Hierarchical fabrication of engineered vascularized bone biphasic constructs via dual 3D bioprinting: integrating regional bioactive factors into architectural design. Adv Healthcare Mater 5(17):2174–2181
Cui H, Nowicki M, Fisher JP, Zhang LG (2017) 3D bioprinting for organ regeneration. Adv Healthcare Mater 6(1):1601118
Dababneh AB, Ozbolat IT (2014) Bioprinting technology: a current state-of-the-art review. J Manuf Sci Eng 136(6)
Dalgard FJ, Gieler U, Tomas-Aragones L, Lien L, Poot F, Jemec GB, ... Evers AW (2015) The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol 135(4):984–991
Daly AC, Critchley SE, Rencsok EM, Kelly DJ (2016a) A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 8(4):045002 (b)
Daly AC, Cunniffe GM, Sathy BN, Jeon O, Alsberg E, Kelly DJ (2016b) 3D bioprinting of developmentally inspired templates for whole bone organ engineering. Adv Healthcare Mater 5(18):2353–2362 (a)
Das S, Pati F, Choi YJ, Rijal G, Shim JH, Kim SW, ... Ghosh S (2015) Bioprintable, cell-laden silk fibroin–gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomaterialia 11:233–246
Das S, Baker AB (2016) Biomaterials and nanotherapeutics for enhancing skin wound healing. Front Bioeng Biotechnol 4:82
Delavary BM, van der Veer WM, van Egmond M, Niessen FB, Beelen RH (2011) Macrophages in skin injury and repair. Immunobiology 216(7):753–762
De Luca AC, Lacour SP, Raffoul W, Di Summa PG (2014) Extracellular matrix components in peripheral nerve repair: how to affect neural cellular response and nerve regeneration? Neural Regen Res 9(22):1943
Demidova-Rice TN, Hamblin MR, Herman IM (2012) Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 2: role of growth factors in normal and pathological wound healing: therapeutic potential and methods of delivery. Adv Skin Wound Care 25(8):349–370
Dorishetty P, Dutta NK, Choudhury NR (2020) Bioprintable tough hydrogels for tissue engineering applications. Adv Colloid Interf Sci 102163
Dussoyer M, Michopoulou A, Rousselle P (2020) Decellularized scaffolds for skin repair and regeneration. Appl Sci 10(10):3435
Dzobo K, Thomford NE, Senthebane DA, Shipanga H, Rowe A, Dandara C, ... Motaung KSCM (2018) Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells Int 2018:2495848
Elias PM, Feingold KR (2005) Stratum corneum barrier function: definitions and broad concepts. In: Skin barrier. CRC Press, pp 21–24
England S, Rajaram A, Schreyer DJ, Chen X (2017) Bioprinted fibrin-factor XIII-hyaluronate hydrogel scaffolds with encapsulated Schwann cells and their in vitro characterization for use in nerve regeneration. Bioprinting 5:1–9
Enoch S, Leaper DJ (2008) Basic science of wound healing. Surgery (Oxford) 26(2):31–37
Erickson JR, Echeverri K (2018) Learning from regeneration research organisms: the circuitous road to scar free wound healing. Dev Biol 433(2):144–154
Fartasch M (1997) Ultrastructure of the epidermal barrier after irritation. Microsc Res Tech 37(3):193–199
Faulkner-Jones A, Fyfe C, Cornelissen DJ, Gardner J, King J, Courtney A, Shu W (2015) Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 7(4):044102
Feingold KR, Elias PM (2014) Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochimica et Biophysica Acta (BBA)-Molecular Cell Biol Lipids 1841(3):280–294
Ferreira AM, Gentile P, Chiono V, Ciardelli G (2012) Collagen for bone tissue regeneration. Acta Biomater 8(9):3191–3200
Freinkel RK, Woodley DT (eds) (2001) The biology of the skin. CRC Press
Frueh FS, Sanchez-Macedo N, Calcagni M, Giovanoli P, Lindenblatt N (2018) The crucial role of vascularization and lymphangiogenesis in skin reconstruction. Eur Surg Res 59(3–4):242–254
Gantwerker EA, Hom DB (2012) Skin: histology and physiology of wound healing. Clin Plast Surg 39(1):85–97
Gao Q, He Y, Fu JZ, Liu A, Ma L (2015) Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 61:203–215
Gautam S, Chou CF, Dinda AK, Potdar PD, Mishra NC (2014) Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mater Sci Eng C 34:402–409
Gelse K, Pöschl E, Aigner T (2003) Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev 55(12):1531–1546
Gonzalez ACDO, Costa TF, Andrade ZDA, Medrado ARAP (2016) Wound healing—a literature review. An Bras Dermatol 91(5):614–620
Gopinathan J, Noh I (2018) Recent trends in bioinks for 3D printing. Biomaterials research 22(1):11
Griffith LG, Swartz MA (2006) Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7(3):211–224
Gudapati H, Dey M, Ozbolat I (2016) A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials 102:20–42
Guillemot F, Souquet A, Catros S, Guillotin B, Lopez J, Faucon M, ... Chabassier P (2010) High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomaterialia 6(7):2494–2500
Guillotin B, Guillemot F (2011) Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol 29(4):183–190
Guo SA, DiPietro LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–229
Gupta SK, Dinda AK, Potdar PD, Mishra NC (2013) Fabrication and characterization of scaffold from cadaver goat-lung tissue for skin tissue engineering applications. Mater Sci Eng, C 33(7):4032–4038
Haas AF (1995) Wound healing. Dermatol Nurs 7(1):28–74
Hagura A, Asai J, Maruyama K, Takenaka H, Kinoshita S, Katoh N (2014) The VEGF-C/VEGFR3 signaling pathway contributes to resolving chronic skin inflammation by activating lymphatic vessel function. J Dermatol Sci 73(2):135–141
Hakam MS, Imani R, Abolfathi N, Fakhrzadeh H, Sharifi AM (2016) Evaluation of fibrin-gelatin hydrogel as biopaper for application in skin bioprinting: an in-vitro study. Bio-Med Mater Eng 27(6):669–682
Hall AH, Mathieu L, Maibach HI (2018) Acute chemical skin injuries in the United States: a review. Crit Rev Toxicol 48(7):540–554
Hart J (2002) Inflammation 2: its role in the healing of chronic wounds. J Wound Care 11(7):245–249
Hemshekhar M, Thushara RM, Chandranayaka S, Sherman LS, Kemparaju K, Girish KS (2016) Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int J Biol Macromol 86:917–928
Hockaday LA, Kang KH, Colangelo NW, Cheung PYC, Duan B, Malone E, ... Chu CC (2012) Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 4(3):035005
Hong S, Sycks D, Chan HF, Lin S, Lopez GP, Guilak F, ... Zhao X (2015) 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv Mater 27(27):4035–4040
Horch RE, Weigand A, Wajant H, Groll J, Boccaccini AR, Arkudas A (2018) Biofabrikation–neue Ansätze für den artifiziellen Gewebeersatz.Handchirurgie·Mikrochirurgie Plastische Chirurgie 50(2):93–100
Horváth L, Umehara Y, Jud C, Blank F, Petri-Fink A, Rothen-Rutishauser B (2015) Engineering an in vitro air-blood barrier by 3D bioprinting. Sci Rep 5(1):1–8
Hribar KC, Soman P, Warner J, Chung P, Chen S (2014) Light-assisted direct-write of 3D functional biomaterials. Lab Chip 14(2):268–275
Huethorst E, Krebber MM, Fledderus JO, Gremmels H, Xu YJ, Pei J, ... Cheng C (2016) Lymphatic vascular regeneration: the next step in tissue engineering. Tissue Eng Part B: Rev 22(1):1–14
Ilhan E, Cesur S, Guler E, Topal F, Albayrak D, Guncu MM, ... Oktar FN (2020) Development of Satureja cuneifolia-loaded sodium alginate/polyethylene glycol scaffolds produced by 3D-printing technology as a diabetic wound dressing material. Int J Biol Macromol 161:1040–1054
Jakus AE, Rutz AL, Shah RN (2016) Advancing the field of 3D biomaterial printing. Biomed Mater 11(1):014102
Jammalamadaka U, Tappa K (2018) Recent advances in biomaterials for 3D printing and tissue engineering. J Funct Biomater 9(1):22
Jang CH, Ahn SH, Yang GH, Kim GH (2016) A MSCs-laden polycaprolactone/collagen scaffold for bone tissue regeneration. RSC Adv 6(8):6259–6265
Janmey PA, Winer JP, Weisel JW (2009) Fibrin gels and their clinical and bioengineering applications. J R Soc Interface 6(30):1–10
Ji S, Guvendiren M (2017) Recent advances in bioink design for 3D bioprinting of tissues and organs. Front Bioeng Biotechnol 5:23
Jia J, Richards DJ, Pollard S, Tan Y, Rodriguez J, Visconti RP, ... Mei Y (2014) Engineering alginate as bioink for bioprinting. Acta Biomaterialia 10(10):4323–4331
Kabirian F, Mozafari M (2020) Decellularized ECM-derived bioinks: prospects for the future. Methods 171:108–118
Kamel RA, Ong JF, Eriksson E, Junker JP, Caterson EJ (2013) Tissue engineering of skin. J Am Coll Surg 217(3):533–555
Kang SW, Kim JS, Park KS, Cha BH, Shim JH, Kim JY, ... Lee SH (2011) Surface modification with fibrin/hyaluronic acid hydrogel on solid-free form-based scaffolds followed by BMP-2 loading to enhance bone regeneration. Bone 48(2):298–306
Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A (2016) A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34(3):312–319
Karimi K, Odhav A, Kollipara R, Fike J, Stanford C, Hall JC (2017) Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg 21(5):425–437
Kesti M, Müller M, Becher J, Schnabelrauch M, D’Este M, Eglin D, Zenobi-Wong M (2015) A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater 11:162–172
Klebe RJ (1988) Cytoscribing: a method for micropositioning cells and the construction of two-and three-dimensional synthetic tissues. Exp Cell Res 179(2):362–373
Knowlton S, Yenilmez B, Anand S, Tasoglu S (2017) Photocrosslinking-based bioprinting: examining crosslinking schemes. Bioprinting 5:10–18
Koch L, Kuhn S, Sorg H, Gruene M, Schlie S, Gaebel R, ... Vogt PM (2010) Laser printing of skin cells and human stem cells. Tissue Eng Part C: Methods 16(5):847–854
Koch L, Gruene M, Unger C, Chichkov B (2013) Laser assisted cell printing. Curr Pharm Biotechnol 14(1):91–97
Kolarsick PA, Kolarsick MA, Goodwin C (2011) Anatomy and physiology of the skin. J Dermatol Nurses’ Assoc 3(4):203–213
Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA (2016) Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci 113(12):3179–3184
Kreimendahl F, Köpf M, Thiebes AL, Duarte Campos DF, Blaeser A, Schmitz-Rode T, ... Fischer H (2017) Three-dimensional printing and angiogenesis: tailored agarose-type I collagen blends comprise three-dimensional printability and angiogenesis potential for tissue-engineered substitutes. Tissue Eng Part C: Methods 23(10):604–615
Kuehn BM (2007) Chronic wound care guidelines issued. Jama 297(9):938–939
Kumar A, Starly B (2015) Large scale industrialized cell expansion: producing the critical raw material for biofabrication processes. Biofabrication 7(4):044103
Langer R, Vacanti JP (1993) Tissue engineering. Sci 260(5110):920–926
Laurens N, Koolwijk P, de Maat MP (2006) Fibrin structure and wound healing. J Thrombosis Haemostasis: JTH 4(5):932–939
Lawrence WT (1998) Physiology of the acute wound. Clin Plast Surg 25(3):321–340
Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Percoraro RE, Rodeheaver G, Robson MC (1994) Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen 2(3):165–170
Le HP (1998) Progress and trends in ink-jet printing technology. J Imaging Sci Technol 42(1):49–62
Lee YB, Polio S, Lee W, Dai G, Menon L, Carroll RS, Yoo SS (2010) Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp Neurol 223(2):645–652
Leirós GJ, Kusinsky AG, Drago H, Bossi S, Sturla F, Castellanos ML, ... Balañá ME (2014) Dermal papilla cells improve the wound healing process and generate hair bud‐like structures in grafted skin substitutes using hair follicle stem cells. Stem Cells Transl Med 3(10):1209–1219
Li J, Chen M, Fan X, Zhou H (2016) Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med 14(1):271
Loss M, Wedler V, Künzi W, Meuli-Simmen C, Meyer VE (2000) Artificial skin, split-thickness autograft and cultured autologous keratinocytes combined to treat a severe burn injury of 93% of TBSA. Burns 26(7):644–652
Lynn AK, Yannas IV, Bonfield W (2004) Antigenicity and immunogenicity of collagen. J Biomed Mater Res Part B: Appl Biomater: Off J Soc Biomater Japanese Soc Biomater Austr Soc Biomater Korean Soc Biomater 71(2):343–354
Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT (2020) Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 226:119536
Mayet N, Choonara YE, Kumar P, Tomar LK, Tyagi C, Du Toit LC, Pillay V (2014) A comprehensive review of advanced biopolymeric wound healing systems. J Pharm Sci 103(8):2211–2230
Melchels FP, Feijen J, Grijpma DW (2010) A review on stereolithography and its applications in biomedical engineering. Biomaterials 31(24):6121–6130
Melchels FP, Domingos MA, Klein TJ, Malda J, Bartolo PJ, Hutmacher DW (2012) Additive manufacturing of tissues and organs. Prog Polym Sci 37(8):1079–1104
Menon GK, Dryer L, Kalafsky R (2009) Approaches to the development of cosmetic products to counter the effects of skin aging. In: Skin aging handbook. William Andrew Publishing, pp 265–290
Metcalfe AD, Ferguson MW (2007) Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interf 4(14):413–437
Mironov V, Kasyanov V, Drake C, Markwald RR (2008) Organ printing: promises and challenges. Future Med 3(1):93–103
Mironov V, Kasyanov V, Markwald RR (2011) Organ printing: from bioprinter to organ biofabrication line. Curr Opin Biotechnol 22(5):667–673
Mohammadi MH, Heidary Araghi B, Beydaghi V, Geraili A, Moradi F, Jafari P, ... Sanati‐Nezhad A (2016) Skin diseases modeling using combined tissue engineering and microfluidic technologies. Adv Healthcare Mater 5(19):2459–2480
Montero FE, Rezende RA, Silva JVL, Sabino MA (2019) Development of a smart bioink for bioprinting applications. Front Mech Eng 5:56
Morgan FL, Moroni L, Baker MB (2020) Dynamic bioinks to advance bioprinting. Adv Healthcare Mater 1901798
Mori H, Shimizu K, Hara M (2013) Dynamic viscoelastic properties of collagen gels with high mechanical strength. Mater Sci Eng, C 33(6):3230–3236
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32(8):773–785
Nguyen D, Hägg DA, Forsman A, Ekholm J, Nimkingratana P, Brantsing C, ... Lindahl A (2017) Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci Rep 7(1):1–10
Ning L, Chen X (2017) A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol J 12(8):1600671
Norouzi M, Boroujeni SM, Omidvarkordshouli N, Soleimani M (2015) Advances in skin regeneration: application of electrospun scaffolds. Adv Healthcare Mater 4(8):1114–1133
Nulty J, Schipani R, Burdis R, Kelly DJ (2019) Bioinks and their applications in tissue engineering. In: Polymer-based additive manufacturing. Springer, Cham, pp 187–218
Osidak EO, Kozhukhov VI, Osidak MS, Domogatskiy SP (2020) Collagen as bioink for bioprinting: a comprehensive review. Int J Bioprint 6(3)
Ozbolat IT (2015) Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol 33(7):395–400
Ozbolat IT, Hospodiuk M (2016) Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 76:321–343
Pajardi G, Rapisarda V, Somalvico F, Scotti A, Russo GL, Ciancio F, ... Trabucchi E (2016) Skin substitutes based on allogenic fibroblasts or keratinocytes for chronic wounds not responding to conventional therapy: a retrospective observational study. Int Wound J 13(1):44–52
Papageorgiou SK, Kouvelos EP, Favvas EP, Sapalidis AA, Romanos GE, Katsaros FK (2010) Metal–carboxylate interactions in metal–alginate complexes studied with FTIR spectroscopy. Carbohyd Res 345(4):469–473
Papini R (2004) Management of burn injuries of various depths. BMJ 329(7458):158–160
Parak A, Pradeep P, du Toit LC, Kumar P, Choonara YE, Pillay V (2019) Functionalizing bioinks for 3D bioprinting applications. Drug Discov Today 24(1):198–205
Park JW, Hwang SR, Yoon IS (2017a) Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 22(8):1259
Park J, Lee SJ, Chung S, Lee JH, Kim WD, Lee JY, Park SA (2017b) Cell-laden 3D bioprinting hydrogel matrix depending on different compositions for soft tissue engineering: characterization and evaluation. Mater Sci Eng: C 71:678–684
Pati F, Jang J, Ha DH, Kim SW, Rhie JW, Shim JH, ... Cho DW (2014) Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 5(1):1–11
Peng W, Unutmaz D, Ozbolat IT (2016) Bioprinting towards physiologically relevant tissue models for pharmaceutics. Trends Biotechnol 34(9):722–732
Peng W, Datta P, Ayan B, Ozbolat V, Sosnoski D, Ozbolat IT (2017) 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomater 57:26–46
Pereira RF, Barrias CC, Granja PL, Bartolo PJ (2013) Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine 8(4):603–621
Pittman RN (2011, April) Regulation of tissue oxygenation. In: Colloquium series on integrated systems physiology: from molecule to function, vol 3, no 3. Morgan & Claypool Life Sciences, pp 1–100
Poldervaart MT, Goversen B, De Ruijter M, Abbadessa A, Melchels FP, Öner FC, ... Alblas J (2017) 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS One 12(6):e0177628
Rahmati M, Blaker JJ, Lyngstadaas SP, Mano JF, Haugen HJ (2020) Designing multigradient biomaterials for skin regeneration. Mater Today Adv 5:100051
Reinke JM, Sorg H (2012) Wound repair and regeneration. Eur Surgical Res. Europaische chirurgische Forschung. Recherches chirurgicales europeennes 49(1):35–43
Rengier F, Mehndiratta A, Von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU, Giesel FL (2010) 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg 5(4):335–341
Rodriguez MJ, Brown J, Giordano J, Lin SJ, Omenetto FG, Kaplan DL (2017) Silk based bioinks for soft tissue reconstruction using 3-dimensional (3D) printing with in vitro and in vivo assessments. Biomaterials 117:105–115
Rutz AL, Hyland KE, Jakus AE, Burghardt WR, Shah RN (2015) A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv Mater 27(9):1607–1614
Saunders R, Bosworth L, Gough J, Derby B, Reis N (2004) Selective cell delivery for 3D tissue culture and engineering. Eur Cells Mater 7(Suppl 1):84–5.ls
Saunders RE, Derby B (2014) Inkjet printing biomaterials for tissue engineering: bioprinting. Int Mater Rev 59(8):430–448
Savoji H, Godau B, Hassani MS, Akbari M (2018) Skin tissue substitutes and biomaterial risk assessment and testing. Front Bioeng Biotechnol 6:86
Schaupper M, Jeltsch M, Rohringer S, Redl H, Holnthoner W (2016) Lymphatic vessels in regenerative medicine and tissue engineering. Tissue Eng Part B Rev 22(5):395–407
Schiele NR, Corr DT, Huang Y, Raof NA, Xie Y, Chrisey DB (2010) Laser-based direct-write techniques for cell printing. Biofabrication 2(3):032001
Seavey JG, Masters ZA, Balazs GC, Tintle SM, Sabino J, Fleming ME, Valerio IL (2016) Use of a bioartificial dermal regeneration template for skin restoration in combat casualty injuries. Regen Med 11(1):81–90
Shah JMY, Omar E, Pai DR, Sood S (2012) Cellular events and biomarkers of wound healing. Indian J Plast Surgery: Off Publ Assoc Plast Surgeons India 45(2):220
Shamloo A, Sarmadi M, Aghababaie Z, Vossoughi M (2018) Accelerated full-thickness wound healing via sustained bFGF delivery based on a PVA/chitosan/gelatin hydrogel incorporating PCL microspheres. Int J Pharm 537(1–2):278–289
Sheridan R (2009) Closure of the excised burn wound: autografts, semipermanent skin substitutes, and permanent skin substitutes. Clin Plast Surg 36(4):643–651
Shi C, Zhu Y, Su Y, Cheng T (2006) Stem cells and their applications in skin-cell therapy. Trends Biotechnol 24(1):48–52
Shim JH, Lee JS, Kim JY, Cho DW (2012). Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J Micromech Microeng 22(8):085014
Shoulders MD, Raines RT (2009) Collagen structure and stability. Ann Rev Biochem 78:929–958
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746
Singh D, Singh D, Han SS (2016) 3D printing of scaffold for cells delivery: advances in skin tissue engineering. Polymers 8(1):19
Sinno H, Prakash S (2013) Complements and the wound healing cascade: an updated review. Plast Surgery Int 2013:146764
Skardal A (2018) Perspective:“Universal” bioink technology for advancing extrusion bioprinting-based biomanufacturing. Bioprinting 10:e00026
Smith MM, Melrose J (2015) Proteoglycans in normal and healing skin. Adv Wound Care 4(3):152–173
Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U (2017) Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res 58(1–2):81–94
Stadelmann WK, Digenis AG, Tobin GR (1998) Physiology and healing dynamics of chronic cutaneous wounds. Am J Surgery 176(2):26S-38S
Stichler S, Böck T, Paxton N, Bertlein S, Levato R, Schill V, ... Groll J (2017) Double printing of hyaluronic acid/poly (glycidol) hybrid hydrogels with poly (ε-caprolactone) for MSC chondrogenesis. Biofabrication 9(4):044108
Stratesteffen H, Köpf M, Kreimendahl F, Blaeser A, Jockenhoevel S, Fischer H (2017) GelMA-collagen blends enable drop-on-demand 3D printablility and promote angiogenesis. Biofabrication 9(4):045002
Talikowska M, Fu X, Lisak G (2019) Application of conducting polymers to wound care and skin tissue engineering: a review. Biosens Bioelectron 135:50–63
Tan R, She Z, Wang M, Fang Z, Liu Y, Feng Q (2012) Thermo-sensitive alginate-based injectable hydrogel for tissue engineering. Carbohyd Polym 87(2):1515–1521
Tanzeglock T, Soos M, Stephanopoulos G, Morbidelli M (2009) Induction of mammalian cell death by simple shear and extensional flows. Biotechnol Bioeng 104(2):360–370
Tasoglu S, Demirci U (2013) Bioprinting for stem cell research. Trends Biotechnol 31(1):10–19
Ter Horst B, Chouhan G, Moiemen NS, Grover LM (2018) Advances in keratinocyte delivery in burn wound care. Adv Drug Deliv Rev 123:18–32
Tirella A, Orsini A, Vozzi G, Ahluwalia ARTI (2009) A phase diagram for microfabrication of geometrically controlled hydrogel scaffolds. Biofabrication 1(4):045002
Traversa B, Sussman G (2001) The role of growth factors, cytokines and proteases in wound management. Primary Intent: Austr J Wound Manag 9(4):161
Ulag S, Ilhan E, Sahin A, Yilmaz BK, Ekren N, Kilic O, ... Gunduz O (2020). 3D printed artificial cornea for corneal stromal transplantation. Eur Polym J 109744
Usui ML, Mansbridge JN, Carter WG, Fujita M, Olerud JE (2008) Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J Histochem Cytochem 56(7):687–696
Xia Z, Jin S, Ye K (2018) Tissue and organ 3D bioprinting. SLAS Technol: Transl Life Sci Innov 23(4):301–314
Xiong S, Zhang X, Lu P, Wu Y, Wang Q, Sun H, ... Ouyang H (2017) A gelatin-sulfonated silk composite scaffold based on 3D printing technology enhances skin regeneration by stimulating epidermal growth and dermal neovascularization. Sci Rep 7(1):1–12
Xu T, Kincaid H, Atala A, Yoo JJ (2008) High-throughput production of single-cell microparticles using an inkjet printing technology. J Manuf Sci Eng 130(2)
Xu X, Jha AK, Harrington DA, Farach-Carson MC, Jia X (2012) Hyaluronic acid-based hydrogels: from a natural polysaccharide to complex networks. Soft Matter 8(12):3280–3294
Xu Y, Wang X (2015) Fluid and cell behaviors along a 3D printed alginate/gelatin/fibrin channel. Biotechnol Bioeng 112(8):1683–1695
Xu J, Zheng S, Hu X, Li L, Li W, Parungao R, ... Song K (2020) Advances in the research of bioinks based on natural collagen, polysaccharide and their derivatives for skin 3d bioprinting. Polymers 12(6):1237
Valkenaers H, Vogeler F, Voet A, Kruth JP (2013) Screw extrusion based 3D printing, a novel additive manufacturing technology. Mater Sci
Varkey M, Visscher DO, van Zuijlen PP, Atala A, Yoo JJ (2019) Skin bioprinting: the future of burn wound reconstruction? Burns Trauma 7:s41038–s42019
Velasco Bayón D, Quílez López C, García Díez M Cañizo López JFD, Jorcano Noval JL (2018) 3D human skin bioprinting: a view from the bio side. J 3D Print Med 2(3): 141–162
Velnar T, Bailey T, Smrkolj V (2009) The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 37(5):1528–1542
Vig K, Chaudhari A, Tripathi S, Dixit S, Sahu R, Pillai S, ... Singh SR (2017). Advances in skin regeneration using tissue engineering. Int Journal Molecular Sci 18(4):789
Vlăsceanu GM, Iovu H, Ioniţă M (2019) Graphene inks for the 3D printing of cell culture scaffolds and related molecular arrays. Compos B Eng 162:712–723
Wahl SM, Wong H, McCartney-Francis N (1989) Role of growth factors in inflammation and repair. J Cell Biochem 40(2):193–199
Wang X, Tolba E, Schröder HC, Neufurth M, Feng Q, Diehl-Seifert B, & Müller WE (2014). Effect of bioglass on growth and biomineralization of SaOS-2 cells in hydrogel after 3D cell bioprinting. PLoS One 9(11):e112497
Wang M, He J, Liu Y, Li M, Li D, Jin Z (2015) The trend towards in vivo bioprinting. Int J Bioprint 1(1)
Wang X, Rijff BL, Khang G (2017) A building-block approach to 3D printing a multichannel, organ-regenerative scaffold. J Tissue Eng Regen Med 11(5):1403–1411
Whitford WG, Hoying JB (2016) A bioink by any other name: terms, concepts and constructions related to 3D bioprinting. Future Sci OA 2(3):141–162
Williams D, Thayer P, Martinez H, Gatenholm E, Khademhosseini A (2018) A perspective on the physical, mechanical and biological specifications of bioinks and the development of functional tissues in 3D bioprinting. Bioprinting 9:19–36
Yan WC, Davoodi P, Vijayavenkataraman S, Tian Y, Ng WC, Fuh JY, ... Wang CH (2018) 3D bioprinting of skin tissue: from pre-processing to final product evaluation. Adv Drug Delivery Rev 132:270-295
Yang X, Lu Z, Wu H, Li W, Zheng L, Zhao J (2018) Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater Sci Eng, C 83:195–201
Yang R, Yang S, Zhao J, Hu X, Chen X, Wang J, ... Xiong K (2020) Progress in studies of epidermal stem cells and their application in skin tissue engineering. Stem Cell Res Therapy, 11(1), 1-13
Yao B, Xie J, Liu N, Yan T, Li Z., Liu Y, ... Fu X (2016) Identification of a new sweat gland progenitor population in mice and the role of their niche in tissue development. Biochemical and Biophysical Res Commun 479(4):670–675
Yeo M, Lee JS, Chun W, Kim GH (2016) An innovative collagen-based cell-printing method for obtaining human adipose stem cell-laden structures consisting of core–sheath structures for tissue engineering. Biomacromol 17(4):1365–1375
Yoo HS, Lee EA, Yoon JJ, Park TG (2005) Hyaluronic acid modified biodegradable scaffolds for cartilage tissue engineering. Biomaterials 26(14):1925–1933
Zhang X, Zhang Y (2015) Tissue engineering applications of three-dimensional bioprinting. Cell Biochem Biophys 72(3):777–782
Acknowledgements
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ilhan, E., Ozerol, E.A., Alpdagtas, S., Sengor, M., Ustundag, C.B., Gunduz, O. (2021). Biofunctional Inks for 3D Printing in Skin Tissue Engineering. In: Kumar, A., Voicu, S.I., Thakur, V.K. (eds) 3D printable Gel-inks for Tissue Engineering. Gels Horizons: From Science to Smart Materials. Springer, Singapore. https://doi.org/10.1007/978-981-16-4667-6_7
Download citation
DOI: https://doi.org/10.1007/978-981-16-4667-6_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4666-9
Online ISBN: 978-981-16-4667-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)