Abstract
Increasing evidence has pinpointed that loss of mitochondrial function or regulation is a critical player toward the pathogenesis of various metabolic, neurodevelopmental, and neurodegenerative disorders, including autism spectrum disorders (ASD) and Alzheimer’s disease (AD). The lacuna in understanding these diseases’ underlying biology is that pathology develops through the interaction of various biological pathways rather than a defined mechanism. Mitochondria are dynamic organelles that perform diverse functions, including cellular energy production, calcium homeostasis, apoptosis, and innate immune regulation. Hence, mitochondria integrate various cellular pathways, and any exogenous or endogenous perturbation may result in their dysfunction. Herein, we explore the latest research insights that have evolved our understanding of ASD and AD pathogenesis with the perspective of mitochondrial dysregulation as the underlying phenomenon. We discuss the pathological relevance of cause and effect of mitochondrial dysregulation, such as increased reactive oxygen species (ROS) production, mitochondrial DNA damage, aberrant immune responses, impaired energy metabolism, and altered gut microbiome in the etiology of ASD and AD. Being at the center stage, mitochondria have emerged as a novel target with considerable therapeutic potential, which can be exploited to delay, manage, or treat ASD, AD, and other neurological disorders. We also discuss the novel therapeutic options such as H2S therapy, dynamic microbiome modulation, ketogenic diet, and cofactor therapy that are emerging as a plausible treatment regimen and have shown favorable outcomes in initial studies. Hence, this article summarizes the current understanding of the functional and structural disturbances in the mitochondria that lead to ASD and AD and could be harnessed for better diagnostic and prognostic outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Mitochondrial dysfunction
- Autism spectrum disorders
- Alzheimer’s disease
- Oxidative stress
- Microbiome
- Bioenergetics
- Immune dysfunction
2.1 Introduction
Mitochondria are a double membrane organelle of approximately 0.75–3 μm2 size present in all cells of the eukaryotes and almost all prokaryotes. They act as the powerhouse of cells by producing energy in the form of adenosine triphosphate (ATP), which is required for cellular functions. Other functions of mitochondria include cellular differentiation, signaling, cell growth, death, and cell-cycle regulation. Mitochondrial structural and functional abnormalities have been demonstrated as a common shared mechanism across multiple neurodegenerative disorders that include diseases/syndromes such as cardiovascular disease; diabetes; schizophrenia; myopathy; stroke; endocrinopathy; bipolar disorder; chronic fatigue syndrome; Pearson syndrome; dementia; Kearns-Sayre syndrome; Parkinson’s disease; Leber’s hereditary optic neuropathy; Barth syndrome; retinitis pigmentosa; Alzheimer’s; Friedreich’s ataxia; mitochondrial encephalopathy, lactic acidosis, and stroke (MELAS) syndrome; Wilson’s disease; progressive external ophthalmoplegia; myoclonic epilepsy with ragged red fibers (MERRF); hereditary spastic paraplegia; etc. But most often, it presents itself in the form of neurological diseases such as ASD and AD. Present epidemiological data confirms that almost 5–80% of children affected by ASD show mitochondrial dysfunction compared to only 0.1% among the general population (Rose et al. 2012; Bayer 2015). Pathophysiological studies have shown a distinct connection between mitochondria and AD; however, an exact epidemiological data is not available thus far.
ASD represents a group of confounding diseases that include autism, Asperger’s syndrome, and pervasive developmental disorders. ASD is marked by developmental and neurological syndromes that lead to impaired social communication abilities and repetitive behavior. ASD symptoms start to manifest during early childhood and last throughout the lifetime. On the other hand, AD is a progressive degradation of brain cells, usually in the elderly, leading to dementia, which affects a person’s social abilities. Though both disorders are at the two ends of the age spectrum, they have common clinical manifestations such as language impairment, a problem in executing functions, dementia, and motor disability (Khan et al. 2016). Understanding ASD and AD from a biological perspective becomes complex due to its diagnosis solely through behavioral criteria directed by the Diagnostic and Statistical Manual of Mental Disorders (DSM), which keeps being revised based on identifying more contemporary patients’ patterns. The identification of ASD and AD through behavioral benchmarks is partly due to an insufficient understanding of the biological processes and the non-availability of quantitative biomarkers for these diseases. The cavities in understanding these diseases’ biology is that the pathology develops through a complex interaction of several biological pathways rather than a defined mechanism. It involves biological components as diverse as bioenergetics, epigenetics, and genetics, besides having environmental effectors.
The pathologies observed in ASD and AD, such as toxic accumulation of protein aggregates in AD, and increased white matter neurons together with a substantial decrease in the GABAergic cerebellar Purkinje cells in ASD, can be due to mutation, rearrangements, or point mutations in mitochondrial DNA (mtDNA). Recent researches indicate that diseases such as Parkinson’s disease, AD, Rett syndrome, and ASD plausibly share a common mechanism of mitochondrial dysfunction leading to disease progression (Frye 2020). Over the last few decades, despite a compelling problem of underdiagnosis, there has been a surge in the prevalence of ASD worldwide (Chiarotti and Venerosi 2020; Maenner et al. 2020). Many studies indicate that mitochondrial dysfunction may have an essential role in the development of ASD. It is also reinforced by the evidence that several comorbidities that develop due to mitochondrial defect such as epilepsy, sleep apnea, gastrointestinal, and immune dysfunction share an association with ASD. Mitochondria are sensitive organelles susceptible to endogenous alterations such as iatrogenic medications, toxicants, immune activation, and metabolic disruption besides the exogenous environment. The general effectors for mitochondrial dysfunction that facilitate progress toward AD and ASD are illustrated in Fig. 2.1. Many of these stressors have also been demonstrated in the etiology of ASD. Hence, there seems a clear link between these two disorders, which points to a possible shared etiological mechanism.
Furthermore, AD also has multiple etiological factors, and aging is considered a significant risk factor. Most studies on AD have focused on τ (tau) and amyloid-beta (amyloid-β) pathology as underlying factors. Some studies have demonstrated that τ pathology contributes to mitochondrial dysfunction leading to neurodegeneration. Amyloid-β protein has been shown to accumulate in the mitochondrial matrix hampering its function, such as failure of energy metabolism, generation of reactive oxygen species (ROS), and permeability transition pore (PTP) formation. Hence, it could be conjectured that brain metabolism in AD may be deranged due to altered mitochondrial functionality. This hypothesis had opened up new frontiers to explore alterations in mitochondrial bioenergetics as a possible cause of ASD, AD, and associated pathologies. Recent research studies have provided insight into novel metabolic targets to treat or prevent ASD and AD pathogenesis.
In this article, we discuss the latest findings that support that mitochondrial dysfunction and functional and structural abnormalities in mtDNA are the central mechanisms toward the pathogenesis of ASD and AD. We will also analyze the association of ASD and AD with respective co-occurring medical conditions in the light of mitochondrial dysfunction at the center stage. The review will also focus on understanding the advancement in the therapeutic approaches used for remodeling and enhancing mitochondrial functions, leading toward novel treatment methodologies for treating or managing ASD and AD.
2.2 Increased Oxidative Stress Linked to Mitochondrial Dysfunction in ASD and AD
The regulation of cellular survival, orchestration of biosynthetic metabolic pathways, and ROS signaling are the canonical functions of mitochondria. These organelles are integral to supporting the energy requirement of our body’s metabolic processes during the resting, active, and stressed state. They populate the cells of the body and harbor their mtDNA (mitochondrial DNA). Besides catabolizing glucose and oxidizing fatty acids to generate ATP, mitochondria play a distinct role in forming reactive oxygen species (ROS), calcium signaling and homeostasis, and the regulation of programmed cell death (Malek et al. 2018).
The accumulation of τ-protein and amyloid-β plaques is a pathological hallmark of AD that consequently progresses toward developing the disease. On the other hand, behavioral challenges mark the identification of ASD. Studies spanning the past few decades have determined that many factors, including biological, environmental, lifestyle, epigenetics, and genetics, influence the development of the two diseases. However, the mechanism of pathogenesis remains elusive. Recent studies have furnished distinctive insights that underscore mitochondrial dysfunction as an early event in ASD and AD pathogenesis (Rossignol and Frye 2012; Frye 2020). Oxidative stress plays a pivotal role in linking mitochondrial dysfunction and neurological disorders. Imbalances in the cellular milieu rendered through the presence of pro-oxidant metabolites, activated immune cells, toxicants, etc. subsequently lead to mitochondrial dysfunction.
The notion that mitochondrial abnormalities may be the cause of ASD came up in 1985, with the observation of lactic acidosis in children with ASD. Presently, ASD is quite common among children and is noted to be affected by different triggering etiologies, one of them being physiological abnormalities of mitochondrial dysfunction widely ranging from 5 to 80% (Rose et al. 2012). Similarly, metabolic changes and increased ROS production in AD brains underscore mitochondrial abnormalities at their nexus. With the advent of more recent techniques, considerable evidence has now accumulated that pinpoints oxidative stress, and calcium homeostasis alterations precede the formation of pathological identifiers in AD, such as plaques and tangles in the brain (Von Bernhardi and Eugenín 2012).
The mitochondrial electron transport chain (ETC) is the underlying source of ROS production. ROS serves as a signaling molecule under normal concentrations, modulating numerous physiological reactions, including the ETC, ion transport, and neurotransmitter receptors. If ROS production exceeds the physiological system’s buffering capacity, it manifests in the form of oxidative stress. The primary cause of oxidative stress is the abnormality in ETC. Increased ROS in the AD brains, as well as a region-specific reduction in the blood flow and oxygen utilization, provide ample evidence implicating compromised mitochondrial physiology in the development and progression of the disease (Bonda et al. 2014). Unregulated ROS production causes damage to cellular lipid, protein, and DNA, leading to derangement in the metabolic processes or development of anatomical lesions. The brain regions that were found to be most vulnerable to high ROS were the frontal, parietal, and temporal lobes. These areas overlap the areas that are found to be affected in AD patients (Wang et al. 2006).
Higher ROS levels induce ASD cascade, which posits mitochondrial abnormalities as the critical origin of neurodevelopmental impairment in ASD. However, these deficits in the mitochondria’s ETC complex activity are distinct from the abnormalities observed in classical mitochondrial diseases. Studies have identified higher ETC complex IV, III, and I activity in ASD animal models and patients (Frye and Naviaux 2011; Delhey et al. 2017; Valiente-Pallejà et al. 2018). Others have linked reduced antioxidant capacity and increased ROS levels at the systemic level in ASD. Molecules of oxidative stress have been shown in the brains of ASD patients and also in their parents (Ohja et al. 2018). Another study demonstrated impairments in the glutathione redox balanced in cerebella and temporal cortices of autistic patients (Rose et al. 2012). Transcriptional profiling of 84 genes of oxidative stress machinery identified a signature pattern of eight genes, namely, glutamate-cysteine ligase modifier (GCLM), superoxide dismutase 2 (SOD2), neutrophil cytosol factor 2 (NCF2), prions (PRNP), prostaglandin-endoperoxide synthase 2 (PTGS2), thioredoxin (TXN), and ferritin heavy chain (FTH1), involved in ROS metabolism which were downregulated in autistic individuals (Bolotta et al. 2018). Further, RBC damage through ROS that causes altered RBC shape and morphology has been a significant feature in autistic patients (Bolotta et al. 2018).
Interestingly, immune dysfunction identified as one of the pathological features of ASD has a close link with oxidative free radicals accumulation. Altered redox balance either in the prenatal or postnatal period is associated with immunological activation, which increases the risk of autism in children. Though prenatal immune dysregulation is not clearly understood, exposure to unhealthy postnatal environments is linked to a distinct immune dysregulation pattern, endogenous autoantibodies, and inflammation observed in autistic patients. Mitochondrial functional abnormalities can initiate specific stress signals leading to an aberrant immune response. Nonetheless, a meticulous approach toward understanding the molecular pathways that trigger inflammasome cascade resulting from mitochondrial dysfunction needs further research (Chen et al. 2018). A thorough understanding of metabolic circuitry that underscores mitochondrial dysfunction in ASD and AD may facilitate novel treatment strategies.
2.3 Mitochondrial DNA Damage Promotes the Development of ASD and AD
Mitochondria are present in every cell at varying numbers depending upon the difference in tissue origin. Cells of tissues with greater metabolic demands like the brain, cardiac, and skeletal muscle tissues have many mitochondria. Each mitochondrion harbors many copies of mtDNA. Moreover, mitochondrial genomes have 10–20-fold high mutation rates compared to nuclear DNA, which renders them inherently heteroplasmic (Stein and Sia 2017). Studies have reported that mitochondria have a skewed concentration of nucleotides that compromise mtDNA polymerase subunit gamma (PolG) enzyme fidelity, corroborating higher mutation rates in mitochondrial DNA. Consequently, these mutations accumulate more in tissue with higher metabolic activity, such as the brain, leading to more pronounced phenotypes.
Additionally, mitochondria are subjected to oxidative stress due to their respiration function, which can induce oxidative lesions in their genome (Sharma and Sampath 2019). ASD and AD, besides other neurodegenerative diseases, have higher ROS concentrations, and reports have demonstrated its association with the deletion of mtDNA. Diminished mitochondrial genome integrity has now been understood to predispose early- and late-onset metabolic diseases such as ASD, Parkinson’s disease, and Alzheimer’s disease.
mtDNA mutations occur across polypeptide mutations, rRNA and tRNA mutations, rearrangement mutations, and mutations in the regulatory region affecting mtDNA replication and transcription. A study on ASD children indicated that around 28.6% of ASD subjects displayed mutations commonly associated with mitochondrial disorders, such as the presence of low mtDNA content and putative pathogenic mtDNA mutations (Varga et al. 2018). mtDNA haplogroup differences can contribute to the modulation of the ASD risk. A cohort study of 1624 patients with ASD identified many mtDNA haplogroups across different clusters in ASD. Hence, mitochondrial haplogroups associated functional variants could be a risk factor for developing ASD (Chalkia et al. 2017). Using databases of mtDNA sequence and variation (comprehensive MITOMAP, A Human Mitochondrial Genome Database initiative), many mutations in mtDNA have been identified that are linked to pathologies and comorbidities associated with ASD and AD (Sharma and Sampath 2019). Several genetic anomalies are related to mitochondrial defects in ASD, including mitochondrial DNA mutations and deletions and chromosomal abnormalities. These abnormalities have been identified in buccal cells and cells of the immune system, fibroblasts, gastrointestinal tissue, and muscle, besides in the brain tissue of patients with ASD.
Studies on AD brains have shown reduced mtDNA content and mass, increased fragmentation of mtDNA, deletion, and apoptotic cell loss, associated with elevated free radicals linked with a reduced cyclooxygenase (COX) level. In a downstream signaling cascade that perpetrates with mitochondrial stress, cell apoptosis is triggered. These innate immune mechanisms are susceptible to nuclear and mtDNA and any other type of DNA from the phagolysosomal compartment. The presence of apoptotic vesicles in the blood from ASD patients, which in turn contain a considerable concentration of mtDNA, is plausibly recognized as an innate pathogen in ASD. These vesicles enter the microglia of ASD patients through blood and lymphatic systems and triggering an immune response (Pangrazzi et al. 2020). Many studies have also observed sporadic mtDNA deletions in brain tissues obtained after the postmortem of late-onset AD patients. In a study, mtDNAΔ4977 showed a 15-fold hike in its occurrence in AD subjects’ cortical neurons (Phillips et al. 2014). Visible chromosomal lesions and slight copy number variants at 16p11, an inverted duplication on one of the domains of chromosome 15q11-q13, are also commonly observed in 10–20% of ASD cases (Cook Jr et al. 1997; Qureshi et al. 2014).
2.4 Calcium Homeostasis Imbalance Perpetrates Mitochondrial Dysfunction in ASD and AD
The function of mitochondria in regulating and buffering cytoplasmic Ca2+ (calcium ion) manifests as a central player in normal neurotransmission, neuronal plasticity, gene transcription regulation, and excitotoxicity (Celsi et al. 2009). Different studies have cited the function of mitochondria in Ca2+ buffering impairment in the aging brain and AD (Camandola and Mattson 2011). Disturbances in the Ca2+ homeostasis are closely connected with mitochondrial permeability transitions potentiated by high ROS generation, elevated phosphate concentrations, and ATP depletion. This eventually leads to the release of pro-apoptotic factors and cell death (Toglia and Ullah 2016; Granatiero et al. 2019). Predominantly, the function of most of the ions is limited to electrical conduction at the cell membrane. However, calcium additionally integrates signaling to cellular transcriptional, translational, metabolic, and biochemical events. The intrinsic function of calcium as a second messenger arbitrates diverse cellular processes through spatial and temporal alteration in concentration (Berridge et al. 2000). This function is carried out by a multitude of calcium-binding proteins, transporters, and voltage-dependent ligand-gated ion channels. Since calcium plays a ubiquitous role in cell physiology, any regulatory defects in the calcium signaling pathway can disrupt neurological function as demonstrated by several pathological conditions, including ASD (Nguyen et al. 2018). Derangement in calcium signaling potentially causes many abnormalities associated with ASD pathogenesis, such as mitochondrial function defects, neurotransmitter signaling, and synaptic plasticity. Mechanistic variants have been identified in calcium signaling pathways related to the endoplasmic reticulum (ER) and mitochondrial organelle dysfunction in ASD and AD. Though organelles are inherent to all cells throughout the body, the central nervous system (CNS) is profoundly affected by organellar diseases (Nguyen et al. 2018). Some genetic studies have also identified ion channel gene mutations in ASD subjects, suggesting it to be a channelopathy and a plausible reason for associated comorbidities (Noebels 2017).
Cognitive functions such as memory, neuron excitability, synaptic plasticity, axon growth, the release of neurotransmitter, and precise modulation of calcium gradients are regulated through intracellular calcium homeostasis (Wen-hong et al. 1998; Hernández-López et al. 2000; Neher and Sakaba 2008). Nerve stimulation is achieved by increasing cytosolic calcium from a resting concentration of ~100 nM by mobilizing calcium from intracellular ER stores or extracellular milieu. Mitochondria play a pivotal role during this signal transduction process by immediately sequestering Ca2+ through its calcium uniporter. Hence, the extracellular signal impulse is swiftly propagated through a tightly regulated mitochondrial process (Giorgi et al. 2012). Mutations in mtDNA affect calcium homeostasis, such as reduced Ca2+ sequestration, which consequently disturb ETC, mitochondrial membrane potential, and ATP production. ATP production is reduced due to the inability of Kreb’s cycle enzymes to function in the absence of calcium. Positive feedback is generated with subsequent loss of ATP synthesis, which affects overall cell physiology. Besides, Ca2+ homeostasis derangement can also lead to intracellular Ca2+ overload within the mitochondria. In AD, the accumulation of amyloid-β facilitates ROS generation, which causes the accumulation of Ca2+ and PTP opening in mitochondria (Giorgi et al. 2012). Vitamin D is a key modulator of calcium homeostasis, which functions through its nuclear receptor, thereby controlling gene expression. It plays a crucial role in cellular proliferation and fine-tuning voltage-gated calcium channels. Furthermore, mitochondria are imperative in producing the active form of vitamin D, D3 (1α,25-dihydroxyvitamin-D3). The deficiency of D3 during fetal life is strongly linked with the pathogenesis of ASD (Vinkhuyzen et al. 2017).
2.5 Neuronal Mitochondrial Dysbiogenesis Underlies the Development of ASD and AD
Many studies have demonstrated that mitochondrial biogenesis is fundamental to neuronal growth. Cellular pathways that are functional during neuronal development also promote mitochondrial biogenesis to arbitrate developing neurons’ energy requirements. Mitochondrial biogenesis is regulated through a concerted mechanism manifested through crosstalk between mitochondrial and genomic DNA counterparts. Mitochondria proliferate and constantly fuse as a part of healthy cellular mechanisms and respond to enhanced energy needs, oxidative stress, and disease conditions. Mitochondrial biogenesis is controlled through checkpoints during transcription, translation, and post-translation. This process is activated by peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) (Jones et al. 2012). Further, sequential activation of nuclear respiratory factor-1 and nuclear respiratory factors-2 (NRF-1 and NRF-2) transcription factors leads to mitochondrial biogenesis. These newly formed mitochondria are integrated into the mitochondrial metabolic machinery, while the ones that are nonfunctional or damaged or demonstrate possible membrane disruption are tagged for degradation and removal. The removal of damaged mitochondria follows an autophagy-dependent mechanism called mitophagy (Lou et al. 2020).
Additionally, mitochondrial dynamics are regulated through fusion and fission processes, which counteract cellular damage by complementing cellular components, besides removing damaged mitochondria through autophagy. The mitochondrial biogenetic pathways functional through NRF 1, NRF 2, PGC-1α, etc. are demonstrated to be impaired in neurological diseases such as ASD and AD. A number of mitochondria and biochemical mediators such as NRF 1, NRF 2, and mitochondrial transcription factor A (TFAM) and PGC-1α are shown to be decreased in the hippocampus of AD brains, which diminish AMP-activated protein kinase (AMPK)-induced neuronal growth. Disturbed mitochondrial biogenesis, demonstrated by altered expression of mitochondrial fission (Fis1 and Drp1) and fusion (Mfn1/2 and Opa1) proteins and in the temporal cortex, indicated its relation to differences in the morphology and function of mitochondria in ASD (Tang et al. 2013). Many studies have correlated this deranged biogenesis potential of mitochondria to the disruption of neuronal plasticity and compromised cellular resilience. This process is demonstrated to eventually regulate psychotic disorders regularly observed in psychiatric comorbidities (Quiroz et al. 2008).
Another feature of mitochondrial biogenesis is an interconnected network of proteases and chaperones that maintains the quality of proteins and remove damaged proteins from the mitochondrial compartment (Folisi 2015). During homeostasis, mitochondrial chaperons facilitate protein folding and translocation, while proteases degrade and remove misfolded and damaged proteins from the mitochondria. Impaired chaperons and proteases consequently lead to the accumulation of protein aggregates, such as τ-protein and amyloid-β in AD, which ensues in the form of mitochondrial dysfunction (Ruan et al. 2013; Deepa et al. 2016). Notably, genetic mutations could cause impaired functions of mitochondrial chaperones and proteases and are extensively demonstrated to precipitate severe neurological diseases (Martinelli and Rugarli 2010; Goo et al. 2013; Strauss et al. 2015). Though few studies have linked the impaired function of proteases and chaperons with AD, others have shown their upregulation to be a priming episode in amyloid progression and τ-pathology in AD (Beck et al. 2016; Sorrentino et al. 2017). Similarly, few researchers have identified impaired inner membrane protease polymorphic forms of IMMP2L in ASD. Recently, the identification of protease malfunction and its reciprocal effect in the development of ASD have also gained momentum.
2.6 Impaired Mitochondrial Energy Metabolism Propels the Development of AD and ASD
Brain function is a composite of neuronal and glial cell function and synaptic efficiency. Healthy neuronal function demand higher energy needs, and hence numerous mitochondria are present in the brain cells (Picard and McEwen 2014). The mitochondria carry out their primary function to synthesize ATP. Coherence with other cellular organelles provides buffering machinery that regulates calcium levels during nerve impulses and signal transduction. Besides, they are instrumental in the biosynthesis of heme and iron-sulfur (Fe-S) clusters required to synthesize presynaptic neuronal transmitters in synapses (Lin and Beal 2006). Hence, it is discernible that disruption of mitochondrial functions is likely to cause nervous system abnormalities leading to neurodegenerative or neurodevelopmental disorders (Alexiou et al. 2018).
The brain is one of the highest energy-consuming organs, which approximately uses 25% of total energy in the resting state. Hence, a deficit in the energy fuel supply, such as the availability of glucose, or metabolic machinery like mitochondrial dysfunction, negatively impacts brain function. Neurons highly depend on oxidative phosphorylation as a source of energy, which renders them susceptible to mitochondrial dysfunction (Cardoso et al. 2016. Studies using fluorodeoxyglucose positron emission tomography (FDG- PET) have identified low glucose metabolic rates in AD patients, especially in the posterior cingulate, temporal, and parietal lobes and the hippocampus (Kapogiannis and Mattson 2011). These regions are dedicated to cognition and memory, and hence defects in energy metabolism could be a proximate source of pathologies in neurological disorders such as AD and ASD. The hypometabolism of glucose in the AD brain is compensated by shifting to amino acid and lipids as energy sources (Toledo et al. 2017). Metabolomics and lipidomic studies have identified at least six central metabolic pathways, including glycerophospholipid and aspartate metabolism in human autopsy samples to be defective.
Similarly, essential amino acids, branched-chain amino acids (BCAAs), polyamine metabolism, and serotonin pathways in the APPswe/PS1deltaE9 double transgenic AD mouse model were found to be altered. Though these studies provide a direct link with AD, not all of these metabolic findings were replicated (Casanova et al. 2016; Pan et al. 2016). Presently, molecular networks, viz., systems biology approach, have identified metabolic connections in varied metabolic pathways, highlighting the dysregulation of biochemical reactions at different disease progression stages (Santiago and Potashkin 2014). This approach provides distinct mechanistic insight into complex diseases such as AD and ASD, which stem from changes in multiple genes, proteins, and metabolites.
Metabolites such as glutamate and glycolytic intermediates, lactate, and pyruvate were observed to be increased. In contrast, carnitine, the fatty acid carrier from the cytosol to the mitochondria, and glutathione were demonstrated to be lower than expected in the serum of ASD and AD patients (Shimmura et al. 2011; Frye et al. 2013; Bjørklund et al. 2020; Oh et al. 2020; Xie et al. 2021). Contrarily, fatty acid palmitate was shown to be increased in ASD plasma samples. Palmitic and stearic acid and omega-6 fatty acids are also demonstrated to cause neuroinflammation and hence τ-phosphorylation and its aggregation in AD disease models. Palmitate is implicated as an intracellular signaling molecule that regulates the progression of several pathologies as diverse as cardiovascular diseases, neurodegenerative diseases, cancer, etc. (Fatima et al. 2019). It has also been proposed that metabolic modifiers precede the onset of neurological symptoms.
2.7 Enteric Microbiome Alterations Modulate Mitochondrial Function in ASD and AD
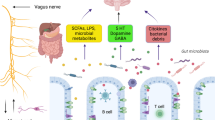
The enteric microbial flora (microbiome) influences the physiological and biochemical status of humans. In the last two decades, understanding the gut microbiome function in influencing health and disease has gained considerable scientific interest. The communities of microbial cells that harbor within the gut are involved in processes as diverse as metabolism, nutrition, and the host’s immune regulation (Guinane and Cotter 2013). Besides a positive effect on human health, some microbial cells also release chemical mediators that can potentially disrupt normal cellular pathways, including mitochondrial functions. Diseases like gastrointestinal complications, diabetes, and autism have been attributed to a microbiome-mediated disruption in addition to other associated mechanisms. The chemical mediators secreted by the microbes residing in the gut can travel through the bloodstream, penetrating the blood-brain barrier (Guinane and Cotter 2013; Burokas et al. 2015). Studies have identified that behavioral symptoms in autism can aggravate with alterations in the diet and changes in the gut microbiota through early antibiotic exposure, perinatal infection, hospitalization, etc. The gut microbe produces short-chain fatty acids (SCFA) upon dietary carbohydrates fermentation, which serve as an essential trigger to modulate mitochondrial functions and other cellular regulatory pathways (Saint-Georges-Chaumet and Edeas 2016). These SCFA produced by gut microbes, most notably propionate, have been concomitant with the development of ASD to affect mitochondrial function (MacFabe 2015) directly. Some microbial products induce a damaging immune response in their immediate vicinity and travel to invade the blood-brain barrier inducing a pro-inflammatory state in the sentinel microglia. This pro-inflammation is associated with derangement in the normal mitochondrial functions and progression toward hypoxia as well as neuroinflammatory and epigenetic modifications (Siniscalco et al. 2013; MacFabe 2015). An SCFA component, propionate, is demonstrated to increase anti-nitrotyrosine immunoreactivity indicating oxidative stress. Propionate is also demonstrated to increase glutamate cysteine ligase modifier (GFA), a marker of immunoreactivity, and reactive astrocytes in the hippocampus of ASD subjects (Edmonson et al. 2014).
The microbiota of the intestinal system is altered as a result of unhealthy lifestyles such as food, sleep problems, circadian rhythm disturbance, and sedentary routines. Multifactorial changes spanning quantitative and qualitative differences have been documented in the gut microbiome of AD patients and are considered a significant risk factor for sporadic pathogenesis of AD. Studies using specific pathogen-free mice and microbiome-reconstituted mice models have revealed increased brain-derived neurotrophic factor (BNDF) in the amygdala and reduced serotonin receptor (5HT1A) mRNA and NR2B subunit of the N-methyl-D-aspartate (NMDA) receptor mRNA expression. Also, in the hippocampus, this decrease is associated with an insufficiency of working and spatial memory (Neufeld et al. 2011). In another study, intestinal dysbiosis induced through ampicillin reduces the mineralocorticoid level and NMDA receptors in the amygdala, impaired spatial memory, and increased test animals’ aggressiveness. However, this was restored through the Lactobacillus fermentum NS9 strain as a part of the intestinal microbiome (Wang et al. 2015). Studies using matched cohorts have also pointed toward an association of microbiome composition with AD (Haran et al. 2019). Metagenomics complemented by clinical data has also confirmed a nexus between microbiome disturbance, neuroinflammation, intestinal disturbances, and AD/ASD disease.
The presence of acute stress and infection with conditional pathogenic bacteria Citrobacter rodentium are also reported to cause memory disorders in C57BL/6 mice (Gareau et al. 2011). A study conducted by Alzheimer’s Disease Research Center (Wisconsin, USA) in 2017 demonstrated marked changes in the gut microbiome of AD patients matched to healthy individuals. This study decreased bacterial numbers belonging to Firmicutes and Actinobacteria phyla (particularly genus Bifidobacterium), and a surge in Bacteroidetes and Proteobacteria phyla bacteria in the gut microflora of AD patients was observed. Hence, the study distinctly demonstrated that intestinal microflora’s functional component and taxonomy influence brain functions (Vogt et al. 2017). The human intestinal microflora has a direct regulatory role that works along the gut-brain-mitochondrial axis, modulating the development of neurological diseases such as ASD and AD.
2.8 Biomarkers of AD and ASD Linked to Mitochondrial Dysfunction
The pathological markers of AD include β-amyloid and τ-accumulation in the brain of AD patients. τ-protein regulates microtubule stability. However, modified τ aggregates in the neurons and is identified as a significant player in neurodegenerative diseases. In mice studies, τ-ablation has been concurrent with the enhanced ATP production and improvement in attentive capacity and recall memory (Jara et al. 2018). Mechanistically, τ-deletion reduced oxidative damage, thereby restoring the mitochondrial pro-fusion state besides inhibiting mitochondrial PTP formation, thus enhancing positive mitochondrial dynamics. There are reports that β-amyloid and τ-protein accumulation and apolipoprotein E (APOE) genes (a sporadic AD risk factor gene) could trigger mitochondrial dysfunction, which exacerbates the pathology.
Another pathological marker of AD, amyloid precursor protein, APP, together with amyloid-β, has a more direct link with mitochondria as compared to τ-protein (Zhang et al. 2021; Mantzavinos and Alexiou 2017). These proteins localize to mitochondrial membranes and interact with other mitochondrial proteins besides disrupting ETC and importing nuclear-encoded mitochondrial proteins. They are shown to increase the production of ROS. However, it is also reported that the declining function of disrupted mitochondria leads to the accumulation of β-amyloid proteins besides several other comorbidities of AD. However, the existence of feedback loops makes a blurred understanding of the cause and effect. Recent researches have revealed that a highly toxic oligomeric form of β-amyloid protein (OAβ) disrupts normal mitochondrial function leading to a cascade mechanism responsible for severe deficit in the energy deficits preceding the development of AD (Sackmann and Hallbeck 2020).
Though ASD is identified mainly through behavioral pattern changes, several pathological markers have recently been correlated with the disease, including biomarkers of fatty acid metabolism, buccal cell enzymology, apoptotic markers, and ROS alteration. Most of these biomarkers are directly related to mitochondrial dysfunction, indicating an active role of mitochondrial disruption in the pathogenesis of ASD (Rose et al. 2018). Environmental exposure to toxicants and microbiome metabolites vis-a-vis oxidizing microenvironment is demonstrated to modulate mitochondrial function in ASD models.
2.9 Mitochondrial Targeting as a Therapeutic Approach for ASD and AD
The current therapy for AD relies on administering either glutamate-NMDA receptor antagonist like memantine or cholinesterase inhibitors, such as galantamine, rivastigmine, and donepezil. However, drug therapy is not approved by the Food and Drug Administration (FDA) for treating symptoms of autism. Autism is usually treated through behavioral management therapy, speech language, nutritional therapy, cognitive behavior therapy, joint attention therapy, physical therapy, etc. Regardless of the underlying mechanism that finally results in the development of ASD and AD, early diagnosis and intervention would lead to better treatment outcomes. As the knowledge of these neurodevelopmental diseases’ pathogenesis is advancing, the prospects of better treatments can be augmented with targeted approaches (Fig. 2.2). Besides the present methodology of the drug-based treatment, many novel therapies are being explored and are continually evolving. Some of the new treatment approaches are discussed here.
2.9.1 H2S Therapy
As observed in AD and ASD, enhanced ROS production affects mitochondrial function, contributing to the onset of neurodegeneration. Immediate consequences of high oxidative stress include lipid and protein oxidation and mtDNA mutation that induces neuronal cell death. Hydrogen sulfide (H2S) has been demonstrated to mitigate these effects of oxidative stress by elevating glutathione (GSH) concentrations through the potassium (KATP/K+) and calcium (Ca2+) ion channels. H2S exerts its antioxidant effect through inorganic and organic compounds that mediate the activities of GSH, glutathione peroxidase, and superoxide dismutase, which in turn neutralizes hydrogen peroxide (H2O2)-induced oxidative damage.
Endogenously, H2S is produced through pyridoxal phosphate-dependent enzymes in tissues, namely, cystathionine β-synthase (CBS), cystathionine γ-lyase (CγL), cysteine aminotransferase (CT), and 3-mercaptopyruvate sulfur transferase (MST). The normal level of H2S for both plasma and tissue is 50–160 μM. CBS expression is very high in the hippocampus and cerebellum areas of the central nervous system (CNS). H2S is a gasotransmitter that functions as a powerful antioxidant during the mitochondrial oxidation process to reduce oxidative stress generated in neurodegenerative diseases. Besides, H2S can exert its protective effects as an anti-inflammatory molecule in the CNS to dissipate neuroinflammation (Zhang et al. 2017). Treatment with H2S donor, sodium hydrosulfide (NaHS), has proven efficacy in suppressing hypoxia-induced neuronal apoptosis by blocking the H2O2-activated Ca2+ signal pathway. H2S could also augment anti-apoptosis through nuclear translocation of nuclear factor kappa B (NF-κB) regulation (Zhang et al. 2017). Furthermore, the enzyme cystathionine γ-lyase (CSE), which produces H2S, binds to τ-protein to exert its catalytic activity. Recently, this enzyme is shown to be depleted in AD human brains and 3xTg-AD mouse models, which leads to lower production and hence diminished concentrations of H2S (Giovinazzo et al. 2021). Therefore, in the absence of H2S, hyperphosphorylation of τ-proteins progresses as observed in AD. On the other hand, H2S prevents this phosphorylation by sulfhydrating the τ-protein kinase, namely, glycogen synthase kinase 3β (GSK3β). This understanding has been furthered in the study by Giovinazzo et al. that demonstrated the amelioration of both motor and cognitive deficits in AD upon administration of the H2S donor sodium GYY4137 to 3xTg-AD mice (Giovinazzo et al. 2021).
2.9.2 Microbiome Modulation and Probiotic Therapy
ASD and AD have a compelling association with mitochondrial dysfunction. Besides, gastrointestinal symptoms are an essential indicator of ASD and are strongly associated with mitochondrial dysfunction. It is noticeable that the gut microbial flora can orchestrate immune modulation and inflammasome activation in both these diseases, as previously described. Furthermore, mitochondrial damage-associated molecular patterns (DAMPs) are signals to activate innate immunity. Hence, a cascade of molecular events triggered by a dysbiotic gut microbiome could stimulate the production of metabolites that target and damage mitochondria. Recent research has indicated that the plasma levels of pro-inflammatory cytokines such as IL-2, IL-4, IL-6, TNF-α, TNF-β, IFN-γ, etc. are significantly high in subjects with ASD. The increase in cytokines TNF-α and IL-6 is distinctly associated with the pathogenic gut microbiome, which constitutes the microbiota unique to ASD individuals in most of the disease cases. Beneficial gut microbiota, which includes Lachnospiraceae and Bacteroides and negatively correlates with pro-inflammatory cytokines, is present at reduced levels or absent in ASD (Cao et al. 2021). Hence, disturbances in plasma cytokine profile that link with alterations in the abundance of healthy gut microbiota in ASD patients could be considered an early diagnostic mechanism for ASD.
On the contrary, a specified microbial population with positive effectors harbors the ability to enhance oxidative capacity and be exploited as treatment strategies. Such a therapeutic approach holds the potential of slowing the onset of several metabolic and neurodegenerative diseases such as ASD and AD. Probiotics have shown promise in improving autistic symptoms by directly restoring intestinal microflora balance with subsequent positive effects in strengthening the gastrointestinal barrier. A study conducted on ASD children used four bacterial strains plus a prebiotic, fructooligosaccharide. It helped to normalize the gut microbiome and gastrointestinal functions, besides ameliorating the typical behaviors in autistic children. The probiotic therapy also increased the bacteria population, such as Bifidobacteriaceae and B. longum, which are beneficial. It reduced the existing potentially disease-causing bacteria such as Clostridium and Ruminococcus associated with autism symptomatology (Wang et al. 2020). Hence, probiotics are being tested as a promising treatment for ASD associated with gastrointestinal symptoms and can be utilized as a safe and effective treatment.
Future AD therapies can also involve the use of probiotics, especially as prophylaxis methodology, when mild cognitive impairment is observed or AD is first diagnosed. A healthy gastrointestinal tract harbors facultative anaerobic or microaerophilic Lactobacillus and Bifidobacterium species, which metabolize glutamate to produce gamma-aminobutyric acid (GABA). GABA is an important inhibitory neurotransmitter in the CNS, and dysfunction of GABA is connected to dysfunction of synaptogenesis, cognitive impairment, and AD (Bhattacharjee and Lukiw 2013). Hence, restoring the typical microbiome in AD patients may have enormous effects and may facilitate customized microbiome manipulative strategies for the therapeutic management of AD and other neurodegenerative disorders.
2.9.3 Ketogenic Diet
A ketogenic diet has shown beneficial effects in children with ASD in improving the primary and associated symptoms of epilepsy. Unlike antiepileptic drugs, they are not related to adverse effects. The ketogenic diet exerts its beneficial effects, possibly through cerebral glucose metabolism, to improve mitochondrial morphology and white matter development in the brain. The ketogenic diet’s positive influence has been observed in children with pyruvate dehydrogenase complex deficiency, with improvement in speech, language, and social functioning. In a case study, early initiation of a ketogenic diet has been associated with longevity and mental growth. From the mitochondrial standpoint, the ketogenic diet seems to be a promising therapy in both ASD and AD. In these neurodegenerative diseases, mitochondrial dysfunction and impaired bioenergetics can be salvaged through the use of ketone bodies. Ketone bodies can serve as the primary energy source for many metabolic processes instead of glucose. Besides, ketone bodies can exert neuroprotective effects by reducing glucose levels and increasing ketone bodies’ formation by the liver. An increase in ketone bodies is mainly through the oxidation of polyunsaturated fatty acids (PUFA). PUFA increases peroxidases and reduces mitochondrial membrane potential and ROS through enhanced mitochondrial uncoupling protein expression (Milder and Patel 2012). A ketogenic diet also enhances the overall anti-injury potential of neurons by increasing global metabolic efficiency even under insufficient energy phases (Henderson et al. 2009). Ketogenic diet therapy seems to be a promising candidate as it can reduce inflammation and ROS generation, delay the progression of AD, and improve cognitive ability in AD patients.
2.9.4 Cofactor Supplementation
Besides many general cofactors that support improvement in the symptomatology of ASD and AD, nicotinamide adenine dinucleotide (NAD), thiamine tetrahydrofurfuryl disulfide (TTFD), biotin (B7), and methylcobalamin (B12) are imperative to healthy mitochondrial functions. Thiamine facilitates normal cellular energy metabolism, production of energy equivalence, and reduction of cellular ROS besides maintaining the structure and function integrity of mitochondria. At the same time, biotin attenuates the loss of mitochondrial membrane potential and reduces ROS production. Methylcobalamin (B12) is an integral cofactor for the regeneration of GSH and GSH/GSSG (James et al. 2004). It is vital for the proper functioning of the brain and nerves and red blood cell production. In a randomized controlled trial, oral supplementation of cofactors (vitamin/minerals) for 3 months has shown improvement in the symptoms of autistic children besides improving methylation, glutathione, ATP, and NAD levels and reducing oxidative stress (Adams et al. 2011).
Additionally, L-carnitine is an amino acid derivative, which affects CNS and mitochondrial physiology. Studies have indicated altered metabolic channeling of L-carnitine in ASD patients (Demarquoy and Demarquoy 2019; Malaguarnera and Cauli 2019). Clinical trials are underway for the use of cofactor therapy in AD, focusing on metabolic improvement through dietary supplementation of L-carnitine tartrate, N-acetylcysteine, nicotinamide riboside, and serine. These studies aim to increase mitochondrial activity in the brain cell types through simultaneous dietary supplementation (ClinicalTrials.gov Identifier: NCT04044131) (Remington et al. 2016; Tardiolo et al. 2018; Peng et al. 2020). The cofactor therapies are well tolerated throughout a person’s pathological status without any significant side effects or long-term detrimental effects. Many of the cofactors are water-soluble vitamin B supplements, which can be eliminated from the body through the kidneys.
2.10 Conclusion
Recent years have observed notable research advances in the field of mitochondrial disease. The development of advanced techniques that provide the ability to uncover novel mitochondrial gene mutations and associated metabolic derangements has immensely improved our understanding of molecular mechanisms that lead to mitochondrial dysfunction, which can influence a plethora of metabolic pathways including amino acid, carbohydrate, and lipid metabolism. Additionally, mitochondrial derangement also affects regulatory networks modulating apoptosis, calcium flux, hormonal and immunologic responses, and oxidative stress, eventually affecting the brain function. Mitochondria posit an inherent tendency to adapt to changing energy demands and microenvironments. However, increased environmental stress such as oxidative stress and diminished defense responses potentially induce structural and functional abnormalities in the mitochondria. Induced or genetic defects in the mitochondria thus act as the precursor of a plethora of neuronal disorders. In this review, we have provided an integrated perception of the major aspects of mitochondrial functional and structural abnormalities such as imbalance in calcium homeostasis, mitochondrial dysbiogenesis, gut microbiome alterations, mtDNA defects, etc. and their implications for neurodevelopmental and neurodegenerative disorders, namely, ASD and AD. Mitochondrial mechanistic failure is presently established as a significant event that impinges upon the progression of these diseases, and hence a potential target for therapeutic intervention.
References
Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, Gehn E, Loresto M, Mitchell J, Atwood S (2011) Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr Metab 8(1):34
Alexiou A, Nizami B, Khan FI, Soursou G, Vairaktarakis C, Chatzichronis S, Tsiamis V, Manztavinos V et al (2018) Mitochondrial dynamics and proteins related to neurodegenerative diseases. Curr Protein Pept Sci 19(9):850–857
Bayer TA (2015) Proteinopathies, a core concept for understanding and ultimately treating degenerative disorders? Eur Neuropsychopharmacol 25(5):713–724
Beck S, Mufson EJ, Counts SE (2016) Evidence for mitochondrial UPR gene activation in familial and sporadic Alzheimer’s disease. Curr Alzheimer Res 13(6):610–614
Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1(1):11–21
Bhattacharjee S, Lukiw WJ (2013) Alzheimer’s disease and the microbiome. Front Cell Neurosci 7:153
Bjørklund G, Tinkov AA, Hosnedlová B, Kizek R, Ajsuvakova OP, Chirumbolo S, Skalnaya MG, Peana M, Dadar M, El-Ansary A (2020) The role of glutathione redox imbalance in autism spectrum disorder: a review. Free Radic Biol Med 160:149–162
Bolotta A, Battistelli M, Falcieri E, Ghezzo A, Manara MC, Manfredini S, Marini M, Posar A, Visconti P, Abruzzo PM (2018) Oxidative stress in autistic children alters erythrocyte shape in the absence of quantitative protein alterations and of loss of membrane phospholipid asymmetry. Oxidative Med Cell Longev 2018
Bonda DJ, Wang X, Lee H-G, Smith MA, Perry G, Zhu X (2014) Neuronal failure in Alzheimer’s disease: a view through the oxidative stress looking-glass. Neurosci Bull 30(2):243–252
Burokas A, Moloney RD, Dinan TG, Cryan JF (2015) Microbiota regulation of the mammalian gut–brain axis. Adv Appl Microbiol 91:1–62
Camandola S, Mattson MP (2011) Aberrant subcellular neuronal calcium regulation in aging and Alzheimer’s disease. Biochim Biophys Acta (BBA) Mol Cell Res 1813(5):965–973
Cao X, Liu K, Liu J, Liu Y-W, Xu L, Wang H, Zhu Y, Wang P, Li Z, Wen J (2021) Dysbiotic gut microbiota and dysregulation of cytokine profile in children and teens with autism spectrum disorder. Front Neurosci 15
Cardoso S, Carvalho C, Correia SC, Seiça RM, Moreira PI (2016) Alzheimer’s disease: from mitochondrial perturbations to mitochondrial medicine. Brain Pathol 26(5):632–647
Casanova R, Varma S, Simpson B, Kim M, An Y, Saldana S, Riveros C, Moscato P, Griswold M, Sonntag D (2016) Blood metabolite markers of preclinical Alzheimer’s disease in two longitudinally followed cohorts of older individuals. Alzheimers Dement 12(7):815–822
Celsi F, Pizzo P, Brini M, Leo S, Fotino C, Pinton P, Rizzuto R (2009) Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochim Biophys Acta (BBA) Bioenerget 1787(5):335–344
Chalkia D, Singh LN, Leipzig J, Lvova M, Derbeneva O, Lakatos A, Hadley D, Hakonarson H, Wallace DC (2017) Association between mitochondrial DNA haplogroup variation and autism spectrum disorders. JAMA Psychiat 74(11):1161–1168
Chen Y, Zhou Z, Min W (2018) Mitochondria, oxidative stress and innate immunity. Front Physiol 9:1487
Chiarotti F, Venerosi A (2020) Epidemiology of autism spectrum disorders: a review of worldwide prevalence estimates since 2014. Brain Sci 10(5):274
Cook EH Jr, Lindgren V, Leventhal BL, Courchesne R, Lincoln A, Shulman C, Lord C, Courchesne E (1997) Autism or atypical autism in maternally but not paternally derived proximal 15q duplication. Am J Hum Genet 60(4):928
Deepa SS, Bhaskaran S, Ranjit R, Qaisar R, Nair BC, Liu Y, Walsh ME, Fok WC, Van Remmen H (2016) Down-regulation of the mitochondrial matrix peptidase ClpP in muscle cells causes mitochondrial dysfunction and decreases cell proliferation. Free Radic Biol Med 91:281–292
Delhey L, Kilinc EN, Yin L, Slattery J, Tippett M, Wynne R, Rose S, Kahler S, Damle S, Legido A (2017) Bioenergetic variation is related to autism symptomatology. Metab Brain Dis 32(6):2021–2031
Demarquoy C, Demarquoy J (2019) Autism and carnitine: a possible link. World J Biol Chem 10(1):7
Edmonson C, Ziats MN, Rennert OM (2014) Altered glial marker expression in autistic post-mortem prefrontal cortex and cerebellum. Mol Autism 5(1):3
Fatima S, Hu X, Gong R-H, Huang C, Chen M, Wong HLX, Bian Z, Kwan HY (2019) Palmitic acid is an intracellular signaling molecule involved in disease development. Cell Mol Life Sci 76(13):2547–2557
Folisi C (2015) Oxidative stress and anti-oxidant response in allergen, virus, and corticosteroids withdrawal-induced asthma exacerbation
Frye RE (2020) Mitochondrial dysfunction in autism spectrum disorder: unique abnormalities and targeted treatments. Semin Pediatr Neurol 35:100829
Frye RE, Naviaux RK (2011) Autistic disorder with complex IV overactivity: a new mitochondrial syndrome. J Pediatr Neurol 9(4):427–434
Frye RE, Melnyk S, MacFabe DF (2013) Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl Psychiatry 3(1):e220–e220
Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, MacQueen G, Sherman PM (2011) Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60(3):307–317
Giorgi C, Agnoletto C, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Poletti F, Rimessi A (2012) Mitochondrial calcium homeostasis as potential target for mitochondrial medicine. Mitochondrion 12(1):77–85
Giovinazzo D, Bursac B, Sbodio JI, Nalluru S, Vignane T, Snowman AM, Albacarys LM, Sedlak TW, Torregrossa R, Whiteman M (2021) Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc Natl Acad Sci U S A 118(4)
Goo H-G, Jung MK, Han SS, Rhim H, Kang S (2013) HtrA2/Omi deficiency causes damage and mutation of mitochondrial DNA. Biochim Biophys Acta (BBA) Mol Cell Res 1833(8):1866–1875
Granatiero V, Pacifici M, Raffaello A, De Stefani D, Rizzuto R (2019) Overexpression of mitochondrial calcium uniporter causes neuronal death. Oxidative Med Cell Longev 2019
Guinane CM, Cotter PD (2013) Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Ther Adv Gastroenterol 6(4):295–308
Haran JP, Bhattarai SK, Foley SE, Dutta P, Ward DV, Bucci V, McCormick BA (2019) Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. MBio 10(3)
Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC (2009) Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab 6(1):31
Hernández-López S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ (2000) D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLCβ1–IP3–calcineurin-signaling cascade. J Neurosci 20(24):8987–8995
James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA (2004) Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 80(6):1611–1617
Jara C, Aránguiz A, Cerpa W, Tapia-Rojas C, Quintanilla RA (2018) Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus. Redox Biol 18:279–294
Jones AW, Yao Z, Vicencio JM, Karkucinska-Wieckowska A, Szabadkai G (2012) PGC-1 family coactivators and cell fate: roles in cancer, neurodegeneration, cardiovascular disease and retrograde mitochondria–nucleus signalling. Mitochondrion 12(1):86–99
Kapogiannis D, Mattson MP (2011) Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol 10(2):187–198
Khan SA, Khan SA, Narendra AR, Mushtaq G, Zahran SA, Khan S, Kamal MA (2016) Alzheimer’s disease and autistic spectrum disorder: is there any association? CNS Neurol Disord Drug Targets 15(4):390–402
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795
Lou G, Palikaras K, Lautrup S, Scheibye-Knudsen M, Tavernarakis N, Fang EF (2020) Mitophagy and neuroprotection. Trends Mol Med 26(1):8–20
MacFabe DF (2015) Enteric short-chain fatty acids: microbial messengers of metabolism, mitochondria, and mind: implications in autism spectrum disorders. Microb Ecol Health Dis 26(1):28177
Maenner MJ, Shaw KA, Baio J (2020) Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill Summ 69(4):1
Malaguarnera M, Cauli O (2019) Effects of l-carnitine in patients with autism spectrum disorders: review of clinical studies. Molecules 24(23):4262
Malek M, Hüttemann M, Lee I (2018) Mitochondrial structure, function, and dynamics: the common thread across organs, disease, and aging, Hindawi
Martinelli P, Rugarli EI (2010) Emerging roles of mitochondrial proteases in neurodegeneration. Biochim Biophys Acta (BBA) Bioenerget 1797(1):1–10
Mantzavinos V, Alexiou A (2017) Biomarkers for Alzheimer’s disease diagnosis. Curr Alzheimer Res 14(11):1149–1154
Milder J, Patel M (2012) Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res 100(3):295–303
Neher E, Sakaba T (2008) Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 59(6):861–872
Neufeld K, Kang N, Bienenstock J, Foster JA (2011) Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 23(3):255-e119
Nguyen RL, Medvedeva YV, Ayyagari TE, Schmunk G, Gargus JJ (2018) Intracellular calcium dysregulation in autism spectrum disorder: an analysis of converging organelle signaling pathways. Biochim Biophys Acta (BBA) Mol Cell Res 1865(11):1718–1732
Noebels J (2017) Precision physiology and rescue of brain ion channel disorders. J Gen Physiol 149(5):533–546
Oh M, Kim SA, Yoo HJ (2020) Higher lactate level and lactate-to-pyruvate ratio in autism spectrum disorder. Exp Neurobiol 29(4):314
Ohja K, Gozal E, Fahnestock M, Cai L, Cai J, Freedman JH, Switala A, El-Baz A, Barnes GN (2018) Neuroimmunologic and neurotrophic interactions in autism spectrum disorders: relationship to neuroinflammation. NeuroMolecular Med 20(2):161–173
Pan X, Nasaruddin MB, Elliott CT, McGuinness B, Passmore AP, Kehoe PG, Hölscher C, McClean PL, Graham SF, Green BD (2016) Alzheimer's disease–like pathology has transient effects on the brain and blood metabolome. Neurobiol Aging 38:151–163
Pangrazzi L, Balasco L, Bozzi Y (2020) Oxidative stress and immune system dysfunction in autism spectrum disorders. Int J Mol Sci 21(9):3293
Peng Y, Gao P, Shi L, Chen L, Liu J, Long J (2020) Central and peripheral metabolic defects contribute to the pathogenesis of Alzheimer’s disease: targeting mitochondria for diagnosis and prevention. Antioxid Redox Signal 32(16):1188–1236
Phillips NR, Simpkins JW, Roby RK (2014) Mitochondrial DNA deletions in Alzheimer’s brains: a review. Alzheimers Dement 10(3):393–400
Picard M, McEwen BS (2014) Mitochondria impact brain function and cognition. Proc Natl Acad Sci U S A 111(1):7–8
Quiroz JA, Gray NA, Kato T, Manji HK (2008) Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology 33(11):2551–2565
Qureshi AY, Mueller S, Snyder AZ, Mukherjee P, Berman JI, Roberts TP, Nagarajan SS, Spiro JE, Chung WK, Sherr EH (2014) Opposing brain differences in 16p11. 2 deletion and duplication carriers. J Neurosci 34(34):11199–11211
Remington R, Bechtel C, Larsen D, Samar A, Page R, Morrell C, Shea TB (2016) Maintenance of cognitive performance and mood for individuals with Alzheimer’s disease following consumption of a nutraceutical formulation: a one-year, open-label study. J Alzheimers Dis 51(4):991–995
Rose S, Melnyk S, Pavliv O, Bai S, Nick T, Frye R, James S (2012) Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry 2(7):e134–e134
Rose S, Niyazov DM, Rossignol DA, Goldenthal M, Kahler SG, Frye RE (2018) Clinical and molecular characteristics of mitochondrial dysfunction in autism spectrum disorder. Mol Diagn Ther 22(5):571–593
Rossignol DA, Frye RE (2012) A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry 17(4):389–401
Ruan Y, Li H, Zhang K, Jian F, Tang J, Song Z (2013) Loss of Yme1L perturbates mitochondrial dynamics. Cell Death Dis 4(10):e896–e896
Sackmann C, Hallbeck M (2020) Oligomeric amyloid-β induces early and widespread changes to the proteome in human ipSc-derived neurons. Sci Rep 10(1):1–12
Saint-Georges-Chaumet Y, Edeas M (2016) Microbiota–mitochondria inter-talk: consequence for microbiota–host interaction. FEMS Pathog Dis 74(1):ftv096
Santiago JA, Potashkin JA (2014) A network approach to clinical intervention in neurodegenerative diseases. Trends Mol Med 20(12):694–703
Sharma P, Sampath H (2019) Mitochondrial DNA integrity: role in health and disease. Cell 8(2):100
Shimmura C, Suda S, Tsuchiya KJ, Hashimoto K, Ohno K, Matsuzaki H, Iwata K, Matsumoto K, Wakuda T, Kameno Y (2011) Alteration of plasma glutamate and glutamine levels in children with high-functioning autism. PLoS One 6(10):e25340
Siniscalco D, Cirillo A, Bradstreet JJ, Antonucci N (2013) Epigenetic findings in autism: new perspectives for therapy. Int J Environ Res Public Health 10(9):4261–4273
Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D’Amico D, Moullan N, Potenza F, Schmid AW, Rietsch S (2017) Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 552(7684):187–193
Stein A, Sia EA (2017) Mitochondrial DNA repair and damage tolerance. Front Biosci (Landmark Ed) 22:920–943
Strauss KA, Jinks RN, Puffenberger EG, Venkatesh S, Singh K, Cheng I, Mikita N, Thilagavathi J, Lee J, Sarafianos S (2015) CODAS syndrome is associated with mutations of LONP1, encoding mitochondrial AAA+ Lon protease. Am J Hum Genet 96(1):121–135
Tang G, Rios PG, Kuo S-H, Akman HO, Rosoklija G, Tanji K, Dwork A, Schon EA, DiMauro S, Goldman J (2013) Mitochondrial abnormalities in temporal lobe of autistic brain. Neurobiol Dis 54:349–361
Tardiolo G, Bramanti P, Mazzon E (2018) Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules 23(12):3305
Toglia P, Ullah G (2016) The gain-of-function enhancement of IP3-receptor channel gating by familial Alzheimer’s disease-linked presenilin mutants increases the open probability of mitochondrial permeability transition pore. Cell Calcium 60(1):13–24
Toledo JB, Arnold M, Kastenmüller G, Chang R, Baillie RA, Han X, Thambisetty M, Tenenbaum JD, Suhre K, Thompson JW (2017) Metabolic network failures in Alzheimer’s disease: a biochemical road map. Alzheimers Dement 13(9):965–984
Valiente-Pallejà A, Torrell H, Muntané G, Cortés MJ, Martínez-Leal R, Abasolo N, Alonso Y, Vilella E, Martorell L (2018) Genetic and clinical evidence of mitochondrial dysfunction in autism spectrum disorder and intellectual disability. Hum Mol Genet 27(5):891–900
Varga NÁ, Pentelényi K, Balicza P, Gézsi A, Reményi V, Hársfalvi V, Bencsik R, Illés A, Prekop C, Molnár MJ (2018) Mitochondrial dysfunction and autism: comprehensive genetic analyses of children with autism and mtDNA deletion. Behav Brain Funct 14(1):1–14
Vinkhuyzen AA, Eyles DW, Burne TH, Blanken LM, Kruithof CJ, Verhulst F, White T, Jaddoe VW, Tiemeier H, McGrath JJ (2017) Gestational vitamin D deficiency and autism spectrum disorder. BJPsych Open 3(2):85–90
Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K (2017) Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7(1):1–11
Von Bernhardi R, Eugenín J (2012) Alzheimer’s disease: redox dysregulation as a common denominator for diverse pathogenic mechanisms. Antioxid Redox Signal 16(9):974–1031
Wang J, Markesbery WR, Lovell MA (2006) Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. J Neurochem 96(3):825–832
Wang T, Hu X, Liang S, Li W, Wu X, Wang L, Jin F (2015) Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benefic Microbes 6(5):707–717
Wang Y, Li N, Yang J-J, Zhao D-M, Chen B, Zhang G-Q, Chen S, Cao R-F, Yu H, Zhao C-Y (2020) Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol Res 157:104784
Wen-hong L, Llopis J, Whitney M, Zlokarnik G, Tsien RY (1998) Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 392(6679):936
Xie K, Qin Q, Long Z, Yang Y, Peng C, Xi C, Li L, Wu Z, Daria V, Zhao Y (2021) High-throughput metabolomics for discovering potential biomarkers and identifying metabolic mechanisms in aging and Alzheimer’s disease. Front Cell Dev Biol 9:602887
Zhang J-Y, Ding Y-P, Wang Z, Kong Y, Gao R, Chen G (2017) Hydrogen sulfide therapy in brain diseases: from bench to bedside. Med Gas Res 7(2):113
Zhang H, Wei W, Zhao M, Ma L, Jiang X, Pei H, Cao Y, Li H (2021) Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int J Biol Sci 17(9):2181–2192. https://doi.org/10.7150/ijbs.57078
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Farhana, A., Khan, Y.S. (2021). Mitochondrial Dysfunction: A Key Player in the Pathogenesis of Autism Spectrum Disorders and Alzheimer’s Disease. In: Md Ashraf, G., Alexiou, A. (eds) Autism Spectrum Disorder and Alzheimer's Disease . Springer, Singapore. https://doi.org/10.1007/978-981-16-4558-7_2
Download citation
DOI: https://doi.org/10.1007/978-981-16-4558-7_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4557-0
Online ISBN: 978-981-16-4558-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)