Abstract
Among the plant nutrients, potassium (K+) is one of the vital elements required for plant growth and physiology, along with nitrogen (N) and phosphorus (P). K+ is a cation present in plants in concentrations ranging from 50 to 150 mM in the liquid parts, the cytoplasm, and the vacuole. The concentration of K+ in the cytoplasm is usually constant about 50 mM, while the concentration in the vacuole may vary quite substantially. It is a component of the plant structure, but also has a regulatory function in several biochemical processes related to protein synthesis, carbohydrate metabolism, and enzyme activation. Numerous physiological processes depend on K+, such as stomatal regulation and photosynthesis. In recent decades, K+ was found to provide abiotic stress tolerance mechanisms, e.g., enzyme activation, protein synthesis, photosynthesis, stomatal movement, turgor regulation, and osmotic adjustment. It also functions in plant signaling systems which assist in defending some stresses by activating antioxidant defense systems. K+ accumulation in plants before stress events such as water deficiency, lodging, cold stress, and salinity stress is a survival strategy for the plant. K+ is needed at high concentrations inside the plants from early stages of vegetative growth phase. High internal K+ concentration can reduce extreme sudden environmental events like cold, frost, late season rains, salinity stress, and heat waves. The whole structure of proteins and protein activity need high concentrations of K+ in the cytosol for optimum plant functions. Yet, to survive osmotic stress and unusual physical burden, prior accumulation of K+ was shown to reduce the damage to plants. Proper use of K+ with other nutrients helps to achieve sustainable productivity and quality of crops and guarantee nutritional food security for animals and humans.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Abiotic stress is defined as environmental conditions that decrease crop growth and yield below optimum levels (Asada et al. 2000). It hampers plant productivity by changing plant growth patterns and physiological responses (Asada et al. 2000). Most cultivated crops undergo abiotic stress or combination of more than one kind of stress through their growth cycle (Asada et al. 2000). Combination of different stresses is becoming more common, for example, drought and high temperature are the most common stress combination (Asada et al. 2000), while in arid and semiarid regions, salinity and high temperature stresses may occur at the same time. These complex stresses generate changes in cropping patterns, crop agricultural practices, and, sometimes, the extinction of plant species (Asada et al. 2000; Vranová et al. 2002).
Food production must be increased by up to 100% by the year 2050 to meet the nutritional needs of the increasing world population (Asada et al. 2000). The area of agricultural lands is very limited and has been declining all over the world; thus, the desirable increases in food production must be achieved on the already cultivated land. However, there is a global decrease in soil productivity and fertility due to degradation and intensive use of soils without consideration of proper soil-management practices (Asada et al. 2000; Vranová et al. 2002). Insufficient and unbalanced mineral nutrient supply and reduced soil fertility are distinct problems, leading to a decrease in global food production, particularly in the developing countries. It is estimated that about 60% of cultivated soils have growth-limiting difficulties associated with mineral deficiencies and toxicities (Asada et al. 2000).
To sustain food security, there is a great need to minimize the harmful effects of abiotic stresses on crop production. Part of the solution can be achieved with balanced supply of mineral nutrients and maintaining soil fertility. This chapter deals with K+ roles in reducing the negative effects of abiotic stress on crop production.
2.2 Potassium in the Soil
Potassium (K+) contains an average of 2.6% of the earth’s crust, making it the seventh most abundant element and the fourth most abundant mineral nutrient in the lithosphere (Asada et al. 2000; Vranová et al. 2002). K+ is a vital element of plant nutrition and is the second largest nutrient assimilated by plants after nitrogen (Asada et al. 2000). The amount of K+ in a given soil reflects the parent materials of the soil, weathering degree, and volume of K fertilizer added, minus losses due to crop removal, soil erosion, and leaching. Crop removal and fertilization are highly important factors in cultivated soils. Cultivating practices used around the world have a strong effect on K+ levels in soils. Any time the crop vegetative portion is removed; a large increase in K+ is expected to be removed as well. In some livestock operations, manure is returned to the soil, which significantly reduces the loss. Where this is not the case, K+ removal is so large that even the best endowed soils cannot withstand the loss forever (Asada et al. 2000; Vranová et al. 2002).
Potassium availability and spatial distribution in agricultural soils are affected by many agro-environmental factors (Biehler and Fock 1996; Cakmak 2000). However, not all K forms are readily available for plants. It is commonly recognized that K+ occurs in soil in four forms: water soluble, exchangeable, non-exchangeable, and structural (Asada et al. 2000). There is a dynamic equilibrium reaction among the K+ different forms that control the release and/or fixation of K+ according to soil biogeochemical properties and processes (Asada et al. 2000; Vranová et al. 2002). Hence, soil K+ form distribution is affected by different agro-environmental factors, such as soil parent materials (Biehler and Fock 1996; Cakmak 2000), soil weathering degree (Asada et al. 2000), topography (Asada et al. 2000; Vranová et al. 2002), and nutrient balance (Bertsch and Thomas 1985).
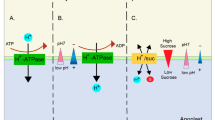
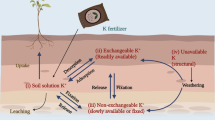
The readily available K+ for plant nutrition exists in the soil solution. K+ concentration in the soil solution is very low at any time; therefore, the replacement of the solution from other K-bearing phases is of great importance in determining a soil’s K fertility status. Once K+ is released to solution, it diffuses into the roots to ensure normal plant uptake. This diffusion process is reliant on several factors, such as soil water content, tortuosity of the diffusion path, temperature, K+ diffusion coefficient in water, and K+ concentration gradient. Plants take up relatively high amounts of K+, thus rapidly reducing the K+ concentration in the root zone (Fig. 2.1) (Biehler and Fock 1996; Cakmak 2000). K cycle in the soil is shown in Fig. 2.2.
Dynamics between the various K phases in soil, from Bertsch and Thomas (1985)
2.3 Potassium Role in Plants
Potassium is an essential nutrient and the most abundant cation in plants. K+ is a unique plant nutrient as it occurs solely in the free ion form (Asada et al. 2000). In sufficiently supplied plants, K+ may make up about 6% of plant dry matter or concentrations of around 200 mM (Asada et al. 2000; Vranová et al. 2002). Highest K+ concentrations are found in young developing tissues and reproductive organs indicative of its high activity in cell metabolism and growth. K+ activates numerous enzymes including those involving energy metabolism, protein synthesis, and solute transport (Biehler and Fock 1996; Cakmak 2000). Other processes where K+ is found to be involved include stomatal movement, osmoregulation and cell extension, photosynthesis, and phloem loading and transport and uptake (Cakmak 2000; Choi et al. 2002). K+ is needed in the plant cells for maintaining transmembrane voltage gradients for cytoplasmic pH homeostasis and in the transport of inorganic anions and metabolites (Asada et al. 2000). K+ is the dominant cation in long-distance transport within the xylem and phloem saps, neutralizing inorganic and organic anions, giving high K+ mobility throughout the whole plant (Asada et al. 2000; Vranová et al. 2002). K+ uptake and accumulation by plant cells is the main driving force for cells’ osmotic expansion (Biehler and Fock 1996; Cakmak 2000).
The most common symptom of K+ deficiency is chlorosis along leaves edges, also known as leaf margin scorching. Chlorosis occurs first in older leaves, due to the high rate of K+ allocation from mature tissues to developing tissues. First, the growth rate is decreasing (known as hidden hunger), and only later chlorosis and necrosis appear in the older leaves. Because K+ is required in photosynthesis and protein synthesis, K+-deficient plants will have slow and stunted growth. In some crops, stems become weak and lodging incidences increase. Seed and fruit size and their production quantity are reduced. Plants with K+ deficiency demonstrate turgor decrease and become flaccid under water stress, especially during midday hours (Choi et al. 2002; Cakmak 2000).
Potassium also contributes to the survival of plants exposed to various abiotic stresses (Asada et al. 2000). Potassium plays vital roles in contributing to the survival of crop plants under environmental stress conditions and many physiological processes, such as protein synthesis, energy transfer, enzyme activation, photosynthesis and translocation of photosynthates into sink organs, osmoregulation, stomatal movement, phloem transport, cation-anion balance, and stress resistance and reducing excess uptake of ions such as Na and Fe in saline and flooded soil (Asada et al. 2000; Vranová et al. 2002).
2.4 Potassium Role in Response to Abiotic Stress
Increasing events of drought and other abiotic stresses rising from loss of soil fertility and global warming will require a specifically high supply of K+ for crop stress reduction (Biehler and Fock 1996; Cakmak 2000). During their evolution, plants have developed a wide range of tolerance mechanisms to cope with a variety of stressed conditions. Increasing evidence suggests that mineral nutrients play a vital role in plant stress tolerance (Cakmak 2000; Choi et al. 2002). Out of all these mineral nutrients, K+ plays a particularly important role in plant growth and metabolism, and it significantly contributes to plant survival under various biotic and abiotic stresses. The importance of K+ fertilizers to the establishing crop production and quality is known. As a result, potash consumption has increased dramatically in most areas of the world (Asada et al. 2000).
2.4.1 Drought
Most crops will be exposed to water shortages at some point during their growing season, resulting in numerous harmful effects on plant growth. Drought stress mitigation is therefore an essential aspect of crop management (Asada et al. 2000; Vranová et al. 2002). Maintaining sufficient K+ status in the plant is vital for drought adaptation (Biehler and Fock 1996; Cakmak 2000), and there is increasing evidence that plants under drought conditions have a higher internal K+ requirement (Cakmak 2000; Choi et al. 2002). Simultaneously, plants’ K+ uptake is significantly decreased by drought stress (Marschner et al. 1996). This reduction is due to both decreased K+ mobility in the soil and reduced transpiration rate and impaired activity of root membrane transporters (Asada et al. 2000).
The reason for the enhanced need for K+ by plants suffering from abiotic stresses is related to the fact that K+ is required to maintain photosynthetic CO2 fixation. High K+ has also been linked to maintaining optimum pH values in the chloroplasts’ stroma and optimal function of photosynthetic mechanisms (Asada et al. 2000; Vranová et al. 2002). Water-stressed chloroplasts were showing increased K+ leakage, resulting in additional photosynthesis suppression (Fig. 2.3) (Biehler and Fock 1996; Cakmak 2000). When water-deficit stressed plants were supplied with higher than optimum quantities of K+, they were able to maintain efficient photosynthetic activity (Cakmak 2000; Choi et al. 2002) and contained higher K+ concentrations compared to plants which received optimal quantities of water (Marschner et al. 1996). This can be explained by K’s ability to maintain CO2 assimilation rates by regulating stomatal function and balancing cell water relations (Asada et al. 2000).
Net photosynthesis of wheat leaves subjected to varied drought stress and K supply, from Cakmak (2000)
Potassium accumulation plays a crucial role in osmotic regulation, contributing on average between 35% and 50% of the cell osmotic potential in crops (Asada et al. 2000; Vranová et al. 2002). Biehler and Fock (1996) and Cakmak (2000) reported that in wheat, differences in shoot K+ content were 84% of the difference in osmotic adjustment among K-sufficient genotypes, while in plants lacking K+ fertilization, K+ accumulation in leaves was only 17–28% of osmotic adjustment. K fertilization was found to increase osmotic adjustment and improved water relations in a wide range of crops (Cakmak 2000; Choi et al. 2002).
The K+ status of the plant also influences on the plant’s ability to extract water from the soil. Plants supplied with sufficiently amounts of K+ can utilize the soil moisture more effectively than K+-lacking plants (Marschner et al. 1996). K+ application was found to enhance cell elongation, the key of plant growth. Cell elongation is initiated by wall relaxation, leading to osmotic potential-driven water uptake and turgor-driven cell expansion (Asada et al. 2000). Enhanced cell expansion and growth set up a pressure gradient between the root and its surrounding which increases water uptake (Asada et al. 2000; Vranová et al. 2002), which might help the plant keep drawing water when the soil is getting dryer.
2.4.2 Cold Stress
Temperature is an important factor determining plants’ geographical distribution in an optimal environment for their survival and development (Biehler and Fock 1996; Cakmak 2000). Cold stress (<20 °C) occurs when low temperatures affect cellular macromolecules, which results in slowing down of metabolism, cell membrane solidification, and losing membrane functions (Cakmak 2000; Choi et al. 2002). Abrupt changes in the plant’s environment result in slower growth and reduced yield due to resource shifting from reproductive processes to metabolic process, in order to attain tolerance (Marschner et al. 1996). Cold stress symptoms include wilting, chlorosis, leaf expansion, and growth reduction (Marschner and Cakmak 1989), which may further develop to necrosis, reduced development of reproductive components, and hindered seed and pod development in sensitive species (Asada et al. 2000). The combination of all these mentioned factors ultimately leads to crop yield reduction.
Plants perform better when exposed to cold stress, when their K concentration is in the range of what Asada et al. (2000) and Vranová et al. (2002) described as “luxury consumption concentration range.” Biehler and Fock (1996) and Cakmak (2000) proposed that K+ accumulation by plants before stress initiation is not luxury but rather an “insurance strategy” to enable the plant to survive an unexpected environmental change (abiotic stress). Lack of K+ in the early developmental stages of plant growth will affect the entire structure of the plant and may lead to lodging and frost damage in a higher ratio than expected from a healthy plant (Cakmak 2000; Choi et al. 2002). When temperatures decline (frost or chilling event), it causes a sudden change in the membrane fluidity. This change differs from cell to cell and dependent on the relative composition of various phospholipids (Marschner et al. 1996). In general, low temperature stress has an effect on the membrane lipid fluidity and thus modifies the membrane structure (Marschner and Cakmak 1989).
The balanced fatty acid ratio is involved as well in reducing plants’ chilling sensitivity. High ratio between unsaturated and saturated fatty acids in the cell membrane makes the tissue more tolerant to low temperatures (Asada et al. 2000). Asada et al. (2000) and Vranová et al. (2002) showed that additional K+ in tomatoes, eggplants, and peppers led to maximum growth response and chilling tolerance. This response was associated with phospholipid increase, membrane permeability, and improving cells’ biophysical and biochemical properties. Biehler and Fock (1996) and Cakmak (2000) studied the effect of increasing K+ concentration on yield and chilling damage in the carnations. Stem brittle incidence percentage increased 5–6 weeks after cold night events (>8 °C), followed by clear sunny days. When K+ concentration in the irrigation water was increased, the weekly loss of broken stems was lower (Fig. 2.4). Likewise, Cakmak (2000) and Choi et al. (2002) reported that high K applications could alleviate cold-induced yield reduction and increases in leaf damage in potato plants under field conditions (Fig. 2.5). K-deficient potato plants might be more vulnerable to frost; Marschner et al. (1996) reported that application of K+ at high rates can efficiently increase frost tolerance of some frost-sensitive potato genotypes. Nitrogen (N) and K effect on two rice cultivars’ spikelet sterility induced by low temperature at the reproductive stage was studied by Marschner and Cakmak (1989). Increasing the K+ supply and the K:N ratio in the leaves reduced the spikelet sterility in one of the tested rice cultivars, but the effect was not clear in the second cultivar. The different response of the rice cultivars to chilling effects might be because they differ in the fatty acid composition of their root membranes.
Broken stems of carnation cv. White candy during the growing season on three on levels of K+ in irrigation water: filled circle 93; filled triangle 252; filled square 378 g K+ m-3. Source: Yermiyahu and Kafkafi (1990)
Potassium supply influence on tuber yield, potassium concentration of leaves, and leaf frost damage in potato. Adapted from Asada et al. (2000)
High K+ content inside cells can increase frost tolerance by regulating osmotic and water potential of the cell sap and decreasing electrolyte leakage caused by low temperatures (Asada et al. 2000; Vranová et al. 2002). Cold stress can also cause photooxidative damage to chloroplasts as a result of impairments in photosynthetic carbon (C) metabolism. Under cold stress, the absorbed light energy surpasses the chloroplast capacity to utilize it in CO2 fixation; instead, the excess energy is used to activate O2 to reactive oxygen species (ROS) (Biehler and Fock 1996; Cakmak 2000).
The main processes damaged by cold stress are the photosynthetic electron transport, rubisco activity, stomatal conductance, and CO2 fixation (Cakmak 2000; Choi et al. 2002). These cellular processes are also being negatively affected by K deficiency. Thus, when K supply is insufficient, low temperature-induced photooxidative damage can be worsened, leading to a further reduction of plant growth and yield. Supplying plants with high amounts of K may protect them against oxidative damage caused by cold stress (Marschner et al. 1996). To summarize, high concentration of K in the tissue can decrease chilling damage and increase cold tolerance, resulting in increased yield production. Frost damage is negatively correlated to K concentration and can be significantly reduced with K fertilization (Marschner and Cakmak 1989). High frost damage was observed in potatoes growing in soils with available K concentration of ≤114 ppm. This concentration might be the critical available K level for frost damage formation in potatoes and also can be used for K fertilizer recommendations (Grewal and Singh 1980).
2.4.3 Salinity Stress
Salinity stress is one of the major abiotic stresses that impact almost every aspect of plant physiology and biochemistry, resulting in yield reduction (Barnes et al. 1995). Thus, it is a serious threat to agricultural productivity, especially in arid and semiarid regions (Asada et al. 2000). It has been estimated that 20% of total cultivated and 33% of irrigated agricultural lands worldwide are afflicted by salinity. Moreover, salinized areas are increasing at a rate of 10% annually. Additionally, brackish water is often used for irrigation, especially in dry climates, due to intensive use of scarce water resources, further increasing salinization of soils and groundwater (Asada et al. 2000; Vranová et al. 2002).
Sodium (Na+) and K nutrition impairment is a great indicator of salt-stressed plants. Thus, K:Na ratio is considered a useful way to estimate salt tolerance in plants (Biehler and Fock 1996; Cakmak 2000). Plants’ ability to maintain high K+ cytoplasmic levels is vital for their survival in saline environments (Cakmak 2000; Choi et al. 2002). In saline soils, Na+ concentration is usually higher than K+ and calcium (Ca2+), which can create a passive accumulation of Na+ in the plant (Marschner et al. 1996). High Na+ levels can cause to Ca2+ relocation from the root membranes, changing their integrity and eventually affecting the selectivity of K+ uptake (Marschner and Cakmak 1989). Potassium uptake is regulating K+ xylem loading (Barnes et al. 1995), suggesting that salinity interferes with K+ translocation from the roots to the shoot, which results in a lower K+ shoot content and a higher K+ root content (Asada et al. 2000). Salinity inhibitory effects on K+ uptake and relocation were found to be stronger when the K+ concentration in the nutrient solution was low (Asada et al. 2000; Vranová et al. 2002). Sodium toxicity happens as a result of Na+ competing with K+ for enzyme activation and protein biosynthesis. Yet, it is not the absolute concentration of Na+ by itself but rather the K+: Na+ ratio in the cytosol that controls the cells’ metabolic capability and ultimately the plant’s ability to survive in saline environments (Biehler and Fock 1996; Cakmak 2000). Numerous studies stated that the cytosolic K+:Na+ ratio plays an important role in salt tolerance of plants (Cakmak 2000; Choi et al. 2002).
In breeding, selecting genotypes with high K:Na ratios can be a solution to minimize the negative effects on plants growing in saline soils (Marschner et al. 1996). A wheat mutant was found to have high capability to accumulate K in the shoot. This mutant showed higher tissue hydration, seed germination, and seedling growth under growing NaCl concentrations, compared with other wheat genotypes (Marschner and Cakmak 1989). Arabidopsis mutant lines showing hypersensitivity to NaCl were found to be hypersensitive to low K supply as well. The high salt sensitivity was associated with very low capacity of the plants to take up K from the growth medium, showing again the importance of K nutrition in salt tolerance (Barnes et al. 1995). Similarly, tomato salt-hypersensitive mutants were found to have malfunctioning K uptake and had a reduced K nutrition (Fig. 2.6) (Zhao et al. 2001). Asada et al. (2000) reported that salinity effect on the growth of maize plant was dependent on K+ concentration in the growth medium. Salinity had no effect on root dry weight, but low K+ concentration in the nutrient solution reduced shoot dry weight significantly. Asada et al. (2000) and Vranová et al. (2002) reported similar responses found in spinach. The differences in shoot growth between plants growing in low and high salinity levels were decreased as a response to an increasing K+ concentration. Shoot/root ratio was lower when plants grew in 100 mmol/L NaCl, but the effect was significant only when the K+ concentration in the nutrient solution was low. These results highlights that sufficient K nutrition has a crucial role in alleviating the detrimental effects of salinity in plants (Biehler and Fock 1996; Cakmak 2000) (Fig. 2.7).
tss1 mutants with hypersensitivity low potassium. From Borsani et al. (2001)
NaCl and potassium concentration effect on shoot and root dry weight of 19-day-old maize plants. Each column represents the mean of four replicates. The error bars represent the standard error (n = 4) (Marschner et al. 1996)
2.4.4 Heat Stress
High temperature stress occurs when the temperature is higher than what was defined as temperature optimum range for plants (Marschner and Cakmak 1989). Heat stress is a major environmental factor which poses limitations on plant growth, metabolism, and productivity all over the world. Plant growth and development include many biochemical responses which are sensitive to temperature (Barnes et al. 1995). Heat stress has a negative impact on different plant processes (e.g., growth, development, physiological) and yield (Zhao et al. 2001). Figure 2.8 is showing the cell signaling associated with low K levels in plants. One of the major heat stresses results in excess production of ROS, which later causes an oxidative stress. Plant responses to heat stress are dependent on the degree and exposure to the stress and the plant type.
Cell signaling associated with low potassium levels in plants (Zhao et al. 2001)
Potassium plays an important role in plant tolerance to heat stress. K+ helps to activate numerous physiological and metabolic processes, e.g., photosynthesis, respiration, and nutrient homeostasis. Additionally, K+ enhances plant tissue’s water potential, which aids in plant tolerance to high temperature stress (Asada et al. 2000). Potassium functions as an osmolyte and assists in maintaining and regulating stomatal conductance and thus preventing cell damage (Asada et al. 2000; Vranová et al. 2002). ROS production was seen in plants with K+ deficiency, which happened via photosynthetic electron transport pathways and NADPH oxidizing enzymatic reactions (Biehler and Fock 1996; Cakmak 2000). K+ protects plants by assisting protein synthesis, participating in numerous enzymatic reactions and carbohydrate biosynthesis, and improving plant cells’ water use efficiency. Under heat stress conditions, wheat heat tolerance was improved with potassium orthophosphate (KH2PO4) foliar application, which protected the leaves from damage (Cakmak 2000; Choi et al. 2002).
When losing significant K+ quantity from the chloroplast, a decrease in photosynthesis is noticed. Applying K+ in this case can assist plant cells to become more resilient to the heat stress by strengthening the photosynthetic capability. Foliar K+ applications lead to increased photosynthate accumulation and translocation, and dry matter as well. Marschner et al. (1996) observed a decline in hydrogen peroxide activity after 21 days of heat stress, after applying foliar KH2PO3 and other nutrients (Fig. 2.9), as part of improved response to heat stress. Heat stress can cause leaf senescence associated with oxidative stress caused by ROS production (like hydrogen peroxide). Reducing ROS overproduction during stress can reduce damage done to cellular components like lipids, protein, DNA, and RNA (Marschner and Cakmak 1989).
2.4.5 Potassium and Lodging
The definition of lodging is stem dislocation from their standing position. Lodging can be partially reversible or permanent, depending on the bending degree. Lodging is more common to occur in cereal crops. There are two kinds of lodging: (1) stem lodging is defined as lower culm internode bending (Marschner et al. 1996; Zhao et al. 2001) and (2) root lodging is defined when the plant is leaning from the crown due to insufficient root growth (Asada et al. 2000). Lodging loss is critical in heading and early grain development stages. Lodging is affected by combined effects of plant type and nutrient management and environmental and soil conditions (Asada et al. 2000; Vranová et al. 2002). Lodging causes yield reduction and financial costs. For example, severe lodging happens once in 3–4 years in the UK and might result in yield losses in cereal crops and oilseed rape of 25–75%, depending on the lodging timing during the growing season (Biehler and Fock 1996; Cakmak 2000). Lodging also affects yield quality, as lodged crop grains are less likely to meet bread-making quality. The estimation for the UK is that lodging can lead to financial losses of 170 M £ in a severe lodging year (50 M £ on average), as a result of yield loss, lower grain quality, and higher grain drying costs (Cakmak 2000; Choi et al. 2002). Lodging increases the risk of mycotoxin development in the grain (Marschner et al. 1996), which can be dangerous to animals and humans.
The stem diameter of K-deficient plants is smaller, which makes the plant more vulnerable to lodging. When K supply is insufficient, sclerenchyma fiber cells and woody parenchyma cells create thin and poorly lignified cell walls which decrease stem diameter (Marschner and Cakmak 1989). Barnes et al. (1995) reported that optimal K nutrition elevated the thickness of sclerenchyma tissue layers in rice. Wheat internode cross section (Fig. 2.10) shows that plants which received more K+ have developed thicker stalk wall. Improved stem stability and thickness can be related to delayed aging of the pith parenchyma, and activation of general defense mechanism when K supply is optimal (Zhao et al. 2001). Adding K+ decreased senescent stalks and stalk lodging percentage in corn grown on K-deficient soils (Marschner et al. 1996; Zhao et al. 2001). Welch and Flannery (1985) reported that applying K+ increased crushing strength and skin.
Cross section of wheat internode with low (left) and high (right) K+ nutrition (Kant and Kafkafi 2002)
Potassium deficiency increases both transpiration and respiration rate and reduces photosynthesis, starch accumulation, and cell wall substances, e.g., holocellulose, which affect stem strength in rice. Potassium content in the culm basal part is highly correlated with the stem breaking strength, suggesting that adequate amount of K+ is related to lignification of vascular bundles and sclerenchyma cells, and strengthening culms, thus increasing the plant lodging resistance (Datta and Mikkelsen 1985). Increasing K+ dose from 50 to 200 kg ha−1 reduced total lodging percentage (Fig. 2.11) (Melis and Farina 1984). Lodging resistance is mainly ruled genetically, and sufficient K application further reduces the likelihood of lodging in different crops (Parks 1985).
K+ application effect on lodging in a susceptible maize cultivar (Melis and Farina 1984)
2.4.6 Potassium and Iron Toxicity
Iron (Fe) toxicity happens when the soil solution contains high concentrations of reduced iron (Fe2+) (Becker and Asch 2005). Iron toxicity is more common in flooded soils and may result in low productivity in affected soils. Because rice is a major crop growing in flooded soils, most of the research is focused on Fe toxicity in this crop. Fe2+ toxicity symptoms occur with uptake of high Fe2+ concentrations, translocating them to the leaves and producing toxic oxygen radicals harming cellular structures and afflicting physiological processes (Wu et al. 2017; Onyango et al. 2019). Consequently, browning symptoms, like bronzing, appear on the leaves, leading to reduced active leaf area and yield. Bronzing developed mostly in older leaves, which transpire more (Suriyagoda et al. 2017).
Potassium application was reported to reduce Fe2+ concentration in rice plants and improve their growth (Yamauchi 1989; Sahrawat 2004). Applying K fertilizer is improving rice root’s oxidizing power and lateral root formation and decreases primary root growth suppression (Fig. 2.12) (Trolldenier 1988; Li et al. 2016). Furthermore, sufficient K supply decreased Fe2+ translocation from roots to shoots (Li et al. 2001) (Fig. 2.13). Under K deficiency conditions, low-molecular-weight metabolite exudation from roots was increased, encouraging the activity of Fe-reducing bacteria in the root zone (Trolldenier 1988). though, by improving K nutritional status, cannot abolish Fe toxicity completely (Ramírez et al. 2002). Furthermore, high K levels in the soil solution might cause a negative effect on bioavailability of other nutrients (Suriyagoda et al. 2020).
Exogenous K+ effect on primary root growth and lateral root formation in Arabidopsis under excess Fe. Values are the means ± SE, n ≥ 4. Different letters represent means statistically different, P < 0.05 (Li et al. 2016)
Fe2+ level effects on shoot and root growth of hybrid rice under three potassium concentrations. Data are means of three replications. The bars are LSD5%, P < 0.05 (Li et al. 2001)
2.4.7 Potassium and Light-Induced Cell Damage
During photosynthesis, ROS are being produced mostly by chloroplasts, e.g., superoxide radical (O2˙−), hydrogen peroxide (H2O2), and singlet oxygen (1O2) (Asada et al. 2000). ROS production in the chloroplasts is especially high when plants are experiencing environmental stresses (Asada et al. 2000; Vranová et al. 2002). ROS are highly toxic, causing damages to the membranes and chlorophyll degradation, leading to leaf chlorosis and necrosis development. Under optimal growing conditions, about 20% of the total photosynthetic electron flux is directed to molecular O2, creating O2˙− and other O2˙− driven ROS (Biehler and Fock 1996; Cakmak 2000). If there is a limiting factor (such as stress) on the absorbed light energy utilization in carbon fixation, the electron flux to O2 is increased, leading to large ROS accumulation in the chloroplasts. Then, excitation energy is being relocated to O2 to form extremely toxic 1O2. ROS production in the chloroplasts turns more noticeable when stressed plants are exposed to high light intensity, which result in photooxidative damage to the chloroplasts (Cakmak 2000; Choi et al. 2002).
Potassium-deficient plants are highly sensitive to intense light. When K supply is low, leaf chlorosis and necrosis will appear in plants growing under high light intensity, but not when growing under low light intensity (Marschner et al. 1996). Partial shading of K-deficient leaves can prevent leaf chlorosis and necrosis development as well. The strong effects of high light intensity on the appearances of chlorosis were not correlated with K concentrations in leaf tissues; the concentrations were similar in shaded and nonshaded leaves with sufficient and deficient K supply (Marschner and Cakmak 1989). These support the concept that photooxidative ROS-induced injury to the chloroplasts has a crucial role in the presence of leaf symptoms typical to K deficiency. There are few explanations for high sensitivity to increasing light intensity in K-deficient plants. K is important to maintain photosynthesis and associated processes.
When plants are exposed to elevated atmospheric CO2 and O3 concentrations, the negative impact of K deficiency on photosynthesis is becoming more distinct (Barnes et al. 1995), suggesting that plants require more K when growing in CO2-enriched conditions. Reduced photosynthesis due to K deficiency (Fig. 2.14) seems to be connected to decreased stomatal conductance, higher mesophyll resistance, and lower activity of the enzyme ribulose bisphosphate carboxylase (Zhao et al. 2001). Maintaining a high rate of photosynthesis at is also reliant on the export and use of photoassimilates inside the plant. When K supply is sufficient, there is a significant increase in sucrose concentration in source leaves and a noticeable decrease in roots when K is insufficient (Marschner et al. 1996; Zhao et al. 2001). Enhanced ROS production is inevitable due to impairment of photosynthetic CO2 fixation and decreased photoassimilate utilization under K deficiency, which then cause photooxidative damage. Increased severity of leaf chlorosis under K deficiency was related to higher activity of enzymes involved in H2O2 detoxification and its utilization in oxidative processes (Fig. 2.15) (Cakmak 1994). In K-deficient leaves, there is an increase in H2O2 detoxification capacity, suggesting that ROS production is enhanced in K-deficient leaves on the account of CO2 fixation. Plants exposed to high light intensity might have larger K necessities at physiological levels compared to plants grown under low light intensity. Higher K supply under high light intensity is required for efficient absorbed light energy utilization in photosynthetic CO2 fixation and photosynthate transport to sink organs (Cakmak 2005).
Photosynthesis rate in leaves sufficient and deficient of potassium supply (Cakmak 2000)
Ascorbate peroxidase and guaiacol peroxidase activity in leaves of bean (Phaseolus vulgaris) plants over 12 days of growth in nutrient solution with 2000 μM and 50 μM potassium (redrawn from Cakmak 1994)
2.5 Conclusions
Potassium deficiency is a crucial nutritional problem, which affects crop yield production and quality. Keeping optimal K nutritional status is vital for plant tolerance to biotic and abiotic stresses. Effective K usage and balanced fertilization combined with other minerals will contribute to sustainable crop growth, yield, and quality, will impact plant health, and decrease environmental risks. Though additional research is needed to fully understand the role of K in whole-plant stress response mechanisms, as they are complex (Fig. 2.16). K is required in high concentrations inside plants, from early developmental vegetative stages. High internal K+ concentration was found to reduce extreme and sudden environmental events like low and high temperature, salinity, drought, and high light intensity stresses. The entire protein structure and protein activity require high K+ concentrations in the cytosol for optimal plant functions.
Potassium roles in resisting all stresses (Wang et al. 2013)
References
Allen DJ, Ort DR (2001) Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci 3:36–42
Amtmann A, Troufflard S, Armengaud P (2008) The effect of potassium nutrition on pest and disease resistance in plants. Physiol Plant 133:682–691
Asada K, Allen J, Foyer CH, Matthijs HCP (2000) The water-water cycle as alternative photon and electron sinks. Philos Trans R Soc Lond B Biol Sci:1419–1431
Ashraf M, Ahmad A, McNeilly T (2001) Growth and photosynthetic characteristics in pearl millet under water stress and different potassium supply. Photosynthetica 39:389–384
Azedo-Silva J, Osório J, Fonseca F, Correia MJ (2004) Effects of soil drying and subsequent re-watering on the activity of nitrate reductase in roots and leaves of Helianthus annuus. Funct Plant Biol 31:611–621
Bagnall D, Wolfe J, King RW (1983) Chill-induced wilting and hydraulic recovery in mung bean plants. Plant Cell Environ 6:457–464
Barnes JD, Pfirrmann T, Steiner K, Lütz C et al (1995) Effects of elevated CO2, elevated O3 and potassium deficiency on Norway spruce [Picea abies (L.) Karst.]: seasonal changes in photosynthesis and nonstructural carbohydrate content. Plant Cell Environ 18:1345–1357
Barré P, Velde B, Fontaine C et al (2008) Which 2:1 clay minerals are involved in the soil potassium reservoir? Insights from potassium addition or removal experiments on three temperate grassland soil clay assemblages. Geoderma 146:216–223
Becker M, Asch F (2005) Iron toxicity in rice—conditions and management concepts. J Plant Nutr Soil Sci 168:558–573
Bergmann E, Bergmann HW (1985) Comparing diagrams of plant/leaf analysis presenting by rapid inspection the mineral nutrient element status of agricultural crop plants. Potash Rev. Sub 5 No 2/1985, pp 1–10
Beringer H, Nothdurft F (1985) Effects of potassium on plant and cellular structures. In: Munson RD (ed) Potassium in agriculture. American Society of Agronomy, Madison, WI, pp 351–367
Beringer H, Troldenier G (1980) The influence of K nutrition on the response of plants to environmental stress. In: Potassium research-review and trends, 11th congress of the International Potash Institute, Bern, Switzerland, pp 189–222
Berkowitz GA, Whalen C (1985) Leaf K+ interaction with water stress inhibition of nonstomatal-controlled photosynthesis. Plant Physiol 79:189–193
Berry PM, Spink J (2012) Predicting yield losses caused by lodging in wheat. Field Crops Res 137:19–26
Berry PM, Sterling M, Spink JH et al (2004) Understanding and reducing lodging in cereals. Adv Agron 84:217–271
Bertsch PM, Thomas GW (1985) Potassium status of temperate region soils. In: Munson RE (ed) Potassium in agriculture. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Madison, WI, pp 131–162
Biehler K, Fock H (1996) Evidence for the contribution of the Mehler-peroxidase reaction in dissipating excess electrons in drought-stressed wheat. Plant Physiol 12:265–272
Blanchet G, Libohova Z, Joost S et al (2017) Spatial variability of potassium in agricultural soils of the canton of Fribourg, Switzerland. Geoderma 290:107–121
Bohra JS, Doerffling K (1993) Potassium nutrition of rice (Oryza sativa L.) varieties under NaCl salinity. Plant Soil 152:299–303
Borlaug NE, Dowswell CR (1993) Fertilizer: to nourish infertile soil that feeds a fertile population that crowds a fragile world. Fert News 387:11–20
Borsani O, Cuartero J, Fernández JA et al (2001) Identification of two loci in tomato reveals distinct mechanisms for salt tolerance. Plant Cell 13:873–887
Botella MA, Martinez V, Pardines J, Cerda A (1997) Salinity induced potassium deficiency in maize plants. J Plant Physiol 150:200–205
Cakmak I (1994) Activity of ascorbate-dependent H2O2-scavenging enzymes and leaf chlorosis are enhanced in magnesium-and potassium-deficient leaves, but not in phosphorus-deficient leaves. J Exp Bot 45:1259–1266
Cakmak I (2000) Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205
Cakmak I (2002) Plant nutrition research: priorities to meet human needs for food in sustainable ways. Plant Soil 247:3–24
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci 168:521–530
Cakmak I, Engels C (1999) Role of mineral nutrients in photosynthesis and yield formation. In: Rengel Z (ed) Mineral nutrition of crops: mechanisms and implications. Haworth Press, New York, pp 141–168
Chen Z, Pottosin II, Cuin TA et al (2007) Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol 145:1714–1725
Choi SM, Jeong SW, Jeong WJ et al (2002) Chloroplast Cu/Zn-superoxide dismutase is a highly sensitive site in cucumber leaves chilled in the light. Planta 216:315–324
Chow WS, Marylin CB, Anderson JM (1990) Growth and photosynthetic responses of spinach to salinity: implications of K+ nutrition for salt tolerance. Aust J Plant Physiol 17:563–578
Colmer TD, Flowers TJ, Munns R (2006) Use of wild relatives to improve salt tolerance in wheat. J Exp Bot 57:1059–1078
Cramer GR, Lynch J, Lauchli A, Epstein E (1987) Influx of Na+, K+, and Ca2+ into roots of salt-stressed cotton seedlings. Effects of supplemental Ca2+. Plant Physiol 83:510–516
Cuin TA, Miller AJ, Laurie SA, Leigh RA (2003) Potassium activities in cell compartments of salt-grown barley leaves. J Exp Bot 54:657–661
Damon PM, Ma QF, Rengel Z (2011) Wheat genotypes differ in potassium accumulation and osmotic adjustment under drought stress. Crop Pasture Sci 62:550–555
Datta SK, Mikkelsen DS (1985) Potassium nutrition of rice. In: Munson RD (ed) Potassium in agriculture. ASA, Madison, WI
Deal KR, Goyal S, Dvorak J (1999) Arm location of Lophopyrum elongatum genes affecting K+ /Na+ selectivity under salt stress. Euphytica 108:193–198
Downton WJS, Grant WJR, Robinson SP (1985) Photosynthetic and stomatal responses of spinach leaves to salt stress. Plant Physiol 78:85–88
Dvorak J, Noaman MM, Goyai S, Gorham J (1994) Enhancement of the salt tolerance of Triticum turgidum L. by the Kna1 locus transferred from the Triticum aestivum L. chromosome 4D by homoeologous recombination. Theor Appl Genet 87:872–877
Dyson T (1999) World food trends and prospects to 2025. PNAS 96:5929–5936
El-Hadi AHA, Ismail KM, El-Akabawy MA (1997) Effect of potassium on the drought resistance of crops in Egyptian conditions. In: Food security in the WANA region, the essential need for balanced fertilization. Proc. of Regional Workshop of IPI, held at Bornova, Izmir, Turkey. IPI, Basel, pp 328–336
Engels C, Marschner H (1992) Adaptation of potassium translocation into the shoot of maize (Zea mays L.) to shoot demand: evidence for xylem loading as a regulating step. Physiol Plant 86:263–268
Foolad MR Recent advances in genetics of salt tolerance in tomato. Plant Cell Tissue Organ Cult 76:101–119
Foyer CH, Vanacker H, Gomézgoméz LD, Harbinson J (2002) Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: review. Plant Physiol Biochem 40:659–668
Fu J, Huang B (2003) Effects of foliar application of nutrients on heat tolerance of creeping bentgrass. J Plant Nutr 26:81–96
Gaxiola R, de Larrinoa F, Villalba JM, Serrano R (1992) A novel and conserved salt-induced protein is an important determinant of salt tolerance in yeast. EMBO J 11:3157–3164
Gething PA (1990) Potassium and water relationships. In: Potash facts. IPI, Bern
Gorham J, Bristol A, Young EM, Jones RGW (1991) The presence of the enhanced K/Na discrimination trait in diploid Triticum species. Theor Appl Genet 82:729–736
Grewal JS, Singh SN (1980) Effect of potassium nutrition on frost damage and yield of potato plants on alluvial soils of the Punjab (India). Plant Soil 57:105–110
Gruhn P, Goletti F, Yudelman M (2002) Integrated nutrient management, soil fertility, and sustainable agriculture: current issues and future challenges. In: Food, Agriculture, and the Environment discussion Paper 32. International Food Policy Research Institute, Washington, DC
Hakerlerker H, Oktay M, Eryuce N, Yagmur B (1997) Effect of potassium sources on the chilling tolerance of some vegetable seedlings grown in hotbeds. In: Proc of Regional Workshop of IPI, held at Bornova, Izmir, TurkeyIPI, Basel, pp 353–359
Haque MZ (1988) Effect of nitrogen, phosphorus and potassium on spikelet sterility induced by low temperature at the reproductive stage of rice. Plant Soil 109:31–36
Hasanuzzaman M, Nahar K, Alam MM et al (2013a) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684
Hasanuzzaman M, Nahar K, Fujita M (2013b) Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ecophysiology and responses of plants under salt stress. Springer, New York, pp 25–87
Hasanuzzaman M, Nahar K, Fujita M (2013c) Extreme temperatures, oxidative stress and antioxidant defense in plants. In: Vahdati K, Leslie C (eds) Abiotic stress—plant responses and applications in agriculture. InTech, Rijeka, Croatia, pp 169–205
Hasanuzzaman M, Bhuyan MHMB, Nahar K et al (2018) Potassium: a vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 8
Hu Y, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci 168:541–549
Hu L, Wang Z, Huang B (2013) Effects of cytokinin and potassium on stomatal and photosynthetic recovery of Kentucky bluegrass from drought stress. Crop Sci 53:221–231
Huner NPA, Öquist G, Sarhan F (1998) Energy balance and acclimation to light and cold. Trends Plant Sci 3:224–230
Jedmowski C, Ashoub A, Momtaz O, Brüggemann W (2015) Impact of drought, heat, and their combination on chlorophyll fluorescence and yield of wild barley (Hordeum spontaneum). J Bot 2015:9. https://doi.org/10.1155/2015/120868
Jeschke WD, Kirkby EA, Peuke AD et al (1997) Effects of P deficiency on assimilation and transport of nitrate and phosphate in intact plants of castor bean (Ricinus communis L.). J Exp Bot 48:75–91
Jewell MC, Campbell BC, Godwin ID (2010) Transgenic plants for abiotic stress resistance. In: Transgenic crop plants. Springer-Verlag, Berlin/Heidelberg, pp 67–132
Kafkafi U (1990) The functions of plant K in overcoming environmental stress situations. In: Proc. 22nd colloquium of IPI, held in Soligorsk, USSR. IPI, Bern, pp 81–93
Kant S, Kafkafi U (2002) Potassium and abiotic stresses in plants. In: Pasricha NS, Bansal SK (eds) Potassium for sustainable crop production. Potash Institute of India, Gurgaon, pp 233–251
Kumar S, Malik J, Thakur P et al (2011) Growth and metabolic responses of contrasting chickpea (Cicer arietinum L.) genotypes to chilling stress at reproductive phase. Acta Physiol Plant 33:779–787
Leigh R, Wyn Jones R (1984) A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol 97:1–13
Li H, Yang X, Luo A (2001) Ameliorating effect of potassium on iron toxicity in hybrid rice. J Plant Nutr 24:1849–1860
Li G, Kronzucker HJ, Shi W (2016) Root developmental adaptation to Fe toxicity: mechanisms and management. Plant Signal Behav 11:e1117722
Liu J, Zhu J-K (1997) An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Plant Biol 94:14960–14964
Maathuis FJM, Amtmann A (1999) K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot 84:123–133
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Marschner H (2011) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, Amsterdam
Marschner H, Cakmak I (1989) High light intensity enhances chlorosis and necrosis in leaves of zinc, potassium, and magnesium deficient bean (Phaseolus vulgaris) plants. J Plant Physiol 134:308–315
Marschner H, Kirkby EA, Cakmak I (1996) Effect of mineral nutritional status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J Exp Bot 47:1255–1263
McKersie BD, Leshem YY (1994) Stress and stress coping in cultivated plants. Kluwer Academic Publishers, Dordrecht, pp 181–193
Melis M, Farina MPW (1984) Potassium effects on stalk strength, premature death and lodging of maize (Zea mays L.). S Afr J Plant Soil 1:122–124
Mengel K, Kirkby EA (2001) Principles of plant nutrition, 5th edn. Kluwer Academic Publishers, Dordrecht
Öborn I, Andrist-Rangel Y, Askegaard M et al (2005) Critical aspects of potassium management in agricultural systems. Soil Use Manag 21:102–112
Ohnishi S, Miyoshi T, Shirai S (2010) Low temperature stress at different flower developmental stages affects pollen development, pollination, and pod set in soybean. Environ Exp Bot 69:56–62
Onyango DA, Entila F, Dida MM et al (2019) Mechanistic understanding of iron toxicity tolerance in contrasting rice varieties from Africa: 1. Morpho-physiological and biochemical responses. Funct Plant Biol 46:93–105
Oosterhuis DM, Loka DA, Raper TB, Paper S (2014) Potassium and stress alleviation: physiological functions and management in cotton. J Plant Nutr Soil Sci 176:331–343
Parks WL (1985) Interaction of potassium with crop varieties or hybrids. In: Munson RD (ed) Potassium in agriculture. ASA, Madison, WI
Parvaiz A, Satyawati S (2008) Salt stress and phyto-biochemical responses of plants—a review. Plant Soil Environ 54:89–99
Pathak J, Ahmed H, Kumari N et al (2020) Role of calcium and potassium in amelioration of environmental stress in plants. In: Roychoudhury A, Kumar Tripathi D (eds) Protective chemical agents in the amelioration of plant abiotic stress: biochemical and molecular perspectives. John Wiley & Sons Ltd., Hoboken, NJ, pp 535–562
Pettigrew WT (2008) Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol Plant:670–681
Pinthus MJ (1974) Lodging in wheat, barley, and oats: the phenomenon, its causes, and preventive measures. Adv Agron 25:209–263
Ramírez LM, Claassen N, Ubiera AA et al (2002) Effect of phosphorus, potassium and zinc fertilizers on iron toxicity in wetland rice (Oryza sativa L.). Plant Soil 239:197–206
Rascio A, Russo M, Mazzucco L et al (2001) Enhanced osmotolerance of a wheat mutant selected for potassium accumulation. Plant Sci 160:441–448
Roberts S, Mc-Dole RE (1985) Potassium nutrition of potatoes. In: Munson RD (ed) Potassium in agriculture. ASA, Madison, WI
Römheld V, Kirkby EA (2010) Research on potassium in agriculture: needs and prospects. Plant Soil 335:155–180
Rymen B, Fiorani F, Kartal F et al (2007) Cold nights impair leaf growth and cell cycle progression in maize through transcriptional changes of cell cycle genes. Plant Physiol 143:1429–1438
Sahrawat KL (2004) Iron toxicity in wetland rice and the role of other nutrients. J Plant Nutr 27:1471–1504
Sangakkara U, Frehner M, Nösberger J (2000) Effect of soil moisture and potassium fertilizer on shoot water potential\ photosynthesis and partitioning of carbon in mungbean and cowpea. J Agron Crop Sci 185:201–207
Santa-María GE, Epstein E (2001) Potassium/sodium selectivity in wheat and the amphiploid cross wheat X Lophopyrum elongatum. Plant Sci 160:523–534
Schroeder D (1978) Structure and weathering of potassium containing minerals. In: Proceedings of the 11th Congress the International Potash Institute, pp 43–63
Sen Gupta A, Berkowitz GA (1987) Osmotic adjustment, symplast volume, and nonstomatally mediated water stress inhibition of photosynthesis in wheat. Plant Physiol 85:1040–1047
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plant 133:651–669
Shabala SN, Lew RR (2002) Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol 129:290–299
Singer SM, El-Tohamy WA, Hadid AFA et al (1996) Chilling and water stress injury in bean (Phaseolus vulgaris L.) Seedlings reduced by pretreatment with CaCI2, Mefluidide, KCl and MgC12. Egypt J Hort Res 23:77–87
Skirycz A, Inzé D (2010) More from less: plant growth under limited water. Curr Opin Biotechnol 21:197–203
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Physiol 125:27–58
Smith AM, Stitt M (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30:1126–1149
Sofia Dias A, Cebola Lidon F (2010) Bread and durum wheat tolerance under heat stress: a synoptical overview. Emir J Food Agric 22:412–436
Sterling M, Joseph G, Gillmeier S, Mohammadi M (2018) Mitigating yield losses due to lodging of cereal crops. International workshop on wind-related disasters and mitigation Tohoku University, Sendai, Japan
Suriyagoda LDB, Sirisena DN, Somaweera KATN et al (2017) Incorporation of dolomite reduces iron toxicity, enhances growth and yield, and improves phosphorus and potassium nutrition in lowland rice (Oryza sativa L.). Plant Soil 410:299–312
Suriyagoda LDB, Tränkner M, Dittert K (2020) Effects of potassium nutrition and water availability on iron toxicity of rice seedlings. J Plant Nutr 43:2350–2367
Syers JK (2003) Potassium in soils: current concepts. In: Johnston AE (ed) Feed the soil to feed the people—the role of potash in sustainable agriculture. Presented at the IPI Golden Jubilee Congress. International Potash Institute, Basel, pp 301–310
Thakur P, Nayyar H (2013) Facing the cold stress by plants in the changing environment: sensing, signaling, and defending mechanisms. In: Plant acclimation to environmental stress. Springer, New York, pp 29–69
Trolldenier G (1988) Visualisation of oxidizing power of rice roots and of possible participation of bacteria in iron deposition. Z Pflanzenernähr Bodenkd 151:117–121
Uchida R (2000) Essential nutrients for plant growth: nutrient functions and deficiency symptoms. In: Silva JA, Uchida R (eds) Plant nutrient management in Hawaii’s soils, approaches for tropical and subtropical agriculture. College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa, Honolulu, pp 31–55
Vaithilingam C, Balasubramanian M (1976) Effect of potash on sclerenchyma thickness and silica content in rice. Indian Potash J 1:17–23
Vranová E, Inzé D, van Breusegem F (2002) Signal transduction during oxidative stress. J Exp Bot 53:1227–1236
Wakhloo JL (1975) Studies on the growth, flowering and production of female sterile flowers as affected by different levels of foliar potassium in Solanum sisymbriifolium Lam. J Exp Bot 26:441–450
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14:7370–7390
Wani SH, Sah SK (2014) Biotechnology and abiotic stress tolerance in Rice. J Rice Res 2:2
Welch LF, Flannery RL (1985) Potassium nutrition of corn. In: Munson RD (ed) Potassium in agriculture. ASA, Madison, WI
White PJ, Karley AJ (2010) Potassium. In: Hell R, Mendel RR (eds) Cell biology of metals and nutrients, plant cell monographs. Springer, Berlin, pp 199–124
Winzeler HE, Owens PR, Joern BC et al (2008) Potassium fertility and terrain attributes in a fragiudalf drainage catena. Soil Sci Soc Am J 72:1311–1320
Wu LB, Ueda Y, Lai SK, Frei M (2017) Shoot tolerance mechanisms to iron toxicity in rice (Oryza sativa L.). Plant Cell Environ 40:570–584
Yamauchi M (1989) Rice bronzing in Nigeria caused by nutrient imbalances and its control by potassium sulfate application. Plant Soil 117:275–286
Yermiyahu U, Kafkafi U (1990) Yield increase and stem brittle decrease in response to increasing concentrations of potassium and NO3−/NH4+ in White carnation CV. Standard. Hassadeh (Hebrew) 90:742–746
Yoshida R, Kanno A, Kameya T (1996) Cool temperature-Induced chlorosis in rice plants II. Effects of cool temperature on the expression of plastid-encoded genes during shoot growth in darkness. Plant Physiol 11:585–590
Zhao DL, Oosterhuis DM, Bednarz CW (2001) Influences of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photo-Dermatology 39:103–199
Zhu J-K, Liu J, Xiong L (1998) Genetic analysis of salt tolerance in arabidopsis: evidence for a critical role of potassium nutrition. Plant Cell 10:1181–1191
Zörb C, Senbayram M, Peiter E (2014) Potassium in agriculture—status and perspectives. J Plant Physiol 171:656–669
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Perelman, A., Imas, P., Bansal, S.K. (2022). Potassium Role in Plants’ Response to Abiotic Stresses. In: Iqbal, N., Umar, S. (eds) Role of Potassium in Abiotic Stress. Springer, Singapore. https://doi.org/10.1007/978-981-16-4461-0_2
Download citation
DOI: https://doi.org/10.1007/978-981-16-4461-0_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4460-3
Online ISBN: 978-981-16-4461-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)