Abstract
The growing concern over potential hazards from chemical pesticide safety among consumers and potential harm to the environment has culminated in consideration of natural management strategies of pests. Because they are complementary to most crop production systems, biopesticides based on plants can be integrated into pest management systems. Plant essential oils (EOs) can replace the more persistent non-natural pesticides in protecting the environment from the accumulation of chemicals reduce resistance and increase crop productivity. In addition, they possess low mammalian toxicity, broad-spectrum activity, and degrade rapidly in foodstuffs. In addition to exhibiting distinctive properties compared with synthetic pesticides, including high levels of pest toxicity and reduced toxicity toward non-target organisms, EOs possess contact, feeding deterrence, fumigant toxicity, oviposition, and repellent properties. In this chapter, we review the sources of EOs, their insecticidal activities, constituents, and mode of action and discuss their synergism and formulation with encapsulation for producing nanoinsecticidal products.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
In spite of its importance in the region, agricultural yields in sub-Saharan Africa (SSA) are generally quite low, leading to food insecurity in the region. One major hindrance to food security in Africa is the insurgence of arthropod pests that are responsible for a huge magnitude of agricultural economic losses both in the field and in storage. Losses as a result of insect pests in SSA countries may result to about 10–88% (Kfir et al. 2002; Ogendo et al. 2004; Ojo and Omoloye 2012; Midega et al. 2016). Losses in the field and during storage result from direct feeding and reproduction, indirectly by insects acting as carriers of other pathogens or raising the humidity and stimulating fungal growth in storage (Tefera et al. 2010; Midega et al. 2016). Furthermore, favorable tropical climatic conditions in the region often favor the rapid population growth of these pests (Bekele et al. 1997; Midega et al. 2016).
In order to overcome food insecurity, there has been need to increase crop production, resulting in escalated and intensified pesticide applications in the last decade. Furthermore, the use of non-moderated applications of pesticides has led to residues in foods above approved limits resulting in detrimental effects on human health. In addition to having widespread insecticide resistance in the field and storage (Georghiou 1990), synthetic insecticides have non-selective action resulting in accumulation and persistence in the environment and food chains, posing risks to human health and imbalance of ecosystems.
Considering that most chemicals are banned, some control methods are either not available or are too costly for most farmers and as the basic requirement to achieve food security, there is an urgent requirement for simple, affordable and effective pest management for the smallholder farmers in SSA who are the majority producers.
The growing concern over potential hazards from chemical pesticide safety among consumers and the possibility of environmental harm has amounted in much deliberation being shifted to the use of natural products for the management of pests in agriculture. Hence, there is a need to seek an array of safe and long-term alternatives to synthetic pesticides that can increase horticultural crop productivity, decrease resistance and protect the environment from insecticidal pollution.
6.2 Plant-Based Biopesticides
Plants are furnished with possible substitutes for insect-control because they contain copius amounts of a wide array of biological compounds, among which are essential oils (EOs) making the application of plant EOs for biological control of economically important insect a subject of interest. In addition, EOs are considered safer than other plant-derived chemicals. Contrary to the problems that arise as a result of using synthetic pesticides, EOs are biodegradable and non-pollutive to the environment, easily accessible, inexpensive, and have appropriately found a promising role as biopesticides in pest management. Furthermore, the utilization of EOs to manage arthropod pests has been used traditionally to protect stored cereals from insect pests and are, therefore, culturally acceptable (Koul et al. 2008). In addition, EOs may be used further to develop pesticidal molecules to target specific insects (Rattan 2010). Thus, the inquisitiveness in the EOs has been revived with recent observations of their bioactivities to a diversity of pests (Isman 2006). Examples of some of these bioactivities are summarized in Table 6.1. One of the advantages of EOs includes the fact that they degrade rapidly in the environment and are more specific, and therefore favor beneficial insects. There are approximately 2000 plant species from Anacardiaceae, Annonaceae, Apiaceae, Araliaceae, Asteraceae, Cannabinaceae, Chenopodiaceae, Cupressaceae, Dipsacaceae, Ericaceae, Euphorbiaceae, Fabaceae, Illiciaceae, Lamiaceae, Lauraceae, Meliaceae, Myrtaceae, Papaveraceae, Pedaliaceae, Piperaceae, Poaceae, Rutaceae, Schisandraceae, Scrophulariaceae, Verbenaceae, Vitaceae and Zingiberaceae plant families have been investigated for the insecticidal potential of their EOs have been found to exhibit lethal and sub-lethal effects such as adulticidal, feeding deterrent, growth and development inhibition, larvicidal, ovicidal, oviposition, progeny production, pupicidal and repellent (Grainge and Ahmed 1988).
6.2.1 Essential Oils
EOs are natural compounds that are volatile in nature and, have aromatic constituents characteristic in plants for various functions. They are synthesized via a combination of secondary metabolic pathways in plants and have a distinctive odour (Ebadollahi et al. 2020), may be composed of complex mixtures of aromatic compounds (Bakkali et al. 2008; Rajendran and Sriranjini 2008) and are present as droplets of fluid in the bark, flowers, fruits, leaves, stems and roots in different plants. Many EOs contain natural antioxidants and natural antimicrobial agents (Dorman et al. 2000). In Lamiaceae, they are produced by glandular trichomes, secretory cavities in Myrtaceae and Rutaceae and resin ducts in Asteraceae, Apiaceae (Fahn 1988). These structures burst open and the compounds are let out in copious amounts when herbivores feed or move on the surface of the plants (Duke et al. 2000).
In addition to their role in triggering the revitalization process for the plant through reproduction processes as attracts of pollinators and seed disseminators, and plant thermotolerance (Zhang et al. 2016), EOs also take part either directly or indirectly in plant defenses against arthropod pests (War et al. 2012).
Direct defense responses against insect pests target the biological systems for example the digestive and nervous systems, the endocrine organs of the insects and may be toxic and repellent, result in antinutrition and reduced digestibility, slowed growth and reduced reproduction (War et al. 2012). While indirect responses are insect-specific, and their compositions vary with the attacking insect. They may also involve the release of chemicals that lure the natural enemies of the herbivore by releasing aromatic compounds that lure or favour another organism(s) that reduce herbivore populations (War et al. 2012; Scholz et al. 2016).
6.2.2 Components of Essential Oils
The volatile compounds of EOs may be grouped into four: benzene derivatives, hydrocarbons, terpenes and other compounds (Haagen-Smit 1949; Ngoh et al. 1998). Terpenes and terpenoids are characterized by low molecular weight terpenes form the main group; C% hemiterpenes, the C10 monoterpenes, C15 sesquiterpenes, C20 diterpenes, C30 triterpenes and C40 tetraterpenes. Monoterpenoids constitute about 90% of the total EOs with a wide variety of functions and structures. Other related compounds are acids (e.g. chrysanthemic acid), acyclic alcohols (e.g. citronellol, geraniol), aldehydes (e.g. citronellal), bicyclic alcohols such as verbenol, cyclic alcohols such as menthol, ketones such as menthone, phenols such as thymol, and oxides (cineole) (Koul et al. 2008).
The chemical compounds of EOs vary within different species of the same genus and may also vary in various plant parts, geographical factors, time of harvest, season, climate and extraction method (Rocha et al. 2014). For instance, the concentration of 1,8-cineole was found to vary in the EOs of Eucalyptus citriodora (18.9%) (Karemu et al. 2013), E. globulus (31%) (Ebadollahi et al. 2010), E. radiata (63.3%) (Toudert-Taleb et al. 2014), and E. saligna (45.2) (Mossi et al. 2011). Similarly, limonene concentrations were reported to vary in Citrus bergamia (38.4%) (Cosimi et al. 2009), C. limonum (54.6%), (Bertuzzi et al. 2013), C. reticulata (64.1%) and C. sinensis (72.7%) (Kamal et al. 2011).
Camphor is well documented for its insecticidal properties (Singh et al. 2014; Tembo et al. 2018). In the same way, camphor concentrations were found to vary in different species of Artemisia (Kordali et al. 2006; Negahban et al. 2007; Shukla et al. 2012).
6.3 Pesticidal Properties of Essential Oils
EOs display a wide range of biopesticidal activities ranging from lethal to sublethal effects against Coleoptera, Diptera, Hemiptera Isoptera and Lepidoptera (Regnault-Roger et al. 2012; Pavela and Benelli 2016; Campos et al. 2019). Table 6.1 shows the pesticidal properties of some EOs from various plants. The differences in constituents of various EOs account for various mechanisms of action that range from antinutritional, developmental inhibitory, acute toxicity to repellency effects (Isman 2006; Pavela 2008; Hernández-Carlos and Gamboa-Angulo 2019). Other than the various patterns of phytochemical activties, toxic effects of EOs have been attributed to several other factors. One of which is the point at which the toxin penetrates the insect. The conventional modes of entry are through inhalation, ingestation or through skin absorption by the insect (Ozols and Bicevskis 1979).
EOs of Artemisia spp. are known to possess repellent and toxicity properties against coleopteran beetles. Examples of which include Sitophilus spp., Tribolium castaneum, and Callosobruchus maculatus. In a similar manner, Nyamador et al. (2010) found that the EOs of Cinnamomum spp possessed contact, fumigant and repellent activity against C. maculatus. The authors reported on adulticidal, antifeed, deterrent, ovicidal and oviposition activities towards C. maculatus and C. subinnotatus as a result of exposure of EO of Cymbopogon giganteus and C. nardus Similarly, Ketoh et al. (2005) reported on development inhibition towards C. maculatus using C. schoenanthus.
EOs of Eucalyptus spp were found to exhibit adulticidal, repellency, oviposition, contact toxicity and fumigant toxicity against coleopteran beetles (Mohan et al. 2011). For instance, Eucalyptus EOs with large amounts of cineole were shown to be insecticidal towards Varroa jacobsoni that is parasitic towards the honeybee (Calderone and Spivak 1995), Tetranychus urticae and Phytoseiulus persimilis (Choi et al. 2004) and Dermatophagoides pteronyssinus (El-Zemity et al. 2006). A similar study by Chagas et al. (2002) reported insecticidal activity against the tick Boophilus microplus using EOs from three Eucalyptus spp., E. citriodora, E. globulus and E. staigeriana.
Taking into consideration the various activities of the EOs against pests of agriculture, and the fact that plant extracts contain compounds that exhibit various bioactivities including ovicidal, repellent, and antifeedant properties, it is feasible to combine the EOs with methods such as gamma radiation (Ahmadi et al. 2008a, b). Monoterpenoids account for a large percentage of the constituents of many plant extracts that display bioinsecticidal activities and the EOs. Citronella, camphor, citral, camphene, geraniol, methyi acetate, linalool, thymol, limonene, eugenol, menthone, carvacrol, trans- anethole 1,8-cineol and α -pinene, are well-known examples of biopesticide compounds (Phillips et al. 2010; Negahban et al. 2007; Isman and Machial 2006; Isman 2006). Furthermore, the fact that monoterpenoids possess antifeedant properties (Sbeghen-Loss et al. 2011; Shukla et al. 2012) acute toxicity repellent (Mediouni-Ben and Tersim 2011; Kim et al. 2010), larvicidal, adulticidal, ovicidal, and pupicidal activities (Yang et al. 2014; Waliwitiya et al. 2009; Murugan et al. 2012) make them potential pest control agents.
6.4 Mode of Activity of Essential Oils
Understanding the mode of activity of available EOs is of importance as it helps qualify the chemical characteristics of novel compounds that may be appropriate for insect pest control and dosage that can be safe and economical in agriculture (Haynes 1988). Given the encouraging results observed with EOs against insect pests, there has been rapid development to evaluate the appropriateness of the formulations of their active ingredient for application in integrated pest control programs.
Being distinctively lipophilic and volatile, EOs can permeate the insects’ cuticle and disrupt their physiological processes (Lee et al. 2002) cause biochemical dysfunction and mortality. Furthermore, this fast action is a demonstration of the neurotoxicity nature of some EOs against some pests (Kostyukovsky et al. 2002). The mechanism of action, lethal doses, time taken to achieve lethal effects and site for bioactivities from plant EOs has been widely studied.
Plant EOs act at multiple levels of insects as fumigants, insect growth regulators, toxicants, repellents, phagodeterrents and synergists (Table 6.1). Neurotoxicity as a result of exposure to EOs in insects is characterized by hyperactivity, hyperexcitation and finally knockdown and immobilization (Enan 2001).
6.4.1 Fumigant Properties of Essential Oils
Current research has established that active ingredients from EOs may be possible alternatives to prevailing fumigants since they are easily changed to vapour at room temperature, including having various activities against a diversity of insects and fast penetrating. Various studies have investigated the probability of the use of components of plant EOs as insect fumigants. EOs of Artemisia spp., Citrus spp., Eucalyptus spp. Lavalandula spp., Mentha spp., have been well documented as fumigants. Table 6.1 shows fumigant activities of various plants.
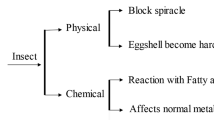
The action of EOs as fumigants against stored product beetles Sitophilus spp. and T. castaneum has been a subject of immense interest (Fang et al. 2010; Ebadollahi et al. 2012; Franca et al. 2012; Germinara et al. 2017; Salem et al. 2017; Idouaarame et al. 2018; Devi et al. 2020). Findings of the various studies demonstrate that the mechanism of action for the oils is predominantly in the gaseous phase and through the respiratory system. Since most insects respire through the trachea, the vapour causes the spiracles to open. Suffocation occurs due of obstruction the tracheal respiration (Schoonhoven 1978) resulting in death of the insect (Pugazhvendan et al. 2012; Wafaa et al. 2017).
Studies by Huang et al. (2000a) reported that diallyl trisulfide and methyl allyl disulfide from garlic possessed fumigant and toxicity properties against the two beetles Sitophilus zeamais and Tribolium castaneum Likewise, Sahaf et al. (2007, 2008) demonstrated that the EOs of Carum copticum possessed fumigant properties against S. zeamais and T. castaneum The fumigant toxicity was accredited to the presence of monoterpenoids especially thymol. Monoterpenoids are basically volatile and induce toxic effects such as fumigants as a result of their ability to penetrate the insect cuticles.
Plant EOs obtained from Cymbopogon (Stefanazzi et al. 2011), Myrtus communis (Bertoli et al. 2012) anise, eucalyptus, cumin, rosemary and oregano were also demonstrated to have fumigant effects resulting in total mortality of the eggs of Tribolium confusum and Ephestia kuehniella (Tunç et al. 2000). Ocimum spp. extracts, and their active ingredients were found to possess insecticidal effects against a diversity of insects (Ebadollahi et al. 2020).
In a separate study, Singh and Pandey (2018) found that linalool, pulegone, limonene, linalayl acetate found in Mentha induced fumigant toxicity to S. oryzae. The apiaceae family have been found to have potential as fumigants agents for insects of stored products. For instance, Kim et al. (2003) reported significant mortalities using Foeniculum vulgare against S. oryzae and Callosobruchus chinensis using. While Chaubey (2008) reported fumigant toxicity using Apium graveolens and Cuminum cyminum against C. chinensis, Park et al. (2006) attributed toxicity of larvae of Lycoriella ingenua to the activie ingredient limonene, menthone and pulegone of Schizonepeta tenuifolia.
6.4.2 Antifeedant Properties
Antifeedant chemicals may deter feeding after contact or act as repellents without making direct contact with the insects (Koul et al. 2008). A significant aspect of the antifeed properties of the EOs of plants is that they have found use in pest management (Table 6.1). Nevertheless, their action on insects is varied and are generally not harmful to the environment .
Indices such as feeding deterrence index (FDI), efficiency of conversion of ingested food (ECI), relative growth rate (RGR) and relative consumption rate (RCR) and are used to determine feeding deterrence. For instance, while the EOs of Artemisia sieberi and A. scoparia displayed antifeeding activity against Tribolium castaneum, EOs of A. sieberi oil had higher efficacy in comparison to those from A. scoparia and significantly decreased RGR and RCR. A. sieberi oil displayed higher efficacy in terms FDI compared to oils from A. scoparia (Negahban et al. 2007). In a separate study by Sahaf and Moharramipour (2008), the efficacy of Carum copticum and Vitex pseudonegundo EOs against C. maculatus was found to increase FDI.
EOs in various plants may disrupt or hinder feeding by making the plant matter unappealing or unappetizing (Talukder 2006; Rajashekar et al. 2012). The insects linger on the plants and ultimately die from starvation. Ebadollahi (2011a) demonstrated antifeed activities of Lavandula against adults of T. castaneum. Melaleuca alternifolia and its constituent compounds manifested antifeedant activities against Helicoverpa armigera (Liao et al. 2017). Similarly, Taghizadeh-Sarikolaei et al. (2014) reported antifeed activities of Thymus daenensis towards Leptinotarsa decemlineata and the prominent constituents as thymol, ρ-cymene and γ-terpinene. Shukla et al. (2012) reported antifeed efficacy of oils of Eupatorium adenophorum aerial parts and the florescence of Artemisia nilagirica against adults of Rhynchophorus ferrugineus. The authors reported significantly higher antifeed activity from E. adenophorum and A. nilagirica from the aerial parts compared to those from E. adenophorum leaves. The differences in activity was attributed to the difference in chemical composition, with the major components in the oils from the florescence and leaves of E. adenophorum showing approximately 41% oxygenated sesquiterpenes and 64% sesquiterpene hydrocarbons, respectively. The principal class of compounds in EOs A. nilagirica aerial parts were composed of monoterpenes (32.92%) and sesquiterpenes (37.02%).
Similarly, the EO constituents citronellal, thymol and α –terpineol were reported to result in feeding deterrence in tobacco cutworm, Spodoptera litura (Hummelbrunner and Isman 2001). Dictamnus dasycarpus rootbark demonstrated feeding inhibition against T. castaneum and S. zeamais (Liu et al. 2002). The authors established that fraxinellone resulted in feeding deterrence in the adults and larvae of T. castaneum and adults of S. zeamais, while dictamnine was responsible for feeding deterrence in adults and larvae of T. castaneum and S. zeamais.
EOs of Salvia mirzayanii displayed a strong feeding deterrence activity towards adults of T. confusum (Soleimannejad et al. 2011). The authors observed an increase in the concentration of the EO, RGR, RCR, whereas ECI were reduced significantly. Therefore, nutritional indices may have been influenced by the EO by interfering with the pre-ingestive and post-ingestive process. Consequently, feeding behavior of an insect may result in a reduction in the consumption and consequently growth rate of the insect.
6.4.3 Repellent Properties
Several studies report on the activities of various plants’ EOs as repellents (Table 6.1). Repellents provide plants with protection gainst insect pests with minimal harm to the ecosystem.
For example, the oils of Laureliopsis philippiana manifested repellent against Sitophilus weevils (Norambuena et al. 2016). Methyleugenol and safrole were established to the compounds responsible for the repellency. Other activities observed during the study were contact toxicity and reduced emergence.
Akrami et al. (2011) reported repellency of Mentha longifolia on C. maculatus and T. castaneum. The authors found significantly more repellency of the EO of A. sieberi at 1.5 ppm towards T. castaneum compared to C. maculatus and S. oryzae (Negahban et al. 2007), and A. scoparia significantly repelled T. castaneum and S. oryzae compared to C. maculatus (Negahban et al. 2006). Strong repellency (100%) was also observed in Anethum graveolens and T. vulgaris, towards P. interpunctella (Rafiei et al. 2009). Repellency may differ between arthropods. For instance, Taghizadeh-Saroukolai et al. (2009) showed that EO of P. acaulis varied in its degrees of repellency against S. oryzae (83.6%), C. maculatus (71.6) and T. castaneum (63.6%).
Nerio et al. (2010) demonstrated that the composition of active components influenced the repellency of the EOs. For instance, feeding deterrence, repellency and toxic activities were exhibited against T. castaneum larvae and adults using EOs extracted from fruits and leaves of Schinus areira (Descamps et al. 2011). The authors reported repellency from the oils obtained from the leaves. The compostions of the EOs of the leaves were predominantly camphene, monoterpenoids, α-phellandrene and 3-carene, whereas 3-carene, α-phellandrene, and β-myrcene were the principal oils obtained from the fruits. All the oils caused mortality of larvae in fumigant and topical bioassays. However, the former was not observed in the adults. Furthermore, both EOs influenced the nutritional index.
6.4.4 Toxicants
The red flour beetle (Tribolium species), rice weevil (S. oryzae) and the maize weevil (S. zeamais) account for more than 60% losses of cereals and pulses during storage in tropical countries (Singh et al. 2012). The use of EOs from plants as toxicants is an attractive option as these are effective and have been used traditionally. Furthermore, studies on plant derivatives have demonstrated that many plant products are toxic to insects that infest stored products (Table 6.1).
Research shows that the efficacy of EOs towards most insects is associated to terpenes. Monoterpenoids and sesquiterpenes account for the major proportion of the major essential constituents (Table 6.1). For instance, carvacrol, 1,8-cineol, thymol, eugenol, α-pinene and limonene, have been reported to have toxic effects against storage insects. While linalool EO of coriander seed was reported to be toxic towards S. oryzae (Knio et al. 2008), limonene, carvone, and (E)-anethole were the principal active components found in the EO of caraway (Fang et al. 2010). High insecticidal toxicity of Carum carvi and Coriandum sativum against Cryptolestes pusillus and Rhyzopertha dominica has been attributed to linalool and camphor-rich fractions (Lopez et al. 2008).
Insecticidal toxicity against Aphis craccivora was observed when faba beans were treated with a neem oil formulation (neemix®) from Azadirachta indica and Ocimum basilicum (Sammour et al. 2011). In addition, the authors also reported cumulative adult mortality of up to 100% after 7 days. Geranial, linalool and methyl chavicol were established as the components for insecticidal activities in basil oil. Similarly, Aslan et al. (2004) reported that geranial, linalool and methyl chavicol were insecticidal towards Tetranychus urticae and Bemisia tabaci.
Insecticidal activity of terpenes (carvone, linalool, terpeniol, phellandrine and citronellol) from garlic and mint were observed on different growth stages of Agrostis ipsilon (Sharaby and El-Nujiban 2015). Similarly, high insecticidal efficacy of Salvia officinalis towards A. ipsilon was reported to result from the presence of sesquiterpenes and terpenes (Sharaby and Al-Dosary 2014). In a parallel study, Sharaby et al. (2012) demonstrated that garlic, eucalyptus, and mint EOs caused toxicity to grasshopper (Heteracris littoralis).
Similarly, toxic effects were observed when EOs from Triaenops persicus were assessed against adults S. oryzae and T. castaneum (Koul et al. 2008). Neurotoxic effects as a result of thymol in thyme were observed when Thymus vulgaris was assayed against Nezara viridula (Koul et al. 2008). Similarly, thymol induced high toxicity to Lipaphis pseudobrasicae (Sampson et al. 2005), Spodoptera litura (Hummelbrunner and Isman 2001) and S. oryzae (Rozman et al. 2006).
6.4.5 Growth Retardants and Inhibitors of Development
Several studies (Table 6.1) have reported effects of plant EOs and their components that disrupt the development and growth of insects, reducing the weight at various stages of growth prolonging the developmental stages (Talukder 2006; Athanassiou et al. 2014; Aziza et al. 2014). The survival rates of larvae, pupae, and adult emergence may also be affected (Koul et al. 2008).
Studies using EOs from azadirachtin and neem seed were reported to increase nymphal mortality of aphids at 80 and 77%, respectively, resulting in prolonged maturation time to adulthood (Kraiss and Cullen 2008). In a similar manner, some botanical biopesticides have been found to especially have dramatized effects during the development and maturation periods, including emergence of adults (Shaalan et al. 2005). Chaubey (2008) found that EOs from Piper nigrum, Myristica Nigella sativa, fragrans, and Trachyspermum ammi influenced changes in the reproduction and growth of C. chinensis. In a separate study, Abbas et al. (2012) found that Citrus reticulata EOs inhibited growth and caused decline in population of Rhyzopertha domonica. In a similar manner, EOs from citrus peels resulted in reduced oviposition of C. maculatus (Elhag 2000). Likewise, Elettaria cardamomum EOs were reported to exhibit deter the oviposition of C. maculatus (Abbasipour et al. 2011).
EOs have also been reported to prolong growth stages. For example, basil oil prolonged the duration of the nymph stage of Aphis craccivora causing a reduction in number of adults (Sammour et al. 2011). Similarly, Anshul et al. (2014) showed that Artemisia annua EOs reduced the weights of Helicoverpa armigera larvae while prolonging the larval stage.
6.4.6 Sterility/Reproduction Inhibitors
Sterility may happen as a consequence of induced insect sterility technique or by the use of a chemosterilant that hinders reproduction (Morrison et al. 2010). Chemosterilants may cause permanent or temporary sterility of either male or female insects or interfere with the development of sexual stages from the young to the adults (Wilke et al. 2009; Navarro-Llopis et al. 2011). Asawalam and Adesiyan (2001) and Shaalan et al. (2005) drew attention to the fact that grains mixed with different parts of plants, extracts, oils or powder had the effect of reducing insect eggs hatchability oviposition, postembryonic or progeny development. For example, Elango et al. (2009) reported on the ovicidal effects against Anopheles subpictus using extracts of Andrographis paniculata, A. lineat, and Tagetes erecta.
Use botanical insecticides as chemosterilants may be at the physiological level for instance azadirachtin has been found to interfere with the synthesis of hormones responsible for molting and release of the same from the prothoracic gland, resulting in incomplete ecdysis in young insects, and sterility in adult insects (Isman 2006).
Constituent compounds from garlic; diallyl disulfide and methyl allyl have been found to display toxicity towards T. castaneum and S. zeamais (Ho et al. 1996; Huang et al. 2000a) at various stages of development. Egg hatching was totally suppressed at 0.32 mg/cm2 using diallyl trisulfide, while at 0.08 mg/cm2 larval and adult emergence were repressed. The food consumption, food utilization and growth rate were significantly reduced by methyl allyl disulfide for adults in both insect species, with feeding deterrence indices of 1.52 mg/g food for T. castaneum and 44% at 6.08 mg/g food for S. zeamais (Huang et al. 2000b). Similarly, Plata-Rueda et al. (2017) demonstrated that the pupal stages of Tenebrio molitor were more susceptible to diallyl disulfide and diallyl sulfide compared to larvae and adult stages. The authors attributed the difference in the developmental stages to the fact that efficacy may have been influenced by the way garlic compounds penetrated of the insect body and the capability of the insect to break down these compounds. Furthermore, the insects exhited changes in movement, muscle contraction and paralysis were they came into contact to the EOs of garlic Muscle contractions and paralysis could have been been as a result of neutotoxicity, coupled with hyperextension and hyperactivity of the abdomen and legs and resulting in an instant knockdown effect or immobilization (Prowse et al. 2006; Zhao et al. 2013).
6.5 Synergistic Action of Essential Oils
Various studies have revealed that mixtures or combinations of various EOs compounds exhibit additive, synergistic, and/or antagonist toxicity effects in different groups of insects (Ntalli et al. 2011; Gallardo et al. 2015; Tak et al. 2016; Wu et al. 2017; Gaire et al. 2020). The synergy observed in EOs may be as a result of the different mechanisms of action of their chemical constituents. For example, using synergistically interacting groups of monoterpenoids found in products allows for the achievement of higher insecticidal activity by using smaller amounts of the active constituents (Tak and Isman 2015; Tak et al. 2016).
The rationale for using combinations of EOs is to produce a superior product with multiple mechanisms of action, bearing in mind that the product has a significant effect than the total effects of the known and unknown chemical components of the individual EOs. Earlier studies proposed that the synergistic role of constituents in the EOs with high camphor content (Gonzalez-Coloma et al. 2006; Nerio et al. 2010). For instance, Tak and Isman (2015) showed that a combination of camphor and 1,8-cineole displayed hightened penetration of the cuticle, resulting in a synergy toxic effect towards the larvae of the cabbage looper. The authors found that these changes increased the ability of the mixture of two oils to penetrate the cuticle, resulting in reduced surface tension and increased solubility. Similarly, Abbassy et al. (2009) demonstrated a higher synergistic insecticidal effect of terpien-4-ol and c-terpinene from EOs of Majorana hortensis against larvae of Spodoptera littoralis than either of the individual compounds.
Faraone et al. (2015) reported increased toxicity (16–20-fold) against Myzus persicae as a result of the synergistic action of imidacloprid and two EOs linalool and thymol of Lavendula angustifolia and Thymus vulgaris, respectively. Mixtures of EO ingredients particularly monoterpenoids exhibited toxic synergy effects against insects as a result of increased ability to penetrate the cuticle (Gaire et al. 2020). The authors observed heightened synergistic effect in toxicity against bed bugs using a mixture of eugenol, thymol and carvacrol. The authors further reported that the synergistic interaction displayed by the mixture was most likely influenced by factors associated to the target site. Including the capability of the monoterpenoids to be operate on various sites within the nervous system of insect.
6.6 Nanoencapsulation
Despite their promising properties, EOs possess problems related to potential for oxidation, solubility in water, volatility, that should be rectified before they can be used effectively (Martin et al. 2010). Turek and Stintzing (2013) explored the factors that influenced EO stability. Besides their being highly volatile, EOs easily decompose in direct heat, exposure to high humidity, light, and/or oxygen. Degradation of the constituents may be as a result of cyclization, oxidation, dehydrogenation or isomerization reactions stimulated chemically or enzymatically (Scott 2005) and may be affected by the conditions during distillation, processing, storage of the plant material, and handling of the final product (Schweiggert et al. 2007).
To achieve high efficacy and stability, EOs are encapsulated and used to deliver EOs in insect pest management programs. Nanoencapsulation uses an approach of encapsulating the active agent in a thin layer of protective membrane in order to cushion it from extreme environmental effects. Nanocapsules consist of a shell, active ingredients that may be adsorbed on the surface or dissolved in the inner core (Khoee and Yaghoobian 2009). The carrier or envelope may be made up of natural polymers such as proteins or polysaccharides or synthetic polymers such as polyamides or melamineformaldehyde, lipids, phospholipids, or inorganic materials such as SiO2 (Nagpal et al. 2001; Kumari et al. 2010), giving EOs high efficacy while cushioning them from the likelihood of evaporation or degradation.
An important characteristic of nanoencapsulated EOs is the controlled release, that is characterized by a a prolonged release that follows a preliminary burst (São Pedro et al. 2013). In addition to minimized evaporation and exposure to extreme environmental conditions, nanoencapsulation of EOs represents a practicable and logical approach that modulates drug release, increases the stability of the active ingredients, decreases their volatility, enhances their bioactivity, and reduces toxicity (Ravi Kumar 2000).
6.7 Essential Oil Nanoformulations and Insect Pest Control
Nanoformulated EOs exhibit distinctive properties including higher pest toxicity. For instance, nanopermethrin had higher larvicidal efficacy towards Culex quinquefasciatus compared to the non-formulated form of permethrin (Anjali et al. 2010). Studies also reveal that when transformed into nanoparticles novel non-precise and biological properties become part of EOs. They gain entry into epithelial and endothelial cells of the pest and move from one cell to another by transcytosis along the axons and dendrites, blood, and lymph, triggering oxidative stress and other reactions (Devi and Maji 2011). For example, geranium oil used as mosquito repellent when transformed into high-quality solid lipid nanoparticle-loading geranium oil (Asnawi et al. 2008). In separate studies by Yang et al. (2009) and Werdin-Gonzalez et al. (2014), EOs of garlic and geranium incorporated into nanoparticles of polyethylene glycol and tested against Tribolium castaneum and Rhyzopertha dominica produced an increase in contact toxicity as a result of the slow and sustained dissemination of the effective terpenes. Furthermore, the nanoformulations increased the ability of the EO contact toxicity and changed the feeding ability of both pests. While the nanoemulsion of EO citronella caused a higher release rate against mosquito (Nuchuchua et al. 2009; Solomon et al. 2012).
Encapsulation of EOs enhances their bioactivity. For instance, Ferreira et al. (2019) demonstrated the efficiency and prolonged activity of chitosan encapsulated EO of Siparuna guianensis against Aedes aegypti larvae as a result of increased contact and slow and controlled release conferred by chitosan nanoparticles. Similarly, chitosan and angico gum nanoparticles containing EOs of Lippia sidoides caused 92% mortality of larvae of the mosquito Aedes aegypti (Paula et al. 2010). Likewise, chitosan and cashew gum nanoparticles containing EO L. sidoides caused 75–100% mortality of A. aegypti after 48 and 72 h respectively (Paula et al. 2011). In a separate study, Christofoli et al. (2015) found that nanoencapsulated EOs from Zanthoxylum rhoifolium displayed great efficacy in reducing the egg numbers and nymphs of Bemisia tabaci populations. The in vitro release was chararacterized by an initial fast release, followed by second sustained slow release. Nanoencapsulated oils display more chemical activity compared to non-encapsulated material, more mobility, allowing entry into the tissues of the insect. This can be achieved by feeding and entry through digestive tract or contact through the insect’s cuticle. The pest cells, Entry into the endothelial and epithelial, is by transcytosis (Devi and Maji 2011).
Campolo et al. (2017) demonstrated significant insecticidal activity against the invasive tomato pest Tuta absoluta using nanoformulations of EOs of citrus peel with polyethylene glycol. Khoobdel et al. (2017) demonstrated insecticidal activity of nanoformulations of EOs of Rosmarinus officinalis towards the red flour beetle, Tribolium castaneum. Louni et al. (2018), showed that nanoemulsion formulation of Mentha longifolia had enhanced contact toxicity on Ephestia kuehniella. Nanoencapsulation mechanism can, therefore serve as novel formulations for the establishment of the EOs and their chemical derivatives with improved functions.
6.8 Conclusion
The use of plant EOs as insecticides offers several advantages over synthetic chemicals. Moreover, their individual components have been determined to have potential for insecticidal activity against several arthropods of economic importance in agriculture. Furthermore, studies have shown that mixtures or combinations of several EO active ingredients exhibit additive, synergistic,and/or antagonist toxicity in various arthropod species. However, despite their promising properties, EOs face obstacles related to solubility in water, possibility of oxidation and volatility, due to exposure air, direct sunlight, high temperatures and moisture, resulting in possible degradation and evaporation of some active components. These setbacks have been solved by encapsulation of EOs resulting in controlled release characterized by a two-phase release; an first burst, then by a prolonged release, minimized evaporation and exposure to extreme environmental conditions, increased stability of the active ingredients, decreased volatility, and enhanced bioactivity.
References
Abbas SK, Ahmad F, Sagheer M et al (2012) Insecticidal and growth inhibition activities of Citrus paradisi and Citrus reticulata essential oils against lesser grain borer, Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae). World J Zool 7:289–294
Abbasipour H, Mahmoudvand M, Fahimeh R, Hosseinpour MH (2011) Fumigant toxicity and oviposition deterrency of the essential oil from cardamom, Elettaria cardamomum, against three stored-product insects. J Insect Sci 11:165. https://doi.org/10.1673/031.011.16501
Abbassy MA, Abdelgaleil SAM, Rabie RYA (2009) Insecticidal and synergistic effects of Majorana hortensis essential oil and some of its major constituents. Entomol Exp Appl 131:225–232
Ahmadi M, Moharramipour S, Mozdarani H, Negahban M (2008a) Combination of medicinal plant essential oils with gamma radiation in management of Tribolium castaneum. In: The 1st international symposium on medicinal plants, their cultivation and aspects of uses, Jordan, Petra, 15–16 Oct 2008, M.A. Ateyyat, p 50
Ahmadi M, Moharamipour S, Zolfagharieh HR (2008b) Comparative fumigant toxicity of Rosmarinus officinalis and Artemisia sieberi against Tribolium castaneum. Integr Prot Stored Prod IOBC/WPRS Bull 40:243–247
Akrami H, Moharramipour S, Imani S (2011) Comparative effect of Thymus kotschyanus and Mentha longifolia essential oils on oviposition deterrence and repellency of Callosobruchus maculatus F. Iran J Med Aromat Plants 27:1–10
Ali A, Tabanca N, Demirci B, Blythe EK, Ali Z et al (2015) Chemical composition and biological activity of four Salvia essential oils and individual compounds against two species of mosquitoes. J Agric Food Chem 63:447–456
Anjali CH, Sudheer Khan S, Margulis-Goshen K et al (2010) Formulation of water-dispersible nanopermethrin for larvicidal applications. Ecotox Environ Safe 73:1932–1936
Anshul N, Kalra A, Singh D (2014) Biological effect of sweet wormwood Artemisia annua methanol extracts and essential oil against Helicoverpa armigera hub. (Lepidoptera: Noctuidae). J Entomol Zool Stud 2:304–307
Asawalam E, Adesiyan S (2001) Potential of Ocimum basilicum (Linn) for the control of maize weevil Sitophilus zeamais (Motsch). Nig Agric J 32:195–201
Aslan I, Özbek H, Çalmaşurand Ö, Şahin F (2004) Toxicity of essential oil vapoura to two greenhouse pests, Tetranychus orticae Koch and Bemisia tabaci Genn. Ind Crop Prod 19:167–173
Asnawi S, Abd Aziz A, Abd Aziz R, Khamis AK (2008) Formulation of geranium oil loaded solid lipid nanoparticles formosquito repellent application. J Chem Nat Resour Eng 2:90–99
Athanassiou CG, Kavallieratos NG, Evergetis E, Kasoula A-M, Haroutounian SA (2012) Insecticidal efficacy of silica gel with Juniperus oxycedrus ssp. oxycedrus (Pinales: Cupressaceae) essential oil against Sitophilus oryzae (Coleoptera: Curculionidae) and Tribolium confusum (Coleoptera: Tenebrionidae). J Econ Entomol 106:1902–1910
Athanassiou CG, Kavallieratos NG, Throne JE, Nakas CT (2014) Competition among species of stored product psocids in stored grains (Psocoptera). PLoS One 9:e102867
Aziza S, Rahman HA, Abdel-Aziz S, Moawad S (2014) Natural plant oils and terpenes as protectors for the potato tubers against Phthorimaea operculella (Zeller) infestation by different application methods. Egypt J Biol Pest Control 24:265–274
Bachrouch O, Ferjani N, Haouel S, Ben Jemâa JM (2015) Major compounds and insecticidal activities of two Tunisian Artemisia essential oils toward two major coleopteran pests. Ind Crop Prod 65:127–133
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils—a review. Food Chem Toxicol 46:446–475
Bassole IHN, Lamien-Meda A, Bayala B et al (2010) Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha piperita L and Ocimum basilicum L essential oils and their major monoterpene alcohols alone and in combination. Molecules 15:7825–7839
Bekele AJ, Obeng-Ofori D, Hassanali A (1997) Evaluation of Ocimum kenyense (Ayobangira) as a source of repellents toxicants and protectants in storage against three major stored product insect pests. J Appl Entomol 121:169e173
Bertoli A, Conti B, Mazzoni V, Meini L, Pistelli L (2012) Volatile chemical composition and bioactivity of six essential oils against the stored food insect Sitophilus zeamais Motsch. (Coleoptera: Dryophthoridae). Nat Prod Res 26:2063–2071
Bertuzzi G, Tirillini B, Angelini P, Venanzoni R (2013) Antioxidative action of Citrus limonum essential oil on skin. Eur J Med Plant 3:1–9
Bittner ML, Casanueva ME, Arbert CC et al (2008) Effect of essential oils from plant species against the granary weevils, Sitophilus zeamais and Acanthoscelides obtectus (Coleoptera). Chil J Agric Res 53:1455–1459
Brito JP, Baptistussi RC, Funichelo M, Oliveira JEM, Bortoli SA (2006) Effect of essential oils of Eucalyptus spp. under Zabrotes subfasciatus (both. 1833) (Coleoptera: Bruchidae) and Callosobruchus maculatus (Fabr. 1775) (Coleoptera: Bruchidae) in two beans species. Bol Sanidad Veg Plagas 32:573–580
Caballero-Gallardo K, Olivero-Verbel J, Stashenko EE (2011) Repellent activity of essential oils and some of their individual constituents against Tribolium castaneum Herbst. J Agric Food Chem 59:690–696
Calderone NW, Spivak M (1995) Plant extracts for control of the parasitic mite Varroa jacobsoni (Acari: Varroidae) in colonies of the western honeybee (Hymenoptera: Apidae). J Econ Entomol 88:1211–1215
Campolo O, Cherif A, Ricupero M, Siscaro G et al (2017) Citrus peel essential oil nanoformulations to control the tomato borer, Tuta absoluta: chemical properties and biological activity. Sci Rep 7:13036
Campos EVR, Proença PLF, Oliveira JL et al (2019) Use of botanical insecticides for sustainable agriculture: future perspectives. Ecol Indic 105:483–495
Chagas ACS, Passos WM, Prates HT et al (2002) Acaricide effect of Eucalyptus spp. essential oils and concentrated emulsion on Boophilus microplus. Braz J Vet Res Anim Sci 39:247–253
Chaubey MK (2008) Fumigant toxicity of essential oil from some common species against pulse beetle, Callosobruchus chinensis (Coleoptera: Bruchidae). J Oleo Sci 57:171–179
Chaubey MK (2012) Biological effects of essential oils against rice weevil Sitophilus oryzae L. (Coleoptera: Curculionidae). J Essent Oil Bear Pl 15:809–815
Choi W, Lee SG, Park HM, Ahn YJ (2004) Toxicity of plant essential oils to Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae). J Econ Entomol 97:553–558
Christofoli M, Costaa ECC, Bicalhoc KU et al (2015) Insecticidal effect of nanoencapsulated essential oils from Zanthoxylum rhoifolium (Rutaceae) in Bemisia tabaci populations. Ind Crop Prod 70:301–308
Cosimi S, Rossi E, Cioni PL, Canale A (2009) Bioactivity and qualitative analysis of some essential oils from Mediterranean plants against stored-product pests: evaluation of repellency against Sitophilus zeamais Motschulsky, Cryptolestes ferrugineus (Stephens) and Tenebrio molitor (L.). J Stored Prod Res 45:125–132
Descamps LR, Sanchez Chopa C, Ferrero AA (2011) Activity of Schinus areira (Anacardiaceae) essential oils against the grain storage pest Tribolium castaneum. Nat Prod Commun 6:887–891
Devi N, Maji TK (2011) Study of complex coacervation of gelatin a with sodium carboxymethyl cellulose: microencapsulation of neem (Azadirachta indica a. Juss.) seed oil (NSO). Int J Polym Mater Polym Biomater 60:1091–1105
Devi MA, Sahoo D, Singh TB, Rajashekar Y (2020) Toxicity, repellency and chemical composition of essential oils from Cymbopogon species against red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). J Consum Prot Food Saf 15:181–191
Dorman HJD, Surai P, Dean SG (2000) In vitro antioxidant of a number of plant essential oils and phytoconstituents. J Essent Oil Res 12:241–248
Duke SO, Canel C, Rimando AM et al (2000) Current and potential exploitation of plant glandular trichome productivity. Adv Bot Res Incorp Adv Plant Pathol 31:121–151
Ebadollahi A (2011a) Antifeedant activity of essential oils from Eucalyptus globulus Labill and Lavandula stoechas L. on Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Biharean Biol 5:8–10
Ebadollahi A (2011b) Chemical constituents and toxicity of essential oil from Agastache foeniculum (Pursh) Kuntze against two stored-product insect pests. Chil J Agric Res 71:212–217
Ebadollahi A, Safaralizadeh MH, Pourmirza AA (2010) Fumigant toxicity of Lavandula stoechas L. oil against three insect pests attacking stored products. J Plant Prot Res 50:56–60
Ebadollahi A, Nouri-Ganbalani G, Hoseini SA, Sadeghi GR (2012) Insecticidal activity of essential oils of five aromatic plants against Callosobruchus maculatus F. (Coleoptera: Bruchidae) under laboratory conditions. J Essent Oil Bear Pl 15:256–262
Ebadollahi A, Jalali Sendi J, Aliakbar A, Razmjou J (2014) Chemical composition and acaricidal effects of essential oils of Foeniculum vulgare mill. (Apiales: Apiaceae) and Lavandula angustifolia miller (Lamiales: Lamiaceae) against Tetranychus urticae Koch (Acari: Tetranychidae). Psyche 2014:427078. https://doi.org/10.1155/2014/424078
Ebadollahi A, Davari M, Razmjou J, Naseri B (2017) Separate and combined effects of Mentha piperata and Mentha pulegium essential oils and a pathogenic fungus Lecanicillium muscarium against Aphis gossypii (Hemiptera: Aphididae). J Econ Entomol 110:1025–1030
Ebadollahi A, Ziaee M, Palla F (2020) Essential oils extracted from different species of the lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules 25:1556
El Nagar TFK, Abdel Fattah HM, Khaled AS, Aly SA (2012) Efficiency of peppermint oil fumigant on controlling Callosobruchus maculatus F. infesting cowpea seeds. Life Sci J 9:375–383
Elango G, Rahuman AA, Bagavan A et al (2009) Laboratory study on larvicidal activity of indigenous plant extracts against Anopheles subpictus and Culex tritaeniorhynchus. Parasitol Res 104:1381–1388
Elhag EA (2000) Deterrent effects of some botanical products on oviposition of the cowpea bruchid Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Int J Pest Manag 46:109–113
El-Zemity S, Hussien R, Saher F, Ahmed Z (2006) Acaricidal activities of some essential oils and their monoterpenoidal constituents against house dustmite, Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J Zhejiang Univ Sci B 7:957–962
Enan E (2001) Insecticidal activity of essential oils: octopaminergic sites of action. Comp Biochem Physiol C 130:325–337
Fahn A (1988) Secretory tissues in vascular plants. New Phytol 108:229–257
Fang R, Jiang CH, Wang XY et al (2010) Insecticidal activity of essential oil of Carum carvi fruits from China and its main components against two grain storage insects. Molecules 15:9391–9402
Faraone N, Hiller NK, Cutler GC (2015) Plant essential oils synergize and antagonize toxicity of different conventional insecticides against Myzus persicae (Hemiptera: Aphididae). PLoS One 10:e0127774. https://doi.org/10.1371/journal.Pone.0127774
Ferreira PT, Haddi K, Corrêa FTR et al (2019) Prolonged mosquitocidal activity of Siparuna guianensis essential oil encapsulated in chitosan nanoparticles. PLoS Negl Trop Dis 13(8):e0007624
Franca SM, Oliveira JV, Esteves Filho AB, Oliveira CM (2012) Toxicity and repellency of essential oils to Zabrotes subfasciatus (Boheman) (Coleoptera, Chrysomelidae, Bruchinae) in Phaseolus vulgaris L. Acta Amazon 42:381–386
Gaire S, Scharf ME, Gondhalekar AD (2020) Synergistic toxicity interactions between plant essential oil components against the common bedbug (Cimex lectularius L.). Insects 11:133
Gallardo A, Picollo MI, Mougabure-Cueto G (2015) Lethal activity of individual and mixed monoterpenoids of geranium essential oil on Musca domestica. Parasitol Res 114:1229–1232
Garcia M, Donadel OJ, Ardanaz CE, Tonn CE, Sosa ME (2005) Toxic and repellent effects of Baccharis salicifolia essential oil on Tribolium castaneum. Pest Manag Sci 61:612–618
Georghiou GP (1990) Overview of insecticide resistance. In: Green MB, Lebaron HM, Moberg WK (eds) ACS symposium series 421. American Chemical Society, Washington, DC, pp 19–41
Germinara G, Stefano M, Acutis L, Pati S et al (2017) Bioactivities of Lavandula angustifolia essential oil against the stored grain pest Sitophilus granarius. Bull Insectol 70:129–138
Gonzalez-Coloma A, Martın-Benito D, Mohamed N, Garcia-Vallejo MC, Soria AC (2006) Antifeedant effects and chemical composition of essential oils from different populations of Lavandula luisieri L. Biochem Syst Ecol 34:609–616
Grainge M, Ahmed S (1988) Handbook of plants with pest-control properties. Wiley-Interscience, Hoboken, NJ
Haagen-Smit AJ (1949) Essential oils-a brief survey of their chemistry and production in the United States. Econ Bot 3:71–83
Haynes KF (1988) Sublethal effects of neurotoxic insecticides on insect behaviour. Annu Rev Entomol 33:149–168
Hedjah-Chehheb M, Toudert-Taleb K, Khoudja ML et al (2013) Essential oils compositions of six conifers and their biological activity against the cowpea weevil, Callosobruchus maculatus Fabricius, 1775 (Coleoptera: Bruchidae) and Vigna unguiculata seeds. Afr Entomol 21:243–254
Hernández-Carlos B, Gamboa-Angulo M (2019) Insecticidal and nematicidal contributions of Mexican flora in the search for safer biopesticides. Molecules 24:897
Ho SH, Koh L, Ma Y, Huang Y, Sim KY (1996) The oil of garlic, Allium sativum L. (Amaryllidaceae), as apotential grain protectant against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Postharvest Biol Technol 9:41–48
Huang Y, Chen SX, Ho SH (2000a) Bioactivities of methyl allyl disulfide and diallyl trisulfide from essential oil of garlic to two species of stored-product pests, Sitophilus Zeamais (Coleoptera: Curculionidae) and Tribolium Castaneum (Coleoptera: Tenebrionidae). J Econ Entomol 93:537–543
Huang Y, Lam SL, Ho SH (2000b) Bioactivities of essential oils from Elletaria cardamomum (L.) Maton. To Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). J Stored Prod Res 36:107–117
Hummelbrunner LA, Isman MB (2001) Acute sublethal, antifeedant and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep. Noctuidae). J Agric Food Chem 49:715–720
Idouaarame S, Abdel-hamid AA, Elfarnini M, Filali OA, Blaghen M (2018) Insecticidal activity of essential oils from five Moroccan plants on three insect pests of stored cereals. GSC Biol Pharm Sci 4:052–057
Isikber AA, Alma MH, Kanat M, Karci A (2006) Fumigant toxicity of essential oils from Laurus nobilis and Rosmarinus officinalis against all life stages of Tribolium confusum. Phytoparasitica 34:167–177
Isman MB (2006) Botanical insecticides, deterrents and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 51:45–66
Isman MB, Machial CM (2006) Pesticides based on plant essential oils: from traditional practice to commercialization. In: Rai M, Carpinella MC (eds) Naturally occurring bioactive compounds. Elsevier BV, Amsterdam, pp 29–44
Kamal GM, Anwar F, Hussain AI, Sarri N, Ashraf MY (2011) Yield and chemical composition of Citrus essential oils as affected by drying pretreatment of peels. Int Food Res J 18:1275–1282
Karemu CK, Ndung’u MW, Githua M (2013) Repellent effects of essential oils from selected eucalyptus species and their major constitutnets against Sitophilus zeamais (coleopteran: Curculionidae). Int J Trop Insect Sci 33:188–194
Ketoh GK, Koumaglo HK, Glitho IA (2005) Inhibition of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) development with essential oil extracted from Cymbopogon schoenanthus L. Spreng. (Poaceae), and the wasp Dinarmus basalis (Rondani) (Hymenoptera: Pteromalidae). J Stored Prod Res 41:363–371
Kfir R, Overholt WA, Khan ZR, Polaszek A (2002) Biology and management of economically important lepidopteran cereal stem borers in Africa. Annu Rev Entomol 47:701–731
Khadria A, Serralheirob MLM, Nogueirab JMF et al (2008) Antioxidant and antiacetylcholinesterase activities of essential oils from Cymbopogon schoenanthus L. Spreng. Determination of chemical composition by GC–mass spectrometry and 13C NMR. Food Chem 109:630–637
Khoee S, Yaghoobian M (2009) An investigation into the role of surfactants in controlling particle size of polymeric nanocapsules containing penicillin-G in double emulsion. Eur J Med Chem 44:2392–2399
Khoobdel M, Ahsaei SA, Farzaneh M (2017) Insecticidal activity of polycaprolactone nanocapsules loaded with Rosmarinus officinalis essential oil in Tribolium castaneum (Herbst). Entomol Res 47:175–184
Kim SII, Park C, Ohh MH, Cho HC, Ahn YJ (2003) Contact and fumigant activities of aromatic plant extracts and essential oils against Lasioderma serricorne (Coleoptera: Anobiidae). J Stored Prod Res 39:11–19
Kim S, Yoon JS, Jung JW et al (2010) Toxicity and repellency of Origanum essential oil and its components against Tribolium castaneum (Coleoptera: Tenebrionidae) adults. J Asia Pac Entomol 13:369–373
Knio KM, Usta J, Dagher S, Zournajian H, Kreydiyyeh S (2008) Larvicidal activity of essential oils extracted from commonly used herbs in Lebanon against the seaside mosquito, Ochlerotatus caspius. Bioresour Technol 99:763–768
Kordali S, Aslan I, Calmasur O, Cakir A (2006) Toxicity of essential oils isolated from three Artemisia species and some of their major components to granary weevil, Sitophilus granarius (L.) (Coleoptera: Curculionidae). Ind Crop Prod 23:162–170
Kostyukovsky M, Rafaeli A, Gileadi C, Demchenko N, Shaaya E (2002) Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: possible mode of action against insect pests. Pest Manag Sci 587:1101–1116
Koul O, Walia S, Dhaliwal GS (2008) Essential oils as green pesticides: potential and constraints. Biopestic Int 4:63–84
Kraiss H, Cullen EM (2008) Insect growth regulator effects of azadirachtin and neem oil on survivorship, development and fecundity of Aphis glycines (Homoptera: Aphididae) and its predator, Harmonia axyridis (Coleoptera: Coccinellidae). Pest Manag Sci 64:660–668
Kumari A, Yadav SK, Yadav SC (2010) Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf 75:1–18
Lee S, Peterson CJ, Coats JR (2002) Fumigation toxicity of monoterpenoids to several stored product insects. J Stored Prod Res 39:77–85
Liang Y, Li JL, Xu S et al (2013) Evaluation of repellency of some Chinese medicinal herbs essential oils against Liposcelis bostrychophila (Psocoptera: Liposcelidae) and Tribolium castaneum (Coleoptera: Tenebrionidae). J Econ Entomol 106:513–519
Liao M, Xiao JJ, Zhou LJ et al (2017) Chemical composition, insecticidal and biochemical effects of Melaleuca alternifolia essential oil on the Helicoverpa armigera. J Appl Entomol 141:721–728
Liu CH, Mishra AK, He B, Tan RX (2001) Composition and antifungal activity of essential oils from Artemisia princeps and Cinnamomum camphora. Int Pest Control 43:72–74
Liu ZL, Xu YJ, Wu J, Goh SH, Ho SH (2002) Feeding deterrents from Dictamnus dasycarpus Turcz against two stored-product insects. J Agric Food Chem 50:1447–1450
Liu CH, Mishra AK, Tan RX et al (2006) Repellent and insecticidal activities of essential oils from Artemisia pinceps and Cinnamomum camphora and their effect on seed germination of wheat and broad bean. Bioresour Technol 97:1969–1973
Lopez MD, Jordan MJ, Pascual-Villalobus MJ (2008) Toxic compounds in essential oils of coriander, caraway and basil active against stored rice pests. J Stored Prod Res 44:273–278
Louni M, Shakarami J, Negahban M (2018) Insecticidal efficacy of nanoemulsion containing Mentha longifolia essential oil against Ephestia kuehniella (Lepidoptera: Pyralidae). J Crop Prot 7:171–182
Maciel MV, Morais SM, Bevilaqua CML et al (2010) Chemical composition of Eucalyptus spp. essential oils and their insecticidal effects on Lutzomyia longipalpis. Vet Parasitol 167:1–7
Mahmoudvand M, Abbasipour H, Basij M et al (2011) Fumigant toxicity of some essential oils on adults of some stored-product pests. Chil J Agric Res 71:83–89
Martin AS, Varona S, Navarrete A, Cocero MJ (2010) Encapsulation and co-precipitation processes with supercritical fluids: applications with essential oils. Open Chem Eng J 4:31–41
Mediouni-Ben JJ, Tersim N (2011) Composition and repellent efficacy of essential oil from Laurus nobilis against adults of cigarette beetle Lasioderma serricorne (Coleoptera: Anobiidae). Tunisian J Plant Prot 6:29–42
Midega CAO, Murage AW, Pittchar JO, Khan ZR (2016) Managing storage pests of maize: Farmers’ knowledge, perceptions and practices in western Kenya. Crop Prot 90:142–149
Mohan M, Haider SZ, Andola HC, Purohit VK (2011) Essential oils as green pesticides: for sustainable agriculture. Res J Pharm Biol Chem Sci 2:100–105
Morrison NI, Franz G, Koukidou M et al (2010) Genetic improvements to the sterile insect technique for agricultural pests. Asia Pac J Mol Biol 18:275–295
Mossi AJ, Astolfi V, Kubiak G et al (2011) Insecticidal and repellency activity of essential oil of Eucalyptus sp. against Sitophilus zeamais Motschulsky (Coleoptera, Curculionidae). J Sci Food Agric 91:273–277
Muñoz O, Christen P, Cretton S et al (2011) Comparison of the essential oils of leaves and stem bark from two different populations of Drimys winteri a Chilean herbal medicine. Nat Prod Commun 6:879–882
Murugan K, Kumar PM, Kovendan K et al (2012) Larvicidal, pupicidal, repellent and adulticidal activity of Citrus sinensis orange peel extract against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 111:1757–1769
Nagpal BN, Srivastava A, Valecha NA, Sharma VP (2001) Repellent action of neem cream against an. Culicifacies and cx. Quinquefasciatus. Curr Sci 80:1270–1271
Navarro-Llopis V, Vacas S, Sanchis J, Primo J, Alfaro C (2011) Chemosterilant bait stations coupled with sterile insect technique: an integrated strategy to control the mediterranean fruit fly (Diptera: Tephritidae). J Econ Entomol 104:1647–1655
Ndomo AF, Ngamo LT, Tapondjou LA et al (2008) Insecticidal effects of the powdery formulation based on clay and essential oil from leaves of Clausena anisata (Willd) J.D. ex. Benth (Rutaceae) against Acanthoscelides obtectus (say) hook (Coleoptera; Bruchidae). J Pestic Sci 81:227–234
Negahban M, Moharramipour S, Sefidkon F (2006) Chemical composition and insecticidal activity of Artemisia scoparia essential oil against three coleopteran stored-product insects. J Asia Pac Entomol 9:381–388
Negahban M, Moharamipour S, Sefidkon F (2007) Fumigant toxicity of essential oil from Artemisia sieberi Besser against three storedproduct insects. J Stored Prod Res 43:123–128
Nenaah GE (2014) Chemical composition, toxicity and growth inhibitory activities of essential oils of three Achillea species and their nanoemulsions against Tribolium castaneum (Herbst). Ind Crop Prod 53:252–260
Nerio LS, Olivero-Verbel J, Stashenko EE (2009) Repellent activity of essential oils from seven aromatic plants grown in Colombia against Sitophilus zeamais Motschulsky (Coleoptera). J Stored Prod Res 45:212–214
Nerio LS, Oliver-Verbel J, Stashenko E (2010) Repellent activity of essential oils: a review. Bioresour Technol 101:372–378
Ngoh SP, Cho LEW, Pang FY et al (1998) Insecticidal and repellent properties of nine volatile constituents of essential oils against the American cockroach, Periplaneta americana (L.). J Pestic Sci 54:261–268
Norambuena C, Silva G, Urbina A, Figueroa I, Rodríguez-Maciel JC (2016) Insecticidal activity of Laureliopsis philippiana (looser) Schodde (Atherospermataceae) essential oil against Sitophilus spp. (Coleoptera Curculionidae). Chilean J Agric Res 76:330–336
Ntalli NG, Ferrari F, Giannakou I, Menkissoglu-Spiroudi U (2011) Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag Sci 67:341–351
Nuchuchua O, Sakulku U, Uawongyart N et al (2009) In vitro characterization and mosquito (Aedes aegypti) repellent activity of essential-oils-loaded nanoemulsions. AAPS Pharm Sci Technol 10:1234–1242
Nyamador WS, Ketoh GK, Amevoin K et al (2010) Variation in the susceptibility of two Callosobruchus species to essential oils. J Stored Prod Res 46:48–51
Ogendo JO, Deng AL, Belmain SR et al (2004) Pest status of Sitophilus zeamais Motschulsky, control methods and constraints to safe maize grain storage in western Kenya. Egert J Sci Tech 5:175–193
Ojo JA, Omoloye AA (2012) Rearing the maize weevil, Sitophilus zeamais, on an artificial maize-cassava diet. J Insect Sci 12:1–9
Ozols G, Bicevskis M (1979) Respects for the use of Ips tyrographus attractant. In: Shumakov EM, Chekmenev SY, Ivanova TV (eds) Biologia Aktualis Veshchestva Zashchiva Rastenij. Izd. Kolos, Moscow, pp 49–51
Palla F, Bruno M, Mercurio F, Tantillo A, Rotolo V (2020) Essential oil as natural biocides in conservation of cultural heritage. Molecules 25:730
Paranagama PA, Gunasekera JJ (2011) The efficacy of the essential oils of Sri Lankan Cinnamomum zeylanicum fruit and Micromelum minutum leaf against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J Essent Oil Res 23:75–82
Park IK, Choi KS, Kim DH et al (2006) Fumigant activity of plant essential oils and components from horseradish (Armoracia rusticana), anise (Pimpinella anisum) and garlic (Allium sativum) oils against Lycoriella ingenua (Diptera: Sciaridae). Pest Manag Sci 62:723–728
Paudyal M, Rajbhandari M, Basnet P, Yahara S, Gewali M (2012) Quality assessment of the essential oils from Nardostachys jatamansi (d. Don) dc and Nardostachys chinensis batal obtained from Kathmandu valley market. Sci World 10:13–16
Paula HCB, Sombra FM, Abre FOMS, Paul RC (2010) Lippia sidoides essential oil encapsulationby angico gum/chitosan nanoparticles. J Braz Chem Soc 21:2359–2366
Paula HCB, Sombra FM, Cavalcante RF, Abreu FOMS, Paula RCM (2011) Preparation and characterization of chitosan/cashew gum beads loaded with Lippia sidoides essential oil. Mater Sci Eng C 31:173–178
Pavela R (2008) Insecticidal properties of several essential oils on the house fly (Musca domestica L.). Phytother Res 22:274–278
Pavela R (2011) Insecticidal and repellent activity of selected essential oils against of the pollen beetle, Meligethes aeneus (Fabricius) adults. Ind Crop Prod 34:888–892
Pavela R, Benelli G (2016) Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci 21:1000–1007
Phillips AK, Appel AG, Sims SR (2010) Topical toxicity of essential oils to the German cockroach (Dictyoptera: Blattellidae). J Econ Entomol 103:448–459
Plata-Rueda A, Martinez LC, Santos MHD et al (2017) Insecticidal activity of garlic essential oil and their constituents against the mealworm beetle, Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Sci Rep 7:46406
Prowse MG, Galloway TS, Foggo A (2006) Insecticidal activity of garlic juice in two dipteran pests. Agr Forest Entomol 8:1–6
Pugazhvendan SR, Ross PR, Elumalai K (2012) Insecticidal and repellent activities of four indigenous medicinal plants against stored grain pest, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Asian Pac J Trop Dis 2:S16–S20
Qin W, Huang S, Li C et al (2010) Biological activity of the essential oil from the leaves of Piper sarmentosum Roxb. (Piperaceae) and its chemical constituents on Brontispa longissima (Gestro) (Coleoptera: Hispidae). Pestic Biochem Phys 96:132–139
Rafiei KZ, Moharramipour S, Rahbarpour A (2009) Investigated repellency effect of some essential oils of 17 native medicinal plants on adults Plodia interpunctella. Am Eurasian J Sustain Agric 3:181–184
Rajashekar Y, Rao LJM, Shivanandappa T (2012) Decaleside: a new class of natural insecticides targeting tarsal gustatory sites. Naturwissenschaften 99:843–852
Rajendran S, Sriranjini V (2008) Plant products as fumigants for stored product insect control. J. Stored Prod Res 44:126–135
Ramzi H, Ismaili MR, Aberchane M, Zaanoun S (2017) Chemical characterization and acaricidal activity of Thymus satureioides C. & B. and Origanum elongatum E. & M. (Lamiaceae) essential oils against Varroa destructor Anderson & Trueman (Acari: Varroidae). Ind Crop Prod 108:201–207
Rattan RS (2010) Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot 29:913–920
Ravi Kumar MN (2000) Nano and microparticles as controlled drug delivery devices. J Pharm Pharm Sci 3:234–258
Regnault-Roger C, Vincent C, Arnason JT (2012) Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol 57:405–424
Rocha RP, Melo EDC, Barbosa LCA et al (2014) Influence of plant age on the content and composition of essential oil of Cymbopogon citratus (DC.) Stapf. J Med Plant Res 8:1121–1126
Romeilah RM, Fayed SA, Mahmoud GI (2010) Chemical compositions, antiviral and antioxidant activities of seven essential oils. J Appl Sci Res 6:50–62
Rozman V, Kalinovic I, Liska A (2006) Insecticidal activity of some aromatic plants from Croatia against granary weevil (Sitophilus granarius L) on stored wheat. Cereal Res Commun 34:705–708
Saei-Dehkordi SS, Tajik H, Moradi M, Khalighi-Sigaroodi F (2010) Chemical composition of essential oils in Zataria multiflora Boiss. From different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem Toxicol 48:1562–1567
Sahaf BZ, Moharramipour S (2008) Fumigant toxicity of Carum copticum and Vitex pseudo-negundo essential oils against eggs, larvae and adults of Callosobruchus maculatus. J Pestic Sci 81:213–220
Sahaf BZ, Moharamipour S, Meshkatassadat MH (2007) Chemical constituents and fumigant toxicity of essential oil from Carum copticum against two stored product beetles. Insect Sci 14:213–218
Sahaf BZ, Moharramipour S, Meshkatalsadat MH (2008) Fumigant toxicity of essential oil from Vitex pseudo- negundo against Tribolium castaneum (Herbst) and Sitophilus oryzae (L). J Asia Pac Entomol 11:175–179
Salem N, Olfa Bachrouch O, Sriti J, Msaada K et al (2017) Fumigant and repellent potentials of Ricinus communis and Mentha pulegium essential oils against Tribolium castaneum and Lasioderma serricorne. Int J Food Prop 20:S2899–S2913
Sammour EA, El Hawary FMA, Abdel-Aziz NF (2011) Comparative study on the efficacy of neemix and basil oil formulations on the cowpea aphid Aphis craccivora Koch. Arch Phytopathol Plant Prot 44:655–670
Sampson BJ, Tabanaca N, Kirimer N et al (2005) Insecticidal activity of 23 essential oils and their major compounds against adults Lipaphis pseudobrassicae (Davis) (Aphididae: Homoptera). Pest Manag Sci 61:1122–1128
São Pedro A, Santo IE, Silva C, Detoni C, Albuquerque E (2013) The use of nanotechnology as an approach for essential oil-based formulations with antimicrobial activity. In: Méndez-Vilas A (ed) Microbial pathogens and strategies for combating them: science, technology and education. Formatex Research Center, Zurbaran, Badajoz, pp 1364–1374
Sbeghen-Loss AC, Mato M, Cesio MV, Frizzo C et al (2011) Antifeedant activity of Citrus waste wax and its fractions against the dry wood termites Cryptotermes brevis. J Insect Sci 11:1–7
Scholz SS, Heyer M, Vadassery J, Mithöfer A (2016) A role for calmodulin-like proteins in herbivore defense pathways in plants. J Endocytobiosis Cell Res 27:1–12
Schoonhoven AV (1978) The use of vegetable oils to protect stored beans from bruchid attack. J Econ Entomol 71:254–256
Schweiggert U, Carle R, Schieber A (2007) Conventional and alternative processes for spice production-a review. Trends Food Sci Technol 18:260–268
Scott RPW (2005) Essential oils. In: Worsfold P, Townshend A, Poole C (eds) Encyclopedia of analytical science, 2nd edn. Elsevier, London, pp 554–561
Sefidkon F (2001) Essential oil composition of Anethum graveolens L. Iranian J Med Arom Plant 8:45–62
Shaalan EAS, Canyon D, Younes MWF, Abdel-Wahab H, Mansour AH (2005) A review of botanical phytochemicals with mosquitocidal potential. Environ Int 31:1149–1166
Shan-Shan G, Chun-Xue Y, Jun-Yu L et al (2015) Chemical composition and bio-activities of the essential oil from Etlingera yunnanensis against two stored product insects. Molecules 20:15735–15747
Sharaby A, Al-Dosary M (2014) An electric air flow olfactometer and the olfactory response of Rhynchophorous ferrugineus weevil to some volatile compounds. J Agric Ecol Res Int 1:40–50
Sharaby A, El-Nujiban A (2015) Evaluation of some plant essential oils against the black cutworm Agrotis ipsilon. Global J Adv Res 2:701–711
Sharaby A, Montasser SA, Mahmoud YA, Ibrahim SA (2012) Natural plant essential oils for controlling the grasshopper (Hetracis littoralis) and their pathological effects on the alimentary canal. Ecol Balk 4:39–52
Sharififard M, Sharififard F, Safdari A, Siahpoush H, Kassiri A (2016) Evaluation of some plant essential oils against the brown-banded cockroach, Supella longipalpa (Blattaria: Ectobiidae): a mechanical vector of human pathogens. J Arthropod Borne Dis 10:528–537
Shukla R, Singh P, Prakash B et al (2011) Efficacy of essential oils of Lippia alba (mill.) N.E. Brown and Callistemon lanceolatus (Sm.) sweet and their major constituents on mortality, oviposition and feeding behaviour of pulse beetle, Callosobruchus chinensis L. J Sci Food Agric 91:2277–2283
Shukla P, Vidyasagar PSPV, Aldosari SA, Abdel-Azim M (2012) Antifeedant activity of three essential oils against the red palm weevil, Rhynchophorus ferrugineus. Bull Insectol 65:71–76
Singh P, Pandey AK (2018) Prospective of essential oils of the genus Mentha as biopesticides: a review. Front Plant Sci 9:1295
Singh P, Pandey AK, Tripathi NN (2012) Essential oils: a renewable source for the management of stored product insects—a review. Agric Rev 33:226–236
Singh P, Jayaramaiah RH, Sarate P et al (2014) Insecticidal potential of defense metabolites from Ocimum kilimandscharicum against Helicoverpa armigera. PLoS One 9:e104377
Soleimannejad S, Moharramipour S, Fathipour Y, Nikooei M (2011) Efficiency of essential oil from Salvia mirzayanii against nutritional indices of Tribolium confusum. Integr Prot Stored Prod IOBC/WPRS Bull 69:299–302
Solomon B, Sahle FF, Gebre-Mariam T, Asres K, Neubert RHH (2012) Microencapsulation of citronella oil for mosquito-repellent application: formulation and in vitro permeation studies. Eur J Pharm Biopharm 80:61–66
Stefanazzi N, Stadler T, Ferrero A (2011) Composition and toxic, repellent and feeding deterrent activity of essential oils against the stored-grain pests Tribolium castaneum (Coleoptera: Tenebrionidae) and Sitophilus oryzae (Coleoptera: Curculionidae). Pest Manag Sci 67:639–646
Suthisut D, Fields PG, Chandrapatya A (2011) Fumigant toxicity of essential oils from three Thai plants (Zingiberaceae) and their majorcompounds against Sitophilus zeamais,Tribolium castaneum and two parasitoids. J Stored Prod Res 47:222–230
Taghizadeh-Saroukolai A, Moharramipour S, Meshkatalsadat MH, Fathipour Y, Talebi AA (2009) Repellent activity and persistence of essential oil extracted from Prangos acaulis to three stored-product beetles. Am Eurasian J Sustain Agric 3:202–204
Taghizadeh-Saroukolai A, Nouri-Ganbalani G, Rafiee-Dastjerdi H, Hadian J (2014) Antifeedant activity and toxicity of some plant essential oils to Colorado potato beetle, Leptinotarsa decemlineata say (Coleoptera: Chrysomelidae). Plant Protect Sci 50:207–216
Tak JH, Isman MB (2015) Enhanced cuticular penetration as the mechanism for synergy of insecticidal constituents of rosemary essential oil in Trichoplusia ni. Sci Rep 5:12690
Tak JH, Jovel E, Isman MB (2016) Comparative and synergistic activity of Rosmarinus officinalis L. essential oil constituents against the larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). Pest Manag Sci 72:474–480
Talukder FA (2006) Plant products as potential storedproduct insect management agents—a mini review. Emirates J Food Agric 18:17–32
Tefera T, Kanampiu F, Groote HD et al (2010) The metal silo: an effective grain storage technology for reducing post-harvest insect and pathogen losses in maize while improving smallholder farmers’ food security in developing countries. Crop Prot 30:240–245
Tembo Y, Mkindi AG, Mkenda PA et al (2018) Pesticidal plant extracts improve yield and reduce insect pests on legume crops without harming beneficial arthropods. Front Plant Sci 9:1425
Toudert-Taleb K, Hedjal-Chehheb M, Hami H, Debras JF, Kellouche A (2014) Composition of essential oils extracted from six aromatic plants of Kabylian origin (Algeria) and evaluation of their bioactivity on Callosobruchus maculatus (Fabricius, 1775) (Coleoptera: Bruchidae). Afr Entomol 22:417–427
Tozlu E, Cakir A, Kordali S et al (2011) Chemical compositions and insecticidal effects of essential oils isolated from Achilleagypsicola, Saturejahortensis, Origanumacutidens and Hypericumscabrum against broadbean weevil (Bruchus dentipes). Sci Hortic 130:9–17
Tripathi NN, Kumar N (2007) Putranjiva roxburghii oil—a potential herbal preservative for peanuts during storage. J Stored Prod Res 43:435–442
Tunç İ, Berger BM, Erler F, Dağlı F (2000) Ovicidal activity of essential oils from five plants against two stored-product insects. J Stored Prod Res 36:161–168
Turek C, Stintzing FC (2013) Stability of essential oils: a review. Compr Rev Food Sci F 12:40–53
Usman LA, Hamid AA, Olawore NO et al (2010) Chemical composition of leaf essential oil of Clausena anisata growing in north-Central Nigeria. J Appl Sci Res 6:891–894
Wafaa MH, Rowida SB, Hussein AHS-AA (2017) Botanical insecticide as simple extractives for pest control. Cogent Biol 3:1404274
Waliwitiya R, Kennedy CJ, Lowenberger CA (2009) Larvicidal and oviposition-altering activity of monoterpenoids, trans-anethole and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Pest Manag Sci 65:241–248
Wang CF, Liu P, Yang K et al (2011) Chemical composition and toxicities of essential oil of Illicium fragesii fruits against Sitophilus zeamais. Afr J Biotechnol 10:18179–18184
War AR, Paulraj MG, Ahmad T et al (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306–1320
Werdin-Gonzalez JO, Gutiérrez MM, Ferrero AA, Band BF (2014) Essential oils nanoformulations for stored-product pest control—characterization and biological properties. Chemosphere 100:130–138
Wilke ABB, Nimmo DD, John O et al (2009) Mini-review: genetic enhancements to the sterile insect technique to control mosquito populations. Asia Pac J Mol Biol Biotechnol 17:65–74
Wu L, Huo X, Zhou X et al (2017) Acaricidal activity and synergistic effect of thyme oil constituents against carmine spider mite (Tetranychus cinnabarinus (Boisduval)). Molecules 22:1873
Yang FL, Li SG, Zhu F, Lei CL (2009) Structural characterization of nanoparticles loaded with garlic essential oil and their insecticidal activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J Agric Food Chem 57:10156–10162
Yang K, Wang CF, You CX et al (2014) Bioactivity of essential oil of Litsea cubeba from China and its main compounds against two stored product insects. J Asia Pac Entomol 17:459–466
Zandi-Sohani N, Hojjati M, Carbonell-Barrachina AA (2013) Insecticidal and repellent activities of the essential oil of Callistemon citrinus (Myrtaceae) against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Neotrop Entomol 42:89–94
Zapata N, Smagghe G (2010) Repellency and toxicity of essential oils from the leaves and bark of Laurelia sempervirens and Drimys winteri against Tribolium castaneum. Ind Crop Prod 32:405–410
Zhang F-P, Yang Q-Y, Wang G, Zhang S-B (2016) Multiple functions of volatiles in flowers and leaves of Elsholtzia rugulosa (Lamiaceae) from southwestern China. Sci Rep 6:27616
Zhao NN, Zhang H, Zhang XC et al (2013) Evaluation of acute toxicity of essential of garlic (Allium sativum) and its selected major constituent compounds against overwintering Cacopsylla chinensis (Hemiptera: Psyllidae). J Econ Entomol 106:1349–1354
Zoubiri S, Baaliouamer A (2012) Chemical composition and insecticidal properties of Lantana camara L. leaf essential oils from Algeria. J Essent Oil Res 24:377–383
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mwamburi, L.A. (2022). Role of Plant Essential Oils in Pest Management. In: Mandal, S.D., Ramkumar, G., Karthi, S., Jin, F. (eds) New and Future Development in Biopesticide Research: Biotechnological Exploration. Springer, Singapore. https://doi.org/10.1007/978-981-16-3989-0_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-3989-0_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3988-3
Online ISBN: 978-981-16-3989-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)