Abstract
The societal claim for a friendly environmental use of pesticides today implicates to promote alternative solutions for a better and relevant using of chemicals. Because they have a broad spectrum of uses, essential oils (EOs) have many industrial applications. They are used now in plant protection and as biocide. They occupy a significant place among insect pest biocontrol agents (BCAs) and represent a consistent part within the market of botanicals used as alternative to chemicals. After phytochemical considerations, this chapter presents the wide range of activities of EOs on insect. Trends and prospects including a discussion about their advantages for an ecological friendly approach but also the factors that impede their commercial development conclude.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Benefit-risk ratio
- commercialization
- essential oils
- insect control
- mechanisms of action
- monoterpenes
- phytochemistry
- regulation
- toxicity
1 Introduction
Because they have a broad spectrum of uses, essential oils (EOs) have many industrial applications in perfumery, cosmetics and detergents, pharmacology, and fine chemistry as well as aromatics for the food industry. They also occupy a significant place to control insect pests and in fact are presently considered to be among most efficient botanicals used as alternative to chemicals. It exists today a societal claim for a friendly environmental use of pesticides that implicates to promote alternative solutions for a better and relevant using of chemicals. EOs take place in the market of biopesticides or BCAs which is expecting to increase to $3.3 billion in 2014 for a 5-year CAGR of 15.6 % (BCC Research; www.bccresearch.com). However, progress has to be done before biopesticides will share with synthetic pesticides the PPP (plant protection products) global market which is estimated at 38,318 billions of USD for 2010 with around 25 % represented by insecticides (9,985 billion USD) [1].
EOs play in fact an important role in the protection of the plants in agricultural and nonagricultural areas (e.g., orchards, gardens). They have a wide range of activities (insecticide, antifeedant, or repellent) against bacteria, virus, and fungi, and also against insects. This chapter is focused on the uses of EOs as biopesticides (BCAs) to control pest insect, the challenges they face, and the factors that nowadays impede their development. After defining the essential oils and their main chemical components, it details their relevant uses to control insect pest insects, including a discussion about their advantages for an ecological friendly approach. Trends and prospects conclude this overview.
2 Essential Oils: Very Complex Natural Mixes
The essential oils have been used since centuries. The first distillation of these products was mentioned during the thirteenth century in Andalusia after which their pharmacological properties induced their inclusion into the very early Pharmacopoeias of several European countries [2].
EOs are biosynthesized by aromatic plants belonging to a few families. They are particularly abundant in conifers, Rutaceae, Umbelliferae, Myrtaceae, Lamiaceae, and Lauraceae. Depending on the species and families, they are localized in specialized histological structures: glandular trichomes (Lamiaceae), secretory canals (Myrtaceae), or resin ducts (Apiaceae). They could be stored in different parts of the plant such as flowers (e.g., Citrus bergamia), leaves (Citronella spp.; Eucalyptus spp.), wood (Santalum spp.), roots (Chrysopogon zizanioides), or seeds (Myristica fragrans) [3].
Essential oils appear to be very complex natural mixes. Terpenoids are major constituents of EOs and, to a lesser amount, phenylpropanoids. EO constituents belong mainly to two phytochemical groups of terpenoids: monoterpenes and sesquiterpenes of low molecular weight. They generally consist of several tens of constituents of which the great majority possess an isoprenoid skeleton. Most of the compounds have ten atoms of carbon (monoterpenes) and 15 atoms of carbon (sesquiterpenes) or more rarely 20 atoms of carbon (diterpenes). Monoterpenes present in EOs may contain terpenes that are hydrocarbons (α-pinene), alcohols (menthol, geraniol, linalool, terpinen-4-ol, p-menthane-3,8-diol), aldehydes (cinnamaldehyde, cuminaldehyde), ketones (thujone), ethers (1,8-cineole e.g., eucalyptol), and lactones (nepetalactone). As the elongation of the chain to 15 carbons increases the number of possible cyclizations, sesquiterpenes have a wide variety of structures (over 100 skeletons). Aromatic compounds are less common and are derived mainly from the shikimate pathway. Some are typical of EOs of particular species, for example, vanillin (Vanillia spp.) or estragol (Artemisia dracunculus L.). Some compounds identified in EOs result from the degradation of fatty acids (jasmonic acid) or are glycosylated volatile compounds (e.g., linalool glucoside) [3].
The majority of EOs contains a limited number of main compounds, but some of the minor compounds play an important role as vectors of fragrance and make up the richness of an extract. It is thus well established that essential oil composition is very variable depending of the species and of chemotypes within the species and also physiological parameters. As examples, the EO of eucalyptus (Eucalyptus globulus Labill.) is characterized by a monoterpene 1,8-cineole and that of coriander (Coriandrum sativum L.) by another monoterpene linalool. Thyme (Thymus vulgaris L.) is a species with numerous chemotypes named according to the major compound, for example, thyme with chemotype thymol or chemotype carvacrol or terpineol or linalool. A typical EO may contain 20–80 phytochemicals. The analyses of EOs of the African basil Ocimum canum Sims contain no less than 80 compounds identified by GS-MS and GS-FID [4].
This complexity of EOs phytochemistry led to a certain inconsistency of the chemical composition of an essential oil. In fact, several factors influence the balance of the compounds within EOs. Terpenoids and isoprenoid are synthesized through secondary metabolism of the plant. Monoterpenes are biosynthesized in plastid via two 5-carbon precursors, that is, isopentenyl pyrophosphate and dimethylallyl pyrophosphate, which condense to give the monoterpenes (10-carbon). The sesquiterpenes (15-carbon) are formed via the mevalonate pathway in the cytosol. Phenylpropanoids are derived mainly from the shikimate pathway [5].
These metabolic pathways do not have the same importance at all stages of the plant development and in all the organs. The physiological development of the plant, its degree of maturity at the harvest, and the choice of the organ to be extracted play a role. Gershenzon et al. [6] showed that limonene and menthone are the major monoterpenes existing in the youngest leaves of peppermint, but limonene content declines rapidly with development of the plant, whereas menthone increases and then declines at later stages. Thus, menthol becomes the dominant constituent. External factors like the climatic and soil conditions or seasonal variations may also change the main compounds identified within an EO. Several studies confirmed that separated geographic areas led to observe different chemotypic races or populations [7]. Isman and Machial [8] reported that rosemary oil extracted from plants harvested in two different areas of Italy contained 1,8-cineole concentrations ranging from 7 % to 55 % and α-pinene concentrations ranging from 11 % to 36 %.
The variability of the composition of an EO is also impacted by the choice of the method of extraction of EOs. One characteristic of EOs is the volatility of their compounds which allows them to be easily extracted by water vapors, in contrast to fixed lipid oils and essences (concrete, absolute, oleoresins, and resinoids) which are extracted by solvents and alcohol. Guenther [9] distinguished three kinds of water and steam distillation methods for obtaining essential oils. These methods are far more restrictive than more recent extraction and separation methods which are mentioned in the European Pharmacopoeia [10] using supercritical fluids, steam distillation, dry distillation, or mechanical cold pressing of plants.
The diversity in the EOs phytochemical composition induces as a consequence that the production of a standardized product is a real challenge for their commercialization.
3 Insecticidal Activities of EOs
An abundant literature, more than 2,000 scientific papers, is devoted to study the EO-insect relationships. Among these, some major reviews would be mentioned [11–15].
3.1 Diversity of Activities on Insect Targets and Routes of Exposure
The insect control by EOs is the result of several kinds of modes of action and depends on the routes of the exposure. EOs develop toxicities by ingestion or contact through cuticle or inhalation for volatile compounds. Some EOs repel the insect or are deterrent or antifeedant. Others disturb oviposition or disrupt the larvae growth or modify the imago’s behavior or physiology. In fact, the activities the EOs exert on an insect could impact several physiological targets at the same or at different stages of the insect development. This complexity in the way they act is illustrated by the numerous following examples.
Early studies were mainly focused to observe the activities of EOs and their volatile constituents on insects of the stored products. Twenty-two essential oils were extracted mainly from Lamiaceae and Umbelliferae families and showed a range of LC50 from 2 to more than 300 mg dm−3 air on the bruchid Acanthoscelides obtectus (Say) imagos [16]. Some EOs produced also ovicidal and larvicidal activities and consequently inhibited the reproduction of the insect [17]. From Lamiaceae were extracted the most efficient of those EOs, that is, thyme (T. vulgaris) and wild thyme (Thymus serpyllum L.), rosemary (Rosmarinus officinalis L.), summer savory (Satureja hortensis L.), oregano (Origanum vulgare L.), and sweet basil (Ocimum basilicum L.), which also had an antifeedant effect on larvae inside artificial seeds [18]. This beetle A. obtectus has been shown to be a convenient model to point out with accuracy which reproductive stage is targeted and which is the speed of the activity of essential oils. Table 138.1 shows that the fumigant toxicity of EOs on imagos and reproduction could be quite different. As an example, parsley Petroselinum sativum L. [Umbelliferae] did not have significant fumigant toxicity on the beetle adults but inhibited strongly its reproduction, whereas the Satureja hortensis presented a high toxicity on adults but inhibited poorly the bruchid reproduction. In the same way, some EOs produced fumigant toxicity and antifeedant effect with variable intensity (e.g., mint Mentha piperita L., bay tree Laurus nobilis L. or dill Anethum graveolens L.).
A lot of EOs protected stored grains against the damages of Coleoptera. EOs of sweet basil, of patchouli (Pogostemon spp.), of Eucalyptus spp., of thyme, and of African basil with its major component linalool were toxic to Mexican bean weevil Zabrotes subfasciatus Boheman, rice weevil Sitophilus oryzae L., lesser grain borer Rhyzopertha dominica Fab., drugstore beetle Stegobium paniceum L., bean weevil Acanthoscelides obtectus Say, red flour beetle Tribolium castaneum Herbst, and pulse beetle Bruchus chinensis L. [19–21]. The toxic effect of essential oils is not only suitable for granary insects but also for flying insects. The Lamiaceae Mentha spp. and Lavandula spp. or Pinus spp. (Pinaceae) EOs were noted to be toxic against the green peach aphid Myzus persicae and the greenhouse white fly Trialeurodes vaporariorum as well as the pear bug Stephanitis pyri [22]. Greek aromatic plants, especially from genus Satureja, Origanum, and Mentha (Lamiaceae), prevented egg hatching and provoked prohibition or malformation of the puparium of the flies Drosophila auraria [23]. And some of these aromatic Mediterranean plant EOs tested on A. obtectus were also toxic to the Mediterranean fruit fly, Ceratitis capitata, and the cereal aphids Rhopalosiphum padi and Metopolophium dirrhodum [24]. More recent studies demonstrate that a wide range of insect taxa are affected by EOs. Park et al. [25] demonstrated the fumigant activity of EOs of Schizonepeta tenuifolia (Lamiaceae) against the sciarid fly Lycoriella america. Papachristos et al. extended the studies of the toxic effect of Mediterranean plants’ EOs on C. capitata with this of citrus peel [26]. In the same way, Liu et al. [27] extended previous works observing the toxicity of EOs to the four major stored-product insects: Tribolium castaneum, Sitophilus zeamais, R. dominica, and sawtoothed grain beetle Oryzaephilus surinamensis [28]. They determined the fumigant toxicity of the water dropwort Ostericum sieboldii (Apiaceae) EOs with LC50 values of 27.4 mg dm−3air (T. castaneum) and 20.9 mg dm−3 air (S. zeamais). Extracted oils from leaves and bark of Chilean laurel Laurelia sempervirens (Atherospermataceae) and Drimys winteri (Winteraceae) carried on fumigant activities of EOs against the aphid Acyrthosiphon pisum [29].
Besides fumigation, other routes of penetration of EOs are effective. Coleopteran insects, maize weevil Sitophilus zeamaïs (Motschulsky), T. castaneum, and larger grain borer Prostephanus americana (Horn) were very sensitive to topical applications of the Citrus spp. essential oils [30]. EOs decimated numerous agricultural pest insects and affected disease-vector insects as well. The Annonaceae Dennettia tripetala EOs decimated a wide range of agricultural pest insects, for example, the American cockroach Periplaneta americana and the grasshopper Zonocerus variegatus [31]. The Myrtaceae Eucalyptus saligna EOs killed lice Pediculus capitis, mosquitoes Anopheles funestus, bed bugs Cimex lectularius, and American cockroach Periplaneta orientalis within 2–30 min [32].
Antifeedant effects could decimate insects too, but the insects’ decision to avoid feeding on a plant could be influenced by such factors as phagodeterrency of the substances, post-consumption physiological stress, or a repellent effect, and it is not always easy to discriminate them. Laboratory choice tests were conducted to evaluate the antifeedant effect of pine EOs and terpenes for the weevil Hylobius pales [33]. Discriminant assays were developed to observe the deterrent activity of EOs of Minthostachys mollis (Lamiaceae) and Melaleuca quinquenervia (Myrtaceae) essential oils on the flour beetle T. castaneum [34]. These assays were conducted to screen the antifeedant activity of Uruguayan plants that belonged to Bignoniaceae (Clytostoma callistegioides, Dolichandra cynanchoides, Macfadyena unguis-cati), Sapindaceae (Dodonaea viscosa, Allophylus edulis, Serjania meridionalis), Lamiaceae (Salvia procurrens, Salvia guaranitica; Solanaceae: Lycium cestroides), and Phytolaccaceae (Phytolacca dioica) against the specialist Coccinellidae Epilachna paenulata and the larvae of the generalist Lepidoptera Spodoptera littoralis [35].
Since the last 25 years, repellent effects of EOs were also fully described with plants of all continents. The Indian plant Adhatoda vasica (Acanthaceae) EOs exhibited repellent activity against S. oryzae and B. chinensis [36]. Essential oils of Kenyan plants Ocimum suave (Lamiaceae) and Lippia spp. (Verbenaceae) repelled S. zeamaïs [37, 38], and Acorus calamus (Araceae) EOs T. castaneum [39]. Wang et al. [40] revisiting the traditional use of a very common weed in China Artemisia vulgaris to protect stored products showed the repellent activity of this EO to the Tenebrionidae Tribolium castaneum. In Europe, Kalemba et al. [41] demonstrated that EOs from the berries of the Cupressaceae Juniperus communis were a very good mosquito repellent. A review recently gathered the numerous studies published during the last 10 years on the repellent effect of EOs [42].
Nevertheless, if a lot of essential oils are repellent, some have been found to be highly attractive [10, 12]. The attractiveness of sandalwood oil, basil oil, and grapefruit oil in yellow sticky traps improved the number of trapped greenhouse whitefly Trialeurodes vaporariorum Westwood [43]. Cade oil, an essential oil produced by destructive distillation of juniper (Juniperus oxycedrus L.) twigs, synergized the attraction of alpha-ionol to tephritid fruit fly Bactrocera latifrons (Hendel) male [44].
Some essential oils develop a combined activity on the insects, for example, EOs of Ocimum spp. exhibited both a repellent and a larvicidal action [45]. Acorus calamus EO and its active ingredients, asarone and its analogues, were both antifeedant and potent growth inhibitors to the variegated cutworm Peridroma saucia (Lepidoptera: Noctuidae) [46]. It has also been shown that some essential oils exert quite opposite effects on different insect species. As an example, the tansy (Tanacetum vulgare L.) EOs impacted in different ways the following three beetles: it was attractive and paralyzing for Rhizoperta dominica, repulsive for Tribolium confusum, and toxic for Sitophilus americana [47].
A difference could be made between accurate and chronic toxicity. A short (24-h) exposure to tansy oil exerted an antifeeding activity in larvae and significantly decreased egg laying in adult females of obliquebanded leafroller (Choristoneura rosaceana), while the chronic (long-time exposure) of this EO mixed into the diet during 75 days decreased significantly larvae survival rate [48].
Besides the variability of the phytochemical composition of EOs mentioned above, the point is that insects vary enormously in their responses to secondary plant products. It is well known that the sensitivity of different insect species could be quite different for the same substance [11]. Oils from Cymbopogon nardus which killed quickly the bruchid A. obtectus [12] only knocked down and disabled the Angoumois grain moth Sitotroga cereallela [49].
From all these observations, it could be deduced that essential oils present a widespread range of activities on insects that necessitate to be sharpened by a case-by-case study before application in pest management. Because of the chemical complexity of essential oils and the variability of sensitivity of the insect species, to be significant, the comparison of the toxicity of essential oils necessitates an evaluation which must be (1) conducted with samples of an EO having a quite similar phytochemical composition at each test and (2) experimented on homogenous insect populations.
3.2 Mechanisms of Action
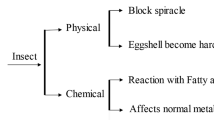
Although the effects of EOs for insect control are abundantly studied, a very few researches are devoted to understand the mechanisms of these observed effect. They involve both physical and chemical properties of EOs. An update was recently published on this point [15].
Because oils are lipophilic, a topical application of EOs laid a film on insect cuticle that modifies the physiology of the insect. The film changed the conditions of penetration inside the body of insect of the air and of substances because of the disruption of lipid bilayers of cell walls. A review on the biological and pharmacologic effects of EOs was recently documented [50].
The second point is linked with volatility of monoterpens. Because they are small volatile molecules, they are involved in the transmission of airborne signals from plants to insects. Detection of bouquets of fragrant and chemosensory-active compounds by insects involves different families of proteins, including OBPs and chemosensory proteins (CSPs). OBPs and CSPs are found on the periphery of the sensory receptors and function in the capture and transport of molecular stimuli [51]. In the sensilla of insects, specialized odorant binding proteins (OBPs) respond to volatile monoterpenes. For example, trichoid sensilla of the female silkworm, Bombyx mori, respond to linalool [52]. In moths, the OBPs include proteins that bind general odorants GOBPs (general odorant binding proteins) such as volatile compounds from plants. The protein identified in tobacco hornworm Manduca sexta, GOBP2, preferentially interacts with floral aromas and green plant odors such as [Z]-3-hexen-1-ol, geraniol, geranyl acetate, and limonene [53]. The different types of GOBPs serve to detect the different categories of odorants released by plants and play an important role in the response of the insect to an EO blend.
Several monoterpenoids (thymol, α-terpineol, linalool, geraniol, eugenol), which have been identified as important components of essential oils, induce a neurotoxicity. The mechanisms of this neurotoxicity have now been explored. They involve receptors of nervous system. Huignard et al. [54] described several different types of receptors which are playing a role. Thymol binds to GABA receptors associated with chloride channels located on the membrane of postsynaptic neurons and disrupts the functioning of GABA synapses [55]. Eugenol acts through the octopaminergic system by activating receptors for the neuromodulator octopamine which increases the concentration of cAMP. This AMPc increase was inhibited in the presence of a mixture of eugenol, α-terpineol, and cinnamic alcohol, but low doses of eugenol and octopamine lead to an increase in adenyl cyclase activity of cells in the nervous system of the cockroach Periplaneta americana [56]. Further studies on cultures of brain cells of P. americana and of Drosophila melanogaster demonstrated that eugenol mimics the action of octopamine with the consequence to increase intracellular calcium levels [57]. The cytotoxicity of EOs through the octopaminergic system was also demonstrated in cultures of epidermal cells of Helicoverpa armigera [58]. Thymol, carvacrol, and α-terpineol influence the production of cAMP and calcium at the cellular level in D. melanogaster. In this insect, tyramine receptors are involved in the recognition of monoterpenes (tyramine is a precursor of octopamine) [59].
Some EOs and their constituents also act on the transmission of the nervous impulse. Price and Berry [60] conducted electrophysiological experiments which showed that eugenol inhibits deeply neuronal activity, whereas citral and geraniol have a biphasic effect that is dose dependent. At low doses, these compounds induce an increase in spontaneous electrical activity but at high doses cause a decrease. Using a similar electrophysiological experimentation, Huignard et al. [54] observed that O. basilicum EOs have a complex neurotoxic activity. The neuronal electrical activity was fully inhibited by the EO which decreased the magnitude of nerve-action current and also reduced the post-hyperpolarization phase and the frequency of nerve-action current firing. The authors hypothesized that this effect could be the result of the combined action of linalool and estragole, two major components of the O. basilicum EO. The mere application of pure linalool in fact produced a reduction in the amplitude of nerve-action current and decreased the post-hyperpolarization while estragole specifically induced a reduction of post-hyperpolarization.
This activity of EOs on nervous system involves also another well known target for neurotoxicity, the enzyme acetylcholinesterase. Tea tree oil inhibited acetylcholinesterase [61]. Lopez and Pascual-Villalobos [62] demonstrated that several types of inhibition are involved with monoterpenes. It could be the result of a reversible competitive inhibition occupying the hydrophobic site of the enzyme’s active site or of a mixed inhibition by linking to a different site from the active site where the substrate bounded.
These studies confirm that the insecticidal activity of EOs and monoterpenes, which are among the major components of EOs, is the consequence of several mechanisms that affect multiple cellular and physiological targets.
4 Trends for the Development of EOs as BCAs
One of the most attractive features of EOs is that they are, in general, low-risk products which provide a friendly environmental pest management. They develop many ecological advantages, but if the pros prevail, some unintended effects have to be mentioned.
4.1 Benefit-Risk Ratio for Environment
On an ecological point of view, EOs present several advantages that could consider them to be useful complementary or alternative method to the intense use of chemical insecticides. Because they are natural and most compounds they included are volatiles, they are biodegradable with short half-life. The AOPWIN Program for alpha-pinene, beta-pinene, camphene, and trans-pinane gave half-life in air ranging from 1.4 to 9.4 h [63]. One of the most common constituents of EOs, α-terpineol, has a short half-life of 4 h in air, 466 h in surface water, and 2 days in soil where it is degradable by soil microflora [64]. This biodegradability induces little persistence in the environment, and in contrast to some synthetic insecticides, no bioaccumulation or biomagnification has been reported to date. As a consequence, this could improve the biodegradability of insecticide treatments and therefore decrease the quantity of toxic insecticide residues not only in the environment but also for food and feed.
EOs generally affect very specific physiological and cellular target. Therefore, they increase insecticide selectivity, and most of the time, by comparison to classical pesticides, the hazards and unintended effects on nontarget species appear to be limited. Very few works, however, were devoted to study the environmental fate and pathways of EOs as well as their effects on agricultural nontarget arthropods. Requiem® is a Chenopodium EO-based biopesticide recently registered in the United States [65]. It is used as insecticide against thrips (Frankliniella occidentalis), green peach aphid, and the greenhouse whitefly (Trialeurodes vaporariorum) and as acaricide against the two-spotted spider mite and the European red mite Panonychus ulmi [66, 67]. Bostanian et al. [68] demonstrated that Requiem® is also operational against two beneficial arthropods used as BCAs, the anthocorid minute pirate bug Orius insidiosus and the micro-Hymenoptera Aphidius colemani which currently control thrips in flower and vegetable greenhouses and also the aphid Rhopalosiphum padi. In Africa, Huignard et al. [69] have shown that EOs of citronella and sweet basil were not only toxic to the bruchid Callosobruchus maculatus but also to its parasitoid Dinarmus basalis, thus compromising its biocontrol.
In same way but on plant species, phytotoxic and allelopathic effects have been described. EOs of wild marigold (Tagetes minuta) and pepper tree (Schinus areira) inhibited roots of maize (Zea mays) and thus have allelopathic effect [70]. Dudai et al. [71] observed that Cymbopogon citratus or Origanum vulgare EOs not only inhibited weed species such as amaranth (Amaranthus palmeri) and Euphorbia hirta but also the growth of crops such as wheat (Triticum aestivum) and tomatoes (Lycopersicon esculentum).
These examples demonstrate that in terms of ecotoxicology, EOs are safe to use but not without potential problems. Because of the variability of species sensitivity to EOs, unintended effects on nontarget species could be observed.
4.2 Benefit-Risk Ratio for Human and Animal Health
It is not because they are natural products that the EOs are without any toxicity when they are used in an inappropriate way. The toxicity of EOs is relatively well studied experimentally and clinically because of their use in human and veterinary medicine. This EOs toxicity may result from the mere toxicity of particular components included therein or from synergistic effects between these numerous compounds being in a same extract.
Their mammalian toxicity of EOs is generally low. Most of EOs (e.g., citronella, lavender, clove, eucalyptus, anise, marjoram, etc.) have an oral LD50 value ranging from 2,000 to 5,000 mg kg−1 in rats. Less than a dozen EOs (e.g., basil, tarragon, hyssop, oregano, savory, tea tree, and sassafras) have LD50 values ranging from 1,000 to 2,000 mg kg−1. But a few are toxic to very toxic. EOs of Pennyroyal (Mentha pulegium mixed with Hedeoma pulegiodes) and Thuja spp. have LD50 values of 400 and 830 mg kg−1. EO of Boldo (Peumus boldus) has LD50 value of 130 mg kg−1 but causes convulsions at a dose of 70 mg kg−1 in rats. The EO of rosemary is also convulsant and can cause epilepsy [2, 72].
Dermal toxicity was observed with some EOs. Tea tree oil is now described since near 15 years to cause skin allergies [73, 74]. Bergamot (Citrus bergamia) and angelica (Angelica archangelica) EOs are identified to be photosensitizing [75] and EOs of wintergreen (Gaultheria procumbens), eucalyptus, clove, and sage to be irritant [76].
In veterinary medicine, some EOs have demonstrated manifestations of toxicity. Dogs dermally exposed to pennyroyal oil at 2 g kg−1 exhibited diarrhea, hemoptysis, and epistaxis 30 h after exposure. A histopathologic examination of liver tissue showed massive hepatocellular necrosis caused by a bioactivation of the major component of this oil pulegone into its hepatotoxic metabolite menthofuran. Commercially available shampoos containing the tea tree (Melaleuca alternifolia) EO and the pure oil have been sold for use on dogs, cats, ferrets, and horses. Clinical signs of three cats dermally exposed to pure melaleuca EO for flea control were described. They included hypothermia, ataxia, dehydration, nervousness, trembling, and coma. Two cats recovered within 48 h following decontamination and supportive care, but one cat died within 3 days following exposure. Terpinen-4-ol, the main constituent of this EO, was detected in the urine of the cats [77].
These examples underline that some EOs need to be handled with caution despite most EOs are not particularly toxic. It is remarkable that these toxicities of some EOs do not coincide with that of the plant from which they are extracted and whose safety is generally recognized [78]. Because risk includes both hazard and exposure, the use of EOs for biocide or for plant protection effects requires that the applicators follow carefully the labeling recommendations given for each situation. EOs are most often delivered by spraying or fogging that may induce a dermal or respiratory exposure. The need of suitable equipment for handling EO products and the treated plant must be observed to avoid accident or chronic intoxication. The EO risk, whatever minimal is it, must not be ignored simply because EOs are natural products.
4.3 Commercial Prospects and Impediments
The insect control by EOs receives attention very soon. In the years 1980–1990, the patents involving essential oils showed that a majority of the inventions focused on household uses. Japanese companies understood very soon the interest to use EOs in such a way. To prevent reinfestation by Blattarias, particularly the German cockroach Blatella germanica, a cleaning solution including clove essential oils and pyrethrinoids [79] and adhesives containing acrylic polymers and high levels of essential oils [80] were commercialized as well as mixtures associated with essential oils and pyrethrinoids to control mosquitoes and flies [81, 82]. Eucalyptus spp. EOs were used as a synergistic insecticide in addition to growth inhibitors [83], and EOs of spearmint, bitter almond, and birch (Betula lenta) bark essential oils were incorporated into a formulation sold for acaricide, insecticide, and insect repellent properties [84]. Domestic uses prevailed. To prevent the clothes from moths and beetles, filter papers or tablets soaked with EO Juniperus rigida were placed in the wardrobe [85]. To protect pet dogs, a flea collar was manufactured by adding essential oils (eucalyptus, cedarwood, citronella, and peppermint) to ethylene-vinyl acetate polymer [86]. To improve resistance to insects, handicraft veneer-faced panels were impregnated with polymer layer and hiba or kinoki EOs [87, 88]. EOs also showed some usefulness for building and hand-manufactured materials. Eucalyptus spp. EOs were mixed with pyrethrinoids and borax in a solution to preserve wooden beams [89].
But the important sector in which EOs present applications as insect BCAs, still is the area of agriculture, the stored-product storage and feed. Mustard essential oil was used very soon into formulation containing insecticide, microbicide, and repellent substances absorbed onto silica used to prevent infestation of mites in feed [90]. In Europe, pine EOs were also incorporated in the 1990s with polymers into sheets to develop attractant adhesive films or coating materials to enhance the control of harmful insects in agriculture, livestock structures, and horticulture [91, 92].
Twenty years after, Arnason [93] underlined that essential oils are now considered to be the most important commercial application of botanical insecticides. He indicated that no less that 88 insect repellent products are actually sold in the United States market containing an essential oil as one of the active ingredients in the formulation. Eight EOs are distinguished. The most commonly used ingredient is citronella oil (45 products), followed by geranium oil-geraniol (33 products), lemongrass oil (24 products), cedar oil (22 products), peppermint oil (16 products), rosemary oil (15 products), soybean oil (15 products), and eucalyptus oil (14 products). According to this author, 57 of these 88 formulations contain a blend of two or more active ingredients. Among reasons that facilitate the use of EOs as BCAs, the need to replace methyl bromide, which is considered to deplete the ozone in the stratosphere, renews interest in essential oils as fumigants.
This enhancement of using EOs to control insect pests in orchards and to protect high-value crops is probably the result of the regulation rules in the USA. Because the procedure for regulatory approval of plant protection products is expensive and the market for BCAs appear to be presently a niche market (about 2 % of pesticides global market), industrial producers of BCAs need a simplified procedure for registration to achieve a reasonable return on investment. As indicated by Regnault-Roger et al. [15]: “A reduced regulation process for these products has existed in the United States since 1996 and it is particularly relevant to EOs. Biopesticides are subject to special procedures outlined in Title 40, Code of Federal Regulations, of FIFRA. A number of natural substances, such as EOs of mint, thyme, rosemary, and lemon grass that did not benefit from this simplified procedure, however, were classified as GRAS (generally regarded as safe). They were placed on a list (FIFRA Section 25[b]), exempting them from the registration process [94].” Paulizt and Bélanger [95] confirmed that this simplified approval procedure has led to a large diversity of EO-based products available in the United States. Moreover, this exemption has become a marketing strategy to promote these products [96, 97]. The company EcoSmartTM was a pioneer, 15 years ago, to develop a line of products on this exemption. These products now are named “EcoEXEMPT® Minimum-Risk & EcoPCO® Products” and are commercialized on a large scale by several suppliers [98].
Compared to United States, only few EO products are available in the European Union [EU] and Canada, where registration is stricter. Pest Management Regulatory Agency [PMRA, Canada] decided in 2004 to deregister citronella products due to a lack of safety data. However, since “Health Canada did not identify any imminent health risks, citronella-based personal insect repellents will remain on the market until a final decision is made” [99, 100], but strict recommendations accompany the use of these products.
In UE, the procedure for reevaluation of plant protection products (PPP) ended in 2008. To be authorized on market, all PPP derived from biological as well as chemical have to be listed in Annex 1. Because they meet the purposes of Directive 2009/128/CE to promote integrated pest management (IPM), some vegetable oils have been recently authorized but for uses that are not insecticidal [101]: tea tree oil for use as a fungicide, citronella oil as an herbicide, clove oil as a fungicide and bactericide, and spearmint oil as a plant growth regulator. The status of EOs extracted from thyme or marigold (Tagetes sp.) for insecticide use is presently pending and should be approved soon. Orange oil is now allowed in France for control of sweetpotato whitefly, Bemisia tabaci, on field pumpkin (Cucurbita pepo) and of greenhouse whitefly on tomato [102].
The situation for using EOs in developing countries is quite different. The tropical flora of many developing countries is remarkably diverse and can be a rich source of potent and valuable EOs. Aromatic plants are traditionally and widely used for stored-product insects or to repel harmful insects in fields. Currently, there is a move to enhance the use of steam-distilled EOs, but the lack of technologic resources leads to use crafty solution like a domestic pressure cooker for extracting EOs by steam distillation. Another point that impedes the proper development of EOs in Africa is that results most of the time are unsupported by scientific experimentation [15]. The promotion of BCAs in developing countries needs more consistent risk analyses as well as stricter regulatory systems.
5 Conclusion
The development of EOs as PPP and biocide to control insect is an alternative or a complementary approach to synthetic insecticides. They are environmentally friendly products, that is, they have natural origin and are biodegradable, and they have diverse physiological targets within insects that may delay the evolution of insect resistance. Thus, they are especially suited to organic farming as well as to integrated pest management. As a result, EOs have been embraced by the public.
Two key points impede their development as BCAs. Because the field of researches is widespread, there is a lack of data on environmental features of EOs and their components and also on plant-insect interrelationships and mechanisms. More fundamental and applied studies are needed; even the publications devoted to EOs improved the last years. The second key point is the difficulty to have a relevant registration both in developed and developing countries. Most of developing countries need to have appropriate and stronger regulation whereas many developed countries need to have adapted regulation. In some countries, it is difficult to meet the consumers demand because of registration requirements. Among the arguments used to seek reduced regulation for EOs is the fact that many active ingredients of EOs are used daily at home or in food. Therefore, it is rather logical to conclude that it would be unreasonable to request heavy and costly registration requirements for these products that have no history of adverse effects [97]. However, the safety of products which are used in a precise context sometimes led to the reality of unintended effects in a new context. As a consequence, an evaluation of the benefit-risk ratio on a case-by-case basis must be required to prevent this undesirable situation and to have a reasonable management of real risk if there is some.
Therefore, most of government policies are now seeking low-risk and alternative plant protection products. The US EPA is the only regulatory regime that has considered reduced risk seriously and, as a result, has allowed a significant number of EOs for commercial use more than 10 years ago. The results of this American position must be checked carefully to determine positive and negative inputs of using EOs in such a way. Because of the numerous advantages of EOs in controlling harmful insects, they certainly have a room in the BCAs approaches which promote sustainable development.
Abbreviations
- AOPWIN:
-
Atmospheric oxidation program for Microsoft Windows
- BCAs:
-
Biocontrol agents
- CAGR:
-
Compound annual growth rate
- cAMP:
-
Cyclic adenosine monophosphate
- CSPs:
-
Chemosensory proteins
- EOs:
-
Essential oils
- GABA:
-
Gamma-aminobutyric acid
- GOBPs:
-
General odorant binding proteins
- GS-FID:
-
Gas chromatography-flame ionization detector
- GS-MS:
-
Gas chromatography–mass spectrometry
- IPM:
-
Integrated pest management
- LC50 :
-
Lethal concentration 50
- LD50 :
-
Lethal dose 50
- OBPs:
-
Odorant binding proteins
- PPP:
-
Plant protection product
- UIPP:
-
Union des industries de la protection des plantes
- USD:
-
US dollar
References
UIPP (2011). Rapport d’activité 2010–2011. http://www.uipp.org/. Accessed 24 Apr 2012
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods – a review International. J Food Microbiol 94:223–253
Bruneton J (1999) Pharmacognosie, 3rd edn. Tec & Doc Lavoisier, Paris
Ngassoum MB, Ousmaila H, Ngamo LT, Maponmetsem PM, Jirovetz L, Buchbauer G (2004) Aroma compounds of essential oils of two varieties of the spice plant Ocimum canum Sims from northern Cameroon. J Food Compos Anal 17(2):197–204
Bernards MA (2010) Plant natural products: a primer. Can J Zool 88:601–614
Gershenzon J, McConkey ME, Croteau RB (2000) Regulation of monoterpene accumulation in leaves of peppermint. Plant Physiol 122:205–213
Pascual-Villalobos MJ, Ballesta-Acosta MC (2003) Chemical variation in an Ocimum basilicum germplasm collection and activity of the essential oils on Callosobruchus maculatus. Biochem System Ecol 31:673–679
Isman MB, Machial CM (2006) Pesticides based on plant essential oils: from traditional practice to commercialization. In: Rai M, Carpinella MC (eds) Naturally occurring bioactive compounds. Elsevier BV, Amsterdam
Guenther E (1972) The production of essential oils. In: Guenther E (ed) The essential oils, vol 1, 2nd edn. Krieger R.E, Malebar Florida
European Pharmacopoeia Communications (2008) European pharmacopoeia, 6th edn. Fr. EDQM, Strasbourg
Regnault-Roger C (1997) The potential of botanical essential oils for insect pest control. Integr Pest Manag Rev 2:25–34
Regnault-Roger C (2008) Recherche de nouveaux biopesticides d’origine végétale à caractère insecticide: démarches méthodologiques et application aux plantes aromatiques méditerranéennes. In: Regnault-Roger C, Philogène BJR, Vincent C (eds) Biopesticides d’Origine Végétale, 2nd edn. Lavoisier Tech & Doc, Paris
Isman MB (2000) Plant essential oils for pest and disease management. Crop Prot 19:603–608
Isman MB (2006) Botanical insecticides, deterrents and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 50:45–66
Regnault-Roger C, Vincent C, Arnason JT (2012) Low-risk products in a high-stakes world. Annu Rev Entomol 57:405–424
Regnault-Roger C, Hamraoui A, Holeman M, Théron E, Pinel R (1993) Insecticidal effect of essential oils from Mediterranean plants upon A. obtectus Say (Coleoptera, Bruchidae), a pest of kidney bean (Phaseolus vulgaris L.). J Chem Ecol 19:1231–1242
Regnault-Roger C, Hamraoui A (1994) Reproductive inhibition of Acanthoscelides obtectus Say (Coleoptera), bruchid of kidney bean (Phaseolus vulgaris L.) by some aromatic essential oils. Crop Prot 13(8):624–628
Regnault-Roger C, Hamraoui A (1994) Antifeedant effect of Mediterranean plant essential oils upon Acanthoscelides obtectus Say (Coleoptera), bruchid of kidneybeans, Phaseolus vulgaris L. In: Highley E, Wright EJ, Banks HJ, Champ BR (eds) Stored product protection. CAB International, Wallingford
Weaver DK, Dubkel FV, Netzububanza L, Jackson LL, Stock DT (1991) The efficacy of linalool, a major component of freshly milled Ocimum canum Sim (Lamiaceae) for protection against post-harvest damage by certain stored Coleoptera. J Stored Prod Res 27:213–270
Deshpande RS, Tipnis HP (1977) Insecticidal activity of Ocimum basilicum Linn. Pesticides 11:11–12
Kurowska A, Kalemba D, Gora J, Majda T (1991) Analysis of essential oils: influence on insects. Part IV. Essential oil or garden thyme (Thymus vulgaris L.). Pestycydy 2:25–29
Mateeva A, Karov S (1983) Studies on the insecticidal effect of some essential oils. Nauchni Trudove–Vissha Selskostopanski Institute ‘Vasil Kolarov’ Plodiv, 28:129–139
Konstantopoulou I, Vassipoulou L, Mauragani-Tsipidov P, Scouras ZG (1992) Insecticidal effects of essential oils. A study of the effects of essential oils extracted from eleven Greek aromatic plants on Drosophila auraria. Experientia 48:616–619
Hamraoui A, Regnault-Roger C (1997) Comparaison des activités insecticides des monoterpènes sur deux espèces d’insectes ravageurs des cultures Ceratitis capitata et Rhopalosiphum padi. Acta Bot Gallica 144:413–417
Park IK, Kim L-S, Choi I-O, Lee Y-S, Shin S-C (2006) Fumigant activity of plant essential oils and components from Schizonepeta tenuifolia against Lycoriella ingenua (Diptera: Sciaridae). J Econ Entomol 99:1717–1721
Papachristos DP, Kimbaris AC, Papadopoulos NT, Polissiou MG (2009) Toxicity of citrus essential oils against Ceratitis capitata (Diptera: Tephritidae) larvae. Ann Appl Biol 155:381–389
Liu ZL, Chu SS, Jiang GH (2011) Insecticidal activity and composition of essential oil of Ostericum sieboldii (Apiaceae) against Sitophilus zeamais and Tribolium castaneum. Rec Nat Prod 5:74–81
Shaaya E, Ravid U, Paster N, Juven B, Lisman U, Pissarev V (1991) Fumigant toxicity of essential oils against four major stored-product insects. J Chem Ecol 7:499–504
Zapata N, Lognay G, Smagghe G (2010) Bioactivity of essential oils from leaves and bark of Laurelia sempervirens and Drimys winteri against Acyrthosiphon pisum. Pest Manag Sci 66:1324–1331
Haubruge E, Lognay G, Marlier M, Danhier P, Gilson JC, Gaspar C (1989) The toxicity of five essential oils extracted from Citrus species with regards to Sitophilus zeamais Motsch (Col., Curculionidae), Prostephanus truncatus (Horn) (Col., Bostrychidae) and Tribolium castaneum Herbst (Col., Tenebrionidae). Medelingen van de Faculteit Landbouwwetenschappen Rijksuniversiteit Gent 54:1083–1093
Iwuala MOE, Osisiogu IUW, Agbakwuru EOP (1981) Dennetia oil, a potential new insecticide: tests with adults and nymphs of Periplaneta americana and Zonocerus variegatus. J Econ Entomol 74:249–252
Kambu K, Di Phanzu N, Coune C, Wauters JN, Angenot L (1982) Study on the insecticidal and chemical properties of Eucalyptus saligna from Zaïre. Plantes Medicinales et Phytothérapie 16(1):34–38
Salom SM, Carlson JA, Ang BN, Grosman DM, Day ER (1994) Laboratory evaluation of biologically-based compounds as antifeedants for the pales weevil (Hylobius pales). J Entomol Sci 29:407–419
Alonso-Amelot ME, Avila JL, Otero LD, Mora F, Wolff B (1994) A new bioassay for testing plant extracts and pure compounds using red flour beetle Tribolium castaneum Herbst. J Chem Ecol 20:1161–1177
Castillo L, González-Coloma A, González A, Díaz M, Santos E, Alonso-Paz E, Bassagoda MJ, Rossini C (2009) Screening of Uruguayan plants for deterrent activity against insects. Ind Crops Prod 29(1):235–240
Kokate CK, D’Cruz JL, Kumar RA, Apte SS (1985) Anti-insect and juvenoidal activity of phytochemicals derived from Adhatoda vasica Nees. Ind J Nat Prod 1:7–9
Hassalani A, Lwande W (1989) Anti pest secondary metabolites from African plants. In: Arnason JT, Philogene BJR, Morand P (eds) Insecticides of plant origin, Am Chem Soc Symp series 387:78–94
Mwangi JW, Addae-Mensah I, Muriuki G, Munavu R, Lwande W, Hassanali A (1992) Essential oils of Lippia species in Kenya IV: maize weevil (Sitophilus zeamais) repellency and larvicidal activity. Int J Pharmacognosy 30:9–16
Jilani G, Saxena RC, Rueda BP (1988) Repellent and growth inhibiting effects of turmeric oil, sweetflag oil, neem oil and Margosan-O on red flour beetle (Coleoptera: Tenebrionidae). J Econ Entomol 81:1226–1230
Wang J, Zhua F, Zhoua XM, Niua CY, Leia CL (2006) Repellent and fumigant activity of essential oil from Artemisia vulgaris to Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J Stored Prod Res 42(3):339–347
Kalemba D, Kurowska A, Gora J, Lis A (1991) Analysis of essential oils: influence of insects. Part V. Essential oil of the berries of Juniper (Juniperus communis L.). Pestycydy 2:31–34
Nerio LS, Olivero-Verbel J, Stashenko E (2010) Repellent activity of essential oils: a review. Bioresour Technol 101(1):372–378
Górski R (2004) Effectiveness of natural essential oils in the monitoring of greenhouse whitefly Trialeurodes vaporariorum Westwood. Folia Horticulturae Ann 16(1):183–187
McQuate GT, Peck SL (2001) Enhancement of attraction of alpha-ionol to male Bactrocera latifrons (Diptera: Tephritidae) by addition of a synergist, cade oil. J Econ Entomol 94(1):39–46
Chokechaijaroenporn O, Bunyapraphatsara N, Kongchensin S (1994) Mosquito repellent activities of Ocimum volatile oils. Phytomedicine 1:135–139
Koul O, Smirle MJ, Isman MB (1990) Asarones from Acorus Calamus oil: their effect on feeding behavior and dietary utilization in Peridroma saucia. J Chem Ecol 16:1911–1920
Kurowska A, Kalemba D, Majda T, Gora J, Mielniczuk Z (1993) Analysis of essential oils in aspects of their influence on insects. Part II. Essential oil of tansy. Zeszyty Naukowe Politechniki Lodzkiej. Technologia I Chemia Spozywcza 589:15–22
Larocque N, Vincent C, Bélanger A, Bourassa JP (1999) Effects of tansy oil, Tanacetum vulgare L., on the biology of the obliquebanded leafroller, Choristoneura rosaceana (Harris) (Lepidoptera: Tortricidae). J Chem Ecol 25:1319–1330
Krishnarajah SR, Gawesalingam VK, Senanayake UM (1985) Repellency and toxicity of some plant oils and their terpene components to Sitotroga cereallela (Oliver) (Lepidoptera, Gelichiidae). Trop Sci 25:249–252
Adorjan B, Buchbauer G (2010) Biological properties of essential oils: an updated review. Flavour Fragr J 25:407–426
Picimbon JF (2005) Synthesis of odorant reception-suppressing agents, odorants-binding proteins (OBPs) and chemosensory proteins (CSPs): molecular targets for pest management. In: Regnault-Roger C, Philogène BJR, Vincent V (eds) Biopesticides of plant origin. Intercept–Lavoisier, Andover-Paris
Picimbon JF, Regnault-Roger C (2008) Composés sémiochimiques volatils, phytoprotection et olfaction: cibles moléculaires pour la lutte intégrée. In: Regnault-Roger C, Philogène BJR, Vincent C (eds) Biopesticides d’Origine Végétale, 2nd edn. Lavoisier Tech & Doc, Paris
Feng L, Prestwich GD (1997) Expression and characterization of a lepidopteran general odorant binding protein. Insect Biochem Mol Biol 27:405–412
Huignard J, Lapied B, Dugravot S, Magnin-Robert M, Ketoh GK (2008) Modes d’action neurotoxiques des dérivés soufrés et de certaines huiles essentielles et risques liés à leur utilisation. In: Regnault-Roger C, Philogène BJR, Vincent C (eds) Biopesticides d’Origine Végétale, 2nd edn. Lavoisier Tech & Doc, Paris
Priestley CM, Williamson EM, Wafford KA, Satelle DB (2003) Thymol, a constituent of thyme essential oils, is a positive modulator of human GABA and a homo-oligosteric GABA receptor from Drosophila melanogaster. Br J Pharmacol 140:1363–1372
Enan EE (2001) Insecticidal activity of essential oils: octopaminergic sites of action. Comp Biochem Physiol 130:325–327
Enan EE (2005) Molecular and pharmacological analysis of an octopamine receptor from American cockroach and fruit fly in response to essential oils. Arch Insect Biochem Physiol 59:161–171
Kostyukovsky M, Rafaeli A, Gileadi C, Demchenko N, Shaaya E (2002) Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants. Pest Manag Sci 58:1101–1106
Enan EE (2005) Molecular response of Drosophila melanogaster tyramine receptor cascade to plant essential oils. Insect Biochem Mol Biol 35:309–321
Price DN, Berry MS (2006) Comparison of effects of octopamine and insecticidal essential oils on activity in the nerve cord, foregut and dorsal unpaired median neurons of cockroaches. J Insect Physiol 52:309–319
Mills C, Cleary BJ, Gilmer JF, Walsh JJ (2004) Inhibition of acetylcholinesterase by tea tree oil. J Pharm Pharmacol 56:375–379
Lopez MD, Pascual-Villalobosa MJ (2010) Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind Crops Prod 31:284–288
Meylan W (1994-1995b) AOPWIN, Syracuse Research Corporation
CHEMWATCH (2009) Material Safety Data Sheet Alpha terpineol sc-291877. http://datasheets.scbt.com/sc-291877.pdf. Accessed 15 Apr 2012
Chiasson H, Delisle U, Bostanian NJ, Vincent C (2008) Recherche, développement et commercialisation de FACINMD, un biopesticide d’origine végétale. Etude d’un cas de réussite en Amérique du Nord. In: Regnault-Roger C, Philogène BJR, Vincent C (eds) Biopesticides d’Origine Végétale, 2nd edn. Lavoisier Tech & Doc, Paris
Chiasson H, Vincent C, Bostanian NJ (2004) Insecticidal properties of a Chenopodium-based botanical. J Econ Entomol 97:1378–1383
Chiasson H, Bostanian NJ, Vincent C (2004) Acaricidal properties of a Chenopodium-based botanical. J Econ Entomol 97:1373–1377
Bostanian NJ, Akalach M, Chiasson H (2005) Effects of a Chenopodium-based botanical insecticide/acaricide on Orius insidiosus (Hemiptera: Anthocoridae) and Aphidius colemani (Hymenoptera: Braconidae). Pest Manag Sci 61:979–984
Huignard J, Dugravot S, Ketoh GK, Thibout E, Glitho AI (2008) Utilisation des composes secondaires pour la protection d’une graine de légumineuse. Conséquences sur les insectes ravageurs et parasitoïdes. In: Regnault-Roger C, Philogène BJR, Vincent C (eds) Biopesticides d’Origine Végétale, 2nd edn. Lavoisier Tech & Doc, Paris
Scrivanti LR, Zunino MP, Zygadlo JA (2003) Tagetes minuta and Schinus areira essential oils as allelopathic agents. Biochem System Ecol 31:563–572
Dudai N, Poljakoff-Mayber A, Mayer AM, Putievsky E, Lerner HR (1999) Essential oils as allelochemicals and their potential use as bioherbicides. J Chem Ecol 25:1079–1089
O’ Neil MJ, Smith A, Heckelman PE, Budavari S (2006) The Merck index: an encyclopedia of chemicals, drugs, and biologicals, 4th edn. Merck & Co, Whitehouse Station
Rubel DM, Freeman S, Southwell IA (1998) Tea tree oil allergy: what is the offending agent? Report of three cases of tea tree oil allergy and review of the literature. Aust J Dermatol 39:244–247
Rutherford T, Nixon R, Tam M, Tate B (2007) Allergy to tea tree oil: retrospective review of 41 cases with positive patch tests over 4.5 years. Aust J Dermatol 48:83–87
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils – a review. Food Chem Toxicol 46:446–475
Hammer KA, Carson CF, Riley TV (1999) Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol 86:985–990
Poppenga RH (2002) Herbal medicine: potential for intoxication and interaction with conventional drugs. Clin Tech Small Anim Pract 17:6–18
Regnault-Roger C, Philogène BJR (2008) Past and current prospects for the use of botanicals and plant allelochemicals in integrated pest management. Pharm Biol 46:1–12
Heinmenberg H (1992) Project XP-11, Patent CA 92–20 77284 920901.
Yamaguchi A, Okubo T, Nanbu H, Ishigaki S, Kawetake M, Okabe T, Saito K, Otomo Y (1989) Insect repelling and antibacterial effect adhesive, Taiyo Chemical Company Ltd. Japan. Patent JP 89–248713 890925
Liang K (1988) People’s Republic of China. Insecticidal composition containing pyrethrinoids for domestic use. Patent CN 88–105917 880711
Kono M, Ono M, Ogata K, Fujimori M, Imai T, Tsucha S (1993) Fuji Flavor Co, Japan & Nippon Tobacco Sangyo. Control of insects with plant essential oils and insecticides. Patent JP 93–70745 930308
Narasaki M, Morita H, Fujisaki T (1987) Synergistic insecticides containing insect growth inhibitors, Mikasa Chemical Industrial Co, Ltd. Japan. Patent JP 87–173368 870711
Matsumoto T, Takaoka K, Watanabe C (1987) Acaricides, insecticides and insect repellents containing benzaldehyde or perilla aldehyde. Patent JP-87 176437 870715
Okano T (1991) Non toxic insect repellent composition for protecting clothes from damage by moth and other insects, Osaka Seiyaku K K, Japan. Patent JP 91–285670 911004
Seto S (1987) Manufacture of pet collars containing pest repellents, Daiichi Yakka Co, Ltd, Japan. Patent JP 87–142137 870609
Akita K (1991) Insect-repellent and mildewcidal laminate boards, Fukubi Kagaku Kogyo K K, Japan. Patent JP 91–242869 910927
Tsubochi K, Sugimoto H (1992) Improved method for extending the insecticidal and mildewicidal performance of impregnated decorative panels, Daiken trade & industry, Japan. Patent JP 92–112272 920403
Urabe C (1992) Insect control in wood, Ohtsu tire Japan. Patent JP 92–308238 921021.
Saijo T (1989) Slow release formulations containing volatile substances useful in industries, Shoko Kagaku Kenkyusho K K, Japan. Patent JP 89–161829 890623
Klerk’s Plastic Industrie BV (1990) Insect control agent for agriculture and horticulture, Netherland. Patent NL 90–1461 900626
Feliu ZM (1990) Manufacture of insecticidal coating materials, Anfel S A, Spain. Patent ES 90–1212 900427
Arnason JT (2011) Natural products from plants as insecticides in agriculture and human health (Chapter 13). In: Pezzuto JM, Kato MJ (eds) Photochemistry and pharmacognosy, Encyclopedia of life support systems (EOLSS), developed under the Auspices of the UNESCO. Eolss, Oxford
Environ Prot Agency (EPA) (2011) Regulating biopesticides. http://www.epa.gov/pesticides/biopesticides. Accessed 22 Apr 2012
Paulitz TC, Bélanger RR (2001) Biological control in greenhouse systems. Annu Rev Phytopathol 39:103–133
Isman MB (2004) Plant essential oils as green pesticides for pest and diseases management. ACS Symp Ser 887:41–51
Regnault-Roger C, Silvy C, Alabouvette C (2005) Biopesticides: r´ealit´es et perspectives commerciales. In: Regnault-Roger C (ed) Enjeux Phytosanitaires pour l’Agriculture et l’Environnement. Lavoisier Tech & Doc, Paris
Bug source (2012) EcoEXEMPT & EcoPCO® Products. http://bugsource.com/ecoexempt__products.html
Health Canada (2009) insect repellents. http://www.hc-sc.gc.ca/hl-vs/iyh-vsv/life-vie/insect-eng.php. Accessed 22 Apr 2012
BC Centre for Disease Control 2012 West Nile Virus. http://www.bccdc.ca/dis-cond/a-z/_w/WestNileVirus/overview/West+Nile+Virus.htm. Accessed 22 Apr 2012
Off J Eur Union (OJEU) (2008) Commission Directive 2008/127/EC of 18 December 2008 amending Council Directive 91/414/EEC to include several active substances. Vol. 51 L 344/89 (20 Dec 2008)
Off J Eur Union (OJEU) (2009) Commission Decision of 8 June 2009 recognising in principle the completeness of the dossier submitted for detailed examination in view of the possible inclusion of orange oil in Annex I to Council Directive 91/414/EEC (notified under document number C(2009) 4232). Vol 52 L145/47 (10 June 2009)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Regnault-Roger, C. (2013). Essential Oils in Insect Control. In: Ramawat, K., Mérillon, JM. (eds) Natural Products. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-22144-6_181
Download citation
DOI: https://doi.org/10.1007/978-3-642-22144-6_181
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-22143-9
Online ISBN: 978-3-642-22144-6
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics