Abstract
We report a defective 1D photonic crystal for real-time sensing of blood hemoglobin concentrations. The proposed structure is configured as adjacent thin films of Na3AlF6 and ZnSe including a defect remain at the middle. The cornerstone of this research is based on the analysis of the transmission spectrum by manipulating the transfer matrix method (TMM). Upon infiltrating the defect layer with blood containing different hemoglobin concentrations, the shift in the resonant mode wavelength is observed within the photonic band gap (PBG). Sensor performance is evaluated by varying the incident light angle and thickness of the defect medium. Numerous sensing characteristics such as sensitivity, figure of merit and signal-to-noise ratio are computed for studying the effectiveness of the proposed sensor. Additionally, the simple structure with notable sensing performance makes the proposed sensor a suitable candidate for biosensing applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Photonic crystals (PhCs) belong to special class of multilayer structures, where the dielectric constant of different layers is repeated in a periodic manner along different dimensions, which result in 1D, 2D and 3D configurations of PhCs [1,2,3]. 1D PhCs are the most investigated owing to their low manufacturing cost and wide range of applications. When electromagnetic (EM) waves interact with PhCs, a distinctive property is perceived known as photonic band gap (PBG), where light of certain frequency ranges is prohibited to pass through the structure [4, 5]. When the incident light frequency matches with the defect mode frequency, the photons are positioned within the band gap, which form a resonant peak [6,7,8]. The resonant peak wavelength \(\left( {\lambda _{{{\text{res}}}} } \right)\) is a strong function of various structure parameters like thickness of dielectric layers, dielectric constant of the constituent materials and number of periods. Reflectance spectrum, transmittance spectrum and absorption spectrum can be vigorously controlled by changing the PBG and resonant mode characteristics [9]. A sensitivity of 428 nm/RIU is achieved by Zhang et al. [10], by deploying a photonic crystal cavity sensor to detect various concentrations of NaCl. A 1D PhC sensor showing a high sensitivity of 2200 nm.RIU−1 is demonstrated by Aly et al. [11], where the structure is realized through an alternate dielectric layers of SiO2 and GaAs. The authors studied a 1D PhC-based transducer for application in sensing of biomolecules, where refractive index of various bioanalytes is sensed by measuring the fluorescence shift [12]. Chen et al. [13] investigated a 1D PhC for realization of gas sensor in terahertz regime. Aly et al. [14] presented a simple 1D structure to detect different creatinine levels present in the blood, where the sensing principle is based on analysis of the transmission spectra.
In the recent decades, researchers have shown keen interest in detection of hemoglobin concentration in blood as any deviation from the normal level may lead to various deadly diseases like anemia, thalassemia, liver disorder, cancer, etc. [15]. Refractive index (RI) is one of the most accepted biophysical parameters to quantify the amount of hemoglobin concentrations in blood. The refractive index of hemoglobin can be effectively measured by fluorescence spectroscopy and optical coherence tomography techniques. Biswas et al. [16] reported an optical resonator configuration designed with periodically arranged air holes for sensing various Hb concentrations present in the blood using FDTD method, but low sensitivity of the structure is a major concern. Natesan et al. [17] investigated transmission spectrum of a dual-core 2D photonic crystal fiber for detection of hemoglobin level in blood. A 1D PhC with central defect has been investigated for sensing hemoglobin concentrations [18], but the authors obtained a sensitivity of only 167 nm/RIU, which is considerably low. Here, a defected PhC with 1D configuration has been investigated with an aim to efficiently detect the hemoglobin concentrations at a wavelength of 680 nm. Table 1 displays the refractive index data of various hemoglobin concentrations [19].
Here, we have picked Na3AlF6 and ZnSe for the design of the proposed 1D PhC. The alternate combination of these material leads to high contrast in refractive index, which results in wide bandgap [20]. Also, these materials show omnidirectional wide band gap over a broad frequency range. Moreover, Na3AlF6 and ZnSe are characterized with feeble absorption loss in the visible and near-infrared wavelengths. This work has couple of benefits such as selection of novel materials for the design of different layers and detail assay of different sensing performances. In addition to this, a thorough investigation is performed to disclose the effects of variation in defect medium thickness and incident angle on the sensing characteristics. Moreover, the simple structure, easy way of analysis and cost-effective fabrication techniques make the proposed structure worth to fabricate.
2 Theoretical Treatment
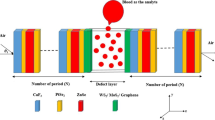
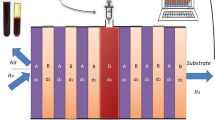
Figure 1 depicts the proposed 1D photonic structure with (A/B)N/D/(A/B)N configuration, where the first layer (A) is designed with Na3AlF6 having refractive index \(n_{A} = 1.34\) and thickness \(d_{A} = 500~\;{\text{nm}}\), whereas the second layer (B) is realized with ZnSe having refractive index \(n_{B} = 2.61~\) and thickness \(d_{B} = 500~\;{\text{nm}}\). The period of the dielectric layer is considered as N = 5. A defect with thickness d is introduced at the center, where blood with different hemoglobin concentrations is infiltrated.
When the EM waves are interacted with the said structure, a certain number of photons are transmitted in the PhC, whereas some photons with certain range of frequencies are prohibited to pass, giving rise to band gap. Here, we have considered that the EM waves are incident at an angle \(\theta _{i}\) and travel along the z-direction, and the dielectric layers lie in the x–y plane. The transmittance characteristic is scrutinized by manipulating transfer matrix method (TMM). We have used TMM because this method is the most efficient and easy to study the reflection/transmission spectrum for multilayer structures, as compared to other existing techniques like PWE and FDTD. In TMM, the complete transfer matrix is obtained by multiplying the transfer matrixes of each discrete layers. The well-known Maxwell equations are manipulated for exploring the transmission characteristics of EM signal in the pth layer, which can be written as below [21],
where \(E_{p} \left( x \right)\) and \(H_{p} \left( x \right)\) denote the field components persisted in the pth layer, \(\omega\) represents angular frequency, and k signifies the wave vector. The transfer matrix for pth layer can be expressed as,
where \(\theta _{p} = d_{p} \cos \theta _{i}\) and \(\Psi _{p} = \sqrt {\mu _{0} /\varepsilon _{0} } ~n_{p} \cos \theta _{i}\). Here, \(\theta _{i}\) denotes the angle of incidence in the pth layer. The characteristics matrix representation of the complete configuration can be mathematically expressed as below,
where \(M_{A}\), \(M_{B}\) and \(M_{D}\) represent the individual transfer matrix of each considered layers. By using Eq. (4), the coefficients of transmittance and reflectance [22] can be computed, namely
where \(\Psi _{0} = \sqrt {\mu _{0} /\varepsilon _{0} } n_{0} \cos \theta _{0}\) and \(\Psi _{P} = \sqrt {\mu _{0} /\varepsilon _{0} } n_{0} \cos \theta _{P}\). Here, \(n_{0}\) is the refractive index of air. The aforementioned equations allow us to evaluate the transmittance and reflectance, which can be expressed as,
3 Results and Interpretations
The mainstay of the current work is the analysis of the resonant modes created in the transmission spectrum of the suggested configuration, in order to detect different concentrations of hemoglobin in blood. We have studied the sensing characteristics for different defect layer thicknesses such as 800, 850 and 900 nm. The TMM technique is used for computation of transmission spectrum of the proposed structure, which is shown in Fig. 2. Upon infiltration of the defect layer with different concentrations of hemoglobin, it is observed that resonant modes are red shifted in wavelength with the rise in hemoglobin concentrations. Besides, it can be witnessed that resonant modes are red shifted upon increasing defect layer thickness. From Fig. 3, it is seen that resonant wavelengths are sifted toward higher wavelengths for higher concentrations of hemoglobin. A remarkable total shifts of 16.4, 14.8 and 15 nm are marked for d = 800 nm, d = 850 nm and d = 900 nm, respectively, for the slight difference in refractive index.
Figure 4 shows the variation in resonant wavelength \(\left( {\lambda _{{{\text{res}}}} } \right)\) for different hemoglobin concentrations for different defect layer thicknesses. Here, it can be clearly seen that \(\left( {\lambda _{{res}} } \right)\) increases with rise in defect layer thicknesses.
From Fig. 4, the relation between the resonant peak \(\left( {\lambda _{{{\text{res}}}} } \right)\) and different hemoglobin concentrations (C) can be mathematically represented as below,
The aforementioned equations are in agreement with linearship, which ensures the precise detection of various hemoglobin concentrations. Sensitivity is one of the key parameters to appraise the performance of the suggested device [49], which can be calculated by the fraction of variation in the resonance wavelength \(\left( {\lambda _{{{\text{res}}}} } \right)\) to the RI contrast (∆n). Sensitivity is expressed as [23],
Sensitivity, which is delineated in Fig. 5, is the utmost significant parameter for appraising the sensor performance, where it is perceived that a maximum sensitivity of 475.6 nm.RIU−1 is acquired for hemoglobin concentration of 87 g/L at d = 850 nm.
Apart from sensitivity, SNR and FOM are other two key parameters to judge the performance of any sensing device. SNR is computed by dividing the change in defect mode wavelength \(\left( {\Delta \lambda _{{{\text{res}}}} } \right)\) by the spectral half-width of the transmission dip \(\left( {\Delta \lambda _{{{\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 2}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{$2$}}}} } \right)\). FOM is explained as the capability of a sensing device to identify a feeble change in the position of the resonance peak. SNR and FOM are computed with the help of references [24, 25]. Table 2 enumerates the numerically calculated values of SNR and FOM for numerous hemoglobin concentrations.
Finally, we analyzed the nature of variation of the transmission spectra with reference to the variation in the incident angle for the projected structure, which is represented in Fig. 6. We have simulated the structure for higher incident angles (\(\theta _{i} = 20^{ \circ } ,~40^{ \circ }\)) and compared the results with the outcomes of \(\theta _{i} = 0^{ \circ }\) (shown in Fig. 3b) with d = 850 nm. Here, it can be envisaged that with an increase in incident angle, resonant modes are blue shifted. The primary reason for this blue shift phenomenon can be described by the Bragg condition, which is expressed as [26, 27],
where \(\lambda _{{{\text{res}}}}\) is the resonant mode wavelength, m denotes the constructive diffraction order, \(\theta _{i}\) represents the incident angle, \(n_{{{\text{eff}}}}\) signifies effective RI of the dielectric layers, and N denotes the period of dielectric layers. So, with an increase in \(\theta _{i}\), the wavelength is blue shifted, to satisfy Bragg condition. Aside this, the aforesaid behavior is perceived in the researches mentioned in the literature.
4 Conclusion
A defect-based 1D PhC is reported in this article for effective sensing of various concentrations of hemoglobin in blood. TMM technique is employed for computation of transmission spectrum for the analysis of shift in the resonant wavelength formed within the PBG. Numerous structure parameters such as dielectric constant of different dielectric layers, thickness of the dielectric layers and defect medium, period of dielectric layers and incident angle play vital role in determination of position of resonant mode wavelengths. Sensing characteristics like sensitivity, SNR, FOM are thoroughly analyzed for different defect layer thicknesses and angles of incident. The obtained performance characteristics indicate that the suggested sensor can be the most apposite contender in biosensing research area.
References
Panda A, Sarkar P, Palai G (2018) Research on SAD-PRD losses in semiconductor waveguide for application in photonic integrated circuits. Optik 154:748–754
Panda A, Pukhrambam PD (2020) Photonic crystal biosensor for refractive index based cancerous cell detection. Opti Fib Technol 54:102123
Panda A, Pukhrambam PD, Keiser G (2020) Performance analysis of graphene-based surface plasmon resonance biosensor for blood glucose and gas detection. Appl Phys A 126(3):153
Aly AH, Ameen AA, Vigneswaran D (2019) Superconductor nanometallic photonic crystals as a novel smart window for low-temperature applications. J Supercond Nov Magn 32(2):191–197
Ramanujam NR et al (2019) Design of one dimensional defect based photonic crystal by composited superconducting material for bio sensing applications. Phys B 572:42
Omar A, El-Aziz A, Elsayed H, Sayed MI (2019) One-dimensional defective photonic crystals for the sensing and detection of protein. Appl Opt 58(30):8309–8315
Ghasemi F, Entezar SR, Razi S (2019) Terahertz tunable photonic crystal optical filter containing graphene and nonlinear electro-optic polymer. Laser Phys 29:056201
Hemmatyar O, Rahmani B, Bagheri A, Khavasi A (2017) Phase resonance tuning and multi-band absorption via graphene-covered compound metallic gratings. IEEEJ Quantum Electron 53:1–10
Panda A, Pukhrambam PD (2021) Investigation of defect based 1D photonic crystal structure for real-time detection of waterborne bacteria. Phys B: Condensed Matter. https://doi.org/10.1016/j.physb.2021.412854
Zhang Y, Han S, Zhang S, Liu P, Shi Y (2015) High-Q and high-sensitivity photonic crystal cavity sensor. IEEE Photonics J 7:1–6
Aly AH, Zaky ZA (2019) Ultra-sensitive photonic crystal cancer cells sensor with a high-quality factor. Cryogenics 104:102991
Frascella F, Ricciardi S, Rivolo P, Moi V, Giorgis F, Descrovi E, Michelotti F, Munzert P, Danz N, Napione L, Alvaro M, Bussolino F (2013) A fluorescent One-dimensional photonic crystal for label-free biosensing based on Bloch surface waves. Sensors 13:2011–2022
Chen T, Han Z, Liu J, Hong Z (2014) Terahertz gas sensing based on a simple one-dimensional photonic crystal cavity with highquality factors. Appl Opt 53:3454–3458
Aly AH et al (2020) Biophotonic sensor for the detection of creatinine concentration in blood serum based on 1D photonic crystal. RSC Adv 10:31765–31772
Yang X et al (2013) Simple paper-based test for measuring blood hemoglobin concentration in resource-limited settings. Clin Chem 59(10):1506–1513
Biswas U, Rakshit JK (2020) Detection and analysis of hemoglobin concentration in blood with the help of photonic crystal based micro ring resonator structure. Opt Quant Electron 52:449. https://doi.org/10.1007/s11082-020-02566-4
Natesan et al (2018) Enhanced sensitivity of hemoglobin sensor using dual-core photonic crystal fiber. Opt Quantum Electron 50(12)
Abadla MM, Elsayed HA (2020) Detection and sensing of hemoglobin using one-dimensional binary photonic crystals comprising a defect layer. Appl Opt 59(2):418–424
Lazareva EN, Tuchin VV (2018) Measurement of refractive index of hemoglobin in the visible/NIR spectral range. J Biomed Opt 23(3):035004
Liu J, Zhang L, Lei Xu (2018) Elastic, electronic structure, and optical properties of orthorhombic Na3AlF6: a first-principles study. Ionics 24:1377–1383
El-Naggar SA (2015) Tunable terahertz omnidirectional photonic gap in one dimensional graphene-based photonic crystals. Opt Quantum Electron 47:1627–1636
Aly AH, Sayed FA, Elsayed HA (2020) Defect mode tunability based on the electro-optical characteristics of the one-dimensional graphene photonic crystals. Appl Opt 59(16)
Panda A, Pukhrambam PD, Keiser G (2020) Realization of sucrose sensor using 1D photonic crystal structure vis-à-vis band gap analysis. Microsyst Technol. https://doi.org/10.1007/s00542-020-05005-2
Barkat O (2015) Theoretical investigation of transmission and dispersion properties of one dimensional photonic crystal. J Electr Electron Eng 3(2):12–18
Shaban M, Ahmed AM, Abdel-Rahman E, Hamdy H (2017) Tunability and sensing properties of plasmonic/1D photonic crystal. Sci Rep 7:419831
Wu F et al (2018) Redshift gaps in one-dimensional photonic crystals containing hyperbolicmetamaterials, Phys Rev Appl 10:064022
Aly AH et al (2020) Theoretical study of hybrid multifunctional one-dimensional photonic crystal as a flexible blood sugar sensor. Phys Scr 95:035510
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Panda, A., Pukhrambam, P.D. (2022). Performance Analysis of Defective 1D Photonic Crystal Structure for Detection of Hemoglobin Concentrations in Blood. In: Lenka, T.R., Misra, D., Biswas, A. (eds) Micro and Nanoelectronics Devices, Circuits and Systems. Lecture Notes in Electrical Engineering, vol 781. Springer, Singapore. https://doi.org/10.1007/978-981-16-3767-4_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-3767-4_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3766-7
Online ISBN: 978-981-16-3767-4
eBook Packages: EngineeringEngineering (R0)