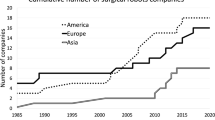
Abstract
This chapter focuses on emerging opportunities and related challenges associated with medical device development. In a rapidly changing healthcare environment and related support structure, it has become essential to develop products and technologies that are not only innovative and affordable, but at the same time, safe and reliable for long-term performance stability. With the advancement of efficient computational platforms and artificial intelligence, newer opportunities in the form of software-driven medical devices and robotic minimally invasive surgical systems have emerged as key thrust areas in medical device manufacturing sector. A lot of research outcomes are being reported worldwide for development of haptic feedback-based robotic manipulators for complex surgical procedures. Leading industry manufacturers are focusing on transdisciplinary synergetic research for faster product development and subsequent market deployment. However, such medical cyber-physical systems have also brought out the need for ensuring product safety and data security for uncompromised privacy and process integrity. There are other challenges related to validation, certification and regulations of healthcare-related mobile applications and software-driven diagnostic and therapeutic tools which need to be handled with highest priority for patient safety. The chapter focuses on the AI-powered and software-driven medical devices as well as various technologies of robotic surgery and the associated research opportunities and challenges in these domains.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The world and its biodiversity are changing at a faster pace due to unprecedented surge in industrialization, rapid economic development and the growing needs of the mankind to meet its unending desire. Though such activities are still considered as an advancement in the quality of life and betterment of mankind, it cannot be denied that all of this has negatively affected the global climate including water and air quality as well as the overall health of most of its inhabitants. With changing lifestyle and related behavioral pattern, the mankind is constantly threatened by newer diseases and pandemics. Though human race has managed to control and limit the spread of some of these diseases earlier like in the case of Flu, Polio, Leprosy, Measles, Mumps, Rubella and Guinea worm, etc., some of the newer ones like Ebola and SARS including the COVID-19 viruses has exposed the limitations of healthcare systems and challenges in dealing with such diseases. The situation becomes further challenging in developing countries and nations with low-resource settings. Consequently, development of medical devices is now required to be more affordable but with higher accuracy and precision. With some of the contagious diseases, it has also become essential to innovate medical devices and tools for remote diagnostics and minimally invasive surgical procedures.
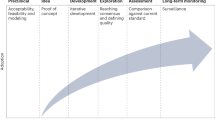
Medical devices play a significant role in the delivery of many health care services [1]. Broadly, medical devices are basically used for the diagnosis, cure, mitigation, treatment or prevention of disease and are not absorbed or metabolized by the body. Such devices range from common medical supplies such as latex gloves and syringes to advanced imaging equipment and implantable devices such as cardiac defibrillators. The development of new medical devices is essential as it can improve the ability to diagnose and treat illness [2, 3]. The future of medical device faces a new world that is full of opportunities, however, many uncertainties lie ahead as well with emerging of new standards and regulations, evolving of health care dynamics with increasingly competitive scenario. The emerging trends in medical device development are shown in Fig. 1. Globally, the manufacturing of medical devices is shifting from transaction-based approach to an approach that focuses on creating value for the patients and practitioners by providing the surgical instruments and the medical tools that are highly innovative and cost-effective.
Over the last few decades, General Surgery has evolved toward using Minimally Invasive Surgery (MIS) than the conventional larger open incisions for various medical procedures. It allows the surgeons to make few small incisions in the patient, rather than making one large incision for access. The incline toward minimally invasive or when possible, non-invasive procedures is greatly because of the patient benefits that come with it such as reduced post-operative pain and faster recovery. With the incisions and access ports becoming lesser and smaller, inclusion of robotics into surgical procedures was initiated. Thus, the development of a series of robotic manipulators to assist the surgeon with medical interventions started [4].
Robotic surgical systems have successfully provided several key advantages over standard minimal access surgery, there are a number of challenges that have prevented this technology from reaching its full potential. Foremost among these is the loss of force feedback (haptics) [5]. Another significant challenge of robotic technology is the extremely high (and recurring) cost of instruments and maintenance. Lastly, the robotic systems are large and bulky and have complex, time-consuming setup, which requires additional specialized training for the entire operating room team.
Artificial intelligence (AI) and machine learning coupled with advancements in electro-mechanical systems have provided a paradigm shift in medical device development through deployment of robotic tools, intelligent patient carts, remote diagnostics and consultation and other automated patient monitoring devices. This chapter mainly focuses on AI-powered and software-driven medical devices and associated research opportunities and challenges in those domains.
2 Opportunities in Medical Device Development
Over the last decade, the medical device manufacturing industry has shifted its focus on developing simple, intuitive and intelligent assistive and therapeutic devices with a faster deployment and commercialization modalities. As a result, software-driven medical devices have emerged as a major thrust area for delivering smart medical solutions for the masses. Starting from wearable health monitoring devices to more sophisticated imaging and surgical medical tools to contact tracing apps, the power of software-driven medical devices has started to touch every aspect of healthcare infrastructure and is expected to remain a key area for times to come [6, 7]. Similarly, surgical robots have recently gained much importance for offering more secure, flexible and efficient manipulators for various complex medical procedures and they provide accuracy levels which are mostly beyond human capability. A description of such fascinating trends in medical device development is presented in this section.
2.1 Software-Driven Medical Devices
Software is becoming increasingly essential as well as pervasive in today’s healthcare system. With increasing availability of technology platforms such as smartphones, tablets, laptops, and network servers and with increasing ease of access with the help of Internet and cloud, software tools customized for medical applications are widely used in today’s healthcare system. Such software-driven and easy to access medical devices primarily encourages healthy lifestyle and promotes digital healthcare system. Nowadays, most life-saving medical devices function using some kind of embedded software for processing of acquired data or for performing a certain assigned assistive task. Such devices include life sustaining implantable pacemakers to life support devices like infusion pumps, defibrillators and insulin pumps [8]. Many of these software tools interface with other hardware equipment or connect to hospital’s information system, thus demanding a higher computational efficiency which eventually pose development challenges for the designers and manufacturers.
As all these devices are directly related to a patient’s safely, developing regulatory framework for such software leading to safe commercialization is another opportunity for medico-legal professionals and certification agencies [9, 10]. In most of the countries, medical devices undergo rigorous scrutiny before they are lunched into the market. Such scrutiny is essential to have assurance and confidence that the product is safe and fit for its purpose [8]. To define the role and interaction of a software with hardware or in stand-alone mode, Software-Driven Medical Devices (SDMD) have been classified as “Software as a Medical devices” (SaMD) and “Software in Medical devices” (SiMD) depending on whether the software operates on stand-alone mode without needing any hardware or it is used in conjunction with a hardware to improve and support the hardware’s functionality respectively [11, 12]. Some examples of SaMDs are the mobile applications installed onto a smartphone such as smartphone-enabled arrhythmia detector, calorie counter, and drug dose reminder as well as all the mobile-enabled fitness trackers also fall under the category of SaMD as the functionality and features of these software codes are not directly related to the operation of the parent hardware system like the smartphone in this case. Similarly, all the computer and mobile applications that maintain and transfer electronic patient care data to the physician come under SaMD category. This includes sending scheduled electronic patient charts to a designated doctor to relaying results of CT scans of the patient from the equipment to the monitor of an observing physician. On the other hand, example of SiMD includes that of a software which controls the motors of an infusion pump or which encrypts the radiation data to digital image form for a CT scanner [13].
2.2 Surgical Robots and Minimally Invasive Surgery (MIS)
An operation technique, Minimally Invasive Surgery (MIS), was established in the 1980s. The surgeon works with long instruments through small incisions without any direct access to the operation field, thereby differentiating it from the open surgery. Usually, four small incisions are made: two for the surgical instrument, one for laparoscope (rigid endoscope) and one for insufflating CO2.
Laparoscopic Surgery
During the late 1980s, breakthroughs in technology brought about an increased development in MIS. This resulted in a shift from traditional open surgeries to laparoscopic procedures. As instrumentation improved, the number of different laparoscopic procedures expanded. Nearly every general procedure previously performed by traditional methods has been performed using laparoscopic techniques. Although more difficult than open surgery, laparoscopic surgery has demonstrated multiple patient benefits. The opportunity in this area still lies in further reducing the invasiveness of surgical procedure by limiting the number and size incisions [14, 15].
Minimally Invasive Robotic Surgery (MIRS)
To overcome the disadvantages of the manual MIS, MIRS plays an important role. MIRS systems help the surgeon to overcome barriers such as the patient’s chest or abdomen, which separate him from the operating area. The distance between the surgeon and patient is overcome even if they are located in different rooms or hospitals. MIRS system is divided into three parts according to physical components of a system: Slave, Master and communication between slave and master. Slave system consists of several sub-systems. The minimally invasive instruments should be very small having diameter less than 10 mm to reduce pain and trauma. To have full manipulability inside the body the instruments should have addition 2° of Freedom (DoF) [5]. The tactile information along with the force should be measurable thereby providing information to the surgeon in order to increase the quality of immersion and for more intelligent control laws for surgical robots. The instruments should be lightweight so that they can be handled by single person in the emergency situation when a person needs to be operated without access to the robotic instruments. The master system has to provide high quality feedback, both in terms of tactile and kinesthetic. The tactile would help the surgeon to find the invisible structures such as blood vessels under the muscles by feeling the palpations whereas the kinesthetic lets the surgeon have a direct access to the forces at the operating area thereby increasing the quality of the operation [16]. The other additional factors that are important include the scaling of the surgeon’s and filtering the surgeon’s tremor to increase the safety and accuracy of the MIRS system.
The communication between the master and the slave should be flexible so that different master stations can be connected so that surgeons can get help from expertise or the training of the surgeons can be enhanced for better usage of the system. So, the communication system should be safe and secure. The communication system should not get affected from the underlying networks or other radiations produced within the premises, thereby acquiring a Quality of Service. Thus, these efforts would make the MIS surgery safer and faster, thereby reducing the cost and post-operative complications for the patients. The tasks such as automatic camera guiding [18, 19], holding needles, positioning of instruments, grasping of tissues, automatic cutting and suturing should be handled by the robot autonomously. To realize the autonomous functions special attention should be made to the organs that are in motion induced by the patient’s respiration and heartbeat. These motions are to be detected and compensated accordingly. With the development of surgical robots like “da Vinci” as shown in Fig. 2, many surgical procedures are being conducted remotely through tele-robotics [17, 20].
Da Vinci Robotic surgical system (from Intuitive Surgical, Inc.): It is designed to facilitate surgery using a minimally invasive approach, controlled by a surgeon from a console [17]
Robotic Cardiac Catheterization
As the human resource in the field of medicine is scared, their welfare is one of the major priorities of healthcare industries. The device manufacturers are striving hard to offer cutting-edge advanced products with more safety and regulatory compliance. For example, the X-ray machines which were developed few decades back are now considered hazardous in view of stringent radiation leakage norms and larger procedural time. Many other medical procedures like surgeries, transplants have also been made safer with the availability of better sterilization methods and higher precision equipment.
But, with such advancements in the field of robotic surgery, there are some procedures which are still being performed manually in hazardous situations and one of them is cardiac catheterization. Cardiac catheterization is a process where a small tubular structure called “catheter” is inserted into a major blood vessel through the groin area and is threaded through the vascular system to reach the human heart. This procedure is used for several diagnostic and treatment purposes such as angiography, angioplasty, valvuloplasty, and arterial fibrillation. The procedure is very effective as it is minimally invasive, safer and with very low recovery time. The only downside of this procedure is that it has to be performed under a fluoroscope. Fluoroscopes is an x-ray imaging device which provides real-time continuous x-ray imaging by which the physician tracks the movement of the catheter and adjust its direction so that it reaches its desired destination. The radiation emitted by the fluoroscope is very harmful for the human body if absorbed in large quantities [21,22,23]. Though for the patients, the radiation dosage is regulated, a physician performing 5–6 procedures in a day is highly vulnerable to radiation side effects. To prevent the radiation absorption, the medical staff uses lead aprons and similar headwear which absorbs most of the leaked radiation and protects the person. But many studies aimed at measuring the level of such protection found that the lead apron and accessory gear is not fully efficient and only protects the torso and forehead leaving eye, throat and palms still vulnerable [24]. To mitigate the radiation side effects, many researchers worldwide have reported development of robotic catheterization systems [25,26,27,28]. Such systems enable the physician to perform the cardiac catheterization procedure away and protected from the hazardous radiation zone. Several multinational companies like Corindus Vascular Robotics Inc. and Hansen medical Inc. have developed commercially available robotic catheterization systems.
However, these robotic devices are designed for a specific procedure only. For example, CorPath GRX of Corindus Inc. (shown in Fig. 3) can only perform angioplasties and angiography primarily by manipulating a hard metal guide wire. Similarly, Sensei X and Magellan of Hansen Medicals can only be used for atrial fibrillation procedure. One of the limitations of the existing systems is the limiting length in linear travel, i.e., the catheter can be moved up to and in between a fixed distance only.
CorPath GRX by Corindus Vascular Robotics Inc. [29]
There is another important component of this robotic cardiac catheterization system that helps in rotating the catheter around its own axis. This turning of the catheter has to be very precise as it determines in which vessel the catheter will enter into. If the movement is not precise, either the catheter will enter into a different artery which can lead to non-desired locations or due to the rotating motion, the catheter can scar or rupture the interiors of the blood vessels which can be life threatening for the patient under procedure. This situation can be resolved by implementing a driving mechanism instead of the conventional geared mechanism as it helps improving the accuracy in rotation of the catheter. Also, there is one more prospect of research which is still relatively untouched as compared to other aspects of such systems, i.e., the catheter tactile sensor. Conventionally, during manual cardiac catheterization procedures, physicians used to rely on the sensation of touch to determine whether the tip of the catheter is hitting any arterial wall or is facing some resistance in travel path. After the movement of the catheter is mechanized, there should be some way to replicate the human sensation judgment during the procedure. Development of this kind of a sensor is a major task in itself because of its size, accuracy and reusability. Development of such haptic feedback system-based robotic systems has already opened up new avenues of robotic research in this field. There is also a need to develop robotic devices that can perform other common procedures like valvuloplasty which uses a soft off-the-shelf catheter.
Haptics in Robotic Systems
Haptic refers to the sense of touch. Ancient psychologists referred haptesthai (Greek origin of word haptic) to tactile sensations. However contemporary psychology treats somatic senses to be working synergistically. Haptic could therefore be effectively referred to proprioception, kinesthesia and the vestibular sense as somatic senses of touch. “Haptic” has been deployed in various contexts (art history, aesthetics and architecture) and most frequently in the psychology of perception and technologies of touch engineering. Various aspects of embodied tactile namely scientific, psychological and engineering aspects find use from the term haptic. However, implications arise while handling spatial access and mobility problems of people with sensory or motor impairments, especially the blind and visually impaired.
Our skin has touch receptors which transfers the sensory information through neuronal firings to brain and help the human being in responding to the information accordingly. This haptic technology is also a mimicking of similar process so as to transfer the information of touch to the central processor. The device mainly measures the stress occurring during any kind of linear or non-linear motion giving signals corresponding to the surface profile on which it is moved. This kind of technology finds its direct application in robotic surgery and several applications which require monitoring the direct interaction of robotic arm/instrument on human subject. The haptic technology consists of device configuration to receive sensory signal corresponding to the sense of touch, and vary with the level of roughness on a surface. It mainly consists of a miniaturized accelerometer and a force sensor, embedded in an elastomeric sheet mimicking the human skin.
Human–machine interaction (HMI) has become an integral component of artificial intelligence and computer vision, required for automation in several applied sectors. However, HMI is not an easy task as still many limitations exist in the behavior of humanoid robots. Mimicking human being completely has always been the target of several researchers, but is a difficult task to make it really possible. Machine vision-based approaches to interact with real world are limited by environment condition, calibration procedures and several other man-made factors. In order to overcome these problems, the sense of touch has been incorporated along with the sense of sight to add on to the amount of information available to a machine for decision-making process. It can be lucidly illustrated through a situation, when a person touches an object to obtain more accurate idea of the shape and texture of the object, when visual clues do not provide enough information, like in dark environments or when object of interest is occluded. Added to it, few researchers have also looked into the aspects of tactile sensor alignment with respect to measured surface and error induced into the system due to the deformation in tactile array [30]. It has been also speculated that a special device if added in between the robot end effector and the tactile sensor to compensate for misalignments would ensure uniform force distribution on the tactile probe. Use of haptic technology to detect geometrical profiles has been used extensively and aids in the task of object recognition, and thus helping in automation-based system [31].
Applications of Haptic Feedback Systems
As haptic systems aim to reproduce tactile sensations and engage the user with “force feedback”, they have found applications in a wide range of areas ranging from the coarse rumble and vibrations in gadgets and domestic technologies such as mobile phones, intuitive robotic surgery and videogame controllers, to the more refined and specialized design and engineering interfaces such as the PHANToM (Personal Haptic iNTerface Mechanism), which more accurately models the surfaces and textures of materials used in computer-aided design and manufacture (CAD/CAM) as shown in Fig. 4 [32].
PHANTOM, commercially available Haptic device, which accurately models the surface and textures of materials used in CAD/CAM [32]
Furthermore, included within an increasing array of consumer technologies, haptics is reaching near ubiquity by being included in everything from vibrating mobile phones, rumbling controllers for videogame consoles, the distribution of touchscreens throughout our urban environments. On the other hand, more specialized haptic technologies are refining the human–computer interface and changing the way that virtual sculpting and virtual prototyping is achieved simply by offering more intuitive, tactile engagement with the computer. Haptic devices are widely used in different applications such as gaming and multimedia.
As mentioned earlier, a lot of activities are being reported worldwide with respect to integration of haptic feedback with robotic systems for better human–machine interaction and ease of remote surgical procedures [33,34,35].
3 Major Challenges in Medical Device Development
3.1 Data Security and Product Safety
With abundant use of software-driven medical devices mow-a-days and over-dependency on such tools at crucial decision-making points, it becomes highly essential to ensure adequate product safety and security, more so when a human life is at stake. Therefore, there are certain regulatory issues specific to SDMDs such as dynamic software development processes, product safety and security, data collection and privacy and payment-related issues. The dynamic software development process mainly deals with the design, development and application of the software. A software is always developed in an evolving environment as it is regularly edited, modified, maintained and updated with deployment of improved versions over a period of time. In such a scenario, there must not be any product rollout or version upgrade at the expense of planning and documentation, both of which are necessary and critical for all the medical devices. Apart of development, distribution of such software is also a concern for regulation [36]. As conventional medical devices are operationalized by formal healthcare system, this software tools can be accessed personally outside the traditional medical supply chain. For example, for a patient, who cannot access traditional medical equipment approved only for usage within a specific geographical area, he/she can easily download and use the tool if it is only based on a software bypassing local regulations and approvals.
The product safety and security also include software vulnerability and bugs that also need to be carefully addressed [37]. As regular updates will be rolled out periodically for a software, there is always a possibility of introduction of a bug or defect with a recent update which can hamper the smooth running of existing medical device. There are also cybersecurity risks associated with the functioning of the SDMDs with extending network capabilities of such software for remote monitoring and control of devices. This also exposes the vulnerability of the device to a cyber-attack to hinder the working of such systems. There is also an issue of maintaining, managing and supporting SDMDs beyond the lifespan of the manufacturer. For example, if a company manufacturing pacemakers and control software application shuts down after a while, then the patients implanted with those specific pacemakers would be adversely affected due to lack of software updates or maintenance of existing software. As such concerns are directly related to a human life, they have to be properly regulated in medical device manufacturing sector [8, 38, 39].
3.2 Process and Product Validation
In a bid to outpace competitors, medical device manufacturers often rush their products into commercialization without much background validation of the process or/and the product. US Food and Drug Administration (FDA) has issued many warnings in the past to such manufacturers for inadequate process validation. According to FDA, process validation is defined as “the collection and evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality product” [40]. Validation refers to the end-product or process and how effectively it does its intended application. Validating a certain process almost always requires a dedicated team of R&D, process and quality assurance engineers along with a target user group. Small and medium scale industries often find it economically untenable to carry out such activities before launching a product. In case of computer-assisted devices, the challenge is to identify the output system parameters to validate the efficacy of a certain procedure with respect to unassisted human procedures. In complex assistive surgical devices having many submodules, several variability like patient anatomy, different data acquisition modalities, differing reference coordinates and subjective surgical approaches and procedures to do a certain task, makes it difficult to evolve a validation protocol to assess the quantitative improvements of maneuverability of the assistive device [41, 42]. As the field of surgical robots and software-driven medical devices are evolving very rapidly, there are also challenges for the regulatory authorities to reach a consensus with the industry regarding the method of validation and the specific parameters to be looked into.
With respect to medical cyber-physical (MCP) systems that are networked and intelligent systems to provide continuous care solutions with remote assistance, the challenges of system validation become further challenging due to data security and privacy issues. MCPs usually have a host of patient data acquisition tools connected to an intelligent sever that continuously monitors the acquired data and issues warnings to the healthcare professionals if any of the monitored data exceeds some preset threshold [43]. Some of the major challenges associated with validation of MCPs are as below [43,44,45,46,47]:
-
Compliance with the EU/US and other local regulatory requirements—Development of a safe and reliable cyber-physical system requires compliance to norms of local regulatory authorities such as US Food and Drug Administration (FDA), European Medicines Agency of the European Union, Health Canada, and The Central Drugs Standard Control Organisation (CDSCO) of India depending on the geographical market of the product or process. Inadequacies and failure to meet the regulatory requirements can attract legal actions leading to suspension or complete ban on product marketing. Actions such as lack of documentation for a software version upgrade, failure to perform monitored pre-clinical and human trials or inadequate process validation methodologies can amount to regulatory violation and can subsequently lead to blacklisting by concerned agencies.
-
Maintaining traceability records—As per USFDA regulations for software validation, it is mandatory that the software codes and any subsequent version changes are required to be mapped to requirement specifications and test cases at each development stages [48]. But, many a time, changes are incorporated in the medical products and associated software codes without proper justification and traceability to the change in requirement specifications. This leads to poor software integrity and other issues related to testing and fixing of probable software bugs.
-
Identifying and recognizing cases where product cannot be validated—There can be disagreements between a certifying agency and the manufacturer regarding the scope and extent of product validation with respect to certain market segments. For example, in additive manufacturing, 3D printing, welding and sterilization of components, it may not be feasible and appropriate to inspect the end-product and it may require the regulators to look into the process itself to validate the outcomes.
-
Verifying and certifying mobile apps for user safety—Of late, smartphone bases applications for monitoring health parameters have emerged as a popular tool among the masses for tracking their daily activities. As per a guidance document released by USFDA in 2011, such apps are defined as software tools that is either used as an accessory to already regulated medical device or transforms a mobile platform into a regulated medical device [44, 49]. To assure consumers of the clinical validity as well as practical utility of medical mobile apps, both the software developers as well as the regulators need to evolve a comprehensive framework for assessing the performance characteristics of such applications. While hardware-based products are relatively easier to assess and validity owing to ease of observability, regulating and validating medical apps for treating and diagnosing diseases becomes challenging.
4 Conclusion
With the rise in infectious diseases and the evolution of chronic comorbidities, the world is constantly looking upon the medical industries with high expectations for affordable and intelligent healthcare solutions. This has given rise to many game-changing medical innovations such as robotics, wearable health monitors, networked medical systems for telemedicine, and remote consultations. The preference and acceptability of minimally invasive or when possible, non-invasive procedures are also increasing rapidly because of the patient benefits and ease of surgical procedures. Thus, the opportunities related to development of a series of robotic manipulators to assist the surgeons during medical interventions are also opening up newer avenues for biomedical researchers and manufacturers. There has been a renewed focus on preventive medication including vaccine research as well as e-healthcare and community care systems. At the same time, stricter regulatory compliance regime has also posed challenges for the manufacturers to get approval and necessary clearances for a product/service launch. The situation becomes trickier when a product is envisaged to launch in multiple global markets as the regulatory framework in each of those constituent countries can be significantly different. Though all such regulations are primarily aimed at ensuring safety and enhanced utility of the products, these can be resource-exhaustive for small industries as the number and type of qualification tests can differ from one country to the other. Considering all such trade-offs between faster product development and product security, it is high time all the stakeholders put their best effort in evolving a dedicated product development methodology for medical devices. Such a methodology should also include post-development market analysis and incorporation of user feedback for enhancing the product efficacy. There is also a need to relook at the global risk management and quality control practices so that they are in all sync with the larger idea of better quality of life with adequate safety measures without unnecessarily delaying a product launch. As former American politician, educator and author Shirley Anita Chisholm, once famously said, “Health is a human right, not a privilege to be purchased”, the medical industries, researchers, regulators and even consumers need to rise to the occasion to cooperate, codevelop, collaborate and formulate a way forward for a better tomorrow.
References
Blumenthal, D., Tavenner, M.: The “meaningful use” regulation for electronic health records. N. Engl. J. Med. 363(6), 501–504 (2010)
Alexandru, A., et al.: Healthcare, big data and cloud computing. Management 1, 2 (2016)
Raghupathi, W., Raghupathi, V.: Big data analytics in healthcare: promise and potential. Health Inf. Sci. Syst. 2(1), 1–10 (2014)
Wall, J., Chandra, V., Krummel, T.: Robotics in general surgery. In: Medical Robotics. IntechOpen (2008)
Ghodoussi, M., Butner, S.E., Wang, Y.: Robotic surgery-the transatlantic case. In: Proceedings 2002 IEEE International Conference on Robotics and Automation (Cat. No. 02CH37292). IEEE (2002)
Gordon, W.J., Stern, A.D.: Challenges and opportunities in software-driven medical devices. Nat. Biomed. Eng. 3(7), 493–497 (2019)
Wang, S., Ding, S., Xiong, L.: A new system for surveillance and digital contact tracing for COVID-19: spatiotemporal reporting over network and GPS. JMIR mHealth uHealth 8(6), e19457 (2020)
Fu, K.: Trustworthy medical device software. Public Health Effectiveness FDA 510, 102 (2011)
Burleson, W., et al.: Design challenges for secure implantable medical devices. In: DAC Design Automation Conference 2012. IEEE
Clark, S.S., Fu, K.: Recent results in computer security for medical devices. In: International Conference on Wireless Mobile Communication and Healthcare. Springer, Berlin (2011)
Group, I.S.W.: Software as a Medical Device (SaMD): key definitions. 9, 9. Published online December (2013)
Digital Health Criteria (FDA, 2018): (2018). Available from: https://www.fda.gov/medical-devices/digital-health/digital-health-criteria
Developing Software Precertification Program: A Working Model US Department of Health and Human Services Food and Drug Administrartion (2018)
Horgan, S., Vanuno, D.: Robots in laparoscopic surgery. J. Laparoendosc. Adv. Surg. Tech. 11(6), 415–419 (2001)
Bass, E.B., Pitt, H.A., Lillemoe, K.D.: Cost-effectiveness of laparoscopic cholecystectomy versus open cholecystectomy. Am. J. Surg. 165(4), 466–471 (1993)
Wagner, C.R., Howe, R.D., Stylopoulos, N.: The role of force feedback in surgery: analysis of blunt dissection. In: International Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems. Citeseer (2002)
Lobontiu, A.: The da Vinci surgical system performing computer-enhanced surgery. Osp Ital Chir 7, 367–721 (2001)
Wei, G.-Q., Arbter, K., Hirzinger, G.: Automatic tracking of laparoscopic instruments by color coding. In: CVRMed-MRCAS’97. Springer, Berlin (1997)
Arbter, K., Wei, G.-Q.: Method of tracking a surgical instrument with a mono or stereo laparoscope. Google Patents (1998)
Wilson, E.: The evolution of robotic general surgery. Scand. J. Surg. 98(2), 125–129 (2009)
Kim, K.P., Miller, D.L.: Minimising radiation exposure to physicians performing fluoroscopically guided cardiac catheterisation procedures: a review. Radiat. Prot. Dosimetry. 133(4), 227–233 (2009)
Sandblom, V., et al.: Evaluation of the impact of a system for real-time visualisation of occupational radiation dose rate during fluoroscopically guided procedures. J. Radiol. Prot. 33(3), 693 (2013)
Kesavachandran, C.N., Haamann, F., Nienhaus, A.: Radiation exposure and adverse health effects of interventional cardiology staff. In: Reviews of Environmental Contamination and Toxicology, pp. 73–91. Springer (2013)
Goni, H., et al.: Investigation of occupational radiation exposure during interventional cardiac catheterisations performed via radial artery. Radiat. Prot. Dosimetry. 117(1–3), 107–110 (2005)
Moll, F.H., et al.: Methods using a robotic catheter system. Google Patents (2010)
Guo, J., Guo, S., Yu, Y.: Design and characteristics evaluation of a novel teleoperated robotic catheterization system with force feedback for vascular interventional surgery. Biomed. Microdevice 18(5), 76 (2016)
Moll, F.H., et al.: Instrument driver for robotic catheter system. Google Patents (2011)
Guo, S., et al.: A novel robot-assisted endovascular catheterization system with haptic force feedback. IEEE Trans. Rob. 35(3), 685–696 (2019)
Rafii-Tari, H., Payne, C.J., Yang, G.-Z.: Current and emerging robot-assisted endovascular catheterization technologies: a review. Ann. Biomed. Eng. 42(4), 697–715 (2014)
Pasca, C., et al.: Intelligent haptic sensor system for robotic manipulation. In: Proceedings of the 21st IEEE on Instrumentation and Measurement Technology Conference. IMTC 04 (2004)
Kirkpatrick, A.E., Douglas, S.A.: Application-based evaluation of haptic interfaces. In: Proceedings of 10th Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems, 2002. HAPTICS 2002. IEEE (2002)
Massie, T.H., Salisbury, J.K.: The phantom haptic interface: A device for probing virtual objects. In: Proceedings of the ASME Winter Annual Meeting, Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems. Kluwer (1994)
Bethea, B.T., et al.: Application of haptic feedback to robotic surgery. J. Laparoendosc. Adv. Surg. Tech. 14(3), 191–195 (2004)
Tiwana, M.I., Redmond, S.J., Lovell, N.H.: A review of tactile sensing technologies with applications in biomedical engineering. Sens. Actuators A 179, 17–31 (2012)
Girão, P.S., et al.: Tactile sensors for robotic applications. Measurement 46(3), 1257–1271 (2013)
Hanna, S., et al.: Take two software updates and see me in the morning: the case for software security evaluations of medical devices. In: HealthSec (2011)
Rakitin, S.R.: Networked medical devices: essential collaboration for improved safety. Biomed. Instrum. Technol. 43(4), 332–338 (2009)
Alsubaei, F., Abuhussein, A., Shiva, S.: Security and privacy in the internet of medical things: taxonomy and risk assessment. In: 2017 IEEE 42nd Conference on Local Computer Networks Workshops (LCN Workshops). IEEE (2017)
Lee, I., et al.: High-confidence medical device software and systems. Computer 39(4), 33–38 (2006)
Guidance for Industry, Process Validation: General Principles and Practices (US Food Drug Administration). Center for Biologics Evaluation and Research. US Department of Health and Human Services, Rockville, MD (2011)
Janda, M., Buch, B.: The challenges of clinical validation of emerging technologies: computer-assisted devices for surgery. JBJS 91(Supplement_1), 17–21 (2009)
Haidegger, T.: Surgical robots: system development, assessment, and clearance. In: Prototyping of Robotic Systems: Applications of Design and Implementation, pp. 288–326. IGI Global (2012)
Lee, I., et al.: Challenges and research directions in medical cyber–physical systems. Proc. IEEE 100(1), 75–90 (2011)
Hrgarek, N.: Certification and regulatory challenges in medical device software development. In: 2012 4th International Workshop on Software Engineering in Health Care (SEHC). IEEE (2012)
Vogel, D.A.: Medical device software verification, validation and compliance. Artech House (2011)
Gatouillat, A., et al.: Internet of medical things: a review of recent contributions dealing with cyber-physical systems in medicine. IEEE Internet Things J. 5(5), 3810–3822 (2018)
McHugh, M., McCaffery, F., Casey, V.: Barriers to adopting agile practices when developing medical device software. In: International Conference on Software Process Improvement and Capability Determination. Springer, Berlin (2012)
General Principles of Software Validation. Final Guidance for Industry and FDA Staff, USFDA, pp. 33–34 (2002)
Shuren, J., Patel, B., Gottlieb, S.: FDA regulation of mobile medical apps. JAMA 320(4), 337–338 (2018)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Khatri, N., Kumar, M., Jha, R. (2022). Opportunities and Challenges in Medical Robotic Device Development. In: Joshi, S.N., Chandra, P. (eds) Advanced Micro- and Nano-manufacturing Technologies. Materials Horizons: From Nature to Nanomaterials. Springer, Singapore. https://doi.org/10.1007/978-981-16-3645-5_13
Download citation
DOI: https://doi.org/10.1007/978-981-16-3645-5_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3644-8
Online ISBN: 978-981-16-3645-5
eBook Packages: EngineeringEngineering (R0)