Abstract
The use of plastic materials is increasing worldwide because of their durability, cost-effectiveness, and light weight. This uncontrolled usage of plastic materials is creating severe disposal and environmental problems. This chapter includes recent and conventional practices for the recovery and recycling of plastic wastes. Brief classifications on the origin of plastic waste, such as thermoplastic, thermosetting, and elastomers, are specified. The sorting of plastic wastes plays a significant role in the overall performance of any recycling method. Various direct and indirect sorting methods are discussed along with their features and limitations. Recycling methods of plastic wastes are mainly classified as primary, secondary, tertiary, and quaternary. Detailed literature analysis including the advantages and technical challenges of each recycling method is discussed. A comprehensive investigation of the literature showed that new and innovative recycling approaches for plastic waste, particularly the scientific and technical inventions in tertiary recycling, present problems that impact their practicality in the industrial and manufacturing sectors. Currently, the recycling of highly contaminated plastic wastes from medical and e-waste are creating new challenges for the recovery and recycling sectors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Plastic waste recycling
- Sorting technologies
- Near-infrared hyperspectral imaging (NIR-HSI)
- Laser-induced breakdown spectroscopy
- Technical challenges
1 Introduction
The plastic industry is one of the developed industries derived from non-renewable fossil fuels. In the past few decades, progress in this industry has reached a remarkable level. Currently, plastic material is a better option than wood, glass, or metal because of its light weight, durability, and cost. Plastics are used in many applications such as household appliances, disposable medical equipment, automotive parts, packaging, construction, bags, containers, mulches, coatings, electronics, greenhouses, and aerospace components. Therefore, the presence of a large amount of plastics in municipal solid waste (MSW) is obvious.
Plastic waste production and disposal have a great impact on the MSW management by incineration and landfilling. Plastic incineration causes the emission of gases and the generation of toxic residues (mainly containing lead and cadmium) (Curlee and Das 1991). Plastic waste mainly goes into landfills and is released into the environment. These wastes, especially plastic packaging, end up in rivers and oceans and are a major threat to aquatic environments. The literature studies suggest that more than 88 kilotons of plastic wastes end up in seawaters yearly (Jambeck et al. 2015). Due to the ongoing global health crisis because of COVID-19, the global plastic packaging market size is projected to reach USD 1 trillion with a CAGR of more than 5.5%. This will ultimately put extra pressure on regular waste management practices. Between 1950 and 2015, 6300 million tons of plastic waste was generated and among these, 12% was incinerated, 9% was recycled, and 80% ended up in landfills or in the open environment.
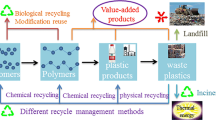
The best way to control the plastic waste management problem is to recycle wastes and produce either recycled materials or useful materials of high value. The major advantages of plastic waste recycling are:
-
1.
Plastic waste recycling leads to a reduction in land and marine pollution; it also significantly reduces CO2 emissions in the environment.
-
2.
Plastic waste recycling makes countries more resource independent and decreases their dependency on non-renewable sources such as oils and natural gas.
-
3.
Developing recycling technologies will create more jobs, which will foster local growth.
-
4.
By setting up systems for plastic waste recycling, local industries recover and value-added products emerge from the recycled materials.
Certainly not all plastic types can be recycled. Polypropylene (PP), high-density polyethylene (HDPE), and polyethylene terephthalate (PET) can be recycled easily; however, the recycling rate of low-density polyethylene (LDPE) is lower due to difficulties in sorting plastics in mixed waste streams. The remaining plastics such as polystyrene (PS) and polyvinyl chloride (PVC) are almost never recycled. The recycling of household plastic wastes and mixed waste streams is particularly difficult as these are highly contaminated. Thus, technologies are needed to sort plastics. Manual sorting technologies are challenging, inefficient, and time-consuming. Therefore, automatic sorting technologies using advanced techniques are required (Becker et al. 2017; Serranti et al. 2011). Innovative sorting technologies using robots help to sort materials with higher yields and efficiency. The artificial intelligence used by sorting robots showed an improved ability to recognize plastic wastes. Also, consumers need to take responsibility to sort their waste properly. The sorting guidelines in place directly impact the quality of streams for recycling. Public authorities need to make guidelines for the sorting of household wastes.
Plastic recycling methods are classified into four parts, from primary to quaternary recycling (Hopewell et al. 2009). The primary or closed-loop recycling method includes the processes in which products like the original plastic are produced. This method can process post-consumer plastics of known origin. Secondary or mechanical recycling can treat plastic materials to produce products that can be used in different forms. The polymers are sorted, ground, washed, and extruded by mechanical means in both primary and secondary recycling methods. These processing techniques lead to varying degrees of polymer degradation which limits their applications for plastic waste recycling at a large scale. Tertiary or chemical recycling uses hydrolysis, solvolysis, thermal and catalytic cracking, gasification, or plasma pyrolysis processes to recover the hydrocarbons in the form of fuels or chemicals, depending on the process configuration. Although this recycling technique produces high-value products, still, it is not implemented on an industrial scale very widely. This process requires high-energy inputs. However, if chemical-conversion processes can produce pure monomers, then they can truly aid in reducing dependence on non-renewable resources. Quaternary recycling includes incineration of the plastic polymers for energy recovery. The energy is recovered in the form of heat, and this process releases hazardous gases in the environment and produces large amount of toxic residues.
The overall efficiency of the plastic waste recycling methods depends on the sorting techniques involved. In fact, different plastics possess different physiochemical properties; therefore, different recycling methods are used. For example, plastics from mixed waste streams needs to be sorted first and contaminants need to be removed because these contaminants lead to the production of unwanted depolymerization reactions. This reaction will ultimately decrease the efficiency of plastic recycling methods. Therefore, fruitful and focused research work is needed not only to reduce and recycle plastic waste but also to find ways to separate it from the rest of the solid wastes. This will be the best way to minimize the technical, environmental, economic, and social impacts associated with plastic waste recycling.
This chapter focuses on different sorting technologies and recycling methods for treating plastic wastes. In addition, technical challenges associated with these techniques are discussed along with future directions.
2 Types of Plastic Wastes
There are several types of developed and commercially available polymers for global applications. Polymer materials are typically catalogued into three types: thermoplastics (also known as “plastics”), thermosets, and elastomers. Table 1 summarizes the types of plastics and their applications. In the thermoplastics type, cross-linking between the polymer chains is not present. Thermoplastics melt upon heating and reversibly become hard when cooling. Polyethylene (PE), PP, PS, and PVC are commonly used thermoplastics that can be recycled. Thermosets are a class of polymers that become hard and infusible upon heating. This type is composed of semi-fluid, small molecular mass units in which a three-dimensional (3D) network of bonds are formed by cross-linking between the polymeric chains. Polyurethanes (PU) and epoxy resins are mainly included in thermosets, and these cannot be recycled. Elastomers are amorphous polymers that have high-degree elasticity. Vulcanization in the elastomers is responsible for the high cross-linked molecular structure. For the recycling process, the capex required for the prior devulcanization process is high; therefore, elastomers have comparatively low recycling rates. The most common elastomers are natural rubber, isoprene rubber, crum rubber, and ethylene propylene rubber. The recycling of elastomers is a difficult process because of cross linking. Therefore, to overcome this problem, thermoplastic elastomers (TPEs) were developed. TPEs have good recyclability and re-moldability properties along with their elasticity and mechanical strength (Naskar and Babu 2014).
Plastic wastes are also classified as municipal and industrial wastes based on their origin. Among these, a major portion of plastic wastes is contributed by municipal wastes. Sources of municipal plastic wastes arise from household items such as foodstuff dishes, one time-use cups, packaging items, compact discs (CD), cassette boxes, fridge liners, cutlery, peddling cups, drainage pipes, sanitation pipes, electronic equipment cases, cushioning fluffs, thermal filling foams, etc.; wires and cable; automobile wrecking; agricultural wastes (fertilizer bags, films, feed bags, and covers for hays, silage, etc.). Therefore, municipal wastes are a mixture of PE, PP, PS, PVC, PET, etc., meaning that this waste has all kinds of polymers, recyclable and non-recyclable. To recycle municipal wastes, sorting and separation of plastics from extra domestic wastes is a necessary step. The most commonly used technique is mechanical separation. For example, in the wet separation technique, mixed plastics can be separated based on their density. Plastics such as PS and PVC have a density greater than water, while PE and PP which have a density inferior to water can be separated. Waste separation at the domestic site is better, and wastes can be segregated into three parts: (i) combustible materials like cloths, newspaper, pantry waste, and timber; (ii) incombustibles such as glass, metallics, and porcelains; and (iii) plastics. In addition, a large amount of plastic waste is also generated as a by-product in industries and farming activities. Industrial plastic wastes (IPWs) are sometimes also called primary wastes. These mainly consist of plastics from demolition and construction companies, electrical and electronics industries, and automotive industry spare parts. IPWs are satisfactorily cleaned and free from impurity and are accessible in large amounts. Municipal and industrial wastes are heterogeneous and homogeneous in nature, respectively. Remolding and repalletization were found to be effective and simple for homogenous plastic waste recycling. However, for heterogeneous plastic wastes, chemical recycling processes are required.
3 Sorting of Plastic Wastes
Plastic recycling is more difficult because of the presence of a mixture of polymers in the waste streams. Plastic materials lose their characteristics because of contaminants present in the waste streams and the aging process in each period of their life series (Ragaert et al. 2017). Therefore, the major obstacle to plastic waste recycling is the availability of capable and precise waste sorting technologies. The sorting plays an important role in recycling and recovery of plastics. Waste sorting technologies are required to:
-
1.
Detect contaminants present in plastics and their technical problems during reprocessing.
-
2.
Improve the efficiency of the separation processes.
-
3.
Recognize the different polymer mixtures to select an appropriate recycling process.
-
4.
Select the additives to reprocess plastics, based on their additive composition.
Manual sorting is the most widely used method, which is effective depending on the identification of plastic constituents. However, it is a challenging, tedious, and time-consuming process. In the following sections, the different methods used to sort plastic waste will be discussed more elaborately.
`
3.1 Spectroscopic Methods
The detection of plastics based on the chemical composition is a crucial step to develop an innovative waste sorting technology. The most used technique is densimetric (float-sink) separation in which plastic materials are separated based on their densities (Pongstabodee et al. 2008). The less dense materials float and heavier ones sink to the bottom. This method is conducted by the dissolution of mixed plastics in suitable solvents.
The use of several spectroscopic methods such as Fourier transform infrared (FT-IR) spectroscopy (Guidelli et al. 2011; Verleye et al. 2001), Raman spectroscopy (Tsuchida et al. 2009), laser-induced breakdown spectroscopy (LIBS), and hyperspectral imaging methods (Serranti et al. 2011) are also reported for detecting plastic materials. FT-IR is a simple, robust, efficient, flexible, and non-destructive technique to distinguish plastic polymers. This technique is based on infrared absorption bands that represent the unique chemical functionalities present in the materials. For instance, LDPE showed a unique band at 1377 cm−1 due to CH3 bending deformations that is not present in HDPE. In this technique, dominant vibrational modes present in the polymers are detected. However, unique identification is challenging due to the weak spectral features. Therefore, the techniques such as near-infrared (NIR) (12,800–4000 cm−1) and mid-infrared (MIR) (4000–600 cm−1) are investigated (Becker et al. 2017). These techniques can record unique vibrational overtones that are mainly observed in plastics such as PS and PVC. NIR analysis gives information about the molecular structure of the plastics and helps to classify them. NIR and MIR techniques are used mainly in the waste industry to recover high-value materials from waste streams.
NIR spectroscopy uses differences in the wavelengths of infrared (IR) light that are reflected by polymers having different chemical structures. The reflected wavelengths depend on the covalent bonds present in polymers. NIR spectroscopy is also used with hyperspectral imaging (HSI) cameras (Serranti et al. 2011) that generate 3D pictures of polymer scarps on a conveyor. The detected scarps are then expelled in two or three altered collecting trays based on their chemical behavior (Fig. 1). The HSI technique can be used for qualitative analysis such as principal component analysis (PCA). NIR spectroscopy has many benefits for detecting and sorting plastic resins; however, this method is not suitable for identification of black-colored or dark-colored plastics.
Principle of near-infrared hyperspectral imaging (NIR-HSI) sorting technology (Serranti et al. 2011).
In the MIR spectral region (4000–600 cm−1), plastic materials show additional vibrational modes spectra such as rocking, deformation, and twisting, depending on the molecular structure of the analyzed samples (Becker et al. 2017). The various functional groups such as O–H, N–H and O–C showed unique spectrums in the range of 2500–600 cm−1 (fingerprint region). The main advantage of this spectroscopy is that it can also analyze dark-colored polymers.
Raman spectroscopy is also used to differentiate between different plastic types because of its spectral range that is well beyond the fingerprint region, its high signal-to-noise ratio, high sensitivity, and better reproducibility. This technique can be used at the industrial level where a massive amount of sorting is required. Raman spectroscopy has advantages over the FT-IR and NIR techniques, including (i) no reference signal is obligatory and (ii) contamination of water and CO2 from the air or on the surface has less of an influence. The mixtures of plastics such as PE/PP, PA/PTFE, and ternary polymer blends and their spatial structures can also be recognized by means of Raman spectroscopic analysis. The effect of fillers and additives on phase separation can also be investigated.
LIB spectroscopy (Noll et al. 2008) uses high-power laser pulse for identification of metallic wastes. LIB spectroscopy provides high-dimensional spectrometric determination for analyzing plastics, metal alloys, and treated wood wastes (Solo-Gabriele et al. 2004; Grzegorzek et al. 2011). The main components of an LBS system are a solid-phase neodymium (Nd)-fixed on yttrium aluminum garnet laser, a charge coupled device (CCD) spectral range spectrometer, and a data analysis processing unit. In the first step, the laser is focused on the bulk waste, leading to cutting out of waste that generates plasma plumes. The cutter portion emits radiations that are captured by the CCD spectrometer. This technique states and discriminates the characteristics of atomic emission streaks and can quickly analyze bulk waste and then detect constituent materials.
Use of the small-angle X-ray scattering technique is also reported in the literature (Jong and Dalmijin 2012; Mesina et al. 2007). It is a powerful technique used to determine the side chains of plastics by diffraction at small angles. The molecular weight of plastics in dilute solutions can be determined by light scattering analysis.
Recently, electronic devices have been used increasingly in our day-to-day lives, as the number of devices per user grows, and product life cycles decrease. Therefore, the amount of waste electrical and electronic equipment (WEEE) plastics is increasing significantly. These materials are more complex, containing co-polymers and composites added to improve the functional properties. These also contain a more intricate set of flame-retardant additives such as ABS-PC, ABS-PMMA or HIPS-PPE. This makes the use of plastic densimetric methods difficult. Beside these challenges, WEEE plastic materials and End-of-Life Vehicles (ELV) are black for photoaging reasons. Carbon black absorbs NIR rays, so NIR-HSI techniques also have a limited use for sorting electronic wastes (Beigbeder et al. 2013). Thus, innovative sorting technologies are required for electronic wastes.
3.2 Automated Sorting Systems
Automatic sorting systems are designed to distinguish plastics like PVC, PET, PS, and PP with ease and identify cellulose-based materials such as cards, paper, wood, cardboards, and natural fibers. Automated waste sorting techniques are classified into two types (i) direct sorting and (ii) indirect sorting. The direct sorting method depends on material properties such as density, electrical conductivity, and magnetic susceptibility by applying exterior fields like gravity, eddy current, and magnets individually (Gaustad et al. 2012; Svoboda 2004). On the contrary, in the indirect sorting method, sensors are used to detect the existence and location of materials in wastes. For this process, automated machines and robots are generally used to sort the identified materials.
Process of automatic sorting of MSW: In the first step, pre-treatment is conducted using disc screen, shredder, screw press, and magnetic techniques. Following the pre-treatment, dry waste is recovered and then subjected to a shredding process or comminution. Further, the ferrous materials are sorted by magnetic drum techniques. Non-ferrous metals are sorted using indirect sorting methods such as X-rays, eddy current, optical sort, LIBS, and hyperspectral imaging.
Comminution techniques: In comminution practices, loose waste solid is pulverized into even-sized particles by utilizing forces that are generated by pressure, cutting, impact, or abrasion (Bonifazi and Serranti 2012). Tools such as rotating drums, ring mills, alligator shears, hammer mills, swing hammer shredders, and impact crushers are used for this process.
Table 2 lists a summary of the different direct and indirect sorting technologies and details of the materials that can be sorted. These sorting technologies are explained in detail in the following sections.
3.2.1 Direct Sorting
In direct sorting, pre-treatment techniques such as magnetic drum, screw press, magnetic head pulley, disc screen, or shredder are used along with a magnet, magnetic crossbelt, eddy current, froth flotation, magnetic density separation (MDS), tribo-electrostatic separation, hydrocyclone, or air separator.
(i) In a screw press, waste materials (organic) are squeezed through narrow slits; thus, lenient and wet portions can be separated from plastic, paper, wood, and metal (Jank et al. 2015). (ii) In the disc procedure, rotating discs are used in which small and heavy waste portions are dropped in among discs, though large and light portions are transported to the edge of discs (Jank et al. 2015). (iii) The shredder along with a magnet is used for sorting paper and carbon-based fractions from MSW. (iv) The magnetic drum and magnetic head pulley techniques separate ferrous portions from non-ferrous and other mixed wastes based on the magnetic susceptibility of wastes (Svoboda and Fujita 2003; Bonifazi and Serranti 2012). (v) The rotary drum type separators are used in the eddy current technique, and the rotary drum is in-line by neodymium iron boron (NdFeB) with alternating north and south magnets. This technique has a low operating cost and highly pure products can be recovered. (vi) Magnetic liquids such as ferrofluid are used in MDS techniques as a separation medium. (vii) The froth flotation technique practices the phenomenon of hydrophobicity of plastic materials for separation. To use this technique, first waste materials are shredded into small particles using the comminution technique (Wang et al. 2015) and then mixed with water. This technique is mainly used for separating plastics from aquatic waste mixtures. (viii) The physical phenomenon, i.e., “contact electrification” or “frictional electrification” are used in the triboelectrostatic separation technique (Lowell and Ross-Innes 1980). (ix) The centrifugal forces are used for density separation in the hydrocyclone technique. This is mainly used for the separation of materials such as acrylonitrile butadiene styrene (ABS), PE, PVC, and HIPS (Al-Salem et al. 2009). (x) An air separator is mainly used for the retrieval of lighter non-metallic materials like foam, polymers, rubber, and fibers after applying techniques such as magnetic sorting and eddy current technologies. In an air separator, a compressed air nozzle is used (Bonifazi and Serranti 2012).
3.2.2 Indirect Sorting
In this sorting technique, sensors are used to identify recyclable materials in the bulk waste and then segregation is performed using various actuators. The techniques such as eddy current-based sorting, LIBS, optical-based sorting, X-ray-based sorting, and spectral imaging-based sorting (summarized in Table 3) are included in indirect sorting technologies.
(i) In eddy current-based sorting, electromagnetic sensors (EMS) are used to detect non-ferrous metal fractions depending on the electrical conductivity and magnetic permeability of the sample (Kutila et al. 2005; Mesina et al. 2003). (ii) The LIBS system uses high-power lasers for the detection of metallic wastes. In this system, segregation of waste materials is done at a comparatively higher speed and volume compared to the eddy current method. However, for LIBS system waste, the sample to be used should be free from paints, lubricants, and oxide layers (Gesing et al. 2008). (iii) The camera-built sensors are used for the identification of waste portions in the optical sorting technique (Rahman and Bakker 2012). In general, the optical sorting technique uses multivariate analysis that comprises an amalgamation of a weight-meter and 3D shape detection camera fixed laterally on the conveyor system. A 3D imaging camera, web camera, optical CCD, linear laser, line scan camera, and ultraviolet are mainly used as sensors in this technique. Non-ferrous metals such as wrought Al, Mg, and cast Al with a sorting efficiency of 85% can be recovered with this technique (Koyanaka and Kobayashi 2011). The efficiency of this technique is not disturbed by surface impurities such as oil, dust, and paint. The hybrid techniques use a combination of an inductive sensor array and color vision to detect metals like zinc, brass, aluminum, copper, and stainless steel (Kutila et al. 2005). The UV sorting procedure is utilized to differentiate between opaque and special glasses (lead glass, ceramic glass, and borosilicate glass) from assorted glass wastes (Huber et al. 2014). The UV technique is independent of the shape and color of the glass specimen. (iv) The X-ray transmission type of indirect sorting is relatively fast and captures X-ray images within a few milliseconds by using a high-intensity X-ray beam. This technique provides data about the atomic density of the material tested. With this technique, copper, chromium, and arsenic were detected with 91, 97, and 98% efficiency, respectively (Hasan et al. 2011).
(v) The spectral imaging-based sorting technique utilizes both spectral reflectance measurement and image processing technologies. This technique uses various methods such as NIR, VIS (visual image spectroscopy), and HSI. In spectroscopic-based technologies like NIR, MIR, and Raman, light is illuminated on the plastic waste sample. Then an exclusive set of wavelengths of light is reflected because of the interaction between light and the tested sample. The reflected wavelength is different for different plastics, and these wavelengths are detected by various sensors such as NIR, MIR, and Raman. Further, processing units are used to sort out the desired material. VIS reflectance spectroscopy was used to detect PP plastic in mixed waste by Safavi et al. (2010). The HSI-based technique is used to classify the polyolefins (PP and PE) from mixed waste with high purity in the NIR region (Serranti et al. 2012).
Among all the discussed automated sorting techniques, the eddy current technique is the most widely used for segregation of metal waste fractions. The spectral- and optical-based techniques have shown improved performance with better coverage of material variations.
For more clarity, an example of a post-consumer, plastic materials sorting system is described as follows. Tsuchida et al. (2009) demonstrated a plastic sorting arrangement using the Raman spectroscopic identification technique, as shown in Fig. 2. In this system, post-consumer plastics were first shredded and added into a preprocess line for removal of wires, metals, labels, and other contaminants. The plastic materials were then moved under a spectrometer using a conveyor. In the final step, an air gun was intended to sort the plastic materials.
Plastic sorting system with Raman spectroscopy (Tsuchida et al. 2009).
3.3 Technical Challenges and Possible Solutions for Sorting Technologies
The most widely used process, manual sorting, depends on the identification of plastic constituents, but it is tedious and time-consuming. On the contrary, the use of automated waste sorting systems is not very common, and these systems are mainly designed for source-segregated wastes. Segregation at the source sites is not commonly practiced because of the very inadequate door-to-door collection and absence of enthusiasm in consumers. Due to this, collected wastes are in an assorted form and discarded in landfills. Then the waste sorting is done manually. In this process, workers are exposed to pathogenic and toxic contaminants. Thus, there is a need to provide automatic tools to the workers to improve the safety and efficiency and specially to eradicate toxic pollutants.
There is a need to grow cheap and persistent computerized waste sorting machinery for cracking waste management problems. Automatic handling of MSW is very challenging because of the mixed nature of the wastes. To mitigate this challenge, researchers need to consider the use of multi-sensor type of systems and find ways to increase the recovery rate. The main technical challenge is the physical integration of more than one sensor in the sorting systems. Hence, sensors should be combined in the system in such a way that cross sensitivity is diminished, and the prototype should be modest.
In addition, an energy-competent computerized robotic system needs to be developed to recover recyclable fractions from landfill sites. These systems must be operated for extended stages, which need long-term self-sufficiency.
The strength of the automated systems could be amended by extending the variety of operating conditions for different robot sub-systems. The major technical challenge for enhancing the robustness is the effect of adverse environmental conditions, such as moisture, dust, wind, and soft terrain, while segregating recyclable portions from MSW in a landfill.
4 Recycling of Plastic Wastes
Plastic recycling is a process of recovering plastic wastes or scraps and reusing the solid for valuable products. The useful products can be either similar or different in forms from virgin plastic. The recovery and recycling of plastics is not a novel methodology. After increasing the rate of production and consumption of plastics every year, many researchers started studying the various types of plastic recovery and recycling processes. Table 4 lists the various plastic waste recycling methods.
Plastics are non-biodegradable; therefore, they cannot be simply converted to the natural carbon cycle. Thus, the life span of plastic wastes ends at waste disposal sites (Luo et al. 2007). The methods for plastic waste disposal are landfilling, incineration, chemical recycling, and material recycling. The largest portion of solid and plastic wastes are landfilled. However, waste disposal to the landfill is highly undesirable because of legislative pressure and the meager biodegradability of packaging polymers (Garforth et al. 2004). Hence, alternative recycling methods need to be explored.
4.1 Types of Plastic Waste Recycling Methods
The recovery and recycling of plastic wastes is broadly divided into four parts as described in detail in the following sub-sections.
4.1.1 Primary Recycling
Primary recycling is also called closed-loop recycling. It is a simple and easy process except for the sorted and cleaned collection of plastic wastes. Primary recycling involves the reprocessing of in-house scrap and industrial residual materials. The industrial residual materials are produced in a plastic manufacturing process such as extrusion and molding. The quality and properties of primary recyclate are similar to regular virgin plastic. Recycled material is either mixed with virgin material or used as a second-grade material. The adaption of the primary recycling process reduces waste production from industries to a great extent. A well-known example of a primary recycling process is PET recovered from postconsumer bottles and used in the production of new bottles (Grigore 2017).
4.1.2 Secondary Recycling
Secondary recycling is also recognized as mechanical recycling. It is one of the most widely used recycling processes because of its cost-effectiveness. This recycling process includes a collective set of different pretreatment and separation steps. Plastic wastes obtained from different sources have different pretreatment preferences (Al-Salem et al. 2009). In general, the following major steps are considered.
-
1.
Polymer waste separation and sorting: The separation and sorting of polymer waste can be carried out simultaneously and step by step. The collection of polymeric material from MSW is part of the separation process, whereas in the sorting process, polymeric waste is segregated based on its type, density, color, and physical properties. The sorting technologies described in Sect. 3 are mainly used.
-
2.
Milling and grinding: In this step, the well-sorted polymeric waste is fragmented into small parts using rotary mills and grinders. After this process, plastics are transformed into powder, granules, and fragments.
-
3.
Washing and cleaning: The plastic material obtained from the separation process is still somewhat contaminated. Therefore, plastic waste is washed with different solvents based on their impurities. For instance, these wastes are washed with water in the cyclones. For the further removal of other impurities, chemical washing is carried out using surfactants and alkaline solvents.
-
4.
Drying: After the washing process, drying of the plastic waste is an important step. The plastic material containing PA and polyester are susceptible to a hydrolysis reaction that leads to degradation of the plastic.
-
5.
Agglutination: After the drying process, plastic waste and binder are mixed, and the homogenization of additives and pigments is carried out. In this process, volume reduction of plastic waste takes place.
-
6.
Reprocessing: In this final step, plastic residue is transformed into polymer pellets of different shapes. This process is performed by extrusion or molding techniques. The recyclate plastics are packaged and transported to manufacturing factories for the production of new products.
The order of these steps depends on the MSW that needs to be processed and the desired product materials.
4.1.2.1 Advantages and Disadvantages of Secondary Recycling
The advantages associated with mechanical recycling are the comparatively lower capex, much less operational complexity, and the capability of existing machines to be used to recycle virgin and recyclate plastics.
The reclaim plastic used for mechanical recycling or secondary recycling contains a large amount of contaminants and other plastic mixtures. The presence of other polymer mixtures creates phase separation; consequently, a compatibility issue arises. The dyes and inks present in plastics restrict the homogeneity of the recyclates. For an efficient large-scale recycling of the plastics, proper segregation and separation from contaminates is highly desirable. Along with a contamination problem in the secondary recycling, the thermochemical degradation of plastic materials is also a major challenge. The over-degradation can limit the number of cycles during reprocessing of the plastic materials.
Products such as packaging materials, grocery bags, shutters, and pipes can be made from recyclates that are obtained by mechanical recycling. But there are always limitations in using recyclate plastic to make food packaging materials. Safety regulations standardize the contamination level in the recyclate plastic.
Initially, primary and secondary recycling were applicable only to thermoplastic plastics. Recently though, mechanical/secondary recycling is also popular for elastomers. The cross-linked rubbers can also be thermo-mechanically recycled after the devulcanization process.
4.1.3 Tertiary Recycling
Tertiary recycling is also identified as chemical or feedstock recycling. This is the most advanced type of recycling technology. In this kind of recycling, plastic waste constituents are changed into smaller molecules, known as chemical intermediates, by using thermal or chemical treatments. The smaller molecules are generally liquids or gases and sometimes solids or waxes. These molecules are used as a raw material or feedstock to produce new plastic and petrochemical materials. That is why it is called feedstock recycling.
Chemical recycling is a reverse process in which long-chain plastic polymers are broken down into monomer units and feedstock by the depolymerization and degradation process. The feedstock obtained after chemical recycling can be used to make comparatively less valuable products based on the level of impurities. Chemical recycling is not restricted to a single type of plastic materials. The mixture of plastic materials can be used in chemical recycling, but for better quality of the final product, sorting and separation technologies are also adopted in the process. Chemical recycling is a better alternative than mechanical recycling of thermosets, fibers, and cross-linked elastomers.
Table 5 describes the mechanisms and significance of different chemical recycling methods. The different methods used for chemical recycling are bio-chemical degradation, chemolysis/solvolysis, gasification or partial oxidation, and cracking. Among these, chemolysis and bio-chemical degradation are considered chemical routes, and gasification and cracking are thermal routes. Biological degradation is a simple and less energy-consuming process, but it has limitations for synthetic plastics. The rate of biological degradation is very slow for the high-molecular-weight hydrophobic plastic materials.
4.1.3.1 Chemolysis
In this process, plastics are chemically treated using chemical agents to break down into monomers. Different processes such as hydrolysis, alcoholysis, methanolysis and glycolysis, are included in chemical recycling.
4.1.3.1.1 Hydrolysis
In the hydrolysis process, plastics are treated with water to recover the original raw materials. Hydrolysable polymers like polyesters, polyureas, polyamides (PA), PU, and PC are resistant to hydrolysis. Hydrolysis of PU foams is of great interest because they have a very low density, i.e., 30 kg m−3, and thus take up considerable space. With this process, high product yields can be obtained. For instance, 90% amine and 100% polyethers can be recovered. The separated materials can be either reprocessed directly or mixed with virgin plastics. The quantity of waste generated is reduced (Ullmann 2003a).
4.1.3.1.2 Alcoholysis
PU can be depolymerized by the alcoholysis method to recover a polyhydroxy alcohol and small urethane fragments. This is an example of a transesterification reaction. If a diol is used as an alcohol, then a urethane fragment with terminal hydroxyl groups can be obtained. The recovered polyhydroxy alcohol can be used to make PU foams by treating with isocyanates (Ullmann 2003b).
4.1.3.1.3 Sub- and Super- critical Solvents
Plastic depolymerization reactions in sub- and super-critical fluids like water and alcohols are also investigated for the chemical recycling process. For instance, monomers such as terephthalic acid (TPA) and ethylene glycol (EG) were obtained by the solvolysis of PET in supercritical water (Al-Sabagh et al. 2016). The hydrolysis of PU in water produces polyol and diamine. In the presence of sub- and super-critical solvents, polymer decomposition proceeds rapidly, and therefore, highly selective products can be obtained.
4.1.3.1.4 Methanolysis and Glycolysis
The polymer degradation in the existence of methanol and glycols is known as methanolysis and glycolysis, respectively. The methanolysis of PET using methanol at a 180–280 °C temperature and 2–4 MPa pressure produces mainly dimethyl terephthalate (DMT) and EG (Scheirs 1998). The glycolysis of PET in the presence of EG produced a chemical, BHET (Chen and Chen 1999). The selectivity depends on the reaction temperature, reaction pressure, catalyst, and EG concentration.
4.1.3.2 Gasification or Partial Oxidation
Gasification or partial oxidation is a well-known process where hydrocarbon containing materials such as plastic or petroleum residues are converted into syngas (CO + H2) in the presence of oxygen. Product quantity and quality are dependent on the polymer type used. Hydrogen production with 60–70% yield is reported by using a two-stage process: pyrolysis followed by partial oxidation. Co-gasification of plastic wastes and biomass is also reported, and an increase in hydrogen yield and decrease in carbon oxide is observed.
Plasma gasification is a method in which plasma torches generate an electric arc via passage of an electric current via a gas. Plasma is created through plasma torches by heating gas (mainly air) to a very high temperature (approx. 3900 °C). In this process, high temperatures 1500 and 5000 °C are used with a very short residence time. On injecting plasma in carbonaceous solids, they are heated and volatile matter is produced and cracked to release CO, H2, CH4, C2H2, and other hydrocarbons. Therefore, plasma technology can be used to produce gaseous fuels. Syngas was produced from waste rubber by using thermal plasma (Huang and Tang 2007). In the near future, plasma gasification can be a very attractive option to reduce greenhouse gas such as CO2 emission. In this plasma gasification, the energy source is non-fossil fuel based and hence, CO2 emission is much less.
4.1.3.3 Cracking
Cracking is a process that includes depolymerization of the plastic materials into smaller hydrocarbons. Thermal cracking, catalytic cracking, and hydrocracking are the processes that are used to depolymerize plastic waste.
4.1.3.3.1 Thermal Cracking/Pyrolysis
This type of cracking is also known as pyrolysis. In thermal cracking, depolymerization of plastic materials is performed by heating without oxygen at temperatures between 550 and 800 °C. The products obtained are a volatile fraction and carbonized char. The volatile portion can be divided into condensable hydrocarbon oil and a high heating value non-condensable gas. Product composition and yields depend on the nature of the plastic waste and the reaction parameters used. Reactor design also plays a crucial role, and various types of reactors such as a batch reactor, fixed-bed reactor, fluidized bed reactor, and screw kiln reactor have been used. The main features of the thermal cracking process are:
-
1.
A high yield of C1 and C2 hydrocarbons in a gaseous product can be obtained.
-
2.
The selectivity of gasoline is very poor.
-
3.
Less branched olefins are formed and a small amount of diolefins can be made at a high temperature.
-
4.
Gas and coke yields are very high.
4.1.3.3.2 Catalytic Cracking
In catalytic cracking, pyrolysis is performed in the presence of a catalyst to improve the quantity and quality of the desired products. Mainly solid acid and bifunctional catalysts are used. With added solid acid catalysts, the molecular weight of the main polymer chains is minimized by consecutive attacks by acid sites on the catalysts. This leads to an increase in yields of low molecular weight products. In addition, the carbonium ion intermediates formed in the catalytic reactions may undergo rearrangement reactions to produce high-quality isomers. The bifunctional catalysts contain both acidic and metal active sites. The metallic sites help to catalyze hydrogenation or dehydrogenation reactions, whereas the acidic sites catalyze the isomerization reactions. These catalysts can enhance the isomerizations of straight-chain paraffins to branched-chain paraffins and promote the dehydrogenation of naphthenes to aromatics and dehydrocyclization of straight-chain paraffins into cycloparaffins. All these catalytic reactions help to increase the octane number in the resulting hydrocarbon oils. The main features of catalytic cracking are:
-
1.
Low reaction rates and reaction temperatures are used.
-
2.
Gaseous products with more C3 and C4 hydrocarbons.
-
3.
Gasoline selectivity is high with more C5–C11 hydrocarbons.
-
4.
The product oil composition and yields can be controlled by using suitable catalysts.
-
5.
The catalysts used are expensive.
-
6.
Naphthene dehydrogenation and olefin cyclization reactions produce more aromatics.
4.1.3.3.3 Hydrocracking
In the hydrocracking process, the larger molecules are cracked into smaller molecules (mainly naphtha or kerosene) in the presence of high hydrogen pressure and catalysts. Typical reaction conditions used are 250–400 °C and 3–10 MPa hydrogen pressure. Hydrocracking is mainly conducted to produce high-quality gasoline using various feeds such as PE, PP, PET, PS, PVC, mixed plastics, and co-pyrolysis of plastics with biomass/coal/VGO/scrap tires. The catalysts mainly include supports such as alumina, silica-alumina, sulfated zirconia, and zeolites and transition metals like Mo, Pt, Fe, and Ni. These catalysts possess functionalities of both cracking and hydrogenation.
4.1.3.3.4 Plasma-Assisted Pyrolysis
Recently, PE depolymerization was performed using plasma technology (Guddeti et al. 2000). The major advantages of plasma pyrolysis over conventional pyrolysis are that plasma provides a high temperature and high energy for the reaction.
All the previously discussed chemical recycling methods can be effectively used for contaminated and mixtures of plastic wastes. Also, the purity and selectivity of the recycled product are good. However, limitations associated with this recycling technique include the high capex of chemical recycling instruments; expensive catalyst systems; energy-intensive processes; and time-consuming development of an efficient catalyst.
4.1.4 Quaternary Recycling
Quaternary recycling mainly includes the incineration of plastic waste to produce energy in the form of heat, steam, and electricity. This recycling process can treat a mixture of plastic wastes containing huge amounts of contaminants. This process is effective compared to other recycling processes. It is a suitable process for medical wastes, cross-linked polymers, and thermosets. In this energy recovery process, the chemical energy of plastics is transformed into electrical and thermal energy. The incineration of high calorific plastic waste is carried out to produce a huge amount of energy. Since in this method only the inherent energy of plastic waste is used, the most contaminated waste plastic can be used without extensive sorting of used plastic.
Thermoplastic and thermosetting plastics are a high-yielding energy resource. For instance, burning 1 ton of organic waste, approximately 2501 kcal kg−1 of heating oil could be saved (Maraghi 1993). The energy recovered depends on the type of plastics used. The energy recovered from PE, PP, PS, phenol–formaldehyde, PVC, and PU is 18720, 18343, 16082, 13179, 7516, and 7014 kcal kg−1, respectively. In a general sense, the average energy contained in plastic is around 10000 kcal kg−1.
There is also a disadvantage of this energy recovery system from plastic waste. During the process, plastics release polyaromatic hydrocarbons (PAHs), soot, pollutant gases, and particulates, and hence, recovering energy from plastic is not suitable at a large scale. Also, various environmental regulation-related concerns are associated with this type of recycling process. There are various modifications that are suggested for the minimization of the emission of gases and particulates such as an addition of ammonia in the incineration chamber, cooling of the flue gas, neutralization by an acid, and adsorption using activated carbon.
The following methods generated during quaternary recycling are examined in the literature to minimize pollutants and particulates in emissions. Wet scrubbing: Gaseous oxides are simultaneously removed by various adsorbents such as NH4OH, Ca(OH)2, NaOH, Mg(OH)2, and Na2SO3.
-
1.
Selective catalytic reduction (SCR): Nitrogen oxides present in the flue gas are chemically converted into nitrogen gas using reducing agents such as urea, ammonia, and hydrocarbons.
-
2.
Adsorption: Nitrogen and sulfur oxides are adsorbed on the surface of adsorbent materials such as alumina, activated carbon, and coke. The metal modified adsorbents showed better catalytic activity.
-
3.
Non-thermal plasma methods: Nitrogen oxides are selectively removed by dielectric barrier, pulsed corona, and radio frequency discharges.
-
4.
Electron beam: Nitrogen and sulfur oxides are converted into chemicals in the presence of ammonia using irradiation of an electron beam.
-
5.
Electrochemical method: In this method, nitrogen oxides are converted into molecular nitrogen by electrochemical redox reactions. Hydrogen acts as an anode and oxygen acts as a cathode for the electrochemical reaction.
4.2 Technical Challenges for Plastic Wastes Recycling
Worldwide, the production of plastics far exceeds plastic recycling. The recycling of plastics is hindered by many factors.
-
1.
Additives: Several additives are used extensively to improve the properties of plastic materials, but they increase the chemical complexities of the final plastic material. Antistatic agents, antiblocking agents, antioxidants, antifogging agents, colorants, flame retardants, blowing agents, impact modifiers, coupling agents, plasticizers, and viscosity depressants are a few examples of additives. Their presence has been reported to create problems in plastic recycling. Some additives present in one plastic material can degrade other plastics. For example, recycled ABS can degrade PC during the recycling process.
-
2.
Compatibility: Plastic material is composed of different chemical units. Therefore, most polymers are immiscible to each other. For example, some coatings and adhesives are made of thermoset materials that make recycling problematic.
-
3.
Contamination: Different types of contamination are present in the recycled plastic based on their usage and sources. Contamination can be from dirt, oils, resins, food items, adhesives, glasses, metals, silica, etc. The presence of these contaminants affects the efficiency and productivity of the recycling process.
-
4.
Discoloring: Often, some plastics are discolored due to the degradation of monomers. This mainly occurs because of the presence of some additives.
5 Conclusions and Future Perspectives
This chapter contributes to the sorting technologies for plastic wastes and recycling techniques used for plastic solid waste management. The recycling of household plastic wastes and mixed waste streams is challenging as these are highly contaminated. Thus, technologies are needed to sort plastics automatically using advanced techniques. The automatic sensor-based sorting technologies such as eddy current-based sorting, LIBS, X-ray-based sorting, optical-based sorting, and spectral imaging-based sorting are highly attractive for the fruitful use of plastic wastes. These methods should be coupled with artificial intelligence so that the process becomes more efficient. This will not only improve the efficiency of the process, but also provide higher yields. Most importantly, these techniques will help in reducing the exposure of manual labors to the contaminants, during sorting of plastic waste. Despite a lot of reported research work, the use of automatic waste sorting technologies is not very common worldwide. Still, there is a need to develop energy-efficient, low cost, simple, safe, and pervasive automatic waste sorting systems. These systems should be designed in such a way that they can be operated for extended periods and under adverse environmental conditions. Also, consumers need to take the responsibility to sort their waste properly. The sorting guidelines in place directly impact the quality of waste streams for recycling. Public authorities need to make guidelines for sorting of household wastes.
The best way to deal with plastic waste disposal is to recycle it. Recycled plastic and useful products obtained from plastic waste will contribute to environmental sustainability and reduce global warming to some extent. Primary and secondary recycling of plastic wastes involves thermal treatments like “melt-and-remold”, while tertiary or chemical recycling techniques include thermal and chemical cracking of plastics into fuels and chemicals. Recycling technologies can only be successful when the appropriate infrastructures and collection systems are implemented. Chemical recycling techniques are gaining momentum for use at the large scale and are being adopted in various countries. This method can easily produce a substitute for fossil fuels as an alternate source of energy. The design of catalytic systems for the chemical recycling of plastics is highly desirable. The catalytic system should work even under mild conditions and have the capacity to produce highly selective compounds. These help to produce high-value products on an industrial scale. In addition, the recycling methods should be designed in such a way that the method must be superior economically and ecologically. The designed method would also manage hazardous plastic wastes and emit negligible or a low amount of pollutants. All these efforts have set the future trends in plastic recycling as an industry.
References
Al-Sabagh AM, Yehia FZ, Eshaq GH, Rabie AM, ElMetwally AE (2016) Greener routes for recycling of polyethylene terephthalate. Egypt J Pet 25(1):53–64
Al-Salem SM, Lettieri P, Baeyens J (2009) Recycling and recovery routes of plastic solid wastes (PSW): a review. Waste Manage 29(10):2625–2643
Becker W, Sachsenheimer K, Klemenz M (2017) Detection of black plastics in the middle infrared spectrum (MIR) using photon up-conversion technique for polymer recycling purposes. Polymers 9:435–444
Beigbeder J, Perrin D, Mascaro JF, Lopez-Cuesta JM (2013) Study of the physico-chemical properties of recycled polymers from waste electrical and electronic equipment (WEEE) sorted by high-resolution near infrared devices. Resour Conserv Recycl 78:105–114
Bonifazi G, Serranti S (2012) Recycling technologies. In: Meyers RA (ed) Encyclopedia of sustainability science and technology. Springer, LLC, pp 8794–8848
Chen JW, Chen LW (1999) The glycolysis of poly(ethylene terephthalate). J Appl Polym Sci 73:35–40
Curlee TR, Das S (1991) Plastic waste (Management, control, recycling and disposal), Noyes data corporation, New Jersey, NJ
Garforth AA, Ali S, Martinez JH, Akah A (2004) Feedstock recycling of polymer wastes. Curr Opin Solid State Mater Sci 8(6):419–425
Gaustad G, Olivetti E, Kirchain R (2012) Improving aluminum recycling: a survey of sorting and impurity removal technologies. Resour Conserv Recycl 58:79–87
Gesing A, Harbeck H (2008) Partcile sorting of light-metal alloys and expanded use of manufacturing scarp in automotive marine, and aerospace markets. In: Proceedings of REWAS 2008, global symposium on recycling, waste treatment, and clean technology, Cancun, Mexico
Grigore ME (2017) Methods of recycling, properties and applications of recycled thermoplastic polymers. Recycling 2(4):24
Grzegorzek M, Schwerbel D, Balthasar D, Paulus D (2011) Automatic sorting of aluminum alloys based on spectroscopy measures. In: Proceeding of OAGM/AAPR workshop
Guddeti RR, Knight R, Grossmann ED (2000) Depolymerization of polyethylene using induction-coupled plasma technology. Plasma Chem Plasma Process 20(1):37–64
Guidelli EJ, Ramos AP, Zaniquelli MED, Baffa O (2011) Green synthesis of colloidal silver nanoparticles using natural rubber latex extracted from Hevea brasilliensis. Spectrochim Acta A Mol. Biomol. Spectrosc. 82(1):140–145
Hasan AR, Solo-Gabriele H, Townsend T (2011) Onlien sorting of recovered waste by automated XRF technology. Part I: detection of preservative-treated wood waste. Waste Manag 31 (4):688–694
Hopewell J, Dvorak R, Kosior E (2009) Plastic recycling: challenges and opportunities. Philos Trans R Soc Lond B Biol Sci 364(1526):2115–2126
Huang H, Tang L (2007) Treatment of organic waste using thermal plasma pyrolysis technology. Enegy Conv Manag 48:1331–1337
Huber R, Leitner K (2014) Method for detecting and sorting glass. US Patent 8,030,589
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perrymen M, Andrady A, Narayan R, Law KL (2015) Marine pollution. plastic waste impacts from land into the ocean. Science 347(6223):768–771
Jank A, Muller W, Schneide I, Gerke F, Bockreis A (2015) Waste Separation Press (WSP): a mechanical pretreatment option for organic waste from source separation. Waste Manag 39:71–77
Jong TPR, Dalmijin WL (2012) X-ray transmission imaging for process optimization of solid resources. In: Proceedings R:02, Congress
Koyanaka S, Kobayashi K (2011) Incorporation of neutral network analysis into a technique for automatically sorting lightweight metal scarp generated by ELV shredder facilities. Resour Conserv Recycl 55(5):515–523
Kutila M, Viitanen J, Vattulainen A (2005) Scrap metal sorting with color vision and inductive sensor array. In Processing of computational intelligence for modelling, control and automation and international conference on intelligent agents, web technologies and internet commerce (CIMCA-IAWTIC), vol 2, pp 725–729
Lowell J, Rose-Innes AC (1980) Contact electrification. Adv Phys 29(6):947–1023
Luo G, Suto T, Yasu S, Kato K (2007) Catalytic degradation of high-density polyethylene and polypropylene into liquid fuel in a powder-particle fluidized bed. Polym Degrad Stab 70:97–102
Maraghi R (1993) Disposal, recycling and reuse. In: Mostafa N, Dekker M (eds) Plastics waste management, New York, USA, pp 223–226
Mesina MB, De Jong TPR, Dalmijn WL (2003) Improvement in separation of non-ferrous scarp metals using an electromagnetic sensor. Phys Sep Sci Eng 12(2):87–101
Mesina MB, De Jong TPR, Dalmijin WL (2007) Automatic sorting of scrap metals with a combined electromagnetic and dual energy X-ray transmission sensor. Int J Miner pro 82(4):222–232
Naskar K, Babu RR (2014) Thermoplastic elastomers (TPEs) and thermoplastic vulcanizates (TPVs). In: Kobayashi S and Mu¨llen K (eds) Encyclopedia of polymeric nanomaterials. Springer, Berlin, pp 1–7
Noll R, Sturm V, Aydin U, Eilers D, Gehlen C, Hohne M, Lamott A, Makowe J, Vrenegor J (2008) Laser-induced breakdown spectroscopy-from research to industry, new frontiers for process control. Spectrochim Acta B 63(10):1159–1166
Pongstabodee S, Kunachitpimol N, Damronglerd S (2008) Combination of three-stage sink-float method and selective flotation technique for separation of mixed post-consumer plastic waste. Waste Manag 28:475–483
Ragaert K, Delva L, Van Geem K (2017) Mechanical and chemical recycling of solid plastic waste. Waste Manag 69:24–58
Rahman MA, Bakker MCM (2012) Hybrid sensor for metal grade measurements of a falling stream of solid waste particles. Waste Manag 32(7):1316–1323
Safavi SM, Masoumi H, Mirian SS, Tabrizchi M (2010) Sorting of polypropylene resins by color in MSW using visible reflectance spectroscopy. Waste Manag 30(11):2216–2222
Scheirs J (1998) Polymer recycling: science, technology, and applications. Wiley, Hoboken, NY, USA
Serranti S, Gargiulo A, Bonifazi G (2011) Characterization of post-consumer polyolefin wastes by hyperspectral imaging for quality control in recycling processes. Waste Manag 31:2217–2227
Serranti S, Gargiulo A, Bonifazi G (2012) Classification of polyolefins from building and construction waste using NIR hyperspectral imaging system. Resour Conserv Recycl 61:52–58
Solo-Gabriele HM, Townsend TG, Hahn DW, Moskal TM, Hosein N, Jambeck J, Jacobi G (2004) Evaluation of XRF and LIBS technologies for on-line sorting of CCS-treated wood waste. Waste Manag 24(4):413–424
Svoboda J (2004) Magnetic techniques for the treatment of materials. Springer
Svoboda J, Fujita T (2003) Recent developments in magnetic methods of material separation. Miner Eng 16(9):785–792
Tsuchida A, Kawazumi H, Kazuyoshi A, Yasuo T (2009) Identification of shredded plastics in mmseconds using Raman spectroscopy for recycling. IEEE SENSORS Conf
Ullmann, F (2003a) ULLMANN’s encyclopedia of industrial chemistry, (6th ed), vol 27. Wiley-VCH, Verlag GmbH & Co, Germany, pp 607–615
Ullmann, F (2003b) ULLMANN’s encyclopedia of industrial chemistry, (6th ed), vol 27. Wiley-VCH, Verlag GmbH & Co, Germany, pp 616–623
Verleye GA, Roeges NP, De Moor MO (2001) Easy identification of plastcis and rubers. Rapra Technology Limited, Shropshire, pp 174
Wang CQ, Wang H, Fu JG, Liu YN (2015) Flotation separation of waste plastics for recycling-a review. Waste Manag 32(7):1297–1305
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Prajapati, R., Kohli, K., Maity, S.K., Sharma, B.K. (2021). Recovery and Recycling of Polymeric and Plastic Materials. In: Parameswaranpillai, J., Mavinkere Rangappa, S., Gulihonnehalli Rajkumar, A., Siengchin, S. (eds) Recent Developments in Plastic Recycling. Composites Science and Technology . Springer, Singapore. https://doi.org/10.1007/978-981-16-3627-1_2
Download citation
DOI: https://doi.org/10.1007/978-981-16-3627-1_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3626-4
Online ISBN: 978-981-16-3627-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)