Abstract
Cerebral aneurysms are abnormal, focal dilatations of cerebral arteries that are usually found at points where vessels branch. In children less than 20 years of age, over 10% of cases of hemorrhagic strokes are caused by spontaneous rupture of cerebral aneurysms. Less than 5% of total intracranial aneurysms are seen in patients who are younger than 18 years. More than 70% of pediatric cerebral aneurysms are found in the anterior circulation with a slight male preponderence. Adult cerebral aneurysms have been studied and reviewed in-depth but much less is known about the pathogenesis, risk factors and optimal treatment modalities of pediatric aneurysms. Even though pediatric aneurysms differ from adult aneurysms in many ways, a lot of the existing information has been extrapolated from adult literature. Even though basic prinicipals of neuroanesthesia and neurocritical care may remain the same, caution must be exercised while extrapolating results from adult trials to pediatric population.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
-
Subarachnoid hemorrhage is the most common presentation in children with cerebral aneurysms.
-
Although both surgical clipping and endovascular techniques are popular, endovascular techniques are increasingly preferred in recent times.
-
During the conduct of anesthesia, it is crucial to maintain stable hemodynamic parameters, perform smooth induction of anesthesia, adequate depth of anesthesia, control intracranial pressure, maintain normothermia, and ensure adequate cerebral oxygenation.
-
Rebleeding, delayed cerebral ischemia, hydrocephalus, seizure, fluid imbalance, and dyselectrolytemia are commonly encountered complications in children with cerebral aneurysms.
-
Timely management of complications is important to prevent secondary neurological injury.
1 Introduction
Cerebral aneurysms are abnormal, focal dilatations of cerebral arteries that are usually found at points where vessels branch. In children less than 20 years of age, over 10% of cases of hemorrhagic strokes are caused by spontaneous rupture of cerebral aneurysms [1].
Less than 5% of total intracranial aneurysms are seen in patients who are younger than 18 years [2]. Although rare, ruptured intracranial aneurysms have been reported even in neonates [3, 4]. More than 70% of pediatric cerebral aneurysms are found in the anterior circulation with a slight male preponderance [2, 5, 6]. Adult cerebral aneurysms have been studied in-depth and discussed in several clinical studies. Still, much less is known about the pathogenesis, risk factors, classification schemes, and optimal treatment modalities of pediatric aneurysms. Even though pediatric aneurysms differ from adult aneurysms in many ways, a lot of the existing information has been extrapolated from adult literature.
2 Etiopathogenesis
The pathogenesis of pediatric intracranial aneurysm is not fully understood. Two main hypotheses have been proposed. The first hypothesis proposes luminal factors such as high blood flow velocity, shear stress, and blood turbulence in the etiogenesis of aneurysms based on the observation that aneurysms are common at sites of arterial bifurcation and arterial-anatomic variants. The second hypothesis suggests abluminal factors such as a morphological abnormality of the vessel wall, functional dysfunction of vessel wall, exogenous risk factors, and systemic diseases as the cause of the development of aneurysms.
It must be noted that many of the established risk factors in adults such as advanced age, chronic hypertension, smoking, drug abuse, and chronic kidney disease are not found in children, underscoring the differences in the formation and natural course of this condition from adults. The underlying cause of aneurysms in children with no systemic diseases remains perplexing; however, several genetic mutations have been implicated, such as the TSC2 and the PKD1 genes (weak vascular wall in tuberous sclerosis and polycystic kidney disease, respectively), the COL3A1 gene (abnormal procollagen in Ehlers-Danlos syndrome), and sickle cell genes (abnormal red cells that cause endothelial injury) [7,8,9,10]. The presence of coarctation of the aorta is a well-established risk factor for the development and rupture of aneurysms in children [11]. Some comorbidities associated with the development of cerebral aneurysms in children are listed in Table 22.1.
3 Types and Location
The four main types of pediatric intracranial aneurysms are saccular (berry), fusiform, traumatic, and infective. Saccular aneurysms are formed because of the disintegration of the elastic layer of the artery. The aneurysmal sac is composed of hyalinized intima and adventitia, with an abnormal tunica media. The internal elastic lamina terminates at the neck of the aneurysm and is absent in the sac. Saccular aneurysms were earlier thought to be congenital or developmental in origin, while recent evidence points toward hemodynamically induced degenerative vascular injury. Fusiform aneurysms are caused by severe atherosclerosis or degenerative changes in childhood. Traumatic aneurysms constitute 5–40% of pediatric aneurysms and are most commonly seen in the distal anterior cerebral artery (ACA) or in the major vessels along the skull base [12]. In the truest sense, traumatic aneurysms are pseudo-aneurysms because they are caused by endothelial damage and thus have a different pathophysiology. Children with traumatic aneurysms have a history of blunt or penetrating head injury or prior intracranial surgery. Infective aneurysms can be multiple and are more common in the anterior circulation, and many children have associated comorbid conditions like congenital or acquired immunodeficiency, endocarditis, and meningoencephalitis. Bacterial infections are more commonly implicated in aneurysms of infective etiology. Dissecting (nontraumatic) aneurysms have the dissection between the tunica intima and the media. These are four times more common in children compared with adults [13]. Posterior circulation aneurysms are overrepresented in children and are three to four times more common than in adults. Complex aneurysms such as giant, multiple, mycotic, or those in unusual locations are more common in children than adults [5, 14]. Nearly one-fourth of pediatric aneurysms can be giant [5].
4 Clinical Presentation
The most common presentation of intracranial aneurysms in symptomatic children is subarachnoid hemorrhage (SAH). Patients may present with headache, features of direct compressive effects, focal neurologic deficits, and seizures. Fusiform aneurysms tend to bleed less. Giant aneurysms commonly present with mass effects. In children, they can sometimes be confused for intracranial tumors on neuroimaging [15]. Seizures or acute hydrocephalus at presentation are twice as common in children compared with adults. Clinical examination and laboratory investigations may reveal a decreased level of consciousness, features of intracranial hypertension, fever, meningism, photophobia, retinal hemorrhage, dyselectrolytemia, and electrocardiographic changes. In young children with elevated ICP and mass effect, additional findings like tense fontanelle, splayed sutures, and opisthotonic posturing may be seen. For unclear reasons, children generally present with better clinical grades than adults and also seem to be less susceptible to developing delayed cerebral ischemia [14].
5 Diagnosis
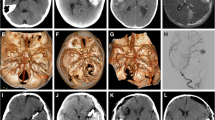
A non-contrast computed tomography (NCCT) of the head is the first investigation in a child with suspected intracranial bleeding. If the NCCT or clinical findings suggest SAH, additional imaging in the form of CT angiogram (CTA) or magnetic resonance angiogram (MRA) of the circle of Willis can be done (Fig. 22.1). The sensitivity of both CTA and MRA to pick aneurysms more than 5 mm in size approaches 100%. With recent advances, the sensitivity to pick even small aneurysms approximates 98–100% [16]. The potential advantage of MRA over CTA is that it does not require iodinated contrast use and limits the exposure of harmful ionizing radiation in young children. Digital subtraction angiography (DSA), while invasive, is the gold standard for the diagnosis of cerebral aneurysms and gives detailed information about the exact site, size, configuration, and neck of the aneurysm, which is important for making treatment decisions (Fig. 22.2).
In a 4-year-old child, axial NCCT head showed (a) well-defined hyperdense lesion (thick white arrow), s/o bleed with perilesional hypodensity (edema) in right temporal lobe with thin SDH in right temporal lobe convexity (thin white arrow). Axial T2 MRI showed (b) well-defined flow void in right distal M1 MCA (thick white arrow) with gliotic changes in the right temporal lobe (thin white arrow). Phase contrast MRI showed (c) intense enhancement within the aneurysm (thick white arrow). Time of flight MRA showed (d) a well-defined aneurysm in right distal M1 MCA (thick white arrow)
Digital subtraction angiography right ICA AP run showed (a) a well-defined lobulated aneurysm in right distal M1 MCA (thick arrow) with paucity of cortical vessels in right MCA territory (thin arrow). DSA 3D images (b–d) showed lobulated aneurysm in right distal M1 MCA, directed antero-supero-laterally (thick white arrow)
6 Clinical Grading
The first popular grading of SAH based on the severity of clinical findings was done by Botterell et al. (into five grades) and was later modified by Hunt and Hess [17, 18]. A further modification added “Grade 0” for unruptured aneurysms (Table 22.2) [19]. The modified Hunt and Hess (H&H) scale is most commonly used to grade the aneurysm at presentation, prognosticate, and guide neurosurgical or interventional therapy. However, it has been criticized for interobserver variation owing to the ambiguity in the terms used while grading, with poor operational definitions. Terms like lethargy, confusion, and stupor may be interpreted differently by different observers. Similarly, some patients may have clinical features that overlap in two different grades (e.g., severe headache in an unruptured aneurysm). No separate clinical grading scale exists in children. Predicted mortality rates based on H&H grades have been calculated in adults and are probably not applicable to children.
The World Federation of Neurological Surgeons (WFNS) scale was introduced in 1988 to improve upon the H&H scale and increase objectivity while assigning grades. The WFNS scale compresses the Glasgow Coma Scale (GCS) into five categories and also incorporates neurological motor deficits [20] (Table 22.2).
Recently, a modified WFNS (mWFNS) scale has been proposed in which adult patients with SAH and a GCS score of 14 are assigned to grade II, and those with a total score of 13 are assigned to grade III, irrespective of the presence of neurological deficit [21]. Although untested in children, the mWFNS score may be better at accurately prognosticating SAH patients than the original score, but further validation is required.
The Fisher grading is based on computed tomography (CT) findings in SAH (Table 22.2). The original Fisher grading was modified by Frontera et al. to account for concomitant intraventricular hemorrhage (IVH) in admission CT scans. It was found to predict symptomatic vasospasm after SAH more accurately than the original Fisher grade (Table 22.2) [22, 23].
Although the Fisher grading is widely used to grade SAH in adults, its applicability in children (using adult CT scan measurement values) is unclear. No separate grading system based on CT findings is available in children.
7 Complications of Aneurysmal SAH
7.1 Rebleeding
Rebleeding of the aneurysm occurs in nearly half of the children with intracranial aneurysm, and for reasons that are not clear, it is consistently more frequent than in adults [24,25,26]. The classic presentation of rebleeding is a child with a deterioration of consciousness, new-onset neurological signs, and abnormal vital signs. Some factors that predispose to rebleeding include poor-grade aneurysm, presence of an intracerebral or an intraventricular hematoma, posterior circulation aneurysm, deranged coagulation parameters, and delayed surgery. Rebleeding has also been described during induction of anesthesia, laryngoscopy, intubation, and during other intraoperative events such as brain retraction and sudden evacuation of the hematoma. The incidence of rebleeding is highest in the first 24–48 h of the first bleed. Studies carried out in adults have found that antifibrinolytic drugs like tranexamic acid protect against rebleeding, especially when administered early in the course of treatment [27]. However, there are conflicting results of ischemic complications with their use [27, 28]. A recently published meta-analysis of ten trials concluded that there is no conclusive evidence to support the use of antifibrinolytics in treating patients with aneurysmal SAH, and further trials are required to evaluate its effectiveness [29]. The same results may also be corroborated to children.
7.2 Vasospasm and Delayed Cerebral Ischemia
Vasospasm is the reactive narrowing of the conducting vessels around the area of the subarachnoid bleeding caused by the irritant effect of blood and its breakdown products. Vasospasm is an important cause of morbidity and mortality in patients before and after the therapeutic procedure. Vasospasm is generally not seen before 3 days of the initial bleed, peaks at the end of 7 days, and wanes by 3 weeks. In adults with aneurysmal SAH, angiographically evident vasospasm is estimated to be present in 40–60% of patients, and clinically significant vasospasm is seen in 20–30% of patients. However, the incidence of delayed cerebral ischemia (DCI) is lower in children (~10%) compared with adults, and children tolerate SAH better [3, 30,31,32,33]. Reasons for better outcomes in children may be related to higher cerebral blood flow, better collateral circulation, and less sensitivity to post-hemorrhagic spasm [30, 34]. Nevertheless, vasospasm causing cerebral ischemia or infarction is an important cause of significant morbidity in children.
7.2.1 Diagnosis of Vasospasm
-
(a)
Clinical: The sudden or gradual appearance of neurological deficits after 3 days of the onset of hemorrhage unexplained by concurrent structural or metabolic abnormalities suggests vasospasm.
Transcranial Doppler (TCD) is the most common noninvasive modality to diagnose vasospastic arteries. Vasospasm narrows the caliber of cerebral vessels and increases the flow velocity through these vessels. A rise of TCD-determined flow velocity more than 50 cm/s in 24 h or a flow velocity >120 cm/s are taken as indicators of vasospasm in adults. However, the cerebral blood flow velocity is lower in children and has a wide variation across age groups compared with adults. TCD studies show that the cerebral blood flow velocity is approximately 24 cm/s in neonates, which increases and peaks at around 7–9 years of age (approximately 100 cm/s) [34]. In older children, the cerebral blood flow velocity gradually decreases to parallel adult values (approximately 50 cm/s) [35]. Thus, it is difficult to establish flow velocity cut-off values in children to diagnose vasospasm. Serial TCD monitoring to establish trends may be more useful than absolute numbers.
-
(b)
Cerebral angiography is the most sensitive tool to diagnose cerebral vasospasm; however, the invasive nature of this modality precludes its frequent use in children. Around one-third of adults with angiographically detected vasospasm develop clinical features. This number is lower in children.
-
(c)
Jugular bulb oximetry: By detecting changes in cerebral oxygen extraction, jugular bulb oximetry is useful in predicting impending vasospasm. Patients who develop clinically significant vasospasm have an elevated cerebral oxygen extraction a day earlier than it can be recognised by the onset of symptoms of cerebral ischemia.
-
(d)
Xenon-enhanced CT scans, single photon emission computed tomography, and several other cerebral blood flow measuring tools, although not common in routine clinical practice, can be used for the diagnosis of vasospasm.
7.2.2 Treatment of Vasospasm and Delayed Cerebral Ischemia
Early endovascular or neurosurgical treatment of the aneurysm is the most important strategy to prevent vasospasm. “Triple H” therapy, including hypertension, hypervolemia, and hemodilution, has long been the mainstay for the management of cerebral vasospasm. This strategy, believed to increase cerebral blood flow (CBF) by expanding intravascular volume and reducing blood viscosity is no longer favored. Hemodynamic augmentation is now considered to be the first-line therapy to treat DCI. This can be achieved by increasing intravascular volume alone or by the use of vasopressors like norepinephrine and phenylephrine. There are practical problems of defining the target threshold for augmentation of blood pressure in children, as normal blood pressure has a wide range in children across different age groups. Hypervolemia increases cardiac output and may result in improved perfusion to the hypo-perfused regions, even though its use has been challenged in studies [36]. Even though in adults with DCI, there is emerging evidence to support euvolemia instead of hypervolemia, this is not clear in children. The risks of hypervolemia include congestive cardiac failure, pulmonary edema, coagulopathy, dilutional hyponatremia, and rebleeding. The benefit of hemodilution is not clear either and is the most controversial element of Triple H. A target hematocrit of 30–35% has been suggested in adults to provide an optimal balance between oxygen-carrying capacity and blood viscosity. The target hemoglobin value to achieve adequate cerebral oxygenation with optimal CBF is not known in children. Blood pressure augmentation should not be done before the aneurysm is secured. Even though there is a paucity of studies in children, no controlled trial has shown improved mortality with Triple H therapy [37, 38].
Nimodipine, which is a calcium channel blocker, is used for the prevention and treatment of vasospastic arteries and is the only proven pharmacological therapy to improve outcome after aneurysmal SAH in adults. The typical pediatric dose of oral nimodipine is 1 mg/kg every 4 h. However, some studies in children with SAH suggest that oral nimodipine use does not eliminate risk of vasospasm and cannot improve prognosis in cases having rebleeding and infarction [39]. In some children, significant hypotension has been seen after oral nimodipine use [39]. Owing to the risk of significant hypotension, the use of intravenous nimodipine in children is uncommon. Large prospective studies examining different dosing regimens and clinical benefits of nimodipine are warranted in children.
Magnesium sulfate has several physiological effects such as cerebral vasodilation, calcium antagonism, inhibition of excitatory postsynaptic potentials, and inhibition of the formation of free radicals after tissue injury. It has shown some promise in reducing vasospasm and improving outcomes with minimal side effects. However, two large randomized trials have found conflicting results on the benefits of magnesium in SAH [40, 41]. A follow-up meta-analysis also failed to demonstrate a favorable neurological outcome of magnesium after SAH [42]. Studies in children are not available. Endothelin receptor antagonists such as clazosentan were found to improve vasospasm in animal models, but the benefits did not translate in human trials [43,44,45,46]. Statins were earlier believed to be of benefit in SAH because of their anti-inflammatory and immunomodulatory effects, but large studies have not shown a significant benefit with their use [47]. Several other drugs that showed early promises, such as tirilazad, which a lipid peroxidation inhibitor, and nicaraven, a hydroxyl radical scavenger, are not useful either. Interventional strategies such as balloon angioplasty for focal stenosis and intra-arterial administration of vasodilators are labor intensive, involve significant risks, and are less commonly performed in children compared with adults.
7.3 Hydrocephalus
Hydrocephalus can be acute, occurring within 72 h of the initial bleeding, or chronic, occurring weeks or sometimes even months after the initial episode. Patients with poor-grade and large-sized aneurysms, thick SAH or intraventricular bleeding, and posterior circulation aneurysms have an increased risk of developing hydrocephalus. Hydrocephalus occurs in 20–30% of adults with aneurysmal SAH and is more common in children, probably because of the greater incidence of posterior circulation and complex aneurysms. Acute hydrocephalus may require the placement of an external ventricular drain (EVD) for decompression, and many children with chronic hydrocephalus require a CSF shunting procedure like ventriculoperitoneal shunt.
7.4 Seizures
Seizures are detrimental and should be promptly terminated because they increase CBF and increase oxygen requirements in an already compromised brain. Up to one-third of children with SAH can have seizures, which is more than in adults [48]. Seizures are more likely in patients with poor-grade SAH, intracerebral bleed, severe vasospasm, cerebral infarct, and rebleeding. There is no high-quality evidence on the benefit of prophylactic anti-seizure medications in adults or children with cerebral aneurysms [49].
7.5 Fluid and Electrolyte Disturbances
Intracranial volume contraction, hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia are common findings in children with SAH. Hyponatremia is extremely common and has been found in up to one-third of patients with SAH. The peak incidence of hyponatremia is between the 2nd and 10th days of SAH. Uncorrected hyponatremia lowers the seizure threshold, worsens cerebral edema, and can alter the level of consciousness. Prompt identification of the cause of hyponatremia is important to determine the appropriate treatment. In cerebral salt-wasting syndrome (CSWS), there is hyponatremia with concomitant extracellular volume contraction. Treatment with isotonic crystalloids to replenish sodium and water is appropriate. If syndrome of inappropriate antidiuretic hormone secretion (SIADH) is present, it is reasonable to restrict fluid intake and administer hypertonic saline. In children with SIADH, the fluid restriction should be done judiciously, as it can lead to rapid intravascular volume depletion and hemodynamic instability, which increases the risk of cerebral ischemia.
7.6 Pulmonary and Cardiac Complications
Pulmonary edema and hospital-acquired pneumonia are common problems in children with aneurysmal SAH. Myocardial dysfunction and arrhythmias are also common. Although 50–100% of adult patients with SAH have ECG changes, such as T-wave inversion, ST depression, U waves, and QT prolongation, the true incidence of electrocardiographic changes in children is not known. Arrhythmia most frequently occurs in the first week of SAH. Possible causes include injury to the posterior pituitary with norepinephrine release resulting in subendocardial injury, and concomitant dyselectrolytemia.
8 Treatment of Cerebral Aneurysm in Children
The treatment of unruptured cerebral aneurysms in children can be divided into surgical, endovascular, and conservative approaches [50]. The management of pediatric intracranial aneurysms differs from adults because of the complexities and heterogeneity of aneurysms seen in children. Children with SAH are most appropriately cared for in dedicated neuro-centers that have experienced neurovascular surgeons, neuroanesthesiologists, neurointerventionalists, and neurointensivists. In any child with SAH, medical management should be immediately started to stabilize the child and prevent secondary neurologic complications. It is important to be cognizant of the child’s intravascular volume status and hemodynamic parameters, which usually require the placement of a urinary catheter and strict charting of fluid balance. In a critically ill child, an indwelling arterial line is useful to optimize blood pressure and titrate vasoactive drugs. The requirement of sedatives and analgesics to treat the child’s anxiety and the headache of SAH should be balanced against the need to perform a frequent neurological examination. In some children with hydrocephalus, CSF flow diversion may be required before or after securing the aneurysm.
The best modality of securing pediatric aneurysms remains a matter of debate. Both endovascular procedures and surgical clipping have been widely performed with good results [51, 52]. There are concerns about the durability of endovascular devices as children’s life expectancy is longer compared with adults [12, 52]. However, a recent meta-analysis found both endovascular and surgical treatments yielded comparable long-term clinical outcomes in children [53]. The International Subarachnoid Aneurysm Trial (ISAT) in adults with ruptured aneurysms showed that endovascular coiling was more likely to result in independent survival than neurosurgical clipping, albeit with a slightly increased risk of rebleeding [54]. A long-term follow-up of the same cohort suggested that even though rebleeding is slightly increased after endovascular coiling, the probability of disability-free survival is higher in the endovascular group compared with neurosurgical clipping at 10 years [55]. Even though endovascular therapeutic options are increasingly preferred, treatment choice in different hospitals seems to be guided by protocols for adults, the preference and expertise of the neurosurgical units, and patient preference.
8.1 Surgical Treatment
Clipping of the aneurysm has been extensively described in the neurosurgical literature. There are reports of several surgical techniques, such as clipping, proximal occlusion with or without bypass, trapping, and wrapping of the aneurysm. The most commonly employed method is to clip the neck of the aneurysm. Aneurysms are increasingly being clipped early, within 24–48 h. The anesthesia goals are tailored for each child primarily based on the preoperative grade of the aneurysm, the proposed surgery, and the need for additional modalities such as intracranial-extracranial bypass procedures. Induced hypotension during the dissection phase of the aneurysm is no longer practiced, and instead many surgeries are carried out using proximal occlusion with temporary clips. Mild hypothermia (32–34 °) and various drugs like mannitol, thiopental, etomidate, propofol, and steroids have been proposed for cerebral protection during temporary clipping with no reliable evidence of their effectiveness [56,57,58].
8.2 Anesthetic Consideration During Surgical Clipping
The goals of neuroanesthesia during surgical clipping are to ensure stable perioperative hemodynamic parameters, perform smooth induction and extubation, provide adequate depth of anesthesia, lower the ICP, and maintain adequate cerebral oxygenation, normothermia, and normocarbia to mild hypocarbia. Unlike in adults, optimal perioperative blood pressure targets are not defined in children. It is reasonable to maintain the intraoperative blood pressure close to the baseline value throughout the conduct of anesthesia. Importantly, the blood pressure should not be allowed to drop below 20% of the baseline value, especially in poor-grade aneurysms, when the brain is already at a critical perfusion threshold. The neuroanesthesiologist should be prepared for a sudden and massive blood loss; it is crucial to have blood and blood products readily available for immediate administration.
8.2.1 Induction of Anesthesia
Propofol (1–2 mg/kg) or thiopental (4–6 mg/kg) in combination with opioids like fentanyl (2–4 μg/kg) or sufentanil (0.3–0.5 μg/kg) is commonly used for induction of anesthesia. Atracurium (0.5–0.8 mg/kg), vecuronium (0.1–0.12 mg/kg), or rocuronium (0.6–1.2 mg/kg) can be used for muscle paralysis. Laryngoscopy and intubation should be quick and smooth. Adjuncts such as lidocaine (1–2 mg/kg), beta-blockers like esmolol (0.25–1 mg/kg), or labetalol (5–20 mg) and additional boluses of fentanyl or propofol are useful to reduce the hemodynamic surge of laryngoscopy and intubation and before other intensely stimulating procedures like insertion of skull pins. A sudden rise of blood pressure will increase the transmural pressure gradient across the aneurysm wall, potentially leading to aneurysm rupture. The intraoperative rupture of the aneurysm carries a very poor prognosis, and it is important to take appropriate preventive measures. Although quite difficult in children, the placement of an arterial catheter before induction of anesthesia is useful for better controlling blood pressure.
8.2.2 Monitoring
Besides standard ASA monitoring (ECG, NIBP, EtCO2, SpO2, and temperature), direct intra-arterial blood pressure monitoring helps in beat-beat monitoring of blood pressure and close titration of vasoactive medications. At least one large-bore intravenous cannula and a central venous catheter are typically inserted. Monitoring the depth of anesthesia (derived electroencephalograph monitor, like bispectral index) and the degree of neuromuscular block (neuromuscular transmission monitor, like train-of-four) may be useful to titrate anesthetics and muscle relaxants. Unlike in adults, the use of intraoperative neuromonitoring like cerebral oximetry, jugular bulb oximetry, transcranial Doppler, and evoked potentials have rarely been described during pediatric aneurysm surgeries.
8.2.3 Maintenance of Anesthesia
With the increasing trend of early surgery for cerebral aneurysms, the challenges of providing a lax brain in an under-prepared patient while at the same time maintaining intraoperative hemodynamics are increasing. Volatile anesthetics like isoflurane, sevoflurane, and desflurane or total intravenous anesthesia with propofol and fentanyl (0.5–2 μg/kg/h) or remifentanil (0.125–0.25 μg/kg/min) can be used. There is no strong evidence to avoid the use of nitrous oxide, and its use varies in different institutions. However, it is prudent to avoid nitrous oxide in patients with high-grade SAH or those with intraoperative “tense brain.” Mannitol (20%), an osmotic diuretic, is frequently used to provide brain relaxation as a slow infusion in dosages of 0.25 mg/kg. Hypertonic saline is an alternative; no intraoperative study has shown a clear outcome benefit of one over the other in children [59,60,61,62]. Experimental models have shown possible benefits of inducing mild hypothermia (33–35 °C) for neuroprotection; however, this has not translated into a clinical benefit in aneurysm surgeries [63]. Normocarbia to mild hypocarbia (PaCO2: 35–40 mmHg) is most commonly employed. Controlled hypotension during the early dissection phase of the surgery is no longer practiced. Instead, occlusion of the feeding vessels using temporary clips may be used to facilitate exposure and prevent aneurysm rupture. The potential problems using temporary clips are focal cerebral infarction and arterial damage because of the clip application. It is important to augment the blood pressure during temporary clipping to maintain regional cerebral perfusion by an increased collateral blood flow.
Sometimes, the surgeon may request the anesthesiologist to administer intravenous indocyanine green (ICG) dye after clipping the aneurysm. ICG is a near-infrared fluorescent dye used to detect major surgical issues like residual filling of the clipped aneurysm and parent or branching artery occlusion, while the surgical field remains exposed intraoperatively so that immediate revision can be done if required [64]. It is available as a lyophilized green-colored powder that is dissolved in sterile water before intravenous administration. The safety and effectiveness of ICG have been established in children, and the maximum permissible dose is 2 mg/kg [65].
8.2.4 Recovery and Extubation
The decision to extubate the trachea at the end of the surgery is based on preoperative patient status and intraoperative surgical or anesthetic events. Good-grade aneurysm patients with an uneventful intraoperative course may be extubated inside the operating room. Others may require a period of postoperative mechanical ventilation. These patients should be shifted to the intensive care unit for ventilation and further management.
8.3 Endovascular Treatment
In the last few years, there has been a tremendous shift in the treatment approach for pediatric aneurysms from open surgery to endovascular techniques and multi-modality therapeutic plans. A growing body of evidence points at the safety of the endovascular approach and its effectiveness in preventing early rebleeding [66, 67]. Several observational and small cohort studies have found the overall clinical outcome of endovascular treatment to be as good or even superior to open surgical methods in children [68,69,70,71]. It is particularly useful when a difficult anatomic location of the aneurysm precludes the use of open surgical methods, such as with posterior circulation aneurysms. Similarly, partially clipped aneurysms and patients with poor-grade aneurysms benefit from endovascular methods. The most popular endovascular technique uses the Guglielmi detachable coil (GDC), which is inserted into the aneurysmal sac, setting up secondary thrombosis. Adjuvant techniques like balloon-assisted coiling or stent-assisted coiling can be used to support a wide-necked aneurysm and prevent coil prolapse into the parent artery [72]. Other endovascular options such as placement of flow-diverting stents (like Pipeline Embolization Device), liquid embolization, or endovascular vessel sacrifice may be required to treat complex aneurysms [53, 73].
8.3.1 Anesthetic Considerations During Endovascular Treatment
The major considerations include providing anesthesia care in a suboptimal anesthetic environment outside the operation rooms, transportation of patients, anticoagulation, management of sudden catastrophic events such as aneurysm rupture and contrast-related anaphylaxis, ensuring early recovery from anesthesia for neurological assessment, protection from radiation hazards, and the care of generally sicker and under-prepared patients, who may have been considered too risky to undergo urgent neurosurgical procedures.
8.3.2 Choice of Anesthesia
General anesthesia with endotracheal intubation is preferred in children as it allows immobility for accurate imaging and intervention, control of PaCO2, and prompt management of possible catastrophic complications. Patient accessibility is always a problem in radiological suites; using two large-bore cannulas with extension tubing is important. Standard ASA monitoring is mandatory. An invasive arterial catheter is useful for beat-to-beat BP monitoring as well as for blood sampling.
8.3.3 Maintenance of Anesthesia
Like in surgical clipping, both total intravenous anesthesia and volatile anesthetics have been used. There is no clear superiority of one anesthetic technique over another. Anticoagulation is achieved using a loading dose of 60–80 units/kg heparin followed by either intermittent boluses or a continuous infusion to maintain activated clotting time (ACT) two to three times the baseline [72].
8.3.4 Recovery and Extubation
The course of recovery from anesthesia and the decision to extubate the trachea at the end of the procedure follows the same basic principles outlined in open surgical methods.
9 Conclusion
Intracranial aneurysms in children are rare. As there are very few studies in pediatric cerebral aneurysms, little is known about their pathogenesis, clinical grading, and treatment modalities. Most of the anesthetic practices have been simply extrapolated from evidences that are derived from adult literature. Even though the basic principles of neuroanesthesia and neurocritical care may remain the same, caution must be exercised when extrapolating such evidences. Large prospective studies in pediatric aneurysm surgeries are clearly warranted.
References
Jordan LC, Johnston SC, Wu YW, Sidney S, Fullerton HJ. The importance of cerebral aneurysms in childhood hemorrhagic stroke: a population-based study. Stroke. 2009;40:400–5.
Hetts SW, Narvid J, Sanai N, Lawton MT, Gupta N, Fullerton HJ, Dowd CF, Higashida RT, Halbach VV. Intracranial aneurysms in childhood: 27-year single-institution experience. AJNR Am J Neuroradiol. 2009;30:1315–24.
Goia A, Garrido E, Lefebvre M, Langlois O, Derrey S, Papagiannaki C, Gilard V. Ruptured intracranial aneurysm in a neonate: case report and review of the literature. World Neurosurg. 2020;140:219–23.
Mohotti JE, Carter NS, Zhang VJW, Lai LT, Xenos C, Asadi H, Chandra RV. Neonatal intracranial aneurysms: case report and review of the literature. J Neurosurg Pediatr. 2018;21:471–7.
Garg K, Singh PK, Sharma BS, Chandra PS, Suri A, Singh M, Kumar R, Kale SS, Mishra NK, Gaikwad SK, Mahapatra AK. Pediatric intracranial aneurysms—our experience and review of literature. Childs Nerv Syst. 2014;30:873–83.
Roche JL, Choux M, Czorny A, Dhellemmes P, Fast M, Frerebeau P, Lapras C, Sautreaux JL. [intracranial arterial aneurysm in children. A cooperative study. Apropos of 43 cases]. Neuro-Chirurgie. 1988;34:243–51.
Brook-Carter PT, Peral B, Ward CJ, Thompson P, Hughes J, Maheshwar MM, Nellist M, Gamble V, Harris PC, Sampson JR. Deletion of the tsc2 and pkd1 genes associated with severe infantile polycystic kidney disease—a contiguous gene syndrome. Nat Genet. 1994;8:328–32.
Halvorson CR, Bremmer MS, Jacobs SC. Polycystic kidney disease: inheritance, pathophysiology, prognosis, and treatment. Int J Nephrol Renov Dis. 2010;3:69–83.
North KN, Whiteman DA, Pepin MG, Byers PH. Cerebrovascular complications in ehlers-danlos syndrome type iv. Ann Neurol. 1995;38:960–4.
Oyesiku NM, Barrow DL, Eckman JR, Tindall SC, Colohan AR. Intracranial aneurysms in sickle-cell anemia: clinical features and pathogenesis. J Neurosurg. 1991;75:356–63.
Donti A, Spinardi L, Brighenti M, Faccioli L, Leoni C, Fabi M, Trossello MP, Gargiulo GD, Bonvicini M. Frequency of intracranial aneurysms determined by magnetic resonance angiography in children (mean age 16) having operative or endovascular treatment of coarctation of the aorta (mean age 3). Am J Cardiol. 2015;116:630–3.
Sanai N, Quinones-Hinojosa A, Gupta NM, Perry V, Sun PP, Wilson CB, Lawton MT. Pediatric intracranial aneurysms: durability of treatment following microsurgical and endovascular management. J Neurosurg. 2006;104:82–9.
Gemmete JJ, Toma AK, Davagnanam I, Robertson F, Brew S. Pediatric cerebral aneurysms. Neuroimaging Clin N Am. 2013;23:771–9.
Mehrotra A, Nair AP, Das KK, Srivastava A, Sahu RN, Kumar R. Clinical and radiological profiles and outcomes in pediatric patients with intracranial aneurysms. J Neurosurg Pediatr. 2012;10:340–6.
Jian Z, Yang Z, Smerin D, Xiong X. Intracranial giant aneurysms in children and adolescents misdiagnosed as intracranial tumors before operation: 2 cases report. Int J Clin Exp Med. 2018;11:6268–75.
Villablanca JP, Jahan R, Hooshi P, Lim S, Duckwiler G, Patel A, Sayre J, Martin N, Frazee J, Bentson J, Vinuela F. Detection and characterization of very small cerebral aneurysms by using 2d and 3d helical ct angiography. AJNR Am J Neuroradiol. 2002;23:1187–98.
Botterell EH, Lougheed WM, Scott JW, Vandewater SL. Hypothermia, and interruption of carotid, or carotid and vertebral circulation, in the surgical management of intracranial aneurysms. J Neurosurg. 1956;13:1–42.
Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20.
Hunt WE, Kosnik EJ. Timing and perioperative care in intracranial aneurysm surgery. Clin Neurosurg. 1974;21:79–89.
Drake CG, Hunt WE, Sano K, Kassell N, Teasdale G, Pertuiset B, et al. Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J Neurosurg. 1988;68:985–6.
Sano H, Satoh A, Murayama Y, Kato Y, Origasa H, Inamasu J, Nouri M, Cherian I, Saito N. Modified world federation of neurosurgical societies subarachnoid hemorrhage grading system. World Neurosurg. 2015;83:801–7.
Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9.
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES Jr, MacDonald RL, Mayer SA. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59:21–7.
Vaid VK, Kumar R, Kalra SK, Mahapatra AK, Jain VK. Pediatric intracranial aneurysms: an institutional experience. Pediatr Neurosurg. 2008;44:296–301.
Proust F, Toussaint P, Garnieri J, Hannequin D, Legars D, Houtteville JP, Freger P. Pediatric cerebral aneurysms. J Neurosurg. 2001;94:733–9.
Storrs BB, Humphreys RP, Hendrick EB, Hoffman HJ. Intracranial aneurysms in the pediatric age-group. Childs Brain. 1982;9:358–61.
Hillman J, Fridriksson S, Nilsson O, Yu Z, Saveland H, Jakobsson KE. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg. 2002;97:771–8.
Fodstad H, Forssell A, Liliequist B, Schannong M. Antifibrinolysis with tranexamic acid in aneurysmal subarachnoid hemorrhage: a consecutive controlled clinical trial. Neurosurgery. 1981;8:158–65.
Baharoglu MI, Germans MR, Rinkel GJ, Algra A, Vermeulen M, van Gijn J, Roos YB. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2013;2013:Cd001245.
Lasjaunias P, Wuppalapati S, Alvarez H, Rodesch G, Ozanne A. Intracranial aneurysms in children aged under 15 years: review of 59 consecutive children with 75 aneurysms. Childs Nerv Syst. 2005;21:437–50.
Aryan HE, Giannotta SL, Fukushima T, Park MS, Ozgur BM, Levy ML. Aneurysms in children: review of 15 years experience. J Clin Neurosci. 2006;13:188–92.
Huang J, McGirt MJ, Gailloud P, Tamargo RJ. Intracranial aneurysms in the pediatric population: case series and literature review. Surg Neurol. 2005;63:424–32.
Beez T, Steiger HJ, Hanggi D. Evolution of management of intracranial aneurysms in children: a systematic review of the modern literature. J Child Neurol. 2016;31:773–83.
Suzuki J, Kodama N. Moyamoya disease—a review. Stroke. 1983;14:104–9.
Udomphorn Y, Armstead WM, Vavilala MS. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr Neurol. 2008;38:225–34.
Lennihan L, Mayer SA, Fink ME, Beckford A, Paik MC, Zhang H, Wu YC, Klebanoff LM, Raps EC, Solomon RA. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2000;31:383–91.
Sen J, Belli A, Albon H, Morgan L, Petzold A, Kitchen N. Triple-h therapy in the management of aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2003;2:614–21.
Egge A, Waterloo K, Sjoholm H, Solberg T, Ingebrigtsen T, Romner B. Prophylactic hyperdynamic postoperative fluid therapy after aneurysmal subarachnoid hemorrhage: a clinical, prospective, randomized, controlled study. Neurosurgery. 2001;49:593–605.
Heffren J, McIntosh AM, Reiter PD. Nimodipine for the prevention of cerebral vasospasm after subarachnoid hemorrhage in 12 children. Pediatr Neurol. 2015;52:356–60.
Wong GK, Poon WS, Chan MT, Boet R, Gin T, Ng SC, Zee BC. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage (imash): a randomized, double-blinded, placebo-controlled, multicenter phase iii trial. Stroke. 2010;41:921–6.
Westermaier T, Stetter C, Vince GH, Pham M, Tejon JP, Eriskat J, Kunze E, Matthies C, Ernestus RI, Solymosi L, Roosen K. Prophylactic intravenous magnesium sulfate for treatment of aneurysmal subarachnoid hemorrhage: a randomized, placebo-controlled, clinical study. Crit Care Med. 2010;38:1284–90.
Wong GK, Boet R, Poon WS, Chan MT, Gin T, Ng SC, Zee BC. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage: an updated systemic review and meta-analysis. Crit Care. 2011;15:R52.
Povlsen GK, Edvinsson L. Mek1/2 inhibitor u0126 but not endothelin receptor antagonist clazosentan reduces upregulation of cerebrovascular contractile receptors and delayed cerebral ischemia, and improves outcome after subarachnoid hemorrhage in rats. J Cereb Blood Flow Metab. 2015;35:329–37.
Laban KG, Vergouwen MD, Dijkhuizen RM, Sena ES, Macleod MR, Rinkel GJ, van der Worp HB. Effect of endothelin receptor antagonists on clinically relevant outcomes after experimental subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2015;35:1085–9.
Ma J, Huang S, Ma L, Liu Y, Li H, You C. Endothelin-receptor antagonists for aneurysmal subarachnoid hemorrhage: an updated meta-analysis of randomized controlled trials. Crit Care. 2012;16:R198.
Vergouwen MD, Algra A, Rinkel GJ. Endothelin receptor antagonists for aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke. 2012;43:2671–6.
Su SH, Xu W, Hai J, Wu YF, Yu F. Effects of statins-use for patients with aneurysmal subarachnoid hemorrhage: a meta-analysis of randomized controlled trials. Sci Rep. 2014;4:4573.
Al-Jarallah A, Al-Rifai MT, Riela AR, Roach ES. Nontraumatic brain hemorrhage in children: etiology and presentation. J Child Neurol. 2000;15:284–9.
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2012;43:1711–37.
Bisson DA, Dirks P, Amirabadi A, Shroff MM, Krings T, Pereira VM, Muthusami P. Unruptured intracranial aneurysms in children: 18 years’ experience in a tertiary care pediatric institution. J Neurosurg. 2019:1–6.
Amelot A, Saliou G, Benichi S, Alias Q, Boulouis G, Zerah M, Aghakhani N, Ozanne A, Blauwblomme T, Naggara O. Long-term outcomes of cerebral aneurysms in children. Pediatrics. 2019;143
Stiefel MF, Heuer GG, Basil AK, Weigele JB, Sutton LN, Hurst RW, Storm PB. Endovascular and surgical treatment of ruptured cerebral aneurysms in pediatric patients. Neurosurgery. 2008;63:859–65.
Yasin JT, Wallace AN, Madaelil TP, Osbun JW, Moran CJ, Cross DT, Limbrick DD, Zipfel GJ, Dacey RG, Kansagra AP. Treatment of pediatric intracranial aneurysms: case series and meta-analysis. J Neurointerv Surgery. 2019;11:257–64.
Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P. International subarachnoid aneurysm trial (isat) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–17.
Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the uk cohort of the international subarachnoid aneurysm trial (isat). Lancet. 2015;385:691–7.
Ogilvy CS, Carter BS, Kaplan S, Rich C, Crowell RM. Temporary vessel occlusion for aneurysm surgery: risk factors for stroke in patients protected by induced hypothermia and hypertension and intravenous mannitol administration. J Neurosurg. 1996;84:785–91.
Lavine SD, Masri LS, Levy ML, Giannotta SL. Temporary occlusion of the middle cerebral artery in intracranial aneurysm surgery: time limitation and advantage of brain protection. J Neurosurg. 1997;87:817–24.
Galvin IM, Levy R, Boyd JG, Day AG, Wallace MC. Cooling for cerebral protection during brain surgery. Cochrane Database Syst Rev. 2015;1:Cd006638.
Raghava A, Bidkar PU, Prakash MV, Hemavathy B. Comparison of equiosmolar concentrations of hypertonic saline and mannitol for intraoperative lax brain in patients undergoing craniotomy. Surg Neurol Int. 2015;6:73.
Gemma M, Cozzi S, Tommasino C, Mungo M, Calvi MR, Cipriani A, Garancini MP. 7.5% hypertonic saline versus 20% mannitol during elective neurosurgical supratentorial procedures. J Neurosurg Anesthesiol. 1997;9:329–34.
Erard AC, Walder B, Ravussin P. [effects of equiosmolar load of 20% mannitol, 7.5% saline and 0.9% saline on plasma osmolarity, haemodynamics and plasma concentrations of electrolytes]. Ann Fr Anesth Reanim. 2003;22:18–24.
Rozet I, Tontisirin N, Muangman S, Vavilala MS, Souter MJ, Lee LA, Kincaid MS, Britz GW, Lam AM. Effect of equiosmolar solutions of mannitol versus hypertonic saline on intraoperative brain relaxation and electrolyte balance. Anesthesiology. 2007;107:697–704.
Nguyen HP, Zaroff JG, Bayman EO, Gelb AW, Todd MM, Hindman BJ. Perioperative hypothermia (33 degrees c) does not increase the occurrence of cardiovascular events in patients undergoing cerebral aneurysm surgery: findings from the intraoperative hypothermia for aneurysm surgery trial. Anesthesiology. 2010;113:327–42.
Ma CY, Shi JX, Wang HD, Hang CH, Cheng HL, Wu W. Intraoperative indocyanine green angiography in intracranial aneurysm surgery: microsurgical clipping and revascularization. Clin Neurol Neurosurg. 2009;111:840–6.
Esposito C, Del Conte F, Cerulo M, Gargiulo F, Izzo S, Esposito G, Spagnuolo MI, Escolino M. Clinical application and technical standardization of indocyanine green fluorescence imaging in pediatric minimally invasive surgery. Pediatr Surg Int. 2019;35:1043–50.
White PM, Lewis SC, Gholkar A, Sellar RJ, Nahser H, Cognard C, Forrester L, Wardlaw JM. Hydrogel-coated coils versus bare platinum coils for the endovascular treatment of intracranial aneurysms (helps): a randomised controlled trial. Lancet. 2011;377:1655–62.
Molyneux AJ, Clarke A, Sneade M, Mehta Z, Coley S, Roy D, Kallmes DF, Fox AJ. Cerecyte coil trial: angiographic outcomes of a prospective randomized trial comparing endovascular coiling of cerebral aneurysms with either cerecyte or bare platinum coils. Stroke. 2012;43:2544–50.
Agid R, Souza MP, Reintamm G, Armstrong D, Dirks P, TerBrugge KG. The role of endovascular treatment for pediatric aneurysms. Childs Nerv Syst. 2005;21:1030–6.
Lasjaunias PL, Campi A, Rodesch G, Alvarez H, Kanaan I, Taylor W. Aneurysmal disease in children. Review of 20 cases with intracranial arterial localisations. Interv Neuroradiol. 1997;3:215–29.
Alawi A, Edgell RC, Elbabaa SK, Callison RC, Khalili YA, Allam H, Alshekhlee A. Treatment of cerebral aneurysms in children: analysis of the kids’ inpatient database. J Neurosurg Pediatr. 2014;14:23–30.
Garg M, Shambanduram S, Singh PK, Sebastian LJD, Sawarkar DP, Kumar A, Gaikwad S, Chandra PS, Kale SS. Management of pediatric posterior circulation aneurysms-12-year single-institution experience. World Neurosurg. 2018;116:e624–e33.
De Sloovere VT. Anesthesia for embolization of cerebral aneurysms. Curr Opin Anesthesiol. 2014;27:431–6.
Lv X, Jiang C, Li Y, Yang X, Wu Z. Endovascular treatment for pediatric intracranial aneurysms. Neuroradiology. 2009;51:749–54.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Ethics declarations
None declared for each author.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Lamsal, R., Mahajan, C. (2021). Anesthetic Management of Cerebral Aneurysm Surgery in Children. In: Rath, G.P. (eds) Fundamentals of Pediatric Neuroanesthesia. Springer, Singapore. https://doi.org/10.1007/978-981-16-3376-8_22
Download citation
DOI: https://doi.org/10.1007/978-981-16-3376-8_22
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3375-1
Online ISBN: 978-981-16-3376-8
eBook Packages: MedicineMedicine (R0)