Abstract
Vector-borne diseases like malaria, dengue, chikungunya, Japanese encephalitis, Zika and others claim millions of lives across the globe annually, and as such their control has become an ardent necessity. Past attempts over the decades have introduced vector control through chemical, biological and environmental means. However, these measures, already in place, failed to completely bring down the mortality rates from vector-borne diseases, most of which lack a vaccine to prevent epidemics or even a specific antidote to treat patients. The modern development of technologies such as the release of insects carrying a dominant lethal (RIDL) gene system, an example of transgenesis; the Wolbachia-based cytoplasmic incompatibility inducing infertility in female insects, an example of paratransgenesis; and the revolutionary gene drive (CRISPR/Cas9) technology, has their roots in the sterile insect technology (SIT), which worked by creating sterilized males through irradiation to compete with their wild counterparts and subsequently mate with females in nature to produce infertile eggs; a technology meant to gradually and finally exterminate the vector population in nature. These technologies have shown great promise, albeit many imperfections, particularly regarding acceptance by the concerned societies. As far as vector control is concerned, we have attempted to simplify their definitions for the common man so that the intricate scientific jargon about these technologies do not instill any fear or doubts to the end users.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Vectors play a pivotal role in dissemination of a large number of deadly and debilitating diseases such as malaria, filariasis, dengue, chikungunya, Zika virus, onchocerciasis, Chagas’ disease, West Nile virus, Japanese encephalitis, etc. that, under the impact of changing climate, are prevalent widely across the tropical and subtropical countries (Gould and Higgs 2009). The control of vector for the global elimination of several diseases like dengue and Zika virus has taken a greater degree of importance due to lack of a specific vaccine or a drug to deal with the infections. Conventionally, insecticides, repellants, biological agents and environmental tools have been applied to keep the vectors under control or to deter them from making contact with human beings, but all the methodologies had several pitfalls in their operation (administrative, operational and storage), and the control of vector was never really achieved to evade infection from mosquito bites (Tyagi 2020). The modern biotechnological advancement has offered several new technologies which are largely sound, economic and practically applicable. While past technologies such as the release of insects having dominant lethal (RIDL) gene system (transgenesis) and Wolbachia-based introduction of cytoplasmic incompatibility in the host body of vector and pathogen to bring about infertility in female insects (paratransgenesis) have been successful in attracting the world’s attention, notwithstanding social, regulatory and operational bottlenecks, the application of recently developed gene drive technologies, encompassing CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9), in vector control has rekindled an altogether new hope in vector control and the diseases they transmit.
7.2 Tools of Genetics: A Potential Game Changer in Vector Management
The introduction of genetic engineering tools in the manipulation of both vectors and disease pathogens has put forth a rejuvenated enterprise globally (Marshall 2010, 2011). Transgenesis involves the direct alteration in the vector through the introduction of a transgene or modified gene, whereas paratransgenesis deals with genetic manipulation of a symbiotic microbe whose protein product interferes with the infective stages of the pathogen. Both these genetic approaches have been preliminarily exploited and have provided considerable insight towards an era of highly promising vector control technologies.
All of the above technologies are, however, developed through highly complicated processes using genetics, genomics, biotechnology and biochemistry as their basic disciplines. In spite of their immense effectiveness, these technologies have left an indelible imprint of a kind of enigmatic maze or jargon of terminologies on the mind of a common man, whereby the end user of the benefits of these technologies finds himself sceptical to accept these tools. We have in this chapter tried to define these technologies in a very simple manner with examples so that even an early student of science can see through the scientific intricacies of these processes and understand, in fine, their practical utility in consonance with the prevailing socioecological considerations. In essence, we are saying, “What are they?”
7.3 Transgenesis
Transgenesis, which literally means transfer of a foreign gene in the hosts body, actually involves the direct alteration in the vector through the introduction of a transgene or modified gene. By introducing a small sequence of foreign DNA into a fertilized egg or developing embryo, animals can be made transgenic. Genetically modified mosquitoes have been created aiming at suppressing target mosquito populations by releasing transgenic males carrying a lethal dominant gene. As described above, an experimentally introduced DNA segment within the genome of a host animal is called a transgene, and it is the transgene which takes the centre stage of host’s biological sense of functionality. A gene of the host at the insertion site can be altered or disrupted and in many cases, a transgene will do both. For example, while on one hand, it will disrupt an endogenous gene expressing a new gene product, it can also impact direct genetic modifications in vector mosquitoes as a novel initiative towards disease control. In Cayman Islands and Brazil, the use of OX513A has been successfully demonstrated to suppress the vector populations of Aedes aegypti, the primary vector of the deadly dengue infection (Harris et al. 2012; Carvalho et al. 2015).
In India, two institutions, e.g. the International Institute of Biotechnology and Toxicology (IIBAT) at Padappai, Tamil Nadu, and Maharashtra Hybrid Seeds Co. (MAHYCO) at Jalna, Maharashtra State, took up laboratory and simulated field studies in the early 2000s, with an objective to release transgenic mosquito, Aedes aegypti OX513A in the field, following approval from the regulators and community acceptance.
7.3.1 International Institute of Biotechnology and Toxicology (IIBAT)
The Coromandel Indag Research Centre, a farmed non-profit research trust/society recognized by the Ministry of Science and Technology, Govt. of India under the DSIR scheme, was established in 1978, and it was subsequently rechristened as the Fredrick Institute of Plant Protection and Toxicology (FIPPAT) in 1985 and eventually the International Institute of Biotechnology and Toxicology (IIBAT) in 2002. It earned the reputation of the first ever India-owned successful institutional venture of pesticide manufacture and evaluation. In 2004, the IIBAT rose to the level of a GLP-certified contract research organization delivering contract services in the areas of toxicology, analytical chemistry, ecotoxicology, genetic toxicology, radioisotope studies, etc.
The IIBAT was permitted officially to import the transgenic strain of Aedes aegypti, OX513A, from Oxitec (Oxford Insect Technologies) Limited, a UK-based company and a spin-off from Oxford University, in 2008, and they were mandated to carry out studies on single objective: “Mating competitiveness of an Asian outcrossed RIDL® strain of mosquito Aedes aegypti under total containment”. In order to carry out experiments, the IIBAT constructed an Arthropod Containment Level II (ACL II) laboratory with all necessary facilities (Fig. 7.1) to conduct experiments with rearing, sexing out, mating, etc. on both the GMM (OX513A) and wild populations of the mosquito, Aedes aegypti.
A lot of biological parameters including mating competitiveness between the RIDL and wild Aedes aegypti populations were found to be satisfactorily matching. However, the IIBAT suddenly aborted the project after a successful duration of nearly 3 years!
7.3.2 Gangabishan Bhikulal Investment and Trading Limited (GBIT)
The laboratory and other paraphernalia on genetically modified Aedes aegypti, imported as eggs from the Oxitec, UK, are located at the site of Maharashtra Hybrid Seeds Co. (MAHYCO), founded in 1964. The MAHYCO is an agricultural Indian company based at Dawalwadi in Jalna (Maharashtra State) and is one of the major seed producers in the country. Its offshoot, Gangabishan Bhikulal Investment and Trading Limited (GBIT), established in 1996, established research and development wing alluding to studies on the biological compatibilities between OX513A Aedes aegypti and wild strain of the mosquito, eventually to utilize the technology for the control of Ae. aegypti—the deadly dengue vector mosquito worldwide (Patil et al. 2015). Based on extensive investigations on the Friendly™ OX513A Aedes aegypti in various situations, including field in Cayman Islands, the following salient features were understood:
-
1.
Friendly™ OX513A is a self-limiting, efficient and cost-effective tool for mosquito control.
-
2.
Friendly™ OX513A is species specific, with no direct impact on non-target organisms.
-
3.
Friendly™ OX513A is environmentally friendly with no adverse effect on human or environmental health.
-
4.
Release in field is made of male mosquitoes which do not bite and cannot transmit disease.
-
5.
Released males seek out wild females and mate; eggs laid by females die before reaching adulthood and so the pest population declines.
-
6.
Released adult mosquitoes do not establish in the environment due to the absence of tetracycline in nature.
With such a prolific background in genetic and hybridization research, the institute had taken up to study at laboratory and simulated field situations the suitability of application in nature after getting green signal from the concerned regulatory authority in the country.
GBIT built a state-of-art Arthropod Containment Level II laboratory facility for phase I research activities on OX513A Ae. aegypti strain and a physically contained facility for conducting phase II field cage trials (Fig. 7.2). They have been working on the Friendly™ OX513A strain since 2011 in collaboration with Oxitec representative in India. Evaluation of the Friendly™ OX513A strain under laboratory conditions during phase I studies and physically contained large field cage during phase II studies simulating natural environmental conditions has shown encouraging results (Patil et al. 2018). They aimed at successful suppression of the wild-type Ae. aegypti population in the field cage trial and are trying to push their studies to stage III, i.e. field release, subject to mandatory regulatory approval. Whenever executed, this experiment will offer a vastly useful database.
7.4 Paratransgenesis
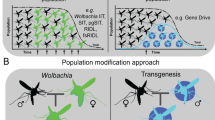
Paratransgenesis deals with genetic manipulation of an intracellular symbiotic microbe whose protein product interferes with the infective stages of the pathogen and/or genome of the host vector. For successful implication of paratransgenesis, the scope of cultivation of the symbiotic microorganisms should be plausible, and their amenability to genetic manipulation within the host body is also necessary. Moreover, they are required to be easily propagated in insects such as mosquitoes to facilitate the propagation of chosen traits. Of several bacteria assayed so far, Wolbachia (many species), naturally prevalent in a large number of arthropod species including mosquitoes though quaintly enough entirely lacking in Aedes aegypti, has shown tremendous potential to bring about control of certain vector mosquitoes. For instance, in China, Wolbachia-induced cytoplasmic incompatibility is being deployed to suppress the Aedes albopictus population at an island village in Guangzhou (Fig 7.2).
Paratransgenesis is a technique applied to replace the vector population and/or exterminate pathogens via introduction of transgenic symbiont bacteria into the vectors. Correct identification of proteins that are capable of preventing the transmission of pathogens within the vector species is the necessary first step. Thereafter, the genes encoding the identified proteins are introduced into the symbiont, so that they can be ultimately expressed within the vector. The final step involves the introduction of the transgenic variants of symbionts into the natural vector populations. In India, the Indian Council of Medical Research’s Vector Control Research Centre, Puducherry, has undertaken a research project on Wolbachia-based control of Aedes aegypti in collaboration with Monash University, Australia.
7.5 Gene Drives and CRISPR
CRISPR, pronounced as “crisper” for convenience of conversation, is among the latest technologies being applied successfully, to a large extent, in agriculture and, in infancy or trial stage, in certain health-related matters such as human disease vectors and animal pests (Marshall and Akbari 2018). Specialized CRISPR-associated protein or Cas proteins like “Cas-9” snip foreign DNA into small fragments and paste them into CRISPR sequence to generate CRISPR RNAs (crRNAs). These crRNAs guide the subsequent recognition and processing of exogenous genetic material by Cas nucleases. A crRNA, or guide RNA (gRNA), is able to cut along specific parts of the DNA sequence, allowing us to edit genes more easily, cheaply and quickly than ever before. The CAS-9 clips off DNA at a pre-decided locus with a precision of a phlebotomist.
The CRISPR is now available in many formats, and CRISPR/Cas-9 is one highly specific moiety or design whose one potential use is gene drives. It can be simply understood from the Mendelian formula that a parent’s traits are usually inherited among 50% of their offspring, but a gene drive can dictate a trait to be inherited greater than 50% of the time. This implies that through gene drive, DNA is manipulable resulting into kinds of individuals and populations which express traits as per designed requirement of science.
Gene drive technologies aim to propagate a trait faster among infective mosquito species and can lead to vector population control or an imminent break to disease transmission. It is a technology that exploits genetic engineering to propagate a particular set of genes throughout a natural vector population by altering the probability of inheritance, such that a specific allele will be transmitted to offspring (instead of the Mendelian 50% probability). Gene drives can be brought about by a variety of mechanisms. Gene drives are expected to provide an effective means of genetically modifying specific populations and/or entire species. The technique can employ adding, deleting, disrupting or modifying genes (Fig. 7.3).
In contrast to their available technology, gene drives have the greatest advantage in effecting manipulation in genome from within the same host DNA where the new trait is finally obtained. Thus, there is no need at all for transferring a gene from outside host, generally a bacterium, or other microbes, and therefore also there are no undue fears of the unseen dangers due to introduction of a foreign gene. In fact, gene drives can be used for a variety of purposes with varied goals in the realms of health, for example, suppression as well as replacement of disease mosquito vector or pest population (Fig. 7.4).
7.6 Conclusion
Vector-borne diseases, notably those spread by mosquitoes, have caused massive havoc globally, with millions of lives being affected and leaving economies grappling with challenges even in the face of slowly emerging solutions. Over the years with innumerate policies in place and programmes encompassing biological, chemical, environmental control of vectors and diseases they transmit put into action, the problem is yet far from over. The novel concepts of genetic modifications in symbiotic gut microbiome such as Wolbachia and Asaia, or identification of innate immune response factors like FREPs, to block pathogenic transmission, are en route to becoming successful models for a one-of-a-kind disease intervention technique in vector biology. The implementation of CRISPR/Cas9 and RNAi-based editing tools has further propagated the trend towards a more holistic control programme. This not only allowed to interpret and intercept vectors and their disease transmission cycles but opened up possibilities to dissect the molecular interactions that occur during the course of infections. Methods such as gene drive, which is a natural process and technology of genome manipulation that propagates a particular suite of genes throughout a population by altering the probability that a specific allele will be transmitted to offspring and adds in the suppression/replacement of pest or vector population, have further opened new vistas of vector control technologies. In fact, both transgenesis and paratransgenesis are two powerful tools of vector control, and several labs across India are involved in experimental implementation of these techniques at various stages of vector and disease control research in animals and plants. One such instance is illustrated through the OX513A Aedes aegypti strain developed as a control measure against dengue. The search for novel ways to curb vector-host interactions and subsequent disease manifestations is, however, multidimensional and has led scientists to take a closer look at the mosquito olfactory receptors. Among them, ionotropic receptors (IRs) are understood to have an important effect on host detection. To verify this, CRISPR/Cas9-based editing has been employed to modify Ir8 receptors in Aedes aegypti. These findings can be applied to generate transgenic mosquitoes with a potential to reduce, suppress or replace the vector population, making way for newer vector control strategies. In essence, the introduction of genetics as a combat tool against the widely prevalent vector-borne diseases has given a directionality in an otherwise bleak disease control scenario, plagued by drug resistance, climate-change-induced behavioural modifications, increasing demographics and mutating viral load.
References
Carvalho DO, McKemey AR, Garziera L et al (2015) Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl Trop Dis 9(7):e0003864
Gould EA, Higgs S (2009) Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg 103(2):109–121
Harris AF, McKemey AR, Nimmo D et al (2012) Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat Biotechnol 30(9):828–830
Marshall JM (2010) The Cartagena protocol and genetically modified mosquitoes. Nat Biotechnol 28:896–897
Marshall JM (2011) The Cartagena protocol in the context of recent releases of transgenic and Wolbachia-infected mosquitoes. Asia Pac J Mol Biol Biotechnol 19:93–100
Marshall JM, Akbari OS (2018) Can CRISPR-based gene drive be confined in the wild? A question for molecular and population biology. ACS Chem Biol 13:424–430
Patil PB, Niranjan Reddy BP, Gorman K et al (2015) Mating competitiveness and life-table comparisons between transgenic and Indian wild-type Aedes aegypti L. Pest Manag Sci 71(7):957–965
Patil PB, Gorman Kevin J, Dasgupta Shaibal K, Seshu Reddy KV, Barwale Shirish R, Zehr Usha B (2018) Self-limiting OX513A Aedes aegypti demonstrate full susceptibility to currently used insecticidal chemistries as compared to Indian wild-type Aedes aegypti. Psyche:7814643, 7 p. https://doi.org/10.1155/2018/7814643
Tyagi BK (2020) Mosquito hunters: a history of hostilities against man’s deadliest foe—the mosquito—since 1881. Scientific Publishers, Jodhpur, 474 pp
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Chatterjee, R., Bhattacharya, S., Tyagi, B.K. (2021). Perspectives into Genetic Manipulations for Control of Dengue Vector (Aedes aegypti Linnaeus, 1762) with Reference to Progress in Indian Experiments. In: Tyagi, B.K. (eds) Genetically Modified and other Innovative Vector Control Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-16-2964-8_7
Download citation
DOI: https://doi.org/10.1007/978-981-16-2964-8_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-2963-1
Online ISBN: 978-981-16-2964-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)