Abstract
Over the last decades, foods contaminated by spoilage fungi and their mycotoxins have become a serious global burden, which not only cause huge loss of food production and quality but also severely threaten human health. Currently, a novel nonthermal technology of cold plasma exhibits great potentials as a cost-effective, efficient, chemical-free and environmental-friendly strategy to inactivate fungi and degrade mycotoxins on foods. Herein, this chapter mainly presents an overview of the decontamination of fungi and their mycotoxins by cold plasma and the possible mechanisms. The published literatures show that cold plasma can effectively inactive various fungi (yeasts and molds) and their biofilms. The reactive species in cold plasma can directly damage the external structure of cell, but also induce oxidative stress in cells, consequently damaging the intercellular components and destroying cell normal physiochemical functions. Besides reducing the mycotoxin production via inactivating fungi, cold plasma can also degrade mycotoxins to less or nontoxic molecules through complex chemical reactions. Furthermore, the last sector summarizes the studies of plasma-activated water (PAW) on fungi inactivation and mycotoxin degradation. Above all, although the effectiveness of cold plasma/PAW for fungi and mycotoxins inactivation has been evidenced, the exact mechanism (especially the degradation pathway of mycotoxin) is still not clear. Meanwhile, studies about the edible safety of plasma/PAW-treated food and the large-scale industrial plasma device development are rare, which need much more attention.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cold plasma
- Plasma-activated water (PAW)
- Fungi
- Mycotoxins
- Inactivation mechanism
- Reactive oxygen and nitrogen species (RONS)
5.1 Introduction
Fungal contamination on crops can cause dramatic crop loss and damage the sensory and nutrient quality of foods, such as changing the color, decreasing the firmness, and producing bad smell (Palm 2001; Rossman 2009). Several types of spoilage fungi are pathogens, which can infect crops at different periods, including seeds, seedlings, and plants, leading to the decrease of germination rate, crop yield, and crop quality (Selcuk et al. 2008; Gonzales 2002). More importantly, most of the fungi can produce mycotoxins on crops and agricultural products, which seriously threatens the health of humans and animals (Lampel et al. 2012; Kuiper-Goodman 1995). Therefore, there is an urgent need to eliminate or minimize the food contamination by fungi and mycotoxins.
Traditionally, various approaches have been developed to inhibit the growth of fungi and decontaminate the mycotoxin on crops and agricultural products, including physical methods (e.g., UV, gamma rays, electron beam, ultrasound, pulsed light, microwave heating, thermal processing, sorting, cleaning, dehulling, and adsorption), chemical methods (e.g., acids and bases compounds, salts, oxidizing agents, chlorinating agents, electrolyzed water, and plant extracts), and biological control methods (e.g., microbial transformation, metabolization, fermentation and degradation, and atoxigenic microbial strains) (Basaran et al. 2008; Khamsen et al. 2016; Pankaj et al. 2018; Wielogorska et al. 2019; Ge et al. 2020). However, most of these methods have their own limitations. For example, some methods require expensive and sophisticated equipment and some approaches are inefficient, time-consuming, and impractical. More seriously, some chemical agents cause environmental pollution and threaten human health (Basaran et al. 2008; Wielogorska et al. 2019). Therefore, there is an urgent need to search for novel alternative control methods, which can overcome the limitations of these classical methods to some extent.
Recently, cold atmospheric plasma (CAP) is a promising green technology with potential applications in food sterilization. Initially, the antimicrobial effects of CAP on the heat-sensitive materials have been first reported at the beginning of the 1990s (Baier et al. 1992; Griffiths 1993; Chau et al. 1996; Laroussi 1996). CAP is an ionized gas, including charged particles, electric fields, ultraviolet photons, and reactive oxygen and nitrogen species (RONS) (Kogelschatz 2004; Laroussi and Leipold, 2004). Among those, RONS are considered as the major antimicrobial agents in CAP, which can inactivate a wide range of microorganisms including spoilage organisms and foodborne pathogens and almost without leaving any residues on foods due to its short lifetime (Ma et al. 2013, 2015; Xu et al. 2019; Du et al. 2020; Korachi et al. 2009; Yasuda et al. 2010; Sun et al. 2012a, b; Pan et al. 2013). RONS composition and concentration in CAP can be regulated via adjusting the voltage, frequency, power, gas type, and flow rate, which in turn can affect the sterilization efficacy of CAP (Xu et al. 2015; Xu et al. 2019). Numerous studies have evidenced the effectiveness of CAP sterilization on various foods (such as fresh fruits and vegetables, grains, nuts, spices, herbs, ready-to-eat meats, dried meats, and seafoods) and its degradation on various mycotoxins in solutions or foods (Misra et al. 2018; Surowsky et al. 2014; Dasan et al. 2016a, b; Khamsen et al. 2016; Devi et al. 2017; Siddiqueab et al. 2018; Go et al. 2019; Scussel et al. 2019; Zhu et al. 2020). Meanwhile, ACP causes minor negative effects on food quality during sterilization. Additionally, air is the most widely used working gas for ACP sterilization, and the energy consumption of ACP discharge is relatively low (Dasan et al. 2016a, b; Khamsen et al. 2016; Devi et al. 2017, Go et al. 2019). Therefore, CAP technology might be a promising efficient, cost-effective and environmental-friendly candidate for the decontamination of fungi and mycotoxins on foods.
More recently, a novel promising alternative disinfection approach based on CAP has been developed, named as plasma-activated water (PAW). PAW is also rich of RONS, which is mainly derived from the delivery of gaseous RONS into the water, the secondary RONS generation in water via complex chemical reactions between gaseous RONS and water, and the UV photolysis of water. These RONS endow the PAW with excellent antimicrobial performance against a broad spectrum of microorganisms, which is similar to CAP (Ma et al. 2015, 2016; Xu et al. 2016). Moreover, compared with CAP treatment, PAW overcomes some drawbacks of practical application, e.g., uniformly treating the objects with irregular shapes and avoiding the adverse effects of CAP-generated electric fields, charged particles, UV photos and electrons on food quality and operators (Ma et al. 2015; Hojnik et al. 2019). Thus, fungi inactivation and mycotoxins degradation by PAW have also attracted much attention and some studies have evidenced its effectiveness.
Therefore, this chapter is mainly to give a detailed description of the effects of CAP/PAW on fungi inactivation and mycotoxins degradation and their possible mechanisms. Firstly, we summarize the antimicrobial effects of CAP against yeast, molds, and their biofilms. Then, the antifungal mechanisms are discussed from two aspects (the major antifungal agents in CAP and cellular response to CAP). Subsequently, we summarize the degradation efficiency of CAP against pure mycotoxins and mycotoxins in real food systems as well as the underlying degradation mechanisms. Lastly, the PAW effects on fungi inactivation and mycotoxins degradation are overviewed.
5.2 Cold Plasma Inactivation of Fungi
Until now, with respect to the antimicrobial effects of CAP, the CAP sterilization against the bacteria has been extensively researched, while the killing effects of CAP against the fungi are relatively less studied. When CAP is applied to eukaryotic microbes, the inactivation becomes less effective as exhibited in many studies compared to that of prokaryotic microbes, which may result from the fact that the eukaryotic microbes have different cellular structures and more elaborate defense mechanisms. Eukaryotic microorganisms possess membranous organelles and cell walls different from those of bacteria. These structural complexity may be able to provide an ability to protect cells from stresses caused by CAP. In addition, the sophisticated defense mechanisms can give eukaryotic microorganisms an ability to survive and maintain homeostasis under CAP stress.
Fungi are common eukaryotic organisms, which can contaminate foods at various media, such as soils, water, and air. Moreover, fungi can be mainly divided into three categories according to the different size and morphology: microscopic single-celled yeasts, microscopic multicellular filamentous molds, and macroscopic filamentous mushroom (Deacon 2013; Kavanagh 2017). Due to the different characteristics of yeasts and molds, this section is divided into two parts: antifungal effects against yeast and antifungal effects against molds.
5.2.1 Antifungal Effects against Yeast
Saccharomyces cerevisiae (S. cerevisiae) is a species of budding yeast and originally isolated from the skins of grapes. S. cerevisiae is not only commonly considered as a safe fermentative yeast in the baking and brewing industry but also used as a probiotic in humans. Various studies have explored the antifungal effects and mechanisms of CAP by using S. cerevisiae as a eukaryotic model organism.
Jin et al. (2006) compared the yeast inactivation treated by dielectric barrier discharge (DBD) plasma with three different working gas (helium (He), nitrogen (N2), and air). The results showed that the DBD with these three gases all exhibited antifungal effects against yeast cells, and He plasma had the maximum inactivation efficiency. Morgan et al. (2009) investigated the effects of plasma discharge current on the inactivation rate against S. cerevisiae by using two kinds of working gases (argon (Ar) and oxygen (O2)). The results show that the D-values of S. cerevisiae by Ar DBD plasma at 0.4, 0.8, and 1.0 mA were 7, 7, and 7.3 min, respectively, while the O2 DBD plasma at 0.4, 0.8, and 1.0 mA led to the D-values of 7, 4.8, and 3.2 min, respectively. Chen et al. (2010) observed that 5-min air DBD plasma led to a 2-log reduction of S. cerevisiae cells in water. Ryu et al. (2013) reported that the antifungal effects of cold plasma against S. cerevisiae cells were closely related with the surrounding media and the inactivation efficiency was in the following order: water > saline solution > yeast extract peptone dextrose (YPD). Xu et al. (2019) found that the antifungal efficiency of He surface micro-discharge (SMD) plasma against S. cerevisiae cells was positively related with the treatment time while negatively related with the treatment distance. 10-min SMD plasma at 1 mm resulted in the best inactivation efficiency of 3.5-log reduction. Xu et al. (2020) reported that He/O2 plasma jet could efficiently inactive the S. cerevisiae cells (about 99%) in water for 5 min. Du et al. (2020) also investigated the influence of working gas on the antifungal effects of SMD plasma against S. cerevisiae cells by using He and air gas. The results demonstrated that air SMD plasma achieved a markedly higher inactivation efficiency compared with that of He SMD plasma.
Different from S. cerevisiae, Candida species (spp) are common pathogenic yeasts resulted in various human mucocutaneous and cutaneous infections (Nield and Kamat 2007; Zaoutis et al. 2005). Sun et al. (2011) investigated the antifungal effects of He/O2 (2%) plasma microjet against Candida species by using three drug-resistant species (Candida albicans (C. albicans), Candida krusei, and Candida glabrata) on agar plate and in water. The results demonstrated that He/O2 plasma achieved a high inactivation efficiency (>90%) in a shorter treatment time in water (1 min) than that on agar plate (10 min). Besides the planktonic fungi, many researches have also concentrated on the antifungal effects of CAP against C. albicans biofilms due to the fact that pathogens in biofilm have high resistance against antimicrobial treatment compared with pathogens in planktonic form, which is considered as a serious issue for C. albicans inactivation in medicine and industry (Cui et al. 2018). Koban et al. (2010) compared the antimicrobial effects of cold plasma on C. albicans biofilms with CHX and NaOCl. The results show that 10-min DBD plasma caused a 5-log reduction of C. albicans biofilms, while 10-min CHX or NaOCl exposure only led to a 1.5-log reduction. Sun et al. (2012b) reported that both He/O2 plasma alone and in combination with common antifungal drugs can efficiently inactivate Candida biofilms. A strong antifungal effect of SMD plasma against Candida biofilms was also reported by Maisch et al., who observed that 6-log reduction was achieved by 8-min plasma treatment. Handorf et al. (2018) reported that cold plasma treatment had obviously negative effects on the cell viability of C. albicans SC5314 biofilms concomitant with the physical destruction of cell membrane and the leakage of intracellular components. In another study, the inactivation effects of cold plasma treatment for C. albicans biofilm are assessed in vitro and in vivo (He et al. 2020). The results show that the microbial counts of C. albicans biofilms in suspensions were reduced from 35.6 × 102 to 4.6 × 102 CFU/ml after 8-min cold plasma treatment and the microbial counts of C. albicans biofilms on OPC mice were deceased from 34.7 × 103 to 34.7 × 103 CFU/g after 4-min plasma treatment. Table 5.1 summarized the recent cold plasma inactivation of yeasts.
Based on the above results, it is obvious that CAP could efficiently inactivate planktonic yeasts (S. cerevisiae and Candida species) in solutions, on agar plate, and other artificial matrix as well as yeast biofilms.
5.2.2 Antifungal Effects against Molds
Phytopathogenic molds can be divided into two groups: field molds (e.g., Fusarium, Cladosporium, and Alternaria) mainly infecting crops in the fields and storage molds (e.g., Aspergillus, Penicillium, and Eurotium) mainly contaminating the crops during postharvest storage (Los et al. 2018). This section mainly summarizes the antifungal effects of cold plasma against the storage molds on foods after harvest (as shown in Table 5.2). For example, Basaran et al. (2008) investigated the influence of working gas on the inactivation efficiency of low-pressure cold plasma (LPCP) against Aspergillus parasiticus (A. parasiticus) inoculated on various nut samples (e.g., hazelnuts, peanuts, and pistachio nuts) by using air and sulfur hexafluoride (SF6) gases. The results demonstrate that SF6 plasma had a higher inactivation efficiency than that of air plasma, which achieved a 5-log reduction of A. parasiticus, while air plasma only led to 1-log reduction for the same treatment time. Moreover, this study also investigated the effects of different nut samples on the plasma inactivation of A. parasiticus and obtained the results that 5-min SF6 plasma achieved an inactivation efficiency ranging from 3 to 6 log CFU/g on various nut samples. In the study of Selcuk et al. (2008), who also applied SF6 cold plasma to treat A. parasiticus inoculated on the nut surfaces, a 5-log reduction of A. parasiticus was achieved after 20-min plasma treatment. Meanwhile, 15-min SF6 plasma treatment caused a 3-log reduction of Aspergillus spp. and Penicillium spp. inoculated on the grains and legumes. Similarly, Suhem et al. (2013) reported that Ar cold plasma at a power of 40 W had an obvious inhibitory effect on the growth of Aspergillus flavus (A. flavus) on the medium after 25-min treatment, and 20-min Ar cold plasma at 40 W could efficiently inhibit A. flavus growth on brown rice cereal bars during 20-day storage at 25 °C and 100% RH. Ouf et al. (2015) investigated the antifungal effects of a double atmospheric pressure argon cold plasma (DAPACP) on Aspergillus niger (A. niger) inoculated on the date palm fruits and found that the A. niger population was decreased from 1000 to 20 CFU 100 mm−2 on date palm disc after 7.5-min treatment at 3.5 L min−1 and 9-min plasma treatment completely inhibit A. niger growth. Pignata et al. (2014) found that the fungi on the cellulose acetate was more easily inactivated by a low-pressure cold plasma compared with that contaminated on the food surface. The results show that the low-pressure cold plasma achieved a 5.4-log reduction of Aspergillus brasiliensis (A. brasiliensis) on cellulose acetate membranes after 15-s treatment at 400 W, 1-min treatment at 300 W, and 5-min treatment at 150 W by using the mixed gas of Ar and O2, while the pure Ar or O2 plasma treatment only led to a 3.45-log reduction. Moreover, with respect to the native fungi on pistachios, 1-min cold plasma treatment at 300 W only resulted in a 2-log reduction. Devi et al. (2017) investigated the effects of the discharge power on the inactivation efficiency against A. parasiticus and A. flavus artificially inoculated on groundnuts at different discharge power by cold plasma and found that 60-W cold plasma caused an inactivation efficiency of 97.9 and 99.3% against A. parasiticus and A. flavus respectively, while the low power led to a weaker antifungal effect. Furthermore, a first study presenting the antifungal effects of cold plasma on A. flavus biofilms was reported by Los et al. (2020), who found that the initial fungal population of the biofilms had a major effect on the inactivation efficiency of cold plasma by using CFU and cell viability assay. When the initial inoculum was 6 log CFU/mL, 20-min cold plasma resulted in a 2.3-log reduction and 11.7% survival rate; while the inactivation efficiency was decreased to 1.5-log reduction and 36.8% survival rate when the initial inoculum was increased to 7 log CFU/mL.
Taken together, although fungi are more resistant than bacteria, CAP still holds great potentials to inactivate various molds on foods with a high efficiency. However, it is still a challenge for cold plasma applied in large-scale industrial food sterilization due to the complex properties of foods, for example, size, shape, topography of food surface, the presence of shell, and solidness of food surface.
5.3 Antifungal Mechanism of Cold Plasma
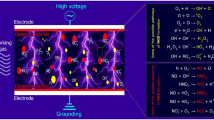
Furthermore, cold plasma is considered as a novel technology for fungi inactivation. However, the fungal mechanisms of CAP have not yet been elucidated in detail. So far, the existing studies to investigate the antifungal mechanism of cold plasma are mainly from the following two aspects (Fig. 5.1). One is to investigate the major antimicrobial agents in cold plasma. The other is to investigate the action of CAP on cell components and functions in the eukaryotic model organism S. cerevisiae and molds, respectively.
5.3.1 The Major Antimicrobial Agents against Fungi in Cold Plasma
Cold plasma is a mixture of ionized gases, which mainly consist of electric fields, heat, charged particles, UV radiation, and RONS (Pankaj et al. 2018). Herein, this sector mainly discusses the roles of different agents in CAP inactivation of fungi. Among all of the agents in cold plasma, although charged particles and UV radiation are involved in the cold plasma inactivation, the RONS are responsible for its antimicrobial effects in the most of existing literatures. The RONS in cold plasma are complex and can be divided into long-lived RONS and short-lived RONS. The long-lived RONS mainly contains hydrogen peroxide (H2O2), nitrite (NO2−), ozone (O3), and nitrate (NO3−), while the short-lived RONS mainly includes hydroxyl radical (·OH), superoxide anion (·O2−), and singlet oxygen (1O2) (Graves 2012). Until now, the inactivation mechanism of individual RONS in cold plasma was not yet fully understood. Thus, it is urgent to investigate the contribution of each RONS to plasma inactivation and its corresponding killing mechanisms.
Lee et al. (2006) suggested that the sterilizing effects of cold plasma against yeast cells were not due to UV, but instead resulted from the action of ROS. Morgan et al. (2009) found that O3 and atomic oxygen (O) play significant roles in the deactivation of S. cerevisiae. Similar results have also been reported by Iseki et al. (2010), who investigated the kinetic analysis of free radicals on fungal spores of Penicillium digitatum (P. digitatum) during plasma treatment including O by using real-time in situ electron spin resonance (ESR) spectroscopy and found that the ESR signal of P. digitatum was decreased only by applying oxygen plasma, which was also accompanied by the change in the shape and color of the mycelia, thereby conforming that O is the key antifungal agent. Iseki et al. (2011) found that O (3Pj) was the major agent in the inactivation of P. digitatum spores by oxygen-radical source cold plasma. Sun et al. (2011) reported that the ·OH and 1O2 detected by ESR in He/O2 plasma-water system were considered as the major inactivation agents against fungi in water. Similar results were also reported by Sun et al. (2012b), who demonstrated that the Candida biofilms inactivation by He/O2 cold plasma was mostly attributed to ·OH, ·O2−, and 1O2. The results reported by Ryu et al. (2013) show that the change trend of ·OH concentration in water, saline, and YPD medium was consistent with that of yeast cell viability in these three solutions, indicating that ·OH was responsible for the yeast inactivation by Ar plasma jet. Atomic nitrogen and ·OH in cold plasma were responsible for microbial inactivation (Surowsky et al. 2014). Ouf et al. (2015) revealed that ·OH and O were the main antifungal agents for A. niger spores on date palm fruit discs by DAPACP due to the fact that the cell viability of A. niger spores was negatively correlated with the quantitative amount of ·OH and O with the extension of DAPACP treatment time. Itooka et al. (2018) demonstrated that the ROS and UV photons in cold plasma had synergistic effects on protein denaturation in yeast cells. Similar results were also obtained by Hojnik et al. (2019), who compared the inactivation efficiency of cold plasma against fungal spores with O3 and UV treatments and found that the cold plasma sterilization was attributed to the synergistic effects of the multiple RONS, UV, and elevated gas temperatures. Xu et al. (2019) evaluated the roles of ·OH, H2O2, oxidation–reduction potential (ORP), and pH in the inactivation of yeast cells by He SMD plasma and revealed that ·OH contributed most to the cold plasma inactivation of yeast cells. Xu et al. (2020) employed specific ROS scavengers (superoxide dismutase (SOD), D-Manitol (D-Man), and L-Histidine (L-His)) to investigate the contribution of ·OH,·O2−, and 1O2 to the He/O2 plasma inactivation of yeast by comparing their inactivation efficiency and found that 1O2 contributed most to the yeast inactivation in this plasma-water system. Du et al. (2020) compared the antifungal effects of air and He SMD plasma against yeast cells and found that air SMD plasma had a stronger inactivation efficiency than He SMD plasma and the main antifungal agent for air and He SMD plasma was ·OH and O3, respectively.
Based on the above results, although the individual agents in cold plasma could inactivate microorganisms to some extent, the antimicrobial effects of cold plasma was mainly attributed to the synergistic effects of all these agents. Moreover, the major antifungal RONS are very different for these studies due to different cold plasma devices, treatment conditions, and fungi species. Therefore, establishment of a unique key antimicrobial agent for all plasma inactivation systems is impossible, and the detailed information of the major antimicrobial agents in every different plasma inactivation system against fungi has been listed in Tables 5.1 and 5.2.
5.3.2 The Mechanism of Eukaryotic Cell Response to Cold Plasma
S. cerevisiae is a well-studied eukaryotic model organism with high homology to humans, and it is genetically tractable and easy to manipulate in the lab; thus numerous studies have investigated the antifungal mechanism of cold plasma and the cellular responses of eukaryotic microorganisms to cold plasma by using S. cerevisiae.
A lot of literatures have evidenced that the RONS generated by cold plasma can cause oxidative stress in yeast cells, consequently resulting in cell dysfunction and death. For instance, it has been reported that the genes in oxidative stress pathway and cell cycle pathway were involved in the yeast response to cold plasma treatment by using a series of single gene mutants of S. cerevisiae (Feng et al. 2010). Chen et al. (2010) reported that cold plasma not only caused physical damage on cell surface of S. cerevisiae, but also induced oxidative stress and cell cycle arrest at G1 phase. Subsequently, similar observations have also been found by Ma et al. (2013), who reported that plasma-generated ROS could lead to cell apoptosis and cell cycle arrest at G1 phase in yeast cells through accumulation of intracellular ROS and calcium ion (Ca2+), depolarization of mitochondrial cell membrane potential, and fragmentation of nuclear DNA. Ryu et al. (2013) reported that Ar cold plasma-induced intracellular RONS and phosphorylation of HOG1 mitogen-activated protein kinase (MAPK) in yeast cells caused damages on cell morphology, membrane lipids, and genetic DNA. Chen et al. (2010) and Ma et al. (2014) revealed that cold plasma can enhance the antioxidant system (such as superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH)) in yeast cells, which also confirmed that the yeast cells suffered from oxidative stress after plasma treatment. Itooka et al. (2016) found that cold plasma not only caused oxidative stress evidenced by the nuclear accumulation of the oxidative stress responsive transcription factor Yap1, mitochondrial fragmentation, and enhanced intracellular oxidation, but also led to protein denaturation, translational repression, and ER stress in yeast cells. Xu et al. (2019) reported that He SMD plasma could effectively inactive the yeast cells by damaging cell membrane integrity and intracellular redox and pH homeostasis. Xu et al. (2020) also found that cold plasma can cause two kinds of cell death (apoptosis or necrosis) dependent on the treatment time. The short-time (3-min) cold plasma treatment caused cell apoptosis due to the accumulation of intracellular ROS, mitochondrial dysfunction, intracellular acidification, and DNA fragmentation, while the long-time (5-min) cold plasma caused cell necrosis due to the severe physical destruction on cell surface.
Besides yeasts, molds also belong to fungi, which comprise two forms (mycelium and spores). The spores have a protective coat to resist the external harsh environmental (Deacon 2013; Kavanagh 2017). Thus, fungi spores were more resistant to antimicrobial treatments. The existing literatures are mainly investigating the antifungal effects of cold plasma on fungi spores and its mechanisms.
For example, Gaunt et al. (2006) reported that the main inactivation mechanism of cold plasma against fungal spores was attributed to the cell wall rupture caused by the charged particles and RONS in cold plasma. Yong et al. (2014) reported that the RONS, UV photos, and other chemical reactive species in cold plasma worked together to destroy the natural defense system of spores, consequently resulting in the damage of intracellular components. Dasan et al. (2016b, 2017) also reported that cold plasma severely disrupted the cell membrane integrity of A. flavus and A. parasiticus on hazelnuts by scanning electron microscopy (SEM). Similarly, Devi et al. (2017) also reported that cold plasma-generated reactive species caused etching effects on the cell membrane of A. flavus spores, resulted in the electroporation and complete disintegration via SEM analysis. Furthermore, Šimončicová et al. (2018) firstly investigated the impact of cold plasma on young undifferentiated hyphae of A. flavus instead of asexual spores. Similar to the results of fungal spores, cold plasma also firstly interacted with the cell wall and membrane of A. flavus hyphae, causing cell membrane lipid peroxidation and damaging cell membrane integrity, followed by the leakage of intracellular contents and intracellular structures disintegration. Moreover, the oxidative stress induced by cold plasma enhanced the activity of intracellular antioxidant enzymes and compounds in A. flavus hyphae, which is consistent with the results in yeast cells.
Taken together, as shown in Fig. 5.1, the proposed antifungal mechanisms of cold plasma are that the active species can initially cause damage to the microbial cell wall and/or membrane, leading to membrane lipid peroxidation, cell membrane depolarization, and loss of membrane permeability, subsequently enabling further RONS or proton to enter the cell and damage the intercellular components such as organelles (mitochondria, nuclear and endoplasmic reticulum) and important biomolecules (DNA, RNA, and proteins) and meanwhile destroy the cellular ROS and pH homeostasis, consequently leading to cell death. Moreover, cold plasma can induce two different death modes (apoptosis and necrosis) dependent on the treatment doses. The detailed damage incidents happening in fungi in every plasma inactivation system have been summarized in Table 5.3. However, until now, most researches have only revealed the cellular physio-biochemical alterations during cold plasma inactivation, which is far from enough to uncover the antifungal mechanism of cold plasma. Thus, future work should employ the genomics, transcriptomics, proteomics, and metabolomics approach to comprehensively study the inactivation mechanisms of cold plasma.
5.4 Mycotoxins Degradation by Cold Plasma
Mycotoxins are secondary metabolites produced by filamentous fungi (such as Aspergillus, Penicillium, Fusarium, Alternaria, Claviceps, and Stachybotrys), which can cause adverse effects (e.g., carcinogenic, mutagenic, estrogenic, nephrotoxic, neurotoxic, hepatotoxic, immunosuppressive, and gastrointestinal toxicity) in human and animal health. Among all mycotoxins, aflatoxin, fumonisins, zearelenone, ochratoxin, and deoxynivalenol are five most toxic mycotoxins severely threatening mammals (Karlovsky et al. 2016).
Traditionally, several strategies have been applied for the reduction of mycotoxins including physical methods (such as cleaning, heating, irradiation, adsorption, ultrasound, pulsed light, sorting, and dehulling), chemical methods (such as acidic and alkaline compounds, salts, oxidizing and chlorinating agents, ammoniation, ozonization, sulphitation, and electrolyzed water), and biological methods (such as applying atoxigenic microbial strains, microbial transformation, microbial metabolization, microbial fermentation, and enzymatic degradation) (Pankaj et al. 2018; Jalili 2015; Hojnik et al. 2017; Diao et al. 2013). However, almost all of the methods have considerable limitations. For example, physical methods are usually expensive, time-consuming, and easy to produce undesirable changes in foods (Khadem et al. 2012). Chemical methods usually produce undesirable toxic residues, which pose a threat to the environment and human health (Mendez-Albores et al. 2007). With respect to the biological method, the degradation efficiency is always low and the culture pigmentation is also the main factor limiting its application (Luo et al. 2014). Thus, it is necessary to seek new mycotoxins decontamination methods to overcome these limitations.
Besides microbial inactivation, cold plasma has also shown promising potential for the degradation of various mycotoxins, which also attracts much attention from researchers in food and agricultural science in the past few years. Thus, this section mainly discusses the degradation effects of cold plasma on the pure mycotoxins and mycotoxins inoculated or produced by fungi on the foods.
5.4.1 Degradation of Pure Mycotoxins
Park et al. (2007) reported that a complete degradation of aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), and aflatoxin G2 (AFG2) on glass substrate was achieved by microwave Ar plasma at atmospheric pressure in 5 s and meanwhile these plasma-treated mycotoxins exhibited no cytotoxicity on mouse macrophage cells. Wang et al. (2015) investigated the effects of cold plasma on AFB1 degradation by using different AFB1 quantity (2, 10, and 50 μg) and plasma discharge power (100, 200, or 300 W). The results show that the initial AFB1 quantity and plasma treatment time almost had no effects on the degradation efficiency, while the plasma discharge power contributed most to the degradation rates, which increased from 58.2 to 88.3% with the discharge power ranging from 100 to 300 W. Similar results were also reported by Siciliano et al. (2016); the degradation efficiency of cold plasma was closely related with plasma discharge power. An increased treatment effectiveness was observed with the plasma discharge power increasing from 400 to 1150 W and the four standard AFs solutions were all completely eliminated after plasma treatment at 400 W for 12 min. Furthermore, AFB1 and AFG1 were more sensitive to plasma treatments compared to AFB2 and AFG2 due to the different molecule structures.
Besides plasma discharge power, Sakudo et al. (2017) also investigated the effects of discharge frequency of cold plasma on AFB1 mycotoxins degradation on a cover glass by using nitrogen cold plasma. The initial AFB1 concentration on cover glass was about 200.01 ppb, while 0.5, 1.0, and 1.5 kilo pulse per second (kpps) reduced the AFB1 content to 135.66, 56.03, and 12.4 ppm, respectively, which indicated that the degradation efficiency of AFB1 by nitrogen cold plasma was dependent on the discharge frequency. Bosch et al. (2007) studied the degradation effects of air cold plasma on six common mycotoxins (deoxynivalenol (DON), zearalenone (ZEN), enniatins A (Enn A), fumonisin B1 (FB1), T2 toxin, and AAL toxin) and found that all of these mycotoxins were almost completely degraded by 60-s cold plasma treatment. Moreover, the author also found a similar phenomenon with that reported by Siciliano et al. (2016), the degradation rates varied with mycotoxin structure. FB1 and structurally related AAL toxin with long aliphatic chains were degraded rapidly, while ST with a compact structure of condensed aromatic rings exhibited the highest resistance to degradation. The other three mycotoxins with intermediate decay rates possessed mixed structures of condensed rings and aliphatic chains. Similar conclusions were also obtained by Wielogorska et al. (2019); six mycotoxins (AFB1, FB1, ochratoxin (OTA), ZEN, DON, and enniatin B (ENB)) in solutions treated by cold plasma presented different degradation efficiencies due to the different structures in the following order: ENB < FB1 < OTA < ZEN < AFB1 < DON. And the author also revealed that He plasma was more effective in the toxin’s degradation than the mixture gas of He/O2 (0.5% and 0.75%). Furthermore, Hojnik et al. (2017) compared the degradation efficiency of cold plasma against AFB1 on glass coverslips with two conventional methods (UV and thermal treatment). The results show that 15-s plasma treatment resulted in more than 80% degradation rate of AFB1, while the UV and thermal treatment could not significantly degrade AFB1, indicating that cold plasma holds great potential as an effective alternative to UV and thermal treatment for AFB1 degradation.
Based on the above results, it is concluded that the degradation efficiency of cold plasma was dependent on various parameters including working gas, treatment time, and discharge power and frequency. More interestingly, the mycotoxins structure can also affect the degradation efficiency by cold plasma. The mycotoxins with long aliphatic chains were more easily degraded than mycotoxins with the condensed aromatic rings (Siciliano et al. 2016; Wielogorska et al. 2019). The in vitro cytotoxicity test evidenced that the cytotoxicity of plasma-treated mycotoxins was significantly reduced. The HPLC-MS results revealed that cold plasma could degrade the mycotoxins to small fragments or only change the structure of chemical bonds in mycotoxins. The detailed degradation mechanisms are discussed in the following sector.
5.4.2 Degradation of Mycotoxin in Real Food Systems
The first evidence of cold plasma degradation of mycotoxins on foods was reported by Basaran et al. (2008), who found that air cold plasma exhibited a better degradation efficiency (about 50%) of total AFs (AFB1, AFB2, AFG1, and AFG2) compared with that of SF6 cold plasma (about 20% decrease). Ouf et al. (2015) investigated the effects of a double atmospheric pressure argon cold plasma jet on fungi spore germination and mycotoxin production in date palm discs. The results show that the amount of fumonisin B2 (FB2) and OTA produced by A. niger was gradually decreased with the extension of plasma treatment time and the mycotoxins were not detected after 6-min treatment for FB2 and 7.5-min treatment for OTA, which may be attributed to the inhibitory effects of cold plasma on the genes involved in the biosynthesis of mycotoxins. This study indicates that besides directly inactivating the fungi to reduce the mycotoxins production, cold plasma may also regulate the genes associated with the biosynthesis of mycotoxins, which should be verified via qRT-PCR or RNA-Seq analysis in the future. Siciliano et al. (2016) investigated the degradation effects of cold plasma on the artificially AFs contaminated hazelnuts without shell at four different discharge powers (400 W, 700 W, 1000 W, and 1150 W) and four different exposure times (1, 2, 4, and 12 min). The results show that the degradation rate of AFs by cold plasma was increased with the plasma discharge power and treatment time. Cold plasma at the highest power (1150 W) achieved the maximum degradation efficiency of AFB1 (29.1%) and total AFs (30.4%) for the longest exposure time (12 min). Devi et al. (2017) investigated the influence of air cold plasma on A. parasiticus and A. flavus growth and AFB1, B2, G1, and G2 production on groundnuts and found that the AFB1 contents were decreased by 70 and 90% in A. parasiticus after 40-W 40-min and 60-W 12-min plasma treatment, while 40-W 15-min and 60-W 12-min plasma treatment resulted in a 65 and 95% reduction in AFB1 in A. flavus samples. Bosch et al. (2007) investigated the effect of matrix on the degradation efficiency of four mycotoxins (FB1, EnnB, sterigmatocystin (ST), and ZEN) by cold plasma. The results show that the mycotoxins in matrix had a lower degradation efficiency than their pure compounds, which was similar to the plasma inactivation on foods. The reason may be that cold plasma-generated RONS can be consumed by the substances of matrix and the matrix could also provide physical shielding for the mycotoxins. Shi et al. (2017) investigated the degradation effects of high-voltage atmospheric cold plasma (HVACP) on AFs in corns by changing various treatment parameters (e.g., gas type (air and MA65 (65% O2, 30% CO2, and 5% N2)), relative humidity (5, 40, and 80% RH), treatment time (1, 2, 5, 10, 20, and 30 min), reaction mode, post-treatment storage, and stirring of corn material). The results show that the MA65 HVACP with longer treatment time and higher relative humidity led to a higher degradation rate of AFs in corns. Moreover, the degradation efficiency was significantly increased when the corns were stirred during HVACP treatment and under post-treatment storage. However, the reaction mode (direct or indirect HVACP treatment) had minor effects on the degradation efficiency. Subsequently, Iqdiam et al. (2019) used two different atmospheric pressure plasma jets (APPJ) to treat the AFTs (AFTB1 and AFTB2) produced by A. flavus inoculated on the peanut kernels and investigated the degradation rate and its effects on the physicochemical quality of peanuts. The results show that 2-min constant APPJ treatment decreased AFTs contents from 62.3 to 48.2 ppb and 5-min agitated APPJ treatment led to a 23% reduction. Both the constant and agitated APPJ treatment could elevate the surface temperature of peanuts, accordingly resulted in the burned peanut kernels. Wielogorska et al. (2019) performed a holistic assessment of cold plasma applicability for the most prevalent toxins on maize by performing degradation optimization, chemical and biological assessment of by-products, and the influence of cold plasma on the matrix molecular integrity. Results show that 10-min cold plasma treatment resulted in more than 66% reduction in AFB1 and FB1 concentration on maize. Degradation products of AFB1 and ZEN were nontoxic to the human hepatocarcinoma cells and small molecular mass components were observed in the cold plasma-treated maize matrix.
In summary, cold plasma could also effectively degrade different mycotoxins on foods, but the degradation rate was reduced compared with their pure mycotoxins due to the RONS consumed by the food matrix. Moreover, the mycotoxins degraded by cold plasma may be due to the following three reasons. Firstly, cold plasma could sterilize the mycotoxin-producing fungi, consequently reducing mycotoxin productions. Secondly, cold plasma may regulate the gene expression associated with the biosynthesis of mycotoxin with a low inactivation efficiency against the fungi. Thirdly, cold plasma could directly degrade mycotoxins on contaminated foods and feedstuffs.
Additionally, to promote the applications of cold plasma in decontamination of AFs on foods, the following issues should be paid more attention in future studies. Firstly, the toxicity of degradation products and the effects of cold plasma on food quality should be further investigated. Secondly, further research should be conducted by using naturally mycotoxins contaminated food. Thirdly, the cold plasma device should be optimized to improve the applicability for mycotoxins inactivation and enhance its economic feasibility.
5.5 Degradation Mechanism of Cold Plasma
Although there are some studies investigating the degradation mechanisms of cold plasma, it is still not well understood due to the highly complex plasma chemistry. For instance, air plasma involves around 500 reactions involving more than 75 reactive species (Pankaj et al. 2014). Thus, it is very difficult to determine the major agents responsible for cold plasma degradation and its degradation products. To our knowledge, most of the existing literatures focus on the degradation mechanisms of AFB1, with only one report investigating the degradation mechanisms of ZEN. In this sector, the degradation mechanisms of cold plasma are discussed from two aspects (the major agents contributed to plasma degradation and the degradation products).
5.5.1 The Major Agents in Cold Plasma Responsible for Mycotoxin Degradation
5.5.1.1 Short-Lived RONS
In the study of Wang et al. (2015), two speculated degradation pathways were obtained by the five degradation products (Fig. 5.2). For the second pathway, AFB1 was degraded through the addition of ·OH and ·H to its double bonds and the damage of carbon monoxide by O· and ·OH. Thus, these short-lived ROS were considered to play important roles in the degradation process by cold plasma. Furthermore, Wielogorska et al. (2019) revealed that the degradation efficiency of cold plasma was decreased with the increase of oxygen ratio in He gas (0%, 0.5%, and 0.75%). And the presented spectra of He and different mixtures of He/O2 (0.5% and 0.75%) clearly showed a decrease in intensity of the hydroxyl radical band, which had a similar change trend to that of degradation efficiency, thereby indicating that ·OH plays an important role in mycotoxins degradation by cold plasma.
The proposed degradation pathway of AFB1 by low-temperature radio-frequency plasma (Wang et al. 2015)
5.5.1.2 Long-Lived RONS
Shi et al. (2017) investigated the degradation efficacy of HVACP treatment on AFs in corn by using different gas type (air and MA65 (65% O2, 30% CO2, 5% N2)) and treatment modes (direct plasma treatment and indirect treatment). The results of different working gas show that MA65 HVACP had a higher degradation efficiency of AFs in corns compared with air HVACP, which was probably due to the higher concentrations of O3 and NOx species in MA65 HVACP. Moreover, with respect to the different plasma treatment modes (direct plasma treatment vs. indirect plasma treatment), the direct plasma treatment with electric fields, charged particles, UV photons, short-lived and long-lived RONS caused a similar degradation efficiency to that of indirect plasma treatment only including long-lived RONS (such as H2O2, O3, and NOx). Based on the above results, it was concluded that the degradation effects of cold plasma on AFs in corns is mainly attributed to the long-lived RONS like O3 or NOx, instead of short-lived RONS, charged particles, electric fields, and UV photons.
5.5.1.3 High Energy Particles (Electrons and Excited Ions and Molecular Species)
Cold plasma is also widely used to degrade chemically stable pollutants in gaseous phase. For the degradation mechanisms of chemical pollutants, it was assumed that the energy dissipated during the cold plasma discharge could induce a series of complex chemical reactions between the plasma-generated RONS (such as O, O3, ∙OH, and NOx) and toxins Hopfe and Sheel 2007; Eliasson and Kogelschatz 1991) and/or decomposition after collision with electrons and ions (Efremov et al. 2004; Coburn and Kay 1979), which led to the cleavage of molecular bonds. Further reactions with plasma species can result in the fragmentation and generation of volatile compounds as no stable residues of toxin degradation could be detected with HPLC-MS. According to the aforementioned mechanisms, Bosch et al. (2007) also speculated that the energy of free electrons and excited ions and molecular species in cold plasma exceeds the dissociation energy of a C-C bond, which can lead to the fragmentation of the mycotoxins. And the mycotoxins molecules in the discharge were subjected to unspecific degradation. This hypothesis was in accordance with the study reported by Sakudo et al. (2017); the HPLC results confirmed the loss of AFB1 after 15-min plasma treatment and the generation of small fragments, possibly originating from the degradation process. Furthermore, the author also assumed that the mycotoxin fragments could be converted into volatile compounds and then eliminated by plasma gas. Therefore, future work will be dedicated to analyze the plasma effluent by mass spectrometry.
5.5.1.4 The Synergistic Effects of Various Reactive Species in Cold Plasma
Hojnik et al. (2017) compared the degradation efficiency of cold plasma against AFB1 on glass coverslips with two conventional methods (UV and thermal treatment) and found that 15-s plasma treatment resulted in more than 80% destruction level of AFB1, while no significant transformation of AFB1 was observed under thermal or UV light treatments, even at the longest exposure times. Thus, it is reasonable to conclude that UV alone is not solely responsible for cold plasma. Furthermore, Wang et al. (2015) revealed that the degradation products (C17H15O7; m/z 331) of AFB1 after cold plasma treatment have also been identified as a major degradation product by exposure to UV and Cobalt-60 gamma irradiation, and among the six cold plasma degradation products, two degradants were ozonized products of AFB1 (Wielogorska et al. 2019), indicating that UV and O3 both contribute to cold plasma degradation. Taken together, the simultaneous exposure to multiple RONS and UV is likely to result in the synergistic effects of mycotoxin degradation by cold plasma.
5.5.2 The Degradation Products of Mycotoxins by Cold Plasma
The first study to investigate the degradation product structure of ABF1 after cold plasma treatment was reported by Wang et al. (2015). According to the deduced structure formulas of the five degradation products, two degradation pathways were proposed. One was that the degradation of AFB1 was initiated by an addition reaction of ∙OH and ∙H to the double bond in the terminal furan ring (C8-C9) and produced an intermediate with m/z 331 (C17H15O7), which was considered as the major degradation pathway of AFB1 by cold plasma. The other was that carbon monoxide could be damaged by the reactive species in cold plasma (Fig. 5.2). Shi et al. (2017) investigated the AFB1 degradation efficiency and mechanism by air HVACP treatment, who proposed a different degradation mechanism of AFB1. As shown in Fig. 5.3, one degradation pathway involved the addition of ∙H, ∙OH, and CHO∙ radicals. The other degradation pathway involved the epoxidation by ∙HO2 radicals and oxidation of AFB1 by the combined effects of the oxidative species OH∙, H2O2, and O3. In a recent study conducted by Wielogorska et al. (2019), the degradation mechanism of AFB1 and ZEN by cold plasma was proposed based on the formulas, structures, and fragmentation spectra of by-products. The results show that the main AFB1 degradation products were generated by the modification of the terminal furan rings and the methoxy group taking place during cold plasma treatment. The most prominent degraded product of AFB1 was AFB1-dihydrodiol (C17H14O8). Singly hydroxylated terminal furan ring produced C16H12O7, followed by the ring cleavage and remaining furan ring dihydroxylation (C15H12O7) and dehydrogenation (C15H10O5). The final degradation product was generated by the remaining furan ring cleavage (C14H12O5) (Fig. 5.4). With respect to ZEN, the cold plasma degraded the ZEN to three main degradation products. One degradation product (C21H24O8) at m/z 405 was produced via the opening of the lactone ring. The other two degradation products had a same molecular formula of C18H23O6 at m/z 335 with different structure, which could potentially be isomers of a mono-hydroxylated product at C7 and C5.
The proposed degradation pathway of AFB1 by air high-voltage atmospheric cold plasma (Shi et al. 2017)
The proposed degradation pathway of AFB1 by He + O2 pulsed DBD jet (Wielogorska et al. 2019)
In summary, the different degradation mechanisms of AFB1 by cold plasma may be due to the different plasma devices and treatment conditions (such as working gas, treatment time, power, frequency, gas flow rate, and so on). To date, there are still few studies investigating the degradation mechanisms of cold plasma; much work should be conducted in the future to realize the degradation pathways, especially considering widespread toxins lacking aromatic rings. Moreover, it is desirable to verify the postulated structural effects on degradation and identify reactive species in the plasma accountable for the degradation.
5.6 PAW for Fungal and Mycotoxin Control
5.6.1 PAW
Recently, a new concept of plasma-activated water (PAW) has been put forward, which is generated by cold plasma reacting with water to produce various RONS. The RONS can be originated from the direct delivery of gas RONS into water, the primary RONS reacting with water or UV photolysis of water. A majority of studies have demonstrated that PAW also exhibited excellent broad-spectrum antimicrobial activity (Burlica et al. 2010; Kamgang-Youbi et al. 2009; Ma et al. 2015; Tian et al. 2015; Zhang et al. 2013). It is generally accepted that PAW inactivation was mainly due to the synergistic effects of RONS and pH, which is different from cold plasma sterilization (Ma et al. 2015; Naitali et al. 2010; Tian et al. 2015; Zhang et al. 2013). More importantly, compared with cold plasma treatment, PAW overcomes some drawbacks of practical application, e.g., uniformly treating the objects with irregular shapes and avoiding the adverse effects of cold plasma-generated electric fields, charged particles, UV photos and electrons on food quality and operators (Misra et al. 2014; Ma et al. 2015; Hojnik et al. 2019). Thus, the fungi inactivation and mycotoxins degradation by PAW have also attracted much attention, and some studies have evidenced its effectiveness. Thus, this section mainly summarized PAW application for the decontamination of fungi and mycotoxins.
5.6.2 PAW Inactivation of Fungi
Recently, although numerous studies have verified the effectiveness of PAW against various bacteria, there still few reports related to the fungi inactivation by PAW due to the high resistance of fungi to antimicrobial agents compared with bacteria. The first evidence demonstrating PAW with antifungal effects was reported by Kamgang-Youbi et al. (2009), who found that 5-min PAW could effectively inactivate Hafnia alvei as well as Staphylococcus epidermidis, Leuconostoc mesenteroides, and S. cerevisiae and 30-min PAW treatment led to a 3-log reduction of S. cerevisiae cells. Souskova et al. (2011) generated PAW by using the direct current corona electric discharge over the water surface and evidenced its effectiveness for the disinfection of molds (Penicillium crustosum, Aspergillus oryzae, Cladosporium sphaerospermum) and yeast cells. The results show that a complete inactivation of yeast cells with an initial concentration of 105 CFU/mL was achieved by 6-min PAW treatment, while 25-min PAW completely inactivated C. sphaerospermum spores and A. oryzae spores were still not completely inactivated after 30-min PAW exposure.
Until 2016, Ma et al. (2015) firstly investigated the antimicrobial effects of PAW against foodborne pathogens on fresh produces. Although there was no inactivation data of fungi in this study, the storage picture showed that almost no visual fungal spoilage was detected on the strawberries treated by PAW after 6-day storage, which directly indicated that PAW may also inhibit fungal growth on fresh produce. Subsequently, Xu et al. (2016) investigated the antimicrobial effects of PAW against the native microorganisms on button mushrooms and its effects on the postharvest quality. The results show that 10-PAW can efficiently inactivate the fungi on button mushrooms and led to a 0.5-log reduction CFU/mL after 7-d storage. Similarly, Ma et al. (2016) applied PAW to preserve the postharvest Chinese bayberry and found that 0.5-min PAW resulted in a 1.1-log CFU/g reduction of fungi on Chinese bayberry after 8-d storage and had minor effects on the postharvest quality of Chinese bayberry. Guo et al. (2017) investigated the antifungal effects of PAW against S. cerevisiae CICC 1374 inoculated on grape berries. The results show that PAW inactivated the yeast cells depending on the PAW treatment time. PAW-30 and PAW-60 achieved a 0.38- to 0.53-log CFU/ml reduction of yeast cells, respectively. Choi et al. (2019) investigated the synergistic effects of PAW and mild heating at 60 °C on the microbial inactivation of the native microorganisms and inoculated foodborne pathogens on the shredded salted Chinese cabbages. The results show that the combined PAW and mild heating treatment efficiently inactivated the mesophilic aerobic bacteria, lactic acid bacteria, yeast and molds on the shredded salted Chinese cabbages, which had a higher inactivation efficiency than that of individual PAW treatment.
In some more recent studies, researchers have compared the antifungal effects of cold plasma and PAW. For instance, in the study of Hojnik et al. (2019), a comparison of plasma-based decontamination techniques (a direct gas cold plasma vs. an indirect treatment using a plasma-activated aqueous broth solution (PAB)) for inactivation of A. flavus spores was reported to highlight their respective efficiencies and corresponding inactivation mechanisms. The results show that the direct gas cold plasma had a stronger antimicrobial effect against A. flavus spores compared with PAB, which was probably due to the fact that the A. flavus spore had a hydrophobic surface, making RONS in PAB hard to contact with the spore surface. Consistent with the above results, Los et al. (2020) also reported that the direct gas plasma caused a higher inactivation efficiency against A. flavus spores than PAW. 20-min direct gas plasma achieved a 2.2-log reduction of A. flavus spores, while PAW only resulted in a 0.6-log reduction after 24-h treatment. The reason may be that PAW inactivation was only derived from the low pH and high contents of RONS, while direct gas plasma also contained various physical effects of high electric field, overpressure shock waves, and intense ultraviolet radiation, which also had antimicrobial effects.
5.6.3 PAW Degradation of Mycotoxin
Until now, according to our knowledge, there was only one study that investigated the degradation effects of PAW on mycotoxin (Chen et al. 2019). The author compared the degradation effects of intense pulsed light (IPL) and PAW treatments on DON in the raw and germinating barley. The results show that IPL treatment can degrade the DON in germinating barley faster than PAW treatment. 180-pulse IPL treatment achieved a 35.5% decrease in the DON contents for 60 s, while PAW treatment caused a 34.6% reduction for 5 min. With respect to the raw barley samples, 180-pulse IPL treatment could also significantly reduce the DON contents to 69.1%, while PAW had a lower degradation efficiency than that in the germinating barley.
This work indicates that PAW holds a great potential as an economical, eco-friendly, nontoxic method to degrade the mycotoxins on foods. However, more studies are needed to investigate its degradation effects on various kinds of mycotoxins and the toxicity of corresponding degradation by-products. Moreover, the effects of PAW on food quality should also be explored.
5.7 Conclusion
Decontamination of fungi and mycotoxins on agricultural products remains a significant challenge for the food industry. The existing literatures show that CAP holds great potential as an effective, economical, chemical-free and eco-friendly alternative to the traditional methods for fungi inactivation on foods and causes less effects on food quality. The proven antifungal activity of CAP may originate from the RONS, which can cause damages to the microbial cell wall and/or membrane, subsequently enabling further reactive species to enter the cell and damage the intercellular components such as organelles and important biomolecules (DNA, RNA, and proteins). Cold plasma can reduce the mycotoxins production via inactivating the mycotoxin-producing fungi, regulating the gene expression associated with mycotoxin biosynthesis, or directly degrading the mycotoxins. For mycotoxin degradation, CAP has exhibited a comparable degradation efficiency to conventional UV, heat, and ozone treatments and also has some similar degradation products. But there are also many new degradation products generated by CAP via other specific chemical pathways due to the complex constituents in CAP, which has not been well understood and needs much more attention. Furthermore, PAW has a weaker antifungal effect compared with CAP probably due to lacking the physical effects of CAP (such as high electric field, overpressure shock waves, and intense ultraviolet radiation). The PAW effects on this subject are only a startup; thus the effectiveness of PAW on fungi and mycotoxins inactivation needs to be verified by much more different fungi and mycotoxins and the exact mechanisms should also be studied further. Additionally, in the future, no matter applying CAP or PAW technologies for decontamination of fungi and mycotoxins on foods, we should pay more attention on their effects on food nutrition and quality as well as the edible safety of CAP/PAW-treated foods. A large-scale industrial CAP device should also be developed to improve their applicability.
References
Baier RE, Carter JM, Sorenson SE, Meyer AE, McGowan BD, Kasprzak SA (1992) Radiofrequency gas plasma (glow discharge) disinfection of dental operative instruments, including handpieces. J Oral Implantol 18:236–242
Basaran P, Basaran-Akgul N, Oksuz L (2008) Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment. Food Microbiol 25(4):626–632
Bosch L, Pfohl K, Avramidis G, Wieneke S, Viol W, Karlovsky P (2007) Plasma-based degradation of mycotoxins produced by Fusarium, Aspergillus and Alternaria species. Toxins 9(97):1–12
Burlica R, Grim R, Shih K, Balkwill D, Locke B (2010) Bacteria inactivation using low power pulsed gliding arc discharges with water spray. Plasma Process Polym 7(8):640–649
Chau TT, Kao KC, Blank G, Madrid F (1996) Microwave plasma for low temperature dry sterilization. Biomaterials 17:1273–1277
Chen H, Bai F, Xiu Z (2010) Oxidative stress induced in Saccharomyces Cerevisiae exposed to dielectric barrier discharge plasma in air at atmospheric pressure. IEEE Trans Plasma Sci 38(8):1885–1891
Chen D, Chen P, Cheng Y, Peng P, Liu J, Ma Y, Liu Y, Ruan R (2019) Deoxynivalenol decontamination in raw and germinating barley treated by plasma-activated water and intense pulsed light. Food Bioprocess Technol 12(4):246–254
Choi EJ, Park HW, Kim SB, Ryu S, Lim J, Hong EJ, Byeon YS, Chun HH (2019) Sequential application of plasma-activated water and mild heating improves microbiological quality of ready-to-use shredded salted kimchi cabbage (Brassica pekinensis L.). Food Control 98:501–509
Coburn J, Kay E (1979) Some chemical aspects of the fluorocarbon plasma etching of silicon and its compounds. IBM J Res Dev 23(1):33–41
Cui HY, Bai M, Rashed M, Lin L (2018) The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157:H7 biofilms on cucumber. Int J Food Microbiol 266:69–78
Dasan BG, Boyaci IH, Mutlu M (2016a) Inactivation of aflatoxigenic fungi (Aspergillus spp.) on granular food model, maize, in an atmospheric pressure fluidized bed plasma system. Food Control 70:1–8
Dasan BG, Mutlu M, Boyaci IH (2016b) Decontamination of Aspergillus flavus and Aspergillus parasiticus spores on hazelnuts via atmospheric pressure fluidized bed plasma reactor. Int J Food Microbiol 216:50–59
Dasan BG, Boyaci IH, Mutlu M (2017) Nonthermal plasma treatment of Aspergillus spp. spores on hazelnuts in an atmospheric pressure fluidized bed plasma system: Impact of process parameters and surveillance of the residual viability of spores. J Food Eng 196:139–149
Deacon JW (2013) Fungal biology. John Wiley & Sons, New York, NY
Devi Y, Thirumdas R, Sarangapani C, Deshmukh RR, Annapure US (2017) Influence of cold plasma on fungal growth and aflatoxins production on groundnuts. Food Control 77:187–191
Diao E, Hou H, Dong H (2013) Ozonolysis mechanism and influencing factors of aflatoxin B1: a review. Trends Food Sci Technol 33(1):21–26
Du M, Xu H, Zhu Y, Ma R, Jiao Z (2020) A comparative study of the major antimicrobial agents against the yeast cells on the tissue model by helium and air surface micro-discharge plasma. AIP Adv 10:025036
Efremov AM, Kim DP, Kim CI (2004) Simple Model for Ion-Assisted Etching Using Coupled Plasma: Effect of Gas Mixing Ratio. IEEE Trans Plasma Sci 32:1344–1351
Eliasson B, Kogelschatz U (1991) Nonequilibrium volume plasma chemical processing. IEEE Trans Plasma Sci 19:1063–1077
Feng H, Wang R, Sun P, Wu H, Liu Q, Fang J, Zhu W, Li F, Zhang J (2010) A study of eukaryotic response mechanisms to atmospheric pressure cold plasma by using Saccharomyces cerevisiae single gene mutants. Appl Phys Lett 97:131501
Fukuda S, Kawasaki Y, Izawa S (2019) Ferrous chloride and ferrous sulfate improve the fungicidal efficacy of cold atmospheric argon plasma on melanized Aureobasidium pullulans. J Biosci Bioeng 128(1):28–32
Gaunt LF, Beggs CB, Georghiou GE (2006) Bactericidal action of the reactive species produced by gas-discharge nonthermal plasma at atmospheric pressure: a review. IEEE Trans Plasma Sci 34(4):1257–1269
Ge M, Zhang L, Ai J, Ji R, He L, Liu C (2020) Effect of heat shock and potassium sorbate treatments on gray mold and postharvest quality of 'XuXiang' kiwifruit. Food Chem 324:126891
Go SM, Park MR, Kim HS, Choi WS, Jeong RD (2019) Antifungal effect of non-thermal atmospheric plasma and its application for control of postharvest Fusarium oxysporum decay of paprika. Food Control 98:245–252
Gonzales P (2002) A handbook of rice seedborne fungi. International Rice Research Institute, Los Banos, Philippines
Graves DB (2012) The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J Phys D Appl Phys 45:263001
Griffiths N (1993) Low temperature sterilization using gas plasma. Med Device Technol 4:37–40
Guo J, Huang K, Wang X, Lyu C, Yang N, Li Y, Wang J (2017) Inactivation of yeast on grapes by plasma-activated water and its effects on quality attributes. J Food Prot 80(2):225–230
Handorf O, Weihe T, Bekeschus S, Graf AC, Schnabel U, Riedel K, Ehlbeck J (2018) Nonthermal Plasma Jet Treatment Negatively Affects the Viability and Structure of Candida albicans SC5314 Biofilms. Appl Environ Microbiol 84(21):e01163–e01118
He M, Duan J, Xu J, Ma M, Chai B, He G, Gan L, Zhang S, Duan X, Lu X, Chen H (2020) Candida albicans biofilm inactivated by cold plasma treatment in vitro and in vivo. Plasma Process Polym 17:e1900068
Hojnik N, Cvelbar U, Tavčar-Kalcher G, Walsh JL, Križaj I (2017) Mycotoxin decontamination of food: cold atmospheric pressure plasma versus “classic” decontamination. Toxins 9(5):151
Hojnik N, Modic M, Ni Y, Filipič G, Cvelbar U, Walsh JL (2019) Effective fungal spore inactivation with an environmentally friendly approach based on atmospheric pressure air plasma. Environ Sci Technol 53(4):1893–1904
Hopfe V, Sheel DW (2007) Atmospheric-pressure PECVD coating and plasma chemical etching for continuous processing. IEEE Trans Plasma Sci 35:204–214
Hosseini SI, Farrokhi N, Shokri K, Khani MR, Shokri B (2018) Cold low pressure O2 plasma treatment of Crocus sativus: An efficient way to eliminate toxicogenic fungi with minor effect on molecular and cellular properties of saffron. Food Chem 257:310–315
Iqdiam BM, Abuagela MO, Boz Z, Marshall SM, Goodrich-Schneider R, Sims CA, Marshall MR, MacIntosh AJ, Welt BA (2019) Effects of atmospheric pressure plasma jet treatment on aflatoxin level, physiochemical quality, and sensory attributes of peanuts. J Food Process Preserv 00(7):e14305
Iseki S, Ohta T, Aomatsu A, Ito M, Kano H, Higashijima Y, Hori M (2010) Rapid inactivation of Penicillium digitatum spores using high-density nonequilibrium atmospheric pressure plasma. Appl Phys Lett 96(15):153704
Iseki S, Hashizume H, Jia F, Takeda K, Ishikawa K, Ohta T, Ito M, Hori M (2011) Inactivation of Penicillium digitatum spores by a high-density ground-state atomic oxygen-radical source employing an atmospheric-pressure plasma. Appl Phys Express 4(11):116201.1–116201.3
Itooka K, Takahashi K, Izawa S (2016) Fluorescence microscopic analysis of antifungal effects of cold atmospheric pressure plasma in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 100(21):9295–9304
Itooka K, Takahashi K, Kimata Y, Izawa S (2018) Cold atmospheric pressure plasma causes protein denaturation and endoplasmic reticulum stress in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 102:2279–2288
Jalili M (2015) A Review on Aflatoxins Reduction in Food. Iran J Health Saf Environ 3(1):445–459
Jin Y, Ren C, Xiu Z, Wang D, Wang Y, Yu H (2006) Comparison of yeast inactivation treated in He, Air and N2 DBD plasma. Plasma Sci Technol 8(6):721–723
Jo YK, Cho J, Tsai TC, Staack D, Kang MH, Roh JH, Shin DB, Cromwell W, Gross D (2014) A non-thermal plasma seed treatment method for management of a seedborne fungal pathogen on rice seed. Crop Sci 54(2):796–803
Kamgang-Youbi G, Herry JM, Meylheuc T, Brisset JL, Bellon-Fontaine MN, Doubla A, Naitali M (2009) Microbial inactivation using plasma-activated water obtained by gliding electric discharges. Lett Appl Microbiol 48(1):13–18
Kang MH, Hong YJ, Attri P, Sim GB, Lee GJ, Panngom K, Kwon GC, Choi EH, Uhm HS, Park G (2014) Analysis of the antimicrobial effects of nonthermal plasma on fungal spores in ionic solutions. Free Radic Biol Med 72:191–199
Kang MH, Pengkit A, Choi K, Jeon SS, Choi HW, Shin DB, Choi EH, Uhm HS, Park G (2015) Differential inactivation of fungal spores in water and on seeds by ozone and arc discharge plasma. PLoS One 10(9):e0139263
Karlovsky P, Suman M, Berthiller F, De Meester J, Eisenbrand G, Perrin I, Oswald IP, Speijers G, Chiodini A, Recker T, Dussort P (2016) Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res 32(4):179–205
Kavanagh K (2017) Fungi: Biology and applications. John Wiley & Sons, New York, NY
Khadem AA, Sharifi SD, Barati M, Borji M (2012) Evaluation of the effectiveness of yeast, zeolite and active charcoal as aflatoxin absorbents in broiler diets. Global Vet 8(4):426–432
Khamsen N, Onwimol D, Teerakawanich N, Dechanupaprittha S, Kanokbannakorn W, Hongesombut K, Srisonphan S (2016) Rice (Oryza sativa L.) seed sterilization and germination enhancement via atmospheric hybrid nonthermal discharge plasma. ACS Appl Mater Interfaces 8:19268–19275
Kim JY, Lee IH, Kim D, Kim SH, Kwon Y-W, Han G-H, Cho G, Choi EH, Lee GJ (2016) Effects of reactive oxygen species on the biological, structural, and optical properties of Cordyceps pruinosa spores. RSC Adv 6(36):30699–30709
Klämpfl TG, Isbary G, Shimizu T, Li YF, Zimmermann JL, Stolz W, Schlegel J, Morfill GE, Schmidt HU (2012) Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl Environ Microbiol 78(15):5077–5082
Koban I, Matthes R, Hübner NO, Welk A, Meisel P, Holtfreter B, Sietmann R, Kindel E, Weltmann KD, Kramer A, Kocher T (2010) Treatment of Candida albicans biofilms with low-temperature plasma induced by dielectric barrier discharge and atmospheric pressure plasma jet. New J Phys 12:073039
Kogelschatz U (2004) Atmospheric-pressure plasma technology. Plasma Phys Controll Fusion 46:63–75
Korachi M, Turan Z, Sentürk K, Sahin F, Aslan N (2009) An investigation into the biocidal effect of high voltage AC/DC atmospheric corona discharges on bacteria, yeasts, fungi and algae. J Electrost 67:678–685
Kuiper-Goodman T (1995) Mycotoxins: risk assessment and legislation. Toxicol Lett 82:853–859
Lampel KA, Khaldi S, Cahill SM (2012) Bad bug book: handbook of foodborne pathogenic microorganisms and natural toxins. U.S. Food and Drug Administration, Washington, DC
Laroussi M (1996) Sterilisation of contaminated matter with an atmospheric pressure plasma. IEEE Trans Plasma Sci 24:1189–1191
Laroussi M, Leipold F (2004) Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int J Mass Spectrom 233(1–3):81–86
Lee K, Paek Kh, Ju WT, Lee Y (2006) Sterilization of bacteria, yeast, and bacterial endospores by atmospheric-pressure cold plasma using helium and oxygen. J Microbiol 44(3):269–275
Liang Jl, Zheng Sh, Ye Sy (2012) Inactivation of Penicillium aerosols by atmospheric positive corona discharge processing. J Aerosol Sci 54:103–112
Liu K, Wang C, Hu H, Lei J, Han L (2016) Indirect treatment effects of water–air MHCD jet on the inactivation of Penicillium Digitatum suspension. IEEE Trans Plasma Sci 44(11):2729–2737
Los A, Ziuzina D, Akkermans S, Boehm D, Cullen PJ, Impe JV, Bourke P (2018) Improving microbiological safety and quality characteristics of wheat and barley by high voltage atmospheric cold plasma closed processing. Food Res Int 106:509–521
Los A, Ziuzina D, Boehm D, Cullen PJ, Bourke P (2020) A comparison of inactivation efficacies and mechanisms of gas plasma and plasma-activated water against Aspergillus flavus Spores and Biofilms. Appl Environ Microbiol:86
Lu Q, Liu D, Song Y, Zhou R, Niu J (2014) Inactivation of the tomato pathogen Cladosporium fulvum by an atmospheric-pressure cold plasma jet. Plasma Process Polym 11(11):1028–1036
Luo X, Wang R, Wang L, Li Y, Bian Y, Chen Z (2014) Effect of ozone treatment on aflatoxin B1 and safety evaluation of ozonized corn. Food Control 37(1):171–176
Ma R, Feng H, Li F, Liang Y, Zhang Q, Zhu W, Zhang J, Becker KH, Fang J (2012) An evaluation of anti-oxidative protection for cells against atmospheric pressure cold plasma treatment. Appl Phys Lett 100:123701
Ma RN, Feng HQ, Liang YD, Zhang Q, Tian Y, Su B, Zhang J, Fang J (2013) An atmospheric-pressure cold plasma leads to apoptosis in Saccharomyces cerevisiae by accumulating intracellular reactive oxygen species and calcium. J Phys D Appl Phys 46:285401
Ma R, Feng H, Guo J, Liang Y, Zhang Q, Tian Y, Zhang J, Fang J (2014) An efficient and specific protection of non-thermal plasma-induced live yeast cell derivative (LYCD) for cells against plasma damage. Plasma Process Polym 11(9):822–832
Ma R, Wang G, Tian Y, Wang K, Zhang J, Fang J (2015) Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J Hazard Mater 300:643–651
Ma R, Yu S, Tian Y, Wang K, Sun C, Li X, Zhang J, Chen K, Fang J (2016) Effect of non-thermal plasma-activated water on fruit decay and quality in postharvest Chinese bayberries. Food Bioprocess Technol 9(11):1825–1834
Maisch T, Shimizu T, Isbary G, Heinlin J, Karrer S, Klämpfl TG, Li YF, Morfill G, Zimmermann JL (2012) Contact-free inactivation of Candida albicans biofilms by cold atmospheric air plasma. Appl Environ Microbiol 78(12):4242–4247
Mendez-Albores A, Del Rio-Garcia JC, Moreno-Martinez E (2007) Decontamination of aflatoxin duckling feed with aqueous citric acid treatment. Anim Feed Sci Technol 135(3-4):249–262
Misra NN, Keener KM, Bourke P, Mosnier JP, Cullen PJ (2014) In-package atmospheric pressure cold plasma treatment of cherry tomatoes. J Biosci Bioeng 118(2):177–182
Misra NN, Yadav B, Roopesh MS, Jo C (2018) Cold plasma for effective fungal and mycotoxin control in foods: mechanisms, inactivation effects, and applications. Compr Rev Food Sci Food Saf 18:106–120
Morfill GE, Shimizu T, Steffes B, Schmidt HU (2009) Nosocomial infections—a new approach towards preventive medicine using plasmas. New J Phys 11:115019
Morgan NN, Elsabbagh MA, Desoky S, Garamoon AA (2009) Deactivation of yeast by dielectric barrier discharge. Eur Phys J Appl Phys 46:31001
Naitali M, Kamgang-Youbi G, Herry JM, Bellon-Fontaine MN, Brisset JL (2010) Combined effects of long-living chemical species during microbial inactivation using atmospheric plasma-treated water. Appl Environ Microbiol 76(22):7662–7664
Nield LS, Kamat D (2007) Prevention, diagnosis, and management of diaper dermatitis. Clin Pediatr (Phila) 46:480–486
Ouf SA, Basher AH, Mohamed AA (2015) Inhibitory effect of double atmospheric pressure argon cold plasma on spores and mycotoxin production of Aspergillus niger contaminating date palm fruits. J Sci Food Agric 95:3204–3210
Palm ME (2001) Systematics and the impact of invasive fungi on agriculture in the United States knowledge of the systematics of plant-inhabiting fungi is fundamental for making appropriate plant quarantine decisions and thereby safeguarding US plant resources. Bioscience 51:141–147
Pan J, Sun K, Liang YD, Sun P, Yang XH, Wang J, Zhang J, Zhu WD, Fang J, Becker KH (2013) Cold plasma therapy of a tooth root canal infected with Enterococcus faecalis biofilms in vitro. J Endod 39:105–110
Pankaj SK, Bueno-Ferrer C, Misra NN, O'Neill L, Jiménez A, Bourke P et al (2014) Surface, thermal and antimicrobial release properties of plasma-treated Zein films. J Renewable Mater 2:77–84
Pankaj SK, Shi H, Keener KM (2018) A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci Technol 71:73–83
Panngom K, Lee SH, Park DH, Sim GB, Kim YH, Uhm HS, Park G, Choi EH (2014) Non-thermal plasma treatment diminishes fungal viability and up-regulates resistance genes in a plant host. PLoS One 9(6):e99300
Park BJ, Takatori K, Sugita-Konishi Y, Kim IH, Lee MH, Han DW, Chung KH, Hyun SO, Park JC (2007) Degradation of mycotoxins using microwave-induced argon plasma at atmospheric pressure. Surf Coat Technol 201:5733–5737
Park G, Baik KY, Kim JG, Kim YJ, Lee KA, Jung RJ, Cho G (2012) Analysis of the biological effects of a non-thermal plasma on Saccharomyces cerevisiae. J Korean Phys Soc 60(6):916–920
Pignata C, Angelo D, Basso D, Cavallero MC, Beneventi S, Tartaro D, Meineri V, Gilli G (2014) Low-temperature, low-pressure gas plasma application on Aspergillus Brasiliensis, Escherichia Coli and Pistachios. J Appl Microbiol 116:1137–1148
Rossman AY (2009) The impact of invasive fungi on agricultural ecosystems in the United States. Biol Invasions 11:97–107
Rupf S, Lehmann A, Hannig M, Schafer B, Schubert A, Feldmann U, Schindler A (2010) Killing of adherent oral microbes by a non-thermal atmospheric plasma jet. J Med Microbiol 59:206–212
Ryu YH, Kim YH, Lee JY, Shim GB, Uhm HS, Park G, Choi EH (2013) Effects of background fluid on the efficiency of inactivating yeast with non-thermal atmospheric pressure plasma. PLoS One 8(6):e66231
Sakudo A, Toyokawa Y, Misawa T, Imanishi Y (2017) Degradation and detoxification of aflatoxin B 1 using nitrogen gas plasma generated by a static induction thyristor as a pulsed power supply. Food Control 73:619–626
Scussel VM, Moecke ES, Da Silva BA, Da Silva JR, Rüntzel CL (2019) Effect of cold plasma on black beans (Phaseolus vulgaris L.), fungi inactivation and micro-structures stability. Emirates J Food Agric 31(11):864–873
Selcuk M, Oksuz L, Basaran P (2008) Decontamination of grains and legumes infected with Aspergillus spp. and Penicillium spp. by cold plasma treatment. Bioresour Technol 99:5104–5109
Shi H, Ileleji K, Stroshine RL, Keener K, Jensen JL (2017) Reduction of Aflatoxin in Corn by High Voltage Atmospheric Cold Plasma. Food Bioprocess Technol 10:1042–1052
Shin YP, Sang DH (2015) Application of cold oxygen plasma for the reduction of Cladosporium cladosporioides and Penicillium citrinum on the surface of dried filefish (Stephanolepis cirrhifer) fillets. Int J Food Sci Technol 50:966–973
Siciliano I, Spadaro D, Prelle A, Vallauri D, Cavallero MC, Garibaldi A, Gullino ML (2016) Use of cold atmospheric plasma to detoxify hazelnuts from aflatoxins. Toxins 8(5):125
Siddiqueab SS, Hardya GESJ, Bayliss KL (2018) Cold plasma: a potential new method to manage postharvest diseases caused by fungal plant pathogens. Plant Pathol 67:1011–1021
Šimončicová J, Kalinakova B, Kovacik D, Medvecka V, Lakatos B, Krystofova S, Hoppanova L, Paluskova V, Hudecova D, Durina P, Zahoranova A (2018) Cold plasma treatment triggers antioxidative defense system and induces changes in hyphal surface and subcellular structures of Aspergillus flavus. Appl Microbiol Biotechnol 102:6647–6658
Souskova H, Scholtz V, Julák J, Kommová L, Savická D, Pazlarová J (2011) The survival of micromycetes and yeasts under the low-temperature plasma generated in electrical discharge. Folia Microbiol 56(1):77–79
Suhem K, Matan N, Nisoa M, Matan N (2013) Inhibition of Aspergillus flavus on agar media and brown rice cereal bars using cold atmospheric plasma treatment. Int J Food Microbiol 161:107–111
Sun P, Sun Y, Wu H, Zhu W, Lopez JL, Liu W, Zhang J, Li R, Fang J (2011) Atmospheric pressure cold plasma as an antifungal therapy. Appl Phys Lett 98:021501
Sun P, Wu HY, Bai N, Zhou HX, Wang RX, Feng HQ, Zhu WD, Zhang J, Fang J (2012a) Inactivation of Bacillus subtilis spores in water by a direct-current, cold atmospheric-pressure air plasma microjet. Plasma Process Polym 9:157–164
Sun Y, Yu S, Sun P, Wu H, Zhu W, Liu W, Zhang J, Fang J, Li R (2012b) Inactivation of Candida biofilms by non-thermal plasma and its enhancement for fungistatic effect of antifungal drugs. PLoS One 7(7):e40629
Surowsky B, Schlüter O, Knorr D (2014) Interactions of non-thermal atmospheric pressure plasma with solid and liquid food systems: a review. Food Eng Rev 7(2):82–108
Tian Y, Ma R, Zhang Q, Feng H, Liang Y, Zhang J, Fang J (2015) Assessment of the physicochemical properties and biological effects of water activated by non-thermal plasma above and beneath the water surface. Plasma Process Polym 12:439–449
Wang SQ, Huang GQ, Li YP, Xiao JX, Zhang Y, Jiang WL (2015) Degradation of aflatoxin B1 by low-temperature radio frequency plasma and degradation product elucidation. Eur Food Res Technol 241:103–113
Wielogorska E, Ahmed Y, Meneely J, Graham WG, Elliott CT, Gilmore BF (2019) A holistic study to understand the detoxification of mycotoxins in maize and impact on its molecular integrity using cold atmospheric plasma treatment. Food Chem 301:125281
Xiong Z, Lu XP, Feng A, Pan Y, Ostrikov K (2010) Highly effective fungal inactivation in He+O2 atmospheric-pressure nonequilibrium plasmas. Phys Plasmas 17:123502
Xu D, Liu D, Wang B, Chen C, Chen Z, Li D, Yang Y, Chen H, Kong M (2015) In situ OH generation from O2− and H2O2 plays a critical role in plasma-induced cell death. PLoS One 10(6):1–14
Xu Y, Tian Y, Ma R, Liu Q, Zhang J (2016) Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem 197:436–444
Xu H, Zhu Y, Cui D, Du M, Wang J, Ma R, Jiao Z (2019) Evaluating the roles of OH radicals, H2O2, ORP and pH in the inactivation of yeast cells on a tissue model by surface micro-discharge plasma. J Phys D Appl Phys 52:395201
Xu H, Ma R, Zhu Y, Du M, Zhang H, Jiao Z (2020) A systematic study of the antimicrobial mechanisms of cold atmospheric-pressure plasma for water disinfection. Sci Total Environ 703:134965
Yasuda H, Miura T, Kurita H, Takashima K, Mizuno A (2010) Biological evaluation of DNA damage in bacteriophages inactivated by atmospheric pressure cold plasma. Plasma Process Polym 7:301–308
Yasui S, Seki S, Yoshida R, Shoji K, Terazoe H (2016) Sterilization of Fusarium oxysporum by treatment of non-thermal equilibrium plasma in nutrient solution. Jpn J Appl Phys 55:01AB01
Ye S, Song X, Liang JL, Zheng S, Lin Y (2012) Disinfection of airborne spores of Penicillium expansum in cold storage using continuous direct current corona discharge. Biosyst Eng 113:112–119
Yong HI, Kim HJ, Park S, Choe W, Oh MW, Jo C (2014) Evaluation of the treatment of both sides of raw chicken breasts with an atmospheric pressure plasma jet for the inactivation of Escherichia coli. Foodborne Pathog Dis 11(8):652e657
Yoshino K, Matsumoto H, Iwasaki T, Kinoshita S, Noda K, Iwamori S (2013) Monitoring of sterilization in an oxygen plasma apparatus, employing a quartz crystal microbalance (QCM) method. Vacuum 93:84–89
Zahoranová A, Hoppanová L, Šimončicová J, Tučeková Z, Medvecká V, Hudecová D, Kaliňáková B, Kováčik D, Černák M (2018) Effect of cold atmospheric pressure plasma on maize seeds: enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem Plasma Process 38:969–988
Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C (2005) The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 41:1232
Zhang Q, Liang Y, Feng H, Ma R, Tian Y, Zhang J, Fang J (2013) A study of oxidative stress induced by non-thermal plasma-activated water for bacterial damage. Appl Phys Lett 102(1–4):203701
Zhu Y, Li C, Cui H, Lin L (2020) Feasibility of cold plasma for the control of biofilms in food industry. Trends Food Sci Technol 99:142–151
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Zhejiang University Press
About this chapter
Cite this chapter
Ma, R., Jiao, Z. (2022). Inactivation of Fungi and Fungal Toxins by Cold Plasma. In: Ding, T., Cullen, P., Yan, W. (eds) Applications of Cold Plasma in Food Safety. Springer, Singapore. https://doi.org/10.1007/978-981-16-1827-7_5
Download citation
DOI: https://doi.org/10.1007/978-981-16-1827-7_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1826-0
Online ISBN: 978-981-16-1827-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)