Abstract
The fungicidal effect of low-temperature plasma generated by positive direct current discharge and its influence on the growth dynamics was evaluated on three micromycete species and yeast in water suspensions. The fungicidal effect was lower than analogous bactericidal effect and differs substantially among various fungal species. Together with the cidal effects, the slower growth of exposed fungal spores was observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The low-temperature plasma generated in various types of electrical discharges is a partially ionized gas where most of energy is stored in the kinetic energy of electrons, whereas ions keep the room temperature. This ionized gas represents a cold mixture of free radicals and charged particles and does not increase the temperature of material applied on. There are numerous works describing the biological effects of this plasma, devoted mainly to the killing of prokaryotic bacteria (for review see Laroussi 2005; Scholtz et al. 2007a; Moreau et al. 2008) or various applications in human medicine (Fridman et al. 2008). Concerning fungi, only Akishev et al. (2008) mentioned the inactivation of Aspergillus niger and Candida lipolytica on agar surface after exposure with the plasma jet device. The possible application of plasma sterilization may be useful, e.g., for treatment of fruits’ surface, preventing the mold overgrowth and bacterial putrefaction. This work presents the fungicidal effect of stabilized positive flashing corona discharge and its influence on the growth dynamics of three micromycete species and yeast in a water suspension.
Materials and methods
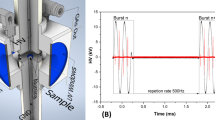
The low-temperature plasma was generated using the previously described simple apparatus of an open-air type (Julák et al. 2006). Briefly, the discharge burns on the point electrode represented by the tip of a syringe needle connected with a serial resistance of 20 MΩ to the positive pole of direct current high-voltage supply. The ground electrode was realized by the surface of water suspension of microorganisms connected with an immersed platinum wire to the negative pole of the supply. The discharge voltage was set to 9.7 kV and the distance between the tip of a needle and water surface was adjusted to 3 mm to get the discharge current of 400 μA. All exposures were performed under laminar flow of HEPA-filtered air to prevent the airborne contamination; an air-conditioning of the laboratory controlled the ambient conditions (Fig. 1).
The conidia of fungal species Aspergillus oryzae (DBM 4002), Cladosporium sphaerospermum (DBM 4282), and Penicilium crustosum (DBM4159) and the cells of yeast Candida albicans were studied. They were cultivated for 5 days on YGCH medium (Bio-Rad, Czech Republic) at 20°C. The fungal conidia were harvested using a bacteriological loop and suspended in sterile PBS (pH 7.4). The suspensions of conidia and of the yeast cells were prepared immediately before the exposure to the discharge. The suspension (0.5 mL) was pipetted into the sterile wells of a dot plate and exposed to the discharge for various time intervals. Following the exposure, the content of each well was spread onto the surface of YGCH agar and, after the cultivation at 20°C, the number of surviving colonies (i.e., CFU) were counted.
Results and discussion
We observed that the number of surviving cells of yeast C. albicans decreased from 5 × 104 CFU/mL to zero after a 6-min exposure to the discharge. The total inactivation of A. oryzae spores does not occur even after a 30-min exposure only the reduction from 105 to 102 CFU/mL could be observed. In the case of C. sphaerospermum, the number of surviving cells was the same as the initial concentration of 105 CFU/mL for the first 10 min, then rapidly decreased up to a total inactivation within further 10 min. Also, the number of surviving cells of P. crustosum decreased during the first 15 min of exposure to one half of initial value (104 CFU/mL); during the next 5 min it decreased to the number of 40 CFU/mL and, finally, to total inactivation after next 5 min of exposure (Table 1).
The dynamics of micromycete growth after exposure with a sublethal dose of plasma was interesting: the surviving exposed fungal spores grow visibly slower than the nonexposed (Table 2). The appearance of the first colonies in exposed micromycetes was delayed 35 h for Cladosporium, 45 h for Aspergillus, and up to 65 h for Penicilium, as compared to nonexposed cultures. On the other hand, the time interval between appearance of growth and sporulation was shortened substantially differing with cultures grown from spores after discharge exposure. The number of fungal colonies on the agar surface was in each case ≈50 CFU/mL, which shows that the above differences could not be attributed to different growing conditions. Thus, it indicates that the exposed micromycetes sporulate faster than the nonexposed ones. In our previous work (Scholtz et al. 2010) with bacteria and yeast, similar effects were not observed.
Comparing these results with those obtained with bacteria, it may be concluded that bacteria are more susceptible than fungi to the low-temperature plasma. Using the less effective negative corona discharge (Scholtz et al. 2010), the complete inactivation of Escherichia coli and Staphylococcus aureus was achieved after 2 and 4 min of exposure, respectively. The bacterial spores of Geobacillus stearothermophilus were also more susceptible than the fungal spores (Scholtz et al. 2007b). The effectiveness of plasma generated by various discharges is apparent from the comparison of yeast inactivation, which occurred after 30 min under the negative corona but after 6 min under the positive discharge used in this work.
The low-temperature plasma generated by the positive corona discharge has the fungicidal effect differing for various species. Whereas the total inactivation of yeast happens in 6 min, the total inactivation of fungal spores needs 20–25 min of exposure for C. sphaerospermum and P. crustosum. A. oryzae spores were not completely inactivated even after 30 min of exposure. The lower resistance of vegetative yeast cells is probably due to the unprotected cell membrane on comparison with the encapsulated fungal spores. The exposure to plasma retards the consecutive fungal growth: while nonexposed spores grow during 45 to 70 h, the exposed ones need 80 to 135 h. This stress also enhances the sporulation in cultures grown from exposed spores. The possible explanation of this effect is the stepwise mechanisms of spore inactivation with partially reversible disruptions in the structure of spore, which retards or inhibits the germination.
Abbreviations
- HEPA:
-
High-efficiency particulate air filter
- PBS:
-
Phosphate-buffered saline
- CFU:
-
Colony forming unit(s)
- YGCH:
-
Yeast extract–glucose–chloramphenicol (medium)
References
Akishev Y, Grushin M, Karalnik V, Trushkin N, Kholodenko V, Chugunov V, Kobzev E, Zhirkova N, Irkhina I, Kireev G (2008) Atmospheric-pressure, nonthermal plasma sterilization of microorganisms in liquids and on surfaces. Pure Appl Chem 80:1953–1969
Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A (2008) Applied plasma medicine. Plasma Process Polym 5:503–533
Julák J, Kříha V, Scholtz V (2006) Corona discharge: a simple method of its generation and study of its bactericidal properties. Czech J Phys 56:B1333–B1338
Laroussi M (2005) Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process Polym 2:391–400
Moreau M, Orange N, Feuilloley MGJ (2008) Non-thermal plasma technologies: new tools for bio-decontamination. Biotech Adv 26:610–617
Scholtz V, Julák J, Kříha V, Mosinger J (2007a) Decontamination effects of low-temperature plasma generated by corona discharge. Part I: an overview. Prague Med Rep 108:115–127
Scholtz V, Julák J, Kříha V, Mosinger J, Kopecká S (2007b) Decontamination effects of low-temperature plasma generated by corona discharge. Part II: new insights. Prague Med Rep 108:128–146
Scholtz V, Julák J, Kříha V (2010) The microbicidal effect of low-temperature plasma generated by corona discharge: comparison of various microorganisms on an agar surface or in aqueous suspension. Plasma Process Polym 7:237–243
This study has been supported by grants MSM ČR 6046137306, MSM ČR 0021620806, and SVV-2010-260506.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soušková, H., Scholtz, V., Julák, J. et al. The survival of micromycetes and yeasts under the low-temperature plasma generated in electrical discharge. Folia Microbiol 56, 77–79 (2011). https://doi.org/10.1007/s12223-011-0005-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-011-0005-5