Abstract
Glass and glass-ceramics play vital roles in today’s technology development. Although glasses are known to mankind from pre-historic period, its wide applicability as a candidate for various technological applications was realized during the past century. Advancement in communication technology was mainly possible with fabrication of high quality glass fibers. Glass-ceramics are derived from glasses and possess both the advantages of glasses and ceramics. In this chapter, initially, the origin of glasses and glass-ceramics are discussed followed by their classification depending up on the nature of glass forming constituents. Synthesis routes for preparation of different types of glasses are discussed in detail. Thermodynamics and kinetics of glass formation and its conversion to glass-ceramics are explained towards end of the chapter. Structure property correlation is an important aspect in any studies involving material development and this aspect is explained in detail in this chapter. Techniques like solid-state NMR, XPS, EXAFS which give mainly information on short-range order in glasses have been explained with examples. Importance of thermal techniques for understanding glass and ceramic science has been brought out in this chapter. Finally, the chapter ends with certain representative applications of glasses and glass-ceramics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
9.1.1 Origin of Glass and Glass-Ceramics
Glasses can be considered as one of the oldest materials used by mankind over several centuries. It is amorphous in nature and is generally formed by mixing several solid substances, heating to melting stage followed by super-cooling to an extent to obtain a single solid-like structure. Despite its appearance as solid form, it is not termed as a solid and is considered as a semi-solid, fluid, or super-cooled liquid with very high viscosity. In a broader sense, glass is defined as a non-crystalline or amorphous solid which exhibits glass transition phenomenon upon heating. Glasses find wide technological applications due to their ease of synthesis and tailor-made properties.
Natural glasses mostly include obsidian (volcanic glass), tektites (meteorite impacts), fulgurites (formed when lightning strikes sand), metamicts (amorphization by radiation from neighboring radioactive atoms), etc. Ancient people used obsidian glasses as ornaments, valuables, and weapons. Scarcity of natural glasses paved the way for the development of man-made glasses. Archaeological excavations and studies revealed that first man-made glasses date to around 3500 BC in Egypt and Eastern Mesopotamia [1]. In medieval ages, around 1300 AD, glass making belonged to Venetian craftsmen who were considered to be expert in this field. George Ravenscroft of England made an important advancement in this field in 1674 when he used the technique of adding lead oxide to molten glass to increase its lifetime. The Industrial Revolution in England paved the way for increasing automation in glass making and converted this art into an industry. The process of making flat glass in the most convenient and reproducible way is credited to Émile Fourcault of Belgium. The method known as Fourcault process was commercialized in 1914. However, modern day glass-making process took shape from Sir Alastair Pilkington’s float glass manufacturing process in 1959 by which more than 90% of glasses are manufactured till date.
Glass-ceramics, on the other hand, are a comparatively newer class of materials with history dates to 1957 AD. Glass-ceramic consists of an amorphous phase (matrix) and one or more embedded crystalline phases produced as a result of controlled crystallization of the matrix. Prominence of glass-ceramic materials in today’s world is highly related to the demands of new technological advancements. An ideal glass-ceramic material should have fabrication advantage of glass coupled with special or required properties of ceramics. Glass-ceramic materials usually contain crystalline phase to the tune of 30–90% with ceramic properties such as opacity, non-brittleness, and high-temperature stability.
Glass-ceramics were discovered accidently by Stanley Donald Stookey [2]. He was working on the development and improvement of properties of Fotoform glasses. However, one night in 1954 he mistakenly heated a piece of experimental Fotoform glass to 900 °C instead of 600 °C. The next morning, he found out that the furnace was overheated. To his astonishment, he noticed an opaque solid which bounced back when accidentally dropped on floor. It was concluded that this may be attributed to presence of microscopic crystals within the matrix. His knowledge of chemistry of crystallization led him to tailor-make the experimental conditions and this resulted in the first glass-ceramic FotoCeram [2]. This accidental discovery led to the development of Corning Ware in the year 1957. Corning Ware (a variety of cookware), the first glass-ceramic material, is considered to be one of the major discoveries by Corning Glass Works and one of Stookey’s million dollar inventions. Till date, major developments in the field of glass-ceramics are being carried out for the design of new types of cookwares and induction oven cooktops. A famous quote by the creator of glass-ceramic goes as:
Why did such an important discovery occur so late in the history of glass, and why was an accident necessary to bring it about?—Donald Stookey, 1977
9.1.2 General Properties of Glass and Glass-Ceramics
Glass-ceramics are primarily synthesized by the method of regulated crystallization of glass melt. However, the controlling step involves heating rate, heating temperature, cooling rate, etc. Variation in each or all of these parameters leads to the generation of a wide variety of physical and chemical properties of glass-ceramics as compared to glass. Thus, it can be said that even we start with same chemical composition to obtain a uniform glass matrix or a glass-ceramic material, and the properties of these two compositions can be entirely different depending upon the process control. Hence, an understanding of some of the general properties of glassy material and its evolution under different experimental conditions is essential for developing materials for different applications.
Some of the general properties of glassy materials are follows:
-
(a)
Most of the glasses used in our daily life are transparent or translucent and allow light to fully or partially transmit through it, depending upon our requirements. Lack of grain boundaries in glasses is responsible for this.
-
(b)
Thermal expansion coefficient (TEC) of most of the glassy materials is of the order of 10−6 K−1.
-
(c)
Glass is a brittle material. This particular property is attributed to the presence of surface flaws or distorted structural units.
-
(d)
Mechanical strength of glass is very high due to the absence of any form of porosity within its structure as it is a super-cooled liquid.
-
(e)
Non-metallic glasses are mostly semiconductors or insulators at ambient temperature. At elevated temperatures, ionic movement within the glass matrix becomes energetically feasible leading to ionic conduction.
-
(f)
The strong bonding involved in glass network makes them chemically durable. Absence of grain boundaries, the primary zones of etching in glassy network, renders their stability toward aqueous or acidic media. However, the presence of alkali or alkaline earth metal ions contributes to leachability of glass.
-
(g)
Glasses can be made entirely recyclable to be reused for indefinite times.
-
(h)
Glasses do not have fixed melting or boiling point. They tend to undergo state change over a range of temperature.
-
(i)
Glasses possess the property of glass transition temperature (Tg) beyond which the glassy or brittle nature is replaced by a viscous or rubbery material. Value of Tg is always lower than the melting point of glass.
Glass-ceramics can circumvent some of the shortcomings of glasses for technological applications. Extensive studies have been carried out in this field in order to fine-tune material properties to satisfy user requirements. A brief description of some of the unique properties of synthesized glass-ceramics is given below.
-
(a)
The thermo-physical property such as thermal expansion coefficient (TEC) can be fine-tuned by modifying the processing condition involved in glass to glass-ceramic conversion [3]. It is now possible to design glass-ceramic materials with very low or even negative thermal expansion coefficient [4, 5]. Glass-ceramic materials also show exotic optical properties. Rare earth elements like Nd-doped glass-ceramics are potential candidates for laser application [6, 7]. Glass-ceramics possess almost zero porosity with lesser extent of surface flaws. Hence, the mechanical strength of glass-ceramic materials is relatively high. Defects present in glass-ceramics make glass-ceramics slightly less brittle. Depending upon the process of fabrication and composition, glass-ceramics can show either higher resistivity or higher conductivity as per requirements. Further, glass-ceramics show less amount of leachability as compared to glassy materials.
In addition to these properties, the glass component of glass-ceramic exhibits glass transition temperature (Tg), whereas the ceramic component will exhibit sharp melting point (Tm). It may however be noted that the Tg and/or Tm may shift to higher or lower temperatures depending upon the chemical environment in these materials as compared to base glass and ceramics. Thus, from the above discussion, it is clear that glass-ceramics are those materials which cover both the advantages of glass and ceramic materials. This eventually led to development of materials with improved functional properties. This aspect is discussed in subsequent part of this chapter.
9.1.3 Functional Glasses and Glass-Ceramics
The word “functional” means “having a specific activity, task, or usefulness”. In general, functional glasses and glass-ceramics mean materials having some useful and characteristic properties enabling them suitable for definite technological applications. Functional glasses and glass-ceramics can be classified into four types based on (a) chemical composition, (b) fabrication, (c) property, and (d) application as can be seen from Table 9.1.
-
(a)
Chemical composition based: Chemical composition-based glasses and glass-ceramics can be classified into two groups, namely the oxide-based and the non-oxide-based. Oxide-based glasses and glass-ceramics are those materials whose network is formed from oxides such as phosphorous pentoxide (P2O5), silica (SiO2), boron trioxide (B2O3), and germanium oxide (GeO2). On the other hand, non-oxide-based glasses and glass-ceramics mostly hover around chalcogen elements (sulfur (S), selenium (Se), tellurium (Te), arsenic (As), and antimony (Sb). Other elements such as germanium (Ge), gallium (Ga), inorganic halides such as fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and metals or alloys are involved in the formation of non-oxide-based glasses. This will be discussed in detail in Sect. 9.2.
-
(b)
Fabrication based: Functional glasses or glass-ceramics can be classified based on their methods of preparation or fabrication. Some important fabrication methods for glass and glass-ceramics are thermal evaporation, sputtering, chemical vapor deposition (CVD), melt-quenching, glow discharge decomposition, radiation-induced damage, etc. These techniques will be discussed in detail in Sect. 9.3.
-
(c)
Property based: Different properties associated with materials are basis for the origin of a class of materials known as “functional materials.” Glasses and glass-ceramics are classified into six broad categories based on optical, photonic, electrical, thermal, electronic, mechanical, and chemical properties.
-
(d)
Application based: Although this is not a criterion for classification of materials, it is closely related to the properties of materials. Many times, properties of materials only decide the application in which the material can be employed. The application or technology includes area such as energy, environment, health care, defense, and space. These points will be dealt in detail in applications part, i.e., Sect. 9.9.
9.2 Different Types of Functional Glasses and Glass-Ceramics
As mentioned earlier, functional glasses and glass-ceramics can be classified under different criteria as shown in Table 9.1. In the following section, different types of functional glasses and glass-ceramics are discussed on the basis of their composition.
9.2.1 Oxide-Based Glasses
Glasses formed by bridging of oxygen atoms (O) with other suitable atoms (X) are commonly referred as oxide glasses. The choice of “X” for oxide glass formation is generally guided by rules proposed by a famous Norwegian-American physicist, Wiliam Houlder Zachariasen, in the year 1932. The rules are summarized below
-
(i)
An O atom should be connected to not more than 2 X atoms.
-
(ii)
There should not be more than 3 to 4 O atoms connected with one X atom.
-
(iii)
The polyhedra formed by the coordination of X–O bond should be only corner shared. Edge sharing and face sharing polyhedra do not result in glass formation.
-
(iv)
Three corners of polyhedra should be shared in order to sustain a 3-D glassy network.
In addition to the above rules, other terminologies like network modifier and intermediates are used to understand and explain glass formation. Network formers are those atoms (X) that can form highly crosslinked 3-D network of covalent chemical bonds. Some common network formers are oxide of silicon (Si), boron (B), phosphorous (P), and germanium (Ge). Network modifiers are mostly metals such as calcium (Ca), lead (Pb), sodium (Na), potassium (K), etc., which prefers to remain in cationic form inside glassy network. The favored cationic forms of these elements alter glass network, whereby a bridging X–O–X bond breaks resulting in the generation of non-bridging O species. There are certain elements such as aluminum (Al), titanium (It), beryllium (Be), and lead (in certain cases) which can act both as network former or network modifier. Hence, these elements are termed as intermediates as their role varies with variation in glass composition. Nomenclature of oxide glasses is made based on their constituent X–O structural units.
-
(a)
Phosphate glasses: Glassy materials based on the network former \({\text{PO}}_{4}^{3 - } ({\text{X}} = {\text{P}})\) are broadly called as phosphate glasses. The phosphate glass network consists of one terminal P = O and three bridging P–O–P bonds. The most common starting material for preparation of this type of glass is P2O5. Highly moisture sensitive nature of P2O5 renders these glasses to be of less technological importance. Hence, suitable network modifiers or intermediates are always required during preparation of phosphate glasses for making them technologically relevant. In general, these glasses possess very high thermal expansion coefficient and melt formation occurs at relatively lower temperatures.
-
(b)
Silicate glass: These are the most versatile glassy materials having \({\text{SiO}}_{4}^{4 - } \left( {{\text{X}} = {\text{Si}}} \right)\) as their constituent building blocks and the starting material for preparation of such glasses is SiO2. This type of glass network mostly consists of four bridging Si–O–Si bonds. The most common types of such glasses include alkali and alkaline earth silicate glasses where introduction of these elements breaks the bridging Si–O–Si bonds resulting in the generation of a negative charge at oxygen (Si–O−) which is balanced by suitable number of mono or divalent cations. Replacement of rigid bridging bonds with less rigid non-bridging linkages results in increase of thermal expansion coefficient and decrease in micro-hardness of these glasses. Higher viscosity of melt and rapid volatilization of alkali metals are problems encountered during preparation of silicate glasses with very low alkali metal concentration.
-
(c)
Borate glass: The most common starting material for single component borate glasses is boron oxide (B2O3) or boric acid (H3BO3), and the main structural building blocks in different borate glasses are BO3 and BO4 units along with boroxol ring. Pure borate glasses are difficult to prepare because of affinity toward moisture and this can be avoided by preparing glasses at very low atmospheric pressure of around 1 mm of Hg. Borate glasses are mostly found to be low melting and hence find use as solder glasses.
-
(d)
Borosilicate glass: The starting materials for the preparation of these glasses are boron oxide (B2O3) or boric acid (H3BO3) and SiO2. Typical network follows with four bridging Si–O–Si bonds and three bridging B–O–B bonds. However, depending upon the ratio of these two starting materials, an average network of B–O–Si is obtained with coordination number between 3 and 4. In order to maintain proper network of four bridging bonds, some network modifiers such as Na2O, BaO, etc., are added during glass formation. Generally, it is considered that at low modifier concentration, Na2O reacts with BO3 to form BO4 structural units, whereas at higher modifier concentration, Na+ is equally shared by BO4- and SiO4 network. At a given alkali content, addition of B2O3/H3BO3 to silicate glasses markedly reduces the thermal expansion coefficient and enhances the chemical durability specially to attack by acids.
-
(e)
Aluminate glass: Alumina (Al2O3) being a refractory material, single component alumina glass is difficult to prepare. However, with addition of suitable alkali or alkaline earth metal oxides binary and ternary glasses with Al2O3 can be formed. Addition of silica or germanium oxide (GeO2) transforms Al2O3-based glasses into technologically relevant material which will be discussed in Sect. 9.9.
-
(f)
Aluminosilicate glass: These glasses mainly consist of around 20 mol% of alumina (Al2O3) with small amounts of calcium oxide (CaO), magnesium oxide (MgO) along with major content of silica (SiO2). Aluminum mainly exists as tetrahedrally and octahedrally coordinated species in these glasses. Further, in these glasses, Si atom is tetrahedrally coordinated with oxygen having different number of Al as the next-nearest neighbors. These glasses can withstand very high temperatures and thermal shock.
-
(g)
Germanate glass: Pure germanium oxide (GeO2) has the ability to readily form glassy phase. However, for practical applications, GeO2 along with SiO2 are added to calcium aluminate glasses. The main building block of these glasses is GeO4 tetrahedra resulting in the formation of an extended network. Introduction of alkali metal ions in low concentration results in transformation of GeO4 tetrahedra to GeO6 octahedra. Under higher concentration, these modifiers result in the formation of non-bridging oxygen atoms attached with Ge in the glass structure.
-
(h)
Tellurite glasses: Tellurite glasses were first prepared and studied by Stanworth in 1952. Single component TeO2 (pure TeO2) forms glass when melted in gold crucible. The most probable reason may be reaction of alumina crucible with the starting material in the former case. Nevertheless, addition of alkali or alkaline earth metal oxides, PbO, WO3, etc., is found to facilitate tellurite glass formation.
-
(i)
Vanadate glasses: Pure V2O5 melts around 660 °C and forms glass only when it is cooled rapidly. Glass formation with V2O5 is quite common upon addition of a number of oxides like P2O5, TeO2, B2O3, and GeO2. Such glasses are potential candidates for different applications.
9.2.2 Non-oxide-Based Glasses
These are the glasses which are mostly formed by bridging of suitable atoms other than oxygen with metal or nonmetals. The major non-oxide-based glasses are discussed in brief as follows:
-
(a)
Chalcogenide glasses: These are glasses consisting of one or more chalcogens such as sulfur (S), selenium (Se), and tellurium (Te) apart from oxygen and polonium (Po). Chalcogenide materials are important due to the following reasons:
-
1.
They can be prepared in the amorphous form in a variety of ways.
-
2.
Glass formation occurs over a wide range of composition and the physical properties vary in a continuous fashion.
-
3.
The bandgaps of chalcogenide glasses are of the order of 1–3 eV, and hence, these materials show semiconducting behavior.
-
4.
Many of these glasses are candidates for technological applications which include infrared transmission, switching devices in computer memories, etc. Representative chalcogenide glasses are given in Table 9.2.
Table 9.2 Representative chalcogen-based glasses
-
1.
-
(b)
Halide glasses: These are glasses consisting of metal halides such as beryllium fluoride (BeF2), zinc chloride (ZnCl2), zirconium fluoride (ZrF4), hafnium fluoride (HfF4), etc. Other glass forming halide systems are mostly based on binary or ternary phases such as AgCl, AgBr, AgI, and PbBr2, PbCl2–BaCl2, SnCl3–PbI2, etc. Ionic radius of Be2+ is quite close to that of Si4+ and that of F− and O2− are almost identical. This is the main reason for similar resemblance of corner connected tetrahedral network of vitreous BeF2 and vitreous SiO2. Glasses based on ZnCl2 are also formed by corner shared tetrahedra of Zn–Cl bonds quite similar to that existing in BeF2 glass. However, the water solubility of zinc chloride glasses renders them ineffective for important technological applications. On the other hand, glasses based on ZrF4 and HfF4 are found to be highly ionic in nature. The basic building blocks in these glasses are not well defined and studies suggest that coordination number between Zr4+/Hf4+ and F− ions varies from 6 to 8. This is the main reason for the ionic nature of these glasses.
-
(c)
Organic glasses: Organic glasses mostly consist of severely entangled C–C chains which prohibits crystallinity upon rapid cooling. The network chains in these glasses are found to be crosslinked and are similar to that existing in chalcogenide glasses. Extent of cross-linking dictates the viscosity of melt and the glass transition temperature of such glasses. Their general properties are mostly similar to those of chain-based inorganic glasses.
-
(d)
Metallic glass: Metallic glasses can be obtained by rapid cooling of alloy (consisting of only metals or both metals and metalloids) melt. Nucleation and growth of crystal become an important competing factor to glass formation in these materials. Metallic glasses can be prepared by preventing nucleation under very fast cooling of the order of 104 Ks−1. Such cooling rates help to generate glass in film or ribbon forms. Some common metallic glasses are Pd80Si20, Ni80P20, Fe40Ni40P14B6, etc. Structural model of these materials can be explained by assuming random packing of nanosized spheres with smaller spheres occupying the interstitial spaces. Addition of metalloid atoms hinders reorganization of the atoms during cooling, thereby preventing unwanted crystalline domains within the glass network.
Most of the above-mentioned types of oxide-based functional glasses can form glass-ceramics by suitable tuning of synthesis procedure. Modulation of heating and cooling rates, heating at an intermediate temperature during processing, etc., can lead to glass-ceramics with tailor-made properties in these types of materials. Preparation of metallic glass-ceramics requires minimal nucleation at distant points within the melt to prevent precipitation of larger crystals. Greater extent of crystallization in metallic glass leads to a composite system of an alloy and glass which may not be compatible with each other, thereby rendering unsuitable for applications.
9.3 Different Routes for Synthesis of Glass
Some of the common methods for preparation of glasses are as follows:
9.3.1 Thermal Evaporation
This technique is one of the most extensively used methods for preparation of amorphous or glassy thin films [8]. Material to be amorphized is kept in powder form inside an evacuated chamber at around ~10−6 torr pressure. A substrate is also kept inside the chamber over the sample to be evaporated. The assembly is then heated by Joule heating (or resistive heating) for low melting materials or using electron beam for refractory materials. The powdered sample becomes mobile at higher temperature and percolates to the substrate. Low temperature of the substrate allows the mobile atoms to freeze on reaching the contact surface resulting in the formation of a glassy or amorphous phase. Schematic diagram of this preparative method is given in Fig. 9.1. Amorphous chalcogenide semiconductors like Si, Ge, GaAs, etc., are prepared by this method. Tunability of properties of the prepared films can be achieved by optimization of various process parameters such as substrate temperature, substrate nature, separation and orientation of base, deposition rate and gas pressure inside the chamber. A major disadvantage of this process is preferential evaporation of low melting component which depletes the source leading to compositional in-homogeneities in the prepared material. Moreover, the volatility of components sometimes results in change of compositional identity in the vapor phase compared to the feed source. A common example in this regard is the presence of As4S4 molecules in vapor phase despite the starting reactant being stoichiometric As2S3 in solid phase.
9.3.2 Sputtering
Sputtering method comprises of bombardment of a target material by high energy ions obtained from plasma generated at low pressure. As a consequence, atoms or cluster of atoms are removed from target and subsequently gets deposited over a substrate in the form of a thin film. The mechanism of synthesis by this process involves the generation of plasma by suitable radio frequency which is struck in between the target material and substrate. During each negative half cycle, the positive ions are attracted toward the target. Since the mobility of electrons is much higher compared to that of corresponding cations generated, accumulation of negative bias takes place. This negative bias attracts the positively charged ions from the plasma as a result of which the positive charged ions are deposited over the substrate. Amorphous thin films of Si, Ge, SiO2, etc., are prepared by this method [9,10,11]. The major advantage of sputtering technique is that relatively more homogenous films of uniform thickness can be prepared by this method as compared to thermal evaporation method. The homogeneity of produced films can be attributed to the sputtering rates which do not have a wide variation among different species. Almost similar sputtering rates preserve the compositional homogeneity of the starting material as compared to thermal evaporation methods. A schematic diagram of sputtering set up is shown in Fig. 9.2.
The only disadvantage of the process lies in optimization of the process control parameters such as ratio of partial pressure of reactive to inert gas, bias voltage applied to the target material, and radio frequency power applied to the target.
9.3.3 Glow Discharge Decomposition
Glow discharge decomposition is quite similar to sputtering technique discussed above. The only difference lies in the fact that a chemical reaction is initiated in the gas phase by creating a radio frequency-induced glow discharge of the reactant gas instead of plasma ejecting material in the sputtering process. Glow discharge results in deposition of solid over a suitable substrate kept inside the chamber. The discharge can be generated not only in a mixture of carrier and reactant gas but also with varied combination of reactant gases along with carrier gas for forming films of different functional materials such as SiO2, Si3N4, etc. [12, 13]. The major disadvantage of this method is optimization of process control parameters for preparation of tailor-made materials. Schematic diagram of glow discharge decomposition setup is shown in Fig. 9.3.
Chemical vapor deposition: Chemical vapor deposition technique is quite similar to glow discharge method of preparation. In this technique, the decomposition of the reactant gas is carried out by applying thermal energy (pyrolytic process). As a consequence, temperatures of the order of 103 K are commonly used and this is one of the major disadvantages of the process. Some of the materials with different functionalities prepared by this process include amorphous hydrogenated Si, B, and P-doped amorphous Si, etc. [14]. Schematic diagram of chemical vapor deposition (CVD) setup is shown in Fig. 9.4.
9.3.4 Melt-Quench Technique
Melt-quench technique is considered the oldest method for the preparation of glassy and amorphous materials. This method involves calcination and melting of glass precursors (mostly oxides, nitrates, acetates, etc.) in compatible or non-reactive crucibles such as platinum, rhodium, alumina, zirconia, sillimanite, followed by pouring of the melt at room temperature or under liquid nitrogen (Fig. 9.5). Depending upon the degree of quenching required, the melting containers are made up of good thermal conducting materials such as copper and graphite. Quenching of the melt is required to avoid nucleation and crystal growth which can lead to phase segregation form the glass matrix. Rate of cooling of the glass melt is an important criterion for glass production by this method. For oxide glasses having glass formers such as B2O3, P2O5, and SiO2, a cooling rate of 1 Ks−1 is found to be sufficient for getting good quality glasses, whereas for metallic glasses, cooling rate must be over the range of 106 Ks−1.
Most of the oxide glasses are prepared by melt-quench method because of simplicity and cost-effectiveness of the process and non-reactive nature of the product under atmospheric conditions. Only minor disadvantage of this process is that for industrial scale preparation of glass, continuous or intermittent stirring is necessary to avoid inhomogeneity of the viscous melt. Covering the reaction crucible will help in preventing evaporation of high volatile components, thereby avoiding significant changes in the composition of final prepared glass. In order to prepare chalcogenide and chalco-halide glass by this technique, melting and pouring of glass melt is required to be carried out under inert atmosphere or in vacuum sealed crucibles [15]. Moreover, the furnace has to be designed with rocking mechanism for proper mixing of components in [15] sealed crucibles for better homogeneity of the melt. It is preferable to have starting precursor materials as oxides since nitrates or acetates will release NOx, acetic acid vapors, respectively, which may damage or even burst the crucible.
9.3.5 Sol-Gel Method
In general, sol is considered as a colloidal suspension of solid in liquid, whereas gel is considered to be a colloidal suspension of liquid in solid. Thus, it is quite evident from the definition itself that gel can be produced from a viscous colloidal solution by facilitating polymerization of the solid particles. The homogenous and amorphous gel is then heated to remove volatile constituents to obtain an amorphous solid. This is then subjected to final sintering process at suitable temperatures to obtain a dense solid material. The oxide particles obtained by this method show fine dispersion and are found to be in nanoscale dimensions. The major advantage of sol-gel processing is the formation of materials containing refractory-based components at relatively lower temperatures. Suitable processing and purification of precursor materials by various methods before sol-gel processing results in formation of products with very high level of purity [16]. Composition and properties of the products obtained can vary depending upon conditions such as temperature, solvent concentration, reaction time, and sintering temperature. Moreover, the process of drying and sintering of gel are time consuming. Availability of suitable metal precursors also limits the applications of this method for preparation of novel materials for catering to the demands of newer technologies.
9.3.6 Electrolytic Deposition
This is one of the most important methods for the preparation of glassy oxide materials. Using metal surface as anode, it can be oxidized to an amorphous oxide layer in an electrolytic cell having suitable electrolytes. On passing a DC voltage through an electrolytic cell, cations migrate toward the cathode and O2− ions migrate toward the metal anode. These anions then react with the metal to yield an amorphous glassy oxide layer over the anode surface which can grow up to ~103Å thickness. This method is widely used to prepare glassy oxide films of Al, Zr, Ti, Nb, and so on [17]. The major disadvantage of this method is the difficulty in preparing non-oxide-based glasses.
9.3.7 Radiation Bombardment
Bombarding by high energy particles such as neutron, alpha, or heavy charged particles (HCP) on crystalline samples leads to formation of amorphous phase. When a particle with very high kinetic energy is impinged over any substrate, enormous amount of local energy deposition takes place followed by rise in local temperature of the order of ~103 K for a very short span of time (typically for 10−10 − 10−11 s). This sudden spurt in temperature results in melting followed by very fast cooling of the substrate. Quartz and cristobalite (allotropes of crystalline silica) are progressively amorphized by radiation resulting in the generation of vitreous silica [18]. Major advantage of this method is incorporation of radioactive species in the material which has immense potential to be used in nuclear medicine and other research applications. Limited experimental facility setup available for carrying out radiation damage studies is the major disadvantage of this method.
The methods discussed above are considered to be the most widely used methods for preparation of glassy or amorphous materials. In addition to these methods, high pressure shock waves, slow mechanical grinding, explosive compaction, hydrogen inclusion, etc., are also used for glass preparation. It may be noted that these methods are specific for certain systems and cannot be extrapolated or optimized for even related systems, thereby lacking a wider applicability in glass manufacturing industry. Till date, melt-quench technique is considered as the best and the most widely used method for glass preparation both in industries as well as in research laboratories worldwide.
9.4 Different Routes for Synthesis of Glass-Ceramics
In spite of the fact that the presence of crystalline phase is undesirable during glass formation, controlled crystallization of desired phases in glass matrix is primary requirement for formation of functional glass-ceramic material. Two most important words in the above sentence are “controlled crystallization” and “desired phase” which are the main guiding factors for preparation of glass-ceramics.
Functional glass-ceramics can be prepared by any of the glass preparation methods followed by modification in heat treatment. The major difference lies in the fact that the one-step process involved in glass preparation has to be converted to a minimum two-step process for glass-ceramic preparation. The most common method is the preparations of glass by a standard glass manufacturing process like melt-quench method. The glass material is then shaped, cooled, and reheated (sometimes repeatedly) above its glass transition temperature to prepare a desired glass-ceramic material. During heat treatments, occasionally nucleating agents such as noble metals, TiO2, and Fe2O3 are added to aid the formation of desired crystals of suitable dimensions required for improving functionality. Synthesis of glass-ceramic materials by this method leads to particle crystallization inside the glass matrix, thereby resulting in a homogenous glass-ceramic material.
The second technique used for preparation of glass-ceramics is induction of controlled internal crystallization during cooling cycle of a glass forming process. This technique involves heating the precursor materials to melting followed by slow cooling above the glass transition temperature followed by quenching below it. This method also involves addition of nucleating agents (sometimes in larger amount) as the reaction kinetics is quite fast. This method generally leads to, relatively coarse glass-ceramics as there exists relatively less control over the whole process. Moreover, the crystalline phase may not be homogeneously distributed within the glass matrix and this leads to reduction in quality of the material for different applications.
The third most common process involves separate preparation of desired glass and ceramic formulations followed by concurrent sinter-crystallization of both the materials to obtain glass-ceramic formulations. The major advantage of this process lies in the fact that one need not be concerned for the formation of “desired ceramic phase” as it is prepared separately. Other advantages are absence of any nucleating agents as the glass and ceramic interface behaves as nucleating sites, leading to faster ceramic particle growth in the matrix. Major disadvantage of the process is residual porosity within the matrix which needs to be eliminated by hot-pressing techniques, thereby making the process costly as compared to others.
9.5 Thermodynamic and Kinetic Aspects of Glass Synthesis
As mentioned earlier in the introduction section, glass is considered as a super-cooled liquid, semi-solid, or fluid in spite of its appearance as a solid form. Thus, technically, it can be said that glass is a liquid having very slow or negligible crystallization kinetics. Crystalline state of any material always has lower energy than liquid state, and hence, the possibility of crystal formation is a thermodynamically favorable process. Crystals are generally obtained on cooling of a liquid melt, whereas the presence of crystals poses a hindrance to glass formation. Hence, the glass fabrication always requires bypassing of stable thermodynamic path. On the other hand, it is also known that glassy materials can exist for centuries without undergoing substantial damage. It is generally accepted that both thermodynamics and kinetics go hand in hand during glass formation. The concept of chemical or thermodynamic stability of glassy materials can be understood with the help of a potential energy diagram (Fig. 9.6). Red balls in Fig. 9.6 can be assumed as a reference glass material. At position A, if the ball is pushed, it will roll down the slope as shown above and settle at position B. Similar will be case for position C and E. In all these three positions (peak), the ball will roll down the slope and settle in a position with lower potential energy (valley). These peak positions (A, C, and E) are thermodynamically called unstable state and any given perturbation leads to change in the energy which is lowered by performing some reactions. Hence, it can be concluded that any material present at elevated potential energy state will be highly reactive. If the ball is at position D, considerable perturbation is required to roll the ball upwards to cross the barrier C or E before it can finally rest. Position D has the lowest potential energy in the above energy profile diagram, and hence, it is difficult for the material being at this position to undergo chemical changes. Position D is termed as the stable state for the reference material.
As the stable state of any liquid upon cooling is the crystalline solid state, glass, which is a super-cooled liquid cannot be called as thermodynamically stable product or thermodynamically controlled product. Assuming a scenario that the ball is rolled from A with very high perturbation will lead to it resting finally at position D. This is the case for all thermodynamically controlled chemical reactions. However, if the reaction is quenched at rapid rate such that the ball cannot rise through barrier C, it will limit its movement up to position B. This position B is called metastable state where the ball is found to be temporarily stable unless the perturbation given makes it crosspoint C. Limited perturbation given at position B can keep the ball chemically unreactive for a long time. This is the condition where a liquid does not convert into crystalline solid but remains stable for longer duration. Any solid obtained at position B will not possess the properties of pure solid (position D) or pure liquid (position A, C and E). It will possess some mixed properties of pure solid and pure liquid, and it can correctly be termed as super-cooled liquid. Thermodynamically, this is termed as the glassy state where the material remains in a metastable equilibrium with its surroundings without undergoing further degradation (devitrification) into the crystalline solid state.
The major question here arises regarding the way to restrict chemical changes to position B. Restriction at this position implies zero or negligible crystal formation in the glass matrix. Formation of crystal in any melt occurs via two major steps namely nucleation and crystal growth. Nucleation occurs when solute molecules dispersed in solvent or melt gather in microscopic domains and arrange themselves in definite geometry to form clusters. However, it needs to be noted that, these cluster formations have to attain a particular size termed as the critical size to become stable nuclei. Crystal growth is the subsequent increase in size or growth of the nuclei after attainment of critical size. Fast cooling or quenching is hence required during glass formation to inhibit nucleation stage within the liquid melt. In nucleation events, a small number of unit cells combine with each other during short timescales, termed as the nucleation time Tnuc. A larger Tnuc implies fluctuations during nucleation stage do not allow the formation of crystals within glass melt. This reasoning is applicable for formation of all types of glasses and is irrespective of preparation methods.
Knowledge of thermodynamic and kinetic control parameters of glass formation can help us in design of functional glasses. It may be noted that both aspects mentioned above change with change in chemical composition and volume of the glass melt. The variation in specific volume as a function of temperature is shown in Fig. 9.7. On gradually lowering the temperature of a liquid, a linear decrease in specific volume is observed till the freezing point (melting point or Tm). At Tm, liquid can undergo either crystallization where liquid is converted to solid or super-cooling in which the material remains in liquid state even below freezing temperature. Crystallization of liquid melt is characterized by a sharp drop in specific volume of the substance at Tm, whereas glass formation or super-cooling is manifested by a gradual decrease in slope of the specific volume. The temperature range over which variation in slope of the cooling curve occurs corresponds to glass transition phenomenon and the onset of such change is the glass transition temperature (Tg). For convenience, glass transition temperature is expressed as fictive temperature (Tf) which is the temperature at the point of intersection of the straight-line regions of cooling curves corresponding to the liquid and the glass. Similar behavior is also observed if the specific volume V is replaced by other thermodynamic parameters such as enthalpy (H) and entropy (S).
It should however be noted that the glass transition temperature is a function of the rate of cooling of liquid melt. Faster or slower cooling of a melt leads to smaller or larger area on the cooling curve in the super-cooled region. Accordingly, there is an increase or decrease in the glass transition temperature. In other words, Tg of a particular glassy material is not an intrinsic property. This is because when a melt is quenched to form a glass, the different degrees of freedom undergo relaxation. When the glass is heated, the different degrees of freedom start relaxing. Since the relaxation rates are different for different degrees of freedom, the Tg values depend on the degree of freedom (property) which is monitored to determine the Tg. Glass transition temperature (Tg) of any material is related to the cooling rate (q) by Eq. 9.1:
where Tm the melting point, q0 and c are constants [19]. Experimentally, the determined values of Tg are also not unique as it depends upon the heating or cooling rates at which measurements are carried out. Hence, an independent parameter Kg (which describes the tendency to form glass) has been derived by Hruby [20] for characterizing glasses (Eq. 9.2).
where Tg is glass transition temperature, Tm is melting temperature and Tc is crystallization temperature. High (Tc − Tg) and low (Tm − Tc) value indicate high Kg values which imply inhibition of nucleation and subsequent crystallization within glass melt, thereby increasing the glass forming tendency of a melt.
Glass transition can also be defined in terms of viscosity of the melt. According to this definition, at glass transition temperature, the viscosity of a melt liquid attains a value of around 1013 poise. Near vicinity of glass transition, viscosity, or the relaxation time suddenly becomes so large, and the equilibrium between thermal state of any material and its surrounding is destroyed. This commonly occurs at around two-third of Tm for most of the oxide glasses. Variation of viscosity of glassy materials as a function of temperature can be explained according to either Arrhenius relaxation law or Vogel-Fulcher law and are represented by Eqs. 9.3 and 9.4. Arrhenius relaxation law states that viscosity (as well as relaxation time) undergoes exponential growth with decrease in temperature according to the relation (Eq. 9.3):
where η0 is constant and A is the activation energy for viscous flow of material.
The second equation of viscosity variation with temperature is Vogel-Fulcher, or Vogel-Fulcher-Tammann-Hesse law (Eq. 9.4) according to which:
where η0, B, and T0 are constants. Divergence in case of the second equation is more than the Arrhenius law. This phenomenon is explained by defining a temperature dependent activation energy \(A = BT/\left( {T - T_{0} } \right)\). For the last few decades, glasses have been characterized based on the above-mentioned nature of temperature dependence of glass transition process, namely strong glasses and fragile glasses. The glasses which exhibit high viscosity above melting temperature are termed as strong glasses and they are found to follow the Arrhenius relaxation law. Consequently, glassy materials following Vogel-Fulcher relaxation law are termed as fragile glasses as they exhibit low viscosity above melting temperature. It may be noted that this classification is based upon the flow characteristics of the melt and not on the mechanical properties of glass.
Thermodynamically, glass transition phenomenon is a second-order phase transition and hence second derivative of the free energy with respect to temperature, i.e., (∂2G/∂T2)p or Cp is discontinuous. Experimentally, it is observed that Cp is discontinuous during glass transition. However, this simple model cannot explain the change in Tg values depending on the thermal history of glass samples.
The phenomenon of glass transition is explained satisfactorily by free volume theory. The basic assumption of this theory is that a glassy material is composed of hard spheres and the total volume of glass is divided into two parts, namely the part which is occupied by the hard spheres (Vocc) and the other part in which the hard spheres or molecules are free to move (Vf). According to this theory, a glassy material is defined as the one having Vf independent of temperature, whereas, for liquids, both Vocc and Vf decrease and undergo redistribution with reduction in temperature. Based on this theory, glass transition takes place when Vf decreases below a critical value (Vfg). The fractional free volume (R) is related to the expression (Eq. 9.5):
-
where Vfg = free volume of glass
-
Vg = volume of glass
and \(\Delta \alpha_{T} = \alpha_{T1} - \alpha_{{T_{\text{g}} }}\) where αTl and αTg represent cubical expansion coefficients of liquid and glass, respectively.
In general, it has been observed that for most of the glasses about 10% of the total volume remains free at Tg. Free volume theory has been adapted for an extensive applicability by incorporation of percolation theory [21]. This theory has extended the free volume theory by accounting for exchange of Vf between nearest neighbors of liquid cells without any concurrent change in volumes of the solid cells. Although many theories exist, the phenomenon of glass transition could not be explained fully on the basis of any single theory, till today [22].
9.6 Kinetics of In Situ Crystallization
In situ crystallization of ceramic phase is a menace for glass formation. On the other hand, nucleation and crystallization of desired phases are mandatory for preparation of functional glass-ceramic materials. In view of this, the kinetics of in situ crystallization within glass matrix becomes an important aspect for synthetic chemists. A proper understanding of in situ crystallization becomes mandatory for design of functional glasses and glass-ceramics. The in situ crystallization kinetics of any glassy material can be understood based on time temperature transformation (TTT) diagrams. The TTT diagrams or isothermal transformation diagrams (as they are called) are plots of temperature versus time (either linear or logarithmic scale). These diagrams are experimentally generated from evaluation of percentage of phase transformation as a function of time. Experimental data at different temperatures are then plotted to yield TTT diagram. This plot is quite informative and helps to understand the crystallization kinetics of glass melts.
Annealing of glass materials is generally carried out to improve their functionality. It is well known that extent of diffusion in glasses becomes maximum near the glass transition temperature. Hence, annealing glasses at temperatures very close to the glass transition temperature reduces thermal stress generated due to fast cooling or quenching. An important strategy for glass-ceramic preparation involves both time and temperature optimization for crystallization of desired phase with required extent of crystal growth as crystal size many times decides the functionality of synthesized material.
The above discussion helps us to realize the importance of annealing and time temperature transformation diagram for developing functional glass or glass-ceramic material. In simpler terms it is a plot of percentage composition of both glassy and crystalline domains of any substance with variation of time. These diagrams are valid only for a particular composition of glass melt. Variation in composition of glass melt will definitely alter the TTT diagram. Let us understand in simple terms, the interpretation of such diagram by using Fig. 9.8. It is already discussed that above melting temperature (Tm), glass melt behaves as a pure liquid and below glass transition temperature (Tg) it behaves as a pure glassy material. Crystallization is not possible in a liquid or glass without undergoing any reaction. Thus, it is clear that the chances of crystallization of discrete phases within glass melt will be limited within the range from Tg to Tm. Experimental data obtained from time variation in glass and crystalline domains are plotted within the range of temperatures at which studies were carried out. (shown in purple color). According to this diagram, for glass formation, the cooling rate of glass melt should not pass through the crystallization domain. Let us assume that the melt is cooled though two different pathways A and B. The pathway A does not cross through the crystalline domain and hence 100% glassy phase can be obtained by cooling at this rate. If pathway B is followed, the formation certain extent of crystalline phases within the formed glass is expected. The slowest cooling rate which helps in the formation of glassy phase is termed as the critical cooling rate for that glass composition. The critical cooling rate that is necessary for preparation of glass can be obtained by drawing a tangent at the nose (Tn, tn) of the crystalline domain where Tn is the temperature coordinate and tn the time coordinate of the nose respectively. Mathematically, it is given by (Eq. 9.6):
The TTT diagram can be utilized for the generation of functional glass-ceramics. Knowledge of precipitation of varying amount of ceramic phase(s) in glass melt with time variation can play a key role in the development of glass-ceramics with tailor-made properties.
9.7 Structural Aspects of Glasses and Glass-Ceramics
Fundamental understanding of structural arrangements of atoms or molecules in any material is of prime importance. This understanding guides researchers to fine-tune physico-chemical properties of materials as well as to induce suitable functionality. This structural understanding becomes relatively easy for crystalline materials as understanding of arrangement of unit cell is only adequate to gain an insight of structural aspects throughout the material. However, as mentioned earlier, glasses lack periodic arrangement of atoms or molecules over long range and hence complete structural analysis of glasses or glass-ceramics is really challenging. Generally, the nature of local or short-range order present in these materials is investigated and the information is used to gain a thorough insight about the extended structure. For example, crystalline quartz has regular arrangement of SiO4 tetrahedra, whereas vitreous silica exhibits randomly connected SiO4 tetrahedra with order over the length scale of few angstroms. Extent of randomness, size of the crystalline domain, effects of one domain upon the neighboring one, etc., are some of the concerns which need to be answered by structural modeling of glassy system. These challenging aspects need to be addressed while undertaking structural analysis of glasses and glass-ceramics.
Structural studies on glasses can be mainly classified into two types depending upon the length scales in which experimental studies are carried out. They are namely (a) macroscopic structure and (b) microscopic structure. Structural studies undertaken by diffraction of X-rays, neutrons, electrons, etc., are sensitive to the variation of few angstroms (microscopic domain), whereas techniques such as optical and scanning electron microscopy are useful for the detection of structural in-homogeneities of the order of thousands of angstroms (macroscopic domain). The microscopic structural domain has been again subdivided into medium-range order involving a length scale of roughly 10–30 Å and a short-range order involving a length scale of few angstroms. Detailed structural aspects of representative systems obtained from different characterization techniques will be discussed in the following section.
9.8 Characterization Techniques
Functional glasses and glass-ceramics are generally characterized for their structural aspects, thermo-physical properties, mechanical, optical, and chemical properties, etc. In this section, we will focus on some of the important techniques used for the characterization of glasses and glass-ceramics.
9.8.1 Structural Analysis
-
(a)
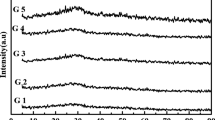
Powder X-ray diffraction: Powder X-ray diffraction (XRD) is one of the most commonly used technique to confirm formation of glassy phases. Since crystalline materials have long-range periodicity of atoms or group of atoms, they are characterized by sharp peaks in the diffraction patterns (Fig. 9.9). Ceramic components in glass-ceramic gives sharp peaks and these peaks are then matched with standard databases to garner information about the nature of embedded crystalline phase. However, glassy samples are characterized by single/multiple broad humps in their diffraction pattern indicating lack of long-range order in the glass (Fig. 9.9).
A powder X-ray diffraction is essentially a plot of intensity as a function of 2θ values (Fig. 9.9). Since glassy materials have random atomic arrangement, there is a considerable distribution in bond lengths and bond angles between the central atom and neighboring atoms giving rise to a broadband of low intensity. However, if a small amount of phase is crystallized within the glass melt, sharp peaks of high intensity (as compared to glassy phase) are observed in the XRD pattern. This helps to identify the nature of phases and also plausible reason for crystallization of phases. In certain cases, the position of first diffraction peak gives idea about medium-range order in glass and this information combined with theoretical simulations can give structural information on extended length scales.
-
(b)
XAFS spectroscopy: X-ray absorption fine structure (XAFS) spectroscopy is an important tool for understanding structural aspects of functional materials. XAFS investigates the modulation of the probability of X-ray absorption at energies in and around the binding energies of core electron of any given atom present in any material. Modulation of X-ray absorption depends upon the chemical environment of a particular atom over long or short range depending upon the energies. X-ray absorption spectrum consists of three ranges: edge region, X-ray absorption near edge structure (XANES), and extended X-ray absorption fine structure (EXAFS), as can be seen from Fig. 9.10. Value of edge gives information regarding the oxidation state of investigated ion. Near edge region (XANES) covering over 50–100 eV energy range around the edge gives information about the band structure, hybridization of molecular orbitals, and extent of long-range order.
EXAFS comprises of Kronig structure, i.e., the oscillatory function spreading over 100 eV past the absorption edge which gives us information about the local structure, coordination number, nearest neighbor distance, and any disorder prevalent in the sample. Compilation of the obtained data and proper processing (beyond the scope of this chapter) is required to simulate the experimental data. Structural models are then calculated comparing with standard (for crystalline phase) or manual modeling (for glassy phase) and fitted with the experimentally obtained and processed data to impart a complete knowledge of structure existing in the sample.
-
(c)
X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy is one of the techniques based on X-rays which can be used for understanding the short-range order around a particular atom/ion in glass and glass-ceramics. In this technique, the material is irradiated with X-rays having energy of few keV and the kinetic energy of the emitted electrons is monitored. The process is schematically as shown in Fig. 9.11.
Once energy (hν) of X-ray is higher than the binding energy (BE) of electrons in a particular level, electrons get ejected out. This ejected electron is called photoelectron and kinetic energy (KE) of the photoelectron can be expressed by the relation (Eq. 9.7)
Here, ϕ is the work function, and the value of which can be obtained from calibration of the machine. From the measured values of kinetic energy and ϕ, binding energy can be calculated which is related to the oxidation state of the corresponding element and its coordination environment. For example, bridging and non-bridging oxygen atoms present in borosilicate and phosphate glasses can be easily distinguished from O1s XPS patterns. Presence of negative charge with the non-bridging oxygen (NBO) atom leads to lower binding energies for O1s electrons for NBO compared to BO. Wang and Zhang used XPS technique confirm the network modifying and network forming action of Pb2+ in binary lead silicate glasses as a function of composition [23].
-
(d)
Vibration spectroscopic techniques: Infrared and Raman spectroscopic techniques belong to the category of vibrational techniques and are extensively used for the characterization of glasses and glass-ceramics. Vibration of groups or bonds which involve change in the dipole moment results in absorption of radiation and this forms the basis of IR spectroscopy. For molecules/bonds or a group to be Raman active, there must be change in the polarisability ellipsoid up on absorption of light. Vibrational spectra of glasses are analyzed by assuming that there exists considerable distribution in bond angles and bond lengths of different structural units constituting glass network/structure. Further, the structural units present are capable of being vibrationally excited independent of the surrounding amorphous matrix or other groups present in the glass structure. Typical FTIR spectrum corresponding to borosilicate glass is shown in Fig. 9.12. The pattern essentially consists of three peaks with maxima around 1400, 996, and 464 cm−1. There is a shoulder peak around 700 cm−1 present along with the broad peak at 996 cm−1. Based on earlier studies [24], it is clear that the peak at 1400 cm−1 is due to the vibration of BO3 structural units. Corresponding vibration for BO4- structural units overlap with the asymmetric stretching vibrations of Si–O–Si linkages, thereby appearing as a broad asymmetric peak around 996 cm−1. Symmetric vibration of Si–O–Si linkages appears around 700 cm−1 and the peak at 464 cm−1 arises due to the bending modes of Si–O–Si linkages. All the peaks are broad indicating a significant extent of disorder (distribution of bond lengths and bond angles) existing in the glass sample.
As the composition of glass changes, all the above modes of vibration undergo change. Weakening and strengthening of the bonds can be clearly seen from the shift in absorption peak maximum and changes in the line shapes. Thus, FTIR spectra can be used to identify and monitor the structural changes taking place with variation in composition of glass samples.
Raman spectroscopy: The technique also gives significant information regarding short and medium-range order existing in glasses and glass-ceramics. In this technique, atoms are excited by photon emitted from laser into a virtual state (lifetime ~10−15 s) which relaxes instantaneously to ground level. The emitted photons may have higher frequency (anti Stoke) or lower frequency (Stoke) as compared to the incident photon. The relative shift is termed as Raman shift which provides information about vibrational, rotational, and certain other low frequency transitions for group of atoms/structural units. Because of the presence of short-range order in glasses, vibrations in a glass are limited to a localized domain unlike in crystalline materials. Many structural groups present in silicate, phosphate, and borate glasses are Raman active. For example, a representative Raman spectrum of PbO–P2O5 glass containing equimolar amounts PbO and P2O5 is shown in Fig. 9.13. The pattern is characterized by sharp peaks around 1150 and 687 cm−1. The peak at 1150 cm−1 is more intense with shoulder peaks placed around 1210 and 1060 cm−1. The intense peaks around 1150 cm−1 are arising due to the symmetric stretching vibrations of Q2 structural units of P (P structural units with 2 bridging oxygen atoms).25,26 The corresponding asymmetric stretching mode is less Raman active (more IR active) and give rise to a shoulder peak around 1210 cm−1. The other shoulder peak around 1060 cm−1 is arising due to pyro-phosphate type of structural units (P2O72−) present in the glass. P–O–P bridges present in the glass are characterized by a peak around 687 cm−1 [25, 26].
In addition to this, Raman spectrum can also give information regarding the medium-range order existing in glass. For example, the presence of boroxol ring in many borate-based glasses is unequivocally confirmed by the observation of Raman peak around 808 cm−1. Recently, Yadav et al. [27] have reviewed Raman studies on a variety of oxide-based glasses.
It may be noted that a complete correlation of theory and experimental data becomes necessary to fully understand and visualize the structural features of a glassy material. Another approach followed by researchers worldwide is calculation of Raman spectra of simple glass samples based on modeling such as molecular dynamics or reverse Monte Carlo simulations followed by comparison of the same with the experimentally obtained data. This approach helps to gain information about both short and even intermediate-range order existing in the glass and shall enable researches for establishing structure property correlations.
-
(e)
Solid-state NMR technique: Solid-state nuclear magnetic resonance (NMR) is an important tool for detailed characterization of structural units present in glasses and glass-ceramics. Contrary to solution NMR, where sharp lines are observed, NMR line shapes of solids are characterized by broad lines due to anisotropic and orientation-dependent interactions present in solids. Unlike in solutions, these interactions do not get averaged out in the solid state. Advent of newer technologies has now enabled researchers to average these interactions to a very small value leading to relatively sharp NMR lines from solids [28, 29]. Alternatively, broad lines observed from solids contain detailed information regarding the anisotropic interactions existing in solids or glass. The internal interactions existing in glass or in the solid state can be classified into three categories, namely (a) magnetic interaction of the nucleus with the surrounding electron cloud (chemical shift interaction) (b) magnetic dipole –dipole interaction among nuclei, and (c) interaction between electric quadrupole moment (for nuclei having spin, I greater than or equal to 1) and the surrounding electric field gradient. These interactions are briefly described below.
-
(i)
Chemical shift interaction: Chemical shift arises because of the effective magnetic field felt around a nucleus and is brought about by the polarization of electron cloud around the nucleus caused by the applied magnetic field. This interaction is sensitive to the configuration of valence electrons, which is governed by the nature of chemical bonding. The interaction is represented by a second rank tensor and can be diagonalized for specific principal axis system with components σ11, σ22 and σ33. For nucleus with axial symmetry, the precession frequency (ωp) of nuclei can be expressed by the relation (Eq. 9.8)
$$\omega_{\text{p}} (\theta ) = \gamma B_{0} [1 - \sigma_{\text{iso}} - \Delta \sigma (3\cos^{2} \theta - 1)/3]$$(9.8)Where ωp(θ) represents the orientation of principal axis with respect to the applied magnetic field direction, σiso = 1/3(σ11 + σ22 + σ33), Δσ = σ33 − σ11. The symbols γ and B0 are the gyro-magnetic ratio and strength of applied magnetic field respectively. It may be noted that when θ = 54.7°, (3 cos2θ − 1) becomes zero and dependence of chemical shift anisotropy term on Larmor frequency gets averaged out to a small value.
-
(ii)
Magnetic dipole-dipole interaction: In this type of interaction, the magnetic moment of one nucleus interacts with the magnetic moment of the other (neighboring nucleus). The nuclei can be either same (homonuclear dipolar interaction) or different (heteronuclear dipolar interaction). The Hamilton corresponding to dipole-dipole interaction is also having an angular dependence of (3 cos2θ − 1), where θ is angle between the inter-nuclear distance vector and applied magnetic field. Hence, this interaction also gets averaged out when inter-nuclear bond vector is having an angle 54.7° with the applied magnetic field.
-
(iii)
Nuclear-quadrupole interaction: Nuclei which are having spin value I > ½ are known as quadrupole nuclei and charge distribution within the nucleus is asymmetric in nature. This results in nuclear electric quadrupole moment, which is represented as eQ where “e” is the charge and Q is the nuclear-quadrupole moment. Interaction of nuclear-quadrupole moment with the surrounding electric field gradient (EFG) leads to broadening of NMR line shapes. Hamiltonian corresponding to quadrupolar interaction consists of both first and second-order terms. The former is having an angular dependence of 3 cos2θ − 1, whereas the latter is having a complex angular dependence. Therefore, the quadrupolar interaction get only partially averaged out when inter-nuclear vector is at an angle 54.7° with the direction of applied magnetic field.
From the above discussion, it is clear all the three types of interactions are having an angular term of 3 cos2θ − 1. Hence, by keeping the sample at an angle of 54.7° with respect to the applied magnetic field, the above-mentioned interaction can be minimized leading to narrowing of NMR line shapes. This aspect is discussed further in detail in the following section.
-
(f)
Magic angle spinning nuclear magnetic resonance (MAS NMR) technique.
The simplest and most popular experimental method for getting high-resolution NMR patterns from solids is MAS NMR technique discovered by Andrew and Lowe [30, 31]. The technique involves rotating the samples at high spinning speeds at an angle of 54.7° with respect to the applied magnetic field. At sufficiently fast spinning speeds, all the interaction vectors get aligned along a direction which is 54.7° with respect to the applied magnetic field. Under this condition, as mentioned above, the angular term 3 cos2θ − 1 becomes very small resulting in sharp NMR peaks. This is schematically as shown in Fig. 9.14.
There are many other experimental strategies to reduce or simplify the line width/shape of solid-state NMR patterns, details of which are reported elsewhere [29].
NMR parameters
In a typical NMR experiment, the important information that can be obtained directly from the spectrum are the chemical shift and coupling constants. Chemical shift is expressed by the following relation (Eq. 9.9):
Where ω and ω0 represent resonance frequency of the nuclei in the sample and in reference respectively. Values of δ are independent of the applied magnetic field and can be used to monitor the structural changes taking place around a particular nucleus. Parameters like dipolar and quadrupolar coupling constants can be obtained by fitting the NMR line shapes obtained under static conditions so that the interactions are not averaged out.
MAS NMR studies on representative glasses and glass-ceramics
As mentioned earlier, unlike crystals, glasses do not have long-range ordering and there is distribution in bond length and bond angles around different ions/atoms constituting the glass. As a result of this, 29Si MAS NMR pattern of silica glass is characterized by a broad peak, whereas crystalline sample of silica (quartz) is characterized by a sharp peak around −109 ppm. Corresponding 29Si MAS NMR patterns are shown in Fig. 9.15a, b.
It may be noted that although both silica glass and quartz crystal contain Q4 structural units of silicon (silicon structural units with 4 bridging oxygen atoms), there is significant distortion around Si structural units in silica glass. This results in broad peak in the corresponding 29Si MAS NMR pattern (Fig. 9.15a). Quartz powder is highly crystalline as revealed by the XRD pattern and there exists perfect ordering around Si structural units in quarts, resulting in sharp 29Si MAS NMR peak (Fig. 9.15b).
Silicate-based glasses
Single component silica glass forms only at high temperatures due to the high melting point of silica. However, with the addition of Na2O, PbO, etc., the melting temperatures drastically reduces. A representative 29Si MAS NMR pattern of binary sodium silicate glass containing around 24% sodium oxide is shown in Fig. 9.16a. The pattern is mainly characterized by a broad asymmetric peak which can be de-convoluted into two peaks around −108 and −99 ppm. Based on earlier 29Si MAS NMR studies, peaks around −108 and −99 ppm are attributed to Q4 and Q3 structural units of silicon [32]. Here, Q4 represents silicon structural units having 4 bridging oxygen atoms and Q3 represents silicon structural units having 3 bridging oxygen atoms. These structural units are schematically as shown in Fig. 9.16b, c.
29Si MAS NMR pattern (a) for sodium silicate glass containing around 25% Na2O. The structural units Q3 and Q4 are schematically as shown in Fig. 9.16 (b and c) respectively
Borosilicate glasses
Although binary silicate glasses are ideal candidates for understanding the structural aspects, such glasses have minimum technological applications. Borosilicate glasses have a wide range of applications because of its improved physico-chemical as well as thermo-physical properties. 11B MAS NMR is an ideal technique to monitor structural units present in such glass samples. Although boron exists in a variety of structural forms/units, BO3 and BO4 are the main structural units in borosilicate glasses. Due to higher symmetry of BO4 structural units compared to BO3 units, the former gives rise to sharp peak and the latter is characterized by a broad peak. A representative 11B MAS NMR pattern of sodium borosilicate glass containing both BO3 and BO4 structural units is shown below (Fig. 9.17) [33].
In the case of glass-ceramics, in addition to the broad peak due to glassy phase, sharp peaks characteristic of crystalline phase is also observed in the 29Si MAS NMR patterns. Figure 9.18 shows the 29Si MAS NMR patterns of magnesium aluminosilicate (MAS) glass-ceramics prepared by addition of around 8 mol% Al2O3. In this glass-ceramics, a fluoro-aluminosilicate phase, namely potassium fluorophlogopite phase is formed, which is having a tile-like structure and is responsible for the machine-able characteristics of the material. The potassium fluorophlogopite phase consists of five different silicon structural units and is characterized by 5 sharp peaks in 29Si MAS NMR pattern. These peaks are superimposed over a broad peak due to the residual glassy phase. Relative concentration of the crystalline phase increases with increase in Al2O3 content in the glass-ceramics [34].
In the recent past number of NMR techniques have been reported which include variable angle spinning NMR, double rotation NMR, multiple quantum MAS NMR, etc.,29 which gives valuable information regarding the short and medium-range order in glasses and glass-ceramics.
9.8.2 Thermo-Physical Analysis
-
(a)
Differential thermal analysis (DTA): DTA technique revolves around the simultaneous heating and cooling of a standard (reference) sample and sample of interest under identical time span. Endothermic or exothermic reactions in the sample material due to absorption or evolution of heat lead to a decrease or increase in temperature, respectively, compared to the standard, which is chosen in such a manner that any sort of thermal event remains absent under experimental conditions. The temperature difference or ΔT is then plotted as a function of time or temperature in a DTA plot. As the change in enthalpy is responsible for a change in temperature, ΔH can also be plotted against temperature in a DTA plot. The major phenomena that can be observed from DTA plots of glasses and glass-ceramics are glass transition temperature characterized by weak and broad endothermic peak, crystallization of a phase characterized by sharp exothermic peak, and melting of residual glass (sometimes crystal) characterized by an endothermic peak. Generally, graphite, alumina, silica, nickel, or platinum crucibles are used for DTA measurements depending upon the temperature range and nature of the material. Disc-shaped thermocouples are used conveniently in DTA to provide optimum thermal contact with bottom of the sample and standard holder material. The only concern for a new experimenter is the choice of suitable heating rate for performing DTA experiments for glassy materials as very fast heating rate leads to overlap of more than one signal, whereas slow heating rate may result in submerged signal. Generally, a heating rate of 10 K min−1 is the optimum rate for observing glass transition temperature in borosilicate glasses.
-
(b)
Differential scanning calorimetry (DSC): DSC technique provides information similar to that of DTA technique. However, DSC is more quantitative mode of measurement as compared to DTA. The main reason for this is that temperature control of the sample and reference pans are more accurate in DSC as compared to DTA. The importance of DSC lies in the fact that it can measure enthalpy change quite accurately during any phase transition occurring with the sample. DSC is mainly of two types: temperature compensated DSC and modulated temperature power compensated DSC. In temperature compensated DSC, during any endothermic event sample is provided with increased heat flow to neutralize the temperature lag, whereas heat is given to the standard for any exothermic event. This principle allows for the calculation of heat flow in or heat flow out as a function of temperature leading to the quantification of the net heat associated with physical or chemical process. In spite of this logic being attractive, users are presently shifting to the newer modulated temperature power compensated DSC. Power compensated DSC uses two independent micro furnaces for heating the sample and standard separately. Initially, the temperature of both the sample and standard are kept at the same value through independent heating. During an endothermic process, power is increased in the furnace corresponding to the sample pan to neutralize the temperature difference, and the increase in power is measured. The procedure is reversed for an exothermic process. Thus, here, the primary signal for calculation of thermal event is the adjusted power which gives us the enthalpy changes involved in the process. The major advantages of this technique are the rapid process of heating or cooling and better resolution for any thermal events.
-
(c)
Thermomechanical analysis (TMA): TMA technique is used for the estimation of coefficient of thermal expansion, dilatometric softening, glass transition temperature, etc. In this method, sample is placed on a holder with a push rod assembly and heated at a constant rate over a particular temperature range in a dilatometer. Expansion of the glass or glass-ceramic sample exerts a force on the push rod as a result of which the push rod starts moving upward. The movement of push rod associated with a scale accounts for the total expansion of the solid sample. However, care is taken to subtract the expansion of the dilatometer assembly itself with temperature. The coefficient of thermal expansion can be calculated based on the equation given below (Eq. 9.10).
$$\alpha_{L} = \left( {\partial L/\partial T} \right)/L$$(9.10)-
where αL is the coefficient of thermal expansion,
-
L is the length of the test sample and T is the temperature.
-
Upon attaining glass transition temperature, test material becomes soft and hence the slope of the curve increases nonlinearly. The temperature at which the onset of nonlinearity is taking place is generally considered as the glass transition temperature. More scientifically, one can say that slope of the curve increases drastically by almost three to four times post glass transition because of softening. The intersection points of the tangents drawn from both these domains are considered as the glass transition temperature. Upon heating above the glass transition temperature, decrease in length of the sample is observed. This is attributed to the softening of glass and subsequent dip of push rod assembly into softened glass which is having lower viscosity. The temperature which marks the onset of the negative expansion of the glass sample is termed as the dilatometric softening temperature.
-
(d)
Micro-hardness characterization: Hardness of any material is defined as its resistance to permanent deformation by any induced force. Hardness is measured for bulk samples by Vickers indentation technique and for thin films by Knoop indenter method. In the Vickers method, a diamond indenter is placed over the surface of a glassy material. Suitable loads are applied on the surface of glass through the indenter till an impression or deformation mark is created on the surface. Typical deformation area or area having dents (impressions) is observed under optical microscope, and the hardness is calculated based on the following formula (Eq. 9.11):
$$H = F/A_{\text{p}}$$(9.11)
where H is the hardness of the material,
F is the applied load, and Ap is the projected deformed surface area.
Upon performing the experiments with a Vicker pyramidal indenter, the formula is modified to Eq. 9.12,
where d is the average length of the diagonal and F is the force applied to the diamond indenter. It must however be noted that based on hardness values, glasses can be classified as normal and anomalous glasses. Normal glasses exhibit flow behavior upon indentation, whereas anomalous glasses exhibit densification. Flow behavior occurs when the pressure applied by diamond indenter becomes sufficient to lower the viscosity of glass up to a certain depth from the glass surface and the material flows [25]. In contrast, the presence of heteroatoms as network modifiers within the glass network inhibits mobility, induces twisting and bending of bonds thereby resulting in densification of glassy structure. Another phenomenon observed is recovery of dent marks in glasses upon sufficient heating.
In the following section, brief description is given regarding applications of glasses and ceramics.
9.9 Application of Glasses and Glass-Ceramics
Glasses and glass-ceramics are extensively used in various technological applications ranging from domestic household materials to materials for nuclear waste immobilization. Although people started making glasses as early as 1500 BC, the extensive use of glass for modern technology was realized in the last century. As we are living in an age of high-speed communication, glass fibers are the obvious material of choice for the above applications. It is necessary that the phonon energy of the glass must be quite low to get optimum luminescence efficiency from lanthanide ions. Erbium-doped fiber amplifiers are extensively used for 1.5 μm window of telecommunication. Such fibers are made by doping lanthanide ions like Er3+ in glass and drawing the glass into fibers at higher temperatures. The major problem observed in the fabrication of efficient lanthanide ions doped fiber amplifiers is the clustering of lanthanide ions and associated reduction in the extent of luminescence. One way to circumvent this problem is to dope lanthanide ions in suitable host with nanosize dimensions prior to its incorporation in glasses.
Another important application of glass is for their use in glass to metal seals. Our earlier studies have demonstrated that lead silicate glasses with SiO2 to PbO ratio 6.9 and containing different amounts of alkali and alkaline earth metal oxides are potential candidates for making compression type glass to metal seals. These glasses have got wide application as feed through materials for various pipelines of plants and holder of bulbs. Photograph of typical glass to metal seal used as feed through material is shown in Fig. 9.19a. In addition to this, glasses are extensively used in nuclear technology for immobilization of high-level nuclear waste. For example, detailed studies on physico-chemical properties of barium borosilicate and sodium borosilicate glasses confirmed that the former is a better candidate for immobilization of nuclear waste to be generated from the proposed advanced heavy water reactors (AHWR). Fabrication of glass-based nuclear waste matrices and their property validation is important for catering the needs of nuclear waste immobilization technology [35, 36].
Glass-ceramics, on the other hand, possesses both the properties of glass and ceramics and they have wide applications catering to the modern needs. Research on glass-ceramics led to the development of products with minimal thermal expansion between room temperature to around 700 °C range. Earlier reports [37, 38] suggest that glass-ceramics-based β-spodumene solid solution and β-quartz solid solution can be obtained with low or even negative thermal expansion (shrinkage type of glass phases). Apart from these materials, glass-ceramics-based on β-eucryptite (LiAlSiO4), Cr-doped mullite (Al6Si2O10), lithium metasilicate (Li2SiO3), etc., developed by Corning Incorporated, USA, are worthwhile to mention as they are used in optical applications in glass fiber-based telecommunication technology [39]. These materials made path breaking developments and gave several epoch making technologies which revolutionized the communication technology. Reports [40, 41] are also there regarding development of spinel-based transparent glass-ceramic materials with embedded TiO2 precipitate for production of photovoltaic substrates. Phosphate-based glass-ceramics have been found to be promising in the field of bone replacement and artificial and restorative dentistry [42, 43]. As glass-ceramics have properties of both glasses and ceramics, they are very widely used for making machine-able glass-ceramics. Magnesium aluminosilicate glass when subjected to programmed crystallization with addition of Al2O3 leads to glass-ceramics with machine-able properties. A variety of tools can be made by adjusting the Al2O3 content and temperature programming. A photograph of nut and bolt fabricated from the machine-able glass-ceramics is shown in Fig. 9.19b.
9.10 Summary
Different aspects of glasses namely synthesis, characterization, properties, etc., are discussed in this chapter. Thermodynamic and kinetic aspects of glass formation are explained in detail to understand the factors that are responsible for glass formation. Conversion of glass to glass-ceramics to achieve both the advantages of glasses and ceramics in the same material and widen the scope of such materials for different applications have been discussed in detail. Representative applications of glasses and glass-ceramics, mentioned at the end of the chapter, clearly establish the importance of carrying out extensive research and development work in this area.
References
https://web.archive.org/web/20110415194738; http://www.glassonline.com/infoserv/history.html
Woodard KL (March 2000) Profiles in ceramics: S. Donald Stookey. Am Ceram Soc Bull 79(3):34–38
Höland W (ed) (2013) G. H. Beall, Handbook of advanced ceramics. Elsevier, Amsterdam, pp 371–381
Hench LL, Freiman SW (1971) Advances in nucleation and crystallization in glasses. Beall GH (eds) Structure, properties, and nucleation of glass-ceramics. Columbus, Ohio USA. The Am Ceram Soc special publ no 5:251–261
Tashiro T, Wada M (1963) Glass-ceramics crystallized with Zirconia. In: Advances in glass tech. Plenum Press, New York, p 18
Cambell J, Suratwala TI (2000) J Non-Cryst Solids 263&264:318
Ehrmann PR, Carlson K, Cambell JH, Click A, Brow RK (2004) Neodymium fluorescence quenching by hydroxyl groups in phosphate laser glasses. J Non-Cryst Solids 349:105
Mattox DM (1998) Handbook of physical vapour deposition processing (PVD): film formation, adhesion, surface preparation and contamination control. Noyes Publications, Oxford, UK, pp 29–54
Soukup RJ, Ianno NJ, Huguenin-Love JL (2007) Solar Energy Mater Solar Cells 91:1383
Pribil G, Hubiˇcka Z, Soukup RJ, Ianno NJ (2001) J Vac Sc Technol A19:1571
Soukup RJ, Ianno NJ, Pribil G, Hubiˇcka Z (2004) Surf Coat Technol 177&178:676
Li L, Lu Q, Fu RKY, Chu PK (2009) Nucl Instrum Methods Phys Res Sect B 267:1696
Binnewies M, Locmelis S, Meyer B, Polity A, Hofmann DM, Von H (2009) Prog Solid State Chem 37:57
Pierson HO (1999) Handbook of chemical vapor deposition (CVD): principles technology and applications. Noyes Publications, pp 217–226, 295–339
Kothiyal GP, Kumar R, Goswami M, Shrikhande VK, Bhattacharya D, Roy M (2007) J Non-Cryst Solids 353:1337
Aegerte MA, Mennig M (2004) Sol-Gel technologies for glass producers and users. Kluver Academic Press, New York
Young L (1961) Anodic oxide films. Academic Press, New York
Primak W (1958) Phys Rev 110:1240
Gibbs JH, Di Marzio EA (1958) J Chem Phys 28:373
Hruby A (1972) J Phys B 22:1187
Turnbull D, Cohen MH (1961) J Chem Phys 34:120
Ngai KL (2007) J Non-Cryst Solids 353:709
Wang PW, Zhang L (1996) J Non-Cryst Solids 194:129
Ross SD (1972) Inorganic infrared and Raman spectra. McGraw-Hill, New York, pp 148, 260
Liu HS, Shih PY, Chin TS (1996) Phys Chem Glasses 37:227
Rouse GB, Miller PJ, Risen WM (1978) J Non-Cryst Solids 28:193
Yadav AK, Singh P (2015) RSC Adv 5:67583
Boolchand P (2000) Insulating and semiconducting glasses. In: Series of directions in condensed matter physics, vol 17. World Scientific, Singapore
Eckert H (1992) Prog NMR Spectroscopy 24:159
Andrew ER, Bradbury A, Eades RG (1959) Nature 183:1802
Lowe IJ (1959) Phys Rev Lett 2:285
Shrikhande VK, Sudarsan V, Kothiyal GP, Kulshreshtha SK (2001) J Non-Cryst Solids 283:18
Sudarsan V, Shrikhande VK, Kothiyal GP, Kulshreshtha SK (2002) J Phys: Condens Matter 14:6553
Goswami M, Kothiyal GP, Sudarsan V, Kulshreshtha SK (2005) Glass Technol 46:341
Thorat VS, Mishra RK, Kumar A, Sudarsan V, Tyagi AK, Kaushik CP (2019) Bull Mater Sci 42:211
Dhara Prakash A, Singh M, Mishra RK, Valsala TP, Tyagi AK, Sarkar A, Kaushik CP (2019) J Non-Cryst Solids 510:172
Beall GH (1971) The Am Ceram Soc 61:251
Tashiro T, Wada M (1963) Adv in Glass Tech 9:18. Plenum Press, New York
Beall GH, Chyung K, Pierson JE (1998) Gratings Proc Int Cong On Glass XVIII (CD-ROM), San Francisco
Beall GH, Pinckney LR (1999) J Am Ceram Soc 82:5
Beall GH, Pinckney LR (1997) J Non-Cryst Solids 219:219
Chevalier J, Gremillard L (2009) J Eur Ceram Soc 29:1245
Kasuga T, Nogami M (2004) Trans Mat Res Soc Japan 29:2933
Acknowledgements
Authors of this chapter thank former and present colleagues in BARC namely Drs. G. P. Kothiyal, V. K. Shrikhande, S. K. Kulshreshtha, C. P. Kaushik, A. K. Tyagi, Madhumita Goswami, Raman Mishra, Dayamoi Banerjee, P. Sengupta, Mrs. Shobha, Dr. Vidya Thorat, Ms. A. Dhara for their active collaboration and scientific interactions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jafar, M., Sudarsan, V. (2021). Synthesis Strategy for Functional Glasses and Glass-Ceramics. In: Tyagi, A.K., Ningthoujam, R.S. (eds) Handbook on Synthesis Strategies for Advanced Materials . Indian Institute of Metals Series. Springer, Singapore. https://doi.org/10.1007/978-981-16-1807-9_9
Download citation
DOI: https://doi.org/10.1007/978-981-16-1807-9_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1806-2
Online ISBN: 978-981-16-1807-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)