Abstract
3D printing nowadays is considered as a very smart method for producing structures with very fine and intricate internal features. The process is now going through a very fast scientific development, and laser based 3D printing tools are already available to the users. Strong and intense laser pulses are bombarded at well-defined locations on a 2D plane covered by particles through a proper alignment process controlled by computers as per the instructions given to it. These intense laser pulses cause the melting of the target which is composed of scattered small particles. Because of this melting and subsequent cooling, these particles start making metallic bonds with the substrate which is already solidified cold metal. This way, three dimensional growth of the structure using fine granules of metals can be achieved. Since the process depends upon the metal granules and intensity of the laser, hence, a fine degree of structure growth control can be obtained in the case of dissimilar material being used for the construction of the model or structure. If alloys are fed as raw material into the printing system then an alloy structure can be obtained through this 3D printing. High power portable lasers which can be focused onto very tiny spots may give additional advantages such as achievement of the temperatures in a very controlled fashion so that almost all the possible combinations of alloy manufacturing can be incorporated into a single system of 3D printing requiring different melting points in constituent metals. Not only laser based printing but also electron beam based printing can be used in this manufacturing process. Vacuum may be an additional requirement of the environment in which such electron beam based additive manufacturing is contemplated. In order to have better control of the structure fabrication process, the computer-based monitoring and controlling of various parameters will be helpful for the advanced manufacturing process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
20.1 Introduction
Production and characterization of nanoparticles produced using electrical explosion of metal wire is well reported by Kotov [1], Das et al. [2]. Nanoparticle production for very interesting application points of view in various media is studied by Nazarenko et al. [3, 4] In another article [6] Nazarenko et al. have reported the effect of various gas environments (reactive gas and non-reactive gas combinations) for the nanoparticle production by electrical exploding wire scheme. They have suggested less than 2% reactive gas for metal nanopowder production which otherwise leads to the oxide formation. Károly Lázár et al. [6] have reported the iron nanopowder reaction in different media using electrical explosion of wire. An interesting, molecular dynamics based simulation is presented by Kryzhevich et al. [7] for exploding wire based nanoparticle production. Earlier Go Kawamura et al. [8] have reported oxygen resistant copper based nanoparticles by wire explosion. Chung et al. [9] have presented a superior oxygen stability copper silver nanoparticle layers sintered by flash lights to achieve the electrical conductivity close to copper. Suitable environment study and findings are reported for tungsten carbide nanopowder production in this method by Ranjan et al. [10]. Taek-Kyun Jung et al. have reported production of nickel nanopowder by exploding wire method [11], and earlier [12] titanium nitride nanopowder production is reported in electrical explosion in nitrogen environment. Beketev et al. [13] have studied magnetic nanoparticles production by electrical explosion of iron wires.
20.1.1 Concept of Pulsed-Power Plasma Method
In order to generate ultrafine nanopowder of the size less than 10 nm, the capacitor bank discharges into the wire has to be optimized such that the energy inside the wire is dissipated at very fast rate which means that before the plasma formation takes place the considerable amount of energy is given to the wire. Because of the conducting nature of the plasma, further resistance (I2R) heating becomes practically impossible and hence the burst phase is relatively less violent. Higher the energy given to the atoms, higher is the kinetic energy or the pressure in plasma state. Because of this generated high pressure, the atoms form very fine coagulation in the form of nanoparticles.
In the case of the generation of micron sized particles, a very simple scheme of melting is followed similar to the electrically exploded nanoparticle system. In this case, the wire is fed longer in the dimensions and is allowed to reach only to the melt state or liquid state, and the thrust is given by the return conductor current to form the droplets of the tens to even hundreds of microns in size. The thrust is J X B thrust (J is the current density, and B is the magnetic field) or the repulsive force or hoop force acting between current carrying conductors with current in opposite directions. In this way, only a single pulse power system can support the fabrication of micron-sized to nano-sized particle synthesis of any metal. As this method of particle synthesis is based on the electrical conductivity and phase-transformation based on heating (resistive), therefore any conducting metal can be used as a feed material and its particle size can be easily controlled and hence well determined. As alloys are constructed mostly from metal atoms, they show finite or considerable electrical conductivity constructed from most of the metals therefore they can also equally efficiently be used for the production of particles from micron to nano size.
We have used copper, zinc, aluminum, Iron and Tungsten for producing nanoparticles during this method.
20.1.2 Merits and Demerits of This Method
Certainly any process of commercial/academic interest needs to be compared with the other processes being used for the similar jobs. Therefore, this process also needs to be compared with other methods.
Now as explained that getting nano-sized particles as well as micron sized particles required different types of approaches to obtain the desired extreme size particles results, a proper experimental approach which is achieved comfortably may give a broad spectrum of the sizes in between these two extremes. For this purpose, in order to meet both the ends a simple and single pulsed power system is used, which is so portable that a single person can handle it and operate it safely. Its mobility also allows it to arrange the whole setup at a place where the particle consumption is taking place. This reduces coagulation probability because of very less transit time especially in the case of very fine nano-powder. Losses during transit, if any, can also be further minimized by connecting powder generator output to its consumption unit directly. For high voltage insulation between two units (particle generator to particle consumption units) conveyor duct or transmission duct of insulator material will be a good choice for the operator. A normal supply available even in households can be used for the purpose because capacitor banks are slowly charged with low wattage power supply, but they deliver higher powers during the electrical discharges. Based on the simplicity, versatility and portability, the device plays a very important role in commercial/academic terms also apart from the safety aspects.
20.2 Characterization and Other Properties
For the characterization of Nano powder X Ray Diffraction (XRD) Scanning electron microscopy (SEM) and Photon Cross Correlation Spectroscopy (PCCS) techniques have been used. The results have revealed that faster discharges are capable of producing nanosized metal particles. Some of the images and the results are attached in the figures in this chapter.
Coating the metal nanopowder on the fabric has been accomplished. A cluster coating is successfully obtained by the method followed and particles produced by this method. The size of the particles forming clusters on the fabric is confirmed in the nano form and can solve the purpose.
Now it has also been shown in the experiments that non optimized dimensions of the wire lead to the production of larger size of particles which implies that the pressure at the burst phase is relatively lower and hence particles of larger size are produced.
In the case of production of larger (micron sized) particles produced by melting of wire and driving by J X B forces, the analysis part has been done mostly on the optical microscopes.
20.3 Application of Advance Materials Prepared By This Route
Various metals like copper, silver, zinc, Iron and tungsten have been used in our experiments. Of course for the largest particles as discussed above the feeder wire dimensions can be increased first in terms of width and then in terms of length also. A challenging metal with conducting nature and not producing nanopowder by this technique is still being found out.
Usually nanoparticles are the particles with the size below 100 nm. There are the methods by which nanoparticles may be generated in quite a good amount of quantity and hence support the construction of materials using additive manufacturing techniques which may be laser based or electron beam based for fusing these particles. Plasma based techniques have successfully been implemented for the production of bulk nanoparticles and the consumption rate of laser based additive manufacturing can be fulfilled easily by the plasma based production technique. In this context Bhabha Atomic Research Centre, Visakhapatnam, has developed a miniaturized machine for the continuous production of nanopowder by using electrically exploding wire technique. It has been understood during the experiments that if the current discharges through the exploding conductor are very quick and intense, then the energy being dissipated in the metal plasma formation can be sufficiently high to generate fine nano particles. For this purpose up to 180 kA (short circuit current) has been dissipated for exploding metal wire of copper, silver, zinc, Iron and tungsten at size of nearly half a millimeter diameter, and the system is compact and portable and can generate nanoparticles, repetitively. Because of this inherent advantage of portability and repeatability of the nanopowder generator, nearly 50 mg of nanopowder is produced every 6 s. In a similar pattern, generating larger size the particles of metal has also been experimented by exploding wire technique but limiting to the liquid state. The system is optimized in such a way that the liquid metal experiences hoop force or JXB force at the time of melting. This ensures that the metal droplets are formed and thrown away from its initial location and are collected on the bottom of the chamber in which the melting or discharge is taking place. With the same electrical parameters of the capacitor bank, which are used for the nanopowder generation of nearly 50 mg material, 500 mg of larger sized (micron sized) particles of the same metal has been produced in a different chamber fitting. The procedure gives the flexibility to the operator or the user so that depending upon the requirement of additive manufacturing one can feed either nanopowder or micron sized powder depending upon the size and the surface finish of the product.
20.4 Nanoparticle Production
It is noteworthy that while producing the nanoparticles from the electrically exploding wire method any metal can be fed which has optimized dimensions according to the pulsed power machine and hence the process is similar for all metals. This fact greatly introduces the flexibility of the process selection for ablative manufacturing feed material. Therefore, pure metals as well alloys can directly be used for ablative manufacturing. One need not worry about the availability of alloys in the form of powder feed. The nanoparticle generator based on the electrically exploding wire is only dependent upon the resistivity parameter of various phases of the material. It has been experimented using this powder generator and is found that copper nanopowder of less than 10 nm particle size is being produced by the machine with good reproducibility.
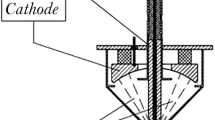
In Fig. 20.1, the repetitive metal nanoparticle generator is shown. Electrical schematic is shown in Fig. 20.2 in which the reaction chamber boundary is shown with the blue circle. Inside this chamber, the repetitive wire loading assembly/mechanism is also fitted.
Figures 20.3 and 20.4 are showing the size distribution of copper nano powder produced by the system. Similarly Figs. 20.5 and 20.6 are showing the particle size distribution produced by aluminum wire in this method. It has been done using the PCCS technique of analysis. Coagulation on nanoparticles is remarkably seen in the case of aluminum metal.
Figure 20.7 is a typical current waveform of the current pulse obtained during capacitor bank discharge inside optimized wire. The calibration of the current measurement probe is 100 kA per Volt for the study undertaken. Single pulse discharge is another indication of optimized wire dimensions being used inside the system.
In recent covid-19 pandemic times, some reports suggested that the coating of Personal Protective Equipment (PPE) fabric material can be conducted using metals in the nanopowder form in order to increase the efficacy of the PPE kit in fight against various infections. For this purpose recently copper, zinc and aluminum nanopowders were produced using this ultra fast system and coating over the clothes was successfully conducted for the demonstration of the technique and process. Clusters of the nanoparticles are distributed over the cloth surface indicating a non-uniform coating by metal particles. Some of the images of the coating conducted by the aforesaid method are shown in Fig. 20.8. SEM analysis of the PPE fabric is also conducted, and similar results are observed.
Zinc oxide nanopowder produced by the generator is shown in Fig. 20.9 which is collected in a plastic bag. This experiment was conducted in a normal air environment and hence no inert gas environment was used. But in the case of Fig. 20.10, the inert gas environment of Argon was used inside the chamber and hence the pure zinc metal nanopowder is visible after opening the chamber. It is collected all over the internal surfaces.
The images of the particles generated in the case of Micron size production are shown in the attached Fig. 20.11 which is obtained from similar discharge of the capacitor bank but with wire heating up to liquid state only.
20.5 Tailoring of Particle Size
The important point is that the action integral data for burst in pure metals are reported by Tucker and Toth [14] in a capacitor bank discharge machine in particular current rise rate. As far as the nanoparticle generator is concerned for the generation of the particles below 10 nm using electrically exploding wire method, the energy dissipation in the form of electrical energy inside the wire material should be conducted at a considerably fast rate. Since the conductivity of the wire reduces, till the wire reaches plasma state through the liquid and the vapor states starting from solid state, the energy dissipation rate or heating rate is quite fast up to the onset of plasma state but as soon as the plasma state is reached, the conductivity of the metal increases abruptly and this reduces further heating of the wire metal and subsequent dissipation of electrical energy in the wire. Hence it becomes very difficult from this stage onwards to further increase the temperature of the wire metal. However, it is reported in the literature [15, 16] that the faster current rates can raise the temperature of the wire metal relatively higher than that compared with the slower current rates. It may be partially considered as a kind of thermal inertia in reaching the plasma state or conducting state at faster current discharge rates and some higher amount of energy compared to the ionization energy of the metal can be given to the metal atoms.
20.6 Effect of Various Parameters on Powder Properties
20.6.1 Effect of Medium
As plasma is highly reactive, the produced metal plasma reacts with the surrounding after the discharge. In order to form oxides, oxygen inside the reaction chamber is required. If pure metal nanopowder is desired then filling the reaction chamber with non-reactive gases is the required condition. It has been observed that experiments in air lead to the formation of oxides while experimenting with aluminum and zinc. Copper in the Argon environment yielded pure metal powder.
Interestingly in the formation of micron size particles it is observed that even in an air atmosphere, copper micro particles are produced in the pure form. It is also noteworthy that since this is a high voltage discharge method of production of particles from the metal wire, the system needs to be filled with some insulating media otherwise the electrical discharge may take place in surrounding media and this may lead to a loss of electrical energy in undesirable processes of discharge. It suggests that the gas pressure of the system should not be in the Paschen-minimum region of the breakdown curve. Rather a higher insulation strength of gas is suggested to be used in the process.
20.6.2 Pressure Control
If high pressure gases of inert nature are filled in the reaction chamber or discharge chamber then the particles produced after explosion will not travel longer distances and this is due to enhanced drag resistance offered by the surrounding media. This way the reaction chamber diameter dimensions may be restricted to the smaller values. Successful operation of the system up to three or four atmospheric pressures inside the reaction chamber has been observed. When inert gas is used in the case of nanoparticle formation, then it has been further observed that the continuity between electrode in repetitive shots is well maintained subsequently helping to conduct repetitive shorts whereas in the case of oxide or nitride nanopowder formation the nanopowder, which gets deposited over the electrodes, may be insulating if the fill gas is reactive. So the accumulation of nanopowder over the electrodes leads to poor electrical connections between the running feed wires in between these two electrodes. Now in this situation, the electrical energy tries to find out some other ways to discharge, which is not through the wire, like from the surfaces or the system starts malfunctioning and gets stopped for the subsequent discharges. This affects the performance of the system. So in repetitive operation it is recommended, based on the experimental evidence, that an inert gas environment is the most suited environment for the production of metal nanopowder. It is also suggested that other reactions of chemical interest may be conducted separately after pure nanopowder is continuously produced in the chamber.
20.6.3 Production of Nanoparticles Inside the Liquid
Since the pulsed power system is very fast and in the sub-microsecond regime of operation, the nanoparticle producing discharges can be done in the liquid submerged conditions as well. This will additionally require the structural integrity analysis in consideration as the pressure generated in the burst phase will be transmitted to the boundaries more intensely and rapidly than that in the case of air/gas. Repetitive wire feed system is the component which is in the close proximity of the metal wire explosion zone and is most vulnerable to the pressures. Hence a proper protection of the system from repetitive shock pressures will be required in the planning and design stage.
20.7 Conclusions
A repetitive operation of a portable and miniaturized capacitor bank is reported earlier [17]. The bank is further augmented with energy and automation in order to be used for the production of metal particles from nanometer size to micron size in a single electrical unit. The discharge chambers are different as per the required size of the particles. This portability leads to further exploration of applications of particles in other fields. The optical isolation of the system from high voltage and confined production of the particles has another feature of enhanced electrical safety chemical safety. In future the particles produced in the bulk quantity are to be fed to the laser or other like electron beam based 3D printing or additive manufacturing setups.
References
Kotov YA (2003) Electric Explosion of Wires as a Method for Preparation of Nanopowders. J Nanopart Res 5:539550. https://doi.org/10.1023/B:NANO.0000006069.45073.0b
Rashmita D, Basanta Kumar Das, Rohit Shukla, T Prabaharan and Anurag Shyam “Analysis of electrical explosion of wire systems for the production of nanopowder” Sadhana Vol. 37, Part 5, October 2012, pp. 629–635.
Nazarenko O (2007) Nanopowders produced by electrical explosion of wires. In: Proceedings of European Congress of Chemical Engineering (ECCE-6) Copenhagen, 16–20 September 2007
Nazarenko OB, Ilyin AP (2008) Nanopowders production by electrical explosion of wires: environmental applications” Proceedings of the 3rd Environmental Physics Conference, 19–23 Feb. 2008, Aswan, Egypt
Nazarenko OB, Ilyin AP, Tikhonov DV (2014) Effect of the Gas Composition at the Electrical Explosion of Wires on the Nanopowders Properties” Advanced Materials Research, Vol. 872 (2014) pp 142–149 Online available since 2013/Dec/19 at www.scientific.net © (2014) Trans Tech Publications. Switzerland
Lázár K, Varga LK, Kis VK, Fekete T, Klencsár Z, Stichleutner S, Szabó L, Harsányi I (2018) Electric explosion of steel wires for production of nanoparticles: Reactions with the liquid media, Journal of Alloys and Compounds, Volume 763:759–770, ISSN 0925–8388,https://doi.org/10.1016/j.jallcom.2018.05.326. (http://www.sciencedirect.com/science/article/pii/S0925838818320632)
Kryzhevich DS, Zolnikov KP, Korchuganov AV, Psakhie SG (2017) Nanopowder synthesis based on electric explosion technology” AIP Conference Proceedings 1893, 030125. https://doi.org/10.1063/1.5007583 Published Online: 26 October 2017
Kawamura G, Alvarez S, Stewart IE, Catenacci M, Chen Z, Ha YC (2015) Production of Oxidation-Resistant Cu-Based Nanoparticles by Wire Explosion. Sci. Rep. 5, 18333. https://doi.org/10.1038/srep18333
Chung WH, Hwang YT, Lee SH, Kim HS. “Electrical wire explosion process of copper/silver hybrid nano-particle ink and its sintering via flash white light to achieve high electrical conductivity.” Nanotechnology. 2016 May 20;27(20):205704. doi: https://doi.org/10.1088/0957-4484/27/20/205704. Epub 2016 Apr 12. PMID: 27070756.
Ranjan P (2020) Formation of tungsten carbide nanoparticles by wire explosion process. Int J Appl Ceram Technol 17:304310. https://doi.org/10.1111/ijac.13350
Jung T-K (2011) Hyo-Soo Lee and Min-Ha Lee “Fabrication of Ni nanopowder using wire explosion process and its characterization” Rev. Adv Mater Sci 28:171–174
Wonbaek Kim, Je-shin Park, Chang-yul Suh, Sung-wook Cho, Sujeong Lee and In-Jin Shon “Synthesis of TiN Nanoparticles by Explosion of Ti Wire in Nitrogen Gas” Materials Transactions, Vol. 50, No. 12 (2009) pp. 2897 to 2899
Beketov IV, Safronov AP, Medvedev AI, Alonso J, Kurlyandskaya GV, Bhagat SM (2012) Iron oxide nanoparticles fabricated by electric explosion of wire: focus on magnetic nanofluids” AIP ADVANCES 2, 022154
Tucker TJ, Toth RP. “EBW1: a computer code for the prediction of the behavior of electrical circuits containing exploding wire elements” Albuquerque (NM): Sandia National Laboratories; 1975 Apr. Report No.: SAND-75–0041.
LChemezova LI, Mesyats GA, Sedoi VS, Semin BN, Valevich VV (1998) The integral of specific current action and the specific energy input under fast electrical explosion. In: IEEE 18th international symposium on discharges and electrical insulation in vacuum Eindhoven 1998
Rohit S, Anurag S (2014) “Note: Compact, reusable inductive-storage-cum-opening-switch based 1.5 GW single-shot pulsed power generator” Review of Scientific Instruments 85, 036101. https://doi.org/10.1063/1.4867079
Shukla R, Shyam A (2008) Note: Repetitive operation of the capacitor bank of the low-voltage miniature plasma focus at 50 Hz. Review of Scientific Instruments 84(10), 106112
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Shukla, R., Sharma, A. (2022). Ultra Fast Electrically Exploding Wire Method for Production of Raw Material for Additive Manufacturing Based 3D Printing. In: Tyagi, A.K., Ningthoujam, R.S. (eds) Handbook on Synthesis Strategies for Advanced Materials. Indian Institute of Metals Series. Springer, Singapore. https://doi.org/10.1007/978-981-16-1803-1_20
Download citation
DOI: https://doi.org/10.1007/978-981-16-1803-1_20
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1802-4
Online ISBN: 978-981-16-1803-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)