Abstract
Melanoma skin cancer is very lethal with high mortality rate. Melanoma disease has a potential to spread in latter stages which make proficient diagnosis of the disease very challenging for medical experts. However, recognition of skin cancer in preliminary stages can be performed efficiently and can save thousands of lives. Therefore, a highly effective lesion segmentation technique based on Adaptive Contour Model (ACM) for the identification of Melanoma disease at preliminary stages is presented. This method is introduced for the proper structure identification of Melanoma disease to form an even border. The contour features are extracted to form a smoother boundary of a lesion using Adaptive Contour Model. Pre-processing methods are utilized along with ADM for the elimination of noise present in Dermoscopic images and faster execution. The proposed technique segregates vital entities from the background of a dermoscopic image and reconstructs actual color information from its neighboring pixels of same class so that an even border can be reconstructed. PH2 dataset is utilized to carry out performance analysis and comparison with other state-of-art-state-segmentation technique. Performance evaluation factors like Jaccard Index (JA) is 88.95 and Dice Coefficient (DI) is 93.97. This validates superiority of proposed lesion segmentation technique in terms of efficiency.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Melanoma is considered as the fastest growing cancer all around the globe. Several statistical reports suggest that skin cancer has highest mortality rate midst all cancer types. Reports suggest that there is a significant enhancement in the cases of skin cancer patients than compared to any other cancer type [1]. According to US statistical reports, every year 5 million cases are identified with skin cancer in United States of America (USA) [2]. The reason of skin cancer is unrestrained abnormal progress of cells. The most common, lethal and destructive form of skin cancer is Melanoma which has highest mortality rate all across the world. For instance, 9000 people lose their life from Melanoma, from 91,000 cases are diagnosed every year in USA [3]. Similarly, Melanoma causes 2000 deaths from 14,000 cases and 22,000 deaths from 100,000 cases diagnosed every year in Australia [4] and Europe [5]. Moreover, most concerning factor is that the growth in number of melanoma patients is very rapid in recent time. For instance, melanoma cases have risen around 225% in last 30 years [1990–2019] in USA [3, 6]. The reports suggest that the survival rate in melanoma cases is reduced from 99% to approximately 14% [7] when diagnosed in later stages. This paper discusses the problem and its solution in the following sections which are as follows. In Sect. 2, background of melanoma and various existing methods are discussed. In Sect. 3, the proposed model for segmentation technique is discussed. In Sect. 4, experimental results are presented and Sect. 5 gives a comparative analysis, and in Sect. 6 conclusion is discussed.
2 Related Background
The human skin contains three types of tissues such as hypodermis, epidermis and dermis. Epidermis consists of melanocytes which are type of a skin surface cells. The abnormal growth of this melanocytes is the reason for deadliest Melanoma cancer disease [8]. The affected region by Melanoma remains same as skin color in initial stages. However, skin color becomes pink, red, purple, blue and ultimately becomes black or dark brown due to melanocytes in later stages [9]. Melanoma is most concerning disease in terms of diagnosis due to its metastasis characteristics which have potential to spread.
However, Melanoma can be cured completely, if detected in early stages of disease. According to several research reports, the survival rate of Melanoma patient becomes 96% in case of detection of Melanoma disease in primary stage [10]. Due to this reason, detection of Melanoma disease in primary stage becomes a crucial priority. However, identification of melanoma in early stages is a challenging process even for skin experts and specialists due to high visual resemblances between malign and benign skin lesions. Detection of Melanoma from naked eye is very complex and challenging procedure. Therefore, various imaging techniques are presented by several researchers. However, Dermoscope imaging technique is one of the most proficient and competent technique for the identification of Melanoma in initial stages. The accuracy of Dermoscopic high resolution and non-invasive imaging technique is quite high which utilizes light magnifying equipment and immersion fluid for enhancing visualization of skin surface [11]. Various Reports states that diagnosis results are improved by 50% for malignant cases in Melanoma using Dermoscopic imaging technique [12]. Skin experts can deeply study the structures of skin surface using Dermoscopy and reduction in surface reflectance provides additional advantage in Melanoma diagnosis. However, understanding of Dermoscope imaging technique is a challenging process even for medical experts and requires a proficient training to study them. Therefore, a proper and effective analysis of Dermoscopic imaging technique is very essential for the proper diagnosis of Melanoma disease.
Study of Dermoscope imaging technique consists of three key aspects such as Dermoscope image segmentation, feature extraction of Dermoscope images and classification of these images. All three stages are very crucial in analysis of Dermoscopy Segmentation is a primary phase for the assessment of Dermoscopic images which is utilized for detecting lesion border in Dermoscopic images. This technique helps to confine grazes effectively. Although segmentation process requires moderate knowledge to perform lesion detection operation but variety of lesion shapes, structures, color and sizes makes this process quite complicated. Moreover, intrinsic factors such as hairs, blood vessels make segmentation process very complex [13]. Therefore, development of a proficient and robust segmentation technique becomes a challenging task. Several researchers have shown interest in developing a proficient segmentation technique for Melanoma diagnosis.
A comprehensive analysis [14] of Dermoscope Imaging Technique is presented in which segmentation, feature extraction and classification of Dermoscope images is explained in brief. Melanoma classification technique is presented in one of the techniques for the proficient diagnosis of digital images. Here, Gaussian filter [15] is utilized for eliminating noise. DERMIS dataset is utilized for performance evaluation. Skin lesion segmentation technique is introduced in one of the method [16] based on adversarial training to extract discriminative features. Here, artifacts are reduced using DenseNet sampling methods. Performance is evaluated on ISIC dataset. In a process described [17], an automated Melanoma detection technique is introduced using Deep-learning methods and this method is used for effective classification of lesion pixels. ISIC dataset is utilized for performance comparison. A statistical analysis is performed on skin lesion detection, their challenges and solutions in one of the technique [18]. Various techniques and their drawbacks are discussed. However, there are several challenges arising while segmenting Dermoscopic images such as skin and non-skin pixel overlap in presence of complex background, color diversity in Melanoma affected region, existence of intrinsic factors in affected area, high overhead, ineffective feature extraction, etc. Therefore, a research on a proficient lesion segmentation technique is very essential to detect Melanoma in early stages.
3 The Proposed Method
3.1 Introduction to ACM
A proficient segmentation method constructed using Adaptive Contour Model (ACM) for the identification of Melanoma disease at primary stages is required. Besides, Gaussian samples are adopted to manage heterogeneous entities. The mean and variance values of Gaussian samples can differ. The contour features of Dermoscopic image lesion can be exploited with the help of Gaussian structures. The contour features are extracted to form a smoother boundary of a lesion using Adaptive Contour Model. The color pixels in demographic images may defer due to varied contour feature extraction for different classes. Pre-processing methods are utilized along with ADM for the elimination of noise present in Dermoscopic images. The proposed Adaptive Contour Model (ACM)-based lesion segmentation technique is evaluated upon PH2 dataset. The performance of proposed Adaptive Contour Model is highly effective and can be applied in diagnosis of Melanoma.
Numerous skin cancer cases have been detected in recent years all across the world because of universally varying temperature. Melanoma is a harmful skin disease whose mortality rate is 1.62% [19]. Australia and USA are the worst hit countries by Melanoma. A survey carried out by World Health Organization (WHO) states that every year 13 million people comes in contact with deadly Melanoma disease [20]. Identification of Melanoma disease at primary phase is extremely essential for the reduction of high mortality rate and diagnosis cost. Therefore, Adaptive Contour Model is introduced for the lesion segmentation of Dermoscopic images in preliminary phase. This section discusses about mathematical modeling of proposed Adaptive Contour Model (ACM) for the segmentation process of Dermoscopic images and methods adopted to extract contour features. Moreover, Pre-processing methods are utilized for the elimination of noise present in Dermoscopic images. The following section discusses about mathematical representation of Adaptive Contour Model (ACM) for effective segmentation process.
3.2 Mathematical Model
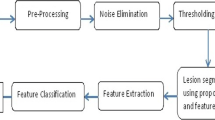
This section discusses mathematical representation of proposed ACM model for image segmentation and contour feature extraction. The effective segmentation of Dermoscopic images helps to identify Melanoma in primary phase. In proposed Adaptive Contour Model after removal of noise with the help of pre-processing block, input image is masked with the ground truth for efficient segmentation of proposed ACM. The steps in ACM model can be represented diagrammatically in the form of a flow chart as given in Fig. 1.
Consider representation of an image area, denoted as \(\delta\) and dermoscope input image can be expressed as \(K\left( m \right): \delta \to X\) and noise exists in the dermoscope images can be represented as \(j\left( m \right): \delta \to X\), unspecified bias region in the image can be indicated as \(Z\left( m \right): \delta \to X\) and finally, reconstructed actual signal can be represented as \(L\left( m \right): \delta \to X\) then color variation in dermoscopic image pixels can be represented as,
Let R entities be present in dermoscopic image domain \(\delta\) then image domain of ath entity can be represented as \(\delta_{a}\). Suppose that the reconstructed actual signal \(L\left( m \right)\) is represented as a piecewise constant for every entity domain, i.e., \(L\left( m \right) = b_{a}\) for \(m \, \epsilon \, \delta_{a}\). Here, \(b_{a}\) can be expressed as a constant. The bias region \(Z\left( m \right)\) is always considered to remain smooth in an image domain \(\delta\).
The noise j exist in the Dermoscopic images is always considered as Gaussian dispersed sample error in which variance and mean can be represented as \(\upsilon_{a}^{2}\) and \(\omega_{a}\), respectively, where mean is considered as zero. Therefore, variance \(\upsilon_{a}^{2}\) and mean \(\omega_{a}\) are the two parameters which can evaluate color pixels of dermoscopic image. Thus, Gaussian dispersed samples can define contour characteristics of image pixels.
However, effective and proficient lesion segmentation of an image cannot be extracted using single Gaussian dispersed sample. Therefore, many Gaussian dispersed samples are required to extract contour characteristics of diverse color pixels for every entity domain. The Gaussian dispersed samples for their respective entity domain \(\delta_{a}\) can be expressed as,
Here, \(\upsilon_{a}\) can be represented as standard deviation and diverse contour mean can be denoted as \(\omega_{a} \left( m \right)\). The bias region \(Z\left( m \right)\) can be considered as zero due its slow variation. Thus, it can be considered that,
where \({\Theta }_{a }\) representation can be denoted as \({\Theta }_{a } = \left\{ {b_{a} , \upsilon_{a} , Z} \right\}\). For every spot m in the image domain \(\delta\), their neighboring central area can be represented as
where Eq. (4) represents small neighborhood. Maximum neighborhood considered is equal to the radius of the entity. Here, d can be expressed as the radius of neighboring central area \(P_{m}\). R, non-overlapping entities are present in the image domain \(\delta_{a}\) of ath entities then the complete set of image domain \(\delta\) can be represented by the following Eq. (5),

where the intersection of \(\delta_{a}\) and \(\delta_{c}\) is represented by ψ where a is not equal to c. Here, a and c are two entities.
The actual color domain \(Y\left( H \right)\) of an image can be transmitted into any other domain X(H) with the help of mapping as,
Here, can be \({\mathbb{R}}\left( {m{|}\Theta_{a} } \right)\) further factorized as,
Let, dispersion of color pixels in an image can be denoted as m and dispersion of pixel color is an independent process and \(i_{a} \left( m \right) = \delta_{a} \cap P_{m}\). Then, its relative probability density operation remains Gaussian for all \({\mathbb{R}}\left( {m{|}\Theta_{a} } \right) \in X\left( H \right)\) and can be represented as,
Let, product of all probability density operation still remains Gaussian for all \(R\left( {n{|}\Theta_{a} } \right)\),
Here, the intersection of \(\delta_{a}\) and \(P_{m}\) is belongs to all n, i.e., \(\delta_{a} \cap P_{m} \in \forall n\). Then, the Gaussian color pixel even function can be represented as
where Eq. 8 is directly proportional to \(R \left( {\omega_{a} , \upsilon_{a}^{2} . \left( {i_{a} \left( m \right)} \right)^{ - 1} } \right)\). Thus,
Suppose that,
where Y is a variable representing ath entity. Then, probability density operation of the ath entity representation can be described as,
Then, the reconstructed combined probability density operation can be performed using ACM for segmentation of images as,
Which is further decomposed in Eqs. (15) and (16) and can be represented as follows,
where \(\phi = \left\{ {\Theta_{a} , a = 1,2, \ldots ,R} \right\}\). and \(\prod\nolimits_{a = 1}^{R} {d = t}\) then,
Which is further factorized as follows,
Form Eqs. (10) and (18), the covariate of Gaussian dispersed samples can be extracted as follows,
where \(t\left( {{\mathbb{R}}\left( {m\left| \phi \right.} \right)} \right)\) is directly proportional to \(R \left( {\omega , \rho } \right)\) and \(\omega\) can be represented as \(\rho \sum\nolimits_{a = 1}^{R} {i_{a} \left( m \right)} . \frac{{\omega_{a} }}{{\left( {\upsilon_{a}^{2} } \right)}}\) and \(\rho\) can be expressed as \(\frac{1}{{\mathop \sum \nolimits_{a = 1}^{R} i_{a} \left( m \right). \left( {\upsilon_{a}^{2} } \right)^{ - 1} }}\).
where the reconstructed combined probability density operation represented by Eq. (16) provides the composition of image pixels with multiple contour class intensities. The actual color pixels are transmitted to another domain which provides the information about same class adjacent pixels of a dermoscopic image from Eq. (7). Therefore, its classification provides noise free output result and an even border can be achieved while segmenting the Dermoscopic images.
4 Results
The analysis of performance for the lesion segmentation of Dermoscopic images using Adaptive Contour Model (ACM) is discussed in this section, to detect Melanoma skin cancer disease in primary stage. Melanoma detection at primary phase is a very challenging process due to the presence of intrinsic factors such as hair, blood and due to high visual resemblances between malign and benign skin lesions. Numerous varieties of lesion shapes, structures, color and sizes make lesion segmentation process even more challenging. Detection of Melanoma is challenging for Experts and medical specialists as well from naked eye. However, primary stage Melanoma detection can be found efficiently. Thus, a proficient segmentation technique based on Adaptive Contour Model (ACM) for the identification of Melanoma disease at primary stages is presented here.
The proposed technique segregates vital entities from the background of an dermoscopic image and reconstructs actual color information from its neighboring pixels of same class so that an even border can be reconstructed. The contour features are extracted to form a smoother boundary of a lesion using Adaptive Contour Model. The performance of proposed Adaptive Counter Model is evaluated based on various factors such as efficient counter feature extraction, noise removal and quality of segmented image. The performance of proposed Adaptive Counter Model is evaluated using PH 2 data set [21]. The PH2 dataset contains 200 dermoscopic diagrams for testing. The dermoscopic image resolution of this dataset is of size 768 × 560. Out of 200 Dermoscopic images present in these PH2 dataset 40 images are of Melanoma type, 80 atypical nevus type and 80 only benign nevi type. The proposed Adaptive Contour Model-based lesion segmentation technique is simulated over windows system. Segmented images and Features are extracted using MATLAB. The comparative analysis of proposed Adaptive Contour model with various state-of-art-technique is presented in the following paragraph.
5 Comparative Analysis
The performance comparison of projected Adaptive Contour Model with various state-of-art segmentation techniques based on the factors like efficient counter feature extraction, noise removal and quality of segmented image is presented here. Pre-processing methods are utilized for the removal of noise from the Dermoscopic images as well as enhances the efficiency of Adaptive Contour Model. The contour features of image lesion can be exploited with the help of Gaussian structures. The contour features are extracted to form a smoother boundary of a lesion.
The proposed Adaptive Contour Model is compared with \({\text{mFCN}} - {\text{PI}}\) [22], Peng el al. [23], DCLPSI [24], FrCN [25], iMSCGnet [26] in terms of Accuracy (AC), Jaccard Index (JA) and Dice Coefficient (DI). This index is utilized for evaluating efficiency of lesion segmentation process. The simulation results of segmentation are very precise and proficient. Here, Table 1 shows the evaluation of proposed.
Adaptive Contour Model with various state of the art segmentation technique in terms of AC, JA and DI. Here, Accuracy (AC) for the segmentation process is achieved quite high as 96.77 compared to other existing techniques. Likewise, Jaccard Index (JA) and Dice Coefficient (DI) factors are obtained using proposed Adaptive Contour Model as 88.95 and 93.97, respectively.
The qualitative analysis of dermoscopic image present in PH2 dataset using Adaptive Contour Model is demonstrated in Fig. 2 and is very proficient and effective. Here, Fig. 2a reveals the input dermoscopy images, Fig. 2b demonstrates the ground truth and Fig. 2c demonstrates the projected even border segmented images.
6 Conclusion
The segmentation of Dermoscopic images to detect Melanoma in primary stage is quite challenging and complex process due to presence of hairs, blood and variety of lesion shapes, structures, color and sizes. Therefore, in this paper, a proficient lesion segmentation technique based on Adaptive Contour Model (ACM) for the identification of Melanoma disease at primary stages is introduced. The contour features are extracted to form a smoother boundary of a lesion using Adaptive Contour Model. The proposed technique segregates vital entities from the background of a dermoscopic image and reconstructs actual color information from its neighboring pixels of same class. Pre-processing methods are utilized along with ADM for the elimination of noise present in Dermoscopic images. A comprehensive mathematical representation of proposed lesion segmentation technique is presented to evaluate contour feature based on probability density operation. The performance of proposed Adaptive Counter Model is evaluated using PH2 dataset. The evaluation of projected Adaptive Contour Model with various state of the art segmentation technique in terms of Accuracy (AC), Jaccard Index (JA) and Dice Coefficient (DI). Here, Accuracy (AC) of lesion segmentation process is quite high than any other state-of-art-techniques which is 96.77. Similarly, factors like Jaccard Index (JA) and Dice Coefficient (DI) are evaluated which are 88.95 and 93.97, respectively. In the future work, efficient feature extraction and classification of lesion segmentation process will be discussed based on proposed Adaptive Contour Model (ACM).
References
Kong B, Sun S, Wang X, Song Q, Zhang S (2018) Invasive cancer detection utilizing compressed convolutional neural network and transfer learning. Lecture notes in computer science. Springer, Cham
American Cancer Society (2018) Cancer facts and figures 2018. [Online]. Available: https://www.cancer.org/research/cancer-factsstatistics/all-cancer-facts-figures/cancer-facts-figures-2018.html
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics. CA: Cancer J Clin 68(1):7–30
Australian Government (2019) Melanoma of the skin statistics. [Online]. Available: https://melanoma.canceraustralia.gov.au/statistics
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 68(6):394–424
Silverberg E, Boring CC, Squires TS (1990) Cancer statistics. CA: Cancer J Clin 40(1):9–26
Rogers HW, Weinstock MA, Feldman SR, Coldiron BM (2015) Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol 151(10):10811086
Feng J, Isern NG, Burton SD, Hu JZ (2013) Studies of secondary melanoma on C57BL/6J mouse liver using 1H NMR metabolomics. Metabolites 3:1011–1035. [CrossRef] [PubMed]
Kasmi R, Mokrani K (2016) Classification of malignant melanoma and benign skin lesions: implementation of automatic ABCD rule. IET Image Process 10(6):448–455
Siegel R, Miller K, Jemal A (2018) Cancer statistics. CA Cancer J Clin 68:7–30. [CrossRef] [PubMed]
Pellacani G, Seidenari S (2002) Comparison between morphological parameters in pigmented skin lesion images acquired by means of epiluminescence surface microscopy and polarized-light videomicroscopy. Clin Dermatol 20:222–227. [CrossRef]
Ali A-RA, Deserno TM (2012) A systematic review of automated melanoma detection in dermatoscopic images and its ground truth data. In: Medical imaging 2012: image perception, observer performance, and technology assessment. International Society for Optics and Photonics, p 8318, Bellingham, WA, USA
Abbas Q, Celebi ME, Garcia IF (2011) Hair removal methods: a comparative study for dermoscopy images. Biomed Signal Process Control 6(4):395–404
Celebi ME, Codella N, Halpern A (2019) Dermoscopy image analysis: overview and future directions. IEEE J Biomed Health Inform 1–1 https://doi.org/10.1109/JBHI.2019.2895803, 2019.
Khan MQ et al (2019) Classification of melanoma and nevus in digital images for diagnosis of skin cancer. IEEE Access 7:90132–90144. https://doi.org/10.1109/ACCESS.2019.2926837
Wei Z, Song H, Chen L, Li Q, Han G (2019) Attention-based dense unet network with adversarial training for skin lesion segmentation. IEEE Access 7:136616–136629. https://doi.org/10.1109/ACCESS.2019.2940794
Berkay M et al (2019) Deep learning based melanoma detection from dermoscopic images. In: Scientific meeting on electrical-electronics and biomedical engineering and computer science (EBBT), Istanbul, Turkey, pp 1–4. https://doi.org/10.1109/EBBT.2019.8741934
Chyad MA, Alsattar HA, Zaidan BB, Zaidan AA, Al Shafeey GA (2019) The landscape of research on skin detectors: coherent taxonomy, open challenges, motivations, recommendations and statistical analysis, future directions. IEEE Access 7:106536–106575. https://doi.org/10.1109/ACCESS.2019.2924989
Tarver T, American Cancer Society (2012) Cancer facts and figures 2014. J Consum Health Internet. 16:366–367. [CrossRef]
Skin cancer, Ultraviolet radiation and the INTERSUN Programme, World Health Organization (WHO)
Mendonça T, Ferreira PM, Marques JS, Marcal ARS, Rozeira J (2013) PH2—a dermoscopic image database for research and benchmarking. In: 35th Annual international conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, pp 5437–5440. https://doi.org/10.1109/EMBC.2013.6610779
Bi L, Kim J, Ahn E, Kumar A, Fulham M, Feng D (2017) Dermoscopic image segmentation via multistage fully convolutional networks. IEEETrans Biomed Eng 64(9):2065–2074
Peng Y, Wang N, Wang Y, Wang M (2019) Segmentation of dermoscopy image using adversarial networks. Multimedia Tools Appl 78(8):10965–10981
Bi L, Kim J, Ahn E, Kumar A, Feng D, Fulham M (2019) Stepwise integration of deep class-specific learning for dermoscopic image segmentation. Pattern Recognit 85:78–89
Al-masni MA, Al-antari MA, Choi M-T, Han S-M, Kim T-S (2018) Skin lesion segmentation in dermoscopy images via deep full resolution convolutional networks. Comput Methods Programs Biomed 162:221–231
Tang Y et al (2020) iMSCGnet: iterative multi-scale context-guided segmentation of skin lesion in dermoscopic images. IEEE Access 8:39700–39712. https://doi.org/10.1109/ACCESS.2020.2974512
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Srikanteswara, R., Ramachandra, A.C. (2022). Segmentation of Skin Lesion Using Adaptive Contours Color Method. In: Shetty, N.R., Patnaik, L.M., Nagaraj, H.C., Hamsavath, P.N., Nalini, N. (eds) Emerging Research in Computing, Information, Communication and Applications. Lecture Notes in Electrical Engineering, vol 790. Springer, Singapore. https://doi.org/10.1007/978-981-16-1342-5_42
Download citation
DOI: https://doi.org/10.1007/978-981-16-1342-5_42
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1341-8
Online ISBN: 978-981-16-1342-5
eBook Packages: EngineeringEngineering (R0)