Abstract
Nanotechnology had a wide potential of its novel applications in the fields of plant nutrition to meet the future demands of the growing population because nanoparticles (NPs) have unique physicochemical properties, i.e., high surface area, high reactivity, tunable pore size, and particle morphology. Management of optimum nutrients for sustainable crop production is a priority area of research in agriculture. In this regard, nanonutrition concerns with the provision of nanosized nutrients for sustainable crop production. The application of nanomaterials for delivery of nutrients and growth-promoting compounds to plants has become more and more popular and their utilization at the proper place, at the proper time, in the proper amount and of the proper composition affects the use efficacy of fertilizers. Using this technology, we can increase the efficiency of micronutrients delivery to plants. In the literature, various NPs and nanomaterials (NMs) have been successfully used for better nutrition of crop plants compared to the conventional fertilizers. This review summarizes the synthesis of nanofertilizers, characterization of nanofertilizers, NPs, and NMs as micronutrient fertilizers and describing their role in improving growth and yield of crops, uptake, translocation, and fate of nanofertilizers in plants and environmental hazard of NPs and NMs application.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nanotechnology is one of the unique technologies of the twenty-first century. In the last decade, a large variety of nanomaterials (NMs) have been developed and used under the umbrella of nanotechnology in multifaceted sectors (Lien et al. 2017). The basis of nanotechnology was laid by Nobel laureate Richard P. Feynman through his popular lecture “There’s Plenty of Room at the Bottom” (Feynman 1960). Taniguchi (1974) first coined the term nanotechnology and stated that nanotechnology consists of the processing, separation, consolidation, and deformation of materials by one atom or one molecule. The term “nanotechnology” is based on the prefix “nano” which hails from the Greek word meaning “dwarf.” It is usually employed for materials having a size ranging from 1 to 100 nm (NNI 2009). Several researches had been awarded Nobel Prize for the development of nanotechnology (Table 26.1).
Nanotechnology, according to Joseph and Morrison (2006), is the modification or self-assembly of individual atoms, molecules, or molecular clusters into structures in order to produce materials devices with new or drastically different properties. Nanotechnology is the design, fabrication, and utilization of materials, structures, devices, and systems through control of matter on the nanometer length scale and exploitation of novel phenomena and properties (physical, chemical, biological) at that length scale in at least one dimension. Table 26.2 enlisted the size distribution of various natural and fabricated nanoparticles (NPs). At nanoscale, the chemical and physical properties of material change and surface area of material are large compared to its volume. This makes material more chemically reactive and changes the strength and electrical properties of material compared to the bulk counterpart. The synthesis protocols for diverse nanoparticles (NPs) were established and advanced to the molecular level (Gugliotti et al. 2004).Generally, it works by following the top-down (includes reducing the size of the smallest structures to the nanoscale) or the bottom-up (comprises manipulating individual atoms and molecules into nanostructures with nearly similar chemistry or biology) approach.
Nanotechnology has emerged as a cutting-edge technology, acting as a convergent science that attracts a plethora of disciplines (environmental science, energy, plant science, agriculture, materials physics, and nanomedicine) and sectors closely linked with human welfare (Gruère 2012; Dasgupta et al. 2016). The application of nanotechnology in various fields anticipated to be advantageous for society and the environment, reduce the cost of input and cause inflation, boost the quality of goods, open opportunities for jobs (Hansen et al. 2008). A wide range of applications of nanotechnology have emerged into the “agrifood sector” which include the nanosensors, tracking devices, targeted delivery of required components, food safety, new product developments, precision processing, smart packaging, nanofertilizers, and others (McClements et al. 2009; Huang et al. 2010; Ranjan et al. 2014; Dasgupta et al. 2016). Nanotechnology can also improve the water solubility, thermal stability, and bioavailability of the functional compounds of food (McClements et al. 2009; McClements and Li 2010). The use of NPs imparted tremendous efficiency compared to bulk particles or particulate matter (PM) because of their large specific surface area, diverse functionalities, easy functionalization, the presence of active sites on the surface, extraordinary electrical and optical properties, extremely high stability, and high adsorption capacity (Boparai et al. 2011; Zhao et al. 2014; Choi et al. 2015; Jiang et al. 2015; Kumar et al. 2015).
2 Applications of Nanotechnology in Agriculture
The present day agriculture is facing many challenges, such as changing climate due to the greenhouse effect and global warming; urbanization due to life pattern changes; non-judicious use of resources like petroleum, natural gas, high-quality rock phosphate, etc., that are non-renewable; and environmental issues like run off, eutrophication related with the application of more chemical fertilizers than required. These problems get more intensified by the world population, which is increasing at an alarming rate and is expected to reach 9.6 billion by the year 2050 (Desa 2008).The demand for global food production has increased during the last two decades. An increase by 70% in global grain production is required to feed this increasing world population (FAO 2009). Agriculture has always been the backbone of most of the developing countries to fuel the growth of economy. According to 2014–2015 estimates, India’s population is 1.27. With the concern of providing food to such a big population, there is a need of new technology in agriculture giving more yields in short period.
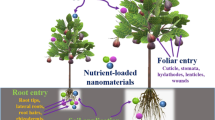
A significant increase in agricultural production could be achieved through utilization of nanotechnology for efficient nutrient management system, good plant protection practices, efficient photocapturing system in plants, precision agriculture, and many others (Tarafdar et al. 2013; Prasad et al. 2014) (Fig. 26.1). Table 26.3 showed the cosmparison between nanofertilizers and conventional products. Applications of nanotechnology in materials science and biomass conversion technologies applied in agriculture are the basis of providing food, feed, fiber, fire, and fuels. Nanotechnology provides a number of cutting-edge techniques for improving precision agricultural practices and allowing precise monitoring at the nanoscale level. In agriculture two types of nanomaterials are mostly used: (1) carbon based single- and multi-walled carbon nanotubes, (2) metal based aluminum, gold, zinc, and metal oxide based ZnO, TiO2, and Al2O3. Single and multi-walled carbon nanotubes are used as nanosensors and plant regulator to enhance plant growth (Khodakovskaya et al. 2012). Nanosilica is used in filtration of food and beverages and packaging. Metal oxides like ZnO, TiO2, and Al2O3 are used in nanofertilizers to boost the crop growth (Gogos et al. 2012; Sabir et al. 2014).
Application of nanotechnology has been regarded as an innovative and promising technology for sustainable agriculture, to feed the ever-increasing population of the world. It has revolutionized agriculture with innovative nutrients in the form of nanofertilizers (NFs), nanopesticides, and efficient water management system (Ditta and Arshad 2016). Conventional fertilizers with low use efficiency (20–50%) and cost-intensive increase in application rates have increased to develop and promote the use of NFs (Aziz et al. 2006). Many scientists worldwide have focused on this innovative field and have developed such NPs and NMs that could serve as nutrients for the plants (Liu and Lal 2015).
For agricultural use, it is preferable to have particle having size less than 20 nm, polydispersity index less than 1, zeta potential value apart from +30 mV and −30 mV, and mostly cubed shaped particle to enter through the plant pores (Tarafdar et al. 2012). Nanoparticles can be synthesized by physical, chemical, physicochemical (aerosol), and biological techniques. Grinding, thermal evaporation, sputtering, and pulse laser deposition technique are important physical methods. Chemical synthesis includes the technique like sol gel, co-precipitation, microwave synthesis, micro-encapsulation, hydrothermal methods, polyvinylpyrrolidone (PVP) method, and sonochemistry.
3 Nanofertilizers
Nanofertilizers are modified fertilizers synthesized by chemical, physical, or biological methods using nanotechnology to improve their attributes and composition, which can enhance the productivity of crops (Singh et al. 2017; Mahto et al. 2021). Nanofertilizers are nanomaterials that can supply one or more nutrients to the plants and enhance plant growth and yields or those that can improve the performance of conventional fertilizers but do not directly provide crops with nutrients. There are several advantage of using nanoformulation of fertilizers in agriculture (Table 26.4). Nanofertilizers can be classified as macronutrient nanofertilizers and micronutrient nanofertilizers (Fig. 26.2). Compared with the conventional fertilizers, these nanofertilizers are expected to significantly improve crop growth and yields, enhance the efficiency of fertilizer use and reduce nutrients losses, and/or minimize the adverse environmental impacts. Various benefits of using nanofertilizers are:
-
Higher product quality with minimum remnants.
-
Eco-friendly synthesis.
-
Custom-made products.
-
Lower-cost production, reducing the amount of fertilizers used.
-
Less negative impacts and toxicity.
-
Controlled release of plant nutrients.
Small size of the NFs facilitate its effective absorption by the plants due to the tremendous increase in the surface area (Fig. 26.3). Moreover, these have the ability to enter into the cells directly as these materials are small sized, which reduces/bypasses the energy-intensive mechanisms of their uptake/delivery into the cell. Similar to the conventional fertilizers, NFs are dissolved in the soil solution and the plants can directly take them up. However, their solubility might be more than that of related bulk solids found in the rhizosphere due to their small size. These are more efficient compared to the ordinary fertilizers, as these reduce nutrient loss due to leaching, emissions, and long-term incorporation by soil microorganisms. Moreover, controlled release NFs may also improve fertilizer use efficiency (FUE) and soil deterioration by decreasing the toxic effects associated with over application of traditional chemical fertilizers (Suman et al. 2010). There are also reports about the use of nanoencapsulated slow release fertilizers. Recently, biodegradable, polymeric chitosan NPs (~78 nm) have been used for controlled release of NPK fertilizer sources such as urea, calcium phosphate, and potassium chloride (Corradini et al. 2010). Other NMs like kaolin and polymeric biocompatible NPs could also be utilized for this purpose (DeRosa et al. 2010).
3.1 Synthesis of Nanofertilizers
Nanofertilizers are synthesized by top-down (physical) or bottom-up (chemical) approaches. Top-down approach is a commonly used method. In top-down approach, the adsorbent or substrate used for synthesis of nanofertilizers such as zeolite or any other carrier is ball milled for several hours to achieve nanodimension. Usually, natural zeolite measures a range of 1000–3000 nm, and grinding using high-energy ball mill reduced the size of the particles. Manik and Subramanian (2014) reported that the ball milling of zeolite at 1, 2, 4, and 6 h had reduced the dimension 1078, 475, 398, 357, and 203 nm, respectively. The size reduction closely coincided with the increase in the respective surface area of 41, 55, 72, 83, and 110 m2 g−1. This phenomenal increase in the surface area provides extensive surface for nutrient adsorption and desorption. Despite the physical method of nanoparticle synthesis is very simple, the product is heterogeneous and particles often get agglomerated. To prevent agglomeration, stabilizing agents such as polymers or surfactants are used. Synthesis, characteristics, and nutrient release capability of some nanofertilizers are presented in Table 26.5.
The studies on slow release fertilizers (SRFs) based on zeolites are limited to nutrients, which can be loaded in cationic forms such as NH4+ and K+. However, if the nutrients are in anionic forms such as SO42−, NO3−, and PO43−, the loading is negligible on unmodified zeolites. Therefore, it is imperative that the material should have adequate affinity for anions so that the anionic nutrients can be efficiently loaded for its use as SRFs. Anionic properties can easily be imparted on the zeolitic surface using the concept of surface modification using surfactant. Surface modification facilitates the loading of anion into the zeolite’s surface by the anion exchange process. Haggerty and Bowman (1994) reported that surfactant modified zeolite (SMZ), a type of inexpensive anion exchanger has been shown to remove anionic contaminants from water. Hexadecyltrimethylammonium bromide (HDTMABr), a cationic surfactant, was used for surface modification of zeolite. It has been found that HDTMABr loading with a maximum of 200 mmol kg−1 corresponds to 200% of the zeolite’s effective cation exchange capacity. A surfactant bilayer forms and the surface reversed to positive (Li and Bowman 1997). Li et al. (1998) revealed that SMZ has been studied extensively in the last 15 years due to its high capacity of sorption and retention of oxyanions. The surfactant molecules (HDTMABr) form bilayers on zeolite external surfaces with the lower layer held by electrostatic interaction between the negatively charged zeolite surface and the positively charged surfactant head groups, while the upper layer is bound to the lower layer by hydrophobic forces between the surfactant tail groups in both layers (Bowman 2003). Surface modified zeolite showed positive results on the retention of chromate (Krishna et al. 2001) and phosphate (Bansiwal et al. 2006). Li and Zhang (2010) reported that the loading capacity of sulfate compared to nitrate on SMZ may be attributed to the charge effect of the anions. Each HDTMABr molecule contributes one positive charge, which needs only one negative charge to balance. Sulfate is divalent and thus needs two HDTMABr molecules to neutralize. Meanwhile, the HDTMABr surface configuration is not rigid because of the surfactant tail–tail interaction. Thus, bridging two HDTMABr molecules with one sulfate may be less favored compared to 1:1 neutralization of HDTMABr by nitrate.
3.2 Characterization of Nanofertilizers
Synthesized nanofertilizers are to be characterized using particle size analyzer (PSA), zeta analyzer, Fourier transform infrared spectroscopy (FTI-IR), Raman spectroscopy, X-ray diffraction (XRD), scanning electron microscope (SEM), energy dispersive X-ray spectroscopy (EDAX), transmission electron microscope (TEM), and atomic force microscope (AFM) to confirm the size, shape, charge distribution, functional groups, elemental composition, and attachment. The synthesized nanofertilizers have been characterized using the set of equipment (Table 26.6). Extensive studies had been undertaken to characterize nitrogenous (Subramanian and Sharmila Rahale 2013; Mohanraj 2013; Manik and Subramanian 2014), phosphatic (Bansiwal et al. 2006; Adhikari 2011; Behnassi et al. 2011), potassic (Subramanian and Sharmila Rahale 2012), sulfatic (Selva Preetha et al. 2014; Thirunavukkarasu 2014), and zinc (Subramanian and Sharmila Rahale 2012) fertilizers.
4 Micronutrient Nanofertilizers
Micronutrients play an important role in many physiological functions of plants. These are required in a very small amount (≤100 ppm) but have a very critical role in various plant metabolic processes. These include chloride (Cl), iron (Fe), boron (B), manganese (Mn), zinc (Zn), copper (Cu), molybdenum (Mo), and nickel (Ni). These are applied to the plants either as Hoagland solution (Hoagland and Arnon 1950) or as foliar or applied in soil depending on crop species and also on the nutrient to be applied. These are also applied to the crop plants with composite fertilizers containing multiple macronutrients like NPK. Micronutrients present in these composites usually provide enough nutrients and cause little environmental risks. However, their availability is severely affected by small changes in pH, soil texture, and organic matter (Fageria 2009). So, it is most likely that under such circumstances, their optimum availability could be achieved through the application of NFs containing these micronutrients. A summary of the studies conducted regarding the investigation of the efficacy of each micronutrient-containing NPs is given in Table 26.7.
4.1 Zinc Nanofertilizer
Many researchers around the world have focused on finding the effect of ZnO-NPs on the growth and productivity of crops. Out of all the micronutrients, it is the most widely studied in plant science worldwide. For example, optimal concentration of ZnO-NPs significantly enhanced the growth and yield parameters of mung bean and chickpea (Mahajan et al. 2011). Optimal concentration of ZnO-NPs to be applied depends on the nature of the crop. With the application of 20 mg L−1 ZnO-NPs to mung bean plants, an increase of 42%, 41%, 98%, and 76% in root length, root biomass, shoot length, and shoot biomass, respectively, was recorded. Moreover, the application of higher doses of ZnO-NPs caused a decrease in the growth rates of mung bean and chickpea. In another greenhouse experiment, the application of ZnO-NPs at the rate of 400 and 800 mg kg−1 caused a significant increase in the growth and yield parameters of cucumber (Cucumis sativus) (Zhao et al. 2014). The results clearly showed an increase of 10% and 60% in plant root dry mass with the application of 400 and 800 mg kg−1, respectively, as compared to control (without ZnO-NPs). However, the same rates caused a slight increase of 0.6% and 6% in the dry fruit weight, respectively, as compared to the control. Similarly, Lin and Xing (2007) reported a significant increase in the root elongation of germinated seeds of radish (Raphanus sativus) and rape (Brassica napus) with the application of ZnO-NPs at 2 mg L−1, in comparison to control (deionized water). The authors also found a significant improvement in the growth parameters of ryegrass (Lolium perenne) with the application rate of 2 mg L−1 metallic Zn-NPs. Seed germination was improved with the application of lower concentrations of ZnO-NPs in peanut (Prasad et al. 2012), soybean (Sedghi et al. 2013), wheat (Ramesh et al. 2014), pearl millet (Tarafdar et al. 2014), tomato (Raliya et al. 2015), and onion (Raskar and Laware 2014). In another experiment, a significant improvement in Cyamopsis tetragonoloba plant biomass, shoot and root growth, root area, chlorophyll and protein synthesis, rhizospheric microbial population, acid phosphatase, alkaline phosphatase, and phytase activity in cluster bean rhizosphere was recorded with the application of ZnO-NPs (Raliya and Tarafdar 2013). Similarly, Helaly et al. (2014) found that ZnO-NPs supplemented with MS-media promoted somatic embryogenesis, shooting, regeneration of plantlets, and also induced proline synthesis, activity of superoxide dismutase, catalase, and peroxidase, thereby improving tolerance to biotic stress. In contrast to these studies, many researchers have reported phytotoxicity of the application of Zn-NPs in various crop plants (Mahajan et al. 2011; Lin and Xing 2007; Lee et al. 2010; López-Moreno et al. 2010). However, phytotoxicity depends on the nature of crop plants. Overall, most of the crop plants usually require merely 0.05 mg L−1 soil solution. The researchers in these studies applied metallic Zn-NPs at a very high rate, ranging from 400 to 2000 mg L−1, which was the main reason for their toxic effects. Even the application of Zn-NPs at 10 mg L−1 to ryegrass proved harmful for normal growth (Lin and Xing 2008). In another study, among cucumber, alfalfa, and tomato, the application of ZnO-NPs only enhanced seed germination of cucumber (de la Rosa et al. 2013).
4.2 Iron Nanofertilizer
In a greenhouse study under a hydroponic system, application of lower concentrations of Fe-NPs (30, 45, and 60 mg L−1) significantly improved the chlorophyll contents of the sub-apical leaves of soybean compared to the regular application of Fe-EDTA (Ghafariyan et al. 2013). The results suggested that Fe-NPs could serve as an efficient source of Fe compared to the regular Fe-EDTA applied at <45 mg L−1 as Fe, thereby reducing the chloratic symptoms caused by its deficiency in soybean. Moreover, the uptake efficiency of Fe-NPs in the plant body was enhanced, which ultimately increased the chlorophyll contents of soybean plants. In another experiment, growth and yield parameters of black-eyed peas were significantly improved when Fe-NPs were applied as foliar at 500 mg L−1 (Delfani et al. 2014). Moreover, the application of Fe-NPs improved the effect of another fertilizer nutrient applied in the form of Mg-NPs. Previously, Hoagland and Arnon (1950) found that most of the plants generally require 1–5 mg L−1 Fe in soil solution.
4.3 Manganese Nanofertilizer
A hydroponic culture experiment was conducted to find out the comparative efficacy of Mn-NPs and commonly used Mn-salt, i.e., MnSO4, on the growth and yield parameters of mung bean (Pradhan et al. 2013). Both were applied at 0.05, 0.1, 0.5, and 1.0 mg L−1. The results showed that application of Mn-NPs at 0.05 mg L−1 significantly improved growth and yield parameters compared to the control with no Mn applied. At higher doses, Mn-NPs did not show toxicity to the bean plants, while MnSO4 applied at 1 mg L−1 showed toxic effects like necrotic leaves, brown roots, and gradual disappearance of the rootlet after 15 days of treatment. Moreover, greater oxygen evolution and photophosphorylation in Mn-NP-treated chloroplasts was noted compared to the control. Greater oxygen evolution was caused by enhanced splitting of water in the oxygen-evolving center located in the chloroplast. The authors concluded that Mn-NPs could serve as a potential modulator of photochemistry in the agriculture sector.
4.4 Copper Nanofertilizer
Previously, it has been clearly found that the application rate of Cu-NPs at Cu 0.02 mg L−1 in Hoagland solution is optimum for normal growth and yield of crops. Scientists around the world have found toxic effects of the application of Cu-NPs, as they have applied them at higher rates than required (Lee et al. 2008; Musante and White 2012). They found that Cu-NPs applied at the rate of 200–1000 mg L−1 caused toxic effects on seedling growth of mung bean, wheat, and yellow squash. Similarly, reduced biomass of zucchini by 90% compared to that of the control (without Cu) after the seedlings were incubated in Hoagland solution for 14 days was recorded with the application of metallic Cu-NPs at 1000 mg L−1. However, researchers like Shah and Belozerova (2009) recorded a significant increase of 40% and 91% in 15-day lettuce seedling growth rate with the application of Cu-NPs at 130 and 600 mg kg−1, respectively. Similarly, a 35% increase in photosynthetic rate of waterweed was recorded in a 3-day incubation study using a low concentration of Cu-NPs applied at ≤0.25 mg L−1 (Nekrasova et al. 2011).
4.5 Molybdenum Nanofertilizer
Molybdenum is essential for legumes as it is involved in biological nitrogen fixation (BNF), being the component part of nitrogenase enzyme. For normal metabolism of crop plants the concentration of soil solution Mo should be ≈0.01 mg L−1. Taran et al. (2014) conducted a pot experiment using different combinations of N-fixing bacteria and Mo-NPs (water, Mo-NPs, microbial inoculation with nitrogen-fixing bacteria, and a combination of the microbes and Mo-NPs). The control was treated with distilled water. Chickpea seeds were soaked in each of the treatments for 1–2 h. The results clearly showed that the combined application of microbes and Mo-NPs significantly improved the microbiological properties of the rhizosphere, including all groups of agronomically important microbes. The same combination significantly improved the root number, nodule number per plant, and nodule mass per plant compared to control.
5 Risk of Nanoparticle Application on Environment
Application of nanomaterials in agriculture is not always beneficial. It has number of negative effects on soil, plant, and aquatic life and most importantly human because of long food chain and easy motion of nanoparticles. Study of behavior of nanoparticles at different sizes with different concentrations in soil, plant, and water is as under:
5.1 Risk of Nanoparticle Application on Soil
Soil is prima facie receiver of fertilizers with nanoparticles. There is harmful chemical reactions and contamination by these nanoparticles to soil ecosystem and change in soil structure due to their large surface area and Brownian motion. Nanoparticles used through fertilizers could be harmful to soil biota and fertility (Ranallo 2013). They affect microbes, microfauna of soil, and digestive system of earthworm. An adverse effect of nanoparticles on soil health is presented in Table 26.8.
The potential harmful effects of nanoparticles Ag, TiO2, ZnO, CeO2, Fe3O4 include reduction in growth, fertility, survival, and increased mortality of earthworm and soil bacteria. Size is the main factor for ecotoxicity. To find out the relationship between size and toxicity, Roh et al. (2010) have initiated a study with TiO2 and CeO2 nanoparticle on Caenorhabditis elegans. It is a free-living, transparent nematode, about 1 mm in length that lives in temperate soil environments. They found that smaller size of TiO2 (7 nm) and CeO2 (15 nm) nanoparticles are more toxic compared to larger size (TiO2 of 20 nm and CeO2 of 45 nm). It has been found that higher doses of ZnO nanoparticle become toxic for soil (Hu et al. 2010). Whereas, the amount of ZnO in the soil is increased from 1 g kg−1 to 5 g kg−1, ZnO nanoparticles bioaccumulate within the earthworm and cause DNA damage.
5.2 Risk of Nanoparticle Application on Plant
Toxicity of nanoparticles depends upon various factors like plant species, size, and concentration of nanoparticles in different stages of crop. Toxic effect of nanoparticles also depends upon their composition and size. Small sized nanoparticles are more reactive and toxic compared to large sized and affect the respiration or photosynthesis process (Navarro et al. 2008). Hund-Rinke and Simon (2006) worked on different sizes of photocatalytic active TiO2 nanoparticles and its ecotoxic effect on algae (EC50: 44 mg L−1) and daphnids with maximum concentration of 50 mg L−1 and found that ecotoxicity of nanomaterials depends upon nature of particles. Toxicity found in algae is more than daphnids. Lin and Xing (2007) worked on phytotoxicity of nanomaterials. They used MWCNT, Al, Al2O3, Zn, and ZnO in their experiment on radish, rape, ryegrass, lettuce, corn, and cucumber and found that seed germination of corn and ryegrass is affected by nanoscale ZnO and Zn, respectively. Aluminum oxide (Al2O3) nanoparticles showed phytotoxicity only on corn, which reduced the root elongation by 35%. Aluminum (Al) improved root growth of rape and radish and inhibited root elongation of ryegrass and lettuce but had no effect on cucumber. Some of the toxicological studies on the effect of nanomaterials are presented in Table 26.9.
The level of toxicity in plants due to nanoparticles is in direct relation with size and nature of the particles. Zinc oxide (ZnO) nanoparticles easily dissolve in soil and uptake by plant and TiO2 nanoparticles accumulate in soil and retain for long time and stick with the cell wall of wheat plant. Both reduced the biomass of wheat crop (Du et al. 2011). Phytotoxicity was studied by Mazumdar and Ahmed (2011) on rice crop. They found that silver nanoparticle accumulated inside the root cell and damage the cell walls during penetration of particles due to complex mechanism and small size of particles, it damaged the external and internal portion of cell wall. The other factor for plant toxicity is the concentration of nanoparticle because a nanoparticle of same size in different concentration changes its chemical properties. Zinc oxide nanoparticle showed great toxicity in different concentrations (Boonyanitipong et al. 2011). They found that ZnO starts showing adverse effect on rice plant from 100 mg L−1 and fully inhabits root growth and biomass at 500–1000 mg L−1 concentration.
5.3 Risk of Nanoparticle Application on Water
The nanoparticles can easily be released in water body or air and uptake by living organisms, create toxic effect for human, animals, and also for aquatic life. Titanium oxide (TiO2) reduced the light to entrap the algal cell and thus reduce the growth (Sharma 2009). The toxicity study of Ag, Cu, Al, Ni, TiO2, and Co nanomaterials on algal species, zebrafish, and daphnids revealed that Ag and Cu nanoparticles cause toxicity to all organisms (Griffitt et al. 2008) and the metal form is less toxic than soluble form of nanoparticles. Table 26.10 describes the aquatic toxicity of use of nanomaterials release in surface water body. It has been proved from different studies that nanoparticles like Ag, Cu, Al, Ni, and TiO2 cause unrecoverable toxic effect on aquatic ecosystem. Silver, iron oxide, and copper nanoparticle adversely affected health of zebrafish. It enhances mortality, hatching, and reduces heartbeat and survival rate affect normal development (Asharani et al. 2008; Griffitt et al. 2007; Zhu et al. 2012). Therefore, the level of nanotoxicity in soil, plant, and water mainly depends on the composition, size (<20 nm), and concentration (>100 ppm) of the nanoparticle.
5.4 Risk of Nanoparticle Application on Human Health
The emerging field of nanotechnology has created an interest on human health risk associated with nanoparticles. These particles create new challenge for researchers to understand and find risk associated with human health. Exposure of these materials occurs through inhalation, ingestion, and dermal exposure during synthesis, manufacturing, and application of these nanomaterials. Table 26.11 shows the adverse effects of nanomaterials on human health.
The most common way of exposure is inhalation of airborne nanoparticles. Greatest emission risk occurs in the manufacturing process with poor filtering and ventilation system (AFSSET 2006). Factors that affect inhaled dose are particle geometry and physiochemical properties, lung morphology, respiration physiology, and environmental condition (Shade and Georgopoulos 2007). Nanoparticles deposit in respiratory traces after inhalation increases the total deposition fraction (TDF) in the lungs with decrease in particle sizes. Nanoparticles can also be taken-up in the brain through the olfactory epithelium (Borm et al. 2006; Jaques and Kim 2000). Ultrafine airborne particles may increase respiratory and cardiovascular morbidity and mortality (Shade and Georgopoulos 2007).
Ingestion is another source of entry of nanoparticles into human body. The nanoparticles entered through gastrointestinal tract directly through intentional ingestion or indirectly via water, food, animal food, and fish (Bergin and Witzmann 2013). Mucociliary escalators may be excreted as inhaled particles or absorbed into the gastrointestinal tract; however, absorption is dependent on particle size and physicochemical characteristics (Hagens et al. 2007). Jani et al. (1990) found that particle size less or equal to 50 nm had more uptake or absorbed across gastrointestinal tract and can be passed to the liver, spleen, blood, and bone marrow by the momentary lymph supply and nodes. Plants have more resistance to prevent translocation of nanoparticles than mammalian barriers (Birbaum et al. 2010).
Dermal exposure is an import route to absorb nanoparticles via the skin. Skin constitutes about 10% of the body’s weight and acts as a buffer against external impurities, as well as shielding, preserving homeostasis, digestion, synthesis, and deposition functions (Crosera et al. 2009). Penetration of nanoparticles depends upon physicochemical characteristics of nanoparticles and medical condition of skin such as eczema, dermatitis, and skin irritation. Absorption between epidermis and dermis or permeability increases in damage skin (Nielsen et al. 2007). Dermal exposure of small size nanoparticles lower than 10 nm is more dangerous. This size of particles may cause erythema, edema, and eschar formation. Further larger size particles cannot penetrate into the skin from transappendageal routes (Gautam et al. 2011).
Thus, it has been established that nanoparticles adversely affect human health and the potential routing could be through inhalation, ingestion, and dermal exposure. It is understood that the nanoparticles show significant health complications in human when exposed to the size of particles less than 50 nm.
5.5 Asian Prospects of Micronutrient Nanofertilizer
Nanotechnology is considered as one of the key technologies in the twenty-first century that promises to advance traditional agricultural practices and offers sustainable development by improving the management and conservation tactics with reduced waste of agricultural inputs (Dubey and Mailapalli 2016; Shang et al. 2019). In 2018, both public and private sectors of worldwide had invested about US $1055.1 million on nanotechnology market which is projected to reach $2231.4 million by 2025. The exponential growth of global investment in nanotechnology research closely coincides with the number of patents relating to nanoproducts. Recent statistics suggests that 88% of the patents are generated from just seven countries comprising US, China, Germany, France, South Korea, Switzerland, and Japan (Subramanian and Tarafdar 2011). The Government of India is currently spending Rs.1000 crores under Nano Science and Technology Mission (Nano Mission) during the Eleventh Five-year Plan period to promote research and development in all flourishing sectors of nanotechnology, and agriculture is one of them. Within the sphere of agricultural sciences, nanotechnology application in relation to soil and crop management is in its nascent stage and over the next few years it is expected to grow exponentially.
Fertilizers play a pivotal role in agricultural production. It has been unequivocally demonstrated that fertilizer contributes to the tune of 35–40% of the productivity of any crops. Without the fertilizer input, it is hardly possible to sustain agricultural productivity of any country. Thus, attempts are being made to synthesize nanofertilizers in order to regulate the release of nutrients demand of crops and overcome the uncertainty of crop production sector with limited natural resources (Godfray et al. 2010). Based on their actions, nanofertilizer could be classified as control or slow release fertilizers, control loss fertilizers, magnetic fertilizers, nanocomposite fertilizers as combined nanodevice to supply wide range of macro- and micronutrients in desirable properties (Panpatte et al. 2016; Lateef et al. 2016). A very few nanofertilizer formulations have been synthesized in China, Taiwan, India, Germany, and the USA and are being tested under laboratory conditions. Liu et al. (2006a, b) an associate from Chinese Academy of Agricultural Sciences (CAAS) have shown that nanocomposites containing organic polymer intercalated in the layers of kaolinite clays can be used as a cementing materials to regulate the release of nutrients from conventional fertilizers. This process increases the nutrient use efficiencies, besides preventing environmental hazard. Bansiwal et al. (2006) reported the use of surface modified zeolite as a carrier of slow release phosphatic fertilizer for the first time in India.
As a promising interdisciplinary research field, nanotechnology has aroused its enormity in agriculture. Micronutrients like zinc (Zn), copper (Cu), iron (Fe), manganese (Mn), boron (B), chlorine (Cl), molybdenum (Mo) also play an integral role in steady increase of crop productivity. However, numerous factors, such as soil pH, cation exchange capacity, soil texture, calcium carbonate content, water content, etc. stimulate their deficiencies in crop production with extensive farming practice (Ghormade et al. 2011). The deficiency of micronutrients decreases not only the productivity of crops, but also affects human health through the consumption of micronutrient-deficient foods (Swaminathan et al. 2013; Monreal et al. 2016). In contrast, the supplementation of nanoformulated or nanoentrapped micronutrients for the slow or controlled release of nutrients would stimulate the uptake process by plants, promote the growth and productivity of crops, and contribute to maintaining soil health as well (Peteu et al. 2010). Although the exact mechanism behind promotion of plant growth and enriched quality is not clear, it may be at least partially explained by the potentialities of nanomaterials to absorb more nutrients and water that in turn helps to enhance the vigor of root systems with increased enzymatic activity (Dubey and Mailapalli 2016; Shojaei et al. 2019). Therefore, the developing countries of Asia come forward to adopt these high potential technologies to ameliorate micronutrient deficiency in crop production and secure the nutritional security to the human being. The government of Myanmar is the first to undertake a program to include micronutrient nanofertilizers in their national fertilizer regimen. Later on, several other Asian countries like, India, Taiwan, Thailand, Malaysia, Iran also approved to commercialize the micronutrient nanofertilizers and Table 26.12 shows some approved micronutrient nanofertilizers currently used in these countries (Dimkpa and Bindraban 2017; Prasad et al. 2017; Elemike et al. 2019).
Nanoform of micronutrients improves their bioavailability to the plants and shows a significant improvement in plant growth and nutrition quality and some recent advancement in micronutrient nanofertilizer research in Asian countries is summarized in Table 26.13. Among the various micronutrients, Zn is the most important one, as it requires for structural component or regulatory co-factor for various enzymes and proteins in plants (Noreen et al. 2018). The foliar application of Zn and B nanofertilizers at 636 and 34 mg tree−1, respectively, increased fruit yield by 30% in pomegranate trees (Khot et al. 2012). Similarly, foliar application of nano Zn and B fertilizers was found to increase fruit yield and quality, including 4.4–7.6% increases in total soluble solids (TSS), 9.5–29.1% decreases in titratable acidity (TA), 20.6–46.1% increases in maturity index, and 0.28–0.62 pH unit increases in juice pH on pomegranate without affecting any physical fruit characteristics (Davarpanah et al. 2016). Cucumber seedlings grown in nutrient solution including rubber type nanomaterial as a Zn source increased shoot and fruit yield compared with those grown in commercial ZnSO4 fertilizer (Mattiello et al. 2015). Application of Zn nanoparticles in pearl millet significantly enhanced grain yield by 38%, which was also associated with an improvement of 15% in shoot length, 4% in root length, 24% in root area, 24% in chlorophyll content, 39% in total soluble leaf protein, and 12% in plant dry biomass compared to the control in a period of 6 weeks (Moghaddasi et al. 2017). It was also observed a considerable yield increase using Zn nanoparticles as a nutrient source in rice, maize, wheat, potato, sugarcane, and sunflower (Monreal et al. 2016; Chhipa 2017). Under Zn deficient soil, application of nano ZnO at low doses positively influences the growth and physiological responses, such as shoot and root elongation, the fresh dry weight, and photosynthesis in many plant species compared to the control (Ali et al. 2019; Asl et al. 2019). Kale and Gawade (2016) reported that application of nano ZnO with other fertilizer in Zn deficient soil not only promotes nutrient use efficiency but also increases barley productivity by 91% compared to the control. Nanoparticles of ZnO showed a significant improvement in biomass, shoot length, root, chlorophyll and protein content, and phosphatase enzyme activity in Vigna radiate, Cicer arietinum, Cucumis sativus, Raphanus sativus, Brassica napus, and Cyamopsis tetragonoloba (Lin and Xing 2007; Mahajan et al. 2011; Zhao et al. 2013; Raliya and Tarafdar 2013).
Iron is also an important nutrient required by plants in minute quantities for maintaining proper growth and development (Palmqvist et al. 2017). Delfani et al. (2014) reported that use of nano Fe on blacked eyed pea recorded 10% increment in chlorophyll content in leaves. In Glycine max chlorophyll content was increased significantly by nano Fe application at 30–60 mg kg−1 (Ghafariyan et al. 2013). Disfani et al. (2017) also found that Fe/SiO2 nanomaterials have significant potential to improve seed germination in barley and maize. Application of 50 mg L−1 nano FeO in Citrus maxima plants significantly improved the chlorophyll contents and root activity by 23% and 24%, respectively, compared to controls (Sharma 2006). Yousefzadeh and Sabaghnia (2016) demonstrated that the application of nano Fe fertilizer not only increased the agronomic traits of Dracocephalum moldavica with sowing density, but also improved essential oil contents of plants. Elfeky et al. (2013) found that foliar application of nano Fe3O4 could significantly enhance total chlorophyll, total carbohydrate, essential oil levels, iron content, plant height, branches per plant, leaves per plant, fresh weight, and dry weight of Ocimum basilicum plants compared to that of soil application. Disfani et al. (2017) demonstrated that 15 mg kg−1 of nano Fe and SiO2 increased shoot length of barley and maize seedlings about 8.25% and 20.8%, respectively.
Application of nano Cu improved photosynthesis in Elodea desaplanch by 35% at low concentration (Nekrasova et al. 2011) and seeding growth up to 40% in lettuce (Shah and Belozerova 2009). Spray of nano Mn on Vigna radiata increased 52% root length, 38% shoot length 71% rootlet, and 38% biomass at 0.05 mg kg−1 concentration in comparison with bulk MnSO4 (Pradhan et al. 2013). However, MnO nanoparticles and FeO nanoparticles were not only less toxic than their ionic counterparts but they also stimulated the growth of lettuce seedlings from 12% to 54%, respectively (Lü et al. 2016). Molybdenum nanoparticle also showed improved microbial activity and seed growth in chickpea after combined treatment with nitrogen fixation bacteria (Taran et al. 2014). In addition to germination, nanomaterials, such as ZnO, FeO, and ZnFeCu-oxide, are reported to increase crop growth and development with quality enhancement in many crop species including peanut, soybean, mung bean, wheat, onion, spinach, tomato, potato, and mustard (Dubey and Mailapalli 2016; Shalaby et al. 2016; Shojaei et al. 2019; Zulfiqar et al. 2019).
The basic economic benefits of the use of micronutrient nanofertilizers are reduced leaching and volatilization associated with the use of conventional fertilizers. Simultaneously, the well-known positive impact on yield and product quality has a tremendous potential to increase growers’ profit margin through the utilization of this technology. Biosynthesized nanoparticles-based fertilizers and nanobiofertilizers should be explored further as a promising technology in order to improve yields while achieving sustainability.
6 Conclusion
The opportunity for application of nanotechnology in agriculture is prodigious. Research on the applications of nanotechnology in agriculture needs to be initiated in all sectors of agriculture. Nanotechnology promises a breakthrough in improving nutrient use efficiency through nanoformulation of fertilizers, breaking yield and nutritional quality barriers through bionanotechnology, surveillance and control of pests and diseases, understanding the mechanism of host–parasite interactions at the molecular scale, development of new-generation pesticides and safe carriers, preservation and packaging of food and food additives, strengthening of natural fiber, removal of contaminants from soil and water bodies, improving the shelf-life of vegetables and flowers, and use of clay minerals as receptacles for nanoresources involving nutrient ion receptors, precision water management, regenerating soil fertility, reclamation of salt-affected soils, checking acidification of irrigated lands, and stabilization of erosion-prone surfaces, to name a few. The use of nanomaterials for delivery of pesticides and fertilizers is expected to reduce the dosage and ensure controlled slow delivery. Nanotechnology has the potential to revolutionize the fertilizer use and has the ability to play an important role in crop nutrition. The usefulness and effectiveness of nanofertilizers to enhance the growth and yield has been clearly demonstrated. Nanomaterials could preferably be used for foliar application but can also be used as seed treatment or for soil application. Nanomaterials perform better under lower concentration and can enhance the nutrient use efficiency and improve soil fertility in an eco-friendly manner. However adverse impact of its use has also been reported. There is very limited knowledge about its long-term adverse effect on soil, plants, and ultimately on human. It is required to study about the non-toxic limit of nanoparticles related to its size and concentration. The positive benefit of nanoparticles should be selected on the basis of their risk related to environment and human.
References
Adhikari T (2011) Nano-particle research in soil science: micronutrients. In: Proceedings of the national symposium on Applications of Clay Science: Agriculture Environment and Industry, pp 18–19
Adhikari T, Kundu S, Biswas AK, Tarafdar JC, Subba Rao A (2015) Characterization of zinc oxide nano particles and their effect on growth of maize (Zea mays L.) plant. J Plant Nutr 38:1505–1515
Adhikari T, Sarkar D, Mashayekhi H, Xing B (2016) Growth and enzymatic activity of maize (Zea mays L.) plant: solution culture test for copper dioxide nano particles. J Plant Nutr 39:99–115
AFSSET (2006) Nanomaterials effects on human health and the environment. http://www.afssa.fr/ET/DocumentsET/afsset-summary-nanomaterials.pdf
Ahmed B, Khan MS, Musarrat J (2018a) Toxicity assessment of metal oxide nano-pollutants on tomato (Solanum lycopersicon): a study on growth dynamics and plant cell death. Environ Pollut 240:802–816
Ahmed B, Shahid M, Khan MS, Musarrat J (2018b) Chromosomal aberrations, cell suppression and oxidative stress generation induced by metal oxide nanoparticles in onion (Allium cepa) bulb. Metallomics 10:1315–1327
Ali S, Rizwan M, Noureen S, Anwar S, Ali B, Naveed M, Abd Allah EF, Alqarawi AA, Ahmad P (2019) Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza sativa L.) plant. Environ Sci Pol 26:11288–11299
Alsaeedi AH, El-Ramady H, Alshaal T, El-Garawani M, Elhawat N, Almohsen M (2017) Engineered silica nanoparticles alleviate the detrimental effects of Na+ stress on germination and growth of common bean (Phaseolus vulgaris). Environ Sci Pol 24:21917–21928
Antisari LV, Carbone S, Gatti A, Vianello G, Nannipieri P (2013) Toxicity of metal oxide (CeO2, Fe3O4, SnO2) engineered nanoparticles on soil microbial biomass and their distribution in soil. Soil Biol Biochem 60:87–94
Asharani PV, Wu YL, Gong Z, Valiyaveettil S (2008) Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 19:255102
Asl KR, Hosseini B, Sharafi A, Palazon J (2019) Influence of nano-zinc oxide on tropane alkaloid production, h6h gene transcription and antioxidant enzyme activity in Hyoscyamus reticulatus L. hairy roots. Eng Life Sci 19:73–89
Atha DH, Wang H, Petersen EJ, Cleveland D, Holbrook RD, Jaruga P, Dizdaroglu M, Xing B, Nelson BC (2012) Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ Sci Technol 46:1819–1827
Aziz T, Rahmatullah MA, Maqsood MA, Tahir IA (2006) Phosphorus utilization by six Brassica cultivars (Brassica juncea L.) from tri-calcium phosphate; a relatively insoluble P compound. Pak J Bot 38:1529–1538
Bai R, Zhang L, Liu Y, Li B, Wang L, Wang P, Autrup H, Beer C, Chen C (2014) Integrated analytical techniques with high sensitivity for studying brain translocation and potential impairment induced by intranasally instilled copper nanoparticles. Toxicol Lett 226:70–80
Bansiwal AK, Rayalu SS, Labhasetwar NK, Juwarkar AA, Devotta S (2006) Surfactant-modified zeolite as a slow release fertilizer for phosphorus. J Agric Food Chem 54:4773–4779
Behnassi M, Shahid AS, D’Silva J (2011) Sustainable agricultural development. Springer, London, pp 171–184
Bergin IL, Witzmann FA (2013) Nanoparticle toxicity by the gastrointestinal route: evidence and knowledge gaps. Int J Biomed Nanosci Nanotechnol 3:1–2
Birbaum K, Brogioli R, Schellenberg M, Martinoia E, Stark WJ, Günther D, Limbach LK (2010) No evidence for cerium dioxide nanoparticle translocation in maize plants. Environ Sci Technol 44:8718–8723
Boonyanitipong P, Kositsup B, Kumar P, Baruah S, Dutta J (2011) Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oryza sativa L. Int J Biosci Biochem Bioinform 1:282
Boparai HK, Joseph M, O’Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J Hazard Mater 186:458–465
Borm PJ, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, Krutmann J (2006) The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol 3:11
Bortolin A, Aouada FA, Mattoso LH, Ribeiro C (2013) Nanocomposite PAAm/methyl cellulose/montmorillonite hydrogel: evidence of synergistic effects for the slow release of fertilizers. J Agric Food Chem 61:7431–7439
Bowman RS (2003) Applications of surfactant-modified zeolites to environmental remediation. Microporous Mesoporous Mater 61:43–56
Burklew CE, Ashlock J, Winfrey WB, Zhang B (2012) Effects of aluminum oxide nanoparticles on the growth, development, and microRNA expression of tobacco (Nicotiana tabacum). PLoS One 7:e34783
Burman U, Saini M, Kumar P (2013) Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol Environ Chem 95:605–612
Calder AJ, Dimkpa CO, McLean JE, Britt DW, Johnson W, Anderson AJ (2012) Soil components mitigate the antimicrobial effects of silver nanoparticles towards a beneficial soil bacterium, Pseudomonas chlororaphis O6. Sci Total Environ 429:215–222
Castiglione MR, Giorgetti L, Geri C, Cremonini R (2011) The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J Nanopart Res 13:2443–2449
Chhipa H (2017) Nanofertilizers and nanopesticides for agriculture. Environ Chem Lett 15:15–22
Chinnamuthu CR, Boopathi PM (2009) Nanotechnology and agroecosystem. Madras Agric J 96:17–31
Choi D, Son B, Park TH, Hong J (2015) Controlled surface functionality of magnetic nanoparticles by layer-by-layer assembled nano-films. Nanoscale 7:6703–6711
Corradini E, De Moura MR, Mattoso LHC (2010) A preliminary study of the incorporation of NPK fertilizer into chitosan nanoparticles. Express Polym Lett 4:509–515
Crosera M, Bovenzi M, Maina G, Adami G, Zanette C, Florio C, Larese FF (2009) Nanoparticle dermal absorption and toxicity: a review of the literature. Int Arch Occup Environ Health 82:1043–1055
Cui HX, Sun CJ, Liu Q, Jiang J, Gu W (2010) Applications of nanotechnology in agrochemical formulation, perspectives, challenges and strategies. In: International conference on Nanoagri, Sao pedro, Brazil, pp 28–33
Das CK, Srivastava G, Dubey A, Roy M, Jain S, Sethy NK, Saxena M, Harke S, Sarkar S, Singh SK (2016) Nano-iron pyrite seed dressing: a sustainable intervention to reduce fertilizer consumption in vegetable (beetroot, carrot), spice (fenugreek), fodder (alfalfa), and oilseed (mustard, sesamum) crops. Nanotechnol Environ Eng 1:2
Dasgupta N, Ranjan S, Rajendran B, Manickam V, Ramalingam C, Avadhani GS, Kumar A (2016) Thermal co-reduction approach to vary size of silver nanoparticle: its microbial and cellular toxicology. Environ Sci Pollut Res 23:4149–4163
Davarpanah S, Tehranifar A, Davarynejad G, Abadía J, Khorasani R (2016) Effects of foliar applications of zinc and boron nano-fertilizers on pomegranate (Punica granatum cv. Ardestani) fruit yield and quality. Sci Hortic 210:57–64
de la Rosa G, López-Moreno ML, de Haro D, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL (2013) Effects of ZnO nanoparticles in alfalfa, tomato, and cucumber at the germination stage: root development and X-ray absorption spectroscopy studies. Pure Appl Chem 85:2161–2174
Delfani M, Baradarn Firouzabadi M, Farrokhi N, Makarian H (2014) Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun Soil Sci Plant Anal 45:530–540
DeRosa MC, Monreal C, Schnitzer M, Walsh R, Sultan Y (2010) Nanotechnology in fertilizers. Nat Nanotechnol 5:91
Desa U (2008) United Nations Department of Economic and Social Affairs. Population division: world population prospects. https://population.un.org/wpp/
Dhoke SK, Mahajan P, Kamble R, Khanna A (2013). Effect of nanoparticles suspension on the growth of mung (Vigna radiata) seedlings by foliar spray method. Nanotechnol Dev 3:e1–e1
Dimkpa CO, Bindraban PS (2017) Nanofertilizers: new products for the industry? J Agric Food Chem 66:6462–6473
Disfani M, Mikhak A, Kassaee MZ, Maghari A (2017) Effects of nano Fe/SiO2 fertilizers on germination and growth of barley and maize. Arch Agron Soil Sci 63:817–826
Ditta A, Arshad M (2016) Applications and perspectives of using nanomaterials for sustainable plant nutrition. Nanotechnol Rev 5:209–229
Du W, Sun Y, Ji R, Zhu J, Wu J, Guo H (2011) TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J Environ Monit 13:822–828
Du W, Yang J, Peng Q, Liang X, Mao H (2019) Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: from toxicity and zinc biofortification. Chemosphere 227:109–116
Dubey A, Mailapalli DR (2016) Nanofertilisers, nanopesticides, nanosensors of pest and nanotoxicity in agriculture. In: Sustainable agriculture reviews. Springer, Cham, pp 307–330
Duhan JS, Kumar R, Kumar N, Kaur P, Nehra K, Duhan S (2017) Nanotechnology: the new perspective in precision agriculture. Biotechnol Rep 15:11–23
El-Temsah YS, Joner EJ (2012) Ecotoxicological effects on earthworms of fresh and aged nano-sized zero-valent iron (nZVI) in soil. Chemosphere 89:76–82
Elemike EE, Uzoh IM, Onwudiwe DC, Babalola OO (2019) The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl Sci 9:499
Elfeky SA, Mohammed MA, Khater MS, Osman YA, Elsherbini E (2013) Effect of magnetite nano-fertilizer on growth and yield of Ocimum basilicum L. Int J Indig Med Plants 46:1286–1293
Fageria NK (2009) The use of nutrıents in crop plants. CRC Press, New York
FAO (2009) How to feed the world in 2050. In: Proceedings of the expert meeting on how to feed the world in 2050. FAO Headquarters, Rome
Federici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84:415–430
Feynman R (1960) There’s plenty of room at the bottom, talk given on December 29th 1959. Sci Eng 23:22
Gautam A, Singh D, Vijayaraghavan R (2011) Dermal exposure of nanoparticles: an understanding. J Cell Tissue Res 11:2703–2708
Ghafariyan MH, Malakouti MJ, Dadpour MR, Stroeve P, Mahmoudi M (2013) Effects of magnetite nanoparticles on soybean chlorophyll. Environ Sci Technol 47:10645–10652
Ghormade V, Deshpande MV, Paknikar KM (2011) Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol Adv 29:792–803
Giorgetti L, Spanò C, Muccifora S, Bellani L, Tassi E, Bottega S, Di Gregorio S, Siracusa G, Sanità TL, Castiglione MR (2019) An integrated approach to highlight biological responses of Pisum sativum root to nano-TiO2 exposure in a biosolid-amended agricultural soil. Sci Total Environ 650:2705–2716
Gliga AR, Skoglund S, Wallinder IO, Fadeel B, Karlsson HL (2014) Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol 11:11
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327:812–818
Gogos A, Knauer K, Bucheli TD (2012) Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. J Agric Food Chem 60:9781–9792
Griffitt RJ, Weil R, Hyndman KA, Denslow ND, Powers K, Taylor D, Barber DS (2007) Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ Sci Technol 41:8178–8186
Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS (2008) Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem 27:1972–1978
Gruère GP (2012) Implications of nanotechnology growth in food and agriculture in OECD countries. Food Policy 37:191–198
Gugliotti LA, Feldheim DL, Eaton BE (2004) RNA-mediated metal-metal bond formation in the synthesis of hexagonal palladium nanoparticles. Science 304:850–852
Hagens WI, Oomen AG, de Jong WH, Cassee FR, Sips AJ (2007) What do we (need to) know about the kinetic properties of nanoparticles in the body? Regul Toxicol Pharmacol 49:217–229
Haggerty GM, Bowman RS (1994) Sorption of chromate and other inorganic anions by organo-zeolite. Environ Sci Technol 28:452–458
Hansen SF, Maynard A, Baun A, Tickner JA (2008) Late lessons from early warnings for nanotechnology. Nat Nanotechnol 3:444
Hedayati A, Shaluei F, Jahanbakhshi A (2012) Comparison of toxicity responses by water exposure to silver nanoparticles and silver salt in common carp (Cyprinus carpio). Glob Vet 8:179–184
Helaly MN, El-Metwally MA, El-Hoseiny H, Omar SA, El-Sheery NI (2014) Effect of nanoparticles on biological contamination of ‘in vitro’ cultures and organogenic regeneration of banana. Aust J Crop Sci 8:612
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil, 2nd edn. California Agricultural Experiment Station, Berkeley, p 347
Hong J, Rico CM, Zhao L, Adeleye AS, Keller AA, Peralta-Videa JR, Gardea-Torresdey JL (2015) Toxic effects of copper-based nanoparticles or compounds to lettuce (Lactuca sativa) and alfalfa (Medicago sativa). Environ Sci Process Impacts 17:177–185
Hossain ST, Mukherjee SK (2013) Toxicity of cadmium sulfide (CdS) nanoparticles against Escherichia coli and HeLa cells. J Hazard Mater 260:1073–1082
Hu CW, Li M, Cui YB, Li DS, Chen J, Yang LY (2010) Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol Biochem 42:586–591
Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A (2010) Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat Nanotechnol 5:602
Hund-Rinke K, Simon M (2006) Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environ Sci Pollut Res 13:225–232
Iavicoli I, Leso V, Fontana L, Bergamaschi A (2011) Toxicological effects of titanium dioxide nanoparticles: a review of in vitro mammalian studies. Eur Rev Med Pharmacol 15:481–508
Jani P, Halbert GW, Langridge J, Florence AT (1990) Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol 42:821–826
Jaques PA, Kim CS (2000) Measurement of total lung deposition of inhaled ultrafine particles in healthy men and women. Inhal Toxicol 12:715–731
Jiang Y, Huo S, Mizuhara T, Das R, Lee YW, Hou S, Moyano DF, Duncan B, Liang XJ, Rotello VM (2015) The interplay of size and surface functionality on the cellular uptake of sub-10 nm gold nanoparticles. ACS Nano 9:9986–9993
Johansen A, Pedersen AL, Jensen KA, Karlson U, Hansen BM, Scott-Fordsmand JJ, Winding A (2008) Effects of C60 fullerene nanoparticles on soil bacteria and protozoans. Environ Toxicol Chem 27:1895–1903
Joseph T, Morrison M (2006) Nanotechnology in agriculture and food. Nanoforum Rept 2:2–3
Jośko I, Oleszczuk P, Futa B (2014) The effect of inorganic nanoparticles (ZnO, Cr2O3, CuO and Ni) and their bulk counterparts on enzyme activities in different soils. Geoderma 232:528–537
Kale AP, Gawade SN (2016) Studies on nanoparticle induced nutrient use efficiency of fertilizer and crop productivity. Green Chem Tech Lett 2:88–92
Khan M, Siddiqui ZA (2018) Zinc oxide nanoparticles for the management of Ralstonia solanacearum, Phomopsis vexans and Meloidogyne incognita incited disease complex of eggplant. Indian Phytopathol 71:355–364
Khodakovskaya MV, De Silva K, Biris AS, Dervishi E, Villagarcia H (2012) Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 6:2128–2135
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW (2012) Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot 35:64–70
Kim S, Choi JE, Choi J, Chung KH, Park K, Yi J, Ryu DY (2009) Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol In Vitro 23:1076–1084
Kim S, Kim J, Lee I (2011) Effects of Zn and ZnO nanoparticles and Zn2+ on soil enzyme activity and bioaccumulation of Zn in Cucumis sativus. Chem Ecol 27:49–55
Kim S, Lee S, Lee I (2012) Alteration of phytotoxicity and oxidant stress potential by metal oxide nanoparticles in Cucumis sativus. Water Air Soil Pollut 223:2799–2806
Komarneni S (2010) Potential of nanotechnology in environmental soil science. In: Proceedings of the 9th International Conference of the East and Southeast, Asia federation of soil science societies, pp 16–20
Kottegoda N, Munaweera I, Madusanka N, Karunaratne V (2011) A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr Sci 101:73–78
Krishna BS, Murty DSR, Prakash BJ (2001) Surfactant-modified clay as adsorbent for chromate. Appl Clay Sci 20:65–71
Kumar V, Chopra A, Arora S, Yadav S, Kumar S, Kaur I (2015) Amperometric sensing of urea using edge activated graphene nanoplatelets. RSC Adv 5:13278–13284
Lateef A, Nazir R, Jamil N, Alam S, Shah R, Khan MN, Saleem M (2016) Synthesis and characterization of zeolite based nano–composite: an environment friendly slow release fertilizer. Microporous Mesoporous Mater 232:174–183
Lee WM, An YJ, Yoon H, Kweon HS (2008) Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environ Toxicol Chem 27:1915–1921
Lee CW, Mahendra S, Zodrow K, Li D, Tsai YC, Braam J, Alvarez PJ (2010) Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ Toxicol Chem 29:669–675
Li Z (2003) Use of surfactant-modified zeolite as fertilizer carriers to control nitrate release. Microporous Mesoporous Mater 61:181–188
Li Z, Bowman RS (1997) Counterion effects on the sorption of cationic surfactant and chromate on natural clinoptilolite. Environ Sci Technol 31:2407–2412
Li Z, Zhang Y (2010) Use of surfactant-modified zeolite to carry and slowly release sulfate. Desalin Water Treat 21:73–78
Li Z, Anghel I, Bowman RS (1998) Sorption of oxyanions by surfactant-modified zeolite. J Dispers Sci Technol 19:843–857
Lien HL, Shih YH, Yan W, Ok YS (2017) Preface: environmental nanotechnology. J Hazard Mater 322:1
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250
Lin D, Xing B (2008) Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol 42:5580–5585
Liu R, Lal R (2015) Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci Total Environ 514:131–139
Liu X, Zhang F, Zhang S, He X, Wang R, Fei Z, Wang Y (2005) Responses of peanut to nano-calcium carbonate. J Plant Nutr Soil Sci 11:385–389
Liu XM, Feng ZB, Zhang FD, Zhang SQ, He XS (2006a) Preparation and testing of cementing and coating nano-subnanocomposites of slow/controlled-release fertilizer. Agric Sci 5:700–706
Liu XM, Feng ZB, Zhang SQ, Zhang FD, Zhang JF, Xiao Q, Wang YI (2006b) Preparation and testing of cementing and coating nano-subnanocomposites of slow-or controlled-release fertilizer. Sci Agril Sin 39:1598–1604
López-Moreno ML, de la Rosa G, Hernández-Viezcas JÁ, Castillo-Michel H, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL (2010) Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ Sci Technol 44:7315–7320
Lü S, Feng C, Gao C, Wang X, Xu X, Bai X, Gao N, Liu M (2016) Multifunctional environmental smart fertilizer based on L-aspartic acid for sustained nutrient release. J Agric Food Chem 64:4965–4974
Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio MM, Catsicas S, Schwaller B, Forró L (2006) Cellular toxicity of carbon-based nanomaterials. Nano Lett 6:1121–1125
Mahajan P, Dhoke SK, Khanna AS (2011) Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J Nanotechnol 2011:696535
Mahto R, Rani P, Bhardwaj R, Singh RK, Prasad SK, Rakshit A (2021) Nanotechnology: a potential approach for abiotic stress management. In: Jogaiah S, Singh HB, Fraceto LF, de Lima R (eds) Advances in nano-fertilizers and nano-pesticides in agriculture a smart delivery system for crop improvement. Woodhead Publishing, Cambridge, pp 249–259
Malekian R, Abedi-Koupai J, Eslamian SS (2011) Influences of clinoptilolite and surfactant-modified clinoptilolite zeolite on nitrate leaching and plant growth. J Hazard Mater 185:970–976
Manik A, Subramanian KS (2014) Fabrication and characterisation of nanoporous zeolite based N fertilizer. Afr J Agric Res 9:276–284
Mattiello EM, Ruiz HA, Neves JC, Ventrella MC, Araújo WL (2015) Zinc deficiency affects physiological and anatomical characteristics in maize leaves. J Plant Physiol 183:138–143
Mazumdar H, Ahmed GU (2011) Phytotoxicity effect of Silver nanoparticles on Oryza sativa. Int J ChemTech Res 3:1494–1500
McClements DJ, Li Y (2010) Structured emulsion-based delivery systems: controlling the digestion and release of lipophilic food components. Adv Colloid Interf Sci 159:213–228
McClements DJ, Decker EA, Park Y, Weiss J (2009) Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit Rev Food Sci Nutr 49:577–606
Miller RJ, Bennett S, Keller AA, Pease S, Lenihan HS (2012) TiO2 nanoparticles are phototoxic to marine phytoplankton. PLoS One 7:e30321
Moghaddasi S, Fotovat A, Khoshgoftarmanesh AH, Karimzadeh F, Khazaei HR, Khorassani R (2017) Bioavailability of coated and uncoated ZnO nanoparticles to cucumber in soil with or without organic matter. Ecotoxicol Environ Saf 144:543–551
Mohanraj J (2013) Effect of nano-zeolite on nitrogen dynamics and green house gas emission in rice soil eco system. M. Tech. (Ag.) Thesis, TNAU, Coimbatore, India, p 307
Monreal CM, DeRosa M, Mallubhotla SC, Bindraban PS, Dimkpa C (2016) Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol Fertil Soils 52:423–437
Moschini E, Gualtieri M, Gallinotti D, Pezzolato E, Fascio U, Camatini M, Mantecca P (2010) Metal oxide nanoparticles induce cytotoxic effects on human lung epithelial cells A549. Chem Eng Trans 22:29–34
Mukherjee A, Pokhrel S, Bandyopadhyay S, Mädler L, Peralta-Videa JR, Gardea-Torresdey JL (2014) A soil mediated phyto-toxicological study of iron doped zinc oxide nanoparticles (Fe@ ZnO) in green peas (Pisum sativum L.). Chem Eng Trans 258:394–401
Musante C, White JC (2012) Toxicity of silver and copper to Cucurbita pepo: differential effects of nano and bulk-size particles. Environ Toxicol 27:510–517
Nair PMG, Chung IM (2015) The responses of germinating seedlings of green peas to copper oxide nanoparticles. Biol Plant 59:591–595
Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS (2010) Nanoparticulate material delivery to plants. Plant Sci 179:154–163
Najafi Disfani M, Mikhak A, Kassaee MZ, Maghari A (2017) Effects of nano Fe/SiO2 fertilizers on germination and growth of barley and maize. Arch Agron Soil Sci 63:817–826
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao AJ, Quigg A, Santschi PH, Sigg L (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–386
Nekrasova GF, Ushakova OS, Ermakov AE, Uimin MA, Byzov IV (2011) Effects of copper (II) ions and copper oxide nanoparticles on Elodea densa Planch. Russ J Ecol 42:458
Nguyen KC, Seligy VL, Massarsky A, Moon TW, Rippstein P, Tan J, Tayabali AF (2013) Comparison of toxicity of uncoated and coated silver nanoparticles. J Phys 429:12–25
Nielsen JB, Nielsen F, Sørensen JA (2007) Defense against dermal exposures is only skin deep: significantly increased penetration through slightly damaged skin. Arch Dermatol Res 299:423–431
NNI (2009) Nanotechnology: big things from a tiny world. The National Nanotechnology Initiative, Arlington
Noreen S, Fatima Z, Ahmad S, Ashraf M (2018) Foliar application of micronutrients in mitigating abiotic stress in crop plants. In: Plant nutrients and abiotic stress tolerance. Springer, Singapore, pp 95–117
Oberdörster E, Zhu S, Blickley TM, McClellan-Green P, Haasch ML (2006) Ecotoxicology of carbon-based engineered nanoparticles: Effects of fullerene (C60) on aquatic organisms. Carbon 44:1112–1120
Okkyoung C, Zhiqiang H (2008) Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol 42:4583–4588
Palmqvist NM, Seisenbaeva GA, Svedlindh P, Kessler VG (2017) Maghemite nanoparticles acts as nanozymes, improving growth and abiotic stress tolerance in Brassica napus. Nanoscale Res Lett 12:1–9
Panpatte DG, Jhala YK, Shelat HN, Vyas RV (2016) Nanoparticles: the next generation technology for sustainable agriculture. In: Microbial inoculants in sustainable agricultural productivity. Springer, New Delhi, pp 289–300
Park S, Lee YK, Jung M, Kim KH, Chung N, Ahn EK, Lim Y, Lee KH (2007) Cellular toxicity of various inhalable metal nanoparticles on human alveolar epithelial cells. Inhal Toxicol 19:59–65
Peteu SF, Oancea F, Sicuia OA, Constantinescu F, Dinu S (2010) Responsive polymers for crop protection. Polymers 2:229–251
Pradhan S, Patra P, Das S, Chandra S, Mitra S, Dey KK, Akbar S, Palit P, Goswami A (2013) Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata: a detailed molecular, biochemical, and biophysical study. Environ Sci Technol 47:13122–13131
Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, Sreeprasad TS, Sajanlal PR, Pradeep T (2012) Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr 35:905–927
Prasad R, Kumar V, Prasad KS (2014) Nanotechnology in sustainable agriculture: present concerns and future aspects. Afr J Biotechnol 13:705–713
Prasad R, Bhattacharyya A, Nguyen QD (2017) Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front Microbiol 8:1014
Raliya R, Tarafdar JC (2013) ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric Res 2:48–57
Raliya R, Nair R, Chavalmane S, Wang WN, Biswas P (2015) Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 7:1584–1594
Ramesh M, Palanisamy K, Babu K, Sharma NK (2014) Effects of bulk and nano-titanium dioxide and zinc oxide on physio-morphological changes in Triticum aestivum L. J Glob Biosci 3:415–422
Ranallo A (2013) Nanomaterials in fertilizer products could threaten soil health, agriculture, moratorium proposed on fertilizing fields with nanomaterials in treated sewage waste. Institute of Agriculture and Trade Policy (IATP). Press release report. https://www.iatp.org/%20fi%20les/2013_04_24_Nanomaterials_PR_0.pdf
Ranjan S, Dasgupta N, Chakraborty AR, Samuel SM, Ramalingam C, Shanker R, Kumar A (2014) Nanoscience and nanotechnologies in food industries: opportunities and research trends. J Nanopart Res 16:2464
Raskar SV, Laware SL (2014) Effect of zinc oxide nanoparticles on cytology and seed germination in onion. Int J Curr Microbiol App Sci 3:467–473
Roh JY, Sim SJ, Yi J, Park K, Chung KH, Ryu DY, Choi J (2009) Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ Sci Technol 43:3933–3940
Roh JY, Park YK, Park K, Choi J (2010) Ecotoxicological investigation of CeO2 and TiO2 nanoparticles on the soil nematode Caenorhabditis elegans using gene expression, growth, fertility, and survival as endpoints. Environ Toxicol Pharmacol 29:167–172
Rossi L, Fedenia LN, Sharifan H, Ma X, Lombardini L (2019) Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol Biochem 135:160–166
Sabir S, Arshad M, Chaudhari SK (2014) Zinc oxide nanoparticles for revolutionizing agriculture: synthesis and applications. Sci World J 2014:925494
Sedghi M, Hadi M, Toluie SG (2013) Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. AWUT-SerBio 16:73
Selva Preetha P (2011) Nano–fertilizer formulation to achieve balanced nutrition in greengram. M. Sc. (Ag.) Thesis, TNAU, Coimbatore, India
Selva Preetha P, Subramanian KS, Sharmila Rahale C (2014) Sorption characteristics of nanozeolite based slow release sulphur fertilizer. Int J Dev Res 4:225–228
Servin AD, White JC (2016) Nanotechnology in agriculture: next steps for understanding engineered nanoparticle exposure and risk. Nano 1:9–12
Shade P, Georgopoulos P (2007) Using inhalation dosimetry models to predict deposition of ultrafine particles, RRC NJDEP poster. http://ccl.rutgers.edu/ccl-files/presentations/2007-01-26_ORC-Workshop-at-DEP/ShadePamela_ORC-NJDEP_poster_2007.01.26.pdf
Shah V, Belozerova I (2009) Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut 197:143–148
Shalaby TA, Bayoumi Y, Abdalla N, Taha H, Alshaal T, Shehata S, Amer M, Domokos-Szabolcsy É, El-Ramady H (2016) Nanoparticles, soils, plants and sustainable agriculture. In: Nanoscience in food and agriculture. Springer, Cham, pp 283–312
Shang Y, Hasan M, Ahammed GJ, Li M, Yin H, Zhou J (2019) Applications of nanotechnology in plant growth and crop protection: a review. Molecules 24:2558
Sharma CP (2006) Plant micronutrients. CRC Press, Boca Raton
Sharma VK (2009) Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment—a review. J Environ Sci Health A 44:1485–1495
Sharma V, Shukla RK, Saxena N, Parmar D, Das M, Dhawan A (2009) DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol Lett 185:211–218
Shojaei TR, Salleh MAM, Tabatabaei M, Mobli H, Aghbashlo M, Rashid SA, Tan T (2019) Applications of nanotechnology and carbon nanoparticles in agriculture. In: Synthesis, technology and applications of carbon nanomaterials. Elsevier, London, pp 247–277
Singh T, Shukla S, Kumar P, Wahla V, Bajpai VK, Rather IA (2017) Application of nanotechnology in food science: perception and overview. Front Microbiol 8:1501
Srivastava G, Das A, Kusurkar TS, Roy M, Airan S, Sharma RK, Singh SK, Sarkar S, Das M (2014) Iron pyrite, a potential photovoltaic material, increases plant biomass upon seed pretreatment. Mater Express 4:23–31
Subramanian KS, Sharmila Rahale C (2012) Ball milled nanosized zeolite loaded with zinc sulfate: a putative slow release Zn fertilizer. Int J Hortic Sci 1:33–40
Subramanian KS, Sharmila Rahale C (2013) Nano-fertilizers–synthesis, characterization and application. Nanotechnology in soil science and plant nutrition. New India Publishing Agency, New Delhi
Subramanian KS, Tarafdar JC (2011) Prospects of nanotechnology in Indian farming. Indian J Agric Sci 81:887–893
Suman PR, Jain VK, Varma A (2010) Role of nanomaterials in symbiotic fungus growth enhancement. Curr Sci 99:1189–1191
Swaminathan S, Edward BS, Kurpad AV (2013) Micronutrient deficiency and cognitive and physical performance in Indian children. Eur J Clin Nutr 67:467–474
Taniguchi N (1974) On the basic concept of nano-technology. In: Proceedings of the international conference on production engineering. British Society of Precision Engineering, London
Tantawy AS, Salama YAM, El-Nemr MA, Abdel-Mawgoud AMR (2015) Nano silicon application improves salinity tolerance of sweet pepper plants. Int J ChemTech Res 8:11–17
Tarafdar JC, Raliya R, Rathore I (2012) Microbial synthesis of phosphorous nanoparticle from tri-calcium phosphate using Aspergillus tubingensis TFR-5. J Bionanosci 6:84–89
Tarafdar JC, Sharma S, Raliya R (2013) Nanotechnology: Interdisciplinary science of applications. Afr J Biotechnol 12:219–226
Tarafdar JC, Raliya R, Mahawar H, Rathore I (2014) Development of zinc nanofertilizer to enhance crop production in pearl millet (Pennisetum americanum). Agric Res 3:257–262
Taran NY, Gonchar OM, Lopatko KG, Batsmanova LM, Patyka MV, Volkogon MV (2014) The effect of colloidal solution of molybdenum nanoparticles on the microbial composition in rhizosphere of Cicer arietinum L. Nanoscale Res Lett 9:289
Thirunavukkarasu M (2014) Synthesis and evaluation of sulphur nano-fertilizer for groundnut. Doctoral dissertation, TNAU, Coimbatore, India
Tirani MM, Haghjou MM, Ismaili A (2019) Hydroponic grown tobacco plants respond to zinc oxide nanoparticles and bulk exposures by morphological, physiological and anatomical adjustments. Funct Plant Biol 46:360–375
Tripathi DK, Singh S, Singh S, Srivastava PK, Singh VP, Singh S, Prasade SM, Singh PK, Dubeya NK, Pandey AC, Chauhan DK (2017) Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol Biochem 110:167–177
Trujillo-Reyes J, Majumdar S, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL (2014) Exposure studies of core–shell Fe/Fe3O4 and Cu/CuO NPs to lettuce (Lactuca sativa) plants: are they a potential physiological and nutritional hazard? J Hazard Mater 267:255–263
Wang Y, Lin Y, Xu Y, Yin Y, Guo H, Du W (2019) Divergence in response of lettuce (var. ramosa Hort.) to copper oxide nanoparticles/microparticles as potential agricultural fertilizer. Environ Pollut Bioavailab 31:80–84
Yousefzadeh S, Sabaghnia N (2016) Nano-iron fertilizer effects on some plant traits of dragonhead (Dracocephalum moldavica L.) under different sowing densities. Acta Agric Slov 107:429–437
Yuvaraj M, Subramanian KS (2015) Controlled-release fertilizer of zinc encapsulated by a manganese hollow core shell. Soil Sci Plant Nutr 61:319–326
Zhao L, Hernandez-Viezcas JA, Peralta-Videa JR, Bandyopadhyay S, Peng B, Munoz B, Keller AA, Gardea-Torresdey JL (2013) ZnO nanoparticle fate in soil and zinc bioaccumulation in corn plants (Zea mays) influenced by alginate. Environ Sci Process Impacts 15:260–266
Zhao L, Peralta-Videa JR, Rico CM, Hernandez-Viezcas JA, Sun Y, Niu G, Servin A, Nunez JE, Gardea MD, Gardea-Torresdey JL (2014) CeO2 and ZnO nanoparticles change the nutritional qualities of cucumber (Cucumis sativus). J Agric Food Chem 62:2752–2759
Zheng L, Hong F, Lu S, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104:83–91
Zhu X, Tian S, Cai Z (2012) Toxicity assessment of iron oxide nanoparticles in zebrafish (Danio rerio) early life stages. PLoS One 7:e46286
Zulfiqar F, Navarro M, Ashraf M, Akram NA, Munné-Bosch S (2019) Nanofertilizer use for sustainable agriculture: advantages and limitations. Plant Sci 289:110270
Zuverza-Mena N, Medina-Velo IA, Barrios AC, Tan W, Peralta-Videa JR, Gardea-Torresdey JL (2015) Copper nanoparticles/compounds impact agronomic and physiological parameters in cilantro (Coriandrum sativum). Environ Sci Process Impacts 17:1783–1793
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, S.K., Patra, A., Verma, Y., Chattopadhyay, A., Rakshit, A., Kumar, S. (2021). Potential and Risk of Nanotechnology Application in Agriculture vis-à-vis Nanomicronutrient Fertilizers. In: Rakshit, A., Singh, S., Abhilash, P., Biswas, A. (eds) Soil Science: Fundamentals to Recent Advances. Springer, Singapore. https://doi.org/10.1007/978-981-16-0917-6_26
Download citation
DOI: https://doi.org/10.1007/978-981-16-0917-6_26
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0916-9
Online ISBN: 978-981-16-0917-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)