Abstract
MicroRNAs (miRNAs) are short endogenous (~ 22 nucleotides long) noncoding RNAs synthesized by RNA polymerase class II enzyme in the nucleus. The exploration of miRNAs has become an emerging field of research due to their epigenetic regulation associated with a wide array of human diseases including cholera, hepatitis, malaria, and leishmaniasis. The miRNAs that are involved in a disease can be used as a biomarker due to their upregulated or downregulated expression level. miRNAs regulate the expression of mRNA through complementary base pairing with its 3-prime untranslated regions (3′ UTRs). miRNAs are categorized according to its processes of precise formation demarcated as the canonical and noncanonical biogenic pathway. Since a huge amount of mRNA and miRNA data have been generated from the past researches, computational methods are needed to provide experimental validation with statistically significant outcomes. Computational approach is considered as one of the robust methods for miRNA target prediction.
In this chapter, we have focused our discussion on different miRNA prediction tools and their features in detail along with its role in infectious diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The world of RNA and its species has widened the scope of scientific research after the two decades of the discovery of various subtypes of nonprotein-coding RNAs (ncRNAs) [1, 2]; long nonprotein-coding RNAs (lncRNAs) [3, 4], small interfering RNAs (siRNAs) [5, 6], piwiRNA [7], circular RNAs (circRNAs) [8], or microRNAs (miRNAs) [9, 10]. miRNA was discovered in 1993 in a nematode worm, Caenorhabditis elegans [11]. Since then, it has been reported in viruses, single-celled eukaryotes, plants, and animals. miRNAs are 22–25-nucleotide-long endogenous noncoding RNAs. It regulates several biological processes through controlling the expression of target genes [12, 13]. Recently, the exploration of miRNAs has become pivotal for the detailed understanding of the alteration of genes in many human diseases [13]. miRNAs have been studied in context with several diseases including infectious as well as noninfectious [14,15,16].
A lot of research has been done in recent years to infer the miRNA targets and their functions which resulted in a huge amount of mRNA and miRNA data; therefore, computational methods provide experimental validation with statistically significant outcomes.
2 Formation of miRNA
miRNAs are synthesized by RNA polymerase class II enzyme in the cell nucleus. Initially, these are formed as a long primary transcript called pri-miRNA. These are formed either from the intronic regions of coding genes or from their noncoding regions of the genome. After its formation, pri-miRNA folds into a hairpin structure. miRNAs are categorized by precise formation processes demarcated as the canonical and noncanonical biogenic pathway (Fig. 14.1). Mostly pri-miRNA follows canonical biogenic pathway distinguished by double processing through the enzymes, namely, Drosha and Dicer, of the ribonuclease III (RNase III) family.
Drosha forms a microprocessor complex with DGCR8 in the nucleus [17,18,19]. This complex cuts the pri-miRNA and forms pre-miRNA. Exportin-5 helps in the transportation of pre-miRNA to the cytoplasm. Further, Dicer carries out the second cleavage of the pre-miRNA in the cytoplasm and forms ~21-nucleotide-long miRNA duplex with 2 nucleotide 3′ overhang [19]. One of the mature strands from the duplex is laden onto an Argonaute (AGO) protein-associated RNA-induced silencing complex (RISC). This multiprotein complex leads to the formation of the effector complex in the cytoplasm. RISC uses miRNA as an antisense strand to recognize and regulate the specific targets. It is also reported that transposable elements are also involved in the miRNA formation [20]. Unlike animals, in plants, miRNA becomes fully mature in the nucleus [21, 22]. Recently, other processes of miRNA biogenesis have been categorized as noncanonical biogenic pathways consisting of Dicer-independent and Drosha-independent mechanism as explained in Fig. 14.1 [23,24,25,26,27,28,29,30,31].
3 Targets of miRNAs
miRNA regulates the expression of mRNA through binding with the 3′ untranslated regions (UTRs) of mRNA through any of the two binding patterns as mentioned below [32]:
-
(a)
In first class, two to seven nucleotides from the 5′ end of the miRNAs have complete Watson-Crick pairing to the 3′ UTR of the mRNA. These nucleotide sequences are known as the seed region. Depending on the number of nucleotides involved in the base pairing, the seed regions are known as 6mer, 7mer, and 8mer accordingly.
-
(b)
While in another class of mRNA expression regulation by miRNA, there is partial base pairing formed in the seed region, but to overcome this insufficient binding, there are some base pairings found in the 3′ side of the miRNA too.
A single mRNA may be regulated by multiple miRNAs and vice versa. Even one miRNA may influence the expression of other miRNAs, and due to this reason, regulatory mechanism becomes more complex.
4 Functions of miRNAs
miRNAs have a significant role in the modulation of gene expression in animals and in plants. In animals, miRNAs play important functions at various physiological and evolutionarily conserved developmental stages. miRNAs mostly show partial complementarity with the specific mRNAs in animals; however, it is adequate to regulate diverse physiological processes at the initiation step accompanied by the degeneration of mRNA [33]. In animals, miRNAs bind partially with the 3′ UTR regulatory elements of the transcript and do not affect the functions of 5′ UTR and target sites [34]. miRNAs affect the stability of the specific mRNAs at the transcriptional stage [35]. According to few studies, miRNAs can enhance translation in some specific cell types under certain conditions [36]. mRNAs seem to be inhibiting miRNAs from regulating the RNAs also. Like animals, plant miRNAs have shown key roles in the facilitation of organ maintenance at several developmental stages. miRNAs in plants have complementary regions to the specific mRNAs. In Arabidopsis thaliana, it has been shown that these regulates negatively also and may be due to their binding to the nonspecific targets in other noncoding RNAs [37].
5 miRNAs in Infectious Diseases
Different miRNAs are involved in a wide array of diseases, and the levels of miRNAs are either upregulated or downregulated based upon the disease. However, our focus centers around the miRNAs involved in the infectious diseases and their expression profiles. miR-93-5p belongs to the miR-106-25 cluster which plays a crucial role in cancer development. HCV-1b core protein enhances the expression of miR-93-5p in Huh7 cells. IFNAR1 is a direct target of miR-93-5p, and upregulation of miR-93-5p inhibits IFN signaling pathway by reducing the phosphorylation status of STAT1, a transcription factor involved in IFN signaling [38]. During HBV, infection level of miR-29 is upregulated in HCC cell line HepG2.2.15. SMARCE1 is a target of miR-29a; an inhibition of SMARCE1 increased the HBV replication and expression [39]. The hepatic miR-122 level is reduced in HCV-infected cultured hepatocytes, and expression of NF-κB-inducing kinase (NIK) is induced [40].
miR-146a plays an important role in immunomodulation of the host response during Vibrio cholerae infection. V. cholerae bacteria releases outer membrane vesicles which upregulate miR-146a. Upregulation of miR-146a allows the pathogen to colonize inside the host due to the reduced strength of an epithelial innate immune defense reaction [41]. miR-155 is another important miRNA that is induced by the V. cholerae to limit the host immune response, thereby reducing the probability of being eliminated. The miR-155 is upregulated in V. cholerae infection [42].
miR-155 acts as a negative regulator of endothelial and blood-brain barrier integrity during severe malaria. Upregulation of miR-155 is associated with increased endothelial activation and blood-brain barrier breakdown [43]. Severe malaria with multiorgan failure includes different miRNAs. Mouse with cerebral malaria shows upregulation of miR-27a, miR-150, and let7i levels in brain tissue compared to a mouse with no cerebral malaria [44]. miR-451 is a negative regulator of the host immune responses to Plasmodium infection. Downregulation of miR-451 induces pathogen clearance of Plasmodium vivax by CD4+ T cells [45]. Levels of plasma miR-451 and miR-16 were significantly downregulated in Plasmodium vivax infection, and these miRNAs can be exploited as biomarkers for malaria infection [46].
Autophagy happens to be one of the key events in the survival mechanism of Leishmania parasite under a stressed condition. The autophagy-related gene BECN1 is a key autophagy-promoting gene. Upon L. donovani infection, the miR-30 family member, miR-30A-3p, modulates the autophagy by targeting BECN1. miR-30A-3p targets BECN1 and decreases its level of expression resulting in decreased autophagic activity and finally parasite elimination [47]. miR-122 is expressed abundantly in the liver and modulates a wide range of liver functions. The L. donovani metalloprotease gp63 targets the DICER1 in human hepatocytes to reduce miR-122 expression [48]. Dendritic cells and macrophages infected with L. donovani show upregulation of let-7a and let-7b miRNAs, while these miRNAs are downregulated in L. major-infected cells. Suppressor of cytokine signaling 4 (SOCS4), a negative regulator of JAK-STAT signaling, plays the role in the regulation of miRNA expression [49]. L. donovani significantly downregulates the expression of miR-494 in THP-1-differentiated human macrophages. L. donovani metalloprotease gp63 degrades c-Jun and specifically upregulates the expression of Rab5a to downregulate miR-494 [50].
6 Fundamental Features Considered for miRNA Target Prediction
miRNAs target the complementary sequences generally present in the 3′ UTR of mRNA. miRNA reduces the translation of mRNA to prevent protein synthesis. There are several computational methods which provide support to predict the possibilities and specificity of a miRNA binding to an mRNA. miRNA target prediction tools are based on a few common features [51], which are the basic features that are often implemented to develop algorithms for the miRNA tools. These features are described as follows.
6.1 Seedmatch
The initial two to eight nucleotides from 5′ end to 3′ end are called as seed sequence. Generally, miRNA target prediction tools are based on the Watson-Crick base pairing between the seed region of miRNA and mRNA. An ideal seed match has no gap in alignment of this region. There are different types of seed sequences that are used in the target prediction tool of miRNA [52]. Commonly used seed matching in miRNA tools are mentioned below:
-
1.
6-mer: It includes the complementary pairing between the second nucleotide of the 5′ side of the miRNA to the sixth nucleotide with the 3′ UTR of the miRNA.
-
2.
7-mer-m8: This is an ideal seed match between the second nucleotide from the 5′ side of the miRNA to the eighth nucleotide with the 3′ UTR of the miRNA
-
3.
7mer-A1: This also is a perfect seed match between the second nucleotide from the 5′ side of the miRNA to the seventh nucleotide with the 3′ UTR of the miRNA in addition to an A across the miRNA first nucleotide.
-
4.
8-mer: This type of seed match is also a perfect seed match between the second nucleotide from the 5′ side of the miRNA to the eighth nucleotide with the 3′ UTR of the miRNA in addition to an A across the miRNA first nucleotide.
6.2 Conservation
Conservation is defined as the measure of the sequence conserved throughout the different species. The functionality of an identified miRNA target is predicted by conservation analysis [51]. This feature is considered for the prediction of miRNA, 3′ UTR, and 5′ UTR and the combination of these three. The seed region in miRNA is considered to be more conserved than any other regions. The 3′-compensatory sites are a small portion of miRNA which has conserved pairing and networks with the target mRNA to compensate the mismatched seed [53].
6.3 Free Energy
The stability of a particular interaction-complex is defined by Gibb’s free energy and is calculated as the variation in free energy change (ΔG). The value of ΔG suggests the stability of a reaction. A larger value of negative ΔG signifies greater stability of the reaction. miRNA binding with a target mRNA leads to the formation of either a stable structure or an unstable one. Formation of most stable interaction-complex is the root base of prediction of the likely target of miRNA.
6.4 Site Accessibility
Site accessibility is the degree of easiness through which a miRNA may work out its target mRNA and interacts with it. Interaction between miRNA and its target mRNA takes place in two steps. First, miRNA interacts with a short accessible part of mRNA, and then unfolding of mRNA takes place. After unfolding of mRNA, binding of miRNA occurs with it [54]. The amount of energy required for making a site in the mRNA accessible for binding of miRNA is required for the determination of site accessibility. Therefore, site accessibility is one of the most common features to find the appropriate target of a miRNA.
6.5 Other Features
There are some other features including target-site abundance, local AU content, GU wobble seed match, 3′-compensatory pairing, seed pairing stability, and position contribution that are often considered in miRNA target prediction algorithms.
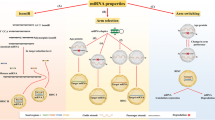
7 Common Tools for miRNA Target Prediction
There are many prediction tools reported for the prediction of the miRNA targets. These all are based on the above-discussed features. Some of the tools are discussed in this section (Fig. 14.2) [55].
7.1 miRanda
It is a target prediction tool of miRNAs in a genome. The algorithm of this tool had been developed in C language. It was developed by the Computational Biology Center, at Memorial Sloan Kettering Cancer Center, USA, and is freely available online. Its algorithm works in the following three steps:
-
1.
A miRNA sequence is entered as an input and is searched for the WC matches against the 3′ UTR provided by the user.
-
2.
The free energy is determined for every miRNA-mRNA target pair, only when the pair surpasses a threshold that matches the score.
-
3.
It applies the conservation analysis and is based on either the score or by having many sites.
7.2 miRanda-mirSVR
It is an online tool that recognizes candidate target sites and then scores them. SVR stands for super vector regression that is much similar to SVM (support vector machine). It comprises the study of AU flanking, site accessibility, and target-site position in the 3′ UTR region. It also offers the miRNA expression analysis and links to the miRBase and miRO [56].
7.3 TargetScan
TargetScan prediction tool permits the operator to search the target by the name of the gene. The targets are predicted by calculating the predicted effectiveness of targeting, i.e., context + score, or by calculating the likelihood of conserved targeting, known as PCT. PCT is the probability of a target being targeted efficiently. No sequence is required to be input for the prediction. For conservation analysis, 3′ UTR conservation is evaluated, and then specific k-mer is analyzed [57].
7.4 DIANA-microT-CDS
It is the latest version of DlANA-microT, first target prediction tool in human [58]. It incorporates data in a way to identify the most related features retrieved from photoactivatable ribonucleoside-enhanced cross-linking and immunoprecipitation (PAR-CLIP) that allows this tool to get an idea about the position of miRNA interaction in the coding sequences and in 3′ UTR region. There are numerous sites in a target that are recognized using microarray expression data. It gives the predicted target location, conservation, score, and external links to miRBase, Ensembl, and PubMed [58].
7.5 MirTarget2
MirTarget2 uses SVM and incorporates a large microarray training dataset in the target search. Its algorithm finds out the seed conservation, base content in the flanking regions, seed match in positions of 2–8 nucleotides, the region of target site in 3′ UTR, and secondary structure [59, 60]. It has a disadvantage that it includes 3′ UTR sequences as a training dataset with only one seed pairing site rather than many sites. It is comprehensible, and miRNA-mRNA interactions can be investigated by either mRNA or miRNA. Since there may be multiple target sites of miRNA in the target mRNA, therefore, there is a target running option that helps to select some specific target sites [59, 60].
7.6 RNA22-GUI
RNA22-GUI is the latest version of RNA22 [61, 62]. It identifies the target islands using pattern discovery and calculation of free energy of these islands and then finally predicts the candidate miRNA [63].
7.7 TargetMiner
TargetMiner identifies the seed sites between the user-provided miRNA and mRNA using an SVM-based classifier. This tool incorporates negative and positive training data to predict more precise seed matches in miRNA and the mRNA [64].
7.8 SVMicrO
SVMicrO is also a machine learning tool that includes a large positive dataset of different species [65]. It identifies the miRNA-miRNA interactions involving features of the miRNA target prediction including seed match, site accessibility, conservation, target-site abundance, and free energy [65].
7.9 Probability of Interaction by Target Accessibility (PITA)
PITA predicts the miRNA target using the target accessibility as a major feature [66]. It is dependent on the key observation that highly accessible regions of the 3′ UTR of mRNAs have privileged and conserved positioning of target sites. This tool first recognizes a probable site through seed match measures. Then it considers site availability by calculating the free energy score depending on the difference between the gain of free energy linked with miRNA-mRNA hybrid target structure and the free energy requirement of dissociating the target to render it reachable. Further, to determine the interaction score for the microRNA and 3′ UTR, the target-site abundance is considered which is computed through accessibility scores of the same miRNA [66].
7.10 RNAhybrid
RNAhybrid has a user-defined seed region and calculates the free energy between miRNA and mRNA. It predicts the target sites by assigning a p-value to the miRNA-mRNA interaction sites [67].
Among all the above-discussed tools miRanda is the most widely accepted tool for miRNA target prediction and also according to the ease of use [51, 68, 69]. But other tools such as RNA22 and GUI offer a graphical representation of the interactions between the miRNA and the mRNA.
References
Ghildiyal M, Zamore PD (2009) Small silencing RNAs: an expanding universe. Nat Rev Genet 10:94–108
Rueda A, Barturen G, Lebrón R, Gómez-Martín C, Alganza Á, Oliver JL, Hackenberg M (2015) sRNAtoolbox: an integrated collection of small RNA research tools. Nucleic Acids Res 43:W467–W473
Khvorova A, Reynolds A, Jayasena SD (2003) Functional siRNAs and miRNAs exhibit strand bias. Cell 115:209–216
Ro S, Park C, Young D, Sanders KM, Yan W (2007) Tissue-dependent paired expression of miRNAs. Nucleic Acids Res 35:5944–5953
Ning S, Zhang J, Wang P, Zhi H, Wang J, Liu Y, Gao Y, Guo M, Yue M, Wang L, Li X (2016) Lnc2Cancer: a manually curated database of experimentally supported lncRNAs associated with various human cancers. Nucleic Acids Res 44:D980–D985
Spielmann N, Wong DT (2011) Saliva: diagnostics and therapeutic perspectives. Oral Dis 17:345–354
Flintoft L (2013) Non-coding RNA: structure and function for lncRNAs. Nat Rev Genet 14:598
Mittal V (2004) Improving the efficiency of RNA interference in mammals. Nat Rev Genet 5:355–365
Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S (2014) circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 56:55–66
Bahn JH, Zhang Q, Li F, Chan TM, Lin X, Kim Y, Wong DT, Xiao X (2015) The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin Chem 61:221–230
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843–854
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5:522–531
Liu ZP, Wu H, Zhu J, Miao H (2014) Systematic identification of transcriptional and post-transcriptional regulations in human respiratory epithelial cells during influenza a virus infection. BMC Bioinform 15:336
Brase JC, Johannes M, Schlomm T, Fälth M, Haese A, Steuber T, Beissbarth T, Kuner R, Sültmann H (2011) Circulating miRNAs are correlated with tumor progression in prostate cancer. Int J Cancer 128:608–616
Srivastava K, Srivastava A (2012) Comprehensive review of genetic association studies and meta-analyses on miRNA polymorphisms and cancer risk. PLoS One 7:e50966
Yang H, Kong W, He L, Zhao JJ, O'Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ (2008) MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res 68:425–433
Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ (2004) Processing of primary microRNAs by the microprocessor complex. Nature 432:231–235
Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R (2004) The microprocessor complex mediates the genesis of microRNAs. Nature 432:235–240
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, Kim VN (2003) The nuclear RNase III Drosha initiates microRNA processing. Nature 425:415–419
Smalheiser NR, Torvik VI (2005) Mammalian microRNAs derived from genomic repeats. Trends Genet 21:322–326
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Park MY, Wu G, Gonzalez-Sulser A, Vaucheret H, Poethig RS (2005) Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci U S A 102:3691–3696
Babiarz JE, Ruby JG, Wang Y, Bartel DP, Blelloch R (2008) Mouse ES cells express endogenous shRNAs, siRNAs, and other microprocessor-independent, dicer-dependent small RNAs. Genes Dev 22:2773–2785
Berezikov E, Chung WJ, Willis J, Cuppen E, Lai EC (2007) Mammalian mirtron genes. Mol Cell 28:328–336
Cheloufi S, Dos Santos CO, Chong MM, Hannon GJ (2010) A dicer-independent miRNA biogenesis pathway that requires ago catalysis. Nature 465:584–589
Cifuentes D, Xue H, Taylor DW, Patnode H, Mishima Y, Cheloufi S, Ma E, Mane S, Hannon GJ, Lawson ND, Wolfe SA, Giraldez AJ (2010) A novel miRNA processing pathway independent of dicer requires Argonaute2 catalytic activity. Science 328:1694–1698
Flynt AS, Greimann JC, Chung WJ, Lima CD, Lai EC (2010) MicroRNA biogenesis via splicing and exosome-mediated trimming in Drosophila. Mol Cell 38:900–907
Okamura K, Hagen JW, Duan H, Tyler DM, Lai EC (2007) The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell 130:89–100
Okamura K, Lai EC (2008) Endogenous small interfering RNAs in animals. Nat Rev Mol Cell Biol 9:673–678
Ruby JG, Jan CH, Bartel DP (2007) Intronic microRNA precursors that bypass Drosha processing. Nature 448:83–86
Yang JS, Lai EC (2011) Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell 43:892–903
Saito T, Saetrom P (2010) MicroRNAs--targeting and target prediction. New Biotechnol 27:243–249
Kato M, Slack FJ (2008) microRNAs: small molecules with big roles – C. elegans to human cancer. Biol Cell 100:71–81
Guo H, Ingolia NT, Weissman JS, Bartel DP (2010) Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466:835–840
Fabian MR, Sonenberg N, Filipowicz W (2010) Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 79:351–379
Vasudevan S, Tong Y, Steitz JA (2007) Switching from repression to activation: microRNAs can up-regulate translation. Science 318:1931–1934
Chitwood DH, Timmermans MC (2007) Target mimics modulate miRNAs. Nat Genet 39:935–936
He CL, Liu M, Tan ZX, Hu YJ, Zhang QY, Kuang XM, Kong WL, Mao Q (2018) Hepatitis C virus core protein-induced miR-93-5p up-regulation inhibits interferon signaling pathway by targeting IFNAR1. World J Gastroenterol 24:226–236
Wu HJ, Zhuo Y, Zhou YC, Wang XW, Wang YP, Si CY, Wang XH (2017) miR-29a promotes hepatitis B virus replication and expression by targeting SMARCE1 in hepatoma carcinoma. World J Gastroenterol 23:4569–4578
Lowey B, Hertz L, Chiu S, Valdez K, Li Q, Liang TJ (2019) Hepatitis C virus infection induces hepatic expression of NF-κB-inducing kinase and lipogenesis by downregulating miR-122. mBio 10:e01617-19
Bitar A, Aung KM, Wai SN, Hammarström ML (2019) Vibrio cholerae derived outer membrane vesicles modulate the inflammatory response of human intestinal epithelial cells by inducing microRNA-146a. Sci Rep 9:7212
Bitar A, De R, Melgar S, Aung KM, Rahman A, Qadri F, Wai SN, Shirin T, Hammarström ML (2017) Induction of immunomodulatory miR-146a and miR-155 in small intestinal epithelium of Vibrio cholerae infected patients at acute stage of cholera. PLoS One 12:e0173817
Barker KR, Lu Z, Kim H, Zheng Y, Chen J, Conroy AL, Hawkes M, Cheng HS, Njock MS, Fish JE, Harlan JM, López JA, Liles WC, Kain KC (2017) miR-155 modifies inflammation, endothelial activation and blood-brain barrier dysfunction in cerebral malaria. Mol Med 23:24–33
Chamnanchanunt S, Fucharoen S, Umemura T (2017) Circulating microRNAs in malaria infection: bench to bedside. Malar J 16:334
Chapman LM, Ture SK, Field DJ, Morrell CN (2017) miR-451 limits CD4(+) T cell proliferative responses to infection in mice. Immunol Res 65:828–840
Chamnanchanunt S, Kuroki C, Desakorn V, Enomoto M, Thanachartwet V, Sahassananda D, Sattabongkot J, Jenwithisuk R, Fucharoen S, Svasti S, Umemura T (2015) Downregulation of plasma miR-451 and miR-16 in Plasmodium vivax infection. Exp Parasitol 155:19–25
Singh AK, Pandey RK, Shaha C, Madhubala R (2016) MicroRNA expression profiling of Leishmania donovani-infected host cells uncovers the regulatory role of MIR30A-3p in host autophagy. Autophagy 12:1817–1831
Ghosh J, Bose M, Roy S, Bhattacharyya SN (2013) Leishmania donovani targets Dicer1 to downregulate miR-122, lower serum cholesterol, and facilitate murine liver infection. Cell Host Microbe 13:277–288
Geraci NS, Tan JC, McDowell MA (2015) Characterization of microRNA expression profiles in Leishmania-infected human phagocytes. Parasite Immunol 37:43–51
Verma JK, Rastogi R, Mukhopadhyay A (2017) Leishmania donovani resides in modified early endosomes by upregulating Rab5a expression via the downregulation of miR-494. PLoS Pathog 13:e1006459
Peterson SM, Thompson JA, Ufkin ML, Sathyanarayana P, Liaw L, Congdon CB (2014) Common features of microRNA target prediction tools. Front Genet 5:23
Akhtar MM, Micolucci L, Islam MS, Olivieri F, Procopio AD (2019) A practical guide to miRNA target prediction. Methods Mol Biol 1970:1–13
Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105
Long D, Lee R, Williams P, Chan CY, Ambros V, Ding Y (2007) Potent effect of target structure on microRNA function. Nat Struct Mol Biol 14:287–294
John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS (2004) Human MicroRNA targets. PLoS Biol 2:e363
Betel D, Koppal A, Agius P, Sander C, Leslie C (2010) Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol 11:R90
Agarwal V, Bell GW, Nam JW, Bartel DP (2015) Predicting effective microRNA target sites in mammalian mRNAs. elife 4:e05005
Paraskevopoulou MD, Georgakilas G, Kostoulas N, Vlachos IS, Vergoulis T, Reczko M, Filippidis C, Dalamagas T, Hatzigeorgiou AG (2013) DIANA-microT web server v5.0: service integration into miRNA functional analysis workflows. Nucleic Acids Res 41:W169–W173
Wang X (2008) miRDB: a microRNA target prediction and functional annotation database with a wiki interface. RNA 14:1012–1017
Wang X, El Naqa IM (2008) Prediction of both conserved and nonconserved microRNA targets in animals. Bioinformatics 24:325–332
Hofacker IL, Fontana W, Stadler PF, Bonhoeffer LS, Tacker M, Schuster P (1994) Fast folding and comparison of RNA secondary structures. Monatshefte für Chemie/Chemical Monthly 125:167–188
Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, Lim B, Rigoutsos I (2006) A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell 126:1203–1217
Loher P, Rigoutsos I (2012) Interactive exploration of RNA22 microRNA target predictions. Bioinformatics 28:3322–3323
Bandyopadhyay S, Mitra R (2009) TargetMiner: microRNA target prediction with systematic identification of tissue-specific negative examples. Bioinformatics 25:2625–2631
Liu H, Yue D, Chen Y, Gao SJ, Huang Y (2010) Improving performance of mammalian microRNA target prediction. BMC Bioinform 11:476
Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E (2007) The role of site accessibility in microRNA target recognition. Nat Genet 39:1278–1284
Krüger J, Rehmsmeier M (2006) RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res 34:W451–W454
Akhtar MM, Micolucci L, Islam MS, Olivieri F, Procopio AD (2016) Bioinformatic tools for microRNA dissection. Nucleic Acids Res 44:24–44
Faiza M, Tanveer K, Fatihi S, Wang Y, Raza K (2019) Comprehensive overview and assessment of microRNA target prediction tools in homo sapiens and drosophila melanogaster. Curr Bioinforma 14:432–445
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rahman, F., Akand, S.K., Faiza, M., Tabrez, S., Rub, A. (2021). miRNA Target Prediction: Overview and Applications. In: Hameed, S., Fatima, Z. (eds) Integrated Omics Approaches to Infectious Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-16-0691-5_14
Download citation
DOI: https://doi.org/10.1007/978-981-16-0691-5_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0690-8
Online ISBN: 978-981-16-0691-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)