Abstract
It is known that visual attention can modulate firing patterns of neurons in visual cortexes and play important functional roles in visual information processes. Despite of several decades of studies, mechanisms of visual attention still remain unclear. Recent neurophysiological studies reported that attention could increase NMDA and AMPA synaptic strength. To understand how neuronal activity is modulated by attention at the synaptic level, we proposed a three-layer neural network in this study. Each neuron receives AMPA and NMDA currents and also GABA currents. In the synapse between every two neurons, neurotransmitters are stochastically bound with receptors in post-synaptic membranes. In the model, it is hypothesized that attention could make the binding process less stochastic, and more neurotransmitters are bound with postsynaptic receptors. Our simulation showed that attention resulted neurons in the model had stronger firing rates and less response variability. We also found that attention had stronger modulation of neuronal activity in higher layers than in lower layers. Inhibitory neurons had stronger attention effects than did excitatory neurons. Overall, our results demonstrated that attention may modulate neuronal activity by controlling the stochastic binding process.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Visual attention has crucial roles in the process of visual information. It could selectively enhance neuronal responses to particular visual stimuli (Reynolds et al. 1999; Treue and Maunsell 1999). Some studies have reported that single neurons could increase its firing rate and reduce its response variability when attention is in work (Anton-Erxleben and Carrasco 2013). It was also found that neuronal activities in higher visual areas are modulated more strongly by attention than those in lower visual areas, and putative interneurons had stronger attention modulation than did putative pyramidal cells (Thiele et al. 2016). A recent experiment further reported that attention increased the efficacy of neuronal communication between neurons, and a presynaptic input could more easily to drive a response of a postsynaptic neuron (Briggs et al. 2013). These experimental observations imply that attention may affect information transmission between neurons at the synaptic level, which thereby could change response patterns of neurons in various cortical regions. It is essential to investigate the underlying mechanism how attention modulates the efficiency of neural signal transmission.
There are lots of proposed computational models to reveal attentional modulation on neuronal activities (Lanyon and Denham 2009). The detail methods to model attentional effects are different in these models. Some models have neurons and their connected microcircuits with detailed biophysical information (Wagatsuma et al. 2013). Some models are constructed with abstract spiking neurons (Itti and Koch 2000) or dynamic rate coded populations (Beuth and Hamker 2015). Some models only have abstract mathematical descriptions (Reynolds and Heeger 2009). However, these models have some limitations to analyze synaptic information transmission modulated by attention. Therefore, the purpose of this study is to reveal how attention controls the binding processing of neurotransmitters to modulate information transmission between neurons.
To investigate this issue, a three-layer network model was proposed to study attentional modulations on neural activities. Each layer has excitatory and inhibitory neurons. Each neuron receives AMPA, NMDA, and GABA currents. Neurotransmitters are released, transmitted in the synaptic cleft, and finally are bound to receptors in the postsynaptic membrane. This binding process is considered as a random process (Dobrunz and Stevens 1997). It is hypothesized that the randomness in the binding process of neurotransmitters with receptors becomes smaller by attention. In effect, attention enables more neurotransmitters to be bound with receptors in the postsynaptic membrane.
The model could simulate attentional modulation on firing rates and response variability. The simulated results are consistent with observations found in experiments. Our model demonstrates that attention could control neurotransmitters transmission in the synapse, which further generates attention-modulated activity patterns.
2 Methods and Materials
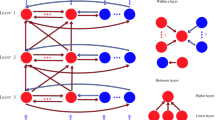
As shown in Fig. 1, a three-layer network is prosed to simulate functions of visual attention. The first layer from the input is labeled as layer 1, the middle layer is labeled as layer 2, and the output layer is labeled as layer 3. There are 20 excitatory and 5 inhibitory neurons in each layer. The connections within a layer or between layers are described as following. Each excitatory neuron has inputs from five inhibitory neurons and inputs from five excitatory neurons selected randomly; one inhibitory neuron receives projections from one randomly selected excitatory neuron. An excitatory neuron in the current layer receives inputs from three randomly selected excitatory neurons in the previous layer. Excitatory neurons transmit information from one layer to next layer. Inhibitory neurons do not relay signals between layers. No signals are feedback from higher layers to lower layers. A sustained external current is inputted to all neurons in layer 1, and there are no external inputs to neurons in other layers.
The Hodgkin-Huxley model of single neurons is used in this study. This single-neuron model could describe in detail spike shapes and properties of excitatory and inhibitory neurons, respectively.
The membrane potential of an excitatory neuron (e) is the following:
The membrane potential of an inhibitory neuron is the following:
In the above equations, \( I_{\text{AMPA}} \) indicates the AMPA current. It is then described by the following equation:
\( I_{\text{NMDA}} \) is the NMDA current. Its dynamic properties is described by the following equation:
The \( {\text{GABA}}_{\text{A}} \) current is given by the following equation:
The kinetics of the ratio of bound receptors r is characterized by the following equation:
where \( \left[ T \right] \) is the concentration of neurotransmitters.
The binding process of neurotransmitter with receptors is stochastic, which was simulated by one stochastic variable shown in the following equation:
where \( \chi_{i} \left( {\text{t}} \right) \) is the Gaussian white noise of zero mean and unit standard deviation, and \( r_{{{\text{noise}}_{ 0} }}^{i} , \sigma_{i} \) are the average and standard deviation of the random sequence (\( i = {\text{AMPA, NMDA, GABA}}_{\text{A}} \)), respectively. \( \tau \) is the time constant.
In Eq. (7), the parameter \( \sigma_{i} \) could control the standard deviation of the stochastic process. This stochastic variable in Eq. (7) is then added to \( r_{i} \) to mimic randomness in the neurotransmitter binding process. Because we hypothesize that randomness in this bind process could be reduced by attention, the parameters of \( \sigma_{\text{AMPA}} \) and \( \sigma_{\text{NMDA}} \) are set lager values when attention is unattended (more randomness). And their values become smaller when attention is attended (less randomness). In this study, we do not take the attentional effect on \( {\text{GABA}}_{\text{A}} \) receptors into account, so the parameter of \( \sigma_{{{\text{GABA}}_{\text{A}} }} \) is set a constant regardless of attention conditions.
3 Results
During simulation, because the binding process was stochastic, the activity pattern of each neuron varied trial by trial even though the external current to the neurons in layer 1 was constant. We calculated neuronal activity in 48 trials for each condition. In each condition, all parameters were set as the same values for each trial, but the variable in Eq. (7) varies trial by trial. We then averaged firing rates and computed response variability for each neuron in the model across these 48 trials.
It was found that the mean firing rate of all neurons decreased when the \( \sigma_{\text{AMPA}} \) increased. \( \sigma_{\text{AMPA}} \) was set different values to indicate different attention conditions. For example, in AMPA channels connecting two excitatory neurons, \( \sigma_{\text{AMPA}} = 0.075 \) in the attention-attended condition and \( \sigma_{\text{AMPA}} = 0.08 \) in the attention-unattended condition. In AMPA channels projecting from excitatory cells to inhibitory cells, \( \sigma_{\text{AMPA}} = 0.075 \) in the attended condition and \( \sigma_{\text{AMPA}} = 0.09 \) in the unattended condition. These specific values were chosen for the two attention conditions. The model with these values could simulate results that were similar to experimental observations.
We did not find that attention significantly alters firing rates of neurons in layer 1 due to the large external current to each neuron (excitatory cells: p = 0.107; inhibitory cells: p = 0.111, Mann-Whitney U-test between the two attentional conditions for each type of cells). But firing rates of both excitatory and inhibitory cells in layer 2 and 3 showed significant differences between the two attentional conditions (p < 0.01, Mann-Whitney U-test).
The Fano factor in layer 1 was very small, indicating that these neurons had stable firing patterns. The Fano factor was not modulated by attention for either excitatory or inhibitory cells (excitatory cells: p = 0.199; inhibitory cells: p = 0.548, Mann-Whitney U-test). In layer 2, Fano factors of excitatory cells had significantly smaller values in the attention-attended condition than in the unattended condition (p < 0.01, Mann-Whitney U-test). Fano factors of inhibitory cells did not affect by attention (p = 0.095, Mann-Whitney U-test). Only five inhibitory cells in layer 2 may not have enough power to reach statistical significance. Fano factors of both excitatory and inhibitory cells in layer 3 were modulated by attention, showing smaller values in the attention-attended condition (excitatory cells: p = 0.019; inhibitory cells: p < 0.01; Mann-Whitney U-test between the two attention conditions for each type of cells).
The randomness in NMDA channels (controlled by \( \sigma_{\text{NMDA}} \)) also modulated firing patterns of neurons in layer 2 and layer 3. The firing rate became smaller when the value of \( \sigma_{\text{NMDA}} \) became larger. Different values were selected for \( \sigma_{\text{NMDA}} \) to indicate different attentional conditions. We chose \( \sigma_{\text{NMDA}} = 0.12 \) in the attention-unattended condition and \( \sigma_{\text{NMDA}} = 0.04 \) in the attended condition for NMDA channels connecting two excitatory cells. \( \sigma_{\text{NMDA}} = 0.16 \) (unattended) and \( \sigma_{\text{NMDA}} = 0.04 \) (attended) were selected for NMDA channels projecting from excitatory cells to inhibitory cells.
Neurons in layer 1 showed significantly stronger firing rates in the attention-attended condition (excitatory cells: p < 0.01; inhibitory cells: p < 0.01; Mann-Whitney U-test). This is the same case in layer 2; firing rates were increased by attention (excitatory cells: p < 0.01; inhibitory cells: p < 0.01; Mann-Whitney U-test). Similar results were found in layer 3, attention increased firing rates of both excitatory and inhibitory cells (excitatory cells: p < 0.01; inhibitory cells: p < 0.01; Mann-Whitney U-test).
We did not found that Fano factors in layer 1 were significantly modulated by attention (excitatory cells: p = 0.561; inhibitory cells: p = 0.548; Mann-Whitney U-test). Fano factors of both excitatory and inhibitory cells in layer 2 showed significant smaller in the attention-attended condition (excitatory cells: p < 0.01; inhibitory cells: p < 0.01; Mann-Whitney U-test to compare the two attentional conditions for each type of cells). Similar results were found in layer 3. Fano factors became significantly smaller in the attended condition (excitatory cells: p < 0.01; inhibitory cells: p = 0.015; Mann-Whitney U-test).
Attention-modulated effects on firing patterns were compared for different types of neurons in different layers. It was found that attention (controlled by \( \sigma_{\text{AMPA}} \) or \( \sigma_{\text{NMDA}} \)) had stronger modulation for inhibitory than excitatory cells in the same layer and stronger modulation for neurons in layer 3 than layer 2.
4 Discussion
We made the hypothesis that attention may reduce stochastic factors in the neurotransmitter binding process, which increase the number of bound receptors located in the post-membrane. The stochastic factors in AMPA and NMDA channels were controlled by the values of \( \sigma_{\text{AMPA}} \) and \( \sigma_{\text{NMDA}} \) in our model, respectively. On the basis of this assumption, the network model of visual attention simulated activity patterns of neurons modulated by attention, increasing their firing rates, reducing their response variability.
Some experiments have shown that the binding process of neurotransmitters with receptors is a stochastic process (Gibb 2001). It also reported that variation of firing rates of neurons was reduced by attention, which might enhance the reliability of information transmission between neurons (Briggs et al. 2013). This result suggested that attention may alter spike patterns of neurons by the method of controlling the stochastic process in neurotransmitter channels. The parameters of \( \sigma_{\text{AMPA}} \) and \( \sigma_{\text{NMDA}} \) in the network model constrain the ratio of bound AMPA or NMDA receptors, respectively. The ratio of bound receptors would increase with decreasing the values of \( \sigma_{\text{AMPA}} \) and \( \sigma_{\text{NMDA}} \). This means that more neurotransmitters were bound with receptors. Therefore, the post-synaptic neuron receives larger synaptic currents and has higher probability to evoke a spike. Meanwhile, when the values of \( \sigma_{\text{AMPA}} \) and \( \sigma_{\text{NMDA}} \) became smaller in the attention-attended condition, stochastic factors in the AMPA or NMDA binding process reduced. This indicated that in the attention-attended condition, the post-synaptic neuron had more stable synaptic inputs to induce spikes in repeated trials, and its response variation reduced. Visual attention may enhance efficiency and reliability of information communication among neurons by reducing stochastic factors in the neurotransmitter binding process.
How does attention control the randomness in the neurotransmitter binding process in the brain? It is an important question. Till now, we did not find experimental observations that directly support that attention controls the randomness in the stochastic binding process. Some reports demonstrated that attention enhanced efficient information transmission, and attention-induced activity modulation was impaired by block of AMPA or NMDA channels, indicating that attention is engaged in the synaptic information processing (Briggs et al. 2013; Herrero et al. 2013). In the literature, computational models of visual attention usually have sophisticated structure of neurons and consider the attentional signal as an external input to the network to generate attention-induced activity (Wagatsuma et al. 2013; Beuth and Hamker 2015). However, this external signal is not explained only as the attentional signal; it may be regarded as other types of signal (e.g., reward signal) but induce attention-like activity. The assumption in this study is that attention could be a process that controls stochastic factors in the synapse. The network model had not sophisticated connection patterns among neurons; the three-layered feed-forward network is enough to generate attention-modulated activity. Our network model proposes a new method to investigate how attention modulates spike activity underlying synaptic mechanisms. One experiment in vivo shows that the single-molecule imaging can measure the dynamic course of bound receptors located in postsynaptic membranes (Ueda and Shibata 2007). It is possible to use this new technology to measure the dynamical course of bound AMPA or NMDA receptors when an animal is performing an attentional task. Statistical properties of bound receptors during the dynamical course are compared between the attention-attended and unattended conditions to verify our hypothesis.
References
Anton-Erxleben, K., & Carrasco, M. (2013). Attentional enhancement of spatial resolution: linking behavioural and neurophysiological evidence. Nature Reviews Neuroscience, 14(3), 188–200.
Beuth, F., & Hamker, F. H. (2015). A mechanistic cortical microcircuit of attention for amplification, normalization and suppression. Vision Research, 116(Pt B), 241–257.
Briggs, F., Mangun, G. R., & Usrey, W. M. (2013). Attention enhances synaptic efficacy and the signal-to-noise ratio in neural circuits. Nature, 499(7459), 476–480.
Dobrunz, L. E., & Stevens, C. F. (1997). Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron, 18(6), 995–1008.
Gibb, A. J. (2001). Neurotransmitter receptors. In Webster, R. (Ed.) Neurotransmitters, drugs and brain function (pp. 57–79). John Wiley & Sons Ltd.
Herrero, J. L., Gieselmann, M. A., Sanayei, M., & Thiele, A. (2013). Attention induced variance and noise correlation reduction in macaque V1 is mediated by NMDA receptors. Neuron, 78(4), 729–739.
Itti, L., & Koch, C. (2000). A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Research, 40(10–12), 1489–1506.
Lanyon, L. J., & Denham, S. L. (2009). Modelling attention in individual cells leads to a system with realistic saccade behaviours. Cognitive Neurodynamics, 3(3), 223–242.
Reynolds, J. H., Chelazzi, L., & Desimone, R. (1999). Competitive mechanisms subserve attention in macaque areas V2 and V4. Journal of Neuroscience, 19(5), 1736–1753.
Reynolds, J. H., & Heeger, D. J. (2009). The normalization model of attention. Neuron, 61(2), 168–185.
Thiele, A., Brandt, C., Dasilva, M., Gotthardt, S., Chicharro, D., Panzeri, S., et al. (2016). Attention induced gain stabilization in broad and narrow-spiking cells in the frontal eye-field of macaque monkeys. Journal of Neuroscience, 36(29), 7601–7612.
Treue, S., & Maunsell, J. H. (1999). Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. Journal of Neuroscience, 19(17), 7591–7602.
Ueda, M., & Shibata, T. (2007). Stochastic signal processing and transduction in chemotactic response of eukaryotic cells. Biophysical Journal, 93(1), 11–20.
Wagatsuma, N., Potjans, T. C., Diesmann, M., Sakai, K., & Fukai, T. (2013). Spatial and feature-based attention in a layered cortical microcircuit model. PLoS ONE, 8(12), e80788.
Acknowledgements
This work was supported National Natural Science Foundation of China (No. 11972159).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Pan, X., Zhang, T., Xu, X., Wang, R. (2021). A Cortical Network Model for Visual Attention. In: Lintas, A., Enrico, P., Pan, X., Wang, R., Villa, A. (eds) Advances in Cognitive Neurodynamics (VII). ICCN2019 2019. Advances in Cognitive Neurodynamics. Springer, Singapore. https://doi.org/10.1007/978-981-16-0317-4_12
Download citation
DOI: https://doi.org/10.1007/978-981-16-0317-4_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0316-7
Online ISBN: 978-981-16-0317-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)