Abstract
Sleep complaints in schizophrenia are fairly frequent. This chapter provides a comprehensive review of various aspects of sleep and schizophrenia. The initial section deals with characteristics of sleep disturbances in the form of subjective sleep complaints and macro- and microstructural sleep abnormalities. Epidemiology and characteristics of individual sleep disorders are also reviewed here. Structural, circuitry, and molecular pathophysiological correlates specific to the interface of sleep and schizophrenia are highlighted. Subsequent section focusses on the directionality of the association between sleep disturbances and schizophrenia; in this section, sleep disturbances across various stages of schizophrenia (from prodrome to residual) are reviewed along with a special emphasis on the mediating role of cognitive dysfunction. Later sections deal with various available treatment strategies and approach to a patient of schizophrenia with sleep complaints.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Schizophrenia is characterized by positive symptoms (delusions, hallucinations, passivity, etc.), negative symptoms (anhedonia, amotivation, apathy, etc.), catatonia, and cognitive symptoms (memory and attentional impairments, executive dysfunction, etc.). Principally, schizophrenia is defined by phenomena (or symptoms) that occur during awake state. However, up to 80% of patients with schizophrenia spectrum disorders have been found to show sleep disturbances, irrespective of the nature of symptoms [1, 2]. Disturbance of sleep in schizophrenia has been long known. Pioneers of modern psychiatry, Emil Kraepelin [3] and Eugen Bleuler [4], both have described sleep to be impaired in schizophrenia, a century years back. Sleep impairment has been understood as a “state” marker, with severity of clinical symptoms strongly correlating with degree of sleep disturbances in both chronic [5] and early phases of illness [6]. On the other hand, impairment of sleep has also been proposed as a “trait” marker. Disturbed sleep has been found to be prevalent during remission states as well; [7, 8] and both clinical [9] and genetic “at-risk” [10, 11] individuals have been reported to have sleep deficits. Moreover, many sleep disorders like obstructive sleep apnea (OSA), circadian rhythm disruption (CRD), restless leg syndrome (RLS), and periodic limb movement syndrome (PLMS) have been found to be comorbid with schizophrenia [2]. With both sleep rhythms and schizophrenia psychopathology being proposed to have common neurobiological underpinnings [12,13,14], study of sleep offers a window into greater understanding of etiopathogenesis, and hence, better management of schizophrenia. Moreover, several treatment options have been proposed with respect to the interface of sleep and schizophrenia [15]. In this chapter, characteristics of sleep disturbances in schizophrenia including various pathophysiological models, the bidirectional relationship between schizophrenia core psychopathology and sleep deficits, and treatment aspects are highlighted.
2 Characteristics of Sleep Disturbances in Schizophrenia
Most common sleep abnormality in schizophrenia patients is insomnia, that is, difficulty initiating and maintaining sleep [8]. Sleep in schizophrenia patients has been described in terms of subjective reports or objective assessments, mainly using polysomnography (PSG). While standard interpretation of PSG recordings describes their “macrostructure” in terms of sleep stages delineated according to Rechtschaffen and Kales [16] scoring criteria, sleep “microstructure” is described in terms of quantification of sleep spindles and slow wave activities, detection of arousals, etc. This subsection highlights impairments in each of these descriptors among schizophrenia patients.
2.1 Subjective Sleep
Subjective reports of sleep are generally assessed in terms of sleep quality. The Pittsburgh Sleep Quality Index [17] has been the most widely used tool. Subjective sleep disturbances have been reported to be present since early psychosis and to be associated with symptom severity [6]. Consistently studies have reported poor sleep quality in patients with schizophrenia [6, 18,19,20,21,22]. About half a proportion of patients with schizophrenia have been labeled as “poor sleepers.” [21] Poor sleep quality also correlates with impaired overall quality of life [19, 21].
Subjective sleep estimation measures have been significantly correlated with objective sleep variables. Rotenberg et al. [22] found that subjective estimation of sleep onset latency, sleep depth, nighttime wakefulness, and dreams significantly correlated with polysomnographic (PSG) macrostructural measures such as sleep onset latency (SOL), percentage duration of slow wave sleep (SWS%), and eye movement density, respectively. Interestingly however, different treatment agents might have diverse effects on subjective versus objective measures. Kajimura et al. [20] reported that soundness of sleep in the subjective sleep assessment is better evaluated during treatment with zopiclone than with benzodiazepines. Baandrup et al. [18] found that melatonin significantly improved self-reported sleep quality, but had no effect on objective sleep efficiency.
Although less often reported, the frequency of subjective sleep disturbances in hospitalized schizophrenia patients has been reported to be statistically comparable to other psychiatric illnesses such as depression, anxiety, and substance use disorders [23].
2.2 Sleep Macrostructure
Generally, sleep macrostructure is classified in terms of sleep continuity (total sleep time, sleep onset latency, sleep efficiency, and wake time after sleep onset) and sleep architectural (percentage duration of each of the sleep stages, REM latency, and REM density) measures. To date, several PSG studies have compared sleep macrostructure between schizophrenia patients and healthy controls. A recent systematic review and meta-analysis by Chan et al. [24] evaluated 31 such studies that included a total of 574 patients and 515 healthy controls. This report concludes that schizophrenia patients have significant impairments in both sleep continuity and sleep architecture. They were found to have reductions in total sleep time, sleep efficiency, slow wave sleep (SWS), duration, and latency of rapid eye movement (REM) sleep and significantly increased sleep onset latency and wake time after sleep onset. A summary of this study’s findings is depicted in Table 20.1.
Chan et al. [24] also highlight that factors like duration of illness, medication status, and duration of medication withdrawal influence these findings. While reduced duration of SWS and REM latency was seen in patients with duration of illness greater than 3 years, decreased REM latency was restricted to patients with short durations of illness. Medication-naïve patients were found to have impairments restricted to sleep continuity measures only. Although sleep continuity measures were similar, schizophrenia patients with antipsychotic withdrawal for longer than 8 weeks were shown to have no significant deficits in any of the sleep architectural measures unlike those with shorter durations of withdrawal, who showed impairments in NREM stage 1 and 2 durations, and REM latency.
Studies have shown that while positive symptoms are associated with both sleep continuity and architectural impairments, deficits restricted to sleep architecture corelate with negative and cognitive symptoms. On one hand, reduced sleep efficiency, prolonged sleep latency, increased REM density, and shorter REM latency have been correlated with positive symptoms [5, 25,26,27]. While on the other hand, short REM latency and SWS deficits correlated with negative symptoms, [28,29,30] and cognitive symptoms correlated with SWS deficits [5, 27].
Longitudinal studies [31] report that in relation to phase of illness and treatment (i.e., during remission), REM parameters tend to normalize while SWS measures remain impaired.
Anecdotal studies have reported that both sleep continuity and architectural measures are useful in discriminating schizophrenia patients from other psychiatric disorders like depression [32]. Though impairments related to SWS have been suggested as being more specific to schizophrenia [33], in recent years, studying macrostructural abnormalities has gone out of favor mainly citing its lack of specificity to schizophrenia [33,34,35].
2.3 Sleep Microstructure
While macrostructure measures describe temporal organization of sleep, microstructure, which is analyzed by scoring phasic events, provides essential information regarding dynamic characteristics of sleep processes that are responsible for the circadian alternation of wake and sleep [36]. Microstructure, most commonly, is described as under-arousals, awakenings, cyclic alternating pattern (CAP), sleep spindles, K-complexes and delta bursts (also called microarousals), rapid eye movements, body movements, atonia, nightmares, etc.
More recently, microstructure assessment has expanded to incorporate “dissociated stages of sleep (DSS),” which includes the phenomena of “intermediate sleep (IS)” [37]. Description of these phenomena involves analysis of electrophysiological sleep patterns that exhibit simultaneous occurrence or rapid oscillation between different sleep stages components. They include: NREM sleep with rapid eye movement (NRSWR), REM sleep without rapid eye movement (RSWR), REM sleep without atonia (RSWA), etc. This preliminary study by Guénolé et al. [37] found that RSWA was significantly increased in drug-naïve first-episode schizophrenia patients.
Although repeated awakenings (fragmented sleep) [38, 39], increased nightmares [40], and increased REM density [24] have been reported, microstructural assessment of sleep spindles and slow waves, which are the main brain oscillations during non-REM sleep, has received majority attention.
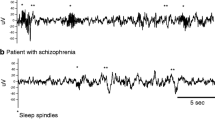
2.3.1 Reduced Sleep Spindles
Reductions in sleep spindles, principally examined during stage 2 NREM, have been repeatedly and consistently observed in schizophrenia patients, albeit in a few studies with very small sample sizes (for review see Manoach et al.) [41]. Measures used as descriptors of sleep spindle activity are spectral power and coherence in the sigma frequency (12–16 Hz) range, number, duration, density, amplitude, morphology of spindles, and integrated spindle activity. Consistent results have been found with respect to reduced number and density of sleep spindles and sigma power (see Table 20.2). Schilling et al. [42] report specific reduction in fast (12–15 Hz) spindle density. Fair consistency has also been seen on reduced spindle duration and amplitude in schizophrenia; Wamsley et al. [43] found these measures not to be significantly different compared to controls. Sigma frequency spectral coherence, spindle morphology, and integrated spindle activity have been reported/assessed only in small number of studies. Interestingly, the integrated spindle activity, a measure derived by dividing the sum of absolute amplitude of each detected spindle by total non-REM sleep duration, has been found to be significantly reduced in schizophrenia patients; and this difference has a very large effect size [44, 45].
These findings do not seem to be influenced by chronicity of illness/duration of illness as schizophrenia patients with both early and chronic stages of illness have shown similar findings [41]. Most of these studies (Table 20.2), with the exception of Manoach et al. [46] have been conducted in patients receiving antipsychotic medications. As of yet, it remains to be explored on how much of a confounding influence does use of long-term medications have on these findings. However, single-dose studies have demonstrated no effect of antipsychotic drugs on sleep spindles [47, 48].
Manoach et al. [41], in their review, also highlight that sleep spindles have significant correlation with cognitive (motor learning, declarative/visual memory, executive functions, intelligence quotient, etc.) and positive symptoms of schizophrenia. Studies have also attempted to address specificity of these findings to schizophrenia patients. Sleep microstructure measures of sleep spindles have been found to validly discriminate schizophrenia patients from depression [44] and non-schizophrenia psychosis [46].
2.3.2 Slow Wave Sleep (SWS) Deficits
A good number of studies have attempted to characterize microstructure of slow wave sleep in schizophrenia by comparing various descriptors in patients compared to non-psychiatry controls (see Table 20.3). Overall, we derived about nine descriptors from various studies—slow wave amplitude, delta wave count/density, wave slope (up/down), frequency of multipeak waves, slow frequency (i.e., delta/theta) spectral power, high frequency (beta and gamma) spectral power, altered distribution, accumulation/dissipation, and rapid eye movements (REMs). Highest evidence, in terms of most studies to show significant difference, is for reduced slow wave amplitude and delta wave count/density. Inconsistent results are seen on delta slow frequency (delta and theta) spectral power and altered distribution. Rest of the descriptors have been limited to a very few studies. Inconsistencies in results and in use of assessment methodology have led to limited attention to slow wave microstructural sleep deficits [10, 49]. Castelnovo et al. [50], in a recent review, emphasize upon use of uniform methodology. Another possible reason, for this lesser attention, has been quoted as the effect of medications. Antipsychotics have been found to induce electroencephalographic (EEG) slowing and reductions in slow frequency spectral power [51, 52].
Association of negative symptoms with reduced delta wave count [53, 54] and of positive symptoms with enhanced gamma power during slow wave sleep [55] has been reported. Recently, Kaskie et al. [56] found a significant negative correlation between slow wave density and positive symptoms. More studies, however, are required to strengthen these assertions.
A few studies have investigated the specificity of slow wave microstructural deficits to schizophrenia. Greater delta wave counts (Ganguly et al. 1987) [53], abnormal accumulation/dissipation of slow waves [57], and lower gamma power during SWS [55] were seen more in patients with depression compared to schizophrenia. However, amplitude of slow waves has been found to be comparable between schizophrenia and depression [57, 58].
2.4 Comorbid Sleep Disorders
Apart from sub-syndromal sleep disturbances and complaints, schizophrenia patients have been found to have many comorbid sleep disorders. It is common to have two or more sleep disorder comorbidities in schizophrenia patients [59, 60]. This subsection describes the characteristics of each of these comorbid sleep disorders.
2.4.1 Insomnia
Insomnia is commonly comorbid with schizophrenia [13]. Studies have reported prevalence rates of insomnia in schizophrenia patients to range from about one-fifths to one-half of the population [61,62,63,64,65]; with about an additional one-third population qualifying for subthreshold insomnia [61]. Moreover, disruption of several of the sleep continuity and architectural measures, especially reduced total sleep time and sleep onset latency (Sect. 20.2.2), implicate greater incidence of insomnia in schizophrenia patients. Among schizophrenia patients with comorbid insomnia, about 20% each have initial and middle insomnia and about 15% have late insomnia (early morning awakening) [62, 63]. About 50% of schizophrenia patients with comorbid insomnia have been found to suffer from severe insomnia [61]. Moreover, other sleep disorders like RLS, PLMS, narcolepsy, and parasomnias have been seen to be significantly associated with insomnia in schizophrenia [59].
There are inconsistencies in the reports showing association of insomnia with clinical symptoms of schizophrenia. Although studies suggest that insomnia is associated with an increase in paranoid thinking, even up to two to threefold [66], some studies on schizophrenia find no independent association of insomnia with positive and negative symptoms [62, 63]. However, recent studies have shown significant association of insomnia with positive symptom and general psychopathology [60, 67]. More significantly, and consistently as well, insomnia has been strongly correlated with suicide risk [64, 68].
2.4.2 Circadian Rhythm Disruption (CRD)
Although small in number, studies show strong evidence for CRD in schizophrenia patients [69,70,71,72,73]; strongly supported by results from animal models as well [74, 75]. The abnormalities range from mild to severely disturbed rhythms with fragmented sleep epochs. About half of the schizophrenia patients have been reported to have severe circadian disruption ranging from phase-advance or phase-delay to non-24-h sleep-wake cycles [73]. However, a recent study by Homabli et al. [59] report very low (i.e., 5%) rates of CRD in schizophrenia patients. Non-24-h melatonin cycles, manifold enhancement in sleep-related prolactin release, and non-inhibition of nocturnal cortisol secretion are some of the related abnormal endocrine rhythms (Van Cauter et al. 1991; Wulff et al. 2012) [72, 73]. The relation between positive and negative symptoms and CRD has been inconsistent and inconclusive. While Afonso et al. [69] found that schizophrenia patients with predominantly positive symptoms have a statistical trend for greater CRD, Bromundt et al. [70] failed to find a significant correlation between CRD and positive and negative symptoms. However, a strong association between CRD and cognitive symptoms has been elicited [70]. Speculations about the confounding role of antipsychotics are still inconclusive and studies addressing this relationship are sparse [13, 71].
Reviews focusing on CRD in schizophrenia suggest that interventions targeting resynchronization circadian rhythms may prove effective in the treatment of schizophrenia symptomatology [13, 76,77,78]. Perhaps, CRD has been found to be comorbid with other illnesses like bipolar disorder and major depressive disorder [76, 77].
2.4.3 Restless Legs Syndrome (RLS)
A series of papers by Kang and colleagues [79,80,81,82,83,84,85,86] report descriptive data on the proportion of schizophrenia patients having comorbid RLS. They report 21.4% of schizophrenia patients to have a comorbid RLS and up to half of them to have at least one RLS symptom, that is, sub-syndromal RLS. They report RLS to be significantly greater in schizophrenia patients compared to controls. Schizophrenia patients with RLS report significant association between psychopathology scores and insomnia [79]. Patients recruited in these studies were hospitalized and were on antipsychotic medications. As antipsychotics are known to cause RLS [87], confounding effects of medications cannot be ruled out. Perhaps, Kang and colleagues use the term antipsychotic-induced RLS in many of their reports.
Antipsychotic-induced akathisia, although a close differential for RLS and challenging to differentiate [13], should be cautiously ruled out before diagnosing comorbid RLS in schizophrenia patients. While, leg paresthesias characterize RLS, inner restlessness has been shown to be a hallmark to akathisia [88]. RLS and akathisia have also been differentiated based on periodic limb movements (PLMs) and long latency flexor reflex (LLFR); RLS has been reported to have greater PLMs and greater LLFR [88, 89].
2.4.4 Periodic Limb Movement Syndrome (PLMS)
Although PLMS is a most common associated feature of RLS and has been considered together with RLS by many authors, this subsection deals with PLMS separately. Reported rates of PLMS in schizophrenia are very inconsistent. While Staedt et al. [90] reported PLMS to be present in all schizophrenia patients, Ancoli-Israel et al. [91] showed PLMS rates to be 14%. Intriguingly, the former study emphasizes the chronicity of antipsychotic use and the latter study reports no significant correlation of PLMS with duration of neuroleptic use. Both these studies included patients in later ages and who were on chronic (mean duration: >25 years) antipsychotic treatment. A very recent study by Hombali et al. [59] reported a prevalence of 14.1% for combined RLS/PLMS symptoms in schizophrenia patients. This study, with a better sample size compared to the earlier two, reported RLS/PLMS to be significantly correlated with age; older aged patients being less likely to have this comorbidity.
2.4.5 Obstructive Sleep Apnea (OSA)
Several reviews and meta-analyses have suggested that OSA has been found to be a common sleep disorder comorbid with schizophrenia [13, 92,93,94,95,96]. The reported prevalence rates of OSA in schizophrenia range from 15 to 48%, with meta-analysis by Stubbs et al. [95] reporting a pooled mean of 15.4%. Increasing age and higher BMI have consistently been reported as significant predictors of OSA in schizophrenia [94, 95]. While some report improvement in psychotic symptoms secondary to treatment of comorbid OSA, significant associations between antipsychotic dose and OSA measures have not been found [93, 94]. The comorbid rates, however, are found to be significantly less than those found in major depressive disorder and bipolar disorder [95].
2.4.6 Narcolepsy
Comorbid occurrence of narcolepsy and schizophrenia like psychosis, is not rare. Walterfang et al. [97] suggested that the association between narcolepsy and schizophrenia is a chance cooccurrence. They found no evidence for a common pathology and hence concluded that the association may be medication related. However, later studies suggested that patients with narcolepsy type 1 (i.e., NT1) present with psychotic symptoms along the course of their illness [98,99,100,101,102]. All these studies assessed for psychotic symptoms in diagnosed cases of narcolepsy. Studies describing narcolepsy among established cases of schizophrenia report prevalence rate of 5–10% [59, 103]. Hombali et al. [59] found these rates to be lower than those seen with depression and anxiety.
Although systematic investigations to delineate the two conditions as either cooccurring or related to a single disease process are required, a possible common autoimmune pathology has been suggested to underlie them [98, 104].
2.4.7 Parasomnias and Sleep State Misperception
Comorbid parasomnias such as sleepwalking, night terrors, nightmares, etc. in schizophrenia patients have been studied sparsely and have not been studied systematically. Hombali et al. [59] report that 9.1% of schizophrenia patients subjectively report parasomnias. Very recently, Reeve et al. [60] found nightmare disorder as a common comorbid sleep disorder in early non-affective psychosis patients, being second only to insomnia. Nightmares have been shown to be associated with an impending relapse, increased delusional severity, and risk of suicide [105]. Moreover, amelioration of nightmares and associated distress has been suggested to reduce psychotic symptoms as well [105].
Another important scenario is when subjective sleep complaints are present but objective measures including PSG do not reveal any abnormalities. This condition, regarded as “sleep state misperception (SSM)” or “paradoxical insomnia,” has been found to be fairly common and has been shown to be associated with severity of negative symptoms [106]. Disturbances in memory and reasoning, core psychopathological elements of schizophrenia, have been attributed to SSM [107].
3 Pathophysiology
While sleep macrostructure provides us with a gross overview of the sleep architecture, microstructural abnormalities help us understand the pathophysiology of sleep dysfunction in schizophrenia better. As described in earlier sections, predominant and consistent dysfunction among various microstructural abnormalities has been sleep spindle deficits and to some extent deficits in slow wave sleep. Therefore, pathophysiology of sleep dysfunction in schizophrenia has mostly been derived and understood based on specific abnormalities in sleep spindles. Moreover, as the basic deficit in sleep spindles has been their reduction in number/density, conceptualization of pathophysiology focusses on generation of sleep spindles specifically.
3.1 Structural Correlates
Thalamus has been primarily implicated in the pathophysiology of sleep spindles. Principally, the thalamic reticular nucleus (TRN), termed as “pacemaker,” has been recognized as the most important structure associated with generation of sleep spindles [12]. Structurally, TRN is a shell-like wrap around the dorsal thalamus. Apart from TRN, the medial dorsal nucleus (MDN) and the lateral geniculate nucleus (LGN) have also been implicated [12] (see Fig. 20.1).
Thalamic nuclei. Nuclei implicated in the pathophysiology of sleep are colored in red. T thalamic reticular nucleus (TRN); M medial dorsal nucleus (MDN); L lateral geniculate nucleus (LGN); (1) ventricular nuclei; (2) anterior nuclei; (3) lateral nuclei; (4) pulvinar nuclei; (5) medial geniculate nucleus; (6) internal medullary lamina; (7) external medullary lamina
Structural changes in all the three regions—TRN [108,109,110], MDN [111] and LGN [112]—have been found to be implicated in schizophrenia, albeit inconsistently reported in postmortem studies [113]. Combining data from postmortem and animal studies, Steullet et al. [109] showed profound abnormalities in parvalbumin (PV) expressing neurons of the TRN in schizophrenia. Volume of MDN, especially the left MDN, has been shown to be reduced in schizophrenia patients. Converging evidence suggests that sensory gating and attentional and emotion processing deficits found in schizophrenia stem from deficits in TRN [12]; deficits in sleep spindles have been suggested to have a mediating influence [108,109,110].
Apart from thalamus, prefrontal cortex (PFC), especially cortical layer VI neurons, has been implicated in the initiation and maintenance of sleep spindles [114]. Specifically, pyramidal cells of the medial PFC have been shown to have a significant role in initiating as well as terminating sleep spindles [115]. Perhaps, PFC is one of the most significant neural areas implicated to be abnormal in schizophrenia; and cognitive dysfunction has been consistently associated with PFC deficits [116, 117]. Intriguingly, deficits in both TRN and PFC have been shown to be developmentally linked [109, 118].
Although not specifically investigated in schizophrenia, dopamine-related areas—ventral tegmental area (VTA), substantia nigra (SN), and ventral periaqueductal gray matter and their projections onto basal forebrain, midbrain, brainstem, and hypothalamus—have been implicated in CRD or sleep-wake cycle disturbances [119]. Dopamine-containing pineal gland, which regulates sleep-wake rhythms through the circadian release of melatonin, also has been implicated [119].
3.2 Circuitry Correlates
TRN, with its distinct structural organization, is the principal modulator of information flow between thalamus and cortex [110]. Specifically, thalamo-cortical (TC) and the cortico-thalamic (CT) circuitry neurons are implicated in the physiology of sleep spindles [114] (see Fig. 20.2).
Flowchart showing the basic physiological model of generation of sleep spindles through the thalamus-cortex-thalamus (TCT) circuit. (Adapted from Clawson et al. (2016)). Digits inside black colored circles depict proposed deficits in schizophrenia patients—structural abnormalities in TRN (1), TC projections (2), and the PFC (3); deficits in Ca+ channel activity in TRN (4); defective depolarization due to reduced GABA activity in TC neurons (5); reduced glutaminergic activity in the TCT circuit (6). TRN thalamic reticular nucleus; GABA gamma-aminobutyric acid; mGluR metabotropic glutamate receptor; NA noradrenergic; 5HT serotonergic; Ca calcium; V voltage; TC thalamo-cortical; CT cortico-thalamic
In line with the hypothesis that dysconnectivity is the core underlying feature of schizophrenia [120], white matter connectivity abnormalities in TC networks, including the thalamic radiation that carries fibers from the thalamus to prefrontal areas, have been reported consistently in schizophrenia. Several lines of evidence, both from functional magnetic resonance imaging (fMRI) and source localizing EEG, suggest reduced thalamus-PFC connectivity [12, 119, 121].
Moreover, MDN and LGN have been showed to serve as “higher-order” and “first-order” relay nuclei, respectively [12, 121]. “High” and “first” in order refer to afferent projections from cortex, specifically layer V, and subcortical structures, respectively. Very recently, Parnaudeau et al. [122] emphasized MDN-PFC connectivity and its role in higher-order cognitive functions. With the focus distinctly falling on MDN, TRN-MDN-PFC circuit dysfunction has been suggested to underlie sleep spindle deficits in schizophrenia in recent studies [122].
3.3 Molecular Correlates
3.3.1 Neurotransmitters
The molecular mechanisms underlying sleep spindles have been shown in Fig. 20.1. A brief summary is given here: The GABAergic neurons in the TRN, which is the ‘pacemaker’ for sleep spindles, are the primary underlying structures. A reduced baseline noradrenergic and serotonergic signaling during NREM maintains a relative hyperpolarized state. Excitatory (glutaminergic) stimulus, predominantly from activation of ionotropic (or activation of gap junction communications from metabotropic receptors in its absence), activates the T type calcium (Ca+) channels. Their subsequent activation of voltage-gated potassium channels leads to membrane afterhyperpolarization and subsequent generation of highly synchronized bursts, that is, spindles. Consequently, “rebound bursts” are then generated by glutaminergic TC neurons after TRN-led GABAergic innervation mediates a series of postsynaptic events and subsequent depolarization. These rebound bursts send excitatory efferents to TRN as well as cortical neurons, which in turn excite TRN through CT neurons; excitatory effects being predominantly glutaminergic [114].
Reduction in the levels of intracellular Ca+ in the TRN, which otherwise has a high content in healthy individuals, has been hypothesized as one of the pathophysiological factors responsible for sleep spindle deficits in schizophrenia. Reduced GABA synthesis, secondary to reduction in glutamate decarboxylase enzyme activity and in GABA membrane transporter density, and therefore impaired GABA mediated depolarization and rebound bursts in the TC neurons, has also been suggested to underpin these deficits. One another molecular mechanism that is implicated to underlie these deficits in the TCT circuit is reduced glutaminergic activity that stems from reduced N-methyl-D-aspartate (NMDA) glutamate receptor activity in TC neurons, MDN, and PFC (for review see Ferrarelli [15]) (see Fig. 20.2). In a gist, this TRN model, with regards to neurotransmitter systems involved, implicates GABA and glutamine dysfunction.
In addition to GABA and glutaminergic neurotransmission, dopaminergic and cholinergic systems have also been implicated. GABA deficits in the TCT, specifically in the TRN, have also been shown to be caused secondary to activation of the dopamine-4 (D4) receptors; they are found presynaptically on GABA-containing projections from the globus pallidus to the TRN. Implying a role in insomnia among schizophrenia patients, abnormal dopaminergic D1 and D2 transmission has been hypothesized in SWS and REM stage impairments as well [13, 56]. Moreover, dopaminergic dysfunction has also been implicated in the CRD comorbid with schizophrenia. Dopamine has been known to promote wake and suppress REM and non-REM (NREM) sleep, and dysfunctional DR4 in the pineal gland leads to impaired melatonin release and subsequent CRD [119]. Recently, Yates [123] suggests a mutual, two-way relationship between dopamine and sleep in schizophrenia—elevated dopamine levels causing CRD and in turn CRD increasing dopamine release and sensitivity. He suggests that interplay between sleep and dopamine is vital to onset and course of schizophrenia. Reduced striatal dopaminergic neurotransmission has been hypothesized in RLS/PLMS. Understandably, RLS/PLMS seen in schizophrenia has been attributed to the effects of anti-dopaminergic agents [81].
With respect to cholinergic neurotransmission, which initiates and coordinates REM sleep, cholinergic super-sensitivity has been suggested as an underlying mechanism for REM-related abnormalities in schizophrenia [119]. Specifically, negative correlation between REM latency and plasma cholinesterase isozyme activity has been demonstrated in schizophrenia [124]. Role of serotonergic neurotransmission in the pathophysiology of sleep deficits in schizophrenia, although suggested anecdotally in SWS deficits [56], is by and large understudied.
3.3.2 Genes
Several genes implicated in spindle physiology have been found to be impaired in schizophrenia patients. As discussed earlier, T type Ca+ channels in the TRN neurons are crucial for generation of sleep spindles. Two genes encode these channels—CaV3.2 (CACNA1H) and the CaV3.3 (CACNA1I). CACNA1I, whose genetic deletion leads to reduction in spindle generation, has been found to be significantly associated with schizophrenia [12, 41]. Excess dopaminergic transmission leading to abnormal activation of D4 receptors, be it in the TRN or in the pineal gland, has been proposed to be caused by impaired degradation of dopamine. The catechol-o-methyl transferase (COMT) gene and its encoding enzyme COMT are responsible for this process and have been implicated in sleep-wake regulation. Polymorphisms in the COMT gene have been consistently found in schizophrenia and implicated in comorbid CRD in these patients [119]. Val81Met polymorphism in the tyrosine hydroxylase (TH) gene, which is also involved in the dopaminergic neurotransmission, has also been found to be associated with RLS in female patients with schizophrenia [86].
Strong evidence for sleep-wake or circadian rhythm disturbances and their association with various endocrine rhythms in schizophrenia (discussed in Sect. 20.2.4.2) has prompted investigations to study molecular rhythmicity (or molecular oscillatory systems) in this disorder. These studies, although preliminary, suggest a role (decreased or loss of rhythmic expression) of several clock genes in schizophrenia [125,126,127]. They are the Circadian Locomotor Output Cycles Kaput (CLOCK), PERIOD (PER) 1, 2 and 3, TIMELESS (TIM), and the Cryptochrome (CRY)-1 genes. Particularly, the CLOCK gene has been suggested to be related with dopaminergic neurotransmission [125]. Interestingly, schizophrenia patients with CLOCK polymorphisms have been suggested to be at a higher risk of RLS as well [128].
Other genes that have been suggested to be associated with RLS in schizophrenia are BTBD9 and GNB3 [129]; their exact role in the pathophysiology of sleep in schizophrenia is yet to be clearly determined.
4 Bidirectional Relationship
Unlike conditions like depression, mania, anxiety, etc., where sleep disturbances are considered primary symptoms of the illness, sleep impairments in schizophrenia are traditionally considered to arise secondary to other core symptoms [130]. Over the course of this chapter, so far, we gather enough evidence to suggest a significant relationship between schizophrenia and sleep disturbances. Understanding the direction of this relationship is also crucial, especially from a treatment point of view. Both directions, schizophrenia psychopathology leading to sleep deficits and vice versa, have been postulated (for understanding sake, these will be referred in this chapter ahead to as “forward” and “backward” directional hypothesis, respectively). In addition, existence of a common pathway that explains or binds the two of them has also been suggested. This subsection describes evidence available on each of these three hypotheses. While reports of schizophrenia like psychotic symptoms in established cases of primary sleep disorders is an area to probe, studies on sleep disturbances across various stages in the schizophrenia illness course also are relevant in this regard. Studies assessing cognition in the context of sleep and schizophrenia are also described here. Endorsing a need to highlight the context of backward hypothesis, initially we discuss occurrence of psychotic symptoms secondary to sleep deprivation in healthy individuals.
4.1 Psychotic Symptoms in Sleep Deprived Healthy Population
Studying psychiatric sequalae of sleep deprivation in healthy individuals is an area that received fair attention in the recent past. The World Health Organization’s World Health Survey (WHS), a 70 country, population-based survey, found a strong association between sleep problems and psychotic symptoms in general population, globally [131]. Two very recent papers [132, 133] systematically reviewed various experimental and observational studies with an aim to assess causal association between sleep disturbances and psychotic and related phenomena. Barton et al. [132] report that insomnia is associated with psychotic-like, dissociative, and hypomanic experiences. They also report an association between hypomanic experiences and evening-ness chronotype and circadian dysrhythmia; evening-ness chronotype, that is, working more efficiently in the evenings, going to sleep late at night, and waking-up late in the morning, was linked to dissociative experiences as well. Experimental sleep-manipulation studies consistently show a potential causal link between sleep loss and psychotic-like phenomena [132, 133]. While complex hallucinations and disordered thinking start to occur by 48 h of sleep deprivation, delusions, hallucinations in all sensory modalities, and a picture resembling acute psychosis occur by the third day without sleep [133]. Very recently, Reeve et al. [134] compared sleep loss condition (restricted to 4 h sleep for 3 nights for 2 consecutive weeks) with standard sleep condition and found that sleep loss is significantly associated with paranoia, hallucinations, and cognitive disorganization, apart from impaired emotional valence and working memory.
4.2 Psychotic (Schizophrenia) Symptoms in Sleep Disorders
Section 20.2 of this chapter describes various sleep disorders encountered in patients with schizophrenia. Various studies described in that section, by and large, report sleep comorbidities in established cases of schizophrenia (forward hypothesis). In this subsection, we attempt to focus on occurrence of schizophrenia/psychotic symptoms in cases of sleep disorders.
Most evidence for a backward directional relationship is available for narcolepsy. Specifically, narcolepsy type 1, that is, NT1, has been consistently found to present with psychotic symptoms along the course of its illness [98,99,100,101,102]. Data on schizophrenia/psychotic symptoms in established cases of insomnia are very sparse. However, very recently Cosgrave et al. [135] found psychotic-like experiences to be significantly greater in persons with insomnia compared to the control group. Although a bidirectional cause-to-effect relationship between RLS and schizophrenia has been suggested by Mackie and Winkelman [136], data to test this hypothesis are largely insufficient.
4.3 Sleep Disturbances Across Various Stages of Schizophrenia
4.3.1 Prior to Illness Onset/Prodromal States/Clinical (or Ultra)-High-Risk (CHR/UHR)
Two sets of studies, that is, those assessing sleep disturbances prior to illness onset retrospectively and those assessing them in CHR/UHR subjects, have been found. Some studies including CHR/UHR assessed the validity of sleep disturbances in predicting psychosis onset as well.
Abnormalities in sleep disturbance (in general), sleep duration (i.e., insomnia), and sleep continuity have been reported prior to illness onset in schizophrenia patients, consistently; some in fact date back sleep disturbances to early childhood (for review, see Davies et al. [6]; Lunsford-Avery and Mittal) [137]. These reports are predominantly retrospective chart reviews or family/parent/self-interviews. Sleep disturbance during remission has also been suggested as an important predictor of relapse in schizophrenia [8, 138].
Studies conducted on CHR/UHR individuals also report disturbances in sleep continuity and architectural measures. While reduced subjective sleep quality, reduced sleep efficiency, increased sleep onset latency, and increased wake time after sleep onset are the sleep continuity disturbances, increased slow wave sleep, greater PLMs, increased REM onset latency, and reduced REM% are the impaired sleep architecture measures (for reviews, see Davies et al. [6]; Lunsford-Avery and Mittal [137]; Zanini et al. [139]). Recently, Poe et al. [140] assessed sleep disturbances in 194 CHR individuals and found that they are significantly greater in this group compared to healthy controls, and that these sleep disturbances were significantly correlated with greater positive and negative symptoms and impaired overall functioning. While most of these studies are either polysomnographic or actigraphic, very recently, Waite et al. [141], using qualitative thematic analysis, found disrupted sleep timing as the characteristic hallmark of sleep problems in UHR individuals.
Interestingly, longitudinal studies assessing onset of psychosis in UHR individuals consistently have found sleep disturbances at recruitment to predict not only transition to psychosis but also increased positive symptom severity [142, 143]. A mediating role of depression and anxiety, however, has been found [138]. While these studies assessed sleep disturbance in general or sleep continuity measures, Lunsford-Avery et al. [144] assessed CRD in UHR individuals and found them to be a possible vulnerability marker for emergence of psychosis. This study findings support the “two-way relationship between dopamine and sleep in schizophrenia” hypothesis by Yates et al. [123], which was based on a moderating role of CRD. A study by Alderman et al. [145], however, did not find sleep disturbances in UHR individuals to be significant predictors of transition to psychosis.
Of note, a few studies that assessed sleep profiles in genetic-high-risk, that is, unaffected first-degree relatives, have shown sleep architectural abnormalities in slow wave sleep [11, 146], REM sleep [146], and integrated spindle activity [10]. These reports suggest that these measures might be trait/endophenotype markers for schizophrenia. Interestingly, across all these studies, it has been seen that sleep continuity measures are unaltered in relatives.
4.3.2 Early/First-Episode Versus Chronic Schizophrenia
Impaired sleep macro- and microstructure (as discussed in Sects. 20.2.1 through 20.2.3) are well documented in early/first-episode schizophrenia patients, [6] therefore suggesting that duration of illness and hence chronicity of symptoms do not necessarily lead to these impairments. In fact, studies comparing sleep profiles across various phases of illness report no substantial difference [27, 147]. Anecdotal evidence, albeit inconsistently, however report poorer sleep quality in chronic schizophrenia cases compared to recent onset cases [148].
A meta-analysis by Chouinard et al. [149] on drug-naïve/untreated patients rules out significant confounding, moderating effect of medications on sleep impairments in schizophrenia. Rather, a clinical review by Monti and Monti [27] report counteracting effects of antipsychotics on sleep impairments in schizophrenia patients, across various phases of illness.
Although significantly impaired sleep profile in schizophrenia patients across various phases of established illness and significant association between impaired sleep measures and various symptom complexes have been demonstrated (also discussed in Sect. 20.2), surprisingly sparsely has there been an attempt to understand the directionality of relationship between sleep and schizophrenia symptomatology. Perhaps only very recently, Reeve et al. [150] using mixed effect models showed that insomnia predicted hallucinations later on than vice versa, and that the association between insomnia and paranoia was bidirectional.
4.3.3 Relapse
Reeve et al. [138], in their systematic review, identified three studies that assessed sleep disruption as a predictor of relapse in schizophrenia. They suggest that sleep disruptions allow us to detect an impending relapse, with acceptable sensitivity and specificity.
4.3.4 Moderating Role of Cognitive Dysfunction
Increasingly, schizophrenia is being conceptualized and treated as a neurocognitive disorder [151], more so with its core symptoms and outcome being understood based on cognitive impairments [152,153,154].
With evolving evidence from neurosciences, the role of sleep in cognitive functions, specifically learning and memory, has changed from being passive to more active. While focus of earlier research was REM sleep, more recently the highlight has been on SWS and sleep spindles. In short, sleep spindles/SWS and REM sleep have been shown to be involved with consolidation of memories and executive functioning, respectively [155,156,157]. As sleep macro- and microstructural abnormalities related to these stages of sleep have been found in schizophrenia patients (Sects. 20.2.2 and 20.2.3), a causal association between sleep and cognitive deficits has been hypothesized [130]. Correspondingly, experimental studies have found associations between sleep spindle deficits and impaired sleep-related memory consolidation [158] and between reduced REM latency and impairment in executive functions [159]. However, some negative results in this regard have also surfaced recently [160]. Moreover, the TRN, the TCT networks, and clock genes implicated in core sleep deficits, that is, sleep spindles and SWS, in schizophrenia have been implicated in memory-related plasticity [41, 119].
Nevertheless, studies assessing the longitudinal course of sleep deficits, cognitive impairments, and other psychotic symptoms are necessary to objectively test the hypotheses proposed.
5 Treatment
Sleep disturbances needs to be treated due to its impact on core psychotic phenomena, quality of life, and social functioning in schizophrenia. Various strategies have been proposed.
5.1 Deep Sleep Therapy
Deep sleep therapy, though only of historical importance, marks an important landmark in the biological therapies for schizophrenia. Zurich-Burghölzli psychiatrist Jakob Klaesi (1883–1980) used a combination of two barbiturates for deep sleep therapy (putting patients into a therapeutic stupor) in 1920. The idea was previously attempted with other barbiturates and with bromine. This therapy was occasionally used as a remedy for psychotic illness in the 1930s and 1940s. Deep sleep therapy lists as one of the early somatic therapies along with insulin coma therapy and electro- and chemical convulsive therapy. Its use ceased, rather completely, after the introduction of chlorpromazine in the early 1950s [161].
5.2 Pharmacological Strategies (I): Insomnia
Currently, there are no clear recommendations regarding the most effective pharmacological treatment approach for insomnia in patients with schizophrenia [162]. Sleep-inducing effects of antipsychotics are largely utilized in clinical settings. Barring eszopiclone, most of the recommended treatments for primary insomnia, such as zaleplon, zolpidem, ramelteon, doxepin, and suvorexant, have not been studied adequately in schizophrenia probands [163]. Interestingly, diazepam, a classical benzodiazepine hypnotic, has been found to be effective in halting psychotic progression in those with early signs of exacerbation in a randomized controlled trial (RCT) by Carpenter et al. [164] Among the off-label options, only melatonin, paliperidone, and sodium oxybate have been systematically studied and are described briefly in this section (for detailed review, please see Stummer et al. [15]).
5.2.1 Antipsychotics
Antipsychotics improve overall quality and efficiency of sleep by reducing psychotic hypervigilance; mostly explained by its anti-dopaminergic actions. Moreover, GABAergic deficits secondary to activation of dopaminergic neurotransmission (discussed in Sect. 20.3.3.1) have been suggested to be undone by dopaminergic antagonism, primarily. Antagonistic effects at histaminergic and alpha-1 adrenergic receptors help enhance sleep, more directly. Anticholinergic effects have been shown to decrease the intensity of REM sleep and lengthen REM latency. Serotonin receptor antagonism has been shown to promote sedation and slow wave sleep as well [15]. A summary of effects on various sleep parameters (continuity and architectural) is provided in Table 20.4 (for review, see Stummer et al. [15] and Katshu et al. [165]). Antipsychotic effects on sleep are discussed in detail in Chap. 41).
Limited but available data on head-to-head comparisons show risperidone to be better than haloperidol, chlorpromazine, and flupentixol and, olanzapine to be better than clozapine (especially with respect to SWS) [15]. However, as a norm, more sedating second-generation antipsychotics such as quetiapine, olanzapine, or risperidone have been utilized for treating insomnia in schizophrenia [166]. Moreover, a switch to second-generation antipsychotic from a first-generation antipsychotic has been shown to improve subjective sleep quality, which also correlates with improvement in negative symptoms [15]. Although investigation into improvement in sleep profile with quetiapine is carried out less systematically, its use in low dosages (25–75 mg) as an augmentation is largely evidenced from practice-based data [15]. Paliperidone has been the only systematically (i.e., randomized, placebo-controlled trial designs) studied antipsychotic for the treatment of insomnia in patients with schizophrenia. Paliperidone (3–12 mg) has been shown to increase increased total sleep time, decreased latency to sleep onset, improved sleep quality, and decreased daytime drowsiness [15, 167].
5.2.2 Melatonin
Ever since Ferrier et al. [168] in 1982 showed that schizophrenia probands with insomnia to have disturbed patterns of melatonin secretion (to the order of 2:3), use of melatonin in this regard has gained attention. Moreover, recent studies also suggest its role in reducing brain oxidative stress [169] and in the neurodevelopmental etiology of schizophrenia [170]. While Stummer et al. [15] in a very recent review identified three trials using melatonin in the treatment of sleep in schizophrenia, Oliveira et al. [167] in their systematic review include two trials using melatonin alongside one each for eszopiclone and paliperidone for treating insomnia in schizophrenia. Both report good tolerability and improvement with melatonin (average dosage 2–3 mg) in sleep efficiency and additional therapeutic benefits in attenuating metabolic adverse effects of second-generation antipsychotics.
5.2.3 Zopiclone/Eszopiclone
Non-benzodiazepine sedatives have been shown to accentuate slow wave sleep with minimal effects on REM sleep in contrast to benzodiazepines. Eszopiclone has been shown to increase sleep spindles during NREM stage 2 in schizophrenia patients, even in those without insomnia, [171] hence holding up promise in improving neuronal plasticity. A recent meta-analysis by Kishi et al. [172], which included three trials that studied the use of eszopiclone for insomnia in schizophrenia, concluded the molecule might be helpful in improving severity of insomnia. This meta-analysis also reports alpidem, another related “Z” drug, to have shown superiority in improving the overall schizophrenia symptoms, compared to placebo. Very recently, Mehta et al. [173] showed that eszopiclone is helpful in ameliorating persistent negative symptoms.
5.2.4 Sodium Oxybate
Sodium oxybate is routinely recommended in the treatment of cataplexy and narcolepsy [174]. Way back in the early 1980s, Levy et al. [175] reported a negative trial on the use of sodium oxybate in the treatment of schizophrenia. However, on a background of a hypothesis that GABA-B receptor agonists increase slow wave sleep, a systematic 4-week open-label trial by Kantrowitz et al. [176] showed that sodium oxybate (4.5 g/night titrated up to 9 g/night) improved subjective sleep quality, slow wave sleep, and total PANSS scores, with no additional benefits in cognition. Potential risk of abuse with this molecule, however, remains a concern over the therapeutic benefits [15]. Also there are anecdotal reports of sodium oxybate inducing or exacerbating psychotic symptoms [177] and cause sleep apnea [178].
5.3 Pharmacological Strategies (II): Other Comorbid Sleep Disorders
Strategies for treatment of comorbid disorders like OSA, narcolepsy, CRD, nightmares, and RLS/PLMS are described in detail elsewhere (Chaps. 16, 17, 18, 19, 20, respectively). Consultation liaison between a psychiatrist and sleep physicians is suggested while managing these comorbid disorders, especially when having to treat with drugs that are routinely not used in psychiatric settings. In the context of schizophrenia, there are certain points that need to be specified for each of these disorders.
-
RLS/PLMS: It is important to understand the role of antipsychotics in the causation of RLS/PLMS and also essential to differentiate it from antipsychotic-induced akathisia (described in Sect. 20.2.4.3). First-generation antipsychotics (FGAs) are less often reported to induce RLS than second-generation antipsychotics (SGAs), especially those with lower dopamine receptor occupancy, [179] an exception being aripiprazole. Aripiprazole not only seems to be less likely among all antipsychotics to cause RLS, but also has been used in the treatment of RLS [180,181,182,183]. However, an anecdotal case of aripiprazole-induced RLS also has been reported [184]. Gabapentin has been successfully used in the treatment of antipsychotic-induced RLS [179]. Caution should always be noted before initiating treatment of RLS with dopamine agonists like ropinirole and pramipexole, as these drugs are known to precipitate psychosis [185, 186].
-
OSA: While on one hand psychotic symptoms have been reported to improve when comorbid sleep apnea is successfully treated; [93] on the other hand, antipsychotics (especially SGAs), mediated with their association with impaired metabolic profile, precipitate OSA [187]. Continuous positive airway pressure (CPAP) therapy remains the mainstay [92].
-
Narcolepsy: Modafinil/armodafinil and sodium oxybate are the mainstay treatment for excessive daytime sleep and cataplexy in narcolepsy [188]. As discussed earlier in the previous section, sodium oxybate is also being used as an adjunct for treating insomnia in schizophrenia. Modafinil/armodafinil, although not successfully, have been studied as adjuncts in the management of cognitive and negative symptoms [189].
-
Nightmares: Prazosin has been suggested for nightmares in patients with schizophrenia [105].
-
Sleep state misperception (SSM): Although some benzodiazepines and Z-drugs have been shown to improve sleep perception in cases of primary insomnia by improving sleep itself [107], no data are available on schizophrenia patients.
5.4 Non-pharmacological Strategies
Waters et al. [162] found that cognitive behavior therapy (CBT) was the most preferred option (even preferred over pharmacotherapy) for treatment of insomnia among schizophrenia patients. While patients perceived pharmacological treatment as a short-term solution only, they saw CBT to have a potential to support and empower them in taking responsibility for their own recovery. Moreover, CBT for insomnia (CBT-I) is considered as a first-line recommended treatment for insomnia, generally [190]. Initially, Myers et al. [191] in a case series demonstrated feasibility and benefits of using CBT-I in schizophrenia with persecutory delusions. A subsequent pilot RCT demonstrated a significant reduction in insomnia (with a large effect size; d = 1.9) among patients with persistent delusions and hallucinations [192]. In a recent open-label trial, Chiu et al. [193] emphasized on the effectiveness of CBT-I for insomnia in schizophrenia; they emphasize that CBT-I ought to be tailored to the person and their insomnia presentation. Waite et al. [194] recommend adaptations in several factors specific to the context of sleep problems in schizophrenia for CBT. These include delusions and hallucinations interfering with sleep, attempts to use sleep as an escape from voices, circadian rhythm disruption, insufficient daytime activity, and fear of the bed based upon past adverse experiences.
Psychological treatment may also be essential for management of nightmares, which have been found to be the second most reported sleep complaint in schizophrenia [60]. Image rehearsal therapy (IRT), apart from prazosin, has been found to be helpful in these patients, especially in the early phases itself as an adjunct to standard treatment [105]. Behavioral approaches, primarily involving corrective feedbacks, have been used in “reversing” misperception of sleep in SSM [107]. While the long-term utility of these techniques are yet to be investigated, no study so far has specifically studied schizophrenia patients.
Brain stimulation therapies also have a role in improving sleep in schizophrenia. Limited but available literature on utility of electroconvulsive therapy (ECT) on sleep in schizophrenia suggests that it improves sleep efficiency, REM latency, and REM density [195]. Interestingly, Kim et al. [196] in an animal model reported electroconvulsive seizure to alter expression and daily oscillation of circadian genes that are implicated in sleep deficits in schizophrenia. Göder et al. [197] investigated the use of transcranial direct current stimulation applied during night in schizophrenia patients. Although they did not report any significant differences in sleep parameters compared to sham condition, they found improvements in certain cognitive variables. While use of transcranial magnetic stimulation in improving sleep profiles has been considered in psychiatric disorders such as depression [198], its use in schizophrenia is sparse.
Among complementary and alternative treatments, acupuncture has shown to improve several impaired sleep parameters in schizophrenia patients—SOL, SE, and WASO [199].
6 Approach to a Patient
As per the literature reviewed over the course of this chapter, we present an algorithm on how to approach a patient of schizophrenia with sleep disturbances (see Fig. 20.3). This algorithm does not represent strict guidelines (which are nonexistent due to limited data) but provides a rough guide in approaching this subset of patients.
Algorithm for approach to assess and manage sleep disturbances in patients of schizophrenia. CRD circadian rhythm disruption; RLS restless leg syndrome; PLMS periodic limb movement syndrome; OSA obstructive sleep apnea; PSG polysomnography; SSM sleep state misperception; TST total sleep time; SE sleep efficiency; SOL sleep onset latency; NREM1 non-rapid eye movement-stage 1; NREM2 non-rapid eye movement-stage 2; SWS slow wave sleep; REM rapid eye movement; WASO wake time after sleep onset; FGA first-generation antipsychotic; SGA second-generation antipsychotic; ECT electro-convulsive therapy; rTMS repetitive transcranial magnetic stimulation; tDCS transcranial direct current stimulation; CPAP continuous positive airway pressure; CBT-I cognitive behavioral therapy for insomnia; IRT image rehearsal therapy
This self-explanatory algorithm is divided into various phases, which define such an approach. They begin with clinical history and assessment, moving on to laboratory assessments (primarily polysomnography) and review of existing treatments corresponding to various phases of illness. Emphasis on reviewing sleep symptoms on each visit has been made, especially where clinical history does not reveal either subjective or objective sleep complaints. The algorithm culminates in management phase, which is classified into those treated on traditional-psychiatry lines and those requiring consultation liaison with sleep physicians.
7 Conclusion
Although sleep and its disturbances do not form a necessary domain in diagnosing schizophrenia according to existing classificatory systems, they are worth evaluating in each case. Sleep disturbances and disorders are commonly comorbid with schizophrenia. Most consistent evidence is in the form of insomnia and specifically sleep spindle and slow wave sleep deficits, apart from macrostructural abnormalities like reduced total sleep time and efficiency, increased sleep onset latency, and nighttime wakefulness. Preliminary data from both animal and human studies point to the thalamic reticular nucleus and abnormalities in the thalamo-cortical-thalamic network as the basic pathophysiological unit for sleep disturbances in schizophrenia. Although traditionally sleep disturbances were thought to be secondary to schizophrenia symptoms, the relationship between the two is bidirectional and rather intricate. Although limited number of studies are available on specific treatment strategies for sleep disturbances/disorders in schizophrenia probands, an accountable guidance for treatment approach could be put forth. Proper attention to assessment of sleep in schizophrenia and treatment of its disturbances can be the key in improving overall prognosis of this disorder. Certainly, data from high quality research (both experimental and clinical) studies are needed for improving the management guidelines.
References
Cohrs S. Sleep disturbances in patients with schizophrenia: impact and effect of antipsychotics. CNS Drugs. 2008;22(11):939–62. https://doi.org/10.2165/00023210-200822110-00004.
Klingaman EA, Palmer-Bacon J, Bennett ME, Rowland LM. Sleep disorders among people with schizophrenia: emerging research. Curr Psychiatry Rep. 2015;17(10):79. https://doi.org/10.1007/s11920-015-0616-7.
Kraepelin E. Dementia praecox and paraphrenia. Edinburgh, Scotland: E.S. Livingston; 1919.
Bleuler EP. Dementia Praecox or the Group of Schizophrenias. Leipzig: Deuticke; 1911. (Translated by Zikkin, J., 1950. International Universities Press, New York).
Yang C, Winkelman JW. Clinical significance of sleep EEG abnormalities in chronic schizophrenia. Schizophr Res. 2006;82(2–3):251–60.
Davies G, Haddock G, Yung AR, Mulligan LD, Kyle SD. A systematic review of the nature and correlates of sleep disturbance in early psychosis. Sleep Med Rev. 2017;31:25–38. https://doi.org/10.1016/j.smrv.2016.01.001.
Afonso P, Viveiros V, Vinhas de Sousa T. Sleep disturbances in schizophrenia. Acta Medica Port. 2011;24(Suppl 4):799–806.
Benson KL. Sleep in schizophrenia: pathology and treatment. Sleep Med Clin. 2015;10(1):49–55. https://doi.org/10.1016/j.jsmc.2014.11.001.
Zanini MA, Castro J, Cunha GR, Asevedo E, Pan PM, Bittencourt L, Coelho FM, Tufik S, Gadelha A, Bressan RA, Brietzke E. Abnormalities in sleep patterns in individuals at risk for psychosis and bipolar disorder. Schizophr Res. 2015;169(1–3):262–7. https://doi.org/10.1016/j.schres.2015.08.023.
D’Agostino A, Castelnovo A, Cavallotti S, Casetta C, Marcatili M, Gambini O, Canevini M, Tononi G, Riedner B, Ferrarelli F, Sarasso S. Sleep endophenotypes of schizophrenia: slow waves and sleep spindles in unaffected first-degree relatives. NPJ Schizophr. 2018;4(1):2. https://doi.org/10.1038/s41537-018-0045-9.
Sarkar S, Katshu MZ, Nizamie SH, Praharaj SK. Slow wave sleep deficits as a trait marker in patients with schizophrenia. Schizophr Res. 2010;124(1–3):127–33. https://doi.org/10.1016/j.schres.2010.08.013.
Ferrarelli F. Sleep in patients with schizophrenia. Curr Sleep Med Rep. 2015;1(2):150–6. https://doi.org/10.1007/s40675-015-0010-3.
Kaskie RE, Graziano B, Ferrarelli F. Schizophrenia and sleep disorders: links, risks, and management challenges. Nat Sci Sleep. 2017;9:227–39. https://doi.org/10.2147/NSS.S121076.
Monti JM, BaHammam AS, Pandi-Perumal SR, Bromundt V, Spence DW, Cardinali DP, Brown GM. Sleep and circadian rhythm dysregulation in schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;43:209–16. https://doi.org/10.1016/j.pnpbp.2012.12.021.
Stummer L, Markovic M, Maroney ME. Pharmacologic treatment options for insomnia in patients with schizophrenia. Medicines (Basel). 2018;5(3):E88. https://doi.org/10.3390/medicines5030088.
Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems of human subjects. Los Angeles, CA: UCLA Information Service, Brain Research Institute; 1968.
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pitts-burgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
Baandrup L, Glenthøj BY, Jennum PJ. Objective and subjective sleep quality: melatonin versus placebo add-on treatment in patients with schizophrenia or bipolar disorder withdrawing from long-term benzodiazepine use. Psychiatry Res. 2016;240:163–9. https://doi.org/10.1016/j.psychres.2016.04.031.
Hofstetter JR, Lysaker PH, Mayeda AR. Quality of sleep in patients with schizophrenia is associated with quality of life and coping. BMC Psychiatry. 2005;5:13.
Kajimura N, Kato M, Okuma T, Sekimoto M, Watanabe T, Takahashi K. A quantitative sleep-EEG study on the effects of benzodiazepine and zopiclone in schizophrenic patients. Schizophr Res. 1995;15(3):303–12.
Ritsner M, Kurs R, Ponizovsky A, Hadjez J. Perceived quality of life in schizophrenia: relationships to sleep quality. Qual Life Res. 2004;13(4):783–91.
Rotenberg P, Indurski R, Kimhi J, Hadjez Y, Gutman E, Shamir Y, Barak A, Elizur V. The relationship between objective sleep variables and subjective sleep estimation in schizophrenia. Int J Psychiatry Clin Pract. 2000;4(1):63–7. https://doi.org/10.1080/13651500050518415.
Müller MJ, Olschinski C, Kundermann B, Cabanel N. Subjective sleep quality and sleep duration of patients in a psychiatric hospital. Sleep Sci. 2016;9(3):202–6. https://doi.org/10.1016/j.slsci.2016.08.004.
Chan MS, Chung KF, Yung KP, Yeung WF. Sleep in schizophrenia: a systematic review and meta-analysis of polysomnographic findings in case-control studies. Sleep Med Rev. 2017;32:69–84. https://doi.org/10.1016/j.smrv.2016.03.001.
Zarcone VP, Benson KL. BPRS symptom factors and sleep variables in schizophrenia. Psychiatry Res. 1997;66(2–3):111–20.
Poulin J, Daoust AM, Forest G, Stip E, Godbout R. Sleep architecture and its clinical correlates in first episode and neuroleptic-naive patients with schizophrenia. Schizophr Res. 2003;62(1–2):147–53.
Monti JM, Monti D. Sleep in schizophrenia patients and the effects of antipsychotic drugs. Sleep Med Rev. 2004;8(2):133–48.
Tandon R, Shipley JE, Eiser AS, Greden JF. Association between abnormal REM sleep and negative symptoms in schizophrenia. Psychiatry Res. 1989;27(3):359–61.
Tandon R, Shipley JE, Taylor S, Greden JF, Eiser A, DeQuardo J, Goodson J. Electroencephalographic sleep abnormalities in schizophrenia. Relationship to positive/negative symptoms and prior neuroleptic treatment. Arch Gen Psychiatry. 1992;49(3):185–94.
Keshavan MS, Miewald J, Haas G, Sweeney J, Ganguli R, Reynolds CF. Slow-wave sleep and symptomatology in schizophrenia and related psychotic disorders. J Psychiatr Res. 1995;29(4):303–14.
Keshavan MS, Reynolds CF 3rd, Miewald JM, Montrose DM. A longitudinal study of EEG sleep in schizophrenia. Psychiatry Res. 1996;59(3):203–11.
Ilanković A, Damjanović A, Ilanković V, Filipović B, Janković S, Ilanković N. Polysomnographic sleep patterns in depressive, schizophrenic and healthy subjects. Psychiatr Danub. 2014;26(1):20–6.
Keshavan MS, Reynolds CF, Kupfer DJ. Electroencephalographic sleep in schizophrenia: a critical review. Compr Psychiatry. 1990;31(1):34–47.
Hudson JI, Lipinski JF, Keck PE Jr, Aizley HG, Vuckovic A, Zierk KC, Pope HG Jr. Polysomnographic characteristics of schizophrenia in comparison with mania and depression. Biol Psychiatry. 1993;34(3):191–3.
McGorry P, Keshavan M, Goldstone S, Amminger P, Allott K, Berk M, Lavoie S, Pantelis C, Yung A, Wood S, Hickie I. Biomarkers and clinical staging in psychiatry. World Psychiatry. 2014;13(3):211–23. https://doi.org/10.1002/wps.20144.
Muzet A. Alteration of sleep microstructure in psychiatric disorders. Dialogues Clin Neurosci. 2005;7(4):315–21.
Guénolé F, Chevrier E, Stip E, Godbout R. A microstructural study of sleep instability in drug-naive patients with schizophrenia and healthy controls: sleep spindles, rapid eye movements, and muscle atonia. Schizophr Res. 2014;155(1–3):31–8. https://doi.org/10.1016/j.schres.2014.03.013.
Benson KL, Zarcone VP. Schizophrenia. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. New York, NY: WB Saunders; 2000. p. 1159–67.
Wooten VD, Buysse DJ. Sleep in psychiatric disorders. In: Chokroverty S, Daroff RB, editors. Sleep disorders medicine: basic sciences, technical considerations, and clinical aspects. Boston, MA: Butterworth-Heinemann Medical; 1999. p. 573–86.
Zarcone VP, Benson KL. Sleep and schizophrenia. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 2nd ed. Philadelphia: WB Saunders; 1994. p. 914–26.
Manoach DS, Pan JQ, Purcell SM, Stickgold R. Reduced sleep spindles in schizophrenia: a treatable endophenotype that links risk genes to impaired cognition? Biol Psychiatry. 2016;80(8):599–608. https://doi.org/10.1016/j.biopsych.2015.10.003.
Schilling C, Schlipf M, Spietzack S, Rausch F, Eisenacher S, Englisch S, Reinhard I, Haller L, Grimm O, Deuschle M, Tost H, Zink M, Meyer-Lindenberg A, Schredl M. Fast sleep spindle reduction in schizophrenia and healthy first-degree relatives: association with impaired cognitive function and potential intermediate phenotype. Eur Arch Psychiatry Clin Neurosci. 2017;267(3):213–24. https://doi.org/10.1007/s00406-016-0725-2.
Wamsley EJ, Tucker MA, Shinn AK, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71(2):154–61. https://doi.org/10.1016/j.biopsych.2011.08.008.
Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483–92.
Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, Bria P, Kalin NH, Tononi G. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167(11):1339–48. https://doi.org/10.1176/appi.ajp.2010.09121731.
Manoach DS, Demanuele C, Wamsley EJ, Vangel M, Montrose DM, Miewald J, Kupfer D, Buysse D, Stickgold R, Keshavan MS. Sleep spindle deficits in antipsychotic-naïve early course schizophrenia and in non-psychotic first-degree relatives. Front Hum Neurosci. 2014;8:762. https://doi.org/10.3389/fnhum.2014.00762.
Göder R, Fritzer G, Gottwald B, Lippmann B, Seeck-Hirschner M, Serafin I, Aldenhoff JB. Effects of olanzapine on slow wave sleep, sleep spindles and sleep-related memory consolidation in schizophrenia. Pharmacopsychiatry. 2008;41:92–9.
Hirshkowitz M, Thornby JI, Karacan I. Sleep spindles: pharmacological effects in humans. Sleep. 1982;5:85–94.
Castelnovo A, D’Agostino A, Casetta C, Sarasso S, Ferrarelli F. Sleep spindle deficit in schizophrenia: contextualization of recent findings. Curr Psychiatry Rep. 2016;18(8):72. https://doi.org/10.1007/s11920-016-0713-2.
Castelnovo A, Graziano B, Ferrarelli F, D’Agostino A. Sleep spindles and slow waves in schizophrenia and related disorders: main findings, challenges and future perspectives. Eur J Neurosci. 2018;48(8):2738–58. https://doi.org/10.1111/ejn.13815.
Knott V, Labelle A, Jones B, Mahoney C. Quantitative EEG in schizophrenia and in response to acute and chronic clozapine treatment. Schizophr Res. 2001;50:41–53. https://doi.org/10.1016/S0920-9964(00)00165-1.
Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2016;33:51–7. https://doi.org/10.1016/j.smrv.2016.05.002.
Ganguli R, Reynolds CF 3rd, Kupfer DJ. Electroencephalographic sleep in young, never-medicated schizophrenics. A comparison with delusional and nondelusional depressives and with healthy controls. Arch Gen Psychiatry. 1987;44(1):36–44.
Sekimoto M, Kato M, Watanabe T, Kajimura N, Takahashi K. Cortical regional differences of delta waves during all-night sleep in schizophrenia. Schizophr Res. 2011;126(1–3):284–90. https://doi.org/10.1016/j.schres.2010.11.003.
Tekell JL, Hoffmann R, Hendrickse W, Greene RW, Rush AJ, Armitage R. High frequency EEG activity during sleep: characteristics in schizophrenia and depression. Clin EEG Neurosci. 2005;36(1):25–35.
Kaskie RE, Gill KM, Ferrarelli F. Reduced frontal slow wave density during sleep in first-episode psychosis. Schizophr Res. 2018; https://doi.org/10.1016/j.schres.2018.10.024.
Hoffmann R, Hendrickse W, Rush AJ, Armitage R. Slow-wave activity during non-REM sleep in men with schizophrenia and major depressive disorders. Psychiatry Res. 2000;95(3):215–25.
Hiatt JF, Floyd TC, Katz PH, Feinberg I. Further evidence of abnormal non-rapid-eye-movement sleep in schizophrenia. Arch Gen Psychiatry. 1985;42(8):797–802.
Hombali A, Seow E, Yuan Q, Chang SHS, Satghare P, Kumar S, Verma SK, Mok YM, Chong SA, Subramaniam M. Prevalence and correlates of sleep disorder symptoms in psychiatric disorders. Psychiatry Res. 2018; https://doi.org/10.1016/j.psychres.2018.07.009.
Reeve S, Sheaves B, Freeman D. Sleep disorders in early psychosis: incidence, severity, and association with clinical symptoms. Schizophr Bull. 2018;45(2):287–95. https://doi.org/10.1093/schbul/sby129.
Freeman D, Pugh K, Vorontsova N, Southgate L. Insomnia and paranoia. Schizophr Res. 2009;108(1–3):280–4. https://doi.org/10.1016/j.schres.2008.12.001.
Xiang YT, Weng YZ, Leung CM, Tang WK, Lai KY, Ungvari GS. Prevalence and correlates of insomnia and its impact on quality of life in Chinese schizophrenia patients. Sleep. 2009;32(1):105–9.
Hou CL, Li Y, Cai MY, Ma XR, Zang Y, Jia FJ, Lin YQ, Ungvari GS, Chiu HF, Ng CH, Zhong BL, Cao XL, Tam MI, Xiang YT. Prevalence of insomnia and clinical and quality of life correlates in Chinese patients with schizophrenia treated in primary care. Perspect Psychiatr Care. 2017;53(2):80–6. https://doi.org/10.1111/ppc.12139.
Li SX, Lam SP, Zhang J, Yu MW, Chan JW, Chan CS, Espie CA, Freeman D, Mason O, Wing YK. Sleep disturbances and suicide risk in an 8-year longitudinal study of schizophrenia-spectrum disorders. Sleep. 2016;39(6):1275–82. https://doi.org/10.5665/sleep.5852.
Chiu VW, Harvey RH, Sloan NB, Ree M, Lin A, Janca A, Waters F. Cognitive and behavioral factors associated with insomnia in inpatients with schizophrenia and related psychoses. J Nerv Ment Dis. 2015;203(10):798–803. https://doi.org/10.1097/NMD.0000000000000370.
Freeman D, Brugha T, Meltzer H, Jenkins R, Stahl D, Bebbington P. Persecutory ideation and insomnia: findings from the second British National Survey of Psychiatric Morbidity. J Psychiatr Res. 2010;44(15):1021–6. https://doi.org/10.1016/j.jpsychires.2010.03.018.
Miller BJ, Parker CB, Rapaport MH, Buckley PF, McCall WV. Insomnia and suicidal ideation in non-affective psychosis. Sleep. 2018; https://doi.org/10.1093/sleep/zsy215.
Pompili M, Serafini G, Innamorati M, Lester D, Shrivastava A, Girardi P, Nordentoft M. Suicide risk in first episode psychosis: a selective review of the current literature. Schizophr Res. 2011;129(1):1–11. https://doi.org/10.1016/j.schres.2011.03.008.
Afonso P, Brissos S, Figueira ML, Paiva T. Schizophrenia patients with predominantly positive symptoms have more disturbed sleep-wake cycles measured by actigraphy. Psychiatry Res. 2011;189(1):62–6. https://doi.org/10.1016/j.psychres.2010.12.031.
Bromundt V, Köster M, Georgiev-Kill A, Opwis K, Wirz-Justice A, Stoppe G, Cajochen C. Sleep-wake cycles and cognitive functioning in schizophrenia. Br J Psychiatry. 2011;198(4):269–76. https://doi.org/10.1192/bjp.bp.110.078022.
Martin JL, Jeste DV, Ancoli-Israel S. Older schizophrenia patients have more disrupted sleep and circadian rhythms than age-matched comparison subjects. J Psychiatr Res. 2005;39(3):251–9. https://doi.org/10.1016/j.jpsychires.2004.08.011.
Van Cauter E, Linkowski P, Kerkhofs M, Hubain P, L’Hermite-Balériaux M, Leclercq R, Brasseur M, Copinschi G, Mendlewicz J. Circadian and sleep-related endocrine rhythms in schizophrenia. Arch Gen Psychiatry. 1991;48(4):348–56.
Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM. Sleep and circadian rhythm disruption in schizophrenia. Br J Psychiatry. 2012;200(4):308–16. https://doi.org/10.1192/bjp.bp.111.096321.
Oliver PL, Sobczyk MV, Maywood ES, Edwards B, Lee S, Livieratos A, Oster H, Butler R, Godinho SI, Wulff K, Peirson SN, Fisher SP, Chesham JE, Smith JW, Hastings MH, Davies KE, Foster RG. Disrupted circadian rhythms in a mouse model of schizophrenia. Curr Biol. 2012;22(4):314–9. https://doi.org/10.1016/j.cub.2011.12.051.
Tam SK, Pritchett D, Brown LA, Foster RG, Bannerman DM, Peirson SN. Sleep and circadian rhythm disruption and recognition memory in schizophrenia. Methods Enzymol. 2015;552:325–49. https://doi.org/10.1016/bs.mie.2014.10.008.
Jagannath A, Peirson SN, Foster RG. Sleep and circadian rhythm disruption in neuropsychiatric illness. Curr Opin Neurobiol. 2013;23(5):888–94. https://doi.org/10.1016/j.conb.2013.03.008.
Jones SG, Benca RM. Circadian disruption in psychiatric disorders. Sleep Med Clin. 2015;10(4):481–93. https://doi.org/10.1016/j.jsmc.2015.07.004.
Pritchett D, Wulff K, Oliver PL, Bannerman DM, Davies KE, Harrison PJ, Peirson SN, Foster RG. Evaluating the links between schizophrenia and sleep and circadian rhythm disruption. J Neural Transm (Vienna). 2012;119(10):1061–75.
Kang SG, Lee HJ, Jung SW, Cho SN, Han C, Kim YK, Kim SH, Lee MS, Joe SH, Jung IK, Kim L. Characteristics and clinical correlates of restless legs syndrome in schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2007a;31(5):1078–83.
Kang SG, Lee HJ, Choi JE, Park JH, Lee SS, Han C, Kim YK, Kim SH, Lee MS, Joe SH, Jung IK, Kim L. Possible association between G-protein β3 subunit C825T polymorphism and antipsychotic-induced restless legs syndrome in schizophrenia. Acta Neuropsychiatr. 2007b;19(6):351–6. https://doi.org/10.1111/j.1601-5215.2007.00240.x.
Kang SG, Lee HJ, Choi JE, Park YM, Park JH, Han C, Kim YK, Kim SH, Lee MS, Joe SH, Jung IK, Kim L. Association study between antipsychotics- induced restless legs syndrome and polymorphisms of dopamine D1, D2, D3, and D4 receptor genes in schizophrenia. Neuropsychobiology. 2008;57(1–2):49–54. https://doi.org/10.1159/000129667.
Kang SG, Park YM, Choi JE, Lim SW, Lee HJ, Lee SH, Kim YK, Kim SH, Cho SN, Kim L. Association study between antipsychotic-induced restless legs syndrome and polymorphisms of monoamine oxidase genes in schizophrenia. Hum Psychopharmacol. 2010;25(5):397–403. https://doi.org/10.1002/hup.1130.
Kang SG, Lee HJ, Park YM, Yang HJ, Song HM, Lee YJ, Cho SJ, Cho SN, Kim L. The BTBD9 gene may be associated with antipsychotic-induced restless legs syndrome in schizophrenia. Hum Psychopharmacol. 2013;28(2):117–23. https://doi.org/10.1002/hup.2287.
Kang SG, Lee HJ, Lee SH, Kim L. MEIS1, a promising candidate gene, is not associated with the core symptoms of antipsychotic-induced restless legs syndrome in Korean schizophrenia patients. Psychiatry Investig. 2015;12(2):263–7. https://doi.org/10.4306/pi.2015.12.2.263.
Kang SG, Lee YJ, Park YM, Kim L, Lee HJ. Haplotype association of the MAP 2K5 gene with antipsychotics-induced symptoms of restless legs syndrome among patients with schizophrenia. Psychiatry Investig. 2018;15(1):84–9. https://doi.org/10.4306/pi.2018.15.1.84.
Cho CH, Kang SG, Choi JE, Park YM, Lee HJ, Kim L. Association between antipsychotics-induced restless legs syndrome and tyrosine hydroxylase gene polymorphism. Psychiatry Investig. 2009;6(3):211–5. https://doi.org/10.4306/pi.2009.6.3.211.
Aggarwal S, Dodd S, Berk M. Restless leg syndrome associated with atypical antipsychotics: current status, pathophysiology, and clinical implications. Curr Drug Saf. 2015;10(2):98–105.
Walters AS, Hening W, Rubinstein M, Chokroverty S. A clinical and polysomnographic comparison of neuroleptic-induced akathisia and the idiopathic restless legs syndrome. Sleep. 1991;14(4):339–45.
Gunduz A, Metin B, Metin SZ, Poyraz BC, Karadeniz D, Kiziltan G, Kiziltan ME. Lower limb flexor reflex: comparisons between drug-induced akathisia and restless legs syndrome. Neurosci Lett. 2017;641:40–4. https://doi.org/10.1016/j.neulet.2017.01.042.
Staedt J, Dewes D, Danos P, Stoppe G. Can chronic neuroleptic treatment promote sleep disturbances in elderly schizophrenic patients? Int J Geriatr Psychiatry. 2000;15(2):170–6.
Ancoli-Israel S, Martin J, Jones DW, Caligiuri M, Patterson T, Harris MJ, Jeste DV. Sleep-disordered breathing and periodic limb movements in sleep in older patients with schizophrenia. Biol Psychiatry. 1999;45(11):1426–32.
Gupta MA, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. J Clin Sleep Med. 2015;11(2):165–75. https://doi.org/10.5664/jcsm.4466.
Kalucy MJ, Grunstein R, Lambert T, Glozier N. Obstructive sleep apnoea and schizophrenia—a research agenda. Sleep Med Rev. 2013;17(5):357–65. https://doi.org/10.1016/j.smrv.2012.10.003.
Myles H, Myles N, Antic NA, Adams R, Chandratilleke M, Liu D, Mercer J, Vakulin A, Vincent A, Wittert G, Galletly C. Obstructive sleep apnea and schizophrenia: a systematic review to inform clinical practice. Schizophr Res. 2016;170(1):222–5. https://doi.org/10.1016/j.schres.2015.11.014.
Stubbs B, Vancampfort D, Veronese N, Solmi M, Gaughran F, Manu P, Rosenbaum S, De Hert M, Fornaro M. The prevalence and predictors of obstructive sleep apnea in major depressive disorder, bipolar disorder and schizophrenia: a systematic review and meta-analysis. J Affect Disord. 2016;197:259–67. https://doi.org/10.1016/j.jad.2016.02.060.
Szaulińska K, Pływaczewski R, Sikorska O, Holka-Pokorska J, Wierzbicka A, Wichniak A, Śliwiński P. Obstructive sleep apnea in severe mental disorders. Psychiatr Pol. 2015;49(5):883–95. https://doi.org/10.12740/PP/32566.
Walterfang M, Upjohn E, Velakoulis D. Is schizophrenia associated with narcolepsy? Cogn Behav Neurol. 2005;18(2):113–8.
Canellas F, Lin L, Julià MR, Clemente A, Vives-Bauza C, Ollila HM, Hong SC, Arboleya SM, Einen MA, Faraco J, Fernandez-Vina M, Mignot E. Dual cases of type 1 narcolepsy with schizophrenia and other psychotic disorders. J Clin Sleep Med. 2014;10(9):1011–8. https://doi.org/10.5664/jcsm.4040.
Cavalier Y, Kothare SV. The association of schizophrenia and narcolepsy in adolescents. Pediatr Neurol. 2018;83:56–7. https://doi.org/10.1016/j.pediatrneurol.2018.01.001.
Huang YS, Guilleminault C, Chen CH, Lai PC, Hwang FM. Narcolepsy-cataplexy and schizophrenia in adolescents. Sleep Med. 2014;15(1):15–22. https://doi.org/10.1016/j.sleep.2013.09.018.
Plazzi G, Fabbri C, Pizza F, Serretti A. Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology. 2015;71(4):218–24. https://doi.org/10.1159/000432400.
Talih FR. Narcolepsy presenting as schizophrenia: a literature review and two case reports. Innov Clin Neurosci. 2011;8(4):30–4.
Sansa G, Gavaldà A, Gaig C, Monreal J, Ercilla G, Casamitjana R, Ribera G, Iranzo A, Santamaria J. Exploring the presence of narcolepsy in patients with schizophrenia. BMC Psychiatry. 2016;16:177. https://doi.org/10.1186/s12888-016-0859-9.
Tsutsui K, Kanbayashi T, Tanaka K, Boku S, Ito W, Tokunaga J, Mori A, Hishikawa Y, Shimizu T, Nishino S. Anti-NMDA-receptor antibody detected in encephalitis, schizophrenia, and narcolepsy with psychotic features. BMC Psychiatry. 2012;12:37. https://doi.org/10.1186/1471-244X-12-37.
Seeman MV. Successful treatment of nightmares may reduce psychotic symptoms in schizophrenia. World J Psychiatry. 2018;8(3):75–8. https://doi.org/10.5498/wjp.v8.i3.75.
Bian Y, Wang ZX, Han XL, Chen L, Zhu Y, Wu CJ. Sleep state misperception in schizophrenia: are negative symptoms at work? Compr Psychiatry. 2016;67:33–8. https://doi.org/10.1016/j.comppsych.2016.02.008.
Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138(1):77–101. https://doi.org/10.1037/a0025730.
Ferrarelli F, Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophr Bull. 2011;37(2):306–15. https://doi.org/10.1093/schbul/sbq142.
Steullet P, Cabungcal JH, Bukhari SA, Ardelt MI, Pantazopoulos H, Hamati F, Salt TE, Cuenod M, Do KQ, Berretta S. The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress. Mol Psychiatry. 2018;23(10):2057–65. https://doi.org/10.1038/mp.2017.230.
Young A, Wimmer RD. Implications for the thalamic reticular nucleus in impaired attention and sleep in schizophrenia. Schizophr Res. 2017;180:44–7. https://doi.org/10.1016/j.schres.2016.07.011.
Buchmann A, Dentico D, Peterson MJ, Riedner BA, Sarasso S, Massimini M, Tononi G, Ferrarelli F. Reduced mediodorsal thalamic volume and prefrontal cortical spindle activity in schizophrenia. NeuroImage. 2014;102(Pt 2):540–7. https://doi.org/10.1016/j.neuroimage.2014.08.017.
Dorph-Petersen KA, Caric D, Saghafi R, Zhang W, Sampson AR, Lewis DA. Volume and neuron number of the lateral geniculate nucleus in schizophrenia and mood disorders. Acta Neuropathol. 2009;117(4):369–84. https://doi.org/10.1007/s00401-008-0410-2.
Dorph-Petersen KA, Lewis DA. Postmortem structural studies of the thalamus in schizophrenia. Schizophr Res. 2017;180:28–35. https://doi.org/10.1016/j.schres.2016.08.007.
Clawson BC, Durkin J, Aton SJ. Form and function of sleep spindles across the lifespan. Neural Plast. 2016;2016:6936381. https://doi.org/10.1155/2016/6936381.
Gardner RJ, Hughes SW, Jones MW. Differential spike timing and phase dynamics of reticular thalamic and prefrontal cortical neuronal populations during sleep spindles. J Neurosci. 2013;33(47):18469–80. https://doi.org/10.1523/JNEUROSCI.2197-13.2013.
Glausier JR, Lewis DA. Mapping pathologic circuitry in schizophrenia. Handb Clin Neurol. 2018;150:389–417. https://doi.org/10.1016/B978-0-444-63639-3.00025-6.
Lewis DA, Glausier JR. Alterations in prefrontal cortical circuitry and cognitive dysfunction in schizophrenia. Neb Symp Motiv. 2016;63:31–75.
Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015;5:e623. https://doi.org/10.1038/tp.2015.115.
Sprecher KE, Ferrarelli F, Benca RM. Sleep and plasticity in schizophrenia. Curr Top Behav Neurosci. 2015;25:433–58. https://doi.org/10.1007/7854_2014_366.
Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35(5):1110–24. https://doi.org/10.1016/j.neubiorev.2010.11.004.
Ferrarelli F, Tononi G. Reduced sleep spindle activity point to a TRN-MD thalamus-PFC circuit dysfunction in schizophrenia. Schizophr Res. 2017;180:36–43. https://doi.org/10.1016/j.schres.2016.05.023.
Parnaudeau S, Bolkan SS, Kellendonk C. The mediodorsal thalamus: an essential partner of the prefrontal cortex for cognition. Biol Psychiatry. 2018;83(8):648–56. https://doi.org/10.1016/j.biopsych.2017.11.008.
Yates NJ. Schizophrenia: the role of sleep and circadian rhythms in regulating dopamine and psychosis. Rev Neurosci. 2016;27(7):669–87. https://doi.org/10.1515/revneuro-2016-0030.
Keshavan MS, Tandon R. Sleep abnormalities in schizophrenia: pathophysiological significance. Psychol Med. 1993;23(4):831–5.
Charrier A, Olliac B, Roubertoux P, Tordjman S. Clock genes and altered sleep-wake rhythms: their role in the development of psychiatric disorders. Int J Mol Sci. 2017;18(5):E938. https://doi.org/10.3390/ijms18050938.
Johansson AS, Owe-Larsson B, Hetta J, Lundkvist GB. Altered circadian clock gene expression in patients with schizophrenia. Schizophr Res. 2016;174(1–3):17–23. https://doi.org/10.1016/j.schres.2016.04.029.
Lamont EW, Coutu DL, Cermakian N, Boivin DB. Circadian rhythms and clock genes in psychotic disorders. Isr J Psychiatry Relat Sci. 2010;47(1):27–35.
Jung JS, Lee HJ, Cho CH, Kang SG, Yoon HK, Park YM, Moon JH, Yang HJ, Song HM, Kim L. Association between restless legs syndrome and CLOCK and NPAS2 gene polymorphisms in schizophrenia. Chronobiol Int. 2014;31(7):838–44. https://doi.org/10.3109/07420528.2014.914034.
Assimakopoulos K, Karaivazoglou K, Skokou M, Kalogeropoulou M, Kolios P, Gourzis P, Patrinos GP, Tsermpini EE. Genetic variations associated with sleep disorders in patients with schizophrenia: a systematic review. Medicines (Basel). 2018;5(2):E27. https://doi.org/10.3390/medicines5020027.
Pocivavsek A, Rowland LM. Basic neuroscience illuminates causal relationship between sleep and memory: translating to schizophrenia. Schizophr Bull. 2018;44(1):7–14. https://doi.org/10.1093/schbul/sbx151.
Koyanagi A, Stickley A. The association between sleep problems and psychotic symptoms in the general population: a global perspective. Sleep. 2015;38(12):1875–85. https://doi.org/10.5665/sleep.5232.
Barton J, Kyle SD, Varese F, Jones SH, Haddock G. Are sleep disturbances causally linked to the presence and severity of psychotic-like, dissociative and hypomanic experiences in non-clinical populations? A systematic review. Neurosci Biobehav Rev. 2018;89:119–31. https://doi.org/10.1016/j.neubiorev.2018.02.008.
Waters F, Chiu V, Atkinson A, Blom JD. Severe sleep deprivation causes hallucinations and a gradual progression toward psychosis with increasing time awake. Front Psych. 2018;9:303. https://doi.org/10.3389/fpsyt.2018.00303.
Reeve S, Emsley R, Sheaves B, Freeman D. Disrupting sleep: the effects of sleep loss on psychotic experiences tested in an experimental study with mediation analysis. Schizophr Bull. 2018a;44(3):662–71. https://doi.org/10.1093/schbul/sbx103.
Cosgrave J, Haines R, van Heugten-van der Kloet D, Purple R, Porcheret K, Foster R, Wulff K. The interaction between subclinical psychotic experiences, insomnia and objective measures of sleep. Schizophr Res. 2018;193:204–8. https://doi.org/10.1016/j.schres.2017.06.058.
Mackie S, Winkelman JW. Restless legs syndrome and psychiatric disorders. Sleep Med Clin. 2015;10(3):351–7, xv. https://doi.org/10.1016/j.jsmc.2015.05.009.
Lunsford-Avery JR, Mittal VA. Sleep dysfunction prior to the onset of schizophrenia: a review and neurodevelopmental diathesis–stress conceptualization. Clin Psychol Sci Pract. 2013;20:291–320.
Reeve S, Sheaves B, Freeman D. The role of sleep dysfunction in the occurrence of delusions and hallucinations: a systematic review. Clin Psychol Rev. 2015;42:96–115. https://doi.org/10.1016/j.cpr.2015.09.001.
Zanini M, Castro J, Coelho FM, Bittencourt L, Bressan RA, Tufik S, Brietzke E. Do sleep abnormalities and misaligned sleep/circadian rhythm patterns represent early clinical characteristics for developing psychosis in high risk populations? Neurosci Biobehav Rev. 2013;37(10 Pt 2):2631–7. https://doi.org/10.1016/j.neubiorev.2013.08.012.
Poe SL, Brucato G, Bruno N, Arndt LY, Ben-David S, Gill KE, Colibazzi T, Kantrowitz JT, Corcoran CM, Girgis RR. Sleep disturbances in individuals at clinical high risk for psychosis. Psychiatry Res. 2017;249:240–3. https://doi.org/10.1016/j.psychres.2016.12.029.
Waite F, Bradley J, Chadwick E, Reeve S, Bird JC, Freeman D. The experience of sleep problems and their treatment in young people at ultra-high risk of psychosis: a thematic analysis. Front Psych. 2018;9:375. https://doi.org/10.3389/fpsyt.2018.00375.
Lunsford-Avery JR, LeBourgeois MK, Gupta T, Mittal VA. Actigraphic-measured sleep disturbance predicts increased positive symptoms in adolescents at ultra high-risk for psychosis: a longitudinal study. Schizophr Res. 2015;164(1–3):15–20. https://doi.org/10.1016/j.schres.2015.03.013.
Ruhrmann S, Schultze-Lutter F, Salokangas RK, Heinimaa M, Linszen D, Dingemans P, Birchwood M, Patterson P, Juckel G, Heinz A, Morrison A, Lewis S, von Reventlow HG, Klosterkötter J. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241–51. https://doi.org/10.1001/archgenpsychiatry.2009.206.
Lunsford-Avery JR, Gonçalves BDSB, Brietzke E, Bressan RA, Gadelha A, Auerbach RP, Mittal VA. Adolescents at clinical-high risk for psychosis: circadian rhythm disturbances predict worsened prognosis at 1-year follow-up. Schizophr Res. 2017;189:37–42. https://doi.org/10.1016/j.schres.2017.01.051.
Alderman T, Addington J, Bearden C, Cannon TD, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Cadenhead KS. Negative symptoms and impaired social functioning predict later psychosis in Latino youth at clinical high risk in the North American prodromal longitudinal studies consortium. Early Interv Psychiatry. 2015;9(6):467–75. https://doi.org/10.1111/eip.12128.
Keshavan MS, Diwadkar VA, Montrose DM, Stanley JA, Pettegrew JW. Premorbid characterization in schizophrenia: the Pittsburgh high risk study. World Psychiatry. 2004;3(3):163–8.
Kamath J, Virdi S, Winokur A. Sleep disturbances in schizophrenia. Psychiatr Clin North Am. 2015;38(4):777–92. https://doi.org/10.1016/j.psc.2015.07.007.
Sharma P, Dikshit R, Shah N, Karia S, De Sousa A. Excessive daytime sleepiness in schizophrenia: a naturalistic clinical study. J Clin Diagn Res. 2016;10(10):VC06–8.
Chouinard S, Poulin J, Stip E, Godbout R. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957–67.
Reeve S, Nickless A, Sheaves B, Freeman D. Insomnia, negative affect, and psychotic experiences: modelling pathways over time in a clinical observational study. Psychiatry Res. 2018b;269:673–80. https://doi.org/10.1016/j.psychres.2018.08.090.
Green MF, Nuechterlein KH. Should schizophrenia be treated as a neurocognitive disorder? Schizophr Bull. 1999;25(2):309–19.
Bell V, Halligan PW, Ellis HD. Explaining delusions: a cognitive perspective. Trends Cogn Sci. 2006;10(5):219–26.
Kaneko K. Negative symptoms and cognitive impairments in schizophrenia: two key symptoms negatively influencing social functioning. Yonago Acta Med. 2018;61(2):91–102.
Tripathi A, Kar SK, Shukla R. Cognitive deficits in schizophrenia: understanding the biological correlates and remediation strategies. Clin Psychopharmacol Neurosci. 2018;16(1):7–17. https://doi.org/10.9758/cpn.2018.16.1.7.
Lau EY, Wong ML, Lau KN, Hui FW, Tseng CH. Rapid-Eye-Movement-Sleep (REM) associated enhancement of working memory performance after a daytime nap. PLoS One. 2015;10(5):e0125752. https://doi.org/10.1371/journal.pone.0125752.
Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93(2):681–766. https://doi.org/10.1152/physrev.00032.2012.
Ulrich D. Sleep spindles as facilitators of memory formation and learning. Neural Plast. 2016;2016:1796715.
Göder R, Graf A, Ballhausen F, Weinhold S, Baier PC, Junghanns K, Prehn-Kristensen A. Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med. 2015;16(5):564–9. https://doi.org/10.1016/j.sleep.2014.12.022.
Das M, Das R, Khastgir U, Goswami U. REM sleep latency and neurocognitive dysfunction in schizophrenia. Indian J Psychiatry. 2005;47(3):133–8. https://doi.org/10.4103/0019-5545.55934.
Baandrup L, Christensen JAE, Fagerlund B, Jennum P. Investigation of sleep spindle activity and morphology as predictors of neurocognitive functioning in medicated patients with schizophrenia. J Sleep Res. 2018;28(1):e12672. https://doi.org/10.1111/jsr.12672.
Shorter E. A historical dictionary of psychiatry. New York: Oxford University Press; 2005. p. 69.
Waters F, Chiu VW, Janca A, Atkinson A, Ree M. Preferences for different insomnia treatment options in people with schizophrenia and related psychoses: a qualitative study. Front Psychol. 2015;6:990. https://doi.org/10.3389/fpsyg.2015.00990.
Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(2):307–49. https://doi.org/10.5664/jcsm.6470.
Carpenter WT Jr, Buchanan RW, Kirkpatrick B, Breier AF. Diazepam treatment of early signs of exacerbation in schizophrenia. Am J Psychiatry. 1999;156(2):299–303.
Katshu MZUH, Sarkar S, Nizamie SH. Effect of olanzapine on clinical and polysomnography profiles in patients with schizophrenia. Schizophr Res Treatment. 2018;2018:3968015. https://doi.org/10.1155/2018/3968015.
Krystal AD, Goforth HW, Roth T. Effects of antipsychotic medications on sleep in schizophrenia. Int Clin Psychopharmacol. 2008;23(3):150–60. https://doi.org/10.1097/YIC.0b013e3282f39703.
Oliveira P, Coroa M, Madeira N. Treatment options for insomnia in schizophrenia: a systematic review. Pharmacopsychiatry. 2018;52(4):165–9. https://doi.org/10.1055/a-0658-1645.
Ferrier IN, Arendt J, Johnstone EC, Crow TJ. Reduced nocturnal melatonin secretion in chronic schizophrenia: relationship to body weight. Clin Endocrinol. 1982;17(2):181–7.
Onaolapo AY, Aina OA, Onaolapo OJ. Melatonin attenuates behavioural deficits and reduces brain oxidative stress in a rodent model of schizophrenia. Biomed Pharmacother. 2017;92:373–83. https://doi.org/10.1016/j.biopha.2017.05.094.
Galván-Arrieta T, Trueta C, Cercós MG, Valdés-Tovar M, Alarcón S, Oikawa J, Zamudio-Meza H, Benítez-King G. The role of melatonin in the neurodevelopmental etiology of schizophrenia: a study in human olfactory neuronal precursors. J Pineal Res. 2017;63(3). https://doi.org/10.1111/jpi.12421.
Wamsley EJ, Shinn AK, Tucker MA, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. Sleep. 2013;36(9):1369–76. https://doi.org/10.5665/sleep.2968.
Kishi T, Inada K, Matsui Y, Iwata N. Z-drug for schizophrenia: a systematic review and meta-analysis. Psychiatry Res. 2017;256:365–70. https://doi.org/10.1016/j.psychres.2017.06.063.
Mehta UM, Ravishankar V, Thirthalli J. Eszopiclone for persistent negative symptoms in schizophrenia—an unintended N-of-1 study. Schizophr Res. 2018;193:438–40. https://doi.org/10.1016/j.schres.2017.06.035.
Ratneswaran C, Sagoo MK, Steier J. Preface for the 3rd Clinical Update Sleep, 23rd February 2018, Royal College of Physicians, London, UK: year in review. J Thorac Dis. 2018;10(Suppl 1):S1–S23. https://doi.org/10.21037/jtd.2017.10.162.
Levy MI, Davis BM, Mohs RC, Trigos GC, Mathé AA, Davis KL. Gamma-hydroxybutyrate in the treatment of schizophrenia. Psychiatry Res. 1983;9(1):1–8.
Kantrowitz JT, Oakman E, Bickel S, Citrome L, Spielman A, Silipo G, Battaglia J, Javitt DC. The importance of a good night’s sleep: an open-label trial of the sodium salt of gamma-hydroxybutyric acid in insomnia associated with schizophrenia. Schizophr Res. 2010;120(1–3):225–6. https://doi.org/10.1016/j.schres.2010.03.035.
Sarkanen T, Niemelä V, Landtblom AM, Partinen M. Psychosis in patients with narcolepsy as an adverse effect of sodium oxybate. Front Neurol. 2014;5:136. https://doi.org/10.3389/fneur.2014.00136.
Hartley S, Quera-Salva MA, Machou M. Sodium oxybate and sleep apnea: a clinical case. J Clin Sleep Med. 2011;7(6):667–8.
Kumar V, Venkatasubramanian G. Gabapentin treatment in clozapine-induced restless legs syndrome: two cases and a review of the literature. Ther Adv Psychopharmacol. 2017;7(1):42–7. https://doi.org/10.1177/2045125316672133.
Kikukawa S. Effectiveness of aripiprazole in treatment of adults with attention deficit disorder and restless legs syndrome. Int J Neuropsychopharmacol. 2008;11(3):439–40. https://doi.org/10.1017/S1461145707008310.
McLean AJ. The use of the dopamine-receptor partial agonist aripiprazole in the treatment of restless legs syndrome. Sleep. 2004;27(5):1022.
Raveendranathan D, Shiva L, Venkatasubramanian G, Rao MG, Varambally S, Gangadhar BN. Clozapine-induced restless legs syndrome treated with aripiprazole. J Neuropsychiatry Clin Neurosci. 2013;25(2):E62–3. https://doi.org/10.1176/appi.neuropsych.12050128.
Virit O, Selek S, Savas HA, Kokaçya H. Improvement of restless legs syndrome and trichotillomania with aripiprazole. J Clin Pharm Ther. 2009;34(6):723–5. https://doi.org/10.1111/j.1365-2710.2009.01051.x.
Bolaños-Vergaray J, Obaya JC, Gonzalez R, Echeverri C, Piquer P. Restless legs syndrome due to aripiprazole. Eur J Clin Pharmacol. 2011;67(5):539–40. https://doi.org/10.1007/s00228-010-0952-9.
Chopra A, Pendergrass DS, Bostwick JM. Mirtazapine-induced worsening of restless legs syndrome (RLS) and ropinirole-induced psychosis: challenges in management of depression in RLS. Psychosomatics. 2011;52(1):92–4. https://doi.org/10.1016/j.psym.2010.11.009.
Signorelli MS, Battaglia E, Costanzo MC, Cannavò D. Pramipexole induced psychosis in a patient with restless legs syndrome. BMJ Case Rep. 2013;2013:bcr2013009716. https://doi.org/10.1136/bcr-2013-009716.
Rishi MA, Shetty M, Wolff A, Amoateng-Adjepong Y, Manthous CA. Atypical antipsychotic medications are independently associated with severe obstructive sleep apnea. Clin Neuropharmacol. 2010;33(3):109–13. https://doi.org/10.1097/WNF.0b013e3181db8040.
Kallweit U, Bassetti CL. Pharmacological management of narcolepsy with and without cataplexy. Expert Opin Pharmacother. 2017;18(8):809–17. https://doi.org/10.1080/14656566.2017.1323877.
Wittkampf LC, Arends J, Timmerman L, Lancel M. A review of modafinil and armodafinil as add-on therapy in antipsychotic-treated patients with schizophrenia. Ther Adv Psychopharmacol. 2012;2(3):115–25. https://doi.org/10.1177/2045125312441815.
Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–41. https://doi.org/10.1016/S0140-6736(11)60750-2.
Myers E, Startup H, Freeman D. Cognitive behavioural treatment of insomnia in individuals with persistent persecutory delusions: a pilot trial. J Behav Ther Exp Psychiatry. 2011;42(3):330–6. https://doi.org/10.1016/j.jbtep.2011.02.004.
Freeman D, Waite F, Startup H, Myers E, Lister R, McInerney J, et al. Efficacy of cognitive behavioural therapy for sleep improvement in patients with persistent delusions and hallucinations (BEST): a prospective, assessor-blind, randomised controlled pilot trial. Lancet Psychiatry. 2015;2(11):975–83. https://doi.org/10.1016/S2215-0366(15)00314-4.
Chiu VW, Ree M, Janca A, Iyyalol R, Dragovic M, Waters F. Sleep profiles and CBT-I response in schizophrenia and related psychoses. Psychiatry Res. 2018;268:279–87. https://doi.org/10.1016/j.psychres.2018.07.027.
Waite F, Myers E, Harvey AG, Espie CA, Startup H, Sheaves B, Freeman D. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273–87. https://doi.org/10.1017/S1352465815000430.
Zhang ZJ, Chen YC, Wang HN, Wang HH, Xue YY, Feng SF, Tan QR. Electroconvulsive therapy improves antipsychotic and somnographic responses in adolescents with first-episode psychosis—a case-control study. Schizophr Res. 2012;137(1–3):97–103. https://doi.org/10.1016/j.schres.2012.01.037.
Kim SH, Park HG, Jeong SH, Kang UG, Ahn YM, Kim YS. Electroconvulsive seizure alters the expression and daily oscillation of circadian genes in the rat frontal cortex. Psychiatry Investig. 2018;15(7):717–26. https://doi.org/10.30773/pi.2018.01.18.2.
Göder R, Baier PC, Beith B, Baecker C, Seeck-Hirschner M, Junghanns K, Marshall L. Effects of transcranial direct current stimulation during sleep on memory performance in patients with schizophrenia. Schizophr Res. 2013;144(1–3):153–4. https://doi.org/10.1016/j.schres.2012.12.014.
Sanchez-Escandón O, Arana-Lechuga Y, Terán-Pérez G, Velazquez-Moctezuma J. Transcranial magnetic stimulation in sleep medicine. JSM Schizophr. 2017;2(2):1010.
van den Noort M, Yeo S, Lim S, Lee SH, Staudte H, Bosch P. Acupuncture as add-on treatment of the positive, negative, and cognitive symptoms of patients with schizophrenia: a systematic review. Medicines (Basel). 2018;5(2):E29. https://doi.org/10.3390/medicines5020029.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tikka, S.K., Garg, S. (2022). Sleep in Schizophrenia. In: Gupta, R., Neubauer, D.N., Pandi-Perumal, S.R. (eds) Sleep and Neuropsychiatric Disorders. Springer, Singapore. https://doi.org/10.1007/978-981-16-0123-1_20
Download citation
DOI: https://doi.org/10.1007/978-981-16-0123-1_20
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0122-4
Online ISBN: 978-981-16-0123-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)