Abstract
Effectiveness of bacteria mediated cancer therapy is gaining momentum as a re-discovered method of cancer treatment. It is principled on homing nature of certain anaerobic bacteria towards tumor microenvironment induced by its inherent hypoxic conditions. However, simple homing phenomenon is of low utility for tumor management, considering the toxicity and infection caused by these very same bacteria to the body. Recently, researchers have developed the ways of conjugation of nanoparticles with selected bacteria towards improving the treatment efficacy. Cargo-laden, expression-based, and antibody-guided approaches have emerged as major routes of application. Cargo-based technique potentiates delivery of chemotherapeutic drug as per tumor type. Genetically engineered microbes can deliver similar results, though long-term efficacy is unseen. Antibody-guided methods exercise specificity of drug-conjugated antibody against antigen-expressed over bacterial wall (located in tumor niche). Nonetheless, multiple challenges and executional limitations remain. In this review, we have focused on enhancement of bacterial functionality by nanoparticles for cancer management. We attempt to identify the challenges ahead and future perspectives of this emerging science. Considering the limited literature, we hope that this review will give its reader the enthusiasm in this field and possibly explore new avenues for precision bacteria-based cancer therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Cancer, regardless of primary or metastatic, tumorigenic or non-tumorigenic, is detrimental to human health and therefore development of its treatment strategies has gained much importance. Transformation of normal cells into tumorigenic may be stimulated by various risk factors—external agents (like smoking, alcohol, pollutants, specific food or high energy waves) or internal agents (like genetic/hereditary causes, age, body weight). Though these external factors may induce cancer, an antagonist role by similar action has been attempted for therapeutic value. Over recent years, association of microbial infection leading to cancer and therapeutic effects of bacteria on cancer have been elucidated (Rai et al. 2020; Ashu et al. 2019; Song et al. 2018).
Microbial role in cancer treatment has been in existence since the nineteenth century. The serendipity, in 1813, of the clostridial infection induced tumor regression made space in treatment approaches of certain cancers (Minton 2003; Vautier 1813). This therapy route gained traction in 1890 when William Coley perfected the therapy, leading to dawn of Coley’s Toxins (Coley 1991). It is principled on tendency of anaerobic bacteria to lodge themselves inside the tumor due to the favorable conditions of hypoxia and availability of nutrients (Dang et al. 2001; Zhao et al. 2005). Unlike Gram-positive bacteria, Gram-negative bacteria elicit the secretion of cytokines like tumor necrosis factor α (TNFα) owing to their endotoxins-lipopolysaccharide in the cell wall (Shear et al. 1943; Wiemann and Starnes 1994). TNFα plays an important role, but itself has limited efficacy for tumor regression (Wiemann and Starnes 1994). This finding brought T-cells into the forefront of active host immune cell-dependent tumor regression. However, as a pre-requisite, the T-cell must be made to respond to tumor before the therapy for effective regression of tumor (Berendt et al. 1978).
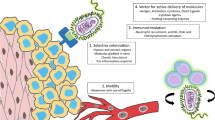
Bacteria after attaching on the certain tumor sites, can stimulate the production of Interleukin-12 (IL-12) (D’Andrea et al. 1992; Macatonia et al. 1995). IL-12 exhibits tumor regression better than TNFα. However, this treatment works well in some cases only. This is due to the requirement of Th1 response to be present in the body for IL-12 to exert its effects, i.e. the patient should have “pre-existing immunity” (Berendt et al. 1978; Iwasaki et al. 2000; Le et al. 2001). The removal of CD4+CD25+T-cells can enable this immunity (Ghiringhelli et al. 2004; Onizuka et al. 1999; Shimizu et al. 1999). Thereon, bacteria mediated cancer therapy has made tremendous progress with development of multitude of approaches (Fig. 14.1). “Targeting of tumor cells” approaches have traditionally involved injection of pro-drugs and bacteria in tumor hosts. However, limitations of routine chemotherapy, i.e. obstruction caused by hypoxic and acidic environment, render the purpose less fruitful.
Some of the major approaches involving bacteria for cancer therapy. (1) Bacteria expressing enzymes to activate anticancer “prodrugs” at tumor sites; (2) Bacteria expressing anticancer agents directly (e.g. anti-angiogenic like VEGFR inhibitor); (3) through bacteria released substances (toxins/enzymes—Bacteriocins/Phenazine 1,6-di-carboxylic acid (PDC)/bacterial enzyme toxin/bacterial spores and vectors as tumoricidal agents); (4) Bacteria transferring eukaryotic expression vectors into tumor cells (plasmid transfection); (5) Bacteria expressing oncogene silencing RNA; (6) delivery of tumor killing nanoparticle conjugated bacteria based therapies; (7) Bacteria expressing tumor specific antigens and antibodies; (8) Targeting of tumor stroma; (9) Bacteria as anticancer agents through biofilms; (10) Genetically Engineered (GE) Bacteria-based microrobot (Bacteriobot); (11) Bacteria as anticancer agents through enhancing immunity (Activating inflammasome pathway/CD4, CD25, CD8 antitumor effector cell response/TNF-α innate immune system in bacteria-based tumor necrosis/Activation of Immune cells)
Unfortunately, these bacteria, although cause regression of tumors, have toxic side effects due to their virulity on the body. Recent studies have shown reduced toxicity by removing certain virulent genes from the bacteria (Berendt et al. 1978). Also, antibiotics can be used to control the effects of bacteria (D’Andrea et al. 1992). The earlier studies thus summarized, exhibit that though the bacteria are efficient in targeting tumors but their efficacy in tumor regression is disputed and bacteria elicits different response in different individuals. This signifies that if external aid is provided, these bacteria can be tuned and manipulated for the treatment of cancer.
Simultaneous and independent development in utility of nanoparticles in cancer diagnosis and management has seen fruitful scientific progress. Application in therapeutics of nanoparticle delivery systems are considered important due to
-
1.
improved targeting of cancer cells,
-
2.
minimal systemic toxicity due to lower dosage,
-
3.
improvement in regulation of circulation time of the intended therapeutic, and
-
4.
conservation of therapeutics’ bioavailability.
Standardizations of nanoparticles for therapy have been inclusive of vesicle dimensions, interaction with cells, binding efficacy, and its sustained release (Yoo et al. 2011).
Despite the above listed and many more advantages, various aspects hinder immediate clinical application. For example, uncertainties in the physical structure of the tumor microenvironment (due to organ of residence), and interstitial fluid pressure loss due to any lymphatic drainage, etc. (Dewhirst and Secomb 2017; Minchinton and Tannock 2006). The complexity is increased with the presence of stromal cells, nature of extracellular matrix of the tumor microenvironment, physical stress generated by growing number of cells within confined space, and expanded intercapillary spaces that impede delivery and/or functionality of chemotherapeutic agents (Jain and Stylianopoulos 2010; Jang et al. 2003; Nia et al. 2017; Saggar et al. 2013). Thus, extermination of cancer cell requires an effective disbursement of optimally medicated therapeutic macromolecules within the tumor.
An approach to combine hypoxic environment targeting bacterial activity with functionality of nanoparticles has been a work that was waiting to happen. Both domains have been critical in targeting and managing tumors either directly (killing tumor cells) or in-directly (targeting stroma, activating immune cells, etc.). However, limited progress made in clinical setting overreaches the limitations these methods have. Hence a potential angle generated by an overlay of these two approaches may have a more impact than either of them alone (Fig. 14.2). Since nanoparticles have been widely utilized for targeted cancer theranostics, evidently, it has also been employed in conjunction with bacteria for cancer therapy. In this chapter, we aim to consolidate the research works carried out on cancer therapy mediated by nanoparticles in concurrence with bacteria mediated tumor regression and targeting.
14.2 Bacteria Associated with Cancer Management
As already summarized in Fig. 14.1, bacteria mediate tumor regression by different ways. Bacteria cause tumor regression by inducing tumor apoptosis and autophagy either by production of toxic substances or depleting nutrients supply to the tumor cells. The bacteria can also activate certain pro-drugs to kill a tumor (Kim et al. 2013). Further, certain bacterial spores have antitumor cytotoxic enzymes which are expressed after spore germination (Bettegowda et al. 2006; Kubiak et al. 2015). Besides, host immunity also plays a crucial role in inducing antitumor activity (Agrawal et al. 2004). The specific mechanisms of tumor regression by some bacteria are listed as follows.
14.2.1 Salmonella
Salmonella shows tropism towards tumor sites due to the presence of receptors of aspartate, serine, ribose/galactose on its surface. These three receptors have characteristic function in the chemotaxis of Salmonella towards the tumor. The aspartate receptor starts the chemotaxis, the serine receptor helps in penetrating the tumor cell wall, and the ribose/galactose receptor guides the bacteria towards necrotic sites. Once settled at the tumor site, the bacteria continue to proliferate leading to overgrowth of bacteria around the tumor. It has been determined that S. typhimurium cells number more than 1 × 1010 CFU/g of tumor tissues after 3 days of administration (Ganai et al. 2011; Uchugonova et al. 2015). Salmonella spp. also induces autophagy of tumor cells by downregulating the AKT/mTOR pathway, which in turn negatively regulates autophagy (Lee et al. 2014; Liu et al. 2016). It induces apoptosis of the tumor cells by increasing the levels of caspase-1, which cleaves pro-IL-β and pro-IL-18, yielding their active forms at tumor sites (Phan et al. 2015). Salmonella upregulates the protein Connexin 43, promoting formation of gap junction between tumor and dendritic cells, and giving access to tumor antigen. Dendritic cells containing antigen peptides are then passed through these junctions into the tumor cell (Shilling et al. 2007). Once inside, these antigen peptides activate CD8+ T-cells for tumor regression. This also lead to reduced production of immunity suppressing enzyme indoleamine2,3-dioxygenase in T-cells causing increased activation of T-cells (Chang et al. 2013; Saccheri et al. 2010). Salmonella also leads to tumor regression by inducing an autoimmune response. The signalling of Flagellin-derived Toll-like receptor 5 not only reduces tumor proliferation but also decreases CD4+ CD25+ T-cells, which stimulates the pre-existing immunity of the body (Cai et al. 2011; Leigh et al. 2014). The Flagellin helps in increasing the levels of interferons, CXCL9 and CXCL10 in Salmonella inhabited tumor sites. It is these cytokines that further recruit natural killer cells and cytotoxic T-cells surrounding the tumor site (Kupz et al. 2014).
14.2.2 Clostridium
The spores of Clostridium find their way to the tumor sites by the undeveloped vasculature of tumor owing to enhanced permeability and retention (EPR) effect that allows entry of macromolecules and nutrients of fixed size (usually in nm to μm) in tumor parenchyma (Fang et al. 2003; Matsumara and Maeda 1986). Also, the hypoxic levels of tumor site provide favorable environment for this anaerobic bacteria to grow and proliferate (Van Mellaert et al. 2006). Once the bacterial spores reach the tumor sites and germinate, they can self-propel around the tumor vasculature (Forbes 2010). The injection of the virgin clostridium bacteria in mice caused tumor lysis but also the death of the mice due to its toxic nature; few mice which survived owing to high antibiotic doses, later died due to regrowth of the tumor (Malmgren and Flanigan 1955). The recent engineered strain of clostridium, C. Novyi-NT, has shown more promising result and is undergoing human clinical trial (Dang et al. 2001; Roberts et al. 2014).
14.2.3 Bifidobacteria
Apart from competing for nutrients with tumor cells, another interesting tumor regression mechanism of this bacterium is biotransformation, i.e. transforming certain compounds into tumor regression compounds. Anticancer drugs like Lapachol and 5-fluorocytosine are converted to active antitumor compounds (Bae et al. 2000; Hidaka et al. 2007; Nakamura et al. 2002; Oliveira Silva et al. 2014). The bacteria also alter the expression of cancerous genes and cytokines, either by suppressing or enhancing them by increasing levels of Interferon-gamma secreting cells (Gu et al. 2016; Reddy 1999; Wu et al. 2016).
14.3 Selected Strategies of Combinatorial Approaches
Tumor is very difficult to penetrate, be it for nutrients or the T-cells. However, in the previous section it is seen that the anaerobic bacteria infiltrate into tumor region with ease. This has led to the rise of the idea to use this phenomenon as a Trojan horse. The conjugation of bacteria with nano-sized particles is of much interest (Lee et al. 2013). The conjugation with the bacteria is done by either modifying receptors on the bacterial cell wall to recognize the nanoparticles or by ligand exchange (Wang et al. 2010; Zhai et al. 2017). The teaming up of bacteria with nanoparticles provides us with advanced imaging and therapeutic strategies, and thereby can be utilized for theranostic purposes. This conjugation opens up various therapeutic avenues for tumor regression. Based on the mode of conjugation and action, nano-bacteria therapy can be broadly classified into cargo-laden, genetically engineered, antibody-guided and co-delivery approaches (Fig. 14.2).
14.3.1 Cargo-Laden Approach
The cargo-laden approach is the most common method in which the bacterium is the carrier and the nanoparticle, quantum dots or anticancer drugs are the cargo. The natural affinity of some anaerobic bacteria, for example, Salmonella, Clostridium, Bifidobacteria, etc. are exploited to carry the cargo either on the surface or inside the tumor. The bacteria may also itself produce the cargo. Liu and colleagues demonstrated the quantum dot-internalized Bifidobacterium bifidum having folic acid on its surface can target the folate receptor expressing tumor cells (Liu et al. 2012). Here, the cadmium-selenium-sulfur quantum dots were encapsulated in lipid layer and internalized in the bacteria by electroporation. To further enhance the targeting efficiency, the bacterial surface was modified with folic acid. The formulation was found to be efficient in targeting the core of solid tumors in mice model and may prove to be an excellent tool for tumor imaging (Fig. 14.3a). However, the side effects and fate of the quantum dots in the body have not been elaborated and are subject to further research. Luo et al. used upconversion nanorods (UCNRs) and bioimaging and photothermal ablation of tumors, respectively (Luo et al. 2016). Considering the deeper penetration using near-infrared (NIR) light in biological tissues, both UCNRs are susceptible of NIR light excitation. The ligand-free UCNRs (LF-UCNRs) were deposited electrostatically on negatively charged surface of Bifidobacterium breve. It was observed that the bacteria-conjugated UCNRs showed significantly better targeting efficiency as compared to the LF-UCNRs (Fig. 14.3b). In a similar study, liposomes loaded with doxorubicin were inserted in Salmonella by electroporation to generate nanoswimmers termed BADOX (Zoaby et al. 2017). The BADOX exhibited an enhanced speed in targeted delivery of doxorubicin to cancer cells. The doxorubicin upon reaching to the cancer cells were released from the liposomes in response to ammonia secreted by the cancer cells and hence creating an osmotic misbalance. Doxorubicin, being both cancer chemotherapeutic and antibiotic drug, kills the tumor cells as well as the bacteria, thereby lowering the probability of any side effects that could be generated by the bacteria. However, the ability of the fabricated nanoswimmers to invade solid tumors has to be clinically proved.
Cargo-laden approach. (a) Fluorescent image showing internalization of quantum dot-B. bifidum-folic acid conjugate by solid tumor at 24 and 72 h post intravenous injection (Liu et al. 2012). (Reproduced with permission). (b) Near infrared (NIR) intensities of tumor site after injection of cargo-laden bacteria-nanoparticle conjugate (Luo et al. 2016). (Reproduced with permission). PBS Phosphate buffer saline, LF-UCNRs Ligand-free upconversion nanorods. Genetically engineered approach. (c) Schematic showing synthesis of nanomaterial-bacteria hybrid, tumor targeting and intratumoral photosynthesis of NO to engender photo-controlled bacterial metabolite therapy (Zheng et al. 2018). (Reproduced with permission)
In another interesting study, E. coli attached with a complex drug system was designed (Park et al. 2017). The cargo was of doxorubicin-loaded polyelectrolyte multilayer (PEM) microparticles including embedded magnetic nanoparticles. The 1 μm wide PEM loaded E. coli were directed using an external magnetic guidance system to 4T1 breast cancer cells. This is unlike the obligate anaerobes like Salmonella and Clostridium which have tendency to migrate towards hypoxic tumor. The microswimmers were easily guided to the desired site and the presence of bacteria resulted in lowering of local pH that led to enhanced uptake of anticancer drugs by the cancer cells. Overall, the platform is highly tunable but needs to be perfected in vivo. In another similar approach, Magnetococcus marinus strain MC-1 was used that naturally produced magnetosome (chain of iron oxide nanoparticles) (Felfoul et al. 2016). Anticancer drug loaded nanoliposomes were covalently attached to the surface of MC-1 bacterial cells and the formulation was guided under magnetic field to HCT116 colorectal xenografts in mice model. Approximately 70 nanoliposomes per MC-1 cells were attached and around 55% of the bacteria-nanoliposome conjugate were able to penetrate the hypoxic region of the xenograft. Apart from imaging and chemotherapeutic studies, the bacteria-nanoparticle conjugate has also been used as a vaccine for cancer immunotherapy. Cationic polymer, β-cyclodextrin-Polyethyleneimine600 (CP), nanoparticles containing plasmid DNA encoding vascular endothelial growth factor receptor 2 (VEGFR2) were coated on liver attenuated Salmonella bacteria (Hu et al. 2015). This coating enabled the bacteria to be administered orally by escaping phagosomes and imparting resistance to the acidic pH of stomach. Upon oral administration of the bacteria-nanoparticle construct, successful T-cell and cytokine activation along with suppression of angiogenesis in tumor was observed leading to tumor necrosis.
14.3.2 Genetically Engineering Bacteria for Expressing Specific Biomolecule
In this approach, a secondary molecule having high affinity to a bacteria-expressed biomolecule is either delivered simultaneously with the bacteria or later. The bacteria itself does not carry the active drug. In one such approach, Park and co-workers modified the facultative anaerobic Salmonella typhimurium to express biotin that enables interaction with streptavidin conjugated microbeads (Park et al. 2014). The “bacteriobot” was seen with the increase of the tumor targeting by the observation of the fluorescent microbeads. Fan and co-workers developed an E. coli MG1655 based vehicle for oral administration (Fan et al. 2018). This non-invasive thermally sensitive programmable bacterial (TPB) therapeutic system expressed TNF-α, and bio-mineralized gold nanoparticles (AuNPs). TPB is transported into internal microcirculation by microfold cells (of Peyer’s patches) followed by homing to tumor microenvironments. Once the TBPs accumulated at the tumor sites, its irradiation by NIR induced expression of TNF-α, eventually inducing apoptotic cell death in tumor treatment. In a very intriguing approach, a metabolic pathway by “charging” facultative anaerobe E. coli with a nano-photocatalyst that strengthens their routine metabolic activities has been proposed (Zheng et al. 2018). Carbon nitride (C3N4), when metabolized by nitric oxide (NO) generation enzymes, enables development of photo-controlled bacterial metabolite therapy (PMT). In the presence of light treatment, C3N4 produced photoelectrons and transferred to E. coli induce enzymatic reduction of cellular NO3− to cytotoxic NO (Fig. 14.3c). Preclinical study involving a tumor-bearing mouse model, C3N4 loaded bacteria collected within the tumor microenvironment and the PMT led to inhibition of tumor progression by 80%. A similar work in non-toxin strains of genetically engineered Salmonella or Clostridium may be used to increase therapeutic efficiency.
14.3.3 Antibody-Guided Approach
As the name suggests, in this approach antibody acts as a guide to deliver the cargo rather than directly loading on the bacteria. Customarily, the bacteria are injected first, allowed to colonize the tumor site and then the antibody conjugated nanoparticles or drugs are injected, leading to regression of tumor. The antibody is against the bacterial antigen; and the microorganisms are not genetically engineered. Apart from guiding the cargo, the approach has also been used to guide bacteria to the tumor specific antigen for augmented colonization and invasion of solid tumor. Probably, the first instance of antibody-guided bacteria mediated delivery of nanoparticles was demonstrated by Akin and co-workers (Akin et al. 2007). Streptavidin-coated polystyrene nanoparticle was first attached to biotinylated antibody against a surface antigen of Listeria monocytogenes; the nanoparticle was then loaded with green fluorescent protein. The cargo was then docked on to bacterial surface giving rise to “microbots.” The microbots were then targeted to desired cells where they were able to deliver the nanoparticle containing plasmid that was further successfully expressed in the target cell. The bacteria were then killed by treatment with antibiotics. Later Kojima and co-workers used a similar biotin-streptavidin approach to fabricate bacteria driven liposomes by raft domain binding method (Kojima et al. 2012). Vibrio alginolyticus mutant strain VIO5 with polar flagella associated with biotinylated antibody was used. The bacteria interacted with biotin-modified liposome via streptavidin.
In a different study discussed in cargo-laden approach by Luo et al., core shell-UCNRs modified with PEG polymers and bound to Clostridium polyclonal antibodies that target the vegetative C. difficile were introduced into mice model post-colonization of tumor sites by C. difficile (Luo et al. 2016). This approach was more efficient than the cargo-laden approach in which nanoparticles were loaded on B. beveri (Fig. 14.4A). Suh and co-workers utilized bioconjugation method based on streptavidin–biotin interaction (Suh et al. 2019). Salmonella enterica serovar Typhimurium VNP20009 surface was modified using a biotinylated antibody targeting tumor. The bacterium with streptavidin-coated poly(lactic-co-glycolic acid) (PLGA) nanoparticles to the outer membrane constituted the NanoBEADS agent (Fig. 14.4B). As the authors did not genetically engineer the bacteria for the construction of NanoBEADS of any specific cargo, the same system can be utilized as a platform for delivery of required drug. Inherent to its nature, S. enterica Typhimurium VNP20009 homes to tumor region through intercellular translocation mode, skipping any external requirement for guidance towards tumor site. Effective tumor targeting and accumulation of therapeutic agent was observed both in vitro and in vivo. The NanoBEADS platform thereby provides a versatile and simple approach to enhance the bacterial colonization and invasion in the tumor and simultaneous delivery of anticancer cargo.
Antibody-guided approach. (A) Near infrared (NIR) intensities of tumor site after injection of antibody-guided bacteria and nanoparticle formulation (Luo et al. 2016). (Reproduced with permission). PBS Phosphate buffer saline, UCNRs upconversion nanorods. (B) Scanning electron microscope image of NanoBEADS agent (Suh et al. 2019). (Reproduced with permission). Co-delivery approach. (C) Schematic showing doxorubicin-loaded triple layered nanogel; (a) Accumulation of bacteria at tumor site; (b) Accumulation of nanoparticles at tumor site; (c) Bacteria degrading the poly(ε-caprolactone) fence triggering release of doxorubicin (Xiong et al. 2013). (Reproduced with permission)
14.3.4 Co-delivery
In this case, a pro-drug and microbe are injected in body independently. Pro-drug is activated either by the microbe or by an external stimulant like light (for example, in photo-dynamic light therapy). Using this approach, Park and colleagues attempted to overcome limitation of remnant tumor cells of primary tumor targeted by any therapy (Park et al. 2020). They formulated an emulsion having two components—multifunctional nanoscintillators (NSs) and C. novyi spores coated with branched gold nanoparticle (for synergistic image-guided combinational treatment). NSs were composed of NaGdF4:Tb,Ce@NaGdF4 core/shell structure that allowed MRI visibility. The emulsion injected into the tumor allowed MRI/CT image guidance and photoactivation of NSs for photo-dynamic therapy (PDT). In an approach similar to pro-drug activation method, attenuated bacteria SBY1 were co-delivered with bacteria-sensitive triple-layered nanogel (TLN) (Xiong et al. 2013). Initially administered SBY1 exclusively accumulated in tumor site after certain duration. The ensuing administration of doxorubicin-loaded TLN was actively degraded by SBY1 to release doxorubicin within the tumor niche. Thus, targeted delivery of doxorubicin was achieved (Fig. 14.4C).
14.4 Conclusion
One can conclude that under careful manifestation, bacterial agents can be employed to manage tumor or cancer. Many of the innovative therapies have excellent efficacy at in vitro and animal model levels but have not been translated to clinical practice due to either incomplete tumor killing or recurrence of treated primary cancer in a short term. An effective combinatorial therapy using advances in nanoparticle-based and bacteria mediated systems for cancer has minimized remnant tumor cells. Many of these concepts can be extended, and cross improvised to generate an entirely new entity that can overcome limitations of many of above systems. Here we have attempted to present the existing systems that have potential for translational studies. We hope that the reader will be encouraged to foresee and undertake research in this domain.
References
Agrawal N, Bettegowda C, Cheong I, Geschwind J-F, Drake CG, Hipkiss EL, Tatsumi M, Dang LH, Diaz LA, Pomper M, Abusedera M, Wahl RL, Kinzler KW, Zhou S, Huso DL, Vogelstein B (2004) Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci U S A 101:15172–15177. https://doi.org/10.1073/pnas.0406242101
Akin D, Sturgis J, Ragheb K, Sherman D, Burkholder K, Robinson JP, Bhunia AK, Mohammed S, Bashir R (2007) Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat Nanotechnol 2:441–449. https://doi.org/10.1038/nnano.2007.149
Ashu EE, Xu J, Yuan ZC (2019) Bacteria in cancer therapeutics: a framework for effective therapeutic bacterial screening and identification. J Cancer 10:1781–1793. https://doi.org/10.7150/jca.31699
Bae EA, Park SY, Kim DH (2000) Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol Pharm Bull 23:1481–1485. https://doi.org/10.1248/bpb.23.1481
Berendt MJ, North RJ, Kirstein DP (1978) The immunological basis of endotoxin-induced tumor regression: requirement for a pre-existing state of concomitant anti-tumor immunity*. J Exp Med 148:1560–1569. https://doi.org/10.1084/jem.148.6.1560
Bettegowda C, Huang X, Lin J, Cheong I, Kohli M, Szabo SA, Zhang X, Diaz LA, Velculescu VE, Parmigiani G, Kinzler KW, Vogelstein B, Zhou S (2006) The genome and transcriptomes of the anti-tumor agent Clostridium novyi-NT. Nat Biotechnol 24:1573–1580. https://doi.org/10.1038/nbt1256
Cai Z, Sanchez A, Shi Z, Zhang T, Liu M, Zhang D (2011) Activation of toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res 71:2466–2475. https://doi.org/10.1158/0008-5472.CAN-10-1993
Chang W-W, Lai C-H, Chen M-C, Liu C-F, Kuan Y-D, Lin S-T, Lee C-H (2013) Salmonella enhance chemosensitivity in tumor through connexin 43 upregulation. Int J Cancer 133:1926–1935. https://doi.org/10.1002/ijc.28155
Coley WB (1991) The treatment of malignant tumors by repeated inoculations of erysipelas. Clin Orthop Relat Res 262:3–11
D’Andrea A, Rengaraju M, Valiante NM, Chehimij J, Kubin M, Aste M, Chan SH, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf SF, Trinchieri G (1992) Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med 176:1387–1398. https://doi.org/10.1084/jem.176.5.1387
Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B (2001) Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci U S A 98:15155–15160. https://doi.org/10.1073/pnas.251543698
Dewhirst MW, Secomb TW (2017) Transport of drugs from blood vessels to tumour tissue. Nat Rev Cancer. https://doi.org/10.1038/nrc.2017.93
Fan JX, Li ZH, Liu XH, Zheng DW, Chen Y, Zhang XZ (2018) Bacteria-mediated tumor therapy utilizing photothermally-controlled TNF-α expression via Oral administration. Nano Lett 18:2373–2380. https://doi.org/10.1021/acs.nanolett.7b05323
Fang J, Sawa T, Maeda H (2003) Factors and mechanism of “EPR”; effect and the enhanced antitumor effects of macromolecular drugs including SMANCS. Adv Exp Med Biol 519:29–49. https://doi.org/10.1007/0-306-47932-X_2
Felfoul O, Mohammadi M, Taherkhani S, de Lanauze D, Zhong Xu Y, Loghin D, Essa S, Jancik S, Houle D, Lafleur M, Gaboury L, Tabrizian M, Kaou N, Atkin M, Vuong T, Batist G, Beauchemin N, Radzioch D, Martel S (2016) Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat Nanotechnol 11:941–947. https://doi.org/10.1038/nnano.2016.137
Forbes NS (2010) Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer 10:785–794. https://doi.org/10.1038/nrc2934
Ganai S, Arenas RB, Sauer JP, Bentley B, Forbes NS (2011) In tumors Salmonella migrate away from vasculature toward the transition zone and induce apoptosis. Cancer Gene Ther 18:457–466. https://doi.org/10.1038/cgt.2011.10
Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F (2004) CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 34:336–344. https://doi.org/10.1002/eji.200324181
Gu Q-Y, Zhang J, Feng Y-C (2016) Role of NLRP3 inflammasome in Bifidobacterium longum-regulated visceral hypersensitivity of postinfectious irritable bowel syndrome. Artif Cells Nanomed Biotechnol 44:1933–1937. https://doi.org/10.3109/21691401.2015.1111238
Hidaka A, Hamaji Y, Sasaki T, Taniguchi S, Fujimori M (2007) Exogenous cytosine deaminase gene expression in Bifidobacterium breve I-53-8w for tumor-targeting enzyme/prodrug therapy. Biosci Biotechnol Biochem 71:2921–2926. https://doi.org/10.1271/bbb.70284
Hu Q, Wu M, Fang C, Cheng C, Zhao M, Fang W, Chu PK, Ping Y, Tang G (2015) Engineering nanoparticle-coated bacteria as Oral DNA vaccines for cancer immunotherapy. Nano Lett 15:2732–2739. https://doi.org/10.1021/acs.nanolett.5b00570
Iwasaki M, Yu WG, Uekusa Y, Nakajima C, Yang YF, Gao P, Wijesuriya R, Fujiwara H, Hamaoka T (2000) Differential IL-12 responsiveness of T cells but not of NK cells from tumor-bearing mice in IL-12-responsive versus - unresponsive tumor models. Int Immunol 12:701–709. https://doi.org/10.1093/intimm/12.5.701
Jain RK, Stylianopoulos T (2010) Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. https://doi.org/10.1038/nrclinonc.2010.139
Jang SH, Wientjes MG, Lu D, Au JLS (2003) Drug delivery and transport to solid tumors. Pharm Res. https://doi.org/10.1023/A:1025785505977
Kim K-A, Jung I-H, Park S-H, Ahn Y-T, Huh C-S, Kim D-H (2013) Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS One 8:e62409. https://doi.org/10.1371/journal.pone.0062409
Kojima M, Zhang Z, Nakajima M, Fukuda T (2012) High efficiency motility of bacteria-driven liposome with raft domain binding method. Biomed Microdevices 14:1027–1032. https://doi.org/10.1007/s10544-012-9711-2
Kubiak AM, Poehlein A, Budd P, Kuehne SA, Winzer K, Theys J, Lambin P, Daniel R, Minton NP (2015) Complete genome sequence of the nonpathogenic soil-dwelling bacterium clostridium sporogenes strain NCIMB 10696. Genome Announc 3. https://doi.org/10.1128/genomeA.00942-15
Kupz A, Curtiss R, Bedoui S, Strugnell RA (2014) In vivo IFN-γ secretion by NK cells in response to Salmonella typhimurium requires NLRC4 inflammasomes. PLoS One 9:e97418. https://doi.org/10.1371/journal.pone.0097418
Le HN, Lee NC, Tsung K, Norton JA (2001) Pre-existing tumor-sensitized T cells are essential for eradication of established tumors by IL-12 and cyclophosphamide plus IL-12. J Immunol 167:6765–6772. https://doi.org/10.4049/jimmunol.167.12.6765
Lee J-M, Yoon T-J, Cho Y-S (2013) Recent developments in nanoparticle-based siRNA delivery for cancer therapy. Biomed Res Int 2013:782041. https://doi.org/10.1155/2013/782041
Lee C-H, Lin S-T, Liu J-J, Chang W-W, Hsieh J-L, Wang W-K (2014) Salmonella induce autophagy in melanoma by the downregulation of AKT/mTOR pathway. Gene Ther 21:309–316. https://doi.org/10.1038/gt.2013.86
Leigh ND, Bian G, Ding X, Liu H, Aygun-Sunar S, Burdelya LG, Gudkov AV, Cao X (2014) A flagellin-derived toll-like receptor 5 agonist stimulates cytotoxic lymphocyte-mediated tumor immunity. PLoS One 9:e85587. https://doi.org/10.1371/journal.pone.0085587
Liu Y, Zhou M, Luo D, Wang L, Hong Y, Yang Y, Sha Y (2012) Bacteria-mediated in vivo delivery of quantum dots into solid tumor. Biochem Biophys Res Commun 425:769–774. https://doi.org/10.1016/j.bbrc.2012.07.150
Liu B, Jiang Y, Dong T, Zhao M, Wu J, Li L, Chu Y, She S, Zhao H, Hoffman RM, Jia L (2016) Blockage of autophagy pathway enhances Salmonella tumor-targeting. Oncotarget 7:22873–22882. https://doi.org/10.18632/oncotarget.8251
Luo CH, Huang CT, Su CH, Yeh CS (2016) Bacteria-mediated hypoxia-specific delivery of nanoparticles for tumors imaging and therapy. Nano Lett 16:3493–3499. https://doi.org/10.1021/acs.nanolett.6b00262
Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O’Garra A (1995) Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol 154(10):5071–5079
Malmgren RA, Flanigan CC (1955) Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res 15:473–478
Matsumara Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 46:6387–6392
Minchinton AI, Tannock IF (2006) Drug penetration in solid tumours. Nat Rev Cancer. https://doi.org/10.1038/nrc1893
Minton NP (2003) Clostridia in cancer therapy. Nat Rev Microbiol 1:237–242. https://doi.org/10.1038/nrmicro777
Nakamura T, Sasaki T, Fujimori M, Yazawa K, Kano Y, Amano J, Taniguchi S (2002) Cloned cytosine deaminase gene expression of Bifidobacterium longum and application to enzyme/pro-drug therapy of hypoxic solid tumors. Biosci Biotechnol Biochem 66:2362–2366. https://doi.org/10.1271/bbb.66.2362
Nia HT, Liu H, Seano G, Datta M, Jones D, Rahbari N, Incio J, Chauhan VP, Jung K, Martin JD, Askoxylakis V, Padera TP, Fukumura D, Boucher Y, Hornicek FJ, Grodzinsky AJ, Baish JW, Munn LL, Jain RK (2017) Solid stress and elastic energy as measures of tumour mechanopathology. Nat Biomed Eng 1. https://doi.org/10.1038/s41551-016-0004
Oliveira Silva E, Cruz de Carvalho T, Parshikov IA, Alves dos Santos R, Silva Emery F, Jacometti Cardoso Furtado NA (2014) Cytotoxicity of lapachol metabolites produced by probiotics. Lett Appl Microbiol 59:108–114. https://doi.org/10.1111/lam.12251
Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E (1999) Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res 59:3128–3133
Park D, Park SJ, Cho S, Lee Y, Lee YK, Min J-J, Park BJ, Ko SY, Park J-O, Park S (2014) Motility analysis of bacteria-based microrobot (bacteriobot) using chemical gradient microchamber. Biotechnol Bioeng 111:134–143. https://doi.org/10.1002/bit.25007
Park BW, Zhuang J, Yasa O, Sitti M (2017) Multifunctional bacteria-driven microswimmers for targeted active drug delivery. ACS Nano 11:8910–8923. https://doi.org/10.1021/acsnano.7b03207
Park W, Cho S, Kang D, Han JH, Park JH, Lee B, Lee J, Kim DH (2020) Tumor microenvironment targeting nano–bio emulsion for synergistic combinational X-ray PDT with oncolytic bacteria therapy. Adv Healthc Mater 9. https://doi.org/10.1002/adhm.201901812
Phan TX, Nguyen VH, Duong MT-Q, Hong Y, Choy HE, Min J-J (2015) Activation of inflammasome by attenuated Salmonella typhimurium in bacteria-mediated cancer therapy. Microbiol Immunol 59:664–675. https://doi.org/10.1111/1348-0421.12333
Rai N, Singh AK, Keshri PK, Barik S, Kamble SC, Singh SK, Kumar R, Mishra P, Kotiya D, Gautam V (2020) Probiotics for Management of Gastrointestinal Cancers. In Probiotic Research in Therapeutics, edited by Indu Pal Kaur and Parmeet Kaur Deol, 1st ed. 373. Singapore: Springer Singapore. https://doi.org/10.1007/978-981-15-8214-1
Reddy BS (1999) Possible mechanisms by which pro- and prebiotics influence colon carcinogenesis and tumor growth. J Nutr 129:1478S–1482S. https://doi.org/10.1093/jn/129.7.1478S
Roberts NJ, Zhang L, Janku F, Collins A, Bai R-Y, Staedtke V, Rusk AW, Tung D, Miller M, Roix J, Khanna KV, Murthy R, Benjamin RS, Helgason T, Szvalb AD, Bird JE, Roy-Chowdhuri S, Zhang HH, Qiao Y, Karim B, McDaniel J, Elpiner A, Sahora A, Lachowicz J, Phillips B, Turner A, Klein MK, Post G, Diaz LA, Riggins GJ, Papadopoulos N, Kinzler KW, Vogelstein B, Bettegowda C, Huso DL, Varterasian M, Saha S, Zhou S (2014) Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med 6:249ra111. https://doi.org/10.1126/scitranslmed.3008982
Saccheri F, Pozzi C, Avogadri F, Barozzi S, Faretta M, Fusi P, Rescigno M (2010) Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci Transl Med 2:44ra57. https://doi.org/10.1126/scitranslmed.3000739
Saggar JK, Yu M, Tan Q, Tannock IF (2013) The tumor microenvironment and strategies to improve drug distribution. Front Oncol 3:154. https://doi.org/10.3389/fonc.2013.00154
Shear BMJ, Turner FC, Perrault A, Shovelton T (1943) Chemical treatment of tumors. V. Isolation of the hemorrhage-producing fraction from Serratia marcescens (Bacillus prodigiosus) Culture Filtratel. J Natl Cancer Inst 4:81–97
Shilling DA, Smith MJ, Tyther R, Sheehan D, England K, Kavanagh EG, Redmond HP, Shanahan F, O’Mahony L (2007) Salmonella typhimurium stimulation combined with tumour-derived heat shock proteins induces potent dendritic cell anti-tumour responses in a murine model. Clin Exp Immunol 149:109–116. https://doi.org/10.1111/j.1365-2249.2007.03393.x
Shimizu J, Yamazaki S, Sakaguchi S (1999) Induction of tumor immunity by removing CD25+CD4+T cells: a common basis between tumor immunity and autoimmunity. J Immunol 163:5211–5218
Song S, Vuai MS, Zhong M (2018) Role of bacteria in cancer therapy. Infect Agent Cancer 13:1–7. https://doi.org/10.1186/s13027-018-0180-y
Suh S, Jo A, Traore MA, Zhan Y, Coutermarsh-Ott SL, Ringel-Scaia VM, Allen IC, Davis RM, Behkam B (2019) Nanoscale bacteria-enabled autonomous drug delivery system (NanoBEADS) enhances intratumoral transport of nanomedicine. Adv Sci 6:1801309. https://doi.org/10.1002/advs.201801309
Uchugonova A, Zhang Y, Salz R, Liu F, Suetsugu A, Zhang L, Koenig K, Hoffman RM, Zhao M (2015) Imaging the different mechanisms of prostate Cancer cell-killing by tumor-targeting Salmonella typhimurium A1-R. Anticancer Res 35:5225–5229
Van Mellaert L, Barbé S, Anné J (2006) Clostridium spores as anti-tumour agents. Trends Microbiol 14:190–196. https://doi.org/10.1016/j.tim.2006.02.002
Vautier AH (1813) General perspectives on the cancer illness. The Áse du Paris 43
Wang S, Chen K-J, Wu T-H, Wang H, Lin W-Y, Ohashi M, Chiou P-Y, Tseng H-R (2010) Photothermal effects of supramolecularly assembled gold nanoparticles for the targeted treatment of cancer cells. Angew Chem Int Ed Engl 49:3777–3781. https://doi.org/10.1002/anie.201000062
Wiemann B, Starnes CO (1994) Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther 64:529–564. https://doi.org/10.1016/0163-7258(94)90023-X
Wu B-B, Yang Y, Xu X, Wang W-P (2016) Effects of Bifidobacterium supplementation on intestinal microbiota composition and the immune response in healthy infants. World J Pediatr 12:177–182. https://doi.org/10.1007/s12519-015-0025-3
Xiong MH, Bao Y, Du XJ, Tan ZB, Jiang Q, Wang HX, Zhu YH, Wang J (2013) Differential anticancer drug delivery with a nanogel sensitive to bacteria-accumulated tumor artificial environment. ACS Nano 7:10636–10645. https://doi.org/10.1021/nn403146t
Yoo JW, Irvine DJ, Discher DE, Mitragotri S (2011) Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. https://doi.org/10.1038/nrd3499
Zhai Y, Su J, Ran W, Zhang P, Yin Q, Zhang Z, Yu H, Li Y (2017) Preparation and application of cell membrane-camouflaged nanoparticles for cancer therapy. Theranostics 7:2575–2592. https://doi.org/10.7150/thno.20118
Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, Xu M, Penman S, Hoffman RM (2005) Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci U S A 102:755–760. https://doi.org/10.1073/pnas.0408422102
Zheng DW, Chen Y, Li ZH, Xu L, Li CX, Li B, Fan JX, Cheng SX, Zhang XZ (2018) Optically-controlled bacterial metabolite for cancer therapy. Nat Commun 9. https://doi.org/10.1038/s41467-018-03233-9
Zoaby N, Shainsky-Roitman J, Badarneh S, Abumanhal H, Leshansky A, Yaron S, Schroeder A (2017) Autonomous bacterial nanoswimmers target cancer. J Control Release 257:68–75. https://doi.org/10.1016/j.jconrel.2016.10.006
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kamble, S.C., Shaikh, F.F., Sarkar, J. (2021). The Evolving Role of Nanoparticles in Bacteria Mediated Cancer Therapy. In: Maddela, N.R., Chakraborty, S., Prasad, R. (eds) Nanotechnology for Advances in Medical Microbiology. Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-15-9916-3_14
Download citation
DOI: https://doi.org/10.1007/978-981-15-9916-3_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9915-6
Online ISBN: 978-981-15-9916-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)