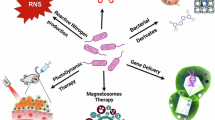
Abstract
Adverse effects and multidrug resistance remain significant obstacles in conventional cancer therapy. Nanomedicines, with their intrinsic properties such as nano-sized dimensions and tunable surface characteristics, have the potential to mitigate the side effects of traditional cancer treatments. While nanomaterials have been widely applied in cancer treatment, challenges such as low targeting efficiency and poor tumor penetration persist. Recent research has shown that anaerobic bacteria exhibit high selectivity for primary tumors and metastatic cancers, offering good safety and superior tumor penetration capabilities. This suggests that combining nanomaterials with bacteria could complement their respective limitations, opening vast potential applications in cancer therapy. The use of bacteria in combination with nanomaterials for anticancer treatments, including chemotherapy, radiotherapy, and photothermal/photodynamic therapy, has contributed to the rapid development of the field of bacterial oncology treatments. This review explores the mechanisms of bacterial tumor targeting and summarizes strategies for synthesizing bacterial-nanomaterial and their application in cancer therapy. The combination of bacterial-nanomaterial hybrids with modern therapeutic approaches represents a promising avenue for future cancer treatment research, with the potential to improve treatment outcomes for cancer patients.
Graphical Abstract

Similar content being viewed by others
Introduction
Cancer remains a leading cause of death globally, posing a significant threat to human health [1]. The development of effective cancer treatments is hampered by the complexity and heterogeneity of tumor biology [2,3,4]. While traditional chemotherapy has played a crucial role in prolonging patient survival, its lack of tumor-specific targeting often leads to suboptimal drug concentrations at the tumor site, diminishing therapeutic efficacy [5,6,7]. Furthermore, the systemic distribution of most chemotherapy drugs results in toxicity and various adverse effects, including myelosuppression, mucositis, and organ dysfunction [8,9,10,11]. These limitations highlight the urgent need for targeted drug delivery methods in cancer therapy. [12]. Current research in cancer therapeutics focuses on enhancing the targeting specificity and delivery efficiency of anticancer agents while simultaneously minimizing adverse effects. The development of nanotechnology over the past few decades has led to the emergence of numerous promising biomedical materials. Nanomaterials have been extensively explored for improving cancer diagnosis and treatment due to their unique physicochemical properties, including nano-scale size, tunable surface characteristics, and capabilities for encapsulation and controlled drug release [2, 13]. This has significantly advanced cancer therapy research. Compared to traditional chemotherapy drugs, nanomedicines offer significant advantages in prolonging drug half-life, enhancing drug stability, bioavailability, and tumor accumulation [14,15,16]. These characteristics can be utilized to design targeted drug delivery systems, improving the bio-distribution and tumor accumulation of free drugs, thereby enhancing the effectiveness of traditional cancer therapy [17, 18]. Research has demonstrated that nanomedicines can significantly enhance drug retention and permeability, effectively targeting tumor tissues [19]. Additionally, nanomedicines enhance drug efficacy by increasing the local drug concentration in tumors while reducing damage to healthy cells [20]. However, the efficiency of nanoparticle-based tumor targeting is reduced due to the unique physiological structures of the tumor microenvironment, such as dense stroma, heterogeneous vascular leakage, and hypoxic conditions [21,22,23]. As the transport of nanoparticles primarily relies on passive accumulation in tumor sites through systemic circulation, lacking active driving forces to penetrate deep into tumors, the application of nanoparticles alone in cancer treatment is limited, thus reducing therapeutic efficacy [24].
In response to the limitations of traditional chemotherapy drugs and nanomedicines, recent research has explored novel strategies for cancer therapy utilizing bacteria [25]. The use of bacteria in cancer treatment dates back decades, with American physician William Coley being the first to document the application of bacteria and bacterial toxins for this purpose [26]. In recent years, bacterial therapy has emerged as a promising antitumor strategy. Whether used alone or in combination with traditional anti-cancer treatments, bacterial therapy has demonstrated promising efficacy in promoting tumor regression and inhibiting cancer cell metastasis [27]. The biocompatibility of bacterial therapy has also been widely validated [28,29,30,31,32]. Bacteria themselves possess certain antitumor effects. For instance, bacterial proliferation at tumor sites can compete with cancer cells for nutrients in the tumor microenvironment, thereby restricting tumor growth [33]. Furthermore, the intrinsic pro-inflammatory capacity of bacteria can stimulate the body’s immune system, enhancing the efficacy of immunotherapy [34]. By introducing specific functional plasmids into bacteria to serve as bioreactors for producing cytotoxins, antigenic substances, etc., bacteria can also exert their antitumor effects [35,36,37]. Additionally, due to the prolonged hypoxic conditions in the tumor microenvironment, obligate or facultative anaerobic bacteria exhibit excellent targeting and colonization abilities towards hypoxic regions of tumors [38, 39]. Many bacteria have been observed to selectively localize and grow at tumor sites, including Escherichia coli, Listeria monocytogenes, Clostridium species, Salmonella species, and Bifidobacterium, demonstrating their colonization capabilities at tumor sites [31, 32, 40,41,42,43,44,45,46]. Isolated native intratumoral bacteria exhibit high biocompatibility in mice and antitumor effectiveness with excellent tumor selectivity through a single administration [47]. Bacteria-mediated tumor targeting offers a promising strategy for cancer therapy, particularly when combined with other anti-cancer therapies. This approach can significantly reduce side effects by improving drug targeting, thereby enhancing the efficacy of cancer treatment.
Researchers have extensively studied the mechanisms of using bacteria as carriers for cancer therapy, confirming that bacteria, when injected into tumors or systemically, can track the tumor microenvironment and colonize at tumor sites, contributing to tumor regression [48]. Therefore, scientists utilize the inherent anaerobic targeting of anaerobic microorganisms as drug delivery carriers to transport therapeutic agents to hypoxic regions of tumors [49, 50]. This approach involves attaching drugs to bacteria, thereby achieving targeted cancer therapy. Commonly used bacterial carriers for tumor therapy include Salmonella typhimurium, Bifidobacterium, and Escherichia coli. Studies have shown that the combination of nanotechnology with attenuated strains of Salmonella has been successfully applied in the treatment of melanoma [26, 51]. Furthermore, liposomes attached to different types of bacteria have also shown promising results in cancer therapy and diagnosis [52]. Due to their unique anaerobic targeting capabilities, anaerobic bacteria-mediated tumor-targeted therapy presents a novel and effective strategy for cancer treatment. Many studies have utilized certain bacteria as drug carriers, including anticancer drugs and transport vehicles for drug-loaded nanomaterials, to more effectively target tumors and enhance the anticancer efficacy of conventional drugs [53]. Therefore, bacterial carriers are emerging as a frontier in drug delivery [42]. The autonomous movement and anaerobic targeting ability of bacteria are utilized to transport nanomedicines to the deep regions of solid tumors, while nanomaterials can be attached to bacteria surfaces in various ways, forming bacteria-nanoparticle hybrids to mediate nanoparticle delivery [54]. This bacteria-nanoparticle hybrid approach for cancer therapy has led to rapid advancements in bacterial research for tumor treatment. The application of bacteria as carriers for nanomedicines is also attracting increasing attention from researchers in the field of new anticancer drug development. This review delves into the mechanisms underlying bacteria-mediated tumor targeting. It summarizes synthetic strategies for nano-bacteria hybrids and elaborates on their applications in cancer therapy (Scheme 1). By providing a comprehensive and integrated overview of nano-bacteria hybrids, this review explores their potential future development directions. Ultimately, it aims to offer valuable insights and guidance for researchers in related fields, facilitating their research and development of bacterial nanomaterial hybrids.
Mechanisms of bacterial targeting and colonization in tumors
Due to their unique properties, bacteria have shown great potential as delivery vectors for targeting drugs and genes to tumor sites [55, 56]. Various bacteria such as Bifidobacterium, Salmonella, Escherichia coli, and Clostridium can selectively accumulate and replicate in hypoxic tumor regions, delivering functional nanoparticles to the target tissue [57, 58]. Table 1 summarizes the bacterial types most investigated for antitumor therapy. Through synthetic biology and genetic engineering, these bacteria can be engineered to precisely deliver anticancer drugs, specific proteins, antibodies, enzymes, antigens, and cytokines [37]. Several factors contribute to the specific targeting and colonization of tumors by bacteria. Solid tumors often exhibit hypoxic regions, a condition prevalent within the tumor microenvironment (TME) [59]. This hypoxic environment is attractive for the colonization and proliferation of anaerobic or facultative anaerobic bacteria [60]. Necrotic areas within tumors are nutrient-rich, providing ample resources for bacterial growth and reproduction [61]. Furthermore, the abnormal structure of blood vessels associated with tumor growth, including tortuous, elongated, or abnormally dilated vessels, leads to a disorganized vascular system. The microstructure of these blood vessels often includes discontinuous endothelial cells and a lack of pericyte coverage, which may facilitate the entry of circulating bacteria into tumor tissue [62, 63]. The immunosuppressive nature of the tumor microenvironment (TME) creates an immune-privileged site, allowing bacteria to accumulate within the tumor [32, 64].
Bacteria may target tumors through various mechanisms, including chemoattractants, signaling molecules released by dying tumor cells, or the hypoxic environment within the TME. Parthenogenetic anaerobic bacteria such as Salmonella, Pseudomonas, and Escherichia coli can sense high nutrient densities within or around the tumor through chemoreceptors, suggesting a strong attraction of bacteria towards the nutrient rich TME [65]. Studies have also shown that Escherichia coli preferentially colonize lung cancer cells that secrete various signaling molecules [66]. Bacterial motility is a crucial factor enabling their deeper penetration into tumor tissue, contrasting with the inherent passive distribution and limited penetration of chemotherapeutic drugs. Bacteria, as living organisms, possess the ability to reproduce through active metabolism and acquire energy from their surroundings, resulting in sustained and effective expression and transport capabilities. Theoretically, after systemic administration, bacteria can utilize their motility to actively exit the vascular system and preferentially migrate towards regions within the tumor tissue that are more conducive to survival and proliferation, such as areas with higher immunosuppression or greater nutrient availability. Studies have shown that Salmonella begins accumulating in colonies within tumors as early as 3 days post-systemic injection and spreads throughout the tumor tissue region [67].
In essence, bacteria exhibit a tropism towards the hypoxic, nutrient-rich, and immunosuppressive TME, endowing them with an inherent tumor-targeting property. Upon successful targeting and entry into the tumor, bacteria can rapidly replicate. While further research is needed to fully elucidate the mechanisms involved, it is undeniable that many therapeutic bacteria can penetrate tumors, presenting immense potential in cancer treatment [68] .
Synthesis methods of bacterial-nanomaterial hybrids
Studies have demonstrated that certain anaerobic bacteria possess anaerobic targeting capabilities, allowing them to actively target and colonize hypoxic tumor microenvironments [104]. In recent years, nanotechnology has significantly advanced the application of bacteria for cancer treatment. Researchers have combined bacteria with nanomaterials to create bacterial-nanomaterial hybrids, enabling bacteria to deliver nanodrugs to tumor sites and thereby enhancing the tumor-targeting capability of the drugs. These bacterial-nanomaterial hybrids leverage the tumor-targeting abilities of bacteria alongside the advantages of nanodrugs [92]. The synthesis of bacterial-nanomaterial hybrids can be achieved through various strategies, including chemical bonding, electrostatic interactions, the streptavidin-biotin system, and other methods (Fig. 1). Table 2 provides a summary of these methods.
Chemical bonding
Covalent bonding between nanomaterials and bacterial cells is a common method for conjugation. The bacterial outer membrane comprises various components, including peptidoglycan, teichoic acid, lipopolysaccharides, lipids, and proteins. These components provide diverse surface properties and functional groups such as thiols, hydroxyls, carboxyls, and amines [109]. Among these, amino, carboxyl, and thiol groups are the most prevalent functional groups used for chemical modification of the bacterial surface [110, 111]. Due to their simplicity, high efficiency, and mild reaction conditions, these groups serve as reactive sites. Amino groups can react with N-hydroxysuccinimide (NHS) esters, isocyanate-activated molecules, or unsaturated aldehydes and ketones [112]. Carboxyl groups can be activated and subsequently conjugated with amino-functionalized compounds using carbodiimide chemistry [113, 114]. Free thiol groups in cysteine side chains can covalently couple with maleimide-containing conjugates under neutral conditions, forming stable thioether bonds [112, 115, 116]. Coupling reactions typically target amino (-NH2) and carboxyl (-COOH) groups. Stable conjugation of bacteria with nanomaterials is primarily achieved through the formation of stable amide bonds between the peptidoglycan of bacterial cell walls or the lipid bilayers of bacterial membranes and the nanomaterials [117]. Therefore, by modifying nanoparticle surfaces with carboxyl or amino groups, amide bonds can be utilized to firmly attach nanoparticles to bacteria. This approach leverages the tumor-targeting ability of bacteria to transport nanodrugs to tumor sites [118]. Numerous studies have utilized amide bonds to link carboxyl groups on bacterial surfaces with amino groups on nanoparticles. For example, Wu et al. designed and prepared Cu2O@Salmonella Typhimurium ΔSt (ΔSt) (Cu2O@ΔSt), a novel microbial-nano drug, by covalently bonding polyethylene glycolated Cu2O nanoparticles’ amino groups with the carboxyl groups on the surface of engineered Salmonella Typhimurium to form amide bonds (Fig. 2A). Additionally, Wu et al. reported LOD/TPZ@Lips-LA, another novel microbial-nano drug [98]. LOD/TPZ@Lips-LA was constructed by anchoring liposomes co-loaded with lactate oxidase (LOD) and the prodrug tirapazamine (TPZ) onto the surface of Lactobacillus (LA) through an amide condensation reaction. The amino groups of the liposomes co-loaded with LOD and TPZ were bonded to the carboxyl groups of the Lactobacillus (LA). By modifying nanoparticles with carboxyl groups, they can also bind to the amino groups on the bacterial surface. For example, poly(lactic-co-glycolic acid) (PLGA), indocyanine green (ICG), and carboxyl-terminated polyethylene glycolated phospholipid (DSPE-PEG-COOH) can self-assemble to form nanoparticles with carboxylated surfaces using the ultrasound emulsification method. These nanoparticles can then react with the amino groups on the surface of Salmonella Typhimurium YB1 (YB1), forming amide bonds and resulting in the bacterium/nanoparticle hybrid (YB1-INP) [58]. Additionally, indocyanine green (ICG) can be encapsulated within human serum albumin (HSA) nanoparticles (HSA/ICG NPs) via intermolecular disulfide bonds. These nanoparticles can be attached to the surface of a natural photosynthetic cyanobacterium, Synechococcus elongatus PCC 7942 (Syne), through amide bonds, creating a biomimetic system (S/HSA/ICG) for triple therapy of metastatic breast cancer [110]. Besides amide bonds, nanoparticles can also covalently bind to bacteria via imine bonds. For instance, methylene blue (MB), a photosensitizer, can be encapsulated in zeolitic imidazolate framework-90 (ZIF-90) nanoparticles that contain surface aldehyde groups. These nanoparticles can react with the amino groups of the facultative anaerobe Shewanella oneidensis MR-1, forming imine bonds under mild conditions [101]. Bacteria and nanoparticles bound by chemical bonds can usually be carried out under physiological conditions and have strong binding forces and high loading rates. Therefore, bacteria-nanoparticle hybrids bound by chemical bonds are relatively stable and not easily dissociated. However, their high stability leads to low drug release efficiency, which often requires more reasonable material design so that the encapsulated drugs can be successfully released, thus hindering the implementation of this method.
Electrostatic interaction
The outer membranes of most bacteria exhibit a negative charge due to the abundance of phosphate and carboxyl groups on their cell membranes, typically resulting in an isoelectric point below 5 [119, 120]. Under physiological conditions, the pH is generally above the isoelectric point of bacterial cells. This negative charge allows for electrostatic interactions with various positively charged nanomaterials, such as polymers, inorganic materials, and liposomes, enabling direct decoration of the bacterial cell surface through simple co-incubation with live bacteria [121,122,123,124,125,126,127,128]. Consequently, by co-incubating positively charged nanomaterials with negatively charged bacteria, stable bacterial-nanomaterial hybrids can be obtained. Numerous studies have utilized the negatively charged bacterial surface to bind various cationic substances through electrostatic methods [57, 80, 87, 129]. Additionally, modifying the surface potential of bacteria allows for further surface decoration with anionic nanomaterials [130, 131]. For example, biocompatible poly(allylamine hydrochloride) (PAH) can be used to modify non-pathogenic Shewanella oneidensis (SO) [132], rendering the bacterial surface positively charged. This allows for the binding of MIL-101 metal-organic framework (MOF) nanoparticles loaded with the anticancer drug doxorubicin (DOX) and coated with negatively charged hyaluronic acid (HA). The DOX-loaded MIL-101 MOF nanoparticles and SO can bind through electrostatic interactions, resulting in the formation of the biohybrid system SO@MIL-101-DOX-HA (SO@MDH) (Fig. 2B). Another example involves labeling anaerobic Clostridium novyi-NT (C. novyi-NT) spores with branched gold nanoparticles (BGNP) for visualization of spore delivery to solid tumors via X-ray computed tomography (CT) following intratumoral injection [87]. Negatively charged C. novyi-NT spores were functionalized with positively charged, low molecular weight branched polyethylenimine (bPEI, 600 Da), increasing the surface charge of the spores to positive values. The synthesized BGNPs were modified with 4-mercaptobenzoic acid (4-MCBA) through ligand exchange, resulting in highly negatively charged BGNPs. Finally, the negatively charged BGNPs were electrostatically deposited onto the positively charged spore walls. In a PC3 prostate tumor mouse model, BGNP-coated spores were injected into the hypoxic center of each tumor, and intratumoral delivery was confirmed via CT. Polyethyleneimine (PEI) is a cationic polymer that can be surface-modified to form positively charged nanoparticles that adsorb onto bacterial surfaces. In the context of bacteria-mediated DNA vaccines, cationic polymers and DNA self-assembled nanoparticles are used for cancer immunotherapy [80]. Fe3O4 nanoparticles modified with amine-functionalized ligands exhibit a positive potential. The negatively charged surface of Magnetospirillum magneticum AMB-1 (AMB-1) allowed the formation of the bio-nanohybrid AMB-1@MNPs through electrostatic interactions [105]. This composite material is referred to as semi-artificial magnetotactic bacteria (SAMTB). The motion of this biohybrid can be regulated by adjusting the size and concentration of Fe3O4 nanoparticles, making it applicable for precise in vivo drug delivery. Furthermore, a research team developed a novel engineered bacterial system capable of targeting hypoxic tumor tissues and effectively mediating photodynamic therapy of these tumors. In this study, Escherichia coli was genetically modified to express catalase (CAT). Black phosphorus quantum dots (BPQD) were then combined with these catalase-expressing E. coli through electrostatic adsorption, producing the engineered E. coli/BPQD (EB) system [133]. This method of bacteria and nanomaterials being adsorbed to each other through positive and negative charges is simple to operate and has good biocompatibility. However, due to factors such as the strength of the charges and external interference, it can easily lead to low binding efficiency and low stability. Moreover, modifying the surface charge of bacteria can negatively affect bacterial activity.
Polydopamine adhesion
Polydopamine (PDA) is well-known for its two key chemical features: a high content of primary and secondary amines and a high content of catechol (3,4-dihydroxybenzene). The coexistence of these functional groups contributes to PDA’s strong interfacial adhesion properties, enabling it to attach to virtually all types of organic and inorganic material surfaces [134]. Inspired by mussel adhesive proteins, dopamine is a small molecule known for its ability to readily form stable PDA nanoparticles. These nanoparticles exhibit high adhesiveness, biocompatibility, negligible cytotoxicity, and good dispersion in aqueous solutions. These advantages have led to widespread applications of PDA in the biomedical field [135]. Numerous researchers have leveraged PDA’s adhesive properties to connect nanomaterials with bacteria, forming bacterial nanobiohybrids for antitumor therapy studies. For instance, a nanomedicine, MCDP, was synthesized by preparing a metal-organic framework (MOF) containing calcium peroxide and doxorubicin (DOX). Subsequently, a polydopamine (PDA) coating was applied to facilitate the attachment of the nanomedicine onto the surface of Bifidobacterium (Bif), resulting in a bacterial nanobiohybrid, MCDP@Bif [93]. MCDP@Bif enables precise delivery of CaO2 nanoparticles and doxorubicin (DOX) to tumor tissues for synergistic chemotherapy and chemodynamic therapy (CDT) (Fig. 2C). Polydopamine (PDA) conjugation can also be employed in immunotherapy as a linker for immunotherapeutic agents. Bacteria with surfaces decorated with mixed immunoactive nanoparticles can induce dual anticancer and antiviral immunity. αPD1 antibody and SARS-CoV-2 spike 1 (S1) protein were chosen as the immune checkpoint inhibitor and virus-specific antigen, respectively. Both αPD1 and S1 protein can be conjugated to PDA nanoparticles, which are then attached to the surface of Escherichia coli Nissle 1917 (EcN) [69]. Beyond its adhesive properties, PDA also exhibits photothermal effects. Under compatible conditions, indocyanine green and PDA nanodeposits can be applied to the surface of bioengineered bacteria expressing natural melanin for tumor photothermal therapy [70]. Additionally, researchers synthesized paclitaxel-loaded nanoparticles (PTX-NPs) using a diblock copolymer of methoxy poly(ethylene glycol)-poly(ε-caprolactone) (mPEG-PCL) as a carrier, with a PDA coating on the surface. These PTX-NPs were then linked to Bifidobacterium infantis, forming Bif@PDA-PTX-NPs hybrids. Upon reaching the hypoxic regions of tumor tissues, the PTX-NPs respond to the reductive tumor environment, releasing the paclitaxel and exerting its antitumor effects [94]. The conjugation of bacteria with nanomaterials via polydopamine has gained increasing popularity in recent years due to its advantages of excellent biocompatibility, ease of operation, strong adhesion, and the ability to dissociate in weakly acidic environments.
Streptavidin-biotin interaction
In addition to chemical bonds and electrostatic interactions, biotin-based bioconjugation interactions have also been employed for conjugating bacteria with various substances. Streptavidin, a protein isolated and purified from bacteria, forms a strong non-covalent interaction with biotin [136]. This non-covalent interaction has been extensively studied in bioanalysis and biomedical applications [137,138,139]. The streptavidin-biotin system has been utilized to conjugate bacteria with various substances, including compounds, nanoliposomes, and metal nanoparticles. One study reported the development of a biohybrid microrobot platform for targeted localization and multi-stimuli-responsive drug release within three-dimensional (3D) biological matrices. Magnetic nanoparticles (mNP) and nanoliposomes (NL) loaded with photothermal agents and chemotherapeutic drugs were integrated into Escherichia coli via non-covalent interactions. This biohybrid system was prepared by exploiting the streptavidin-biotin interaction between a biotin-expressing engineered strain of E. coli MG1655 and streptavidin-functionalized mNPs. Subsequently, biotinylated NLs were directly conjugated, forming a biotin-streptavidin-biotin system [140] (Fig. 2D). Red blood cell membranes can be combined with bacteria using the biotin-streptavidin-biotin system. Engineered motile bacteria and red blood cells form biohybrid microswimmers, containing the model anticancer drug doxorubicin (DOX) and superparamagnetic iron oxide nanoparticles (SPIONs). These microswimmers offer autonomous propulsion, untethered magnetic steering, and efficient drug encapsulation and release. DOX molecules and SPIONs were first loaded into red blood cells, which were then attached to genetically engineered E. coli MG1655 via a biotin-streptavidin-biotin complex [71]. Research has also utilized anaerobic Salmonella VNP20009 to bind with nanoparticles through streptavidin-biotin interactions. Streptavidin was modified onto PLGA nanoparticles, and biotin-treated bacterial antibodies were attached to the bacterial surface. Through the streptavidin-biotin interaction, PLGA nanoparticles and bacteria formed bacterial/nanomaterial composites (NanoBEADS). NanoBEADS can enhance the retention and distribution of nanoparticles in solid tumors by up to 100-fold [56]. Additionally, biotinylated Listeria monocytogenes monoclonal antibodies were used to attach streptavidin-coated polystyrene nanoparticles to the bacterial surface. Biotinylated GFP plasmids were then attached to the remaining streptavidin sites on the nanoparticles [103]. The biotin-streptavidin binding method is a more traditional choice. This strategy often has strong interactions and high stability, usually does not interfere with the activity of bacteria, and more importantly, has high specificity. However, it also often leads to low drug release efficiency.
Other methods
Other reported methods include the use of acid-labile linkers and antigen-antibody interactions to connect bacteria and nanoparticles [72, 95, 141]. Additionally, nanoparticles can be introduced into bacteria through incubation and electroporation methods [142]. Electroporation utilizes electric pulses to transiently increase the permeability of the cell membrane, allowing nanoparticles or other substances to enter the cell [143]. Research has successfully employed electroporation to introduce phosphatidylcholine-coated quantum dots into folate-modified short Bifidobacteria, leveraging the high-quality luminescent properties and photostability of quantum dots to significantly improve in vivo imaging and therapeutic effects on tumors [144]. Cell-penetrating peptides (CPPs) can also facilitate nanoparticle entry into cells without the need for an external electric field. CPPs possess the ability to transfer large molecules from the extracellular environment to the cytoplasm, a feature that has been utilized to deliver gold nanoparticles into bacterial cells [145]. Biomineralization, an extensively explored strategy involving the in-situ biosynthesis of nanoparticles, has emerged as a promising method for producing bacterial-nanoparticle hybrids. Biomineralization phenomena have been observed in various microorganisms. For instance, Bacillus subtilis has been reported to biomineralize gold ions into gold nanoparticles and deposit them on its cell wall surface [146]. Biomineralization serves as a detoxification mechanism for microorganisms, reducing heavy metal ions into nanoparticles. Numerous types of metal nanoparticles have been reported to be formed through biomineralization [147, 148]. Through a simple biomineralization process, a biocompatible and biodegradable calcium carbonate (CaCO3) shell was coated on the surface of Salmonella, forming SAL@CaCO3 [149].
The construction of bacteria-nanoparticle conjugates can be achieved through various methods, including chemical bonding, electrostatic interactions, and others. Each method possesses its own advantages and disadvantages. Consequently, a comprehensive consideration of both bacterial and nanomaterial modifications is crucial when designing these conjugates.
(A) Schematic diagram of the construction of Cu2O@ΔSt microbiotic nanomedicine by bonding Cu2O onto the surface of ΔSt. Modified with permission [118]. (B) Synthesis of the self-driven, tumor tropism SO@MDH Biohybrid. Modified with permission [132]. (C) Schematic illustration showing the fabrication process of the biomotor MCDP@Bif. Modified with permission [93]. (D) Schematic illustration of the bacterial biohybrid microrobots, conjugated with NLs and mNPs. NLs are loaded with DOX and ICG, and both NLs and mNPs are conjugated to bacteria via biotin-streptavidin interactions. Modified with permission [140]
Bacteria-mediated nanomaterials for antitumor therapy
The application of nanotechnology in bacterial-based cancer therapy has advanced rapidly in recent years. Researchers have discovered that hybrid materials combining bacteria and nanomaterials can enhance tumor targeting. Numerous studies have integrated bacteria-nanomaterial hybrids with traditional cancer treatments, including radiotherapy, chemotherapy, immunotherapy, photothermal therapy, and photodynamic therapy, leading to effective eradication of tumor cells. We will elaborate from two perspectives: bacterial-mediated tumor monotherapy and combination therapy (Fig. 3).
Monotherapy
Bacteria-mediated nanomaterials for monotherapy in tumor treatment include chemotherapy, radiotherapy, photothermal therapy, photodynamic therapy, chemodynamic therapy, and immunotherapy. Monotherapy only requires loading one therapeutic drug, making the system design simple and easy to implement, with fewer side effects. Simple photothermal therapy, even only used for subcutaneous tumors, has limited model selection and cannot be extended to other in situ models. However, the system is simple, more feasible for development, and conducive to large-scale production. Table 3 summarizes the research on the application of bacteria and nanomaterials for cancer monotherapy.
Chemotherapy
Chemotherapy is a primary treatment modality for metastatic cancer, as it allows drugs to circulate through the bloodstream and reach various metastatic sites, enabling systemic therapy [161]. Chemotherapy drugs target the growth and division processes of cancer cells, preventing their normal replication and ultimately leading to cell death. However, chemotherapy is often associated with severe side effects [162]. Recent studies have highlighted the potential of bacteria as carriers for chemotherapeutic drugs in cancer treatment, enabling specific targeting of both primary and metastatic tumors [163]. For example, a study reported the development of a biocompatible bacteria/nanoparticle hybrid (Bif@DOX-NPs) designed to enhance the efficacy of chemotherapy in breast cancer [92]. Bif@DOX-NPs selectively accumulated in tumors due to the hypoxic targeting ability of Bifidobacterium and the affinity of the bovine serum albumin (BSA) layer to the albumin-binding secreted protein acidic and rich in cysteine (SPARC), which is expressed on many solid tumors. This selective accumulation enhances the antitumor efficacy of the chemotherapeutic drug while reducing side effects (Fig. 4A). Notably, Bif@DOX-NPs significantly prolonged the survival of mice by over 30 days compared to the saline group.
Radiotherapy
Radiotherapy utilizes high-energy rays to irradiate the tumor area, killing tumor cells by inducing DNA damage. However, the hypoxic microenvironment within solid tumors significantly reduces the efficacy of radiotherapy [164]. Furthermore, studies have shown that radiotherapy can induce protective autophagy in tumor cells, enabling them to maintain homeostasis and continue proliferating and metastasizing [161]. The combination of bacteria and nanomaterials has the potential to improve the hypoxic conditions of tumors and synergistically enhance the effects of radiotherapy. Integrating bacteria with radiosensitizing nanomaterials can result in more pronounced therapeutic outcomes for tumor treatment. For instance, a study developed an integrated nanosystem composed of engineered bacteria (Bacillus) and Bi2S3 nanoparticles (BNPs) (Bac@BNP) to enhance radiosensitivity [150]. The engineered bacteria specifically colonize tumor sites and overexpress the cytolysin A (ClyA) protein, which modulates the cell cycle from the radiation-resistant phase to the radiation-sensitive phase. Meanwhile, the peptide-modified BNPs, serving as a radiosensitizer with high atomic (Z) elements, are released from the Bacillus surface in response to matrix metalloproteinase-2 (MMP2) present in the tumor microenvironment. Upon X-ray irradiation, the BNPs trigger intracellular reactive oxygen species (ROS) production, leading to DNA damage and increased radiosensitivity (Fig. 4B). Notably, mice in the PBS, PBS + X-ray, BNP, and Bacillus groups exhibited increasing mortality before day 30. In contrast, mice in the Bac@BNP group exhibited a 75% survival rate at day 60. In this study, Bac@BNP under X-ray irradiation significantly inhibited breast cancer in mice while reducing toxic side effects.
Immunotherapy
The advancement of cancer immunotherapy has brought new opportunities to oncology, making immune-based anticancer strategies increasingly significant [165,166,167]. Cancer immunotherapy harnesses the body’s own immune system to recognize and attack cancer cells, ultimately achieving therapeutic goals. In recent years, several studies have proposed methods using bacterial nanocarriers for immunomodulation [54]. Ma et al. utilized Fe3O4@liposome nanocomposites to genetically modify engineered Escherichia coli, developing magnetically manipulable bacteria targeted to tumors. Alternating magnetic fields (AMF) are an ideal signal for manipulating bacteria due to their virtually limitless tissue-penetrating capability and excellent biosafety. The study describes an AMF-manipulated tumor-homing bacteria (AMF-Bac), constructed by modifying genetically engineered E. coli BL21 attenuated strain with a Fe3O4@lipid nanocomposite. The magnetic engineered bacteria enable consistent magnetic field-controlled movement to enhance tumor targeting and improve therapeutic efficacy, achieving tumor-specific CD47 blockade and precise tumor immunotherapy [157] (Fig. 4C).
Photothermal therapy
Photothermal therapy (PTT) involves the absorption of photon energy by a photothermal agent, causing a transition from the ground state to the excited state. The excited photothermal agent returns to the ground state upon collision with surrounding molecules, releasing energy and generating heat [168]. Nanomaterials, including inorganic and polymer nanoparticles, serve as the primary photothermal agents [169, 170]. These nanomaterials can be combined with bacteria to form antitumor nano-bacterial complexes. In bacteria-mediated PTT therapy, tumor treatment exhibits high specificity. Bacteria carrying photosensitizers preferentially accumulate within solid tumors. Upon exposure to near-infrared laser, bacteria with photosensitizers demonstrate excellent efficacy in destroying cancer cells and eradicating solid tumors [171, 172]. Research has connected nano photosensitizers (indocyanine green nanoparticles, INPs) to the surface of Yersinia enterocolitica strain YB1, forming a bio/non-bio cross-linked system (YB1-INPs) for precise tumor treatment [173]. This YB1-INPS treatment strategy demonstrates specific hypoxia targeting against solid tumors, excellent photothermal conversion, and efficient fluorescence (FL) imaging properties. Under near-infrared laser irradiation (NIR), YB1-INPS exhibits a reliable, efficient photothermal ability to eradicate large solid tumors without recurrence (Fig. 4D). Additionally, researchers have exploited the advantages of using Salmonella VNP20009 biotherapy in combination with dopamine-mediated photothermal therapy to enhance the antitumor efficacy against malignant melanoma [92]. VNP20009 is coated with dopamine through oxidation and self-polymerization and then injected intravenously into tumor-bearing mice. The dopamine-coated VNP20009 targets the hypoxic regions of solid tumors, and upon near-infrared laser irradiation, the tumor heats up due to the presence of dopamine. This combined approach eliminates tumors with a single injection and laser irradiation, with no recurrence or metastasis.
Photodynamic therapy
Photodynamic therapy (PDT) utilizes photosensitizers, which, upon activation, generate cytotoxic reactive oxygen species (ROS), inducing apoptosis, necrosis, and autophagy in tumor cells [174]. While extensively researched as a non-invasive treatment for tumors, PDT is limited by the drawbacks of photosensitizers, including their short half-life, low solubility, and poor stability [175]. Precise delivery of sufficient photosensitizers to the tumor site remains a significant challenge [176]. Numerous studies have explored the use of nano-bacterial biohybrids for delivering photosensitizers for PDT [177]. Ding et al. developed a novel engineered bacterial system capable of targeting hypoxic tumor tissues and effectively mediating PDT for these tumors. Escherichia coli was genetically modified to express peroxidase, and black phosphorus quantum dots (BPQDs) were attached to the bacterial surface via electrostatic adsorption, generating the engineered E. coli/BPQD (EB) system [156]. Laser irradiation at 660 nm drove EB to generate ROS and disrupt the bacterial cell membrane, leading to the release of catalase, followed by its degradation to produce oxygen. Increased oxygen levels alleviate intratumoral hypoxia, thereby enhancing BPQD-mediated PDT (Fig. 4E).
Chemodynamic therapy
Chemodynamic therapy (CDT) disrupts cancer cells by converting hydrogen peroxide and superoxide in the tumor microenvironment (TME) into reactive oxygen species (ROS). However, its antitumor efficacy is limited by tumor-targeting capability. Moreover, high levels of ROS can promote the exposure of tumor-associated antigens, facilitating the engulfment of dead cells and fragments by antigen-presenting cells (APCs), thereby further eliciting a systemic immune response [27]. In recent years, bacteria integrated with functional nanoparticles have achieved precise antitumor effects through therapeutic reactions, especially Fenton-like reactions [178]. The strategy of bacteria-mediated Fenton-like reactions for tumor therapy primarily utilizes genetic engineering techniques to introduce genes encoding catalase and iron reductase into microbial cells, enabling them to produce hydrogen peroxide and ferrous ions. In Fenton-like reactions, hydrogen dioxide (H2O2) can be converted into highly reactive hydroxyl radicals (•OH) with the presence of metal catalysts [179]. As a kind of the reactive oxygen species (ROS), hydroxyl radical is of highly oxidative activity towards organic molecules and induces severe oxidative damage of tumor cells. Researchers developed an integrative bioreactor based on engineered bacteria for tumor therapy via Fenton-like reaction with localized H2O2 generation. Nonpathogenic bacterium Escherichia coli MG1655 (Ec) was engineered with respiratory chain enzyme II (NDH-2) overexpression (Ec-pE). Amino-functionalized magnetic Fe3O4 nanoparticles (MNP) were modified onto the engineered bacteria via amide bonds, constructing a Fenton-like bioreactor (Ec-pE@MNP) [159]. Under the anaerobic targeting of bacteria and the influence of an external magnetic field, Ec-pE carrying MNP reaches the tumor microenvironment with abundant spontaneous H2O2 production, generating many hydroxyl radicals to kill tumor cells (Fig. 4F).
(A) Schematic diagram of the antitumor process of bacterial nanohybrids. Modified with permission [92]. (B) When self-targeted to tumor sites, the bacteria-based nanosystem could secrete ClyA protein to arrest the cell cycle to the radiosensitive phase and release BNP to realize radiotherapy sensitivity by ROS generation and DNA damage. Modified with permission [150]. (C) Illustration of potential immune responses induced by AMF-Bac. Modified with permission [157]. (D) YB1-INPs with hypoxia-targeting and photothermal-assisted bioaccumulation for tumor penetrative therapy. Modified with permission [173]. (E) Schematic illustration of a novel engineered bacterium/black phosphorus quantum dot hybrid system for hypoxic tumor targeting and efficient photodynamic therapy. Modified with permission [156]. (F) The scheme of bacteria-based Fenton-like bioreactor and its chemodynamic therapy process for antitumor therapy. Modified with permission [159]
Synergistic therapy
Given the high rates of metastasis, recurrence, individual variability, and low survival rates associated with cancer, combination therapy is particularly important and urgent [180]. Extensive research has led to the engineering of various bacterial cells as carriers for delivering chemotherapeutic drugs, plasmids, proteins, and other composite nanomedicines for tumor treatmentt [89, 181,182,183]. These composite nanosystems endow bacterial cells with multiple therapeutic functions, enabling multidirectional treatment approaches such as Fenton-like catalytic therapy [184], photothermal therapy [185] and biotherapy [186]. Bacteria-mediated nanomaterials for the synergistic treatment of tumors have also been extensively studied preclinically. Synergistic therapy, which employs multiple treatment methods simultaneously to combat tumors, achieves comprehensive tumor killing and exhibits enhanced therapeutic efficacy. Table 4 summarizes the research on bacteria-mediated synergistic therapy for tumor treatment.
Photothermal therapy-based synergistic therapies
Photothermal therapy (PTT), a non-invasive treatment method, offers precise spatial and temporal control in antitumor therapy [204]. PTT converts light into heat energy through photothermal agents under specific wavelengths of near-infrared irradiation, killing tumor cells in an oxygen-independent manner. While both PDT and PTT induce cell death by generating free radicals, each has inherent limitations, making it difficult to achieve sufficient therapeutic effects with a single modality. However, the combined use of PTT and PDT not only retains the advantages of low toxicity and minimal side effects associated with phototherapy but also produces additive or even synergistic therapeutic effects, thereby enhancing efficacy and reducing side effects [205,206,207,208]. PTT can enhance immune activation, and combining PTT with immunotherapy can yield potent antitumor effects. For instance, tumor-specific antigens (OVA) and checkpoint blockade α-PD-1 antibodies can be in situ precipitated with polydopamine (PDA) to form PDA nanoparticles, which are then conjugated onto the surface of Escherichia coli (EcN). PDA exerts a photothermal effect, inducing the repolarization of M2 macrophages to M1 macrophages. The attached OVA promotes the maturation of dendritic cells (DCs), while α-PD-1 can induce the activation of cytotoxic T lymphocytes [73] (Fig. 5A). Combining chemotherapy with PTT can increase the sensitivity of tumor cells to chemotherapeutic drugs and enhance drug penetration into deeper tumor regions [204, 209]. The facultative anaerobic bacterium Escherichia coli Nissle 1917 (EcN) was functionalized to form a self-propelled microrobot, actively delivering chemotherapeutic drugs and photosensitizers to the deep hypoxic regions of tumors [197]. Doxorubicin (Dox) and gold nanorods (AuNRs) were conjugated onto the surface of EcN. Cellular co-localization of EcN-Dox-Au conjugates in MCF-7 cells was observed using CLSM. The nuclei appeared red fluorescence of Dox, and the fluorescence signals enhanced with the increase of co-incubation time, demonstrating that the conjugates could be internalized into nuclei and exert antitumor efficacy. Upon near-infrared laser irradiation near the tumor, the AuNRs converted light into heat, significantly enhancing the permeability of tumor cell membranes and increasing the penetration of both Dox and EcN, thus achieving an optimized synergistic effect of PTT and chemotherapy (Fig. 5B). Additionally, bacterial-nanomaterial hybrids can be employed for a combinatorial approach integrating chemotherapy, PTT, and CDT. A “Trojan nanobacteria hybrid,” comprising facultative anaerobic Escherichia coli MG 1655 and prodrug based HINCs (highly integrated nanocapsules), has been successfully constructed. The hybrid, termed E. coli@HINCs (HINE-Hybrid), utilizes HINCs, which are covalently cross-linked through pillararene derivatives and cisplatin prodrug linkers. These HINCs can be endocytosed and cleaved to release therapeutic agents. Under near-infrared (NIR) light irradiation (808 nm), HINCs significantly increase the system’s temperature, which further leads to the efficient production of reactive oxygen species (ROS). Moreover, in both in vitro and in vivo studies, HINE-Hybrid demonstrates significant antitumor effects and promotes immune cell infiltration and antitumor cytokine expression within the tumor microenvironment (TME) [198]. Additionally, bacterial-mediated PTT combined with radiotherapy has also been reported. A hybrid system of black phosphorus quantum dots (BPQD) and Escherichia coli (E. coli) (BE) has been developed. BPQD can directly enhance X-ray-mediated tumor radiosensitization [199], achieving significant radiotherapy efficacy at lower radiation doses. This system effectively destroys hypoxic tumor tissues, thereby inhibiting tumor growth (Fig. 5C).
Nanoparticles encapsulating the photosensitizer Ce6 and Au NPs (Au-Ce6) were conjugated to the native photosynthetic bacterium Synechococcus elongatus PCC 7942 (Syne) [190]. The engineered bacteria loaded with Au-Ce6 (Bac@AuCe6) retained the photosynthetic characteristics and tumor-targeting abilities of Syne. When a 660 nm laser irradiated the tumor site, the photosynthetic autotrophic Syne continuously released photosynthetic O2, which was immediately activated into 1O2 by the loaded photosensitizer. Subsequently, 808 nm laser irradiation was applied for PTT to further enhance tumor cytotoxicity (Fig. 5D).
(A) Decorated bacteria-mediated reversal of the tumor immunosuppressive microenvironment via repolarization of TAMs, maturation of dendritic cells, and activation of cytotoxic T lymphocytes. Modified with permission [73]. (B) Schematic illustration of the developed EcN-Dox-Au microrobots for synergistic photothermal-chemotherapy and immunomodulatory effect of breast tumor. Modified with permission [197]. (C) Schematic illustration of BPQDs/bacteria system that synergistically facilitates PTT against hypoxic tumors and complementary low-dose radiotherapy. Modified with permission [199]. (D) Synthesis of Bac@Au-Ce6 and schematic diagram of PDT and PTT tumor therapy in vivo. Modified with permission [190]
Chemotherapy-based synergistic therapies
The clinical efficacy of chemotherapeutic drugs is often compromised due to poor drug selectivity, off-target effects, and drug resistance. Bacterial vectors can deliver chemotherapeutic drugs to deeper tumor regions [49, 50], and can be combined with other therapeutic modalities to enhance antitumor effects. Bacteria-mediated synergistic chemotherapy and CDT have demonstrated promising antitumor efficacy. For example, by functionalizing MCDP nanoparticles containing calcium peroxide (CaO2) and doxorubicin (DOX) onto polydopamine-coated metal-organic frameworks (MOFs), synergistic chemotherapy and chemodynamic therapy can be achieved with anaerobic Bifidobacterium infantis (Bif) [93]. 4T1 cells readily took up MIL + DOX + PDA (MDP) and MCDP NPs compared to free DOX, similar uptake was observed in A549 and CT26 cells. This hybrid material, composed of MIL framework + CaO2 + DOX + polydopamine (MCDP)@Bif, leverages the innate targeting ability of Bif to actively home in on the hypoxic regions of solid tumors. In the acidic tumor microenvironment (TME), MCDP generates hydroxyl radicals through an enhanced Fenton-like reaction between Fe2+ and the hydrogen peroxide produced by CaO2. The disruption of Ca2+ homeostasis and the resulting mitochondrial Ca2+ overload induce apoptosis and enhance oxidative stress, thereby promoting tumor cell death (Fig. 6A). Studies have also explored the use of anaerobic bacteria to mediate the synergistic effects of chemotherapy and immunotherapy. Probiotic Bifidobacterium bifidum (Bi) combined with DOX-loaded CaP/SiO2 nanoparticles (DNPs@Bi) has been utilized for tumor treatment. Studies have also explored the use of anaerobic bacteria to mediate the synergistic effects of chemotherapy and immunotherapy. Probiotic Bifidobacterium bifidum (Bi) combined with DOX-loaded CaP/SiO2 nanoparticles (DNPs@Bi) has been utilized for tumor treatment [96]. DNPs@Bi was able to enhance the cellular uptake. The DNPs@Bi system releases DOX to exert chemotherapeutic effects and induce immunogenic cell death (ICD). Furthermore, DNPs@Bi stimulates the maturation of dendritic cells (DCs) and the infiltration of cytotoxic T lymphocytes (Fig. 6B). Additionally, the integration of the chemotherapeutic drug doxorubicin (DOX) onto the surface of non-pathogenic Escherichia coli (E. coli) with high expression of glucose dehydrogenase (GDH) has been employed to enhance its antitumor efficacy. The nicotinamide adenine dinucleotide phosphate (NADPH) produced by E. coli facilitates the generation of toxic reactive oxygen species (ROS) within tumors. These ROS are subsequently catalyzed by NADPH oxidase activated by DOX. Importantly, the hybrid bacteria augment the systemic antitumor immune response, leading to effective tumor eradication [187].
Photodynamic therapy-based synergistic treatment
The tumor microenvironment (TME) is typically characterized by significant hypoxia, with partial oxygen pressure (pO2) below 20 mmHg [210,211,212,213,214,215]. This local hypoxia severely impedes the effectiveness of cancer treatments, particularly photodynamic therapy (PDT) [216,217,218,219]. Anaerobic bacteria have excellent tumor-targeting capabilities, and attaching photosensitizers to bacteria can enhance the efficacy of photodynamic therapy (PDT). For example, iridium(III) photosensitizers covalently labeled onto the surface of genetically engineered bacteria (Ir-HEcN) selectively target and penetrate deep into tumor tissues [200](Fig. 6C). Under irradiation, Ir-HEcN can induce tumor cell pyroptosis and immunogenic cell death, leading to the regression of solid tumors through the combination of PDT and immunotherapy. Additionally, the combination of photodynamic therapy (PDT) and sonodynamic therapy (SDT) can achieve superior antitumor effects. Chlorin e6 (Ce6) nanoparticles can be incorporated into bifidobacteria. Under laser or ultrasound irradiation, Ce6 generates reactive oxygen species (ROS) to kill cancer cells [201] (Fig. 6D).
(A) Schematic illustration showing antitumor mechanism of the biomotor MCDP@Bif. Modified with permission [93]. (B) Bifidobacterium bifidum (Bi) was covalently modified with DOX-loaded CaP/SiO2 nanoparticles (DNPs@Bi) for tumor Therapy. Modified with permission [96]. (C) Schematic illustration of the elimination of solid tumors by enhanced photodynamic immunotherapy. The injected photosensitizer-bacteria hybrid induces photodynamic therapy at tumor tissues under irradiation and evokes innate and adaptive immune responses, thereby leading to the final tumor regression. Modified with permission [200]. (D) Combination of laser and US exposure exerted a synergistic effect on ROS generation of Ce6–B. bifidum–anti-DR5 Ab (a) and Combined 671 nm laser and US targeted therapy (b). Modified with permission [201]
Others
In addition to the common bacterial-mediated combination therapies, bacterial vectors can activate the immune system or synergize with other treatments such as immunotherapy, chemotherapy, or radiotherapy to enhance antitumor effects [55, 220,221,222,223,224,225,226]. For instance, engineered Salmonella typhimurium strains secreting Vibrio vulnificus flagellin B (SLpFlaB) have been used to activate immune responses and, in combination with radiotherapy, enhance the treatment of intracranial sarcomas [227, 228]. Moreover, another study designed a bacterial vector coated with gold-platinum bimetallic nanozymes (Au-Pt@VNP20009, APV). This system leverages the intense tumor inflammatory response induced by low-dose X-rays, significantly enhancing the tumor-targeting delivery capacity of CD11b + immune cells hitchhiking on APV. This approach effectively alleviates tumor hypoxia and immune suppression, thereby inhibiting tumor growth and metastasis [229]. Synergistic therapy has better therapeutic efficacy. It uses multiple treatment methods simultaneously to fight tumors, achieving comprehensive tumor killing. Especially after combining with immunotherapy, the recurrence rate is lower in the long term. However, the system synthesis is composed of two or more therapeutic agents, leading to high complexity and potentially increased side effects compared to monotherapy. Drug development becomes more difficult and costly.
Challenges and outlook
Tumor-targeting bacteria can specifically penetrate primary tumors and metastases throughout the body and proliferate within necrotic areas [230]. This unique proliferation mechanism effectively overcomes the limitations of current clinical cancer treatments, such as chemotherapy, molecular targeted therapy, cell therapy, and antibody therapy, which cannot penetrate the interior of tumors, and radiotherapy, surgery, and other physical therapies that cannot target potential metastatic sites. However, clinical trial results have shown that bacterial monotherapy has not yet achieved significant antitumor effects [33, 231]. This is primarily because the cytotoxicity of tumor-targeting engineered bacteria is insufficient to eliminate tumor cells, and the secreted therapeutic agents cannot diffuse throughout the entire solid tumor. Additionally, the proliferation of some bacteria may promote tumor growth and metastasis, posing significant challenges for clinical application [232]. Therefore, combining bacterial targeted therapy with the advantages of existing clinical treatments is crucial for its ultimate clinical application. The application of bacteria-nanoparticle hybrids has been shown to successfully promote tumor regression and prolong survival in mouse models. Although many published studies on bacteria-based biotherapy have shown promising results in experimental models, the use of tumor-targeting bacteria in clinical practice as therapeutic drugs still faces significant hurdles. There are still some challenges that need to be addressed.
First, potential risks associated with bacteria must be carefully considered. Given that these conjugates contain live bacterial cells, addressing biosafety concerns is paramount. Rapid introduction of a large number of bacterial cells into the human body can trigger a strong immune response [233, 234]. Researchers have proposed several solutions to address the safety concerns of using bacteria as bioactive drug carriers. Firstly, it is recommended to use biocompatible probiotics or non-pathogenic bacteria for treatment. E coli Nissle 1917, a non-pathogenic intestinal probiotic, has been used to treat gastrointestinal diseases [235, 236]. Bifidobacterium is also known for its oral probiotic use. Second, reducing the pathogenicity of bacteria by deleting virulence-related genes can alleviate immune responses. For example, genetically engineered Salmonella strains lacking ppGpp genes elicit weaker immune responses and have been applied in antitumor therapy [237]. Additionally, attenuation strategies can be employed to reduce the virulence of pathogenic bacteria. These approaches can enhance the safety and efficacy of bacterial-nanoparticle conjugates, facilitating their clinical development. Second, the binding stability of nanoparticles and bacteria during in vivo transport needs improvement, and conducting clinical trials also presents significant difficulties. Furthermore, limited drug loading efficiency is another challenge hindering the anti-cancer efficacy of bacteria. Besides further optimizing the preparation methods, genetically modifying bacteria to produce anti-cancer drugs may enhance the therapeutic effect. Finally, the production process for live bacteria is more complex than that for small molecule anti-cancer drugs. Unlike small molecules or other non-active clinical drugs, live therapeutic bacteria cannot be sterilized by filtration or heat treatment, which would be the greatest challenge for producing products that pass Good Manufacturing Practice (GMP) grade testing. Traditional sterility testing standards will no longer apply. Therefore, real-time supervision, production, purification, and harvesting of live bacteria under strict aseptic procedures are practical methods to ensure the quality of the final product. Bacterial seed banks and storage systems should also be further optimized. Finally, the potential environmental impact when live bacteria can be used in a clinical setting is another issue that needs to be carefully addressed.
Bacteria can deliver a variety of drugs, amino acids, and proteins. Their excellent tumor-targeting properties make them play a crucial role in cancer treatment. It is noteworthy, however, that there are also some issues that need to be addressed in future work in bacterial-mediated synergistic cancer therapy. To facilitate the clinical application of bacterial synergistic therapy, more resources need to be invested in researching large-scale production, sterilization techniques, management plans, production equipment, storage, and transportation methods. Tumor therapy with bacteria-mediated nanomaterials also requires further research, which will provide a solid theoretical basis and new development directions for promoting tumor treatment. Although this novel drug delivery system requires improvement, bacteria-nanoparticle conjugates have demonstrated their potential in enhancing nanomaterial targeted delivery through bacterial tumor colonization. This innovative therapeutic approach has shown promising preliminary results. Since the initial attempts to use bacteria for tumor treatment, the concept of bacterial biotherapy and its effectiveness as a drug delivery system for various diseases have been widely recognized. The powerful tumor colonization ability of bacteria makes them ideal carriers for tumor-targeted drug delivery. By combining the antitumor advantages of nanomedicine with the targeting ability of bacteria, this novel delivery system can specifically enhance tumor therapeutic efficacy while significantly reducing side effects. Combining bacteria-nanoparticle conjugates with traditional or novel antitumor therapies may represent a new direction for future cancer treatment. This approach holds great potential and broad prospects for development in the biomedical field, especially in oncology.
Conclusions
This review summarizes the mechanisms potentially involved in bacterial targeting of tumors, as well as the construction strategies for bacteria-nanoparticle conjugates and their applications in antitumor therapy. Bacteria used for antitumor therapy could target tumors and colonize hypoxic tumor regions, making them ideal nanocarriers for delivering drugs to tumor areas. Therefore, constructing novel drug delivery systems by combining bacteria with nanomaterials occupies an important position in antitumor therapy research. Bacteria are attracted to the TME, which is characterized by hypoxia, nutrient richness, and immunosuppression. Some chemoattractants, as well as the active movement ability of bacteria, are also key factors in enabling bacteria to target tumor sites. Bacterial targeting offers higher targeting efficiency and penetration compared to traditional ligand-modified targeting. Additionally, bacteria can carry a wide variety of therapeutic agents, such as anticancer drugs, immunomodulators, and gene therapy vectors. Bacteria-nanoparticles can be applied in various cancer treatment modalities. In conclusion, this anti-cancer method utilizes the active targeting ability of bacteria and the high efficiency of nanomaterials in killing cancer cells, achieving enhanced targeting effects in tumor treatment. Although bacteria-based therapies are still in their infancy, they have immense potential. The combination of bacterial therapy with other treatments to generate effective cancer therapies remains a topic worthy of further research. One of the main focuses currently is to effectively combine bacteria-based therapies with nanomedicine. Utilizing bacterial tumor colonization can lead to the development of novel antitumor drugs for use in routine clinical applications.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- PTT:
-
Photothermal therapy
- PDT:
-
Photodynamic therapy
- CDT:
-
Chemodynamic therapy
- TME:
-
Tumor microenvironment
- DOX:
-
Doxorubicin
- PTX:
-
Paclitaxel
- PLGA:
-
Poly (lactic-co-glycolic acid)
- PDA:
-
Polydopamine
- ICG:
-
Indocyanine green
- ROS:
-
Reactive oxygen species
- 1O2:
-
Singlet oxygen
- NPs:
-
Nanoparticles
- mNP:
-
Magnetic nanoparticles
- NL:
-
Nanoliposomes
- Ce6:
-
Chlorin e6
- OVA:
-
Ovalbumin
- APCs:
-
Antigen-presenting cells
- DCs:
-
Dendritic cells
- TAMs:
-
Tumor-associated macrophages
- AMF:
-
Alternating magnetic fields
- BSA:
-
Bovine serum albumin
- 4-MCBA:
-
4-mercaptobenzoic acid
- PEI:
-
Polyethyleneimine
- SPIONs:
-
Superparamagnetic iron oxide nanoparticles
- NIR:
-
Near-infrared laser irradiation
References
Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer, Statistics. 2021. CA Cancer J Clin. 2021;71(1):7–33.
Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37.
Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Zare P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18(1):59.
Blanco E, Shen H, Ferrari M. Principles of Nanoparticle Design for Overcoming Biological barriers to Drug Delivery. Nat Biotechnol. 2020;9:941–51.
Guo Q, Jiang C. Delivery strategies for macromolecular drugs in cancer therapy. Acta Pharm Sin B. 2020;10(6):979–86.
Manzari M, Shamay Y, Kiguchi H, Rosen N, Scaltriti M, Heller D. Targeted drug delivery strategies for precision medicines. Nat Rev Mater. 2021;6(4):351–70.
Alqahtani MS, Alqahtani A, Syed R, Thabit A, Roni M. Novel lignin nanoparticles for oral drug delivery. J Mater Chem B. 2019;7(28).
Landgraf M, Lahr C, Kaur I, et al. Targeted camptothecin delivery via silicon nanoparticles reduces breast cancer metastasis. Biomaterials. 2020;240:119791.
Florea AM, Büsselberg D. Cisplatin as an Anti-tumor Drug: Cellular mechanisms of Activity, Drug Resistance and Induced Side effects. Cancers. 2011;3(1):1351–71.
Hartmann J, Lipp H. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4(6):889–901.
Baldo BA, Pham NH. Adverse reactions to targeted and non-targeted chemotherapeutic drugs with emphasis on hypersensitivity responses and the invasive metastatic switch. Cancer Metastasis Rev. 2013;32(3–4):723–61.
Witzigmann D. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–57.
Klein J. Probing the interactions of proteins and nanoparticles. Proc Natl Acad Sci U S A. 2007;104(7):2029–30.
Jia L, XinLiu. HuiXia, JindongShi, XiangyangShen, Mingwu. Ultrasound-enhanced precision tumor theranostics using cell membrane-coated and pH-responsive nanoclusters assembled from ultrasmall iron oxide nanoparticles. Nano Today. 2021;36(1):9.
Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42(6):463–78.
Feliciano CP, Tsuboi K, Suzuki K, Kimura H, Nagasaki Y. Long-term bioavailability of redox nanoparticles effectively reduces organ dysfunctions and death in whole-body irradiated mice. Biomaterials. 2017;129:68–82.
B AGAA, C VP, C DTLBC. Tumor-targeted nanomedicines for cancer theranostics. Pharmacol Res. 2017;115:87–95.
van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder W, Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14(11):1007–17.
Prabhakar U, Maeda H, Jain RK, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73(8):2412–7.
Yetisgin AA, Cetinel S, Zuvin M, Kosar A, Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Molecules. 2020;25(9):2193.
Tearney GJ, Fukumura D, Jain RK, et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med. 2009;15(10):1219–23.
Smoody A, Adayton P, Zamboni WC. Imaging methods to evaluate tumor microenvironment factors affecting nanoparticle drug delivery and antitumor response. Cancer Drug Resist. 2021;4(2):382–413.
Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–64.
Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410.
Becerra-Báez E, Meza-Toledo S, Muñoz-López P, et al. Recombinant attenuated Salmonella enterica as a delivery system of Heterologous molecules in Cancer Therapy. Cancers. 2022;14(17):4224.
Awan BN, Fatima N, Riaz S, Malik S, Ahmad W. Bacterial and liposomal vector guided Drug Delivery System via TumorMarkers Carrier Gene to treat Neoplasm. J App Pharm. 2015;8:1.
Xie Y, Huang M, Li D, et al. Bacteria-based nanodrug for anticancer therapy. Pharmacol Res. 2022;182:106282.
Jain R. Normalization of Tumor vasculature: an emerging Concept in Antiangiogenic Therapy. Science. 2005;307(5706):58–62.
Danquah MK, Zhang XA, Mahato RI. Extravasation of polymeric nanomedicines across tumor vasculature. Adv Drug Deliv Rev. 2011;63(8):623–39.
Forbes N. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10(11):785–94.
Yu YA, Shabahang S, Timiryasova TM, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22(3):313–20.
Kim S, Castro F, Paterson Y, Gravekamp C. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 2009;69(14):5860–6.
Toso J, Gill V, Hwu P, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20(1):142–52.
Charbonneau M, Isabella V, Li N, Kurtz C. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat Commun. 2020;11(1):1738.
Afkhami-Poostchi A, Mashreghi M, Iranshahi M, Matin MM. Use of a genetically engineered E. Coli overexpressing β-glucuronidase accompanied by glycyrrhizic acid, a natural and anti-inflammatory agent, for directed treatment of colon carcinoma in a mouse model. Int J Pharm. 2020;579:119159.
Hayashi K, Zhao M, Yamauchi K, et al. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem. 2009;106(6):992–8.
Zheng J, Nguyen V, Jiang S, et al. Salmonella typhimuriumtwo-step enhanced cancer immunotherapy with engineered secreting heterologous flagellin. Sci Transl Med. 2017;9(376):eaak9537.
Liu S, Minton N, Giaccia A, Brown J. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Ther. 2002;9(4):291–6.
Broadway K, Suh S, Behkam B, Scharf B. Optimizing the restored chemotactic behavior of anticancer agent Salmonella enterica Serovar Typhimurium VNP20009. J Biotechnol. 2017;251:76–83.
Feng X, He P, Zeng C, et al. Novel insights into the role of Clostridium novyi-NT related combination bacteriolytic therapy in solid tumors. Oncol Lett. 2021;21(2):110.
Staedtke V, Roberts N, Bai R, Zhou S. Clostridium novyi-NT in cancer therapy. Genes Dis. 2016;3(2):144–52.
Pan Z, Weiskirch L, Paterson Y. Regression of established B16F10 melanoma with a recombinant Listeria monocytogenes vaccine. Cancer Res. 1999;59(20):5264–5249.
Parker R, Plummer H. Effect of histolyticus infection and toxin on transplantable mouse tumors. Proc Soc Exp Biol Med. 1947;66(2):461–7.
Malmgren R, Flanigan C. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 1955;15(7):473–8.
Pawelek J, Low K, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57(20):4537–44.
Lambin P, Theys J, Landuyt W, et al. Colonisation of Clostridium in the body is restricted to hypoxic and necrotic areas of tumours. Anaerobe. 1998;4(4):183–8.
Goto Y, Iwata S, Miyahara M, Miyako E. Discovery of Intratumoral oncolytic Bacteria toward targeted Anticancer Theranostics. Adv Sci. 2023;10(20):e2301679.
Mostaghaci B, Yasa O, Zhuang J, Sitti M. Bioadhesive Bacterial microswimmers for targeted drug delivery in the urinary and gastrointestinal tracts. Adv Sci. 2017;4(6):1700058.
Cronin M, Morrissey D, Rajendran S, et al. Orally administered bifidobacteria as vehicles for delivery of agents to systemic tumors. Mol Ther. 2010;18(7):1397–407.
Fan J, Li Z, Liu X, Zheng D, Chen Y, Zhang X. Bacteria-mediated tumor therapy utilizing photothermally-controlled TNF-α expression via oral administration. Nano Lett. 2018;18(4):2373–80.
Zhu C, Ji Z, Ma J, Ding Z, Shen J, Wang Q. Recent advances of nanotechnology-facilitated Bacteria-based drug and gene Delivery systems for Cancer Treatment. Pharmaceutics. 2021;13(7):940.
Kojima M, Zhang Z, Nakajima M, Ooe K, Fukuda T. Construction and evaluation of bacteria-driven liposome. Sens Actuat B-Chem. 2013;183:395–400.
Allemailem KS. Innovative approaches of Engineering Tumor-Targeting Bacteria with different therapeutic payloads to Fight Cancer: a Smart Strategy of Disease Management. Int J Nanomed. 2021;16:8159–84.
Moreno V, Baeza A. Bacteria as Nanoparticle Carriers for Immunotherapy in Oncology. Pharmaceutics. 2022;14(4):784.
Cao Z, Liu J. Bacteria and bacterial derivatives as drug carriers for cancer therapy. J Control Release. 2020;326:396–407.
Suh S, Jo A, Traore M, et al. Nanoscale Bacteria-Enabled Autonomous Drug Delivery System (NanoBEADS) enhances Intratumoral Transport of Nanomedicine. Adv Sci. 2019;6(3):1801309.
Zheng D, Chen Y, Li Z, et al. Optically-controlled bacterial metabolite for cancer therapy. Nat Commun. 2018;9(1):1680.
Chen F, Zang Z, Chen Z, et al. Nanophotosensitizer-engineered Salmonella bacteria with hypoxia targeting and photothermal-assisted mutual bioaccumulation for solid tumor therapy. Biomaterials. 2019;214:119226.
Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004:4–9.
Pan H, Zheng M, Ma A, Liu L, Cai L. Cell/Bacteria-Based bioactive materials for Cancer Immune Modulation and Precision Therapy. Adv Mater. 2021;33(50):e2100241.
Yifan X, Feng X, Xiaoxue Z et al. Microbiota in tumors: from understanding to application. Adv Sci 2022;9(21).
Pries A, Cornelissen A, Sloot A, et al. Structural adaptation and heterogeneity of normal and tumor microvascular networks. PLoS Comput Biol. 2009;5(5):e1000394.
Gong X-T, Zhuang J, Chong KC et al. Far-Red Aggregation-Induced Emission Hydrogel-Reinforced Tissue Clearing for 3D Vasculature Imaging of Whole Lung and Whole Tumor. Adv Mater. n/a(n/a):2402853.
Céline AB, Edwin RM, Teodora IK et al. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res. 2012;72(24).
Jochen S, Stephanie W, Philip JH, Tobias AO, Werner G, Aladar AS. Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int J Med Microbiol. 2007;297(3).
Rachel WK, Neil SF. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res. 2007;67(7).
R B A SG. J P S, B B, N S F. In tumors Salmonella migrate away from vasculature toward the transition zone and induce apoptosis. Cancer Gene Ther. 2011;18(7).
Ming Z, Meng Y, Xiao-Ming L et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci U S A. 2005;102(3).
Liu Y, Zhang M, Wang X, et al. Dressing Bacteria with a hybrid Immunoactive Nanosurface to Elicit Dual Anticancer and antiviral immunity. Adv Mater. 2023;35(11):e2210949.
Guo H, Cao Z, Li J, et al. Integrating Bacteria with a Ternary combination of photosensitizers for monochromatic irradiation-mediated photoacoustic imaging-guided synergistic Photothermal Therapy. ACS Nano. 2023;17(5):5059–71.
Alapan Y, Yasa O, Schauer O, et al. Soft erythrocyte-based bacterial microswimmers for cargo delivery. Sci Robot. 2018;3(17):eaar4423.
Xie S, Chen M, Song X, et al. Bacterial microbots for acid-labile release of hybrid micelles to promote the synergistic antitumor efficacy. Acta Biomater. 2018;78:198–210.
Li J, Xia Q, Guo H, et al. Decorating Bacteria with Triple Immune Nanoactivators generates Tumor-Resident Living Immunotherapeutics. Angew Chem Int Ed Engl. 2022;61(27):e202202409.
Fan Ju P, Mu, Wang H, Zheng, Ha. Zhang Xh. Engineered Bacterial Bioreactor for Tumor Therapy via Fenton-Like reaction with localized H2O2 generation. Adv Mater. 2019;31(16).
Deng X, Yang W, Shao Z, Zhao Y. Genetically modified bacteria for targeted phototherapy of tumor. Biomaterials. 2021;272:120809.
Feng X, Yang G, Liu L, et al. A convenient preparation of multi-spectral microparticles by bacteria-mediated assemblies of conjugated polymer nanoparticles for cell imaging and barcoding. Adv Mater. 2012;24(5):637–41.
Wu M, Wu W, Duan Y, Li X, Liu B. Photosensitizer-Bacteria bio-hybrids promote photodynamic Cancer cell ablation and intracellular protein delivery. Chem Mater. 2019;31(18):7212–20.
Akolpoglu M, Alapan Y, Dogan N, et al. Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli-responsive cargo delivery. Sci Adv. 2022;8(28):eabo6163.
Dong H, Sarkes D, Rice J, Hurley M, Fu A, Stratis-Cullum D. Living Bacteria-nanoparticle hybrids mediated through surface-displayed peptides. Langmuir. 2018;34(20):5837–48.
Hu Q, Wu M, Fang C, et al. Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy. Nano Lett. 2015;15(4):2732–9.
Chen W. Bacteria-Driven Hypoxia Targeting for Combined Biotherapy and Photothermal Therapy. ACS Nano. 2018;12(6):5995–6005.
Ektate K, Munteanu M, Ashar H, Malayer J, Ranjan A. Chemo-immunotherapy of colon cancer with focused ultrasound and Salmonella-laden temperature sensitive liposomes (thermobots). Sci Rep. 2018;8(1):13062.
Zheng S, Han, et al. Active tumor-therapeutic liposomal bacteriobot combining a drug (paclitaxel)-encapsulated liposome with targeting bacteria (Salmonella Typhimurium). Sens Actuat B-Chem. 2016;3:217–24.
Zoaby N, Shainsky-Roitman J, Badarneh S, et al. Autonomous bacterial nanoswimmers target cancer. J Control Release. 2017;257:68–75.
Wang C, Zhong L, Xu J et al. Oncolytic mineralized bacteria as potent locally administered immunotherapeutics. Nat Biomed Eng. 2024.
Guo L, Ding J, Zhou W. Converting bacteria into autologous tumor vaccine via surface biomineralization of calcium carbonate for enhanced immunotherapy. Acta Pharm Sin B. 2023;13(12):5074–90.
Park W, Cho S, Huang X, Larson A, Kim D. Branched gold nanoparticle coating of Clostridium novyi-NT spores for CT-Guided Intratumoral Injection. Small. 2017;13(5).
Luo C, Huang C, Su C, Yeh C. Bacteria-mediated hypoxia-specific delivery of nanoparticles for Tumors Imaging and Therapy. Nano Lett. 2016;16(6):3493–9.
Felfoul O, Mohammadi M, Taherkhani S, et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat Nanotechnol. 2016;11(11):941–7.
Samira T. Covalent binding of nanoliposomes to the surface of magnetotactic bacteria for the synthesis of self-propelled therapeutic agents. ACS Nano. 2014;8(5):5049–60.
Ye P, Li F, Zou J, et al. In situ Generation of Gold nanoparticles on Bacteria-derived magnetosomes for imaging-guided Starving/Chemodynamic/Photothermal synergistic therapy against Cancer. Adv Funct Mater. 2022;32(17):2110063.
Xiao S, Shi H, Zhang Y, et al. Bacteria-driven hypoxia targeting delivery of chemotherapeutic drug proving outcome of breast cancer. J Nanobiotechnol. 2022;20(1):178.
Li J, Dai J, Zhao L, et al. Bioactive Bacteria/MOF hybrids can achieve targeted synergistic chemotherapy and chemodynamic therapy against breast tumors. Adv Funct Mater. 2023;33(42):2303254.
Shi H, Chen L, Liu Y, et al. Bacteria-driven Tumor Microenvironment-Sensitive nanoparticles Targeting Hypoxic regions enhances the Chemotherapy Outcome of Lung Cancer. Int J Nanomed. 2023;18:1299–315.
Li Y, Li Y, Leng Q, et al. Anaerobic bacteria mediated ‘smart missile’ targeting tumor hypoxic area enhances the therapeutic outcome of lung cancer. Chem Eng J. 2022;438:135566.
He T, Wang L, Gou S, et al. Enhanced immunogenic cell death and Antigen Presentation via Engineered Bifidobacterium bifidum to Boost Chemo-Immunotherapy. ACS Nano. 2023;17(11):9953–71.
Luo Y, Xu D, Gao X, et al. Nanoparticles conjugated with bacteria targeting tumors for precision imaging and therapy. Biochem Biophys Res Commun. 2019;514(4):1147–53.
Wu W, Pu Y, Yao H, Lin H, Shi J. Microbiotic nanomedicine for tumor-specific chemotherapy-synergized innate/adaptive antitumor immunity. Nano Today. 2022;42:101377–101377.
Wang W, Zhang L, Zhang Y, et al. A self-adaptive pyroptosis inducer: optimizing the Catalytic Microenvironment of nanozymes by membrane-adhered microbe enables Potent Cancer Immunotherapy. Adv Mater. 2024;36(14):2310063.
Haosheng S, Changyu Z, Shengjie L et al. Prodrug-conjugated tumor-seeking commensals for targeted cancer therapy. Nat Commun. 2024;15(1).
Chen Q-W, Liu X-H, Fan J-X, et al. Self-mineralized photothermal Bacteria hybridizing with Mitochondria-targeted metal–Organic frameworks for Augmenting Photothermal Tumor Therapy. Adv Funct Mater. 2020;30(14):1909806.
Xi M, Deng Z, Zhang C et al. FeS-based cascade bioreactors driven by Shewanella oneidensis MR-1 for efficient chemodynamic therapy with augmented antitumor immunity. Nano Today. 2024/04/01/ 2024;55:102165.
Akin D, Sturgis J, Ragheb K, et al. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat Nanotechnol. 2007;2(7):441–9.
Dróżdż M, Makuch S, Cieniuch G, Woźniak M, Ziółkowski P. Obligate and facultative anaerobic bacteria in targeted cancer therapy: current strategies and clinical applications. Life Sci. 2020;261:118296.
Li Q, Chen H, Feng X, et al. Nanoparticle-regulated semiartificial magnetotactic Bacteria with tunable magnetic moment and magnetic sensitivity. Small. 2019;15(15):e1900427.
Chen W, Zhu Y, Chen J, et al. Potentiating the systemic immunity by Bacteria-delivered Sting activation in a Tumor Microenvironment. Adv Funct Mater. 2023;33:2307001.
Park S, Park S, Cho S, et al. New paradigm for tumor theranostic methodology using bacteria-based microrobot. Sci Rep. 2013;3:3394.
Wang X, Niu M, Fan J, Chen Q, Zhang X. In situ photoelectric Bacteria enhance the production of tetrodotoxin for Antitumor Therapy. Nano Lett. 2021;21(10):4270–9.
Beveridge TJ, Graham LL. Surface layers of bacteria. Microbiol Rev. 1992;55(4):684–705.
Liu L, He H, Luo Z, Zhou H, Cai L. In situ photocatalyzed oxygen generation with photosynthetic Bacteria to Enable Robust Immunogenic Photodynamic Therapy in Triple-negative breast Cancer. Adv Funct Mater. 2020;30(10).
Bi X, Yin J, Guanbang AC, Liu CF. Chemical and enzymatic strategies for bacterial and mammalian cell Surface Engineering. Chemistry. 2018;24(32):8042–50.
Theile CS, Witte MD, Blom AEM, Kundrat L, Ploegh HL, Guimaraes CP. Site-specific N-terminal labeling of proteins using sortase-mediated reactions. Nat Protoc. 2013;8(9):1800–7.
Han L, Terasaki O, Che S. Carboxylic group functionalized ordered mesoporous silicas. J Mater Chem. 2011;(30).
Xie S, Zhao L, Song X, Tang M, Mo C, Li X. Doxorubicin-conjugated Escherichia coli Nissle 1917 swimmers to achieve tumor targeting and responsive drug release. J Control Release. 2017;268:390–9.
Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16(9):1035–41.
Luo H, Chen Y, Kuang X, et al. Chemical reaction-mediated covalent localization of bacteria. Nat Commun. 2022;13(1):7808.
Wei X, Du M, Chen Z, Yuan Z. Recent advances in Bacteria-based Cancer Treatment. Cancers. 2022;14(19):4945.
Wu W, Pu Y, Gao S, et al. Bacterial metabolism-initiated nanocatalytic Tumor Immunotherapy. Nanomicro Lett. 2022;14(1):220.
HARDEN V, HARRIS J. The isoelectric point of bacterial cells. J Bacteriol. 1953;65(2):198–202.
Lin S, Wu F, Zhang Y, et al. Surface-modified bacteria: synthesis, functionalization and biomedical applications. Chem Soc Rev. 2023;52(19):6617–43.
Akolpoglu M, Dogan N, Bozuyuk U, Ceylan H, Kizilel S, Sitti M. High-yield production of Biohybrid Microalgae for On-Demand Cargo Delivery. Adv Sci. 2020;7(16):2001256.
Park J, Yang S, Lee J, Ko E, Hong D, Choi I. Nanocoating of single cells: from maintenance of cell viability to manipulation of cellular activities. Adv Mater. 2014;26(13):2001–10.
Das S, Liang J, Schmidt M, Laffir F, Marsili E. Biomineralization mechanism of gold by zygomycete fungi Rhizopus oryzae. ACS Nano. 2012;6(7):6165–73.
Yuan H, Liu Z, Liu L, Lv F, Wang Y, Wang S. Cationic conjugated polymers for discrimination of microbial pathogens. Adv Mater. 2014;26(25):4333–8.
Li L, Liu L, Li L et al. Chitosan coated bacteria responsive metal-polyphenol coating as efficient platform for wound healing. Compos Part B-Eng. 2022:234.
YufeiTang ZL, KangZhao SF. Positively charged and flexible SiO2@ZrO2 nanofibrous membranes and their application in adsorption and separation. RSC Adv. 2018;8(23):13018–25.
Li Z, Ma J, Ruan J, Zhuang X. Using positively charged magnetic nanoparticles to capture Bacteria at Ultralow Concentration. Nanoscale Res Lett. 2019;14(1):195.
Cao Z, Wang X, Pang Y, Cheng S, Liu J. Biointerfacial self-assembly generates lipid membrane coated bacteria for enhanced oral delivery and treatment. Nat Commun. 2019;10(1):5783.
Li J, Sun W, Yang Z, et al. Rational design of self-assembled Cationic Porphyrin-based nanoparticles for efficient photodynamic inactivation of Bacteria. ACS Appl Mater Interfaces. 2020;12(49):54378–86.
Feng P, Cao Z, Wang X, Li J, Liu J. On-Demand bacterial reactivation by restraining within a Triggerable Nanocoating. Adv Mater. 2020;32(34):e2002406.
Hou W, Li J, Cao Z, et al. Decorating Bacteria with a therapeutic nanocoating for synergistically enhanced Biotherapy. Small. 2021;17(37):e2101810.
Wang J, Chen Q, Luo G, et al. A self-driven Bioreactor based on Bacterium-Metal-Organic Framework Biohybrids for Boosting Chemotherapy Cyclic Lactate Catabolism. ACS Nano. 2021;15(11):17870–84.
Ding S, Liu Z, Huang C, Zeng N, Li Q. Novel Engineered Bacterium/Black Phosphorus Quantum Dot Hybrid System for Hypoxic Tumor Targeting and efficient photodynamic therapy. ACS Appl Mater Interfaces. 2021;13(8):10564–73.
Cheng W, Zeng X, Chen H, Li Z, Zhao Y. Versatile polydopamine platforms: synthesis and promising applications for Surface Modification and Advanced Nanomedicine. ACS Nano. 2019;13(8).
Pan C, Li J, Hou W, et al. Polymerization-mediated multifunctionalization of living cells for enhanced cell-based therapy. Adv Mater. 2021;33(13):e2007379.
Hyre DE, Le TI, Merritt EA, et al. Cooperative hydrogen bond interactions in the streptavidin-biotin system. Protein Sci. 2006;15(3):459–67.
Park SJ, Park SH, Cho S, et al. New paradigm for tumor theranostic methodology using bacteria-based microrobot. Sci Rep. 2013;3:3394.
Dundas CM, Demonte D, Park S. Streptavidin–biotin technology: improvements and innovations in chemical and biological applications. Appl Microbiol Biotechnol. 2013;97(21):9343–53.
Chivers CE, Koner AL, Lowe ED, Howarth M. How the biotin–streptavidin interaction was made even stronger: investigation via crystallography and a chimaeric tetramer. Biochem J. 2011;435(1):55–63.
Akolpoglu MB, Alapan Y, Dogan NO, et al. Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli-responsive cargo delivery. Sci Adv. 2022;8(28):eabo6163.
Luo CH, Huang CT, Su CH, Yeh CS. Bacteria-mediated hypoxia-specific delivery of nanoparticles for Tumors Imaging and Therapy. Nano Lett. 2016;16(6):3493–9.
Nour Z. Autonomous bacterial nanoswimmers target cancer. J Control Release. 2017;257:68–75.
Tsong TY. Electroporation of cell membranes. Adv Anat Embryol Cell Biol. 1991;60(2):297–306.
Liu Y, Zhou M, Luo D, et al. Bacteria-mediated in vivo delivery of quantum dots into solid tumor. Biochem Biophys Res Commun. 2012;425(4):769–74.
Kumar M, Tegge W, Wangoo N, Jain R, Sharma R. Insights into cell penetrating peptide conjugated gold nanoparticles for internalization into bacterial cells. Biophys Chem. 2018;237:38–46.
Beveridge T, Murray R. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980;141(2):876–87.
Yin T, Diao Z, Blum N, Qiu L, Ma A, Huang P. Engineering Bacteria and bionic bacterial derivatives with nanoparticles for Cancer Therapy. Small. 2022;18(12):e2104643.
Park T, Lee S, Heo N, Seo T. In vivo synthesis of diverse metal nanoparticles by recombinant Escherichia coli. Angew Chem Int Ed Engl. 2010;49(39):7019–24.
Lina G, Jinsong D, Wenhu Z. Converting bacteria into autologous tumor vaccine via surface biomineralization of calcium carbonate for enhanced immunotherapy. Acta Pharm Sin B. 2023;13(12).
Pan P, Dong X, Chen Y, Zeng X, Zhang X. Engineered Bacteria for enhanced radiotherapy against breast carcinoma. ACS Nano. 2022;16(1):801–12.
Xiande L, Shengnan, et al. Radiotherapy combined with an engineered Salmonella typhimurium inhibits tumor growth in a mouse model of colon cancer. Exp Anim. 2016;65(4):413–8.
Dinesh C. 32-Phosphorus selectively delivered by listeria to pancreatic cancer demonstrates a strong therapeutic effect. Oncotarget. 2017;8(13):20729–40.
Jin-Xuan F. Bacteria-mediated tumor therapy utilizing photothermally-controlled TNF-α expression via oral administration. Nano Lett. 2018;18(4):2373–80.
Wang L, Qin W, Xu W, et al. Bacteria-mediated tumor therapy via Photothermally-programmed Cytolysin A expression. Small. 2021;17(40):e2102932.
Chu B, Yang Y, Tang J, Song B, He Y, Wang H. Trojan Nanobacteria System for Photothermal Programmable Destruction of Deep Tumor Tissues. Angew Chem Int Ed Engl. 2022;61(36):e202208422.
Ding S, Liu Z, Huang C, Zeng N, Jiang W, Li Q. Novel Engineered Bacterium/Black Phosphorus Quantum Dot Hybrid System for Hypoxic Tumor Targeting and efficient photodynamic therapy. ACS Appl Mater Interfaces. 2021;13(8):10564–73.
Ma X, Liang X, Li Y, et al. Modular-designed engineered bacteria for precision tumor immunotherapy via spatiotemporal manipulation by magnetic field. Nat Commun. 2023;14(1):1606.
Guo L, Chen H, Ding J, Rong P, Sun M, Zhou W. SalmonellaSurface engineering with pH-responsive polyserotonin and self-activated DNAzyme for better microbial therapy of tumor. Exploration. 2023;3(6):20230017.
Fan J, Peng M, Wang H, et al. Engineered Bacterial Bioreactor for Tumor Therapy via Fenton-Like reaction with localized H O generation. Adv Mater. 2019;31(16):e1808278.
Zhang W, Liu J, Li X, et al. Precise chemodynamic therapy of Cancer by Trifunctional Bacterium-Based Nanozymes. ACS Nano. 2021;15(12):19321–33.
Hou X, Jiang J, Tian Z, Wei L. Autophagy and Tumour Chemotherapy. Adv Exp Med Biol. 2020;1207:351–74.
Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79.
Song S, Vuai M, Zhong M. The role of bacteria in cancer therapy - enemies in the past, but allies at present. IInfect Agent Cancer. 2018;13:9.
Horsman M, Mortensen L, Petersen J, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9(12):674–87.
Pan R, Ryan J, Pan D, Wucherpfennig K, Letai A. Augmenting NK cell-based immunotherapy by targeting mitochondrial apoptosis. Cell. 2022;185(9):1521–38.
Van Leent MMT, Priem B, Schrijver DP et al. Regulating trained immunity with nanomedicine. Nat Rev Mater. 2022;(6):7.
Garland K, Sheehy T, Wilson J. Chemical and Biomolecular strategies for STING pathway activation in Cancer Immunotherapy. Chem Rev. 2022;122(6):5977–6039.
Xingshu L, Jonathan F, et al. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–74.
Lv S, Miao Y, Liu D, Song F. Recent development of Photothermal agents (PTAs) based on small Organic Molecular dyes. ChemBioChem. 2020;21(15):2098–110.
Li C, Cheng Y, Li D, et al. Antitumor Applications of Photothermal agents and Photothermal Synergistic therapies. Int J Mol Sci. 2022;23(14):7909.
Nam J, Son S, Ochyl L, Kuai R, Schwendeman A, Moon J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun. 2018;9(1):1074.
Lou X, Chen Z, He Z, Sun M, Sun J. Bacteria-mediated synergistic Cancer therapy: small Microbiome has a big Hope. Nanomicro Lett. 2021;13(1):37.
Fuming C, Zhongsheng Z, Ze C, et al. Nanophotosensitizer-engineered Salmonella bacteria with hypoxia targeting and photothermal-assisted mutual bioaccumulation for solid tumor therapy. Biomaterials. 2019;214:119226.
Celli J, Spring B, Rizvi I, et al. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010;110(5):2795–838.
Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle D, Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38(8):2628–35.
Chen J, Zhu Y, Wu C, Shi J. Nanoplatform-based cascade engineering for cancer therapy. Chem Soc Rev. 2020;49(24):9057–94.
Lu H, Niu L, Yu L, et al. Cancer phototherapy with nano-bacteria biohybrids. J Control Release. 2023;360:133–48.
Yunlu D, Zhen Y, Siyuan C et al. Toxic reactive oxygen species enhanced synergistic combination therapy by self-assembled metal-phenolic network nanoparticles. Adv Mater. 2018;30(8).
Xiao Y, Deng Y, Huan W, Li J, Zhang J, Xing M. Hollow-structured Fe2O3/Au/SiO2 nanorods with enhanced and recyclable photo-Fenton oxidation for the remediation of organic pollutants. Mater Today Chem Mar. 2019;11:86–93.
Gu Z, Zhu S, Yan L, Zhao F, Zhao Y. Graphene-based Smart platforms for Combined Cancer Therapy. Adv Mater. 2019;31(9):e1800662.
Qinglian H, Min W, Chun F et al. Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy. Nano Lett. 2015;15(4).
Dezhi N, Shuang Q, Hui D et al. Biomimetically Engineered demi-Bacteria potentiate vaccination against Cancer. Adv Sci 2017;4(10).
Hong D, Deborah AS, Jeffrey JR, Margaret MH, Adele JF. Dimitra N S-C. Living Bacteria-Nanoparticle Hybrids Mediated through surface-displayed peptides. Langmuir. 2018;34(20):5837–48.
Jin-Xuan F, Meng-Yun P, He W, et al. Engineered Bacterial Bioreactor for Tumor Therapy via Fenton-Like reaction with localized H(2) O(2) generation. Adv Mater. 2019;31(16):e1808278.
Jin-Xuan F, Zi-Hao L, Xin-Hua L, Di-Wei Z, Ying C, Xian-Zheng Z. Bacteria-mediated tumor therapy utilizing photothermally-controlled TNF-α expression via oral administration. Nano Lett. 2018;18(4):2373–80.
Di-Wei Z, Ying C, Zi-Hao L, et al. Optically-controlled bacterial metabolite for cancer therapy. Nat Commun. 2018;9(1):1680.
Sun M, Ye H, Shi Q, et al. Both-In-One hybrid Bacteria suppress the Tumor Metastasis and Relapse via Tandem-Amplifying reactive oxygen species-immunity responses. Adv Healthc Mater. 2021;10(21):e2100950.
Kalyani E, Cristina MM, Harshini A, Jerry M, Ashish R. Chemo-immunotherapy of colon cancer with focused ultrasound and Salmonella-laden temperature sensitive liposomes (thermobots). Sci Rep. 2018;8(1):13062.
Zhang H, Wang Y, Zhu L, et al. A bulldozer driven by anoxic bacteria for pancreatic cancer chemo-immunotherapy. J Control Release Aug. 2023;360:660–71.
Yin C, Wang Z, Dai C, et al. Light-triggered photosynthetic engineered bacteria for enhanced-photodynamic therapy by relieving tumor hypoxic microenvironment. Theranostics. 2023;13(5):1632–48.
B XDA, A WY AZS. Genetically modified Bacteria for targeted phototherapy of Tumor. Biomaterials. 2021;272:120809.
Self-Mineralized Photothermal Bacteria. Hybridizing with Mitochondria‐targeted metal–Organic frameworks for Augmenting Photothermal Tumor Therapy. Adv Funct Mater. 2020;30(14).
Reghu S, Miyako E. Bifidobacterium bifidumNanoengineered with optical activity for Photothermal Cancer Immunotheranostics. Nano Lett. 2022;22(5):1880–8.
Chen W, He C, Qiao N, et al. Dual drugs decorated bacteria irradiate deep hypoxic tumor and arouse strong immune responses. Biomaterials. 2022;286:121582.
Reghu S, Iwata S, Komatsu S, Nakajo T, Miyako E. Cancer immunotheranostics using bioactive nanocoated photosynthetic bacterial complexes. Nano Today. 2023/10/01/ 2023;52:101966.
Luo W, Zhang Z, Zhou D, et al. Deep tumor penetration of CRISPR-Cas System for Photothermal-Sensitized Immunotherapy via Probiotics. Nano Lett. 2023;23(17):8081–90.
Wu D, Zhao Z, Liu H, et al. Escherichia coli Nissle 1917-driven microrobots for effective tumor targeted drug delivery and tumor regression. Acta Biomater. 2023;169:477–88.
Zang M, Ji Y, Ding X et al. Trojan nanobacteria hybridized with prodrug nanocapsules for efficient combined tumor therapy. Nano Res. 2023/07/01 2023;16(7):9651–9662.
Ji P, Chen J, Wang H, Shi L, Tang X, Duo Y. Bacteria-targeted delivery of black phosphorus quantum dots facilitates photothermal therapy against hypoxic tumors and complementary low-dose radiotherapy. Biomater Sci. 2023;11(13):4727–40.
Lin W, Liu Y, Wang J, et al. Engineered Bacteria labeled with Iridium(III) Photosensitizers for enhanced photodynamic immunotherapy of solid tumors. Angew Chem Int Ed Engl. 2023;62(43):e202310158.
Li W, Zhang Z, Liu J, et al. Nanodrug-loaded Bifidobacterium bifidum conjugated with anti-death receptor antibody for tumor-targeted photodynamic and sonodynamic synergistic therapy. Acta Biomater. 2022;146:341–56.
Li H, Pei P, He Q, et al. Nanozyme-coated Bacteria Hitchhike on CD11b + Immune cells to Boost Tumor Radioimmunotherapy. Adv Mater. 2024;36(8):2309332.
Wang W, Zhang L, Zhang Y, et al. A self-adaptive pyroptosis inducer: optimizing the Catalytic Microenvironment of nanozymes by membrane-adhered microbe enables Potent Cancer Immunotherapy. Adv Mater. 2024;36(14):e2310063.
Chen H, Zhao Y. Applications of light-responsive systems for Cancer Theranostics. ACS Appl Mater Interfaces. 2018;10(25):21021–34.
Han D, Liu X, Wu S. Metal organic framework-based antibacterial agents and their underlying mechanisms. Chem Soc Rev. 2022;51(16):7138–69.
Li Y, Liu X, Li B, Zheng Y, Wu S. Near-Infrared Light Triggered Phototherapy and Immunotherapy for Elimination of Methicillin-Resistant Staphylococcus aureus Biofilm infection on Bone Implant. ACS Nano. 2020;14(7):8157–70.
Zheng Q, Liu X, Zheng Y, et al. The recent progress on metal-organic frameworks for phototherapy. Chem Soc Rev. 2021;50(8):5086–125.
Zhang W, Ding X, Cheng H, et al. Dual-targeted gold nanoprism for recognition of early apoptosis, dual-model imaging and Precise Cancer Photothermal Therapy. Theranostics. 2019;9(19):5610–25.
Wu D, Xu S, Zhang X, et al. A Near-Infrared laser-triggered size-shrinkable nanosystem with in situ drug release for Deep Tumor Penetration. ACS Appl Mater Interfaces. 2021;13(14):16036–47.
Overchuk M, Zheng G. Overcoming obstacles in the tumor microenvironment: recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials. 2018;156:217–37.
Dai Y, Xu C, Sun X, Chen X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem Soc Rev. 2017;46(12):3830–52.
Yuan Z, Liu X, Ling J, et al. In situ-transition nanozyme triggered by tumor microenvironment boosts synergistic cancer radio-/chemotherapy through disrupting redox homeostasis. Biomaterials. 2022;287:121620.
Sun D, Wang Z, Zhang P, et al. Ruthenium-loaded mesoporous silica as tumor microenvironment-response nano-fenton reactors for precise cancer therapy. J Nanobiotechnol. 2021;19(1):98.
Xu Q, Lan X, Lin H, et al. Tumor microenvironment-regulating nanomedicine design to fight multi-drug resistant tumors. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023;15(1):e1842.
Pitt J, Marabelle A, Eggermont A, Soria J, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27(8):1482–92.
Casas A, Di Venosa G, Hasan T, Batlle A. Mechanisms of resistance to photodynamic therapy. Curr Med Chem. 2011;18(16):2486–515.
Maas A, Carter S, Wileyto E, et al. Tumor vascular microenvironment determines responsiveness to photodynamic therapy. Cancer Res. 2012;72(8):2079–88.
Tang W, Zhen Z, Wang M, et al. Red blood cell-facilitated photodynamic therapy for Cancer Treatment. Adv Funct Mater. 2016;26(11):1757–68.
Correia J, Rodrigues J, Pimenta S, Dong T, Yang Z. Photodynamic therapy review: principles, photosensitizers, applications, and future directions. Pharmaceutics. 2021;13(9):1332.
Fu S, Zhang R, Gao Y, et al. Programming the lifestyles of engineered bacteria for cancer therapy. Natl Sci Rev. 2023;10(5):nwad031.
Pei P, Zhang Y, Jiang Y, et al. Pleiotropic immunomodulatory functions of Radioactive inactivated bacterial vectors for enhanced Cancer Radio-Immunotherapy. ACS Nano. 2022;16(7):11325–37.
Canale F, Basso C, Antonini G, et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature. 2021;598(7882):662–6.
Abedi M, Yao M, Mittelstein D, et al. Ultrasound-controllable engineered bacteria for cancer immunotherapy. Nat Commun. 2022;13(1):1585.
Savage T, Vincent R, Rae S, et al. Chemokines expressed by engineered bacteria recruit and orchestrate antitumor immunity. Sci Adv. 2023;9(10):eadc9436.
Min J, Kim H, Park J, et al. Noninvasive real-time imaging of tumors and metastases using tumor-targeting light-emitting Escherichia coli. Mol Imaging Biol. 2008;10(1):54–61.
Min J, Nguyen V, Kim H, Hong Y, Choy H. Quantitative bioluminescence imaging of tumor-targeting bacteria in living animals. Nat Protoc. 2008;3(4):629–36.
Liu X, Guo Y, Sun Y, et al. Comparison of Anticancer activities and Biosafety between Salmonella enterica Serovar Typhimurium ∆ppGpp and VNP20009 in a Murine Cancer Model. Front Microbiol. 2022;13:914575.
Liu Z, Lim SH, Min JJ, Jung S. Synergistic Antitumor Effect of Combined Radiotherapy and Engineered Salmonella typhimurium in an intracranial sarcoma mouse model. Vaccines Jul. 2023;23(7):1275.
Li H, Pei P, He Q, et al. Nanozyme-coated Bacteria Hitchhike on CD11b Immune cells to Boost Tumor Radioimmunotherapy. Adv Mater. 2024;36(8):e2309332.
Zhang Y, Ji W, He L, et al. E. Coli Nissle 1917-Derived minicells for targeted delivery of Chemotherapeutic Drug to hypoxic regions for Cancer Therapy. Theranostics. 2018;8(6):1690–705.
Cunningham C, Nemunaitis J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Hum Gene Ther. 2001;12(12):1594–6.
Bullman S, Pedamallu C, Sicinska E, et al. Fusobacterium Analysis of persistence and antibiotic response in colorectal cancer. Science. 2017;358(6369):1443–8.
Bao Y, Cheng Y, Liu W, Luo W, Zhou P, Qian D. Bacteria-based synergistic therapy in the Backdrop of Synthetic Biology. Front Oncol. 2022;12:845346.
Hosseinidoust Z, Mostaghaci B, Yasa O, Park B, Singh A, Sitti M. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv Drug Deliv Rev. 2016;106:27–44.
Schultz M. Clinical use of E. Coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(7):1012–8.
Różańska D, Regulska-Ilow B, Choroszy-Król I, Ilow R. The role of Escherichia coli strain Nissle 1917 in the gastro-intestinal diseases. Postepy Hig Med Dosw. 2014;68:1251–6.
MALMGREN R. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 1955;15(7):473–8.
Acknowledgements
Authors are thankful to all those provided support for this study. All figures have been drawn using a platform of Biorender.com.
Funding
None.
Author information
Authors and Affiliations
Contributions
Susu Xiao and Min Mu wrote the main manuscript text , Chenqian Feng prepared Figs. 1, 2, 3, 4, 5 and 6 and Shulin Pan prepared Tables 1, 2 and 3. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
We have included 4 figures (Figs. 2, 4, 5 and 6) from previously published literature with required copyright permission from the copyright owners. The graphical abstract, Scheme 1, Figs. 1 and 3 were created with Biorender.com. We have mentioned this in the manuscript with appropriate citations. All authors approved the final manuscript for the submission to this journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiao, S., Mu, M., Feng, C. et al. The application of bacteria-nanomaterial hybrids in antitumor therapy. J Nanobiotechnol 22, 536 (2024). https://doi.org/10.1186/s12951-024-02793-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-024-02793-x