Abstract
The circular bioeconomy is one development strategy that has at their center the use and management of the biomass. Biomass could transform the bases of a global economy highly dependent on non-renewable raw materials and fossil origin, to a mostly bio-based economy that can simultaneously address the three main global challenges: provision of safe water, accessible energy for all, and climate change mitigation. In order to the circular bioeconomy not to be just a popular global goal, it is necessary to identify concrete measures that can make the concept operational, so that politicians, decision makers and stakeholders can see its practical implications. In this chapter, is reported an experience of an Integrated Waste Management Technology (IWMT) with microalgae in Argentina. The proposed IWMT includes microalgae as a complementary treatment of sewage effluents in waste stabilization pond systems, with the triple objective of wastewater purification, recovery of nutrients in biomass, and mitigation of greenhouse gases by using bioenergy from the biomass generated. Experiments were carried out with the Scenedesmus quadricauda microalgae, growing it in four concentrations of effluents (T25%, T50%, T75%, and T100%). Microalgae productivity parameters and energy and environmental qualities were studied. The species was able to grow successfully in T25% and T50% treatments, but not in T75% and T100%. This implies that it is still necessary to dilute the effluents to reduce their organic load, which at some times of the year (mainly the dry autumn-winter season) may exceed the limits allowed for their discharge. The crop has been able to grow without temperature control. The maximum CD in the treatments was around 70% higher than the control (only culture medium). The organic load reduction capacity was on average 83.4% ± 5% and 74.55% ± 4.2% (for T25% and T50%, respectively). The removal of phosphates and nitrates was 57.6% and 58.7% in T25%, and 54.6% and 76.9% in T50%. Total coliforms and fecal coliforms were reduced by 89.6% and 77.4% for T25%, and 86.6% and 68.7% by T50%. In all cases, it was possible to confirm the ability of the microalgae to remove nutrients and reduce the organic load and pathogens. The water treated with microalgae has reached permitted values for be discharged. The biomass generated has a high energy potential in comparison with other fuels, close to 4.41 ± 0.43 kWh/kg. The integration of algae in tertiary systems could improve the treatment of wastewater and water cleaning, with the possibility of achieving the reuse of water. The proposed system was simple, and can be easily replicated on larger scales, including some optimization factors if necessary. Under the pressure of climate change, the IWMT will be essential technologies particularly in regions with low water and energy availability, mitigating GHG emissions and strengthening local communities.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
The “biomass” includes a very heterogeneous set of materials that have organic matter as the main component (Sherwood 2020), excluding those that have been incorporated in geological formations undergoing a mineralization process (i.e., fossil fuels). An observation to the elemental composition of the biomass resources allows to assume that about 50% of the dry weight of them is carbon (Grobbelaar et al. 1988). On this basis, it is stated that the energy obtained from any of these resources is carbon neutral: although there will be CO2 emissions (main greenhouse gas, GHG), that carbon had already been fixed by the plant previously, through photosynthesis. So the use of biomass does not generate emissions or contribute to global warming (which may not apply in all cases, Agostini et al. 2014; Haberl et al. 2012; Wiloso et al. 2016; Khan et al. 2020a, b). It is a renewable resource, although it will depend on the intensity of use (Keegan et al. 2013). It is widely available (possibly, the sites with the lowest biomass presence are the deserts and arid areas of the world) and accessible as it does not require specific equipment and qualified personnel to be able to locate and access resources (such as fossil fuels) (Bilgili et al. 2017; Pleissner 2020).
The great diversity of materials that are included under this term (forest, agricultural, livestock, agro and forestry industries, sewage effluents, energy crops, microalgae, urban wastewater, urban solid waste, sedimentation sludge, etc.) makes it a versatile energy source, from which solid, liquid, or gaseous biofuels can be obtained, using more or less complex processes and for various applications (REN-21 2019; Hidalgo et al. 2019; Ubando et al. 2020). An energy system based on biomass is intimately linked to each territory: from the production or generation of biomass, the recollection, processing, transportation, energy conversion, and final application (Pfau et al. 2014; Manrique 2017; Meena and Lal 2018). In these biomass supply chains, each stage involves different organic resources or residues, actors and sectors of the territory (such as the forestry, energy, agricultural, economic, industrial, governmental sector and many others). (Carus and Dammer 2018; Manrique et al. 2020). A bioenergy project will, therefore, impact in multiple aspects of the territory (economy, social, politician, institutional, environmental) not only in the provision of renewable energy or mitigation of GHGs, which are the most recognized advantages worldwide (Kraxner et al. 2003; Keegan et al. 2013; Agostini et al. 2014; Rocca et al. 2015; Paletto et al. 2019; Linser and Lier 2020).
In rural or marginal urban areas, where modern energy services do not reach, biomass constitutes one of the most accessible fuel sources in its traditional form (REN-21 2019). In these sectors, the use of abundant residual resources can promote employment opportunities and niches for micro- or medium-sized companies (collection, treatment, commercialization and diverse equipment, among others), mobilizing regional development. As there is the possibility of introducing management practices and more efficient technologies, the energy obtained from biomass will result in greater benefits not only at the local level, but also at the national level (diversified primary matrix and less demand for imported fuels) (Demirbas et al. 2009; Heimann 2018; Pleissner 2020). Also, it can contribute to the fight against desertification, since it makes possible the productive use of marginal lands, on slopes or semi-arid lands and the implantation of energy crops in abandoned lands, which could prevent soil erosion and degradation (Kraxner et al. 2003; Manrique 2017; Jhariya et al. 2018a, 2018b). It can also be integrated with environmental recovery processes, mainly when they correspond to the use of by-products of productive processes or waste o sewage from human activities (Abdel-Raouf et al. 2012; Nagarajan et al. 2020).
For the characteristics mentioned, the biomass resources could transform the bases of a global economy highly dependent on non-renewable raw materials and fossil origin, to a mostly bio-based economy (Bilgili et al. 2017; Meena et al. 2018) and simultaneously address the three main global challenges: provision of safe water, accessible energy for all, and climate change mitigation (International Energy Agency (IEA) 2016; Mouratiadou et al. 2016; Del Borghi et al. 2020; Leivas et al. 2020). This chapter analyzes through a concrete case study how could be possible providing a comprehensive territorial strategy that is compatible with these current world goals towards sustainability (United Nations (UN) 2019a). Firstly, the role of biomass within this triple global challenge and the emergence of the new paradigm of the circular bioeconomy are discussed. Then, it is examined how the biomass can optimize communities’ ecological footprint (EFP) and finally, a proposal for the use and management of biomass, and its potential in sanitation, recovery of nutrients, and energy generation, is evaluated.
6.2 Water, Energy, and Climate Change: The Top Three Global Challenges
6.2.1 What Role Does Biomass Play?
Climate change and access to water and energy represent three of the main challenges that will determine the sustainable development in the coming decades (Fig. 6.1) (Mouratiadou et al. 2016; Del Borghi et al. 2020; United Nations (UN) 2019a; Leivas et al. 2020). Since a few years ago, these aspects have been concurrently tackled from a nexus approach under the understanding that one aspect influences and is influenced by the other and all three are fundamental for the subsistence of human life (Food and Agriculture Organization of the United Nations (FAO) 2014; International Energy Agency (IEA) 2016).
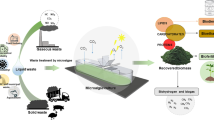
Contrast between organizational models of the world economy: (a) fossil fuel dependent economy and (b) circular bioeconomy, and potential linkages between water, energy, and climate change (see in the text). Where: the downward spiral of (a) refers to the depletion of finite resources and (1–3) imply high dependence of one sector on the other, and high negative impact on each other. (1) Increased use of fossil energy will demand more water and (3) greater amount of GHG emissions. Greater change in the climate will imply less availability of water (2) and will require more energy to access water (3). The continuous spiral in (b) refers to renewability of the raw material and (4–6) propose ideal relationships between the elements, with optimization of their use, minimization of impacts, and multiple advantages of biomass utilization. Microalgae biomass could allow simultaneously water recovery, renewable energy generation, and climate change mitigation
In a fossil-dependent world economy such as the current one, energy and water are intricately connected (Bilgili et al. 2017; Leivas et al. 2020; Teotónio et al. 2020) (Fig. 6.1a). The energy sector requires 15% of global water withdrawals for energy production (including from resources extraction to their transformation into energy) (World Water Assessment Program (WWAP) 2017). By 2035, global energy consumption will increase 50%, implying an increase in water consumption of 85% (International Energy Agency (IEA) 2016). The water access demands energy for pumping water extraction, transportation, treatment, and desalination as well as for irrigation. The climate change will affect both the energy and water sectors (Mouratiadou et al. 2016; Meena et al. 2020a). Indeed, as the temperature rises as projected, there will be places with greater demand for water in the face of drought and reduced rainfall, so access to water will require greater energy expenditure (Leivas et al. 2020). For example, the surface water pumping requires 30% less energy than the underground water pumping, but because the water level could decrease in some territories, it is very probable that the groundwater demand increases (World Water Assessment Program (WWAP) 2017). The provision of cleaner water and energy services is also linked to the health, economic activities, and family life; therefore, climate change will mostly affect the most vulnerable sectors (Mouratiadou et al. 2016; Bilgili et al. 2017). Finally, the emissions derived from the use of fossil fuels for the provision of energy imply the greatest impact on the warming of the Earth’s atmosphere (mainly from electricity generation and transport). In this way, the GHG emissions into the atmosphere are strongly dependent on the current ways of organizing the world economy: almost 80% of these emissions are related to the energy sector (FAO Food and Agriculture Organization of the United Nations (FAO) 2014; Intergovernmental Panel Climate Change (IPCC) 2006).
In a context of probable modification of the average conditions of the global atmosphere, which will affect the availability of water and access to energy sources (Siddiqi and Anadon 2011; Leivas et al. 2020) the bioeconomy represents an opportunity to rethink the development of the territories, as well as to face the commitments against the climate change (El-Chichakli et al. 2016; Del Borghi et al. 2020) (Fig. 6.1b). The base of the bioeconomy is the use and management of biomass in its multiple forms getting food and animal feeds, bio-products, and biofuels (Birner 2018; European Commission (EC) 2018; Sherwood 2020). It appears as a new model towards sustainability and seeks to highlight the biological origin of economic processes, and the problems humanity faces in relying on a limited amount of fossil resources, which are unevenly distributed and whose intensive use is affecting atmospheric GHG balances (Rodriguez et al. 2017; Birner 2018). It is not a new word, but its concept has been and is being redefined over the years, in the search for a conceptual and operational framework that leads the transformation of the global economy highly dependent on non-renewable raw materials to a sustainable “biological or biobased economy” (Bilgili et al. 2017; Sanders and Langeveld 2020; Pleissner 2020).
On the other hand, the so-called circular economy, driven by environmental problems and resources scarcity, has also gained strength since the late 1970s (Wautelet 2018). It emerges from different schools of thought, and proposes a different look at the current resource management schemes in the various territorial production chains, where there are large amounts of waste and effluents, which in marginal economies are largely untreated. This new model of intervention on the resources of nature emphasizes a measured and rational use of the raw material of origin, and a follow-up from its collection, its transportation, its processing, and its conversion into some type of final product or service. It seeks the benefits maximization that each resource can provide, and the minimization of material and energy losses in the production circuit. In this sense, each portion of matter or energy removed from a stage pursues to be reinserted in a new cycle of use and, therefore, is described as an economy with closed material loops. It is thus presented as a model that overcomes the traditional economic scheme of linear use of resources (Ellen Macarthur Foundation (EMF) 2015; Kirchherr et al. 2017; Kalmykova et al. 2018; Del Borghi et al. 2020). The key to this model is that nothing is lost but that the different production cycles are intertwined with each other, and, therefore, the residues of one cycle constitute value resources for the following cycle (Ellen Macarthur Foundation (EMF) 2015). Sharing, maintaining, reusing, redistributing, remanufacturing, recovering, and recycling are some of the basic principles that underpin the new model (Carus and Dammer 2018).
The bio- and circular economies are currently considered as two complementary policy strategies that have biomass and its derivatives (organic waste, effluents, and wastewater) as the basis that supports their actions (De Schoenmakere et al. 2018). The term “circular bioeconomy” integrates the principles of bioeconomy into a broader set of policies that promote the circular economy (Carus and Dammer 2018; Sherwood 2020). Although this current of thought is in the initial stage of conceptualization and practice (Kirchherr et al. 2017; Linser and Lier 2020), some characteristics have reached consensus in the world community to define the circular bioeconomy, where the biomass is the cornerstone (Keegan et al. 2013; Nagarajan et al. 2020; Pleissner 2020). This new model includes a comprehensive, efficient, and prioritized use of resources (“cascade use,” Keegan et al. 2013; Erajaa 2016; Pleissner 2020); substitution of materials and energy from fossil sources, with materials and energy derived from biomass (biomaterials and bioenergy) (Kalmykova et al. 2018); efficiency of processes and introduction of environmentally friendly technologies (bioprocesses, appropriate and clean technologies) (Vanhamäki et al. 2020); reintegration of waste into new productive cycles (circular economy), and less demand for fresh materials (BIORES 2015; Von Braun 2015; Carus and Dammer 2018; Sherwood 2020; Del Borghi et al. 2020) among the main concepts.
In the context of the global challenges posed, cascading chains with long-term carbon sequestration must be prioritized for the mitigation of climate change while promoting an efficient and minimized use of water resources (Vanhamäki et al. 2020). The energy use of biomass is the one that gives the least added value to the resource, so it must be included as the last option for use within the chain, so that the material has been previously used in all possible ways (Carus and Dammer 2018).
6.2.2 Ecological Footprint and Circular Bioeconomy
From a broader perspective, the circular bioeconomy can have a high impact on reducing the EFP. This concept was continuously matured by one group of scientists (Global Footprint Network, GFN) since the 90s. It is a measure of human demand on the Earth’s ecosystems (including food, wood, fiber, carbon sequestration, and infrastructure housing) and the Earth’s ability to meet these demands (“biocapacity”) and shows this into one number (Borucke et al. 2013). The GFN also estimates the “Earth Overshoot Day,” day when humanity will have used the year-round budget for nature’s resources (Monfreda et al. 2004). The date has been advanced 2 months in the last 20 years (Wackernagel et al. 2019). On July 29 the last year (2019) the global human demand for resources and services was 75% greater than the supply (1.75 planets), implying a value that is 2.5 times higher than the estimated six decades ago (1961). This implies that we are depleting our natural capital and this can be seen through the overexploitation, deforestation, contamination, loss of biodiversity, climate change, among others (Jhariya et al. 2019a, 2019b). Avoiding ecological collapse and therefore humanity requires a rigorous immediate action plan.
In this context a global conversion of the economy is necessary: from fossil-fuel-driven cultures to biomass-driven cultures (Galli et al. 2012; Pleissner 2020). This phenomenon has already occurred previously in historical times but in the opposite direction: societies highly dependent on biomass resources reorganized their economies with a focus on new fossil energy resources in the eighteenth century (with the advent of coal) and in the 19th and 20th centuries (with the advent of oil). In this time, the technical and technological developments allow more efficient ways of using available and renewable biomass and even get benefits that go beyond energy (Matamoros et al. 2016; Tang et al. 2020), mainly in rural areas (Pleissner 2020).
The joint application of both concepts of “bioeconomy” and “circular economy” (“circular bioeconomy”), from a competitive approach that guides practical strategies on the regions, reduces the assimilation pressure of residues within ecosystems, but also the over-extraction of resources (De Schoenmakere et al. 2018). Since the circular bioeconomy avoids using fossil carbon, the carbon footprint (CFP) will be low as a direct consequence of its implementation (Carus and Dammer 2018) contributing to climate mitigation targets. Moreover, there are some key areas in which the circular bioeconomy promoting could have a significant impact for the EFP decreasing in the communities (Pfau et al. 2014):
-
Resource use optimization: lower demand for natural resources due to a more comprehensive use of them, and reduction in the waste discharge and rubbish generation (Ellen Macarthur Foundation (EMF) 2015; Pleissner 2020; Del Borghi et al. 2020).
-
Carbon reserves: in its natural forms, biomass fixes carbon in the tissues, keeping it there for long periods of time, with which by forming ecosystems, it not only constitutes an important reserve of carbon not emitted into the atmosphere, but also the associated biodiversity is preserved as a source of future resources (Intergovernmental Panel Climate Change (IPCC) 2006; Manrique 2017; Carus and Dammer 2018; Giuntoli et al. 2020; Manrique et al. 2020).
-
Energy: renewable energy can be obtained from biomass residues and/or use of biomass in those ways in which it does not compete for the use of land, water, or affect biodiversity (residual biomass). Although biomass combustion generates carbon emissions, it is assumed that this carbon was previously fixed by the biomass plant structures and, therefore, does not contribute to global overheating, making it possible to partially reduce the use of fossil fuels (Agostini et al. 2014; BIORES 2015; European Commission (EC) 2018). It is fundamental because currently, the carbon emissions from burning fossil fuels constitute 60% of humanity’s EFP (Wackernagel et al. 2019). From another angle, every time a material is recovered, reused, or recycled, there is indirectly an energy saving by avoiding the energy demand for the production of a new product unit (Kirchherr et al. 2017; Carus and Dammer 2018; Vanhamäki et al. 2020).
There is still a fourth aspect in which biomass can contribute to reducing EFP (in a similar sense to that analyzed for energy) and is linked to water use (Qadir et al. 2020). On the one hand, the use of some forms of biomass can imply water savings: for example, taking advantage of existing residues for food, feed, energy, or substances purposes, instead of growing a new biomass unit with the same purpose, which will demand more water. On the other hand, wastewater and effluents can be recovered and cleaned, through phytoremediation, enabling this same water to be reused (Prajapati et al. 2013; Singh et al. 2016; European Commission (EC) 2018; Nagarajan et al. 2020).
For a growing world population, in a finite world, only a circular bioeconomy will be able to sustain the growing demand for subsistence (European Commission (EC) 2018; De Schoenmakere et al. 2018). Linser and Lier (2020) argue that the circular bioeconomy is the pathway to meet the Sustainable Development Goals (SDG) by 2030 (United Nations (UN) 2019a). The promotion of a circular bioeconomy does not imply assuming that biomass can potentially achieve the complete replacement of fossil fuels and non-renewable materials that are currently used (like as minerals and metals, for example, Carus and Dammer (2018). At least not with the current technological and production means (Sherwood 2020). However, the society in general agrees about the need to reduce the quantity and quality of waste generated in order to ensure the capacity of natural systems to remain productive in time (Qadir et al. 2020; Vanhamäki et al. 2020). Achieving greater efficiency in resources use, limiting the use of finite feedstock, and promoting renewable and less polluting systems than current ones are the fundamental principles of circular bioeconomy. However, attaining circularity requires concrete alternatives that give multiple solutions to the needs of the territories and their communities (Kalmykova et al. 2018; Sherwood 2020).
6.2.3 Territorial Comprehensive Management Waste
It is easy to understand that in primeval cultures the solid wastes were dumped or buried outside their settlements, while aqueous discharges were made directly into the ground or into local water courses, and gaseous emissions were simply released into the atmosphere (Meena et al. 2020b, c). However, as the communities increased in number and quantity of demands, the amount of waste generated also grew in volume, quality, and speed of generation, requiring increasingly organized forms of management to avoid sources of contamination (Seadon 2006; Rodríguez and Aramendis 2019). Indeed, global solid waste generation is expected to grow a 61% by 2050 (from 2.1 billion tons per year) (Kaza et al. 2018) and wastewater volumes are projected rise up to 573.8 billion cubic meters by 2050 (Qadir et al. 2020), involving twice the current generation. These huge quantities of waste and effluents generated from the productive activities contain materials, water, and energy that are annually wasted, and they generate environmental impacts and economic costs (Tarallo et al. 2015; World Water Assessment Program (WWAP) 2017; Nagarajan et al. 2020; Rajesh Banu et al. 2020).
From the outlined outlook, it is worth asking: What are the practical tools that the circular bioeconomy paradigm can provide in this context? Which concrete strategies can contribute to face the three global challenges water–energy–climate change while taking care of converting waste into resources? Perhaps one of the first proposals that have a place within this new paradigm is those related to the proper management of waste. The “integrated waste management” was first mentioned in the 1990s, as a new starting point for waste treatment with efficient material and energy management and reduction of environmental impacts (UNEP 1996; Morselli et al. 2008). Integrated Waste Management Technologies (IWMTs) include comprehensive and hierarchical management proposals (cascade use) to treat most of the discarded residual fractions (Seadon 2006; Gouveia et al. 2016; Morselli et al. 2008; Hidalgo et al. 2019; Rajesh Banu et al. 2020) and where the ideal is the total and absolute reduction of waste (Tarallo et al. 2015; Sanders and Langeveld 2020).
In a context of future scarcity, all residual flows have become part of a new category of interest, where water, energy, and materials are abundant resources (Abdel-Raouf et al. 2012; Acien Fernández et al. 2017; Barkia et al. 2019). Its use not only implies a thoughtful and committed involvement in safeguarding the natural terrestrial and aquatic environment, but a potential source of income and engine of initiatives with local impact (Drira et al. 2016; Gouveia et al. 2016; Hidalgo et al. 2019). The IWMT might be one of the most successful strategies for efficient resource management. Although IWMTs are not standardized technologies, they basically include all the concepts of the circular economy and there are still successful examples in the circular bioeconomy, such as those in which microalgae are included as one of the most versatile biomass resources. In these new management schemes, microalgae could become the star of this circular bioeconomy (Xiao et al. 2011; Gouveia et al. 2016; Acien Fernández et al. 2017; Nagarajan et al. 2020; Rajesh Banu et al. 2020). Microalgae can easily adapt to different conditions and are practically ubiquitous in all kinds of environments. They have high efficiency in the sunlight use, a fast growth, and higher productivity than other agricultural crops (Sydney et al. 2011; Han et al. 2015). Their potential role as suppliers of different types of fuels (biomethane, biodiesel, bioethanol, biohydrogen, among others) makes them a multiple alternative for the global energy supply (Anand and Arumugam 2015; Zuliani et al. 2016). Although the cultivation of algae like any other crop requires the addition of nutrients (Slade and Bauen 2013), it is possible to grow algae in nutrient-rich effluents with the dual purpose of cleaning the water and capturing those nutrients as inputs for the multiplication of algal biomass (Kothari et al. 2012; Nagarajan et al. 2020). This water purification capacity is known as “phytoremediation” and it makes microalgae an excellent environmental resource, since once the generated biomass is harvested, it can be used for industrial, food, medicinal, cosmetic, or energy purposes (Prajapati et al. 2013; Singh et al. 2016; Rajesh Banu et al. 2020). Successful global experiences encourage in this regard (Andrade et al. 2009; Abdel-Raouf et al. 2012; Wong et al. 2015; Zuliani et al. 2016; Barkia et al. 2019; Tang et al. 2020).
6.2.4 Circular Bioeconomy: Alternative or Need?
Currently, more than 80% of the world’s wastewater does not receive any type of treatment, a figure that reaches 95% in some less developed countries. The wastewater sanitation services that cover rural and urban sectors can only be considered as safe (no contact with human excreta) in 26% and 35%, respectively (World Water Assessment Program (WWAP) 2017). In addition, the presence of emerging pollutants, many of which have toxic effects is increasingly frequent and in higher quantities because hardly the traditional wastewater treatment plants have the technical capacity to remove these new substances (Matamoros et al. 2016). Likewise, wastewaters contain many nutrients which can produce eutrophication in the bodies of water (Andrade et al. 2009; Singh et al. 2016). Although there are physical or chemical processes that could be applied for the purification of the effluents, this implies a high investment of money and energy, and not in all cases they are efficient systems (Drira et al. 2016). Systems such as the wastewater stabilization ponds (WSP), which are the most common and widely used due to their simplicity, absence of mechanical elements and the low cost of investment, operation and maintenance; commonly do not remove in a 100% the load of incoming waste and many organisms still remain in treated effluents. Even when discharges are made in compliance with the standards that regulate the discharge limits, they often pollute the receiving waters due to the variation in the flow of water and the cumulative effects on the environment (World Water Assessment Program (WWAP) 2017).
The integration of algae in tertiary systems could improve the treatment of wastewater and water cleaning, with the possibility of achieving even the reuse of water if the conditions for its use were reached (Gouveia et al. 2016). Moreover, the culture medium implies a high cost in microalgae cultivation (Kothari et al. 2012), so the nutrient-rich wastewaters are an opportunity and not a problem for the microalgae production (Menger-Krug et al. 2012; Nagarajan et al. 2020).
Given that the circular bioeconomy pursues the utopia of maximizing efficiency in the use of resources and zeroing waste (Clark et al. 2016) by returning them to use (Von Braun 2015; Vanhamäki et al. 2020), it is necessary to identify concrete measures that can make the concept operational and that politicians, decision makers, and stakeholders can observe its practical implications, in order to include them in a specific regional implementation strategy (Vanhamäki et al. 2020). However, there are still not enough experiences or guidelines, which can lead this popular global and current goal into practice (Sherwood 2020; Pleissner 2020). The IWMT with microalgae may be one of the oldest and best known of the strategies that must be studied, adapted, and promoted in the territories (Oswald et al. 1953).
This work contributes in this direction, focusing on one IWMT with microalgae in Argentina, where there are few experiences yet, but with promising results (Méndez et al. 2011; Codina et al. 2012). IWMT from microalgae can become an efficient technical, environmental, and productive practical strategy, of immediate application, with impact on the water security, health ecosystems, conserving energy and mitigating the GHG emissions. However, given that many factors define the growth and composition of microalgae biomass and their purification capacities, the performance must be evaluated in local conditions (Park et al. 2011). An IWMT system is described in the next section, where its qualities, potentials, and needs for further research and development can be appreciated, for a broader promotion and impact.
6.3 Biomass Production from Wastewater: A Win–Win Strategy?
Biomass energy recovery is an efficient alternative to the urgent need to reorient the production model towards a circular model based on the bioeconomy. Not only does it mean obtaining a renewable fuel, neutral in terms of CO2 emissions and competitive in price with fossil fuels, but it also has a fundamental part in the ecosystem management and opportunity of development of world rural areas. This chapter reports the results of a study of productivity, energy, and environmental qualities of microalgae growing in urban sewage effluents, highlighting some aspects of performance optimization, which was developed as part of the National Microalgae Network (https://www.magyp.gob.ar/site/areas/microalgas/). This Network was created in 2015 in Argentina, through an initiative promoted by the Ministry of Agriculture, Livestock and Fisheries and the Ministry of Science, Technology and Productive Innovation, and works to join public and private efforts for integrated development of technology and its application to specific problems in the country. The context of opportunity for the application of the proposed IWMT system is reviewed below.
6.3.1 National Context for the Promotion of Integrated Waste Management Technologies
At the present, around 50 governments of the world (developed countries and many in development), have defined formal strategies for the development of their Bioeconomy and are making progress in the design of specific programs and policies for their consolidation (German Bioeconomy Council (GBC) 2018; Linser and Lier 2020). Argentina has joined this trend, with the creation of its Bioeconomy Promotion Program under the Secretariat of Aggregate Value of the Ministry of Agribusiness, given the abundance of biomass resources in the country, industrial capacities, services, and of the quaternary sector (of information and knowledge) existing (Trigo et al. 2017). As in other countries of the region, the strategies developed around the water, energy, and climate change will enable the strengthening of regional economies and territorial sustainability.
The greater challenge in the country is the water quality and not the quantity, since the national coverage of sewage services is 40% of the population, compared to 82% covered by the public water network (Instituto Nacional de Estadísticas y Censos (INDEC) 2010), with differences between urban and rural sectors. For the year 2015, about 40 million inhabitants of the country are registered in urban areas, sector where there is still a lack of 13% in the access to the public network water and 42% to sewers (Bereciartua 2017). Some sources estimate that the level of wastewater treatment is between 15 and 20% of the collected water. The situation in rural areas is more disadvantageous, although it is currently not possible to have reliable statistics. National statistics allow identifying and dimensioning some key areas where the country should concentrate efforts (InterAmerican Network of Academies of Sciences (IANAS) 2019), to achieve 100% safe water coverage.
From the energy point of view, the total renewable power capacity was doubled from 2007 to 2017, and without considering the hydropower, the capacity of renewables was multiplied by six (REN-21 2019). However, excluding the hydroelectric power, the share of renewable sources is still low (barely 2%) although Law 27,191 provides that in 2025, 20% of all Argentina’s energy generation will be renewable. The energy diversification together with the promotion of energy efficiency measures is key to achieving international environment commitments but above all, to ensure one environmental quality that allow sustain the country economy and their people (KPMG 2019).
Finally, in terms of climate change, between 1961 and 2018, the temperature increased on average 1 degree Celsius in the country. Rainfall also increased significantly and the trend, in the medium term, will be even worse (Secretaría de Ambiente y Desarrollo Sustentable de la Nación (SAyDS) 2015). In particular, extreme temperatures and water scarcity are expected in the northwest region. Argentina presented emission reduction commitments as National Determined Contributions (NDCs), which differ in that they contain goals that depend on external financing (conditional) and others that do not (unconditional). The country has proposed to unconditionally—that is, without receiving financing and technological support—reduce 18% of GHG emissions by 2030. The country could reduce an additional 19%, which would be conditional on some type of international support, be it financial, technological or capacity development. In total, the reduction would be 37% until 2030. 93% of the Argentine reductions involve the transport sectors (through changes in the forms of mobility), energy (boosting energy efficiency and renewable energies), and forests (through its conservation and recovery). There is great potential in the implementation of bioeconomy strategies to achieve the proposed objectives. This study reflects some of these possibilities.
6.3.2 Microalgae Growth in Sewage Effluents
The strain used for this study was from the Microalgae Laboratory of the Faculty of Natural Sciences of the Trelew, belong to the National University of Patagonia San Juan Bosco. For the selection of the species to be used, a sampling was carried out in the receiving freshwater body of the treatment plant, characterizing the phyco-flora that was present in the sample concentrated by phytoplankton net of mesh size of 30 μm, by means of a qualitative analysis. The determination was made in the Laboratory of Water Quality of the Faculty of Natural Sciences of the National University of Salta.
Scenedesmus quadricauda was selected since it was recognized as having optimal qualities for use in wastewater treatment for its ability to withstand high concentrations of nutrients, have high metabolic activity, and ability to resist environmental variations (Xiao et al. 2011; Anand and Arumugam 2015). Scenedesmus quadricauda (Turp.) De Breb., Var. Longispina is described in Guerrero (1941) as microscopic, tetracellular colonies or with fewer cells; the distal with two powerful straight stingers, longer than them. The habitat where it found is freshwater puddles. According to the Biodiversity Information System (Biodiversity Information System (BIS) 2019), the species has been found in eight provinces of the country, including the Province of Salta.
The microalgae were kept in modified Detmer culture medium—DM—(Accorinti 1960) in a chamber under lighting conditions with 12:12 photoperiod (3000 lux), daily agitation. A system of continuous tests was designed to allow the progressive acclimatization of the microalgae by growing it in sewage effluents. Dilutions of the effluent were made at 25, 50, and 75% with the DM until 100% effluent was reached (Table 6.1). The tests were done with sewage effluent pre-treated with coarse filtration using cotton and gauze as a filter medium, with an initial Scenedesmus cell density (CD) of 250,000 cells/mL. Triplicate tests were performed, with a volume of 3000 mL in each case, and in parallel, control cultures were started only containing the DM medium with the microalgae without effluent (T0%). The first set-up tests were made by growing the strain in DM medium (control) with and without temperature control. After these first experiments, a better response of the crop was observed growing at room temperature, so the rest of the treatments were performed at room temperature.
The sewage effluents for the tests were obtained from samples taken at the exit of the third pond of the sewage effluent plant called the North Purification Plant, which is located in the north of the municipality of Salta capital (left bank of the Mojotoro River). Sampling campaigns were carried out in the different seasons, observing great variability in the samples (Table 6.2). The trials began with samples from the fall and winter campaign and were repeated twice. 50 litters of sample were collected each time in plastic drums from the outlet duct of the tertiary ponds. Samples were stored at 5 °C in a refrigerator for testing. Subsamples were taken for physical–chemical analysis in an external Laboratory, with certification.
Microalgae productivity parameters and energy and environmental qualities were studied. CD was estimated with a 0.1 mm deep Neubauer chamber until the start of the stationary phase. Specific growth rate (day-1) was estimated as: μ = (ln Nf- ln Ni/ (tf-ti)); Ni = cells density at the start of the exponential phase (ti), and Nf = CD at the end of the exponential phase (tf). Nmax (cell/mL) was considered by convention the highest μ observed for each treatment. The doubling time (DT), time needed for the population to double, was calculated as DT (day) = (lnNt-lnN0/0.639)/(t-t0), where 0.639 = Ln2. Sampling was done every day. Before entering the stationary phase, the agitation was stopped to induce the sedimentation of the biomass for 3 days. Finally it was calculated the total biomass (TBH) harvested by means of a centrifuge (5000 rpm) and dried at 70 °C ± 0.5, until it reached a constant weight.
Given the enormous amount of biochemical components that microalgae possess, it is possible to obtain practically any liquid, gaseous, or solid fuel (Ubando et al. 2020). However, the most basic form of energy generation from algal biomass is the direct combustion of the same generating heat and electricity (Kadam 2002). Therefore, the calorific value of algal biomass in its solid fuel form (dry biomass) was explored. Higher calorific value (HCV) was determined by Parr 1108 Oxygen Combustion Bomb. Specific experimental procedures and calculation formulae are detailed by the European Standard EN 14918: 2009. Following Grobbelaar et al. (1988), a 7.45% participation of elemental hydrogen in microalgal biomass was assumed for the Lower Calorific Value (LCV). On the other hand, in general terms, it was considered that the purification capacity of the algae was efficient, if the effluents were able to meet the discharge limits required by current legislation. In addition, RE between the initial situation and the final situation was evaluated to know the magnitude of the change (both in COD and in nitrates and phosphates). The Removal Efficiency (RE) was estimated considering: RE = (Cf-Ci)/Ci * 100%, where Cf is the final concentration at time tf and Ci is the initial concentration at the time ti. Laboratory determinations were made following the standard SM (Standard Methods for the Examination of Water and Wastewater) as follows: COD (mg/L) according to SM 5220 D Ed22, nitrates and phosphates (mg/L) according to SM 4110 B Ed22, and total and fecal coliforms according to SM 9221 B/C Ed 22. All values are summarized as mean ± SD. The student t-test was used for statistical analysis (significance: α = 0.05).
6.3.3 Results and Discussion on the Experience
It was interesting to observe the response of the tests, with the lower investment of energy resources and the lower costs, in view of the possibility of their easy replication in different locations. The first trial with the control culture in DM medium was carried out to compare its response to ambient temperature conditions (13.3 ± 3.1 °C) and controlled (18 ± 0.1 °C), considered as half of the safe thermal limit (Zargar et al. 2006). It was interesting to know how microalgae cultivation could respond to climatic variations in the region, with low temperatures. Figure 6.2 shows a better crop yield of S. quadricauda when it was subject to the temperature dynamics of the autumn-winter season. This could imply that the crop responds better when it is subjected to thermal stress, with an incidence of temperatures higher and lower than the control temperature (Sonmez et al. 2016). In both cases, the point of maximum growth was obtained on day 13.
On the other hand, the temperature records inside the laboratory where the tests were performed using thermocouples stored in data logger for later analysis. These data were compared with temperature records outside the building (exterior) (Table 6.3). The amplitude of the temperature range recorded outside was greater than that achieved in the laboratory, and extremes of about 1 °C and up to 30 °C can be observed, achieving possibly more favorable conditions for the growth of the crop inside the laboratory. The next tests were, therefore, carried out at room temperature. This is an advantage by replicating the trials in open ponds, enabling perhaps this IWMT in regions of the world where there are high temperature fluctuations (diurnal and seasonal). Likewise, avoiding temperature control implies a decrease in operational costs. Sonmez et al. (2016) effectively observed that Scenedesmus sp can adapt successfully to daily temperature fluctuations in a range of 10–50 °C, even increasing lipid production when the species is subjected to the variable temperature regime (between 16 °C and 30 °C).
Figure 6.3 shows the cell growth parameters for the four treatments performed. For 25% of effluent, the culture was reproduced until doubling the initial concentration between day 2 and 3 (DT = 2.87 d), with a DT less than the control (DT = 3.38 d), associated with the greater availability of nutrients. On day 7, the greatest difference in CD was recorded with respect to the control, reaching a 95% higher value (value = 2.75 × 106 cell/mL). In later days, this difference was decreasing. On day 12, the aftershocks reached a stabilized value of 4.51 × 106 with the lowest coefficient of variation (CV = 0.5%), which was still higher than the control (65% higher). The maximum CD (5.77x 106 cell/mL ± 0.42 × 106) implied a 69% higher value in the treatments. A TBH of 0.526 g was achieved, implying a production efficiency of 0.173 g/L, achieving 1.7 times the amount of the control.
For the T50% treatment, the cultures had behavior similar to the first treatment. The cell DT was also faster than in the control (2.78 d vs 2.92 d) and in turn, it was also shorter than for T25%, possibly because the algae achieved an acclimatization process during the first treatment. In this case, the greatest difference in the cellular density of the treatment with respect to the control was reached at day 5 (113% higher). On day 12 the repetitions reached a stabilized value similar to the first treatment (with a value of 4.97 × 106 cell/mL and a CV = 1.46%). The maximum CD achieved was higher than in T25% (7.52 × 106 cell/mL ± 0.37 × 106). The TBH achieved was 0.687 g, implying a production efficiency of 0.226 g/L, achieving twice the control culture, and a total of 0.16 g more than in T25%.
The treatments T75% and T100% practically remained stationary and the microalgae could not reproduce. After a week they began to disappear, possibly due to the presence of other microorganisms of greater tolerance and aggressiveness such as the group of rotifers, which were detected in the trials, since the effluent was not sterilized. Although there are various treatments to combat them (Park et al. 2011), the objective was to know the response of the IWMT system with the lowest level of manipulation, energy investment, and resources. The T75% treatment reached day 7 a maximum CD of 4.15 × 105 cell/mL ± 1.42 × 105 cell/mL, after which it began to decrease dramatically. In the case of the T100%, the maximum value reached was 3.95 × 105 ± 1.73 × 105 cell/mL after which it also began to decrease its existence. The TBH was only 11% and 9% of the total value achieved in the control, whose average was 0.34 g ± 0.04 g.
The RE is shown in Fig. 6.4. The purification capacity of S. quadricauda could only be evaluated in the first two treatments that effectively fulfilled the growth cycle until the start of the stationary phase, while the species could not thrive in treatments T75% and T100%. The COD reduction capacity was on average 83.4% ± 5% for T25%, and 74.55% ± 4.2% for T50%. The removal of phosphates and nitrates was 57.6% and 58.7% in T25%, and 54.6% and 76.9% in T50%. Total and fecal coliforms were reduced by 89.6% and 77.4% for T25% and 86.6% and 68.7% by T50%. This implies that the microalgae have contributed to the sanitation of the effluents that, at some times of the year, exceed the organic load as well as the concentration of pathogens, allowed for discharges into watercourses. In this case, the waters treated with microalgae have reached permitted values for be discharged. The study of nutrient removal by microalgae is the basis for the design of hydraulic retention time prediction models, necessary to adapt effluents to the discharge standards (Han et al. 2015).
The RE found is within the range of one of the few studies in the country on this species, carried out by Méndez et al. (2011), who indicate a COD removal capacity of 73.7%. Other authors report a COD removal of 55.7% (Chacón et al. 2006), 35.59% (Andrade et al. 2009) in fish farm effluents and 91.4% in WSP treatments plants (León and Chaves 2010). Regarding nutrients, nitrogen and phosphorus are the main chemical constituents of the dry weight of the microalgae biomass (Grobbelaar et al. 1988). Nitrogen is incorporated as nitrate (NO3 -) or as ammonium (NH4 +) (Abdel-Raouf et al. 2012). It is a critical factor in regulating the lipid content of microalgae, since nitrogen limitation stimulates lipid accumulation in algae cells, but decreases algal biomass production, so they are mutually exclusive mechanisms (Park et al. 2011; Anand and Arumugam 2015). Although the phosphorus content of microalgae is around 1%, it is essential in nucleic acid formation and energy transfer and it is one of the greatest growth limitations (Slade and Bauen 2013). In both cases its assimilation by algal biomass, therefore, is a fundamental nutrient recycling. Méndez et al. (2011) indicate removal of 40% nitrates, 93.8% phosphates, and total and fecal coliforms of 84.8% and 85.9%. Andrade et al. (2009) report removal efficiencies of 94.44% for ammoniacal nitrogen and 77.54% for phosphates. Xiao et al. (2011) point out that the removal of the total phosphorus and nitrogen in the digested wastewater after 8 days cultivation was more than 94%. Hammouda et al. (1995), in laboratory cultures using Chlorella sp. and Scenedesmus sp., obtained 100% removal of nitrate, ammonium, and phosphorus after 36, 42, and 48 days. Furthermore, the microalgae reduce the pathogens present, probably by raising the temperature, the pH, and the dissolved oxygen concentration (Schumacher et al. 2003). It has been estimated that for every kg of microalgae biomass generated from sewage effluents, between 1.5 and 2 kg of O2 may be released (Grobbelaar et al. 1988; Muñoz et al. 2004). In all cases it was possible to confirm the capacity of the microalgae to remove nutrients and reduce the organic load and pathogens. Further research is necessary to delve specifically into the recovery of other toxic ions or ions of economic interest, the feasibility of which has already been observed since the 1990s for this same species (Harris and Ramellow 1990). Abdel-Raouf et al. (2012) points to numerous trials with dangerous ions where the algae have been successful.
In recognition that each effluent that can be used as a culture medium will have a different physical and chemical composition (Anand and Arumugam 2015; Matamoros et al. 2016), it is necessary to evaluate the particular energy qualities of biomass in each of these effluents. The calorific value of the generated biomass is shown in Fig. 6.5. The results obtained are highly promising, and located within the range from 14 MJ / kg to 24 MJ /kg already mentioned by other authors (Chen et al. 2014). As in the previous case, given that the last two treatments could not be completed, the biomass harvest and subsequent processing by a combustion bomb were only performed to treatments T25% and T50%, as well, to the control culture.
The biomass obtained from the T25% tests has an energy content that was 11.19% and 12.33% higher than the control value (for HCV and LCV, respectively); while for E50% the biomass shows values 6.96% and 7.67% lower than the control (for HCV and LCV, respectively). Comparing between treatments, the energy content was higher for T25%, which was even more advantageous than the HCV obtained for the control.
In any case, the biomass generated has a high energy potential (from 16.04 to 19.17 MJ/kg), in relation to other solid, liquid, and gaseous fuels (Table 6.4) being located at the same level as wood pellets with 10% humidity. The available energy is equivalent to an average of 4.41 ± 0.43 kWh/kg. Coimbra et al. (2019), who worked with Chlorella sorokiniana, found higher values but in algal biomass growing in synthetic wastewater (HCV0% = 22.9 MJ/kg), suggesting that this biomass could be mixed without problems in carbon combustion systems, without notable effects in their energy performance (co-combustion processes). Chen et al. (2014) obtained a similar HCV to that obtained for T50% in this study (HCV = 16.1 MJ/kg) from the same Scenedesmus genus.
6.3.4 System Optimization Aspects
The proposed IWMT system could be optimized with the management of some fundamental variables of the process of cultivation, harvesting, drying, and use of biomass. In general terms, the cultivation of photoautotrophic microalgae can be carried out through two basic designs (Slade and Bauen 2013; Sonmez et al. 2016; Martins et al. 2018): open raceway pond (ORP), which are open ponds and with little or no control of the microalgae growth processes; and closed photobioreactors (PBRs) where it is possible to control the process conditions. PBRs are the only ones feasible for large-scale biomass production although currently commercial production is limited to a few hundred tonne and it is carried out in large ORP or lagoons. The ORP is simpler, cheaper, and longer lasting systems than the PBR (Garofalo 2009; Rodolfi et al. 2009), and they are the main system used to produce human nutritional products (Barkia et al. 2019). The difficulty of controlling crop parameters makes this system less efficient, but more accessible in different local communities. In addition, the ORP is the only systems that can meet the double objective of sanitation and production of useful biomass (Abdel-Raouf et al. 2012; Kothari et al. 2012; Gouveia et al. 2016). However, in the proposed IWMT from microalgae, the economic exploitation of biomass for products with high added value (biodiesel for example) is unfeasible given the high investment costs that this would entail by the variable quality of the substrate and the handling of large volumes of water (Rocca et al. 2015).
Even so, there are certain crop management parameters that could be optimized with relative ease for greater system productivity, adaptation to a change of scale, or its re-adaptation in other communities. Among these parameters are temperature, light, nutrients, pH, and agitation (Zargar et al. 2006; Han et al. 2015; Zuliani et al. 2016). Some authors mention that the optimum range the temperature is between 16 and 27 °C (Suh and Lee 2003): lower temperatures decreases the efficiency of microalgae-based treatments and higher temperatures can lead to energy losses. The selection of the species is fundamental. In this study, S. quadricauda managed to survive the autumn-winter conditions of the region, grown in a laboratory at room temperatures. Bakuei et al. (2015) study the performance of Scenedesmus sp in the face of different types of lights, water sources, and pH, under controlled temperature and light intensities. The higher biomass concentration was obtained by applying tungsten lamps (versus led and fluorescent lamp) with more red light emissions and lower blue light led to; distilled water or diluted seawater versus tap water (the presence of chloride ions might be inhibitors for microorganism growth); and alkaline medium (8.2–8.7). Gas exchange must ensure the contribution of CO2 and the removal of photosynthetic O2. The exchange across the surface of the crop is insufficient so that it is necessary to provide aeration and/or to have an adequate system of agitation and mixing (Suh and Lee 2003). Because the reduction of nitrate or the assimilation of CO2 supposes an elevation of the pH of the culture medium, this should be corrected by means of buffered media, adding acid in a controlled way or injecting in the air stream an adequate proportion of CO2 (Park et al. 2011). Logically, this will necessarily imply an energy expense and higher costs. Abu-Ghosh et al. (2015) suggest an energy expenditure of around 27 kJ to pump CO2 into the system for every 1 kg of dry algal biomass.
Furthermore, microalgae crops are susceptible to grazing by some zooplanktonic groups, such as cladoceros, rotifers, or nematodes, especially in open systems (Rocca et al. 2015). However, there are practical and economical methods of controlling these microorganisms like as adjust pH to a value of 11 (Park et al. 2011). Several investigations have shown that in addition to the availability of nutrients, the relationship in which they are found influences the correct growth of microalgae, such as N: P rate: P below the optimum, will be limited to nitrogen; while in greater than optimal relationships, the limit will be phosphorus (Rocca et al. 2015). S. quadricauda has shown a clear response to inhibitory processes caused by nitrogen deficit, increasing 2.27 times the production of lipids in dry weight, but decreasing 27 times the production of biomass (Anand and Arumugam 2015). Finally, the availability of light is one of the determining aspects for the optimal microalgae growth, which should be considered as another nutrient since it will become biomass. The optimum range the light intensity is between 200 and 400 μE/m2.s, when the photosynthetic apparatus becomes saturated (Pancha et al. 2015).
The harvest and drying stages can also be optimized. There are many chemical, biological, and physical or combinations harvested techniques that have been studied (filtration and flotation; coagulation or flocculation; gravity sedimentation or centrifugation). The final decision of the method that will be used, many times will be made based on costs, recovery efficiency, and availability of technology (Kothari et al. 2012; Rocca et al. 2015; Barros et al. 2015; Drira et al. 2016; Koutra et al. 2018). Bagchi et al. (2015) highlight that the drying process is also a highly expensive stage (up to 30% of the total cost) and demands a lot of energy. They designed a drying oven that saves 50% (0.017 kWh) of the energy generally used in this process. If the algae are harvested with energy fines, part of the calorific value of the algae biomass could be used for drying the algae to acceptable solids content (Azari et al. 2019).
Nevertheless, there is still no agreement on which methods of harvesting and dewatering might be more advantageous and less energy-demanding for microalgae production (Slade and Bauen 2013; Tedesco et al. 2014; Rocca et al. 2015; Azari et al. 2019). In the proposed IWMT, the drying and harvesting process must still be optimized, reducing energy expenditure. Solar dryers are being developed as complementary to the system under experimentation, and could imply significant energy savings. On the other hand, there are numerous examples of energy use of effluents through anaerobic biodigestion processes, whereby the energy demand for drying would be greatly reduced (Prajapati et al. 2013; Zuliani et al. 2016; Koutra et al. 2018; Olsson 2018). Although this energy application would allow bypassing the biomass drying stage, it is undoubtedly a use with fewer added values, which would only allow a marginal energy benefit. In addition, it would imply having some conditions of the system, for which only some plants have currently achieved internal energy self-sufficiency (Tarallo et al. 2015).
The next challenge is to continue expanding the scale of work, and gain experience in the ORP, which will involve a critical evaluation with its corresponding environmental impact, in recognition of the need for space (land use), handling of large volumes of water, and danger of contamination of operators (Azari et al. 2019). Negotiations with the responsible Company of the treatment plant have already begun. Since the maximum biomass production capacity with microalgae (photosynthetic) is determined—among other factors—by the availability of solar radiation (Suh and Lee 2003; Pancha et al. 2015; Acien Fernández et al. 2017) which is a function of geographical location, the north of the country has great advantages (WB 2020). Due to the high levels of solar radiation registered in this area, so it provides the optimal conditions for algal growth, although this advantage has not yet been sufficiently exploited. The simultaneous effort at the National Network will make it possible to expand the successful results to numerous communities and promote concrete technologies for the strengthening of the circular bioeconomy.
6.3.5 Towards Mitigating Carbon Footprint through the Algal Biomass
The previous results allow visualizing the practical application of the circular bioeconomy through an IWMT incorporating microalgae. In particular, applying the IWMT reduces the CFP (Wiedmann and Minx 2007), that becomes especially important as it is the fraction of the global EF that has the greatest impact on climate change (more than 60% currently) (Wackernagel et al. 2019). Globally, there are two main strategic approaches to achieve the reduction of CO2 present in the atmosphere (which has been recognized as the main GHG, Intergovernmental Panel Climate Change (IPCC) 2018): a) capture del gas that has already been released, through the use of “negative emission technology,” NETs (IPCC 2018); or b) avoid the release of various emissions, or offset them by achieving carbon neutrality.
In the first group (item a), the capture or removal of atmospheric CO2, three main strategies are distinguished (Molazadeh et al. 2019): (1) the use of chemical methods (Kraxner et al. 2003) (2) capture and retain CO2 and insert it into the ocean or geological structures or CCS technologies (Ajayi et al. 2019), and (3) biogenic carbon sequestration through photosynthesis (Wiloso et al. 2016). The absorption (through physical or chemical solvents) is the most widely used chemical method to clean the combustion gases (Kraxner et al. 2003). Other options are the use of gas separation membranes or methods of adsorption and cryogenics, although greater effort must be made to achieve the least environmental impacts of this type of application, beyond the demand for space and investment to apply those (Molazadeh et al. 2019).
The CCS technologies are still highly expensive and there is much to know regarding leaks over the years (IPCC 2018; Ajayi et al. 2019; Molazadeh et al. 2019). Last, biological CO2 fixation is an inexpensive, safe, and non-polluting method of capturing CO2 (Kumar et al. 2010), and occurs naturally through photosynthetic land and aquatic plants. In this sense, it is assumed that 50% of the biomass generated is carbon (Intergovernmental Panel Climate Change (IPCC) 2006), although the elemental composition of the microalgae biomass can vary in a range from 37% C to 54% C (Coimbra et al. 2019). In IWMT autotrophic systems (where essential metabolites are photosynthesized from inorganic substances), microalgae take easily existing CO2 from the atmosphere. Although the CO2 capture capacity of these microorganisms is faster than land plants (up to 50 times), even this capture capacity is limited (Xiao et al. 2011; Bilanovic et al. 2009). However, in the case of the proposed IWMT, and as already mentioned among the optimization aspects, it is possible to inject inorganic carbon to achieve a more efficient system. CO2 can be bubbled as well as a higher dose of nutrients if these are scarce. At this point, not only will greater efficiency be achieved, but also power plants’ emissions as a source of CO2 can be used (or cement plants, fermentation industries, among others, Azari et al. 2019; Molazadeh et al. 2019), with an extra benefit.
In the second group (item b), considering that the global energy sector is the main generator of CO2 emissions (Intergovernmental Panel Climate Change (IPCC) 2018), bioenergy is the main strategy to achieve carbon neutrality. The use of energy from biomass releases an equivalent fraction of fossil energy that will not be used, and, therefore, will imply a reduction in emissions linked to the carbon emission factor of the substituted fuel (Intergovernmental Panel Climate Change (IPCC) 2006). In this work, algal biomass has energy content per unit of matter that means between one third and one half of fossil fuel energy (Table 6.4). On the other hand, each biomass feedstock for bioenergy has a different impact of the CFP reduction (Haberl et al. 2012). The proposed IWMT system has the advantage of using a “type” of biomass resource that does not imply competition for the use of land or water, nor will it lead to new deforestation indirectly, nor will it affect the prices of local food or feed (Menger-Krug et al. 2012; Martins et al. 2018). This is of fundamental importance since numerous authors have questioned the neutrality of bioenergy for these causes (Haberl et al. 2012; Wiloso et al. 2016; Agostini et al. 2014; Paletto et al. 2019), pointing out that said neutrality is only fulfilled under certain conditions. Haberl et al. (2012) mention that biomass only compensates for emissions from fossil fuels while it is growing and storing carbon (in vegetation or soil). However, if that biomass is harvested intentionally for energy purposes, there is a new amount of emissions generated, and, therefore, there is more fossil carbon that will not be lost but instead biogenic carbon will be emitted. Agostini et al. (2014) point out that if the biomass source is stemwood from dedicated plantation for bioenergy, this would cause, in the short term, an increase in GHG emissions compared to a scenario with the use of fossil fuels, and only it could be beneficial in the long term. Therefore, according to these authors, biomass has a positive effect when it is growing but not when it is used as source of bioenergy. Now, if the biomass source for bioenergy is residual feedstocks not used for any other purpose and which would also release CO2 when decomposing, then there would be a true GHG mitigation impact (Haberl et al. 2012).
Another important aspect to consider in the CFP is the type of conversion energy technology that will be used in the IWMT (Paletto et al. 2019; Coimbra et al. 2019). Thermochemical conversion routes are the most effective and promising options, including pyrolysis, gasification, and combustion (Coimbra et al. 2019). Processes that improve the energy value of biomass, like as the torrefaction could be an alternative option (Chen et al. 2014). Although they involve a higher level of complexity, other biochemical conversion routes (in addition to the anaerobic digestion already mentioned) that can achieve high-value biofuels (alcoholic fermentation and transesterification) could also be explored (Singh et al. 2016; Barkia et al. 2019) given the forecast high demand for liquid fuels (biodiesel and bioethanol) in the future (Global Bioeconomy Summit (GBS) 2018; Azari et al. 2019; Intergovernmental Panel Climate Change (IPCC) 2018). The bioenergy that could be generated from this microalgae biomass is not only renewable, but would also imply a reduction effect of GHG emissions into the atmosphere since 1 kg dry mass was generated from 1.83 kg of CO2 sequestered from the atmosphere (Slade and Bauen 2013; Bakuei et al. 2015; Azari et al. 2019). By last a new trend known as climate positive solution proposes an alternative that overcomes the previous ones: Bioenergy & CCS (BECCS): this implies the utilization of biomass as a fuel for industrial or power generation processes and the capture and storage of the CO2 released in the process (Kraxner et al. 2003; Realmonte et al. 2019) into geological formations (or their removal using some of the chemical methods already described). Even these processes are still being studied currently (Intergovernmental Panel Climate Change (IPCC) 2018; Realmonte et al. 2019). The CO2 biofixation and bioenergy are the two main strategies promoted by the circular bioeconomy (Pfau et al. 2014).
6.3.6 Application of Algal Biomass for Reducing Water Footprint
An analysis of the implication of the proposed system on water demand (known as the “water footprint” WFP, Hoekstra et al. 2011) can contribute to a better understanding of the importance of promoting this type of alternative IWMT. The WFP is an indicator equivalent to EFP: while it calculates the demand for productive land of a given population to supply itself with products and services, the WFP calculates the demand for water to cover those same needs. Likewise, this indicator can also be useful to the product or service analysis in a particular way. It includes three categories of water use: (1) Green WFP: amount of rainwater accumulated in the soil within reach of crops; (2) Blue WFP: amount of water used from natural or artificial sources; and (3) Gray WFP: dirty water generated during the production cycle (assessed as the quantity of necessary water to adjust the effluent to allowable tipping limits) (Hoekstra et al. 2011).
In the case of the IWMT analyzed, although it is not the main objective, biomass is grown from microalgae. Like all crops, the production of algae for energy generation will involve the stages of cultivation, harvesting, drying, and energy conversion (Azari et al. 2019). The demand for water, therefore, will be associated with each of these stages, which in turn will involve energy expenditure. For each tonne of generated biomass, it is necessary to remove an amount of water that is up to 250 times higher in weight, which implies the management of large volumes of wastewater and a great associated cost (Barros et al. 2015). Here the importance of a right choice of algae harvesting techniques that allows an efficient use of an abundant volume of water and its recycling (Rocca et al. 2015). However, the volume of water involved depends upon the type of cultivation system and its geometric characteristics (ORP or PBR system) (World Water Assessment Program (WWAP) 2017; Azari et al. 2019). The main difference in water demand between ORP systems (operating from fresh water and effluents) and PBR lies in the loss of water from the ORP system through evaporation and leaks (Azari et al. 2019), which will depend fundamentally of the geographical location. In a context of water scarcity and high temperature sites, this loss may be unacceptable, so PBR systems may be convenient (Martins et al. 2018). However, the costs will also be different: US$ 494/t algae generated for open ponds have been estimated, and a range from US$ 639 to US$ 1737/t algae in PBR of different characteristics. In other words, the ORP system implies an investment that is 30% less than the more economical PBR system (Clippinger and Davis 2019). The demand for water in the cultivation stage in ORP systems reaches up to 13,000 m3 /ha/year (Chinnasamy et al. 2010) or up to 200 m3/GJ of energy from microalgae biodiesel obtained (Gerbens-Leenes et al. 2014). Therefore, the use of municipal and agricultural wastewater can minimize the amount of fresh water necessary for the cultivation, constituting a great environmental advantage and contributing to sustainability objectives (Martins et al. 2018). In effect, Azari et al. (2019) found that the WFP is 96.80% lower (just a water consumption of 117.8 kg per 1 kg of biodiesel) when the ORP system uses effluents than when the same system uses fresh water to obtain biodiesel from algae.
Although the demand for water from the cultivation stage is excluded from the WFP analysis-because wastewater is used instead of clean water (either fresh or salt water)-, other demands for water through the process must be considered (Azari et al. 2019). It is worth mentioning, for example, the washing and cleaning of the facilities, biomass processing, or the need for dilution when the effluent is highly concentrated. Indeed, the results of the IWMT analyzed in this work show that the successful growth of algae was only possible when the effluent concentration was up to 50% in the culture medium; although perhaps the algae support a lower concentration of effluents when the mixtures are made from fresh water and not with culture medium. The recommendations of the analyzed system are to dilute the effluent mainly in the dry seasons of the year, where it arrives more concentrated. Other indirect water demands are generated as a consequence of the production of electricity necessary for the process (Martins et al. 2018). Therefore, the reduction of WFP is in turn associated with lower energy demand and lower CFP. For example, the CFP in the ORP system with wastewater (67 g CO2 eq. per MJ of produced energy) is 55% lower than other ORP from clean water and these emissions are basically associated with the electricity demanded in the different steps of the process (about 85% of this demand) (Azari et al. 2019), which can include the operation of mixers (to keep microalgae suspended and facilitate their contact with light and atmospheric CO2), thermal regulation processes or harvest, drying and energy conversion (Martins et al. 2018).
Finally, the optimization of the proposed system from the point of view of the WFP would imply the reuse of the water treated by the microalgae, for different purposes (World Water Assessment Program (WWAP) 2017). Global perspectives of the possibilities of these strategies can be seen in countries such as Israel, a leading country in water recycling (more than 80%) which implies a great effort to achieve highly treated effluents and not cause damage to crops and soils (Tal 2016). The influence of IWMT systems could be global, if a systematic action plan was promoted in all regions. For this, awareness and evaluation is necessary, which allows its promotion from a conscious strategy and on the basis of adequate investments and political framework. Early regional planning and corresponding regulations and control schemes will allow to take advantage “upstream,” that is, from the points of demand for clean water and effluent generation, to endorse efficient use and decrease the volumes of effluents to be treated, with the implication of savings in water, energy, and costs, reducing environmental impacts (mainly in the most vulnerable communities).
Within this effort, it is important to agree on an appropriate definition that can be coined identically worldwide, since there are discrepancies and inaccuracies in the concepts of “reuse” and “recycling” (in some cases synonymous and in other cases with a different scope), within which there are no precise limits for what is considered fully, partially, or untreated/treated water (World Water Assessment Program (WWAP) 2017). This effort is essential to observe the level of achievement of the stated goal and record progress in the same way among countries. Likewise, it is necessary to enable adequate registry systems for this goal, since the lack of primary data registries and the lack of updating of information are common problems in third world countries, not only associated with the subject of water and effluents (Manrique et al. 2020).
6.3.7 Conclusions about the Experience
In the study reported, microalgae growing in different concentrations of sewage effluents, with minimized controls during the cultivation process, had good growth, achieving removal efficiencies of organic matter, nutrients, and pathogens greater than 50% and reaching the maximum CD in a maximum time of 17 days. The maximum biomass yields were achieved at effluent concentrations not exceeding 50%. A higher concentration of effluent was limiting for growth, so, in the beginning, treatment for bioremediation should include a stage of mixing of the effluent (with river water, for example) at the discharge point of the purification pond, so that the microalgae can thrive. The proposed system was simple, and can be easily replicated on larger scales, including some optimization factors, such as pH control, lighting, agitation, among others, if necessary. IWMT will be essential technologies particularly in regions with low water and energy availability (since the proposed system involves a reduction in the WFP with respect to its non-implementation), mitigating GHG emissions (CFP reduction) and strengthening local communities in the face of climate change.
6.4 Future Perspectives in the Biomass Sector
6.4.1 Current and Future Challenges for Achieving Sustainability from the Circular Bioeconomy
From the year 2000 onwards, two perspectives of bioeconomy can be recognized. The first, the fossil to biomass substitution perspective, which recognizes that new technological developments for biomass utilization will allow access to large feedstock supplies of biomass for new bioprocesses or biofuels, it was the one that has prevailed during the first decade of this century. Probably, this trend was promoted by the predictions of depletion of fossil fuels and their high price, and later, by the commitments made in the fight against climate change that found a milestone in the Paris Agreement.
The second perspective places its emphasis on a knowledge-based bioeconomy and biotechnology. It promotes the new technologies and processes developed from the latest advances in chemistry, mechanics, systems engineering, life sciences, and information technologies, with numerous applications (such as precision farming, healthier biochemical products, new recyclable materials, synthetic biology, digitization, and advanced manufacturing) (Birner 2018; Global Bioeconomy Summit (GBS) 2018; Linser and Lier 2020). This perspective is the one that has begun to predominate in recent years (Birner 2018). Biorefineries are one of the maximum exponents of what the circular bioeconomy promotes (Keegan et al. 2013; Rajesh Banu et al. 2020; Ubando et al. 2020).
Currently, both approaches appear mixed and with different variants in the national strategies proposed, from the concept to the scope, depth and type of activities, and areas that formed the bioeconomy (Global Bioeconomy Summit (GBS) 2018; Birner 2018). This is not only a technical option, but the result of different country characteristics, political preconditions, circumstances, priorities, settings, technological development, resource base, and public demands (Linser and Lier 2020). Even so, since approximately 2015, there is a clear reference that these strategies will contribute to meeting the sustainability objectives and those related to climate agreements (European Commission (EC) 2018; Global Bioeconomy Summit (GBS) 2018; United Nations (UN) 2018). However, these proclamations lack empirical evidence given the short existence of these national strategies, and most of them do not have goals or quantitative indicators that allow this evaluation to be carried out over time (German Bioeconomy Council (GBC) 2018). So, can it be simply assumed that compliance with the bioeconomy strategy will necessarily imply the achievement of sustainability goals? From the academic scientific field there is no consensus in this regard, and conflicting visions can be identified.
Some proponents of this circular bioeconomy paradigm assume innate sustainability and base their argument only on the analysis of some specific contributions of it (renewable resources and energy or some physical and ecological benefits of biomass use: Jenkins 2008; Navia and Mohanty 2012; Barkia et al. 2019). Intermediate positions recognize that the bioeconomy could bring benefits under certain conditions (Garofalo 2009; Demirbas et al. 2009; Bilgili et al. 2017; Scheiterle et al. 2018). Others more skeptical, distrust the new umbrella of the bioeconomy as a new green make-up, and point to problems associated with its promotion as the land use antagonism: food vs feed biomass production (Rosegrant et al. 2013); but also the threat to biodiversity or ecosystems that the production of biomass feedstocks may imply; or the negative balance of GHG emissions in the processing of biomass for energy (Agostini et al. 2014; Raj et al. 2020; Banerjee et al. 2020). At the other extreme, detractors point out that the implementation of the bioeconomy paradigm is disconnected from regional realities (Marsden 2013); it lacks sufficient regulatory frameworks to verify the fulfillment of goals and progress towards sustainability (Sheppard et al. 2011) and is basically promoted from a neoliberal capitalist ideology that moves everything under the rules of the market (Birch 2006; Birch et al. 2010; Gottwald 2016).
This current controversy should draw attention to the need to specifically consider sustainability as a central objective of the bioeconomy itself (Chisti 2010; Marsden 2013; Pfau et al. 2014; Scheiterle et al. 2018; Heimann 2018). If sustainability objectives are not clearly defined in each territory, the bioeconomy will not necessarily contribute to the achievement of the SDGs, since it will be guided by economic benefit and market interests (Birch et al. 2010). The 2030 Agenda still calls for more vigorous and urgent action (UN 2019b) particularly with regard to the goals of climate change (SDG 13), safe water (SDG 6), and access to energy (SDG 7), always considering an adequate management of resources. Indeed, there are still major challenges in terms of: (1) climate change: despite international agreements, carbon markets, and other incentives, the temperature has risen 1 °C from the reference scenario (United Nations (UN) 2019b); (2) water: a quarter of the world population faces extremely high water stress (WRI 2019); around 10% world population still did not have basic drinking water services (United Nations (UN) 2019b) while 380 billion m3 were eliminated as effluents across the world (Qadir et al. 2020); and (3) energy: about 39% of the world population lack clean fuels and technologies. Within a delicate framework of support for terrestrial and aquatic environments (risk of extinction of species and ocean acidification) (United Nations (UN) 2019b), further discussion is necessary for both goals of the bioeconomy and sustainable development, converge favorably and be verified. The last requires a specific action plan with quantitative aims (Global Bioeconomy Summit (GBS) 2018) based on concrete strategies custom-made for the territories and a regulatory framework that considers the different areas of action of the bioeconomy (Pfau et al. 2014; Rodríguez and Aramendis 2019).
6.4.2 Policies, Legal Framework, and Financing for the Circular Bioeconomy
Although the circular bioeconomy is under construction and redefinition of its bases and foundations (Carus and Dammer 2018), this new paradigm of production and consumption must be strengthened from a new institutional political framework that allows its development. Existing rules and regulations correspond to a fossil-dependent world economy based on the industrial revolution and need to be reviewed, modified, or adapted to the new bio-circular context (Bilgili et al. 2017; Rodríguez et al. 2017; Rodríguez and Aramendis 2019).
One of the challenges of the bioeconomy lies in the possibility of structuring the different value chains (which includes all the economic activities carried out in a territory, being the harvest of available raw materials the first link in the chain) in interconnected processes that simultaneously meet the dual objective of efficient management of feedstock: recirculation of energy and the cascade use of resources (BIORES 2015; Sherwood 2020). This challenge will be overcome with an appropriate territorial planning policy, based on stakeholder’s consultation and consensus (from public and private sectors), and regulations that promote responsible behavior in the productive processes, with a view to reducing environmental and social impacts (Chisti 2010; Rodríguez et al. 2017; Paletto et al. 2019; Vanhamäki et al. 2020).
The cascade use of a resource (taking advantage of the raw material for the elaboration of products, by-products, extraction of substances, and finally energy, Carus and Dammer 2018; Del Borghi et al. 2020), can be a difficult path to follow in small domestic economies (Demirbas et al. 2009; Pleissner 2020). While some world economies have resources, infrastructure, technology, and capital to reach the simplest level of plant cells to take advantage of each of their functional chemical groups (Ubando et al. 2020), in other regions (and even within the countries there are these differences), there are still unsatisfied basic demands that are those that should mobilize all attention, resources, and efforts towards more sustainable communities and territories (Food and Agriculture Organization of the United Nations (FAO) 2014; World Water Assessment Program (WWAP) 2017, Linser and Lier 2020; Pleissner 2020). In this sense, it is necessary, on the one hand, to generate more discussion on how the bioeconomy can reduce these inequalities within and among countries (disparities also observed between urban and rural areas) (Linser and Lier 2020) and, on the other, to promote differential bioeconomy strategies that allow optimal levels of circular bioeconomy to be reached within territories (involving both public and private sectors) (Vanhamäki et al. 2020).
Assumed that rural development is one of the aims of the bioeconomy (Pfau et al. 2014), a set of policy instruments focused on different regions must be developed. Public policies are those that will allow to model the character of circular bioeconomy in the territories, articulating the different ministries in a new space the interaction (production, health, environment, agribusiness, science, and technology, etc.) and the jurisdictional hierarchies (nation, province, municipality). These policies should start from the recognition of the characteristics and local individual needs (small family farming, small-scale enterprises, regulation of links and intermediaries in value chains), promoting infrastructure investments, and ensuring the participation of all levels in decision-making processes (Von Braun 2015; Rodríguez and Aramendis 2019; Manrique et al. 2020).
In other words, the bioeconomy of each country must start from the recognition of the bioeconomy of its regions, its different capacities, and its territorial vocation (particularly local feedstock offer). Each region should recognize their comparative advantages, and specialize and focus on them to create added value to their local productions (Global Bioeconomy Summit (GBS) 2018). Marketing within defined spaces will not only grant identity and empowerment to small local economies by promoting jobs and generation of incomes, but will also demand less fuel consumption for transportation and therefore a reduction in GHG emissions (Seadon 2006; Heimann 2018). It is also possible to boost the cascade use of biomass in developing economies, which will depend on clear organizational and political aspects, territorial planning and synergistic work in the territories (Rodríguez et al. 2017). The regulations should contemplate, for example, the addition of value through product origin seals, quality standards, and certifications of local manufactures or fair trade. Likewise, incentives for investment and innovation that support the creation of new small-businesses or new value added small-companies in the different productive chains of the territories. The advancement of communication and information technologies is contributing to being able to implement circular and cascade processes, opening the space to the introduction of multiple companies that provide new creative services (Carus and Dammer 2018; Global Bioeconomy Summit (GBS) 2018; Rodríguez and Aramendis 2019). There is great potential ahead, which must be deployed within ethical agreements and sustainability limits clearly defined in each site.
6.4.3 Research and Development for Integrated Waste Management Technologies Implementation
To the extent that the circular bioeconomy has motivating principles on which society agrees (basically generation and efficient use of biomass resources and diminish rubbish and refuse) (Heimann 2018; Hidalgo et al. 2019; Sherwood 2020), concrete strategies are required to guide efforts to implement these principles in practice. These will also become evaluation and monitoring points for the quantification of results (Linser and Lier 2020).
The definition and achievement of these strategies must be supported by a strong component of R&D at the local level (Scheiterle et al. 2018; Dietz et al. 2018; Kalmykova et al. 2018), generating integrative information across value chains (Sanders and Langeveld 2020). It must be considered that the bioeconomy can be approached from multiple fields of the science, whose knowledge of different nature will imply a high complexity at the time of its integration for decision-making in the regions. Therefore, interdisciplinary approaches, in ad hoc teams for solving specific problems in the territory, with the integration of contributions from the people involved in the biomass value chains, will allow the knowledge generated to be effectively applied, bridging the gap between science and society (Chisti 2010; Rodríguez et al. 2017; European Commission (EC) 2018).
For example, the solutions that focus on trinomial water–energy–climate change are what will consolidate the social and environmental networks, by mobilizing small domestic economies with practical solutions of the IWMT type (Nagarajan et al. 2020; Rajesh Banu et al. 2020). This will be of great impact in emerging economies, developing countries, or marginal sectors, in which the treatment systems are inefficient but in turn there is a high need for basic resources (water, energy, nutrients) (World Water Assessment Program (WWAP) 2017; Qadir et al. 2020). Qadir et al. (2020) estimated that globally, a total of 25.9 Tg of three essential nutrients (N, P, K) are lost annually in sewage effluents and Tarallo et al. (2015) recognize that the potential energy in the wastewater is around 500% the energy used for treatment processes. However, current technologies do not allow 100% recovery of the nutrient load and few systems recover nutrients and energy (Abdel-Raouf et al. 2012; Barkia et al. 2019; Qadir et al. 2020). The inclusion of microalgae as natural accumulators of nutrients, achieving greater system efficiency, should be promoted and tested in pilot plants and even replicated in real conditions at the local level, which makes it possible to define the limitations and real opportunities for co-benefits (benefits secondary) beyond the effluent sanitation and ecological benefits in receiving water bodies (Park et al. 2011; Prajapati et al. 2013; Matamoros et al. 2016; Rajesh Banu et al. 2020; Nagarajan et al. 2020). Likewise, is currently possible to achieve a neutral energy balance in the wastewater treatment cycle through the onsite renewable bioenergy generation (Tarallo et al. 2015). Numerous and more or less efficient bioenergy technologies are available on the market, and those have potential for local manufacturing in small economies (Slade and Bauen 2013; Prajapati et al. 2013; REN-21 2019; Manrique et al. 2020; Vanhamäki et al. 2020).
IWMT could be one of the fundamental tools of the circular economy, taking advantage of a greater portion of the resources and giving value to the wastes (Hidalgo et al. 2019). Although the IWMT do not fully incorporate the concept of cascade use, do bring the return of matter and energy to the system and provide solutions to specific local demands. In this line, there is still much to explore, defining solutions adapted to the territories according to existing biomass resources, but above all identifying sources of residual biomass that could be reused in circular processes (Jenkins 2008; León and Chaves 2010; Kaza et al. 2018; Pleissner 2020).
Scientific and technological networks will be of fundamental value in the territories and will make it possible to generate information on the new relevant variables (biomass, potential, distribution, functionalities); promote discussion tables; facilitate the dissemination of strategies and awareness of the population about the new products and services; socialize successful experiences and the failure analysis as an engine of innovation; constitute points of connection between multiple stakeholders: producers, consumers, government, academia, industry (Von Braun 2015; Clark et al. 2016; Rodríguez et al. 2017; Kalmykova et al. 2018; Vanhamäki et al. 2020). Undoubtedly, there is still a long way to go in developing IWMT strategies, but the benefits are more than promising.
Abbreviations
- CD:
-
Cell density (cell/mL)
- CFP:
-
Carbon footprint (t CO2eq)
- CO2:
-
Carbon dioxide
- COD:
-
Chemical Oxygen Demand (mg/L)
- DM:
-
Detmer culture medium
- DT:
-
Doubling time (day)
- EFP:
-
Ecological footprint
- GHG:
-
Greenhouse gas
- HCV:
-
Higher calorific value (MJ/kg)
- IWMT:
-
Integrated waste management technology
- LCV:
-
Lower calorific value (MJ/kg)
- ORP:
-
Open raceway pond
- PBR:
-
Photobioreactor
- RE:
-
Removal efficiency (%)
- TBH:
-
Total biomass harvested (grams)
- TN:
-
Total nitrogen (mg/L)
- TP:
-
Total phosphorus (mg/L)
- WFP:
-
Water footprint (m3 per unit of manufactured product or service consumed)
- WSP:
-
Wastewater stabilization ponds
References
Abdel-Raouf N, Al-Homaidan AA, Ibraheem ABM (2012) Microalgae and wastewater treatment. Saudi J Biol Sci 19:257–275
Abu-Ghosh S, Fixler D, Dubinsky Z, Iluz D (2015) Energy-input analysis of the life-cycle of microalgal cultivation systems and best scenario for oil-rich biomass production. Appl Energy 154:1082–1088
Accorinti J (1960) Cultivo unialgal y masivo de Scenedesmus obliquus Turp. KTZ. Técnicas de obtención. Museo Argentino de Ciencias Naturales, Ciencias Botánicas 1(9):21–29
Acien Fernández FG, Fernández Sevilla JM, Molina Grima E (2017) Microalgae: the basis of mankind sustainability. In: Llamas Moya B, Storch de Gracia MD and Mazadiego LF (eds.) Case study of innovative projects - successful real cases. IntechOpen. https://doi.org/10.5772/67930. https://www.intechopen.com/books/case-study-of-innovative-projects-successful-real-cases/microalgae-the-basis-of-mankind-sustainability. Accessed 13 Jan 2020
Agostini A, Giuntoli J, Boulamanti A (2014) Carbon accounting of forest bioenergy. In: Marelli L (ed.) JRC technical reports. European Commission, Joint Research Centre, Institute for Energy and Transport, Ispra. http://iet.jrc.ec.europa.eu/bf-ca/sites/bf-ca/files/files/documents/eur25354en_online-final.pdf. Accessed 10 Feb 2020
Ajayi T, Salgado Gomes J, Bera A (2019) A review of CO2 storage in geological formations emphasizing modeling, monitoring and capacity estimation approaches. Petroleum Sci 16:1028–1063
Anand J, Arumugam M (2015) Enhanced lipid accumulation and biomass yield of Scenedesmus quadricauda under nitrogen starved condition. Bioresour Tech 188:190–194
Andrade CE, Vera AL, Cárdenas CH, Morales ED (2009) Biomass production of microalga Scenedesmus sp. with wastewater from fishery. Revista Técnica de Ingeniería de la Universidad de Zulia 32(2):126–134
Azari A, Noorpoor AR, Bozorg-Haddad O (2019) Carbon footprint analyses of microalgae cultivation systems under autotrophic and heterotrophic conditions. Int J Environ Sci Technol 16:6671–6684
Bagchi SK, Rao PS, Mallick N (2015) Development of an oven drying protocol to improve biodiesel production for an indigenous chlorophycean microalga Scenedesmus sp. Bioresour Tech 180:207–213
Bakuei N, Amini G, Njafpour GD, Jahanshahi M, Mohammadi M (2015) Optimal cultivation of Scenedesmus sp microalgae in a bubble column photobioreactor. Indian J Chem Tech 22:20–25
Banerjee A, Jhariya MK, Yadav DK, Raj A (2020) Environmental and sustainable development through forestry and other resources. Apple Academic Press, CRC Press, Taylor and Francis Group, p 400. https://doi.org/10.1201/9780429276026
Barkia I, Saari N, Manning SR (2019) Review microalgae for high-value products towards human health and nutrition. Mar Drugs 17:304
Barros AI, Gonçalves AL, Simões M, Pires JC (2015) Harvesting techniques applied to microalgae: a review. Renewable Sustain Energy Rev 41:1489–1500
Bereciartua (2017) Los objetivos de desarrollo sostenible y el plan del agua en Argentina. Avances en materia de agua potable, saneamiento y tratamiento de efluentes. Serie n°1. Secretaría de Infraestructura y Política Hídrica. Buenos Aires, Argentina. https://www.argentina.gob.ar/sites/default/files/doc._2_-_objetivos_de_desarrollo_sostenible_y_el_pna.pdf. Accessed 22 Jan 2020
Bilanovic D, Andargatchew A, Kroeger T, Shelef G (2009) Freshwater and marine microalgae sequestering of CO2 at different C and N concentrations–response surface methodology analysis. Energy Convers Manag 50:262–267
Bilgili F, Koçak E, Bulut U, Kuşkaya S (2017) Can biomass energy be an efficient policy tool for sustainable development? Renewable Sustain Energy Rev 71:830–845
Biodiversity Information System (BIS) (2019) Scenedesmus quadricauda. https://sib.gob.ar/especies/scenedesmus-quadricauda. Accessed 27 Jan 2020
BIORES (2015) Sustainable regional supply chains for woody bioenergy. Status report, 34 p. http://bioresproject.eu/wp-content/uploads/2017/09/D5.1-Status-report-on-sustainability-in-forestry.pdf. Accessed 3 Mar 2020
Birch K (2006) The neoliberal underpinnings of the bioeconomy: the ideological discourses and practices of economic competitiveness. Genom Soc Policy 2(3):1–15
Birch K, Levidow L, Papaioannou T (2010) Sustainable capital? The neoliberalization of nature and knowledge in the European “knowledge-based bio-economy”. Sustainability 2(9):2898–2918
Birner R (2018) Bioeconomy concepts. In: Lewandowski I (ed) Bioeconomy. Springer, Cham. https://doi.org/10.1007/978-3-319-68152-8_3
Borucke M, Moore D, Cranston G, Gracey K, Iha K, Larsona J, Lazarus E, Morales JC, Wackernagel M, Galli A (2013) Accounting for demand and supply of the biosphere’s regenerative capacity: the National Footprint Accounts’ underlying methodology and framework. Ecol Indic 24:518–533
Carus M, Dammer L (2018) The “Circular Bioeconomy” – concepts, opportunities and limitations. Nova paper #9 on bio-based economy. Nova-Institut, Hürth. www.bio-based.eu/nova-papers. Accessed 30 Apr 2020
Chacón C, Andrade C, Cárdenas C, Araujo I, Morales E (2006) Uso de Chlorella sp y Scenedesmus sp en la remoción de nitrógeno, fósforo y DQO de aguas residuales urbanas de Maracaibo. Scielo 38:1–13
Chen WH, Wub ZY, Chang JS (2014) Isothermal and non-isothermal torrefaction characteristics and kinetics of microalga Scenedesmus obliquus CNW-N. Bioresour Technol 155:245–251
Chinnasamy S, Bhatnagar A, Hunt RW, Das K (2010) Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour Technol 101:3097–3105
Chisti Y (2010) A bioeconomy vision of sustainability. Biofuels Bioprod Biorefin 4:359–361
Clark JH, Farmer TJ, Herrero-Davila L, Sherwood J (2016) Circular economy design considerations for research and process development in the chemical sciences. Green Chem 18:3914–3934
Clippinger J, Davis R (2019) Techno-economic analysis for the production of algal biomass via closed Photobioreactors: future cost potential evaluated across a range of cultivation system designs. NREL/TP-5100-72716, National Renewable Energy Laboratory, Golden. https://www.nrel.gov/docs/fy19osti/72716.pdf. Accessed 17 May 2020
Codina MF, García CB, Barón JH, Da Silva SM, Bosch JP (2012) Planta piloto de microalgas para mejoramiento del tratamiento efluentes urbanos en Catamarca, Argentina. Revista de la Red de Expertos de Energía 8:15–17
Coimbra RN, Escapa C, Otero M (2019) Comparative thermogravimetric assessment on the combustion of coal, microalgae biomass and their blend. Energies 12:2962
de Schoenmakere M, Hoogeveen Y, Gillabel J, Manshoven S (2018) The circular economy and the bioeconomy: partners in sustainability. EEA report no 8/2018, European Environment Agency, 2018. https://www.eea.europa.eu/publications/circular-economy-and-bioeconomy. Accessed 20 Mar 2020
Del Borghi A, Moreschi L, Gallo M (2020) Circular economy approach to reduce water energy-food nexus. Curr Opin Environ Sci Health 13:23–28
Demirbas MF, Balat M, Balat H (2009) Potential contribution of biomass to the sustainable energy development. Energy Conv Manage 50:1746–1760
Dietz T, Börner J, Förster JJ, von Braun J (2018) Governance of the bioeconomy: a global comparative study of National Bioeconomy Strategies. Sustainability 10:3190
Drira N, Piras A, Rosa A, Porcedda S, Dhaouadi H (2016) Microalgae from domestic wastewater facility’s high rate algal pond: lipids extraction, characterization and biodiesel production. Bioresour Tech 206:239–244
El-Chichakli B, Von Braun J, Lang C, Barben D, Philp J (2016) Five cornerstones of a global bioeconomy. Nature 535:221–223
Ellen Macarthur Foundation (EMF) (2015) Towards a Circular economy: business rationale for an accelerated transition. https://www.ellenmacarthurfoundation.org/assets/downloads/TCE_Ellen-MacArthur-Foundation_9-Dec-2015.pdf. Accessed 21 Mar 2020
Erajaa S (2016) Cascading use of biomass: opportunities and obstacles in EU policies. Policy briefing. BirdLife Europe and the European Environmental Bureau. http://www.birdlife.org/sites/default/files/attachments/cascading_use_memo_final.pdf. Accessed 12 Feb 2020
European Commission (EC) (2018) A sustainable bioeconomy for Europe: Strengthening the connection between economy, society and the environment. In: Updated bioeconomy strategy. European Commission Directorate-General for Research and Innovation Unit F—Bioeconomy; Publications Office of the European Union: Luxembourg, p 105
Food and Agriculture Organization of the United Nations (FAO) (2014) Walking the talk: assessing the water-energy-food Nexus. Food and Agriculture Organization of the United Nations, Rome
Galli A, Wiedmann T, Ercin E, Knoblauch D, Ewing B, Giljum S (2012) Integrating ecological, carbon, and water footprint into a “Footprint Family” of indicators: definition and role in tracking human pressure on the planet. Ecol Indic 16:100–112
Garofalo R (2009) Algae and aquatic biomass for a sustainable production of 2nd generation biofuels from Scenedesmus. Journal Aquafuels FP7-241301-2 2009: 77-82
Gerbens-Leenes PW, Xu L, deVries GJ, Hoekstra AY (2014) The blue water footprint and land use of biofuels from algae. Water Resour Res 50:8549–8563
German Bioeconomy Council (GBC) (2018) Bioeconomy policy (part III) update report of national strategies around the world. Office of the Bioeconomy Council, Bonn. p 124. https://biooekonomierat.de/fileadmin/Publikationen/berichte/GBS_2018_Bioeconomy-Strategies-around-the_World_Part-III.pdf. Accessed 19 Nov 2019
Giuntoli J, Robert N, Ronzon T, Sanchez Lopez J, Follador M, Girardi I, Barredo Cano J, Borzacchiello M, Sala S, M’Barek R (2020) Building a monitoring system for the EU bioeconomy. EUR 30064 EN, Publications Office of the European Union, Luxembourg
Global Bioeconomy Summit (GBS) (2018) Conference report. Global Bioeconomy Summit: Berlin. https://gbs2018.com/fileadmin/gbs2018/GBS_2018_Report_web.pdf. Accessed 2 Jan 2020
Gottwald FT (2016) Bioeconomy – a challenge to integrity. In: Westra L, Gray J, D’Aloia A (eds) The common good and ecological integrity: human rights and the support of life. Earthscan, Routledge, London, New York, pp 22–35
Gouveia L, Graça S, Sousa C, Ambrosano L, Ribeiro B, Botrel EP, Castro Neto P, Ferreira AF, Silva CM (2016) Microalgae biomass production using wastewater: treatment and costs scale-up considerations. Algal Res 16:167–176
Grobbelaar JU, Soeder CJ, Groeneweg ES, Hartig P (1988) Rates of biogenic oxygen production in mass-cultures of microalgae, absorption of atmospheric oxygen and oxygen availability for waste- water treatment. Water Res 22:1459–1464
Guerrero PG (1941) Algas de agua dulce de la República Argentina. http://www.rjb.csic.es/jardinbotanico/ficheros/documentos/pdf/anales/1941/Anales_01(1)_141_171.pdf. Accessed 08 Feb 2020
Haberl H, Sprinz D, Bonazountas M, Cocco P, Desaubies Y, Henze M, Hertel O, Johnson RK, Kastrup U, Laconte P, Lange E, Novak P, Paavola J, Reenberg A, van den Hove S, Vermeire T, Wadhams P, Searchinger T (2012) Correcting a fundamental error in greenhouse gas accounting related to bioenergy. Energy Policy 45:18–23
Hammouda O, Gaber A, Abdel-Raouf N (1995) Microalgae and wastewater treatment. Ecotox Environ Saf 31:205–210
Han L, Pei H, Hu W, Jiang L, Ma G, Zhang S, Han F (2015) Integrated campus sewage treatment and biomass production by Scenedesmus quadricauda SDEC-13. Bioresour Tech 175:262–268
Harris PO, Ramellow GJ (1990) Binding of metal ions by particulate biomass derived from Chlorella vulgaris and Scenedesmus quadricauda. Environ Sci Technol 24(2):220–228
Heimann T (2018) Bioeconomy and sustainable development goals (SDGs): does the bioeconomy support the achievement of the SDGs? Earth’s Future 7:43–57
Hidalgo D, Martín-Marroquín JM, Corona F (2019) A multi-waste management concept as a basis towards a circular economy model. Renewable Sustain Energy Rev 111:481–489
Hoekstra AY, Chapagain AK, Aladaya MM, Mekonnen MM (2011) The water footprint assessment manual - setting the global standard. Earthscan, London
Instituto Nacional de Estadísticas y Censos (INDEC) (2010). http://www.Indec.Gov.Ar/Webcenso/Index.Asp. Accessed 20 Dec 2019
InterAmerican Network of Academies of Sciences (IANAS) (2019) Calidad del Agua en las Américas Riesgos y Oportunidades. México, 661 pp. https://www.ianas.org/images/books/wb09.pdf. Accessed 21 Jan 2020
Intergovernmental Panel Climate Change (IPCC) (2006) Land use, land use change, and forestry. Cambridge University Press, Cambridge
Intergovernmental Panel Climate Change (IPCC) (2018) Summary for Policymakers. In: Masson-Delmotte V, Zhai P, Pörtner H-O, Roberts D, Skea J, Shukla PR, Pirani A, Moufouma-Okia W, Péan C, Pidcock R, Connors S, Matthews JBR, Chen Y, Zhou X, Gomis MI, Lonnoy E, Maycock T, Tignor M, Waterfield T (eds.) Global Warming of 1.5°C. An IPCC special report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty
International Energy Agency (IEA) (2016) World energy outlook 2016 - water energy nexus. IEA, Paris. https://doi.org/10.1787/weo-2016-en
Jenkins T (2008) Toward a biobased economy: examples from the UK. Biofuels Bioprod Biorefin 2:133–143
Jhariya MK, Banerjee A, Meena RS, Yadav DK (2019a) Sustainable agriculture, forest and environmental management. Springer, Singapore, p 606. https://doi.org/10.1007/978-981-13-6830-1
Jhariya MK, Banerjee A, Yadav DK, Raj A (2018b) Leguminous trees an innovative tool for soil sustainability. In: Meena RS, Das A, Yadav GS, Lal R (eds) Legumes for soil health and sustainable management. Springer, Berlin, pp 315–345. https://doi.org/10.1007/978-981-13-0253-4_10
Jhariya MK, Yadav DK, Banerjee A (2018a) Plant mediated transformation and habitat restoration: phytoremediation an eco-friendly approach. In: Gautam A, Pathak C (eds) Metallic contamination and its toxicity. Daya Publishing House, A Division of Astral International Pvt. Ltd, New Delhi, pp 231–247
Jhariya MK, Yadav DK, Banerjee A (2019b) Agroforestry and climate change: issues and challenges. Apple Academic Press, CRC Press, Taylor and Francis Group, pp 335. https://doi.org/10.1201/9780429057274
Kadam KL (2002) Environmental implications of power generation via coal- microalgae cofiring. Energy 27(10):905–922
Kalmykova Y, Sadagopan M, Rosado L (2018) Circular economy – from review of theories and practices to development of implementation tools. Resour Conserv Recycl 135:190–201
Kaza S, Yao L, Bhada-Tata P, van Woerden F (2018) What a waste 2.0: a global snapshot of solid waste management to 2050. Urban Development. World Bank, Washington. https://openknowledge.wor ldbank.org/handle/10986/30317. Accessed 30 Apr 2020
Keegan D, Kretschmer B, Elbersen B, Panoutsou C (2013) Cascading use: a systematic approach to biomass beyond the energy sector. Biofuels Bioprod Biorefin 7:193–206
Khan N, Jhariya MK, Yadav DK, Banerjee A (2020a) Herbaceous dynamics and CO2 mitigation in an urban setup- a case study from Chhattisgarh, India. Environ Sci Pollut Res 27(3):2881–2897. https://doi.org/10.1007/s11356-019-07182-8
Khan N, Jhariya MK, Yadav DK, Banerjee A (2020b) Structure, diversity and ecological function of shrub species in an urban setup of Sarguja, Chhattisgarh, India. Environ Sci Pollut Res 27(5):5418–5432. https://doi.org/10.1007/s11356-019-07172-w
Kirchherr J, Reike D, Hekkert M (2017) Conceptualizing the circular economy: an analysis of 114 definitions. Resour Conserv Recycl 127:221–232
Kothari R, Pathak VV, Kumar V, Singh DP (2012) Experimental study for growth potential of unicellular alga Chlorella pyrenoidosa on dairy waste water: an integrated approach for treatment and biofuel production. Bioresour Tech 116:466–470
Koutra E, Economou CN, Tsafrakidou P, Kornaros M (2018) Bio-based products from microalgae cultivated in digestates. Trends Biotechnol 36:819–833
KPMG (2019) Development of renewable energy in Argentina. Energy and natural resources. KPMG. https://assets.kpmg/content/dam/kpmg/ar/pdf/development-renewable-energy-argentina-2019.pdf. Accessed 28 Nov 2019
Kraxner F, Nilsson S, Obersteiner M (2003) Negative emissions from BioEnergy use, carbon capture and sequestration (BECS)—the case of biomass production by sustainable forest management from semi-natural temperate forests. Biomass Bioenergy 24:285–296
Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J, Malcata FX, van Langenhove H (2010) Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol 28:371–380
Leivas R, Laso J, Abejón R, Margallo M, Aldaco R (2020) Environmental assessment of food and beverage under a NEXUS water-energy-climate approach: application to the spirit drinks. Sci Total Environ 720:137576
León C, Chaves D (2010) Tratamiento de residual vacuno utilizando microalgas, la lenteja de agua Lemna aequinoctiales y un humedal subsuperficial en Costa Rica. Revista Latinoamericana de Biotecnología Ambiental Algal 1(2):155–177
Linser S, Lier M (2020) The contribution of sustainable development goals and Forest-related indicators to National Bioeconomy Progress Monitoring. Sustainability 12:2898
Manrique SM (2017) Biomasa con fines energéticos: recursos, potencialidad y cambio climático. Ciudad Autónoma de Buenos Aires. EdUTecNe, 220 p
Manrique SM, Torreiro Villarino Y, Contreras Rodríguez ML, Sánchez Hervás JM, Garrido SM, Curbelo Alonso AJ (2020) Recursos, tecnologías, transferencia y políticas: Una mirada desde múltiples perspectivas y dimensiones a los sistemas de bioenergía en Iberoamérica. CYTED (Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo), Madrid, España, 270 p
Marsden T (2013) Sustainable place-making for sustainability science: the contested case of Agri-food and urban—rural relations. Sustain Sci 8:213–226
Martins AA, Marques F, Cameira M, Santos E, Badenes S, Costa L, Verdelho Vieira V, Caetano NS, Mata TM (2018) Water footprint of microalgae cultivation in photobioreactor. 5th international conference on energy and environment research, ICEER 2018. Energy Procedia 153:426–431
Matamoros V, Uggetti E, García J, Bayona JM (2016) Assessment of the mechanisms involved in the removal of emerging contaminants by microalgae from wastewater. J Hazard Mater 301:197–205
Meena RS, Kumar S, Datta R, Lal R, Vijaykumar V, Brtnicky M, Sharma MP, Yadav GS, Jhariya MK, Jangir CK, Pathan SI, Dokulilova T, Pecina V, Marfo TD (2020a) Impact of agrochemicals on soil microbiota and management: a review. Land (MDPI) 9(2):34. https://doi.org/10.3390/land9020034
Meena RS, Kumar V, Yadav GS, Mitran T (2018) Response and interaction of Bradyrhizobium japonicum and arbuscular mycorrhizal fungi in the soybean rhizosphere: a review. Plant Growth Regul 84:207–223
Meena RS, Lal R (2018) Legumes for soil health and sustainable management. Springer, Singapore, p 541. https://doi.org/10.1007/978-981-13-0253-4_10
Meena RS, Lal R, Yadav GS (2020b) Long term impacts of topsoil depth and amendments on soil physical and hydrological properties of an Alfisol in Central Ohio, USA. Geoderma 363:1141164
Meena RS, Lal R, Yadav GS (2020c) Long-term impact of topsoil depth and amendments on carbon and nitrogen budgets in the surface layer of an Alfisol in Central Ohio. Catena 194:104752
Méndez L, Albarracín I, Cravero M, Salomón R (2011) Scenedesmus quadricauda growth in sewage effluents of Trelew City, Chubut, Argentina. Rev Cuba Investig Pesq 28(1):36–41
Menger-Krug E, Niederste-Hollenberg J, Hillenbrand T, Hiess H (2012) Integration of microalgae systems at municipal wastewater treatment plants: implications for energy and emission balances. Environ Sci Technol 46:11505–11514
Molazadeh M, Ahmadzadeh H, Pourianfar HR, Lyon S, Rampelotto PH (2019) The use of microalgae for coupling wastewater treatment with CO2 biofixation. Front Bioeng Biotechnol 7:42
Monfreda C, Wackernagel M, Deumling D (2004) Establishing national natural capital accounts based on detailed ecological footprint and biocapacity assessments. Land Use Policy 21:231–246
Morselli L, Vassura I, Passarini F (2008) Integrated waste management. Technologies and environmental control. In: Clini C, Musu I, Gullino ML (eds) Sustainable development and environmental management. Springer, Dordrecht
Mouratiadou I, Biewald A, Pehl M, Bonsch M, Baumstark L, Klein D, Popp A, Luderer G, Kriegler E (2016) The impact of climate change mitigation on water demand for energy and food: an integrated analysis based on the shared socioeconomic pathways. Environ Sci Pol 64:48–58
Muñoz R, Köllner C, Guieysse B, Mattiasson B (2004) Photosynthetically oxygenated salicylate biodegradation in a continuous stirred tank photobioreactor. Biotechnol Bioeng 87(6):797–803
Nagarajan D, Lee DJ, Chen CY, Chang JS (2020) Resource recovery from wastewaters using microalgae-based approaches: a circular bioeconomy perspective. Bioresour Tech 302:122817
Navia R, Mohanty AK (2012) Resources and waste management in a bio-based economy. Waste Manage Res 30:215–216
Olsson J (2018) Co-digestion of microalgae and sewage sludge: a feasibility study for municipal wastewater treatment plants. Mðlardalen University Doctoral Dissertation 262. Mðlardalen University Press Dissertations. ISBN 978-91-7485-386-5. ISSN 1651-4238. Printed by E-Print AB, Stockholm, Sweden
Oswald WJ, Gotaas HB, Ludwig HF, Lynch V (1953) Algae symbiosis in oxidation ponds: III. Photosynthetic oxygenation. Sewage Ind Wastes 25(6):692–705
Paletto A, Bernardi S, Pieratti E, Teston F, Romagnoli M (2019) Assessment of environmental impact of biomass power plants to increase the social acceptance of renewable energy technologies. Heliyon 5(7):e02070
Pancha I, Chokshi K, Mishra S (2015) Production potential with nutritional stress amelioration through optimization of carbon source and light intensity in Scenedesmus sp CCNM 1077. Bioresour Tech 179:565–572
Park J, Craggs R, Shilton A (2011) Wastewater treatment high rate algal ponds for biofuel production. Bioresour Tech 102:35–42
Pfau SF, Hagens JE, Dankbaar B, Smits AJM (2014) Visions of sustainability in bioeconomy research. Sustainability 6:1222–1249
Pleissner D (2020) Chances and challenges of the biologization of the economy of rural areas. Curr Opin Green Sustain Chem 23:46. https://doi.org/10.1016/j.cogsc.2020.02.008. Accessed 30 Apr 2020
Prajapati SK, Kaushik P, Malik A, Vijay VK (2013) Phycoremediation coupled production of algal biomass, harvesting and anaerobic digestion: possibilities and challenges. Biotechnol Adv 31:1408–1425
Qadir M, Drechsel P, Jiménez Cisneros B, Kim Y, Pramanik A, Mehta P, Olaniyan O (2020) Global and regional potential of wastewater as a water, nutrient and energy source. Nat Res Forum:1–12
Raj A, Jhariya MK, Yadav DK, Banerjee A (2020) Climate change and agroforestry systems: adaptation and mitigation strategies. Apple Academic Press, CRC Press, Taylor and Francis Group, p 383. https://doi.org/10.1201/9780429286759
Rajesh Banu J, Preethi S, Kavitha M, Gunasekaran G, Kumar G (2020) Microalgae based biorefinery promoting circular bioeconomy-techno economic and life-cycle analysis. Bioresour Tech 302:12282
Realmonte G, Drouet L, Gambhir A, Glynn J, Hawkes A, Köberle AC, Tavoni M (2019) An inter-model assessment of the role of direct air capture in deep mitigation pathways. Nat Commun 10:3277
Renewable Energy Network for the 21st Century (REN-21) (2019) Renewables global status report. Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ) GmbH
Rocca S, Agostini A, Giuntoli J, Marelli L (2015) Biofuels from algae: technology options, energy balance and GHG emissions. Insights from a literature review EUR 27582. https://publications.jrc.ec.europa.eu/repository/bitstream/JRC98760/algae_biofuels_report_21122015.pdf. Accessed 28 Jan 2020
Rodolfi L, ChiniZittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Rodríguez AG, Aramendis RH (2019) El financiamiento de la bioeconomía en América Latina: identificación de fuentes nacionales, regionales y de cooperación internacional, serie Recursos Naturales y Desarrollo, N° 193 (LC/TS.2019/82), Santiago, Comisión Económica para América Latina y el Caribe (CEPAL)
Rodríguez AG, Mondaini AO, Hitschfeld MA (2017) Bioeconomía en América Latina y el Caribe Contexto global y regional y perspectivas. CEPAL - Serie Desarrollo Productivo N° 215. Naciones Unidas, Santiago. https://www.cepal.org/es/publicaciones/42427-bioeconomia-america-latina-caribe-contexto-global-regional-perspectivas. Accessed 9 Mar 2020
Rosegrant MW, Ringler C, Zhu T, Tokgoz S, Bhandary P (2013) Water and food in the bioeconomy: challenges and opportunities for development. Agric Econ 44(1):139–150
Sanders JPM, Langeveld JWA (2020) Development perspectives for the bio-based economy. Chapter 2. In: Biobased products and industries. pp 41–78
Scheiterle L, Ulmer A, Birner R, Pyka A (2018) From commodity-based value chains to biomass-based value webs: the case of sugarcane in Brazil’s bioeconomy. J Cleaner Prod 172:3851–3863
Schumacher G, Blume T, Sekoulov I (2003) Bacteria reduction and nutrient removal in small wastewater treatment plants by an algal biofilm. Water Sci Tech 47:195–202
Seadon JK (2006) Integrated waste management – looking beyond the solid waste horizon. Waste Manag 26(12):1327–1336
Secretaría de Ambiente y Desarrollo Sustentable de la Nación (SAyDS) (2015) Tercera Comunicación Nacional (TCN) a la CMNUCC. http://3cn.cima.fcen.uba.ar/docs/3Com-Resumen-Ejecutivo-de-la-Tercera-Comunicacion-Nacional.pdf. Accessed 28 Feb 2020
Sheppard AW, Raghu S, Begley C, Richardson DM (2011) Biosecurity in the new bioeconomy. Curr Opin Environ Sustain 3:1–3
Sherwood J (2020) The significance of biomass in a circular economy. Bioresour Tech 300:122755
Siddiqi A, Anadon LD (2011) The water–energy nexus in Middle East and North Africa. Energy Policy 39(8):4529–4540
Singh V, Tiwari A, Das M (2016) Phyco-remediation of industrial waste-water and flue gases with algal-diesel engenderment from micro-algae: a review. Fuel 173:90–97
Slade R, Bauen A (2013) Microalgae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 53:29–38
Sonmez C, Elcin E, Akın D, Avni HO, Yucel M (2016) Evaluation of novel thermo-resistant Micractinium sp and Scenedesmus sp for efficient biomass and lipid production under different temperature and nutrient regimes. Bioresour Tech 211:422–428
Suh IS, Lee CG (2003) Photobioreactor engineering: design and performance. Biotechnol Bioprocess Eng 8:313–321
Sydney EB, Da Silva TE, Tokarski A, Novaka AC, de Carvalhoa JC, Woiciecohwskia AL, Laroche C, Soccol CR (2011) Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl Energy 88:3291–3294
Tal A (2016) Rethinking the sustainability of Israel's irrigation practices in the Drylands. Water Res 90(1):387–394
Tang DYY, Khoo KS, Chew KW, Tao Y, Ho SH, Show PL (2020) Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour Tech 304:122997
Tarallo S, Shaw A, Kohl P, Eschborn R (2015) A guide to net-zero energy solutions for water resource recovery facilities (ENER1C12). Water Environment Research Foundation (WERF), Alexandria. http://www.werf.org/downloads/ener1c12.pdf. Accessed 2 May 2020
Tedesco S, Marrero Barroso T, Olabi A (2014) Optimization of mechanical pre- treatment of Laminariaceae spp. biomass-derived biogas. Renew Energy 62:527–534
Teotónio C, Rodríguez M, Roebeling P, Fortes P (2020) Water competition through the ‘water-energy’ nexus: assessing the economic impacts of climate change in a Mediterranean context. Energy Econ 85:104539
Trigo E, Vera Morales E, Grassi (2017) Bioeconomía Argentina: Visión desde Agroindustria. https://www.agroindustria.gob.ar/sitio/areas/bioeconomia/_archivos//000000_Bioeconomia%20Argentina.pdf. Accessed 25 Feb 2020
Ubando AT, Felix CB, Chen WH (2020) Biorefineries in circular bioeconomy: a comprehensive review. Bioresour Tech 299:122585
United Nations Environmental Programme (UNEP) (1996) International source book on environmentally sound technologies for municipal solid waste management. International environmental technology centre technical publication series (6). United Nations environmental programme
United Nations (UN) (2018) Global Indicator Framework for the Sustainable Development Goals and targets of the 2030 Agenda for Sustainable Development. In: A/RES/71/313 E/CN.3/2018/2, United Nations, New York
United Nations (UN) (2019a) Circular economy for the SDGs: from concept to practice. General Assembly and ECOSOC Joint Meeting Draft Concept. https://www.un.org/en/ga/second/73/jm_conceptnote.pdf. Accessed 11 Nov 2019
United Nations (UN) (2019b) Informe sobre el cumplimiento de ODS 2019. https://unstats.un.org/sdgs/report/2019/The-Sustainable-Development-Goals-Report-2019_Spanish.pdf. Accessed 2 May 2020
Vanhamäki S, Virtanen M, Luste S, Manskinen K (2020) Transition towards a circular economy at a regional level: a case study on closing biological loops. Resour Conserv Recycl 156:104716
von Braun J (2015) El concepto de bioeconomía en perspectiva y su relevancia para la Agenda Global de Políticas de desarrollo. http://conferencias.cepal.org/Conferencia_bioeconomia/Miercoles%207/Pdf/Joachim%20von%20Braun.pdf. Accessed 2 Mar 2020
Wackernagel M, Beyers B, Rout K (2019) Ecological footprint: managing our biocapacity budget. New Society Publishers, p 288
Wautelet T (2018) The concept of circular economy: its origins and its evolution. Working paper. https://doi.org/10.13140/RG.2.2.17021.87523
Wiedmann T, Minx J (2007) A definition of ‘carbon footprint’. ISA Reino Unido research report, 07-ISA Reino Unido Research and Consulting
Wiloso EI, Heijungs R, Huppes G, Fang K (2016) Effect of biogenic carbon inventory on the life cycle assessment of bioenergy: challenges to the neutrality assumption. J Cleaner Prod 125:78–85
Wong YK, Yung KK, Tsang YF, Xia Y, Wang L, Ho KC (2015) Scenedesmus quadricauda for nutrient removal and lipid production in wastewater. Water Environ Res 87(12):2037–2044
World Water Assessment Program (WWAP) (2017) Informe Mundial de las Naciones Unidas sobre el Desarrollo de los Recursos Hídricos 2017. Aguas residuales: El recurso desaprovechado. UNESCO, París. https://unesdoc.unesco.org/ark:/48223/pf0000247647. Accessed 12 Dec 2019
Xiao R, Chen R, Zhang HY, Li H (2011) Microalgae Scenedesmus quadricauda grown in digested wastewater for simultaneous CO2 fixation and nutrient removal. J Biobased Mater Bioenergy 5:234–240
Zargar S, Krishnamurthi K, Saravana Devi S, Ghosh TK, Chakrabarti T (2006) Temperature-induced stress on growth and expression of hsp in freshwater alga Scenedesmus quadricauda. Biomed Environ Sci 19(6):414–421
Zuliani L, Frison N, Jelic A, Fatone F, Bolzonella D, Ballottari M (2016) Microalgae cultivation on anaerobic digestate of municipal wastewater, sewage sludge and agro-waste. Int J Molecular Sci 17(10):1692
Acknowledgments
The study here reported was funded by the Research Council of the National University of Salta, through the Project N°2152. The participation of the following students in the project is especially appreciated: D. Díaz, R. Fernández, and C. Zárate.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Manrique, S.M. (2021). Biomass as a Cornerstone of a Circular Economy: Resources, Energy, and Environment. In: Banerjee, A., Meena, R.S., Jhariya, M.K., Yadav, D.K. (eds) Agroecological Footprints Management for Sustainable Food System. Springer, Singapore. https://doi.org/10.1007/978-981-15-9496-0_6
Download citation
DOI: https://doi.org/10.1007/978-981-15-9496-0_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9495-3
Online ISBN: 978-981-15-9496-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)