Abstract
Malignant mesothelioma is an aggressive tumor that has been associated with exposure to asbestos fibers. The discovery that germline heterozygous mutations of the gene encoding the deubiquitylase BRCA-associated protein 1 (BAP1) leads to inheritable higher susceptibility to mesothelioma underscores the relevance of gene x environment (GxE) interactions. Carriers of BAP1 germline mutations are affected by the BAP1 cancer syndrome, a high penetrance Mendelian disorder, characterized by earlier development of mesothelioma and specific types of other cancers. Numerous next-generation sequencing (NGS) analyses have been recently conducted searching for both germline and somatic alterations in patients affected by mesothelioma and associated cancers, and their relatives. BAP1 resulted in the more frequently germline mutated gene; however, other genes involved in DNA repair and homologous recombination were also identified. The pattern of chromothripsis, or chromosome staggering, which has been somatically identified in mesothelioma by several groups, may explain the frequent occurrence of noncontiguous biallelic genome alterations. Moreover, transcriptome studies in mesothelioma showed also the occurrence of fusion transcripts involving tumor suppressor genes. The complete knowledge of the genetic background associated with the GxE interactions involved in the pathogenesis of mesothelioma will be further improved by future genetic and genomic studies, allowing to develop better strategies for the prevention and treatment of this malignancy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Malignant mesothelioma is an aggressive tumor whose pathogenesis is associated closely with occupational exposure to asbestos. The populations of workers handling asbestos, such as miners, manufacturing, or shipyard workers displayed a higher incidence of mesothelioma than the general population [1, 2].
The latency period between the exposure to mineral fibers to the development of asbestos-associated pleural mesothelioma is on average of 30–60 years [3]. Therefore, the incidence of mesothelioma is still increasing despite the legal bans on the use of asbestos in the Western countries at the end of the last century [4]. The majority of emerging countries are still using asbestos in their manufacturing activities, thus mesothelioma incidence in these counties is expected to keep increasing in the future [5].
Asbestos refers to a family of six mineral fibers that were used commercially until the 1970s and 1980s, which are classified into two subgroups: the amphiboles, a group of rod-like fibers including amosite, or brown asbestos, crocidolite or blue asbestos, anthophyllite, actinolite, and tremolite; and the serpentine group, consisting of chrysotile or white asbestos [6]. Exposure to the naturally occurring asbestos-like mineral fibers, such as erionite, antigorite, and others, as well as irradiation, account for further environmental risk factors for mesothelioma.
It has been observed that human mesothelial cells are particularly susceptible to cytotoxicity induced by asbestos, of which major mechanism of cell death appears to be in the form of necrosis rather than apoptosis. Then, a large amount of high mobility group box 1 (HMGB1) protein that belongs to the damage-associated molecular protein (DAMP) family, gets released by mesothelial cells, recruiting macrophages to sustain chronic inflammation [7]. Owing to the prolonged chronic inflammation microenvironment, surviving mesothelial cells accumulate genetic alterations after prolonged asbestos exposure. The accumulation of such genetic alterations might cause those mesothelial cells to develop mesothelioma after long latency [8]. However, the observation that among the workers with a long history of exposure to asbestos, only ~5% developed mesothelioma led to speculate that genetic component may also confer addition to occupational and environmental risks [5].
2 Germline Mutations of the BAP1 Gene
About 20 years ago, Michele Carbone discovered apparent autosomal dominant transmission of mesothelioma susceptibility in some Turkish families, who have resided and have been exposed to erionite in the soil for a long time [9, 10]. Furthermore, Carbone and coworkers discovered germline mutations in the gene encoding the BRCA1-associated Protein 1 (BAP1), located in chromosome 3p21.3, in families with a high incidence of both pleural and peritoneal mesothelioma as well as uveal melanomas (UVMs), cutaneous melanoma, and clear cell renal carcinoma [11]. Subsequently, families of similar phenotypes with BAP1 germline mutations have been reported in various ethnicities with an elevated risk of developing several other malignancies, such as cholangiocarcinoma, basal cell carcinoma, meningioma (reviewed in [12]). These findings established the concept of the “BAP1 cancer syndrome,” as an autosomal familial cancer syndrome. An extended family with over nine generations inheriting mesothelioma, UVM, and other cancers since the 1700s established the inheritance mode of BAP1 cancer syndrome [11].
BAP1 encodes a nuclear ubiquitin carboxy-terminal hydrolase (UCH) functioning as a deubiquitinating enzyme. BAP1 is unique among UCH family members because of its long C-terminal tail, which contains two nuclear localization signals [13]. Both nuclear localization and deubiquitinating activity of BAP1 protein are postulated to be necessary for the maintenance of tumor suppressor activity [14]. BAP1 is implicated in the regulation of cell cycle, cellular differentiation, gluconeogenesis, chromatin remodeling, gene transcription, and DNA repair [12].
At the clinical level, the discovery of the BAP1 cancer syndrome emphasizes the necessity for genotyping the DNA of patients with mesothelioma for mutations, to determine the presence of germline mutations in the BAP1 gene and other yet unidentified additional genes to acquire more complete information on the inherited predisposition to cancers like mesothelioma.
3 NGS Analysis in the Search for Germline Mutations in Other Genes
Several Next-Generation Sequencing (NGS) studies have been performed following the identification of BAP1 in mesothelioma and other cancers to investigate germline variants individuals at risk of mesothelioma or in patients with this aggressive cancer [12]. Patients (n = 89) who developed pleural mesothelioma because of ascertained cumulative exposure to asbestos were screened for the presence of germline pathogenic truncating nonsense or frameshift variants (PTVs), by targeting 94 genes known for predisposition to cancer. BAP1 germline PTVs were identified in four patients with mesothelioma, while germline PTVs were found also in CDKN2A or DNA repair genes. The asbestos exposure was significantly higher in patients with familial mesothelioma and PTVs in tumor suppressor genes than the patients with no germline variants in the 94 cancer-predisposing genes [15, 16].
A different approach, aimed at studying the inheritance of germline mutations of BAP1 or other genes, was used to select a cohort of 79 individuals to be investigated. This population consisted of 52 unrelated probands with familial mesothelioma and their 27 first- and second-degree relatives, and was selected for possible genetic predisposition, based on the following four criteria: (1) mesothelioma in first- or second-degree relatives; (2) diagnosis of cancers typical of BAP1+/−carriers (uveal melanoma, cutaneous melanoma, clear-cell renal cell carcinoma) in the probands or at least one first- or second-degree relative; (3) family history of multiple cancers; and (4) early cancer onset less than 50 years old. BAP1 Sanger sequencing and tNGS of more than other additional 50 cancer susceptibility genes were performed in this population. The results of this study showed that most of the patients were carriers of BAP1+/− with familial mesothelioma (43/79). Germline PTVs involving the following cancer susceptibility genes other than BAP1 were also identified in this group: ARID1A, ARID2, BAP1, CREBBP, KDR, MLH1, NCOR1, RAD50, RBM6, SETD2, SMARCA2, SMARCA4, SMARCE1, SMO, TP53. Survival of 77 patients were compared with data from the mesothelioma in general, using dataset of the Surveillance, Epidemiology, and End Results (SEER) cohort (https://seer.cancer.gov), revealing a significant improvement of survival and earlier age at diagnosis (5 years and 54 years of age, respectively) in the selected population compared with the SEER cohort (8 months and aged 72 years, respectively). In the selected patients with familial mesothelioma and wild-type BAP1, survival was even more favorable (9 years) and diagnosis occurred earlier (45 years). These data point at the selected criteria as helpful in identifying patients and family members who are more susceptible to develop additional cancers [17].
Another study performed targeted NGS (tNGS) in 198 germline DNAs from patients with different types of mesothelioma, analyzing 85 cancer susceptibility genes. Germline mutations of BAP1 other genes involved in homologous recombination (HR) and DNA repair were found in 12% of cases. Age, cancer diagnosis, and asbestos exposure were examined by multivariate analysis, revealing that young age and a second diagnosis of cancer were significantly associated with the occurrence of germline mutations in cancer susceptibility genes, for which minimal or no asbestos exposure turned out to be the most significant predictor [18].
The joint effort of two large centers of the National Cancer Institute (NCI) and the University of Chicago (UC) allowed studying the relationship of germline mutations in tumor suppressor or DNA repair genes with responsiveness to platinum-based chemotherapy in 385 patients with different types of mesothelioma. A multi-gene panel BROCA v10, containing 73 target genes associated with DNA repair and/or with inherited predisposition to develop solid cancers was used for genotyping. The analysis of the NCI/UC cohort identified at least a mutation in one of the targeted genes in 12% of patients. BAP1 was the most altered gene (16 mutations), while the other 12 mutations involved the following genes: CHEK2, PALB2, BRCA2, MLH1, POT1, TP53, and MRE11A. In patients with pleural mesothelioma (not with peritoneal type) mutated BAP1, or a mutation in the other targeted genes, was significantly associated with improved overall survival (OS), compared with wild-type patients [19].
Interestingly, within a large exon tNGS study of 168 genes associated with hereditary cancer in a cohort of more than 600 patients with different cancers, the results obtained in 12 mesotheliomas revealed the highest frequency of pathogenic variants (7/12, 58%) in genes regulating HR DNA repair, with the genes of the pathway of Fanconi anemia (BRCA2 or FANCD1, FANCA, FANCC, FANCD2, and FANCM) particularly represented [20].
The results of all these studies (summarized in Table 12.1) clearly indicate that at least 10%–12% of mesothelioma cases were associated with germline mutations in BAP1 or in other HR genes and displayed better prognosis and chemosensitivity than patients with wild-type genetic background.
4 Somatic Mutations of BAP1
Frequent somatic mutations in BAP1 have been observed in highly metastatic uveal melanomas, 26 of 31 (84%) metastasizing tumors [21]. The majority (63.6%) of sporadic mesotheliomas contain somatic BAP1 mutations/inactivation [22]. These findings confirmed our previous data on BAP1 inactivation in epithelioid type mesothelioma accompanied by loss of heterozygosity (LOH) [23], and are supported by two NGS studies of the mesothelioma genome that revealed that BAP1 was somatically mutated in 41% [24] and 58% [25] of mesotheliomas, respectively. Therefore, the BAP1 gene undergoes biallelic inactivation in tumors, thus, meeting the criteria of classical two-hit inactivation theory for tumor suppressor genes.
5 Chromothripsis in Mesothelioma Genome
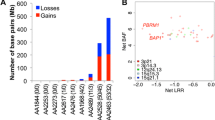
Frequent observation of loss of heterozygosity on 3p21 in malignant mesothelioma led us and others to focus on BAP1 as a target gene of somatic inactivation. In 2011 a study found that BAP1 was inactivated by somatic mutations in mesothelioma [26], while in metastatic clear cell renal carcinoma the minimal common deletion region at 3p21.1 contained BAP1 and PBRM1 at 3p21 [27]. The genomic pattern of peritoneal mesothelioma is similar to that of pleural mesothelioma [28]. We performed a comprehensive tumor genome analysis targeting the 3p21 region by performing high-density array comparative genomic hybridization (CGH; average probe interval: 254 bp) detecting multiple minute simultaneous biallelic deletions in this region, especially in BAP1 (8/33, 24%), SETD2 (7/33, 21%), PBRM1 (3/33, 9%), and SMARCC1 (2/33, 6%) [29]. Overall, 46 genes in this region were found to contain biallelic deletions in at least one biopsy specimen out of 33 mesothelioma specimens examined. Breakpoints of these genomic deletions were different in different cases. Many of these deletions were not contiguous but alternated with segments showing oscillating copy number changes along the 3p21 region. This may be because of chromothripsis (derived from the Greek word “chromos” for chromosome and “thripsis” for shattering into pieces) [30], a phenomenon characterized by numerous genomic rearrangements caused by a single catastrophic event in multiple cancer samples. The catastrophic genetic event known as chromothripsis consists of the fragmentation of a segregated single chromosome that is then rearranged leading to incorrect reassembling or loss of certain DNA sequences. Therefore, a single chromothripsis event may cause a high number of alterations in the genome after a short number of cell replications, leading to oncogenic activations or to loss of tumor suppressor functions, eventually favoring tumorigenesis [30].
Interestingly, noncontiguous biallelic genome alterations with the characteristic pattern of chromothripsis have been observed in mesothelioma [29], later confirmed by other groups [31], also with the potential consequence of neoantigen expression and tumor immunogenicity [31].
NGS alone hardly detects larger-sized DNA deletions (>30 bp). Conventional array CGH alone cannot detect smaller-sized deletions (<3000 bp). In other words, these analyses overlook genomic alteration in the size range of 30–3000 bp. Our comprehensive genome analysis combining high-density array CGH (average probe interval: 254 bp in the 3p21 region) and targeted NGS disclosed to or at higher frequencies than frequencies of sequence-level mutations [29]. Genomic alterations in mesothelioma usually include genomic rearrangements that induce complex and multiple deletions. Digital MLPA, which analyzes the copy number of approximately 600 exons simultaneously by using NGS-based MLPA, shall become a reliable method for high-throughput detection of multiple segmental deletions in small amounts of DNA in mesothelioma specimens to complement NGS analysis.
6 LOH, CDKN2A, NF2
The chromosomal changes of malignant mesothelioma are complex and heterogeneous, and more losses than gains of genetic material are observed. Losses of chromosomes 1p, 3p, 4q, 6q, 9p, 13, 14q, and 22 were detected in the majority of the abnormal cases [32,33,34]. Homozygous deletion of 9p21.3 is most frequently detected for the genetic alteration of mesothelioma and occurs in more than 90% of established cell lines. Deletion region involves CDKN2A, CDKN2B (cyclin-dependent kinase inhibitor 2B), and often adjacent MTAP (methylthioadenosine phosphorylase) and MIR31 genes. The CDKN2A gene generates at least three alternatively spliced variants encoding distinct proteins: p16INK4A, p16gamma, and p14ARF. These products encoded by this gene play an essential role in cell cycle and senescence regulation through two major tumor-suppressing pathways of retinoblastoma protein (RB) and p53 in the cell. Fluorescence in situ hybridization (FISH) of CDKN2A would be useful for the diagnosis of mesothelioma because this analysis could differentiate pleural mesothelioma cells from reactive mesothelial cells [35, 36]. Accumulating information shows that the homozygous deletion of CDKN2A is a predictor of poor survival [37].
The NF2 (Neurofibromin 2) gene responsible for neurofibromatosis type 2 familial cancer syndrome was shown to be the target gene of 22q12 loss. This gene is inactivated by homozygous deletion or heterozygous deletion/point mutation in a total of 40–50% of mesotheliomas [38, 39]. NF2 protein acts upstream of SAV1, LATS1/2, and yes-associated protein (YAP) in the Hippo tumor suppressor pathway. In addition to NF2 inactivation, deletions/mutations in SAV1 and LATS2 genes are found in mesothelioma [40]. Hippo tumor suppressor pathway plays a vital role in controlling proper organ sizes, cell contact inhibition, stem cell function, and regeneration. Studies with this pathway would hide the possibility of causing a new therapeutic strategy.
7 Fusion Transcripts, Altered Splicing, MicroRNA
Transcriptome analysis by next-generating sequencing (n = 211) showed fusion transcripts involving tumor suppressor genes in mesothelioma: 13 fusions in NF2, 7 in BAP1, 8 in SETD2, 7 in PBRM1, 2 in PTEN, and 6 in others [41]. The reports on fusion transcripts in mesothelioma have been accumulating [42, 43], but the gene pairs of fusion and the braking-region of these transcripts were different among patients with mesothelioma. Then the detection of fusion transcripts has not yet to be exploited as a diagnostic tool. Many of these fusions and aberrant splicing variants are derived from the genes in chromosomes 3p21, 9p21.3, 13q12, and 22q12, frequently deleted regions in mesothelioma. These gene regions might be fragmented by chromothripsis and lead to extensive rearrangements causing fusion genes or aberrant splicing variants. In addition, the mutation of the SF3B1 gene, encoding subunit 1 of the splicing factor 3b protein complex, was found at ~2% of frequency (4/216) [41] and the mutations in this splicing factor gene were associated with specific alterations in mRNA splicing.
Because mutations in the genes encoding proteins associated with histone modification and chromatin remodeling, including BAP1, SETD2, and PBRM1, occur predominantly in mesothelioma, diverse gene expression changes induced by aberrant epigenetic regulation are estimated. Most of the deregulated genes in mesothelioma belong to the following pathways: angiogenesis, cell adhesion, p53 signaling, integrin signaling, MAPK signaling, apoptosis, and cell cycle regulation [44]. A special set of genes could differentiate mesothelioma from others. The set of 26 genes could distinguish pleural mesothelioma from others, normal pleura, sarcomas, renal cell carcinoma, and thymoma, with high sensitivity and specificity [45]. It was also reported that two gene sets, one including 22 genes and the other 40 genes, narrowed down from 117 genes selected from previous reports could be discriminate malignant from benign pleural proliferations [44].
MicroRNAs (miRNAs) are short noncoding RNAs of approximately 18–22 nucleotides in length, which function as posttranscriptional regulators of gene expression. It is known that miRNA expression is dysregulated in human cancer through various mechanisms, including amplification or deletion of miRNA genes, abnormal transcriptional control of miRNAs, dysregulated epigenetic changes, and defects on biogenesis components. MiR-31 expression was shown to be reduced in mesotheliomas in most cases via deletion combined with the CDKN2A gene at 9p21.3. MiR-34b and miR-34c, sharing a common primary transcript, were silenced by methylation in the majority (85%) of mesothelioma tumors. The miR-15/16 family has also been shown to be significantly downregulated in mesothelioma compared with those from normal pleura. MiR-193a-3p and the miR-200 family showed a statistically significant down-expression in mesothelioma tumors compared to normal pleura. The miRNAs including let-7 and miR-21 have been reported several times from different groups. These findings are reviewed in [46]. MiRNA mimics are small, double-stranded RNA molecules, designed to mimic endogenous mature miRNA molecules when transfected into cells. In order to deliver miRNAs, the minicells, known as EDVTMnanocells (EDVs) derived from asymmetric bacterial cell division were used. The therapy, dubbed TargomiRs, comprises patented miRNA mimics based on the miR-15/107 consensus sequences, packaged in EDVs that are targeted with an anti-EGFR-specific antibody. The trial was designed to test TargomiRs in patients with pleural MM or advanced NSCLC (ClinicalTrials.gov Identifier: NCT02369198). The drug showed early signs of activity [47].
Comprehensive molecular profiling, including exome sequencing, copy-number arrays, mRNA sequencing, noncoding RNA profiling, DNA methylation, and reverse-phase protein arrays, identified four distinct integrated subtypes of mesothelioma [48]. The results of the study (summarized in Table 12.2) indicate that survival was significantly different across the 4 clusters (P < 0.0001) [48]. Cases in the poor-prognosis subset showed higher AURKA mRNA expression and upregulation of the PI3K and mTOR signaling pathways. This study showed a strong expression of the immune-checkpoint gene VISTA in epithelioid pleural mesothelioma. These new findings integrated into the biology of mesothelioma could lead to new therapeutic strategies.
8 Conclusions
Since the discovery of BAP1 as a predisposition gene to mesothelioma and a number of other different cancers, grouped in the BAP1 cancer syndrome, numerous germline analyses were performed in patients with mesothelioma and in subjects individuals who have experienced environmental or occupational exposure to carcinogenic fibers and are therefore at high risk of developing mesothelioma. The knowledge of the molecular mechanisms underlying the pathogenesis of malignant mesothelioma will benefit from the future results of further studies required to complete the information on the prevalence of germline and somatic variants present in cancer susceptibility genes.
References
Franke K, Paustenbach D. Government and navy knowledge regarding health hazards of asbestos: a state of the science evaluation (1900 to 1970). Inhal Toxicol. 2011;23(Suppl 3):1–20. https://doi.org/10.3109/08958378.2011.643417.
Sluis-Cremer GK, Liddell FD, Logan WP, Bezuidenhout BN. The mortality of amphibole miners in South Africa, 1946–80. Br J Ind Med. 1992;49(8):566–75.
Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J Occup Med. 1992;34(7):718–21.
Linton A, Vardy J, Clarke S, van Zandwijk N. The ticking time-bomb of asbestos: its insidious role in the development of malignant mesothelioma. Crit Rev Oncol Hematol. 2012;84(2):200–12. https://doi.org/10.1016/j.critrevonc.2012.03.001.
Carbone M, Adusumilli PS, Alexander HRJ, Baas P, Bardelli F, Bononi A, et al. Mesothelioma: scientific clues for prevention, diagnosis, and therapy. CA Cancer J Clin. 2019;69:402–29. https://doi.org/10.3322/caac.21572.
Baumann F, Ambrosi JP, Carbone M. Asbestos is not just asbestos: an unrecognised health hazard. Lancet Oncol. 2013;14(7):576–8. https://doi.org/10.1016/S1470-2045(13)70257-2.
Yang H, Rivera Z, Jube S, Nasu M, Bertino P, Goparaju C, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci U S A. 2010;107(28):12611–6. https://doi.org/10.1073/pnas.1006542107.
Carbone M, Yang H. Mesothelioma: recent highlights. Ann Transl Med. 2017;5(11):238. https://doi.org/10.21037/atm.2017.04.29.
Carbone M, Emri S, Dogan AU, Steele I, Tuncer M, Pass HI, et al. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer. 2007;7(2):147–54. https://doi.org/10.1038/nrc2068.
Roushdy-Hammady I, Siegel J, Emri S, Testa JR, Carbone M. Genetic-susceptibility factor and malignant mesothelioma in the Cappadocian region of Turkey. Lancet. 2001;357(9254):444–5. https://doi.org/10.1016/S0140-6736(00)04013-7.
Carbone M, Flores EG, Emi M, Johnson TA, Tsunoda T, Behner D, et al. Combined genetic and genealogic studies uncover a large BAP1 Cancer syndrome kindred tracing Back nine generations to a common ancestor from the 1700s. PLoS Genet. 2015;11(12):e1005633. https://doi.org/10.1371/journal.pgen.1005633.
Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G. BAP1 and cancer. Nat Rev Cancer. 2013;13(3):153–9.
Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16(9):1097–112.
Ventii KH, Devi NS, Friedrich KL, Chernova TA, Tighiouart M, Van Meir EG, et al. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 2008;68(17):6953–62. https://doi.org/10.1158/0008-5472.CAN-08-0365.
Betti M, Aspesi A, Ferrante D, Sculco M, Righi L, Mirabelli D, et al. Sensitivity to asbestos is increased in patients with mesothelioma and pathogenic germline variants in BAP1 or other DNA repair genes. Genes Chromosomes Cancer. 2018;57(11):573–83. https://doi.org/10.1002/gcc.22670.
Betti M, Casalone E, Ferrante D, Aspesi A, Morleo G, Biasi A, et al. Germline mutations in DNA repair genes predispose asbestos-exposed patients to malignant pleural mesothelioma. Cancer Lett. 2017;405:38–45. https://doi.org/10.1016/j.canlet.2017.06.028.
Pastorino S, Yoshikawa Y, Pass HI, Emi M, Nasu M, Pagano I, et al. A subset of mesotheliomas with improved survival occurring in carriers of BAP1 and other Germline mutations. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36:3485–94. https://doi.org/10.1200/jco.2018.79.0352.
Panou V, Gadiraju M, Wolin A, Weipert CM, Skarda E, Husain AN, et al. Frequency of Germline mutations in cancer susceptibility genes in malignant mesothelioma. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(28):2863–71. https://doi.org/10.1200/jco.2018.78.5204.
Hassan R, Morrow B, Thomas A, Walsh T, Lee MK, Gulsuner S, et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc Natl Acad Sci U S A. 2019; https://doi.org/10.1073/pnas.1821510116.
Bertelsen B, Tuxen IV, Yde CW, Gabrielaite M, Torp MH, Kinalis S, et al. High frequency of pathogenic germline variants within homologous recombination repair in patients with advanced cancer. NPJ Genom Med. 2019;4:13. https://doi.org/10.1038/s41525-019-0087-6.
Flores RM. Induction chemotherapy, extrapleural pneumonectomy, and radiotherapy in the treatment of malignant pleural mesothelioma: the memorial Sloan-Kettering experience. Lung Cancer. 2005;49(Suppl 1):S71–4. https://doi.org/10.1016/j.lungcan.2005.03.015.
Nasu M, Emi M, Pastorino S, Tanji M, Powers A, Luk H, et al. High incidence of somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol. 2015;10(4):565–76. https://doi.org/10.1097/JTO.0000000000000471.
Yoshikawa Y, Sato A, Tsujimura T, Emi M, Morinaga T, Fukuoka K, et al. Frequent inactivation of the BAP1 gene in epithelioid-type malignant mesothelioma. Cancer Sci. 2012;103(5):868–74. https://doi.org/10.1111/j.1349-7006.2012.02223.x.
Guo G, Chmielecki J, Goparaju C, Heguy A, Dolgalev I, Carbone M, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res. 2015;75(2):264–9. https://doi.org/10.1158/0008-5472.can-14-1008.
Lo Iacono M, Monica V, Righi L, Grosso F, Libener R, Vatrano S, et al. Targeted next-generation sequencing of cancer genes in advanced stage malignant pleural mesothelioma: a retrospective study. J Thorac Oncol. 2015;10(3):492–9. https://doi.org/10.1097/jto.0000000000000436.
Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43(7):668–72. https://doi.org/10.1038/ng.855.
Eckel-Passow JE, Serie DJ, Cheville JC, Ho TH, Kapur P, Brugarolas J, et al. BAP1 and PBRM1 in metastatic clear cell renal cell carcinoma: tumor heterogeneity and concordance with paired primary tumor. BMC Urol. 2017;17(1):19. https://doi.org/10.1186/s12894-017-0209-3.
Chirac P, Maillet D, Lepretre F, Isaac S, Glehen O, Figeac M, et al. Genomic copy number alterations in 33 malignant peritoneal mesothelioma analyzed by comparative genomic hybridization array. Hum Pathol. 2016;55:72–82. https://doi.org/10.1016/j.humpath.2016.04.015.
Yoshikawa Y, Emi M, Hashimoto-Tamaoki T, Ohmuraya M, Sato A, Tsujimura T, et al. High-density array-CGH with targeted NGS unmask multiple noncontiguous minute deletions on chromosome 3p21 in mesothelioma. Proc Natl Acad Sci U S A. 2016;113(47):13432–7. https://doi.org/10.1073/pnas.1612074113.
Ly P, Cleveland DW. Rebuilding chromosomes after catastrophe: emerging mechanisms of chromothripsis. Trends Cell Biol. 2017;27(12):917–30. https://doi.org/10.1016/j.tcb.2017.08.005.
Mansfield AS, Peikert T, Smadbeck JB, Udell JBM, Garcia-Rivera E, Elsbernd L, et al. Neoantigenic potential of complex chromosomal rearrangements in mesothelioma. J Thorac Oncol. 2019;14(2):276–87. https://doi.org/10.1016/j.jtho.2018.10.001.
Bjorkqvist AM, Tammilehto L, Anttila S, Mattson K, Knuutila S. Recurrent DNA copy number changes in 1q, 4q, 6q, 9p, 13q, 14q and 22q detected by comparative genomic hybridization in malignant mesothelioma. Br J Cancer. 1997;75(4):523–7. https://doi.org/10.1038/bjc.1997.91.
Hagemeijer A, Versnel MA, Van Drunen E, Moret M, Bouts MJ, van der Kwast TH, et al. Cytogenetic analysis of malignant mesothelioma. Cancer Genet Cytogenet. 1990;47(1):1–28. https://doi.org/10.1016/0165-4608(90)90258-c.
Taguchi T, Jhanwar SC, Siegfried JM, Keller SM, Testa JR. Recurrent deletions of specific chromosomal sites in 1p, 3p, 6q, and 9p in human malignant mesothelioma. Cancer Res. 1993;53(18):4349–55.
Hu Q, Akatsuka S, Yamashita Y, Ohara H, Nagai H, Okazaki Y, et al. Homozygous deletion of CDKN2A/2B is a hallmark of iron-induced high-grade rat mesothelioma. Lab Investig. 2010;90(3):360–73. https://doi.org/10.1038/labinvest.2009.140.
Nabeshima K, Matsumoto S, Hamasaki M, Hida T, Kamei T, Hiroshima K, et al. Use of p16 FISH for differential diagnosis of mesothelioma in smear preparations. Diagn Cytopathol. 2016;44(9):774–80. https://doi.org/10.1002/dc.23501.
Hamasaki M, Matsumoto S, Abe S, Hamatake D, Kamei T, Hiroshima K, et al. Low homozygous/high heterozygous deletion status by p16 FISH correlates with a better prognostic group than high homozygous deletion status in malignant pleural mesothelioma. Lung Cancer. 2016;99:155–61. https://doi.org/10.1016/j.lungcan.2016.07.011.
Bianchi AB, Mitsunaga SI, Cheng JQ, Klein WM, Jhanwar SC, Seizinger B, et al. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci U S A. 1995;92(24):10854–8. https://doi.org/10.1073/pnas.92.24.10854.
Sekido Y, Pass HI, Bader S, Mew DJ, Christman MF, Gazdar AF, et al. Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res. 1995;55(6):1227–31.
Murakami H, Mizuno T, Taniguchi T, Fujii M, Ishiguro F, Fukui T, et al. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 2011;71(3):873–83. https://doi.org/10.1158/0008-5472.CAN-10-2164.
Bueno R, Stawiski EW, Goldstein LD, Durinck S, De Rienzo A, Modrusan Z, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48(4):407–16. https://doi.org/10.1038/ng.3520.
Panagopoulos I, Thorsen J, Gorunova L, Haugom L, Bjerkehagen B, Davidson B, et al. Fusion of the ZC3H7B and BCOR genes in endometrial stromal sarcomas carrying an X;22-translocation. Genes Chromosomes Cancer. 2013;52(7):610–8. https://doi.org/10.1002/gcc.22057.
Hung YP, Dong F, Watkins JC, Nardi V, Bueno R, Dal Cin P, et al. Identification of ALK rearrangements in malignant peritoneal mesothelioma. JAMA Oncol. 2018;4(2):235–8. https://doi.org/10.1001/jamaoncol.2017.2918.
Bruno R, Ali G, Giannini R, Proietti A, Lucchi M, Chella A, et al. Malignant pleural mesothelioma and mesothelial hyperplasia: a new molecular tool for the differential diagnosis. Oncotarget. 2017;8(2):2758–70. https://doi.org/10.18632/oncotarget.13174.
De Rienzo A, Richards WG, Yeap BY, Coleman MH, Sugarbaker PE, Chirieac LR, et al. Sequential binary gene ratio tests define a novel molecular diagnostic strategy for malignant pleural mesothelioma. Clin Cancer Res. 2013;19(9):2493–502. https://doi.org/10.1158/1078-0432.CCR-12-2117.
Lo Russo G, Tessari A, Capece M, Galli G, de Braud F, Garassino MC, et al. MicroRNAs for the diagnosis and management of malignant pleural mesothelioma: a literature review. Front Oncol. 2018;8:650. https://doi.org/10.3389/fonc.2018.00650.
van Zandwijk N, Pavlakis N, Kao SC, Linton A, Boyer MJ, Clarke S, et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 2017;18(10):1386–96. https://doi.org/10.1016/S1470-2045(17)30621-6.
Hmeljak J, Sanchez-Vega F, Hoadley KA, Shih J, Stewart C, Heiman D, et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov. 2018;8(12):1548–65. https://doi.org/10.1158/2159-8290.Cd-18-0804.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Emi, M., Gaudino, G., Yoshikawa, Y., Ohmuraya, M. (2021). Recent Advances in the Genomic and Proteomic Researches on Mesothelioma: What Are Novel Insights into Mesothelioma Biology?. In: Nakano, T., Kijima, T. (eds) Malignant Pleural Mesothelioma. Respiratory Disease Series: Diagnostic Tools and Disease Managements. Springer, Singapore. https://doi.org/10.1007/978-981-15-9158-7_12
Download citation
DOI: https://doi.org/10.1007/978-981-15-9158-7_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9157-0
Online ISBN: 978-981-15-9158-7
eBook Packages: MedicineMedicine (R0)