Abstract
The concept of wastewater treatment is generally to allow anthropoid and industrial effluents to be inclined of without hazard to human health or impermissible harm to the natural environment. Conventional wastewater treatment is a combination of physical, chemical, and biological processes to remove suspended solids, dissolved solids, biological decomposition of organic matter, and nutrients from wastewater. Broadly, there are various degrees of treatment in sequence to increase the treatment level, which are preliminary, primary, secondary, and tertiary and/or advanced wastewater treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Water is an inorganic, colorless chemical substance. It is available in three states, that is, liquid, solid, and gaseous, and is also a major constituent of our Earth’s hydrosphere. It is also an essential fluid of most of the living forms. It covers almost 70% of Earth’s surface (Gleick 1993). It is estimated that about 97% of the total water is in the oceans and inland seas with giant salt content. This water is insufficient to anthropoid consumption. Around 2% of water is found in glaciers and ice caps. Only 1% of water on this Earth is available for human consumption as ground water, lakes, rivers, and soil moisture (Satyanarayana 2008). Day by day, human need for water is increasing due to which availability of water is becoming scarce.
Water is an essential nutrient on earth. It is absolutely essential for the existence of life (animal and plant). Life does not exist without water. It is needed for multiple purposes such as cooking, bathing, washing, and other human activities. It should be noted, water which we consume and use for additional purposes should be clean, that is, it must be free from germs and other harmful chemicals. Water that is safe for drink or to use for food preparation is called as potable water. Water that is not safe for drinking is called as non-potable water whose consumption leads to many infectious diseases (Griffiths et al. 1991).
Water that has been polluted by human use can be considered wastewater. Water is used for many purposes like domestic, industrial, commercial, and agricultural activities of mankind. Wastewater carries any kind of physical, chemical, and biological pollutants (Tilley et al. 2014).
2 Types of Wastewater
The characteristics of wastewater differ depending upon the source and release. The major wastewater contaminants and their significance are shown in Table 17.1.
Types of wastewater include the following:
-
Domestic wastewater (released from households).
-
Municipal wastewater (released from communities).
-
Industrial wastewater (released from industries) (Tilley et al. 2014).
The main aim of wastewater treatment is to eliminate suspended solids as much as possible and the remaining water, called effluent, is discharged back to the environment. This process can be termed as water reclamation as treated wastewater can be used for other purposes (Metcalf and Eddy 2003). The purpose of wastewater treatment is to remove suspended solids (less than 50 mg/L), biodegradable organic matter (BOD5 at 20 °C less than 30 mg/L), and to kill pathogens.
3 Process of Wastewater Treatment
There are four major steps involved in the wastewater treatment processes, which include preliminary treatment, primary treatment, secondary treatment, and tertiary or advanced treatment.
3.1 Preliminary Treatment
Preliminary treatment involves the removal of floating materials such as leaves, papers, wood pieces, sticks, tree branches, diapers, napkins, glass bottles, plastics, dead animals, and other heavy settleable inorganic solids such as sand, grit, fats, oils, grease substances, etc. There are some specified devices designed particularly for performing preliminary treatment including, screeners, grit chambers, and skimming tanks.
Screeners
Screening is an essential step in wastewater treatment in order to remove floating objects such as sticks, dead animals, wood pieces, tree branches, papers, clothes, diapers, napkins, glass bottles, plastic bottles, coconut shells, and other large-sized floating materials. It is the first step in treatment processes; screener is a device with a uniform size opening for removal of floating matter in the wastewater. Floating objects retained by screens are called screenings. Objects which are not removed clog and damage the pumps, valves, pipe lines, and other appurtenances. The screening equipment has parallel bars, rods gratings, wire meshes, or perforated plates, and the openings may be of any shape mostly of circular or rectangular depending upon the size (Srinivas 2008). Depending upon the size of opening, screeners are classified into course screens, medium screens, and fine screens. Design aspects of these screens are shown in Table 17.2.
Grit Chambers
Grit chambers are long, narrow tanks designed to slow down the flow so that solids such as sand, eggshells, coffee grounds, etc. will settle out of the water (Ambulkar and Nathanson 2019). The heavy inorganic materials like sand, ash, etc., are removed by grit chambers. This technique is based on the principle of sedimentation due to gravitational forces (Satyanarayana 2008). Grit causes excessive wear and tear on pumps and equipment. Its removal is particularly important in cities with combined sewer systems, which carry a good deal of silt, sand, and gravel that wash off streets or land during a storm (Ambulkar and Nathanson 2019). Grit chambers are usually located ahead of pumps or comminuting devices and, if mechanically cleaned, should be preceded by coarse bar rack screens.
Skimming Tanks
Skimming tank is arranged to remove several greasy and oily materials (fats, oils, waxes, soaps, etc.) from the sewage. These oily and greasy materials from domestic and industrial outlets are released into the sewage. A skimming tank is fitted with baffle walls that divide the tank. It is divided into three compartments that are interconnected. The compressed air is pushed from the floor of the tank, leading to the coagulation of raising air bubbles and solidification of the oily and greasy materials present in the sewage. This removed material is pushed to the side compartment, referred to as stilling compartment, and from there it is removed either manually or mechanically.
3.2 Primary Treatment
It is carried out majorly in order to remove the small-sized inorganic matter and large-sized settleable organic matter, 60–65% of suspended solids, and 30–35% of BODs. It is a physical process that basically includes sedimentation and coagulation.
Sedimentation
It is a physical treatment process using gravity to eliminate suspended solids from water. The primary purpose is to produce a clarified effluent, and it is the mostly used unit operation in wastewater treatment process. Sedimentation process is carried out twice: once before secondary treatment, called as primary sedimentation, and then after secondary treatment, called as secondary sedimentation. In this process, solid particles present in the sewage get settled down due to the gravitational force. Majority of the solid particles are organic compounds and maintain the suspended state in a flowing sewage. This flow of sewage is stopped and is stored in a tank known as sedimentation tank, facilitating the solid particles to settle down at the bottom. According to the tendency of particles, the process of settling is of four types: discrete settling, flocculant settling, zone or hindered settling, and compression.
-
1.
Discrete settling: The constant particles that do not change their size, shape, and weight are called as discrete or granular particles.
Example: Use of grit in sewage.
-
2.
Flocculant settling: The particles that change their size, shape, and weight losing their original identity are called as flocculant particles.
Example: Settling of bioflocs in secondary sedimentation tanks.
-
3.
Zone or hindered settling: The mass of these particles can settle as a unit or zone even after flocculation. The particles remain in a fixed position with respect to each other. In this process of settling, the congregation of particles increases from top to bottom resulting in thickening of sludge.
Example: Secondary sedimentation tank (activated sludge process).
-
4.
Compression: Settlement of particles in the lower layers occur by compressing the weight of the particles on the upper layers, which facilitates the thickening of the sludge at the bottom.
Example: Sludge thickening (wastewater treatment plant).
Several ways of classifying sedimentation tanks include the following:
-
Based on shape: Rectangular, circular, and square.
-
Based on flow of sewage: Longitudinal, vertical, radial, and spiral.
-
Based on purpose and position: Primary, secondary, coagulation-cum-sedimentation tanks, grit chambers, septic and Imhoff tanks.
-
Based on operation: Batch and continuous flow type.
Coagulation
It is a chemical process in which charged particles are destabilized by the addition of chemical agents. Every time it is not possible to remove the colloidal wastes in sewage by plain sedimentation. Sometimes it is mandatory to use chemical coagulants to help sedimentation. This procedure is termed as chemical precipitation or coagulation-aided sedimentation or coagulation. By using this technique, about 60–80% of the suspended particles can be easily removed. Types of chemical agents used in coagulation: coagulants and coagulant-aids.
-
1.
Coagulants: Coagulants (positively charged) are chemicals that form insoluble and gelatinous precipitates with the colloidal particles (negatively charged). Example: alum (aluminum sulfate), iron salts, lime and soda ash, sodium silicate, and sodium aluminate.
-
2.
Coagulant-aids: These chemicals facilitate the coagulation process. They enhance the action of coagulants and thus reducing the amount of sludge formed. Example: activated silica, weighting agents such as silica, powdered limestone, and polyelectrolytes (Ambulkar and Nathanson 2019).
3.3 Secondary Treatment
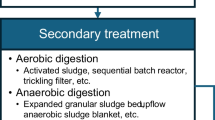
Secondary treatment is also known as biological treatment of wastewater. If the BOD/COD ≥0.6, then biological treatment can be applied for industrial wastewater. Secondary treatment is useful for the removal of 95% of suspended solids, 5 day BOD and decomposition of organic matter. Various types of microorganisms such as bacteria, algae, fungi, and protozoa are used for the decomposition of organic matter. For balance growth of microbes in a biological treatment, the ratios of BOD:N:P for aerobic systems and anaerobic systems are 100:5:1 and 100:2.5:0.5. Microbes can feed on the unstable organic matter and decomposed to solid inorganic forms. Secondary treatment or biological treatment processes of wastewater are broadly classified as aerobic, anaerobic and pond processes shown in Fig. 17.1. Depending upon the nature and use of microorganisms, the biological processes are further classified as “suspended growth systems” and “attached growth systems” (Satyanarayana 2008).
3.3.1 Aerobic Process
Aerobic treatment of wastewater requires oxygen to break down organic contaminants and other pollutants such as nitrogen and phosphorous. It is further classified into two sub categories.
(a) Aerobic suspended growth treatment: There are many methods involved under this treatment process. The most important suspended growth biological systems used for this treatment are below:
-
1.
Activated sludge process
-
2.
Aerated lagoons
-
3.
Sequencing batch reactor
-
4.
Aerobic digestion
(1) Activated sludge process: This process is considered to be the most used modern biological treatment process. Aeration of the sewage containing organic matter with microbes is carried out in an aeration tank with the help of a mechanical aerator. The contents of the reactor are called as “mixed liquor” (Satyanarayana 2008). The microorganisms during the onset of favorable aerobic conditions metabolize the suspended particulate matter. Microbes utilize a portion of the organic matter and synthesize new bacterial cells, and the remaining of the organic matter gets converted into CO2 (carbon dioxide) and H2O (water). These newly formed bacterial cells aggregate together to form flocs, which is technically called as sludge. Now this separated sludge loses connection with the organic matter and becomes activated. This activated sludge is segregated from the settling tank to aeration tank and recycled. This recycled activated sludge serves as an inoculum, whereas the excess sludge gets eliminated (Fig. 17.2). In order to carry out this activated sludge process in an efficient way, a continuous oxygen supply needs to be maintained. This is achieved either by using rotating paddles or through mechanical aeration. The growth of protozoa is an indicator of activated sludge performance. The waste sludge that is eliminated can be used as a fertilizer in various agricultural lands. The activated sludge process can be categorized as conventional activated sludge process and modified activated sludge process.
Conventional activated sludge process
In this process, a separation tank (settling or sedimentation tank) and sludge removal line are required. Following the primary treatment, the sewage is released into head of the tank, and oxygen is supplied throughout the tank uniformly.
Modified activated sludge process
In this process, several modifications have been done to improve aeration efficiency. Aeration can be provided by step aeration, tapered aeration, high rate aeration by complete mixing, and extended aeration.
Factors affecting the activated sludge process:
-
Type of reactor
-
Inadequate air supply in aeration tank
-
Food/microorganism ratio
-
Nutrients
-
Sludge recirculation rate
-
Low pH value
-
Temperature
-
Prolonged detention of sludge in secondary settling tank
-
Industrial sewage which favors the growth of filamentous fungi
Advantages:
-
It is a low cost, very compact, and an efficient biological sewage treatment process.
-
Under ideal conditions, 95% of BOD, 98% of bacteria, and 95% of suspended organic matter can be removed through this process.
-
Even the waste sludge can be used as an effective fertilizer in many agricultural lands.
Disadvantages:
-
Large amount of sludge production becomes difficult to handle sometimes.
-
Require high amount of power supply.
(2) Aerated lagoons: These are also called as aerated ponds. It is simply a pond with artificial aeration. In these aerated lagoons, surface aerators are inaugurate. These aerators can control the bad odors released due to the organic materials. Continuous nitrification can be possibly carried out in these aerated lagoons depending upon the temperature. The microbial treatment of this process is compared to activated sludge process. It has the large surface area when compared to the activated sludge process and is more susceptible to temperature effects (Satyanarayana 2008). The aerated lagoons can be divided as facultative aerated lagoons and aerobic aerated lagoons.
Facultative aerated lagoons
In such type of lagoons, the lower part is feasibly anaerobic, whereas the top layers are aerobic. Hence, these are termed as facultative aerated lagoons. In these, few solids depart from the effluent stream, whereas some solids settle down in the lagoon due to insufficient aeration power input. These are also known as partially mixed type aerated lagoons as they can be operated at low rate of aeration.
Aerobic aerated lagoons
These are entirely oxygenic from top to bottom. In these lagoons, the aeration power input is sufficient to keep all the solids of the suspension. There is no settling of solids in these lagoons, and parallelly new solids are formed in this system are equivalent to the number of solids leaving. Further treatment is provided after these lagoons in order to lower the concentration of solids in the effluent (Oliveira and von Sperling 2011).
Advantages of aerated lagoons
-
These are simple in operating as the aerator is the only moving piece of the equipment.
-
These are majorly used for treating industrial wastes.
-
They require only 5–10% as much land as stabilization ponds.
Disadvantages:
-
Slighter competent in cold climates.
-
If not properly maintained, they supply a birth site area for mosquitoes and other insects.
(3) Sequencing batch reactors: In this process, aeration and sedimentation processes are implemented one by one respectively in the same tank. Simply, it is a modified activated sludge system as activated sludge processes both aeration and sedimentation are carried out in separate tanks. Hence, it is a fill and draw activated sludge process (Satyanarayana 2008). These reactors treat the sewage in batches. Oxygen is sent in the form of bubbles through the mixture of wastewater and activated sludge in sequence to eliminate the organic matter. Effluent can be discharged into surface waters after the treatment. This treatment can be carried out in various stages as follows:
-
Filling—This is the first stage; during this stage the inlet valve is opened and the tank gets filled in followed by mechanical mixing. As this stage does not involve air, it is said to be anoxic.
-
Aerating or reacting—This is the second stage; at this stage the above mixed liquor is aerated through mechanical pumps or fine bubbles.
-
Settling or sedimentation—This is the third stage; the above aerated liquor is allowed to be maintained in an undisturbed state so that the suspended solids starts to settle.
-
Decanting—This is the fourth stage; now the outlet valve is opened, through which effluent is discharged out.
-
Idle—This is the final stage; the excess sludge can be removed (Satinder Ahuja et al. 2014).
Advantages:
-
The effluent produced during the process contains low organic compounds.
-
It can effectively remove nutrients such as nitrogen and phosphorous (Satinder Ahuja et al. 2014).
Disadvantages:
-
It requires a skilled person for carry out this process.
-
This process can eliminate some pathogens (Ghodeif 2013).
(4) Aerobic digestion: In this process of sewage treatment, the volume of sludge is reduced so that it can be further used (Water Environment Federation 2008). The organic sludge produced in the previously discussed systems are undergone aerobic digestion in a specific reactor called aerobic digester (Satyanarayana 2008). It is usually a batch process. Air is sent through the tank followed by constant stirring as to keep the contents assorted. Gases such as carbon dioxide and hydrogen sulfide are given off as they are required to be reduced in odors. Continuous digestion is carried out until the degradable solids percentage fall between 20% and 10% according to local conditions. The non-sewage waste can be further processed by removing wastes like food, cardboard, etc. (Water Environment Federation 2008).
Advantages:
-
It is a less time-consuming process.
-
This process is less complex when compared to anaerobic digestion.
Disadvantages:
-
As blowers and pumps are used for the supply of oxygen, their operating costs are beyond anaerobic digestion.
-
Lower energy yield when compared to anaerobic digestion (Water Environment Federation 2008).
(b) Aerobic attached growth treatment: These are the processes mostly used for the elimination of organic matter present in the wastewater or sewage. These processes can also favor nitrification. There are many processes involved under this treatment, which include the following:
-
1.
Trickling filters
-
2.
Roughing filters
-
3.
Rotating biological contactors
-
4.
Packed bed reactors
(1) Trickling filters: Trickling filters are mostly used for the secondary treatment of wastewater released from the domestic sources such as households and industrial sewage. They are also called as sprinkling filters (Satyanarayana 2008). A typical trickling filter unit has been fixed with a bed of rocks (porous media), coke, gravel, peat moss, etc. Wastewater is allowed to pass through by spraying (Fig. 17.3). The sewage flows downward as spray and due to which a microbial slime grows and covers the bed of the media (Parker et al. 1989). This slime rich in microbes such as Pseudomonas, Alcaligenes, yeasts, and fungi (Satyanarayana 2008). The whole unit is maintained in a perfect aerobic condition through splashing and diffusion, etc. (Parker et al. 1989). As the sewage is allowed to pass through the newly formed microbial slime layer, organic matter used by the microbes. Microbes present on the upper layer of the film carry out the process of oxidation. Accordingly, with the time, the biofilm raise its width and settles at the bottom of the tank (Satyanarayana 2008).
Based on organic loading, trickling filters are classified into
-
Low-rate trickling filters: These are mostly used for the treatment of domestic sewage. These are simple units that can produce a consistent effluent quality independent of the influent strength. There is no recirculation of the effluent. It can remove up to 80–85% of the applied BOD (Parker et al. 1989).
-
High-rate trickling filters: High-rate trickling filters used for treating industrial waste. Hence, the organic loading per unit area in the filter can be increased due to which flow velocity increases causing continuous sloughing of excess growth. It can remove 65–85% of BOD from the sewage (Parker et al. 1989).
-
Super-rate trickling filters: These are also useful for the industrial waste treatment (Satyanarayana 2008).
Factors Affecting the Trickling Filters:
-
Type of media
-
Organic loading
-
Recirculation rate
-
Flow distribution
-
Filter staging
Advantages:
-
These are simple and operated at low costs.
-
Effectively works in hot climate.
Disadvantages:
-
Excess sludge should be disposed of necessarily.
-
Raw sewage cannot be handled by these filters until unless primary sedimentation is done (Satyanarayana 2008).
(2) Roughing filters: These are the filters that are mostly used to reduce the suspended solid organic matter from the wastewater. They not only reduce the pathogens but also reduce the amount of iron and manganese in the wastewater (Nkwonta and Ochieng 2009). They usually operate in downstream processing; if the organic loading is very high, then there will be a continuous shedding of the microbial slime. The planning of the treatment process is increased by recycling the unsettled effluent (Satyanarayana 2008). Based on flow of directions, roughing filters can be classified as follows:
-
Downflow roughing filters: Filters have downward flow direction; type of filter medium is sand.
-
Upflow roughing filters: Filters have upward flow direction; type of filter medium is gravel.
-
Horizontal flow roughing filters: Filters have horizontal flow direction; type of filter medium used in this kind of filter is coconut husk fiber. Among three types, upflow roughing filters are most widely used as they are cheap and easily maintained (Nkwonta and Ochieng 2009).
(3) Rotating biological contactors (RBC): It is the most important system used for the treatment of municipal wastewater or used for the bioprocessing of industrial wastes like vegetable industry. RBC’s recent devices work on the principle of aerobic attached growth system performed on the moving or rotating media (Satyanarayana 2008). This process is executed after the completion of primary treatment; flowing wastewater comes in contact with biological film or contactor. During this contact, the pollutants present in the wastewater are eliminated; now water free from pollutants is released into the environment, which can be reused (Fujie et al. 1983).
A typical RBC consists of some parallel discs that are closely spaced and are placed on a rotating shaft (Fig. 17.4). As the wastewater is allowed to flow through this RBC, the organic matter present in that water is degraded by the microbes that grow on these parallel discs (Fujie et al. 1983). The rotating bio discs are generally made up of polystyrene or polyvinylchloride (PVC). The shaft aligned with these discs is allowed to rotate at slow speed. About 40% of the discs are submerged in water. The microbial growth gets attached to the disc, and a biofilm is formed as a result. This bio disc contacts the wastewater with the atmospheric air and carries out oxidation in its rotating motion. Aeration for this system is provided by the rotating motion of the discs due to which the media get exposed to the air after the contact with wastewater, and hence pollutants get eliminated (Fujie et al. 1983).
Factors Affecting RBC (Satyanarayana 2008):
-
Rotating speed of the shaft
-
Temperature
-
Wastewater retention time
-
Submergence of disc
-
Organic loading
Advantages (Satyanarayana 2008):
-
This process is very effective because it can remove about 90% of BOD.
-
Low operating costs.
Disadvantages:
-
Frequent occurrence of odor.
-
Sludge disposal becomes difficult.
(4) Packed bed reactors: These are also called as fluidized bed reactors. They not only serve the purpose of removing BOD but also help in the nitrification. A specific reactor is used in this system, which is filled or packed with a medium (Fig. 17.5). Aeration is induced from the bottom of reactor. When the sewage is allowed to pass through this reactor, the microbes get freely attached on to the medium, thereby forming a biofilm. This formed biofilm removes the suspended organic matter from the sewage by feeding on that organic matter.
3.3.2 Anaerobic Process
Anaerobic process does not require oxygen, that is, decomposition of organic matter takes place anaerobically, which leads to formation of end products such as methane and carbon dioxide. It is further classified into two subcategories.
(a) Anaerobic suspended growth treatment: Suspended processes occur in a specialized airtight reactor, called an aerobic digester. Sludge is introduced continuously/intermittently. Mostly used systems under this treatment are
-
1.
Anaerobic digestion
-
2.
Anaerobic contact digesters
-
3.
Upflow anaerobic sludge blankets (UASB)
(1) Anaerobic digestion: Anaerobic digestion is mostly used to treat industrial sewage. Anaerobic digesters are classified into two types, standard-rate digester and the high-rate digester. Digesters are basically designed to treat high-strength organic wastes. The standard-rate digester reacts without any external heat supply; due to this reaction, time gets prolonged and detention time will be increased. Whereas in high-rate digester, heat is supplied including stirring, which will reduce the detention time. Depending upon the condition, both reactors are arranged in a combined set up to be used. Such process is termed as a two-stage system (Ebrahimi and Najafpour 2016). The process of anaerobic digestion are of three steps.
Hydrolysis: It is an enzyme-catalyzed reaction, in which high-molecular weight compounds (polysaccharides, proteins, lipids, and nucleic acids) are converted to low-molecular weight compounds (monosaccharides, amino acids, fatty acids, purines, and pyrimidines). These compounds serve as substrates for microbial growth and energy supply.
Acidogenesis: The low-molecular weight compounds are further changed into acidic products (lactate, butyrate, and propionate).
Methanogenesis: It is the final stage that involves the production of methane and carbon dioxide from the intermediates formed in the acidogenesis.
Microorganisms to degrade organic matter of sludge or sewage: Consortium of anaerobic microorganisms associated together for degradation of sludge or sewage. It is categorized into two types:
-
Acidogenic bacteria: These are also called as acidogens. Various types of acidogenic bacteria such as Clostridium sp, Corynebacterium sp, Lactobacillus sp, Actinomyces sp, staphylococcus sp, Peptococcus sp, and Escherichia coli convert low-molecular weight compounds into acidic forms.
-
Methanogenic bacteria: These are also called as methanogens and are accountable for converting acid and hydrogen into methane and carbon dioxide, for example, methanobacterium, methanobacillus, methanococcus, and methanosarcina (Ebrahimi and Najafpour 2016).
(2) Anaerobic contact digesters: An anaerobic contact process that is mostly used to treat industrial waste containing high BOD. This process is carried out in a set of reactors that are sequenced in a series. In these reactors, the recycled sludge with the sewage is pumped and then digested under anaerobic conditions. After this, two layers get separated by the use of a clarifier, and the formed supernatant is discharged while sludge is settled and again set for recycling (Satyanarayana 2008).
(3) Upflow anaerobic sludge blanket: These digesters are mainly used to overcome the problems caused: filter clogging, low loading rate difficulty, etc. About 200 operating plants were present worldwide. These are mostly used to treat food processing wastes, sugar beet waste, brewery waste, winery waste, etc. (S N Jogdand 2010). It is a strict anaerobic process and forms a blanket of granular sludge. This granular sludge can be processed further by anaerobic microorganisms (Tilley et al. 2014). It is basically a three-phase separator that enables to separate solid (sludge), liquid (water), and gas (methane) under high turbulence conditions (Fig. 17.6). Methane gas formed can be separated through multiple gas hoods of UASB. During treatment, substrate is enabled to pass through an expanded sludge bed that consists of biomass concentration at a very high concentration. Later the remaining substrate is allowed to pass through a less dense biomass called sludge blanket (Tilley et al. 2014). The influent is introduced from the bottom of the digester. Its level increases upward and meets with the biomass in the sludge bed. The movement of the influent continues upwards, and the remaining substrate acts with biomass present in sludge blanket. To ensure the quality of effluent, the volume of sludge blanket must be maintained at sufficient quantity. After the three-phase separator, the sludge blanket will separate solid particles from the mixture and set a way out for liquid and gas through which they leave the UASB reactor. Now the treated waste was discharged into water bodies, and gas was collected and reused as a biofuel (Tilley et al. 2014).
(b) Anaerobic attached growth process: Attached growth system is a process in which microbes treating the wastes are attached to the media in a reactor. Anaerobic attached growth treatment mainly involves
-
1.
Anaerobic filter process
-
2.
Expanded bed process
(1) Anaerobic filter process: Anaerobic filter process takes place in a special anaerobic digestion tank that consists of filter medium on to which anaerobic microbes attached and establishes themselves. In this process, wastewater flow upwards through the filter media; organic matter present in the wastewater is degraded by active anaerobic microbial population attached to the filter media (Satyanarayana 2008).
(2) Expanded bed process: Reaction occurs in a reactor that consists of a tank filled with a bed of sand, coal, or other aggregates. Bed acts as a medium on to which anaerobic microbes attach and form a biofilm. Wastewater or influent is pumped into the reactor and passes through the bed. As the influent comes into contact with biofilm, the organic matter from the influent is degraded by the anaerobes on the bed. The effluent is again recycled for maintaining the flow rate (Satyanarayana 2008).
3.3.3 Pond Treatment Processes
Pond treatment is a biological process used for treatment of sewage. This process is carried out in special ponds constructed by human in order to reduce the organic waste and remove pathogens from wastewater by using both bacteria and algae (Satyanarayana 2008). These ponds are usually large, shallow basins and are called as stabilization ponds, frequently used for industrial effluents or municipal water treatment. Influent enters one side of the stabilization pond for treatment and exits from other side as effluent after treatment. After treatment, it can be introduced into water bodies or can be used for irrigation purposes. It is a time-consuming process as it is a naturally occurring one. Pond treatment may involve either single pond or a series of ponds in a system each having individual roles in pathogen removal (Ho et al. 2017).
Various types of stabilization ponds:
-
(a)
Aerobic ponds
-
(b)
Anaerobic ponds
-
(c)
Facultative ponds
(a) Aerobic ponds: Aerobic ponds are maintained completely in aerobic conditions. These ponds consist of bacteria and algae in suspension through which organic wastes can be treated. Ponds have a depth of about 0.5–1.5 feet (150–450 mm) and allow the penetration of light throughout the liquid depth. Usually oxygen can be produced by an alga that is grown in the pond. Besides this, it is also maintained in a continuous atmospheric diffusion by pumps. In these ponds, bacteria and algae exhibit symbiotic association. Photosynthesis process is carried out by algae and releases oxygen to keep oxygenic conditions in the pond, while the bacteria degrade organic matter to produce carbon dioxide and nutrients utilized by algae. Growth of protozoa and rotifers are responsible for polishing of effluent (Satyanarayana 2008).
Factors affecting aerobic ponds: species of bacteria and algae, degree of pond mixing, availability of nutrients, sunlight, pH, and temperature.
(b) Anaerobic ponds: Ponds maintained under anaerobic conditions and mainly employed for treatment of the sewage containing high-strength organic solids. Heat conservation is possible because they are very deep (up to 30 feet, i.e., about 9 m). When sewage is introduced into the pond, precipitation and anaerobic conversion of organic waste into carbon dioxide, methane, organic acids, etc. occur. Under favorable conditions, about 75% of BOD removed in anaerobic treatment (Satyanarayana 2008). Factors affecting anaerobic ponds: temperature, HRT, pH, sulfide, toxic compounds, degree of mixing, etc. (Ho et al. 2017).
(c) Facultative ponds: These are the stabilization ponds that are mainly employed in the biological treatment of industrial and domestic wastewater. In these ponds, both aerobic and anaerobic conditions are maintained. Three types of microorganisms are employed in facultative ponds, that is, aerobic, anaerobic, and facultative (both aerobic and anaerobic). A typical facultative pond has three zones, which include the following (Fig. 17.7):
-
Surface zone (aerobic zone): Zone having aerobic bacteria and algae existing in a symbiotic relationship.
-
Bottom zone (anaerobic zone): Zone having only anaerobic bacteria that decompose the organic matter present in the sewage.
-
Intermediate (facultative zone): Zone having partly aerobic, anaerobic, and both types (aerobic and anaerobic) of bacteria.
Process that occurs in facultative ponds: the algae in the aerobic zone execute photosynthesis and release oxygen taken up by aerobes and facultative anaerobes for the oxidation of organic matter. Anaerobes degrade the organic compounds into dissolved organic solids and gases such as carbon dioxide, methane, hydrogen sulfide, etc. The released carbon dioxide is utilized by the algae to carry out photosynthesis, and then hydrogen sulfide combines with the oxygen to form sulfuric acid (Satyanarayana 2008).
3.4 Tertiary Treatment
Tertiary treatment removes total suspended solids, total dissolved solids, organic and inorganic matter present in the secondary effluent. It can also remove the specific organic and inorganic constituents, nutrients, and kill the pathogens.
Tertiary treatments are of four types that include the following:
-
1.
Ion exchange
-
2.
Membrane separation techniques
-
3.
Electrochemical techniques
-
4.
Advanced oxidation process
(1) Ion exchange: Ion exchange is one of the water treatment method in which one (or) more undesirable ionic contaminants are removed from water by exchange with another non-objectionable or less objectionable ionic substances. Ion exchange is one of the most appropriate technologies to remove dissolved inorganic ions effectively. In wastewater treatment plant, it is widely used for water softening and removal of nitrogen, heavy metals, and total dissolved solids (Fig. 17.8). Ion exchange resins have the capacity to exchange soluble cations or anions with electrolyte solutions by transferring into the sludge. Resins possess specific metal uptake capacity and hence can easily carry out ion exchange process. In this process, ion exchange resin is suspended in an electrolyte. As water passes through resin bed, these ions get attached to the resin beads due to which the loosely held solution gets released into the water. After some time, the beds get saturated and lose its power so it should be recharged. As a part of this recharge, the resin is flushed with a salt brine solution due to which exchange of ions occurs between salt brine solutions and flushed out water (Keller 2005).
The water softening and recharge process. Modified from Skipton et al. (2008)
Applications of ion exchange resins:
-
For controlling nitrogen: Natural zeolite such as clinoptilolite can be used as ion exchange resin as it has greater affinity for ammonium ions and is inexpensive. It also creates a problem as they form calcium carbonate precipitates within the filter.
-
For controlling heavy metals: Natural zeolite such as chabazite is used for removing heavy metals. Chelating resins such as aminophosphonic resins have capacity to remove metals such as copper, nickel, and so on.
-
For controlling total dissolved solids: Cationic and anionic exchange resins are used for removing total suspended solids.
(2) Membrane filtration techniques: Membrane filtration work on the principle of filtration. In membrane filtration process, wastewater is allowed to flow through a semipermeable membrane. Membrane filters remove dissolved particles up to 0.0001 to 1 μm. Under high-pressure conditions, membrane acts as a selective barrier that will allow the passage of certain components and will retain other components present in the liquid. It is mostly used in the treatment of seawater, brackish water desalination, etc. (Nqombolo et al. 2018). Membrane filtrations are of various types based on material used for membrane, nature of motive power, detachment mechanism, and minimal size of separation achieved (Fig. 17.9).
Separation of compounds by membrane filtration processes. Modified from Mo Mukiibi and Feathers (2009)
(a) Microfiltration (MF)
(b) Ultrafiltration (UF)
(c) Nanofiltration (NF)
(d) Reverse Osmosis (RO)
-
(a)
Microfiltration: It is a physical separation to remove suspended solids and bacteria from water streams through a membrane porosity of between 0.1 and 10 μm. Microfiltration technique is mostly used for treatment of potable water, industrial, and municipal wastewater.
-
(b)
Ultrafiltration: It is used for the removal of particulates and macromolecules from raw water to produce potable water. Filters have membranes containing pore size between 100 and 10 nm. They are usually supplied with pressure between 1 and 10 bar in order to facilitate the separation process.
-
(c)
Nanofiltration: It is used for treating wastewater that consists of low amount of total dissolved solid particles, for example, surface water treatment. This technology is mostly being used in the dairy industries for partial demineralization of products. This process mainly softens the water and removes the organic by-products. Nanofilters have pore size from 1 to 2 nm and require 3–20 bar pressure for the particle separation.
-
(d)
Reverse osmosis: This process utilizes a partially permeable membrane in order to remove unwanted molecules, ions, and salts from the water. It is mostly used to treat fresh water and plays a major role in desalination of seawater. The membranes used in this process have pore size less than 1 nm. As this process uses membranes with less porosity, it is operated usually at high-pressurized conditions (up to 80 bars).
(3) Electrochemical Techniques: This method can be achieved by direct oxidation and reduction reactions by releasing chemicals that can physically remove all the pollutants from the wastewater or through producing chemical species that are reactive. Electrochemical techniques are of two types: electrodialysis and electrocoagulation (Feng et al. 2016).
-
(a)
Electrodialysis: In this technique, transport of salt ions is achieved from the wastewater. This transport occurs over ion-exchange membranes in an applied electric potential difference. This process usually occurred in an electrodialysis cell. This cell usually has a diluent section and a brine part formed by an anion exchange membrane. The cationic membrane is between the two electrodes, and due to electric potential contrast, the negatively charged ions migrate toward anode whose further migration is prevented by the anionic membrane (Fig. 17.10). The positively charged ions migrate toward the cathode whose further migration is prevented by the cationic membrane. This migration of ions occurs equally. Due to this migration, electric current flows between cathode and anode. Electrodialysis results in the increase of ion concentration in the brine compartment with the depletion of ions in dilute compartment (Tedesco et al. 2016).
-
(b)
Electrocoagulation (EC): Electro means to apply an electrical charge to water, and coagulation means the process of changing the particles surface charge, allowing suspended matter to form an agglomeration. It is an advanced, economical, and broad spectrum treatment technology. EC removes the suspended solids, heavy metals, emulsified oils, total petroleum hydrocarbons, grease, latex, bacteria, and other contaminants from water (Tedesco et al. 2016).
Electrodialysis process (ED). Modified from Michele Tedesco et al. (2016)
(4) Advanced Oxidation Process: Advanced oxidation process is used to oxidize complex organic constituents found in wastewater that are difficult to degrade biologically into simpler end products. It involves generation and use of hydroxide free radical (HO) (Nick Nicholas 2018). Most commonly used advanced oxidation processes are:
-
(a)
Ozone/UV
-
(b)
Ozone/hydrogen peroxide
-
(c)
Hydrogen peroxide/UV
(a) Ozone/UV process: In this process, the photons present in the UV spectrum splits ozone to oxygen and peroxide in the presence of water. The formed peroxide now reacts with the ozone and releases hydroxyl free radical (Tichonovas et al. 2017).
where O3 = = ozone
UV = Ultraviolet radiation
O2 = oxygen
O (1D) = excited oxygen atom
HO· = Hydroxyl radical
(b) Ozone/hydrogen peroxide process: In this process, the hydrogen peroxide is used with the ozone in order to enhance the release or formation of hydroxyl radicals (Mansouri et al. 2019). Hydrogen peroxide is a weak acid and dissociates into additional hydrogen peroxide ions, reacts with the ozone, and results in the formation of hydroxyl free radicals (Kuo et al. 1999). It is effective in reducing trichloroethylene (TCE) and perchloroethylene (PCE).
(c) Hydrogen peroxide/UV: This process is effective for removal of N-nitrosodimethylamine (NDMA) and other compounds of concern in treated waste water such as PPCPs/ micro constituents (steroidal hormones, veterinary and human antibiotics, human prescription and nonprescription drugs, industrial and house hold wastewater products).
Applications:
-
Used for low COD wastewater because of the cost of ozone and/H2O2 required to generate the hydroxyl radicals.
-
Used for high-level disinfection and treatment of refractory organic compounds.
-
Used for removal of estrogenic, antibiotic, herbicide, pesticides, and viruses.
4 Conclusion
The following points were concluded in conventional wastewater treatment processes:
-
Preliminary treatment is useful for elimination of coarse solids and other large objects present in wastewater. Hence, this removal increases the operation and maintenance of subsequent treatment units.
-
Primary treatment is useful for elimination of settleable inorganic and organic solids, approximately 20–50% of the incoming BOD, 50–70% total suspended solids, and 65% of oil and grease materials.
-
Secondary or biological treatment removes the 95% of suspended solids, BOD, and residual organics.
-
Tertiary treatment removes the total suspended solids, total dissolved solids, specific organic and inorganic constituents, and nutrients removal (nitrogen and phosphorous).
References
Ahuja S, Larsen MC, Eimers JL, Patterson CL, Sengupta S, Schnoor JL (eds) (2014) Comprehensive water quality and purification. Elsevier, Amsterdam
Amina T (2017) Wastewater Screening & Classification of screens (complete list) wastewater treatment. Environ Eng 17:2017
Ambulkar A, Nathanson JA (2019) Wastewater treatment. https://www.britannica.com/technology/wastewater-treatment. Accessed 17 July 2019
Ebrahimi A, Najafpour GD (2016) Biological treatment processes: suspended growth vs. attached growth. Iranica J Energ Environ 7(2):114–123
Feng Y, Yang L, Liu J, Logan BE (2016) Electrochemical technologies for wastewater treatment and resource reclamation. Environ Sci Water Res Technol 2(5):800–831
Fujie K, Bravo HE, Kubota H (1983) Operational design and power economy of a rotating biological contactor. Water Res 17(9):1153–1162
Ghodeif K (2013) Baseline assessment study for wastewater treatment Plant for Al Gozayyera Village, West Kantara City. Ismailia Governorate, Egypt
Ho LT, Van Echelpoel W, Goethals PL (2017) Design of waste stabilization pond systems: a review. Water Res 123:236–248
Keller MC (2005) Basic ion exchange for residential water treatment. Water Conditioning & Purification
Kuo CH, Zhong L, Zappi ME, Hong AP (1999) Kinetics and mechanism of the reaction between ozone and hydrogen peroxide in aqueous solutions. Can J Chem Eng 77(3):473–482
Mansouri L, Tizaoui C, Geissen S-U, Bousselmi L (2019) A comparative study on ozone, hydrogen peroxide and UV based advanced oxidation processes for efficient removal of diethyl phthalate in water. J Hazard Mater 363:401–411
Metcalf and Eddy (2003) Wastewater engineering: treatment and reuse, 4th edn. McGraw-Hill, New York
Mukiibi M, Feathers R (2009) Membrane technology: a break through in water treatment. http://wcponline.com/2009/02/10/membrane-technology-break-water-treatment, Accessed 10 Feb 2009
Nicholas N (2018) How to choose the appropriate advanced oxidation process for wastewater treatment. https://www.wateronline.com Accessed 20 Jun 2019
Nkwonta O, Ochieng G (2009) Roughing filter for water pre-treatment technology in developing countries: a review. Int J Phys Sci 4(9):455–463
Nqombolo A, Mpupa A, Moutloali RM, Nomngongo PN (2018) Wastewater treatment using membrane technology. In: Wastewater and water quality. IntechOpen, Sine Loco, p 29
Oliveira SC, von Sperling M (2011) Performance evaluation of different wastewater treatment technologies operating in a developing country. J Water Sanitation Hygiene Develop 1(1):37–56
Griffiths O, Henderson H, Simpson M (1991) Environmental health for aboriginal communities: a training manual for aboriginal environmental health workers. Health Promotion Services Branch, Health Dept. of Western Australia, Perth. https://www1.health.gov.au. Accessed Nov 2010
Parker D, Lutz M, Dahl R, Bernkopf S (1989) Enhancing reaction rates in nitrifying trickling filters through biofilm control. J Water Pollut Cont Federat 6:618–631
Gleick PH (1993) Water in crisis a guide to the World’s fresh water resources, 1st edn. Oxford University Press, Oxford, p 504
Jogdand SN (2010) Environmental biotechnology (industrial pollution management), 3rd edn. Himalaya Publishing House, Mumbai
Satyanarayana U (2008) Text book of biotechnology. Books & Allied Ltd, Kolkata
Shon HK, Vigneswaran S, Kandasamy J (2011) Characteristics of Effluent Organic Matter in Wastewater. Eolss Publishers, Oxford
Skipton S, Dvorak BI, Niemeyer S (2008) G08–1491 Drinking Water Treatment: Water Softening (Ion Exchange). DigitalCommons@University of Nebraska, Lincoln
Srinivas T (2008) Environmental biotechnology. New Age International (P) Ltd., Hyderabad
Tedesco M, Hamelers HVM, Biesheuvel PM (2016) Nernst-Planck transport theory for (reverse) electrodialysis: I. effect of co-ion transport through the membranes. J Membr Sci 510:370–381
Tichonovas M, Krugly E, Jankunaite D, Racys V, Martuzevicius D (2017) Ozone-UV-catalysis based advanced oxidation process for wastewater treatment. Environ Sci Pollut Res 24(21):17584–17597
Tilley E, Ulrich L, Lüthi C, Reymond P, Zurbrügg C (2014) Compendium of sanitation systems and technologies, 2nd edn. Switzerland Eawag, Dübendorf, p 175
Water Environment Federation (2008) Operation of municipal wastewater treatment plants (No. 11). WEF Press, New York
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ranjit, P., Jhansi, V., Reddy, K.V. (2021). Conventional Wastewater Treatment Processes. In: Maddela, N.R., García Cruzatty, L.C., Chakraborty, S. (eds) Advances in the Domain of Environmental Biotechnology. Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-15-8999-7_17
Download citation
DOI: https://doi.org/10.1007/978-981-15-8999-7_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-8998-0
Online ISBN: 978-981-15-8999-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)