Abstract
Recently, the development of biodegradable polymer-based film/composites has acknowledged more attention due to their environment-friendly properties. Polyvinyl alcohol (PVA) is a biodegradable, non-toxic and biocompatible polymer with major limitation of water solubility in water. The present work is an attempt to decrease the solubility limitation with improvement in mechanical properties. In the present work, citric acid was used as cross-linking agent to reduce the water solubility of PVA. Concentration of citric acid and curing time was considered as the important parameter, and their effect on fabricated films was evaluated. Further, water absorption test was performed to test the water solubility. Mechanical characterization of films was done by tensile test which determines the ultimate tensile strength, % elongation and Young’s modulus. It was observed that cross-linking reaction was optimum when the curing time for preparing films was kept more than 48 h. It was also observed that maximum UTS was found for 30 wt% CA when curing time was kept 96 h. The maximum UTS was increased up to 300% as compared to neat PVA. ANOVA result states that curing time was the major critical factor which affects the mechanical properties of cross-linked films.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Polyvinyl alcohol (PVA) is a biodegradable, synthetic, thermoplastic and non-toxic polymer that is soluble in water. It is creamy or whitish, biocompatible, thermostable, non-toxic, colourless, odourless and semi-crystalline polymer. Moreover due to its resistant to grease, oils and solvents researcher gain interest in PVA for many application. For commercially purpose, as the raw material PVA is either in granular or powdered form. Polymerization technique is not employed for fabrication of PVA unlike the other vinyl polymers. Hydrolysis is employed to obtain PVA by hydrolyzing the polyvinyl acetate by removal of an acetate group, (replaced with hydroxide group –OH) which is prepared through the polymerization of vinyl acetate. Thus, key raw material to create PVA is the vinyl acetate monomer. Physical, thermal and mechanical properties of PVA depend upon the degree of hydrolysis [1]. Important parameter which affects the hydrolysis process is the time period in the reaction.
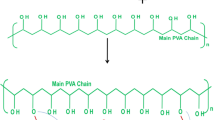
Semi-crystalline nature of PVA occurs due to the formation of hydrogen bonding between the PVA chains due to the presence of hydroxyl group. Hydroxyl group also plays an important role in mechanical, optical and electrical properties of PVA-based films/composites [2]. Properties of PVA had been modified by different researcher in past few years. González-Guisasola and Ribes-Greus [3] prepared PVA composite membrane by using different proportion of graphene oxide (GO) as a reinforcement material. During preparation of composite, it was cross-linked with sulfosuccinic acid (SSA) to reduce the water solubility and has application in proton exchange membrane fuel cells (PEMFCs). GO dispersion was confirmed by TEM image, and cross-linking was confirmed by FTIR spectroscopy. Moreover, thermal stability of membrane is also increased by reinforcing GO which is desirable for fuel cell application. Sonkar et al. [4] fabricated PVA-based cross-linked composites using suberic and terephthalic acid as cross-linking agent. Both the acid are dicarboxylic acids contain two carboxyl group (molecular formula: HO2C–R–CO2H, where R can be aliphatic or aromatic). Tensile and thermal properties of the cross-linked PVA were found. It was noted that suberic acid gives better properties than terephthalic acid. Prepared sample was also kept for biodegradation which shows desirable results. Sonker et al. [5] prepared PVA-based composite film using bacterial cellulose nanowhiskers [BCNW] as a reinforcement material. In 2017, they have used citric acid, whereas in 2018 tartaric acid [6] was as cross-linking agent to reduce water uptake. Due to the covalent bond formation, cross-linked PVA composite film showed better mechanical properties and improved thermal stability. With citric acid, composite film shows 10 times percentage reduction in water swelling test. It was observed that as the concentration of citric acid and cross-linking time (in hour) increases, there is significant reduction in swelling percentage. With tartaric acid, the composite film was prepared by two methods, viz. microwave (MW)-assisted rapid synthesis and by conventional hot air oven heating (CH). Cross-linked composite prepared by MW method took 1/8th time as compared to CH method. Cross-linked PVA with citric acid was also used as coating purpose on to the empty fruit bunch paper by Shakir et al. [7]. Cross-linked PVA solution was applied to the dried hand sheet paper using a spray gun. Swelling test and dimensional changes of paper were observed. It was concluded that with the increase in cross-linked PVA coating reduces volume of water absorbed and slows down the speed of water absorption in the paper. Aparicio et al. [8] fabricated PVA/TiO2-based nano-composite membranes which are cross-linked by glutaraldehyde solution (GA) as an agent. Impedance spectroscopy was used to measure ionic conductivity. With increase in the reaction time, the proton conductivity also increases significantly. The prepared membrane was suggested to be used as proton exchange membrane for fuel cells.
2 Material and Method
2.1 Material
PVA in powdered form having molecular weight 2000–96,000, pH value ranging from 5.0 to 8, having viscosity (4% aqueous solution at 20 °C) 35–50 cP and 87% degree of hydrolysis and was purchased from Thermo Fisher Scientific India Pvt Ltd., India. Citric acid (C6H8O7) was also purchased from Thermo Fisher Scientific India Pvt Ltd. It was received in fine granular form. It was anhydrous, 99.9% pure, having molecular weight 192.12 g/mol.
2.2 Method
All samples were prepared by solution casting method. In this method, aqueous solution PVA, citric acid and distilled water were prepared on magnetic stirrer and then casted into polypropylene moulds. The mould is then dried in an oven at 70 °C for different time. To prepare neat PVA film, 5 g PVA is poured into 100-ml-distilled water and the solution is left for approximately 1 h 30 min on magnetic stirrer at 70 °C and 250 RPM. To prepare different cross-linked PVA films, 1 g, 1.5 g and 2 g citric acid are mixed in above solution for another 1 h on magnetic stirrer. Each casted film was dried in oven for 24, 48, 72 and 96 h detail shown in Table 1. After completion of respective time in oven, films were peeled off from the mould. To avoid moisture absorption, all prepared films were kept in a close glass box containing calcium chloride at the bottom without touching the films. Nomenclature of fabricated films was presented in Table 1.
2.3 Water Absorption Test
In the present work, cross-linking of PVA film was done with citric acid to overcome the major drawback of PVA, i.e. solubility in water and some organic solvent. To verify the cross-linking reaction, water absorption test was performed on cross-linked PVA-based film. For performing the test, 40 × 10 mm2 sample film was cut from each prepared cross-linked PVA-based film. Weight of each cut sample (W1) was measured by an electronic weighting machine (having least count 0.001). Each sample was dipped into 100 ml distilled water and left to absorbed water for 24 h at room temperature. Afterwards, all samples were withdrawn from water. Tissue paper was used to wipe out extra amount of water present on the surface of the film. After that, weight of water absorbed sample was measured (W2) and compared with initial weight of cut samples. Water absorption percentage is calculated by the following expression [9, 10].
where
W1 = initial weight of samples and
W2 = specimen weight after 24 h of water absorption.
2.4 Tensile Test
Tensile testing is considered as an essential step to determine the suitable application in different field. In the present work, mechanical properties such as ultimate tensile strength, Young’s modulus and percentage of elongation had been studied by tensile test. The tensile tests were carried out on the 5 kN servo hydraulic universal testing machine (model AMT-SC). All of the specimens for tensile testing were prepared according to the ASTM D638 [11,12,13] type IV standard having rectangular sample of size 60 mm × 10 mm with gauge length of 40 mm and thickness less than 1 mm approximately. Thickness of films was measured at three points from span of gauge length for calculating cross-sectional area. Test was conducted at room temperature with speed of 2 mm/min.
3 Result and Discussions
3.1 Water Absorption Test
Results of water absorption percentage were presented in Fig. 1 and Table 2. Water absorption test was performed to analyse the effect of cross-linking agent CA on to solubility of PVA in water medium. Results show that fabricated samples which were dried for 24 h (in the oven at 70 °C) found completely soluble in water [14]. This concluded that no cross-linking reaction had been done between PVA chain and CA irrespective of the concentration of CA. With increase in cross-linking curing time water absorption percentage of PVA-based samples were restricted. Samples which were dried for 48 h (in the oven at 70 °C) at 10 wt% of CA was completely soluble, 20 wt% had large amount of water absorption and 30 wt% had 193%. As water absorption percentage is very high, it can be analysed that degree of cross-linking was very low even after 48 h curing time. Further, it was found that the degree of cross-linking further increases with increase in dried time from 48 to 72 h and all films were insoluble in water. Above result shows that time has major effect on the cross-linking reaction as compared to concentration of CA. With further increase in the dried time from 72 to 96 h, water absorption percentage almost become same for 20 and 30 wt% cross-lined composites. Restriction in solubility of PVA/CA films conformed the cross-linked reaction, i.e. CA react to PVA to remove hydroxyl group (–OH).
3.2 Tensile Test
Values of ultimate tensile strength (UTS), percentage elongation and Young’s modulus are evaluated from tensile tests for PVA and CA cross-linked PVA film and shown in Table 3. Behaviour of material under tensile loading is represented in Fig. 2. Neat PVA has ultimate tensile strength of about 30 MPa with percentage elongation of about 10%. From Table 3, it can be observed that maximum strength among cross-linked PVA films is 90.1 MPa for PVA30CA-IV, which is about 300% more that neat PVA. Both curing time and concentration of CA act as the major factors which affect the mechanical properties of PVA-based films. It was observed that UTS, % elongation and Young’s modulus of fabricated cross-linked films varies with respect to curing time (film dried time in oven) and weight percentage of citric acid. It is observed from the results that with respect to curing time, UTS of PVA/CA cross-linked film increases, whereas % elongation decreases. On the other end with increase in wt% of CA, % elongation increases in each case. Whereas UTS first increases up to 30 wt% CA, then decreases for 40 wt% CA. In 10 wt% CA films UTS and YM almost become constant after 72 h curing time. It was observed that maximum UTS and Young’s modulus were achieved for 30 wt% of CA and 96 h curing time.
From the water absorption test, this can also be analysed that cross-linking with CA was achieved after 48 h of curing time. Water absorption percentage significantly reduces for the 72 h curing time which shows successful cross-linking reaction. Further, UTS decreases for 40% concentration of CA which shows incomplete cross-linking reaction. This shows that CA molecules got stuck between PVA chains which hamper the cross-linking reaction partially and cause reduction in UTS. This fact can also be confirmed by observing UTS value of PVA40CA-I sample (19.5 MPa), which shows no cross-linking reaction. From the ANNOVA analysis Table 4, it is observed that curing time has major influence on the obtained values of UTS (68.61%), percentage elongation (74.47%) and YM (49.26%) as compared to wt% CA. So curing time is the most important control parameter which affects tensile properties of all sample films. Also in case of UTS value, wt% of citric acid affects significantly (24.99% contribution).
4 Conclusions
The effect of curing time and concentration of cross-linking agent CA on the water absorption and tensile properties of sample films was studied. In water absorption test, it was observed that curing time has major impact. Water uptake percentage decreases with respect to increase in curing time as degree of cross-linking also increases; similar observation is also seen for concentration of citric acid. The sample which was dried from 72 to 96 h in oven was found insoluble in water with minimum water absorption percentage. In tensile test, it was observed with increasing curing time, UTS was increased and % elongation was reduced while with increase in concentration of CA, UTS increases only up to 30 wt% of CA. It shows that degree of cross-linking reduces when CA was added beyond 40 wt% which conclude that more curing time is required for cross-linking reaction between PVA and CA. But on the other hand, it is undesirable on the basic of manufacturing point of view. Maximum UTS and maximum YM were obtained for 96 h curing time and at 30 wt% of CA which is 300% higher than the neat PVA.
References
Aslam M, Kalyar MA, Raza ZA (2018) Polyvinyl alcohol: a review of research status and use of polyvinyl alcohol based nanocomposites. Polym Eng Sci. https://doi.org/10.1002/pen.24855
Reddy N, Yang Y (2009) Citric acid cross-linking of starch films. Food Chem. https://doi.org/10.1016/j.foodchem.2009.05.050
González-Guisasola C, Ribes-Greus A. Dielectric relaxations and conductivity of cross-linked PVA/SSA/GO composite membranes for fuel cells. Polym Test. https://doi.org/10.1016/j.polymertesting
Sonker AK, Rathore K, Nagarale RK, Verma V (2017) Crosslinking of polyvinyl alcohol (PVA) and effect of crosslinker shape (aliphatic and aromatic) thereof. J Polym Environ. https://doi.org/10.1007/s10924-017-1077-3
Sonker AK, Teotia AK, Rajaram AK, Nagarale K, Verma V (2017) Development of polyvinyl alcohol based high strength biocompatible composite films. Macromol Chem Phys J. https://doi.org/10.1002/macp.201700130
Sonker AK, Rathore K, Teotia AK, Kumar A, Verma V (2018) Rapid synthesis of high strength cellulose–poly(vinyl alcohol) (PVA)biocompatible composite films via microwave crosslinking. J Appl Polym Sci. https://doi.org/10.1002/app.47393
Shakir MA, Yhaya MF, Ahmad MI (2017) The effect of crosslinking fibers with polyvinyl alcohol using citric acid. Imperial J Interdisc Res (IJIR) 3(4)
Aparicioa GM, Vargasb RA, Buenoc PR (2019) Protonic conductivity and thermal properties of cross-linked PVA/TiO2 nanocomposite polymer membranes. J Non-Cryst Solids. https://doi.org/10.1016/j.jnoncrysol.2019.119520
Jain N, Ali S, Singh VK, Kumar N, Chohan S (2019) Creep and dynamic mechanical behaviour of cross-linked polyvinyl alcohol reinforced with cotton fiber laminate composites. J Polym Eng. https://doi.org/10.1515/polyeng-2018-0286
Jain N, Verma A, Singh VK. Dynamic and creep-recovery analysis of polyvinyl alcohol based cross-linked composite reinforced with basalt fiber. Mater Res Express. https://doi.org/10.1088/2053-1591/ab4332
Verma A, Jain N, Singh VK (2019) Fabrication and characterization of chitosan coating sisal fiber reinforced phytagel modified soy protein based green composite. J Compos Mater. https://doi.org/10.1177/0021998319831748
Jain N, Singh VK, Chauhan S (2018) Dynamic and creep analysis of polyvinyl alcohol based films blended with starch and protein. J Polym Eng 39(1):26–35. https://doi.org/10.1515/polyeng-2018-0032
Deepmala K, Jain N, Singh VK, Chauhan S (2018) Fabrication and characterization of chitosan coated human hair reinforced phytagel modified soy protein-based green composite. J Mech Behav Mater. https://doi.org/10.1515/jmbm-2018-0007
Jain N, Singh VK, Chauhan S (2017). A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J Mech Behav Mater. https://doi.org/10.1515/jmbm-2017-0027
Acknowledgements
The authors would like to thank the Department of Mechanical Engineering, MIET, Meerut, for proving the necessary facilities for fabricating the cross-linked films. Authors also express gratitude to AKTU for providing necessary found under TEQIP-III for procurement of raw material and testing/analysing the fabricated films.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Jain, N., Deep, G., Madan, A.K., Dubey, M., Tomar, N., Gupta, M. (2021). Fabrication and Characterization of PVA-Based Films Cross-Linked with Citric Acid. In: Singari, R.M., Mathiyazhagan, K., Kumar, H. (eds) Advances in Manufacturing and Industrial Engineering. ICAPIE 2019. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-8542-5_64
Download citation
DOI: https://doi.org/10.1007/978-981-15-8542-5_64
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-8541-8
Online ISBN: 978-981-15-8542-5
eBook Packages: EngineeringEngineering (R0)