Abstract
A biosensor is a measurement device for the detection of an analyte that contains a biological material with a signal transducer. Current advances in nanotechnology and nanomaterial synthesis have created a new biosensor called nanobiosensor. In this sensor, the biological molecule is immobilized on the nanomaterial to form a compact probe. The reaction between the biomolecule and the analyte is heterogeneous in nature; therefore, the surface of this biointerface is very crucial in the performance of the nanobiosensor. To further improve their performance, various kinds of nanomaterials have been designed. Due to their large surface area, nanomaterials possess high sensitivity and thus, enhance its application in the detection and diagnosis of various diseases. This chapter provides an overview of various types of nanobiosensors that have been developed for biological, environmental, and medical applications. Different types of biosensors fabricated using various biomolecules are discussed in detail and a brief idea on the mode of transducer reaction is also highlighted. The later part of the chapter gives information about applications of nanobiosensor in diverse fields, mostly in the health sector.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
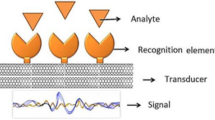
The biosensor is an analytical device that senses biological materials and estimates it by biological signals. These signals are then evaluated and converted into readable form using transduction and electromechanical interpretation. Figure 4.1 represents a model of a biosensor and gives information about its components. There are three major components of a biosensor, namely bioreceptor, transducer, and detector. Their main function is to sense a biological material like immunological molecules, biomolecules, and enzymes.
Nanomaterials have unique properties; therefore, they can be exploited for the development of sensitive nanobiosensor. The nanomaterial-based sensors are economical, reasonable, robust, and reproducible (Rai et al. 2012). Thus, nanobiosensor is used to determine a biochemical or biological event through a compact probe (Di Giusto et al. 2005; Gullberg et al. 2004; Prasad 2014). At present, it is considered that nanobiosensor technology can revolutionize the health care industry because of applications for human health like monitoring of diabetes, measurement of metabolites, detection of cancer biomarkers, and in forensic medicine, etc. In the victuals industry, nanobiosensor is used for remote sensing of water quality, monitoring the presence of drug residues in food. They can also be used for the detection of pesticides in the environment, heavy metals in river water, and genome analysis of organisms. The nanomaterials like silver, gold, magnetic nanoparticles, carbon nanotubes, and quantum dots have been vigorously investigated for their application as biosensors. Several studies reported that the silver and other metal nanoparticles have significant applications in the field of biolabeling (Kulesza et al. 2009), drug delivery system (Lv et al. 2009; Marcato and Durán 2008), filters, and as antimicrobial drugs (Durán et al. 2010; Rai et al. 2009) and sensors (Baruah and Dutta 2009).
4.2 Nanobiosensor and Their Properties: Fusion of Nanotechnology with Biosensor
Nanotechnology has contributed a lot in the field of biosensor development due to its unique electronic, magnetic, and optical properties of the nanomaterials used. The special characteristics like submicron size and large surface area to volume ratio enhance the sensitivity of the nanosensor for the analyte. Nanomaterials are considered environmentally sustainable and thus can be utilized in sensing technology. Nanobiosensor is defined as a device that is used to detect analyte quantitatively with the help of a biologically active element attached to a suitable nanostructured transducer (Fan et al. 2008; Velasco-Garcia 2009; You et al. 2009). Nanobiosensor is a modified version which has biologically sensitive molecule immobilized onto the surface of the physico-chemical transducer. It is the effort of researchers working in diverse fields like electronics, biologists, material chemist, and physicists (Turner 2000). It is a compact analytical device that has enhanced inherent specificity, quick response time, and reliability. The important property of a biosensor is the specificity and it should be high enough to determine analyte from the rest of the unwanted material. The interaction between the target analyte and sensor should not be affected by physical factors like temperature and pH. Also, the sensor should provide analysis results with precision, accuracy, and linearity without the disturbance of electrical noise. Stability is another important criterion for an ideal nanobiosensor under normal storage conditions. It should be economical, inexpensive, manageable and should be conveniently used by semi-skilled operators (Rai et al. 2012). Although nanobiosensor is still in the developing stage but it has shown promising results in bioanalytical applications and has gained popularity because of its ability to detect ultra-low concentrations of any analyte that are observed to be a potent threat to the living being. It works at the atomic level with great efficiency, and due to its high sensitivity, sometimes it is considered to be an error-prone system (Rai et al. 2012).
The biologically active or recognition element, the transducer, and the detector are three important components to formulate any nanobiosensor. The biological elements used are bioreceptor probes that are highly selective for target analytes like antibodies, nucleic acids, pathogens, and metabolites. Molecular recognition elements include biologically derived materials or bio-mimic components such as receptors, enzymes, nucleic acids, antibodies, molecular imprints, lectins, tissue, microorganisms, and organelles (Razavi and Janfaza 2015; Sharma and Rogers 1994). The working of nanobiosensor starts with the binding of targeted bioanalytes with the bioreceptors and this binding generates a physicochemical signal which is modulated by the receptor. The transducer can be electrochemical, mass, optical, and thermal. The transducer measures changes which occur as a result of heterogeneous reaction at bioreceptor/element and thus acts as a borderline between receptor and detector. It captures and transforms the signal into measurable electrical output. This electrical signal is trapped by the detector component which is further amplified and observed by the microprocessor (Prasad 2014).
In nanobiosensor, the change in the signal like electric potential, current, impedance, intensity, current, and phase of electromagnetic radiation is measured. These variations are analyzed and confirm the presence or absence of bioanalytes. The properties of transducers and biorecognition element determine the sensitivity of a nanobiosensor. The nanostructured materials are located between the biological agents and detector component and thus behave as intermediary phase and this nanomaterial is immobilized on the transducer in order to fabricate a nanobiosensor. Several nanomaterials have been screened for the development of nanobiosensor (Gomes et al. 2015; Sharma and Rogers 1994). The nanostructured materials of different chemical nature are discussed in the next section.
4.2.1 Nanomaterials Used in Biosensing Devices
Recent years have seen tremendous growth in using nanoscale materials for developing electrochemical biosensing devices as the properties of nanomaterials offer an excellent outlook in constructing high performance, novel sensing systems. The nanomaterials are incorporated into the transducer which can send the observable signal to the recorder. Controlling the size and morphology of nanomaterials can enhance the power of detection, sensitivity, and transducing capability to a certain degree (Pak et al. 2001). There are few factors which are kept in mind while selecting a particular kind of nanoscale material.
Materials at the nanoscale level are integrated with highly sensitive electrical and electromechanical properties when engineered with the nanoelectromechanical systems (NEMS). It has enabled the materials to gain complex electrical, mechanical, fluidic, thermal, optical, and magnetic properties. NEMS is a class of devices and as the name suggests, the device is in the nanometer range and thus having novel features like low mass, high mechanical resonance frequencies, more surface-to-volume ratio, and large quantum mechanical effects such as zero-point motion. NEMS and MEMS (microelectromechanical system) devices have resulted in mechanical materials to perform in a much better and sophisticated way as the mechanical property of a material determines its size. MEMS is a mini device with electrical and mechanical components. Using these devices, surface forces like adhesion, cohesive, friction, and viscous drag force can be regulated precisely which helps in biochemical interaction taking part in sensing technology. Incorporating nanomaterials with these devices increases the response to a wide range of stimuli (Bhushan 2007).
Optimizing the optical properties of nanomaterials is also one of the important criteria considered for its selection. Plasmon surface resonance is an interesting feature of nanomaterials which maximizes the optical response of the sensing materials with the incident light. Ionic and charged species are used to excite the surface of sensing materials and cause excitation of the fluidic state of charged particles. Due to this phenomenon, nanoparticles have photonic character and can be used as fluorophores. The refractive index of a medium is a vital property that governs the flow of light through a medium and also affects the surface plasmon resonance. A nanobiosensor is thus able to detect the infinitesimal biological interactions and results in a much better and reliable degree of estimation. So, for the implementation of nanomaterials, they are to be optimized for their performance and effect to be used in biosensing devices as per the required goal (Haes and Van Duyne 2002; Kelly et al. 2003). Tailoring of physical/chemical properties like shape, size, structure, and composition of nanomaterials is done to alter the specific absorptive, emissive, and light-scattering features. Various nanostructured materials have been modified and utilized with specific forms such as 0D (quantum dots, nanoparticles), 1D (nanowires or carbon nanotubes), or 2D (metallic platelets or graphene sheets) orientation that reflects in their final properties (Pandit et al. 2016). These nanomaterials are described below.
4.2.1.1 Metallic Nanoparticles
Due to their microscopic size, high surface to volume ratio, electrical and optical properties, metallic nanoparticles have been a huge success in diverse fields. They have been exploited in bioanalytical applications for the development of biosensors, diagnostics, imaging, drug delivery, and therapy. Among metal NPs (1–100 nm in size), gold nanoparticles (GNPs) have been widely employed for such applications. They have a large surface area, strong adsorption ability, scattering properties, and facile conjugation to various biomolecules and are considered as nontoxic, biocompatible, and inert core nanomaterials. Gold NPs are being extensively used for the detection of various analytes by fabricating it into immunoassays, diagnostics, and biosensors. They can act as nanocarriers for the delivery of drugs, DNA, and genes in the therapy of cancer and other diseases (Kim et al. 2002; Park et al. 2013). After GNPs, magnetic NPs (MNPs) are the second most common NPs which have been used in the development of nanobiosensor and detection of analytes such as proteins, enzymes, DNA, mRNA, drugs, metabolites, pathogens, and tumor cells. The MNPs are being extensively used by industries to develop diagnostic magnetic resonance (DMR) technology and nuclear magnetic resonance detector (μNMR) which can detect a sensitive analyte in microliter volumes (Sahoo et al. 2017). Silver nanoparticles (AgNPs) are also very commonly synthesized and considered to be a noble metal having attractive physicochemical properties including the surface plasmon resonance and large effective scattering cross-section of individual silver nanoparticles. They have been used in diverse applications effectively including the detection of biological macromolecules. Hydrophobic Ag–Au composite nanoparticles have been observed to show strong adsorption and good electrical conducting properties and thus are being used in biosensing devices (Link et al. 1999; Rai et al. 2012).
4.2.1.2 Carbon Nanotubes (CNTs)
Since the discovery of carbon nanotubes (CNTs) in the 90s, its application has been heavily investigated in biosensing devices. The first CNT-based nanosensor was fabricated in 2003 by Wang and Musameh (2003) and Yun et al. (2009). CNTs are an exciting, one-dimensional, and a new form of carbon-based nanostructured material that has already been exploited in many fields like diagnostics, tissue engineering, cell tracking and labeling, and delivery of drugs and biomolecules (Pandit et al. 2016). CNTs are cylindrical in shape and consist of one, two, or several concentric graphene sheets seamlessly wrapped into a tube, capped by fullerenic hemisphere. There are two main types of CNTs—single-walled and multi-walled. CNTs have gained attention due to their unique properties such as structure-dependent electronic and mechanical properties, high thermal conductivity, excellent biocompatibility, high chemical stability, extraordinary electrocatalytic activity, very low surface fouling, low overvoltage, and high surface to volume ratio (Pandit et al. 2016). The surface of CNTs can be modified by adsorbing biomolecules such as protein, DNA, etc., electrostatically or it can be attached to the functional group present on the CNTs. CNT-based nano biosensor has been used in the diagnosis of analytes in healthcare, industries, environmental monitoring, and food quality analysis.
4.2.1.3 Graphene
Graphene is made up of a thin layer of sp2-hybridized carbon. Due to its exquisite properties such as electron transfer ability, high mechanical strength, high thermal conductivity, tunable optical properties, tunable bandgap, high elasticity, very high room temperature electron mobility, and demonstration of the room temperature quantum Hall effect, graphene is becoming more popular than other nanomaterials in bioanalytical and bioimaging applications. Their superior performance facilitates them to be widely used in electrochemical, impedance, fluorescence, and electrochemiluminescence biosensors for the detection of a wide range of analytes such as glucose, cytochrome c, NADH, hemoglobin, cholesterol, ascorbic acid, dopamine, uric acid, hydrogen peroxide, horseradish peroxidase, catechol, DNA, heavy metal ions, and gases. They also have low production costs and minimize harmful environmental effects (Pandikumar et al. 2014).
4.2.1.4 Quantum Dots (QDs)
QDs have unique spectral properties and thus recently, they have been exploited as a new generation of fluorophores in bioimaging and biosensing. They are nanostructured materials with size ranging from 1 to 10 nm. QDs have unique optical properties of broad excitation, narrow size-tunable emission spectra, high quantum yield and molar extinction coefficients, high photochemical stability, and negligible photobleaching (Androvitsaneas et al. 2016). Optical biosensors have been developed using QDs (inorganic nanocrystals) as nanomaterials exclusively for the detection of ions, organic compounds, pharmaceutical analytes, and biomolecules such as nucleic acids, proteins, amino acids, enzymes, carbohydrates, and neurotransmitters (Androvitsaneas et al. 2016; Bakalova et al. 2004; Bulovic et al. 2004).
4.2.1.5 Nanowires and Nanorods
Nanowires are semi-conductor nano-structured materials having unique optical and electronic properties with size ranging from several tens to over 100 μm length and are sensitive enough to detect the binding events of small molecules, peptide nucleic acid (PNA)–DNA as well as DNA–DNA hybridization. Due to their structure, nanowires also show distinctive absorption and photocurrent characteristics. Nanowire-based nanobiosensor shows the sensing procedure when there is a change in charge density (inducing the change in the electric field on the nanowire surface) upon binding of biomolecules (negatively charged molecules bind to the n-type field-effect transistor) (Ambhorkar et al. 2018; Panpradist and Lai 2016).
Nanorods are nanoscale objects having dimensions range of 1–100 nm. Due to their optoelectronic properties, they are gaining momentum in developing different designs of nanosensors. Gold nanorods (GNRs) are considered an excellent candidate to be used in sensing devices as the absorbance band changes with the refractive index of local material and thus allows extremely accurate sensing. GNRs were used in detecting target sequences of infecting agents of many dangerous diseases, for example, an HIV-1 (He et al. 2008).
4.3 Types of Nanobiosensor
Nanobiosensor has been classified broadly based on bioreceptor used and the types of the transducer. Different biomolecules are being immobilized on the surface of the transducer as a bioreceptor which can be divided into several classes such as enzymatic biosensor, DNA/RNA biosensor, immunosensor, aptasensor, and microbial biosensor (Razavi and Janfaza 2015). The classification can also be done based on transducer used for sensing such as electrochemical, optical, etc.
4.3.1 Classification Based on Bioreceptor
4.3.1.1 Enzymatic Biosensor
Enzymes that are specific for an analyte to convert it into a product can be immobilized with a suitable transducer. Figure 4.2 represents a schematic diagram of an enzymatic biosensor. Enzymatic biosensor measures the activity of enzymes selectively upon interaction with a specific target and generates a biological signal proportional to the target analyte concentration. These biosensors have been used to detect analytes at nanoscale level especially with an electrochemical transducer. There is a heterogeneous electron transfer occurring between the electrode and the protein redox center which governs the performance of this biosensor. The enzymatic biosensor is the most common nanobiosensor developed till now, for e.g., glucose nanobiosensor for the rapid self-diagnosis of blood glucose levels (Lad et al. 2008; Trojanowicz 2002; Wang et al. 2014).
Schematic representation of the enzymatic biosensor. Reprinted with permission from Kurbanoglu et al. (2017). © 2018, Springer
4.3.1.2 Oligonucleotide (DNA/RNA) Biosensor
Oligonucleotide biosensors are diagnostic devices that consist of a probe (single-stranded DNA/RNA) that is in association with or integrated within a transducer or transducing micro nanosystem as shown in Fig. 4.3. The transducer employed for achieving high sensitivity can be electrochemical, optical, thermometric, piezoelectric, magnetic, or micromechanical (del Valle and Bonanni 2014). DNA based biosensor is a current approach and researchers are trying to develop DNA biosensors for low-cost detection of specific DNA sequences in human, viral, and bacterial nucleic acids, several attempts have been made to increase sensitivity and selectivity of the sensor (Zhao et al. 2014). Nanomaterials play an important role in DNA biosensor, i.e. it is used as a substrate for DNA attachment as well as amplifies the signal for hybridization, an enhanced amount of DNA immobilization occurs on nanomaterial and another advantage is that DNA maintains its biological activity. Nanoparticles like gold, cadmium sulfide, nanowires like silicon, nanotubes like carbon nanotubes, etc., are being employed for DNA biosensor (Sheehan and Whitman 2005; Shi et al. 2013). Electrochemical based DNA biosensor has been fabricated to detect genetic mutations. It can also be applied for gene analysis, detection of genetic disorders, clinical diagnostics, tissue matching, and forensic investigation (Odenthal and Gooding 2007).
General design of DNA biosensor. Reproduced from Asal et al. (2018). © 2018 (CC BY 4.0)
4.3.1.3 Immunosensor
Immunosensors are based on immobilized antibodies as a biological recognition element that is highly specific and has been widely applied in clinical analysis; a typical immunosensor is shown in Fig. 4.4. These have been generally used to detect disease at a molecular level. Microorganisms like Escherichia coli, Salmonella, Staphylococcus aureus, pesticides, herbicides, etc., have been detected with high accuracy (Shirale et al. 2010). Viral antigens of tumor have been detected upon the interaction of antigen with the antibody receptor by measuring the response in conductivity across the immunosensor surface and change in the resistance (Bahadır and Sezgintürk 2015; Cruz et al. 2002; Shirale et al. 2010).
Schematic diagram of immunosensor binding configuration. (a) Sandwich structure formation, (b) competitive style immunoassays and (c) extended sandwich structure formation (d) sandwich structure formation on a (micro-nanoparticle) surface. Reproduced from Asal et al. (2018). © 2018, (CC BY 4.0)
4.3.1.4 Aptamer Biosensor
Aptamers are a new class of oligonucleotide/nucleic acid recognition elements as they have high selectivity and affinity towards their target. These are single-stranded nucleic acid or peptide molecules having a size less than 25 kDa with a natural or synthetic origin. They can be used for the development of a sensor for DNAs, proteins, and small molecules. The nucleic acid molecules are selected by an in vitro selection process called SELEX (Systematic Evolution of Ligands by Exponential Enrichment) from random sequence libraries (Radko et al. 2007; Sassolas et al. 2009). They have been developed widely for diagnostic applications, e.g. detection of a wide range of non-nucleic acid analytes. DNA aptamer biosensor has also been used for the separation or capture of pathogens and small molecules (Nguyen et al. 2009). These biosensors have been created for a variety of targets such as proteins, peptides, small organics, and whole cells. It has been immobilized on a variety of transducers for the detection of proteins (Liu et al. 2011).
4.3.1.5 Microbial Biosensor
Microbial nanobiosensor is an analytical device which comprises nanomaterials as transducer and an immobilized viable or non-viable microorganism or whole cell. This type of nanobiosensor generates a measurable signal proportional to the concentration of analytes (Shin 2011). Several microbial nanobiosensors have been developed for the detection of glucose on the basis of oxygen consumption of the respiratory activity in the microbes (D’souza 2001). Electrochemical and optical techniques have been commonly used for the development of such biosensors. The selectivity of such biosensors can be enhanced by blocking or inhibiting undesired metabolic pathways and transport mechanisms and inducing targeted metabolic activities (Gäberlein et al. 2000).
4.3.2 Classification on the Basis of Transducer
4.3.2.1 Electrochemical
Most of the nanobiosensors developed so far are based on electrochemical detection as they have many advantages over other sensing techniques such as fast response time, highly sensitive, low cost, low-interference characteristics, and compatible with microfabrication technology, small size, economical cost, minimum power requirement, ease of use, and low maintenance (Bertók et al. 2013; Lad et al. 2008). In this technique, biochemical reactions between the nanofabricated biomolecule or biological element and target analyte are being analyzed with the help of electrical means (Chaubey and Malhotra 2002; Cai et al. 2001). It works on the principle of electrochemistry. The readable electrochemical signals are detected during a bio interaction when an electron is consumed or produced and are measured by the electrochemical method (Chaubey and Malhotra 2002). The electrical signals quantitatively correspond with the concentration of analyte present in the sample (Ronkainen et al. 2010). In a study by Sistani et al. (2014), biochemical detection of penicillin was done successfully using penicillinase enzyme immobilized on nanoparticles (Sistani et al. 2014). Electrochemical nanosensors can be categorized into potentiometric, conductometric, amperometric, and impedimetric biosensors according to their working principle.
Potentiometric nanosensor is based on measuring the potential of a system at a working electrode with respect to a sensitive and accurate reference electrode under zero current flow (Koncki et al. 2000). This potential is generated by converting the biorecognition process and obtaining analytical information of a system (Dzyadevych et al. 2004). The potential signal is generated due to the accumulation of ions at ion-selective electrodes and ion-sensitive field-effect transistors at equilibrium (Koncki et al. 2000). It has been well-established and utilized in the biomedical field, in the detection of various analytes such as antibiotics, preservatives, heavy metals, and pesticides in agricultural and food industries (Durán and Marcato 2013).
Conductometric nanosensor measures change in conductivity of the solution once the interaction of target and immobilized analyte is achieved. The principle is based on a change occurring in electrical resistance between two parallel electrodes during a biochemical reaction (Mikkelsen and Rechnitz 1989; Muhammad-Tahir and Alocilja 2003).
Amperometric nanosensor allows subsequent measurement of current produced by oxidation or reduction of an electroactive species in an electrochemical reaction. They have been mostly used in medical devices and offer many advantages over other electrochemical sensors as they are highly sensitive, fast, precise, accurate, and economical and have a wide linear range (Wang 1999). It has been used in fields of health and diagnostics like development of ATP sensor (Kueng et al. 2004) and beta-HCG sensor (Santandreu et al. 1999) for pregnancy test, in environmental and agriculture like detection of organophosphates, ractopamine, sulfonamides, and hydrogen peroxide (Lin et al. 2013; Xu et al. 2013; Yan et al. 2013).
Impedimetric nanosensors have not been used frequently as compared to other electrochemical sensors. It basically measures the electrical impedance of a particular biological system and gives analytical information about that system (Chuang et al. 2011; Huang et al. 2008).
4.3.2.2 Optical
Optical nanobiosensor measures the change in optical signal and this change is recorded in resonant frequency after the interaction between the analyte and a resonator which oscillates a light within a cavity (Vo-Dinh 2005). They are a powerful and versatile detection tool and highly sensitive to biomolecular targets and provide a quick response. They are insensitive to electromagnetic interference. Optical methods include surface plasmon resonance (SPR), localized surface plasmon resonance (LSPR), surface-enhanced Raman scattering, fluorescence spectroscopy, colorimetric spectroscopy, total internal reflectance, light rotation, and polarization (Borisov and Wolfbeis 2008).
Surface plasmon resonance (SPR) is an optical sensing technique that involves the interaction of light with the electrons of metal and hence causes transfer of energy to electrons present at the surface of the metal (Haes and Duyne 2004). SPR based biosensors have been successfully utilized in fundamental biological studies, drug discovery, health science research, and clinical diagnosis and can detect a wide range of analytes such as proteins, small molecules, antibody–antigen, DNA and RNA hybridization, concanavalin A, antibiotics, mycotoxins, and pathogen like E. coli (Liedberg et al. 1983).
In localized surface plasmon resonance (LSPR), a local oscillation occurs in the close proximity of metallic nanoparticles (MNPs). Light interacting with the MNPs are much smaller than the incident wavelength. This technique has been utilized successfully due to its label-free method as they do not require labeling of the target molecule with any kind of reagent and high sensitivity favors the method (Jia et al. 2012).
Fluorescence biosensor measures change in fluorescence which recognizes and gives information on the presence, activity, or conformation of a given target specifically and quantitatively and hence provides the dynamic molecular behavior. They are known to have high sensitivity, quick response and have the ability to achieve high spatial resolution through spectroscopic and imaging methods. Detection of some analytes such as nitrite, reactive oxygen species, pathogenic bacteria such as S. aureus, V. parahaemolyticus, S. typhimurium, and E. coli have been done by this technique (Chen et al. 2016; Dasary et al. 2008; Hu et al. 2014; Wu et al. 2014).
Colorimetric biosensor measures the change in absorption when a reaction proceeds and forms a colored product. It has been applied to a diverse field such as environmental detection of the toxic metal, clinical diagnosis of analytes, such as glucose, cancer biomarkers, and viruses (Cao et al. 2014; Chen et al. 2014; Sener et al. 2014; Zhang et al. 2014).
4.3.2.3 Piezoelectric
The piezoelectric sensor measures the change in the resonant frequency of piezoelectric quartz which oscillates under the effect of electric field and this frequency is proportional to surface adsorbed mass which changes due to absorption or desorption of molecules from the surface of the quartz. The crystals contain biorecognition element on its surface which selectively binds with the target and causes a change in mass. It has been applied for the detection of viruses, bacteria, proteins, and nucleic acids due to their great sensitivity and selectivity (Borman 1987; Durmuş et al. 2015; Tilmaciu and Morris 2015).
4.4 Applications of Nanobiosensor
Nanobiosensor has diverse applications in various fields like biomedical, environmental, and agricultural industry, etc., as highlighted in Fig. 4.5. Blood glucose sensing is the most common and major application in biosensing due to its plentiful market potential. Biosensors have incredible market value in other fields, but commercial adoption was slow due to several technological difficulties. Biosensor contamination is a major problem, due to the presence of biomolecules along with semiconductor materials.
Current developments in the field of nanoelectronics, biological, and information technology present way to develop medical nanorobots which are implanted and incorporated devices. The nanorobotics consists of main sensing, actuation, data transmission, remote control uploading, and coupling power supply subsystems (Appenzeller et al. 2002; Cavalcanti et al. 2007; Freitas 1999; Lavan et al. 2003; Liu and Shimohara 2007; Shi et al. 2007). Nanorobots should offer new tools for common medical treatments because it has nanoscopic quality (Leary et al. 2006; Patel et al. 2006). Table 4.1 lists different nanomaterials used for fabricating nanobiosensor with various sensing techniques and applications in diverse fields.
4.4.1 Detection of Glucose/Other Metals
Conventional methods are often tiresome in diabetes detection and monitoring. To eliminate this problem, the nanorobot sensor should be used to sense biological changes linked with hyperglycemia as it generates proteomic-based information (Cavalcanti et al. 2008). This will also make it possible to treat diabetes more quickly and effectively (Cash and Clark 2010; Gordijo et al. 2011; Samuel et al. 2010). Recently, Yang et al developed a very sensitive colorimetric detection method to detect early diabetes analysis by glucose detection in urine as an alternative of the blood sample, which can prevent the painful blood collection and infection risks. They projected a method to couple Fe/Pd/rGO with a portable paper sensor for urine glucose detection. From this method urine glucose was sensitively detected in a broad range of 1–200 μM with a limit of detection of 1.76 μM. The color difference with increasing concentration of urine glucose was easily visualized by naked eyes, which is significant to its realistic usage in screening and diagnosis of diabetes in early-stage (Yang et al. 2019).
Song et al. developed a novel strategy for clinical detection of Zn2+ in the human body based on the “on–off–on” ratiometric fluorescent nanosensor using the coupled quantum dots-carbon dots (Song et al. 2018). Copper is directly associated with liver damage; therefore, it is necessary to develop a simple and sensitive strategy to detect copper ions (Cu2+) in liver cells.
Lu et al. developed hydrophobic carbon dots (HCDs)-based dual-emission fluorescent probe for Cu2+ detection. The developed probe showed high sensitivity and selectivity to Cu2+ above former substances, and it was used to determine the changes of Cu2+ level in liver cells (Lu et al. 2017). Qian et al. developed a QDs nanosensor for the detection of 2,4,6-trinitrotoluene (TNT) explosives concentration over a range of 10 nM to 8 μM with a low detection limit of 3.3 nM. One can perform onsite visual determination of TNT with a high resolution because the ratiometric fluorescence nanosensing system exhibited visible fluorescence color changes (Qian et al. 2016).
4.4.2 Detection of Biomolecular Interaction
The elevated sensitivity of the localized surface plasmon resonance (LSPR) spectrum of nanomaterials to adsorbate induced changes in the local refractive index is being used to develop a different class of nanobiosensors or chemosensor (Baida et al. 2009; Brockman et al. 2000; Huang et al. 2009; Nguyen et al. 2015).The optical biosensor detects changes in local refractive index prototypical immunoassay involving biotin (B) and anti-biotin (AB) by monitoring the LSPR (Riboh et al. 2003). Liu et al. (2018b) studied an improved biomolecular interaction analysis probing with an incorporated array fluorescent biosensor with a wide range of low to high concentrations of anti-bisphenol A (BPA) antibody. This scheme is characterized by its high-throughput cross-reactivity analysis between antigens and antibodies on the patterned waveguide with a shortened time-to-result (Liu et al. 2018b). Recently, Zhang et al. prepared biosensor by Ag nanocubes/chitosan composite for the detection of mouse IgG and used to amplify the SPR signal (Zhang et al. 2016). A direct reasonable enzyme-linked immunosorbent assay format was developed and optimized on the surface of a carbon electrode by immobilizing the antibody using an electro-deposition of gold nanoparticles conjugated with polyclonal anti-tetracycline antibodies for detection of tungro disease in paddy plantations (Uda et al. 2019).
4.4.3 Pathogenic Bacteria Detection
Traditionally, bacteria identification in culture and biochemical testing are based on morphology. However, these methods are tiresome and occasionally all bacteria do not grow in culture and therefore the method of detection of bacteria in clinical samples needs to be developed. Magnetic nanoparticles have been used for the identification of Mycobacterium avium spp. paratuberculosis (MAP) through magnetic relaxation by Kaittanis et al. (2007). Nanobiosensor can be used for direct detection of the pathogenic agent while indirect detection is achievable by evaluating the pathogen’s metabolic activity by monitoring the nutrient utilization rate in solution. The dextran-coated gold nanoparticle-based technique is used for the measurement of antimicrobial susceptibility (Nath et al. 2008). Salmonella was detected by a gold/silicon nanorods immobilized with dye molecules (Fu et al. 2008). The dye molecules immobilized with silicon nanorods produced fluorescence when it comes in contact with Salmonella. This method has remarkable potential in biomedical diagnostics.
Some researcher has also developed a protocol for a simpler diagnostic technique for bacteria. These techniques have unique features that combine magnetic and fluorescent parameters in a nanoparticle-based platform. The magneto-fluorescent nanosensor (MFnS) has been developed that detected E. coli O157:H7 contamination with very high sensitivity, 1 colony-forming unit present in water can be detected (Banerjee et al. 2016; Shelby et al. 2017). This method has also been used to detect and quantify the pathogen contamination in both early- and late-stage contamination (Song et al. 2016).
4.4.4 Application in Cancer Biology
Early cancer detection and cure is a rising and attractive ground for research in plasmonic nanobiosensor. Telomerase is a specific reverse transcriptase enzyme containing catalytic subunit and RNA component that maintains the length and function of the telomer together with the proteins associated with the telomere (Greider and Blackburn 1985; Van Steensel and De Lange 1997; Van Steensel et al. 1998). Eventually, a critical telomere length is reached in normal cells, inducing cellular senescence and ultimately leading to apoptosis. In most malignancies, high levels of telomerase activity are found and are thought to play an important role in tumorigenesis (Bodnar et al. 1998; Counter et al. 1998; Kim et al. 1994; Shay and Bacchetti 1997). Telomere dysfunction also causes genetic instability with complex cellular and molecular responses involving checkpoints and apoptosis pathways for the retinoblastoma gene/p53 (Lan et al. 2003; Leri et al. 2003). Grimm et al have developed a magnetic-based nanobiosensor for fast screening of telomerase activity in biological samples (Gullberg et al. 2004). The telomerase-synthesized telomeric repeats (TTAGGG) annealed upon nanoparticles to change their magnetic state (a phenomenon readily detectable by magnetic readers), the developed magnetic nanosensor can determine telomerase activity. Recently, an optical fiber nanobiosensor was built to efficiently sense a broad-spectrum cancer biomarker, telomerase with its nanoscale tip at a single cell level (Zheng and Li 2010).
Injectable biosensors might be providing a new concept for prostate cancer biomarkers by querying the status of the prostate via a non-invasive readout. Proteases enzyme plays an important role in every characteristic of cancer; its activities could act as biomarkers. Dudani et al. developed a nanosensor library by a panel of prostate cancer proteases through transcriptomic and proteomic analysis that detect protease activity in vitro and in vivo using fluorescence and urinary readouts (Dudani et al. 2018). The impact of nanomaterials on the accuracy of biosensors in early detection of cancer such as lung, prostate, breast, and other cancers was discussed and reviewed by Sharifi et al. The modification of electrode performance by nanomaterials, however, is relatively complicated, resulting in limitations on the use of certain nanomaterials in biosensor applications (Sharifi et al. 2018).Wang et al. developed a small device that facilitated fast and straight analysis of the specific binding of small molecules to proteins using silicon nanowire (SiNW) field-effect transistor (FET) devices (Wang et al. 2005). Chandra et al. designed a sensor probe made-up by immobilizing monoclonal permeability glycoprotein antibody on the gold nanoparticles (AuNPs) conducting polymer composite to detect cancer cells between 50 and 100,000 cells/ml with the detection limit of 23 ± 2 cells/ml (Chandra et al. 2015). Thus, nanosensor can help in the diagnosis of fatal diseases like cancer at an early stage.
4.5 Conclusions and Future Prospects
There is a great demand of analytical devices for rapid, reliable, and economical detection of substances in biological fluids. These devices will be commercialized only if they can be used by a common man rather than by centralized labs or doctor’s clinics; biosensors are competent enough to solve these issues. Although biosensors have applications in various fields, their use in health care monitoring is the most important. Nanobiosensor is probed containing immobilized biological molecules on the nanomaterial. They can be used in the detection of microorganisms, pollutants, molecular biomarkers, and monitoring of metabolites in body fluids. Different kinds of nanomaterials like metal, QD, graphene, carbon nanotubes, etc., can be used in the biosensor, each having its own characteristic property. For example, the SPR property of metal nanoparticles is exploited in optical biosensors. Most of the biosensors which are commercialized are enzyme-based sensor as enzymes are highly specific biomolecules. Developments of fabrication methods for nanomaterials which are nontoxic and economically viable are required. Research is required to improve the properties of the nanobiosensor so that it can detect and quantify biological fluids without multiple calibrations using clinical samples. Efforts should be made to improve the sensitivity of detection with high sensitivity. Thus, nanobiosensor should be low cost, disposable, reliable, and easy to use that can be utilized for in-home medical diagnosis of diseases.
Abbreviations
- MNPs:
-
Magnetic nanoparticles
- AgNPs:
-
Silver nanoparticles
- Au:
-
Gold nanoparticles
- QDs:
-
Quantum dots
- CNTs:
-
Carbon nanotubes
- NW:
-
Nanowires
- NR:
-
Nanorods
- SiNW:
-
Silicon nanowire
- FET:
-
Field-effect transistor
- SPR:
-
Surface plasmon resonance
- LSPR:
-
Localized surface plasmon resonance
- SELEX:
-
Systematic evolution of ligands by exponential enrichment
- DMR:
-
Diagnostic magnetic resonance
- GNRs:
-
Gold nanorods
- MEMS:
-
Microelectromechanical system
- NEMS:
-
Nanoelectromechanical systems
- ml:
-
Microliter
- mM:
-
Millimolar
References
Ali ME, Hashim U, Mustafa S, Che Man YB, Yusop MH, Kashif M, Dhahi TS, Bari MF, Hakim MA, Latif MA (2011) Nanobiosensor for detection and quantification of DNA sequences in degraded mixed meats. J Nanomater 2011:32–43
Ambhorkar P, Wang Z, Ko H, Lee S, Koo KI, Kim K, Cho DI (2018) Nanowire-based biosensors: from growth to applications. Micromachines 9:679
Androvitsaneas P, Young AB, Schneider C, Maier S, Kamp M, Höfling S, Knauer S, Harbord E, Hu CY, Rarity JG, Oulton R (2016) Charged quantum dot micropillar system for deterministic light-matter interactions. Phys Rev B 93:241409
Appenzeller J, Martel R, Derycke V, Radosavljević M, Wind S, Neumayer D, Avouris P (2002) Carbon nanotubes as potential building blocks for future nanoelectronics. Microelectron Eng 64:391–397
Asal M, Ozen O, Şahinler M, Polatoğlu İ (2018) Recent developments in enzyme, DNA and immuno-based biosensors. Sensors 18:1924
Bahadır EB, Sezgintürk MK (2015) Applications of electrochemical immunosensors for early clinical diagnostics. Talanta 132:162–174
Baida H, Billaud P, Marhaba S, Christofilos D, Cottancin E, Crut A, Lermé J, Maioli P, Pellarin M, Broyer M, Del Fatti N (2009) Quantitative determination of the size dependence of surface plasmon resonance damping in single Ag@ SiO2 nanoparticles. Nano Lett 9:3463–3469
Bakalova R, Ohba H, Zhelev Z, Ishikawa M, Baba Y (2004) Quantum dots as photosensitizers? Nat Biotechnol 22:1360–1361
Bamrungsap S, Treetong A, Apiwat C, Wuttikhun T, Dharakul T (2016) SERS-fluorescence dual mode nanotags for cervical cancer detection using aptamers conjugated to gold-silver nanorods. Microchim Acta 183:249–256
Banerjee T, Sulthana S, Shelby T, Heckert B, Jewell J, Woody K, Karimnia V, McAfee J, Santra S (2016) Multiparametric magneto-fluorescent nanosensors for the ultrasensitive detection of Escherichia coli O157: H7. ACS Infect Dis 2:667–673
Barahona F, Bardliving CL, Phifer A, Bruno JG, Batt CA (2013) An aptasensor based on polymer-gold nanoparticle composite microspheres for the detection of malathion using surface-enhanced Raman spectroscopy. Ind Biotechnol 9:42–50
Baruah S, Dutta J (2009) Nanotechnology applications in pollution sensing and degradation in agriculture: a review. Environ Chem Lett 7:191–204
Bertók T, Katrlík J, Gemeiner P, Tkac J (2013) Electrochemical lectin based biosensors as a label-free tool in glycomics. Microchim Acta 180:1–13
Bhushan B (2007) Nanotribology and nanomechanics of MEMS/NEMS and BioMEMS/BioNEMS materials and devices. Microelectron Eng 84:387–412
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–352
Borisov SM, Wolfbeis OS (2008) Optical biosensors. Chem Rev 108:423–461
Borman S (1987) Optical and piezoelectric biosensors. Anal Chem 59:1161A–1164A
Brockman JM, Nelson BP, Corn RM (2000) Surface plasmon resonance imaging measurements of ultrathin organic films. Annu Rev Physiol 51:41–63
Bulovic V, Mandell A, Perlman A (2004) Molecular memory device. U.S. Patent No. 6,781,868. Washington, DC: U.S. Patent and Trademark Office
Cai H, Xu C, He P, Fang Y (2001) Colloid Au-enhanced DNA immobilization for the electrochemical detection of sequence-specific DNA. J Electroanal Chem 510:78–85
Cao Z, Zeng M-X, Zhang L, Huang X-X, Wang M-X, Gong F-C, Tan S-Z (2009) Novel optical nanobiosensor assembled with silver nanoparticles on gold surface. In: 2009 4th IEEE international conference on nano/micro engineered and molecular systems. IEEE, Piscataway, pp 987–990
Cao K, Jiang X, Yan S, Zhang L, Wu W (2014) Phenylboronic acid modified silver nanoparticles for colorimetric dynamic analysis of glucose. Biosens Bioelectron 52:188–195
Cash KJ, Clark HA (2010) Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol Med 16:584–593
Cavalcanti A, Shirinzadeh B, Freitas R Jr, Hogg T (2007) Nanorobot architecture for medical target identification. Nanotechnology 19:015103
Cavalcanti A, Shirinzadeh B, Kretly LC (2008) Medical nanorobotics for diabetes control. Nanomed Nanotechnol 4:127–138
Chandra P, Noh H-B, Pallela R, Shim Y-B (2015) Ultrasensitive detection of drug resistant cancer cells in biological matrixes using an amperometric nanobiosensor. Biosens Bioelectron 70:418–425
Chaubey A, Malhotra B (2002) Mediated biosensors. Biosens Bioelectron 17:441–456
Chen S, Hai X, Chen X-W, Wang J-H (2014) In situ growth of silver nanoparticles on graphene quantum dots for ultrasensitive colorimetric detection of H2O2 and glucose. Anal Chem 86:6689–6694
Chen C, Yuan Z, Chang H-T, Lu F, Li Z, Lu C (2016) Silver nanoclusters as fluorescent nanosensors for selective and sensitive nitrite detection. Anal Methods 8:2628–2633
Chuang Y-H, Chang Y-T, Liu K-L, Chang H-Y, Yew T-R (2011) Electrical impedimetric biosensors for liver function detection. Biosens Bioelectron 28:368–372
Counter CM, Meyerson M, Eaton EN, Ellisen LW, Caddle SD, Haber DA, Weinberg RA (1998) Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene 16:1217–1222
Cruz HJ, Rosa CC, Oliva AG (2002) Immunosensors for diagnostic applications. Parasitol Res 88:S4–S7
D’souza S (2001) Microbial biosensors. Biosens Bioelectron 16:337–353
Darestani-Farahani M, Faridbod F, Ganjali MR (2018) A sensitive fluorometric DNA nanobiosensor based on a new fluorophore for tumor suppressor gene detection. Talanta 190:140–146
Dasary SS, Rai US, Yu H, Anjaneyulu Y, Dubey M, Ray PC (2008) Gold nanoparticle based surface enhanced fluorescence for detection of organophosphorus agents. Chem Phys Lett 460:187–190
del Valle M, Bonanni A (2014) Impedimetric DNA biosensors based on nanomaterials. Biosens Nanotechnol 18:81–110
Devi R, Yadav S, Nehra R, Yadav S, Pundir C (2013) Electrochemical biosensor based on gold coated iron nanoparticles/chitosan composite bound xanthine oxidase for detection of xanthine in fish meat. J Food Eng 115:207–214
Di Giusto DA, Wlassoff WA, Gooding JJ, Messerle BA, King GC (2005) Proximity extension of circular DNA aptamers with real-time protein detection. Nucleic Acids Res 33:e64–e64
Dudani JS, Ibrahim M, Kirkpatrick J, Warren AD, Bhatia SN (2018) Classification of prostate cancer using a protease activity nanosensor library. Proc Natl Acad Sci U S A 115:8954–8959
Durán N, Marcato PD (2013) Nanobiotechnology perspectives. Role of nanotechnology in the food industry: a review. Int J Food Sci Technol 48:1127–1134
Durán N, Marcato PD, Conti RD, Alves OL, Costa F, Brocchi M (2010) Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J Braz Chem Soc 21:949–959
Durmuş NG, Lin RL, Kozberg M, Dermici D, Khademhosseini A, Demirci U (2015) Acoustic-based biosensors. In: Encyclopedia of microfluidics and nanofluidics. Springer, New York, pp 8–40
Dzyadevych SV, Arkhypova VN, Martelet C, Jaffrezic-Renault N, Chovelon JM, El'skaya AV, Soldatkin AP (2004) Potentiometric biosensors based on ISFETs and immobilized cholinesterases. Electroanalysis 16:1873–1882
Eissa S, Alshehri N, Rahman AMA, Dasouki M, Abu-Salah KM, Zourob M (2018) Electrochemical immuno sensors for the detection of survival motor neuron (SMN) protein using different carbon nanomaterials-modified electrodes. Biosens Bioelectron 101:282–289
Fan X, White IM, Shopova SI, Zhu H, Suter JD, Sun Y (2008) Sensitive optical biosensors for unlabeled targets: a review. Anal Chim Acta 620:8–26
Feng X, Gan N, Zhang H, Li T, Cao Y, Hu F, Jiang Q (2016) Ratiometric biosensor array for multiplexed detection of microRNAs based on electrochemiluminescence coupled with cyclic voltammetry. Biosens Bioelectron 75:308–314
Frasconi M, Tel-Vered R, Riskin M, Willner I (2010) Surface plasmon resonance analysis of antibiotics using imprinted boronic acid-functionalized Au nanoparticle composites. Anal Chem 82:2512–2519
Freitas RA (1999) Nanomedicine, volume I: basic capabilities vol 1. Landes Bioscience, Georgetown
Fu J, Park B, Siragusa G, Jones L, Tripp R, Zhao Y, Cho Y-J (2008) An Au/Si hetero-nanorod-based biosensor for Salmonella detection. Nanotechnology 19:155502
Gäberlein S, Spener F, Zaborosch C (2000) Microbial and cytoplasmic membrane-based potentiometric biosensors for direct determination of organophosphorus insecticides. Appl Microbiol Biotechnol 54:652–658
Gomes RC, Abib Pastore VA, Martins OA, Biondi GF (2015) Nanotechnology applications in the food industry: a review. Braz J Hyg Anim Sanity 1:1–8
Gordijo CR, Koulajian K, Shuhendler AJ, Bonifacio LD, Huang HY, Chiang S, Ozin GA, Giacca A, Wu XY (2011) Nanotechnology-enabled closed loop insulin delivery device: in vitro and in vivo evaluation of glucose-regulated insulin release for diabetes control. Adv Funct Mater 21:73–82
Greider CW, Blackburn EH (1985) Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43:405–413
Gullberg M, Gústafsdóttir SM, Schallmeiner E, Jarvius J, Bjarnegård M, Betsholtz C, Landegren U, Fredriksson S (2004) Cytokine detection by antibody-based proximity ligation. Proc Natl Acad Sci U S A 101:8420–8424
Haes AJ, Duyne RPV (2004) Preliminary studies and potential applications of localized surface plasmon resonance spectroscopy in medical diagnostics. Expert Rev Mol Diagn 4:527–537
Haes AJ, Van Duyne RP (2002) A nanoscale optical biosensor: sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. J Am Chem Soc 124:10596–10604
He W, Huang CZ, Li YF, Xie JP, Yang RG, Zhou PF, Wang J (2008) One-step label-free optical genosensing system for sequence-specific DNA related to the human immunodeficiency virus based on the measurements of light scattering signals of gold nanorods. Anal Chem 80:8424–8430
Hernández-Ibáñez N, García-Cruz L, Montiel V, Foster CW, Banks CE, Iniesta J (2016) Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens Bioelectron 77:1168–1174
Hu L, Deng L, Alsaiari S, Zhang D, Khashab NM (2014) “Light-on” sensing of antioxidants using gold nanoclusters. Anal Chem 86:4989–4994
Huang Y, Bell MC, Suni II (2008) Impedance biosensor for peanut protein AraH1. Anal Chem 80:9157–9161
Huang C, Bonroy K, Reekman G, Verstreken K, Lagae L, Borghs G (2009) An on-chip localized surface plasmon resonance-based biosensor for label-free monitoring of antigen–antibody reaction. Microelectron Eng 86:2437–2441
Jia K, Bijeon JL, Adam PM, Ionescu RE (2012) Sensitive localized surface plasmon resonance multiplexing protocols. Anal Chem 84:8020–8027
Kaittanis C, Naser SA, Perez JM (2007) One-step, nanoparticle-mediated bacterial detection with magnetic relaxation. Nano Lett 7:380–383
Kelly KL, Coronado E, Zhao LL, Schatz GC (2003) The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B 107(3):668–677
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PD, Coviello GM, Wright WE, Weinrich SL, Shay JW (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2015
Kim F, Song JH, Yang P (2002) Photochemical synthesis of gold nanorods. J Am Chem Soc 124:14316–14317
Kim JH, Ahn JH, Barone PW, Jin H, Zhang J, Heller DA, Strano MS (2010) A luciferase/single-walled carbon nanotube conjugate for near-infrared fluorescent detection of cellular ATP. Angew Chem Int Ed 49:1456–1459
Ko S, Park TJ, Kim H-S, Kim J-H, Cho Y-J (2009) Directed self-assembly of gold binding polypeptide-protein A fusion proteins for development of gold nanoparticle-based SPR immunosensors. Biosens Bioelectron 24:2592–2597
Koncki R, Radomska A, Głąb S (2000) Potentiometric determination of dialysate urea nitrogen. Talanta 52:13–17
Kueng A, Kranz C, Mizaikoff B (2004) Amperometric ATP biosensor based on polymer entrapped enzymes. Biosens Bioelectron 19:1301–1307
Kulesza A, Mitrić R, Bonačić-Koutecký V (2009) Silver cluster induced absorption enhancement and conformation control of peptides. Eur Phys J D 52:203–206
Kurbanoglu S, Rivas L, Ozkan SA, Merkoçi A (2017) Electrochemically reduced graphene and iridium oxide nanoparticles for inhibition-based angiotensin-converting enzyme inhibitor detection. Biosens Bioelectron 88:122–129
Lad U, Khokhar S, Kale GM (2008) Electrochemical creatinine biosensors. Anal Chem 80:7910–7917
Lan J, Xiong Y-Y, Lin Y-X, Wang B-C, Gong L-L, Xu H-S, Guo G-S (2003) Helicobacter pylori infection generated gastric cancer through p53-Rb tumor-suppressor system mutation and telomerase reactivation. World J Gastroenterol 9:54–58
Landry MP, Ando H, Chen AY, Cao J, Kottadiel VI, Chio L, Yang D, Dong J, Lu TK, Strano MS (2017) Single-molecule detection of protein efflux from microorganisms using fluorescent single-walled carbon nanotube sensor arrays. Nat Nanotechnol 12:368–377
Lao Y-H, Chi C-W, Friedrich SM, Peck K, Wang T-H, Leong KW, Chen L-C (2016) Signal-on protein detection via dye translocation between aptamer and quantum dot. ACS Appl Mater Interfaces 8:12048–12055
Lavan DA, McGuire T, Langer R (2003) Small-scale systems for in vivo drug delivery. Nat Biotechnol 21:1184–1191
Leary SP, Liu CY, Apuzzo ML (2006) Toward the emergence of nanoneurosurgery: part III--nanomedicine: targeted nanotherapy, nanosurgery, and progress toward the realization of nanoneurosurgery. Neurosurgery 58:1009–1026
Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F, Nadal-Ginard B, Kajstura J, Anversa P, Blasco MA (2003) Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J 22:131–139
Li X, Zhao C, Liu X (2015) A paper-based microfluidic biosensor integrating zinc oxide nanowires for electrochemical glucose detection. Microsyst Nanoeng 1:15014
Li S, Fu Y, Ma X, Zhang Y (2017) Label-free fluorometric detection of chymotrypsin activity using graphene oxide/nucleic-acid-stabilized silver nanoclusters hybrid materials. Biosens Bioelectron 88:210–216
Liao QG, Wei BH, Luo LG (2017) Aptamer based fluorometric determination of kanamycin using double-stranded DNA and carbon nanotubes. Microchim Acta 184:627–632
Liedberg B, Nylander C, Lunström I (1983) Surface plasmon resonance for gas detection and biosensing. Sensors Actuators 4:299–304
Lin Y, Lu F, Tu Y, Ren Z (2004) Glucose biosensors based on carbon nanotube nanoelectrode ensembles. Nano Lett 4:191–195
Lin K-C, Hong C-P, Chen S-M (2013) Simultaneous determination for toxic ractopamine and salbutamol in pork sample using hybrid carbon nanotubes. Sensors Actuators B Chem 177:428–436
Lin B, Yu Y, Li R, Cao Y, Guo M (2016) Turn-on sensor for quantification and imaging of acetamiprid residues based on quantum dots functionalized with aptamer. Sensors Actuators B Chem 229:100–109
Link S, Wang ZL, El-Sayed M (1999) Alloy formation of gold− silver nanoparticles and the dependence of the plasmon absorption on their composition. J Phys Chem B 103:3529–3533
Liu J-Q, Shimohara K (2007) Molecular computation and evolutionary wetware: a cutting-edge technology for artificial life and nanobiotechnologies. IEEE Trans Syst 37:325–336
Liu Y, Yan J, Howland MC, Kwa T, Revzin A (2011) Micropatterned aptasensors for continuous monitoring of cytokine release from human leukocytes. Anal Chem 83:8286–8292
Liu C, Hu Y-L, Deng W-J, Pan Q-S, Yi J-T, Chen T-T, Chu X (2018a) A graphene oxide nanosensor enables the co-delivery of aptamer and peptide probes for fluorescence imaging of a cascade reaction in apoptotic signaling. Analyst 143:208–214
Liu L, Zhou X, Ma R, He M, Shi H, Yi Q (2018b) High-throughput biomolecular interaction analysis probing by an array fluorescent biosensor platform. Sensors Actuators B Chem 259:888–893
Lu L, Feng C, Xu J, Wang F, Yu H, Xu Z, Zhang W (2017) Hydrophobic-carbon-dot-based dual-emission micelle for ratiometric fluorescence biosensing and imaging of Cu2+ in liver cells. Biosens Bioelectron 92:101–108
Lv Y et al (2009) Silver nanoparticle-decorated porous ceramic composite for water treatment. J Membr Sci 331:50–56
Marcato PD, Durán N (2008) New aspects of nanopharmaceutical delivery systems. J Nanosci Nanotechnol 8:2216–2229
Mikkelsen SR, Rechnitz GA (1989) Conductometric transducers for enzyme-based biosensors. Anal Chem 61:1737–1742
Muhammad-Tahir Z, Alocilja EC (2003) A conductometric biosensor for biosecurity. Biosens Bioelectron 18:813–819
Nath S, Kaittanis C, Tinkham A, Perez JM (2008) Dextran-coated gold nanoparticles for the assessment of antimicrobial susceptibility. Anal Chem 80:1033–1038
Nguyen T, Hilton JP, Lin Q (2009) Emerging applications of aptamers to micro-and nanoscale biosensing. Microfluid Nanofluid 6:347
Nguyen H, Park J, Kang S, Kim M (2015) Surface plasmon resonance: a versatile technique for biosensor applications. Sensors 15:10481–10510
Odenthal KJ, Gooding JJ (2007) An introduction to electrochemical DNA biosensors. Analyst 132:603–610
Pai J-H, Yang C-T, Hsu H-Y, Wedding AB, Thierry B (2017) Development of a simplified approach for the fabrication of localised surface plasmon resonance sensors based on gold nanorods functionalized using mixed polyethylene glycol layers. Anal Chim Acta 974:87–92
Pak SC, Penrose W, Hesketh PJ (2001) An ultrathin platinum film sensor to measure biomolecular binding. Biosens Bioelectron 16:371–379
Pandikumar A, How GT, See TP, Omar FS, Jayabal S, Kamali KZ, Yusoff N, Jamil A, Ramaraj R, John SA, Lim HN (2014) Graphene and its nanocomposite material based electrochemical sensor platform for dopamine. RSC Adv 4:63296–63323
Pandit S, Dasgupta D, Dewan N, Prince A (2016) Nanotechnology based biosensors and its application. Pharm Innov 5:18
Pang Y, Wang C, Wang J, Sun Z, Xiao R, Wang S (2016) Fe3O4@ Ag magnetic nanoparticles for microRNA capture and duplex-specific nuclease signal amplification based SERS detection in cancer cells. Biosens Bioelectron 79:574–580
Panpradist N, Lai JJ (2016) Point-of-care diagnostics. In: Biomaterials nanoarchitectonics. Elsevier, London, pp 139–156
Park K, Drummy LF, Wadams RC, Koerner H, Nepal D, Fabris L, Vaia RA (2013) Growth mechanism of gold nanorods. Chem Mater 25:555–563
Patel GM, Patel GC, Patel RB, Patel JK, Patel M (2006) Nanorobot: a versatile tool in nanomedicine. J Drug Target 14:63–67
Pavlov V, Xiao Y, Shlyahovsky B, Willner I (2004) Aptamer-functionalized Au nanoparticles for the amplified optical detection of thrombin. J Am Chem Soc 126:11768–11769
Prasad S (2014) Nanobiosensors: the future for diagnosis of disease? Nanobiosens Dis Diagn 3:1–10
Qian J, Hua M, Wang C, Wang K, Liu Q, Hao N, Wang K (2016) Fabrication of l-cysteine-capped CdTe quantum dots based ratiometric fluorescence nanosensor for onsite visual determination of trace TNT explosive. Anal Chim Acta 946:80–87
Radko S, Rakhmetova SY, Bodoev N, Archakov A (2007) Aptamers as affinity reagents for clinical proteomics. Biochemistry 1:198–209
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Rai M, Gade A, Gaikwad S, Marcato PD, Durán N (2012) Biomedical applications of nanobiosensors: the state-of-the-art. J Braz Chem Soc 23:14–24
Razavi H, Janfaza S (2015) Medical nanobiosensors: a tutorial review. Nanomed J 2:74–87
Reza KK, Ali MA, Srivastava S, Agrawal VV, Biradar A (2015) Tyrosinase conjugated reduced graphene oxide based biointerface for bisphenol A sensor. Biosens Bioelectron 74:644–651
Riboh JC, Haes AJ, McFarland AD, Ranjit Yonzon C, Van Duyne RP (2003) A nanoscale optical biosensor: real-time immunoassay in physiological buffer enabled by improved nanoparticle adhesion. J Phys Chem B 107:1772–1780
Ronkainen NJ, Halsall HB, Heineman WR (2010) Electrochemical biosensors. Chem Soc Rev 39:1747–1763
Sahoo SK, Misra R, Parveen S (2017) Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed Cancer 8:73–124
Samuel D, Bharali D, Mousa SA (2010) The role of nanotechnology in diabetes treatment: current and future perspectives. Int J Nanotechnol 8:53–65
Santandreu M, Alegret S, Fabregas E (1999) Determination of β-HCG using amperometric immunosensors based on a conducting immunocomposite. Anal Chim Acta 396:181–188
Sassolas A, Blum LJ, Leca-Bouvier BD (2009) Electrochemical aptasensors. Electroanalysis 21:1237–1250
Sener G, Uzun L, Denizli A (2014) Colorimetric sensor array based on gold nanoparticles and amino acids for identification of toxic metal ions in water. ACS Appl Mater Interfaces 6:18395–18400
Shariati M (2018) The field effect transistor DNA biosensor based on ITO nanowires in label-free hepatitis B virus detecting compatible with CMOS technology. Biosens Bioelectron 105:58–64
Sharifi M, Avadi MR, Attar F, Dashtestani F, Ghorchian H, Rezayat SM, Saboury AA, Falahati M (2018) Cancer diagnosis using nanomaterials based electrochemical nanobiosensors. Biosens Bioelectron 126:773–784
Sharma A, Rogers KR (1994) Biosensors measurement. Sci Technol 5:461
Shay J, Bacchetti S (1997) A survey of telomerase activity in human cancer. Eur J Cancer 33:787–791
Sheehan PE, Whitman LJ (2005) Detection limits for nanoscale biosensors. Nano Lett 5:803–807
Shelby T, Sulthana S, McAfee J, Banerjee T, Santra S (2017) Foodborne pathogen screening using magneto-fluorescent nanosensor: rapid detection of E. coli O157: H7. J Vis Exp 127:e55821
Shi H, Xia T, Nel AE, Yeh JI (2007) Part II: coordinated biosensors–development of enhanced nanobiosensors for biological and medical applications. Nanomedicine 2:599–614
Shi S, Wang X, Sun W, Wang X, Yao T, Ji L (2013) Label-free fluorescent DNA biosensors based on metallointercalators and nanomaterials. Methods 64:305–314
Shin HJ (2011) Genetically engineered microbial biosensors for in situ monitoring of environmental pollution. Appl Microbiol Biotechnol 89:867–877
Shirale DJ, Bangar MA, Park M, Yates MV, Chen W, Myung NV, Mulchandani A (2010) Label-free chemiresistive immunosensors for viruses. Environ Sci Technol 44:9030–9035
Sistani P, Sofimaryo L, Masoudi ZR, Sayad A, Rahimzadeh R, Salehi B (2014) A penicillin biosensor by using silver nanoparticles. Int J Electrochem Sci 9:6201–6212
Song C, Li J, Liu J, Liu Q (2016) Simple sensitive rapid detection of Escherichia coli O157: H7 in food samples by label-free immunofluorescence strip sensor. Talanta 156:42–47
Song Q, Ma Y, Wang X, Tang T, Song Y, Ma Y, Xu G, Wei F, Cen Y, Hu Q (2018) “On-off-on” fluorescent system for detection of Zn2+ in biological samples using quantum dots-carbon dots ratiometric nanosensor. J Colloid Interface Sci 516:522–528
Spain E, Gilgunn S, Sharma S, Adamson K, Carthy E, O’Kennedy R, Forster RJ (2016) Detection of prostate specific antigen based on electrocatalytic platinum nanoparticles conjugated to a recombinant scFv antibody. Biosens Bioelectron 77:759–766
Tilmaciu C-M, Morris MC (2015) Carbon nanotube biosensors. Front Chem 3:59
Trojanowicz M (2002) Determination of pesticides using electrochemical enzymatic biosensors. Electroanalysis 14:1311–1328
Turner AP (2000) Biosensors--sense and sensitivity. Science 290:1315–1317
Uda MN, Hasfalina CM, Samsuzanaa AA, Faridah S, Gopinath SC, Parmin NA, Hashim U, Mat M (2019) A disposable biosensor based on antibody-antigen interaction for tungro disease detection. In: Nanobiosensors for biomolecular targeting. Elsevier, London, pp 147–164
Vaishanav SK, Korram J, Nagwanshi R, Ghosh KK, Satnami ML (2017) Mn2+ doped-CdTe/ZnS modified fluorescence nanosensor for detection of glucose. Sensors Actuators B Chem 245:196–204
Van Steensel B, De Lange T (1997) Control of telomere length by the human telomeric protein TRF1. Nature 385:740–743
Van Steensel B, Smogorzewska A, De Lange T (1998) TRF2 protects human telomeres from end-to-end fusions. Cell 92:401–413
Velasco-Garcia M (2009) Optical biosensors for probing at the cellular level: a review of recent progress and future prospects. In: Seminars in cell & developmental biology, vol 1. Elsevier, London, pp 27–33
Vo-Dinh T (2005) Optical nanosensors for detecting proteins and biomarkers in individual living cells. In: Protein nanotechnology. Springer, Cham, pp 383–401
Wang J (1999) Amperometric biosensors for clinical and therapeutic drug monitoring: a review. J Pharmaceut Biomed 19:47–53
Wang J, Musameh M (2003) Carbon nanotube/teflon composite electrochemical sensors and biosensors. Anal Chem 75:2075–2079
Wang WU, Chen C, Lin K-H, Fang Y, Lieber CM (2005) Label-free detection of small-molecule–protein interactions by using nanowire nanosensors. Proc Natl Acad Sci U S A 102:3208–3212
Wang Z, Luo X, Wan Q, Wu K, Yang N (2014) Versatile matrix for constructing enzyme-based biosensors. ACS Appl Mater Interfaces 6:17296–17305
Weerathunge P, Ramanathan R, Shukla R, Sharma TK, Bansal V (2014) Aptamer-controlled reversible inhibition of gold nanozyme activity for pesticide sensing. Anal Chem 86:11937–11941
Wilkins MD, Turner BL, Rivera KR, Menegatti S, Daniele M (2018) Quantum dot enabled lateral flow immunoassay for detection of cardiac biomarker NT-proBNP. Sens Biosens Res 21:46–53
Wu S, Duan N, Shi Z, Fang C, Wang Z (2014) Simultaneous aptasensor for multiplex pathogenic bacteria detection based on multicolor upconversion nanoparticles labels. Anal Chem 86:3100–3107
Xu Y, Ding J, Chen H, Zhao Q, Hou J, Yan J, Wang H, Ding L, Ren N (2013) Fast determination of sulfonamides from egg samples using magnetic multiwalled carbon nanotubes as adsorbents followed by liquid chromatography–tandem mass spectrometry. Food Chem 140:83–90
Xu S, Zhang Y, Dong K, Wen J, Zheng C, Zhao S (2017) Electrochemical DNA biosensor based on graphene oxide-chitosan hybrid nanocomposites for detection of Escherichia coli O157: H7. Int J Electrochem Sci 12:3443–3458
Yan J, Guan H, Yu J, Chi D (2013) Acetylcholinesterase biosensor based on assembly of multiwall carbon nanotubes onto liposome bioreactors for detection of organophosphates pesticides. Pestic Biochem Physiol 105:197–202
Yang C, Feng W, Li Y, Tian X, Zhou Z, Lu L, Nie Y (2019) A promising method for diabetes early diagnosis via sensitive detection of urine glucose by FePd/rGO. Dyes Pigments 164:20–26
You C, Bhagawati M, Brecht A, Piehler J (2009) Affinity capturing for targeting proteins into micro and nanostructures. Anal Bioanal Chem 393:1563–1570
Yun YH, Shanov VN, Bange A, Heineman WR, Halsall HB, Seth G, Pixley SK, Behbehani M, Bhattacharya A, Dong Z, Yarmolenko S (2009) Carbon nanotube smart materials for biology and medicine. In: Nanoscience in biomedicine. Springer, New York, pp 451–484
Zhang Z, Lin M, Zhang S, Vardhanabhuti B (2013) Detection of aflatoxin M1 in milk by dynamic light scattering coupled with superparamagnetic beads and gold nanoprobes. J Agric Food Chem 61:4520–4525
Zhang LN, Deng HH, Lin FL, Xu XW, Weng SH, Liu AL, Lin XH, Xia XH, Chen W (2014) In situ growth of porous platinum nanoparticles on graphene oxide for colorimetric detection of cancer cells. Anal Chem 86:2711–2718
Zhang D, Sun Y, Wu Q, Ma P, Zhang H, Wang Y, Song D (2016) Enhancing sensitivity of surface plasmon resonance biosensor by Ag nanocubes/chitosan composite for the detection of mouse IgG. Talanta 146:364–368
Zhao X, Hilliard LR, Mechery SJ, Wang Y, Bagwe RP, Jin S, Tan W (2004) A rapid bioassay for single bacterial cell quantitation using bioconjugated nanoparticles. Proc Natl Acad Sci U S A 101:15027–15032
Zhao W-W, Xu J-J, Chen H-Y (2014) Photoelectrochemical DNA biosensors. Chem Rev 114:7421–7441
Zheng XT, Li CM (2010) Single living cell detection of telomerase over-expression for cancer detection by an optical fiber nanobiosensor. Biosens Bioelectron 25:1548–1552
Acknowledgements
The author is thankful to the University Grant Commission (UGC) for providing financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Noori, R., Ahmad, R., Sardar, M. (2020). Nanobiosensor in Health Sector: The Milestones Achieved and Future Prospects. In: Mohsin, M., Naz, R., Ahmad, A. (eds) Nanobiosensors for Agricultural, Medical and Environmental Applications. Springer, Singapore. https://doi.org/10.1007/978-981-15-8346-9_4
Download citation
DOI: https://doi.org/10.1007/978-981-15-8346-9_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-8345-2
Online ISBN: 978-981-15-8346-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)