Abstract
The human microbiota is a concoction of bacteria, archaea, fungi, and other microorganisms. It is necessary to maintain a partnership between the host and the microbiota in order to maintain the different aspects of human physiology, such as nutrient absorption, immune function and metabolism. The microbiota can contribute to both progression and suppression of the disease, including cancer. A disturbance in this interspecies balance called microbiome dysbiosis becomes a reason for the host to be more prone to issues such as immunodeficiency and cancer. Gut microbiota could potentially influence the factors that govern cancer susceptibility and progression through mechanisms such as immunomodulation, by producing metabolites, such as, bacteriocins, antimicrobial peptides involved in tumor suppression, and short-chain fatty acids (SCFA), and through enzymatic degradation. It is now an established fact that the host physiology as well as risk of diseases such as cancer could be greatly modulated by these commensal microbes and the regulation of cancer development, progression as well as response to anticancer therapy is greatly dependent on the host microbiota. Therefore, it is being envisaged that by the involvement of microbiome in augmenting antitumor responses to therapeutic approaches, potentially a new era of research with potentially broad implication on cancer treatment could be established. Better cancer treatment responsiveness can be achieved by understanding the role of the “tumor microbiome” in shaping the tumor microenvironment. This will help us to develop personalized anticancer solution with the goal to discover a bacterial species or a combination of species that decreases systemic toxicity and helps in anticancer therapy. This chapter is written in same context, which focuses on the association of the gut microbiome with the suppression and progression of cancers, the role of the immune system in this interaction, the utilization of these organisms for the treatment of cancers, and future perspectives.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
There are several different types of bacteria and other microorganisms present in the human body, which comprise the human microbiota. They inhabit in the epithelial barrier surfaces of the body exhibiting commensalism with the host. About 3×1013 bacterial cells are present in the gut microbiota whose composition is shaped by colonization at the time of birth, host genetics, type of delivery, incidences of diseases, exposure to antibiotics as well an individual’s lifestyle. The composition of the microbiota changes during early years of life, which remains relatively constant throughout life. The gut microbiota impacts various aspects of human physiology, such as nutrient absorption, metabolism, and immune function. A continuous crosstalk between the gut microbiota, immune cells, and the mucosal barrier is necessary to maintain a healthy body (Roy and Trinchieri 2017). There are strong scientific indications that gut microbiota plays a shielding role against cancer in animal models and an imbalance of the gut microbiota (dysbiosis) might result in the development of many disorders, including cancer.
Probiotics are used to address the problem of gut microbiota imbalance, which when given in balanced quantity can provide health benefits to the host. They are known to have direct and indirect benefits on host well-being. While the direct benefit involves gut health improvement, these are also known to indirectly help with prevention and treatment of cancer, reduction in tumor formation and metastasis by modulating the microbiota, immune response, reduction in bacterial translocation, enhanced gut barrier function, and anti-inflammatory antipathogenic activity (Yu and Li 2016). Therefore, it has variously been suggested that probiotics could be used as a dietary supplement against neoplastic predisposition by influencing both the local and systemic immune processes of the host. It gives the hope that some probiotic strains can also be developed and used to prevent or treat cancers by functioning as adjuvants by modulating intestinal microbiota and immune responses. Owing to the ability of gut microbiota to modulate host metabolism, inflammation, and immunity as well as its involvement in the initiation and/or progression of different cancers, this chapter is written with the aim to discuss various aspects of gut microbiota and gut dysbiosis and its association with cancer. Additionally, we have also discussed the various anticancer therapies based on gut microbiota.
1.2 Gut Microbiota, Gut Dysbiosis, and Cancer
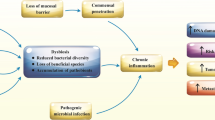
Gut microbiota has a significant local as well as systemic effect on the nutrient absorption, metabolism, and immune function. Maintenance of the epithelial barriers is crucial for the health of the organism as it provides the surface for microbiota to reside on (Scott et al. 2013). The epithelial barrier and the commensal microorganisms maintain a peaceful relationship that mediates the protection of the host from pathogens and pathobionts. The physiological relationship between epithelial cells and the microbiota is disrupted by the alteration in the composition of the microbiota, a condition called dysbiosis (Fig. 1.1). Dysbiosis has been linked to the breach of the barriers, induction of inflammatory responses as well as initiation and progression of cancerous conditions (Roy and Trinchieri 2017). These organisms that comprise the microbiome are also believed to colonize tumors, and there are several models that suggest the role of the microbiome as a contributor to carcinogenesis. A healthy individual is said to be associated with high diversity of gut microbiota, which critically influences bacterial dysbiosis, pathogenesis, genotoxin production, and host metabolism disruption that controls the host immune system. Regulation of systemic function by the microbiota is crucial for the survival and health of the host (Yang et al. 2009). A lot of studies have been done to understand the metabolic functions of the associated microbes. However, the focus has shifted toward understanding the interconnections between physiologies of microbial communities, their host, and the impact of the gut microbiota to maintain health and disease (Hooper et al. 2007).
Mechanism of gut microbiota in development and inhibition of carcinogenesis. Bacterial translocation happens because of imbalance in bacterial diversity (dysbiosis) causing chronic inflammation, resulting in the overexpression of proinflammatory cytokines and generation of reactive oxygen species causing oxidative stress and DNA damage resulting in carcinogenesis. In the presence of healthy microbial community (eubiosis), short-chain fatty acids (SCFAs) such as acetate, butyrate, and propionate are secreted, which create an immune homeostasis state influencing the process of cancer cell attenuation by limiting c-myc expression and by regulating P57 levels
Decoding and sequencing of the microbiome have helped the researchers to get a clear sight of extending the benefits of manipulating the gut microbiota to treat diseases. Whole-genome shotgun sequencing and 16S ribosomal RNA amplicon sequencing help to deduce the diversity of particular taxa present in the gut microbiome. The information gathered will aid in reconstructing the potential metabolic capacity of the microbiome at strain, species, genus, and taxonomic levels (Saus et al. 2019). Advancement in metagenomic analyses has provided more direction to differentiate the gut microbiota present in diseased and healthy individuals. The past two or three decades have provided a sizable functional data relating to the presence of gut microbes in numerous physiological processes, including digestion of food substances and maturation of the immune system (Qin et al. 2010; Wong et al. 2019; Sender et al. 2016). Imbalance of microbiota or an impaired microbiota can result in the development of cancer, disturbing the host physiological functions through the interference with the immune system. Modulation of cancer treatment can be done by certain factors like antibiotic ingestion, defined microbiome transplantation, and change in lifestyle (Raza et al. 2018). The mechanisms using which these organisms affect the systemic function are less understood when compared to the localized functions (Belkaid and Naik 2013). Following section briefly deals with an overview of gut microbiota and gut dysbiosis and its role in cancer.
1.2.1 Gut Microbiota and Its Interaction with Host
The human gut is a concoction of different bacteria, archaea and protozoa, which collectively constitute the microbiota (Gharaibeh and Jobin 2019). Constant crosstalk between these microbes, the mucosal barrier, and the immune system results in an efficient gut epithelial barrier (Ma et al. 2019). The central nervous system (CNS) and the “gut brain axis” (GBA) communicate and connect bidirectionally through the “gut brain axis” (GBA). Various components of GBA include (1) the autonomic nervous system (ANS), (2) the central nervous system (CNS), (3) the enteric nervous system (ENS), (4) the entero-endocrine system (EES), and (5) the hypothalamic–pituitary–adrenal (HPA) axis (Vivarelli et al. 2019). Here the gut is a part of an interface between the resident microbiota of the gastrointestinal tract and the human body. In a bidirectional crosstalk between the human body and GBA, the gut microbiota acts as doorkeeper for such communications to happen (Neuman et al. 2015). It has variously been reported that the gut microbiome composition gets modified based on the host’s hormones and neurohormones. For example, there are several peptide hormones secreted by the gastrointestinal entero-endocrine cells that could be sensed by the gut bacteria and in turn the gut microbiota composition is tuned. Similarly, gut microbiota also secretes some active molecules that are sensed by host’s gut cells and translation of corresponding signals to GBA. It has been reported that gut microbiota can (1) produce vitamin K, vitamin B, and linoleic acid, (2) produce short-chain fatty acids, and (3) transform molecules such as glutamate to gamma-aminobutyric acid or histidine to histamine, affecting various aspects of the host health such as (1) modulation of host’s immune system, (2) maintenance of host’s gut barrier integrity, (3) modulation of host’s metabolism, (4) xenobiotic and drug metabolism by host, and (5) host’s protection against gastrointestinal pathogens (Vivarelli et al. 2019).
This partnership between the microbiota and the host is an essential element of health, and this interspecies balance is termed as eubiosis (Lazar et al. 2018). A disturbance in eubiosis termed microbiome dysbiosis is the alteration of the microbiota composition, which is associated with disrupting the microbiota–epithelial cell interaction. Switching of eubiosis to dysbiosis in the host becomes a reason for the host to be more prone to issues, such as immunodeficiency and cancer (Lazar et al. 2018, 2019). The role of gut microbiome in cancer is dual in nature as they play a role both in tumorigenesis and in the prevention and treatment of cancer. Cancer progression may alter the microbiome, and microbiome may also affect the progression of cancer. In diseases such as colorectal cancer, composition of bacteria in the host intestine was shown to be different in patients with colorectal cancer compared to healthy subjects (Gharaibeh and Jobin 2019). Intestinal epithelial cells function to provide mechanical protection and to regulate immunity by secreting chemokines, cytokines, and antimicrobial peptides (Wu and Wu 2012). Cytokines are soluble signaling proteins produced in immune cells such as macrophages, neutrophils, and B and T cells for the regulation of immune responses (Stenken and Poschenrieder 2015). They are also associated with maintaining a microbiota homeostasis. The cytokine interleukin-18 (IL-18) facilitates the protection of the intestinal mucosa, and mice deficient in IL-18 demonstrate dysbiosis that increases susceptibility to colon carcinogenesis (Roy and Trinchieri 2017). In a similar study carried out by Elinav et al. (2011), wild-type mice showed symptoms of dysbiosis after fecal microbiota of mice deficient in IL-18 were transferred to the wild-type mice.
1.2.2 Gut Microbiota as Cancer Promoter
The first bacterial protein to have been associated with human cancer is CagA produced by Helicobacter pylori (Vivarelli et al. 2019). Fusobacterium nucleatum (Fn), when present in abundance, has been associated with colorectal carcinoma (Zhou et al. 2018). Fn contributes to colorectal cancer by using its FadA adhesin to bind to E-cadherin and causes the activation of host β-catenin–WNT signaling. Thus, FadA is a potential diagnostic and therapeutic target (Rubinstein et al. 2013). Certain bacterial pathogens make the host prone to cancer by promoting dysbiosis and altering the host’s immune system and thus triggering the growth of tumor. Metalloproteinase toxin (MP toxin) by Bacteroides fragilis also plays a role by disrupting intercellular junctions and activating β-catenin signaling (Vivarelli et al. 2019). Bacteria such as Bacteroides fragilis, Escherichia coli, and Peptostreptococcus anaerobius have been associated with colorectal cancer through the activation of Th17 cell response and direct DNA damage (Wong et al. 2019). Helicobacter hepaticus has been reported to activate the WNT/β-catenin pathway as well as nuclear factor-kappa B (NF-κB)-regulated and Th-1 immune network resulting in hepatocellular carcinoma (Fox et al. 2010). Injuries to the epithelial barrier, inflammation, and chronic infections can trigger carcinogenesis in individuals. Infections due to various pathogenic microorganisms in the gut have been correlated with an increased risk of tumor development. Individuals with a Salmonella typhi infection are at the risk of developing gallbladder carcinoma; similarly, chronic Streptococcus bovis infection may lead to the development of colon cancer (Hooper et al. 2007). In the case of H. pylori and S. typhi infections, correlation between microorganisms and their tendency to initiate cancer in the hosts varies for different individuals (Mager 2006).
On the contrary, there are other organisms in the gut microbiota that are of great interest to the cancer researchers to mediate the effects of anticancer therapies (Wong et al. 2019). H. pylori has been reported to increase the risk of gastric cancer in some people and reduce the risk of esophageal cancer in others; however, the cause is still unclear (Whiteman et al. 2010). Salmonella typhimurium has been associated with gallbladder cancer, and it has also been used as a carrier of therapeutic agents for different types of cancers; as being a facultative anaerobe, it can easily survive in the anoxic environment often found in tumors (Mager 2006). They are made to migrate toward the tumor sites by rendering them auxotrophic for compounds found in high concentrations at the tumor sites such as by the removal of metabolic gene purI from mutants such as VNP20009. This forces the organism to move toward the tumor for survival (Low 2004). S. typhimurium destroys tumors by (1) using bacterial toxins to activate Caspase-3 for apoptosis, (2) delivering anticancer compounds, and (3) sensitizing the immune system to the tumors (Wall et al. 2010).
Several preclinical, clinical, and meta-analyses of clinical studies have explored the possibilities of manipulating the microbiota to change the host’s response to different diseases, including cancer. One of the key mechanisms that scientists have tried to explore is immunomodulation (Ma et al. 2019). Immunomodulators change the way the immune system responds to the tumors by increasing (immunostimulators) or decreasing (immunosuppressive) antibody production (Bascones-Martinez et al. 2014). Recent studies have discovered that the bacteria in the gut impact the way cancer patients respond to immune checkpoint blockade therapy by using antibodies targeting co-inhibitory receptors to enhance the activity of T cell response (Gharaibeh and Jobin 2019)
1.2.3 Gut Microbiota as Cancer Suppressor
Several researchers have attempted to describe the mechanism by which gut microbiome influences the host physiology. The gut bacteria show anticancer effect either by increasing host immunity or by preventing gut dysbiosis. The bacteria L. rhamnosus GG (LGG) can counteract cancer growth in tumor models of ovarian, colorectal, breast, hepatic, cervical, and oral squamous cancers through its influence on mTOR or WNT pathways (Vivarelli et al. 2019; Nagy et al. 1998). One of the mechanisms involves short-chain fatty acids (SCFA), which are products of bacterial fermentation of undigested dietary fibers (Nagpal et al. 2018; Mager et al. 2005). They are associated with several functions such as intestinal repair, maintenance of intestinal homeostasis, inhibition of cancer cell proliferation, activation of G-protein-coupled receptors (GPCRs), etc. (Lazar et al. 2018; Nagpal et al. 2018; Arun et al. 2019). Butyrate and propionates show anticancer effect by inhibiting the histone deacetylases (Vivarelli et al. 2019; Ohland and Jobin 2015).
Probiotic bacteria such as lactic acid bacteria help the growth of SCFA-producing gut bacteria (Wang et al. 2019). Reduced production of SCFAs has been associated with an increase in the incidence of colorectal cancer (CRC) (Nagpal et al. 2018). Butyrate is an essential SCFA as it plays a key role in homeostasis (Lazar et al.2018). By inhibiting histone deacetylase (HDAC), it increases the acetylation of histone, which in turn regulates the transcriptional activity of tumor suppressors, resulting in a reduction of inflammation and CRC risk (Wang et al. 2019). Escherichia coli Nissle 1917 administered as mutaflor in combination with intestinal antibiotic rifaximin shows anti-inflammatory activity. Other probiotics such as L. casei trigger apoptosis and inhibit tumor growth by secreting a ferrichrome metabolite (Vivarelli et al. 2019). The association between microbiome and cancer is complex and has not been completely characterized. Additionally, various factors such as lifestyle, diet, and host immune system strongly influence the activity of the microbiota. Hence, it is difficult to conclude if their role is as promoters of cancer or as inhibitors.
1.2.4 Gut Microbiota as Immune Checkpoint Inhibitors
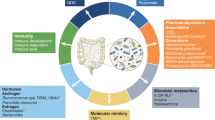
The gut microbiota helps the host immune system to develop tolerance toward beneficial microbiota and prompt an immune response against the gut pathogens as indicated in Fig. 1.2 (Vivarelli et al. 2019). The role of recognizing and attacking tumor cells is played by many cells of the immune system including T cells (Sharma and Allison 2015). In the presence of an antigen, T cells receive stimulatory signals for proliferation. T cells also receive inhibitory signals to downregulate their population once the infection is under control. Inhibitory signals can limit the response of T cells against cancer and hinder the process of tumor eradication (Andersen et al. 2006). CTLA-4 gene, programmed cell death protein 1 (PD-1) and its ligand (PD-L1) modulate down regulation of T cell response (Seidel et al. 2018). Thus, by targeting this inhibitory interaction, they can cause the T cells to remain activated for a period long enough for tumor eradication. The U.S. Food and Drug Administration (FDA) had approved antibodies such as ipilimumab, pembrolizumab, and nivolumab that target CTLA-4 and PD-L1, respectively (Sharma and Allison 2015). By targeting the inhibitory signals, the patient’s own immune response is reactivated against the cancer. Although this strategy appears straightforward, the responses to these checkpoint inhibitors among patients are varied. Several human clinical studies have shown that the key players in influencing the checkpoint inhibitor response are the individuals’ gut microbiota that have shown to affect the antitumor immunity and the efficacy of immunotherapy (Matson et al. 2018). Understanding the interaction between the microbiota and modulators of the immune system will pave the way to develop better therapeutic agents to treat cancer.
A study carried out in Jackson Laboratory (JAX) mice and the Taconic mice showed different rates of tumor growth and varied response to anti-PD-L1 antibodies due to the presence of Bifidobacterium sp. that has shown to possess antitumor activity by reactivating dendritic cells that in turn improve CD8-positive T cell against tumors (Sivan et al. 2015). When fecal matter from the mice that responded to treatment with anti-PD-L1 antibodies was transplanted into germ-free mice that would otherwise show no response to treatment, it was observed that there was an enhanced response to anti-PD-L1 therapy. The same was not observed when the fecal microbiota transplantation (FMT) was from mice that did not respond to therapy. This suggests the role of microbiota in the response (Gharaibeh and Jobin 2019). In a similar study carried out by Routy et al. (2018), Akkermansia muciniphila and E. hirae were found to be dominant in those patients responding to treatment with anti-PD-L1, while Corynebacterium aurimucosum and Staphylococcus haemolyticus were seen predominantly among patients who did not respond to treatment. A. muciniphila when given individually or when combined with E. hirae resulted in an improved response to PD-1 blockade in an IL-12-dependent manner by increasing the number of CCR9+, CXCR3+, and CD4+ T cells in the tumor beds (Routy et al. 2018). Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium were also seen to be abundantly present in another cohort of patients of metastatic melanoma who responded to immunotherapy using anti PD-L1 (Matson et al. 2018). Several other studies provide evidence to support the fact that the gut microbiota is an important variable in cancer immunotherapy (Vétizou et al. 2015; Gopalakrishnan et al. 2018; Matson et al. 2018). While studying the role of the microbiota in patients with metastatic melanoma, Gopalakrishnan et al. (2018) found that the Faecalibacterium prausnitzii was in abundance among the responders to anti-PD-1 therapy. These findings suggest that the composition of the microbiota possesses clinical significance for the treatment of cancer via immune checkpoint blockade therapy. Although the exact mechanism through which it is possible is not clear yet, manipulating these organisms may enhance patient’s response to treatment (Gharaibeh and Jobin 2019)
1.2.5 Gut Microbiota, Inflammasomes, and Tumorigenesis
The gut microbiota makes use of multiprotein intracellular complexes called “inflammasomes” in order to interact with immune cells and gut cells (Vivarelli et al. 2019). They possess a subset of cytoplasmic pattern recognition receptors (PRRs) called NOD-like receptors (NLRs), using which they detect pathogenic and nonpathogenic microorganism-derived molecules and sterile stressors molecules. The inflammasomes are termed as the guardians of cellular and tissue integrity, as they are capable of playing an active role in responding to commensals and pathogens. Any imbalance in these complexes can result in a variety of diseases ranging from autoimmunity to cancer. These inflammasomes have been associated with both tumor promotion and suppression in different scenarios depending on the nature of the tumor and its microenvironment. The outcome of the inflammasome activation depends on factors such as (1) its expression pattern, (2) effector molecule, (3) tumor nature, (4) tumor stage, and (5) gut microbiome. While inflammasome-dependent IL-18 production plays an important role in suppressing colitis-associated CRC, inflammasome-dependent IL-1ß activation results in pro-inflammatory and tumor-promoting trigger resulting in the development of lung, skin, breast, and pancreatic cancer (Zaki et al. 2010; Salcedo et al. 2010).
Inflammasomal NLRs are a kind of innate receptors present in epithelial and innate immune cells, which aid in the detection of commensal microbiota and their bioproducts. These commensals and their bioproducts induce inflammasomal activation and IL-18 production in the gut, which helps in preventing intestinal barrier disruption and dysbiosis. Therefore, the hosts, which are deficient in inflammasomal components, are prone to reduced production of IL-18, leading to intestinal barrier impairment followed by larger penetration by commensal bacteria, increased inflammation, and finally trigger tumorigenesis (McLoed et al. 2016; Kolb et al. 2016; Daley et al. 2017). NLRs play a key role in regulating susceptibility to intestinal inflammation through its microbiome-modulatory activity (Vivarelli et al. 2019).
1.2.6 Gut Microbiota and Gastric Malignancies
Gastric cancer is a multifactorial disease affected by the environment, H. pylori infection, and other genetic factors. Gastric cancer is an inflammation-associated cancer (Meng et al. 2018). An infection with H. pylori triggers the initial steps of carcinogenesis through a decrease in acid production that allows other bacterial communities to grow, leading to increased inflammation and degradation of the epithelial barrier (Ferreira et al. 2018). Studies also suggest that along with inducing inflammation these microbes interfere with anticancer agents (Meng et al. 2018). Although H. pylori is the lead player in gastric carcinomas, it cannot be described under gut microbiota, since it is a pathogen and not a commensal. The microbiome has its influence on the cancers of the GI tract including pancreatic, liver, colorectal, and gastric cancers (Meng et al. 2018). Several bacteria other than H pylori are also associated with carcinogenesis through mechanisms like (1) inflammation promotion, (2) modification of the action of stem cells, and (3) stimulation of cell proliferation and production of toxic metabolites (Petra et al. 2017). In contrast, study conducted to compare the microbiota of patients suffering from gastric cancer and normal patients revealed that there was no significant difference in compositions between the two suggesting that microbiota are just bystanders in the progression of cancer (Dicksved et al. 2009). A study carried out by Maldonado-Contreras et al. (2011) compared the gastric microbiota of H. pylori positive and negative individuals. The study showed that H. pylori positive individuals are having an increased count of Acidobacteria, Proteobacteria, and Spirochaetes and reduction in Firmicutes, Bacteroidetes, and Actinobacteria. Similarly, H. pylori negative individuals showed an increase in Firmicutes, Bacteroidetes, and Actinobacteria (Maldonado-Contreras et al. 2011; Bik et al. 2006). Difference in the organisms associated with esophageal cancer and a healthy esophagus was noted. Firmicutes, Proteobacteria, and Bacteroidetes were the common phyla in samples of esophageal squamous cell carcinoma (ESCC) and esophageal squamous dysplasia (ESD) patients indicating their association with tumorigenic process (Yang et al. 2012; Aghazadeh et al. 2017; Nasrollahzadeh et al. 2015).
Colorectal cancer (CRC) is one of the leading causes of cancer mortality in the world (Fleming et al. 2012; Sasaki et al. 2005). Exposure of microorganism is continuous from the mouth to the anus mostly because the gastrointestinal epithelium is connected to the environment. One of the most preferable study sites for microbial diversity is colon as bacterial load is more and it follows the hierarchy of jejunum, duodenum, and least is at the oral cavity. Understanding of the recent advancement in the microbiome study has established a huge impact on human health. The association between the microbial abundance and cancer incidence has indicated the significant role of microbiota in colorectal cancer (Wong et al. 2019).
Experimental, geographical variation, and migration studies have provided compelling evidence that both environmental and genetic alteration is the reason for the formation of CRC. Development of CRC is basically due to the epigenetic modification of several genes and accumulation of mutation. According to the definition provided by Louis et al. (2014), CRC mostly occurred by the transition of normal mucosa to premalignant lesions due to sequential genetic alteration and mutation. The reason thought to drive this mutation is by the origin of mutation in the adenomatous polyposis coli tumor suppressor gene, which encodes a protein that plays a significant role in WNT pathway, intercellular adhesion, regulation of the cell cycle, and apoptosis (Louis et al. 2014). Another risk that is specified for the commencement of the CRC is the association with diet and lifestyle. Epidemiological studies have pointed out that consumption of excessive protein and fat from red and processed meat can escalate the risk of development of colorectal tumorigenesis (Yang and Yu 2018). The colonic health is maintained by residues of diet such as complex carbohydrates, protein residues, and primary bile acids, which are absorbed in the intestine. Hence ensuring a balanced diet can critically protect from the risks of CRC as the saccharolytic fermentation of complex carbohydrate will produce short-chain fatty acids (SCFAs) and butyrate, which pose anti-inflammatory and antineoplastic properties through the acceleration of cellular metabolism, microbiota homeostasis, antiproliferation, immunomodulatory, and genetic and epigenetic regulation (O’Keefe 2016).
Clinical and epidemiological studies show the mechanism of gut microbiota interaction and its vital relationship to human health. Administering adequate amount of probiotics (live organisms) is of much interest for researchers as supplementation of probiotics in the right amount would improve the ecological health of microbiota, which can convert dysbiosis to eubiosis and can be an alternative option for treatment of antibiotics (Neish 2009). Many researchers have suggested the significance of probiotics and its administration as an alternative. Bacteria like Proteobacteria, Verrucomicrobia, Actinobacteria, Fusobacteria, and Cyanobacteria help in metabolizing complex carbohydrates into short-chain fatty acids, which increase apoptosis in the colon (Singh et al. 1997; Berdanier 2018). As the large intestine comprises the majority of the microbial wealth, researchers are keen in understanding the intestinal environment and the abundance of the type of bacteria that contribute to carcinogenesis and/or tumor protection. Interaction of tumor with its local microenvironment and its systemic effects on the host revealed the imbalance and destruction of gut microbiota (Lin et al. 2019). According to Lin et al. (2019), Fusobacterium nucleatum, E. coli, Bacteroides fragilis, and Peptostreptococcus anaerobius are the bacteria that were abundant in the tumor tissues of the patients, which have the ability to promote CRC.
Lactobacillus casei BL23 has shown to increase the apoptosis rate by inducing the production of Caspase-9, Caspase-7, and Bik that help in inhibiting the cell proliferation of CRC (Jacouton et al. 2017; Yang and Yu 2018; Lenoir et al. 2016). Lactobacillus pentosus B281 and Lactobacillus plantarum B282 have been reported for their ability to arrest the G1 phase of the cell cycle resulting in the downregulation of certain cyclin genes, thereby inhibiting the growth of colon cancer (Saxami et al. 2016). Pediococcus pentosaceus GS4 (Dubey et al. 2016), Lactobacillus BCRC1710 (Saber et al. 2017; Nekouian et al. 2017), L. acidophilus CL1285, L. casei LBC80R, and L. rhamnosus CLR2 are other few bacterial strains that have been found to control colorectal cancer (Desrouillères et al. 2015). LAB strains prevent the formation of aberrant crypt foci, a precursor of colorectal cancer by the induction of hepatic detoxifying enzymes and by inducing apoptotic proteins Bax/Bcl-2 causing significant damage to the cancer cells. Pediococcus controls proliferation of the tumor cells that cause colon cancer by triggering apoptosis by downregulating NF-kB and p-Akt. Evidence suggests that several LAB strains possess antioxidant activity that can reduce the oxygen-centered free radicals in the gastrointestinal tract (Yeh et al. 2013; Kanmani et al. 2013). L. salivarius, which has been isolated from fecal samples, has been reported to suppress 4NQO-related spread of cancer in rats by decreasing induced apoptosis and the expression of proliferating cell nuclear antigen in a dose-dependent style (Zhang et al. 2013). Yeh et al. (2013) have reported an improvement in the serum albumin, prealbumin, and body weight of patients treated with cancer medicine and were given nutritional supplements containing probiotics (Yeh et al. 2013). Bifidobacteria, which produces butyric acid, could initiate apoptosis in the colon and also inhibit 2-amino-3-methylimidazo-induced colon, liver, and mammary carcinogenesis (Reddy 1997).
Fermentation of dietary nondigestible carbohydrates is the source of energy for gut microbiota. SCFAs, such as propionate, butyrate, and acetate, exhibit anti-inflammatory properties in human monocytes, whereas butyrate constitutes a major energy source for colonocytes (Neish 2009). Inulin-type fructans reduce the growth of hepatic BaF3 cells and lessens the inflammation by a cAMP level-dependent pathway. The gut microbiota has influenced BaF3 cell progression by varying its metabolome (Bindels et al. 2012). Gut microbiota improves the potential of manipulating the efficiency of treatment for cancer by reducing the side effects (Qin et al. 2010). Proteolytic enzymes of lactic acid bacteria help in cleaving the milk protein during fermentation, and the resulting biological peptides can trigger apoptosis activation and inhibition of cancer cells via cell membrane disruption without having a negative effect on the healthy cells. The reports of a study suggested that use of four proteolytic strains of L. helveticus grown under the skim milk has the capability of releasing bioactive compounds (Elfahri et al. 2016). However far too little attention has been paid toward the etiologic role of chronic infections in carcinogenesis. Diversity of pathogens extracted from the tissue samples of lung cancer patients has pointed out that mycoplasma strains were spotted in all samples; more often the strains of Staphylococcus epidermidis, Streptococcus mitis, and bacterial strains like candida, listeria, and chlamydia have shown a descending frequency (Apostolou et al. 2011). L. casei SR4 and L. paracasei SR2 strains show anticancer activity by the upregulation of BAX, BAD, Caspase-3, Caspase-8, and Caspase-9 genes and by downregulating the Bcl-2 gene, hence preventing cervical cancer. Streptococcus thermophilus M17PTZA496 and Streptococcus thermophilus TH982 act against cancer cells by releasing folic acid, histamine, and tyramine possessing high cytotoxic effects against cancer cells (Tarrah et al. 2018). Secretions of Kluyveromyces marxianus AS41 can cause the activation of extrinsic and intrinsic pathways for cancer cell apoptosis by upregulation of BAD, Fas R, CASP 9, CASP 8, and CASP 3 (Saber et al. 2017).
1.3 Gut Microbiota and Anticancer Therapies
The final goal of anticancer therapies is to be efficient in eradicating targeted malignancies. Radiotherapy, chemotherapy, and immunotherapy treatments are the pillars of the currently applied cancer treatment system, and almost all of these available treatments have detrimental effects toward normal cells including the normal gut microbiota leading to gut dysbiosis. Eventually, such altered microbiome composition can significantly affect the patient’s response toward applied anticancer therapies (Roy and Trinchieri 2017; Vivarelli et al. 2019). Therefore, novel strategies to manipulate the gut microbiome needs to be evaluated and identified so as to maintain the intact gut microbiome to finally improve the patient’s therapeutic outcome. In the following section, we will discuss various aspects of gut microbiota in relation to their role and application in various anticancer therapies.
1.3.1 The Tumor Microbiome and Its Application in Anticancer Treatment
Several studies have demonstrated that certain microorganisms preferentially colonize and replicate in the tumor microenvironment. Presence of such microbes in tumor tissue may be a direct cause for tumorigenesis, as seen in the case of H. pylori colonization in gastric cancer. Pockets of necrosis and hypoxia due to insufficient blood supply and increased oxygen demand from rapidly growing tumor cells along with immune suppressed microenvironment formed within the tumor niche provide favorable environment for proliferation of certain bacterial communities (Bashirdas et al. 2017). This could result from coinciding infection or bacterial translocation from the gut lumen due to epithelial barrier disruption. This local colonization of bacterial communities in tumor environments is termed as “tumor microbiome,” and various reports have suggested a complex interaction between the microbiome of the tumor and tumor immunity (Rubinstein et al. 2013; Gur et al. 2015). Tumor microbiome may play roles in the (1) development of tumor resident microbes and (2) reduction of treatment-related systemic adverse effects by distribution of therapeutics specifically at tumor sites (Bashirdas et al. 2017).
There are various antitumor immune stimulatory effects that could be mediated by structural components of tumor-associated bacteria such as flagellin, peptidoglycan, LPS, and other pathogen-associated molecular patterns (PAMs), which could be applied to elicit antitumor immune response. Additionally, affinity of anaerobes such as clostridial spores to germinate in hypoxic regions of solid tumors is also being experimented as a therapeutic approach. Attenuated clostridial spores have been tested in canine tumors (Roberts et al. 2014). Similarly, attenuated Salmonella strain has also been utilized as agents against cancer due to their ability to colonize tumors. The antitumor activity of these attenuated bacterial strains is known to be facilitated by bacteremia-induced TNF-alpha secretion. Due to the vasoactive property of these secreted products, they facilitate the entry of bacteria into the tumor microenvironment, which results in activation of CD8+ T cell numbers for improved tumor surveillance and clearance (Leschner et al. 2009; Stern et al. 2015; Bashirdas et al. 2017).
The nonspecific toxicity of systemically administered therapeutic agents has been reported to be reduced by using bacteria as tumor-specific targeted drug delivery platforms. Various tumor-targeting agents such as bacterial toxins, cytokines, and immune activating proteins have been designed for specific delivery at tumor sites using bacterial vehicles (Bashirdas et al. 2017). Similarly, a quorum sensing-based interbacterial communication system has been applied for targeting regions that are hypoxic and inaccessible to chemotherapy agents (Ryan et al. 2009). These approaches are elegant and optimistic, which require further finetuning and optimization to make them effective.
1.3.2 Gut Microbiota and Modulation of Chemotherapy and Immunotherapy Efficiency
Cancer pathogenesis along with its therapeutic outcome could be significantly impacted by dysbiosis in the composition of gut microbiota, as the gut microbiota has the ability to (1) metabolize antitumoral compounds, (2) modulate the immune response of host, and (3) modulate inflammation pathway (Vivarelli et al. 2019). With reference to chemotherapy, it has been observed that efficacy of certain anticancer drugs, e.g., cisplatin and cyclophosphamide, was altered depending on the presence or absence of fully functional gut microbiota or coupling the treatment with certain probiotic strains such as L. johnsonii and E. hirae (Iida et al. 2013; Gui et al. 2015; Viaud et al. 2013; Daillère et al. 2016). Similar effects have been observed with respect to immunotherapeutic treatments when administration of CpG oligodeoxynucleotide (immunotherapeutic agent that is synthetic molecule mimicking bacterial DNA) along with Alistipes shahii resulted in improved immunotherapeutic outcome as compared to the condition where CpG was administered alone (Iida et al. 2013).
Patient’s gut microbiome composition has also been linked to the intrinsic efficacy of immune checkpoint inhibitor-based immunotherapy, where the immune inhibitory pathway was blocked by use of therapeutic agents so as to modulate the T cell activation against tumor target cells (Vivarelli et al. 2019). It was observed that enrichment of gut microbiome with Bacteroides fragilis and Burkholderia cepacia significantly increased the efficacy of anticytotoxic T lymphocyte-associated protein 4 (CTLA4) antibodies, which were used for reducing sarcoma tumor growth in mice. Similarly, administration of Bifidobacterium sp. was found to improve the efficacy of programmed death ligand 1 (PD-L1) targeting antibody in mice model (Vétizou et al. 2015; Sivan et al. 2015). In line with these reports, Gopalakrishnan et al. (2018) has demonstrated that the microbiome of anti-PD-L1 responders is significantly different from nonresponders. Similarly, Matson et al. (2018) reported the significance of E. faecium, Bifidobacterium longum, and Collinsella aerofaciens in ameliorating the anti-PD-L1 efficacy (Matson et al. 2018). PD-L1 therapy is done to generate the antitumor immunity, which works by prevention of the interaction between PD1 protein and PD-L1. It has been found that the prevention of this interaction is enhanced by the various components of gut microbiota. Therefore, it was concluded that the bacterial immune synergy for response to anti-PD-L1 therapy is facilitated by intestinal microbial communities (Sivan et al. 2015; Roy and Trinchieri 2017; Jobin 2018). Contrary to this, there are specific microorganisms whose presence, in vicinity or even at a distant site, can interfere with the treatment of cancer. For example, presence of Escherichia coli (Enterobacteriaceae) strains have been reported to negatively interfere with tumor response against the chemotherapeutic agent gemcitabine, whose efficacy was compromised by metabolization or deactivation of the active form of the drug (Jobin 2018). Another example is that of Firmicutes such as Faecalibacterium, which when present in increased numbers can result in toxic side effects after anti-CTLA4 antibody treatment. A decrease in the abundance of Bacteroides also has a similar effect. Similarly, it was found that the introduction of Akkermansia muciniphila reversed the low response to PD-1 blockade in mice receiving human nonresponder FMT. This showed an improvement in antitumor immune cell infiltration and activity in tumors indicating that these microbes can be used to improve the precision of cancer medicines (Jobin 2018).
1.3.3 Use of Probiotics in Cancer Treatment
Because of their ability to preserve gut homeostasis, probiotics are tested against gut dysbiosis in cancer patients undergoing chemotherapy and radiotherapy (Table 1.1). Probiotics work locally as well systemically and exert their antitumor properties by a combination of events such as (1) antioxidant activity improvement, (2) host’s immune response modulation that includes both gut associated and systemic immune responses, (3) improvement of gut homeostasis and bacterial translocation, (4) carcinogen degradation, etc. (Reid et al. 2003; Yu and Li 2016).
Probiotic lactobacilli have variously been reported to significantly reduce the prevalence of colon cancer. It was found that the administration of these probiotic bacteria leads to modification of the enteric flora of mice and by influencing the overgrowth of bacteria and their translocation in Wistar rats after 80% gut resection (Yu and Li 2016) (Table 1.2). In one such study conducted by Konishi et al. (2016), it was found that ferrichrome produced by L. casei ATCC334 acts as a tumor-suppressive molecule, responsible for its observed tumor-suppressive effect. This molecule when used on colon cancer cells showed a strong tumor-suppressive effect by activating c-jun N-terminal kinase (JNK) signaling pathway. Another study showed a positive impact on colon cancer reduction by lowering the activity of certain enzymes when the organism L. rhamnosus LC705 was used in combination with Propionibacterium freudenreichii sp. Shermanii JS (Hatakka et al. 2008; Brady et al. 2000). Similarly, probiotic yogurt has been found to be effective in controlling Bacteroides fragilis (ETBF) associated with inflammatory bowel disease and colorectal cancer (Odamaki et al. 2012).
Probiotics positively influence intestinal health by protecting the intestinal barrier and by minimizing the DNA damage in intestinal tissues. During the initial stages of colorectal cancer, there is a disruption of the tight junctions that causes loss of integrity across the intestinal barrier. It has been shown that the introduction of certain prebiotic and probiotic (Lactobacillus plantarum) can prevent the disruption of the epithelial barrier (Commane et al. 2005; Ko 2007). Similarly, permeability across intestinal barrier as well as attenuation of inflammatory response was reported by the administration of certain probiotics in patients undergoing biliary drainage (Jones et al. 2013). Similarly, DNA damage or adduct formation by mutagen was found to be reduced by administration of probiotic molecules (Horie et al. 2003; Yu and Li 2016). In this context, it has been reported that similar to the tumor-suppressor protein p53, probiotics exert their functions by channelizing the cell apoptosis during elevated DNA damage levels (Zhang et al. 2009).
It has been reported that daily intake of L. casei has a positive effect on natural killer (NK) cell activity (Takeda and Okumura 2007). Similarly, in animal models a decrease in the occurrence of colon cancer was observed through immunomodulatory effects of probiotics and/or synbiotic administration (Yu and Li 2016). Enhanced NK cell number or cell cytotoxicity, CD4/CD8-positive lymphocytes, or phagocytic activity of macrophages were found in rats or mice treated with probiotic products (Roller et al. 2004; de Moreno de LeBlanc et al. 2005; Yu and Li 2016; Guha et al. 2019). B. lactis sp 420 when tested on human colon carcinoma cell line Caco-2, it was found to exert anti-inflammatory and anticarcinogenic properties by modulating cyclooxygenase expression profile (Nurmi et al. 2005). Probiotics exert their anti-inflammatory effect by regulation of inflammatory mediators such as interferons, interleukins, and cytokines. Regulation of anti-inflammatory activity results in beneficial effects such as effective control of inflammation and carcinogenesis. Improving the functioning of antioxidative enzymes has also been reported to be exerted by probiotics, which is known to help against carcinogen-induced damage (Yu and Li 2016). A list of bacterial products and their anticancer mechanism has been presented in Table 1.3. Hence, it could be concluded that probiotics developed based on gut-microbiota could be developed as a potential anticancer therapy.
The problem of chemotherapy-associated gastrointestinal toxicity has also been addressed by use of probiotics (Lactobacillus spp.) as supportive treatment strategy, pertaining to their anti-inflammatory activity within the intestinal microenvironment. L. rhamnosus GG (LGG) is one of the first studied probiotic model species used in cancer-related studies (Chen et al. 2017). Several clinical trials have also attempted to study the role of LGG administration in order to prevent the toxic effects of anticancer therapies as well as its potential role in the direct modulation of cancer development (Tables 1.4 and 1.5) (Vivarelli et al. 2019).
1.3.4 Use of Fecal Microbiota Transplantation (FMT) in Cancer Treatment
Fecal microbiota transplantation (FMT) has variously been projected as an alternate strategy used to cure pathogen infection or in treatment of gut diseases, e.g., recurrent Clostridium difficile duodenal infection has been cured by FMT (van Nood et al. 2013). Similarly, the efficacy of FMT in reducing colon tumorigenesis has been seen during preclinical studies done in mice. Clinical trials are in progress to evaluate and establish the use of FMT in treatment of cancer as well as in preventing the intestinal side effects of anticancer treatment (Vivarelli et al. 2019) (Table 1.5). Once established, FMT could be developed as an efficient antitumor therapeutic strategy.
1.4 Conclusion and Future Prospect
The gut microbiome and the respective host share a complex relationship among themselves. The gut microbiota is inherited by people and changes depending on factors such as age, diet, and environment. This microbiota footprint changes during the lifetime of each individual. The gut microbiota has been studied in great detail for its performance with respect to a number of important functions, such as protection from infections, pathogen colonization control, dietary compound hydrolysis, and vitamin production. It is now an established fact that the host physiology as well as risk of diseases, such as cancer, could be greatly modulated by these commensal microbes. Regulation of cancer development, progression as well as response to anticancer therapy is greatly dependent on the host microbiota. And therefore, a potentially new era of research with potential broad implication on cancer treatment could be envisaged by the involvement of microbiome in augmenting antitumor responses to therapeutic approaches. Better cancer treatment responsiveness can be achieved by understanding the role of the “tumor microbiome” in shaping the tumor microenvironment. Researchers are considering personalized cancer treatment by modifying the patient’s microbiota as a possibility. The individuals’ microbiota composition could be used as a biomarker, a diagnostic tool, and possibly a therapeutic target due to its resilience, stability, and responsiveness to environmental, physiological, and pathological changes (Lee et al. 2017). This will help us to develop personalized anticancer solutions with the ultimate goal to discover a bacterial species or a combination of species that decreases systemic toxicity and helps in anticancer therapy. To make it a success, we need to apply modern scientific advancements for microbiome-based patient stratification rather than relying on population-based data or frequently used “trial-and-error” approaches. In this direction, modern advancement in data sciences like artificial intelligence and machine learning approaches may enable us to tailor treatment combinations so as to more optimally achieve therapeutic efficiency while minimizing adverse effects. Overall, this approach represents a new and exciting frontier toward future harnessing of microbiome as a diagnostic tool (Bashirdas et al. 2017).
Therefore, it is expected that targeting the microbiota is likely to become one of the next frontiers for personalized medicine (Roy and Trinchieri 2017). Targeted interventions on microbiome by supplementation of prebiotic and/or probiotic might be used as preventive healthcare solutions for cancer as well as to improve the efficacy of the existing cancer treatments such as chemotherapy, radiotherapy, and immunotherapy.
References
Aghazadeh Z, Pouralibaba F, Khosroushahi AY (2017) The prophylactic effect of Acetobacter syzygii probiotic species against squamous cell carcinoma. J Dent Res Dent Clin Dent Prospect 11(4):208
Andersen MH, Schrama D, Thor Straten P, Becker JC (2006) Cytotoxic: T cells. J Invest Dermatol 126(1):32–41
Apostolou P, Tsantsaridou A, Papasotiriou I, Toloudi M, Chatziioannou M, Giamouzis G (2011) Bacterial and fungal microflora in surgically removed lung cancer samples. J Cardiothorac Surg 6:1
Arun KB, Madhavan A, Thomas S, Nisha P (2019) Short chain fatty acids enriched fermentation metabolites of soluble dietary fibre from Musa paradisiaca drive HT29 colon cancer cells to apoptosis. PLoS ONE 14(5):e0216604
Bascones-Martinez A, Mattila R, Gomez-Font R, Meurman JH (2014) Immunomodulatory drugs: oral and systemic adverse effects. Med Oral Patol Oral Cir Bucal 19(1):e24–e31
Bashirdas S, Tuganbaev T, Federici S, Elinav E (2017) The microbiome in anti-cancer therapy. Semin Immunol 32:74–81
Belkaid Y, Naik S (2013) Compartmentalized and systemic control of tissue immunity by commensals. Nat Immunol 14(7):646–653
Berdanier C (2018) The human microbiota. Nutr Today 53(3):125–131
Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Relman DA (2006) Molecular analysis of the bacterial microbiota in the human stomach. PNAS 103(3):732–737
Bindels L, Porporato P, Dewulf E, Verrax J, Neyrinck A, Martin J, Scott K, Buc Calderon P, Feron O, Muccioli G, Sonveaux P, Cani P, Delzenne N (2012) Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br J Cancer 107(8):1337–1344
Brady L, Gallaher D, Busta F (2000) The role of probiotic cultures in the prevention of colon cancer. J Nutr 130(2):410S–414S
Chen Z, Hsieh Y, Huang C, Tsai C (2017) Inhibitory effects of probiotic Lactobacillus on the growth of human colonic carcinoma cell line HT-29. Molecules 22(1):107
Chondrou P, Karapetsas A, Kiousi D, Tsela D, Tiptiri-Kourpeti A, Anestopoulos I, Kotsianidis I, Bezirtzoglou E, Pappa A, Galanis A (2018) Lactobacillus paracasei K5 displays adhesion, anti-proliferative activity and apoptotic effects in human colon cancer cells. Benefic Microbes 9(6):975–983
Commane D, Shortt C, Silvi S, Cresci A, Hughes R, Rowland I (2005) Effects of fermentation products of pro- and prebiotics on trans-epithelial electrical resistance in an in vitro model of the colon. Nutr Cancer 51(1):102–109
Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, Duong C, Flament C, Lepage P, Roberti M, Routy B, Jacquelot N, Apetoh L, Becharef S, Rusakiewicz S, Langella P, Sokol H, Kroemer G, Enot D, Roux A, Eggermont A, Tartour E, Johannes L, Woerther P, Chachaty E, Soria J, Golden E, Formenti S, Plebanski M, Madondo M, Rosenstiel P, Raoult D, Cattoir V, Boneca I, Chamaillard M, Zitvogel L (2016) Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide induced therapeutic immunomodulatory effects. Immunity 45(4):931–943
Daley D, Mani V, Mohan N, Akkad N, Pandian G, Savadkar S, Lee K, Torres-Hernandez A, Aykut B, Diskin B, Wang W, Farooq M, Mahmud A, Werba G, Morales E, Lall S, Wadowski B, Rubin A, Berman M, Narayanan R, Hundeyin M, Miller G (2017) NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J Exp Med 214(6):1711–1724
de Moreno de LeBlanc A, Matar C, Thériault C, Perdigón G (2005) Effects of milk fermented by R389 on immune cells associated with mammary glands in normal and a breast cancer model. Immunobiology 210(5):349–358
Desrouillères K, Millette M, Vu K, Touja R, Lacroix M (2015) Cancer preventive effects of a specific probiotic fermented milk containing Lactobacillus acidophilus CL1285, L. casei LBC80R and L. rhamnosus CLR2 on male F344 rats treated with 1,2-dimethylhydrazine. J Funct Foods 17:816–827
Dicksved J, Lindberg M, Rosenquist M, Enroth H, Jansson JK, Engstrand L (2009) Molecular characterization of the stomach microbiota in patients with gastric cancer and in controls. J Med Microbiol 58(4):509–516
Dubey V, Ghosh A, Bishayee K, Khuda-Bukhsh A (2016) Appraisal of the anti-cancer potential of probiotic Pediococcus pentosaceus GS4 against colon cancer: in vitro and in vivo approaches. J Funct Foods 23:66–79
Elfahri K, Vasiljevic T, Yeager T, Donkor O (2016) Anti-colon cancer and antioxidant activities of bovine skim milk fermented by selected Lactobacillus helveticus strains. J Dairy Sci 99(1):31–40
Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Flavell RA (2011) NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145(5):745–757
Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, Costa JL, Carneiro F, Machado JC, Figueiredo C (2018) Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 67(2):226–236
Fleming M, Ravula S, Tatishchev SF, Wang HL (2012) Colorectal carcinoma: Pathologic aspects. J Gastrointest oncol 3(3):153–173
Fox JG, Feng Y, Theve EJ, Raczynski AR, Fiala JL, Doernte AL, Tannenbaum SR (2010) Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut 59(01):88–97
Gharaibeh RZ, Jobin C (2019) Microbiota and cancer immunotherapy: in search of microbial signals. Gut 68(3):385–388
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Cogdill AP (2018) Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359(6371):97–103
Guha D, Banerjee A, Mukherjee R, Pradhan B, Peneva M, Aleksandrov G, Suklabaidya S, Senapati S, Aich P (2019) A probiotic formulation containing L. bulgaricus DWT1 inhibits tumour growth by activating pro-inflammatory responses in macrophages. J Funct Foods 56:232–245
Gui Q, Lu H, Zhang C, Xu Z, Yang Y (2015) Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet Mol Res 14(2):5642–5651
Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S, Shussman N, Almogy G, Cuapio A, Hofer E, Mevorach D, Tabib A, Ortenberg R, Markel G, Miklić K, Jonjic S, Brennan C, Garrett W, Bachrach G, Mandelboim O (2015) Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42(2):344–355
Hatakka K, Holma R, El-Nezami H, Suomalainen T, Kuisma M, Saxelin M, Poussa T, Mykkänen H, Korpela R (2008) The influence of L. rhamnosus LC705 together with Propionibacterium freudenreichii ssp. Shermanii JS on potentially carcinogenic bacterial activity in human colon. Int J Food Microbiol 128(2):406–410
Heydari Z, Rahaie M, Alizadeh A (2019) Different anti-inflammatory effects of Lactobacillus acidophilus and Bifidobactrum bifidioum in hepatocellular carcinoma cancer mouse through impact on microRNAs and their target genes. J Nutr Intermed Metabol 16:100096
Hooper SJ, Crean SJ, Fardy MJ, Lewis MA, Spratt DA, Wade WG, Wilson MJ (2007) A molecular analysis of the bacteria present within oral squamous cell carcinoma. J Med Microbiol 56(12):1651–1659
Horie H, Zeisig M, Hirayama K, Midtvedt T, Möller L, Rafter J (2003) Probiotic mixture decreases DNA adduct formation in colonic epithelium induced by the food mutagen 2-amino-9H-pyrido [2,3-b]indole in a human-flora associated mouse model. Eur J Cancer Prev 12(2):101–107
Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS (2013) Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342(6161):967–970
Jacouton E, Chain F, Sokol H, Langella P, Bermúdez-Humarán L (2017) Probiotic Strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Front Immunol 8:1553
Jobin C (2018) Precision medicine using microbiota. Science 359(6371):32–34
Jones C, Badger S, Regan M, Clements B, Diamond T, Parks R, Taylor M (2013) Modulation of gut barrier function in patients with obstructive jaundice using probiotic LP299v. Eur J Gastroenterol Hepatol 25(12):1424–1430
Kanmani P, Satish Kumar R, Yuvaraj N, Paari K, Pattukumar V, Arul V (2013) Probiotics and its functionally valuable products—a review. Crit Rev Food Sci Nutr 53(6):641–658
Kaur S, Kaur S (2015) Bacteriocins as potential anticancer agents. Front Pharmacol 6:272. https://doi.org/10.3389/fphar.2015.00272
Ko J (2007) Lactobacillus plantarum inhibits epithelial barrier dysfunction and interleukin-8 secretion induced by tumor necrosis factor-α. World J Gastroenterol 13(13):1962
Kolb R, Phan L, Borcherding N, Liu Y, Yuan F, Janowski A, Xie Q, Markan K, Li W, Potthoff M, Fuentes-Mattei E, Ellies L, Knudson C, Lee M, Yeung S, Cassel S, Sutterwala F, Zhang W (2016) Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat Commun 7:1
Konishi H, Fujiya M, Tanaka H, Ueno N, Moriichi K, Sasajima J, Ikuta K, Akutsu H, Tanabe H, Kohgo Y (2016) Probiotic-Derived Ferrichrome Inhibits Colon Cancer Progression via JNK-Mediated Apoptosis. Nat Commun 7:12365
Kumeria T, Maher S, Wang Y, Kaur G, Wang L, Erkelens M, Forward P, Lambert M, Evdokiou A, Losic D (2016) Naturally derived iron oxide nanowires from bacteria for magnetically triggered drug release and cancer hyperthermia in 2D and 3D culture environments: Bacteria biofilm to potent cancer therapeutic. Biomacromolecules 17(8):2726–2736
Lazar V, Ditu L, Pircalabioru G, Gheorghe I, Curutiu C, Holban AM, Chifiriuc CM (2018) Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology and cancer. Front Immunol 9:1830
Lazar V, Ditu LM, Pircalabioru GG, Picu A, Petcu L, Cucu N, Chifiriuc MC (2019) Gut microbiota, host organism, and diet trialogue in diabetes and obesity. Front Nutr 6(21):1–20
Lee N, Son S, Jeon E, Jung G, Lee J, Paik H (2015) The prophylactic effect of probiotic Bacillus polyfermenticus KU3 against cancer cells. J Funct Foods 14:513–518
Lee WH, Chen HM, Yang SF, Liang C, Peng CY, Lin FM, Yang T (2017) Bacterial alterations in salivary microbiota and their association in oral cancer. Sci Rep 7(1):16540
Lenoir M, del Carmen S, Cortes-Perez N, Lozano-Ojalvo D, Muñoz-Provencio D, Chain F, Langella P, de Moreno de LeBlanc A, LeBlanc J, Bermúdez-Humarán L (2016) Lactobacillus casei BL23 regulates Treg and Th17 T-cell populations and reduces DMH-associated colorectal cancer. J Gastroenterol 51(9):862–873
Leschner S, Westphal K, Dietrich N, Viegas N, Jablonska J, Lyszkiewicz M, Lienenklaus S, Falk W, Gekara N, Loessner H, Weiss S (2009) Tumor invasion of Salmonella typhimurium is accompanied by strong hemorrhage promoted by TNF-α. PLoS ONE 4(8):6692
Lin C, Cai X, Zhang J, Wang W, Sheng Q, Hua H, Zhou X (2019) Role of gut microbiota in the development and treatment of colorectal cancer. Digestion 100:72–78
Louis P, Hold G, Flint H (2014) The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 12(10):661–672
Low KB (2004) Construction of VNP20009: a novel genetically stable antibiotic sensitive strain of tumor targeting Salmonella for parenteral administration in humans. Methods Mol Med 90:47–60
Ma W, Mao Q, Xia W, Dong G, Yu C, Jiang F (2019) Gut microbiota shapes the efficiency of cancer therapy. Front Microbiol 10:1050
Mager DL (2006) Bacteria and cancer: cause, coincidence or cure? A review. J Transl Med 4(1):14
Mager DL, Haffajee AD, Devlin PM, Norris CM, Posner MR, Goodson JM (2005) The salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med 3(1):27
Maldonado-Contreras A, Goldfarb KC, Godoy-Vitorino F, Karaoz U, Contreras M, Blaser MJ, Dominguez-Bello MG (2011) Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J 5(4):574
Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Gajewski TF (2018) The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 359(6371):104–108
McLoed A, Sherrill T, Cheng D, Han W, Saxon J, Gleaves L, Wu P, Polosukhin V, Karin M, Yull F, Stathopoulos G, Georgoulias V, Zaynagetdinov R, Blackwell T (2016) Neutrophil-derived IL-1β impairs the efficacy of NF-κB inhibitors against lung cancer. Cell Rep 16(1):120–132
Meng C, Bai C, Brown TD, Hood LE, Tian Q (2018) Human gut microbiota and gastrointestinal cancer. Genomics Proteomics Bioinformatics 16(1):33–49
Nagpal R, Wang S, Ahmadi S, Hayes J, Gagliano J, Subashchandrabose S, Yadav H (2018) Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci Rep 8(1):12649
Nagy KN, Sonkodi I, Szöke I, Nagy E, Newman HN (1998) The microflora associated with human oral carcinomas. Oral Oncol 34(4):304–308
Nasrollahzadeh D, Malekzadeh R, Ploner A, Shakeri R, Sotoudeh M, Fahimi S, Islami F (2015) Variations of gastric corpus microbiota are associated with early esophageal squamous cell carcinoma and squamous dysplasia. Sci Rep 5:8820
Neish A (2009) Microbes in gastrointestinal health and disease. Gastroenterology 136(1):65–80
Nekouian R, Rasouli B, Ghadimi-Darsajini A, Iragian G (2017) In vitro activity of probiotic Lactobacillus reuteri against gastric cancer progression by downregulation of urokinase plasminogen activator/urokinase plasminogen activator receptor gene expression. J Cancer Res Ther 13(2):246
Neuman H, Debelius J, Knight R, Koren O (2015) Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol Rev 39(4):509–521
Nurmi J, Puolakkainen P, Rautonen N (2005) Bifidobacterium lactis sp. 420 up-regulates cyclooxygenase (Cox)-1 and down-regulates Cox-2 gene expression in a Caco-2 cell culture model. Nutr Cancer 51(1):83–92
O’Keefe S (2016) Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol 13(12):691–706
Odamaki T, Sugahara H, Yonezawa S, Yaeshima T, Iwatsuki K, Tanabe S, Tominaga T, Togashi H, Benno Y, Xiao J (2012) Effect of the oral intake of yogurt containing Bifidobacterium longum BB536 on the cell numbers of enterotoxigenic Bacteroides fragilis in microbiota. Anaerobe 18(1):14–18
Ohland CL, Jobin C (2015) Microbial activities and intestinal homeostasis: a delicate balance between health and disease. Cell Mol Gastroenterol Hepatol 1(1):28–40
Petra CV, Rus A, Dumitrascu DL (2017) Gastric microbiota: tracing the culprit. Clujul Med 90(4):369–376
Qin J, Li R, Raes J, Arumugam M, Burgdorf K, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende D, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto J, Hansen T, Le Paslier D, Linneberg A, Nielsen H, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich S, Wang J (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464(7285):59–65
Raza M, Gul K, Arshad A, Riaz N, Waheed U, Rauf A, Aldakheel F, Alduraywish S, Rehman M, Abdullah M, Arshad M (2018) Microbiota in cancer development and treatment. J Cancer Res Clin Oncol 145(1):49–63
Reddy B (1997) Effect of dietary oligofructose and inulin on colonic preneoplastic aberrant crypt foci inhibition. Carcinogenesis 18(7):1371–1374
Reid G, Jass J, Sebulsky MT, McCormick JK (2003) Potential uses of probiotics in clinical practice. Clin Microbiol Rev 16:658–672
Riaz Rajoka M, Zhao H, Lu Y, Lian Z, Li N, Hussain N, Shao D, Jin M, Li Q, Shi J (2018) Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct 9(5):2705–2715
Roberts NJ, Zhang L, Janku F, Collins A, Bai RY, Staedtke V, Rusk AW, Tung D, Miller M, Roix J, Khanna KV, Murthy R, Benjamin RS, Helgason T, Szvalb AD, Bird JE, Roy-Chowdhuri S, Zhang HH, Qiao Y, Karim B, Zhou S (2014) Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med 6(249):249. https://doi.org/10.1126/scitranslmed.3008982
Roller M, Femia A, Caderni G, Rechkemmer G, Watzl B (2004) Intestinal immunity of rats with colon cancer is modulated by oligofructose-enriched inulin combined with Lactobacillus rhamnosus and Bifidobacterium lactis. Br J Nutr 92(6):931–938
Routy B, Le Chatelier E, Derosa L, Duong CP, Alou MT, Daillère R, Fidelle M (2018) Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 359(6371):91–97
Roy S, Trinchieri G (2017) Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer 17(5):271
Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW (2013) Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14(2):195–206
Ryan R, Green J, Williams P, Tazzyman S, Hunt S, Harmey J, Kehoe S, Lewis C (2009) Bacterial delivery of a novel cytolysin to hypoxic areas of solid tumors. Gene Ther 16(3):329–339
Saber A, Alipour B, Faghfoori Z, Yari Khosroushahi A (2017) Secretion metabolites of dairy Kluyveromyces marxianus AS41 isolated as probiotic, induces apoptosis in different human cancer cell lines and exhibit anti-pathogenic effects. J Funct Foods 34:408–421
Sadhu A, Ganguly KK (2017) Lactobacillus sp.—a threat to pathogenic microorganisms and tumor cells. J Cancer Ther 8(2):96–111. https://doi.org/10.4236/jct.2017.82009
Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai R, Wang E, Ma W, Haines D, O'hUigin C, Marincola F, Trinchieri G (2010) MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med 207(8):1625–1636
Sasaki M, Yamaura C, Ohara-Nemoto Y, Tajika S, Kodama Y, Ohya T, Kimura S (2005) Streptococcus anginosus infection in oral cancer and its infection route. Oral Dis 11(3):151–156
Saus E, Iraola-Guzmán S, Willis J, Brunet-Vega A, Gabaldón T (2019) Microbiome and colorectal cancer: roles in carcinogenesis and clinical potential. Mol Asp Med 69:93–106
Saxami G, Karapetsas A, Lamprianidou E, Kotsianidis I, Chlichlia A, Tassou C, Zoumpourlis V, Galanis A (2016) Two potential probiotic lactobacillus strains isolated from olive microbiota exhibit adhesion and anti-proliferative effects in cancer cell lines. J Funct Foods 24:461–471
Scott K, Gratz S, Sheridan P, Flint H, Duncan S (2013) The influence of diet on the gut microbiota. Pharmacol Res 69(1):52–60
Seidel JA, Otsuka A, Kabashima K (2018) Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 8:86
Sender R, Fuchs S, Milo R (2016) Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164(3):337–340
Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348(6230):56–61
Singh J, Hamid R, Reddy BS (1997) Dietary fat and colon cancer: modulating effect of types and amount of dietary fat on ras-p21 function during promotion and progression stages of colon cancer. Cancer Res 57(2):253–258
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Chang EB (2015) Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 350(6264):1084–1089
Stenken JA, Poschenrieder AJ (2015) Bioanalytical chemistry of cytokines–a review. Anal Chim Acta 853:95–115
Stern C, Kasnitz N, Kocijancic D et al (2015) Induction of CD4 (+) and CD8(+) antitumor effector T cell responses. Int J Cancer 137:2019–2028
Takeda K, Okumura K (2007) Effects of a fermented milk drink containing Lactobacillus casei strain shirota on the human NK-Cell Activity. J Nutr 137(3):791–793
Tarrah A, Castilhos J, Rossi R, Duarte V, Ziegler D, Corich V, Giacomini A (2018) In vitro probiotic potential and anti-cancer activity of newly isolated folate-producing Streptococcus thermophilus strains. Front Microbiol 9:2214
Todorov S, de Melo Franco B, Tagg J (2019) Bacteriocins of gram-positive bacteria having activity spectra extending beyond closely-related species. Benefic Microbes 10(3):315–328
van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal E, de Vos W, Visser C, Kuijper E, Bartelsman J, Tijssen J, Speelman P, Dijkgraaf M, Keller J (2013) Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368(5):407–415
Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Poirier-Colame V (2015) Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350(6264):1079–1084
Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot D, Pfirschke C, Engblom C, Pittet M, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther P, Eberl G, Berard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson C, Dore J, Kroemer G, Lepage P (2013) The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342(6161):971–976
Vivarelli S, Salemi R, Candido S, Falzone L, Santagati M, Stefani S, Libra M (2019) Gut microbiota and cancer: from pathogenesis to therapy. Cancer 11(1):38
Wall DM, Srikanth CV, McCormick BA (2010) Targeting tumors with Salmonella typhimurium-potential for therapy. Oncotarget 1(8):721
Wang G, Yu Y, Wang YZ, Wang JJ, Guan R, Sun Y, Fu XL (2019) Role of SCFAs in gut microbiome and glycolysis for colorectal cancer therapy. J Cell Physiol 234(10):17023–17049
Whiteman DC, Parmar P, Fahey P, Moore SP, Stark M, Zhao ZZ, Study AC (2010) Association of Helicobacter pylori infection with reduced risk for esophageal cancer is independent of environmental and genetic modifiers. Gastroenterology 139(1):73–83
Wong S, Kwong T, Wu C, Yu J (2019) Clinical applications of gut microbiota in cancer biology. Semin Cancer Biol 55:28–36
Wu HJ, Wu E (2012) The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3(1):4–14
Yang J, Yu J (2018) The association of diet, gut microbiota and colorectal cancer: what we eat may imply what we get. Protein Cell 9(5):474–487
Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z (2009) Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 137(2):588–597
Yang L, Francois F, Pei Z (2012) Molecular pathways: pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin Cancer Res 18(8):2138–2144
Yeh KY, Wang HM, Chang JW, Huang JS, Lai CH, Lan YJ, Wu TH, Chang PH, Wang H, Wu CJ, Hsia S, Wang CH (2013) Omega-3 fatty acid-, micronutrient-, and probiotic-enriched nutrition helps body weight stabilization in head and neck cancer cachexia. Oral Surg Oral Med Oral Pathol Oral Radiol 116(1):41–48. https://doi.org/10.1016/j.oooo.2013.01.015
Yu A, Li L (2016) The potential role of probiotics in cancer prevention and treatment. Nutr Cancer 68(4):535–544
Zaki M, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti T (2010) IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol 185(8):4912–4920
Zhang X, Liu F, Cheng Z, Wang W (2009) Cell fate decision mediated by p53 pulses. PNAS 106(30):12245–12250
Zhang M, Wang F, Jiang L, Liu R, Zhang L, Lei X, Li J, Jiang J, Guo H, Fang B, Zhao L, Ren F (2013) Lactobacillus salivarius REN inhibits rat oral cancer induced by 4-nitroquioline 1-oxide. Cancer Prev Res 6(7):686–694
Zhou Z, Chen J, Yao H, Hu H (2018) Fusobacterium and colorectal cancer. Front Oncol 8:371
Acknowledgment
Dr. Alok Malaviya is thankful to Centre for Research Projects, CHRIST (Deemed to be University), Bangalore, for the generous research grant (MRPDSC—1829) on Probiotic development.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Malaviya, A., Paari, K.A., Malviya, S., Kondapalli, V.K., Ghosh, A., Samuel, R.A. (2021). Gut Microbiota and Cancer Correlates. In: Deol, P.K. (eds) Probiotic Research in Therapeutics. Springer, Singapore. https://doi.org/10.1007/978-981-15-8214-1_1
Download citation
DOI: https://doi.org/10.1007/978-981-15-8214-1_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-8213-4
Online ISBN: 978-981-15-8214-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)