Abstract
The aphids are soft-bodied small insects (< 7 mm) and feed by sucking plant sap. They usually live in colonies on the undersides of leaves or tender terminal shoots. Aphids excrete considerable amount of sugary liquid, honeydew, on which sooty mould usually turns them black and also serves as food for ants, bees and their parasitic wasps. The aphids are unique on the account of their peculiar mode of reproduction, development and polymorphism. They may reproduce either by parthenogenesis, zygogenesis or paedogenesis. They may either be oviparous or viviparous and alatae or apterae, the male often being wanting and frequently rare in certain generations. Parthenogenetic reproduction allows rapid increase in numbers and results in populations consisting of clones. Some species reproduce both parthenogenetically and sexually (holocyclic species), whereas only few reproduce parthenogenetically (anholocyclic species). In parthenogenetic reproduction, life cycle completes within 10 days in temperate regions. The aphids are polymorphic, and both winged (alate) and wingless (aptera) morphs may be found in the same colony. Several factors, both biotic and abiotic, have effect on the formation of different phenotypes. Each morph performs different ecological roles in the life history which is characteristic of aphids. This trait coupled with the ability to breed by means of diploid parthenogenesis and viviparity for a major part of the life cycle in aphids has enabled them to produce a large number of clones in different kinds of plants even under adverse conditions. Aphids are frequently engaged in mutualistic associations with bacterial endosymbionts that not only provide essential amino acids to them but also grant them protection from natural enemies, protection from extreme temperatures, development of resistance to a fungal pathogen and the ability to use a greater diversity of resources. Out of globally 5110 species of aphids described, about 250 species are major agricultural and horticultural pests. They damage the crops directly by sucking their nutrients, making galls and hampering photosynthesis and respiration by the growth of sooty moulds on the honeydew deposited thereon. Aphids also damage the crop indirectly by transmitting hundreds of plant viruses. Because of their economic importance, their population must be controlled to save the crops. In this contribution, several aspects of aphid systematic and biology such as endemism, host–plant association, diversity, morphology, feeding behaviour, life history, polymorphism and factors affecting it, migration, defence, aphid–ant association, endosymbiosis, economic importance and their population management have been described in detail.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Aphid, also known as greenfly, blackfly, plant lice, ant cow, etc., is a common name for all the bugs that belong to the family Aphididae (order Hemiptera, suborder Sternorrhyncha, infraorder Aphidomorpha, superfamily Aphidoidea). They are small (1–10 mm), soft-bodied, sap-sucking insects infesting both aerial and subaerial parts of a variety of plant species, few of which are notorious pests of agricultural and horticultural crops. They possess a proboscis which originates between and behind the forelegs; their antennae have two thick basal segments and a flagellum composed of up to four segments, the last of which is divided into a proximal part and a thinner distal part called a ‘process terminalis’. They possess two compound eyes and two ocular tubercles made up of three lenses each which are situated behind and above the compound eyes. They have two tarsal segments; the wings when present have only one prominent longitudinal vein. The fifth abdominal segment bears a pair of upward and backward pointing tubes on the dorsal surface called siphunculi or cornicles, and a cauda is usually present below and between them on the last abdominal segment. They are cosmopolitan but are most abundant in temperate climates. They are unique on account of their peculiar mode of reproduction, development and polymorphism. They may reproduce either by parthenogenesis, zygogenesis or paedogenesis. They may either be oviparous or viviparous. The sexes may be unequally represented (males are frequently rare) in certain generations. Parthenogenetic reproduction allows rapid increase in numbers and results in populations consisting of clones. Some species reproduce both parthenogenetically and sexually (holocyclic species), whereas only a few reproduce solely through parthenogenesis (anholocyclic species) (Dixon 1977, 1998; Singh and Ghosh 2002) (Fig. 3.1).
Aphids are related to members of other Aphidomorpha, e.g. adelgids (conifer aphids, pine aphids, spruce aphids) (family Adelgidae, superfamily Adelgoidea) and phylloxerids (family Phylloxeridae, superfamily Phylloxeroidea) (Heie and Wegierek 2009), all of which probably evolved about 280 million years ago (MYA) in the Carboniferous; they probably bred on nonflowering plants such as Cordaitales and Cycadophyta (Capinera 2008). The oldest known fossil aphid is Triassoaphis cubitus from the Triassic about 220 MYA (Evans 1956). Aphids did not always look like they do now; the characteristic shape and wing venation and the structure of their proboscis had evolved by the Jurassic (e.g. Juraphis crassipes), whereas the cauda and the siphunculi evolved later in the Cretaceous about 55 MYA (Shaposhnikov 1979).
3.2 Systematics and Biology
There have been different opinions regarding the nomenclature of the aphid taxonomy. The classification of aphids is still not well established that may be accepted all over the world. There were various opinions regarding the classification of the Aphidoidea. Aphid taxonomy is often frustrated by the host alternation and extensive polyphenism displayed by many species. In the literature, some aphidologists (Remaudiere and Remaudiere 1997; Blackman and Eastop 2007) refer to the families of Heie (1987) as subfamilies. There is no extremely good reason to prefer one categorisation over the other, and fortunately this dichotomy in the literature has led to little confusion. However, the use of family designations has the only single advantage of allowing slightly more details in taxonomic hierarchies. Favret and Eades (2020) in aphid.speciesfile.org kept all the extant Aphididae under 24 subfamilies, viz. Aiceoninae, Anoeciinae, Aphidinae, Baltichaitophorinae, Calaphidinae, Chaitophorinae, Drepanosiphinae, Greenideinae, Eriosomatinae, Hormaphidinae, Israelaphidinae, Lachninae, Lizeriinae, Macropodaphidinae, Mindarinae, Neophyllaphidinae, Phloeomyzinae, Phyllaphidinae, Pterastheniinae, Saltusaphidinae, Spicaphidinae, Taiwanaphidinae, Tamaliinae and Thelaxinae. Table 3.1 summarises the species diversity of aphids in different taxa of Aphididae in the world and in India.
3.2.1 Endemism in India
Globally, 5110 species of aphids are described under 528 genera. Out of 24 subfamilies of Aphididae, only 16 subfamilies are represented in India (Table 3.1). In India, 809 species of aphids under 208 genera are reported, out of which about half of them (at least 385 species) are endemic, and among them almost all are represented in the Himalayas region. The most species diversity was observed in the subfamily Aphidinae (444 species) (Singh et al. 2014, 2015a; Singh and Singh 2016a, b, c, 2017a, b, c) followed by Greenideinae (96 species) (Singh and Singh 2017d), Eriosomatinae (64 species) (Singh and Singh 2017e) and Hormaphidinae (57 species) (Singh and Singh 2018). Singh and Singh (2019) summarised the diversity of Indian aphids. At least 32 endemic genera are represented in India. Except Aspidophorodon Verma, Indiaphis Basu, Neomasonaphis Ghosh and Raychaudhuri, Myzakkia Basu and Brachyunguis Das, all other endemic genera are monotypic. All the eight species under Aiceoninae, three species of Taiwanaphidinae and one species of Lizeriinae are endemic. The percentage of endemism in other subfamilies is as follows: Thelaxinae (75%), Greenideinae (71.9%), Calaphidinae (62.5%), Drepanosiphinae (50%), Hormaphidinae (47.4%), Aphidinae (44.5%), Chaitophorinae (44.4), Lachninae (39.0%) and Eriosomatinae (34.4%). Only eight endemic species are present in the peninsular area including two species, viz. Eutrichosiphum davidi and Paoliella nirmalae, which are exclusive of this area. The high percentage of endemism shows that the Himalayan areas provide congenial ecological conditions for the active speciation of aphids, while the peninsular region which is a part of the Gondwanaland is very old and stable landmass with distinct flora and fauna quite different from the northern parts.
3.2.2 Host–Plant Association in India
Singh and Singh (2016a, b, c, d, 2017a, b, c, d, e, f, 2018; Singh et al. 2014, 2018, 2015a) recently catalogued the Indian aphids and their food plants. Of all plant species, 25 % are used as food plant by the aphids, and though it is believed that the speciation of aphids has followed that of plants, not all groups of plants are equally infested (Mahr 2018). The Asteraceae, the third most specious plant family, supports the most aphid species (696 species) (Singh et al. 2015b), but the Orchidaceae, the second most specious plant family, supports only nine species of aphids, while the Rosaceae which is only the 22nd most specious plant family supports the third highest number of aphid species (293 species) in India. The plant family which supports the second highest number of aphids is Coniferae (includes several families, viz. Pinaceae, Araucariaceae, Podocarpaceae, Sciadopityaceae, Cupressaceae, Cephalotaxaceae, Taxaceae [363 species]), but these are nonflowering plants. Most aphids are monophagous, though some species are highly polyphagous, e.g. Myzus persicae which feed on more than 500 different plant species worldwide; in India, it alone infests 293 plant species under 64 plant families (Singh et al. 2015a).
Few aphids are known to make gall on the plants. The aphid galls are abnormal outgrowths of the plant tissues that serve as their own microhabitats as well as food. Galls may also provide the aphid with physical protection from parasitoids and predators (Fig. 3.2). In Northwest Himalayas, about 76 gall-inducing aphid species are reported under Eriosomatinae, Hormaphidinae, Aphidinae and Calaphidinae in these areas, and all of them are present in Northwest Himalaya. But Northeast Himalaya supports only eight gall-inducing species (10.52%), and most of them belong to Aphidinae. Of Eriosomatinae, 93% can induce galls in Northwest Himalaya (Chakrabarti 2007). In general, the gall-forming aphid species are heteroecious, i.e. alternate between their primary and secondary hosts in different periods of the year. However, a few have been found to be autoecious, i.e. monophagous. These aphids are also highly polymorphic in nature. Unless morphs from both of their primary and secondary hosts are available, their identities in some cases are difficult (Chakrabarti 1987).
A gall on a leaf made by gall making aphid (a) and a gall cut to show the aphids inside (b). Courtesy bugguide.net
Availability and diversification of host–plants has direct influence on the diversification of aphids. The major host–plant subclasses that harbour more aphid species are Rosidae, Asteridae, Dilleniidae and Colelinidae. More than 150 aphid species are found on these plants. Out of about 696 plant species described under the family Asteraceae in India, only 207 species were found to be infested by 199 aphid species. Among the aphid species, Aphis gossypii was recorded feeding 77 species of Asteraceae, followed by Brachycaudus helichrysi (72 species), Aphis spiraecola (70 species), Myzus persicae (45 species), Myzus ornatus (35 species), Aphis fabae (25 species) and Aphis craccivora (23 species). Artemisia and Sonchus were observed to be attacked by 60 and 25 aphid species, respectively (Singh et al. 2015b).
There are many examples of special host associations in this area. The nonavailability of specific primary host has influenced the life cycle patterns in many species especially under the subfamily Eriosomatinae. Many species continued anholocyclic parthenogenesis for a long time, such as species of Fordini and Hormaphidinae. Recently, Singh and Singh (2019) have summarised the diversity and food plant associations of Indian aphids.
3.2.3 Diversity in India
The great diversity and abundance of aphids usually occur in the higher altitudes where subtropical to warm temperate climate prevails which represents transition area between Oriental and Palaearctic realms. The biological diversity of India is well reflected in the distribution and abundance of fauna and flora and also in aphid–host association. The richness and diversity of aphid fauna is largely due to diverse flora in different kinds of ecosystem present in India. The food plants of Indian aphids cover over 1250 species belonging to 137 plant families and 86 orders and suborders (Agarwala and Ghosh 1985) against a total plant species of about 45,000 found in India. Among the areas enjoying subtropical to warm temperate climate in India, diversity and concentration of aphids are more pronounced in Northwest and Northeast Indian states, whereas hotter and drier areas of Indo-Gangetic and Peninsular India have less species diversity and poor prevalence. Chakrabarti (2009) analysed the aphid diversity, its distribution and endemism, host–plant association and the life cycle pattern from subregion of the Oriental region and observed that all these are different in each zone. Out of the total Indian aphid species, the Himalayas represent 808 species in 219 genera under 17 subfamilies indicating high species richness. The Northwest Himalayas has 573 species in 177 genera of 16 subfamilies, and the Eastern Himalayas has 464 species in 147 genera of 14 subfamilies, while Central Himalayas is represented by only 67 species in 56 genera of 8 subfamilies. The Peninsular India has 126 species in 56 genera, and the Gangetic plain has 64 species in 32 genera, while the Indus valley has only 27 species in 15 genera (Chakrabarti 2009).
Subfamily-wise breakup of the taxa in the said biogeographical areas reveals that in Northeast India, subfamily Anoeciinae represents 8 species, Aphidinae 270 species, Chaitophorinae 7 species, Drepanosiphinae (including Calaphidinae, Lizeriinae, Phyllaphidinae, Saltusaphidinae and Taiwanaphidinae) 24 species, Greenideinae 49 species, Hormaphidinae 29 species, Lachninae 11 species and Eriosomatinae 16 species. Similarly, in Northwest India, subfamily Anoeciinae represents 8 species, Aphidinae 168 species, Chaitophorinae 13 species, Drepanosiphinae (including Calaphidinae, Lizeriinae, Phyllaphidinae, Saltusaphidinae and Taiwanaphidinae) 28 species, Greenideinae 24 species, Hormaphidinae 12 species, Lachninae 23 species and Eriosomatinae 34 species. On the other hand, Gangetic plain represents Aphidinae 42 species, Greenideinae 2 species and Calaphidinae 1 species only. Also, Indus plain represents three subfamilies, viz. Aphidinae 30 species, Calaphidinae 1 species and Greenideinae 1 species. Lastly, in Peninsular India, Aphidinae represents 50 species, Calaphidinae 4 species, Greenideinae 10 species, Hormaphidinae 5 species, Lachninae 1 species and Eriosomatinae 3 species. Thus, among five biogeographical areas, Northeast India represents the maximum number of aphid species and subspecies (414), followed by Northwest India (310), Peninsular India (73), Gangetic plain (45) and Indus plain (32). Also, the subfamily Aphidinae among other subfamilies (Anoeciinae, Drepanosiphinae, Calaphidinae, Lizeriinae, Phyllaphidinae, Saltusaphidinae, Taiwanaphidinae, Greenideinae, Hormaphidinae, Lachninae and Pemphiginae) has higher frequency of occurrence than any other subfamilies. The Peninsular India having Eastern and Western Ghats and Vindhya Range as well as Gangetic and Indus plains has comparatively poor diversity of aphid fauna. It may be mentioned here that both Northeast and Northwest India represent the genera belonging to all subfamilies. The subfamily Anoeciinae is apparently yet to be recorded from Peninsular India. Likewise, the subfamilies Aphidinae, Greenideinae and Calaphinae are on record from both Peninsular and Gangetic plain regions. It is worthwhile to mention that the genera belonging to subfamily Aphidinae are found to occur in all the regions of India and its genera are most abundant in all the biogeographical areas in comparison to other subfamilies of the family Aphididae (Ghosh and Singh 2000).
3.2.4 Morphology
The body of the aphid is usually divisible into the head, thorax and abdomen. However, in some species, it is very difficult to divide the body due to tendency of fusion of the segments. The external morphology of an aphid is provided in Figs. 3.3 and 3.4. The head is usually dorsoventrally flattened. The number of antennal segments varies between one and six. The last antennal segment has a stout base and a short to very long slender terminal portion, the processus terminalis, with at least three terminal hairs. The primary rhinarium is placed at the junction of the base. The eyes are always well developed and larger in the winged morph than wingless ones. At the posterior margin of the eye protrudes an ocular tubercle, or triommatidion. Alate aphids bear three ocelli, one on the front of the head and the other two laterally near the anterior part of each eye. The proboscis, which is laid back beneath the body when not in use, may be so long, especially in the species that live on trees that it sticks out beyond the end of the abdomen. The stylet bundle with which aphids take up plant sap consists of two pairs of needlelike stylets, the inner pair of maxillary stylets and the outer pair of mandibular stylets (Fig. 3.3).
The prothorax or the entire thorax may be fused variably with the head. Each thoracic segment bears a pair of legs having usually five segments: coxa, trochanter, femur, tibia and two-segmented tarsi. The segments are partly or completely fused together or atrophied in some species, especially in Pemphiginae and Hormaphidinae. The alatae bear a pair of wings; these are of similar consistency, but the forewing is always longer and broader than the hindwing. The forewings have two longitudinal veins: one is the costa, which is a weak vein running along the frontal edge of the wing, and the other is the strong main vein which runs just behind the costa (Fig. 3.3).
The abdomen consists of nine visible segments, the ninth being the cauda (Fig. 3.4). The first segment may be fused with the thorax or may remain distinct; other segments except the segment 8 may be clearly or indistinctly demarcated from each other or may get fused. Aphids bear nine pairs of spiracles (or stigma) on their bodies: one pair on the meso- and metathorax and on the first seven abdominal segments laterally. Wax plates or pores may be present on the dorsum of the thorax and abdomen, the position and size being variable in wax plates. Because of this, the aphids are often more or less pulverulent with white waxy exudates, wither of powdery, filamentous, plate-like or rod-like appearance. Small papilla-like tubercles are often observed in Aphididae. They occur singly as a rule on each segment and are arranged on the body in a marginal row and less often also occur in mesal and pleural rows. The ventral hairs may be short to long, and they are usually with cute apices. Dorsolaterally on the abdominal segments 5 or 6 usually occurs a pair of siphunculi or cornicles which are typically tube-like structure through which alarm pheromones exudate (Behura 1996a). These may be altogether absent, but when present they may be of variable shapes and sizes, viz. ring-like, mammiform, cone-shaped, cylindrical, truncate, tapering, slightly to distinctly clavate or cigar-shaped, and may be with a flange near its apex; the siphuncular opening may be placed right at the apex or may be shifted laterally; and the surface may be smooth or warty. At the posterior end of the abdominal sternum, there are two sclerotised plates, the anal plate which represents the tenth abdominal sternite and the genital plate which represents the eighth. Subanal plate is situated ventral to the cauda which may be entire, indented, elongate, oval, semioval, crescent-shaped, knobbed or bilobed, semicircular, broadly triangular, semicircular, etc. The subgenital plate bears many hairs. The cauda is short, either crescent-shaped, semicircular, broadly triangular or shortly tongue-shaped, and bears two to many hairs (Fig. 3.4).
3.2.5 Feeding Behaviour
Both nymph and adult aphids feed in the same way. They are phloem feeder. They find the phloem vessels from the stems, leaves and roots of the plant. In most cases, they feed passively by means of high pressure within the sieve elements of the plant (Fig. 3.5). The maxillae and mandibles are elongated into a stylet bundle that penetrates the plant tissues to reach the feeding site in the phloem. At this time, the distal tip of the labium helps stylet penetration from the outside, acting as a guide. The stylets, enclosed within the proboscis when the aphid is not feeding, are very thin and could break during insertion into the plant. Therefore, aphids secrete a substance from the tips of their stylets which begin to harden forming a hard protective covering around the stylets as they are slowly pushed into the plant in search of the phloem tubes (Miles 1999; Will and Vilcinskasa 2015). The saliva also isolates plant tissues from the mouthparts avoiding plant reaction at the feeding site (Felton and Eichenseer 1999). When the stylets reach a phloem tube, the aphid injects saliva into it. The saliva helps prevent the plant cell from sealing the puncture (i.e. the mouthparts of the aphids) with special proteins which are the plants’ normal defence mechanism (Will and van Bel 2006; Pettersson et al. 2017). Aphids pierce their stylets very slowly, and it may take half an hour to 24 hours from beginning to prick the stylets to actually sucking the phloem juice. This feeding habit causes little mechanical damage to the plant as compared to that of biting and chewing insects. The phloem sap also contains high sugar level that causes high osmotic pressure inside the stomach of the aphid due to which water transfers from haemolymph to the stomach causing hyperosmotic stress that may cause the death of the aphid. Therefore, the aphids avoid this situation by several osmoregulatory mechanisms (Ashford et al. 2000). Excess sugars are excreted through anus called honeydew which is used by other insects as food.
Schematic representation of a feeding aphid (Guerrieri and Digilio 2008)
3.2.6 Life History
The life history of aphids is highly complicated including parthenogenetic and sexual generations, elaborate polyphenism and obligate shifting between unrelated host–plant taxa. These and other unusual life cycle traits occur in a variety of combinations among the approximately 5100 extant species within the family. The aphids have prolific breeding, polyphagy, advanced degree of polymorphism, anholocyclic/holocyclic reproduction, host alternation and high potential for rapid evolutionary changes because of parthenogenesis and polyvoltinism (Minks and Harrewijn 1987; Behura 1994).
Some aphids are anholocyclic in which males are totally absent, and the parthenogenetic diploid females reproduce only by viviparity, while others living in temperate climates are holocyclic that produce males and oviparae, which mate to produce eggs for overwintering. Anholocyclic ones overwinter with viviparous females in protected locations, and no sexual morphs and eggs emerged. According to the aphid evolution theory, anholocycly originated from holocycly during the fourth glacial epoch (Moran 1992). Dixon (1998) and Hardie (2017) illustrated the generalised life cycle pattern of aphids and discussed the role of nutrition on the production of morph. Aphid life cycles are complex and may be either monoecious or dioecious, involving holocycly or anholocycly. In the simple and generalised monoecious holocyclic aphid life cycle (Fig. 3.6a), the aphids feed on a single host–plant species throughout the year. The sexual morphs are produced in the following autumn, in response to decreasing photoperiod. Then, mating takes place between males and females (oviparae) producing genetically recombinant eggs that overwinter on the host–plant. In the spring, fundatrix emerges from the eggs that overwinter on the host–plant and mature parthenogenetically and gives births to nymphs that mature to viviparae and continue the reproduction by this way in the summer. If the aphid group produces plant galls, the fundatrix is responsible for their production. The viviparae may be apterae (wingless) or alate (winged), but in some groups all viviparae are alate. The parthenogenetic reproduction of viviparae allows very rapid buildup of numbers and collapse of generation time. Apterae produce more offspring per female than do alatae. Once an aphid population reached its maximum, due to crowding effect among apterae or lack of nutrient levels, they turn to produce alatae, which migrate to better situations. During migration, a number of individuals die off because of landing on unsuitable food plants. In the situation, when alatae find a suitable host–plant, they fed for a short period, and they reproduce viviparidae generation. The production of viviparae continues until autumn when the conditions stimulate them to produce sexuals.
Evolutionary development of generalised aphid life cycles. Initially, aphids developed monoecious holocycly on an ancestral woody primary host, where aestivation occurred because sap amino acids were unavailable during summer growth cessation (a). Next, multiple subfamilies independently evolved dioecious holocycly, where viviparae moved to summer-growing herbaceous secondary hosts but returned to their ancestral host in autumn (b). In some aphids, secondarily monoecious holocycly developed on the secondary host when the primary host was lost (c). Often in warm areas, where selection for an overwintering egg is not imposed, some populations of dioecious and secondarily monoecious holocyclic aphids may lapse into facultative anholocycly on their secondary hosts; this condition may become obligate anholocycly if the ability to produce sexuals is lost (d) (Sorensen, 2009)
A second, more complicated dioecious life cycle (Fig. 3.6b) has independently evolved among several different aphid groups that show seasonal alternation between differing hosts. This dioecious cycle probably evolved in response to the seasonally inadequate supply of nutrients, especially amino acids, on their primary host. Woody deciduous plants normally transport amino acids in quantity only during the spring. Aphid groups feeding on and confined to such plants face a nitrogen deficit during the summer, when active plant growth retards and phloem sap is low or lacks nitrogen. In this situation, some groups of aphids, e.g. Periphyllus spp., may develop an aestivating nymph that halts growth until autumn, while others, e.g. Aphidinae, have developed to escape those primary hosts during the late spring. Their spring alatae migrate to herbaceous fast-growing secondary hosts as emigrants during the summer. In the autumn, when the secondary hosts disappeared, the aphids come back to their woody primary host by producing migrating males and gynoparae. The male and oviparae mate to lay their overwintering eggs. Depending on the aphid or its group, their secondary host–plants are either specific or of several plant species, but the primary hosts are often specific to a plant genus. However, in warmer climates, the aphid populations do not need an egg for overwintering survival. Under such conditions, otherwise holocyclic dioecious or monoecious populations may drop facultatively into anholocycly on their secondary hosts. If such populations remain anholocyclic for a longer period, they evolve into obligate anholocycly by losing the capacity to produce sexual morphs (Dixon 1998).
Some aphid groups have evolved beyond dioecious holocycly, entirely leaving their primary host to remain on their secondary host, in secondarily monoecious holocycly (Fig. 3.6c). These aphid groups do not require eggs to overwinter survival. Under such conditions, otherwise holocyclic dioecious or monoecious populations may lapse facultatively into anholocycly on their secondary hosts (Fig. 3.6d). If such populations remain anholocyclic long enough, they may eventually evolve into obligate anholocycly by losing the ability to produce sexual morphs, despite undergoing environmental conditions that normally trigger their production (Dixon 1998; Hardie 2017).
About 80% of the described species from India are parthenogenetic virginoparous for most of the year but are capable of sexual reproduction with production of eggs (Singh and Ghosh 2012). They develop in parthenogenetic female without fertilisation. Even embryos inside parthenogenetic females may contain embryos, i.e. a mother can have in its ovarioles developing embryos which in turn also contain embryos, the future granddaughters. Thus, there is a telescopic generation due to parthenogenesis and viviparity in aphids (Minks and Harrewijn 1987). This results in reduced postnatal development periods and generation time. All aphids have diploid parthenogenesis, and there is no reduction division, and development starts from germinal cells with full complement of chromosomes including XX chromosome. Sexual females, like asexual ones, have two sex chromosomes, i.e. XX. Males have only one sex chromosome, i.e. OX. In theory, this means males could produce sperm with either no sex chromosomes, i.e. an O, or one sex chromosome, i.e. an X. However, in reality, sperm with an O sex chromosome degenerate very rapidly and never contribute to an embryo. This means that all offspring of a sexual mating must have XX as their sex chromosomes, because females always contribute an X chromosome, and therefore all aphids resulting from sexual matings are female. Eggs are laid during the autumn as the overwintering stage in many temperate forms and, as explained above, give rise to females whether they are the result of sexual mating or not. In other species, a special overwintering form develops in the autumn called a ‘hiemalis’, while in some species the adults are the overwintering stage. Ova within a viviparously reproducing female start to develop immediately after ovulation; this occurs long before birth (even human females are born with all the ova they will ever need throughout their life, though they remain undeveloped for many years). This means that an embryo can exist inside another larger and more mature embryo. In fact, a newly born summer aphid can contain within herself not only the developing embryos of her daughters but also those of her granddaughters which are developing within her daughters. Parthenogenesis combined with this ‘telescoping of generations’ gives aphids an exceedingly rapid turnover of generations, meaning they can build up immense populations very quickly. There is a more or less regular cyclic or anholocyclic alternation of parthenogenetic oviparous and viviparous generations associated with polymorphism, changes of food plants and mode of life. Several generations often succeed each other, in which the males are extremely rare or are totally absent. Individuals of the same generation often differ considerably from one another. Some have fully developed wings, others have atrophied wings and still others are apterous (Singh and Singh 2016).
A generalised life cycle pattern of heteroecious life cycle of bird cherry aphid, also known as oat aphid, Rhopalosiphum padi, is illustrated in Fig. 3.7 whose several populations in temperate regions that have cold winters reproduce combining sexual and asexual phases (holocycly), alternating between primary winter tree hosts (bird cherry) and secondary summer host–plants that are grasses (Poaceae). In contrast, the reproduction of populations in areas where the winters are mild, and also in some temperate countries where the primary hosts are less available or absent, the life cycle is completely parthenogenic (anholocyclic) on poaceous plants.
Although low temperature, short day length and physical condition of host–plants are regarded as important factors governing the production of sexuales, the discovery of apterous oviparous females of the mustard aphid Lipaphis erysimi on mustard in the arid and semiarid region of Jaipur (Rajasthan) (Ghosh and Rajendran 1988) suggests that some other factors are also operative in the phenomenon. Complete life histories of Indian aphids are not known. The possible life cycle of Lipaphis erysimi (Fig. 3.8) in India is illustrated. Medda et al. (1997) studied different modes of life cycles of some aphid species infesting Salix in India (Fig. 3.9).
Possible life cycle of the mustard aphid, Lipaphis erysimi in India (Singh and Singh 2016)
Life cycle pattern of Salix-infesting aphids: Host alternating and cyclical parthenogenetic (a), non-host alternating and cyclical parthenogenetic (b) and non-host alternating and permanently parthenogenetic (c) (Medda et al. 1997)
3.2.7 Polymorphism
Aphids are remarkable on account of their peculiar mode of development and the polymorphism, i.e. occurrence of two or more morphologically distinct morphs in a population having the same genotype, exhibited in different generations of the same species. Each morph performs different ecological roles in the life history which is characteristic of aphids (Hille Ris Lambers 1966). This trait coupled with the ability to breed by means of diploid parthenogenesis and viviparity for a major part of the life cycle in aphids has enabled them to produce a large number of clones in different kinds of plants even under adverse conditions (Agarwala 2007). Genetically identical individuals living in different environments may be different in form, physiology or behaviour. Such individuals demonstrate phenotypic plasticity in response to environmental factors like seasonality of their host–plants, food quality, climate and natural enemy association, etc., that vary in space and lime. Agarwala (2007) nicely reviewed this phenomenon in aphids and the factors that cause such variations.
Females may have up to eight genetically identical distinct phenotypes that differ in morphology, physiology, numbers, timing of production, progeny sizes, developmental periods, longevity, host preferences and ability to locate and utilise the alternative host–plants. During the life cycles of a typical migratory aphid, the following sequence of polymorphism is usually met with.
3.2.7.1 Fundatrices: The Stem Mother or Foundress
These are usually apterous, viviparous, viriginoparae or parthenogenetic females which emerge in spring from the overwintered eggs (Fig. 3.10a–c). This morph is characteristic of egg-laying holocyclic aphids. The sense organs, legs and antennae are not so well developed as in succeeding apterous generations; the antennae, for example, are shorter and may comprise a smaller number of segments. The reduction of the parts is apparently correlated with increased reproductive capacity. The eyes are often smaller or consist of fewer facets than in the succeeding generations, and there may be differences in the siphunculi. In Drepanosiphon platanoides and some others, the fundatrices are exceptionally alate.
3.2.7.2 Fundatrigeniae or Viriginoparae: Apterous Viviparous Female
These are apterous, parthenogenetic, viviparous females which are the progeny of the fundatrices and live on the primary host (Fig. 3.10d). They are also known as ‘virginoparae’ due to their being virgin mothers, which are prolific breeders under favourable conditions. In heteroecious species where sexual and asexual generations are spent on plants of unrelated taxa, this morph is distinguishable into fundatrigeniae proper and alienicolae. The alienicolae is produced by fundatrigeniae on the secondary hosts.
3.2.7.3 Migrantes: Alate Viviparous Female
The migrantes usually develop in the second, third or later generations of fundatrigeniae and consist of alate parthenogenetic viviparous females (Fig. 3.10e, g). The wings of aptera are sometimes incompletely developed due to local adaptations called brachypterae. The antennae of these morphs are longer than aptera having more sensoria; the eyes are also prominent including ocelli. They develop on the primary host in the beginning of spring, called spring migrants or emigrants, and subsequently fly to the secondary host. In Drepanosiphon platanoides, all the viviparous females are winged and consequently fundatrigeniae are wanting. The return migrantes to the primary hosts are the sexuparae or sexuales (Dixon 1998).
Compared to the apterous phenotype, the alate aphids have a longer nymphal development period, lower offspring production and higher longevity (Tsumuki et al. 1990). Moreover, alate aphids are able to tolerate starvation (Hazell et al. 2005). The morphological and physiological characteristics of winged aphids enable them to survive in harsh conditions, have the chance to disperse and clone to a new environment (Dixon et al. 1993).
3.2.7.4 Alienicolae
Alienicolae are parthenogenetic, viviparous females (also known as exule) developing for the most part on the secondary host. They often differ markedly from the fundatrices and migrantes; many generations may be produced comprising both apterous and winged forms.
3.2.7.5 Sexuparae
The sexupara is used to specialised phenotypes that would produce sexual phenotypes in the next generation in holocyclic species (Miyazaki 1987). These are parthenogenetic viviparous females which usually develop on the secondary host, the alate forms migrating to the primary host at the end of the summer. The sexuparae terminate the generations of alenicolae by giving rise to the sexuales.
3.2.7.6 Sexuales
These usually appear only once in the life cycle and consist of sexually reproducing males (androparae) and females (gynoparae), the latter being oviparous (oviparae). The females with rare exceptions are apterous and distinguishable from the apterous viviparous generations of the same sex by the thickened tibiae of the hind legs and the greater body length (Fig. 3.10f). The males are either alate or apterous (Fig. 3.10h). Intermediates between alate and apterous (brachypterous) forms also occur. The sexuales exhibit various types of specialisation among different genera. Apterous parthenogenetic viviparous females may overwinter in several species, e.g. Brevicoryne brassicae (Fig. 3.10) and Myzus persicae.
With nonmigratory species, the terms migrantes and alienicolae are not applicable. In these cases, the winged and wingless viviparous females are more conveniently referred to as fundatrigeniae alatae or apterae as the case may be, and either one or the other may give rise to the sexuparae.
3.2.7.7 Morphological Changes in Phenotypes
Most of the holocyclic aphids overwinter in egg stage, but in some species, e.g. Colophina arma, the apterae viviparae produce very small and stout nymphs, ‘midget’ that hide themselves in the bark and overwinter without moulting until next spring (Aoki 1980). Similarly, there are several aphid species which aestivate during summer to tide over adverse condition of food and temperature by producing dormant first instar nymph, which remain glued to the leaves until autumn, e.g. Periphyllus spp. (Miyazaki 1987). This phenotype has a flattened body covered with plates (Fig. 3.11a) unlike normal ones (Fig. 3.11b).
First instar nymph aestivating in summer (a) and first instar normal nymph produced in autumn (b) of Periphyllus sp. infesting acer plants in the Himalayas; sterile first instar soldier phenotype of a bamboo-feeding aphid. Ceratovacuna sp. showing frontal horns in the head (Agarwala 2007)
Some species of aphid in divergent taxa, e.g. Eriosomatinae (Eriosoma, Colopha, Paracolopha, Colophina) and Hormaphidinae (Astegopteryx, Pseudoregma, Ceratovacuna), have evolved ‘soldier’ phenotype to defend aphid colonies from attacks from enemies and ants (Aoki 1982). However, these morphs do not contribute to reproduction, dispersal, etc., but increase the survival value of parental colonies. Soldiers are not moulting first instar nymphs characterised by sclerotised legs, prehensile forelegs and long and pointed frontal horns.
3.2.7.8 Colour Polymorphism
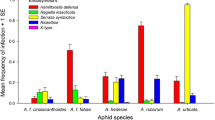
In colonies of several aphid species, the individuals may be of different colours, such as yellow, brown, red, green, black, pink and purple and various shades of these colours, which match with the coloration of the leaf, flower, fruit and stem of the host–plants on which they feed (Forsman et al. 2008). This affords them certain amount of camouflage. The colour variations may appear irregularly among members of the colony or may be associated with a particular sex or generation (Fig. 3.12). The colour of the aphids is due to a pigment present in their haemolymph, which is derived from the sap obtained from the food plants. The water-soluble pigment known as protoaphin is a glucoside, and the proportion of its constituents determines the various colourations of the aphid. Detail chemistry of the pigments was reviewed by Behura (1996b). Not only do aphids display a bewildering complexity of colour, but their bodies are frequently covered entirely with white or grey wax. In certain species, this wax is secreted only by definite body regions and may be in the form of flakes, ribbons or other shapes. Colour variation is also associated with relative susceptibility to its predators and parasitoids. Müller (1962) had shown that the aphid body colour is genetically determined, with red being dominant over green, and Losey et al. (1997) demonstrated that ladybird beetles tend to devour red aphids on green plants, while the parasitoid wasps preferentially attack green aphids. On these accounts, Losey et al. (1997) concluded that the predation and parasitism pressures appear to regulate the colour variation in natural aphid populations. Moran and Jarvik (2010) discovered that the aphid genome contains several genes for carotenoid synthesis not found in animal genomes and the genes are of fungal origin and seem to have been acquired in the evolutionary history of aphids via ancient lateral transfer. One of the genes is concerned in synthesis of red colour pigments, and its presence or absence is accountable for the red or green coloration of the aphids. Simultaneously, Tsuchida et al. (2010) reported an endosymbiont bacterium, Rickettsiella viridis, that modifies body colour of pea aphids in natural populations which is expected to influence prey–predator interactions, as well as interactions with other endosymbionts. Watanabe et al. (2016) demonstrated that ant attendance appears to regulate the proportion of red and green morphs of mugwort aphid, Macrosiphoniella yomogicola, in fields. Even the colour polymorphism in aphids appears irregularly among members of the colony or associated with a particular sex or generation.
3.2.7.9 Factors Influencing Morph Determination
There are several factors that influence the aphid to develop into a definite morph. Some of them are associated with their same generation, and others are associated with dispersal. The major factors that determine the gamic forms are the photoperiodism, temperature and the quality of food. In summer season, the factors that always influence aphids to determine their forms are the quality of food, crowding, semiochemicals or pesticides, influence of parasitoids and predators and many other biotic as well as abiotic stresses (Singh and Ghosh 2002). So far as polymorphism is concerned, several factors operate directly or indirectly and interact in various ways (Behura 1994). There are likely to be many different mechanisms, and in only a few species the processes have been analysed experimentally. Agarwala (2007) summarised the factors that could trigger the phenotype plasticity in aphids.
3.2.7.9.1 Photoperiod and Temperature
The photoperiods and temperatures are associated with each other. During shorter photoperiod, temperature is usually low (winter season) and vice versa (summer season). The production of sexual morphs is known in some species to be under photoperiodic control (Dixon 1977; Chen et al. 2019). In Megoura viciae, exposure to short-day conditions induces the viviparous females to produce oviparae; there is a critical photoperiod of 15 h at 15 °C, shortening slightly as the temperature rises to a maximum of 23 °C above which the effect does not operate. Photoperiod is perceived directly by the mother (rather than through the host–plant), and this can occur while she is herself developing within the grandmother (Lees 1966). Other species conform to a similar pattern, with differences in the length of the critical photoperiod and of the upper temperature limit, e.g. Acyrthosiphon pisum (Lamb and Pointing 1972), Myzus persicae (Blackman 1975) and Rhopalosiphum padi (de Barro 1992) in which the photoperiod also differs for the production of male and female progeny. Helden et al. (1994) reported a high degree of phenotypic plasticity both within and between morphs of Sitobion avenae. Increased production of alate phenotype in response to longer day length and higher temperature has been reported for Myzus persicae (Matsuka and Mittler 1978), Nasonovia ribisnigri (Diaz and Fereres 2005) and Rhopalosiphum maidis (Chen et al. 2019). However, low temperature was reported to induce wing form in Myzus persicae, Lipaphis erysimi, Brevicoryne brassicae, Aphis glycines and Macrosiphoniella sanborni and high temperature to inhibit wing dimorphism (Lee, 1966; Lv and Chen 1993). Higher temperature also reduced the size of the apterous aphids, e.g. Myzus persicae (Tiwari and Singh 2018), as well as their demographic parameters (Pal et al. 2008; Singh and Singh 2015).
3.2.7.9.2 Host–Plant: Food Quality
The cultivated plants vary in species, varieties and cultivars. All such plants have seasonality in development, and content of nutrition varies between crop varieties, growth stages, different parts of the plant, etc. The food quality is one of the important factors that determine the phenotype of aphids (Mittler and Sutherland 1969). Aphids can perceive changes in the quality of their food through gustatory mechanisms and can respond by morphogenetic changes (Mittler 1973). Aphis gossypii grown on unsuitable host–plants produces yellow dwarf phenotypes (Watt and Hales 1996). Apterous viviparous phenotype of several aphid species differs in morphometry and growth rates, e.g. Aphis gossypii (Singh and Singh 2015a), Aphis spiraecola (Dubey and Singh 2008), Lipaphis erysimi (Agarwala and Das 1998), Myzus persicae (Tiwari and Singh 2016) and Sitobion miscanthi (Srivastava and Singh 2008) in response to food plants. Myzus persicae produces more apterae on a deficient artificial diet, while Phorodon humuli may increase the production of alates when the host–plant grows under unfavourable conditions. Rhopalosiphum padi feeding on reduced quality food produce more alate morphs (de Barro 1992). However, Johnson (1966) reported prolonged periods of starvation both of parent aphids and of young nymph induce apterous development in case of Aphis craccivora. Secondary metabolites of the host–plants have also been observed to induce alate production (Harrewijn 1978).
3.2.7.9.3 Crowding
Crowding is one of the major biotic factors that induce the production of winged morphs among aphids. Higher aphid densities have been always found to lead to more tactile stimulations between individual aphids, triggering wing induction (Lees 1967; Martínez and Costamagna 2018). Winged morph production has been considered a driver of density regulation in aphids, and in many species, the production of winged individuals is strongly density dependent (Lees 1967; Purandare et al. 2014). However, in few species, e.g. Metopeurum fuscoviride, crowding had no effect on the production of winged morphs (Mehrparvar et al. 2013). The production of winged morphs among aphid colonies is crucial in their life history and is the best possible tactic for their dispersal and colonisation in new optimal environments (Müller et al. 2001). However, the stage in the life cycle of the aphid at which crowding has the most influence differs between species (Shaw 1970). The sensitivity of Rhopalosiphum padi (Noda 1958), Therioaphis trifolii (Toba et al. 1967) and Brevicoryne brassicae (Kawada 1965) to crowding seems to be confined almost entirely to the first instar. Thus, crowding tends to promote the appearance of alate virginoparae, though the effect is produced differently in different species. In Megoura viciae Buckton, apterous viviparous produces only apterous progeny when reared singly, but when crowded they give rise to alate offspring; the effect is prenatal and not due to nutritional factors (Lees 1966). It is likely that the ultimate causes of the morphogenetic changes that underlie polymorphism are alterations in the endocrine balance during embryonic and postembryonic development.
3.2.7.9.4 Predator–Parasitoid Mediation
The prey phenotypic response to predators is little known. Earlier, it was observed that the magnitude and direction of transgenerational phenotypic responses to predators vary among individuals and/or populations of the same species (Weisser et al. 1999). Acyrthosiphon pisum exposed to predator produce offspring developing winged dispersing forms (Dixon and Agarwala 1999; Mondor et al. 2005; Balog et al. 2013; Sentis et al. 2019). The presence of a predator, the larvae of Chrysoperla carnea, significantly increased the percentage of winged individuals among the offspring of Macrosiphoniella tanacetaria; however, the presence of predators had no effect on the production of winged individuals of Metopeurum fuscoviride (Mehrparvar et al. 2013). The antennae of aphids play a major role in perceiving the presence of predators/parasitoids that induce the production of dispersal morph (Kunert and Weisser 2005). Sloggett and Weisser (2002) reported that the parasitoid, Aphidius ervi, induced the production of alate morph of Acyrthosiphon pisum. Rios Martinez and Costamagna (2017) suggested that these facultative morphological changes may be adaptive as they reduce the probability of predation. Weisser et al. (1999) reported that the kairomones emitted by predators cause plasticity in the morphology, life cycle and behaviour of their prey.
3.2.8 Effects of Inbreeding and Outbreeding
Cyclic parthenogenesis in aphids leads to the peculiar mode of population structures and adaptations to the host–plants. One outcome of this reproductive mode is the frequent incidence of inbreeding; male and female members of the same clone can mate to produce fertilised eggs (Helden and Dixon 1997). This mode of mating is comparable to self-fertilisation in helminths and some annelids. Aphid populations often consist of sexual, asexual and intermediate clones in temperate regions. In such mixed populations, the effects of inbreeding may be restricted. However, a high level of inbreeding may have influenced the local adaptations and the evolution of sex ratios and mating systems in holocyclic populations of aphids. The extent of inbreeding is affected by the life cycle and taxonomic group of the aphid species. As in other insects, inbreeding is expected to occur frequently in species with low migratory ability (Thornhill 1993). In this respect, aphid species without host alternation are likely to inbreed (Komatsu and Akimoto 1995). Furthermore, in the Eriosomatinae and Hormaphidinae, inbreeding may arise easily because the alates of these groups in autumn (sexuparae) have fully grown male and female embryos in their abdomen (Dixon 1998); the sexuals are stout, remain on the host trunk after birth and mate without feeding. Akimoto (2006) observed that when males and females of the same clone of Prociphilus oriens are confined in a small cage, they mate readily and produce selfed eggs and, by comparing the hatch rates of selfed and outbred eggs, estimated the effect of inbreeding. These studies indicate that eggs from intra-clonal mating (selfed eggs) hatch less successfully than do eggs from inter-clonal mating, suggesting inbreeding depression. However, the impact of inbreeding depression varied largely among aphid species. Huang and Caillaud (2012) documented the existence of severe inbreeding depression upon selfing in the cyclic parthenogenetic aphid, Acyrthosiphon pisum, and opined that the inbreeding avoidance take place sometime between copulation and sperm transfer that suggest that cryptic female choice may play a role in the process. Akimoto (2006) observed that enforced selfing led to a large variation in the hatching time and morphology of first instars. The nymphs hatched out from selfed eggs had longer antennae and tibiae than that hatched out from outbred eggs. Also, their gonads were much smaller in size.
The inbreeding may also influence aphid sex ratios and mating systems. Sexuparae of Prociphilus oriens have female-biased sex ratios (Yamaguchi 1985), and Foster (2002) explained it by local mate competition. In the situation where local mate competition occurs, inbreeding is also expected (Hamilton 1967). Akimoto (2006) observed that Prociphilus oriens sexuparae consist of two types: one type produces males and females simultaneously in the abdomen (‘M + F’ type), while the other type produces females only (‘F’ type), and the proportions of the ‘M + F’ and ‘F’ types varied from year to year, and accordingly the sex ratio also varied greatly. It is possible that ‘F’-type sexuparae have the advantage of avoiding inbreeding. For understanding the evolution of aphid mating systems, it is necessary to focus on the incidence of inbreeding in the field.
3.2.9 Migration
Johnson (1969) defined insect migration as their periodic flight beyond the boundaries of their old breeding habitats into new ones. Here, the migrants are relatively not distracted during flight by the feeding as well as oviposition stimuli that normally lead. Aphids are important vectors of plant viruses attacking several crops. Therefore, the knowledge of their migration and seasonal presence is necessary to make decisions regarding the time of their control to prevent virus transmission to crops. Aphids fly rather slowly and heavily, but with the help of the wind, they occasionally make astonishing extensive migrations and are capable of very long distance movement. Air current may carry them to altitude of about 1000 m a.s.l. On calm, warm and humid days, thousands of them float in and out among one another, all moving in the same direction by the gentle wind. Most of the aphids are airborne twice a year in India, once in winter (November to January) and the other during spring (March to April). Their aerial activity is minimum during the monsoons (July–August). During winter, the peak hour for alate flight is about noon, while two peak periods are observed in spring, one at noon and the other in less number during afternoon (Ghosh and Raychaudhuri 1980). Several workers have reviewed the ecology of aphid flight (Kring 1972; Parry 2013; Fereres et al. 2017). Most of the aphid migration is related with the search of mates and food and is influenced by a wide range of factors (Johnson 1954).
Pemphigus bursarius is one of the migratory aphid species which occurs on poplar and migrates to the roots of various Poaceae and returns back to poplar in autumn; Myzus persicae, in winter, feeds on its primary host, peach, and after winter it migrates to a number of secondary host species (van Emden et al. 1969). Aphis fabae overwinters as the egg in autumn on the spindle tree (Euonymus), and in May and June, it flies to beans, sugar beet, etc., returning to the spindle tree in October. In Nepal, Brachycaudus helichrysi alternates from peach as primary host to Calendula and others as secondary hosts. Similarly, Rhopalosiphum nymphaeae migrates from the primary host, plum, to the secondary host, water lily. Beginning of the autumn, the sexuparae migrate to the tender leaves of primary host where gynoparae develop which produce oviparous females as well as males. The aphids have potentiality to switch over the plants during unfavourable season on other food plants (Fereres et al. 2017).
3.2.10 Alarm Pheromones: The Defence Chemicals
The sesquiterpene, (E)-β-farnesene, is the alarm pheromone of most of the aphid species (Mondor and Roitberg 2000; El-Sayed 2019). An aphid grabbed by forceps, mimicking a predator, or by an actual predator emits droplets from its siphunculi that contain (E)-β-farnesene (Kislow and Edwards 1972; Pickett and Griffiths 1980). Byers (2005) reported that all life stages and sizes of Aphis gossypii reared on cotton plants secrete (E)-β-farnesene in amounts ranging from 0.1 to 1.5 ng/individual. When any predator arrives the aphid colony, (E)-β-farnesene stops aphids from feeding and induces them to escape or drop off the leaf (Wohlers 1982; Pickett et al. 1992). These alarm pheromones also induce the production of wing morph to escape the feeding site for survival (Kunert and Weisser 2005; Kunert et al. 2005; Hatano et al. 2010). Since aphid colonies are composed of genetically identical individuals, therefore, genes for synthesis and recognition of an alarm pheromone in the colony members would increase its complete fitness by warning them to avoid the predator. Few studies have quantified the amounts of (E)-β-farnesene in aphids (Vandermoten et al. 2011) and ecological impact of these chemicals on natural enemies of the aphids (Pickett et al. 2017).
3.2.11 Ant Associations
The honeydew of the aphids is the main source of food for many nectar-feeding insects, viz. ants, bees, wasps, parasitoids, dipterans and others. Most of the ants not only lick up the honeydew but also move over the aphid colonies. The aphids produce honeydew more rapidly when the ants tap their antennae on them. In turn, the ants protect the aphids from their natural enemies. Sometimes, the ants carry the aphids from one plant to others if the earlier one begins to wilt. The workers of jet black ant, Lasius fuliginosus, carry newly hatched fundatrices of the aphids from the base of oak trees where they overwinter as eggs to new growing leaves at the top of the tree (Ślipiński et al. 2014). The common meadow ants, Lasius flavus, even collect aphid eggs in the autumn and early winter and keep them in its nests. In spring, these eggs are transferred nearby the plant roots, so that upon hatching, newly hatched fundatrices find food (Ivens et al. 2012). Some ants are almost aphid dependent for food, while few aphids are obligate myrmecophiles and excrete honeydew only when ants stimulate them to do so. Other ants build roofs of rotten wood over aphid herds that live in cracks in bark; extensions of the tunnels formed in this way are connected directly with the ants’ nest. Many ants feed more or less exclusively on honeydew. An amusing sight is an ant helping an aphid to pull its long proboscis out of a plant; evidently, the aphid finds it troublesome to withdraw the rostrum only when some danger requires it to run away as quickly as possible. Sometimes aphids are fed by ants. Flatt and Weisser (2000) observed that the aphids suffer from strong fitness losses if they are not tended by ants.
The number of ants associated with a given species of aphid and the number of aphid species associated with a given species of ant varies from place to place; up to 14 aphid species have been recorded in Lasius flavus nests (Depa and Węgierek 2011). Sometimes different ant species which live in similar habitats foster different aphid species, i.e. Lasius niger with Anoecia corni and Lasius flavus with Anoecia nemoralis. However, aphid species that have evolved close relationships with ants may have broader environmental tolerances than their hosts; hence, the aphid Forda formicaria is attended by Lasius spp. in the lowlands and by Formica spp. in the highlands (Seckbach and Dubinsky 2011). Ants are not always good with aphids; if aphid numbers increase, the ants used to kill a few off and devour them (Dixon and Hemptinne 2001). Although studies on the mutualism between ants and Indian aphids are scanty, Veeravel and Baskaran (1994) reported that the population of Aphis gossypii on brinjal, Solanum melongena, is more when the black ant, Lasius niger, attended them. Some aphid species are not attended by ants, apparently because their honeydew has a disagreeable quality.
The protection that ants give the aphids they attend is not always excellent and varies depending on the type of predator or parasitoid (Douglas and van Emden 2017). Generally speaking, the ants are better at dealing with ladybird larvae and anthocorid bugs than they are at dealing with lacewing larvae and hover fly larvae. They not only remove the larvae, sometimes killing them, but also remove the eggs of hover flies and ladybirds. Interestingly, by herding aphids onto the tops of the plants, ants render them more vulnerable to attack from some parasitoids (Seckbach and Dubinsky 2011). Detail account of aphid–ant associations has been dealt by Dixon (1985) and Stadler and Dixon (2005).
3.2.12 Endosymbionts
Aphids are frequently engaged in mutualistic associations with bacterial endosymbionts like other insects. Both kinds of symbionts, primary (obligate) and secondary (facultative), occur in aphids.
3.2.12.1 Obligate Endosymbionts
In the obligate relationship, neither aphid nor bacteria is able to survive without the other. Such obligate symbiosis is found in sap-feeding insects like aphids. Aphids feed on the phloem sap of the plants which is an unbalanced diet for them, as it is deprived of essential amino acids. Like other animals, the aphids cannot synthesise these amino acids. For that, aphids harbour certain bacteria symbionts (e.g. Buchnera aphidicola) in 60–80 special cells, called bacteriocytes. Buchnera is entirely symbiotic and remains viable only inside it (Douglas 1998). This symbiotic relationship was established 200–250 MYA and led to co-speciation of the hosts and their symbionts (Moran et al. 1993). These bacteria are vertically transmitted to eggs and embryos through host generations (Braendle et al. 2003).
3.2.12.2 Facultative Endosymbionts
Facultative symbionts are of two kinds: reproductive manipulators that affect the reproduction of host aphid to maximise their own transmission and the mutualists that can affect a wide range of life history and ecological traits (Oliver et al. 2010; Douglas and van Emden 2017). These symbionts are typically transmitted from mother to offspring, although horizontal transfer occurs at lower frequencies (Gehrer and Vorburger 2012). Among the aphids, Acyrthosiphon pisum are the best studied insect–symbiont systems which alone is known to host at least eight different facultative symbiont species (Sandström et al. 2001; Tsuchida et al. 2010). Guo et al. (2017) have described the functions of nine facultative symbionts (Serratia symbiotica, Hamiltonella defensa, Regiella insecticola, Rickettsia, Rickettsiella, PAXS [pea aphid X-type symbiont], Spiroplasma, Wolbachia and Arsenophonus) of aphids and discussed the associations between these symbionts and aphids, plants and environment. The facultative symbionts grant a wide range of benefits to their hosts, e.g. protection from natural enemies (Oliver et al. 2003; Vorburger et al. 2010), protection from extreme temperatures (Montllor et al. 2002), development of resistance to a fungal pathogen (Scarborough et al. 2005; Łukasik et al. 2013) and the ability to use a greater diversity of resources (Tsuchida et al. 2004). Tsuchida et al. (2011) reported that Rickettsiella-Hamiltonella coinfections of Acyrthosiphon pisum changed red aphids to green. The red/green aphid body colour has ecological, evolutionary and biochemical relevance. In Europe and the USA, red and green aphids commonly coexist within the same populations of Acyrthosiphon pisum. It has already been demonstrated that ladybird beetles consume red aphids on green plants (Losey et al. 1997) and that parasitoid wasps Aphidius ervi preferentially oviposited into green aphids (Bilodeau et al. 2013), suggesting that these natural enemies with different colour preferences may contribute to the colour polymorphisms in natural aphid populations.
3.3 Aphid–Plant Interaction
Host–plant specificity in aphids varied from extreme monophagy, e.g. Astegopteryx spp. on bamboos (Singh and Singh 2018), to highly polyphagy, e.g. the green peach aphid, Myzus persicae, whose summer generations develop on about 300 plant species in India (Singh et al. 2015a).
3.3.1 Response to Plant Attributes
The first phase of host–plant selection by aphids is to assess the suitability of the plant as food. For this, the alate aphids use visual and olfactory cues while landing to decide its suitability. Webster et al. (2008) identified chemical cues involved in the long-distance recognition of a host–plant by Aphis fabae. Upon landing on the plant, its physical features such as trichomes, simple or glandular, and their density influence the selection behaviour and success of attack of the aphids. Several cultivated crops are resistant against aphids because of these features of the plants. Crops, such as resistant variety of tomato having high density of glandular trichomes, always interfere with the movement and feeding the aphid, Macrosiphum euphorbiae. The gustatory receptors located at the back of the mouth help in the recognition of host and non-host–plant. The plants may have inherent toxic chemicals, or upon insertion of the stylet into plant tissue, the non-host–plants secrete toxic chemicals preventing aphid feeding (Schoonhoven et al. 2007). However, few aphids have evolved mechanisms that defend the toxic chemicals present in the plants. For example, brassica crops are rich in glucosinolates (e.g. singrin) to defend insects, but the cabbage aphid (Brevicoryne brassicae), mustard aphid (Lipaphis erysimi) and Myzus persicae evolve mechanisms to defend these chemicals and also use them for host–plant selection (Gols et al. 2008). Similarly, Macrosiphum euphorbiae and Myzus persicae evolve physiological defence mechanisms from tomatine and solanine (glycosidic alkalosis) of tomato. The glandular trichomes of few plants like Solanum berthaultii also secrete (E)-farnesene, the aphid alarm pheromone that prevents the colonisation by inducing dispersal behaviour in winged aphis (Gibson and Pickett 1983).
3.3.2 Plant Response to Aphid Attack
Plant responses to aphid attack are different to aphids which are associated or not associated with transmission of viruses. Plant responses to aphids not associated with transmission of viruses are extremely variable. Response of tomato plants to attack of its aphid, Macrosiphum euphorbiae, is not evident except the deprivation of plant nutrients making them weak and also susceptible for other insects and pathogens (Guerrieri and Digilio 2008). Similar response of plants to aphid attack can be observed following infestation by generalist aphids, e.g. Aphis fabae and Macrosiphum rosae. However, in case of Dysaphis plantaginea, the injection of aphid saliva may be extremely toxic, leading to localised chlorosis near the feeding site and around the stylet tracks, caused by chloroplast disruption on apple fruits (Miles 1999). Attack of Aphis spiraecola on citrus causes growth distortions of its leaves, while peach trees can be curled by Myzus varians. The injection of aphid saliva also alters the hormonal balance of the plant, leading to the gall formation that also helps aphid protecting them from their natural enemies and insecticides (Wool 2004).
The aphids transmit phytopathogenic viruses; different kinds of plant responses were noticed after aphid attack. Sometimes, after aphid attack, most virus-infected plants become yellowish in colour that attracts most of the alate aphids. Also, the amount of free amino acids in plant sap is higher in case of virus-infected plants that enhance the development and reproduction of aphids that induces crowding. The crowding induces aphids to differentiate alate morphs that migrate to colonise new healthy plants, thus dispersing the virus. In addition to the above benefit to the aphids that virus infection provides, the plant viruses have an indirect beneficial effect on aphid fitness, by reducing plant defence response. The aphids, in turn, benefit viruses by transmitting them in favourable host for replication; however, few viruses also circulate inside the aphid without replication. Both circulative and replicative viruses make the aphid infective for the rest of its life, with disastrous consequences for agricultural crops (Guerrieri and Digilio 2008).
3.3.3 Indirect Defence Response to Aphid Attack: Tritrophic Context
Singh (2003) reviewed the tritrophic interactions between host–plant, aphids and their natural enemies. These interactions between three trophic levels are physically, chemically and semiochemically mediated responses.
3.3.3.1 Physically Mediated Interactions
It has been demonstrated that the plant architectures influence interactions over several trophic levels. Singh et al. (2000b) have reported that the searching efficiency of Binodoxys indicus is highly influenced by foliar pubescence of the food plants supporting its host, Aphis craccivora and Aphis gossypii. However, sometimes, the physical and chemical influences are not clear, e.g. the decrease in adult survival of the aphid parasitoid, Aphidius matricarae, with increasing glandular trichome densities was observed (Obrycki and Tauber 1984).
3.3.3.2 Chemically Mediated Interactions
The direct and indirect effects of plants on herbivores and their natural enemies at the chemical levels have received considerable attention in the literature in recent past. It has been established that after aphid attack, plants release certain volatile chemicals that attract predators and parasitoids of aphids (López Pérez et al. 2007). For example, broad bean plants infested by the pea aphid Acyrthosiphon pisum are six times more attractive than uninfested plants towards the parasitoid Aphidius ervi Haliday (Guerrieri et al. 1993). In many plant–aphid systems, methyl salicylate is released by aphid infestation that attracts both aphid parasitoids (Sasso et al. 2007) and predators (Zhu and Park 2007). These chemicals are specific for aphid–parasitoid/predator interactions. Singh et al. (2000b) have observed that parasitoid Binodoxys indicus parasitises Aphis gossypii differently on different host–plants, and Omkar and Pervez (2002) observed the influence of food plants on the predatory potential of an aphid predator, Coccinella septempunctata. Infestation of Prunus persica by the aphid Brachycaudus helichrysi caused a change in the level of different foliar chemicals (soluble sugars and nitrogen, polyphenol, lipid) that provide ovipositional stimulus to the adults of aphid predator, Eupeodes corollae (=Metasyrphus corolla) (Chakrabarti and Chakrabarti 2002).
3.3.3.3 Semiochemically Mediated Interactions
The plants have an incredibly diverse array of secondary compounds that perform allelochemical functions either as allomones or as kairomones. These chemicals sometimes pass as such into herbivore insect and remain unchanged. Such chemicals or the chemicals synthesised in herbivore insects by modifying the precursors derived from the plants help in their detection by their natural enemies. The literature on host selection by the aphid parasitoids is full of evidence that plant odours attract herbivores as well as their natural enemies (Albittar et al. 2016). The aphid Brevicoryne brassicae uses sinigrin (present in brassica plants) as a signal to find host–plants, while its parasitoid Diaeretiella rapae uses a related compound allyl isothiocyanate (mustard oil) to find the plant and then the aphid. Therefore, the intrinsic defence of the plant has direct and indirect effects on natural enemies that may be important in biological control and extrinsic plant defence.
3.4 Nature of Damage
Globally, more than 250 species of aphids are pests of both agricultural and horticultural crops (Verma 2000). This figure is only about 5% of the estimated world fauna of over 5110 species (Favret and Eades 2020). Aphids have been reported as one of the devastating insect pests in realising the productivity of many cultivated crops throughout the world. The list of some major aphid pests are displayed in Table 3.2. The damage in some crops is to the extent that nothing remains to harvest such as some cereals, potatoes and rapeseed mustard.
3.4.1 Direct Damage
Aphids attack all parts of the plants including roots. Some of them directly damage the plants by sucking their nutrient that causes a lack of vigour in the plant. The aphid saliva is toxic to plants. Such infested plants have a variety of symptoms such as decreased growth rates, mottled leaves, yellowing, stunted growth, curled leaves, browning, wilting, low yields and ultimately death (Behura and Das 1976). The young seedlings die, the inflorescences fail to blossom and fruits fail to develop normally showing various malformations like twisting of pods, impaired developments of seeds, etc. The subaerial infestations by aphids also cause yellowing of foliages and stunted general growth. Some aphids make different kinds of leaf and stem galls (Chakrabarti 2007). These symptoms are observed on perennial forest trees.
3.4.2 Indirect Damage
In spite of these direct effects, aphids have also some indirect effects. Some species of aphids excrete a high amount of honeydew that covers the stomatal openings hampering their normal physiological processes like photosynthesis, transpiration and respiration. The honeydew also allows the growth of black sooty mould (Capnodium spp.) onto the leaves which in turn proves detrimental to the normal activity of plant life. Also, the honeydew has been observed to reduce the effectiveness of fungicides by obstructing their absorption (Dika and Van Pelt 1992). The honeydew also contaminates cotton lint, reducing its quality and economic value. The presence of honeydew on fruit crops can significantly reduce their marketability. Honeydew, as food source, may attract to other crop pest insects. However, the honeydew has some beneficiary act to aphids. It attracts bees, wasps and ants that may provide their protection from their natural enemies such as parasitoids and predators.
The honeydew also provides a valuable food source for beneficial insects such as parasitoids and predators involved in their natural control (Singh et al. 1996, 2000a). The excess honeydew may also nourish soil microorganisms, including nitrogen fixers (Owen and Wiegert (1976). In a nitrogen-poor environment, this could provide an advantage to an infested plant over a noninfested plant (Stadler and Muller 1996), but this does not appear to be supported by the observational evidence (Choudhury 1984).
3.4.3 Damage Through Virus Transmission
Most of the damage caused for the crop by the aphids is their ability to transmit viruses (Eastop 1977). The major viruses transmitted by aphid pests of cultivated plants are given in Table 3.2. Aphid-transmitted viruses account for approximately 50% of the 600 known viruses with an invertebrate vector (Hooks and Fereres 2006). The detail account of transmission mechanisms of aphids as virus vectors was described by Harris and Maramorosch (1977) and Stevens and Locomme (2017). The subfamily Aphidinae includes the majority of aphid vectors (Blackman and Eastop 2000). There are a number of unique features that contribute to the success of aphids as vectors of plant viruses. These include their polyphagous nature (Myzus persicae, about 500 plant species worldwide), the ability to undergo parthenogenetic reproduction that facilitates their rapid production and the possession of a needlelike stylet capable of piercing plant cell walls that deliver viruses into host cell. Feeding behaviour and host–plant selection by an aphid affect its potential as a vector (Harris and Maramorosch 1977; Pettersson et al. 2017). The extents to which these factors influence virus transmission depend on the specific virus and its mechanism of transmission (Ng and Perry 2004).
Eight genera of the plant viruses, viz. Potyvirus, Macluravirus, Babuvirus, Bymovirus, Luteovirus, Cucumovirus, Comovirus and Closterovirus, are transmitted by aphids in India. All three modes of transmission, viz. nonpersistent, semi-persistent and persistent, are observed in India. Nonpersistent virus transmission (stylet-borne) is characterised by very short acquisition and inoculation time in which aphid stylet does not usually pierce beyond the epidermal cells. Because of this, the vectors hardly colonise the host–plant. The aphids begin to lose the infectivity immediately after acquisition. Alfamovirus, Cucumovirus, Fabavirus, Macluravirus and Potyvirus are important genera transmitted by aphids in this manner. In semi-persistent, transmission is characterised by few minutes to hours acquisition time with few hours of retention period. Caulimovirus and Closterovirus are genera transmitted by aphids in this manner. In the persistent-circulative type of transmission, the virus has to be ingested by the aphids and reach the salivary gland via haemolymph. Acquisition period generally ranges from hours to days, and the aphid remains viruliferous for weeks or lifelong. Among the aphid-transmitted viruses, Luteovirus and Babuvirus are transmitted by aphids in this manner. Ghosh et al. (2017) very nicely reviewed the role of several aphid species as vector of plant viruses of economically important crops.
The green peach aphid (Myzus persicae) being highly polyphagous is a vector for more than 110 plant viruses. Cotton aphid (Aphis gossypii) transmits more than 75 plant viruses and often infects chilli, potato, sweet potato, brinjal, sugarcane, papaya and groundnuts with viruses. Most of the nonpersistent viruses (e.g. tobacco mosaic virus [TMV]) are transmitted only by aphids. Few semi-persistent viruses (e.g. beet yellow stunt virus [BYV]) and persistent viruses (e.g. potato leafroll virus [PLRV]) are also transmitted by them (Ghosh 1980). A list of virus pathogens transmitted by aphids and aphid species which are regarded as vector of one or more plant viruses has been listed by Ghosh et al. (2017).
The aphids are of great agricultural significance, and nowadays they are being considered as serious pests of agriculture and horticulture. Usually in the absence of primary agricultural crops, i.e. when harvesting of one crop is over, they tide over in unfavourable season on other economic crops; in lieu of these latter crop plants, they just thrive upon many wild plants. Thus, the aphids affect the yield and quality of the seed of several crops which also affect the reproductive potential of the crops.
3.5 Management Measures
3.5.1 Integrated Pest Management (IPM)
Concerns about the risks that the chemical poisons pose to the environment, human health and increased costs of pesticides have increased the urgency for more research into the alternative methods of crop and food protection. The current pest control approaches aim to maximise productivity and approaches that emphasise efficiency and the long-term sustainability of agroecosystems. Indeed, pest management was thought to be a new terminology evolving from pest control to plant protection or crop protection and then pest management and/or integrated pest management (IPM). A broader definition of IPM was given by Stern et al. (1959) as follows: ‘Integrated Pest Control is a pest management system that, in the context of the associated environment and the population dynamics of the pest species, utilises all suitable techniques and methods in as compatible a manner as possible and maintains the pest population at levels below those causing economic injury’. Thus, IPM is an effective and eco-friendly approach to pest management that trusts on a combination of common-sense practices and uses current, full information on the life cycles of pests and their interaction with the ecological factors. This approach of pest management is not only cost-effective but also ecologically safe. The IPM takes advantage of all appropriate pest management practices including, but not limited to, the judicious use of pesticides. In practicing IPM, growers should follow a four-tiered approach, viz. monitoring and identification of pests, estimation of economic injury level (EIL) and economic threshold level (ETL), prevention and control measure.
3.5.1.1 Monitoring and Identification
The monitoring and identification of pests removes the possibility that pesticides will be used when they are not really required. The identification of aphid infestation on plants is not difficult. The stunted plants and plants with curled or deformed leaves are likely to have aphid infestations. Feeding aphids usually occur in clusters on succulent shoots, under leaves or in other suitable feeding sites. The presence of honeydew or sooty mould is often an excellent clue that aphids are present. Plants should be examined closely on a regular basis to detect aphids before damage is evident. The evidence of natural enemies such as ladybird beetles, lacewings, syrphid fly larvae, the mummies (parasitised aphids) and disease-killed aphids should be observed. Considerable numbers of any of these natural control means demonstrate that the aphid population may be reduced rapidly without the use of any control practice. Otherwise, some plant protection measure should be applied.
3.5.1.2 Economic Injury Level (EIL) and Economic Threshold Levels (ETLs)
Integrated pest management (IPM) is a well-established strategy for managing agronomically important insect pests (Pedigo et al. 1986) and has been identified as the most cost-efficient tool to reduce aphid outbreaks. The economic injury level (EIL) and economic threshold level (ETL) are key IPM concepts. Stern et al. (1959) defined the ETL as the density at which control measures should be determined to prevent an increasing pest population from reaching the economic injury level and the EIL as the lowest population that will cause economic damage. The value of ETL is about 75% of the value of EIL. While theoretically there are EIL values for decision-makers to consider in evaluating an ETL, they are largely, if not completely, ignored. That is partly due to the fact that most EIL and ETL differ with aphid species, plant species/varieties, growing stage of the plant, etc.
In other words, the ETL is the population level of insect or extent of crop damage at which the value of the crop damaged exceeds the cost of controlling the pest (Zalom 2010). It is expressed in several ways including the number of insects per plant or per square metre, the amount of leaf surface damage, etc. ETLs have been developed for few aphid species. In the cotton aphid IPM under irrigation conditions, 70% of plants attacked are suggested by Almeida (2001) as the recommended level to start chemical control. In most of the estimates depending on the crop, it ranges from 5 aphids to 300 aphids/plant depending on the plant phenology (Table 3.3). Therefore, EIL and ETL are not fixed values for a pest species, but they varied considerably in relation to the plant growth stages, plant varieties, sowing time and irrigation and also in relation to the cropping seasons. It is essential to know the EIL and ETL of each pest species before control measure on the crop to save the excess cost of pesticide and labour and safe to the other fauna and environment from excess toxicity.
3.5.2 Prevention and Control Measures
High reproductive rate and multiple host sequences provide optimal conditions for aphid population development. The varied habitats, seasonal population development and intra- and intercrop and wild host movement present an extremely complex and difficult challenge requiring new approaches for formulating control and suppression methodology for aphids (Singh 2015). There is really no easy way of controlling these vector insects. In the past, adults were easily killed with insecticides, but insecticide resistance in their populations is a common problem. These insects have become resistant to chemical insecticides quite rapidly, and the wisdom of relying only on chemical insecticides is questioned. Therefore, an integrated approach becomes essential to manage their population. This approach combines the cultural and biological practices with the application of selective insecticides. The cultural control helps in the prevention of aphid infestation on the crop.
3.5.2.1 Mechanical Control
Aphid populations on strong plant may be reduced by knocking them off with a strong spray of water. Most dislodged aphids will not be able to return to the plant, and their honeydew will also be washed off as well. Using water sprays early in the day allows plants to dry off rapidly in the sun and be less susceptible to fungal diseases.
3.5.2.2 Cultural Control
The cultural control involves changes in crop production practices to make the crop less suitable for the pest or to make it more suitable for the natural enemies or to enhance the ability of the crop to resist pest attack (Norris et al. 2003). Cultural control is an environmentally friendly approach and more of prophylactic in nature than of curative and is frequently first line of defence against pest populations. The following are the important agronomical practices that directly or indirectly affect the aphid biology and keep their population at low level (Sachan 1997; Chang et al. 2017).
3.5.2.2.1 Host–Plant Resistance
The very first event in the farming is the selection of seeds. The seeds of resistant crop varieties should be used for crop production. Crop cultivars resistant to major pests and diseases have been developed in cowpea against Aphis craccivora (Omoigui et al. 2017), soybean against Aphis glycine (Hill et al. 2004), melon against Aphis gossypii (Chen et al. 1997), rice against Rhopalosiphum padi (Sun et al. 2017), wheat against Sitobion avenae (Hu et al. 2011; Liu et al. 2012), maize against Rhopalosiphum padi (Hance et al. 1994) and sorghum against Melanaphis sacchari (Sharma et al. 2014) and to a limited extent in pulse and oilseed crops. Host–plant resistance is an efficient and environmentally friendly means of controlling insects, including aphids, but resistant-breaking biotypes have occurred in several plant–aphid systems (Dogimont et al. 2010). There are three mechanisms by which plants become resistant, antibiosis (adverse effect on herbivore biology), antixenosis (induce non-preference behaviour in herbivores) and tolerance (plant traits to withstand herbivore injury), and all these plant traits are regulated by resistant genes (R). In recent years, there has been an increase in the knowledge on R genes, but only a few R genes that cause resistance against aphids have been identified. Some of them include virus aphid transmission (Vat) that makes resistance to Aphis gossypii in melon (Chen et al. 1997; Martin et al. 2003), recombination-activating gene (Rag1) in soybean that provides resistance to soybean aphid, Aphis glycines (Li et al. 2007) and Mi-1.2 gene in tomato that makes resistance to Macrosiphum euphorbiae (Linda and Walling 2008). The Vat gene in melon enhances the sieve element (SE) wound healing and thus confers resistance to Aphis gossypii (Kaloshian et al. 1997). The cloning of Mi-1.2 gene has been a milestone in plant resistance to aphids (Goggin et al. 2001). Insect-resistant transgenic cotton does not interfere in the performance of aphids (Burgio et al. 2007), but transgenic Indian mustard (Brassica juncea) was found resistant against the mustard aphid, Lipaphis erysimi (Kanrar et al. 2002).
3.5.2.2.2 Planting Time
This practice is more meaningful if plating of crop is done on the basis of information on the population dynamics of aphid(s) as this is purely based on the phenological asynchrony of the crop with aphid. It is now established that early sown crop either escapes aphid attack or has less degree of infestation. Brassica campestris var. toria escapes the attack of Lipaphis erysimi if sown in mid-September (Sachan 1990). Other brassica oilseed crops suffer less if they are sown between the middle of October to the first week of November depending on the ecoclimatic belt (Upadhyay 1995). Sowing of mustard can be advanced by 10–15 days for escaping from attack of aphids without any appreciable loss in yield (Singh et al. 1984). Eruca sativa suffers less due to Myzus persicae if planted in October (Singh and Singh 1985). Information is also available on the time of planting of various other crops where they suffer least with aphids. Among the cereals, barley sown between mid-October and mid-November showed less incidence of Rhopalosiphum maidis (Singh 1982). Safflower when sown early escapes the attack of Uroleucon carthami, particularly at early stage of the crop (Jakhmola 1986). Coriander also suffered less due to Hyadaphis coriandri when planted before mid-October (Jain and Yadava 1986). Similarly, lentil planted in early November also showed higher population of Aphis craccivora Koch as compared to crop sown in late November or early December (Hossain et al. 2008).
3.5.2.2.3 Manual Removal of Infested Twigs
It is essential to nip the early infestation of aphids in the buds or twigs as aphids after appearance settle on the twigs and multiply from where they disperse to adjoining plants and field. In cotton, removal of the top leaves by hand, using a pruning knife where aphids fed, reduced contamination of bolls below these leaves (Deguine et al. 2000). Plucking and destruction of twigs should be carried out at 15 days interval (Singh et al. 1993).
3.5.2.2.4 Crop Geometry
The primary objective of this cultural method is to maximise yield per unit area without reducing crop quality, so that yield advantages override pest incidence reduction. Plant density has direct influence on the plant growth as well as yield of the crop. Each plant competes with other for nutrients, moisture, sunlight, etc. Dense population may be congenial for some insects, whereas it may be unfavourable to others. Modifying the plant spacing affects the incidence and population development of insect pests in general. A’Brook (1968) demonstrated that Aphis craccivora and Aphis gossypii were trapped more often over widely spaced than over close-spaced groundnuts. Similarly, in chickpea, wider spacing (60 x 20 cm) or low plant population per unit area resulted in higher population of A. craccivora (Lal et al. 1989).
3.5.2.2.5 Intercropping
It includes mixed intercropping, row intercropping, strip cropping, relay cropping and passageway intercropping. Intercropping is preferred over monoculture to avoid risk of crop failure, better utilisation of farm resources and labour and to protect the crop from insect pests. Intercrop reduces the attraction of pest to the host and adversely modifies the microclimate of the pest habitat which may result in impeded dispersal, increased emigration and reduced survival of the pest in the intercrop (Mumford and Baliddawa 1983). Potts and Gunadi (1991) reported a decrease in Aphis gossypii populations in potatoes that are intercropped with Allium cepa or Allium sativum. It has been shown that infestation of Aphis gossypii is less in pure crops of green gram, black gram and sunflower as compared to the main crop in combination with cotton. Chamuene et al. (2007) reported that sorghum and pigeon pea intercropped with cotton had fewer Aphis gossypii-infested plants and contained abundant population of natural enemies like syrphids, green lacewings and spiders. When beans intercropped with older and taller maize plants interfered with aphid colonisation and only small proportions of beans were infested by the aphid Aphis fabae (Ogenga-Latigo et al. 1993). Girma et al. (2000) reported that maize associated with hedge row experienced significantly lower infestation of Rhopalosiphum maidis than pure maize. Intercropping of groundnut with pearl millet reduced the incidence of Aphis craccivora on main crop (Kennedy et al. 1990). Less population of Aphis craccivora was observed on peas when barley and lentil were used as intercrops (Prasad et al. 1987).
3.5.2.2.6 Water Management
Water management is one of the most important factors responsible for proper growth and development of plant and higher yield. Under drought and/or rainfed conditions, plant loses turgidity as well as sap pressure which may result in reduction of feeding, reproduction and survival in aphids. These conditions also stimulate dispersal of aphids. Drought condition increases the solute concentration and sap viscosity to such an extent that feeding by aphid is drastically hampered (Bakhetia and Brar 1988). Population of Lipaphis erysimi increases on mustard crop, Brevicoryne brassicae on cabbage and Aphis craccivora on lentil and groundnut under irrigated conditions. Mustard crop should be irrigated twice to avoid heavy aphid infestation (Gangasaran and Giri 1986). Samuel et al. (2006) observed that short watering intervals (regimes) increase the population of Aphis craccivora on cowpea. Regarding water management in various crops, under no irrigation, Brassica carinata suffered heavily and succumb to injury of Lipaphis erysimi (Bakhetia and Brar 1988). On the contrary, Prasad et al. (1987) could not find any difference in the population of Aphis craccivora and Acyrthosiphon pisum on irrigated and unirrigated peas.
3.5.2.2.7 Fertility Management
There are 20 essential plant elements which are needed for the growth and development of the plants (Barker and Pilbeam 2016). Out of these, N, P and K are major nutrients. In general, high nitrogen supply results in increased tissue softness and water content as carbohydrates making the plant more susceptible to attack by aphids (Nevo and Coll 2001). Excess nitrogen in the plant limits proteolysis which results in poor nitrogen level in sap, whereas poor level of soil nitrogen leads to reduced concentration of soluble amino acids and amides in the plants (Tingey and Singh 1980). High dose of nitrogen increases the population of Lipaphis erysimi (Sidhu and Kaur 1977; Singh et al. 1995), Aphis craccivora (Sridharan et al. 1990) and Myzus persicae (Kashyap and Bhanot 1987). The presence of higher level of phosphorus makes the plant less susceptible for aphids. However, potassium has balancing effects on nitrogen and phosphorus. Deficiency of potassium results in the accumulation of soluble nitrogen and carbohydrates owing to inhibition of protein synthesis and increase in the rate of proteolysis (Tingey and Singh 1980). The presence of potassium causes toughening of plant tissues which might be due to decrease in protein by corresponding increase in carbohydrate content. In general, P and K application decreased the population of Lipaphis erysimi on mustard (Singh et al. 1995). Decreasing level of K led to greater fecundity of Myzus persicae and Brevicoryne brassicae on brussels sprout (van Emden 1966). The lowest aphid population was noticed at 80 kg/ha of DAP. Higher proportion of N:P:K (80:40:30) showed higher population of Lipaphis erysimi, whereas 40:80:40 ratio reduced aphid infestation (Singh et al. 1995). Similarly, high N:P:K (225:90:45) increased population of Brevicoryne brassicae on cauliflower (Sinha et al. 2018).
3.5.2.2.8 Removal of Alternate Hosts
Important aphids like Myzus persicae, Aphis craccivora and Aphis gossypii are polyphagous in nature and thrive well on cultivated as well as on wild plants. These wild plants and weeds provide suitable habitat and food for the aphid during off season. Wheatgrass, Agropyron cristatum, and Canada wildrye, Elymus canadensis, were observed as alternative hosts of the Russian wheat aphid, Diuraphis noxia, and serve as hosts between the time winter wheat was harvested and planted (Armstrong et al. 1991). Removal of such plants prevents the initial population ready for attack on the main crop, wheat. Destruction of stray groundnut plants and weeds has been recommended for the management of Aphis craccivora. Similarly, the destruction of yellow flowering weeds has been found useful against Myzus persicae in potato field (Raman 1985).
3.5.2.2.9 Trap Crop
Trap crop is generally used to ward off the insects from the main crop. It prevents the insects from reaching the main crop. Trap crop is more attractive and susceptible than the main crop. The planting of trap crop is done in such a time that its susceptible stage coincides with peak activity of the insect. Mustard as trap crop has been found very useful in the management of Lipaphis erysimi and Brevicoryne brassicae on cabbage when planted in mustard/cabbage (2:9) ratio (Srinivasan and Krishana Moorthy 1991). Firstly, it can attract aphids and draw them away from their host–plants. Secondly, it can alter the recognition of the host–plant. This effect is mostly attributed to companion plant volatiles since they disturb the aphid host–plant location, and additionally they may react chemically and physiologically with the host–plant, making it an unsuitable host for aphids. Thirdly, it can attract natural enemies by providing shelter and food resources (Ben-Issa et al. 2017).
3.5.2.2.10 Distance from Other Crops
Closely related or crops grown for different purposes should be planted distantly so that insects from one crop may not be able to reach other crop where physiological conditions suitable for aphid deteriorate. Toria and Sarson should be sown away from mustard and other long duration brassicas. Seed plot of potato should be away and located upwind from the commercial potato (Raman 1985).
3.5.2.2.11 Rogueing and Avoidance of Ratooning
Rogueing of aphid infested plants and avoidance of ratooning have been found very useful in the management of banana aphid, Pentalonia nigronervosa, a vector of bunchy top virus, and Aphis gossypii which transmits cucumber mosaic virus (Tandon 1994).
3.5.2.2.12 The Use of Reflective Mulches
Reflective plastic mulch, or silver-coloured plastic mulch, is covering material placed in fields when the plants are young and initially aphid-free. This mulch reflects light that interferes with the ability of flying aphids to locate plants which delay or reduce the extent of infestation of young plants by winged aphids (Stapleton and Summers 2002). It works better for small horticultural and vegetable crops that are especially sensitive to viral diseases transmitted by aphids. As plants grow larger, reflective mulch becomes less effective. Reflective mulch ceases to repel insects by the time the plant canopy covers more than about half of the soil surface. In addition to above benefit, the reflective mulch also enhances growth of the plants by increasing photosynthesis and reducing heat and water stress by keeping the plant and soil cooler (Pramanik et al. 2015).
3.5.2.3 Biological Control
Biological control of aphids in the fields has been successfully achieved in several parts of the world because their predators and parasitoids have great potential in managing their populations in spite of certain limitations. Indeed, biological control has been a central core around which IPM has commonly been developed (Dent 2000). The reason for this is that natural enemies constitute the major natural control factors, which can be manipulated. Singh (2001), Joshi et al. (2010), Boivin et al. (2012), Singh and Singh (2016) and Hance et al. (2017) have given a detailed account of biological control of aphids in India and abroad. In addition, entomopathogenic fungi have also been found to control few aphid species (Meyling and Eilenberg 2007).
3.5.2.3.1 Predators
Aphid predators belong to four orders of insects: Coleoptera (families Coccinellidae and Carabidae), Diptera (families Chamaemyiidae, Syrphidae and Cecidomyiidae), Hemiptera (families Anthocoridae and Geocoridae) and Neuroptera (family Chrysopidae). Ladybird beetles are most common aphid predators encountered throughout the world (Fig. 3.13a and c). The common genera predaceous on aphids are Adalia, Adonia, Brumoides, Coccinella, Cheilomenes, Exochomus, Hippodamia, Oenopia, Micraspis, Scymnus, etc. Aphidophagous coccinellids have a long history of importation in classical biocontrol with only few recognised successes (Obrycki and Kring 1998). Dixon (2000) judged only one to be substantially successful after 155 tallied intentional introductions of coccinellid species worldwide that specifically targeted aphids. The aphid midge, Aphidoletes aphidimyza, is a cecidomyiid fly whose larvae are effective predators of aphids, an important component of biocontrol for greenhouse crops (Boulanger et al. 2019), and is commercially available (e.g. APHIDEND®, Koppert B.V., The Netherlands). Several species of syrphid flies have been evaluated as biocontrol agents against aphids (Fig. 3.13b) (Joshi and Ballal 2013), and few species are commercially available, e.g. Episyrphus balteatus (SYRPHIDEND®, Koppert B.V., The Netherlands). Green lacewings, particularly members of the genera Chrysopa, Chrysoperla and Mallada (Chrysopidae) (Pappas et al. 2011) (Fig. 3.13d), and brown lacewing (Hemerobiidae) are major biocontrol agents of aphids among Neuroptera and have been used against aphids in several parts of the world (Rocca and Messelink 2017).
3.5.2.3.2 Parasitoids
Most aphid parasitoids belong to Hymenoptera (Braconidae, Fig. 3.14, and Aphelinidae). Most of the aphidiine parasitoids used in biocontrol belong to the genera: Aphidius, Binodoxys, Diaeretiella, Ephedrus, Praon and Trioxys. They are cosmopolitan in distribution so that one species, Diaeretiella rapae, parasitises about 98 species of the aphids distributed in 87 countries throughout the world (Singh and Singh 2015b). More than 100 biocontrol programmes have been monitored against at least 30 species of aphids, and about 50% of them proved successful. These programmes include the introduction of about 25 species of parasitoids. Singh and Singh (2016) enlisted and summarised major biocontrol attempts against the aphids throughout the world. The parasitoids become established in 34 out of 57 attempts. The introduction of Aphelinus mali in France to control the woolly aphid, Eriosoma lanigerum, in apples was probably the first attempt of biocontrol of aphids (Howard 1929). It was then introduced in several European countries, Australia, New Zealand and India. The Indian species Aphidius smithi quickly established in Mexico, Canada and the USA in the fields of alfalfa (Mackauer 1971). Similarly, Aphidius eadyi successfully controlled the pea aphid, Acyrthosiphon pisum, in New Zealand, and Trioxys complanatus and Trioxys tenuicaudus suppressed the population of alfalfa aphid, Therioaphis trifolii, and elm aphid, Tinocallis platani, respectively, in the USA (Hughes 1989). Active biocontrol attempts have been made by the introduction of Diaeretiella rapae against the Russian wheat aphid Diuraphis noxia with partial success (Brewer and Elliott 2004). Singh and Agarwala (1992) and Singh and Rao (1995) demonstrated successful control of Aphis craccivora on pigeon pea and Aphis gossypii on cucurbits by introducing the indigenous parasitoid Binodoxys indicus. Levie et al. (2005) showed that the release of 20,000 Aphidius rhopalosiphi per hectare in wheat crops, twice at 1-week intervals, allowed the control of the aphid, Sitobion avenae. In China, mass release of Aphidius gifuensis was used to control Myzus persicae in tobacco crops (Yang et al. 2009). In apple orchards, the inundative release of two parasitoid species, Ephedrus persicae and Aphidius matricariae, controlled the population of rosy apple aphid, Dysaphis plantaginea (Boivin et al. 2012). Waterhouse (1998) summarised the attempts of biocontrol of Aphis craccivora and Aphis gossypii using their parasitoids in several countries such as Australia, China, Columbia, Cuba, East Asia, France, India, Iraq, Israel, Italy, Japan, Korea, Malaysia, Netherlands, Pakistan, Philippines, USA, Russia, Vietnam, etc.
Glasshouse crop cultivation is a striking example of recent development in the field of biocontrol. Around 55 years ago, even specialists had serious doubt about the success of biocontrol in the glasshouses because this method of crop raising is economically vulnerable. Parr and Scopes (1970) described the problems associated with biocontrol of glasshouse pests. According to them, biocontrol gives more predictable control lasting several weeks to months despite being cheaper and eco-friendly. Paprikas, tomatoes, lettuces, chrysanthemums and other ornamental pot plants are cultivated in glasshouses mostly in Europe. All these plants severely suffer with Myzus persicae. Successful biocontrol of Myzus persicae was achieved by introducing Aphidius matricariae (Hussey and Scopes 1985) and Ephedrus cerasicola (Hofsvang and Hågvar 1980; Hågvar and Hofsvang 1990). Biocontrol through inundative or inoculative releases is applied in greenhouses where it gives the best results (van Lenteren 2000). During the year 2006, more than 37,000 ha of greenhouses was under biocontrol programmes (Parrella 2008). However, the augmentative use of parasitoids for aphid biocontrol requires the release of thousands of individuals. For instance, under greenhouse cultivation, quantities of parasitoids released for aphid control range from 2500 to 10,000 individuals per ha (van Lenteren 2003). Table 3.4 summarises the attempts and success of biocontrol of Myzus persicae and Myzus ascalonicus in glasshouses worldwide.
3.5.2.3.3 Pathogenic Fungi
The entomopathogenic fungi are different kinds of biocontrol agents of insect pests (Rehner 2005). Twenty-eight mycopesticides using seven species of entomopathogenic fungi, such as Beauveria bassiana, Metarhizium anisopliae and Lecanicillium spp., are commercially available in several countries for control of aphid pests (Goettel et al. 2005; de Faria and Wraight 2007). Sabbour (2019) demonstrated the promising control of Myzus persicae by using destruxins which is isolated from Metarhizium anisopliae. It is a cyclic hexadepsipeptide and causes paralysis and a speedy death to the insects, and also it causes suppression of the insect immune system. The destruxins are also used as synergist to enhance the efficiency of other biopesticides used against aphids (Yi et al. 2012).
These mycopesticides mainly use propagules such as conidia, blastospores or hyphae which have advantages of direct mortality of the target aphid pest (Shan and Feng 2010). However, the conidia of entomopathogenic fungi are highly affected by environmental factors, such as temperature and relative humidity, and are slow in causing mortality. These factors have prevented wider application and use of these biocontrol agents. In spite of that, these species are being applied in agriculture and forestry in temperate regions (Meyling and Eilenberg 2007; Kim et al. 2013). However, these pathogens adversely affect the potential of aphid parasitoids. For example, Beauveria bassiana have been found to reduce the emergence and longevity of females of Diaeretiella rapae against Myzus persicae (Silva et al. 2014). Moreover, González-Mas et al. (2019) could not observe any detrimental effect on the predator Chrysoperla carnea and the parasitoid Aphidius colemani of Aphis gossypii on melon crop when inoculated by Beauveria bassiana in Spain. Therefore, application of these two kinds of biological control agents in combination of the control of aphids requires effective time management to avoid antagonistic interactions. The use of entomopathogenic fungi in the management of insect pests of field crops has been reviewed by Maina et al. (2018).
3.5.2.3.4 Bacteria
The biosurfactants produced by Bacillus atrophaeus L193 were reported to control the aphid, Rhopalosiphum padi, in order to suggest a friendly alternative to chemical pesticides. These surfactants contain lipopeptides, such as surfactins, fengycins, bacillomycins and iturins, that cause aphid death by affecting cuticle membranes (Rodríguez et al. 2018). A multifunctional endophytic bacterial strain Bacillus velezensis YC7010 has been found to induce systemic resistance against bacterial and fungal pathogens of rice. Rashid et al. (2017) demonstrated that root drenching of the brassica plant Arabidopsis seedlings with this bacterial strain induces higher accumulation of hydrogen peroxide, cell death and callose deposition in leaves that reduced settling, feeding and reproduction of Myzus persicae on its leaves. Foliar spray of a bacterium, Pseudomonas poae, to plants before aphid colonisation was found to reduce the infestation by Myzus persicae (Paliwal 2017). Similarly, field application of another species, Pseudomonas fluorescens, was observed to control Aphis gossypii on Bt and non-Bt cotton (Manjula et al. 2018).
3.5.2.3.5 Interactions Between Host–Plant Resistance and Biological Control
The development of resistant crop cultivars offers a sound and very practical approach to the long-range control of certain agricultural pests. However, the varietal breeding is traditionally in the botanical field of plant breeding that falls rather outside the scope of the biological control workers. Recent advancement of our knowledge about its compatibility with biological control necessitates interdisciplinary action in achieving good pest management. Nonlethal plant defences may potentiate the role of predation, parasitism and pathogen infection in regulating herbivore population. It is usually assumed that host–plant resistance is generally or highly compatible with integrated pest management and biological control strategies (Kogan and Jepson 2007). Host–plant resistance can affect the natural enemies of insects in several ways. In some situations, plant resistance can reduce the performance of natural enemies, while in others it can act in a complimentary way (Price 1986). For example, while aphids feed on plants, plants produce semiochemicals, which may act as repellents to aphids or attractants to natural enemies of these aphids (Biswas and Singh 1998). Presumably, this control results because the resistant variety facilitates the searching behaviour of the enemy, reduces the vigour of the host to avoid parasitisation, delays development of the host so that the pest and the enemy populations are temporarily synchronised and/or among other things modifies the behaviour of the host so that it is more easily parasitised (Price et al. 1980).
Each and every plant defends themselves by herbivore insects in general either by producing chemicals, such as toxin, or digestibility reducers, or through physical defence by trichomes or toughness, or by a combination of the two, as with glandular trichomes or resins (intrinsic defence of the plants, resistance of host–plant), and by benefiting natural enemies of the herbivores (extrinsic defence of the plants) (Singh 2003). It is now recognised that almost every mechanism of the intrinsic defence of a plant has an effect on the trophic system and that intrinsic defence may impact positively or negatively upon the third trophic level as well as on those factors involved with extrinsic defence (Price et al. 1980; Singh et al. 2000b). The intrinsic and extrinsic defences of plants reduce the colonisation rate of the herbivores.
Plant trichomes are one of the bases of host–plant resistance against a number of aphids. The presence of trichomes in potatoes confers resistance against the aphid Myzus persicae and Macrosiphum euphorbiae (Tingey et al. 1982).
Du et al. (1998) observed that the plant Vicia faba synthesises some chemicals particularly 6-methyl-5-hepten-2-one after the infestation by the pea aphid Acyrthosiphon pisum, and this chemical was observed most attractive for its parasitoid Aphidius ervi. Price et al. (1980) suggested the melding of these two approaches is essential in evaluating the roles of natural enemies in population control of herbivores. Several workers have studied the resistance of cereal crops to a variety of pests, particularly to biotypes of Schizaphis graminum. The synergistic ability of host–plant resistance with the parasitoid Lysiphlebus testaceipes was demonstrated by several workers in the past (Burton and Starks 1977; Starks and Burton 1977). They have observed that greater degree of parasitism by Lysiphlebus testaceipes on resistant variety of sorghum and oats resulted from increased degree of movement of the aphids, which made them several times more susceptible to parasitisation. They observed that resistant varieties of sorghum and oats enhance the killing efficiency of the parasitoid Lysiphlebus testaceipes in reducing the population of the aphid Schizaphis graminum. Biswas and Singh (1998) observed increased efficiency of Lysiphlebus delhiensis against Melanaphis sacchari on resistant corn cultivar. Fuentes-Contreras and Niemeyer (1998) observed that hydroxamic acids, plant secondary metabolites associated with aphid resistance in wheat, influence the host acceptance and suitability of the aphid, Sitobion avenae, to its parasitoid Aphidius rhopalosiphi. Similarly, Fuentes-Contreras and Niemeyer (2000) reported significant reductions of population growth rate of aphids with the joint action of wheat resistance and its natural enemies. Farid et al. (1998a, b) observed that the plant resistance against the Russian wheat aphid, Diuraphis noxia, did not have an adverse affect on the percentage of its parasitism by Diaeretiella rape.
Cultural control practices may also be integrated with biological control and host–plant resistance (van Emden 2017).
3.5.2.3.6 Interaction Between Cultural Control and Biological Control
Increasing the diversity within the agricultural fields by introducing multiple cropping, intercropping, strip harvesting, selective retention of weeds within the crop or conservation of wild plants at field margins also promotes the conservation of natural enemies of aphids and also enhances their natural control ability. Increasing diversity within crops is predicted to provide a greater number of opportunities for natural enemies to survive in agricultural systems and also tends to increase natural enemy abundance and diversity, providing a system more resilient to pest population increase (Rodriguez-Saona et al. 2012). Vegetational diversity also provides support for insect biocontrol at local and landscape levels. The plants serve as a reservoir of the alternative host species, and flowering plants are important sources for food as the adult parasitoids do not necessarily feed only on honeydews but also on pollen, nectar and other sugary plant secretions.
Provision of food resources, such as floral (nectar and pollen), extrafloral (nectaries, exudates and fruits) and insect products (honeydew) in the fields, had significantly increased the longevity and potential fecundity (egg load) in the aphid parasitoids Aphidius rhopalosiphi (Budenberg et al. 1992), Diaeretiella rapae (Tylianakis et al. 2004), Aphidius colemani (Charles and Paine 2016) and Aphidius ervi (Hogervorst et al. 2007); searching activity of Aphidius nigripes (Bouchard and Cloutier 1984) and Lysiphlebus testaceipes (Grasswitz and Paine 1993); and intrinsic rate of increase of Lysiphlebia mirzai (Singh et al. 1996) and Lipolexis scutellaris (Singh et al. 2000a). Costello and Altieri (1995) reported that Diaeretiella rapae highly parasitised Myzus persicae on clean cultivated broccoli, and Shlyakhovoi and Bobonich (1975) observed that parasitism of Brevicoryne brassicae on cabbage by Diaeretiella rapae was high if nectar-bearing plants are grown in its neighbourhood. These positive effects on the parasitoid’s reproductive activity improve its effectiveness of both conservation and augmentation biological control of aphids.
3.5.2.3.7 Interaction Between Transgenic Plants and Biological Control
Crop plants transformed to express toxin genes derived from Bacillus thuringiensis (Bt) provide high levels of resistance to certain pest species, which is likely to have consequent effects on parasitoids specialising on such pests. A better understanding of the interaction between transgenic plants, pests and parasitoids is important to limit disruption of biological control and to provide background knowledge essential for implementing measures for the conservation of parasitoid populations (Singh 2003). It is also essential for investigations into the potential role of parasitoids in delaying the buildup of Bt-resistant pest populations. Introducing genetically modified insect-protected crops into the agricultural landscape has a profound effect on target herbivore abundance and distribution. Populations of specialised natural enemies are expected to be reduced because vast acreages of crops will no longer contain appropriate hosts. However, hosts should still be abundant in refuge plantings designed to prevent the spread of resistance in the target herbivore populations.
Behavioural choice tests with maize expressing the Cry1Ab toxin of Bt and larvae of the predatory lacewing Chrysoperla carnea demonstrated that the predator preferentially feeds on aphids rather than on lepidopteran larvae (the targets of the Bt toxin) (Meier and Hilbeck 2001). This preference will reduce the exposure of Chrysoperla carnea to Cry1Ab toxin since aphids do not ingest the toxin when feeding on Bt maize (Raps et al. 2001). Population-scale laboratory experiments with the aphid Myzus persicae and its parasitoid, Diaeretiella rapae, showed that the parasitoid was as effective in controlling this nontarget pest on Bt and proteinase inhibitor oilseed rape plants as on untransformed plants (Schuler et al. 2001).
3.5.2.4 Chemical Control
As far as possible, chemical control of aphid pests should be avoided as most of them are fatal for honey bees and other pollinators and other beneficial insects, particularly the parasitoids and predators of aphids. Only when the use of insecticide becomes inevitable, then insecticides may be used to control the aphid population in different agroecosystems (Dewar and Denholm 2017). The main aim with insecticide use should be to select such insecticides that have minimal detrimental effect on pollinators and natural enemies but are still effective on the insect mortality.
According to the crop, multiple applications of insecticides may be needed. However, additional applications are only needed if live aphids are still present. It is necessary that before purchasing the suitable insecticide and using it, all label directions must be read and followed, specially the formulation and dosage because the label is the law; therefore, the product label is the final authority on what crop or areas the product can be applied and at what rate. Before purchasing an insecticide, one should be sure to look on the package for active ingredient and select the product with the proper active ingredient to control the pest. Spray should always be done mid- to late evening for best result and to protect beneficial insects and also to avoid any potential plant damage.
3.5.2.4.1 The Use of Insecticidal Soap or Horticultural Oil
The first choice of spray should be insecticidal soap (potassium salt of long-chain fatty acids, 1–2% soap mixed with water) or horticultural oil as the application of these covers the body of the aphids to close the spiracles that results to its suffocation and death. The fatty acids also penetrate the body wall of the aphids and disrupt the cell membranes due to which the cell contents leak out causing the insect to dehydrate and die (Puritch 1981). These solutions when dry after the spray do not leave residue, and any beneficial insect that arrives thereafter will not be contacted with these soaps or oils and remains alive. However, insecticidal soaps are generally ineffective in controlling aphid populations, e.g. leaf curl plum aphid (Brachycaudus helichrysi) or the woolly ash aphid (Prociphilus fraxinifolii), which are protected inside distorted foliage or galls. Also, the use of soaps or oils should not be applied on water-stressed plants or when the temperature exceeds 32 °C as these may be phytotoxic to some plants. Common aphid species controlled with these types of oils include the woolly apple aphid (Eriosoma lanigerum), green apple aphid (Aphis pomi), rosy apple aphid (Dysaphis plantaginea), mealy plum aphid (Hyalopterus pruni), black cherry aphid (Myzus cerasi), etc.
3.5.2.4.2 The Use of Biopesticides
The effective step-up from the soaps and oils is application of biopesticides which are derived from plants (botanicals) and are in use in modern agriculture due to their upper hand over synthetic insecticides as usually they are not toxic to nontarget animals and are easily degradable. The use of pyrethrins, nicotine, azadirachtin, rotenone, etc., is time-honoured insecticides; all are commercially available.
3.5.2.4.2.1 Nicotine
Commercially, nicotine (Black Leaf 40) is a formulation of nicotine alkaloid (95%) and nicotine sulphate (40%). The alkaloid is derived from tobacco (Nicotiana tabacum and N. rustica). It is highly toxic to a great number of insects including aphids as a nerve poison. Since it is also very toxic to humans, it is banned in India but is manufactured for export only. However, the household aqueous preparation of tobacco leaves, garlic and neem gives promising result against cowpea aphid (Bahar et al. 2007).
3.5.2.4.2.2 Pyrethrum
The pyrethrum is extracted from the flowers of Chrysanthemum spp. and Tanacetum cinerariifolium. It is composed of four compounds: pyrethrins I and II and cinerins I and II. The cinerins are more stable than the pyrethrins. This biopesticide can be very effective in providing a relatively quick knockdown of aphids. This chemical attacks the insects’ peripheral nervous system and for this reason has a rapid knockdown; however, the insects soon recover to full activity. Therefore, some synergists are added in the formulation. It is available as spray and dust for use against aphids on fruit trees, vegetables and flowers. Commercial ‘Pyrethrum FS’ is based on pyrethrin (sesame oil is added as a synergist) against aphids and other sucking insects. The active ingredients are rapidly broken down by sunlight and are only effective for a short time. It has been used against aphids in tomato (Verghese 2015).
3.5.2.4.2.3 Rotenone
Rotenone is found in the roots of several species of plants in the genera Derris, Millettia, Tephrosia and Lonchocarpus. It is probably the second most used botanical. Rotenone is a white to yellowish white crystal and is readily detoxified by the action of air and light. It is a metabolic inhibitor (i.e. inhibits the respiratory chain, the oxidation of NADH-linked substrate) and is a broad-spectrum contact and stomach poison that affects insect nerve and muscle cells, causing the insects to stop feeding and die anywhere from a few hours to a few days after ingestion. Rotenone has been shown to be an effective control agent of many pest species, including aphids (Isman et al. 2011). Yi et al. (2012) have observed effective control of Aphis gossypii by the spray of a mixture of rotenone and destruxins. The insecticidal activities of destruxin have been observed against aphids (Robert and Riba 1989).
3.5.2.4.2.4 Ryania
Ryania is extracted from the stem and roots of a woody South American plant Ryania speciosa. The active ingredient is an alkaloid ryanodine. Ryania is a stomach poison that causes insects to stop feeding soon after ingestion. It is reported to be most effective when used in hot weather. Ryania has been suggested to use against aphids on trees (Veena 2009).
3.5.2.4.2.5 Azadirachtin
Azadirachtin is the most active compound found in neem (Azadirachta indica) plants and is highly toxic to several insect pests such as cotton aphids, cotton bollworms, brown plant hopper, cabbage butterfly, etc. Indeed, the neem plants contain thousands of chemical constituents. Of special interest are the terpenoids that are unique to neem. More than a hundred terpenoids are known from different parts of the neem plant. Azadirachtin is one of the terpenoids. Several different kinds of azadirachtins (A to K) have been isolated, the most abundant of which is azadirachtin A. The neem terpenoids are present in all parts of the plant, in the living tissues especially in the seed kernels. The commercial products of neem (Neem®, Nimbicide®, Achook®, BioNeem®, Neemix®, Azatin®, etc.) work on the metamorphosis of insects. Neem has been used with variable results to manage aphids and other insects. The extract of neem reduces the population of several aphid species causing high mortality and decreasing fecundity, as well as inhibiting population growth in many crops, e.g. Acyrthosiphon pisum on pea (Stark and Rangus 1994), Elatobium abietinum on spruce (Partridge and Borden 1997), Aphis craccivora on cowpea (Ulrichs et al. 2001), Aphis (Toxoptera) citricidus on citrus (Tang et al. 2002), Myzus persicae on pepper (Shannag et al. 2014), Sitobion avenae on wheat (Shah et al. 2017; Matharu and Tanwa 2019) and other aphid pests.
3.5.2.4.3 The Use of Synthetic Insecticides
Both contact and systemic insecticides are available for the control of aphids, primarily on ornamentals, although there are formulations for cereals, legumes, vegetables and fruits. If an insecticide is to be applied on vegetables or fruits, the label on the container must be seen as it will give specific directions as to when the product can be applied prior to harvest. There are hundreds of chemical insecticides with many formulations, but when it is established that chemical control is necessary, a suitable insecticide should be selected. Such insecticides should have following characteristics: (i) it should be safe to nontarget organisms but be highly efficient to kill the target insects; (ii) it should not be phytotoxic nor should it impair the germination of seeds and cause damage to flowers and fruits; (iii) it should not impart off-flavour of food materials; (iv) it should kill the target insects very quickly; (v) it should be persistence in toxicity, i.e. it should maintain lethal action for a longer period; (vi) it should be quickly degradable if persistence is not required; (vii) it should be stable during longer storage; (viii) it should be cheaper and within the reach of poor farmers; etc. (Dewar and Denholm 2017). However, these attributes differ in different situations, and no one insecticide possesses all these desirable attributes. In addition, one should be very careful in applying insecticides in agricultural crops to control aphids.
It should be taken into account that not all insecticides can wipe out aphids. Often the success of any insecticide depends on the timing and way of its application rather than its chemistry. However, different kinds of insecticides are needed to work well for different crops and different aphid species. It has been observed that sometimes combinations of conventional insecticides, such as orthene, endosulfan, metasystox-R, dimethoate and pyrethroids, may be superior. The use of carbaryl against aphids must be avoided as it can be much more detrimental to their natural enemies. Any insecticide should be applied only after proper monitoring the incidence of aphids and its level of infestation exceeding ETL. Table 3.5 displays the type of active ingredients of representative insecticides used nowadays, its formulation, mode of action and possible danger.
Most of the insecticides used to control aphid population are systemic in nature. Chemical control measures for following major agricultural aphid pests are given below.
3.5.2.4.3.1 Aphis gossypii
In case of Aphis gossypii on cotton, the following steps should be applied to control its population. Seed treatment with imidacloprid 60 FS @ 10 ml/kg seed or with thiamethoxam 70 WS @ 5 g/kg seed keeps the crop free from aphids for a month. If the plant is infested with aphids, spray of following insecticides controls the aphid population: NSKE 5% @ 2.0 ml/l, clothianidin 50 WDG @ 0.075 g/l, imidacloprid 17.8 SL @ 0.25 ml /l, acetamiprid 20 WP @ 0.2 g/l and thiamethoxam 25 WP @ 0.2 g/l.
3.5.2.4.3.2 Lipaphis erysimi
The most effective treatments against Lipaphis erysimi infesting a seed crop of radish, menazon at 0.17 and 0.34 kg a.i./ha and oxydemeton-methyl at 0.34 kg a.i./ha were recommended, which reduced the aphid population (Sekhon et al. 1980). On cabbage, the treatment of thiamethoxam 0.01%, imidacloprid 0.01%, acetamiprid 0.004% and methyl-o-demeton 0.025% provides considerable control of Lipaphis erysimi (Vermora et al. 2010). On cauliflower, it can be controlled by applying cypermethrin 10EC 400 ml/ha and chlorpyriphos 20EC 1000 ml/ha (Krishna et al. 2009) or imidacloprid 17.8 SL @ 0.2 g/l and fipronil 5 SC @ 1.0 ml/l (Dotasara et al. 2017).
The spray of flonicamid 0.02% at seedling stage, flubendamide 0.014% at pre-flowering stage, azadirachtin 0.15% at 50% flowering stage and acephate + fenvalerate 0.028% at 50% pod formation stage keeps the population of Lipaphis erysimi on mustard crop (Kalasariya 2016). Shukla and Mishra (2010) observed promising control of Lipaphis erysimi on taramira by applying dimethoate 0.03%, monocrotophos 0.05%, methyl demeton 0.03% and acephate 0.02% proved to be highly effective followed by dimethoate 0.02%, acephate 0.01% and methyl.
3.5.2.4.3.3 Myzus persicae
On cauliflower, Myzus persicae can be controlled by applying cypermethrin 10EC 400 ml/ha, flufenoxuron (400 ml/ha) and chlorpyriphos 20EC 1000 ml/ha (Krishna et al. 2008). The following insecticides also give satisfactory results when applied in proper concentration and formulation: chinimix, dichlorvos, endosulfan, M.I.P.C., malathion, methyl-o-demeton, phosphamidon, quinalphos, bifenthrin, cyfluthrin, dicrotophos, ethiofencarb, fenvalerate, furadan, methamidophos, parathion, permethrin and pirimicarb (Singh 2015).
3.5.2.4.3.4 Aphis craccivora
In case of Aphis craccivora on cowpea, the following insecticides give promising result: spray of methyl demeton 25 EC 500 ml/ha or dimethoate 30 EC 500 ml/ha. Three sprays of chlorpyriphos 50% EC and cypermethrin 5% EC @ 2 ml/l after 15 days interval yield considerable control of the aphid (Dhakal et al. 2019).
3.5.2.4.3.5 Cereal Aphids
Worldwide, six species of aphids, viz. bird cherry oat aphid (Rhopalosiphum padi), corn aphid (Rhopalosiphum maidis), English grain aphid (Sitobion avenae) and Indian grain aphid (Sitobion miscanthi), attack several cereal crops, such as barley, corn, millets, sorghum and wheat in India. The aphid damage is seen during grain filling stage when both nymphs and adults damage the crops by sucking cell sap from leaves and maturing grains. The infested leaves turn pale, wilt and have a stunted appearance and cause heavy grain yield loss. A summary of registered insecticides for use in cereal crops for controlling these aphids is given in Table 3.6.
3.6 Conclusions
The aphids as a group of sucking insects are very fascinating with regard to their morphology, life cycle, behaviour, host–plant interaction, etc. Out of about 5110 species described, about 250 species are considered as notorious pests of hundreds of agricultural and horticultural crops. Some aphids are monophagous, while few are highly polyphagous feeding on hundreds of host–plants. Aphids excrete considerable amount of sugary liquid, honeydew, on which sooty mould usually turns them black that hampers photosynthesis and respiration. The honeydew also serves as food for ants, bees and their parasitic wasps. The aphids are unique on the account of their peculiar mode of reproduction, development and the polymorphism. They may reproduce either by parthenogenesis or zygogenesis. They may either be oviparous or viviparous. Parthenogenetic reproduction allows rapid increase in numbers and results in populations consisting of clones. Some species reproduce both parthenogenetically and sexually (holocyclic species), whereas only few reproduce parthenogenetically (anholocyclic species). The aphids are polymorphic, and both winged (alate) and wingless (aptera) morphs may be found in the same colony. Several factors, both biotic and abiotic, have effect on the formation of different phenotypes, and each phenotype performs different ecological roles in the life history. Aphids are frequently engaged in mutualistic associations with bacterial endosymbionts that not only provide essential amino acids to them but also grant them protection from natural enemies, protection from extreme temperatures, development of resistance to a fungal pathogen and the ability to use a greater diversity of resources.
About 250 species of aphids are major agricultural and horticultural pests of several crops. They damage the crops directly by sucking their nutrients, making galls and hampering photosynthesis and respiration by the growth of sooty moulds on the honeydew deposited thereon. Aphids also damage the crop indirectly by transmitting hundreds of plant viruses. Because of their economic importance, their population must be controlled to save the crops. There are several ways by which the aphids are controlled both in glass houses and fields.
References
A’Brook J (1968) The effect of plant spacing on the number of aphids trapped over groundnut crop. Ann Appl Biol 61:289–294
Agarwala BK (2007) Phenotypic plasticity in aphids (Homoptera: Insecta): components of variation and causative factors. Curr Sci 93:308–313
Agarwala BK, Das A (1998) Population diversity in aphids: the influence of host-plants on morphology, biology and ecological performance of the mustard aphid Lipaphis erysimi (Kaltenbach). J Aphidol 12:21–32
Agarwala BK, Ghosh AK (1985) Biogeographical considerations of India Aphididae (Homoptera). Insecta Matsumurana (New series) 31:81–96
Akashe VB, Mehtre SP, Shewale MR (1997) Estimation of economic threshold level of safflower aphid (Uroleucon compositae Theobald) on Bhima. In: Proceedings of IVth international safflower conference, Bari (Italy), June 2–7, pp 317–319
Akimoto S (2006) Inbreeding depression, increased phenotypic variance, and a trade-off between gonads and appendages in selfed progeny of the aphid Prociphilus oriens. Evolution 60:77–86
Albert R (1990) Experience with biological control measures in glasshouses in Southwest Germany. SROP/WPRS Bull 13:1–5
Albittar L, Ismail M, Bragard C, Hance T (2016) Host-plants and aphid hosts influence the selection behaviour of three aphid parasitoids (Hymenoptera: Braconidae: Aphidiinae). Eur J Entomol 113:516–522
Almeida RP (2001) Effect of the population levels of Aphis gossypii on cotton agronomic traits and fibre quality. Proc Section Exp Appl Entomol Nev Amsterdam 12:97–100
Anand S, Biradar VK, Neharkar PS, Gawande RW (2017) Determination of economic threshold level (ETL) of safflower aphid, Uroleucon compositae (Theobald). International journal of researches in bioscience, agriculture and technology. Special Issue 2(5):274–276
Aoki S (1980) Life cycles of two Colophina aphids (Homoptera: Pernphigidae) producing soldiers. Kontyu 48:464–476
Aoki S (1982) Pseudoscorpion-like second instar larvae of Pseudoregma shitosanensis (Homoptea: Aphidoidea) found on its primary host. Kontvu 50:445–453
Armstrong JS, Porter MR, Peairs FB (1991) Alternate hosts of the Russian wheat aphid (Hornoptera: Aphididae) in northeastern Colorado. J Econ Entomol 84(6):1691–1694
Ashford DA, Smith WA, Douglas AE (2000) Living on a high sugar diet: the fate of sucrose ingested by a phloem-feeding insect, the pea aphid Acyrthosiphon pisum. J Insect Physiol 46(3):335–341
Bahar MH, Islam MA, Mannan MA, Uddin MJ (2007) Effectiveness of some botanical extracts on bean aphids attacking yard-long beans. J Entomol 4:136–142
Bakhetia DRC, Brar KS (1988) Effect of water stress in Ethiopian mustard (Brassica carinata) and Indian mustard (B. juncea Subsp. juncea) on infestation by Lipaphis erysimi. Indian J Agric Sci 58:638–640
Balog A, Mehrparvar M, Weisser WW (2013) Polyphagous predatory rove beetles (Coleoptera: Staphylinidae) induce winged morphs in the pea aphid Acyrthosiphon pisum (Hemiptera: Aphididae). Eur J Entomol 110:153–157
Barker AV, Pilbeam DJ (2016) Handbook of plant nutrition. CRC Press, Boca Raton, p 632
Basak S, Das SS, Pal S (2017) Economic threshold level of aphid on mustard crop at Pundibari (a part of Coochbehar district): it’s determination by application of probability and statistics. Int J Zool Stud 2(4):10–13
Behura BK (1994) The mystery of aphid life-history. J Aphidol 8:1–18
Behura BK (1996a) The structure and function of the cornicles of aphids (Homoptera: Aphididae). J Aphidol 10:1–12
Behura BK (1996b) The colour of aphids (Homoptera: Aphididae): a mini-review and bibliography. J Aphidol 10:61–66
Behura BK, Das MM (1976) Aphid and their role in agriculture. Proc Natl Acad Sci India 46:261–265
Ben-Issa R, Gomez L, Gautier H (2017) Companion plants for aphid pest management. Insects 28(4):112
Bilodeau E, Guay J-F, Turgeon J, Cloutier C (2013) Survival to parasitoids in an insect hosting defensive symbionts: a multivariate approach to polymorphic traits affecting host use by its natural enemy. PLoS One 8:e60708
Biswas S, Singh R (1998) Interaction between host-plant resistance and the biocontrol of a cereal aphid: a laboratory study. Biol Agric Hortic 16:25–36
Blackman RL (1975) Photoperiodic determination of the male and female sexual morphs of Myzus persicae. J Insect Physiol 21:435–453
Blackman RL, Eastop VF (2000) Aphids on the World’s crops: an identification and information guide, 2nd edn. Wiley, London, p 476
Blackman RL, Eastop VF (2007) Aphids on the world’s herbaceous plants and shrubs. In: The aphids, vol 2. Wiley, Chichester, pp 1025–1456
Boivin G, Hance T, Brodeur J (2012) Aphid parasitoids in biological control. Can J Plant Sci 92(1):1–12
Bouchard Y, Cloutier C (1984) Honeydew as a source of host searching kairomones for the aphid parasitoid Aphidius nigripes (Hym.: Aphidiidae). Can J Zool 62:1513–1520
Boulanger FX, Jandricic S, Bolckmans K, Wäckers FL, Pekas A (2019) Optimizing aphid biocontrol with the predator Aphidoletes aphidimyza, based on biology and ecology. Pest Manag Sci 75(6):1479–1493
Braendle C, Miura T, Bickel R, Shingleton AW, Kambhampati S, Stern DL (2003) Developmental origin and evolution of bacteriocytes in the aphid-Buchnera symbiosis. PLoS Biol 1:70–76
Brewer MJ, Elliott NC (2004) Biological control of cereal aphids in North America and mediating effects of host-plant and habitat manipulations. Annu Rev Entomol 49:219–242
Budenberg WJ, Powell W, Clark SJ (1992) The influence of aphids and honeydew on the leaving rate of searching aphid parasitoids from wheat plants. Entomol Exp Appl 63:259–264
Burgio G, Lanzoni A, Accinelli G, Dinelli G, Bonetti A, Marotti I, Ramilli F (2007) Evaluation of Bt-toxin uptake by the non-target herbivore, Myzus persicae (Hemiptera: Aphididae), feeding on transgenic oilseed rape. Bull Entomol Res 97:211–215
Burton RL, Starks KJ (1977) Control of a primary parasite of the greenbug with a secondary parasite in greenhouse screening for plant resistance. J Econ Entomol 70:219–220
Buxton JH, Jacobsen R, Saynor M, Storer R, Wardlow L (1990) An integrated pest management programme for peppers, three years trials experience. SROP/WPRS Bull XIII/5:45–50
Byers JA (2005) A cost of alarm pheromone production in cotton aphids, Aphis gossypii. Naturwissenschaften 92:69–72
Capinera JL (2008) Encyclopedia of entomology. Springer Science & Business Media, Heidelberg, pp 193–194
Carter N, Powell W, Wright AF, Ashby JE (1989) Effectiveness of different insecticides applied at various growth stages to control aphids on winter wheat. Crop Prot 8:271–276
Chakrabarti S (1987) Biosystematics of gall aphids (Aphididae, Homoptera) of western Himalaya, India. Proc Indian Acad Sci (Animal Science) 96:561–572
Chakrabarti S (2007) Diversity and biosystematics of gall-inducing aphids (Hemiptera: Aphididae) and their galls in the Himalaya. Orient Insects 41:35–54
Chakrabarti S (2009) Diversity, distribution and endemism of aphids (Hemiptera) in Indian subregion of oriental realm. Redia 42:78–85
Chakrabarti S, Chakrabarti S (2002) Tritrophic relationship: aphidophaga, aphid and its host plant - a study on Brachycaudus helichrysi (Kalt.) (Homoptera: Aphididae). J Aphidol 16:39–44
Chamuene A, Ecole C, Freire M, Macuácua R, Maposse I, Santos L, Sidumo A, Sousa H (2007) Cropping systems and pest management strategies in the Morrumbala region of Mozambique: enhancing smallholders cash crop production and productivity. Afr Crop Sci Conf Proc 8:1045–1047
Chang MG, Gurr GM, Tylianakis JM, Wratten SD (2017) Cultural control. In: van Emden HF, Harrington R (eds) Aphids as crop pests. Oxford University Press, Oxford, pp 494–514
Charles JJ, Paine TD (2016) Fitness effects of food resources on the polyphagous aphid parasitoid, Aphidius colemani Viereck (Hymenoptera: Braconidae: Aphidiinae). PLoS One 11(1):e0147551
Chen JQ, Rahbé Y, Delobel B, Sauvion N, Guillaud J, Febvay G (1997) Melon resistance to the aphid Aphis gossypii: behavioral analysis and chemical correlations with nitrogenous compounds. Entomol Exp Appl 85:33–44
Chen Y, Verheggen FJ, Sun D, Wang Z, Francis F, He K (2019) Differential wing polyphenism adaptation across life stages under extreme high temperatures in corn leaf aphid. Sci Rep 9:8744
Choudhury D (1984) Aphid honeydew: a re-appraisal of the hypothesis of Owen and Wiegert. Oikos 45(2):287–290
Costello M, Altieri MA (1995) Abundance, growth rate and parasitization of Brevicoryne brassicae and Myzus persicae (Hom.: Aphididae) on broccoli growth in living mulshes. Agric Ecosyst Environ 52:187–196
de Barro PJ (1992) The role of temperature, photoperiod, crowding and plant quality on the production of alate viviparous females of the bird cherry-oat aphid, Rhopalosiphum padi. Entomol Exp Appl 65(3):205–214
de Faria MR, Wraight SP (2007) Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control 43:237–256
de Mendoza AH, Belliure B, Carbonell EA, Real V (2001) Economic thresholds for Aphis gossypii (Hemiptera: Aphididae) on Citrus clementina. J Econ Entomol 94(2):439–444
de Mendoza AH, Arouni R, Belliure B, Carbonell EA, Pérez-Panadés J (2006) Intervention threshfduolds for Aphis spiraecola (Hemiptera: Aphididae) on Citrus clementina. J Econ Entomol 99(4):1273–1279
Deguine JP, Goze E, Leclant F (2000) The consequences of late outbreaks of the aphid Aphis gossypii in cotton growing in Central Africa: towards a possible method for the prevention of cotton stickiness. Int J Pest Manage 46(1):85–89
Dent D (2000) Insect pest management, 2nd edn. CABI Publishing, Wallingford, p 410
Depa L, Węgierek P (2011) Aphid fauna (Sternorrhyncha, Aphidinea) in the nests of Lasius flavus (Fabricius, 1781) (Hymenoptera: Formicidae) of various plant communities. Aphids Other Hemipterous Insects 17:73–79
Dewar AM, Denholm I (2017) Chemical control. In: van Emden HF, Harrington R (eds) Aphids as crop pests. Oxford University Press, Oxford, pp 398–425
Dhakal R, Ghimire R, Sapkota M, Thapa S, Bhatta AK, Regmi R (2019) Bioefficacy of different insecticides on cowpea aphid (Aphis craccivora Koch). Int J Entomol Res 7(1):1–7
Diaz BM, Fereres A (2005) Life table and population parameters of Nasonovia ribisnigri (Homoptera: Aphididae) at different constant temperatures. Environ Entomol 34:527–534
Dika J, Van Pelt JA (1992) Interaction between phyllosphere yeasts, aphid honeydew and fungicide effectiveness in wheat under field conditions. Plant Pathol 41(6):661–675
Dixon AFG (1977) Aphid ecology: life cycles, polymorphism and population regulation. Annu Rev Ecol Evol Syst 8:329–353
Dixon AFG (1985) Aphid ecology. Blackie, Glasgow, London, p 157
Dixon AFG (1998) Aphid ecology: an optimization approach. Chapman and Hall, London, p 300
Dixon AFG (2000) Insect predator-prey dynamics, ladybird beetles and biological control. Cambridge University Press, Cambridge, p 257
Dixon AFG, Agarwala BK (1999) Ladybird-induced life-history changes in aphids. Philos Trans R Soc B 266:1549–1553
Dixon AFG, Hemptinne JL (2001) Body size distribution in predatory ladybird beetles reflects that of their prey. Ecology 82:1847–1856
Dixon AFG, Horth S, Kindlmann P (1993) Migration in insects: cost and strategies. J Anim Ecol 62:182–190
Dogimont C, Bendahmane B, Chovelon V, Boissot N (2010) Host-plant resistance to aphids in cultivated crops: genetic and molecular bases, and interactions with aphid populationsLa résistance des plantes cultivées aux pucerons: bases génétiques et moléculaires et interaction avec les populations de pucerons. C R Biol 333(6–7):566–573
Dotasara SK, Agrawal N, Singh N, Swami D (2017) Efficacy of some newer insecticides against mustard aphid Lipaphis erysimi (Kalt.) in cauliflower. J Entomol Zool Stud 5(2):654–656
Douglas AE (1998) Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu Rev Entomol 43:17–37
Douglas AE, van Emden HF (2017) Nutrition and symbiosis. In: van Emden HF, Harrington R (eds) Aphids as crop pests, 2nd edn. CABI, Wallingford, pp 114–131
Du Y, Poppy GM, Powell W, Pickett JA, Wadhams LJ, Woodcock CM (1998) Identification of semiochemicals released during aphid feeding that attracts parasitoid Aphidius ervi. J Chem Ecol 24:1355–1368
Dubey S, Singh R (2008) Host-plant induced variations in life-table statistics of Aphis spiraecola patch (Homoptera: Aphididae). J Aphidol 23:53–60
Dubey VK, Yadu YK (1998) Determination of economic threshold level of aphid (Lipaphis erysimi Kalt.) on mustard crop. J Soils Crops 8(2):123–126
Eastop VF (1977) World wide importance of aphids as virus vectors. In: Harris F, Marmorosch K (eds) Aphids as virus vectors. Academic Press, London, pp 4–62
El-Heneidy AH, Ibraheem MM, Megahed HE, Attia AA, Magdy AA, Abdel-Awal WM, Hassan MM (2003) Assessment of economic injury and threshold levels for key cereal aphid species in Egyptian wheat regions. Bull Entomol Soc Egypt Econ Ser 29:43–56
El-Sayed, A.M., 2019. The pherobase: database of insect pheromones and semiochemicals. http://www.pherobase.com
Evans JW (1956) Palaeozoic and Mesozoic Hemiptera (Insecta). Aust J Zool 4(2):165–258
Farid A, Johnson JB, Shafii B, Quisenberry SS (1998a) Tritrophic studies of Russian wheat aphid, a parasitoid, and resistant and susceptible wheat over three parasitoid generations. Biol Control 12:1–6
Farid A, Quisenberry SS, Johnson JB, Shafii B (1998b) Impact of wheat resistance on Russian wheat aphid and a parasitoid. J Ecol Entomol 91:334–339
Farooq A, Tasawar Z (2008) Evaluation of integrated management of aphid pests, Brevicoryne brassicae and Lipaphis erysimi on canola crop in southern Punjab, Pakistan. Pak J Zool 40(1):13–17
Favret C, Eades DC (2020). www.aphid.speciesfile.org retrieved on 31 March, 2020
Felton GW, Eichenseer H (1999) Herbivore saliva and its effects on plant defense against herbivores and pathogens. In: Agrawal AA, Tuzun S, Bent E (eds) Induced plant defenses against pathogens and herbivores. Biochemistry, ecology, and agriculture. APS Press, St Paul, pp 19–36
Fereres A, Irwin ME, Kampmeier GE (2017) In: van Emden HF, Harrington R (eds) Aphids as crop pests, 2nd edn. CABI, Wallingford, pp 196–224
Flatt T, Weisser WW (2000) The effects of mutualistic ants on aphid life history traits. Ecology 81(12):3522–3529
Forsman A, Ahnesjö J, Caesar S, Karlsson M (2008) A model of ecological and evolutionary consequences of color polymorphism. Ecology 89:34–40
Foster WA (2002) Aphid sex ratios. In: Hardy ICW (ed) Sex ratios: concepts and research methods. Cambridge University Press, Cambridge, pp 254–265
Fuentes-Contreras E, Niemeyer HM (1998) DIMBOA-glucoside, a wheat chemical defense, affects host acceptance and suitability of Sitobion avenae (Hemiptera: Aphididae) to the cereal aphid parasitoid Aphidius rhopalosiphi (Hymenoptera: Braconidae). J Chem Ecol 24:371–381
Fuentes-Contreras E, Niemeyer HM (2000) Effects of wheat resistance, the parasitoid Aphidius rhopalosiphi, and the entomopathogenic fungus Pandora neoaphidis, on population dynamics of the cereal aphid Sitobion avenae. Entomol Exp Appl 97:109–114
Gangasaran, Giri J (1986) Growth and yield of mustard as influenced by irrigation and plant population. Ann Agric Res 7(1):68–74
Gehrer L, Vorburger C (2012) Parasitoids as vectors of facultative bacterial endosymbionts in aphids. Biol Lett 8:613–615
Ghosh AK (1980) The Fauna of India and adjacent countries. Homoptera, Aphidoidea. I. Subfamily Chaitophorinae. Zoological Survey of India, Kolkata, p 124
Ghosh LK, Rajendran TP (1988) First record of an aphid sexual (Homoptera: Aphididae) from Rajasthan, India. J Aphidol 2:51–58
Ghosh MR, Raychaudhuri DN (1980) Some aspects of population ecology of aphids (Homoptera:Aphididae) in India. Bull Entomol 19:25–44
Ghosh LK, Singh R (2000) Biodiversity of Indian insects with special reference to aphids (Homoptera: Aphididae). J Aphidol 14:113–123
Ghosh A, Chakrabarti S, Mandal B, Krishna Kumar NK (2017) Aphids as vectors of the plant viruses in India. In: Mandal B, Rao G, Baranwal V, Jain R (eds) A century of plant virology in India. Springer, Singapore, pp 515–536
Gibson RV, Pickett JA (1983) Wild potato repels aphids by release of aphid alarm pheromone. Nature 302:608–609
Gilkson LA (1990) Biological control of aphids in glasshouse sweet peppers and tomatoes. SROP/WPRS Bull 13(5):64–70
Girma H, Rao MR, Sithanantham S (2000) Insect pests and beneficial arthropod populations under different hedgerow intercropping systems in semiarid Kenya. Agrofor Syst 50(3):279–292
Godwal B (2010) Population dynamics and varietal preference of aphid, Aphis craccivora Koch on Indian bean, Lalab purpureus (Linn.) Sweet. Ph.D. thesis, S.K. Rajasthan Agricultural University, Bikaner, Campus – Jobner
Goettel MS, Eilenberg J, Glare T (2005) Entomopathogenic fungi and their role in regulation of insect populations. In: Gilbert LI, Iatrou K, Gill SS (eds) Comprehensive molecular insect science, Control, vol 6. Elsevier, Amsterdam, pp 361–405
Goggin FL, Williamson VM, Ullman DE (2001) Variability in the response of Macrosiphum euphorbiae and Myzus persicae (Hemiptera: Aphididae) to the tomato resistance gene mi. Environ Entomol 30:101–106
Gols R, Wagenaar R, Bukovinszky T, van Dam NM, Dicke M, Bullock JM, Harvey JA (2008) Genetic variation in defense chemistry in wild cabbages affects herbivores and their endoparasitoids. Ecology 89:1616–1626
González-Mas N, Cuenca-Medina M, Gutiérrez-Sánchez F, Quesada-Moraga E (2019) Bottom-up effects of endophytic Beauveria bassiana on multitrophic interactions between the cotton aphid, Aphis gossypii, and its natural enemies in melon. J Pest Sci 92:1271–1281
Grasswitz TR, Paine TD (1993) Influence of physiological state and experience on the responsiveness of Lysiphlebus testaceipes (Cresson) (Hymenoptera, Aphididae) to aphid honeydew and to host-plants. J Insect Behav 6:511–528
Guerrieri E, Digilio MC (2008) Aphid-plant interactions: a review. J Plant Interact 3(4):223–232
Guerrieri E, Pennacchio F, Tremblay E (1993) Flight behaviour of the aphid parasitoid Aphidius ervi Haliday (Hymenoptera: Braconidae) in response to plant and host volatiles. Eur J Entomol 90:415–421
Guo J, Hattab S, He K, Chen J, Francis F, Wang Z (2017) Nine facultative endosymbionts in aphids. Review J Asia-Pacific Entomol 20(3):794–801
Hågvar EB, Hofsvang T (1990) The aphid parasitoid Ephedrus cerasicola, a possible candidate for biological control in glasshouses. SROP/WPRS Bull XIII(5):87–90
Hamilton WD (1967) Extraordinary sex ratios. Science 156:477–488
Hance T, Delannoy O, Foucart G (1994) The screening of maize resistance to aphids as a contribution to integrated pest management. In: Struik PC, Vredenberg WJ, Renkema JA, Parlevliet JE (eds) Plant production on the threshold of a new century. Developments in plant and soil sciences, vol 61. Springer, Dordrecht, pp 407–409
Hance T, Kohandani-Tafresh F, Munaut F (2017) Biological control. In: van Emden HF, Harrington R (eds) Aphids as crop pests. Oxford University Press, Oxford, pp 448–493
Hansen LM (2000) Establishing control threshold for bird cherry-oat aphid (Rhopalosiphum padi L.) in spring barley (Hordeum vulgare L.) by aphid-days. Crop Prot 19(3):191–194
Hansen LM (2003) Kornbladlus (Sitobion avenae) i vinterhvede-et beslutnings-stöttesystem. Mark Theory 280:2–6
Hardie J (2017) Life cycle and polyphenism. In: van Emden HF, Harrington R (eds) Aphids as crop pests, 2nd edn. CABI, Wallingford, pp 81–97
Harrewijn P (1978) The role of plant substances in polymorphism of the aphid Myzus persicae. Entomol Exp Appl 24:198–214
Harris KF, Maramorosch K (1977) Aphids as virus vectors. Academic Press, New York, p 576
Hatano E, Kunert G, Weisser WW (2010) Aphid wing induction and ecological costs of alarm pheromone emission under field conditions. PLoS One 5:e11188
Hazell S, Gwynn D, Ceccarelli S, Fellowes M (2005) Competition and dispersal in the pea aphid: clonal variation and correlations across traits. Ecol Entomol 30:293–298
Heie OE (1987) Palaeontology and phylogeny. In: Harrewijn P, Minks AK (eds) Aphids: their biology, natural enemies and control. In the series world crop pests, vol 2A. Elsevier, Amsterdam, pp 367–391
Heie OE, Wegierek (2009) A classification of the Aphidomorpha (Hemiptera: Sternorrhyncha) under consideration of fossil taxa. Redia 92:69–77
Helden AJ, Dixon AFG (1997) Inbreeding and egg hatching success in Sitobion avenae. Ecol Entomol 22(1):124–126
Helden AJ, Dixon AFG, Carter N (1994) Environmental factors and morphological discrimination between spring and summer migrants of the grain aphid, Sitobion avenae (Homoptera: Aphididae). Eur J Entomol 91:23–28
Hemagirish MB, Goud KB, Mallapur CP (2001) Utilization of Chrysoperla carnea Stephens in the management of safflower aphid, Uroleucon compositae Theobald. Karnataka J Agric Sci 14(3):806–808
Hill CB, Li Y, Hartman GL (2004) Resistance to the soybean aphid in soybean germplasm. Crop Sci 44:98–106
Hille Ris Lambers D (1966) Polymorphism in Aphididae. Annu Rev Entomol 11:47–78
Hofsvang T, Hågvar EB (1980) Use of mummies of Ephedrus cerasicola Starý to control Myzus persicae (Sulzer) in small glass houses. J Appl Entomol 90:220–226
Hogervorst PAM, Wackers FL, Romeis J (2007) Effects of honeydew sugar composition on the longevity of Aphidius ervi. Entomol Exp Appl 122:223–232
Holz F, Wetzel T, Freier B (1994) Drei bis fünf Blattläuse pro Ähre im Winterweizen-eine neue Bekämp fungs schwelle? Gesunde Pflanzen 46(1):8–12
Hooks CRR, Fereres A (2006) Protecting crops from non-persistently aphid-transmitted viruses: a review on the use of barrier plants as a management tool. Virus Res 120:1–16
Hossain M, Ferdous J, Salim M (2008) Relative abundance and yield loss assessment of lentil aphid, Aphis craccivora Koch in relation to different sowing dates. J Agric Rural Dev 4(1):101–106
Howard LO (1929) Aphelinus mali and its travel. Ann Entomol Soc Am 22:341–368
Hu XS, Liu XF, Hu ZQ, Zhang YH, Zhao HY, Zhang GS (2011) The resistance of 10 wheat varieties to Sitobion avenae (Homoptera: Aphididae) in wheat seedlings phase in lab. Plant Prot 37:81–85
Huang MH, Caillaud MC (2012) Inbreeding avoidance by recognition of close kin in the pea aphid, Acyrthosiphon pisum. J Insect Sci 12:39
Hughes RD (1989) Biological control in the open field. In: Minks AK, Herrewijn P (eds) World crop pests, Aphids: their biology, natural enemies and control, vol C. Elsevier, Amsterdam, pp 167–198
Hussey NW, Scopes N (1985) Biological Pest control: the glasshouse experience. Blanferd Press, Pooe, Dorset, p 240
Isman MB, Miresmailli S, Machial C (2011) Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem Rev 10:197–204
Ivens ABF, Kronauer DJC, Pen I, Weissing FJ, Boomsma JJ (2012) Ants farm subterranean aphids mostly in single clone groups - an example of prudent husbandry for carbohydrates and proteins? BMC Evol Biol 12:106
Jain PC, Yadava CPS (1986) Effect of dates of sowing on the incidence of insect pests of coriander. Indian J Agric Sci 56:56–59
Jakhmola SS (1986) Bionomics and management of key pests of pulses and oilseeds. In: Proceedings of the Nat. Con. Key Pests Agric. Crops, C. S. A. U., T. Kanpur, p 7
Jeon HY, Kang TJ, Kim HH, Yang CY, Kim DS (2008) Economic injury level of Myzus persicae (Homoptera: Aphididae) at Chinese cabbage. Korean J Appl Entomol 47(4):407–411
Johnson CG (1954) Aphid migration in relation to weather. Biol Rev 29:87–118
Johnson B (1966) Wing polymorphism in aphids III. The influence of the host-plant. Entomol Exp Appl 9:213–222
Johnson CG (1969) Migration and dispersal of insects by flight. Methuen & Co. Ltd., London, p 766
Joshi S, Ballal CR (2013) Syrphid predators for biological control of aphids. J Biol Control 27(3):151–170
Joshi S, Rabindra RJ, Rajendran TP (2010) Biological control of aphids. J Biol Control 24:185–202
Kalasariya RL (2016) Management of aphid, Lipaphis erysimi in mustard with different spray schedules. Indian J Plant Prot 44(1):16–23
Kaloshian I, Kinsey DE, Ullman DE, Williamson VM (1997) The impact of meul-mediated resistance in tomato on longevity, fecundity, and behavior of the potato aphid, Macrosiphum euphorbiae. Entomol Exp Appl 83:181–187
Kamath SP, Hugar PS (2001) Determination of economic threshold level of safflower aphid Uroleucon compositae (Theobald). Karnataka J Agric Sci 13(2):349–353
Kanrar S, Venkateswari J, Kirti PB, Chopra VL (2002) Transgenic Indian mustard (Brassica napus) with resistance to the mustard aphid (Lipaphis erysimi Kalt.). Plant Cell Rep 20:976–981
Kashyap RK, Bhanot JP (1987) Effect of different biochemical factors on the development of Myzus persicae (Sulzer) on various potato cultures. In: Labeyrie V, Fabres G, Lachaise D (eds) Insects-plants proceedings of 6th international symposium of insect-plant relationship (PAU, 1986). Junk Publishers, Dordrecht, Netherlands, Dr. W, p 398
Kawada K (1965) The development of winged forms in the cabbage aphid Brevicoryne brassicae Linnaeus II. The period of determination of wing development. Berichte de Ohara Inst 13(1):1–5
Kennedy FJH, Rajamanickam K, Raveendran TS (1990) Effect of Intercroppong on insect pests of groundnut and their natural enemies. J Biol Control 4:63–64
Kim JJ, Jeong G, Han JH, Lee S (2013) Biological control of aphid using fungal culture and culture filtrates of Beauveria bassiana. Mycobiology 41(4):221–224
Kislow CJ, Edwards LJ (1972) Repellent odours in aphids. Nature 235:108–109
Kogan M, Jepson P (2007) Perspectives in ecological theory and integrated pest management. Cambridge University Press, Cambridge, p 570
Komatsu T, Akimoto S (1995) Genetic differentiation as a result of adaptation to the phenologies of individual host trees in the galling aphid Kaltenbachiella japonica. Ecol Entomol 20:33–42
Kring JB (1972) Flight behaviour of aphids. Annu Rev Entomol 17:461–492
Krishna G, Kumar S, Singh BP, Kumari M (2008) Evaluation of different insecticides and intercropping against Myzus persicae (Sulzer) (Homoptera: Aphididae). J Aphidol 22(1&2):21–24
Krishna G, Kumar S, Singh BP, Kumari M (2009) Impact of different insecticides and inter cropping against Lipaphis erysimi (Kalt.) (Homoptera: Aphididae) on cauliflower. J Aphidol 23(1&2):77–80
Kulkarni NS (2016) Loss estimation and economic threshold level for aphids (Acyrthosiphon pisum Harris) in lucerne. Range Manage Agroforestry 37(1):113–115
Kumawat KC, Khinchi SK (2016) Estimation of economic decision levels of aphid, Aphis craccivora Koch on cowpea, Vigna unguiculata (Linn.) Walp. Resonance 5(3):6–9
Kunert G, Weisser WW (2005) The importance of antennae for pea aphid wing induction in the presence of natural enemies. Bull Entomol Res 95:125–131
Kunert G, Otto S, Rose USR, Gershenzon J, Weisser WW (2005) Alarm pheromone mediates production of winged dispersal morphs in aphids. Ecol Lett 8:596–603
Lal SS, Yadav CP, Dias CAR (1989) Effect of planting density and chickpea cultivars on the infestation of black aphid, Aphis craccivora Koch. Madras Agric J 76:461–462
Lamb RJ, Pointing PJ (1972) Sexual morph determination in the aphid, Acyrthosiphon pisum. J Insect Physiol 18:2029–2042
Larsson H (1986) Damage threshold for aphids in barley and winter wheat, weed and plant protection conference 1986, Växtskyddsrapporter, Jordbruk 39. Swedish University of Agricultural Sciences, pp 201–210
Larsson H (1991) Economic importance of cereal aphids of different cost levels. In: Proceedings of the 4th Swedish crop protection conference, Uppala, 30–31 January 1991
Larsson H (2005) A crop loss model and economic thresholds for the gain aphid, Sitobion avenae (F.), in winter wheat in southern Sweden. Crop Prot 24:397–405
Lees AD (1966) The control of polymorphism in aphids. Adv Insect Physiol 3:207–277
Lees AD (1967) The production of the apterous and alate forms in the aphid Megoura viciae Buckton, with special reference to the role of crowding. J Insect Physiol 13:289–318
Levie A, Legrand MA, Dogot P, Pels C, Baret PV, Hance T (2005) Mass releases of Aphidius rhopalosiphi (Hymenoptera: Aphidiinae), and strip management to control of wheat aphids. Agric Ecosyst Environ 105:17–21
Li Y, Hill C, Carlson S, Diers B, Hartman G (2007) Soybean aphid resistance genes in the soybean cultivars Dowling and Jackson map to linkage group M. Mol Breed 19:25–34
Li-Jiping JS, Guonfang H, AnMing W, Jp L, Sl J, Gf H, Ann W (1995) A preliminary study on population dynamics and economic threshold of wheat aphids in Gangy County, Gansu province. Plant Prot 21(2):2–4
Linda L, Walling LL (2008) Avoiding effective defenses: strategies employed by phloem-feeding insects. Plant Physiol 146(3):859–866
Liu XL, Yang XF, Wang CY, Wang YJ, Zhang H, Ji WQ (2012) Molecular mapping of resistance gene to English grain aphid (Sitobion avenae F.) in Triticum durum wheat line C273. Theor Appl Genet 124:287–293
Loginova, E., Atanassov, N., Georgiev, G., 1987. Biological control of pests and diseases in glasshouses in Bulgaria today and in the future. SROP/WPRS Bulletin X/2, 101–105
López Pérez M, Fernández Argudín M, Powell W (2007) Foraging behaviour of the parasitoid Lysiphlebus testaceipes (Hymenoptera: Braconidae) in response to plant volatiles, with reference to biocontrol of aphids in peri-urban vegetable production systems. Biocontrol Sci Tech 17:677–686
Losey JE, Harmon J, Ballantyne F, Brown C (1997) A polymorphism maintained by opposite patterns of parasitism and predation. Nature 388:269–272
Łukasik P, van Asch M, Guo H, Ferrari J, Godfray HCJ (2013) Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol Lett 16:214–218
Lv L, Chen R (1993) Study on the production of alatae in soybean aphid Aphis glycines. Acta Entomol Sin 36:143–149
Mackauer M (1971) Acyrthosiphon pisum (Harris), pea aphid (Homoptera: Aphididae). In: Biological control programmers against insects and weeds in Canada (1959–1968), 4. Technical Communication, pp 3–10
Mahr S (2018) Aphids. https://wimastergardener.org/article/aphids, published on October 8
Maina UM, Galadima IB, Gambo FM, Zakaria D (2018) A review on the use of entomopathogenic fungi in the management of insect pests of field crops. J Entomol Zool Stud 6(1):27–32
Manjula TR, Kannan GS, Sivasubramanian P (2018) Field efficacy of Pseudomonas fluorescens against the cotton aphid, Aphis gossypii glover (Hemiptera: Aphididae) in Bt and non Bt cotton. Int J Curr Microbiol App Sci 6:11–24
Marchi-Werle L, Baldin ELL, Fischer HD, Heng-Moss TM, Hunt TE (2017) Economic injury levels for Aphis glycines Matsumura (Hemiptera: Aphididae) on the soybean aphid tolerant KS4202 soybean. J Econ Entomol 110(5):2100–2108
Markkula M, Tittanen K (1985) Biology of the midge Aphidoletes and its potential for biological control. In: Hussey NW, Scopes NEA (eds) Biological Pest control - the glasshouse experience. Blandford, Poole, Dorset, pp 74–81
Martin B, Rahbe Y, Fereres A (2003) Blockage of stylet tips as the mechanism of resistance to virus transmission by Aphis gossypii in melon lines bearing the vat gene. Ann Appl Biol 142:245–250
Martínez AFR, Costamagna AC (2018) Effects of crowding and host-plant quality on morph determination in the soybean aphid, Aphis glycines. Entomol Exp Appl 166:53–62
Matharu KS, Tanwa PS (2019) Efficacy of different insecticides and biopesticide against wheat aphid. J Entomol Zool Stud 7(3):521–524
Matsuka M, Mittler TE (1978) Enhancement of alata production by an aphid, Myzus persicae, in response to increase in daylength. Bull Fac Agric Tamagawa Univ Tokyo 18:1–7
McCarville MT, Kanobe C, Macintosh GC, O’neal M (2011) What is the economic threshold of soybean aphids (Hemiptera: Aphididae) in enemy-free space? J Econ Entomol 104(3):845–852
Medda PK, Sarkar S, Chakrabarti S (1997) Willow infesting aphids (Homoptera: Aphididae) of India and adjacent countries. J Aphidol 11(1):83–97
Mehrparvar M, Zytynska SE, Weisser WW (2013) Multiple cues for winged morph production in an aphid metacommunity. PLoS One 8:e58323
Meier MS, Hilbeck A (2001) Influence of transgenic Bacillus thuringiensis corn-fed prey on prey preference of immature Chrysoperla carnea (Neuroptera: Chrysopidae). Basic Appl Ecol 2:35–44
Meyling NV, Eilenberg J (2007) Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: potential for conservation biological control. Biol Control 43:145–155
Miles PW (1999) Aphid saliva. Biol Rev 74(1):41–85
Minks AK, Harrewijn P (1987) Aphids: their biology, natural enemies and control, vol A. Elsevier Science Publisher B.V., Amsterdam, p 450
Mittler TE (1973) Aphid polymorphism as affected by diet. In: Lowe AD (ed) Perspectives in aphid biology, Bull. No. 2. Entromological Society, pp 65–75
Mittler TE, Sutherland ORW (1969) Dietary influences on aphid polymorphism. Entomol Exp Appl 12:703–713
Mittnacht A (1986) Blattlausbekämpfung in Winterweizen nach Schaden-schwellen. Gesunde Pflanzen 38(4):186–189
Miyazaki M (1987) Forms and morphs of aphids. In: Minks AK, Harrewijn P (eds) Aphids: their biology, natural enemies and control, vol 2A. Elsevier, Amsterdam, pp 27–50
Mondor EB, Roitberg BD (2000) Has the attraction of predatory coccinellids to cornicle droplets constrained aphid alarm signaling behavior? J Insect Behav 13:321–329
Mondor EB, Rosenheim JA, Addicott JF (2005) Predator-induced transgenerational phenotypic plasticity in the cotton aphid. Oecologia 142:104–108
Montllor CB, Maxmen A, Purcell AH (2002) Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol Entomol 27:189–195
Morales I, Diaz BM, de Mendoza AH, Nebreda M, Fereres A (2013) The development of an economic threshold for Nasonovia ribisnigri (Hemiptera: Aphididae) on lettuce in Central Spain. J Econ Entomol 106(2):891–898
Moran NA (1992) The evolution of aphid life-cycles. Annu Rev Entomol 37:321–348
Moran NA, Jarvik T (2010) Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 328(5978):624–627
Moran NA, Munson MA, Baumann P, Ishikawa H (1993) A molecular clock in endosymbiotic bacteria is calibrated using the insect hosts. Proc R Soc Lond B 253:167–171
Müller FP (1962) Biotypen und Unterarten der Erbsenlaus’ Acyrthosiphon pisum (Harris). Z Pflanzenkrankh Pflanzenschutz 69:129–136
Müller CB, Williams IS, Hardie J (2001) The role of nutrition, crowding and interspecific interactions in the development of winged aphids. Ecol Entomol 26:330–340
Mumford JD, Baliddawa CW (1983) Factors governing insect occurrence in various cropping systems. Insect Sci Appl 4:59–64
Naga KC, Kumawat KC (2015) Estimation of economic decision levels of aphid, Acyrthosiphon pisum (Harris) on Fenugreek, Trigonella foenum-graecum Linn. Ann Plant Prot Sci 23(1):37–42
Nebreda M (2005) Dinámica poblacional de insectos homópteros en cultivos de lechuga y bróculi, identificación de parasitoides asociados y evaluación de alternativas fisicas de control. Thesis. Universidad Complutense de Madrid, Madrid, Spain
Nevo E, Coll M (2001) Effect of nitrogen fertilization on Aphis gossypii (Homoptera: Aphididae): variation in size, color, and reproduction. J Econ Entomol 94(1):27–32
Ng JCK, Perry KL (2004) Transmission of plant viruses by aphid vectors. Mol Plant Pathol 5(5):505–511
Noda I (1958) The emergence of winged viviparous female in aphid. III. Critical period of determination of wing development in Rhopalosiphum prunifoliae. Jpn J Entomol 2:53–58
Norris RF, Caswell-Chen EP, Kogan M (2003) Concepts in integrated Pest management. Prentice Hall, Upper Saddle River, p 586
Obrycki JJ, Kring TJ (1998) Predaceous coccinellidae in biological control. Annu Rev Entomol 43:295–321
Obrycki JJ, Tauber MJ (1984) Natural enemy activity on glandular pubescent potato plants in the green house: an unreliable predictor of effects in the field. Environ Entomol 13:679–683
Ogenga-Latigo MW, Baliddawa CW, Ampofo JK (1993) Factors influencing the incidence of the black bean aphid Aphis fabae Scop., on common beans intercropped with maize. Afr Crop Sci J 1(1):49–58
Oliver KM, Russell JA, Moran NA, Hunter MS (2003) Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci USA 100:1803–1807
Oliver KM, Degnan PH, Burke GR, Moran NA (2010) Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55:247–266
Omkar, Pervez A (2002) Ecology of aphidophagous ladybird beetle, Coccinella septempunctata Linn. (Coleoptera: Coccinellidae). J Aphidol 16:175–201
Omoigui L, Ekeuro G, Kamara A, Bello L, Timko M, Ogunwolu G (2017) New sources of aphids [Aphis craccivora (Koch)] resistance in cowpea germplasm using phenotypic and molecular marker approaches. Euphytica 213(8):178–192
Owen DF, Wiegert RG (1976) Do consumers maximize plant fitness? Oikos 27:488–492
Pal M, Singh R, Srivastava PN (2008) Thermal influence on the life-table statistics of the cabbage aphid, Brevicoryne brassicae (Linn.) (Homoptera: Aphididae). J Aphidol 22:73–80
Paliwal D (2017) Identification and characterisation of new aphid killing bacteria for use as biological pest control agents. PhD thesis, University of Reading
Pappas ML, Broufas GD, Koveos DS (2011) Chrysopid predators and their role in biological control. J Entomol 8:301–326
Parr WJ, Scopes NEA (1970) Problems associated with biological control of glasshouse pests. NAAS Quarterly Rev 89:113–121
Parrella MP (2008) Biological control in protected culture: will it continue to expand? Phytoparasitica 36:3–6
Parry HR (2013) Cereal aphid movement: general principles and simulation modeling. Mov Ecol 1(1):14
Partridge M, Borden JH (1997) Evaluation of neem seed extract for control of the spruce aphid, Elatobium abietinum (Walker) (Homoptera: Aphidae). Can Entomol 129:899–906
Pawar AD (2002) Integrated Pest management package for Citrus, IPM Package No. 28. Directorate of Plant Protection, Quarantine & Storage, Government of India, New Delhi, pp 1–26
Pedigo LP, Hutchins SH, Higley LG (1986) Economic injury levels in theory and practice. Ann Teview Entomol 31:341–368
Pettersson J, Tjallingii WF, Hardie J (2017) In: van Emden HF, Harrington R (eds) Aphids as crop pests, 2nd edn. CABI, Wallingford, pp 173–195
Pickett JA, Griffiths DC (1980) Composition of aphid alarm pheromones. J Chem Ecol 6:349–360
Pickett JA, Wadhams LJ, Woodcock CM, Hardie J (1992) The chemical ecology of aphids. Annu Rev Entomol 37:67–90
Pickett JA, Bruce TJA, Glinwood RT (2017) Chemical ecology. In: van Emden HF, Harrington R (eds) Aphids as crop pests, 2nd edn. CABI, Wallingford, pp 148–172
Polgar L (1987) Induced diapause for long term storage of Aphidius matricariae. SROP/WPRS Bull 10:152–154
Popov NA, Belousov YV, Zabudskaya IA, Khudyakova OA, Shevtscenko VB, Shijko ES (1987) Biological control of glasshouse pests in the South of the USSR. SROP/WPRS Bull X/2:155–157
Potts MJ, Gunadi N (1991) The influence of intercropping with Allium on some insect populations in potato (Solanum tuberosum). Ann Appl Biol 119:207–213
Pramanik P, Bandyopadhyay KK, Bhaduri D, Bhattacharya D, Aggarwal P (2015) Effect of mulch on soil thermal regimes - a review. Int J Agric Environ Biotechnol 8(3):645–658
Prasad D, Singh KM, Katiyar RN, Singh RN (1987) Impact of intercropping on the plant growth, pest incidence and crop yield of pea, Pisum sativum Linn. Ind J Entomol 49:153–172
Price PW (1986) Ecological aspects of host-plant resistance and biological control: interactions among three trophic levels. In: Boethal DJ, Eikenbary RD (eds) Interactions of plant resistance and parasitoids and predators of insects. Wiley, New York, pp 11–30
Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weise AE (1980) Interaction among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Evol Syst 11:41–65
Purandare SR, Bickel RD, Jaquiery J, Rispe C, Brisson JA (2014) Accelerated evolution of morph-biased genes in pea aphids. Mol Biol Evol 31:2073–2083
Puritch GS (1981) Pesticidal soaps and adjuvants-what are they and how do they work? In: Proceedings of the 23rd annual lower mainland horticultural improvement association growers short course, Abbotsford, B.C., February 11–13
Rabasse JM, Lafont JP, Delpuech I, Silvie P (1983) Progress in aphid control in protected crops. SROP/WPRS Bull VI/3:151–162
Ragsdale DW, Mccornack BP, Venette RC, Potter BD, Macrae IV, Hodgson EW, O’neal ME, Johnson KD, O’neil RJ, Difonzo CD, Hunt TE, Glogoza PA, Cullen EM (2007) Economic threshold for soybean aphid (Hemiptera: Aphididae). J Econ Entomol 100(4):1258–1267
Ramakers PLJ (1989) Biological control in green house. In: Mink AK, Harriwijn P (eds) World crop Pest: aphids their biology, natural enemies and control, vol C. Elsevier, Amsterdam, pp 199–208
Raman KV (1985) Transmission of potato viruses by aphids, Technical Information Bulletin 2. International Potato Center (CIP), Lima-Peru, p 23
Raps A, Kehr J, Gugerli P, Moar WJ, Bigler F, Hilbeck A (2001) Detection of Cry1Ab in phloem sap of Bacillus thuringiensis corn and in the non-target herbivores Rhopalosiphum padi (Homoptera: Aphididae) and Spodoptera littoralis (Lepidoptera: Noctuidae). Mol Ecol 10:525–533
Rashid MH, Khan A, Hossain MT, Chung YR (2017) Induction of systemic resistance against aphids by endophytic Bacillus velezensis YC7010 via expressing Phytoalexin Deficient4 in Arabidopsis. Front Plant Sci 8:211
Regar R, Kumawat KC, Khinch SK (2016) Estimation of economic decision levels of aphid, Aphis craccivora Koch on cowpea, Vigna unguiculata (Linn.) Walp. (grown for vegetable). Asian Resonance 5(3):6–9
Rehner SA (2005) Phylogenetics of the insect pathogenic genus Beauveria. In: Vega FE, Blackwell M (eds) Insect-fungal associations: ecology and evolution. Oxford University Press, Oxford, pp 3–27
Remaudiere G, Remaudiere M (1997) Catalogue des Aphididae du monde/catalogue of the world’s Aphididae. Homoptera Aphidoidea. INRA Editions, Paris, p 474
Rios Martinez AF, Costamagna AC (2017) Dispersal to predator-free space counterweighs fecundity costs in alate aphid morphs. Ecol Entomol 42:645–656
Robert P, Riba G (1989) Toxic and repulsive effects of spray, ‘per os’ and systemic applications of destruxin E to aphids. Mycopathologia 108:179–183
Robert L, Burton D, Simon K, Starks J, Robert M (1985) Seasonal damage by green bugs (Homoptera: Aphididae) to a resistant and a susceptible variety of wheat. J Econ Entomol 78:395–401
Rocca M, Messelink GJ (2017) Combining lacewings and parasitoids for biological control of foxglove aphids in sweet pepper. J Appl Entomol 141:402–410
Rodríguez M, Marín A, Torres M, Béjar V, Campos M, Sampedro I (2018) Aphicidal activity of surfactants produced by Bacillus atrophaeus L193. Front Microbiol 9:3114
Rodriguez-Saona C, Blaauw BR, Isaacs R (2012) Manipulation of natural enemies in agroecosystems: habitat and semiochemicals for sustainable insect pest control. In: Larramendy M, Soloneski S (eds) Integrated Pest management and Pest control – current and future tactics. Intech, Rijeka, Croatia, pp 89–126
Sabbour MM (2019) Effect of destruxin on the population reduction of green peach aphid Myzus persicae (Hemiptera: Aphididae) and the predator Coccinella undecimpunctata (Coleoptera: Coccinellidae) in tomato fields. Bull Nat Res Centre 43:132
Sachan GC (1990) Problem of insect pests in brassicas and research work at Pantnagar. In: Omran A (ed) Proceedings of the IDRC three meetings held at Pantnagar and Hyderabad, India, 4–17 January, 1989, pp 56–65
Sachan GC (1997) Cultural control of aphids: a review and bibliography. J Aphidol 11(1):25–35
Samota RP, Kumawat KC, Samota RG (2014) Economic decision for levels of aphid, Myzus persicae (Sulz.) on cumin, Cuminum cyminum Linn. Ann Biol 30(4):738–742
Samuel A, Ofuya T, James PO (2006) Effects of watering regimes on aphid infestation and performance of selected varieties of cowpea (Vigna unguiculata L. Walp) in a humid rainforest zone of Nigeria. Crop Prot 25(1):73–78
Sandström JP, Russell JA, White JP, Moran NA (2001) Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol Ecol 10:217–228
Sasso R, Iodice L, Digilio MC, Carretta A, Ariati L, Guerrieri E (2007) Host locating response by the aphid parasitoid Aphidius ervi to tomato plant volatiles. J Plant Interact 2:175–183
Saunakiya AK, Tiwari N (2014) Economic injury and threshold level of Lipaphis erysimi (Kalt.). Int J Life Sci Res 2(4):178–184
Scarborough CL, Ferrari J, Godfray HCJ (2005) Aphid protected from pathogen by endosymbiont. Science 310:1781
Schoonhoven LM, van Loon JJA, Dicke M (2007) Insect-plant biology. Oxford University Press, Oxford, p 448
Schuler TH, Denholm I, Jouanin L, Clark SJ, Clark AJ, Poppy GM (2001) Population-scale laboratory studies of the effect of transgenic plants on non-target insects. Mol Ecol 10:1845–1853
Seckbach J, Dubinsky Z (2011) All flesh is grass: plant-animal interrelationships. Springer Science & Business Media, Dordrecht, p 531
Seiter N (2018) Integrated pest management: what are economic thresholds, and how are they developed? Farmdoc Daily 8:197
Sekhon BS, Bakhetia DRC (1991) Economic threshold of mustard aphid, Lipaphis erysimi Kaltenbach. Proc 8th Int Rapeseed Congr 2:502–505
Sekhon SS, Sajjan SS, Kanta U (1980) Chemical control of mustard aphid, Lipaphis erysimi on seed crop of radish. Indian J Plant Prot 8(2):151–153
Sentis A, Bertram R, Dardenne N, Ramon-Portugal F, Louit I, Le Trionnaire G, Simon JC, Magro A, Pujol B, Hemptinne JL, Danchin E (2019) Different phenotypic plastic responses to predators observed among aphid lineages specialized on different host-plants. Sci Rep 9:9017
Shah FM, Razaq M, Ali A, Han P, Chen J (2017) Comparative role of neem seed extract, moringa leaf extract and imidacloprid in the management of wheat aphids in relation to yield losses in Pakistan. PLoS One 12(9):e0184639
Shan LT, Feng MG (2010) Evaluation of the biocontrol potential of various Metarhizium isolates against green peach aphid Myzus persicae (Homoptera: Aphididae). Pest Manag Sci 66:669–675
Shannag HS, Capinera JL, Freihat NM (2014) Efficacy of different neem-based biopesticides against green peach aphid, Myzus persicae (Hemiptera: Aphididae). Int J Agric Policy Res 2(2):61–68
Shaoyou L, Stoltz RL, Xinzhi N (1986) Damage to wheat by Macrosiphum avenae in Northwest China. J Econ Entomol 79:1688–1691
Shaposhnikov GC (1979) Late Jurassic and early cretaceous aphids. Paleontol J 1979(4):66–78
Sharma KK, Dutta SK, Borah BK (2000) Economic injury level of Aphis craccivora Koch in green gram var. AAU-34. Crop Res 23:463–468
Sharma HC, Bhagwat VR, Daware DG, Pawar DB, Munghate RS, Sharma SP, Kumar AA, Reddy BVS, Prabhakar KV, Ambekar SK, Gadakh SR (2014) Identification of sorghum genotypes with resistance to the sugarcane aphid Melanaphis sacchari under natural and artificial infestation. Plant Breed 133(1):36–44
Shaw MJP (1970) Effects of population density on alienicolae of Aphis fabae Scop. Ann Appl Biol 65:191–196
Shlyakhovoi NA, Bobonich VM (1975) Natural regulators of the numbers of pests. Zashchita Rastenii 5:31
Shukla A, Mishra VP (2010) Efficacy of insecticides against Lipaphis erysimi (Kalt.) and Myzus persicae (Sulz.) (Homoptera: Aphididae) on taramira (Eruca sativa Linn.). J Aphidol 24(1&2):33–36
Sidhu HS, Kaur P (1977) Influence of nitrogen application to the host-plant on the fecundity of mustard aphid, Lipaphis erysimi (Kalt.). J Res Punjab Agric Univ 14:445–448
Sigsgaard L, Enkegaard A, Eilenberg J, Kristensen K, Jensen NL (2013) Biological control of tortricids and aphids in strawberries, Pesticide research no. 150. The Danish Environmental Protection Agency, Denmark
Silva RJ, Alencar JR, Silva KP, Cividanes FJ, Duarte RT, Agostini LT, Polanczyk RA (2014) Interactions between the entomopathogenic fungi Beauveria bassiana (Ascomycota: Hypocreales) and the aphid parasitoid Diaeretiella rapae (Hymenoptera: Braconidae) on Myzus persicae (Hemiptera: Aphididae). J Econ Entomol 107(3):933–938
Singh V (1982) Effect of sowing time on incidence of corn leaf aphid, Rhopalosiphum maidis (Fitch.) on barley. Ind J Entomol 44:89–92
Singh R (2001) Biological control of the aphids by utilising parasitoids. In: Upadhyay RK, Mukerji KG, Chamola BP (eds) Biocontrol potential and its exploitation in sustainable agriculture, vol 2. Kulwer Academic/Plenum Publishers, USA, pp 57–73
Singh R (2003) Tritrophic interactions with reference to biological control of insect pests. Rev Biol Memoirs 29(2):55–70
Singh R (2015) Elements of entomology, 2nd edn. Rastogi Publications, Meerut, India, p 564
Singh R, Agarwala BK (1992) Biology, ecology and control efficiency of the aphid parasitoid Trioxys indicus: a review and bibliography. Biol Agric Hortic 8:271–298
Singh R, Ghosh S (2002) The glimpses of Indian aphids (Insecta: Hemiptera, Aphididae). Proc Natl Acad Sci Allahabad 72B:215–234
Singh R, Ghosh S (2012) Sexuales of aphids (Insecta: Homoptera: Aphididae) in India. Lap Lambert Academic Publishing, Saarbrücken, p 414
Singh SV, Malik YP (1998) Population dynamics and economic threshold of Lipaphis erysimi (Kaltenbach) on mustard. Ind J Entomol 60:43–49
Singh R, Rao SN (1995) Biological control of Aphis gossypii glover (Homoptera: Aphididae) on cucurbits by Trioxys indicus Subba Rao and Sharma (Hymenoptera: Aphidiidae). Biol Agric Hortic 12:227–236
Singh G, Singh G (1985) Effect of date of sowing on the appearance and abundance of Myzus persicae (Sulzer) and yield of taramira crop. Indian J Agric Sci 55:237–289
Singh K, Singh R (2015) Effect of temperature on the life history traits of Aphis gossypii glover (Homoptera: Aphididae) on bottle gourd, Lagenaria siceraria (Molina) Standl. (Cucurbitaceae). Int J Life Sci Biotechnol Pharma Rese 4(4):179–183
Singh R, Singh K (2015a) Life history parameters of Aphis gossypii glover (Homoptera: Aphididae) reared on three vegetable crops. Int J Res Stud Zool 1(1):1–9
Singh R, Singh G (2015b) Systematics, distribution and host range of Diaeretiella rapae (McIntosh) (Hymenoptera: Braconidae, Aphidiinae). Int J Res Stud Biosci 3(1):1–36
Singh R, Singh G (2016) Aphids and their biocontrol. In: Omkar (ed) Ecofriendly pest management for food security. Academic Press, London, pp 63–108
Singh G, Singh R (2016a) Food plant records of Aphidini (Aphidinae: Aphididae: Hemiptera) in India. J Entomol Zool Stud 5(2):1280–1302
Singh G, Singh R (2016b) Distribution of Aphis (Aphis) spiraecola patch 1914 (Aphidini: Aphidinae: Aphididae: Hemiptera) and its food plants recorded in India. Int J Recent Adv Multidiscip Res 3(12):2100–2111
Singh G, Singh R (2016c) Distribution and economic importance of Aphis (Aphis) craccivora Koch (Aphidini: Aphidinae: Aphididae: Hemiptera) and its food plants in India. J Recent Adv Multidiscip Res 4(2):2274–2289
Singh G, Singh R (2016d) Food plant records of aphids (Aphididae: Sternorrhyncha: Hemiptera) in India belonging to subfamilies Aiceoninae, Anoeciinae, Chaitophorinae and Drepanosiphinae. Int J Zool Investig 2(2):281–295
Singh G, Singh R (2017a) Updated checklist of food plants of Macrosiphini (Aphididae: Hemiptera) in India - 1. Int J Res Stud Zool 3(1):6–33
Singh G, Singh R (2017b) Updated checklist of food plants of Macrosiphini (Aphididae: Hemiptera) in India - 2. Int J Res Stud Zool 3(1):42–76
Singh G, Singh R (2017c) Updated checklist of food plants of Macrosiphini (Aphididae: Hemiptera) in India - 3. Int J Res Stud Zool 3(2):1–31
Singh G, Singh R (2017d) Updated checklist of Greenideinae (Aphididae: Hemiptera) and its host-plants in India. Int J Contemp Res Rev 8(3):20191–20219
Singh G, Singh R (2017e) Updated check-list of Indian Eriosomatinae (Aphidinae: Aphididae: Hemiptera) and their food plants. J Entomol Zool Stud 5(1):921–936
Singh G, Singh R (2017f) Updated checklist of host-plants of Calaphidinae (Aphididae: Hemiptera) in India. Int J Contemp Res Rev 8(2):20171–20190
Singh G, Singh R (2018) Updated check-list of Indian Hormaphidinae (Aphididae: Hemiptera) and their food plants. J Entomol Zool Stud 6(2):1345–1352
Singh R, Singh G (2019) Species diversity of Indian aphids (Hemiptera: Aphididae). Int J Biol Innov 1(1):27–33
Singh H, Rohilla HR, Kalra VK, Yadava TP (1984) Response of brassica varieties sown on different dates to the attack of mustard aphid, Lipaphis erysimi (Kalt). J Oilseeds Res 1:49–56
Singh H, Singh H, Rohilla HR, Singh D (1993) Integrated pest management in rapeseed-mustard crops in Haryana. National Seminar Oilseeds Research and Development in India-Status and Strategies, Aug. 2–5, 1993, Hyderabad, p 117
Singh RP, Yazdani SS, Verma GD, Singh VN (1995) Effect of different levels of nitrogen, phosphorus and potash on aphid infestation and yield of mustard. Ind J Entomol 57:18–21
Singh R, Biswas S, Pandey S (1996) Dietary role of honeydew on the life-table parameters of a cereal aphid parasitoid, Lysiphlebia mirzai Shuja-Uddin (Hymenoptera: Braconidae). J Appl Zool Res 7:102–103
Singh R, Singh K, Upadhyay BS (2000a) Honeydew as a food source for an aphid parasitoid Lipolexis scutellaris Mackauer (Hymenoptera: Braconidae). J Adv Zool 21:76–83
Singh R, Singh A, Pandey S (2000b) Ability to switch over alternative host-complexes by an aphid parasitoid Binodoxys indicus (Hymenoptera: Braconidae). Entomol Generalis 25:53–66
Singh G, Singh NP, Singh R (2014) Food plants of a major agricultural pest, Aphis gossypii glover (Homoptera: Aphididae) from India: an updated checklist. Int J Life Sci Biotechnol Pharm Res 3(2):1–26
Singh R, Singh G, Tiwari AK, Sharma A, Patel S, Pratibha (2015a) Myzus (Nectarosiphon) persicae (Sulzer, 1776) (Homoptera: Aphididae): updated check list of host-plants in India. Int J Zool Investig 1:8–25
Singh R, Singh G, Tiwari A, Agrawal R, Sharma A (2015b) Host-plant diversity of aphids (Homoptera: Aphididae) infesting Asteraceae in India. Int J Zool Investig 1(2):137–167
Singh G, Prasad M, Singh R (2018) Updated check-list of Lachninae, Lizeriinae, Mindarinae, Phyllaphidinae, Saltusaphidinae, Taiwanaphidinae and Thelaxinae (Aphididae: Hemiptera) and their food plants in India. J Entomol Zool Stud 6(2):3157–3166
Sinha R, Singh B, Rai PK, Kumar A, Jamwal S, Sinha BK (2018) Soil fertility management and its impact on mustard aphid, Lipaphis erysimi (Kaltenbach) (Hemiptera: Aphididae). Cogent Food Agric 4:1450941
Ślipiński P, Markó B, Rzeszowski K, Babik H, Czechowski W (2014) Lasius fuliginosus (Hymenoptera: Formicidae) shapes local ant assemblages. North-Western J Zool 10(2):404–412
Sloggett JJ, Weisser WW (2002) Parasitoids induce production of the dispersal morph of the pea aphid, Acyrthosiphon pisum. Oikos 98:323–333
Sridharan S, Venkatesan S, Prakasan V, Thamburaj S (1990) Influence of nitrogen fertilization on the incidence of sucking pests and pod borer in french bean. South Indian Hortic 38:226–227
Srinivasan K, Krishana Moorthy PN (1991) Indian mustard as a trap crop for management of Lepidopterous pests on cabbage. Trop Pest Manage 37:26–32
Srivastava A, Singh R (2008) Effect of host-plants on the life-table of Sitobion miscanthi (Takahashi) (Homoptera: Aphididae). J Aphidol 23:1–8
Stadler B, Dixon AFG (2005) Ecology and evolution of aphid-ant interactions. Annu Rev Ecol Evol Syst 36:345–372
Stadler B, Muller T (1996) Aphid honeydew and its effect on the phyllosphere microflora of Picea abies (L.) Karst. Oecologia 108(4):771
Stam PA, Abdelrahman AA, Munir B (1994) Comparisons of control action thresholds for Heliothis armigera, Bemisia tabaci and Aphis gossypii on cotton in the Sudan Gezira and Rahad regions. Crop Prot 13(7):503–512
Stapleton JJ, Summers CG (2002) Reflective mulches for management of aphids and aphid-borne virus diseases in late-season cantaloupe (Cucumis melo L. var. cantalupensis). Crop Prot 21:891–898
Stark JD, Rangus TM (1994) Lethal and sublethal effects of the neem insecticide formulation, ‘Margosan-O’, on the pea aphid. Pestic Sci 41:155–160
Starks KJ, Burton RL (1977) Greenbugs: Determining biotypes, culturing, and screening for plant resistance, with notes on parasitoids. USDA Technical Bulletin, Washington, p 1556
Stern VM, Smith RF, van den Bosch R, Hagen KS (1959) The integrated control concept. Hilgardia 29:81–101
Stevens M, Locomme C (2017) Transmission of plant viruses. In: van Emden HF, Harrington R (eds) Aphids as crop pests, 2nd edn. CABI, Wallingford, pp 323–361
Sun Y, Huang X, Ning Y, Jing W, Bruce TJA, Qi F, Xu Q, Wu K, Zhang Y, Guo Y (2017) TPS46, a rice terpene synthase conferring natural resistance to bird cherry-oat aphid, Rhopalosiphum padi (Linnaeus). Front Plant Sci 8:110
Tandon PL (1994) Problems and prospects of inset pest management in fruit trees. In: Dhaliwal GS, Arora R (eds) Trends in agricultural insect Pest management. Commonwealth Publishers, India, p 376
Tang YQ, Weathersbee Iii AA, Mayer RT (2002) Effect of neem extract on the brown citrus aphid (Homoptera: Aphididae) and its parasitoid Lysiphlebus testaceipes (Hymenoptera: Aphididae). Environ Entomol 31:172–176
Thornhill NW (1993) The natural history of inbreeding and outbreeding. University of Chicago Press, Chicago, p 575
Tingey WM, Singh SR (1980) Environmental factors influencing the magnitude and expression of resistance. In: Maxwell FG, Jennings PR (eds) Breeding plants resistant to insects. Wiley, New Delhi, pp 95–96
Tingey WM, Plaisted RL, Laubengayer JE, Mehlenbacher A (1982) Green peach aphid resistance by glandular trichomes in Solanum tuberosum X S. berthaultii hybrids. Amer Potato J 59:241–251
Tiwari AK, Singh R (2016) Effect of host-plants on the morphology of green peach aphid, Myzus persicae (Sulzer) (Homoptera: Aphididae). Int J Zool Investig 2(1):133–146
Tiwari AK, Singh R (2018) Effect of temperature on the morphology of green peach aphid, Myzus persicae (Sulzer) (Homoptera: Aphididae). Bioinf Pharm Chem Sci 4(5):53–70
Toba HH, Paschke JD, Friedman S (1967) Crowding as the primary factor in the production of the agamic alate form of Therioaphis maculata (Homoptera: Aphididae). J Insect Physiol 13:381–396
Tsuchida T, Koga R, Fukatsu T (2004) Host-plant specialization governed by facultative symbiont. Science 303:1989
Tsuchida T, Koga R, Horikawa M, Tsunoda T, Maoka T, Matsumoto S, Simon J, Fukatsu T (2010) Symbiotic bacterium modifies aphid body color. Science 330(6007):1102–1104
Tsuchida T, Koga R, Matsumoto S, Fukatsu T (2011) Interspecific symbiont transfection confers a novel ecological trait to the recipient insect. Biol Lett 7:245–248
Tsumuki H, Nagatsuka H, Kawada K, Kanehisa K (1990) Comparison of nutrient reservation in apterous and alate pea aphids, Acyrthosiphon pisum (Harris): 1. Developmental time and sugar content. Appl Entomol Zool 25:215–221
Tylianakis JM, Didham RK, Wratten D (2004) Improved fitness of aphid parasitoids receiving resource subsidies. Ecology 85(3):658–666
Ulrichs CH, Mewis I, Schnitzler WH (2001) Efficacy of neem and diatomaceous earth against cowpea aphids and their deleterious effect on predating Coccinellidae. J Appl Entomol 125:571–575
Upadhyay S (1995) Influence of sowing dates and fertilizer levels on the incidence of aphid (Lipaphis erysimi Kalt.) on Indian mustard. Ind J Entomol 57:294–296
van Emden HF (1966) Studies on the relations of the insect and host-plant. III. A comparison of the reproduction of Brevicoryne brassicae and Myzus persicae (Hemiptera:Aphididae) on Brussels sprout plants supplied with different rates of nitrogen and potassium. Entomol Exp Appl 9:444–460
van Emden HF (2017) Integrated Pest management and introduction to IPM case studies. In: van Emden HF, Harrington R (eds) Aphids as crop pests. Oxford University Press, Oxford, pp 533–544
van Emden HF, Eastop VF, Hughes RD, Way MJ (1969) The ecology of Myzus persicae. Annu Rev Entomol 14:197–270
van Lenteren JC (2000) Measures of success in biological control of arthropods by augmentation of natural enemies. In: Gurr G, Wratten S (eds) Measures of success in biological control. Kluwer Academic, Dordrecht, pp 77–103
van Lenteren JC (2003) Environmental risk assessment of exotic natural enemies used in inundative biological control. BioControl 48:3–38
van Lenteren JC, Woets J (1988) Biological and integrated pest control in greenhouses. Annu Rev Entomol 33:239–269
van Lenteren JC, Roskam MM, Timmer R (1997) Commercial mass production and pricing of organisms for biological control of pests in Europe. Biol Control 10:143–149
Vandermoten S, Mescher MC, Francis F, Haubruge E, Verheggen FJ (2011) Aphid alarm pheromone: an overview of current knowledge on biosynthesis and functions. Insect Biochem Mol Biol 42(3):155–163
Veena (2009) Understanding ecology. Discovery Publishing House, New Delhi, p 344
Veeravel R, Baskaran P (1994) Effect of ant attendance on the multiplication levels of aphid (Aphis gossypii glover) in brinjal ecosystem. J Aphidol 8:131–135
Verghese PS (2015) Control of pyrethrum against the tomato disease CMV caused by aphids. Int J Curr Res Chem Pharm Sci 2(10):40–44
Verma KD (2000) Economically important aphids and their management. In: Upadhyay RK, Mukerji KG, Dubey OP (eds) IPM system in agriculture, vol 7. Aditya Books Private Ltd., New Delhi, pp 143–168
Vermora JM, Raghvani KL, Joshi MD, Makadia RR, Boricha HV, Dalwadi NG (2010) Chemical control of aphid Lipaphis erysimi (Kalt.) on cabbage. Int J Plant Prot 3(1):101–103
Vorburger C, Gehrer L, Rodriguez P (2010) A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol Lett 6(1):109–111
Watanabe S, Murakami T, Yoshimura J, Hasegawa E (2016) Color polymorphism in an aphid is maintained by attending ants. Sci Adv 2(9):e1600606
Waterhouse DF (1998) Biological control of insect pests: southeast Asian prospects. ACIAR Monograph 51:548
Watt M, Hales DF (1996) Dwarf phenotype of the cotton aphid, Aphis gossypii glover (Hemiptera: Aphididae). Aust J Entomol 35:153–159
Webster B, Bruce T, Dufour S, Birkemeyer C, Birkett MA, Hardie J, Pickett JA (2008) Identification of volatile compounds used in host location by the black bean aphid, Aphis fabae. J Chem Ecol 34:1153–1161
Weisser W, Braendle C, Minoretti N (1999) Predator-induced morphological shift in the pea aphid. Proc R Soc B 266:1175–1181
Wetzel T (1995) Getridblattlause in Pflanzenschutz und im Agrookosystem. Archiv für Phytopathologie und Pflanzenschutz 29:437–469
Will T, van Bel AJE (2006) Physical and chemical interactions between aphids and plants. J Exp Bot 57(4):729–737
Will T, Vilcinskasa A (2015) The structural sheath protein of aphids is required for phloem feeding. Mol Biol 57:34–40
Wise IL, Lamb RJ (1990) Economic injury level of the potato aphid in flax (1990 Annual Report). Winnipeg Research Station, pp 26–27
Wise IL, Lamb RJ, Kenaschuk EO (1995) Effects of the potato aphid Macrosiphum euphorbiae (Thomas) (Homoptera: Aphididae) on oilseed flax, and stage-specific thresholds for control. Can Entomol 127(2):213–224
Wohlers P (1982) Effect of alarm pheromone (E)-β-farnesene on aphid behaviour during flight and after landing on plants. Z Angew Entomol 93:102–108
Wool D (2004) Galling aphids: specialization, biological complexity, and variation. Annu Rev Entomol 49(1):175–192
Wyatt IJ (1985) Aphid control by parasites. In: Hussey NW, Scopes N (eds) Biological pest control. The Glasshouse experience. Blanford Press, Poole, pp 134–137
Xibei W, Yihao F, Shizhong L, Lirong Z, Huadi W (1994) A study on the damage and economics threshold of the soybean aphid at the seedling stage. Plant Prot 20:12–13
Yamaguchi Y (1985) Sex ratios of an aphid subject to local mate competition with variable maternal condition. Nature 318:460
Yang S, Yang SYY, Zhang CP, Wei J, Kuang RP (2009) Population dynamics of Myzus persicae on tobacco in Yunnan province, China, before and after augmentative releases of Aphidius gifuensis. Biocontrol Sci Tech 19:219–228
Yi F, Zou C, Hu Q, Hu M (2012) The joint action of destruxins and botanical insecticides (rotenone, azadirachtin and paeonolum) against the cotton aphid, Aphis gossypii glover. Molecules 17:7533–7542
Zalom FG (2010). Pesticide use practices in integrated pest management. Hayes’ handbook of pesticide toxicology
Zhu J, Park K-C (2007) Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J Chem Ecol 30:1733–1746
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, R., Singh, G. (2021). Aphids. In: Omkar (eds) Polyphagous Pests of Crops. Springer, Singapore. https://doi.org/10.1007/978-981-15-8075-8_3
Download citation
DOI: https://doi.org/10.1007/978-981-15-8075-8_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-8074-1
Online ISBN: 978-981-15-8075-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)