Abstract
The need for sustained ocular drug delivery systems grows with the increasing number of people with visual impairments. Drug delivery to the posterior segment poses unique challenges that a variety of systems have been developed to overcome. Currently, small-molecule drugs and protein therapeutics are mainly administered as topical solutions and intravitreal injections, respectively. Small-molecule drugs have a short half-life and diffuse easily throughout the eye, making it challenging to deliver a localized, and maintained, therapeutic dose. The main challenge of delivery protein therapeutics is maintaining a sustained therapeutic level of protein during manufacture and release since most protein therapeutics depend on their complex structural integrity to be effective. Due to these limitations, repeated doses of small-molecule drugs and protein therapeutics are given to maintain a therapeutic range. Thus, developing new drug delivery systems (DDSs) that have a controlled, extended, and localized release of drug and maintain drug stability would reduce the frequency of administration. This chapter discusses new DDSs for small-molecule and macromolecular biological drugs, particularly protein therapeutics, in the form of ocular implants, ocular inserts, microneedles, cell-based systems, injectable nano/microparticles, injectable hydrogels, and composite systems. Many of these advances in drug delivery will help to play a role in improving anatomic and visual outcomes for various forms of macular surgery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Posterior segment delivery
- Small-molecule drug delivery
- Protein delivery
- Injectable hydrogel
- Ocular implants
- Visual impairment
- Sustained release
- Microneedles
1 Introduction
Visual impairment is a global health concern that has a significant impact on patients’ physical and mental health. Leading causes of visual impairment are age-related macular degeneration (AMD), diabetic retinopathy (DR), glaucoma, eye inflammations, and retinal vein occlusion (RVO). A significant number of macular abnormalities that require surgery are also quite common in the elderly (macular holes, epiretinal membranes, vitreomacular adhesions, diabetic macular traction detachments, etc.). The number of people affected by visual problems is increasing due to an overall aging population. For example, the projected number of people with age-related macular degeneration in 2020 is 196 million, increasing to 288 million in 2040 worldwide [1]. Likewise, the number of people with DR and vision-threatening DR (VTDR) has been estimated to rise to 191.0 million and 56.3 million, respectively, by 2030 [2]. The total global economic burden of eye diseases and visual impairment was estimated at $3 trillion in 2010 and projected to increase by approximately 20% by 2020 [3].
With better understanding of ocular physiology and pathology, there have been numerous pharmacological agents such as dexamethasone for ocular inflammation, travoprost and bimatoprost for glaucoma, and anti-vascular endothelial growth factor (anti-VEGF) drugs for ocular neovascularization. The success of treatment not only depends on drug potency and patients’ genetic response, but also relies heavily on the delivery method used, tissue barriers, and physicochemical properties of pharmacological agents involved [4]. Ocular barriers and clearance mechanisms make delivering therapeutic doses to the posterior segment of the eye challenging. These barriers include the ocular surface that causes drug loss from lacrimal fluid, lacrimal fluid-eye barriers that limit drug absorption, and blood-ocular barriers that prevent distribution of drug [5].
The eye offers multiple entry routes through which pharmacological agents can be delivered, including topical, systemic, periocular, and intraocular routes [4]. Although the optimal dose and delivery routes can usually be chosen for each drug, an issue facing most existing treatment regimen is frequent administration due to fast clearance, low bioavailability, or low stability of pharmacological agents [4, 5]. This frequent application may increase risks of complications, lower patient compliance, and cause significant socioeconomic burden on healthcare systems [4]. Therefore, designing and developing new ocular drug delivery systems (DDSs) that can achieve controlled and extended drug release to targeted location, improve drug bioavailability, and preserve drug stability over both manufacture and release is in great demand. It was estimated that the total current market size for sustained ocular drug delivery system is worth more than $9.3 billion and continuously growing [6].
Targeted and sustained ocular drug delivery is a rapidly developing area and has great potential to achieve more effective treatment regimen for most eye diseases. This chapter presents current advancements in ocular drug delivery systems, classified into small-molecule and macromolecular biological drugs (particularly protein therapeutics). An emphasis is on systems currently in clinical studies and promising to be available on the market.
2 Drug Delivery Systems for Small-Molecule Drugs
Small molecules are molecules with a molecular weight less than 900 daltons [7]. Small molecules have a short half-life requiring frequent dosing and can result in poor patient compliance. Small-molecule ocular drugs, such as dexamethasone for inflammation; triamcinolone acetonide (TA) for uveitis, retinal vein occlusion (RVO), and diabetic macular edema (DME); bimatoprost for glaucoma and ocular hypertension; travoprost for glaucoma and ocular hypertension; and fluocinolone acetonide (FA) for glaucoma and ocular hypertension, are commonly prescribed to combat posterior segment diseases and symptoms.
Small-molecule drugs can be challenging to delivery to the posterior segment due to the ocular barriers and clearance mechanisms of the eye previously mentioned. Topical DDSs for small molecules, such as ophthalmic solutions and eye drops, can circumvent these challenges due to their ability to diffuse across many ocular barriers. Since they diffuse easily throughout the eye, however, small-molecule DDSs can be challenging to localize; additionally, the delivery is limited by the clearance mechanisms of the eye and administering a high initial dose to counter the clearance and diffusion of drug may become toxic [8]. Furthermore, most treatments for posterior segment disease cannot be systemically administered since they will not reach a therapeutic dose within the eye itself [8, 9]. Intraocular injections may also be used to bypass some of these barriers, but the invasiveness of injection increases the risks of complication, such as retinal detachment, retinal hemorrhage, endophthalmitis, and increased ocular pressure [10,11,12]. A minimally invasive, localized, controlled, and extended release of small molecules would provide the safest and most effective DDS.
With the growing number of patients with posterior segment diseases (both medical and surgical), an increasing number of systems are being developed that allow for more effective treatment. Advances in DDSs address current issues with delivering small molecules to the posterior segment. Microneedles, hydrogels, topical treatment, injectable substances, and implants will be addressed in the following sections. Table 41.1 summarizes small-molecule DDSs currently in clinical trials or recently approved by the U.S. Food and Drug Administration (FDA).
2.1 Ocular Implants
One of the main benefits of solid ocular implants is that they eliminate the need for repetitive treatments by administering drug for extended periods. EyePoint Pharmaceuticals developed a solid polymer implant called Durasert™. This implant can release small molecules for up to 3 years. The implant is only 3.5 mm in length and 0.37 mm in diameter, allowing it to be injected through a needle. The Durasert™ technology has been approved by the FDA for the following products: Iluvien® (fluocinolone acetonide intravitreal implant), Retisert® (fluocinolone acetonide intravitreal implant), and Vitrasert® (ganciclovir). The properties of this system allow for the customization of release duration, linear release kinetics, and high drug loading [13, 14].
Allergan has also developed Bimatoprost SR, a biodegradable polylactic intracameral implant that releases drug for 4–6 months for the treatment of open-angle glaucoma and ocular hypertension. Bimatoprost SR is injected directly into the anterior chamber, allowing a much lower loading dose as compared to ophthalmic bimatoprost 0.03% solution. Furthermore, the elimination of total drug exposure improves the safety of treatment by leading to fewer adverse events. After a completed phase 3 study that showed a similar reduction in IOP by 30% as compared to daily topical treatment, the new drug application (NDA) for Bimatoprost SR was approved in mid-2019 [15, 16].
Envisia Therapeutics created ENV515 Travoprost, another degradable polylactic intracameral implant that showed promising results as a treatment for glaucoma and ocular hypertension. A phase 2 study showed that its release of travoprost for 6 months lowers IOP similarly to ophthalmic solution [17, 18].
2.2 Injectable Micro/Nanoparticles
Due to the wide availability of different materials that can be tailored for specific drugs and applications, micro/nanoparticles have also been an attractive drug delivery method for therapeutic agents. Synthetic, biodegradable polymers are commonly used to develop DDSs due to their biocompatibility, adjustable degradation, and reproducible release [19]. While intravitreal injections are more invasive than other administration routes, they allow for more direct delivery to the targeted site with well-controlled doses.
Poly(d,l-lactate-co-glycolide) (PLGA) has evolved into most widely used copolymer for making micro- and nanoparticles since its approval by FDA for medical and biological applications. Graybug Vision’s GB-102, which recently started phase 2 clinical trials, consists of sunitinib malate-loaded poly(lactic-co-glycolic acid) (PLGA) microspheres that are injected into the inferior vitreous for the treatment of neovascular AMD (nAMD), diabetic macular edema (DME), and RVO. Administering by intravitreal injection allows for a localization of the microspheres, which does not disrupt vision since they are injected inferiorly. The PLGA microspheres allow for controlled, extended release of drug and high drug loading. Thus, the annual number of injections could be reduced to twice per year [20]. On the other hand, PLGA microspheres have some disadvantages such as low drug encapsulation and generation of acidic environments upon degradation [4].
Dexycu®, recently approved by the FDA, is an intracameral injection using Verisome® technology to deliver dexamethasone for 21 days for inflammation associated with cataract surgery. Developed by EyePoint Pharmaceuticals, Verisome® technology is a drug delivery system that has a wide range of applications including peptides, small molecules, and proteins. This system can be formulated into a biodegradable solid, gel, or liquid substance that provides a controlled, extended release of drug. It serves as an alternative to corticosteroid drops post-cataract surgery [21]. The Verisome® technology has application for delivery to the posterior segment.
2.3 Injectable Hydrogels
Hydrogels have become one of the most useful materials for biomedical applications including drug delivery, tissue-engineering scaffolds, and cell transplantation since their development in 1960s [22]. Their high water content and mild preparation conditions make them inherently good candidates as drug delivery carriers for cells and many labile therapeutic agents such as small molecules, peptides, proteins, and nucleic acids [23,24,25]. Some hydrogels can modulate their gelation behavior upon changes of environment, such as temperature, pH, and ionic strength [26, 27]. This makes them injectable through small-gauge needles as either aqueous solution or liquid-like form, and then they solidify into hydrogels at physiological conditions [26, 27]. These injectable hydrogels are easily fabricated and have gained significant interests as drug delivery candidate into small spaces within the body, specifically the ocular structures.
Ocular Therapeutix’s hydrogel technology is a versatile platform employed to create sustained-release therapies, including OTX-TP, OTX-TIC, and OTK-TKI, that may expand treatment options across multiple ocular conditions. The easy-to-inject, bioresorbable hydrogel has an adjustable and consistent release rate [28, 29]. OTX-TP, which releases travoprost on the ocular surface for up to 90 days, is recruiting patients for its phase 3 clinical trial [30]. Similarly, OTX-TIC releases travoprost for 3–4 months for the treatment of glaucoma and ocular hypertension. Preclinical studies in beagles have demonstrated sustained intraocular pressure lowering and maintenance of drug levels in the aqueous humor [31]. Lastly, OTX-TKI is administered via intravitreal injection and releases tyrosine kinase inhibitors (TKIs) for 6 months. Preclinical studies in Dutch belted rabbits demonstrate that a therapeutic level of drug was maintained for up to 12 months with efficacy in a VEGF-induced vascular leakage model [32]. While this technology seems effective, safety and biocompatibility need to be further investigated.
Huu et al. have developed a light-responsive hydrogel that releases nintedanib-loaded nanoparticles for the treatment of macular degeneration and DR for up to 30 weeks [33]. This system allows for a more controlled release of drug than bolus injections since drug release is triggered by UV exposure. Preliminary studies have shown that the light-sensitive nanoparticles are safe and biocompatible [33]. Further research must be conducted before starting clinical trials.
2.4 Ocular Inserts
Eye drops are commonly prescribed due to the ease of use and noninvasive qualities. Even when patients follow dosage regimens, they are less effective than other routes due to low absorbance and fast clearance within the eye. DDSs that increase the contact time of drug with the cornea allow for better delivery. Figure 41.1 shows Allergan’s bimatoprost ring, an externally applied ring that releases for up to 6 months for the treatment of open-angle glaucoma and ocular hypertension. It consists of an outer silicone ring that covers a polypropylene support structure shown in Fig. 41.2. The concentration gradient allows drug to diffuse passively from the silicon matrix to the tear film. A completed phase 2 study showed similar results to eye drop treatment [15, 16]. The bimatoprost ring was able to reduce IOP with clinical relevance over less than 19 months when applied every 6 months [15, 16, 34]. A major challenge with this design is determining proper loading dose of drug and achieving a controlled drug release due to the variability of the ocular surface conditions.
External application of bimatoprost ring onto the upper fornix [34]
Structure of bimatoprost ring [34]
Ocular Therapeutix’s resorbable hydrogel technology, as previously mentioned, is also used as an ocular insert. Dextenza® releases dexamethasone on the ocular surface for up to 30 days as a treatment for postsurgical pain and inflammation [28, 29]. It is placed in the canaliculus through the punctum, activated by moisture, and reabsorbed in the nasolacrimal system [35]. In mid-2019, the FDA accepted the resubmission of the NDA for Dextenza®.
2.5 Microneedles
Micron-size needles, or microneedles, enable minimally invasive delivery of free or encapsulated drug. Clearside Biomedical developed a microneedle and injector that administers a suprachoroidal injection of corticosteroid triamcinolone acetonide (CLS-TA), which is Clearside Biomedical’s proprietary suspension of TA. It is used for the treatment of uveitis, RVO, and DME. The injector allows for consistent insertion of microneedle into the suprachoroidal space. Thus, this method reduces the risks commonly associated with intravitreal injections, including the potential for retinal damage [36]. Due to the small surface area of the microneedle, this system is limited to small molecules and microneedles cannot always deliver a therapeutic dose. Currently, the use of microneedles for the treatment of uveitis is undergoing a phase 3 clinical trial. Around 46.9% of patients receiving treatment had an increase in visual acuity from baseline as compared to only 15.6% of the control patients [36, 37]. As for safety, around 11.5% of treated patients had increased IOP but the control patients did not have any increases. Clearside Biomedical plans to submit a new drug application to the FDA and other regulatory agencies outside of the USA by the end of 2019 [36]. Clearside Biomedical is also conducting a phase 3 clinical trial for a combination therapy of suprachoroidal CLA-TA with one or two intravitreal injections of anti-VEGF [37]. Reported in a phase 2 study, the combination treatment group had a 61% reduction in the number of re-treatments as compared to the group treated with aflibercept alone [38].
With the increasing demand for effective treatments, there are many microneedle DDSs in preclinical stages for the delivery of small molecules. An example of microneedle in preclinical stages includes a fenestrated microneedle made from titanium deep-reactive ion etching developed by Khandan et al. [39]. The design of the fenestrated needle increases the surface area, allowing for a higher dose of drug. It may also allow for the delivery of some larger molecules [39]. Another example is a rapidly dissolving microneedles fabricated from polyvinylpyrrolidone (PVP) designed by Thakur et al. [40]. Moreover, the rapidly dissolving microneedle is less invasive than previously developed microneedles. It also has the potential to deliver macromolecules [40].
3 DDS for Macromolecular Biological Drugs
With the rapid progress in biotechnology in the last several decades, there has been a growing number of macromolecular biological drugs such as peptides, proteins, and nucleic acids developed for prevention, diagnosis, or treatment of a variety of diseases [41]. Ophthalmology is one field that has benefited enormously from the development of biological therapeutics. For example, the introduction of anti-VEGF therapy has revolutionized the clinical management of ocular neovascular diseases and eventually evolved into the standard of care for wet AMD and PDR [42,43,44]. Despite its great success, a major challenge of anti-VEGF drugs and other protein therapeutics is their fast clearance and relatively short half-life [10, 45]. Therefore, frequent and repeated intravitreal (IVT) injections (every 4–6 weeks) are required for management of chronic eye diseases. However, these repeated IVT injections usually present increased risk of potential complications including endophthalmitis, retinal detachment, intravitreal hemorrhage, and cataract [10, 12]. Additionally, the significant socioeconomic burden upon patients, families, and healthcare systems cannot be ignored. In 2017, it was estimated that there were 22.3 million IVT injections performed globally and 5.4 million injections in the USA alone [12]. Thus, a sustained DDS for protein drugs like anti-VEGF is greatly needed to reduce the frequency of IVT injections and the associated societal burdens.
Recent years have seen a variety of drug delivery systems being developed for controlled and extended delivery of anti-VEGF and other protein drugs in the form of ocular implants, cell-based systems, injectable nano/microparticles, injectable hydrogels, and composite systems [4]. However, only a limited number of systems are advancing in clinical trials. The main challenge of these DDSs is maintaining a sustained therapeutic level of protein bioactivity during both manufacture and release stages, since biological drugs like proteins rely heavily on their complex structural integrity to perform their biological activities (e.g., neutralization of a cytokine or a growth factor). Numerous stress factors during DDS fabrication, release, and degradation such as presence of catalyst, changes in temperature and pH, and polymer-induced aggregation/adsorption [5, 46, 47] can contribute to loss of protein bioactivity. In this section, some of the most promising ocular DDSs for biological drugs, both in clinical studies and preclinical studies, are discussed. And Table 41.2 summarizes the DDSs currently in clinical studies.
3.1 Ocular Implants
Refillable rigid port delivery system (RPDS) is an intravitreal implant that is the size of a grain of rice (Fig. 41.3). It consists of a subconjunctival refill port outside of the eye and an intravitreal reservoir preloaded with drug. It was first developed by Forsight Vision4, and then licensed exclusively to Genentech as the platform offering ranibizumab (Lucentis®) in a sustained-release format. One of the biggest advantages of RPDS is that on-demand refills can be performed in an in-office procedure using a customized exchange needle. The replacement with new drug not only maintains drug potency, but also provides reproducible and predictable drug release after each refill. However, since the drug reservoir is nonbiodegradable, both surgical implantation and removal are required which may increase risks of complications [48]. Recent phase 2 data showed that the median time to refill was 8.7, 13.0, and 15.0 months in PDS 10 mg/mL, PDS 40 mg/mL, and PDS 100 mg/mL [49]. The data also demonstrated that the PDS 100 mg/mL arm had visual and anatomic outcomes comparable with monthly intravitreal ranibizumab group based on the adjusted mean BCVA [49].
The ranibizumab refillable port delivery system (RPDS) [49]
Replenish Inc.’s posterior micropump (PMP) system is a mini programmable drug pump fabricated using principles of microelectromechanical system (MEMS) technology. The pumping mechanism is based on electrolysis and the pump includes a drug refill port as well as a check valve to control drug delivery [50]. Recently, the system has been investigated for safety and surgical feasibility to deliver ranibizumab for treatment of diabetic macular edema (DME) in a phase 1 study. It was reported that the PMP was safely implanted subconjunctivally in 11 diabetic patients with DME, and capable of delivering a programmable microdose of ranibizumab into the vitreous cavity for 90 days [51].
Recently, a nanoporous film device has been developed by Zordera Inc. for controlled delivery of anti-VEGF for ocular neovascularization (Fig. 41.4). This intravitreal implant consists of capsule, preloaded with drug pellet, sandwiched between a nanoporous thin film and a second impermeable thin-film layer made from biodegradable polycaprolactone (PCL). The device is only 40 μm thick, which allows intravitreal implantation through a syringe. The pore size can match the drug molecule diameter to achieve sustained, zero-order release over the course of several months before polymer complete degradation. Recent studies have established in vitro release of ranibizumab for 16 weeks, and safety and in vivo release on New Zealand rabbit model for 3 months [52].
Encapsulated cell technology (ECT) is essentially a cell-based delivery system and has been proven to be a very effective strategy for long-term delivery of biologically active proteins and polypeptides to both central nervous system (CNS) and retina [53]. Neurotech Pharmaceuticals, Inc. has been developing its ECT platform targeting retinal degenerative diseases like AMD, glaucoma, retinitis pigmentosa, and macular telangiectasia (MacTel). The basis of this ECT platform is the genetically engineered human retinal pigment epithelial cell line, known as NTC-200 cell line, capable of continuously secreting ciliary neurotrophic factor (CNTF). The cells are packaged into a nonbiodegradable polymeric capsule device consisting of internal scaffolding and a semipermeable exterior membrane, which allows for controlled cell growth and continuous protein production and delivery for over 2 years. Despite its invasive implantation and surgical removal procedure, this system is theoretically versatile for different cell lines and protein therapeutics with controlled, continuous, and sustained release [54]. Neurotech’s NT-501 ECT for CNTF delivery is undergoing phase 2 clinical studies for the treatment of MacTel and glaucoma [55, 56].
Although the above ocular implant systems have shown promising results in preclinical studies and are currently advancing to/in clinical stages, the invasive implantation and sometimes surgical removal procedures (if nondegradable material used) required for these devices can increase risks of complications. Therefore, injectable delivery systems such as micro/nanoparticles, hydrogels, and particle-hydrogel composite systems have emerged recently as promising strategies for controlled delivery of protein drug in a minimally invasive manner.
3.2 Injectable Micro/Nanoparticles
Since most macromolecular biological drugs like protein are hydrophilic, they can usually be encapsulated into polymeric capsules by the solvent/evaporation method from emulsifications such as water-in-oil-in-water (w/o/w) or solid-in-oil-in-water (s/o/w) emulsions [19]. To preserve the protein stability during manufacture, additional protein stabilizers like albumin, sugars, and surfactant are commonly employed. After identifying bovine serum albumin (BSA) as a major protective agent for bevacizumab during emulsification procedure, Varshochian et al. developed an albuminated PLGA nanoparticle for sustained delivery of bevacizumab [57]. The nanoparticles were fabricated by w/o/w double emulsion using albumin as a stabilizer, with a size of ~200 nm. Their recent studies in rabbits showed that vitreous concentration of bioactive bevacizumab was maintained above 500 ng/mL for about 8 weeks after single intravitreal injection [58]. Bevacizumab-loaded PLGA microparticles were also fabricated using s/o/w method by Zhou Ye et al. [59]. The size of particles was 2–7 μm. A significantly prolonged half-life of bevacizumab in vitreous (9.6 days) and aqueous humor (10.2 days) has been achieved in New Zealand albino rabbits, compared to 3.91 days and 4.1 days for free drug, respectively [59]. However, low protein encapsulation efficiency (usually <60% for microparticles and <30% for nanoparticles, respectively), high initial bursts (20–50% of encapsulated protein in the first 24 h), incomplete release of the entrapped proteins, and loss of protein drug bioactivity during release are major challenges for PLGA particulate systems [19, 60, 61].
Among the above challenges, preserving protein drug stability and bioactivity during manufacture and release remains the biggest challenge for PLGA micro/nanoparticle systems. Protein stability is mainly affected by moisture-induced aggregation caused by initial fast water uptake, nonspecific protein adsorption to polymer surface, and covalent/non-covalent aggregation due to acidic microenvironment after polymer degradation [5, 47]. Although stabilizers are added to counter these stress factors, their primary effects are during manufacture and initial release. Significant low protein bioactivity is usually seen after 2–3 months of release [62,63,64]. Recent studies showed that more hydrophilic polymers such as PEG-based PLGA diblock/triblock copolymers, alginate, and polyethylene glycol-polybutylphthalate (PEG-PBT) may improve release and protein bioactivity during release [65,66,67]. For example, Adamson et al. investigated different formulations of their PEG-PBT microparticles (based on PEG length, PEG:PBT ratio, and water/polymer ratio) for sustained release of their newly designed anti-VEGF dual antibody [66]. It was reported that more than 50% of protein bioactivity can be maintained throughout 6 months of release in vitro. Inhibition of laser-induced choroidal neovascularization by single intravitreal injection of drug-loaded microparticles was also found in primates [66]. Despite progress in polymers, a more important concern for these injectable particulate systems is that migration of these particles after injection into the eye can cause faster clearance by phagocytes, ocular lymphatic, and vascular circulation and sometimes even lead to complications such as glaucoma and ocular inflammation [66, 68]. This movement of particles in the eye may limit their applications as sustained DDS by IVT injections.
3.3 Injectable Hydrogels
Ocular Therapeutix’s injectable and bioresorbable hydrogel technology is recently also seeking application for sustained anti-VEGF delivery. They are collaborating with Regeneron (manufacturer for aflibercept) to investigate the feasibility of OTX-IVT for sustained delivery of aflibercept for 4–6 months targeting wet AMD and other serious retinal neovascular diseases [69].
Thermoresponsive hydrogels have been particularly attractive candidates as injectable ocular DDS in a minimally invasive manner because they used temperature change as the trigger for their gelation and changes in swelling. At room temperature, these hydrogels either are in solution form or have a fluidlike consistency. After injection into the eyes, they either in situ cross-link or solidify into a solid form upon reaching the body temperature [26, 27]. These hydrogels usually have a sharp volume phase transition temperature at ~30–33 °C, which makes them ideal candidates for localized and extended drug delivery. Recently, Kang-Mieler et al. have developed a poly(N-isopropylacrylamide) (PNIPAAm)-based thermoresponsive hydrogel by cross-linking PNIPAAm with poly(ethylene glycol) diacrylate (PEG-DA) or poly(ethylene glycol)-co-(l-lactic acid) diacrylate (PEG-PLLA-DA) through free radical polymerization [70, 71]. It was shown that this system is capable of localized release of proteins such as bevacizumab and ranibizumab for about a month and induced no long-term effects on retinal function [71, 72]. Additionally, controlled degradation and complete release from these hydrogels were achieved by incorporating biodegradable copolymer and other additives [71]. Other than PNIPAAm, thermoresponsive copolymer poly(2-ethyl-2-oxazoline)-b-poly(ε-caprolactone)-b-poly(2-ethyl-2-oxazoline) (PEOz-PCL-PEOz) was also synthesized and used to make biodegradable thermoresponsive hydrogels for extended release of bevacizumab by Wang et al. [73]. Good in vitro and in vivo biocompatibility was achieved using human retinal pigment epithelial cell line and rabbit for 2 months, respectively [73]. An extended release of bioactive bevacizumab from hydrogels for 1 month in vitro was established, although no in vivo efficacy data on animal models have been reported [73]. Poly(ethylene glycol)-poly-(serinol hexamethylene urethane), or ESHU, thermoresponsive hydrogel was developed by Rauck et al. to encapsulate and extend the delivery of bevacizumab after IVT injection into New Zealand white rabbit eyes [74]. It was reported that the system was easily injected through a 31-gauge needle with a force required less than for a 1% solution of hyaluronic acid, a commonly injected ophthalmic material. The system was proved to be biocompatible in rabbit eyes and a significant higher bioactive bevacizumab concentration was detected in eyes receiving bevacizumab-loaded hydrogel IVT injections than those receiving the bolus injections [74].
Instead of preforming a hydrogel with phase transition properties as injectable DDS, alternative strategy is developing in situ-forming hydrogels, which usually consist of polymers in aqueous solution form and are spontaneously chemically or physically cross-linked into solid hydrogels upon physiological conditions after injection [26]. Loading with adjustable drug dosage could usually be easily achieved for these hydrogels since most protein drugs are hydrophilic and can be completely dissolved into the aqueous precursor at desirable concentrations. In addition, since usually no initiator is necessary, better protein stability and biocompatibility are anticipated [26]. Recently, a vinyl sulfone-functionalized hyaluronic acid (HA-VS)-thiolated dextran (Dex-SH) in situ-forming hydrogels have been developed by Chau et al. for controlled delivery of bevacizumab [75]. The bevacizumab-containing polymer solution was injected into rabbit eyes and then chemically cross-linked into transparent hydrogels at physiological condition. Binocular indirect ophthalmoscope (BIO) images, full-field electroretinogram (ERG), and histology showed that the hydrogels were safe for rabbit eyes after injections. It was also promising that a concentration of bioactive bevacizumab, about 107 times higher than bolus injection, was reportedly maintained 6 months after injection [75].
Although injectable hydrogels are very promising vehicles for use in developing new extended ocular DDSs, challenges such as (1) difficulty sterilizing, especially when biomolecules such as proteins are encapsulated [76, 77]; (2) potential for toxic effects caused by residual initiators after polymerization [78]; and (3) most-of-the-time relatively faster release of protein drugs (less than 2–3 months) due to their inherently higher water content [73,74,75], have limited their application as extended ocular DDS for protein therapeutics.
3.4 Composite DDSs
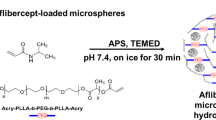
As discussed before, although injectable polymeric micro/nanoparticles usually can provide controllable and sustained drug release through adjustments made to their polymer composition, difficulties to localize them to the injection site in the eyes can be problematic for long-term release applications. Due to their either thermoresponsive or in situ gelation properties, injectable hydrogels can be good candidates as second carrier for micro/nanoparticles to obtain localized and extended drug release after injection [4]. This composite DDS may offer advantages over both particles and hydrogels alone by further extending release and reducing initial burst. In addition, both proteins and small molecules can be encapsulated into particles and hydrogels in a variety of ways to enhance delivery potential. More recently, this strategy has been validated by Kang-Mieler et al. who have combined their injectable PNIPAAm-based thermo-responsive hydrogel with PLGA microspheres to create a microsphere-hydrogel composite DDS [79,80,81]. Their DDS was able to encapsulate ranibizumab or aflibercept and release them in a controlled manner for ~200 days [80, 81]. In vitro bioactivity during release and in vivo efficacy in laser-induced CNV rodent model have been established [64, 82]. By controlling the amounts of microspheres suspended within the hydrogel, total amount of drug delivered to the retina can be controlled without changing the volume and injectability of the system. More recently, by introducing hydrolytically degradable polymer poly(ethylene glycol)-co-(l-lactic acid) diacrylate to PNIPAAm-based hydrogels, they were able to make their hydrogel also biodegradable. It was found that this microsphere-hydrogel DDS was more biocompatible and at the same time capable of releasing bioactive aflibercept for 6 months in vitro [64].
4 Conclusions
Due to the increased number of patients afflicted by a variety of ocular disorders, sustained DDSs providing better treatment and management of the medical and surgical diseases previously discussed in this chapter are currently in high clinical demand. Comparing to traditional methods of ocular drug delivery such as eye drops, periocular/intraocular injections, and systemic delivery, sustained DDSs are designed to encapsulate and deliver the specific therapeutic agents to the target sites for an extended period of time at a therapeutic level without any detriment to the drug. Since most of the small-molecule ophthalmic drugs are more stable than protein therapeutic drugs in terms of maintaining activity during DDS manufacture and release, there are numerous DDSs for small-molecule drugs available in the market or advancing in clinical studies. However, utilization of full clinical potential of novel sustained DDSs for protein drug (e.g., anti-VEGF) has been limited by protein instability during DDS manufacture and release. Overall, with the advancement in drug discovery and development, biomaterials, and microfabrication techniques, novel sustained DDSs for a variety of drugs targeting for ocular diseases are expected to grow fast and achieve better management and control of ocular diseases in future.
References
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:106–16.
Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44:260–77.
Gordois A, Cutler H, Pezzullo L, Gordon K, Cruess A, Winyard S, Hamilton W, Chua K. An estimation of the worldwide economic and health burden of visual impairment. Glob Public Health. 2012;7:465–81.
Kang-Mieler JJ, Dosmar E, Liu W, Mieler WF. Extended ocular drug delivery systems for the anterior and posterior segments: biomaterial options and applications. Expert Opin Drug Deliv. 2017;14:611–20.
Radhakrishnan K, Sonali N, Moreno M, Nirmal J, Fernandez AA, Venkatraman S, Agrawal R. Protein delivery to the back of the eye: barriers, carriers and stability of anti-VEGF proteins. Drug Discov Today. 2017;22:416–23.
PR Newswire. Ocular drug delivery market to be worth US$18.124 billion by 2025. 2017. https://www.prnewswire.com/news-releases/ocular-drug-delivery-market-to-be-worth-us18124-billion-by-2025-manufacturers-collaborating-with-hospitals-to-bolster-positions-632232553.html. Accessed 7 Nov 2019.
Yang NJ, Hinner MJ. Getting across the cell membrane: an overview for small molecules, peptides, and proteins. In site-specific protein labeling. New York: Humana Press; 2015. p. 29–53.
Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–5.
Del Amo EM, Rimpelä AK, Heikkinen E, Kari OK, Ramsay E, Lajunen T, Schmitt M, Pelkonen L, Bhattacharya M, Richardson D, Subrizi A. Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res. 2017;57:134–85.
Peyman GA, Lad EM, Moshfeghi DM. Intravitreal injection of therapeutic agents. Retina. 2009;29:875–912.
Bande MF, Mansilla R, Pata MP, Fernandez M, Blanco-Teijeiro MJ, Pineiro A, Gomez-Ulla F. Intravitreal injections of anti-VEGF agents and antibiotic prophylaxis for endophthalmitis: a systemic review and meta-analysis. Sci Rep. 2017;7:18088.
Williams GA, Mich RO. IVT injections: health policy implications. Rev Ophthalmol. 2014. https://www.reviewofophthalmology.com/article/ivt-injections-health-policy-implications.
Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126:1191–201.
Haghjou N, Soheilian M, Abdekhodaie MJ. Sustained release intraocular drug delivery devices for treatment of uveitis. J Ophthalmic Vis Res. 2011;6:317–29.
Chen MY, Sall KN, Tepedino M, McLaurin E, Olander K, Wirta D, Flynn W, Walker G, Ling J, Yang J, Goodkin M. Patient-reported outcomes of bimatoprost ocular ring in an open-label extension study in patients with open-angle glaucoma or ocular hypertension. Invest Ophthalmol Vis Sci. 2018;59:9,1231.
Lee SS, Almazan A, Decker S, Zhong Y, Ghebremeskel AN, Hughes P, Robinson MR, Burke JA, Weinreb RN. Intraocular pressure effects and mechanism of action of topical versus sustained-release bimatoprost. Transl Vis Sci Technol. 2019;8:15.
Mansberger SL, Conley J, Verhoeven RS, Blackwell K, Depenbusch M, Knox T, Walters TR, Ahmad I, Yerxa BR, Navratil T. Interim analysis of low dose ENV515 travoprost XR with 11 month duration followed by dose escalation and 28 day efficacy evaluation of high dose ENV515. Invest Ophthalmol Vis Sci. 2017;58:2110.
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02371746, safety and efficacy of ENV515 travoprost extended release (XR) in patients with bilateral ocular hypertension or primary open angle glaucoma; 2015 Feb 20 [cited 2019 Nov 5]; [about 4 screens]. https://clinicaltrials.gov/ct2/show/record/NCT02371746?term=ENV515&rank=1.
Herrero-Vanrell R, Bravo-Osuna I, Andres-Guerrero V, Vicario-de-la-Torre M, Molina-Martinez IT. The potential of using biodegradable microspheres in retinal diseases and other intraocular pathologies. Prog Retin Eye Res. 2014;42:27–43.
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03249740, A depot formulation of sunitinib malate (GB-102) in subjects with neovascular (wet) age-related macular degeneration; 2017 Aug 2 [cited 2019 Nov 5]; [about 4 screens]. https://clinicaltrials.gov/ct2/show/record/NCT03249740?term=graybug+vision&rank=1.
Kiss S, Pena J. Ocular implants–a novel approach in ocular drug delivery. Rec Patents Nanomed. 2012;2:113–25.
Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12.
Lin CC, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26:631–43.
Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46.
Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18:1345–60.
Agrawal AK, Das M, Jain S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin Drug Deliv. 2012;9:383–402.
Klouda L. Thermoresponsive hydrogels in biomedical applications a seven-year update. Eur J Pharm Biopharm. 2015;97:338–49.
Talamo JH, Tyson SL, Bafna S, Joseph GP, Goldberg DF, Jones JJ, Jones MP, Kim JK, Martel JB, Nordlund ML, Piovanetti IK. Results of a phase 3, randomized, double-masked, vehicle controlled study (phase 3c) evaluating the safety and efficacy of DEXTENZA ™ (dexamethasone insert) 0.4 mg for the treatment of ocular inflammation and pain after cataract surgery. Invest Ophthalmol Vis Sci. 2017;58:1825.
Gira JP, Sampson R, Silverstein SM, Walters TR, Metzinger JL, Talamo JH. Evaluating the patient experience after implantation of a 0.4 mg sustained release dexamethasone intracanalicular insert (Dextenza™): results of a qualitative survey. Patient Prefer Adherence. 2017;11:487.
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02914509, OTX-16-002: a phase 3 study evaluating the safety and efficacy of OTX-TP in subjects with open-angle glaucoma and ocular hypertension; 2016 Sept 21 [cited 2019 Nov 5]; [about 4 screens]. https://clinicaltrials.gov/ct2/show/study/NCT02914509?term=Ocular+Therapeutix&rank=3.
Driscoll A, Blizzard CD, Desai A, D’Abbraccio S, Langh J, Mangano J, Buff N, Metzinger JL, Goldstein MH, Gelormini A. Safety analysis of a sustained release travoprost intracameral hydrogel implant in beagle dogs. Invest Ophthalmol Vis Sci. 2018;59:1250.
Jarrett PK, Elhayek RF, Jarrett T, Lattrell Z, Kahn E, Takach S, Metzinger JL, Goldstein MH, Sawhney A. Efficacy & Tolerability of OTX-TKI, a sustained hydrogel delivery system for a tyrosine kinase inhibitor, in a VEGF induced retinal leakage model through 12 months. Invest Ophthalmol Vis Sci. 2018;59:3465.
Huu VA, Luo J, Zhu J, Zhu J, Patel S, Boone A, Mahmoud E, McFearin C, Olejniczak J, de Gracia Lux C, Lux J. Light-responsive nanoparticle depot to control release of a small molecule angiogenesis inhibitor in the posterior segment of the eye. J Control Release. 2015;28:71–7.
Brandt JD, Sall K, DuBiner H, Benza R, Alster Y, Walker G, Semba CP. Six-month intraocular pressure reduction with a topical bimatoprost ocular insert: results of a phase II randomized controlled study. Ophthalmology. 2016;123:1685–94.
Ocular Therapeutix. Dextenza® (dexamethasone ophthalmic insert) 0.4 mg for intraocular use. https://ocutx.com/products/dextenza. Accessed 7 Nov 2019.
Campochiaro P, Wykoff C, Brown D, Boyer D, Barakat M, Taraborelli D, Noronha G. Suprachoroidal triamcinolone acetonide for retinal vein occlusion: results of the tanzanite study. Retina. 2018;2:320–8.
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03203447, suprachoroidal injection of triamcinolone acetonide with IVT anti-VEGF in subjects with macular edema following RVO (TOPAZ); 2017 June 28 [cited 2019 Nov 5]; [about 5 screens]. https://clinicaltrials.gov/ct2/show/study/NCT03203447?term=ocular+microneedle&rank=9.
Thrimawithana TR, Young S, Bunt CR, Green C, Alany RG. Drug delivery to the posterior segment of the eye. Drug Discov Today. 2011;16:270–7.
Khandan O, Kahook MY, Rao MP. Fenestrated microneedles for ocular drug delivery. Sensors Actuators B Chem. 2016;223:15–23.
Thakur R, Tekko I, Al-Shammari F, Al A, McCarthy H, Donnelly R. Rapidly dissolving polymeric microneedles for minimally invasive intraocular drug delivery. Drug Deliv Transl Res. 2016;6:800–15.
Zelikin AN, Ehrhardt C, Healy AM. Materials and methods for delivery of biological drugs. Nat Chem. 2016;8:997–1007.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S. Ranibizumab versus Verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Kim LA, D’Amore PA. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol. 2012;181:376–9.
Park SJ, Oh J, Kim YK, Park JH, Park JY, Hong HK, Park KH, Lee JE, Kim HM, Chung JY, Woo SJ. Intraocular pharmacokinetics of intravitreal vascular endothelial growth factor-Trap in a rabbit model. Eye. 2015;29:561–8.
Giteau A, Venier-Julienne MC, Aubert-Pouessel A, Benoit JP. How to achieve sustained and complete protein release from PLGA-based microparticles. Int J Pharm. 2008;350:14–26.
Mohammadi-Samani S, Taghipour B. PLGA micro and nanoparticles in delivery of peptides and proteins; problems and approaches. Pharm Dev Technol. 2015;20:385–93.
Rubio RG. Long-acting anti-VEGF delivery. Retina Today. 2014;78–80. https://retinatoday.com/articles/2014-july-aug/long-acting-anti-vegf-delivery.
Campochiaro PA, Marcus DM, Awh CC, Regillo C, Adamis AP, Bantseev V, Chiang Y, Ehrlich JS, Erickson S, Hanley WD, Horvath J, Maass KF, Singh N, Tang F, Barteselli G. The port delivery system with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 LADDER clinical trial. Ophthalmology. 2019;126:1141–54.
Saati S, Lo R, Li PY, Meng E, Varma R, Humayun MS. Mini drug pump for ophthalmic use. Curr Eye Res. 2010;35:192–201.
Humayun M, Santos A, Altamirano JC, Ribeiro R, Gonzalez R, de la Rosa A, Shih J, Pang C, Jiang F, Calvilo P, et al. Implantable MicroPump for drug delivery in patients with diabetic macular edema. Transl Vis Sci Technol. 2014;3:5.
Lance KD, Bernards DA, Ciaccio NA, Good SD, Mendes TS, Kudisch M, Chan E, Ishikiriyama M, Bhisitkul RB, Desai TA. In vivo and in vitro sustained release of ranibizumab from a nanoporous thin-film device. Drug Deliv Transl Res. 2016;6:771–80.
Tao W. Application of encapsulated cell technology for retinal degenerative diseases. Expert Opin Biol Ther. 2006;6:717–26.
Kauper K, McGovern C, Sherman S, Heatherton P, Rapoza R, Stabilia P, Dean B, Lee A, Borges S, Bouchard B, et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative disease. Invest Ophthalmol Vis Sci. 2012;53:7484–91.
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02862938, study of NT-501 encapsulated cell therapy for glaucoma neuroprotection and vision restoration; 2016 Aug 11 [cited 2019 Nov 4]; [1 page]. https://clinicaltrials.gov/ct2/show/NCT02862938?cond=NT-501&rank=2.
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03071965, Extension study of NT-501 ciliary neurotrophic factor (CNTF) implant for macular telangiectasia (MacTel); 2017 Mar 7 [cited 2019 Nov 4]; [1 page]. https://clinicaltrials.gov/ct2/show/NCT03071965?cond=NT-501&rank=1.
Varshochian R, Jeddi-Tehrani M, Mahmoudi AR, Khoshayand MR, Atyabi F, Sabzevari A, Esfahani MR, Dinarvand R. The protective effect of albumin on bevacizumab activity and stability in PLGA nanoparticles intended for retinal and choroidal neovascularization treatments. Eur J Pharm Sci. 2013;50:341–52.
Varshochian R, Riazi-Esfahani M, Jeddi-Tehrani M, Mahmoudi AR, Aghazadeh S, Mahbod M, Movassat M, Atyabi F, Sabzevari A, Dinarvand R. Albuminated PLGA nanoparticles containing bevacizumab intended for ocular neovascularization treatment. J Biomed Mater Res A. 2015;103:3148–56.
Ye Z, Ji YL, Ma X, Wen JG, Wei W, Huang SM. Pharmacokinetics and distributions of bevacizumab by intravitreal injection of bevacizumab-PLGA microspheres in rabbits. Int J Ophthalmol. 2015;8:653–8.
Yeo Y, Park K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch Pharm Res. 2004;27:1–12.
Manoharan C, Singh J. Insulin loaded PLGA microspheres: effect of zinc salts on encapsulation, release, and stability. J Pharm Sci. 2009;98:529–42.
Marquette S, Peerboom C, Yates A, Denis L, Langer I, Amighi K, Goole J. Stability study of full-length antibody (anti-TNF alpha) loaded PLGA microspheres. Int J Pharm. 2014;470:41–50.
Moreno MR, Tabitha TS, Nirmal J, Radhakrishnan K, Yee CH, Lim S, Venkatraman S, Agrawal R. Study of stability and biophysical characterization of ranibizumab and aflibercept. Eur J Pharm Biopharm. 2016;108:156–67.
Liu W, Lee BS, Mieler WF, Kang-Mieler WF. Biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of bioactive aflibercept in vitro. Curr Eye Res. 2019;44:264–74.
Zhang K, Tang X, Zhang J, Lu W, Lin X, Zhang Y, Tian B, Yang H, He H. PEG-PLGA copolymers: their structure and structure-influenced drug delivery applications. J Control Release. 2014;183:77–86.
Adamson P, Wilde T, Dobrzynski E, Sychterz C, Polsky R, Kurali E, Haworth R, Tang CM, Korczynska J, Cook F, et al. Single ocular injection of a sustained-release anti-VEGF delivers 6 months pharmacokinetics and efficacy in a primate laser CNV model. J Control Release. 2016;244:1–13.
Jay SM, Saltzman WM. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. J Control Release. 2009;134:26–34.
Thackaberry EA, Farman C, Zhong F, Lorget F, Staflin K, Cercillieux A, Mieller AP, et al. Evaluation of the toxicity of intravitreally injected PLGA microspheres and rods in monkeys and rabbits: effects of depot size on inflammatory response. Invest Ophthalmol Vis Sci. 2017;58:4274–85.
Ocular Therapeutix. OTX-IVT (anti-VEGF antibody implant). https://ocutx.com/research/otx-ivt/. Accessed 7 Nov 2019.
Drapala PW, Brey EM, Mieler WF, Venerus DC, Kang-Derwent JJ, Perez-Luna VH. Role of thermo-responsiveness and poly(ethylene glycol) diacrylate cross-link density on protein release from poly(N-isopropylacrylamide) hydrogels. J Biomater Sci Polym Ed. 2011;22:59–75.
Drapala PW, Jiang B, Chiu YC, Mieler WF, Brey EM, Kang-Mieler JJ, Perez-Luna VH. The effect of glutathione as chain transfer agent in PNIPAAm-based thermo-responsive hydrogels for controlled release of proteins. Pharm Res. 2014;31:742–53.
Turturro SB, Guthrie MJ, Appel AA, Drapala PW, Brey EM, Perez-Luna VH, Mieler WF, Kang-Mieler JJ. The effect of cross-linked thermo-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials. 2011;32:3620–6.
Wang CH, Hwang YS, Chiang PR, Shen CR, Hong WH, Hsiue GH. Extended release of bevacizumab by thermosensitive biodegradable and biocompatible hydrogel. Biomacromolecules. 2012;13:40–8.
Rauck BM, Friberg TR, Medina-Mendez CA, Park D, Shah V, Bilonick RA, Wang Y. Biocompatible reverse thermal gel sustains the release of intravitreal bevacizumab in vivo. Invest Ophthalmol Vis Sci. 2014;55:469–76.
Yu Y, Lau LC, Lo AC, Chau Y. Injectable chemically crosslinked hydrogel for the controlled release of bevacizumab in vitreous: a 6-month in vivo study. Transl Vis Sci Technol. 2015;4:5.
Kanjickal D, Lopina S, Evancho-Chapman MM, Schmidt S, Donovan D. Effects of sterilization on poly(ethylene glycol) hydrogels. J Biomed Mater Res A. 2008;87:608–17.
Chang D, Park K, Famili A. Hydrogels for sustained delivery of biologics to the back of the eye. Drug Discov Today. 2019;24:1470–82.
Moreau MF, Chappard D, Lesourd M, Montheard JP, Basle MF. Free radicals and side products released during methylmethacrylate polymerization are cytotoxic for osteoblastic cells. J Biomed Mater Res. 1998;40:124–31.
Osswald CR, Kang-Mieler JJ. Controlled and extended release of a model protein from a microsphere-hydrogel drug delivery system. Ann Biomed Eng. 2015;43:2609–17.
Osswald CR, Kang-Mieler JJ. Controlled and extended in vitro release of bioactive anti-vascular endothelial growth factors from a microsphere-hydrogel drug delivery system. Curr Eye Res. 2016;41:1216–22.
Liu W, Borrell MA, Venerus DC, Mieler WF, Kang-Mieler JJ. Characterization of biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of ranibizumab. Transl Vis Sci Technol. 2019;8:12.
Osswald CR, Guthrie MJ, Avila A, Valio JA Jr, Mieler WF, Kang-Mieler JJ. In vivo efficacy of an injectable microsphere-hydrogel ocular drug delivery system. Curr Eye Res. 2017;42:1293–301.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kang-Mieler, J.J., Rudeen, K.M., Liu, W., Mieler, W.F. (2020). New Ocular Drug Delivery Systems. In: Chang, A., Mieler, W.F., Ohji, M. (eds) Macular Surgery. Springer, Singapore. https://doi.org/10.1007/978-981-15-7644-7_41
Download citation
DOI: https://doi.org/10.1007/978-981-15-7644-7_41
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7642-3
Online ISBN: 978-981-15-7644-7
eBook Packages: MedicineMedicine (R0)