Abstract
Increasing public attention to health issues and disease prevention has created a favorable global market for naturally derived nutraceuticals. This has led to a dramatic increase in demand for carotenoids in general and fucoxanthin (FX) in particular. This orange-colored compound has an array of health stimulating properties, including antioxidant, anticancer, anti-obesity, and anti-diabetic. It is currently isolated from seaweeds, but fast-growing diatoms, a class of microalgae, synthesize FX at higher levels, making them a promising candidate for sustainable FX production. Still, to produce diatom FX cost-effectively at large scale, significant improvements in productivity are quintessential. In the present chapter we provide an overview of FX biosynthesis by diatoms and the effect of various abiotic growth factors on FX production. Eventual commercial deployment of diatoms will depend on genetically constructing superior FX producing strains and optimizing the diatom cultivation conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
The new health paradigm towards well-being and disease prevention has led to an expansion of naturally derived compounds for the nutraceuticals and functional foods industry. Nutraceuticals have dual benefits of nutritional and pharmaceutical value and are intended to provide long-term health benefits, thereby improving a person’s quality of life. Among nutraceuticals, carotenoids are a rapidly emerging class that is naturally synthesized by algae, plants, yeasts, and bacteria (Mikami and Hosokawa 2013). Carotenoids (tetraterpenes) are pigment compounds with a characteristic eight isoprene (5-carbon) unit creating a linear 40 carbon backbone with up to 11 conjugated double bonds (Novoveská et al. 2019). Based on the presence of oxygen in their molecular structure, carotenoids are divided into two main categories: (1) Carotenes made of carbon and hydrogen, including α/β carotene and lycopene; and (2) Xanthophylls, which are oxygenated carotenes, such as fucoxanthin, astaxanthin, zeaxanthin, and lutein (Mohamadnia et al. 2019). In algae, xanthophylls are further classified as primary xanthophylls, which are located in the chloroplasts, and secondary xanthophylls, which are located in lipid vesicles inside either the plastid or cytosol (Bauer et al. 2019). The primary xanthophylls serve as structural and functional components of the algal cell photosynthetic apparatus with light harvesting antennas, whereas the secondary xanthophylls, including fucoxanthin, are metabolites synthesized by the algal cells in response to environmental stressors, such as nutrient depletion, light intensity, temperature, and salinity (Mohamadnia et al. 2019).
Among xanthophylls, fucoxanthin is a pro-vitamin A (can be converted to vitamin A by the human body) carotenoid with reportedly numerous biological activities and health stimulating properties, including antioxidant, anti-inflammatory, cardiovascular, anti-obesity, anti-diabetic, anti-angiogenic, and anti-malarial activity (Mohamadnia et al. 2019; Aslanbay Guler et al. 2020). It has also demonstrated protective effects against dermal, ophthalmic, bone, cerebrovascular, and cardiovascular disorders and is even consumed as a dietary supplement for weight loss (McClure et al. 2018). Moreover, it has been reported to exhibit broad anticancer and anti-proliferative activity in leukemic (H-60), epithelial colorectal adenocarcinoma, prostate cancer, urinary bladder cancer, breast cancer, lung cancer, and gastric cancer cell lines (Karpiński and Adamczak 2019). These biological activities of FX are due to its unique molecular structure that includes an allenic bond, a conjugated carbonyl with a 5,6-momoepoxide, and an acetyl group (Mohamadnia et al. 2019). Thanks to the broad spectrum of health benefits of fucoxanthin (FX), the global FX market is expected to increase from $88 million in 2019 to over $100 million over the next 5 years (Report, Global Fucoxanthin Market 2020).
In nature, FX is the orange-colored pigment found in Chromophyta (Heterokonphyta or Ochrophyta), including brown seaweeds (Phaeophyceae) and diatoms (Bacillarophyta) (Peng et al. 2011). Presently, FX is commercially produced from the brown macroalgae (seaweeds) Saccharina japonica, Undaria pinnatifda, Sargassum fusiforme, and Eisenia bicyclis, which contain 0.1–1.0 mg of FX per gram of macroalgal mass (Bauer et al. 2019; Sahin et al. 2019). However, brown seaweeds cannot meet the global demand for FX due to their slow growth, low yield, need for cell growth regulators, and quality concerns associated with heavy metal contamination of the oceans (Gómez-Loredo et al. 2016; Guo et al. 2016). As a result, diatoms are seen as far more promising organisms for the production of FX, since they yield 2.0–26.6 mg/g of FX, which is more than 10× higher than brown seaweeds, have shorter doubling time, can be cultivated in closed and controlled systems free of heavy metals, and are not affected by seasonal variations unlike seaweeds (Bauer et al. 2019).
The present chapter analyzes the studies reported to date on the production of FX from diatoms with emphasis on the effects that key abiotic factors, like light intensity, nutrient availability, and carbon sources, have on production of the pigment. Moreover, we review efforts to improve FX production in diatoms via genetic engineering of these algae.
10.2 Fucoxanthin Biosynthesis in Diatoms
Diatoms encompass a taxonomic group of about 200,000 different species responsible for an astounding 40% of natural carbon fixation via photosynthesis (Ikeda et al. 2008). They are the major ecological players in the biogeochemical cycling of carbon, nitrogen, and silicon (Depauw et al. 2012). Since their first appearance approximately 180 million years ago as a result of endosymbiosis of red algae and heterotrophic flagellates, they inhabit both marine and freshwater ecosystems (Veith et al. 2009; Zulu et al. 2018). Diatoms belong to the Stramenopile and Heterokont phyla under the Bacillariophyceae class (Zulu et al. 2018). Their most extraordinary feature is their ability to precipitate soluble silicic acid and incorporate it into a highly patterned cell wall (frustule) composed of silica, (SiO2)n(H2O), organized as nanostructured valves (Siaut et al. 2007; Veith and Büchel 2007; Baldisserotto et al. 2019). Compared to plants, they possess additional genes and a complex plastid with four membranes instead of two (Zulu et al. 2018).
Based on their frustule morphology, diatoms can be broadly classified into pennate (fresh water), radial centric (marine with circular valves), and bipolar centric (marine with bipolar valves) (Baldisserotto et al. 2019). Furthermore, although the photosynthetic apparatus of diatoms resembles that of higher plants and cyanobacteria, it has distinct thylakoids, which are not divided into discrete grana and stroma lamellae, but are instead organized into bands containing three thylakoids each with no distinction between photosystem I (PSI) and photosystem II (PS II) (Veith and Büchel 2007). Whereas in plants the core of PSII consists of 25 subunits surrounded by membrane proteins referred to as light harvesting centers (LHC), in diatoms PSII consists of fucoxanthin-chlorophyll (a and c)proteins (FCPs) responsible for harvesting sunlight and conducting energy transfer during photosynthesis (Büchel 2003; Bauer et al. 2019). FX absorbs visible light at 450–570 nm, which is blue green to yellow green with maximum absorbance between 510 and 525 nm, giving the diatoms their characteristic golden-brown color (Peng et al. 2011; Telussa et al. 2019). Three different classes of FCP genes have been characterized to date in diatoms: (1) fcp1-5 in Cyclotella cryptica and fcpA-F in Phaeodactylum tricornutum; (2) fcp4 in C. cryptica and a homologue gene in Thalassiosira pseudonana; and (3) i818 in Chlamydomonas reinhardtii and fcp6, 7, and 12 in C. cryptic (Veith and Büchel 2007). Among the diatoms, only a few genera have been studied to date, including Thalassiosira, Chaetoccros, Coscinodiscus, Skeletonema, Phaeodactylum, Nitzcia, and Cyclotella (Baldisserotto et al. 2019). However, only two of them, P. tricornutum (pennate) and T. pseudonana (centric), have been completely sequenced.
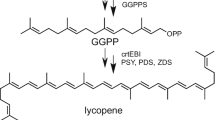
The biosynthesis of FX in the diatom P. tricornutum reportedly occurs via three different putative pathways: (1) the violaxanthin (Vlx) cycle; (2) the diadinoxanthin (Ddx) cycle; and (3) the β-cryptoxanthin cycle (Fig. 10.1). P. tricornutum utilizes the methylerythritol-4-phosphate (MEP) plastidic pathway, also known as the non-mevalonate pathway, for isoprenoid biosynthesis (Bauer et al. 2019). The pathway starts with the formation of 1-deoxy-d-xylulose 5-phosphate from glucose-derived glyceraldehyde-3-phosphate, catalyzed by 1-deoxy-d-xylulose 5-phosphate synthase (DXS). This is followed by the formation of isopentenyl diphosphate (IPP) and dimethyl allyl diphosphate (DMAPP). Then, the formation of geranylgeranyl phosphate (GGP) initiates biosynthesis of β-carotene, which is the starting molecule for all the three hypothesized pathways for FX biosynthesis. Phytoene synthase (psy) catalyzes the conversion of GGP to phytoene, followed by desaturation of phytoene to ξ-carotene using phytoene desaturase (pds). Then, ξ-carotene desaturase (zds) catalyzes the formation of prolycopene followed by conversion to lycopene using lycopene β-cyclase and then to β-carotene (β-Car), which is the actual precursor molecule for FX biosynthesis, as depicted in Fig. 10.1 (Depauw et al. 2012).
The Violaxanthin (Vlx) cycle starts with the sequential conversion of β-Carotene to Zeaxanthin (Zea), followed by reversible conversion to Antheraxanthin (Atx) and Violaxanthin (Vlx) with both reactions being catalyzed by Zeaxanthin epoxide (ZEP). Vlx is subsequently converted to Neoxanthin (Nex) and then either to FX or to Diadinoxanthin (Ddx) (Cui et al. 2019). The Ddx cycle follows the same pathway as the Vlx cycle, i.e. β-Car→Zea→Atx→Vlx. Subsequently, Vlx gets converted to Ddx, which can then be converted to FX or diatoxanthin (Dtx). The third pathway results in the conversion of β-Car to β-cryptoxanthin (β-Cpx) and then to β-cryptoxanthin 5, 6-epoxide followed by conversion to Vlx, thereby resulting in the formation of Ddx, which in turn is converted to FX or Dtx (Cui et al. 2019). However, these pathways have not be fully elucidated or experimentally validated yet due to missing information and lack of characterization of several enzymes involved in the conversion of intermediate products, as depicted in Fig. 10.1.
10.3 Abiotic Factors Affecting Fucoxanthin Production
The cultivation conditions significantly impact the growth characteristics and biochemical composition of diatoms. Important abiotic factors are light intensity and wavelength, nutrient depletion/deprivation, and carbon utilization mode (autotrophic, heterotrophic or mixotrophic). Other factors include temperature, agitation speed, salinity, and mode of cultivation (batch, continuous or fed batch). Carotenoids in diatoms are potent antioxidant molecules that are synthesized in large quantities during any environmental stress in order to enable diatom cells to quench the formation of reactive oxygen species (ROS) and re-establish cellular homeostasis (De Jesus Raposo et al. 2015). To this end, various abiotic stress strategies have been utilized to augment FX production in a range of diatoms (Table 10.1).
Since FX is involved in light harvesting and photoprotection of diatoms, light intensity and spectra significantly impact its biosynthesis. It is under low light intensity that biosynthesis of FX is activated in order to help the cellular metabolism capture and transport photon energy as efficiently as possible (Nymark et al. 2009). FX binds to specific proteins present in the photosynthetic apparatus (Li et al. 2019). In contrast, under high light intensity, the FCPs become supersaturated, resulting in photoinhibition and oxidative stress (Nymark et al. 2009). This stimulates synthesis of secondary carotenoids, such as astaxanthin and β-carotene, at the expense of FX through modulation of the Ddx cycle (Fig. 10.1). Hence, low light intensity is favorable to FX production, but the exact intensity for maximum FX production varies among diatom species. In P. tricornutum, maximum FX yield (productivity) of 42.8 mg/g was reported when the culture was illuminated with 100 μmol photons m−2 s−1, whereas at 150 μmol photons m−2 s−1 FX productivity dropped to half (23.2 mg/g) (Table 10.1).
Although cultivation at low light intensity increases FX yield, it slows down the overall metabolism and growth of diatoms reducing biomass productivity and hence resulting in a trade-off between biomass production (cell growth) and FX biosynthesis. For diatoms to serve at large scale as a viable source for commercial FX production, the use of outdoor cultivation systems will be imperative from a process and economic standpoint, necessitating the use of diatom strains that can synthesize high levels of FX under natural high light intensities. One such promising strain is Odontella aurita, which produced 12.5 mg/g of FX when cultivated under 300 μmol photons m−2 s−1at a peak biomass concentration of 5.84 g/L (Li et al. 2019).
In addition to light intensity, nitrogen also has a major effect on FX yield of diatoms, as nitrogen is an essential nutrient involved in the biosynthesis and regulation of cellular metabolites (Guo et al. 2016). In algae, including diatoms, nitrogen is assimilated in the form of ammonium (NH4+) by glutamate synthase/glutamine synthetase leading to formation of glutamate, which is the precursor for amino acids and chlorophyll synthesis (Alipanah et al. 2018). The genomes of both P. tricornutum and T. pseudonana contain transporter proteins for the uptake of inorganic and organic nitrogen compounds. The most common nitrogen source used for the cultivation of diatoms is nitrate, which is first reduced to nitrite by nitrate reductase and is then converted to ammonium by nitrite reductase (Arora et al. 2019).
A directly proportional relationship has been established between nitrogen availability and FX production in diatoms, as increasing the nitrogen concentration in the growth media resulted in enhanced FX productivity (Table 10.1). This could be due to the upregulation of chlorophyll biosynthesis in high nitrogen presence, thereby also promoting FX production (Guo et al. 2016). When the effect of nitrogen depleted (0 g/L) and nitrogen-rich media (2 g/L) was studied with two diatom strains, Nitzschia sp. and Nanofrustulum shiloi, Nitzschia sp. was not able to grow under nitrogen depleted conditions, while N. shiloi grew, but both its biomass production and FX yield were significantly lower than in nitrogen-rich media (Sahin et al. 2019). Moreover, the combined effect of nitrogen and light intensity has been studied in various diatom strains as listed in Table 10.1. In Odontella aurita, five different combinations of light and nitrogen were studied: low light with low nitrogen, high light with high nitrogen, and high light with high nitrogen and supplementary nitrogen (Table 10.1). Among these combinations, the latter with supplementary nitrogen resulted in maximum FX productivity of 25 mg/g (Xia et al. 2018). These results confirm that continuous supply of nitrogen boosts FX biosynthesis in diatoms and should be used at large scale to achieve high FX yields.
Besides nitrogen, phosphorous and iron may also have an effect on FX production as they are essential components of the photosynthetic and cellular mechanisms of diatoms (Table 10.1). Phosphorous is an important nutrient aiding in nitrate absorption, photosynthetic respiration, energy transfer, signal transduction, and biosynthesis of nucleic acids, lipids, and other metabolites (Arora et al. 2015; Alipanah et al. 2018). To date, only one study has studied the effect of phosphorous on FX production in Isochrysis sp. (Sun et al. 2019b). The authors reported no significant changes in biomass or FX production irrespective of phosphate concentration in the growth media (1.13, 2.25, and 4.50 mg/L) (Table 10.1). However, this could be a species-specific response and more detailed studies are imperative to establish the possible effect of phosphorous on FX biosynthesis in other diatom species.
Iron (Fe) is responsible for the biosynthesis of a protoporphyrin precursor, δ-aminolevuline acid, which is involved in chlorophyll synthesis (Kosakowska et al. 2004). Furthermore, iron is a component of both cytochromes b and c, the electron transport chain (ferrodoxine), photorespiration, enzymes involved in nitrogen assimilation, and activators of peroxidase and catalase (Kosakowska et al. 2004).Similar to nitrogen, deficiency in Fe led to a decrease in both biomass and FX production in Nitzschia sp. and N. shiloi (Sahin et al. 2019). Iron deficiency appears to result in photooxidation and activation of a photoprotective cycle triggering the biosynthesis of secondary carotenoids and Dtx, as opposed to FX and light harvesting pigments.
Another important factor in FX production is the mode of carbon utilization by diatoms. Diatom growth can be autotrophic (assimilation of inorganic carbon (CO2) + light), heterotrophic (utilization of organic carbon + dark), and mixotrophic (uptake of both CO2 and organic carbon + light). The preferable mode for maximum cell growth and FX production is species-specific, but overall it appears that algae grown mixotrophically achieve higher biomass productivity, since photorespiration does not impact growth. Cultivation of Nitzschia laevis under mixotrophic conditions resulted in higher biomass and FX yield as compared to heterotrophic conditions (Table 10.1). Interestingly, C. cryptica could grow heterotrophically with similar FX production, 7.7 mg/g, as autotrophically (Guo et al. 2016). However, when the authors also explored the synergistic effect of nitrogen and heterotrophy, higher FX productivity was achieved in the nitrogen-rich media under light conditions indicating that for this particular strain concentration of nitrogen is more crucial than carbon utilization mode for FX production (Table 10.1).
In order to reduce growth media cost, some new low-cost media were recently tested with diatoms in an attempt to improve the economics of future scale up and commercial production of FX (Table 10.1). The list of media included palm oil mill effluent, sea water, and spent yeast cell hydrolysate (Ishika et al. 2019; Nur et al. 2019; Yuan et al. 2019). Unfortunately, none of these media was able to match the FX productivity obtained when diatoms were cultivated in the costlier standard media F/2.
10.4 Genetic Engineering Strategies to Improve Fucoxanthin Productivity
Among the numerous diatom strains producing FX, only a few have been successfully genetically engineered, including P. tricornutum, Cylindrotheca fusiformis, C. cryptica, Navicula saprophila, Fistulifera solaris, T. pseudonana, and Halamphora coffeaeformis (Poulsen and Kröger 2005; Velmurugan and Deka 2018). Furthermore, although there is extensive literature on the overproduction of lipids from P. tricornutum, very few studies have attempted to specifically enhance FX production (Lavaud et al. 2012; Kadono et al. 2015; Eilers et al. 2016). In order to successfully introduce heterologous (foreign DNA) or overexpress the endogenous genes in the genome of diatoms, the development of an appropriate genetic toolbox is quintessential (Huang and Daboussi 2017). Such a toolbox should include: (1) an expression vector system with all the essential elements, namely promoters, ribosome binding sites, terminators, and 5′ UTR (untranslated region) and 3′ UTR sites; (2) selectable markers for isolation and identification of transformed cells; and (3) efficient transformation techniques for DNA delivery, homologous recombination, and strain stability.
Both constitutive and inducible promoters have been identified and tested for the overexpression of desired genes in diatoms. To date, various endogenous constitutive promoters have been identified in diatoms, such as the promoters associated with the expression of numerous genes, including the light harvesting complex protein (lhcf 1-15), the histone gene (h4), the elongation factor 2 (ef2) gene, the ammonium transporter (amt) gene, and the purine permease (pup) and diacylglycerol acyltransferase (dgat1) genes (Huang and Daboussi 2017; Adler-Agnon (Shemesh) et al. 2018; Watanabe et al. 2018). The Lhcf promoters, in particular, have been widely used for overexpression of various genes in several diatom strains, but their dependence on light availability makes them unsuitable for FX gene expression under dark conditions (Huang and Daboussi 2017). On the other hand, although the h4 promoter is light independent, it yields low levels of expression compared to the Lchf promoters. The ef2 promoter does not require light and has proven to be effective in terms of expression (1.2 fold higher than Lchf2) (Huang and Daboussi 2017).
Endogenous inducible promoters include those for nitrate reductase (nr), iron starvation induced protein 1 (Isi 1), ferrichrome binding protein 1(fbp1), flavodixin (fld genes), and a CO2 responsive promoter derived from the carbonic anhydrase gene (ca1) (Huang and Daboussi 2017). Although inducible promoters offer the advantage of controlled gene expression, their use at industrial scale is problematic because of operational issues (extensive cleanliness needed to remove any trace metals that could untimely induce the promoters) and the need to cultivate the diatoms in inducer-free media, which may generate stress on the cells. Unlike the plethora of promoters, only a few endogenous terminators have been identified in diatoms, namely Lhcf1, Lhcf9, Lhcr14, nr, and rubisco small subunit (rbcL) (Huang and Daboussi 2017).
The genetic transformation methods available for diatoms include biolistic, electroporation, and conjugation (Bozarth et al. 2009). Among them, the biolistic method is the most successful one achieving ~90% transformation efficiency in the chromosome of diatoms, when using Zeocin as selection marker (Velmurugan and Deka 2018). Apart from the cell nucleus, chloroplast transformation has also been successfully reported in P. tricornutum, particularly for overexpressing genes of prokaryotic origin (Bozarth et al. 2009).
In another genetic attempt to enhance FX production, the violaxanthin De-epoxide gene (VDE) was silenced in P. tricornutum (Lavaud et al. 2012). The VDE gene catalyzes the de-epoxidation of violaxanthin (Vlx) to Zeaxanthin (Zea) and then its subsequent conversion to Dtx, thereby channelling the flux of Zea eventually to FX (Fig. 10.1). However, this knockdown did not result in overproduction of FX, possibly due to the presence of multiple pathways for FX production, as outlined earlier (Lavaud et al. 2012). In another study, the psy gene obtained from P. tricornutum and dxs1 gene obtained from corn were overexpressed in P. tricornutum (Eilers et al. 2016). The authors reported an increase in FX yield to 24.2 mg/g in the psy transformed cells and 18.4 mg/g in dxs1 transformed lines, as compared to the wild type (10 mg/g). In contrast, when the psy gene was overexpressed in P. tricornutum, no FX augmentation was observed (Kadono et al. 2015).
10.5 Conclusion and Future Perspectives
The health-boosting benefits of FX supplementation in several preclinical and a few clinical studies have significantly increased its demand worldwide. Among the potential sources for FX production, diatoms represent one of the most promising groups of microorganisms that could be commercially exploited thanks to their fast growth rate, high FX yield, and ability to grow in enclosed controlled systems compared to currently used seaweeds. Indicative of the diatom potential is the recent expansion of commercial FX production from P. tricornutum by an Israeli biotechnology company that cultivates the diatom in closed photobioreactors under natural light conditions to meet global demand (algatech.com). Over the last few years, researchers have filled crucial gaps in diatom physiology and FX biosynthesis. Nevertheless, there is still (1) from a genetic standpoint, a need for full elucidation of the carotenoid and FX biosynthesis mechanisms in diatoms, and (2) from a process engineering standpoint, a need for better understanding of the effect of additional abiotic factors, such as temperature, inoculum size, bioreactor operation, and salinity, on FX production. All this knowledge is imperative to propel diatom-based FX production from lab scale to commercial scale.
Although there are about 200,000 diatom species, identification of efficient FX producing strains and optimization of nutrient and abiotic parameters remain a big challenge. Progress on those issues can be achieved by screening and down-selecting more diatom strains possessing high growth rate, high FX yield, resistance to algal predators, ability to grow in saline water or wastewater, and ability to easily flocculate for cost-effective downstream processing and extraction and purification of FX. It is rather unlikely that a single diatom strain will naturally possess all these characteristics, hence genetic engineering tools will be required to further enhance promising natural strains. Recent advances in genetics, such as suppression/knockdown of target genes using RNAi silencing, CRISPR-Cas9, and transcription activator-like effect or nuclease, present a great opportunity to design improved diatoms. However, such tools can be best used once there is in-depth understanding of carotenoid and FX biosynthesis, which can benefit from the integration of OMICS technologies, such as genomics, transcriptomics, proteomics, metabolomics, and fluxomics. In parallel with genetic engineering approaches, we recommend the use of genome modification techniques, such as chemical mutations and adaptive laboratory evolution (ALE), to select for high FX yield diatoms among both natural and genetically modified strains.
References
Adler-Agnon (Shemesh) Z, Leu S, Zarka A et al (2018) Novel promoters for constitutive and inducible expression of transgenes in the diatom Phaeodactylum tricornutum under varied nitrate availability. J Appl Phycol 30:2763–2772. https://doi.org/10.1007/s10811-017-1335-8
Alipanah L, Winge P, Rohloff J et al (2018) Molecular adaptations to phosphorus deprivation and comparison with nitrogen deprivation responses in the diatom Phaeodactylum tricornutum. PLoS One 13:1–24. https://doi.org/10.1371/journal.pone.0193335
Arora N, Patel A, Pruthi PA, Pruthi V (2015) Synergistic dynamics of nitrogen and phosphorous influences lipid productivity in Chlorella minutissima for biodiesel production. Bioresour Technol 213:79–87. https://doi.org/10.1016/j.biortech.2016.02.112
Arora N, Tripathi S, Pruthi PA et al (2019) Assessing the robust growth and lipid-accumulating characteristics of Scenedesmus sp. for biodiesel production. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-07023-8
Aslanbay Guler B, Deniz I, Demirel Z et al (2019) Comparison of different photobioreactor configurations and empirical computational fluid dynamics simulation for fucoxanthin production. Algal Res 37:195–204. https://doi.org/10.1016/j.algal.2018.11.019
Aslanbay Guler B, Deniz I, Demirel Z et al (2020) A novel subcritical fucoxanthin extraction with a biorefinery approach. Biochem Eng J 153:107403. https://doi.org/10.1016/j.bej.2019.107403
Baldisserotto C, Sabia A, Ferroni L, Pancaldi S (2019) Biological aspects and biotechnological potential of marine diatoms in relation to different light regimens. World J Microbiol Biotechnol 35:1–9. https://doi.org/10.1007/s11274-019-2607-z
Bauer CM, Schmitz C, Corrêa RG et al (2019) In vitro fucoxanthin production by the Phaeodactylum tricornutum diatom. Stud Nat Products Chem 63:211–242
Bozarth A, Maier UG, Zauner S (2009) Diatoms in biotechnology: modern tools and applications. Appl Microbiol Biotechnol 82:195–201. https://doi.org/10.1007/s00253-008-1804-8
Büchel C (2003) Fucoxanthin-chlorophyll proteins in diatoms: 18 and 19 kDa subunits assemble into different oligomeric states. Biochemistry 42:13027–13034. https://doi.org/10.1021/bi0349468
Cui H, Ma H, Cui Y et al (2019) Cloning, identification and functional characterization of two cytochrome P450 carotenoids hydroxylases from the diatom Phaeodactylum tricornutum. J Biosci Bioeng 128:755–765. https://doi.org/10.1016/j.jbiosc.2019.06.008
De Jesus Raposo MF, De Morais AMMB, De Morais RMSC (2015) Carotenoids from marine microalgae: a valuable natural source for the prevention of chronic diseases. Mar Drugs 13:5128–5155. https://doi.org/10.3390/md13085128
Depauw FA, Rogato A, D’Alcalá MR, Falciatore A (2012) Exploring the molecular basis of responses to light in marine diatoms. J Exp Bot 63:1575–1591. https://doi.org/10.1093/jxb/ers005
Eilers U, Bikoulis A, Breitenbach J et al (2016) Limitations in the biosynthesis of fucoxanthin as targets for genetic engineering in Phaeodactylum tricornutum. J Appl Phycol 28:123–129. https://doi.org/10.1007/s10811-015-0583-8
Global Fucoxanthin Market (2020) Business opportunities, current trends, market challenges & global industry analysis by 2026. Global Fucoxanthin Market. Reterived 1 Sept 2020
Gómez-Loredo A, Benavides J, Rito-Palomares M (2016) Growth kinetics and fucoxanthin production of Phaeodactylum tricornutum and Isochrysis galbana cultures at different light and agitation conditions. J Appl Phycol 28:849–860. https://doi.org/10.1007/s10811-015-0635-0
Guo B, Liu B, Yang B et al (2016) Screening of diatom strains and characterization of Cyclotella cryptica as a potential fucoxanthin producer. Mar Drugs 14. https://doi.org/10.3390/md14070125
Huang W, Daboussi F (2017) Genetic and metabolic engineering in diatoms. Philos Trans R Soc B Biol Sci 372. https://doi.org/10.1098/rstb.2016.0411
Huang JJ, Lin S, Xu W, Cheung PCK (2018) Enhancement of the production of bioactive microalgal metabolites by ultraviolet radiation (UVA 365 nm). J Agric Food Chem 66:10215–10224. https://doi.org/10.1021/acs.jafc.8b03789
Ikeda Y, Komura M, Watanabe M et al (2008) Photosystem I complexes associated with fucoxanthin-chlorophyll-binding proteins from a marine centric diatom, Chaetoceros gracilis. Biochim Biophys Acta Bioenerg 1777:351–361. https://doi.org/10.1016/j.bbabio.2008.01.011
Ishika T, Laird DW, Bahri PA, Moheimani NR (2019) Co-cultivation and stepwise cultivation of Chaetoceros muelleri and Amphora sp. for fucoxanthin production under gradual salinity increase. J Appl Phycol 31:1535–1544. https://doi.org/10.1007/s10811-018-1718-5
Kadono T, Kira N, Suzuki K et al (2015) Effect of an introduced phytoene synthase gene expression on carotenoid biosynthesis in the marine diatom Phaeodactylum tricornutum. Mar Drugs 13:5334–5357. https://doi.org/10.3390/md13085334
Karpiński TM, Adamczak A (2019) Fucoxanthin—an antibacterial carotenoid. Antioxidants 8:1–8. https://doi.org/10.3390/antiox8080239
Kosakowska A, Lewandowska J, Stoń J, Burkiewicz K (2004) Qualitative and quantitative composition of pigments in Phaeodactylum tricornutum (Bacillariophyceae) stressed by iron. Biometals 17:45–52. https://doi.org/10.1023/A:1024452802005
Lavaud J, Materna AC, Sturm S et al (2012) Silencing of the violaxanthin de-epoxidase gene in the diatom Phaeodactylum tricornutum reduces diatoxanthin synthesis and non-photochemical quenching. PLoS One 7. https://doi.org/10.1371/journal.pone.0036806
Li Y, Sun H, Wu T et al (2019) Storage carbon metabolism of Isochrysis zhangjiangensis under different light intensities and its application for co-production of fucoxanthin and stearidonic acid. Bioresour Technol 282:94–102. https://doi.org/10.1016/j.biortech.2019.02.127
Lu X, Sun H, Zhao W et al (2018) A hetero-photoautotrophic two-stage cultivation process for production of fucoxanthin by the marine diatom Nitzschia laevis. Mar Drugs 16. https://doi.org/10.3390/md16070219
Lu X, Liu B, He Y et al (2019) Novel insights into mixotrophic cultivation of Nitzschia laevis for co-production of fucoxanthin and eicosapentaenoic acid. Bioresour Technol 294:122145. https://doi.org/10.1016/j.biortech.2019.122145
McClure DD, Luiz A, Gerber B et al (2018) An investigation into the effect of culture conditions on fucoxanthin production using the marine microalgae Phaeodactylum tricornutum. Algal Res 29:41–48. https://doi.org/10.1016/j.algal.2017.11.015
Mikami K, Hosokawa M (2013) Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int J Mol Sci 14:13763–13781. https://doi.org/10.3390/ijms140713763
Mohamadnia S, Tavakoli O, Faramarzi MA, Shamsollahi Z (2019) Production of fucoxanthin by the microalga Tisochrysis lutea: a review of recent developments. Aquaculture 734637. https://doi.org/10.1016/j.aquaculture.2019.734637
Novoveská L, Ross ME, Stanley MS et al (2019) Microalgal carotenoids: a review of production, current markets, regulations, and future direction. Mar Drugs 17:1–21. https://doi.org/10.3390/md17110640
Nur MMA, Muizelaar W, Boelen P, Buma AGJ (2019) Environmental and nutrient conditions influence fucoxanthin productivity of the marine diatom Phaeodactylum tricornutum grown on palm oil mill effluent. J Appl Phycol 31:111–122. https://doi.org/10.1007/s10811-018-1563-6
Nymark M, Valle KC, Brembu T et al (2009) An integrated analysis of molecular acclimation to high light in the marine diatom Phaeodactylum tricornutum. PLoS One 4. https://doi.org/10.1371/journal.pone.0007743
Peng J, Yuan JP, Wu CF, Wang JH (2011) Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Mar Drugs 9:1806–1828. https://doi.org/10.3390/md9101806
Poulsen N, Kröger N (2005) A new molecular tool for transgenic diatoms: control of mRNA and protein biosynthesis by an inducible promoter-terminator cassette. FEBS J 272:3413–3423. https://doi.org/10.1111/j.1742-4658.2005.04760.x
Sahin MS, Khazi MI, Demirel Z, Dalay MC (2019) Variation in growth, fucoxanthin, fatty acids profile and lipid content of marine diatoms Nitzschia sp. and Nanofrustulum shiloi in response to nitrogen and iron. Biocatal Agric Biotechnol 17:390–398. https://doi.org/10.1016/j.bcab.2018.12.023
Siaut M, Heijde M, Mangogna M et al (2007) Molecular toolbox for studying diatom biology in Phaeodactylum tricornutum. Gene 406:23–35. https://doi.org/10.1016/j.gene.2007.05.022
Sun P, Wong CC, Li Y et al (2019a) A novel strategy for isolation and purification of fucoxanthinol and fucoxanthin from the diatom Nitzschia laevis. Food Chem 277:566–572. https://doi.org/10.1016/j.foodchem.2018.10.133
Sun Z, Wang X, Liu J (2019b) Screening of Isochrysis strains for simultaneous production of docosahexaenoic acid and fucoxanthin. Algal Res 41:101545. https://doi.org/10.1016/j.algal.2019.101545
Telussa I, Rusnadi, Nurachman Z (2019) Dynamics of β-carotene and fucoxanthin of tropical marine Navicula sp. as a response to light stress conditions. Algal Res 41:101530. https://doi.org/10.1016/j.algal.2019.101530
Veith T, Büchel C (2007) The monomeric photosystem I-complex of the diatom Phaeodactylum tricornutum binds specific fucoxanthin chlorophyll proteins (FCPs) as light-harvesting complexes. Biochim Biophys Acta Bioenerg 1767:1428–1435. https://doi.org/10.1016/j.bbabio.2007.09.004
Veith T, Brauns J, Weisheit W et al (2009) Identification of a specific fucoxanthin-chlorophyll protein in the light harvesting complex of photosystem I in the diatom Cyclotella meneghiniana. Biochim Biophys Acta Bioenerg 1787:905–912. https://doi.org/10.1016/j.bbabio.2009.04.006
Velmurugan N, Deka D (2018) Transformation techniques for metabolic engineering of diatoms and haptophytes: current state and prospects. Appl Microbiol Biotechnol 102:4255–4267
Wang S, Verma SK, Hakeem Said I et al (2018) Changes in the fucoxanthin production and protein profiles in Cylindrotheca closterium in response to blue light-emitting diode light. Microb Cell Factories 17:1–13. https://doi.org/10.1186/s12934-018-0957-0
Watanabe Y, Kadono T, Kira N et al (2018) Development of endogenous promoters that drive high-level expression of introduced genes in the model diatom Phaeodactylum tricornutum. Mar Genomics 42:41–48. https://doi.org/10.1016/j.margen.2018.06.003
Xia S, Wang K, Wan L et al (2013) Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar Drugs 11:2667–2681. https://doi.org/10.3390/md11072667
Xia S, Gao B, Fu J et al (2018) Production of fucoxanthin, chrysolaminarin, and eicosapentaenoic acid by Odontella aurita under different nitrogen supply regimes. J Biosci Bioeng 126:723–729. https://doi.org/10.1016/j.jbiosc.2018.06.002
Yuan X, Liang L, Liu K et al (2019) Spent yeast as an efficient medium supplement for fucoxanthin and eicosapentaenoic acid (EPA) production by Phaeodactylum tricornutum. J Appl Phycol. https://doi.org/10.1007/s10811-019-01909-3
Zulu NN, Zienkiewicz K, Vollheyde K, Feussner I (2018) Current trends to comprehend lipid metabolism in diatoms. Prog Lipid Res 70:1–16. https://doi.org/10.1016/j.plipres.2018.03.001
Acknowledgments
The authors wish to thank the Patel College of Global Sustainability at the University of South Florida for its financial support.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Arora, N., Philippidis, G.P. (2021). Fucoxanthin Production from Diatoms: Current Advances and Challenges. In: Mandotra, S.K., Upadhyay, A.K., Ahluwalia, A.S. (eds) Algae. Springer, Singapore. https://doi.org/10.1007/978-981-15-7518-1_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-7518-1_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7517-4
Online ISBN: 978-981-15-7518-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)