Abstract
Polymeric insulators are being extensively used for outdoor insulation due to their excellent hydrophobic property. Several studies have been reported to evaluate the performance of these insulators as outdoor insulation with micro and nanofillers. These studies indicate, aged insulators under different environmental conditions exhibit considerably poor performance due to reduced tracking and erosion resistance. The present paper discusses the development of micro-filled magnesium oxide silicone rubber (MgOSiR) specimen and its performance for high voltage insulation under various environmental conditions. In the present study, 24 h water absorbance test, inclined plane test (IPT), flashover experiments have been conducted on the developed 30 weight percent (30 wt%) micromagnesium oxide-filled Silicone Rubber specimens (30 MMg) and commercially available Silicone Rubber specimens. The characterization using Fourier Transform infrared spectroscopy (FTIR) indicates micro-filled sample has better resistance to degradation and good hydrophobic recovery. The experimental results reveal that, developed specimens show superior hydrophobic property, tracking and erosion resistance and higher flashover voltage under dry and wet conditions compared to commercial silicone rubber specimen.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Polymeric insulators are being extensively used in outdoor insulation application over conventional ceramic counterparts due to several advantages. Various environmental and electrical stresses are the prime factors affecting its long-term performance under contaminated conditions. Accelerated ageing and surface degradation affecting the electrical withstand ability of these insulators make it fail at normal operating voltage leading to outages, causing huge financial loss [1].
The property evaluation in terms of tracking and erosion resistance is one of the prime factors to be considered to access its performance under contaminated conditions. The improvement in this property can be achieved by incorporating micro- /nano-inorganic oxide. The fillers are added to improve polymers specific properties such as resistance to degradation, hydrophobicity, and thermal properties. The properties improvement depends on size, shape, structure, degree of dispersion, orientation in matrix and degree of adhesion with polymer chains.
In the high voltage power networks, insulation must possess excellent pollution performance, better dielectric strength, long tracking time, and desirable mechanical properties. Partial discharges in these systems may cause physical damage and change in chemical structure. This may lead to failure of the insulators. In this regard, author have carried out work in [2] to improve the above-mentioned parameters in EPDM co-filled with Boron nitride particles at microlevel/nanolevel. The co-filled composites show better dielectric strength, high volume resistivity, and longer tracking time. Enhanced surface area of the fillers, improved thermal conductivity, and better thermal stability are the factors responsible for its improvement under contaminated conditions.
High-temperature-vulcanized silicone rubber is a kind of material used in insulation application due to its self extinguishing property. By incorporation of Alumina Trihydrate filler at microlevel, its flame retardant property is improved. But occasional tracking phenomena on these ATH-filled insulator lead to degradation affecting its pollution performance. So researchers in [3] have carried out a study to see the effect of different halogen-free flame retardant additives on tracking and erosion resistance. An inclined plane test set up was designed according to the standards. Melamine Cyanurate as an additive was used as a filler along with ATH which showed least dielectric loss and permittivity. The arc quenching mechanism of melamine is seen as the major factor for the improvement of tracking and erosion resistance of these specimens.
Researchers in [4] have discussed the use of various inorganic fillers such as alumina-trihydrate, zinc oxide, titanium dioxide and barium titanate to improve electrical, mechanical, and thermal properties of outdoor insulation. The concentration level of microfillers with 30 and 40 weight percent is proposed. The excessive addition of the fillers at microlevel/nanolevel over certain percentage is said to improve electrical property but may hinder hydrophobic and mechanical properties. The problem encountered with these nanoparticles as they tend to agglomerate easily because of their high surface energy.
The presence of nanofillers affects the space charge accumulation in the polymer matrix. The accumulation of space charge could affect the internal electric field this may ultimately cause in failure of the insulation system due to partial discharges and electrical treeing. So the inorganic fillers provide reduced space charge accumulation affecting the dielectric properties, enhancing the lifetime of the polymers. In [5], the uses of various inorganic oxides in the field of high voltage applications have been reported. Few insulating oxides such as Al2O3 provide high thermal conductivity, TiO2 gives photo catalytic property, Calcium Carbonate can be employed for low cost applications, SiC yields good mechanical strength, and ZnO used to control electric stress. These properties can be achieved only when there is homogeneous distribution in the polymer base matrix.
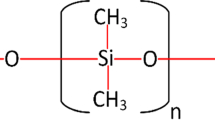
Room temperature-vulcanized rubber (RTV) SiR is frequently recommended for outdoor insulation due to hydrocarbons and Si–O bond. These chemical structures make their insulating and hydrophobicity excellent. As they are organic in nature, they may get degraded due to environmental and electrical stress. Authors in [6] have carried out studies to investigate the long-term performance of RTV SiR insulating specimens under multiple stress environments. They prepared composites by incorporating micro/nanosilica on to the RTV base. The specimens were subjected to 9000 h of aging in a multi-stress chamber with UV, temperature, humidity, acid rain, and heat and fog conditions. From the characterization results, it was concluded that the samples with micro/nano-additives were least aged compared to virgin RTV specimens. The leakage current was found to be less and micro- /nano-filled samples were found to possess good hydrophobic property.
High voltage polymeric insulator's surface gets deteriorated with deposition of contaminants. It further degrades when these contaminated insulators are subject to temperature, humidity, and sand storms. But in the case of desert environments, frequent sandstorms increases the surface roughness cause chemical changes on its surface affecting its flashover voltage. So author in [7] have carried out study on electrical, mechanical, and thermal properties of silicone rubber specimens filled with alumina trihydrate (ATH), Boric acid, and Magnesium Hydroxide. The result showed that the flashover voltage is improved by incorporation of ATH and Boric acid. ATH fillers is seen to have better thermal and flame retardant properties. Mechanical properties of ATH fillers were found to be better due to improvement in the tensile strength.
In [8], researchers have carried out series of experiments to study the effect of micro/nanoSiO2 on mechanical, electrical, and thermal properties. The SiR, EPDM, and Epoxy composites were prepared with micro/nano-additives. Thermal stability was found to be better in SIR composites. Highest dielectric strength was found in epoxy composites. EPDM showed better tensile and elongation at break. Authors concluded that the silicone micro/nanocomposites suit insulation applications. The researchers have carried out the pollution flashover (FoV) experiments on micro- /nano-filled silicone rubber specimen with SiO2, Boric acid, and Mg(OH)2 for different levels of concentration. Authors have carried out study to understand the fillers influence on mechanical and thermal properties.
The use of insulating oxides such as ZnO, MgO used at microlevel/nanolevel to improve the electrical properties of the polymeric specimens is reported in [5]. The MgO has high melting point, better dielectric strength, and good hydrophobicity than the ATH-filled silicone rubber. The mixing of MgO into polymer base may lead to better breakdown strength. But its performance evaluation of silicone rubber specimen under contaminated conditions incorporating MgO oxides at microlevel is yet to be investigated. Further flashover tests of these micro-Mgo-filled SiR is necessary for the outdoor insulation applications. This will help high voltage engineers to choose good material among the alternatives which performs better under contaminated conditions.
In the present work, 30 weight percentage micro-filled Magnesium Oxide Silicone Rubber specimens have been developed to evaluate its performance for high voltage insulation. Inclined plane test and flashover experiments have been conducted as per the standards on developed and commercial Silicone Rubber specimens. Characterization was carried out on the specimen using Fourier Transform Infrared Spectroscopy (FTIR), Performance has been analysed based on Tracking and erosion resistance, percentage weight change, surface roughness, eroded length, hydrophobicity and flashover voltage under dry and wet conditions.
2 Preparation of Sample
The silicone rubber, ATH, and magnesium oxide of 30 weight percentage having a crystal size of 10 micrometer were thoroughly mixed using Sigma Kneader equipment. The researchers have taken different weight percentage at microlevel from 10 to 50%, but optimum mixture is around 30 wt%. This composition was moulded into a specimen of 150 mm × 50 mm × 6 mm size using rubber injection moulding at high pressure and temperature. Then, the specimens were cooled until they become stiff.
3 Evaluation Techniques
Commercially available specimens and 30 MMg specimens were evaluated for various performance parameters which are explained below.
3.1 Surface Roughness
Surface roughness is a function of surface texture. The deviation in the direction of the normal vector of a real surface from its ideal form is used to quantify surface roughness. The surface is considered to be smooth, if this deviations is small and rough if it is large. The instrument used for measuring surface roughness in this work is Mitutoyo SJ-201 P portable surface roughness tester.
3.2 Contact Angle (CA)
Hydrophobicity is the resistance to the formation of conducting water tracks. This will result in flashover due to increase in leakage current by deterioration of the surface. In this work, static hydrophobicity was measured with constant magnitude of stress using Image-J software. Water droplets are put on the surface and images were captured with 20 MP camera.
3.3 Flashover Studies
Flashover studies under dry and wet condition on the polymeric insulating specimens when subjected to HVAC stress were performed to determine the surface flashover strength during normal and rainy conditions. An experimental setup has been used to carry out these studies according to IEC 383 [9].
3.4 Tracking and Erosion Studies Using Inclined Plane Test (IPT) Setup
The inclined plane test setup as per IEC60587 [10] has been used to evaluate the tracking and erosion resistance of the polymeric specimens. The tracking and erosion resistance were evaluated in terms of change in weight of specimens, eroded area, and depth.
4 Experimental Setup
4.1 Experimental Setup for Conducting Dry and Wet Flashover Tests
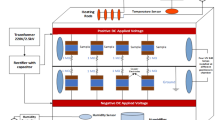
HV transformer of 5kVA, 230/50 kV, 50 Hz with 4.5% impedance has been used as source to energize the polymeric specimen. The schematic arrangement is shown in Fig. 1. The lab temperature was recorded, it was between 25 and 33 °C. The pressure is 1013 mbar.
An acrolyte fog chamber of dimension 30 cm × 30 cm × 30 cm has been used to conduct flashover experiment. Inside the chamber, proper copper electrode arrangement has been made to hold the silicone rubber specimens. For one end of copper strip, positive voltage was applied and other end was grounded. The arrangement is shown in Fig. 2a, b.
The flashover experiments have been conducted according to the IEC 60583 [11] standard. Tracking and erosion studies were performed using IPT inclined place test setup which is explained in the next subsection.
4.2 IPT Test Setup
The IPT setup shown in Fig. 3 comprises of 3 kVA, 230 V/30 kV, single phase transformer, a 30 kΩ current limiting resistor, and a stand which can provide an inclination of 45° angle to the specimen mounted on it, along with two electrodes to be placed on the surface of the specimen, a 1 Ω standard resistor, and a DSO connected across it. A constant voltage of 3.5 kV was maintained across the specimen and the contaminant flow rate was kept at 0.3 ml/min. The filter paper was inserted in-between the specimen and the electrode in order to filter the crystals in saline water (Fig. 4).
The IPT test arrangement and the procedure followed as per IEC 60,587 [9] standard.
5 Results and Discussions
5.1 Dry Flashover Test
The polymeric specimens have been placed on the electrode arrangement as shown in Fig. 2a and energized with HV transformer as discussed in the experimental setup in Sect. 4.1. Figure 5 shows the average dry flashover voltage of 5 trials conducted on commercial and developed specimens.
5.2 Wet Flashover Test
The polymeric samples were kept in pollution chamber made of acrolyte (refer Fig. 2b) with steam being generated from the steam generator. The steam was filled in the chamber until relative humidity of 90–95% was achieved. The flashover test was done on the specimen under wet condition. Figure 5 shows the average wet flashover voltage of 5 trials conducted on commercial and developed specimens.
From Fig. 5, it can be observed that under dry condition, developed 30MMg Specimen has a flashover voltage of 32.12 kV compared to commercial specimens 30.2 kV. In the case of wet condition, 30 MMg has better breakdown voltage of 14.28 kV comparing to commercial specimen 13.39 kV. This indicates developed polymeric material is better in dry and rainy conditions.
5.3 Contact Angle
The water droplets were placed on the surface of the specimens using syringe. Its images were captured and contact angle was measured with the help of Image-J software. The recorded values were 92° in the case of commercial specimen and 96 degrees in the case of developed 30 MMg specimen indicating micro-filled specimen has considerably better hydrophobicity.
5.4 Length and Depth of Eroded Area
IPT test was conducted on all the polymeric samples. Due to flow of leakage current with contamination flowing on to the surface, it gets eroded. The eroded areas length and depth have been measured and plotted in Fig. 6.
From Fig. 6, eroded length of 0.4 cm and depth of 0.2 cm have been recorded in case of 30 MMg specimens which is lesser than commercial specimen.
Weight Change: Weight change indictaes the erosion of the metrial due to the flow of saline water on its surface during energization. The weight of the commercial and 30 MMg specimens was measured before and after IPT test. It was found that 30 MMg has 0.56 mg of weight erosion compared to 0.66 mg for commercial specimen. The erosion length, depth, and weight parameters were found to be less in 30 MMg specimens indicating that it has better erosion resistance compared to the commercial specimen.
5.5 Surface Roughness
The change in the surface roughness indicates the degradation or erosion of the material due to influence of electric or environmental stress. The surface roughness was measured using Mitutoyo instruments in micrometers. Commercial and 30 MMg specimens’ surface smoothness was measured. The measurement was done before and after the IPT test and plotted in Fig. 7
Figure 7 indicates that the surface roughness is 12.18 μm after IPT test in case of 30MMg specimen and 20.88 μm for commercial specimen. This infers that the surface is least degraded in developed micro-filled specimen and its erosion resistance is significantly high under contaminated environment.
5.6 24 h Water Absorbance Test
The polymeric specimen of 5 cm × 1 cm × 0.6 cm is kept in distilled water with conductivity of 22 Micro siemens for 24 h water absorbance test as per the ASTM 570. The weight of the specimen before and after the test was measured to the accuracy of three decimal places. Test arrangement and results in terms of % weight change of both the specimens are shown in Figs. 8 and 9.
From the chart, it indicates weight gain is 4 mg in commercial specimen compared to 1.1 mg gain in 30 MMg sample. It is evident that micro-filled specimens absorbed least water during the 24 h, making it ideal to work under rainy conditions.
5.7 Fourier Transform Infrared Spectroscopy (FTIR)
The severity in material degradation can be accessed by difference in infrared absorbance spectra. Presence of Si–O–Si bonds suggests the stability of the specimen. In the present case, absorption bands in the range of 1000–1100 cm−1can be attributed to the Si–O–Si bond in the silicone rubber. Appearance of these bands corresponding to Si–O–Si suggests that specimens are resistance to degradation. Absorption bands in the wavenumber range 1255–1270 are due to Si–CH3 bond and it depicts hydrophobicity property of the material. Figure 10 shows FTIR graph for the all the specimen before and after the IPT test under normal contamination.
In order to analyse its resistance to bond breaking or scission, its % variation in the absorption values are calculated for both the specimens before and after the IPT test. The drop in absorption percentage is due to affect of normal contamination on Si–O–Si bond. It is found out that 30 MMg has least drop in % of variation, i.e., 6.9 compared to commercial 37.7%. This indicates it has better resistance to formation of oligomores, silica, and free active Si–O–bonds [11].
The percentage variation in absorbance values are calculated for the wave number 1250 Si–CH3 bond indicates 30 MMg has 16.06% of improvement in its absorption peak compared to Commercial (10.68%). This signifies both the specimens are able to regain the hydrophobicity even after the normal contamination exposure. 30 MMg specimen was able to regain marginally higher due to increase in CH3 functional groups in Silicone backbone. This has improved the surface hydrophobicity, due to the migration of low molecular weight from the polymer base to the surface.
6 Conclusions
Present paper discusses the development of micro-filled MgO silicone rubber specimen with 30 weight percentage and comparison of its performance with the commercial silicone rubber specimen. Following conclusions can be drawn from the present experimental results:
-
Dry and wet flashover voltages of 30 MMg specimen are marginally high compared to commercial specimen
-
From the IPT test, the eroded length, depth, surface roughness, and weight change have been found low in 30 MMg sample
-
Hydrophobicity is better in 30 MMg specimen based on contact angle measurement and water absorbance test
-
FTIR results indicate 30 MMg specimen has superior resistance to degradation and better hydrophobic recovery
-
Developed specimen has lesser tracking length, superior erosion resistance, and good hydrophobicity.
Developed micro-MgO-filled polymeric specimen seems to be one of the alternatives for the high voltage application.
Future work can be carried out by incorporating MgO at microlevel with different weight percentage into the polymer base. Experimental studies can be conducted to find the optimal weight percentage, which may further enhance its performance parameters for outdoor insulation applications.
References
PK Agarwal (2010) Indian blackout 2010 watch [Online]. Available https://www.nrldc.in/docs/documents/ArticlesIndian_Blackout.pdf
Tariq Nazir M, Phung BT, Li S, Akram S, Ali Mehmood M, Yeoh GH, Hussain S (2019) Effect of Micro-Nano additives on breakdown, surface tracking and mechanical performance of ethylene propylene diene monomer for high voltage insulation. J Mater Sci Mater Electron 30(15):14061–14071
Schidt LE, Kornmann X, Hillborg H, Krivda A (2008) Tracking and Erosion Resistance of High Temperature vulcanizing Silicone Rubber. IEEE Electr Insul Conf 17(2):533–540
Momen G, Farzaneh M (2011) Survey of micro/nano filler use to improve silicone rubber for outdoor insulators. Adv Material Sci J 27(1):1–13
Plesa I, Notingher PV, Schlogl S, Sumereder C, Muhr M (2016) Properties of polymer composites used in high-voltage applications. Polymers 8(5)
Amin M, Khattak A, Ali M (2016) Accelerated aging investigation of silicone rubber/silica composites for coating of high voltage insulators. J Electr Eng 100(1):217–230
Nasrat LS, Hamed AF, Hamiod MA, Mansour SH (2013) Study the flashover voltage for outdoor polymer insulators under desert climatic conditions. Egyptian J Petrol 32(1):1–8
Khan H, Amin M, Yusuf M, Iqbal M (2016) Effect of micro/nano SiO2 on mechanical, Thermal, Electrical Properties of silicone rubber, epoxy and EPDM composites for outdoor electrical applications. Tourkish J Electr Eng Comput Sci 25(2):1426–1435
Insulator string and insulator set for AC system—definition, test methods and acceptance criteria. IEC 383 (1993)
Electrical insulating materials used under severe ambient conditions—test methods for evaluating resistance to tracking and erosion. IEC 60587 (2007)
Tariq Nazir M, Phung BT, Yu S, Li S (2018) Resistance against AC corona discharge of micro–ATH/Nano-Al2O3 co-filled silicone rubber composites. IEEE Trans Dielectr Insul 25(02):157–167
Acknowledgement
The Authors would like to thank Management, Principal, and HOD, Department of Electrical and Electronics Engineering, Ramaiah Institute of Technology, Bangalore, for their constant support and for providing facility to carry out the research work. They sincerely thank Mr. Raghavendra Shanbag, Innovative Silicone, Bangalore, for his contribution to the experimental work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Rao, V.V. et al. (2020). Development and Performance Evaluation of Micromagnesium Oxide Filled Silicone Rubber for High Voltage Insulation. In: Siano, P., Jamuna, K. (eds) Advances in Smart Grid Technology. Lecture Notes in Electrical Engineering, vol 687. Springer, Singapore. https://doi.org/10.1007/978-981-15-7245-6_35
Download citation
DOI: https://doi.org/10.1007/978-981-15-7245-6_35
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7244-9
Online ISBN: 978-981-15-7245-6
eBook Packages: EnergyEnergy (R0)