Abstract
Therapeutic hypothermia has been used for millennia, but more recently, targeted temperature management has caught physician’s interest as the main neuroprotective strategy for cardiac arrest patients who remain comatose after return of spontaneous circulation. Randomized clinical trials have shown benefits in neurologic and mortality outcomes when lowering body’s core temperature to mild-to-moderate ranges of hypothermia, in conjunction with strict hyperthermia prevention measurements. The International Liaison Committee on Resuscitation recommends in their current guidelines to use a target temperature between 32 °C and 36 °C, for at least 24 h, in post-cardiac arrest patients, regardless of their initial rhythm (shockable vs. non-shockable). Therapeutic hypothermia consists of three well-defined phases: induction, maintenance, and rewarming. Each of these phases has very specific physiologic and clinical considerations for optimal patient management. The optimal dose, the induction and maintenance method, and the temperature monitoring technique remain unclear and are the focus of future research. Despite the overwhelmingly positive data regarding the benefits of therapeutic hypothermia, this technique remains underused. Clinicians should be familiar with this therapeutic intervention.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Therapeutic hypothermia
- Targeted temperature management
- Cardiac arrest
- Reperfusion injury
- Induced hypothermia
1 Overview
Cardiac arrest (CA) remains as one of the main causes of death, accounting for over 500,000 deaths per year just in North America [1]. Overall, CA survival seems to be improving due to advances in cardiopulmonary resuscitation and post-cardiac arrest care. Targeted temperature management (TTM) or therapeutic hypothermia (TH) has demonstrated to improve survival and neurologic outcome when used as a postresuscitation technique in patients with postanoxic/hypoxic encephalopathy [2,3,4,5]. For its utilization in CA, support came from the results of two multicenter randomized controlled trials (RCTs) in 2002 and 45 nonrandomized studies. The American Heart Association and the European Resuscitation Council recommend TTM use since 2003, and despite the overwhelming positive results, the implementation rate of TTM in many countries remains low [6].
Nielsen and coworkers in 2013 reported results from a large randomized clinical trial in 2013, in which they cooled down CA patients to either 33 °C or 36 °C and found no survival or neurological outcome difference. This trial remains highly controversial with people accepting Nielsen’s study results and others remaining convinced that 32–34 °C are adequate target temperatures. The TTM Trial by Nielsen has been criticized for its possible selection bias, delays in cooling initiation of up to 4 h, up to 10 h to reach a target temperature of 33 °C, and excessively fast rewarming rate, among other issues [7].
Following Nielsen’s findings, a leading center in CA conducted a retrospective cohort study after changing their TTM approach from 33 °C to 36 °C, and they found their fever rates increased (0% vs 19%), and there was a decrease in alive discharges (71% vs 58%) and home discharge with good neurological outcome (71% vs 56%) [8].
In this chapter, the authors will review the history of TH, pathophysiology of ischemic-reperfusion injury, protective mechanisms of TTM, physiology of cooling, current indications for TH, controversies, and future directions.
2 Historical Aspects of Therapeutic Hypothermia
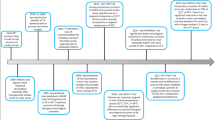
Therapeutic hypothermia dates back millennia. Hippocrates recommended the use of snow and ice packing to diminish blood loss in the injured [9]. The modern clinical use has been documented for the last 217 years [10]. In 1803, the “Russian method of resuscitation” was described as burying in snow CA patients in an attempt to resuscitate them [11]. The surgeon Baron Dominique-Jean Larrey was intrigued by how injured soldiers could sustain the pain in cold temperatures and described how injuries exposed to either ice or cold temperatures bled less when compared to those in warmer weather. In 1812, Baron Larrey, as Napoleon’s chief surgeon, during the Russian campaign, used TH to better preserve wounded limbs and as an anesthesia method prior to amputations [12].
Dr. Temple Fay, the head of the Neurosurgery Department at Philadelphia’s Temple University Hospital, is attributed as having introduced TH, both localized and whole body, to the modern medical setting. Fay conducted a series of experiments in the 1990s to assess the effects of varying temperatures on tissue growth of both normal and cancerous cells. He reported that cellular differentiation in chicks practically stopped at 32.2 °C. With these findings, he felt confident applying his knowledge at the bedside, thus beginning the largest series of hypothermia experiments in humans of its time, which would eventually end up catalyzing hypothermia usage in mainstream modern medicine [9, 13, 14].
Fay’s first hypothermia patient was a woman with metastatic breast cancer and neoplasm-related pain. He started his therapy by applying cold locally to the breast tumor area for weeks. After repeat biopsies, regression was noted, and the local infection seemed cured. With this evidence at hand and concerned of the persistent generalized pain, he decided to attempt to reach deeper structures. In 1938, Fay “cooled” this lady to 32 °C for 24 h, utilizing what he called “generalized refrigeration” (ice packs), and successfully rewarmed her. He reported a reduction in pain symptoms in 95.7% of surviving patients with a mortality rate of 10% [14, 15].
Bigelow and McBirnie found a possible use for TH during cardiac surgery in an animal model. They reported that during deep hypothermia (<25 °C), the area of neurological injury had less bleeding, cerebral edema, and inflammatory response [16]. One year later, in 1954, Rosomoff and collaborators demonstrated a direct relationship between core body temperature and intracranial pressure. These researchers proved that hypothermia decreased the cerebral blood flow and the metabolic rate in dogs as they were cooled from 35 °C to 26 °C [17, 18].
Benson, a professor of anesthesia at Johns Hopkins, conducted in 1958 the first clinical trial of TH outside the operating room, specifically in comatose post-cardiac arrest patients. This therapeutic measure showed 50% survival in the hypothermia group (33 °C) vs. 14% in the normothermic group [19].
Although studies in the 1970s showed promising results, between the 1960s and 1990s, TH research slowed down due to the recognition of clinical complications (mainly cold-induced coagulopathies). True resurgence of interest for TH occurred until the late 1980s, with advances in management of coagulopathies [14, 20].
It was not until 2002 that the American Heart Association (AHA), followed by the European Resuscitation Council in 2003, recommended TH as a post-resuscitative measure for out-of-hospital cardiac arrest patients that remained comatose after return of spontaneous circulation (ROSC). This recommendation was based on the results from two multicenter, prospective, randomized, controlled, clinical trials published in 2002 [21, 22]. One of the studies was conducted in Europe, in which 275 patients with cardiac arrest secondary to ventricular fibrillation were enrolled; 137 patients were managed at mild hypothermic ranges (32–34 °C) for 24 h, and 138 were normothermic control patients. The hypothermia group showed improved favorable outcomes in 55% of patients vs. 39% in the normothermic group [3].
The other 2002 study was done by Bernard and colleagues in Australia, in which they enrolled 77 patients, all of which had an initial rhythm of ventricular fibrillation. They were divided into two groups: a hypothermia group (a target temperature of 33 °C for 12 h) with 43 patients and a normothermia control group with 34 patients. Forty-nine percent of patients in the hypothermia group had a favorable neurological outcome vs. 26% in the normothermia group [2].
These two randomized, clinical trials once again sparked physician’s interest in TH, not only inside the operating room, or in post-cardiac arrest patients, but also in many other instances. However, before talking about present-day indications, it is imperative to review the pathophysiology of ischemic/reperfusion injury and the physiologic rationale behind the beneficial effects that hypothermia confers when used promptly in postanoxic/hypoxic patients.
3 Pathophysiology of Ischemic Insult and Reperfusion Injury
The return of spontaneous circulation after a CA in which the patient was subject to prolonged whole-body ischemia will give place to a complex pathophysiological state, originally coined postresuscitation disease by Dr. Vladimir Negovsky in 1970 [23]. Although appropriate for its time, “postresuscitation” implies that resuscitation has been achieved, due to which in 2008 the term was changed to post-cardiac arrest syndrome (PCAS) [24].
Even though prolonged whole-body ischemia will cause global tissue and organ damage, there will be additional insult during and after ROSC due to reperfusion injury. Ischemia/reperfusion response, post-cardiac arrest brain injury, post-cardiac arrest myocardial stunning, and persistent damage by the underlying pathology precipitating the cardiac arrest are the main components of PCAS [24,25,26]. The clinical presentation of PCAS resembles a systemic inflammatory response syndrome (SIRS)/sepsis-like state which will vary individually depending on the severity of the ischemic injury, the underlying cause of the CA, and the patient’s comorbidities [27,28,29]. Making this even more complex, determining the exact incidence and prevalence of this phenomenon is a challenging task, as there is no simple diagnostic test; hypotension, reduced cardiac output, usage of vasopressors, and laboratory abnormalities, among others, have been used as screening methods for PCAS but have failed to be reliable [28, 30, 31].
A pathophysiological approach to PCAS would be to define the phases according to time. The immediate post-arrest phase occurs in the first 20 min after ROSC. Early post-arrest phase lasts from 20 min to the first 12 h after ROSC, a period in which early therapeutic interventions might be the most beneficial. The intermediate phase is between 12 h and 72 h post-ROSC, during which ischemia/reperfusion injuries are still ongoing and aggressive treatment can still be initiated. Lastly, the recovery phase extends beyond 3 days, when prognostication is easier and outcome is more predictable [24, 25]. Understanding the pathophysiology behind the ischemia/reperfusion injury that occurs during the CA and afterward during PCAS will be the deciding factor for adequate individualized treatment for patients.
The ischemic injury starts seconds after the arrest even if the patient experiencing the CA is lucky enough to have a bystander who can initiate cardiopulmonary resuscitation (CPR) in an early fashion. Under perfect conditions, CPR generates 1.3 l/min of forward flow (15–25% of normal cardiac output), which can only maintain about 30% of the baseline cerebral blood flow (CBF). This flow rate usually is not able to generate a coronary flow that keeps up with the demands of a dysfunctional heart [32,33,34]. Due to this, brain injury and myocardial instability management have proved to be the main survival determinants after a CA [35]. In 2005, Laver and company identified that two-thirds of post-cardiac arrest patients admitted to the intensive care units died due to neurological injury [36].
The central nervous system (CNS) receives about 30% of total cardiac output [37]. During CA, the CBF stops completely, reducing the delivery of oxygen and glucose. Consciousness is lost within the first 10 s, and by 20 s, the neurological oxygen reserve is depleted, followed by isoelectric electroencephalographic activity in the next couple of seconds [20, 34]. As previously mentioned, even with CPR, the CBF is only 15–30% of the baseline [37]. The CNS is only suited to tolerate brief periods of ischemia as glucose and adenosine triphosphate (ATP) stores are completely lost by 5 min of circulatory arrest, making this a critical period during which tissue can still be salvaged [19, 38, 39]. Energy depletion enhances anaerobic metabolism increasing lactate, hydrogen, and phosphate production, promoting tissue acidosis [34, 40]. The pathological mechanisms that ensue after cardiac arrest will lead to cell death by both necrosis and apoptosis.
During ischemia, due to the depletion of oxygen and glucose, there will be a rapid reduction/depletion of ATP production and ATP reserves. By not having enough oxygen available to be used as the final electron acceptor in the mitochondria, there is a REDOX shift toward reduction, causing an increase of electron-rich metabolites which cannot follow the regular mitochondrial cytochrome oxidase pathway. This begins an electron “leakage” within the mitochondria, thus starting the production of reactive oxygen species (ROS), also known as free radicals, during the ischemia phase [41,42,43]. At the same time, ATP deficiency generates a disruption of ion homeostasis by affecting the transmembrane transport structures like Na+/K+-ATPase and Ca2+-ATPase pumps. Inhibition of these pumps leads to an ion gradient shift, which causes an increase in interstitial K+ and intracellular Ca2+, giving rise to an excitotoxic release of extracellular glutamate from presynaptic nerve terminals and astrocytes [44,45,46].
Even in the subacute phase of ischemic/reperfusion insult, after restoration of circulation and replenishment of energy reserves, tissue injury will persist. The damage will come from nocive pathways that started during the acute ischemia phase, plus secondary reperfusion mechanisms, all happening simultaneously [47].
N-Methyl-D-aspartate (NMDA) receptors are activated by the, previously described, increase of extracellular glutamate, as well as the increase of body temperature that occurs after ROSC [19, 20]. Activation of NMDA receptors further increases intracellular Ca2+ levels via glutamate receptor-gated ion channels [48]. By activating NMDA receptors, the postsynaptic cells will have an influx of Na+ and Cl−, thus generating an intracellular hyperosmolar state with consequent influx of water to the cell. The end result of this influx will be a decrease of the inhibitory effect that Mg2+ has on NMDA receptors, thus making them more sensitive to glutamate levels, which allows more Ca2+ entrance, promoting cellular edema and eventual neuronal necrosis [40, 49]. It’s important to understand that this increase in intracellular Ca2+ creates a harmful positive feedback loop that dictates glutamate release, all of which is completely dependent on the severity of the ischemia [50]. Overall, the elevation of intracellular Ca2+ will activate a cascade of calcium-dependent enzymatic pathways (proteases, nucleases, and lipases) that contribute to cell membrane disintegration and eventual tissue necrosis [37, 48].
In normal conditions, the endoplasmic reticulum and the mitochondria can sequester Ca2+ if the intracellular levels increase, but during anoxia and ischemia, this regulatory mechanism is impaired [40]. Calcium regulation by the mitochondria is ATP and oxygen dependent, and as explained, within seconds to minutes after the CA2+, the ATP and oxygen reserves are completely depleted [19, 20, 34]. During ischemia, as Ca2+ influx is first happening, the mitochondria are able to buffer some of it by sequestering some of the Ca2+, which leads to mitochondrial depolarization and overall mitochondrial dysfunction [9, 40].
The mitochondrial dysfunction is mainly caused by an increase in the permeability of the inner mitochondrial membrane (IMM) that occurs due to both the electron leakage and the opening of permeability transition pores (PTPs) [42, 49]. These pores are multiprotein complexes that create nonselective pores in the IMM that contribute to the IMM depolarization, which transforms the mitochondria from an ATP producer organelle to an ATP consumer by reversing the ATP synthase pump action, in an attempt to maintain the membrane potential [51]. Mitochondrial PTPs appear to be sensitive to Ca2+, pH, voltage changes, and REDOX reactions. With reperfusion, mitochondria are at high risk of PTP opening due to the increase of intracellular Ca2+ levels, the alkalosis seen as the acidosis is washed away, and the increase of ROS that first started with the electron leakage and the synthesis of nitric oxide due to glutamate excess [9, 50, 52, 53].
After ROSC, due to the sudden oxidative stress experienced with reperfusion and reoxygenation, there will be a secondary rapid increase of ROS, mainly in highly metabolically active organs, like the brain and heart [43, 41, 54]. These free radicals are a result of different pathways, mainly mitochondrial dysfunction, uncoupled nitric oxide synthase, glutamate and consequent activation of NMDA receptors, arachidonic acid, and catecholamines [40, 41, 54, 55].
Since 1995, Globus and colleagues demonstrated that ROS production and glutamate release into extracellular space are temperature dependent [40, 56]. This becomes clinically relevant when we take into consideration the pyrexia that patients experience post-ROSC, as this pyrexia enhances the damaging effects of free radicals, when patient’s temperature increase by >0.5 °C over 37 °C [19, 20]. Free radicals will cause oxidative damage, post-ROSC, by acting in DNA, lipid membranes, proteins, and enzymes, eventually leading to cell death [40, 41, 54].
Both ischemia and reperfusion mechanisms play a big role in the cell’s fate. Ischemia primes mitochondria for PTP opening, and reperfusion triggers PTP opening. Depending on the severity of PTP opening, the mitochondrial membrane depolarization will be either transient or sustained. If the depolarization is severe, cytochrome C, along with other apoptogenic factors (apoptosis-inducing factor, Smac/DIABLO, and Apa-1), can leak from the intermembrane space into the cytosol, activating both caspase-dependent and caspase-independent pathways which culminate in mitochondrial-mediated apoptosis days to weeks after the ischemia episode [40, 51, 52, 54, 57].
The combination of elevation of intracellular Ca2+ and production of ROS due to ischemia/reperfusion mechanisms contributes to cell membrane disintegration and eventual neuronal death by either necrosis or apoptosis, mainly observed in neurologic and myocardial tissue [19, 20, 43, 58].
Due to the nature of the pathophysiology described in PCAS, the main goals of treatment in post-cardiac arrest patients should be identifying and treating the precipitating cause of the cardiac arrest, minimizing the brain injury, controlling the cardiovascular dysfunction, and managing any metabolic issues that arise from the ischemic/reperfusion insult [24]. One therapeutic approach that allows healthcare providers to address multiple of these potential issues is TH, as it has proven to have beneficial effects in multiple of the damaging pathways of the ischemic/reperfusion insult.
4 Protective Mechanisms and Effects of Therapeutic Hypothermia
Therapeutic hypothermia was first used under the rationale that its protective mechanism was completely due to slowing the metabolic rate, specifically the cerebral metabolism [59]. Cerebral oxygen consumption, glucose utilization, and lactate production are all temperature-dependent processes which benefit from hypothermia induction [19]. Cerebral metabolic rate is estimated to decrease 6–7% for each 1 °C below a body core temperature of 37 °C [60].
During the ischemic episode, CBF has either completely stopped or is significantly reduced. As circulation is restored (ROSC), hyperemia occurs in cerebral vessels, followed by a steady decline of blood flow over a period of hours. Therapeutic hypothermia has shown to blunt the hyperemia episode seen in the immediate post-ROSC phase. Avoiding this increase of blood flow allows the brain to preserve its energy reserves of ATP and to maintain the pH [50].
As previously explained, ATP is needed to maintain ion gradients, and when reserves are depleted, as it happens during ischemia, there is a rapid calcium influx which leads to the increased release of glutamate and other excitotoxic amino acids. Hypothermia plays a role in preventing this excitotoxic cascade by limiting the calcium influx by multiple mechanisms. Preserving ATP levels minimizes ion pump malfunction, reducing intracellular calcium entrance. One study showed that hypothermia decreases the ischemia-induced downregulation of the glutamate receptor 2 (GluR2) subunit of the AMPA receptor, which is thought to play a role in limiting calcium influx [61]. Hypothermia further prevents calcium influx by affecting NMDA receptors. N-Methyl-D-aspartate receptors are temperature dependent, so as patients are cooled down, these receptors are inhibited [20, 48]. Also, hypothermia reduces brain glycine levels which makes NMDA receptors more responsive to glutamate levels, so by reducing glycine levels, the NMDA-glutamate pathway is blunted [40].
The protection TH confers to post-cardiac arrest patients cannot be fully explained by the prevention of ATP depletion or blunting of the excitotoxic cascade due to a decrease of intracellular calcium influx and glutamate release. Still during the acute ischemic phase, TH affects early gene expression. Expression of 70-kDa inducible heat shock protein (HSP70) increases during hypothermia, thus enhancing its neuroprotective properties [50].
During the subacute phase of PCAS (1–7 days post-ischemia), reperfusion pathways in conjunction with the ischemic mechanisms can widen the tissue injury [47]. Free radical formation is the main pathway by which reperfusion injures organs [41]. Therapeutic hypothermia is useful in this subacute phase as ROS production is another temperature-dependent process which slows down from cooling the patient [19]. Additionally, inflammatory responses activate during this period, which lead to cell death by both necrosis and apoptosis. Hypothermia affects these cell death pathways in a way that favors cell survival [4].
In an ischemia setting, necrosis is caused because of the intracellular swelling due to the ion pump malfunction. By allowing ion pumps to work, hypothermia decreases cellular necrosis. The other cell death pathway is apoptosis, which consists of two main pathways. The intrinsic pathway is found at mitochondrial level and is initiated in response to “death signals” (DNA damage, hypoxia, and mitochondrial membrane instability). This pathway is highly regulated by antiapoptotic molecules, such as those of BCL-2 family; apoptotic signals lead to a release of cytochrome c from within the mitochondria to the cytosol, where it will activate the caspase 9, which in turn activates caspase 3, eventually leading to apoptosis [40, 50]. Cooling can interfere with the intrinsic pathway by modifying the expression of BCL-2 family members, decreasing cytochrome c release by the mitochondria and decreasing caspase activation [62].
The extrinsic pathway starts outside the cell, by the activation of death receptors, the FAS (apoptosis-inducing receptor) and FASL (ligand) being the most studied extrinsic signaling pathway. Activation of death receptors leads to caspase 8 activation, which, same as in the intrinsic pathway, activates caspase 3, leading to apoptosis. Apoptosis has shown to suppress the expression of both FAS and FASL, thus decreasing activation of the extrinsic pathway [50].
At the same time, while blunting cell death pathways, hypothermia has shown to promote neuronal growth. Brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), and neurotrophin all help preserve neuronal integrity, and hypothermia significantly increases its levels[40, 50]. The synergy between hypothermia and BDNF has been reported to decrease neuronal damage and reduce infarction size up to 40% [63].
In a way, TH decreases the magnitude of the damaging pathways that ensue during and after a CA. The protective mechanisms come from preventing or decreasing the energy depletion, thus diminishing the overall metabolic malfunction that develops afterward. In addition, TH promotes cell survival while blunting the cell death pathways. Each of the three separate phases of injury seen in PCAS can potentially benefit from TTM. The therapeutic usefulness will vary accordingly to the severity of the ischemia, the cause of the CA, and the baseline comorbidities of different patients, but overall, TTM will allow the body’s metabolism to slow down, preventing further tissue damage and allowing, to some extent, the injured cells to heal and regain function [64].
5 Physiology of Cooling and Clinical Considerations
As core body temperature decreases, for each 1 °C below 37 °C, the cerebral metabolism decreases 6–7%, along with brain oxygen and glucose consumption [60]. Therapeutic hypothermia improves the oxygen delivery to the ischemic areas of the brain. Intracranial pressure (ICP) is reduced with TH because of a hypothermia-induced vasoconstriction that decreases cerebral blood flow, thus decreasing ICP and providing an anticonvulsant effect on the patient (which can be used to treat refractory status epilepticus) [40].
The main physiologic responses to mild-to-moderate TH in the cardiovascular system are a decrease in heart rate and an increase of the systemic vascular resistance [65]. Recent studies have associated bradycardia during TH with better outcome [66, 67]. This bradycardia is attributed to a prolonged action potential, as well as less spontaneous repolarization of the cardiomyocyte [68]. In 2002, Lewis and associates analyzed heart contractility of 10 patients who were under hypothermia, at 33 °C, and reported that patients had an impaired contractility when the heart rate was “artificially” sustained during hypothermia, thus demonstrating that the heart’s contractility is directly related to a low heart rate [69]. Hypothermia will be accompanied by a release of catecholamine, which will cause an increase of the stroke volume and initially an increase in cardiac output. As core temperature continues to decrease, the effect over cardiac output will shift, but the mean arterial pressure will be preserved due to the increase of the peripheral vascular resistance [58].
The catecholamine surge will also cause coronary artery vasodilation, improving myocardial perfusion and oxygenation. This is clinically important, as TH not only diminishes the overall metabolic rate and the oxygen demand but also increases oxygen delivery to both brain and heart [50, 70].
When core temperature is 32 °C, the metabolic rate is 50–60% of baseline. The oxygen consumption and carbon dioxide production shift in a parallel manner, giving rise to important respiratory considerations. Mechanical ventilation settings need to be adjusted frequently because if left unchanged, patients are at risk of hyperventilating [19], which could in turn cause cerebral vasoconstriction and affect the oxygen delivery to an already injured brain. To manage ventilator settings appropriately, blood gases should be taken frequently, especially during the induction phase [4].
Therapeutic hypothermia causes increased renal blood flow, which in turn causes what is known as “cold diuresis.” This diuresis is especially relevant during induction phase, as the diuresis can cause hypovolemia, manifesting as hypotension. During this same phase, potassium will shift intracellularly, leading to hypokalemia. Correction of the hypokalemia during induction phase can be harmful later on during the maintenance and rewarming phases as the potassium will exit the cell and cause rebound hyperkalemia. From an acid-base perspective, for every 1 °C below 37 °C, the intracellular pH increases 0.016 points [65].
The ischemic injury in conjunction with hypothermia causes intestinal dysfunction. Patients will have a delayed gastric emptying due to a decrease of peristalsis [71]. Traditionally, enteral feeding has been deferred until rewarming phase, but recent studies have shown that enteral nutrition is feasible in patients undergoing TH. It is important to keep in mind that predictive equations will most likely overestimate the patient’s nutritional needs due to the decrease in metabolic rate. Further research is needed to define optimal feeding rates [72, 73].
Some associations between hypothermia and coagulopathies have been reported [74]. At temperatures ≤35 °C, there will be mild platelet dysfunction, with some inhibition of the coagulation cascade, prolonging both prothrombin and partial thromboplastin times [75]. At the same time, with hypothermia, there is an increased incidence of neutropenia, being pneumonia a common infection seen during TH [47].
6 Applications and Indications of Targeted Temperature Management
In the past decades, CA research has focused on methods to obtain more easily and at a higher rate ROSC [24]. Cardiopulmonary resuscitation continues being the treatment of choice to counteract CA, and despite the huge effort put into promoting early initiation of CPR, mortality and neurologic morbidity remain high in post-cardiac arrest patients with ROSC [46]. So, how do we manage post-cardiac arrest patients? Therapeutic hypothermia with TTM is a therapeutic intervention that has been implemented in an attempt to minimize the mortality and the neurologic damage experienced in the PCAS. The initial rationale behind the clinical implementation of TTM was that it decreases the metabolic rate, thus allowing the injured brain to heal [64].
In 2002, two multicenter, prospective, controlled RCTs showed a favorable neurological outcome in post-cardiac arrest patients with ROSC who underwent hypothermia vs. those who were managed with a normothermia approach [2, 3]. One year after these clinical trials, the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation (ILCOR) wrote an advisory statement, recommending for the first time that comatose adult patients with ROSC after out-of-hospital cardiac arrest (OHCA) should undergo TH with TTM (32–34 °C for 12–24 h) when initial rhythm, during the arrest, was ventricular fibrillation [21].
Nielsen and colleagues, in 2013, published an RCT that compares TTM at 33 °C with TTM at 36 °C in OHCA patients, focusing in survival and neurological outcomes [76]. The results showed no difference in either outcome between both target temperature groups, leading to a debate regarding expanding target temperature to 36 °C instead of 32 °C–34 °C, as the 2010 guidelines recommended. Many centers understood Nielsen’s results as TTM no longer being required or that fever control would suffice post-cardiac arrest management, which is not at all the case [6, 59].
The ILCOR does not take part in the debate regarding ideal target temperature that sparked after Nielsen’s TTM Trial. Instead, they continue to recommend TTM use and expanded their previous temperature range recommendation of 32–34 °C to a constant target temperature between 32 °C and 36 °C [77]. Specific information about the ideal duration of TTM is lacking [59], due to which current recommendation is temperature control for at least 24 h since achievement of selected target temperature, as it was done in the two largest randomized clinical trials in 2002 [77].
Traditionally, TTM had been reserved only for patients who remained comatose after OHCA with an initial shockable rhythm [21, 64, 78, 79]. In 2015, the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation published an advisory statement with the most recent and current recommendations for TH with TTM in post-cardiac arrest patients [77]. Between 2010 and 2015, clinical trials showed a neurological benefit when TTM was used for both OHCA with initial non-shockable rhythm and in-hospital cardiac arrest (IHCA) regardless of initial rhythm [80,81,82,83,84], which prompted a change to prior guidelines. Current guidelines recommend to:
-
Initiate TTM for adult patients who remain comatose after ROSC in OHCA which initially had a shockable rhythm.
-
Initiate TTM for adult patients who remain comatose after ROSC in OHCA which initially had a non-shockable rhythm.
-
Initiate TTM for adult patients who remain comatose after ROSC in IHCA, regardless of initial rhythm.
By making these strong recommendations, the task force group is stating that essentially there are no patients in whom temperature control between 32 °C and 36 °C is contraindicated [85]. After 24 h of temperature control in between that range, slow rewarming is recommended (≤0.25 °C/h), followed by strict hyperthermia management [6, 77, 85]. This way, the ILCOR supports TTM as a protective intervention from a neurologic and cardiovascular standpoint [77, 86].
There is conflicting data on benefit and risk of cooling induction in a prehospital setting [87,88,89,90,91,92]. Current guidelines recommend against usage of large volumes of cold fluid in a prehospital setting for induction of TH right after ROSC and make no comment on other prehospital cooling techniques [85].
One year after the release of the current guidelines, in 2016, Schenone et al. presented a meta-analysis and systematic review in which they reviewed the mortality and neurologic outcome when utilizing expanded criteria for targeted temperature in OHCA survivors [93]. Fifty-one percent of the patients included in the meta-analysis had initially a shockable rhythm, with arrest times ranging from 20 to 34.6 min (a mean of 24.6) [93], which compares with the downtimes in the TTM trial, which excluded patients with downtimes of more than 20 min [76]. Schenone showed that the use of TH correlates with a higher survival rate and a better neurological outcome after OHCA, regardless of initial rhythm and with a more flexible downtime window. These results seem to help elucidate the questions regarding TTM usage, favoring TTM use in all cardiac arrest patients, even when patients had a longer downtime or a delay in initiation of therapy [94].
Despite the overwhelming positive evidence of TTM in post-cardiac arrest patients, TTM remains underutilized [86, 95,96,97,98,99]. It is our opinion that we should initiate TTM in all cardiac arrest patients with ROSC, regardless of their downtime and initial rhythm, when feasible.
Success utilizing TH in cardiac arrest’s RCTs, coupled with physiologic knowledge on the hypothermia topic, has motivated physicians on expanding the utilization of TH to other pathologies apart from PCAS [64]. There is data of benefit in diseases like ischemic stroke [100,101,102], traumatic brain injury [103], near-drowning [104], neonatal hypoxic-ischemic encephalopathy [105], hepatic encephalopathy [106,107,108], and acute respiratory distress syndrome [109], among others. Therapeutic hypothermia protocols might differ in target temperature and duration of TTM in these other clinical indications.
7 Time Window and Timing
The extent of neurologic damage seen in post-cardiac arrest patients is primarily related to the duration of ischemia [20]. The first 5 min after the circulatory arrest delimits the classic threshold in which brain tissue can still be completely salvaged [38, 39]. For this reason, reaching the selected target temperature (32–36 °C) as quickly as possible after ROSC is the main goal in post-cardiac arrest care [19]. Laboratory analysis has shown that the therapeutic window for hypothermia is initiation in less than 6 h after ROSC; however, clinical trials do not support this finding, and currently, we remain without a defined therapeutic window [110].
Animal studies have shown neurologic benefit with very early initiation of TTM after ROSC [111, 112]. However, human studies have failed to provide definitive data regarding prehospital induction of TH [47, 113]. Physicians have even looked into initiating hypothermia during the intra-arrest phase, utilizing different techniques, with inconclusive results [88, 114]. Nonetheless, clinical studies have shown a remarkable therapeutic benefit from TTM even when cooling initiation was delayed for several hours [93, 104]. Taking all this into consideration, the reality is that TTM remains being a neuroprotective intervention, which ideally should be started as early as possible, in a hospital environment. The traditional time window to achieve target temperature after obtaining ROSC is 4 h, but patients with delayed induction of hypothermia can still benefit from being cooled down [94].
The ideal duration of TTM in order to obtain a good neurological outcome remains unknown. The ILCOR originally had recommended 12–24 h of TTM at 32–34 °C [21, 78, 79]. A recent multicenter RCT, in 2017, compared TTM use for 48 h with TTM use for 24 h and reported no significant differences between both groups in neurologic outcome or survival rate at 6-month follow-up [115]. Current resuscitation guidelines recommend at least 24 h of TTM between 32 °C and 36 °C [85]. However, a clinical trial has yet to prove benefit from TTM for durations longer than 24 h [115]. Then again, dose-finding RCTs cannot assume there is an optimal fixed duration that can be applied to all the post-cardiac arrest patient population [116].
8 Cooling Techniques
There are multiple techniques to induce TH [60, 117]. Current recommendations do not specify the optimal method for initiating and/or maintaining TH, as the ideal cooling device has yet to be developed, and there is insufficient evidence to recommend a specific cooling method [85, 118]. The ideal cooling device would have a high cooling rate with short induction times, preferentially would be directed toward target organs (brain and heart), would be easy to transport (lightweight, portable, and durable), and could be used in a prehospital setting, maybe even during cardiopulmonary resuscitation. The main modalities used nowadays are surface and invasive cooling [47, 119].
Surface cooling methods are relatively easy to use but take a longer time to cool down patients to the desired target temperature (2–8 h). They include circulating cold-water blankets, cold air-forced blankets, self-adhesive hydrogen-coated pads, and less sophisticated methods like ice packs and cold-water immersion.
Immersing patients in cold water is an option but should be avoided as it compromises monitoring temperature, and it is troublesome to defibrillate patients, as it takes time to dry them off [47]. Ice-pack usage remains a viable cost-effective option that can be used in conjunction with more advanced cooling techniques. Main disadvantages of ice packs, other than being labor intensive, are that unintentional overcooling past target temperature is common and if applied directly to the skin, they can cause skin necrosis [117]. One of the major drawbacks from surface techniques is that the shivering response seen during the induction phase is increased [20]. Due to this, appropriate prevention and/or treatment of the shivering is needed as the increased oxygen consumption could mitigate the protective mechanisms that TH confers [4].
Invasive endovascular cooling methods were developed mainly because of the major drawbacks of surface cooling [47]. Methods included in this modality are infusion of cold intravenous fluid (4 °C), cold carotid infusions, single-carotid artery perfusion with extracorporeal cooled blood circulation, ice water nasal lavage, cardiopulmonary bypass, cold peritoneal lavage, nasogastric and rectal lavage, and esophageal cold-water circulating device [47, 120]. Cold intravenous fluid infusion has shown to be easily available and cost-effective in inducing hypothermia, but not in maintaining it. Up to two liters of cold intravenous fluid is safe to administer in post-cardiac arrest patients [117].
Overall, endovascular cooling methods have shown to have faster cooling rates and, more importantly, more time within target temperature with less temperature fluctuations. Tight temperature control, limiting temperature fluctuations, might become more important with the newer recommendations to manage patients at temperatures up to 36 °C, as a shivering episode could lead to a 1.5 °C increase in temperature, placing the patient in the febrile territory [121].
A newer method is through an esophageal device. This device maintains standard orogastric tube functions while cooling down patients at a rate of 1.3 °C/h, with minimal temperature fluctuations [120].
In many cases, a combination of hypothermia induction methods is used to reach target temperature in a faster manner [4]. Maintenance of TH is usually done based on the characteristics of the patient, in conjunction with the physician’s expertise with different devices, as well as the availability of different methods [120].
9 Monitoring Temperature
Therapeutic hypothermia levels are classically divided into four: mild, 34–36 °C; moderate, 28–32 °C; deep, 17–27 °C; and profound, 4–16 °C [19]. Induced TH is currently recommended in a mild-to-moderate (32–36 °C) fashion, as in between this temperature range the protective mechanisms have shown to outweigh the possible complications [85].
Regardless of the hypothermia induction method and the TTM maintenance technique, reliable continuous temperature monitoring is imperative for adequate neuroprotection [110]. Even though the brain is the main end-organ target of TTM, core temperature is usually the one measured in order to have tight temperature control [122]. Despite it being more than 15 years since the release of the first TTM recommendations, current guidelines do not recommend a specific site for temperature measurement [21, 85].
Monitoring can be done with a variety of invasive or noninvasive probes, including pulmonary artery catheter, nasopharyngeal, esophageal, tympanic membrane, bladder, and rectal [19]. Blood in the pulmonary artery catheter is considered the “gold standard” of core temperature measurement, as it has a higher accuracy and precision than other monitoring methods [123], but its use in an emergency setting can be impractical.
Physicians need to take into consideration that the devices used to measure temperature during a hypothermia regimen were not designed to detect quick shifts in temperature. Inevitably, these temperature monitoring devices will be “lagging” behind in time when compared to “true” core temperature. This time lag will be greater when cooling is being done at fast rates (as seen in newer cooling devices). Due to this, during the induction phase, the measured temperature will be constantly behind the actual core temperature. It means that the cooling device will keep cooling down the patient even if the target core temperature has been achieved, and by the time the measured temperature reflects the target temperature, the patient’s core temperature may be significantly below the therapeutic range [71].
Nasopharyngeal and esophageal temperatures have shown to correlate better with brain temperature than other body site temperatures further away from the brain, during TH [124, 125]. Tympanic membrane temperature is readily available in a noninvasive manner and seems to correlate well with brain temperature [75]; however, measurement can be impaired when there is an ear canal obstruction (i.e., ear wax). If tympanic temperature is the choice, cooling of the head should be avoided to reduce the chance of “false” readings [126,127,128]. Rectal probes are not ideal, as fecal impaction can disrupt measurements when compared to intracranial temperature [47]. Bladder probe is another option that seems to correlate well with pulmonary artery temperatures, making it an easy-to-use option [20].
10 Therapeutic Hypothermia Phases
A TH cycle is clinically divided into three phases [60]:
-
1.
Induction phase, defined as the period since the patient is first being cooled down until reaching the selected target temperature. The aim is to get the patient’s core temperature to a desired target temperature between 32 °C and 36 °C as quickly as possible.
-
2.
Maintenance phase starts as soon as target temperature has been reached. The main goal is to manage core temperature strictly, with minimal or no fluctuation in body core temperature (maximum fluctuation of 0.2–0.5 °C).
-
3.
Rewarming phase occurs after at least 24 h of TH. Rewarming should be done at a slow rate, ranging from 0.25 °C to 0.5 °C/h.
Each phase has their own clinical considerations that need to be kept in mind. When attempting to cool down a patient, counter-thermoregulatory mechanisms will activate [71]. At a core temperature of 36.5 °C, there will be vasoconstriction of the skin in an attempt to decrease the body’s heat loss. In this case, the vasoconstriction of skin vessels makes more difficult inducing hypothermia, especially when using surface cooling methods. In addition, about 1 °C below skin vessels vasoconstriction, shivering will kick in attempting to maintain body temperature by increasing heat production [129]. If left untreated, shivering will increase the systemic oxygen consumption, decrease the neurologic oxygenation, increase the ICP, and will interfere with the cooling rate, prolonging induction’s phase duration [110, 130].
Patient’s shivering while inducing hypothermia can be treated with sedatives, anesthetics, opiates, magnesium, neuromuscular blockade, etc. Despite neuromuscular paralytic agents being an option, they should probably be avoided, if possible [130]. First, when utilizing paralytic agents, there will be muscular blunting, but centrally, there won’t be any effect. Second, paralytic agents can mask seizure activity. Third, sedatives in conjunction with anesthetics can usually manage the shivering and can help with the cooling rate as they can cause vasodilation. Lastly, paralysis may mask insufficient sedation [4].
Short-term paralysis seems appropriate as a first-line option to treat shivering in patients in whom hemodynamic stability is a concern (in whom sedatives and/or analgesics could cause hypotension) [131]. It appears reasonable that paralysis should be used only when appropriate sedation and analgesia failed to manage shivering. But, even then, shivering response decreases past the 33.5 °C mark. For this reason, the sedation strategy should aim for a high bolus dose during the induction phase, continued by a relatively low dose during maintenance phase, as there will be a decreased drug clearance [71].
The induction phase represents the period during which the patient is at highest risk for short-term side effects like hypovolemia, electrolyte imbalances, and hyperglycemia [65]. Hypovolemia can develop due to hypothermia-induced “cold-diuresis” or a fluid shift toward the extravascular space. Electrolyte disorders occur by a combination of an increased renal excretion (by both cold diuresis and tubular dysfunction) and an intracellular shift. Magnesium and K+ depletion are especially important [4]. The recommendation is to keep K+ at a level between 3.0 and 3.5 mmol/l during the induction phase (and maintenance phase) to avoid the risk of rebound hyperkalemia during rewarming phase (see later) [110]. Magnesium supplementation is important, as several studies have shown a relationship between hypomagnesemia and increased mortality in the intensive care unit (ICU) [132, 133]. Giving Mg2+ to these patients is also useful as Mg2+ happens to be an NMDA receptor antagonist that increases the shivering threshold [60]. Hyperglycemia happens due to the decrease in insulin sensitivity and a reduction of insulin secretion by the pancreatic islet cells, caused by the hypothermia [47].
Management during the induction phase is critical for the patient’s outcome, with frequent adjustments to ventilator settings, sedation, insulin and vasopressor dosage, and fluids and electrolyte management. A way to minimize these side effects is by shortening the induction phase duration by reaching the maintenance phase as quickly as possible, which can be obtained by combining cooling techniques [4].
The maintenance phase is characterized by patient “stability” when compared to the induction phase. The shivering response diminishes past the 33.5 °C mark [71]. The risk for hypovolemia or electrolyte imbalance is less of a concern during this phase. Attention should be given to the prevention of infections, pressure ulcers, and deep venous thrombosis prophylaxis [71].
After completing at least 24 h of TH, the best approach for rewarming and continuing temperature management remains unknown [85]. Rewarming after TH should be done at a slow rate, between 0.25 °C and 0.5 °C/h, as plasma electrolytes, intravascular volume, and metabolic rate can shift quickly during rewarming [60, 134]. Studies have shown that a fast rewarming rate is associated with a worse outcome [135, 136].
During the rewarming phase, it is common for patients to present shivering and hypotension [20]. Shivering should be controlled as it can eliminate the protective effects of TH by increasing the overall metabolic rate. Hypotension is caused by a redistribution of intravascular fluid, as skin vessels dilate with the temperature going up [137].
In patients in whom intracranial hypertension is being controlled with TH, the rewarming rate should be even slower; recommended rewarming rate is 0.5–1 °C/day [47]. After achieving normothermia, in the post-therapeutic hypothermia phase, studies have noted that hyperthermia presents in a considerable amount of patients [138, 139]. Rebound hyperthermia management is key in the post-rewarming period, as it also is associated with worse neurological outcome [140]. Further research is required to define a maximum safe target temperature post-rewarming and the duration of hyperthermia prevention post-cardiac arrest [118].
11 Complications
Hypothermia causes physiologic changes in all the organs of the body [141]. It is important to differentiate between these physiologic changes that occur and the true side effects and complications of TH. But, even after making this distinction, some of these normal physiologic changes are unwanted in critically ill patients, requiring prompt medical management. Other side effects are expected but will not endanger patient’s lives [71]. The most important complications of TH will be discussed below.
Bradycardia is the most common heart dysrhythmia during TH. However, it is important to remember that myocardial contractility is highly related with a low heart rate (at least in this temperature setting), due to which patients should not receive chronotropic medications in order to keep the heart rate up. Forty beats per minute can be considered a normal heart rate for patients being managed at 32 °C. Overall, the risk of developing life-threatening dysrhythmias is very low at temperatures above 30 °C [58]. In a more profound hypothermia regimen, patients usually start with atrial fibrillation, which can shift to any dysrhythmia, most commonly being to either ventricular fibrillation or ventricular tachycardia. A major clinical consideration to keep in mind is that at profound hypothermia levels, dysrhythmias are harder to manage with anti-arrhythmic drugs because myocardial tissue is less responsive [71]. Appropriate care involves constant reliable temperature monitoring not only to diminish temperature fluctuations but also to prevent going below the recommended therapeutic range [110].
A major concern with hypothermia is the supposed predisposition for bleeding. Hypothermia causes some degree of platelet dysfunction, decreases platelet count, and affects kinetics within the coagulation cascade, affecting both prothrombin and partial thromboplastin times [20, 47]. Clinically, this does not represent a major concern in patients who are not actively bleeding when hypothermia is induced. However, the situation changes when patients are actively bleeding prior to cooling them down. In this case, the bleeding should be stopped prior to initiating temperature management. When bleeding is a concern, it is worth knowing that platelet dysfunction does not happen until the temperature is ≤35 °C and the coagulation cascade is not blunted until the temperature is ≤33 °C [4, 142]. These temperature levels are the basis for some authors recommending higher temperatures of TH for patients in whom bleeding is a concern, but this bleeding risk is not a reason to not initiate TH [110].
One of the major protective attributes of TH is the inhibition of various inflammatory pathways [40]. With this inhibitory effect, its inherent that there will also be impairment of the immune system response. Inhibition of proinflammatory cytokines, leukocyte migration, and phagocytosis make the body more susceptible to infections [71]. Other factor that further enables infection development is the hypothermia-induced hyperglycemia, caused by both increased insulin resistance and diminished insulin secretion [142].
The most common infection seen in TH patients is pneumonia. Information regarding what aspect of TH (duration, temperature, method, etc.) causes the increased risk for infection remains unknown. An increased incidence of infection during hypothermia opens up the debate regarding prophylactic administration of antibiotics, but guidelines remain neutral on the topic [143]. Even if antibiotics are used prophylactically or not, prevention of pressure ulcers during hypothermia should be a big priority as hypothermia increases the risk of wound infection. Special attention should be given to surgical wounds or catheter insertion sites [71, 144].
A clinical tie-in with the increased risk of wound infection is the possibility of skin necrosis if direct cold injury occurs. As previously explained, hypothermia induction can be done through multiple methods. If surface cooling is the option selected, one possibility is that if cold substances (i.e., ice, pads with hydrogel) are applied directly on top of the skin, there will be severe peripheral vasoconstriction which can lead to skin necrosis [145]. Again, wound prevention is very important during TH.
12 Controversies
In 2013, Nielsen and colleagues published the TTM Trial. This international RCT reported that TTM at 33 °C conferred no benefit regarding survival rate and neurologic outcome when compared to a target temperature of 36 °C [76]. This publication sparked a debate within the hypothermia community that threatened TTM usage.
The results from the Nielsen’s TTM Trial are usually interpreted as if TH only confers protective mechanisms by controlling/preventing fever in post-cardiac arrest patients. This hypothesis is partially true in the sense that fever in post-cardiac arrest patients has been shown to be harmful in those who had an ischemic event [146]. For years authors have stated the detrimental effect that hyperthermia has on prognosis, but hyperthermia prevention is not the only mechanism by which TH confers beneficial effects [118]. The question that Nielsen was seeking to answer with this trial is very valid, but the execution of the study was probably suboptimal.
The study design was an international RCT, which involved 36 intensive care units (ICUs) in Europe and Australia. During 26 months, patients 18 years or older, who suffered an OHCA, who remained comatose at least 20 min after ROSC were screened to form part of the trial. The main exclusion criteria were that more than 240 min had passed since ROSC until screening and asystole as initial rhythm [76].
Between November 2010 and January 2010, 950 patients were enrolled in the TTM Trial (476 assigned to 33 °C and 474 assigned to 36 °C). These numbers mean that on average each center was enrolling only one patient per month into the trial. Were all post-cardiac arrest patients with ROSC screened for this study? We believe physicians were subconsciously preselecting patients who they thought could benefit from TH [146].
Additionally, a major concern within the design of this study is the time allowance for screening of up to 4 h [147]. Animal studies and pathophysiologic knowledge support that early induction of hypothermia correlates with better protective effectiveness [111, 112]. Thus, with the design of the study, patients could go up to 4 h, before initiating the cooling process, with some patients not reaching target temperature after up to 10 h post-ROSC. This skews their proposed analysis and results, as medical literature states that the time window to reach target temperature to have the greatest effectiveness is 4 h [7].
Clinical data and patient characteristics play a big role when comparing both groups (33 °C vs. 36 °C). The patients in the 33 °C group were already hypothermic upon admission, which in this setting means a more severe brain injury. This alone might define the study patient population as heterogeneous, thus affecting the interpretation of results [76].
Rewarming rate has shown to affect the outcome of patients. Studies have shown that fast rewarming is associated with poorer outcomes and that fast rewarming can even negate the benefits that TH provides. In the TTM Trial, patients were rewarmed at a rate of 0.5 °C/h, which could translate to a mitigation of the beneficial effects of patients cooled down to either 33 °C or 36 °C [148]. Literature has stress that rewarming should be slow, and guidelines state that the rate of 0.5 °C/h was based on clinical surveys, rather than a scientifically proven benefit [60].
After the release of the TTM Trial, some major medical centers in the world stopped inducing TH on their post-cardiac arrest patients or changed target temperature to 36 °C [6]. This has become one of the major concerns after Nielsen’s trial; due to the misunderstanding that strict normothermia and hyperthermia avoidance would apparently yield the same results as a hypothermia regimen, there has been some trend to stop using hypothermia, between the mild-to-moderate range, in the post-cardiac arrest patient population. Temperature control at 36 °C is harder as the shivering response is increased and a single shivering episode could increase temperature to 37.5 °C, putting the patient in the febrile territory, and more importantly, brain temperature is usually 0.34–2.0 °C above body core temperature, and avoiding brain hyperthermia is one of the main post-cardiac arrest goals [121]. It is imperative to debunk the misinterpreted results from the TTM Trial that can lead to more harm than good.
There is no doubt that the TTM Trial shows the benefits from strict hyperthermia control in diminishing postanoxic brain injury in cardiac arrest patients. But, is this information sufficient to state that TH confers no additional benefit than strict fever prevention? We believe that the study has shown the importance of strict hyperthermia control after a TH regimen, but that does not mean that fever control is the only method by which TH confers benefits to CA patients. The results from the TTM Trial show that fever control could suffice, to some extent, in some CA patients, but we do not believe 36 °C should become the new ideal target temperature. This trial raises important questions regarding ideal target temperature to specific patient population [7].
Recently, an open-label RCT, which included 25 French ICUs, compared moderate TH at 33 °C for 24 h with targeted normothermia at 37 °C for 48 h. The study included 581 patients, 284 in the hypothermia group and 297 in the normothermia group. They assessed neurological and mortality outcome on day 90 after randomization. Lascarrou and colleagues reported that 10.2% of patients in hypothermia group had a favorable neurologic outcome, compared to the normothermia group which had a good neurologic outcome in 5.7% of patients. Mortality at 90 days after randomization did not differ in both groups [149].
This RCT has some important limitations. First, the primary outcome was assessed through a phone interview. Second, an important proportion of patients were hyperthermic, despite the TTM, especially those in the normothermia group. Third, patients in the hypothermia group had TTM for up to 64 h, compared to only 48 h in the normothermia group. Lastly, as stated in the paper, an outcome change in one patient would make a difference in dictating if hypothermia was statistically significant, compared to normothermia, regarding the primary outcome [149].
Despite the important limitations of this study, it demonstrates the importance of TH in post-cardiac arrest patient. Patients in the normothermia group had a much higher incidence of hyperthermia, which correlates with the data that states that normothermia is more difficult to maintain than hypothermia [7]. Many questions remain unanswered, which should be the focus on future research.
13 Future Directions
Despite the overwhelmingly positive evidence regarding TTM, this therapy remains underused [95,96,97,98]. Focus should be put into promoting and educating healthcare providers in the usage of TH. Standardization of guidelines and recommendations is required to achieve a higher adequate usage of this beneficial therapy. The ILCOR has attempted to standardize TH usage through their recommendations which are updated every 5 years [21, 78, 60, 85]. Despite all the research in the TH field, many questions remain unanswered. As it has been explained, there still is confusion on the target temperature during induced hypothermia, remaining as one of the main challenges that researchers have: How low should we go in temperature for the optimal protection? We know that mild-to-moderate hypothermia confers organic protection in patients with ischemic events, but an ideal temperature has yet to be defined [59].
Traditionally, hypothermia is maintained for at least 24 h in post-cardiac arrest patients, but this time duration needs to be looked into, as the recommendation stems from the time used in the major RCTs. Research is required to identify if certain patient population could benefit from a longer or shorter hypothermia regimen [115].
A major area of interest is the post-rewarming period. It is known that a slower rewarming rate is better than a fast one, but there is no definite study that shows what should be the optimal target temperature in the post-rewarming phase and for how long should patients have strict hyperthermia avoidance. This aspect is very relevant, as there is evidence that fast rewarming and/or poor temperature control after rewarming can mitigate the beneficial effects of TH [135, 150].
Studies have shown that early hypothermia induction is associated with better outcomes in post-cardiac arrest patients [88, 113]. But, how early can we start cooling down patients? Data has shown that the initiation of hypothermia during the intra-arrest phase is possible and might be feasible. Cooling down patients this way would not only shorten the time until the desired target temperature is reached but would also promote TTM usage [151].
Targeted temperature management could expand to other clinical scenarios in the next couple of years. Study after study has shown the positive effects of hypothermia in the body. Healthcare providers need to acknowledge the beneficial effects of induced hypothermia for certain patient populations and start integrating it as part of their therapeutic options [19, 20].
References
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. https://doi.org/10.1161/CIR.0000000000000659.
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63. https://doi.org/10.1056/NEJMoa003289.
Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56. https://doi.org/10.1056/NEJMoa012689.
Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 Suppl):S186–202. https://doi.org/10.1097/CCM.0b013e3181aa5241.
Girotra S, Chan PS, Bradley SM. Post-resuscitation care following out-of-hospital and in-hospital cardiac arrest. Heart. 2015;101(24):1943–9. https://doi.org/10.1136/heartjnl-2015-307450.
Polderman KH, Varon J. Confusion around therapeutic temperature management hypothermia after in-hospital cardiac arrest? Circulation. 2018;137(3):219–21. https://doi.org/10.1161/circulationaha.117.029656.
Polderman KH, Varon J. How low should we go? Hypothermia or strict normothermia after cardiac arrest? Circulation. 2015;131(7):669–75. https://doi.org/10.1161/circulationaha.114.012165.
Bray JE, Stub D, Bloom JE, Segan L, Mitra B, Smith K, et al. Changing target temperature from 33 degrees C to 36 degrees C in the ICU management of out-of-hospital cardiac arrest: a before and after study. Resuscitation. 2017;113:39–43. https://doi.org/10.1016/j.resuscitation.2017.01.016.
Karnatovskaia LV, Wartenberg KE, Freeman WD. Therapeutic hypothermia for neuroprotection: history, mechanisms, risks, and clinical applications. Neurohospitalist. 2014;4(3):153–63. https://doi.org/10.1177/1941874413519802.
Varon J. Therapeutic hypothermia in cardiac arrest: 206 years later! Resuscitation. 2009;80(12):1335. https://doi.org/10.1016/j.resuscitation.2009.08.021.
Liss HP. A history of resuscitation. Ann Emerg Med. 1986;15(1):65–72. https://doi.org/10.1016/s0196-0644(86)80490-5.
Remba SJ, Varon J, Rivera A, Sternbach GL. Dominique-Jean Larrey: the effects of therapeutic hypothermia and the first ambulance. Resuscitation. 2010;81(3):268–71. https://doi.org/10.1016/j.resuscitation.2009.11.010.
Alzaga AG, Salazar GA, Varon J. Resuscitation great. Breaking the thermal barrier: Dr. Temple Fay. Resuscitation. 2006;69(3):359–64. https://doi.org/10.1016/j.resuscitation.2006.02.014.
Bohl MA, Martirosyan NL, Killeen ZW, Belykh E, Zabramski JM, Spetzler RF, et al. The history of therapeutic hypothermia and its use in neurosurgery. J Neurosurg. 2018:1–15. https://doi.org/10.3171/2017.10.JNS171282.
Fay T. Observations on prolonged human refrigeration. NY State J Med. 1941;2(3):347.
Bigelow WG, McBirnie JE. Further experiences with hypothermia for intracardiac surgery in monkeys and groundhogs. Ann Surg. 1953;137(3):361–5. https://doi.org/10.1097/00000658-195303000-00010.
Rosomoff HL, Holaday DA. Cerebral blood flow and cerebral oxygen consumption during hypothermia. Am J Phys. 1954;179(1):85–8. https://doi.org/10.1152/ajplegacy.1954.179.1.85.
Bonaventura J, Alan D, Vejvoda J, Honek J, Veselka J. History and current use of mild therapeutic hypothermia after cardiac arrest. Arch Med Sci. 2016;12(5):1135–41. https://doi.org/10.5114/aoms.2016.61917.
Alzaga AG, Cerdan M, Varon J. Therapeutic hypothermia. Resuscitation. 2006;70(3):369–80. https://doi.org/10.1016/j.resuscitation.2006.01.017.
Varon J, Acosta P. Therapeutic hypothermia: past, present, and future. Chest. 2008;133(5):1267–74. https://doi.org/10.1378/chest.07-2190.
Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW, Kloeck WG, Billi J, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108(1):118–21. https://doi.org/10.1161/01.Cir.0000079019.02601.90.
Nolan JP, Hazinski MF, Steen PA, Becker LB. Controversial Topics from the 2005 International Consensus Conference on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2005;67(2–3):175–9. https://doi.org/10.1016/j.resuscitation.2005.09.008.
Negovsky VA. The second step in resuscitation—the treatment of the ‘post-resuscitation disease’. Resuscitation. 1972;1(1):1–7.
Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Bottiger BW, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79(3):350–79. https://doi.org/10.1016/j.resuscitation.2008.09.017.
Binks A, Nolan JP. Post-cardiac arrest syndrome. Minerva Anestesiol. 2010;76(5):362–8.
Madder RD, Reynolds JC. Multidisciplinary management of the post-cardiac arrest patient. Cardiol Clin. 2018;36(1):85–101. https://doi.org/10.1016/j.ccl.2017.08.005.
Bougouin W, Cariou A. Management of postcardiac arrest myocardial dysfunction. Curr Opin Crit Care. 2013;19(3):195–201. https://doi.org/10.1097/MCC.0b013e3283607740.
Polderman KH, Varon J. Cool hemodynamics—the intricate interplay between therapeutic hypothermia and the post-cardiac arrest syndrome. Resuscitation. 2014;85(8):975–6. https://doi.org/10.1016/j.resuscitation.2014.06.002.
Oksanen T, Skrifvars M, Wilkman E, Tierala I, Pettila V, Varpula T. Postresuscitation hemodynamics during therapeutic hypothermia after out-of-hospital cardiac arrest with ventricular fibrillation: a retrospective study. Resuscitation. 2014;85(8):1018–24. https://doi.org/10.1016/j.resuscitation.2014.04.026.
Kleinman ME, Perkins GD, Bhanji F, Billi JE, Bray JE, Callaway CW, et al. ILCOR scientific knowledge gaps and clinical research priorities for cardiopulmonary resuscitation and emergency cardiovascular care: a consensus statement. Resuscitation. 2018;127:132–46. https://doi.org/10.1016/j.resuscitation.2018.03.021.
Jones AE, Shapiro NI, Kilgannon JH, Trzeciak S. Emergency medicine shock research network I. Goal-directed hemodynamic optimization in the post-cardiac arrest syndrome: a systematic review. Resuscitation. 2008;77(1):26–9. https://doi.org/10.1016/j.resuscitation.2007.10.021.
Lurie KG, Nemergut EC, Yannopoulos D, Sweeney M. The physiology of cardiopulmonary resuscitation. Anesth Analg. 2016;122(3):767–83. https://doi.org/10.1213/ANE.0000000000000926.
Lewis LM, Stothert JC Jr, Gomez CR, Ruoff BE, Hall IS, Chandel B, et al. A noninvasive method for monitoring cerebral perfusion during cardiopulmonary resuscitation. J Crit Care. 1994;9(3):169–74.
Maramattom BV, Wijdicks EF. Postresuscitation encephalopathy. Current views, management, and prognostication. Neurologist. 2005;11(4):234–43. https://doi.org/10.1097/01.nrl.0000159985.07242.22.
Ferreira Da Silva IR, Frontera JA. Targeted temperature management in survivors of cardiac arrest. Cardiol Clin. 2013;31(4):637–55. https://doi.org/10.1016/j.ccl.2013.07.010. ix
Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30(11):2126–8. https://doi.org/10.1007/s00134-004-2425-z.
Tahsili-Fahadan P, Farrokh S, Geocadin RG. Hypothermia and brain inflammation after cardiac arrest. Brain Circ. 2018;4(1):1–13. https://doi.org/10.4103/bc.bc_4_18.
Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363(13):1256–64. https://doi.org/10.1056/NEJMct1002402.
Eleff SM, Maruki Y, Monsein LH, Traystman RJ, Bryan RN, Koehler RC. Sodium, ATP, and intracellular pH transients during reversible complete ischemia of dog cerebrum. Stroke. 1991;22(2):233–41. https://doi.org/10.1161/01.str.22.2.233.
Gonzalez-Ibarra FP, Varon J, Lopez-Meza EG. Therapeutic hypothermia: critical review of the molecular mechanisms of action. Front Neurol. 2011;2:4. https://doi.org/10.3389/fneur.2011.00004.
Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–51. https://doi.org/10.1016/j.redox.2015.08.020.
Han F, Da T, Riobo NA, Becker LB. Early mitochondrial dysfunction in electron transfer activity and reactive oxygen species generation after cardiac arrest. Crit Care Med. 2008;36(11 Suppl):S447–53. https://doi.org/10.1097/ccm.0b013e31818a8a51.
Patil KD, Halperin HR, Becker LB. Cardiac arrest: resuscitation and reperfusion. Circ Res. 2015;116(12):2041–9. https://doi.org/10.1161/CIRCRESAHA.116.304495.
Vaagenes P, Ginsberg M, Ebmeyer U, Ernster L, Fischer M, Gisvold SE, et al. Cerebral resuscitation from cardiac arrest: pathophysiologic mechanisms. Crit Care Med. 1996;24(2 Suppl):S57–68.
Kuffler DP. Maximizing neuroprotection: where do we stand? Ther Clin Risk Manag. 2012;8:185–94. https://doi.org/10.2147/TCRM.S16196.
Kuschner CE, Becker LB. Recent advances in personalizing cardiac arrest resuscitation. F1000Res. 2019;8:F1000 Faculty Rev-915. https://doi.org/10.12688/f1000research.17554.1.
Varon J, Marik PE, Einav S. Therapeutic hypothermia: a state-of-the-art emergency medicine perspective. Am J Emerg Med. 2012;30(5):800–10. https://doi.org/10.1016/j.ajem.2011.03.007.
Zipfel GJ, Babcock DJ, Lee JM, Choi DW. Neuronal apoptosis after CNS injury: the roles of glutamate and calcium. J Neurotrauma. 2000;17(10):857–69. https://doi.org/10.1089/neu.2000.17.857.
Small DL, Morley P, Buchan AM. Biology of ischemic cerebral cell death. Prog Cardiovasc Dis. 1999;42(3):185–207.
Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13(4):267–78. https://doi.org/10.1038/nrn3174.
Hurst S, Hoek J, Sheu SS. Mitochondrial Ca(2+) and regulation of the permeability transition pore. J Bioenerg Biomembr. 2017;49(1):27–47. https://doi.org/10.1007/s10863-016-9672-x.
Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005;1047:248–58. https://doi.org/10.1196/annals.1341.022.
Arun S, Liu L, Donmez G. Mitochondrial biology and neurological diseases. Curr Neuropharmacol. 2016;14(2):143–54.
Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, et al. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010;41(2–3):172–9. https://doi.org/10.1007/s12035-010-8102-z.
Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, et al. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12(7):857–63. https://doi.org/10.1038/nn.2334.
Globus MY, Busto R, Lin B, Schnippering H, Ginsberg MD. Detection of free radical activity during transient global ischemia and recirculation: effects of intraischemic brain temperature modulation. J Neurochem. 1995;65(3):1250–6. https://doi.org/10.1046/j.1471-4159.1995.65031250.x.
Li Y, Tang Q, Wang P, Qin J, Wu H, Lin J, et al. Dynamic changes of mitochondrial fusion and fission in brain injury after cardiac arrest in rats. Biomed Res Int. 2017;2017:1948070. https://doi.org/10.1155/2017/1948070.
Chavez LO, Leon M, Einav S, Varon J. Editor’s choice- inside the cold heart: a review of therapeutic hypothermia cardioprotection. Eur Heart J Acute Cardiovasc Care. 2017;6(2):130–41. https://doi.org/10.1177/2048872615624242.
Walker AC, Johnson NJ. Targeted temperature management and postcardiac arrest care. Emerg Med Clin North Am. 2019;37(3):381–93. https://doi.org/10.1016/j.emc.2019.03.002.
Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation. 2010;81(10):1305–52. https://doi.org/10.1016/j.resuscitation.2010.08.017.
Colbourne F, Grooms SY, Zukin RS, Buchan AM, Bennett MV. Hypothermia rescues hippocampal CA1 neurons and attenuates down-regulation of the AMPA receptor GluR2 subunit after forebrain ischemia. Proc Natl Acad Sci U S A. 2003;100(5):2906–10. https://doi.org/10.1073/pnas.2628027100.
Liu L, Yenari MA. Therapeutic hypothermia: neuroprotective mechanisms. Front Biosci. 2007;12:816–25. https://doi.org/10.2741/2104.
Berger C, Schabitz WR, Wolf M, Mueller H, Sommer C, Schwab S. Hypothermia and brain-derived neurotrophic factor reduce glutamate synergistically in acute stroke. Exp Neurol. 2004;185(2):305–12. https://doi.org/10.1016/j.expneurol.2003.10.008.
Perman SM, Goyal M, Neumar RW, Topjian AA, Gaieski DF. Clinical applications of targeted temperature management. Chest. 2014;145(2):386–93. https://doi.org/10.1378/chest.12-3025.
Varon J. Therapeutic hypothermia: implications for acute care practitioners. Postgrad Med. 2010;122(1):19–27. https://doi.org/10.3810/pgm.2010.01.2095.
Staer-Jensen H, Sunde K, Olasveengen TM, Jacobsen D, Draegni T, Nakstad ER, et al. Bradycardia during therapeutic hypothermia is associated with good neurologic outcome in comatose survivors of out-of-hospital cardiac arrest. Crit Care Med. 2014;42(11):2401–8. https://doi.org/10.1097/CCM.0000000000000515.
Thomsen JH, Hassager C, Bro-Jeppesen J, Soholm H, Nielsen N, Wanscher M, et al. Sinus bradycardia during hypothermia in comatose survivors of out-of-hospital cardiac arrest—a new early marker of favorable outcome? Resuscitation. 2015;89:36–42. https://doi.org/10.1016/j.resuscitation.2014.12.031.
Kiyosue T, Arita M, Muramatsu H, Spindler AJ, Noble D. Ionic mechanisms of action potential prolongation at low temperature in guinea-pig ventricular myocytes. J Physiol. 1993;468:85–106. https://doi.org/10.1113/jphysiol.1993.sp019761.
Lewis ME, Al-Khalidi AH, Townend JN, Coote J, Bonser RS. The effects of hypothermia on human left ventricular contractile function during cardiac surgery. J Am Coll Cardiol. 2002;39(1):102–8. https://doi.org/10.1016/s0735-1097(01)01694-1.
Frank SM, Satitpunwaycha P, Bruce SR, Herscovitch P, Goldstein DS. Increased myocardial perfusion and sympathoadrenal activation during mild core hypothermia in awake humans. Clin Sci (Lond). 2003;104(5):503–8. https://doi.org/10.1042/CS20020256.
Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37(3):1101–20. https://doi.org/10.1097/CCM.0b013e3181962ad5.
Dobak S, Rincon F. “Cool” topic: feeding during moderate hypothermia after intracranial hemorrhage. JPEN J Parenter Enteral Nutr. 2017;41(7):1125–30. https://doi.org/10.1177/0148607116655448.
Williams ML, Nolan JP. Is enteral feeding tolerated during therapeutic hypothermia? Resuscitation. 2014;85(11):1469–72. https://doi.org/10.1016/j.resuscitation.2014.08.018.
Varon J, Acosta P. Coagulopathy during therapeutic hypothermia: where are the data? Resuscitation. 2009;80(7):726–7. https://doi.org/10.1016/j.resuscitation.2009.04.036.
Zeiner A, Holzer M, Sterz F, Behringer W, Schorkhuber W, Mullner M, et al. Mild resuscitative hypothermia to improve neurological outcome after cardiac arrest. A clinical feasibility trial. Hypothermia After Cardiac Arrest (HACA) Study Group. Stroke. 2000;31(1):86–94. https://doi.org/10.1161/01.str.31.1.86.
Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369(23):2197–206. https://doi.org/10.1056/NEJMoa1310519.
Donnino MW, Andersen LW, Berg KM, Reynolds JC, Nolan JP, Morley PT, et al. Temperature management after cardiac arrest: an advisory statement by the advanced life support task force of the international liaison committee on resuscitation and the American heart association emergency cardiovascular care committee and the council on cardiopulmonary, critical care, perioperative and resuscitation. Circulation. 2015;132(25):2448–56. https://doi.org/10.1161/CIR.0000000000000313.
Part 7.5: postresuscitation support. Circulation. 2005;112(24_supplement):IV-84–IV-8. https://doi.org/10.1161/circulationaha.105.166560.
Morrison Laurie J, Deakin Charles D, Morley Peter T, Callaway Clifton W, Kerber Richard E, Kronick Steven L, et al. Part 8: advanced life support. Circulation. 2010;122(16_suppl_2):S345–421. https://doi.org/10.1161/circulationaha.110.971051.
Nichol G, Huszti E, Kim F, Fly D, Parnia S, Donnino M, et al. Does induction of hypothermia improve outcomes after in-hospital cardiac arrest? Resuscitation. 2013;84(5):620–5. https://doi.org/10.1016/j.resuscitation.2012.12.009.
Testori C, Sterz F, Behringer W, Haugk M, Uray T, Zeiner A, et al. Mild therapeutic hypothermia is associated with favourable outcome in patients after cardiac arrest with non-shockable rhythms. Resuscitation. 2011;82(9):1162–7. https://doi.org/10.1016/j.resuscitation.2011.05.022.
Dumas F, Grimaldi D, Zuber B, Fichet J, Charpentier J, Pene F, et al. Is hypothermia after cardiac arrest effective in both shockable and nonshockable patients?: insights from a large registry. Circulation. 2011;123(8):877–86. https://doi.org/10.1161/circulationaha.110.987347.
Vaahersalo J, Hiltunen P, Tiainen M, Oksanen T, Kaukonen KM, Kurola J, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in Finnish intensive care units: the FINNRESUSCI study. Intensive Care Med. 2013;39(5):826–37. https://doi.org/10.1007/s00134-013-2868-1.
Mader TJ, Nathanson BH, Soares WE 3rd, Coute RA, McNally BF. Comparative effectiveness of therapeutic hypothermia after out-of-hospital cardiac arrest: insight from a large data registry. Ther Hypothermia Temp Manag. 2014;4(1):21–31. https://doi.org/10.1089/ther.2013.0018.
Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, et al. Part 8: post-cardiac arrest care: 2015 American heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):S465–82. https://doi.org/10.1161/CIR.0000000000000262.
Guterman EL, Kim AS, Josephson SA. Neurologic consultation and use of therapeutic hypothermia for cardiac arrest. Resuscitation. 2017;118:43–8. https://doi.org/10.1016/j.resuscitation.2017.06.025.
Kim F, Nichol G, Maynard C, Hallstrom A, Kudenchuk PJ, Rea T, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA. 2014;311(1):45–52. https://doi.org/10.1001/jama.2013.282173.
Lyon RM, Van Antwerp J, Henderson C, Weaver A, Davies G, Lockey D. Prehospital intranasal evaporative cooling for out-of-hospital cardiac arrest: a pilot, feasibility study. Eur J Emerg Med. 2014;21(5):368–70. https://doi.org/10.1097/mej.0000000000000100.
Nie C, Dong J, Zhang P, Liu X, Han F. Prehospital therapeutic hypothermia after out-of-hospital cardiac arrest: a systematic review and meta-analysis. Am J Emerg Med. 2016;34(11):2209–16. https://doi.org/10.1016/j.ajem.2016.09.007.
Scales DC, Cheskes S, Verbeek PR, Pinto R, Austin D, Brooks SC, et al. Prehospital cooling to improve successful targeted temperature management after cardiac arrest: a randomized controlled trial. Resuscitation. 2017;121:187–94. https://doi.org/10.1016/j.resuscitation.2017.10.002.
Schenfeld EM, Studnek J, Heffner AC, Nussbaum M, Kraft K, Pearson DA. Effect of prehospital initiation of therapeutic hypothermia in adults with cardiac arrest on time-to-target temperature. CJEM. 2015;17(3):240–7. https://doi.org/10.2310/8000.2014.141307.
Uray T, Mayr FB, Stratil P, Aschauer S, Testori C, Sterz F, et al. Prehospital surface cooling is safe and can reduce time to target temperature after cardiac arrest. Resuscitation. 2015;87:51–6. https://doi.org/10.1016/j.resuscitation.2014.10.026.
Schenone AL, Cohen A, Patarroyo G, Harper L, Wang X, Shishehbor MH, et al. Therapeutic hypothermia after cardiac arrest: a systematic review/meta-analysis exploring the impact of expanded criteria and targeted temperature. Resuscitation. 2016;108:102–10. https://doi.org/10.1016/j.resuscitation.2016.07.238.
Surani S, Varon J. The expanded use of targeted temperature management: time for reappraisal. Resuscitation. 2016;108:A8–9. https://doi.org/10.1016/j.resuscitation.2016.09.002.
Patel PV, John S, Garg RK, Temes RE, Bleck TP, Prabhakaran S. Therapeutic hypothermia after cardiac arrest is underutilized in the United States. Ther Hypothermia Temp Manag. 2011;1(4):199–203. https://doi.org/10.1089/ther.2011.0015.
Jena AB, Romley JA, Newton-Cheh C, Noseworthy P. Therapeutic hypothermia for cardiac arrest: real-world utilization trends and hospital mortality. J Hosp Med. 2012;7(9):684–9. https://doi.org/10.1002/jhm.1974.
Dresden SM, O’Connor LM, Pearce CG, Courtney DM, Powell ES. National trends in the use of postcardiac arrest therapeutic hypothermia and hospital factors influencing its use. Ther Hypothermia Temp Manag. 2015;5(1):48–54. https://doi.org/10.1089/ther.2014.0023.
Morrison LJ, Brooks SC, Dainty KN, Dorian P, Needham DM, Ferguson ND, et al. Improving use of targeted temperature management after out-of-hospital cardiac arrest: a stepped wedge cluster randomized controlled trial. Crit Care Med. 2015;43(5):954–64. https://doi.org/10.1097/CCM.0000000000000864.
Varon J, Acosta P. Therapeutic hypothermia use among health care providers in 2 developing countries. Am J Emerg Med. 2008;26(2):244. https://doi.org/10.1016/j.ajem.2007.05.025.
Guluma KZ, Hemmen TM, Olsen SE, Rapp KS, Lyden PD. A trial of therapeutic hypothermia via endovascular approach in awake patients with acute ischemic stroke: methodology. Acad Emerg Med. 2006;13(8):820–7. https://doi.org/10.1197/j.aem.2006.03.559.
Geurts M, Petersson J, Brizzi M, Olsson-Hau S, Luijckx GJ, Algra A, et al. COOLIST (cooling for ischemic stroke trial): a multicenter, open, randomized, phase II. Clin Trial Strok. 2017;48(1):219–21. https://doi.org/10.1161/STROKEAHA.116.014757.
Kurisu K, Yenari MA. Therapeutic hypothermia for ischemic stroke; pathophysiology and future promise. Neuropharmacology. 2018;134(Pt B):302–9. https://doi.org/10.1016/j.neuropharm.2017.08.025.
Fox JL, Vu EN, Doyle-Waters M, Brubacher JR, Abu-Laban R, Hu Z. Prophylactic hypothermia for traumatic brain injury: a quantitative systematic review. CJEM. 2010;12(4):355–64.
Varon J, Marik PE. Complete neurological recovery following delayed initiation of hypothermia in a victim of warm water near-drowning. Resuscitation. 2006;68(3):421–3. https://doi.org/10.1016/j.resuscitation.2005.07.020.
Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;(1):CD003311. https://doi.org/10.1002/14651858.CD003311.pub3.
Rose C, Michalak A, Pannunzio M, Chatauret N, Rambaldi A, Butterworth RF. Mild hypothermia delays the onset of coma and prevents brain edema and extracellular brain glutamate accumulation in rats with acute liver failure. Hepatology. 2000;31(4):872–7. https://doi.org/10.1053/he.2000.5923.
Vaquero J, Rose C, Butterworth RF. Keeping cool in acute liver failure: rationale for the use of mild hypothermia. J Hepatol. 2005;43(6):1067–77. https://doi.org/10.1016/j.jhep.2005.05.039.
Stravitz RT, Lee WM, Kramer AH, Kramer DJ, Hynan L, Blei AT. Therapeutic hypothermia for acute liver failure: toward a randomized, controlled trial in patients with advanced hepatic encephalopathy. Neurocrit Care. 2008;9(1):90–6. https://doi.org/10.1007/s12028-008-9090-y.
Villar J, Slutsky AS. Effects of induced hypothermia in patients with septic adult respiratory distress syndrome. Resuscitation. 1993;26(2):183–92. https://doi.org/10.1016/0300-9572(93)90178-s.
Madden LK, Hill M, May TL, Human T, Guanci MM, Jacobi J, et al. The implementation of targeted temperature management: an evidence-based guideline from the neurocritical care society. Neurocrit Care. 2017;27(3):468–87. https://doi.org/10.1007/s12028-017-0469-5.
Che D, Li L, Kopil CM, Liu Z, Guo W, Neumar RW. Impact of therapeutic hypothermia onset and duration on survival, neurologic function, and neurodegeneration after cardiac arrest. Crit Care Med. 2011;39(6):1423–30. https://doi.org/10.1097/CCM.0b013e318212020a.
Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21(9):1348–58. https://doi.org/10.1097/00003246-199309000-00019.
Cabanas JG, Brice JH, De Maio VJ, Myers B, Hinchey PR. Field-induced therapeutic hypothermia for neuroprotection after out-of hospital cardiac arrest: a systematic review of the literature. J Emerg Med. 2011;40(4):400–9. https://doi.org/10.1016/j.jemermed.2010.07.002.
Bernard SA, Smith K, Finn J, Hein C, Grantham H, Bray JE, et al. Induction of therapeutic hypothermia during out-of-hospital cardiac arrest using a rapid infusion of cold saline: the RINSE Trial (Rapid Infusion of Cold Normal Saline). Circulation. 2016;134(11):797–805. https://doi.org/10.1161/circulationaha.116.021989.
Kirkegaard H, Soreide E, de Haas I, Pettila V, Taccone FS, Arus U, et al. Targeted temperature management for 48 vs 24 hours and neurologic outcome after out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2017;318(4):341–50. https://doi.org/10.1001/jama.2017.8978.
Callaway CW. Targeted temperature management after cardiac arrest: finding the right dose for critical care interventions. JAMA. 2017;318(4):334–6. https://doi.org/10.1001/jama.2017.8977.
Vaity C, Al-Subaie N, Cecconi M. Cooling techniques for targeted temperature management post-cardiac arrest. Crit Care. 2015;19:103. https://doi.org/10.1186/s13054-015-0804-1.
Fukuda T. Targeted temperature management for adult out-of-hospital cardiac arrest: current concepts and clinical applications. J Intensive Care. 2016;4:30. https://doi.org/10.1186/s40560-016-0139-2.
Glover GW, Thomas RM, Vamvakas G, Al-Subaie N, Cranshaw J, Walden A, et al. Intravascular versus surface cooling for targeted temperature management after out-of-hospital cardiac arrest—an analysis of the TTM trial data. Crit Care. 2016;20(1):381. https://doi.org/10.1186/s13054-016-1552-6.
Tommasi E, Lazzeri C, Bernardo P, Sori A, Chiostri M, Gensini GF, et al. Cooling techniques in mild hypothermia after cardiac arrest. J Cardiovasc Med (Hagerstown). 2017;18(7):459–66. https://doi.org/10.2459/JCM.0000000000000130.
Sonder P, Janssens GN, Beishuizen A, Henry CL, Rittenberger JC, Callaway CW, et al. Efficacy of different cooling technologies for therapeutic temperature management: a prospective intervention study. Resuscitation. 2018;124:14–20. https://doi.org/10.1016/j.resuscitation.2017.12.026.
Coppler PJ, Marill KA, Okonkwo DO, Shutter LA, Dezfulian C, Rittenberger JC, et al. Concordance of brain and core temperature in comatose patients after cardiac arrest. Ther Hypothermia Temp Manag. 2016;6(4):194–7. https://doi.org/10.1089/ther.2016.0010.
Akata T, Setoguchi H, Shirozu K, Yoshino J. Reliability of temperatures measured at standard monitoring sites as an index of brain temperature during deep hypothermic cardiopulmonary bypass conducted for thoracic aortic reconstruction. J Thorac Cardiovasc Surg. 2007;133(6):1559–65. https://doi.org/10.1016/j.jtcvs.2006.11.031.
Stone JG, Young WL, Smith CR, Solomon RA, Wald A, Ostapkovich N, et al. Do standard monitoring sites reflect true brain temperature when profound hypothermia is rapidly induced and reversed? Anesthesiology. 1995;82(2):344–51. https://doi.org/10.1097/00000542-199502000-00004.
Markota A, Palfy M, Stozer A, Sinkovic A. Difference between bladder and esophageal temperatures in mild induced hypothermia. J Emerg Med. 2015;49(1):98–103. https://doi.org/10.1016/j.jemermed.2014.12.059.
Zweifler RM, Voorhees ME, Mahmood MA, Parnell M. Rectal temperature reflects tympanic temperature during mild induced hypothermia in nonintubated subjects. J Neurosurg Anesthesiol. 2004;16(3):232–5.
McIlvoy L. Comparison of brain temperature to core temperature: a review of the literature. J Neurosci Nurs. 2004;36(1):23–31.
Cabanac M, Germain M, Brinnel H. Tympanic temperatures during hemiface cooling. Eur J Appl Physiol Occup Physiol. 1987;56(5):534–9. https://doi.org/10.1007/bf00635366.
Lopez M, Sessler DI, Walter K, Emerick T, Ozaki M. Rate and gender dependence of the sweating, vasoconstriction, and shivering thresholds in humans. Anesthesiology. 1994;80(4):780–8. https://doi.org/10.1097/00000542-199404000-00009.
Choi HA, Ko SB, Presciutti M, Fernandez L, Carpenter AM, Lesch C, et al. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocrit Care. 2011;14(3):389–94. https://doi.org/10.1007/s12028-010-9474-7.
Howes D, Gray SH, Brooks SC, Boyd JG, Djogovic D, Golan E, et al. Canadian Guidelines for the use of targeted temperature management (therapeutic hypothermia) after cardiac arrest: a joint statement from The Canadian Critical Care Society (CCCS), Canadian Neurocritical Care Society (CNCCS), and the Canadian Critical Care Trials Group (CCCTG). Resuscitation. 2016;98:48–63. https://doi.org/10.1016/j.resuscitation.2015.07.052.
Rubeiz GJ, Thill-Baharozian M, Hardie D, Carlson RW. Association of hypomagnesemia and mortality in acutely ill medical patients. Crit Care Med. 1993;21(2):203–9. https://doi.org/10.1097/00003246-199302000-00010.
Chernow B, Bamberger S, Stoiko M, Vadnais M, Mills S, Hoellerich V, et al. Hypomagnesemia in patients in postoperative intensive care. Chest. 1989;95(2):391–7. https://doi.org/10.1378/chest.95.2.391.
Arrich J, European Resuscitation Council Hypothermia After Cardiac Arrest Registry Study G. Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35(4):1041–7. https://doi.org/10.1097/01.CCM.0000259383.48324.35.
Zhu SZ, Gu Y, Wu Z, Hu YF, Pan SY. Hypothermia followed by rapid rewarming exacerbates ischemia-induced brain injury and augments inflammatory response in rats. Biochem Biophys Res Commun. 2016;474(1):175–81. https://doi.org/10.1016/j.bbrc.2016.04.095.
Naito H, Isotani E, Callaway CW, Hagioka S, Morimoto N. Intracranial pressure increases during rewarming period after mild therapeutic hypothermia in postcardiac arrest patients. Ther Hypothermia Temp Manag. 2016;6(4):189–93. https://doi.org/10.1089/ther.2016.0009.
Bro-Jeppesen J, Annborn M, Hassager C, Wise MP, Pelosi P, Nielsen N, et al. Hemodynamics and vasopressor support during targeted temperature management at 33 degrees C Versus 36 degrees C after out-of-hospital cardiac arrest: a post hoc study of the target temperature management trial*. Crit Care Med. 2015;43(2):318–27. https://doi.org/10.1097/CCM.0000000000000691.
Bro-Jeppesen J, Hassager C, Wanscher M, Soholm H, Thomsen JH, Lippert FK, et al. Post-hypothermia fever is associated with increased mortality after out-of-hospital cardiac arrest. Resuscitation. 2013;84(12):1734–40. https://doi.org/10.1016/j.resuscitation.2013.07.023.
Winters SA, Wolf KH, Kettinger SA, Seif EK, Jones JS, Bacon-Baguley T. Assessment of risk factors for post-rewarming "rebound hyperthermia" in cardiac arrest patients undergoing therapeutic hypothermia. Resuscitation. 2013;84(9):1245–9. https://doi.org/10.1016/j.resuscitation.2013.03.027.
Monsieurs KG, Nolan JP, Bossaert LL, Greif R, Maconochie IK, Nikolaou NI, et al. European Resuscitation Council Guidelines for Resuscitation 2015: section 1. Executive summary. Resuscitation. 2015;95:1–80. https://doi.org/10.1016/j.resuscitation.2015.07.038.
Karcioglu O, Topacoglu H, Dikme O, Dikme O. A systematic review of safety and adverse effects in the practice of therapeutic hypothermia. Am J Emerg Med. 2018;36(10):1886–94. https://doi.org/10.1016/j.ajem.2018.07.024.
Saigal S, Sharma JP, Dhurwe R, Kumar S, Gurjar M. Targeted temperature management: current evidence and practices in critical care. Indian J Crit Care Med. 2015;19(9):537–46. https://doi.org/10.4103/0972-5229.164806.
Geurts M, Macleod MR, Kollmar R, Kremer PH, van der Worp HB. Therapeutic hypothermia and the risk of infection: a systematic review and meta-analysis. Crit Care Med. 2014;42(2):231–42. https://doi.org/10.1097/CCM.0b013e3182a276e8.
Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334(19):1209–15. https://doi.org/10.1056/nejm199605093341901.
Liu YM, Ibrahim A, Jan T, Chang P, Fagan S, Goverman J. Skin necrosis as a complication of therapeutic hypothermia. J Burn Care Res. 2014;35(3):e184–e6. https://doi.org/10.1097/BCR.0b013e3182a22730.
Polderman KH, Varon J. Interpreting the results of the targeted temperature management trial in cardiac arrest. Ther Hypothermia Temp Manag. 2015;5(2):73–6. https://doi.org/10.1089/ther.2014.0031.
Stub D. Targeted temperature management after cardiac arrest. N Engl J Med. 2014;370(14):1358. https://doi.org/10.1056/NEJMc1401250.
Varon J, Polderman K. Targeted temperature management after cardiac arrest. N Engl J Med. 2014;370(14):1358–9. https://doi.org/10.1056/NEJMc1401250.
Lascarrou J-B, Merdji H, Le Gouge A, Colin G, Grillet G, Girardie P, et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N Engl J Med. 2019;381(24):2327–37. https://doi.org/10.1056/NEJMoa1906661.
Cariou A, Payen JF, Asehnoune K, Audibert G, Botte A, Brissaud O, et al. Targeted temperature management in the ICU: guidelines from a French expert panel. Ann Intensive Care. 2017;7(1):70. https://doi.org/10.1186/s13613-017-0294-1.
Stratil P, Holzer M. Is hypothermia indicated during cardiopulmonary resuscitation and after restoration of spontaneous circulation? Curr Opin Crit Care. 2016;22(3):212–7. https://doi.org/10.1097/mcc.0000000000000299.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Araiza, A., Varon, J. (2021). Hypothermia Therapy in Sudden Death. In: Zhu, H. (eds) Sudden Death. Springer, Singapore. https://doi.org/10.1007/978-981-15-7002-5_17
Download citation
DOI: https://doi.org/10.1007/978-981-15-7002-5_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7001-8
Online ISBN: 978-981-15-7002-5
eBook Packages: MedicineMedicine (R0)