Abstract
It is recognized that lactic acid bacteria (LAB) provide a wide range of benefits primarily in host health and food, which is why they are considered to be probiotics; i.e., living microorganisms that administered in adequate amounts confer benefits on the host health. The most representative probiotic strains of LAB include members of the genus Pediococcus, Lactobacillus, Bifidobacterium, and Enterococcus. In terms of benefits, it has become evident that the consumption of probiotics has been shown to be useful in treating various clinical conditions ranging from childhood diarrhea, antibiotic-associated diarrhea, relapse of Clostridium difficile colitis, Helicobacter pylori infections, inflammatory bowel disease leading to cancer and urogenital infections. Other beneficial effects of probiotics include increasing nutrient utilization, lowering serum cholesterol, improving lactose intolerance, and decreasing antibiotic use. Its hypocholesterolemic, antimutagenic, antiosteoporotic, antihypertensive, and immunomodulatory effects have also been recognized. This chapter will describe the generalities of probiotics, the main metabolites by which they exert a beneficial effect, as well as the mechanisms by which they protect against various diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
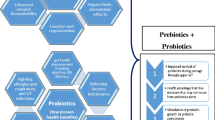
The intestinal microbiota forms a relatively stable and highly diverse ecosystem that is increasingly being recognized for its impact on human health. Deviation from its normal structure is often related to systemic and localized diseases. Modulation of the intestinal microbiota could be helpful to improve health and could be achieved through different nutritional concepts, ranging from food-specific ingredients to complex diets or by ingestion of special living microorganisms such as probiotics.
Probiotics are defined as “living microorganisms which, when administered in appropriate dosage, provide health benefits to the host.” In the last decades the contribution to the modulation of respiratory, gastrointestinal, and immunological functions that probiotics have has begun to be fully appreciated and scientifically evaluated. To this day, most commercially available probiotics are lactic acid bacteria (LAB), especially those of the genus Lactobacillus, but also bifidobacteria. A much smaller number of bacteria of the genus Lactococcus, Leuconostoc, Pediococcus, Streptococcus, and Enterococcus are also used as probiotics.
It has been identified that the foods and beverages in which a fermentation process has been carried out contain various nutritional and therapeutic properties; in this sense, fermented products such as milk are benefited by LAB since they give them positive health effects. Such foods, beverages, and powders are highly acceptable to consumers due to their high nutritional values and flavors. Potential health benefits of fermented milk include anti-tumor and antimutagenic activity, reduction of serum cholesterol levels, and prevention of gastrointestinal diseases. Probiotics are commonly used for their gastrointestinal effects, of which the best demonstrated clinical benefits of probiotics are the treatment and prevention of antibiotic-related diarrhea; their use can also be extended to oral, skin, and vaginal health, as well as treatment for liver disorders, allergies, and metabolic diseases. Some examples of the health benefits of probiotics are shown in Table 6.1.
Normally, probiotics first colonize the intestinal tract and then strengthen host defense systems by inducing a generalized immune response from the mucosa, including dendritic cell modulation/interaction with natural killer cells, a balanced auxiliary T-lymphocyte response, polymeric immunoglobulin A (IgA) secretion, and self-limiting inflammatory response. Many studies have shown that LABs, mainly Lactobacillus, as well as Bifidobacterium and its fermentation products, could improve innate and acquired immunity, alleviate allergies, prevent lesions in the gastric mucosa, and create a defense against intestinal infections. In addition, probiotic LABs are distinguished by being functional foods because they contain specific characteristics in order to potentially prevent or treat a variety of intestinal diseases including constipation, colon cancer, and inflammatory bowel disease (Mozzi 2016).
6.2 LABs as Probiotics
It is recognized that LABs provide a wide range of benefits primarily in host health and food, which is why they are considered probiotics. The most representative probiotic strains of LAB include members of the genus Pediococcus, Lactobacillus, Bifidobacterium, and Enterococcus (Buntin et al. 2008).
6.3 Generalities of LAB
Lactic acid bacteria (LAB) have been used since ancient times for the fermentation of various foods and is one of the oldest methods used to preserve food. The use of LAB dates back to 6000 B.C., with fermentation specifications in dairy products and 300 B.C. plants and 1500 B.C. meat. The conventional or typical LABs are positive Gram, immobile, negative catalase, non-spore formers, devoid of cytochromes, bacilli, or aerotolerant anaerobic coconuts, which are acid-resistant and generate lactic acid as the main final product of carbohydrate fermentation.
LABs can be located in any product, such as fermented foods and plants, and ecosystem and on the mucosal surfaces of the human body. Plants are preferred as a source for the isolation of LABs because of their metabolic activities and because of their specific flavor-forming properties. Each specific plant species provides a unique environment according to the competitiveness of microorganisms, natural plant antagonists, as well as the type, accessibility, and concentration of the substrate in various physical factors. These conditions allow the development and growth of a typical epiphytic microflora through which a population emerges and a series of fermentative processes begin after the plant material is prepared for fermentation. Strains derived from lactobacteria have demonstrated tolerance to high salt and pH concentrations, a high level of stress resistance compared to those from milk, and the ability to ferment various types of carbohydrates. Likewise, no really significant differences were observed in fermentation characteristics and enzyme profiles, such as peptidases, phosphatases, and lipases, needed to acquire various fermented dairy products from commercial lactobacteria plants and strains (Venugopalan and Dinesh 2010).
LABs have a general condition recognized as safe. They are very important within the dairy industry and are necessary for the production of several new and traditional dairy products. LABs (Lactobacillus, Lactococcus, Leuconostoc, Enterococcus, Streptococcus, and Pediococcus) are known to have probiotic activity, which beneficially influences diseases of the gastrointestinal tract (Benyacoub et al. 2005). In addition to probiotic activity, recent research has shown their ability to produce different biologically active metabolites (lactic acid, ɣ-aminobutyric acid, conjugated linoleic acid, bacteriocin and reutericycline, bioactive peptides, and exopolysaccharides). Food products supplemented with strains of LAB which contain probiotic properties (various biological activities) are functional foods with indisputable benefits for human health (Randazzo et al. 2016).
Phylogenetically, only bacteria called lactic acid bacteria (Holzapfel 2014) are within the order of Lactobacillales although some genera are more distant, they share similar physiological characteristics. Bifidobacteria and some Bacilli of the order Bacillales are examples of bacteria considered with similarities but these are not lactic acid bacteria. Bifidobacteria belonging to another phylum have frequently been considered as LAB.
However, the metabolism of LABs is different and unique and they do not share any genetic relationship. Similarly, some Bacilli, such as Bacillus coagulans, are used to generate lactic acid as the only fermentation product from pentoses and hexoses (Pleissner et al. 2016). However, in this case the metabolism is also different.
The strong adaptation of LABs to nutrient-rich niches, such as meat, fruit, or milk, leads to a truly significant reduction in their metabolic capacities. They are classified as nuisance microorganisms because they depend on external or exogenous sources for vitamins, precursor nucleic acids, and amino acids. This is an obvious disadvantage for its production in a chemical industry, since the necessary components are very expensive and in general, it is very complicated to carry out the purification of the products from the growing medium. These microorganisms have a quite efficient metabolism with only a few defined products and high flows. Moreover, this efficiency, which is thanks to adaptation, is reflected in their genomes.
6.4 Genome of Lactic Acid Bacteria: Low Redundancy
LAB have small genomes, ranging from 2.0 megabases (Mb) to 3.4 megabases (Holzapfel 2014). However, its genetic diversity is impressive despite the narrow functional definition (Sun et al. 2015). The historical definition of LAB is based on a comparable metabolism of carbohydrates (lactic acid production). In fact, the central genome of 213 Lactobacilli strains and closely associated species comprise 73 genes, none of which is related to carbohydrate metabolism (typically the LAB genome encompasses 1300–3000 genes and most of which have between 2000 and 2500) (Holzapfel 2014). On the other hand, pangenome has more than 44,000 gene families. 48 members of 133 known families of glycosyl hydrolases have been found, which at present have not been recognized for their biotechnological potential (Sun et al. 2015). The detailed analysis of the evolution of the central metabolism of carbon exposes an incredibly complex relationship between a large number of lactic acid bacteria (Salvetti et al. 2013), but it also exposes a great diversity based on the environment in which they are found. The process of reductive evolution was a consequence of the adaptation of LABs to nutrient-rich habitats, which led to the simplification of their metabolism through the accumulation of multiple auxotrophs (Makarova et al. 2006). Immediate adaptation to its environmental niche also requires less redundancy and less high level genetic control systems compared to other organisms, which makes it easier to have control of its metabolism. A correlation has been suggested between the reduced size of the genome and two components of the signal transduction system involved in stress signaling (Zúñiga et al. 2011).
6.5 Carbon Metabolism in LABs
The fermentation pathways of LAB are not favorable for obtaining energy (one or two ATP molecules per sugar molecule), which are mitigated by high conversion rates, therefore, the overall biomass gain is not high. In addition, the biosynthetic pathways and central carbon metabolism required for cell growth are highly decoupled in LABs because they adapt to nutrient-rich niches. These niches make it easier for bacteria to occupy several building cell structures, rather than synthesizing them from carbon sources. As a consequence of the aforementioned, energy is obtained from carbon sources but is inefficient. This is why, huge amounts of sugar are fermented and not much of the sugar is lost for biomass generation. This combination results in optimal yields during the fermentation process, making them interesting for the industrial area, and some applications have already been suggested.
It should be noted that LABAs are classified into three groups: obligate homolactic fermenters (lactic acid as the sole fermentation product); obligate heterolactic fermentation organisms; and obligate heterolactic fermenters (which generate lactic acid, CO2, and ethanol/acetate) (Gänzle 2015). In general, ATP is earned when sugar is oxidized to pyruvate. To reduce pyruvate, it is necessary to close the balance through the removal of electrons. The direct reduction of pyruvate produces lactic acid, which is a product of industrial importance and there are various processes for its formation, based on the diversity of LAB (Hofvendahl, and Hahn-Hägerdal 2000).
Another important aspect is the ability of many LABs to make use of various pentoses and hexoses, which makes it the perfect starting condition to take advantage of lignocellulosic sugars. Such sugars are the most abundant renewable resources on our planet and have been researched as the oil of the future (Abdel-rahman et al. 2011; Boguta et al. 2014).
A particularly interesting feature of LAB’s carbon metabolism is the flexibility of electron acceptors to carry out metabolism. Commonly, electron acceptors incorporate a significant variety to the configuration of metabolism. One of the fundamental electron acceptors is oxygen, but sometimes there is no presence of oxygen and replaces it with pyruvate. If oxygen is present, it is reduced to water without any energy conservation. Glycerol or fructose are compounds that accept other molecules that benefit the chemical industry: many heterofermentative LABs have the ability to grow in the presence of fructose, and that on the one hand fructose itself is used as a carbon source and is reduced to mannitol on the other hand. For the production of biomass and energy gain, growth in the presence of glucose is used, with fructose as an electron acceptor (Tyler et al. 2016). But, when both carbon sources are present, microorganisms use glycerol as an electron acceptor, which significantly increases the efficiency of sugar energy production, and this transforms glycerol to 1,3-propanediol with high efficiency (Pflügl et al. 2014). The first step in the use of glycerol as an electron sink is the redox-neutral action of glycerol dehydrate, generating 3-hydroxypropionaldehyde by dehydration. The second step is the reduction of aldehyde. This is the actual reaction for electron dissipation.
It is important to mention that this and similar reactions (such as the conversion of 1,2-propanediol to propionaldehyde or 2,3-butanediol to 2-butanone (Ghiaci et al. 2014)) are not linked to either energy or redox metabolism. In nature, these ketone or aldehyde intermediates never accumulate in cells that are growing due to their high toxicity (Doleyres et al. 2005).
6.6 Search for New Probiotics
Among the characteristics that a microorganism must present for it to be considered a probiotic is the ability to resist passage through the different parts of the gastrointestinal system since these microorganisms will interact with substances such as bile acids and acid pH, as well as the ability to adhere to the intestinal mucosa as this increases the benefits that these microorganisms provide to the body (Lee et al. 2008).
Probiotic microorganisms and LABs are generally associated with dairy foods; however, some foods are developed every day and marketed as functional foods, which contain probiotic microorganisms, which are generally added or supplemented to the food to obtain the benefits of this type of microorganisms. After dairy products, fruit or vegetable juices are the most technologically developed foods in relation to probiotic microorganisms, mainly this interest is associated with the potential to provide probiotic strains to people intolerant to lactose with reduced cholesterol content, which makes it of interest to many regular consumers. As mentioned above, fruits and vegetables are generally considered potential matrices for containing probiotics because they are rich in nutrients such as vitamins, dietary fiber, sugars, minerals, and polyphenolic compounds (antioxidants). Microorganisms such as Lactobacillus rhamnosus, L. fermentum, L. reuteri, and L. plantarum have been found in commercial fruit products containing a pH of 4.2 and stored 80 days at 4 °C; these strains showed resistance to storage, while the L. acidophilum strain showed low resistance in the juice matrix. This shows that the acid pH of some fruit juices represents a technological change for the formulation of foods containing LAB. Similarly, high levels of viable probiotic microorganism cells have been found in fruit juices at levels between 109 and 1010 CFU/mL after 28–50 days of storage at 4 °C in carrot, peach, and orange juices, demonstrating that fruit juices are a good matrix for delivering an adequate concentration of these microorganisms to consumers without the use of dairy additives (Freitas et al. 2015).
During the process of deterioration of fresh meat a wide variety of LAB can also be obtained; the main genera are Leuconostoc, Carnobacterium, Lactococcus, Weissella, Enterococcus, and Lactobacillus. Species derived from this genus (L. curvatus, L. algidus, L. fuchuensis, L. oligofermentans) are associated with severe acidification, emission of unpleasant odors, among others (Pothakos et al. 2015). The main producer of organic acids, such as acetic acid, is the genus Leuconostoc and is found mainly in all types of meat and meat packaging conditions (Pothakos et al. 2015). They have also been obtained from the study of fermented milk, where it has been found that they provide health benefits due to the metabolic products generated by the biological activities of lactic acid bacteria (Domínguez Gonzáleza et al. 2014).
LABs that perform the yogurt fermentation process can be produced in different environments, especially in plant materials (de Almeida Júnior et al. 2015). LAB strains to generate prebiotic foods, which are inert substances that stimulate the growth of probiotic organisms (Quigley 2012), are isolated from humans, since they have a greater capacity to adhere to the intestinal wall and colonize it; but on the other hand, also strains of animal origin can be used since they have positive effects on the human organism (Ruiz et al. 2017). One of the reasons for the increased consumption of fermented dairy products is that they contain probiotics and are readily available on the market (Savadogo et al. 2006). In yogurt, the main species are Streptococcus, such as S. lactis, S. cremoris (cheese, yogurt, butter) and S. thermophilus in yogurt and cheese (Ramírez et al. 2011). Finally, some LAB genera such as Lactobacillus, Bifidobacterium, and Pediococcus are commonly found in the intestinal microbiota of mammals (Porto et al. 2017). They have also been isolated from microbiota from freshwater fish, ocean or saltwater fish, as well as from minimally processed aquatic products (Ghanbari et al. 2013). Benavides et al. (2016) also isolated them from mature and immature subtropical fruits, such as guava, berries, and other inflorescences.
6.7 Resistance of Probiotics to Food Processing: The Case of High Hydrostatic Pressures
High hydrostatic pressures (HHP) technology has been used in food products that contain lactic acid bacteria or probiotics, such as dairy products. However, one of the main functions of high pressure as food treatment is the reduction of microbial load in order to increase the shelf life of products, so there is no selectivity between the destruction of pathogens and probiotic bacteria. In this sense, some techniques have been proposed that help these microorganisms to be used in foods treated with HHP. For example, methods of attenuation of lactic acid bacteria have been proposed for use as adjuvants in cheese making. Such is the case of Lactobacillus lactis spp. lactis treated at 300 MPa and Lactobacillus casei spp. casei treated at 350 MPa, which can be added to cheese during processing, which does not allow microorganisms to produce acid during processing, but provide an extra contribution of enzymes that accelerate the maturation of cheeses; likewise, the attenuation of Lactobacillus lactis ssp. cremoris, treated at 200 MPa for 20 min at 20 °C, can be used in combination with primary strains in the production of cheddar cheese, producing high levels of free amino acids and accelerating secondary proteolysis in cheese (Juan et al. 2016).
The effect of HHP on products containing conventional probiotic microorganisms has been widely studied. However, few studies have focused on the effects on the microflora of final products in this type of dairy foods (i.e., yogurt). In the processing of this type of products with HPP, it was found that 400 MPa was the minimum pressure necessary to inactivate Lactobacillus delbrueckii sp. bulgaricus; however, at these same conditions, Streptococcus thermophilus was resistant to pressure, and during storage of yogurt after pressurization a decrease in the concentration of the microorganism was observed, likewise, no acidification of the product was observed during cold storage (Juan et al. 2016).
Within the probiotic microorganisms, some endospore-producing bacteria are known (these forms of cell maintenance are very resistant to different environmental conditions), which gives it a high survival capacity under different environmental conditions. Thus, some bacteria belonging to the genus Bacillus sp. have been classified as probiotic microorganisms with high potential for use in the food industry.
Unlike lactic acid bacteria (LAB) used in dairy products such as those belonging to the genera Lactobacillus sp., Streptococcus sp., among others, the genera Bacillus sp. present a greater resistance to HHP processes. Bacterial spores require higher pressures (>1200 MPa) to be inactivated; for this process of inactivation, or inhibition of the germination of bacterial spores to be carried out, a combination with moderate heat pre-treatments or other treatments such as ultrasound is required. For example, for the inactivation of Clostridium botulinum spore-forming bacteria, a thermal process of 90–121 °C is required in conjunction with a pressure of 500–800 MPa (Rastogi and Shukla 2013).
Foods with high acidity content (low pH) are the best candidates for HHP technology; however, their stability may be compromised because the treatment does not destroy bacterial spores, so it will be necessary to establish coupled processes to meet the requirement to eliminate spore-producing pathogens (Rastogi and Shukla 2013). However, this same behavior can be used as a benefit to obtain food containing spore-forming bacteria (SFB) and considered as probiotics. By not destroying SFB with conventional high hydrostatic pressure (600 MPa), it is possible to take advantage of this characteristic of microbial resistance and inoculate food with a certain number of microorganisms, and in this way, it will be possible to destroy the detrimental microorganisms of food and pathogens and maintain a minimum acceptable level of the content of spore-producing probiotics. Thus, food containing probiotics can be obtained without compromising the safety and security of the food.
An advantage of acidic foods treated with HHP is that the acid pH (<4.5) is that, at this level of acidity, bacterial pathogen spores are not able to initiate growth. For this reason, this food group represents the majority of the products on the market.
6.8 Probiotics as a Functional Food
Probiotics exert their effects by mechanisms still unclear; however, there are hypotheses that explain how these microorganisms provide these benefits to the host (O’Toole and Cooney 2008). The competition between probiotics and pathogenic bacteria to colonize the gastrointestinal tract is one of the mechanisms of action that probiotics exert to prevent harmful microorganisms from causing some pathology adhering to the intestinal mucosa. This mechanism was tested with bacteria of the genus Lactobacillus (plantarum and rhamnosus) as they showed the ability to inhibit the adhesion of E. coli to the epithelium of the intestine (Mack et al. 1999).
Another possible mechanism is the secretion of antimicrobial substances that keep the intestinal microbiota in balance (Rolfe 2000). One of the substances secreted by these microorganisms are bacteriocins, in addition to other substances such as hydrogen peroxide, diacetyl and short-chain acids burn to be secreted by probiotics, modifying the intestinal microbiota for a proper balance. Probiotics also improve the immune response by stimulating the secretion of a greater amount of immunoglobulins, especially the IgA type (Link-Anster et al. 1994), natural killer cells, and the phagocytic action of macrophages, since through this increase in cells defending the immune system can inhibit the gastrointestinal tract colonization by pathogenic bacteria, thus benefiting the composition of the microbiota (Fuller and Gibson 1997). Probiotics also compete with pathogenic microorganisms for nutrients (Vandenplas and Benninga 2009), as in the case of Clostridium difficile which needs carbohydrates for its growth and colonization; this is where probiotics act using the largest amount of monosaccharides leaving this and many pathogens without nutrients (Wilson and Perini 1988) thus avoiding the incidence of diseases caused by the spread and colonization of pathogens.
6.9 Metabolites Produced by Probiotics
Probiotics are commonly used in foods and also act as preservatives and enhancers of texture, flavor, and odor. These properties result from the ability of these bacteria to produce different types of sugars and metabolites such as ethanol, organic acids (lactic, acetic), diacetyl, acetone, exopolysaccharide, specific proteases, and bacteriocins (Porto et al. 2017).
6.9.1 Polysaccharides
Some probiotics have the ability to synthesize glycosidic polymers. Usually, such molecules are secreted and can be kept covalently attached to the cell surface, typically in a capsule form; released into the environment; or remain slightly attached to the surface (Kumar et al. 2007; Viana de Souza and Silva Dias 2017). Probiotics belonging to the genera Lactococcus, Lactobacillus, Enterococcus, Leuconostoc, Oenococcus, Pediococcus, Weissella, and Streptococcus have been identified as producers of exopolysaccharides (EPS) (Amari et al. 2013).
The EPS obtained by these bacteria can be different in: (1) composition, since they have different monosaccharides attached by different types of bonds; (2) molecular weight; (3) structure, since they contain several degrees and types of branching; and (4) overall structural conformation.
The role of EPS in food is determined by the characteristics mentioned above, as well as the concentration of polymeric compounds and their way of interacting with food matrices. Taking into account its composition, the EPS are classified into: (1) homopolysaccharides (HoPS), consisting of monosaccharides of a single type or (2) heteropolysaccharides (HePS), consisting of monosaccharides of two or more types. Based on the different links that it contains and the carbon involved in the link, HoPS are separated into four groups: α-d-glucans, β-D-glucans, β-D-fructans, and others (i.e., polygalactans) (Pérez-Ramos et al. 2015).
The interest in EPS has increased over the years because of the diversity generated by probiotics. The isolated forms of EPS are not yet used industrially because the prices of polysaccharides of bacteria are high compared to polysaccharides isolated from plants, animal sources (pectin, alginate, galactomannan, starch, among others), and algae (Zannini et al. 2016). However, EPS produced by LABs are industrially used as stabilizers, thickeners, emulsifiers, viscous agents, gelling agents, and water-binding agents.
Despite the above, in many food industries, the production of EPS by LABs with probiotic attributes is carried out by in situ fermentation and this is decisive in the organoleptic characteristics that it presents as they influence the consistency and rheology of fermented products (Notararigo et al. 2013), so they are used as viscosifiers, stabilizers, emulsifiers, or gelling agents (Viana de Souza and Silva Dias 2017). EPS also contribute to water retention in certain products such as cheese, which benefits the product in texture and calorie reduction (da Silva Ferrari et al. 2016). In addition, some EPS have anticholesterolemic and anti-tumor activity. They have the capacity to decrease the formation of biofilms by pathogens and the produced β-glucans have immunomodulatory, antitumorigenic, antiviral, and antiulcerous activities (Notararigo et al. 2014; Nwodo et al. 2012). In addition, they can also act as prebiotics by increasing the growth of beneficial microorganisms (de Moreno de LeBlanc et al. 2018).
It is evident that EPS synthesized by LABs have greater potential for the production of cereal and dairy products (Badel et al. 2011). Table 6.2 shows a list of LABs, the EPSs they produce and the foods in which they are found.
6.9.2 Aromatic Compounds
Some probiotics enhance the organoleptic characteristics of fermented foods by producing various compounds that are involved in the formation of other aromas and flavors. They can influence cheese maturation by transforming milk components into aromatic and flavored compounds through the metabolism of carbohydrates, proteins, citrate, and milk lipids.
The aroma formed in fermented foods is due to the action of lysed and intact LABs. Cytoplasmic enzymes are released during cell lysis, which continue their activity even outside the cell, producing metabolites in the fermented food matrix (Lortal and Chapot-Chartier 2005). Different amino acids are presented in food matrices or can be obtained in proteolysis, their catabolism also results in the synthesis of aromatic compounds. Isoleucine, leucine, and valine (branched-chain amino acids) give sweet and fruity flavors; phenylalanine, tryptophan, and tyrosine (aromatic amino acids) through catabolism generate floral flavors; aspartate is catabolized into butter flavors; and cysteine and methionine (sulfur amino acids) are metabolized into compounds flavored with meat, garlic, and boiled cabbage (Ardö 2006). Through citrate metabolism, LABs also generate aromatic compounds.
6.9.3 Aromatic Compounds with Four Carbons
Few probiotics generate aromatic four-carbon compounds from citrate such as L. lactis subspecies lactis biovar. diacetylactis (L. diacetylactis) (Hugenholtz 1993), some from Leuconostoc (Hemme and Foucaud-Scheunemann 2004), Lactobacillus plantarum (Minervini et al. 2010), a few of Enterococcus (Martino et al. 2016), Oenococcus oeni (Bartowsky and Henschke 2004), and Weissella paramesenteroides (García-Quintáns et al. 2008).
Citric acid is an organic acid present in fermentable food products, such as vegetables, fruits, fruit juice, cheese, and milk. Under acidic and anaerobic conditions, bacteria have the ability to metabolize citrate through the pyruvate pathway to produce aromatic four-carbon compounds such as acetoin, diacetyl, ethanol, and 2,3-butanediol (Smid and Kleerebezem 2014). The Leuconostoc and L. diacetylactis species are the main citrate fermenting bacteria found in milk starters (Drider et al. 2004).
The efficiency of the synthetic routes of diacetyl will depend on the activity of the enzymes responsible for transforming citrate to pyruvate, and the concentration gradient is influenced by the rate of citrate disappearance in an intracellular medium (Laëtitia et al. 2014). Most of the pyruvate generated is converted to lactate by the action of the enzyme lactate dehydrogenase (LDH) (Ko et al. 2016).
6.9.4 Antimicrobial Compounds
LABs have been shown to produce antimicrobial compounds as a form of defense against pathogens, thus protecting food from spoilage. LABs generate a variety of compounds, such as organic acids (acetic acid and lactic acid), hydrogen peroxide, diacetyl, bacteriocins, and bactericidal proteins, all of which have bacteriostatic or antibacterial properties. These metabolic products have the function of extending the shelf life and inhibiting the growth of pathogenic microorganisms in the food, additionally also positively impacting the color, taste, smell, and texture of the food.
There is some concern about manufacturing processes involving heat treatment (sterilization, heating, and pasteurization), dehydration, acidification, and the addition of preservatives (nitrite, antibiotics, and sulfur dioxide) and organic compounds such as sorbate, benzoate, acetate, lactate, and propionate. Although the above procedures are generally effective, there is growing public demand for naturally occurring and microbiologically safe products that give consumers positive health effects (Zacharof and Lovitt 2012). Consequently, bacteriocins are becoming increasingly interesting as they can be found in natural sources and contribute to food safety (Yang et al. 2014).
6.9.5 Bacteriocins
Bacteriocins are defined as ribosomally synthesized antimicrobial peptides and are commonly used as preservatives in foods (Chikindas et al. 2018) because they inhibit or annihilate other microorganisms that are closely related to the producer, without harming the harmless microbiota (Cotter et al. 2013). There is a greater amount and structural variability of bacteriocins produced by Gram-positive bacteria compared to those obtained from Gram-negative bacteria. LABs are a group of gram-positive bacteria identified with a high potential to generate bacteriocins. Most LAB bacteriocins are non-toxic to eukaryotic cells and are activated in the nanomolar range (Messaoudi et al. 2013).
Bacteriocins cause cell death in bacteria at much lower concentrations compared to antimicrobial peptides in prokaryotic cells, and this is probably because bacteriocins interact with specific receptors in target cells. Therefore, interest in its potential use in food products has increased. Bacteriocins are inactivated by the action of digestive proteases, which causes them to have a minimal effect on the intestinal microbiota, a property that supports their use as food preservatives. Some of its characteristics are that they are heat and pH tolerant, have not been related to antibiotic resistance, and are usually encoded by genes that are located in a plasmid, which facilitates genetic manipulation (Gálvez et al. 2007). Bacteriocins may act as bacteriostatic or bactericidal. Their action will depend on various factors such as, for example, the dose of bacteriocin and the degree of purification and experimental conditions such as pH, temperature, or the existence of agents that modify the integrity of the cell wall (Juodeikiene et al. 2002).
The incorporation of bacteriocins for food preservation could have several advantages: (1) to minimize the risk of diseases caused by pathogens present in foods such as food poisoning; (2) to provide extra protection during excessive temperature conditions and to decrease the use of chemical preservatives; (3) to extend the shelf life of food products; (4) to allow lower intensity physical treatments, thereby preserving nutrients, organoleptic properties of foods, and vitamins, as well as lowering processing costs; (5) to reduce waste due to food spoilage; and (6) to provide alternative preservation barriers to “novel” foods in the hope of meeting consumer demands for safe, ready-to-eat, fresh-tasting, minimally processed foods (Messaoudi et al. 2013).
Most LAB produce bacteriocins, such as Lactococcus, Streptococcus, Lactobacillus, Pediococcus, Leuconostoc, Enterococcus, Carnobacterium, Aerococcus, Oenococcus, Tetragenococcus, Vagoccus, and Weissella (Ananou et al. 2007; Gálvez et al. 2007; Gálvez et al. 2008; Leistner and Gorris 1995; Oliveira et al. 2008); however, only bacteriocin obtained from Lactococcus lactis, named nisin, which has been approved by the FDA (Sobrino-López and Martín-Belloso 2008; Zacharof and Lovitt 2014), is commercially permitted, so research on new bacteriocins is of paramount importance, given the benefits they bring.
6.9.6 Hydrogen Peroxide (H2O2)
Some LABs contain flavoprotein oxidases, which gives them the ability to produce and accumulate H2O2 in the presence of molecular oxygen. This compound has bactericidal and sporicidal action, due to its strong oxidizing effect, which is generated by the tendency of H2O2 to form highly reactive metabolites, such as hydroxyl and superoxide radicals, to which damage to membrane lipids, DNA, and other essential cellular compounds is attributed (Holzapfel et al. 2003).
A food product in which H2O2 has great importance is in raw milk, since H2O2 exerts its characteristic bactericidal and sporicidal action, which benefits this product by extending its shelf life. These antagonistic actions are easier to perform in raw milk because it has the enzyme lactoperoxidase that has the function of catalyzing the oxidation of thiocyanate inherent in the presence of H2O2, obtaining from this reaction a compound capable of producing the desired effect. Therefore, in places without optimal refrigeration equipment, milk or cheese is stored by adding H2O2 (Martin et al. 2014). However, the addition of H2O2 is not allowed in the USA and several places around the world, with the exception of certain applications in cheese, mainly because this compound can cause gastrointestinal problems, the degradation of vitamins (folic acid) and certain essential amino acids (methionine) (Antelmann and Helmann 2011; Rutala and Weber 2008).
H2O2 has the advantage of having a broad spectrum of inhibition since it acts against viruses, bacteria, yeasts, and bacterial spores, having antagonistic effect on Gram-positive and Gram-negative bacteria (Viana de Souza and Silva Dias 2017), which is used in the food industry using this compound during bottling, transport and packaging (Imlay 2003).
6.9.7 Diacetyl
Diacetyl is obtained through citrate metabolism in which LABs such as Lactococcus lactis subsp. lactis biovar diacetylactis, Leuconostoc spp., Lactobacillus paracasei, and Lactobacillus rhamnosus are involved. Its obtained form is non-enzymatic, by means of the oxidative reaction of the acetate, which originated from pyruvate (Díaz-Muñiz et al. 2006; Hugenholtz 1993; Tamang et al. 2015).
In its pure form, diacetyl is a yellow liquid with a strong butter aroma (Starek-Swiechowicz and Starek 2014). It is used in products such as wine, dairy products, roasted coffee, and beer (Shibamoto 2014). Because of its characteristic aroma and flavor, this compound is used in a variety of applications, such as microwave popcorn (Brass and Palmer 2017). It is also used as an antagonist agent, by inhibiting pathogenic microorganisms, generating this effect by having the ability to penetrate bacterial membranes and interfere with the functions of essential metabolites. It is known that the antagonistic effect caused by diacetyl is through the blocking of the catalytic site of the enzymes responsible for the utilization of arginine, causing cells to be unable to synthesize this essential protein (Hor and Liong 2014).
Microorganisms that inhibit diacetyl and have been reported are: Yersinia enterocolitica, Aeromonas hydrophila, Escherichia coli, and Salmonella anatum (Naidu 2000). According to Ferrari (2016), LABs isolated from goat’s milk have a strong potential to produce this antimicrobial compound.
6.9.8 Organic Acids
During the fermentation process, a wide variety of organic acids are produced: lactic acid, acetic acid, succinic acid, propionic acid, formic acid, and butyric acid (Haller et al. 2001; Özcelik et al. 2016; Ross et al. 2002). The use of these acids is highly regarded by the food industry for their ability to improve food quality and safety (Hwanhlem et al. 2011). The antimicrobial effect that characterizes these acids is due to their ability to reduce pH below the optimal growth range of various microorganisms and to metabolic inhibition by undissociated organic acid molecules (Crowley and Mahony 2013). The decrease in pH caused by organic acids also has the objective of giving them greater liposolubility, allowing this to interfere with the conservation of the potential of the cell membrane. As a result, the active transport is inhibited, the cell membrane is crossed, and the cytoplasm is reached, thus causing the reduction of intracellular pH and the inhibition of several metabolic functions in the pathogenic microorganism (Haller et al. 2001; Özcelik et al. 2016; Ross et al. 2002).
6.9.9 Lactic Acid
Lactic acid is mainly used in the pharmaceutical, chemical, cosmetic, and food industries (Li et al. 2015). It can be obtained through fermentation or chemical synthesis. However, 90% of this product is preferably produced worldwide by bacterial fermentation (Vijayakumar et al. 2008). The identified LABs involved in this process are those belonging to the genera Lactobacillus, Sporolactobacillus, Enterococcus, Lactococcus, Bacillus, Streptococcus, Pediococcus, Leuconostoc, and Bifidobacteria (Naidu 2000).
About 70% of the lactic acid produced is used in the food sector (Martinez et al. 2013). It is found naturally or as a fermentation product in: olives and pickled vegetables, yogurt, butter and other fermented milk, fermented dough bread, and other fermented products. It is also used as an acidulant, condiment, pH buffer of the decomposition generated by bacteria of various processed products such as sweets, bread and bakery products, non-alcoholic beverages, soups, sorbets, dairy products, beer, jams and jellies, mayonnaise, processed eggs, among others (Datta and Henry 2006).
The mechanism used by lactic acid against pathogenic microorganisms focuses on its ability to cross its cell membrane, resulting in intracellular pH reduction and the failure of the transmembrane proton motive force. During the process, the acid dissociation of the molecules is carried out, as well as the incorporation of a set of factors that benefit in the antimicrobial capacity of the lactic acid like the accumulation of toxic anions, the stress provoked in the cellular homeostasis, the inhibition of the metabolic reactions and the inhibitory effect on the division that comes to cause the death of the microorganism (Smith et al. 1993; Pisoschi and Negulescu 2011).
LAB such as Lactobacillus and Bidifobacterium come to generate lactic acid and has been observed the inhibition of this against Escherichia coli O157:H7 (Reis et al. 2012). It also has this effect against Salmonella typhimurium, Enterobacteriaceae, Salmonella bareilly, and Listeria monocytogenes (Mani-López et al. 2012; Naidu 2000), in addition to Mycobacterium tuberculosis, Bacillus coagulans, Yersinia enterocolitica, Aeromonas hydrophila, Clostridium botulinum, Enterobacteriaceae, Lactobacillaceae, Aspergillus sp., Pseudomonas fragi, Clostridium sporogenes, Vibrio vulnificus, Helicobacter pylori, and Pseudomonas sp. (Naidu 2000).
Applications of lactic acid in non-food are in the production of plastics, dyes, pesticides, synthetic polymers, glue (acetic acid), and even in the washing of corpses (Reis et al. 2012).
6.9.10 Acetic Acid
Acetic acid is an another product derived from the fermentation of some substrates by LAB. It is used in almost all countries as vinegar (Panda et al. 2016). It is used in the food industry as an additive to control pH levels and improve taste (Supharoek et al. 2017). As well as for the preservation of various foods, as this product has the effect of inhibiting growth and reducing the viability of Gram-positive and Gram-negative bacteria, yeasts, and fungi. It presents bacteriostatic activity at 0.2% and bactericidal activity at 0.3% (Ray 2004), so it is said that acetic acid has a more lethal effect against pathogenic microorganisms than lactic acid. Due to its pungent odor and taste, the use of this product in food is limited (Erginkaya et al. 2014); for example, in Canada it is only permissible to have a concentration of 4.1–12.3% acetic acid in vinegar (Panda et al. 2016). Excessive consumption or application of this organic acid is also not recommended, because its corrosive properties can cause stomach diseases (Supharoek et al. 2017).
6.9.11 Proteolytic Capacity
LABs are commonly used as a source of carbon the sugars and citrate and as a source of nitrogen the caseins. They are unable to synthesize several amino acids and have to be able to degrade peptides and proteins in order to meet their amino acid requirements.
One of the essential biochemical processes for obtaining fermented dairy products is the proteolysis that is involved in the organoleptic characteristics of the final product (Chaves-López et al. 2011). The proteolytic systems of the LABs are made up of: (1) proteinases that hydrolyze caseins to originate peptides; (2) transport systems to translocate peptides to the cytoplasm; and (3) peptidases that transform peptides into amino acids. For example, the taste of cheese during ripening is formed by the action of L. lactis, this is mainly caused by the proteolysis of casein-generating free amino acids, which behave as substrates for catabolic reactions generating aromatic compounds (Smid and Kleerebezem 2014).
It is also known that proteolysis can activate bioactive peptides (Gobbetti et al. 2002), metabolites of great importance since they show a wide range of desirable effects such as antimicrobial activity, antithrombotic antihypertensive, immunomodulatory, etc. (de Moreno de LeBlanc et al. 2018). According to Pescuma et al. (2009) this biochemical event has come to prevent and reduce frequent allergies in children under 3 years because of poor digestion of milk proteins.
6.10 Beneficial Effects of Probiotics
6.10.1 Immunology
Probiotics can modulate the immune response in animals and humans not only at the level of the intestinal mucosa, but also at the systemic level (Manzano et al. 2012). The probiotic lactic acid bacteria (LAB) to modulate their actions through the intestinal mucosal immune system must also interact with the normal intestinal flora and the food that is ingested. The interaction of probiotics with intestinal epithelial cells (enterocytes) generates cytokines and chemokines that initiate immunomodulatory events. Several studies have shown that numerous lactobacilli can alert the intestinal immune system and secondarily favor the rejection of potentially harmful infectious microorganisms; this can be done through the production of specific type A immunoglobulins (Kaila et al. 1992) or the activation of K cells (“natural killer”) (Gill et al. 2001). Other immunomodulatory effects of these probiotics derive from their ability to increase the phagocytic activity of intestinal leukocytes, promote increased proliferation of B lymphocytes along with increased secretion of immunoglubulins A and G, as well as stimulate cytosines such as interleukin IL-2, IL-6, or tumor necrosis factor (TGB). The probiotics Lactobacillus casei, L. rhamnosus, Bifidobacterium breve, Lactobacillus gasseri are some of the examples studied to demonstrate this action, as well as the general activation of B lymphocytes.
Given their immunomodulatory properties, the usefulness of probiotics in the preventive or therapeutic management of inflammatory diseases is currently being evaluated. The consumption of probiotics could have a positive effect on human health in some situations that may alter the balance of the intestinal microbiota and influence the immune response of the individual, such as feeding with infant formulas, treatment with antibiotics, physiological changes related to aging, gastrointestinal diseases, and stress.
In summary, the different studies show the viability of using probiotics to modulate the immune system, prevent infections, and control the inflammatory process, but the results are diverse, so it is necessary to conduct research to reduce the gap between the differences found with factors such as: strain or species used, dose of probiotic, supplementation time, and characteristics of the subjects studied. This will allow comparisons to be made and conclusions to be drawn that will benefit the therapeutic and preventive use of probiotics on the immune system.
Recently, the function of probiotics as modulators of the immune system has been identified where mucosal immunity is of primary importance (Donkor et al. 2010; Koninkx et al. 2010). The effects of probiotics on the immune system can be classified into two major categories, involving the activation of cells of the innate immune system, such as phagocytes and natural killer cells, and the inhibition of abnormal immune responses. The former is expected to have an inhibitory effect against infections and cancer, and the latter may have an inhibitory effect against inflammatory bowel diseases, allergies, and autoimmune diseases (Chiba et al. 2010; Rook and Brunet 2005).
6.10.2 Respiratory Infections
In many occasions, probiotics are used for the prevention of certain infections, such is the case of respiratory infections, where a study carried out by Di Pierro et al. (2014) demonstrated the beneficial effects they produce. The study included 61 children diagnosed with recurrent oral streptococcal disorders, who were given the S. salivarius K12 strain through a tablet. Only 30 children completed the test, consuming the probiotic for 90 days in order to prevent tonsillitis. The results showed a significant reduction in episodes of streptococcal pharyngeal infection compared to the previous year’s infection rates. It should also be noted that the product supplied was well tolerated and had no side effects.
Reported strains with the potential to reduce the load of pathogens in the respiratory system are Lactobacillus and Bifidobacterium. Their mechanism is linked to the action of immune cells such as natural killer and macrophages (Hardy et al. 2013).
6.10.3 Diabetes
In diabetes mellitus, satisfactory results from the use of probiotics have been perceived, since it has been observed, in experimentation with rats, that strains such as Lactobacillus reuteri GMN-32 reduce blood glucose levels from 4480 to 3620 mg/L when applied at a concentration of 107 CFU/d. In addition, the changes in the heart that cause this disease were also reduced (Lin et al. 2014).
Most LABs, especially Lactobacillus species, have great antidiabetic potential. A higher content of Lactobacillus acidophilus and Lactobacillus casei has been found in fermented milk products such as Dahi, which improves hyperglycemia, hyperinsulinemia, dyslipidemia and reduces oxidative stress in diabetic rats (Yadav et al. 2008). Lactobacillus casei has an important preventive effect against diabetes, being tested in non-obese diabetic mice that elevates plasma glucose and reduces plasma insulin levels due to the destruction of pancreatic cells β.
6.10.4 Cancer
The use of probiotic therapy for treatment and prevention of cancer through probiotics has increased the interest of clinical nutritionists, scientists, and industry (Soa et al. 2017; Vafaeie 2016), as it has been recognized the beneficial effect they come to generate against different types of cancer: colon and rectum, breast, blood, cervical, prostate and bladder, skin, esophagus, liver, gallbladder, head, and neck (Dasari et al. 2016). The advantage of probiotics over other cancer treatments is their absence or minimal presence of side effects (Soa et al. 2017; Vafaeie 2016). Isolated strains of fermented milk with antitumor activity are Bifidobacterium infantis, B. bifidum, B. animalis, L. acidophilus, and L. paracasei. When studied on the growth of a breast cell line it was demonstrated that the most effective species were B. infantis and L. acidophilus (Biffi et al. 1997).
However, only the anti-cancer effect of LABs has been proven to be in vitro studies (George Kerry et al. 2018), so it is necessary to apply the research carried out to confirm this activity in humans.
6.11 The Role of Probiotics in Intestinal Diseases
6.11.1 Diseases Associated with Imbalances of the Intestinal Microbiota
The intestine is formed by a complex microbial ecosystem that has specific and protective metabolic functions (Parra 2012). The intestinal microbiota is the community of living microorganisms residing in the digestive tract (Icaza-Chávez 2013). It is composed of microorganisms that are classified as pathogenic, neutral, or beneficial to the host. This last group of bacteria is commonly included mainly in dairy derivatives (Parra 2012). That is why it is essential for proper body growth, the development of immunity and nutrition as it exercises nutritional, metabolic, and protective functions that make it indispensable for the host while it delivers nutrients and adequate conditions for growth (Morales and Brignardello 2010). The intestinal microbiota participates mainly in multiple functions such as the metabolism of some carbohydrates, specialization and activation of the immune system, regulation of intestinal cell growth, and synthesis of certain vitamins (K and B). Some bacteria in the intestinal microbiota have enzymes that are capable of digesting certain carbohydrates that cannot otherwise be processed. More or less complex polysaccharides, such as those that make up insoluble dietary fiber, undergo fermentation processes that give rise to products such as short-chain fatty acids, which have beneficial effects on the metabolism of carbohydrates and cholesterol. This fermentation also produces gases and flatulences with odors characteristic of feces.
Imbalances in their composition or a lack of microbial richness have been found to be risk factors for certain pathologies such as obesity, diabetes, asthma, or some types of cancer and may play an important role in others such as Parkinson’s or autism (Sáez 2015). Several acute diarrheal diseases are due to pathogens that proliferate and have invasive characteristics or produce toxins. Diarrhea associated with antibiotics is due to an imbalance in the composition of the intestinal flora with the proliferation of pathogenic species, such as some strains of Clostridium difficile that produce toxins that cause pseudomembranous colitis (Guarner 2007).
6.11.2 The Role of Probiotics in Colon Cancer
The colon is the first part of the large intestine that absorbs water and nutrients from food and serves as a storage place for solid waste. Colon-rectal cancer develops in the digestive system, which is the primary mechanism for food processing, energy production, and solid waste disposal (Parra 2012). Currently experimental models have shown that intestinal bacteria can play a role in the initiation of colon cancer through the formation of carcinogenic products (Guarner 2007). The high number and diversity of the human gut microflora are reflected in its wide and varied metabolic capacity, especially in relation to the biotransformation of xenobiotics and the synthesis and activation of carcinogens (Burns and Rowland 2003).
The molecular genetic defects that appear in human colorectal cancer are well known, and appear to be a consequence of the genotoxicity of products generated in light from the intestine. Epidemiological data suggest that environmental factors such as diet play an important role in the development of colon cancer. Consumption of animal fat and red meat, particularly processed meat, is associated with higher risk (Guarner 2007). On the other hand, some probiotics are able, by mechanisms not completely clarified, to degrade mutagenic substances present in the intestine decreasing their genotoxicity, to bind to them (as to heterocyclic amines), to decrease levels of enzymes (such as azoreductase, nitroreductase, ß-glucuronidase, ß-glucosidase, and 7-a-dehydrolase) that can regenerate toxic substances that had been detoxified in the liver and excreted for disposal (Wollowski et al. 2018).
Evidence from a wide range of sources supports the view that colonic microflora intervenes in the etiology of cancer. It follows that modification of the intestinal microflora would interfere with the carcinogenesis process, and this opens up the possibility of dietary modifications to reduce the risk of colon cancer (Burns and Rowland 2003).
This type of cancer is a major cause of death in the Western world. Approximately 70% of people with this type of cancer are associated with environmental factors, probably mostly diet. Most colon cancers arise from polyps that begin to grow in the inner lining of the colon or rectum. In this regard, studies have shown that fermented dairy products prevent rectal colon cancer (Parra 2012). Probiotic lactic-acid bacteria have been shown to deactivate carcinogenic genotoxic substances. while in in vitro model systems they can prevent mutations. While in in vivo colon tissues they can prevent DNA damage and stimulate protective systems. These bacteria have potential as chemoprotective agents against genotoxic chemicals and further research is needed to clarify and quantify their beneficial effect on the prevention of colon cancer in humans (Wollowski et al. 2018). Other studies indicate that probiotics would be useful in preventing colon cancer, through the production of short-chain fatty acids, which acidify the environment (Parra 2012), exert anti-inflammatory and apoptotic effects of cells that could become carcinogenic associated with lower cancer risk.
6.12 Conclusion
The main application of these bacteria occurs in the fermentation process of food. Due to the multiple metabolites they produce, probiotics have been a very effective way for natural preservation. One of its applications are food additives that provide flavors, odors, textures, and even nutritional value. They have long been used in industrial applications mainly as starters for food fermentation, biocontrol agents, or as probiotics. They are widely used by the chemical industry, due to the production potential of polylactides as biodegradable plastic polymers and biocompatible derivatives of petrochemical products. Several LAB strains have proven probiotic properties, and their biomass can be considered a high value product (Mazzoli et al. 2014).
Since LABs are also found within the gastrointestinal microbiota, they have the ability to produce different substances to avoid some adverse effects produced by some microorganisms, only by modifying their metabolism without destroying it and thus their population is diminished. As mentioned before, LABs have a great fermentative capacity of which organic acids are produced from simple carbohydrates, which determines an increase in intestinal acidity that limits the growth of bacteria, especially Gram-negative bacteria. The function of LABs is very useful in the food industry: the preservation of food by bacteriocins produced by Lactobacillus has obtained successful results in meat foods, to control pathogenic microorganisms that can cause alterations in food, such as Salmonella spp. and E. coli (Ruiz et al. 2017).
Probiotics have a highly developed defense against stress factors; they can survive in environments with a large number of changes or conditions. It is necessary to know the response of these bacteria to stress conditions for a good selection of them (D’Angelo et al. 2017) because their different applications depend on what conditions they can be found, and through research, can be found more fields in which can be used this large group of bacteria.
References
Abdel-rahman MA, Tashiro Y, Sonomoto K (2011) Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: overview and limits. J Biotechnol 156(4):286–301. https://doi.org/10.1016/j.jbiotec.2011.06.017
Amari M, Fernanda L, Arango G, Gabriel V, Robert H, Morel S et al (2013) Characterization of a novel dextransucrase from Weissella confusa isolated from sourdough. Appl Microbiol Biotechnol 97:5413–5422. https://doi.org/10.1007/s00253-012-4447-8
Ananou S, Maqueda M, Martínez-Bueno M, Valdivia E (2007) Biopreservation, an ecological approach to improve the safety and shelf-life of foods. In: Communicating current research and educational topics and trends in applied microbiology. FORMATEX, Badajoz, pp 475–486
Antelmann H, Helmann JD (2011) Thiol-based redox switches and gene regulation. Antioxid Redox Signal 14(6):1049–1063. https://doi.org/10.1089/ars.2010.3400
Ardö Y (2006) Flavour formation by amino acid catabolism. Biotechnol Adv 24(2):238–242. https://doi.org/10.1016/j.biotechadv.2005.11.005
Badel S, Bernardi T, Michaud P (2011) New perspectives for Lactobacilli exopolysaccharides. Biotechnol Adv 29(1):54–66. https://doi.org/10.1016/j.biotechadv.2010.08.011
Bartowsky EJ, Henschke PA (2004) The ‘buttery’ attribute of wine—diacetyl—desirability, spoilage and beyond. Int J Food Microbiol 96(3):235–252. https://doi.org/10.1016/j.ijfoodmicro.2004.05.013
Benavides AB, Ulcuango M, Yépez L, Tenea GN (2016) Evaluación de las propiedades bioactivas in vitro de bacterias ácido lácticas aisladas de nichos ecológicos nativos del Ecuador. Rev Argent Microbiol 48(3):236–244. https://doi.org/10.1016/j.ram.2016.05.003
Benyacoub J, Pérez PF, Rochat F, Saudan KY, Reuteler G, Antille N et al (2005) Enterococcus faecium SF68 enhances the immune response to giardia intestinalis in mice 1. J Nutr 135:1171–1176
Biffi A, Coradini D, Larsen R, Riva L, Di Fronzo G (1997) Antiproliferative effect of fermented milk on the growth of a human breast cancer cell line. Nutr Cancer 28(1):93–99. https://doi.org/10.1080/01635589709514558
Boguta AM, Bringel F, Martinussen J, Jensen PR (2014) Screening of lactic acid bacteria for their potential as microbial cell factories for bioconversion of lignocellulosic feedstocks. Microb Cell Factories 13:97. https://doi.org/10.1186/s12934-014-0097-0
Brass DM, Palmer SM (2017) Models of toxicity of diacetyl and alternative diones. Toxicology 388:15–20. https://doi.org/10.1016/j.tox.2017.02.011
Buntin N, Chanthachum S, Hongpattarakere T (2008) Screening of lactic acid bacteria from gastrointestinal tracts of marine fish for their potential use as probiotics. Songklanakarin J Sci Technol 30:141–148
Burns AJ, Rowland IR (2003) Prebióticos y probióticos en la prevención del cáncer de colon. Gastroenterol Hepatol 26(Supl 1):73–84
Chaves-López C, Serio A, Martuscelli M, Paparella A, Osorio-cadavid E, Suzzi G (2011) Microbiological characteristics of kumis, a traditional fermented Colombian milk, with particular emphasis on enterococci population. Food Microbiol 28(5):1041–1047. https://doi.org/10.1016/j.fm.2011.02.006
Chiba Y, Shida K, Nagata S, Wada M, Bian L, Wang C et al (2010) Well-controlled proinflammatory cytokine responses of Peyer’s patch cells to probiotic Lactobacillus casei. Immunology 130(3):352–362. https://doi.org/10.1111/j.1365-2567.2009.03204.x
Chikindas ML, Weeks R, Drider D, Chistyakov VA, Dicks LM (2018) Functions and emerging applications of bacteriocins. Curr Opin Biotechnol 49:23–28. https://doi.org/10.1016/j.copbio.2017.07.011
Cotter PD, Ross RP, Hill C (2013) Bacteriocins—a viable alternative to antibiotics? Nat Rev Microbiol 11(2):95–105. https://doi.org/10.1038/nrmicro2937
Crowley S, Mahony J (2013) Current perspectives on antifungal lactic acid bacteria as natural. Trends Food Sci Technol 33(2):93–109. https://doi.org/10.1016/j.tifs.2013.07.004
D’Angelo LD, Cicotello J, Zago M, Guglielmotti D, Suárez V (2017) Leuconostoc strains isolated from dairy products: response against food stress conditions. Food Microbiol 66:28. https://doi.org/10.1016/j.fm.2017.04.001
da Silva Ferrari I, de Souza JV, Ramos CL, da Costa MM, Schwan RF, Dias FS (2016) Selection of autochthonous lactic acid bacteria from goat dairies and their addition to evaluate the inhibition of Salmonella typhi in artisanal cheese. Food Microbiol 60:29–38. https://doi.org/10.1016/j.fm.2016.06.014
Dasari S, Kathera C, Janardhan A, Praveen Kumar A, Viswanath B (2016) Surfacing role of probiotics in cancer prophylaxis and therapy: a systematic review. Clin Nutr 36:1465. https://doi.org/10.1016/j.clnu.2016.11.017
Datta R, Henry M (2006) Lactic acid: recent advances in products, processes and technologies - a review. J Chem Technol Biotechnol 82:1115–1121. https://doi.org/10.1002/jctb
de Almeida Júnior WLG, da Silva Ferrari Í, de Souza JV, da Silva CDA, da Costa MM, Dias FS (2015) Characterization and evaluation of lactic acid bacteria isolated from goat milk. Food Control 53:96–103. https://doi.org/10.1016/j.foodcont.2015.01.013
de Moreno de LeBlanc A, Luerce TD, Miyoshi A, Azevedo V, LeBlanc JG (2018) Functional food biotechnology. In: Omics technologies and bio-engineering. Academic Press, London, pp 105–128. https://doi.org/10.1016/B978-0-12-815870-8.00007-3
Di Pierro F, Colombo M, Zanvit A, Risso P, Rottoli AS (2014) Use of Streptococcus salivarius K12 in the prevention of streptococcal and viral pharyngotonsillitis in children. Drug Healthc Patient Saf 6:15–20
Díaz-Muñiz I, Banavara DS, Budinich MF, Rankin SA, Dudley EG, Steele JL (2006) Lactobacillus casei metabolic potential to utilize citrate as an energy source in ripening cheese: a bioinformatics approach. J Appl Microbiol 101(4):872–882. https://doi.org/10.1111/j.1365-2672.2006.02965.x
Doleyres Y, Beck P, Vollenweider S, Lacroix C (2005) Production of 3-hydroxypropionaldehyde using a two-step process with Lactobacillus reuteri. Appl Microbiol Biotechnol 68:467–474. https://doi.org/10.1007/s00253-005-1895-4
Domínguez Gonzáleza KN, Cruz Guerrero AE, Márquez HG, Gómez Ruiz LC, García-Garibay M, Rodríguez Serrano GM (2014) El efecto antihipertensivo de las leches fermentadas. Rev Argent Microbiol 46(1):58–65. https://doi.org/10.1016/S0325-7541(14)70050-1
Donkor ON, Shah NP, Apostolopoulos V, Vasiljevic T (2010) Development of allergic responses related to microorganisms exposure in early life. Int Dairy J 20(6):373–385. https://doi.org/10.1016/j.idairyj.2009.12.017
Drider D, Bekal S, Prévost H (2004) Genetic organization and expression of citrate permease in lactic acid bacteria. Genet Mol Res 3(2):273–281
Erginkaya Z, Ünal E, Kalkan S (2014) Food processing: strategies for quality assessment. Springer, New York. https://doi.org/10.1007/978-1-4939-1378-7
Freitas DR, Campos JMS, Marcondes MI, Valadares SC, Franco MO, Martins EC et al (2015) Levedura seca integral na alimentação de vacas lactantes. Arq Bras Med Vet Zootec 67:211–220
Fuller R, Gibson GR (1997) Modification of the intestinal microflora using probiotics and prebiotics. Scand J Gastroenterol 32(sup222):28–31. https://doi.org/10.1080/00365521.1997.11720714
Gálvez A, Abriouel H, López RL, Omar NB (2007) Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol 120(1–2):51–70. https://doi.org/10.1016/j.ijfoodmicro.2007.06.001
Gálvez A, López RL, Abriouel H, Valdivia E, Omar NB (2008) Application of bacteriocins in the control of foodborne pathogenic and spoilage bacteria. Crit Rev Biotechnol 28(2):125–152. https://doi.org/10.1080/07388550802107202
Gänzle MG (2015) Lactic metabolism revisited: metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr Opin Food Sci 2:106–117. https://doi.org/10.1016/j.cofs.2015.03.001
García-Quintáns N, Blancato V, Repizo G, Magni C, López P (2008) Citrate metabolism and aroma compound production in lactic acid bacteria. In: Mayo B, López P, Pérez-Martín G (eds) Molecular aspects of lactic acid Bacteria for traditional and new applications. Kerala, Research Signpost, pp 64–88
George Kerry R, Patra JK, Gouda S, Park Y, Shin H-S, Das G (2018) Benefaction of probiotics for human health: a review. J Food Drug Anal 26:927–939. https://doi.org/10.1016/j.jfda.2018.01.002
Ghanbari M, Jami M, Domig KJ, Kneifel W (2013) Seafood biopreservation by lactic acid bacteria - a review. LWT Food Sci Technol 54(2):315–324. https://doi.org/10.1016/j.lwt.2013.05.039
Ghiaci P, Lameiras F, Norbeck J, Larsson C (2014) Production of 2-butanol through meso -2,3-butanediol consumption in lactic acid bacteria. FEMS Microbiol Lett 360(1):70–75. https://doi.org/10.1111/1574-6968.12590
Gill HS, Rutherfurd KJ, Cross ML (2001) Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J Clin Immunol 21(4):264–271
Gobbetti M, Stepaniak L, De Angelis M, Corsetti A, Di Cagno R, Fitzgerald D (2002) Latent bioactive peptides in milk proteins: proteolytic activation and significance in dairy processing. Crit Rev Food Sci Nutr 42(3):223–239. https://doi.org/10.1080/10408690290825538
Guarner F (2007) Papel de la flora intestinal en la salud y en la enfermedad. Nutr Hosp 22:14–19
Haller D, Colbus H, Ganzle M, Scherenbacher P, Bode C, Hammes WP (2001) Metabolic and functional properties of lactic acid bacteria in the gastro-intestinal ecosystem: a comparative in vitro study. Syst Appl Microbiol 226(24):218–226. https://doi.org/10.1128/AEM.02063-08
Hardy H, Harris J, Lyon E, Beal J, Foey AD (2013) Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutrients 5:1869. https://doi.org/10.3390/nu5061869
Hemme D, Foucaud-Scheunemann C (2004) Leuconostoc, characteristics, use in dairy technology and prospects in functional foods. Int Dairy J 14(6):467–494. https://doi.org/10.1016/J.IDAIRYJ.2003.10.005
Hofvendahl K, Hahn-Hägerdal B (2000) Factors affecting the fermentative lactic acid production from renewable resources1. Enzym Microb Technol 26(2–4):87–107. https://doi.org/10.1016/S0141-0229(99)00155-6
Holzapfel WH (2014) Lactic acid bacteria: biodiversity and taxonomy. Wiley, Chichester
Holzapfel WH, Schillinger U, Gelsen R, Lücke F (2003) Starter and protective cultures. In: Russell NJ, Gould GW (eds) Food preservatives. Springer, Boston, MA, p 291
Hor YY, Liong MT (2014) Use of extracellular extracts of lactic acid bacteria and bifidobacteria for the inhibition of dermatological pathogen Staphylococcus aureus. Dermatol Sin 32(3):141–147. https://doi.org/10.1016/j.dsi.2014.03.001
Hugenholtz J (1993) Citrate metabolism in lactic acid bacteria. FEMS Microbiol Rev 12(1–3):165–178. https://doi.org/10.1016/0168-6445(93)90062-E
Hwanhlem N, Buradaleng S, Wattanachant S, Benjakul S, Tani A, Maneerat S (2011) Isolation and screening of lactic acid bacteria from Thai traditional fermented fish (Plasom) and production of Plasom from selected strains. Food Control 22(3–4):401–407. https://doi.org/10.1016/j.foodcont.2010.09.010
Icaza-Chávez ME (2013) Microbiota intestinal en la salud y la enfermedad. Rev Gastroenterol Mex 78(4):240–248. https://doi.org/10.1016/j.rgmx.2013.04.004
Imlay JA (2003) Pathways of oxidative damage. Annu Rev Microbiol 57(1):395–418. https://doi.org/10.1146/annurev.micro.57.030502.090938
Juan B, Zamora A, Quevedo JM, Trujillo AJ (2016) Proteolysis of cheese made from goat milk treated by ultra high pressure homogenisation. LWT - Food Sci Technol 69:17–23. https://doi.org/10.1016/j.lwt.2015.12.013
Juodeikiene G, Bartkiene E, Viskelis P, Urbonaviciene D, Eidukonyte D, Bobinas C (2002) Fermentation processes using lactic acid bacteria producing bacteriocins for preservation and improving functional properties of food products. In: Advances in applied biotechnology. IntechOpen, London
Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H (1992) Enhancement of the circulating antibody secreting cell response in human diarrhea by a human lactobacillus strain. Pediatr Res 32(2):141
Ko J, Nam S, Park J, Wee Y, Kim D, Lee WS et al (2016) Synthesis and characterization of glucosyl stevioside using Leuconostoc dextransucrase. Food Chem 211:577. https://doi.org/10.1016/j.foodchem.2016.05.046
Koninkx JFJG, Tooten PCJ, Malago JJ (2010) Probiotic bacteria induced improvement of the mucosal integrity of enterocyte-like Caco-2 cells after exposure to Salmonella enteritidis 857. J Funct Foods 2(3):225–234. https://doi.org/10.1016/j.jff.2010.06.001
Kumar AS, Mody K, Jha B (2007) Bacterial exopolysaccharides—a perception. J Basic Microbiol 47:103–117. https://doi.org/10.1002/jobm.200610203
Laëtitia G, Pascal D, Yann D (2014) The citrate metabolism in homo- and heterofermentative LAB: a selective means of becoming dominant over other microorganisms in complex ecosystems. Food Nutr Sci 05(10):953–969. https://doi.org/10.4236/fns.2014.510106
Lee K, Lee H-G, Pi K, Choi Y-J (2008) The effect of low pH on protein expression by the probiotic bacterium Lactobacillus reuteri. Proteomics 8(8):1624–1630. https://doi.org/10.1002/pmic.200700663
Leistner L, Gorris LGM (1995) Food preservation by hurdle technology. Trends Food Sci Technol 6(2):41–46. https://doi.org/10.1016/S0924-2244(00)88941-4
Li X, Chen Y, Zhao S, Chen H, Zheng X, Luo J, Liu Y (2015) Efficient production of optically pure l-lactic acid from food waste at ambient temperature by regulating key enzyme activity. Water Res 70:148–157. https://doi.org/10.1016/j.watres.2014.11.049
Lin C-H, Lin C-C, Shibu MA, Liu C-S, Kuo C-H, Tsai F-J et al (2014) Oral Lactobacillus reuteri GMN-32 treatment reduces blood glucose concentrations and promotes cardiac function in rats with streptozotocin-induced diabetes mellitus. Br J Nutr 111(04):598–605. https://doi.org/10.1017/S0007114513002791
Link-Anster H, Rochat F, Saudan KY, Mignot O, Aeschlimann JM (1994) Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol Med Microbiol 10:55–63
Lortal S, Chapot-Chartier M (2005) Role, mechanisms and control of lactic acid bacteria lysis in cheese. Int Dairy J 15:857–871. https://doi.org/10.1016/j.idairyj.2004.08.024
Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA (1999) Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol Gastrointest Liver Physiol 276(4):G941–G950. https://doi.org/10.1152/ajpgi.1999.276.4.G941
Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, Koonin E et al (2006) Comparative genomics of the lactic acid bacteria. Proc Natl Acad Sci 103:15611
Mani-López E, García HS, López-Malo A (2012) Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res Int 45(2):713–721. https://doi.org/10.1016/j.foodres.2011.04.043
Manzano C, Estupiñán D, Poveda E (2012) Clinical efects of probiotics: what does the evidence says. Rev Chil Nutr 39:98–110
Martin N, Friedlander A, Mok A, Kenet D, Martin W, Boor K (2014) Peroxide test strips detect added hydrogen peroxide in raw milk at levels affecting bacterial load. J Food Prot 76(2):333–337. https://doi.org/10.4315/0362-028X
Martinez FA, Balciunas EM, Salgado JM, González JM, Converti A, de Souza Oliveira RP (2013) Lactic acid properties, applications and production: a review. Trends Food Sci Technol 30:70–83. https://doi.org/10.1016/j.tifs.2012.11.007
Martino G, Quintana IM, Espariz M, Blancato VS, Magni C (2016) Aroma compounds generation in citrate metabolism of Enterococcus faecium: genetic characterization of type I citrate gene cluster. Int J Food Microbiol 218:27–37. https://doi.org/10.1016/j.ijfoodmicro.2015.11.004
Mazzoli R, Bosco F, Mizrahi I, Bayer EA, Pessione E (2014) Towards lactic acid bacteria-based biorefineries. Biotechnol Adv 32(7):1216–1236. https://doi.org/10.1016/j.biotechadv.2014.07.005
Messaoudi S, Manai M, Kergourlay G, Prévost H, Connil N, Chobert J, Dousset X (2013) Lactobacillus salivarius: bacteriocin and probiotic activity. Food Microbiol 36:296. https://doi.org/10.1016/j.fm.2013.05.010
Minervini F, De Angelis M, Di Cagno R, Pinto D, Siragusa S, Rizzello CG, Gobbetti M (2010) Robustness of Lactobacillus plantarum starters during daily propagation of wheat flour sourdough type I. Food Microbiol 27(7):897–908. https://doi.org/10.1016/j.fm.2010.05.021
Morales P, Brignardello J (2010) La microbiota intestinal: un nuevo actor en el desarrollo de la obesidad. Rev Med Chil 138:1020–1027
Mozzi F (2016) Lactic acid bacteria. In: Encyclopedia of food and health. Academic Press, Amsterdam, pp 501–508. https://doi.org/10.1016/B978-0-12-384947-2.00414-1
Naidu AS (2000) Natural food antimicrobial systems. CRC Press, London
Notararigo S, Nácher-Vázquez M, Ibarburu I, Werning M, De Palencia PF, Dueñas MT et al (2013) Comparative analysis of production and purification of homo- and hetero-polysaccharides produced by lactic acid bacteria. Carbohydr Polym 93(1):57–64. https://doi.org/10.1016/j.carbpol.2012.05.016
Notararigo S, de las Casas-Engel M, de Palencia PF, Corbí AL, López P (2014) Immunomodulation of human macrophages and myeloid cells by 2-substituted (1–3)-β-d-glucan from P. parvulus 2.6. Carbohydr Polym 112:109–113. https://doi.org/10.1016/j.carbpol.2014.05.073
Nwodo UU, Green E, Okoh AI (2012) Bacterial exopolysaccharides: functionality and prospects. Int J Mol Sci 13(11): 14002–14015. https://doi.org/10.3390/ijms131114002
O’Toole PW, Cooney JC (2008) Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip Perspect Infect Dis 2008:1. https://doi.org/10.1155/2008/175285
Oliveira RBP, Oliveira ADL, Glória MBA (2008) Screening of lactic acid bacteria from vacuum packaged beef for antimicrobial activity. Braz J Microbiol 39(2):368–374. https://doi.org/10.1590/S1517-83822008000200031
Özcelik S, Kuley E, Özogul F (2016) Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. LWT Food Sci Technol 73:536–542. https://doi.org/10.1016/j.lwt.2016.06.066
Panda SK, Mishra SS, Kayitesi E, Ray RC (2016) Microbial-processing of fruit and vegetable wastes for production of vital enzymes and organic acids: biotechnology and scopes. Environ Res 146:161–172. https://doi.org/10.1016/j.envres.2015.12.035
Parra R (2012) Yogur en la salud humana. Rev Lasallista Invest 9(2):162–177
Pérez-Ramos A, Nácher-Vázquez M, Notararigo S, López P, Mohedano ML (2015) Current and future applications of bacterial extracellular polysaccharides. In: Watson RR, Preedy VR (eds) Probiotics, prebiotics, and synbiotics. Elsevier, Oxford, pp 329–344
Pescuma M, Hébert EM, Dalgalarrondo M, Haertlé T, Mozzi F, Chobert JM, De Valdez GF (2009) Effect of exopolysaccharides on the hydrolysis of ß-lactoglobulin by lactobacillus acidophilus CRL 636 in an in vitro gastric/pancreatic system. J Agric Food Chem 57(12):5571–5577. https://doi.org/10.1021/jf9006505
Pflügl S, Marx H, Mattanovich D, Sauer M (2014) Heading for an economic industrial upgrading of crude glycerol from biodiesel production to 1, 3-propanediol by Lactobacillus diolivorans. Bioresour Technol 152:499–504. https://doi.org/10.1016/j.biortech.2013.11.041
Pisoschi AM, Negulescu GP (2011) Methods for total antioxidant activity determination: a review. Biochem Anal Biochem 1(1):1–10. https://doi.org/10.4172/2161-1009.1000106
Pleissner D, Neu A-K, Mehlmann K, Schneider R, Puerta-Quintero GI, Venus J (2016) Fermentative lactic acid production from coffee pulp hydrolysate using Bacillus coagulans at laboratory and pilot scales. Bioresour Technol 218:167–173. https://doi.org/10.1016/j.biortech.2016.06.078
Porto MCW, Kuniyoshi TM, Azevedo POS, Vitolo M, Oliveira RPS (2017) Pediococcus spp.: an important genus of lactic acid bacteria and pediocin producers. Biotechnol Adv 35(3):361–374. https://doi.org/10.1016/j.biotechadv.2017.03.004
Pothakos V, Devlieghere F, Villani F, Björkroth J, Ercolini D (2015) Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci 109:66–74. https://doi.org/10.1016/j.meatsci.2015.04.014
Quigley EMM (2012) Prebiotics and probiotics. Nutr Clin Pract 27(2):195–200. https://doi.org/10.1177/0884533611423926
Ramírez JC, Ulloa P, Velázquez M, Ulloa J, Arce F (2011) Bacterias lácticas: importancia en alimentos y sus efectos en la salud. Rev Fuente 2(7):16. Retrieved from http://www.hablemosclaro.org/repositorio/biblioteca/b_305_bacterias_lacticas_importancia_en_alimentos.pdf
Randazzo C, Restuccia C, Mancini A, Muccilli S, Gatti M, Caggia C (2016) Ragusana donkey milk as a source of lactic acid bacteria and yeast strains of dairy technological interest. Int J Dairy Sci Process 3:38–46
Rastogi A, Shukla S (2013) Amaranth: a new millennium crop of nutraceutical values. Crit Rev Food Sci Nutr 53(2):109–125. https://doi.org/10.1080/10408398.2010.517876
Ray B (2004) Fundamental food microbiology. CRC Press, New York. https://doi.org/10.1007/s13398-014-0173-7.2
Reis JA, Paula AT, Casarotti SN, Penna ALB (2012) Lactic acid bacteria antimicrobial compounds: characteristics and applications. Food Eng Rev 4(2):124–140. https://doi.org/10.1007/s12393-012-9051-2
Rolfe RD (2000) The role of probiotic cultures in the control of gastrointestinal health. J Nutr 130(2):396S–402S. https://doi.org/10.1093/jn/130.2.396S
Rook GAW, Brunet LR (2005) Microbes, immunoregulation, and the gut. Gut 54(3):317–320. https://doi.org/10.1136/gut.2004.053785
Ross R, Morgan S, Hill C (2002) Preservation and fermentation: past, present and future. Int J Food Microbiol 79(1–2):3–16. https://doi.org/10.1016/S0168-1605(02)00174-5
Ruiz MJ, Colello R, Padola NL, Etcheverría AI (2017) Efecto inhibitorio de Lactobacillus spp. sobre bacterias implicadas en enfermedades transmitidas por alimentos. Rev Argent Microbiol 49:174. https://doi.org/10.1016/j.ram.2016.10.005
Rutala W, Weber D (2008) Disinfection and sterilization in health care facilities: what clinicians need to know. Clin Infect Dis 39(5):702–709. https://doi.org/10.1086/423182
Sáez C (2015) Desequilibrios en la microbiota intestinal, posible origen de algunos cánceres. La vanguardia
Salvetti E, Fondi M, Fani R, Torriani S, Felis GE (2013) Evolution of lactic acid bacteria in the order Lactobacillales as depicted by analysis of glycolysis and pentose phosphate pathways. Syst Appl Microbiol 36:291. https://doi.org/10.1016/j.syapm.2013.03.009
Savadogo A, Ouattara C, Bassole I, Traore S (2006) Bacteriocins and lactic acid bacterias - a mini review. J Biotechnol 5(9):678–683
Shibamoto T (2014) Diacetyl: occurrence, analysis, and toxicity. J Agric Food Chem 62(18):4048–4053. https://doi.org/10.1021/jf500615u
Smid EJ, Kleerebezem M (2014) Production of aroma compounds in lactic fermentations. Annu Rev Food Sci Technol 5(1):313. https://doi.org/10.1146/annurev-food-030713-092339
Smith DR, Smyth AP, Strauss WM, Moir DT (1993) Incorporation of copy-number control elements into yeast artificial chromosomes by targeted homologous recombination. Mamm Genome 4:141–147
Soa SSY, Wana MLY, El-Nezami H (2017) Probiotics-mediated suppression of cancer. Curr Opin Oncol 29(1):62–72. https://doi.org/10.1097/CCO.0000000000000342
Sobrino-López A, Martín-Belloso O (2008) Use of nisin and other bacteriocins for preservation of dairy products. Int Dairy J 18(4):329–343. https://doi.org/10.1016/j.idairyj.2007.11.009
Starek-Swiechowicz B, Starek A (2014) Diacetyl exposure as a pneumotoxic factor: a review. Rocz Panstw Zakl Hig 65(2):87–92
Sun Z, Harris HMB, McCann A, Guo C, Argimón S, Zhang W et al (2015) Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat Commun 6(1):8322. https://doi.org/10.1038/ncomms9322
Supharoek S, Ponhong K, Siriangkhawut W, Grudpan K (2017) Employing natural reagents from turmeric and lime for acetic acid determination in vinegar sample. J Food Drug Anal 26:583–590. https://doi.org/10.1016/j.jfda.2017.06.007
Tamang J, Thapa N, Tamang B, Rai A, Chettri R (2015) Microorganisms in fermented foods and beverages. In: Health benefits of fermented foods and beverages. CRC Press, Boca Raton, pp 1–110. https://doi.org/10.1201/b18279-2
Tyler CA, Kopit L, Doyle C, Yu AO, Hugenholtz J, Marco ML (2016) Polyol production during heterofermentative growth of the plant isolate Lactobacillus florum 2F. J Appl Microbiol 120(5):1336. https://doi.org/10.1111/jam.13108
Vafaeie F (2016) Critical review on probiotics and its effect on cancer. Cancer Press 2(2):30–34
Vandenplas Y, Benninga M (2009) Probiotics and functional gastrointestinal disorders in children. J Pediatr Gastroenterol Nutr 48(Suppl 2):S107–S109. https://doi.org/10.1097/MPG.0b013e3181a1603a
Venugopalan V, Dinesh MS, Geetha KS (2010) Enhancement of antimicrobial potential of Phyllanthus niruri by fermentation. J Herb Med Toxicol 4:167–175
Viana de Souza J, Silva Dias F (2017) Protective, technological, and functional properties of select autochthonous lactic acid bacteria from goat dairy products. Curr Opin Food Sci 13:1–9. https://doi.org/10.1016/j.cofs.2017.01.003
Vijayakumar J, Aravindan R, Viruthagiri T (2008) Recent trends in the production, purification and application of lactic acid. Chem Biochem Eng Q 2(2):245–264
Wilson KH, Perini F (1988) Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect Immun 56(10):2610–2614
Wollowski I, Rechkemmer G, Pool-zobel BL (2018) Protective role of probiotics and prebiotics in colon cancer. Am J Clin Nutr 73:451s
Yadav H, Jain S, Sinha PR (2008) Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J Dairy Res 75(2):189–195. https://doi.org/10.1017/S0022029908003129
Yang S, Lin C, Sung CT, Fang J (2014) Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol 5:1–10. https://doi.org/10.3389/fmicb.2014.00241
Zacharof MP, Lovitt RW (2012) Bacteriocins produced by lactic acid bacteria a review article. APCBEE Proc 2:50–56. https://doi.org/10.1016/J.APCBEE.2012.06.010
Zacharof MP, Lovitt W (2014) Bacteriocins produced by lactic acid bacteria - a review. Acta Period Technol 45:271–283. https://doi.org/10.2298/APT1445271V
Zannini E, Waters DM, Coffey A, Arendt EK (2016) Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl Microbiol Biotechnol 100:1121–1135. https://doi.org/10.1007/s00253-015-7172-2
Zarour K, Vieco N, Pérez-ramos A (2017) Lactic acid bacteria. In: Microbial production of food ingredients and additives. Elsevier, Saint Louis. https://doi.org/10.1016/B978-0-12-811520-6/00004-0
Zúñiga M, Gómez-escoín CL, González-candelas F (2011) Evolutionary history of the OmpR/IIIA family of signal transduction two component systems in Lactobacillaceae and Leuconostocaceae. BMC Evol Biol 11:34. https://doi.org/10.1186/1471-2148-11-34
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Cruz Casas, D.E. et al. (2021). Probiotics as Functional Foods. In: Goel, G., Kumar, A. (eds) Advances in Probiotics for Sustainable Food and Medicine. Microorganisms for Sustainability, vol 21. Springer, Singapore. https://doi.org/10.1007/978-981-15-6795-7_6
Download citation
DOI: https://doi.org/10.1007/978-981-15-6795-7_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6794-0
Online ISBN: 978-981-15-6795-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)